Abstract
Background
The growing resistance of bacteria to antimicrobial medicines is a global issue and a direct threat to human health. Despite this, antibiotic prophylaxis is often still routinely used in dental implant surgery to prevent bacterial infection and early implant failure, despite unclear benefits. There is a lack of sufficient evidence to formulate clear clinical guidelines and therefore there is a need for well‐designed, large‐scale randomized controlled trials to determine the effect of antibiotic prophylaxis.
Purpose
To compare the effect of a presurgical antibiotic regimen with an identical placebo regimen in healthy or relatively healthy patients receiving dental implants.
Materials and Methods
The 474 patients participating in the study were recruited from seven clinics in southern Sweden. We randomized the patients into a test and a placebo group; the study was conducted double‐blinded. Preoperatively, the test group received 2 g of amoxicillin and the control group, identical placebo tablets. The primary outcome was implant failure; secondary outcomes were postoperative infections and adverse events. Patients were evaluated at two follow‐ups: at 7–14 days and at 3–6 months.
Results
Postoperative evaluations of the antibiotic (n = 238) and the placebo (n = 235) groups noted implant failures (antibiotic group: six patients, 2.5% and placebo group: seven patients, 3.0%) and postoperative infections (antibiotic group: two patients, 0.8% and placebo group: five patients, 2.1%). No patient reported any adverse events. Between‐group differences in implant failures and postoperative infections were nonsignificant.
Conclusion
Antibiotic prophylaxis in conjunction with implant placement is likely of small benefit and should thus be avoided in most cases, especially given the unabated growth in antibiotic‐resistant bacteria. Clinical trial registration number: NCT03412305.
Keywords: antibiotic prophylaxis, dental implants, multicenter placebo‐controlled randomized clinical trial
What is known
Antibiotic resistance is a large threat to modern health care.
Overuse of antibiotics leads to increasing rates of antibiotic resistance.
In addition, short‐term antibiotic treatments may select for resistance.
The use of prophylactic antibiotics in implant dentistry is controversial.
There is a need to reduce inappropriate use of antibiotics.
What this study adds
The effect of antibiotic prophylaxis in conjunction with uncomplicated dental implant surgery in healthy or relatively healthy patients seems to be small and clinically irrelevant.
This study helps to create a basis for strict guidelines regarding antibiotic prophylaxis in dental implant surgery.
1. INTRODUCTION
The growing resistance to antimicrobial medicines is a global issue and a direct threat to human health. The consequence of bacteria developing resistance is a threat to modern treatment methods in healthcare and means that many diseases become more difficult to treat. This affects society and all of humanity. 1 It is important to use antibiotics only when necessary since overuse of antibiotics encourages the development of antibiotic‐resistant bacteria. 2 , 3 Thus, antibiotic use must be clearly defined in line with verified scientific evidence so that overuse is avoided. 1
Dental implant surgery is a common treatment for replacing missing teeth and has a high success rate. 4 To prevent postoperative infection and early implant failure, the technique initially included a presurgical antibiotic regimen. Penicillin V orally 1 h before surgery and for 10 days postoperatively was recommended. 5 The literature still cites no evidence to support this recommendation, nor any established protocol for a systemic prophylactic antibiotic regimen in conjunction with dental implant surgery.
In 2013, a Cochrane systematic review 6 and a meta‐analysis of six randomized controlled trials (RCTs) 7 , 8 , 9 , 10 , 11 , 12 showed that amoxicillin, given orally 1 h preoperatively, significantly reduced early implant failure, and routine use of a single dose of 2 g amoxicillin directly before dental implant placement was suggested. Absolute risk reduction, however, was low (4%) and the number needed to treat (NNT) to prevent one patient having an implant failure was 25.
Another systematic review and meta‐analysis, 13 which unlike the Cochrane report only included RCTs comparing antibiotic prophylaxis with placebo, 7 , 8 , 9 , 14 reported an absolute risk reduction of 2%, yielding an NNT of 50, and suggested that antibiotic prophylaxis in uncomplicated implant surgeries was of no benefit in healthy patients. In a consensus report from the European Association for osseointegration (EAO) in 2015, 15 it was stated that antibiotic prophylaxis had no beneficial effect in straightforward cases of implant surgeries.
In recent years, several additional systematic reviews have been carried out, 16 , 17 , 18 , 19 , 20 , 21 but unfortunately, no new large‐scale well‐conducted, placebo‐controlled RCTs have been published. All of the previously mentioned systematic reviews report that antibiotic prophylaxis associated with implant placement significantly reduces the risk of implant loss. However, the risk reduction is modest, and the results can therefore be interpreted differently. Some conclude that antibiotic prophylaxis is indicated to prevent early implant failure in healthy patients 21 while others point out that antibiotic‐associated risks must be considered and the results for implant failure outcomes may not warrant the indiscriminate use of antibiotics in patients who are healthy. 17 Unlike the statement of EAO 2015, 15 a recent consensus report by the Italian Academy of Osseointegration 22 advocates the administration of a unique dose of antibiotics in straightforward implant cases.
Despite the large number of systematic reviews, it is clear that there are differing views on the benefits of antibiotic prophylaxis in implant surgery. It is obvious that there is a need for additional well‐designed, large‐scale RCTs to determinate the efficacy of preoperative antibiotics in prevention of dental implant failure or postoperative infections.
The aim of the present study was to conduct a large‐scale, multicenter RCT to compare the effect of 2 g amoxicillin with identical placebo tablets taken 1 h preoperatively in healthy or relatively healthy patients undergoing dental implant surgery. The hypothesis was that the difference in early implant failure and postoperative infections between patients receiving prophylactic antibiotics and those receiving placebo is minor, with low clinical relevance.
2. MATERIALS AND METHODS
2.1. Study design and setting
The present study is a prospective, randomized, placebo‐controlled, double‐blinded clinical trial conducted in the southern part of Sweden. The study protocol was registered in EudraCT (EudraCT number: 2012‐002213‐19), approved by the Medical Products Agency in Sweden (Approval number: 5.1‐2013‐56 072), and registered in Clinical Trials.gov (Registration number: NCT03412305). The Regional Ethics Review Board in Lund, Sweden, approved the present study (Dnr: 2013/257), which follows the ethical principles of the Declaration of Helsinki and the CONSORT guidelines for clinical trials. Patients were recruited and treated between November 2014 and April 2018. All patients signed informed‐consent forms. Figure 1 presents the study flowchart.
FIGURE 1.
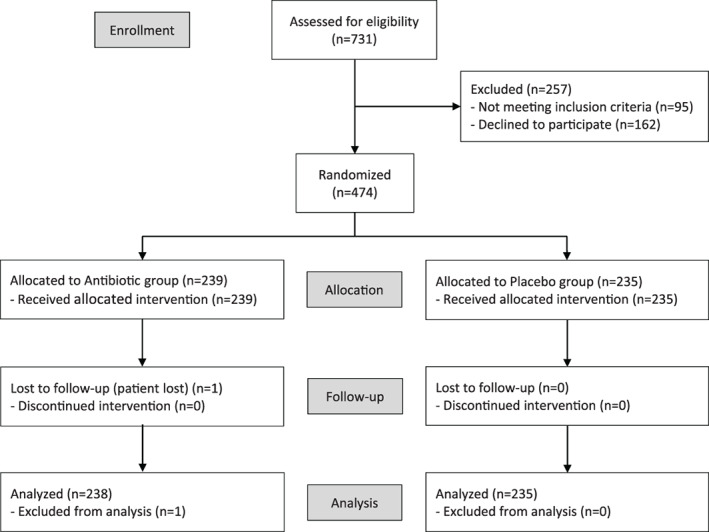
Flowchart diagram of the study protocol according to Consort guideline
Seven dental clinics in Sweden participated:
Department of Oral and Maxillofacial Surgery and Oral Medicine, Faculty of Odontology, Malmö University, Malmö
Public Dental Service, Väster Vall, Varberg
Department of Dental and Maxillofacial Surgery, Public Dental Service, Karlskrona
Department of Oral and Maxillofacial Surgery, Skåne University Hospital, Lund
Department of Periodontology, Public Dental Service, Kalmar
Maxillofacial Department, Hallands Hospital, Halmstad
The Department of Periodontology, Public Dental Service, Växjö
All treating clinicians were oral and maxillofacial surgeons, periodontists, or general dental practitioners who routinely performed implant procedures.
2.2. Study population
Patients aged >18 years and in need of a dental implant were eligible for inclusion. The exclusion criteria were (i) immunodeficiency or immunosuppressing treatments; (ii) severe diabetes; (iii) severe systemic disease (ASA group > 2) 23 ; (iv) previous radiotherapy to the head and neck area; (v) acute or chronic oral infection (including ongoing periodontal infection); (vi) current antibiotic treatment; (vii) allergy to penicillin; (viii) previous treatment with bisphosphonates; (ix) implant surgery requiring substantial bone augmentation (implant placement that involve autogenous bone blocks or extensive amount of particulate autogenous bone harvested from extraoral or intraoral donor sites); and (x) implants planned for immediate loading (within 2 weeks).
2.3. Presurgical medication and randomization
Recipharm Pharmaceutical Development AB (Gårdsvägen 10A, 169 70 Solna, Sweden) supplied 1000 doses (the intended number of study participants according to the power analysis) of the amoxicillin and placebo tablets in plastic containers, one dose per container. A dose was either four 500‐mg tablets of amoxicillin (500 doses) or four identical‐looking placebo tablets (500 doses). Recipharm had no financial or other interest in the study.
Patients were randomized to one of two groups:
Antibiotic (test) group: Patients were administered 2 g amoxicillin (four 500‐mg tablets) 1 h before implant surgery.
Placebo (control) group: Patients were administered the placebo (four tablets identical in appearance to the antibiotic tablets) 1 h before implant surgery.
The randomization was performed by consecutively assigning each included patient a medicine container with a code number according to a computer‐generated list of random numbers with block‐size six. In this way, the patients were randomly divided into one of two groups: test or control group.
The code number of each medicine container was registered in each patient's study record. The list that revealed which code number was test versus control group remained sealed until the clinical trial was finished, and all data had been collected.
2.4. Clinical procedures
Before surgery, each patient received one of the medicine containers. The time when the tablets were consumed was recorded. The referring prosthodontist or the surgeon chose the implant system for each patient. Implant manufacturer recommendations were followed, as were the surgical routines for each clinic. Surgical techniques such as open or closed sinus lift, implant placement with guided bone regeneration (GBR) and minor bone augmentation were done if the surgeon considered it appropriate. Bone augmentation was limited to autogenous bone chips and bone debris, harvested adjacent to the actual surgical site, with simultaneous implant placement. The surgeon decided whether to use a one‐ or two‐stage surgery protocol. Each patient received single or multiple implants in the maxilla and/or the mandible. The duration of the implant surgery was recorded. An aseptic technique was used to prevent microorganisms being introduced to anything that has a direct or indirect contact with the operative field.
Four implant systems were used: Straumann® SLA, Straumann Implants, Switzerland; Astra Tech Dental Implant Systems®, Dentsply Sirona, Sweden; Nobel Biocare®, Sweden; and Southern Implants®, Ltd, South Africa.
Each surgeon prescribed preoperative and/or postoperative chlorhexidine 0.2% according to the surgical protocols and routines in place at their clinics. Patients were examined postoperatively at 7–14 days (first follow‐up) and at 3–6 months (second follow‐up). The timing of the follow‐ups depended on surgical method and clinic routines.
2.5. Outcome variables
The primary outcome measure was implant failure, defined as implants lost during follow‐up or unstable implants discovered during hand tightening of the implant abutment at the second follow‐up. Secondary outcome measures were postoperative infections and adverse events. Postoperative infections were defined as any sign of bacterial infection such as wound dehiscence, swelling, fistula, abscess, or redness. Adverse events were defined as nausea, vomiting, diarrhea, or urticaria. Secondary outcomes were recorded at the first and second follow‐ups.
2.6. Statistical methods
Sample size was calculated on the basis of previous study reports on early implant loss of 2% with an antibiotic prophylaxis and 5% without. Thus, for a type one error of 0.05 and a power of 80%, 500 patients would be required in each of the antibiotic and placebo groups in order to detect a significant difference between the groups, if one existed. To show equivalence, that is, the same outcome in the antibiotic group as in the placebo group, the difference between the two groups will be described with the relative risk with a 95% confidence interval. Proportional differences between the antibiotic group and the placebo group were analyzed using Pearson's chi‐squared test (χ2). The multiple logistic regression model used implant failure as the dependent variable; smoking (yes/no), and age (<50 years; 50–64; and ≥65) as dichotomous indicator variables; and bone augmentation (yes/no), number of implants (1, 2–3, and ≥4), and treatment group (antibiotic/placebo) as independent variables. All analyses were done using STATA 15 SE. P‐values less than 0.05 or 95% confidence intervals for ratios not including 1 were considered statistically significant.
3. RESULTS
3.1. Patient characteristics and interventions
The intention was to include 1000 patients in the study. Because the trial drugs expired before all patients could be recruited, patient recruitment ceased at 731 patients; of these, 474 could be included. One patient in the antibiotic group was lost after the first follow‐up due to change of clinic, leaving 473 patients for analysis (Figure 1). The lost patient had no implant failure or sign of infection at the first follow‐up. Data from the lost patient are presented in Table 1 however not in any of the other tables.
TABLE 1.
Patient and intervention characteristics of the two study groups in a comparison of antibiotic prophylaxis (n = 238) with a placebo (n = 235) before implant surgery
| Variable | Group | |
|---|---|---|
| Placebo | Antibiotics | |
| Gender | ||
| Male | 118 (50.2%) | 121 (50.6%) |
| Female | 117 (49.8%) | 118 (49.4%) |
| Age (mean years ± SD) | 57.1 ± 13.7 | 57.7 ± 14.2 |
| Smoking | ||
| Yes | 40 (17.0%) | 46 (19.2%) |
| No | 170 (72.3%) | 165 (69.0%) |
| Unknown | 26 (11.0%) | 28 (11.7%) |
| Jaw receiving implants | ||
| Mandible | 82 (34.9%) | 94 (39.3%) |
| Maxilla | 141 (60.0%) | 131 (54.8%) |
| Both | 12 (5.1%) | 12 (5.0%) |
| Implant system | ||
| Straumann | 141 (60.0%) | 140 (58.6%) |
| stra | 76 (32.3%) | 64 (26.8%) |
| Nobel | 18 (7.7%) | 31 (13.0%) |
| Southern | 0 (0.0%) | 3 (1.3%) |
| Surgical protocol | ||
| One stage | 184 (78.3%) | 189 (79.1%) |
| Two stage | 49 (20.9%) | 49 (20.5%) |
| Both | 2 (0.9%) | 1 (0.4%) |
| Sinus lift surgery | 10 (4.3%) | 11 (4.6%) |
| Bone augmentation | 43 (18.3%) | 35 (14.7%) |
| Implant placement and GBR surgery | 15 (6.4%) | 14 (5.9%) |
| Duration of intervention (mean minutes ± SD) | 32.6 ± 18.8 | 31.3 ± 14.6 |
| Number of implants/patient | ||
| 1 | 140 (59.6%) | 144 (60.3%) |
| 2–3 | 82 (34.9%) | 78 (32.6%) |
| 4–6 | 13 (5.5%) | 17 (7.1%) |
| Total number of implants | 373 | 384 |
| Chlorhexidine rinse | ||
| Never | 4 (1.7%) | 3 (1.3%) |
| Before surgery | 2 (0.9%) | 1 (0.4%) |
| After surgery | 76 (32.3%) | 79 (33.1%) |
| Before and after surgery | 152 (64.7%) | 156 (65.3%) |
Among the excluded patients, 162 declined participation and 95 met at least one exclusion criterion. The most common reasons for those who met an exclusion criterion was systemic disease (62 patients, 65.3%) or ongoing infection (13 patients, 13.7%). Six patients (3.7%) were excluded due to the complexity of the planned operation, including major bone augmentation procedures, and severe atrophy of the mandible/maxilla.
The study cohort (n = 474), comprising the antibiotic group (n = 239) and the placebo group (n = 235), received 757 implants. Gender distribution was fairly equal, 239 males and 235 females, and mean age in the entire cohort at time of surgery was 57.4 (±13.9) years. Gender distribution was also fairly equal in each group, and the mean age of the two groups did not differ significantly (Table 1).
Of the entire study cohort, 281 patients (59.3%) received implants from Straumann; 149 (31.4%), Astra; 49 (10.3%), Nobel BioCare; and 3 (0.6%), Southern. No difference in primary outcome was noted regarding the different implant systems that were used. The majority were one‐stage surgeries (373 implants, 78.7%). Some surgeries included a sinus lift (21 patients, 4.4%), minor bone augmentation (78 patients, 16.5%) or implant placement with guided bone generation (GBR) (29 patients, 6.1%; Table 1).
3.2. Implant failures
During follow‐up, implants failed in 13 patients (2.7%): six patients (2.5%) in the antibiotic group and seven (3.0%) in the placebo group. The between‐group difference in implant failure was nonsignificant at the patient level (RR 0.85; 95% CI: 0.29–2.48, p = 0.75; Table 2). Three patients lost two implants each, two patients in the antibiotic group and one patient in the placebo group; the remaining 10 patients lost one implant each. Thus, of 757 implants placed, 16 failed, an overall survival rate of 97.9% at the implant level. All lost implants were replaced after the study concluded. Table 3 overviews the primary outcome in four subgroups (straightforward implant surgery, implant placement and simultaneous minor bone augmentation, sinus lift, implant placement and guided bone regeneration (GBR) surgery). Table 4 presents a multivariate logistic regression analysis of the exposure variables. No variable was significantly associated with implant failure.
TABLE 2.
Primary and secondary outcomes during postoperative implant surgery follow‐up (first at 7–14 days; second at 3–6 months) in the two study groups comparing preoperative antibiotic prophylaxis (n = 238) a with a placebo (n = 235)
| Outcome | Group | p value | |
|---|---|---|---|
| Placebo | Antibiotics | ||
| Primary outcome | |||
| Implant failure, first and second follow‐ups | 7 (3.0%) | 6 (2.5%) | 0.75 |
| Secondary outcome | |||
| Postoperative infection, first follow‐up | 5 (2.1%) | 2 (0.8%) | 0.25 |
| Postoperative infection, second follow‐up | 7 (3.0%) | 5 (2.1%) | 0.54 |
| Adverse events, first and second follow‐ups | 0 | 0 | 0 |
One patient experienced implant failure at first follow‐up and was thus not included in the second follow‐up.
TABLE 3.
Primary outcomes presented in four subgroups: Straightforward implant surgery, implant placement and simultaneous minor bone augmentation, sinus lift, implant placement and GBR surgery
| Subgroups | Group | p value | |
|---|---|---|---|
| Placebo | Antibiotics | ||
| Straightforward implant surgery | |||
| Implant failure | 5 (2.8%) | 3 (1.8%) | 0.18 |
| No implant failure | 179 (97.2%) | 167 (98.2%) | |
| Implant placement and simultaneous minor bone augmentation a | |||
| Implant failure | 0 (0.0%) | 1 (2.9%) | 1.00 |
| No implant failure | 43 (100.0%) | 34 (97.1%) | |
| Implant placement and sinus lift b | |||
| Implant failure | 1 (10.0%) | 1 (10.0%) | 1.00 |
| No implant failure | 9 (90.0%) | 9 (90.0%) | |
| Implant placement and GBR surgery | |||
| Implant failure | 1 (6.7%) | 1 (7.1%) | 0.96 |
| No implant failure | 14 (93.3%) | 13 (92.9%) | |
Sinus lift or GBR surgery not included.
With or without simultaneous minor bone augmentation.
TABLE 4.
Multivariate analysis of implant failure including the two study groups and potential confounding variables
| Variable | Odds ratio | 95% CI |
|---|---|---|
| Smoking | ||
| No | Ref | |
| Yes | 1.4 | 0.4–5.6 |
| Age (years) | ||
| <50 | Ref | |
| 50–64 | 4.3 | 0.5–37.0 |
| ≥65 | 4.4 | 0.5–38.5 |
| Number of implants | ||
| 1 | Ref | |
| 2–3 | 2.2 | 0.6–7.3 |
| ≥4 | 2.9 | 0.5–16.0 |
| Bone augmentation | ||
| No | Ref | |
| Yes | 1.7 | 0.5–5.5 |
| Treatment group | ||
| Antibiotics | Ref | |
| Placebo | 1.2 | 0.4–3.6 |
Abbreviations: CI , confidence interval; Ref, reference.
3.3. Postoperative infections
At the first follow‐up, 7–14 days after implant surgery, infection was observed in seven patients (1.5%): two (0.8%) in the antibiotic group and five (2.1%) in the placebo group. The between‐group difference in postoperative infections at the patient level was not significant (RR: 0.29; 95% CI: 0.08–2.01, p = 0.25; Table 2).
At the second follow‐up, 3–6 months (mean: 16 weeks ± 6.1) after implant surgery, infection was observed in 12 patients (2.5%): 5 (2.1%) in the antibiotic group and 7 (3.0%) in the placebo group. The between‐group difference at the patient level was not significant (RR: 0.70; 95% CI: 0.23–2.18, p = 0.54; Table 2).
One patient in the placebo group received antibiotics at the first follow‐up due to infection with healing observed at the second follow‐up. Three patients received antibiotics at the second follow‐up; two of these patients were in the placebo group and one patient was in the antibiotic group. None of these patients showed any signs of implant failure but had abscesses with fistulas; thus, postoperative antibiotics were indicated. All three patients had healed at a later follow‐up, after the study had concluded, and their implants remained stable.
4. DISCUSSION
The present RCT observed no significant difference in the primary outcome measure between the antibiotic (implant failure rate = 2.5%) and the placebo (implant failure rate = 3.0%) group. Absolute risk reduction was 0.46% with an NNT of 219. This means that only about one in every 219 patients will benefit from antibiotic treatment.
One limitation of this study is that we were only able to recruit 474 eligible patients of the 1000 which the power analysis indicated would be needed to detect a significant difference, if there was one. However, the difference between the two groups was smaller than we had expected. To be able to demonstrate that such a small difference is statistically significant, more than 8000 patients in each group would have been required. Such a large RCT would have been difficult to conduct. One reason we could not include as many patients as we had initially planned was that 3 of the 10 participating clinics delivered no patient data to our research team, and one of the participating clinics only sent data for six patients. The remaining clinics could not cover this loss in recruitment before the expiration date of the trial drugs. Another limitation was that the follow‐ups had no fixed timepoints. Instead, they were scheduled according to the routines in place at the clinic. Follow‐up time was also individual, based on the patient and surgical procedure. No other important shortcomings were identified in the design or execution of the study.
Use of identical‐looking antibiotic and placebo tablets, all produced by the same manufacturer, allowed the study to remain double‐blinded to the patients, surgeons, and researchers until data acquisition was complete. The randomization process was able to distribute the baseline characteristics evenly between the two groups (Table 1). Out of all the included patients in the study, only seven did not use chlorhexidine rinse and none of these seven patients had any implant loss.
The scientific basis for determining which antibiotic has the best effect in preventing postoperative infection in the oral cavity is insufficient. However, since approximately 90% of the oral microflora in immunocompetent patients are sensitive to penicillin, 24 , 25 amoxicillin is an appropriate choice of antibiotic. The regimen of 2 g amoxicillin 1 h before the implant surgery was chosen because it has been used in previous RCT studies on antibiotic prophylaxis in dental implant surgery. 7 , 8 , 9 , 10 Furthermore, amoxicillin 2.0 g for 1 h before the dental procedures is also a recommended regimen for prevention of infective endocarditis. 26 , 27
The present study not only included patients receiving standardized, uncomplicated implant surgeries but also patients who would receive more than one implant and patients who needed minor bone augmentation and/or an uncomplicated sinus lift. Our aim was to include generally healthy individuals representative of patients who receive dental implants.
The implant failure rate was generally low. Experienced surgeons, performing the surgeries in well‐established surgical environments and using agreed‐upon routines, may explain the low failure rate. Most surgeons also prescribed a pre‐ and/or postoperative chlorhexidine rinse, which could well have contributed to the overall high success rate. Studies have shown that the surgical competence of the surgeon and the ability to keep the implant and surgical area sterile during the operation are clear factors in the successful osseointegration of an implant in atraumatic surgery. 28 , 29
Outcomes measures did not differ substantially between the seven clinics participating in the present study. The logistic regression analysis showed no significant associations of smoking, age, number of implants, or bone augmentation with implant failure or postoperative infection. At present, there is no clear consensus on whether generally healthy individuals undergoing routine implant surgery need prophylactic antibiotics. Thus, additional studies, like the present RCT, are beneficial in helping to establish an evidence‐based consensus in the literature. 15 , 22
No previously published placebo‐controlled RCT has been able to show a significant difference between antibiotic and placebo groups in early implant failure. In the present trial, six patients in the antibiotic group and seven in the placebo group lost implants. Anitua and colleagues 9 reported similar results: two patients in each group lost implants; Tan and colleagues 30 observed: no patient in the antibiotic group had an implant failure, and only one patient in the placebo group lost implants. Furthermore, a recent placebo‐controlled RCT 31 reported that three patients in the antibiotic group and one patient in the placebo group lost an implant. This is in contrast to the results in two earlier RCTs, 7 , 8 which reported more patients with implant failure in the placebo than the antibiotic group (8 vs. 2 and 12 vs. 5 patients).
The present study reports a low incidence of patients with implant failure in the placebo group (3.0%). This agrees well with reports of 1.3%–5.1% in other placebo‐controlled RCTs. 7 , 8 , 30 In contrast, a recently published non‐placebo‐controlled trial found a significantly higher incidence of implant loss in the patient group not receiving antibiotic prophylaxis compared with the test group (12.9% vs. 4.9%). Despite the higher incidence of implant loss in the test group, the between‐group difference was still significant. The reason why this study reports such high incidences of implant losses in both groups is unclear. 32
Since the development of antibiotic‐resistant bacteria is directly correlated with the frequency of antibiotic use, 33 antibiotic use should clearly be constrained to occasions where it is truly needed and benefits patient health. Khalil and colleagues 34 showed in their study that just one dose of 2 g amoxicillin could disturb the ecology of the oral microflora, causing resistant strains to occur.
The rapid development of bacteria resistant to antibiotics is a worldwide problem endangering the lives of millions. Europe alone reported that 25 000 people died from deadly infection caused by antibiotic‐resistant bacteria in 2013. 35 To avoid a post‐antibiotic era, fundamental shifts within the medical and agricultural fields must be implemented where antibiotic use is strictly regulated through clear guidelines informed by solid, evidence‐based research. 36 The correlation of overuse and misuse of antibiotics with the development of resistant bacteria is strongly connected, and many reports unfortunately detail wide misusage within the medical and agricultural fields. 35 This growing global problem should be considered in every field that handles antibiotics, and each dose should be prescribed based on evidence‐based guidelines.
In conclusion, the results of the present study suggest that the effect of antibiotic prophylaxis in conjunction with dental implant surgery in preventing implant loss is small and may not be clinically relevant.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
The concept and design of this study was performed by Bengt Götrick and Gunnar Tobin. Data was collected by Palwasha Momand. Data analysis and interpretation of data was performed by Palwasha Momand, Bengt Götrick, Aron Naimi‐Akbar and Jonas P. Becktor. Statistics was executed by Aron Naimi‐Akbar. Funding was secured by Bengt Götrick and Gunnar Tobin. Writing and critical revision was performed by all authors.
ACKNOWLEDGMENTS
This study was supported by grants from Skåne Regional Council, Sweden and Magn. Bergvalls Stiftelse, Sweden. The authors thank the participating surgeons and staff at the following Swedish institutions: Department of Oral and Maxillofacial Surgery and Oral Medicine, Faculty of Odontology, Malmö University, Malmö; Public Dental Service, Västra Vall, Varberg; the Department of Dental and Maxillofacial Surgery, Public Dental Service, Karlskrona; the Department of Oral and Maxillofacial Surgery, Skåne University Hospital, Lund; the Department of Periodontology, Public Dental Service, Kalmar; Maxillofacial Department, Hallands Hospital, Halmstad; and the Periodontal Clinic, Public Dental Service, Växjö.
Momand P, Becktor JP, Naimi‐Akbar A, Tobin G, Götrick B. Effect of antibiotic prophylaxis in dental implant surgery: A multicenter placebo‐controlled double‐blinded randomized clinical trial. Clin Implant Dent Relat Res. 2022;24(1):116‐124. doi: 10.1111/cid.13068
Funding information Magnus Bergvalls Stiftelse; Region Skåne
DATA AVAILABILITY STATEMENT
Data available on request from the authors
REFERENCES
- 1. World Health Organization . Global Action Plan on Antimicrobial Resistance. World Health Organization; 2015. [DOI] [PubMed] [Google Scholar]
- 2. Bell BG, Schellevis F, Stobberingh E, Goossens H, Pringle M. A systematic review and meta‐analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis. 2014;14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health. 2015;109(7):309‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moraschini V, Poubel LA, Ferreira VF, Barboza ES. Evaluation of survival and success rates of dental implants reported in longitudinal studies with a follow‐up period of at least 10 years: a systematic review. Int J Oral Maxillofac Surg. 2015;44(3):377‐388. [DOI] [PubMed] [Google Scholar]
- 5. Brånemark P‐I, Zarb GA, Albrektsson T. Tissue‐Integrated Prostheses : Osseointegration in Clinical Dentistry. Quintessence Publ. Co. Inc; 1985. [Google Scholar]
- 6. Esposito M, Grusovin MG, Maghaireh H, Worthington HV. Interventions for replacing missing teeth: different times for loading dental implants. Cochrane Database Syst Rev. 2013;2013(3):Cd003878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Esposito M, Cannizzaro G, Bozzoli P, et al. Efficacy of prophylactic antibiotics for dental implants: a multicentre placebo‐controlled randomised clinical trial. Eur J Oral Implantol. 2008;1(1):23‐31. [PubMed] [Google Scholar]
- 8. Esposito M, Cannizzaro G, Bozzoli P, et al. Effectiveness of prophylactic antibiotics at placement of dental implants: a pragmatic multicentre placebo‐controlled randomised clinical trial. Eur J Oral Implantol. 2010;3(2):135‐143. [PubMed] [Google Scholar]
- 9. Anitua E, Aguirre JJ, Gorosabel A, et al. A multicentre placebo‐controlled randomised clinical trial of antibiotic prophylaxis for placement of single dental implants. Eur J Oral Implantol. 2009;2(4):283‐292. [PubMed] [Google Scholar]
- 10. Caiazzo A, Casavecchia P, Barone A, Brugnami F. A pilot study to determine the effectiveness of different amoxicillin regimens in implant surgery. J Oral Implantol. 2011;37(6):691‐696. [DOI] [PubMed] [Google Scholar]
- 11. Nolan R, Kemmoona M, Polyzois I, Claffey N. The influence of prophylactic antibiotic administration on post‐operative morbidity in dental implant surgery. A prospective double blind randomized controlled clinical trial. Clin Oral Implants Res. 2014;25(2):252‐259. [DOI] [PubMed] [Google Scholar]
- 12. Abu‐Ta'a M, Quirynen M, Teughels W, van Steenberghe D. Asepsis during periodontal surgery involving oral implants and the usefulness of peri‐operative antibiotics: a prospective, randomized, controlled clinical trial. J Clin Periodontol. 2008;35(1):58‐63. [DOI] [PubMed] [Google Scholar]
- 13. Lund B, Hultin M, Tranaeus S, Naimi‐Akbar A, Klinge B. Complex systematic review ‐ perioperative antibiotics in conjunction with dental implant placement. Clin Oral Implants Res. 2015;26(Suppl 11):1‐14. [DOI] [PubMed] [Google Scholar]
- 14. Arduino PG, Tirone F, Schiorlin E, Esposito M. Single preoperative dose of prophylactic amoxicillin versus a 2‐day postoperative course in dental implant surgery: a two‐centre randomised controlled trial. Eur J Oral Implantol. 2015;8(2):143‐149. [PubMed] [Google Scholar]
- 15. Klinge B, Flemming T, Cosyn J, et al. The patient undergoing implant therapy. Summary and consensus statements. The 4th EAO consensus conference 2015. Clin Oral Implants Res. 2015;26(Suppl 11):64‐67. [DOI] [PubMed] [Google Scholar]
- 16. Rodríguez Sánchez F, Rodríguez Andrés C, Arteagoitia I. Which antibiotic regimen prevents implant failure or infection after dental implant surgery? A systematic review and meta‐analysis. J Craniomaxillofac Surg. 2018;46(4):722‐736. [DOI] [PubMed] [Google Scholar]
- 17. Braun RS, Chambrone L, Khouly I. Prophylactic antibiotic regimens in dental implant failure: a systematic review and meta‐analysis. J Am Dent Assoc. 2019;150(6):e61‐e91. [DOI] [PubMed] [Google Scholar]
- 18. Romandini M, De Tullio I, Congedi F, et al. Antibiotic prophylaxis at dental implant placement: which is the best protocol? A systematic review and network meta‐analysis. J Clin Periodontol. 2019;46(3):382‐395. [DOI] [PubMed] [Google Scholar]
- 19. Jain A, Rai A, Singh A, Taneja S. Efficacy of preoperative antibiotics in prevention of dental implant failure: a meta‐analysis of randomized controlled trials. Oral Maxillofac Surg. 2020;24(4):469‐475. [DOI] [PubMed] [Google Scholar]
- 20. Canullo L, Troiano G, Sbricoli L, et al. The use of antibiotics in implant therapy: a systematic review and meta‐analysis with trial sequential analysis on early implant failure. Int J Oral Maxillofac Implants. 2020;35(3):485‐494. [DOI] [PubMed] [Google Scholar]
- 21. Roca‐Millan E, Estrugo‐Devesa A, Merlos A, Jané‐Salas E, Vinuesa T, López‐López J. Systemic antibiotic prophylaxis to reduce early implant failure: a systematic review and meta‐analysis. Antibiotics (Basel). 2021;10(6).698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Caiazzo A, Canullo L, Pesce P. Consensus report by the Italian Academy of Osseointegration on the use of antibiotics and antiseptic agents in implant surgery. Int J Oral Maxillofac Implants. 2021;36(1):103‐105. [DOI] [PubMed] [Google Scholar]
- 23. Doyle DJ, Goyal A, Bansal P, Garmon EH. American society of anesthesiologists classification. StatPearls. StatPearls Publishing LLC; 2021. [PubMed] [Google Scholar]
- 24. Isla A, Canut A, Rodríguez‐Gascón A, et al. Pharmacokinetic/pharmacodynamic analysis of antibiotic therapy in dentistry and stomatology. Enferm Infecc Microbiol Clin. 2005;23(3):116‐121. [DOI] [PubMed] [Google Scholar]
- 25. Chunduri NS, Madasu K, Goteki VR, Karpe T, Reddy H. Evaluation of bacterial spectrum of orofacial infections and their antibiotic susceptibility. Ann Maxillofac Surg. 2012;2(1):46‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis. Kardiol Pol. 2015;73(11):963‐1027. [DOI] [PubMed] [Google Scholar]
- 27. Dajani AS, Taubert KA, Wilson W, et al. Prevention of bacterial endocarditis recommendations by the American Heart Association. JAMA. 1997;277(22):1794‐1801. [PubMed] [Google Scholar]
- 28. Jinno Y, Jimbo R, Hjalmarsson J, Johansson K, Stavropoulos A, Becktor JP. Impact of surface contamination of implants with saliva during placement in augmented bone defects in sheep calvaria. Br J Oral Maxillofac Surg. 2019;57(1):41‐46. [DOI] [PubMed] [Google Scholar]
- 29. Chrcanovic BR, Kisch J, Albrektsson T, Wennerberg A. Factors influencing early dental implant failures. J Dent Res. 2016;95(9):995‐1002. [DOI] [PubMed] [Google Scholar]
- 30. Tan WC, Ong M, Han J, et al. Effect of systemic antibiotics on clinical and patient‐reported outcomes of implant therapy ‐ a multicenter randomized controlled clinical trial. Clin Oral Implants Res. 2014;25(2):185‐193. [DOI] [PubMed] [Google Scholar]
- 31. Payer M, Tan WC, Han J, et al. The effect of systemic antibiotics on clinical and patient‐reported outcome measures of oral implant therapy with simultaneous guided bone regeneration. Clin Oral Implants Res. 2020;31(5):442‐451. [DOI] [PubMed] [Google Scholar]
- 32. Kashani H, Hilon J, Rasoul MH, Friberg B. Influence of a single preoperative dose of antibiotics on the early implant failure rate. A randomized clinical trial. Clin Implant Dent Relat Res. 2019;21(2):278‐283. [DOI] [PubMed] [Google Scholar]
- 33. WHO . Global Action Pan on Antimicrobial Resistance. World Health Organization; 2015. [Google Scholar]
- 34. Khalil D, Hultin M, Rashid MU, Lund B. Oral microflora and selection of resistance after a single dose of amoxicillin. Clin Microbiol Infect. 2016;22(11):949.e941‐949.e944. [DOI] [PubMed] [Google Scholar]
- 35. Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P t. 2015;40(4):277‐283. [PMC free article] [PubMed] [Google Scholar]
- 36. Spellberg B, Gilbert DN. The future of antibiotics and resistance: a tribute to a career of leadership by John Bartlett. Clin Infect Dis. 2014;59(Suppl 2):S71‐S75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors


