Abstract
Aim
Cardiovascular mortality risk evolves over the lifespan of kidney failure (KF), as patients develop comorbid disease and transition between treatment modalities. Absolute cardiovascular death rates would help inform clinical practice and health‐care provision, but are not well understood across a continuum of dialysis and transplant states. We aimed to characterize cardiovascular death across the natural history of KF using a lifespan approach.
Methods
We performed a population‐based cohort study of incident patients commencing kidney replacement therapy in Australia and New Zealand. Cardiovascular deaths were identified using data linkage to national death registers. We estimated the probability of death and kidney transplant using multi‐state models, and calculated rates of graft failure and cardiovascular death across demographic factors and comorbidities.
Results
Among 60 823 incident patients followed over 381 874 person‐years, 25% (8492) of deaths were from cardiovascular disease. At 15 years from treatment initiation, patients had a 15.2% probability of cardiovascular death without being transplanted, but only 2.3% probability of cardiovascular death post‐transplant. Females had a 3% lower probability of cardiovascular death at 15 years (15.3% vs. 18.6%) but 4% higher probability of non‐cardiovascular death (54.5% vs. 50.8%). Within the first year of dialysis, cardiovascular mortality peaked in the second month and showed little improvement across treatment era.
Conclusion
Despite improvements over time, cardiovascular death remains common in KF, particularly among the dialysis population and in the first few months of treatment. Multi‐state models can provide absolute measures of cardiovascular mortality across both dialysis and transplant states.
Keywords: cardiovascular disease, chronic, dialysis, kidney failure, mortality
SUMMARY AT A GLANCE
In this population‐based cohort study using multi‐state models (alive without kidney transplant [KT], CV death without KT, non‐CV death without KT, alive after first KT, CV death after first KT and non‐CV death after first KT), the probability of CV death was higher in non‐KT than KT patients at 15 years from treatment. In patients on dialysis, CV mortality was highest from the second month after commencing dialysis and remained high thereafter. Thus, the use of multi‐state models provides helpful information on impacts of different treatments with respect to serious outcomes.
1. INTRODUCTION
Cardiovascular disease is a leading cause of mortality in patients with kidney failure (KF). In 2018, cardiovascular disease was the most common cause of death in the Australian and New Zealand population receiving kidney replacement therapy (KRT), representing 31% of all deaths. 1 The United States Renal Data System reported over half of all deaths in patients with KF in 2015 were due to cardiovascular causes. 2 Although cardiovascular mortality risk is reduced following kidney transplantation, it remains elevated compared to the general population and is a major contributor to graft loss. 1 , 2 , 3 , 4
Progression of KF and changes in comorbidity burden may result in varying cardiovascular mortality risk over a patient's lifespan. Pathological changes that occur in association with declining kidney function, such as left ventricular hypertrophy and vascular calcification, may accelerate on dialysis and decrease the effectiveness of conventional therapies. 5 , 6 , 7 For instance, there is no evidence that statins have significant mortality benefit in patients with KF, 8 while warfarin and aspirin may increase the risk of major bleeding in patients with chronic kidney disease. 9 , 10 , 11 Moreover, the lived experience of KF patients often involves transitions between dialysis and kidney transplant, and it is unclear how cardiovascular mortality varies across these modality changes. Although kidney transplantation slows the progression of cardiovascular disease, patients with higher‐risk cardiovascular comorbidities may be excluded from transplantation. 12 , 13 , 14 , 15 Guidelines for cardiac screening and transplant eligibility vary internationally, and there is a lack of high‐quality evidence for specific screening practices. 12 , 13 , 14 , 15
It is important for clinicians, patients and researchers to understand how cardiovascular prognosis evolves over the lifespan of a person with KF and varies with KF treatment. Trials of new treatments require estimates of event rates and health states, while insights into lifetime risk can inform shared decision making, research and health service planning. Our study objective was to describe the epidemiology of cardiovascular death (specifically cardiac and peripheral vascular deaths excluding stroke) across the lifespan of KF. In particular, we aimed to understand the effects of multimorbidity and KRT modality on cardiovascular mortality rates. We further aimed to characterize changes in cardiovascular death across treatment era.
2. MATERIALS AND METHODS
2.1. Study population
We performed a population‐based cohort study on incident patients with KF receiving KRT from the Australian and New Zealand Dialysis and Transplant registry (ANZDATA). ANZDATA prospectively collects data on people receiving dialysis and kidney transplants in Australia and New Zealand centres. Data collection and validation methods have been described in detail elsewhere. 16 Briefly, information collected includes patient demographics (date of birth; sex; ethnicity; body mass index; and smoking status), comorbidities (diabetes; cerebrovascular disease; coronary artery disease; peripheral artery disease; and malignancies) and KRT history (KRT modality; date of treatment initiation and modality switches; and cause of KF). Body mass index and smoking status were not routinely collected before 1990.
2.2. Death outcome and data linkage
We determined the date and cause of death of patients receiving KRT using data linkage with respective national death registers in Australia and New Zealand. We linked Australian patients with the National Death Index, which is maintained by the Australian Institute of Health and Welfare (AIHW) and has collected data on all deaths since 1980. Linkage was performed using probabilistic methods, matching on identifiers including full name, sex, date of birth and date of death. For New Zealand, we linked patients to the Mortality Collection database, which is maintained by the New Zealand Ministry of Health and records information on all deaths from 1988. New Zealand patients were linked using deterministic record linkage based on National Health Index number. There is data lag, and when linkage was performed, the most recent data available in death registries was until 2013 in Australia and 2012 in New Zealand. Therefore, our analysis included incident patients commencing KRT in 1980–2013 for Australia and 1988–2012 for New Zealand.
Data linkage was performed by the AIHW and New Zealand Ministry of Health using best‐practice privacy‐preserving protocols. All linked data was de‐identified before being made available to researchers. Ethical oversight was granted for this study from the University of Sydney (Project No.: 2014/917), AIHW (Reference No.: EO2015/3/181) and the New Zealand Ministry of Health (Reference No.: 14/NTB/171).
2.3. Cause of death
Australian and New Zealand national death registers provide ICD‐10‐AM diagnosis codes for each individual's underlying cause of death, based on the main disease or injury which led to death, and contributing causes of death, based on other diseases or injuries that contributed to the death. ICD‐10 is an internationally agreed global health information standard for mortality and morbidity statistics, used by over 100 countries to report and compare health outcomes. 17 Cardiovascular deaths were identified from each individual's underlying cause of death, as obtained via data linkage with national death registers. They were defined by the following ICD‐10‐AM codes: hypertensive heart disease (I11), coronary heart disease (I20‐I25), valve disease (I34‐I37), cardiomyopathy (I42‐I43), arrhythmias (I44‐I49), heart failure (I50), peripheral vascular disease (I70‐I74, I77‐I79) and stroke deaths (I60‐64, G45).
2.4. Statistical analyses
The patient follow‐up period was from date of first KRT to the earliest of either the date of death, 31 December 2013 (Australian patients) or 31 December 2012 (New Zealand patients). Patient demographics were summarized using absolute counts and percentages. We estimated cardiovascular mortality rates from KRT initiation, stratified by patient factors including age, sex, calendar year and pre‐existing comorbidities (cerebrovascular disease, coronary artery disease, peripheral artery disease). Graft failure rates were calculated post‐transplant, stratified by age, sex and pre‐existing cardiovascular comorbidities. To provide estimates on patient survival with a functioning graft, we also determined rates of composite graft failure and death by censoring patients at the earliest of either event.
Multi‐state models were used to estimate the probability of patients being in health states over time since KRT. Our multi‐state model included six possible states: alive without transplant, cardiovascular death without transplant, non‐cardiovascular death without transplant, alive after first transplant, cardiovascular death after first transplant and non‐cardiovascular death after first transplant. Probability of transitions was determined using Aalen‐Johansen estimates, while SEs were computed using an infinitesimal jack‐knife. 18 Probabilities of cardiovascular and non‐cardiovascular death were also estimated in the study population and in those with a kidney transplant, stratified by sex.
We used cause‐specific hazard models to evaluate risk factors associated with cardiovascular and non‐cardiovascular death, and cardiovascular sub‐causes including cardiac, peripheral vascular and valvular deaths. Covariates were selected a priori based on data available in ANZDATA, and included: age/year at KRT initiation, sex, country, BMI category (underweight, ≤18.4 kg/m2; Normal, 18.5–24.9 kg/m2; overweight, 25.0–29.9 kg/m2; and obese, ≥30.0 kg/m2), ethnicity, comorbidities, smoking history and cause of KF. Missing values for BMI and smoking history in patients diagnosed ≤1990 were imputed using chained equations with five iterations. Covariates used for the imputation included death outcome, log‐time of follow up, and all variables in the cause‐specific hazards model.
Data were analysed using Stata version 15 (Stata Corporation, College Station, TX, United States).
3. RESULTS
3.1. Patient characteristics
The study included 60 823 incident patients receiving KRT in Australia (1980–2013) and New Zealand (1988–2012) (Figure S1). Median follow‐up time was 4.0 years (IQR:1.7–8.4), with 381 874 person‐years of observation. By the end of the study period, 26 505 (44%) patients were still alive and 34 318 (56%) patients had died (Table 1). During follow up, 43 190 (71%) patients were treated with dialysis alone and 17 633 (29%) received at least one kidney transplant, of which 1649 were treated pre‐emptively. The majority of patients (69%) were aged ≥50 at KRT initiation (median age 59 years, IQR: 46–69). Most patients were male (58%), of European ethnicity (76%), and began KRT after 1995 (74%). Diabetes was the most common cause of KF (28%), followed by glomerulonephritis/IgA nephropathy (27%), other causes (26%), hypertension/renal artery disease (12%) and polycystic kidney disease (7%).
TABLE 1.
Characteristics of patients receiving kidney replacement therapy in Australia and New Zealand
| Characteristics | Cardiac/vascular deaths | Non‐cardiac/vascular deaths | Alive | Total | ||||
|---|---|---|---|---|---|---|---|---|
| n | (%) a | n | (%) a | n | (%) a | n | (%) a | |
| Total (%) b | 8492 | (14) | 25 826 | (42) | 26 505 | (44) | 60 823 | (100) |
| Age at KRT (years) | ||||||||
| ≤29 | 151 | (2) | 1024 | (4) | 3913 | (15) | 5088 | (8) |
| 30–49 | 1212 | (14) | 4364 | (17) | 8231 | (31) | 13 807 | (23) |
| 50–64 | 3045 | (36) | 8701 | (34) | 8234 | (31) | 19 980 | (33) |
| 65–74 | 2562 | (30) | 7357 | (28) | 3923 | (15) | 13 842 | (23) |
| ≥75 | 1522 | (18) | 4380 | (17) | 2204 | (8) | 8106 | (13) |
| Median [IQR] | 64 | [55, 72] | 63 | [52, 71] | 51 | [38, 63] | 59 | [46, 69] |
| Sex | ||||||||
| Female | 3125 | (37) | 11 248 | (44) | 10 669 | (40) | 25 042 | (41) |
| Male | 5367 | (63) | 14 578 | (56) | 15 836 | (60) | 35 781 | (59) |
| Year of KRT | ||||||||
| ≤1995 | 3220 | (38) | 8674 | (34) | 3488 | (13) | 15 382 | (25) |
| 1996–2005 | 3627 | (43) | 11 440 | (44) | 7048 | (27) | 22 115 | (36) |
| 2006–2013 | 1645 | (19) | 5712 | (22) | 15 969 | (60) | 23 326 | (38) |
| Country | ||||||||
| Australia | 7242 | (85) | 21 750 | (84) | 22 459 | (85) | 51 451 | (85) |
| New Zealand | 1250 | (15) | 4076 | (16) | 4046 | (15) | 9372 | (15) |
| Ethnicity c | ||||||||
| European | 6898 | (81) | 20 125 | (78) | 19 002 | (72) | 46 025 | (76) |
| East Asian | 388 | (5) | 1355 | (5) | 2612 | (10) | 4357 | (7) |
| Aboriginal Australian | 481 | (6) | 1862 | (7) | 1904 | (7) | 4247 | (7) |
| Maori | 467 | (6) | 1619 | (6) | 1154 | (4) | 3240 | (5) |
| Pacific Island peoples | 211 | (2) | 728 | (3) | 1320 | (5) | 2259 | (4) |
| African and Middle Eastern | 41 | (0.5) | 117 | (0.5) | 334 | (1) | 492 | (1) |
| Peoples of the Americas | 0 | (<0.1) | 4 | (<0.1) | 15 | (<0.1) | 19 | (<0.1) |
| Other | 6 | (<0.1) | 16 | (<0.1) | 165 | (1) | 187 | (0.3) |
| Body mass index (mg/kg2) | ||||||||
| Underweight (<18.5) | 254 | (3) | 1052 | (4) | 1396 | (5) | 2702 | (4) |
| Normal (18.5–24.9) | 2632 | (31) | 8383 | (32) | 8851 | (33) | 19 866 | (33) |
| Overweight (25.0–29.9) | 2227 | (26) | 6652 | (26) | 7762 | (29) | 16 641 | (27) |
| Obese (≥30) | 1608 | (19) | 5085 | (20) | 7079 | (27) | 13 772 | (23) |
| Not collected | 1771 | (21) | 4654 | (18) | 1417 | (5) | 7842 | (13) |
| Comorbidities at KRT | ||||||||
| Cerebrovascular disease | 1561 | (18) | 4078 | (16) | 2037 | (8) | 7676 | (13) |
| Diabetes | 2855 | (34) | 10 321 | (40) | 8541 | (32) | 21 717 | (36) |
| Coronary artery disease | 4364 | (53) | 10 318 | (40) | 6103 | (23) | 20 785 | (34) |
| Peripheral artery disease | 2628 | (31) | 7111 | (28) | 3634 | (14) | 13 373 | (22) |
| Previous malignancy | 1819 | (21) | 8096 | (31) | 5873 | (22) | 15 788 | (26) |
| Smoking status | ||||||||
| Current/former | 4235 | (50) | 12 259 | (47) | 11 895 | (45) | 28 389 | (47) |
| Never | 2683 | (32) | 9617 | (37) | 13 561 | (51) | 25 861 | (43) |
| Not collected | 1574 | (19) | 3950 | (15) | 1049 | (4) | 6573 | (11) |
| Cause of kidney failure | ||||||||
| Diabetes | 2151 | (25) | 8023 | (31) | 6735 | (25) | 16 909 | (28) |
| Hypertension/renal artery disease | 1853 | (22) | 3079 | (12) | 2332 | (9) | 7264 | (12) |
| Glomerulonephritis/IgA nephropathy | 1923 | (23) | 5914 | (23) | 8795 | (33) | 16 632 | (27) |
| Polycystic kidney disease | 415 | (5) | 1279 | (5) | 2309 | (9) | 4003 | (7) |
| Other d | 2150 | (25) | 7531 | (29) | 6334 | (24) | 16 015 | (26) |
| Incident KRT modality | ||||||||
| Peritoneal dialysis | 2644 | (31) | 8134 | (32) | 7069 | (27) | 17 847 | (29) |
| Haemodialysis | 5825 | (69) | 17 562 | (68) | 17 946 | (68) | 41 333 | (68) |
| Transplant | 24 | (0.3) | 131 | (0.5) | 1494 | (6) | 1649 | (3) |
| Transplanted during study | 1181 | (14) | 3951 | (15) | 10 852 | (41) | 15 984 | (26) |
| Transplanted ever | 1205 | (14) | 4082 | (16) | 12 346 | (47) | 17 633 | (29) |
Abbreviation: KRT, kidney replacement therapy.
Column percentages.
Row percentages.
As defined by the Australian Standard Classification of Cultural and Ethnic Groups, 2019.
Primarily uncertain diagnosis (7%), analgesic nephropathy (5%), reflux nephropathy (5%).
Of 34 318 patients who had died, 8492 deaths (25%) were due to cardiovascular disease while 25 826 (75%) were from other causes. Patients who died from cardiovascular disease were more commonly male (63% vs. 56%) and of European ethnicity (81% vs. 78%). Haemodialysis was the most common KRT modality at time of death (62%), followed by peritoneal dialysis (28%) and transplant (9%).
3.2. Likelihood of cardiovascular deaths since KRT (multi‐state probabilities)
Cardiovascular death occurred in 2.7% (95% CI:2.5–2.8%) of dialysis patients at 1 year from KRT initiation, growing to 10.3% (95% CI:10.1%–10.6%) at 5 years and 15.2% (95% CI:14.9%–15.6%) at 15 years (Figure 1). Cardiovascular mortality after first transplant was less common, occurring in 0.4% (95% CI: 0.3%–0.4%) of all patients receiving KRT at 5 years and slowly increasing to 2.3% (95% CI:2.2%–2.5%) at 15 years. Non‐cardiovascular mortality was more common in both dialysis and transplant patients, occurring in over half of patients by 15 years. Concurrently, the proportion transitioning to kidney transplant increased from 10.6% (95% CI:10.3%–10.8%) at 1 year to 27.7% (95% CI:27.3%–28.1%) at 10 years. The multi‐state probability of being alive without transplant decreased from 78.7% (95% CI:78.4%–79.0%) at 1 year to 10.3% (95% CI:10.0%–10.6%) at 10 years after KRT. After 15 years of KRT, over 80% of living patients had received a transplant.
FIGURE 1.
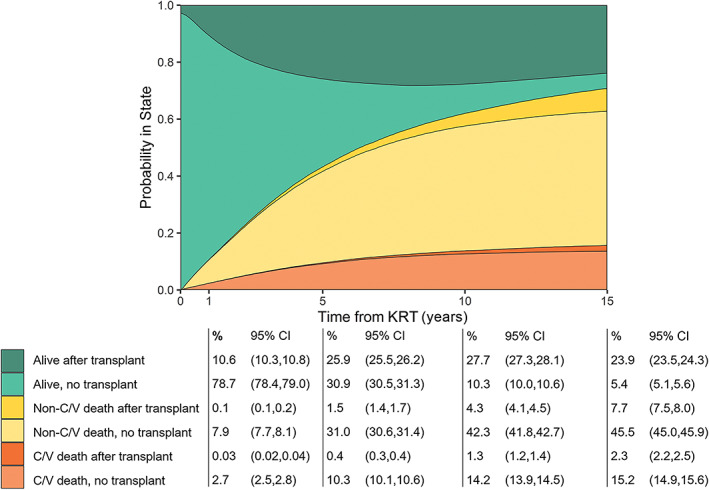
Multi‐state model demonstrating probability of cardiovascular and non‐cardiovascular death with or without transplant, over time from kidney replacement therapy
At 15 years from treatment initiation, females had a 3% lower probability of cardiovascular death at 15 years (15.3% vs. 18.6%), but 4% higher probability of non‐cardiovascular death (54.5% vs. 50.8%) (Figure S2A). Similarly, females had lower rates of cardiovascular death post‐transplant compared to males at 15 years (7.1% vs. 9.1%) but higher rates of non‐cardiovascular death (28.6% vs. 27.7%) (Figure S2B). The proportion of cardiovascular death was lower in transplant recipients compared to general KRT patients across the lifespan of KF. At 5 years from treatment initiation, the percentage of cardiovascular death was four times lower in transplant patients (2.0% vs. 9.3% in females), becoming three times lower at 10 years (4.8% vs. 13.5%) and twice as low at 15 years (7.1% vs. 15.3%).
3.3. Mortality rates
The overall cardiovascular mortality rate in patients receiving dialysis was 3524 (95% CI:3445–3606)/100 000 person‐years (pys). Within the first year of dialysis, mortality rates peaked at 2 months from treatment (4312 (95% CI:3611–5149)/100 000 pys in males; 3821 (95% CI:3052–4783)/100 000 pys in females) (Figure 2A). Rates then remained steady in the first year of dialysis treatment before gradually increasing over the next 5 years, reaching 5090 (95% CI:4572‐5668)/100 000 pys in males and 3936 (95% CI:3434‐4513)/100 000 pys in females at 6 years from dialysis commencement. Transplant recipients had consistently lower cardiovascular mortality rates compared to patients on dialysis, at 688 (95% CI:650‐728)/100 000 pys overall (Figure 2B). Rates were highest in the first month from transplant, at 2591 (95% CI:1722‐3899)/100 000 pys in males and 1397 (95% CI:699‐2793) in females, before declining to 286 (95% CI:196‐417)/100 000 pys in males and 229(95% CI:136‐387)/100 000 pys in females at 2 years from transplant. Rates then increased gradually to 981 (95% CI:668‐1440)/100 000 pys in males and 323 (95% CI:145‐718)/100 000 pys in females at 15 years from transplant.
FIGURE 2.
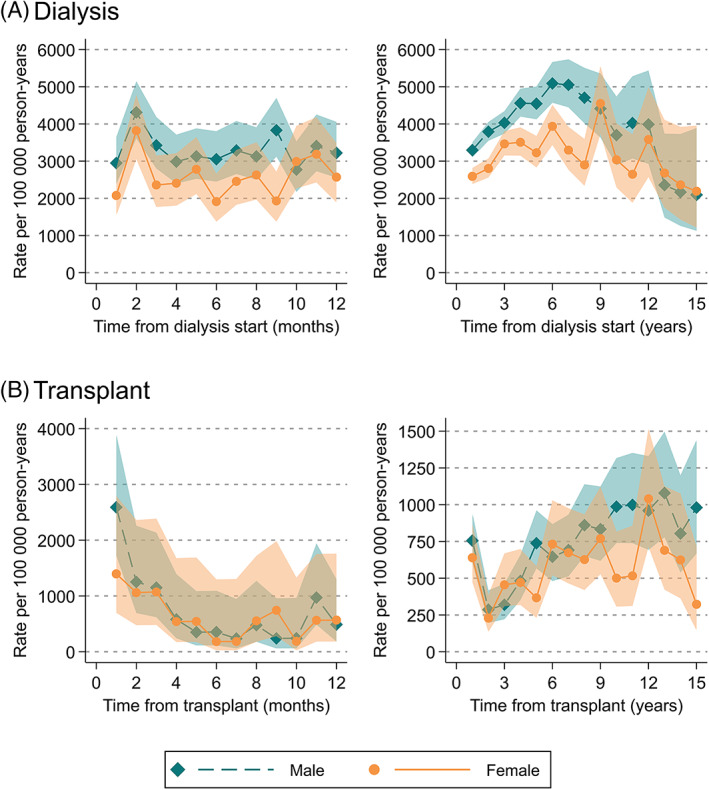
Mortality rates from cardiovascular death stratified by sex, within (A) 12 months and 15 years after dialysis initiation, (B) 12 months and 15 years after kidney transplant
Cardiovascular death rates were lower in patients who initiated dialysis more recently, from 3816 (95% CI:3661‐3978)/100 000 pys in ≤1995 to 2867 (95% CI: 2731‐3010)/100 000 pys in 2006–2013. However, this disparity was only evident after the first 9 months of dialysis (Figure 3A). Cardiovascular death rates improved significantly in transplant patients, with an overall rate of 929 (95% CI:873‐989)/100 000 pys in ≤1995 decreasing to 166 (85% CI:108‐254)/100 000 pys in 2006–2013. These improved mortality rates were evident from the first month of treatment and sustained across follow up (Figure 3B).
FIGURE 3.
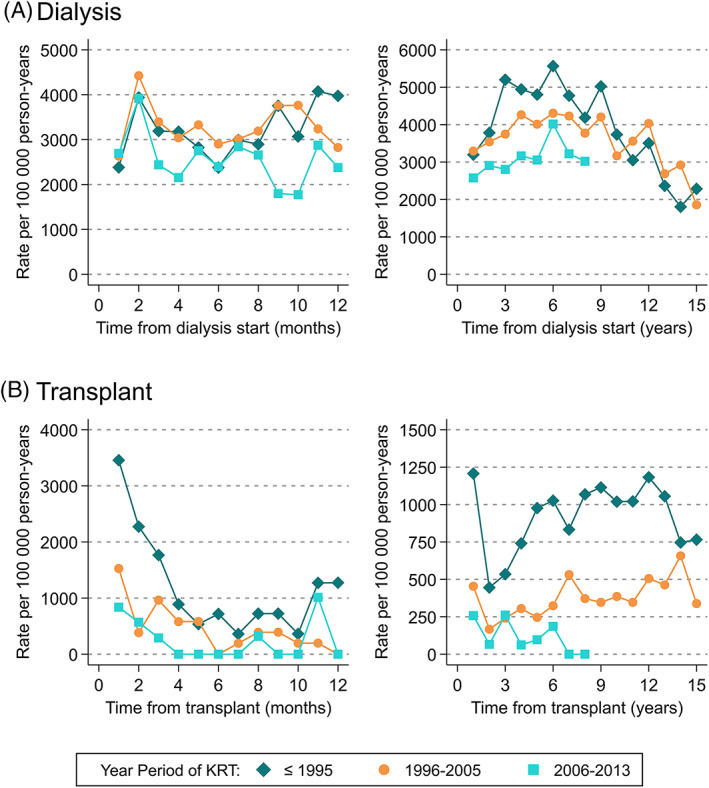
Mortality rates from cardiovascular death stratified by calendar year period of kidney replacement therapy, within (A) 12 months and 15 years after dialysis initiation, (B) 12 months and 15 years after kidney transplant
Cardiovascular death rates among dialysis patients were higher with older age, in males and in the presence of comorbidities including peripheral vascular disease, cerebrovascular disease and coronary artery disease (Figure S3). In transplant recipients, cardiovascular death rates increased with older age only. However, transplant recipients consistently had lower cardiovascular mortality compared to dialysed patients in similar age group, sex and comorbidity status.
3.4. Graft failure
In the first year post‐transplant, those with cardiovascular comorbidities had higher composite rates of graft failure or death, but not for graft failure alone (Figure 4). In females, composite rates of graft failure or death were 13.8 (95% CI:11.0–17.2)/100 pys in those with comorbidities and 9.8 (95% CI:9.0–10.6)/100 pys in those without comorbidities. In males, composite rates of graft failure or death were 12.0 (95% CI:10.3–14.0)/100 pys in those with comorbidities and 8.8 (95% CI:8.2–9.5)/100 pys in those without comorbidities. After 1 year, patients with and without comorbidities had similar rates of composite graft failure and death until ≥6 years post‐transplant, where rates were higher in patients with comorbidities.
FIGURE 4.
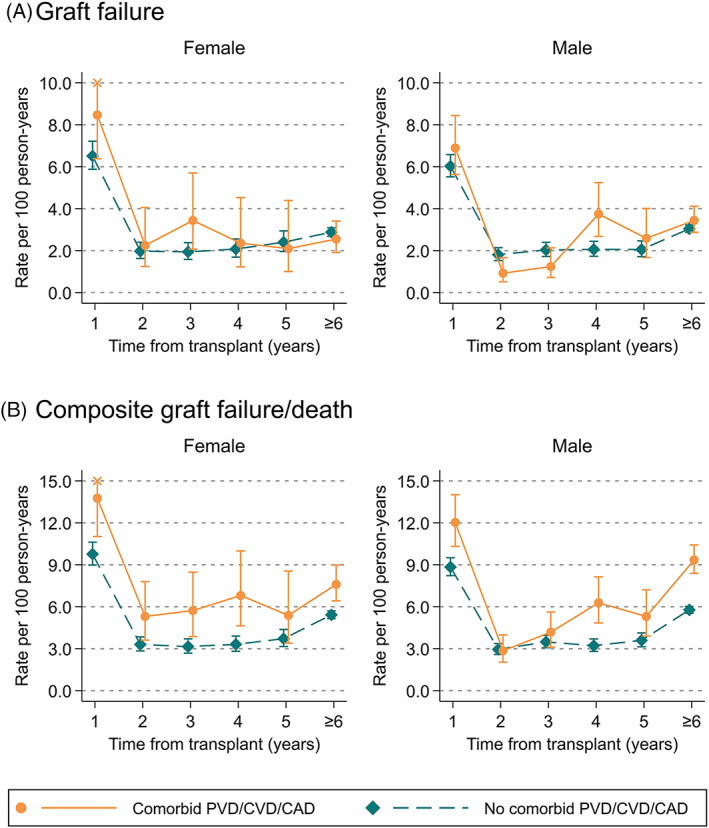
Rates of (A) graft failure, and (B) composite graft failure and death post‐transplant, stratified by sex, time from transplant and pre‐existing cardiovascular disease
3.5. Risk factors for cardiovascular mortality
In the multivariate analysis, there was increased risk of cardiovascular mortality with older age, in males, earlier calendar periods, Aboriginal Australians, obese BMI, cardiovascular comorbidities and current/former smoker (Table S1). Patients who started KRT in 2006–2013 had a 79% lower risk of death compared to those first treated pre‐1995 (HR 0.21, 95% CI:0.20–0.22). Aboriginal Australians had 11% greater risk of death (HR 1.11, 95% CI:1.01–1.23) and East Asians had 17% lower risk of death (HR 0.83, 95% CI:0.75–0.92). Patients with obesity (HR 1.17, 95% CI:1.09–1.26), pre‐existing coronary artery disease (HR 1.72, 95% CI:1.63–1.81), peripheral artery disease (HR 1.06, 95% CI:1.01–1.12) and cerebrovascular disease (HR 1.19, 95% CI:1.12–1.27) had increased cardiovascular mortality. However, previous malignancy appeared ‘protective’ for cardiovascular death (HR 0.53, 95% CI:0.51–0.56), likely due to selection bias and competing risks among cancer patients with KF transitioning to dialysis. Among causes of KF, hypertension/renal artery disease (HR 1.68, 95% CI:1.57–1.80), diabetes (HR 1.13, 95% CI:1.05–1.21) and other causes of KF (HR: 1.08, 95% CI:1.02–1.15) were associated with increased risk of cardiovascular death, while polycystic kidney disease (HR 0.87, 95% CI:0.78–0.96) was associated with lower risk.
Within cardiovascular deaths, 6884 deaths (81%) were due to cardiac causes, including 315 valvular deaths (4%), while 667 (8%) were due to peripheral vascular disease and 941 (11%) were due to stroke (Table S2). Risk factors for subtypes of cardiovascular deaths were similar to the main results. Of note, male sex was associated with increased cardiac death (HR 1.32, 95% CI:1.25–1.39) but was protective for stroke death (HR 0.73, 95% CI:0.64–0.84). Patients with overweight BMI had higher cardiac mortality (HR 1.11, 95% CI:1.03–1.19) but lower peripheral vascular (HR 0.81, 95% CI:0.67–0.97) and stroke mortality (HR 0.83, 95% CI:0.69–0.98). Similarly, diabetes as a cause of KF was a risk factor for cardiac deaths (HR 1.24, 95% CI:1.15–1.34) and stroke deaths (HR 1.51, 95% CI:1.22–1.87) but appeared slightly protective for peripheral vascular deaths (HR 0.70, 95% CI:0.53–0.94), possibly due to competing risks.
4. DISCUSSION
In this binational population‐based study, we characterized cardiovascular deaths among a cohort of 60 823 patients across 381 000 person‐years of follow up among the Australian and New Zealand KRT population. We took a patient‐centred approach, estimating probabilities over a treatment lifespan to account for transitions from dialysis to transplant, which are typical for patients receiving KRT. These indicate that after 15 years, the probability of cardiovascular death after transplant was relatively low at 2.3% compared to 15.2% among those without transplant. Transitions from dialysis to transplant were common, with 80% of living patients receiving a transplant at 15 years of follow up. After commencement of KRT, cardiovascular mortality rates varied over time, peaking in the first few months before declining, then gradually increasing again over years. Similarly, composite graft failure and death rates were elevated during the first year post‐transplant in those with comorbid cardiovascular disease. Improvements have occurred over time, with lower rates and risk of cardiovascular death in the most recent era. Whether this represents improvements in care, selection bias for transplantation, or both is less clear.
Our multi‐state models provide absolute measures of both cardiovascular and non‐cardiovascular mortality, allowing clinicians and patients to compare treatment impacts and make prognostic estimates over time. Moreover, these probabilities account for changes in risk profile across different treatment states. To our knowledge, cause‐specific mortality has not previously been described across treatment states in general KF populations. Our approach of modelling probabilities over a lifespan of KRT is novel and translational for several key reasons. Clinically, absolute mortality estimates are important for clinicians to communicate lifetime risks and may be implemented in patient decision aid tools. To advance care, clinical research design requires specific inputs for calculating power and outcome estimates, which this work provides. Our multi‐state model estimates could be further applied in health economic evaluation, where average time spent in certain treatment states and mortality risk over time can inform resource use and cost projections. Transitions between disease states are also useful in planning prospective studies and calculating sample sizes based on treatment changes during follow up.
The first few months of KRT were a high‐risk period for cardiovascular death in dialysis patients. This is consistent with international studies, which show high rates of cardiovascular mortality immediately following dialysis initiation. 19 , 20 , 21 In particular, cardiovascular disease is associated with the highest elevation in mortality risk within the first 120 days from haemodialysis. 19 Sub‐cause analysis of subarachnoid haemorrhages, intracerebral haemorrhages, intracranial haemorrhages and ischaemic strokes have previously been studied and reveal similar trends. 22 , 23 The risk of cardiovascular mortality in dialysis patients may be exacerbated by dialysis‐induced cardiac injury, 24 endothelial dysfunction 25 and oxidative stress 26 in new patients. Treatment agents for cardiovascular disease have not been extensively studied in patients with KF, despite differences in disease progression compared to general populations. Statins have not been shown to reduce mortality or cardiac events in dialysis patients, 8 while randomized controlled trials investigating benefits of beta‐blockers are limited by small sample size. 27 , 28 The ISCHEMIA‐CKD trial found that an initial invasive strategy for coronary artery disease provided no significant benefit compared to initial medical therapy in advanced chronic kidney disease. 29 A focus on earlier recognition and mitigation of cardiovascular disease may be a more effective strategy to improve outcomes in early KF. Kidney Disease Improving Global Outcome guidelines recommend lipid‐lowering and antihypertensive therapies in patients with chronic kidney disease not requiring dialysis, in line with clinical trial evidence that these reduce mortality and cardiovascular events. 30 , 31 , 32 , 33 A holistic management approach combining individualized blood pressure targets and lifestyle interventions may be extended to patients who recently begin KRT. Several risk factors for cardiovascular death in our study, such as older age, female sex and hypertension, have previously been identified as risk factors for cerebrovascular mortality, allowing for identification of high‐risk groups and opportunities for targeted prevention. 22 , 23 Improved awareness of cardiovascular risk in chronic kidney disease may further facilitate intervention research aimed at improving risk profile before KF onset.
Overall cardiovascular mortality in KF has continued to improve over time, with adjusted mortality risk decreasing by 74% from ≤1995 to 2013. Increased access to kidney transplantation in Australia and New Zealand, where transplants doubled between 1994 and 2013, likely contributed to improved cardiovascular outcomes. 34 While possible that selection bias is at play, with improved post‐transplant outcomes a consequence of higher risk dialysis patients not being listed for transplantation, this seems unlikely given the rising age and comorbidity burden of transplant recipients in Australia and New Zealand. International guidelines generally recommend screening for cardiovascular risk in transplant candidates, but there is a lack of high‐quality data to support specific screening practices. 12 , 13 , 14 , 15 Previous studies have found that coronary artery disease in kidney transplant candidates does not predict post‐operative mortality, 35 and perfusion studies do not predict rates of major cardiac events or death post‐kidney transplant after adjusting for other patient factors. 36 , 37 Moreover, kidney transplantation may provide a survival benefit even in patients with high cardiovascular risk. 38 Economic modelling in Australian and New Zealand patients on waitlist for kidney transplantation found that practising no cardiac screening after waitlist entry may be cost‐effective and improves overall survival. 39 As availability of kidney transplantation continues to improve, extending access to patients with higher cardiovascular risk may be key to improving clinical outcomes at both an individual and population level.
Strengths of this study include its use of a large, contemporary, population‐based binational dataset. Cause‐of‐death information was recorded using internationally standardized ICD‐10 codes, allowing for generalization of data to other countries. However, there were still limitations. It was difficult to consider further mortality differences relating to racial and social determinants, such as socioeconomic status, due to lack of general population mortality data stratified by these factors. The use of probabilistic linkage for Australian patients may lead to false linkages with the National Death Index, although false positive linkage rates are low at an estimated ≤0.5%. 40 Overseas deaths were not captured in national death registers, though these are rare due to limited international travel post‐dialysis initiation. Our study had high agreement for fact of death between ANZDATA and death registers, reaffirming the low risk of incorrect linkages and missed overseas deaths. Our multi‐state model did not include transitions between dialysis modalities to reduce complexity and computational requirements. Further studies could investigate whether cardiovascular mortality is similarly elevated following these modality changes.
Despite improvements over time, cardiovascular disease is still a common cause of death in KF, particularly in dialysis patients. The first few months of dialysis initiation and transplant are a high‐risk period for cardiovascular death, highlighting the importance of early prevention strategies. Multi‐state probabilities are a useful addition that can be used to inform clinicians and patients about likely outcomes over a lifetime, allowing for KRT changes. Further research into cardiovascular disease prevention, transplant screening practices and equitable health care access may identify opportunities for further improvement.
CONFLICT OF INTEREST
The authors declared no conflicts of interest. The results presented in this paper have not been published previously in whole or part, except in abstract format.
AUTHOR CONTRIBUTIONS
Concept development: Angela C. Webster, Rachael L. Morton, Patrick J. Kelly. Statistical design: Victor Khou, Nicole L. De La Mata. Statistical analysis: Victor Khou, Nicole L. De La Mata. Result interpretation: Victor Khou, Nicole L. De La Mata, Patrick J. Kelly, Philip Masson, Emma O'Lone, Rachael L. Morton, Angela C. Webster. Draft manuscript preparation: Victor Khou. Manuscript revision: Victor Khou, Nicole L. De La Mata, Patrick J. Kelly, Philip Masson, Emma O'Lone, Rachael L. Morton, Angela C. Webster. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. The corresponding author has had full access to the data in the study and the final responsibility to submit for publication.
Supporting information
TABLE S1 Summary of hazard ratio estimates from cause‐specific hazard model for cardiovascular and non‐cardiovascular death.
TABLE S2. Summary of hazard ratio estimates from cause‐specific hazard model for cardiac and peripheral vascular deaths.
FIGURE S1. Patient flow chart of data linkage process and inclusion into analysis.
FIGURE S2. Multi‐state model demonstrating A) probability of cardiovascular and non‐cardiovascular death with time from kidney failure, stratified by sex; B) probability of cardiovascular and non‐cardiovascular death with time from transplant, stratified by sex.
FIGURE S3. Mortality rates from cardiovascular death in people receiving kidney replacement therapy, stratified by age at treatment initiation, sex, treatment modality (dialysis or transplant) and comorbid cardiovascular disease.
ACKNOWLEDGEMENTS
The data reported here has been supplied by the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA), the Australian Institute of Health and Welfare (AIHW) and the New Zealand Ministry of Health. We would like to acknowledge the assistance provided by all these entities in the data linkage process. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of interpretation of ANZDATA, AIHW or the New Zealand Ministry of Health. Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
Khou V, De La Mata NL, Kelly PJ, et al. Epidemiology of cardiovascular death in kidney failure: An Australian and New Zealand cohort study using data linkage. Nephrology. 2022;27(5):430-440. doi: 10.1111/nep.14020
Funding information The data linkage on which this study was based was supported by Kidney Health Australia, Grant/Award Number: PG3815. Kidney Health Australia had no role in study design, analysis, or interpretation of data, writing the report, or the decision to submit the report for publication.
REFERENCES
- 1. Bikbov B, Purcell CA, Levey AS, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. United States Renal Data System : 2018 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2018. [Google Scholar]
- 3. UK Renal Registry . UKRenal Registry 21st Annual Report – Data to 31/12/2017. UK Renal Registry; 2019. Accessed February 19, 2020 https://www.renalreg.org/patient-info [Google Scholar]
- 4. Ojo AO, Hanson JA, Wolfe RA, Leichtman AB, Agodoa LY, Port FK. Long‐term survival in renal transplant recipients with graft function. Kidney Int. 2000;57(1):307‐313. [DOI] [PubMed] [Google Scholar]
- 5. Lindner A, Charra B, Sherrard DJ, Scribner BH. Accelerated atherosclerosis in prolonged maintenance hemodialysis. N Engl J Med. 1974;290(13):697‐701. [DOI] [PubMed] [Google Scholar]
- 6. London GM, Guérin AP, Marchais SJ, Métivier F, Pannier B, Adda H. Arterial media calcification in end‐stage renal disease: impact on all‐cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18(9):1731‐1740. [DOI] [PubMed] [Google Scholar]
- 7. Matsushita K, Ballew SH, Coresh J. Influence of chronic kidney disease on cardiac structure and function. Curr Hypertens Rep. 2015;17(9):581. [DOI] [PubMed] [Google Scholar]
- 8. Palmer SC, Navaneethan SD, Craig JC, et al. HMG CoA reductase inhibitors (statins) for dialysis patients. Cochrane Database Syst Rev. 2013;(9):Cd004289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harel Z, Chertow GM, Shah PS, et al. Warfarin and the risk of stroke and bleeding in patients with atrial fibrillation receiving dialysis: a systematic review and meta‐analysis. Can J Cardiol. 2017;33(6):737‐746. [DOI] [PubMed] [Google Scholar]
- 10. Nochaiwong S, Ruengorn C, Awiphan R, Dandecha P, Noppakun K, Phrommintikul A. Efficacy and safety of warfarin in dialysis patients with atrial fibrillation: a systematic review and meta‐analysis. Open Heart. 2016;3(1):e000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Palmer SC, Di Micco L, Razavian M, et al. Antiplatelet agents for chronic kidney disease. Cochrane Database Syst Rev. 2013;4:5‐15. [DOI] [PubMed] [Google Scholar]
- 12. Abramowicz D, Cochat P, Claas FH, et al. European Renal Best Practice Guideline on kidney donor and recipient evaluation and perioperative care. Nephrol Dial Transplant. 2015;30(11):1790‐1797. [DOI] [PubMed] [Google Scholar]
- 13. Campbell S, Pilmore H, Gracey D, Mulley W, Russell C, McTaggart S. KHA‐CARI guideline: recipient assessment for transplantation. Nephrology (Carlton). 2013;18(6):455‐462. [DOI] [PubMed] [Google Scholar]
- 14. Lentine KL, Kasiske BL, Levey AS, et al. KDIGO clinical practice guideline on the evaluation and care of living kidney donors. Transplantation. 2017;101(8S suppl 1):S1‐S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lentine Krista L, Costa Salvatore P, Weir Matthew R, et al. Cardiac disease evaluation and management among kidney and liver transplantation candidates. Circulation. 2012;126(5):617‐663. [DOI] [PubMed] [Google Scholar]
- 16. McDonald SP, Russ GR. Australian registries‐ANZDATA and ANZOD. Transplant Rev (Orlando). 2013;27(2):46‐49. [DOI] [PubMed] [Google Scholar]
- 17. World Health Organisation . International Classification of Diseases (ICD) Information Sheet. Accessed July 2, 2020. https://www.who.int/classifications/icd/factsheet/en/.
- 18. Therneau T, Crowson C, Atkinson E. Multi‐state models and competing risks. Accessed July 17, 2019 https://cran.r-project.org/web/packages/survival/vignettes/compete.pdf.
- 19. Bradbury BD, Fissell RB, Albert JM, et al. Predictors of early mortality among incident US hemodialysis patients in the dialysis outcomes and practice patterns study (DOPPS). Clin J Am Soc Nephrol. 2007;2(1):89‐99. [DOI] [PubMed] [Google Scholar]
- 20. Collins AJ, Foley RN, Gilbertson DT, Chen SC. The state of chronic kidney disease, ESRD, and morbidity and mortality in the first year of dialysis. Clin J Am Soc Nephrol. 2009;4(suppl 1):S5‐S11. [DOI] [PubMed] [Google Scholar]
- 21. de Jager DJ, Grootendorst DC, Jager KJ, et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. Jama. 2009;302(16):1782‐1789. [DOI] [PubMed] [Google Scholar]
- 22. De La Mata NL, Alfaro‐Ramirez M, Kelly PJ, et al. Absolute risk and risk factors for stroke mortality in patients with end‐stage kidney disease (ESKD): population‐based cohort study using data linkage. BMJ Open. 2019;9(2):e026263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De La Mata NL, Masson P, Al‐Shahi Salman R, et al. Death from stroke in end‐stage kidney disease. Stroke. 2019;50(2):487‐490. [DOI] [PubMed] [Google Scholar]
- 24. Selby NM, McIntyre CW. The vicious cycle of dialysis‐induced cardiac injury—are dynamic changes in diastolic function involved? Am J Kidney Dis. 2013;62(3):442‐444. [DOI] [PubMed] [Google Scholar]
- 25. Oleśkowska‐Florek W, Połubinska A, Baum E, et al. Hemodialysis‐induced changes in the blood composition affect function of the endothelium. Hemodial Int. 2014;18(3):650‐656. [DOI] [PubMed] [Google Scholar]
- 26. Pupim LB, Himmelfarb J, McMonagle E, Shyr Y, Ikizler TA. Influence of initiation of maintenance hemodialysis on biomarkers of inflammation and oxidative stress. Kidney Int. 2004;65(6):2371‐2379. [DOI] [PubMed] [Google Scholar]
- 27. Jin J, Guo X, Yu Q. Effects of beta‐blockers on cardiovascular events and mortality in dialysis patients: a systematic review and meta‐analysis. Blood Purif. 2019;48(1):51‐59. [DOI] [PubMed] [Google Scholar]
- 28. Weir MA, Herzog CA. Beta blockers in patients with end‐stage renal disease‐evidence‐based recommendations. Semin Dial. 2018;31(3):219‐225. [DOI] [PubMed] [Google Scholar]
- 29. Bangalore S, Maron DJ, O'Brien SM, et al. Management of coronary disease in patients with advanced kidney disease. N Engl J Med. 2020;382(17):1608‐1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kidney Disease: Improving Global Outcomes (KDIGO) Lipid Work Group . KDIGO clinical practice guideline for lipid management in chronic kidney disease. Kidney Int Suppl. 2013;3:259‐305. [Google Scholar]
- 31. Tonelli M, Wiebe N, Culleton B, et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17(7):2034‐2047. [DOI] [PubMed] [Google Scholar]
- 32. Cheung AK, Rahman M, Reboussin DM, et al. Effects of intensive BP control in CKD. J Am Soc Nephrol. 2017;28(9):2812‐2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Palmer SC, Navaneethan SD, Craig JC, et al. HMG CoA reductase inhibitors (statins) for people with chronic kidney disease not requiring dialysis. Cochrane Database Syst Rev. 2014;(5):Cd007784. [DOI] [PubMed] [Google Scholar]
- 34. Warnock DG, Delanaye P, Glassock RJ. Risks for all‐cause mortality: stratified by age, estimated glomerular filtration rate and albuminuria. Nephron. 2017;136(4):292‐297. [DOI] [PubMed] [Google Scholar]
- 35. De Lima JJ, Gowdak LH, de Paula FJ, et al. Coronary artery disease assessment and intervention in renal transplant patients: analysis from the KiHeart cohort. Transplantation. 2016;100(7):1580‐1587. [DOI] [PubMed] [Google Scholar]
- 36. Doukky R, Fughhi I, Campagnoli T, et al. Validation of a clinical pathway to assess asymptomatic renal transplant candidates using myocardial perfusion imaging. J Nucl Cardiol. 2018;25(6):2058‐2068. [DOI] [PubMed] [Google Scholar]
- 37. Helve S, Nieminen T, Helanterä I, et al. The value of myocardial perfusion imaging in screening coronary artery disease before kidney transplantation. Clin Transplant. 2020;34(8):e13894. [DOI] [PubMed] [Google Scholar]
- 38. Jeloka TK, Ross H, Smith R, et al. Renal transplant outcome in high‐cardiovascular risk recipients. Clin Transplant. 2007;21(5):609‐614. [DOI] [PubMed] [Google Scholar]
- 39. Ying T, Tran A, Webster AC, et al. Screening for asymptomatic coronary artery disease in waitlisted kidney transplant candidates: a cost‐utility analysis. Am J Kidney Dis. 2020;75(5):693‐704. [DOI] [PubMed] [Google Scholar]
- 40. Chantrel F, de Cornelissen F, Deloumeaux J, Lange C, Lassalle M, registre REIN . Survival and mortality in ESRD patients. Nephrol Ther. 2013;9(Suppl 1):S127‐S137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Summary of hazard ratio estimates from cause‐specific hazard model for cardiovascular and non‐cardiovascular death.
TABLE S2. Summary of hazard ratio estimates from cause‐specific hazard model for cardiac and peripheral vascular deaths.
FIGURE S1. Patient flow chart of data linkage process and inclusion into analysis.
FIGURE S2. Multi‐state model demonstrating A) probability of cardiovascular and non‐cardiovascular death with time from kidney failure, stratified by sex; B) probability of cardiovascular and non‐cardiovascular death with time from transplant, stratified by sex.
FIGURE S3. Mortality rates from cardiovascular death in people receiving kidney replacement therapy, stratified by age at treatment initiation, sex, treatment modality (dialysis or transplant) and comorbid cardiovascular disease.


