Abstract
The life expectancy of the world’s elderly population (65 and older) continues to reach new milestones with older individuals currently comprising greater than 8.5% (617 million) of the world’s population. This percentage is predicted to approach 20% of the world’s population by 2050 (representing 1.6 billion people). Despite this amazing feat, many healthcare systems are not equipped to handle the multitude of diseases that commonly manifest with age, including most types of cancers. As the world’s aging population grows, cancer treatments continue to evolve. Immunotherapies are a new drug class that has revolutionized our ability to treat previously intractable cancers; however, their efficacy in patients with compromised immune systems remains unclear. In this review, we will discuss how aging-associated losses in immune homeostasis impact the efficacy and safety of immunotherapy treatment in preclinical models of aging. We will also discuss how these findings translate to elderly patients receiving immunotherapy treatment for refractory and relapsed cancers, as well as, strategies that could be explored to improve the efficacy of immunotherapies in aged patients.
Keywords: aging, anti-inflammatory drugs, clinical trials, immunity, immunotherapies, preclinical models
1 |. INTRODUCTION
1.1 |. Aging and cancer
The world’s elderly population, those older than 65, is projected to grow to 1.6 billion individuals in the next three decades.1 By 2035, a “silver tsunami” is expected to occur in the United States, with the number of Americans 65 and older predicted to surpass those 18 and younger.2 This exceptional feat is in part attributed to the development of vaccines and antibiotics, which has increased the number of children surviving deadly childhood diseases such polio, smallpox, measles, and pneumonia,3,4 converging with declining birth rates. Other modern advances, such as a better understanding of nutrition, the implementation of routine exercise, more stringent clean water standards, and improvements in sewage treatment have also significantly contributed to extending the global life expectancy.5,6
Society greatly benefits from the presence of elderly people and we would all like to reach our golden years. However, the increased prevalence of older people places an enormous burden on global healthcare systems, in part, due to our inability to effectively treat elderly patients with various aging-associated malignancies.2,7 Aging and cancer are closely intertwined with 60% of new cancer diagnoses being made in adults aged 65 and older, and 70% of cancer deaths occurring in this population. Therefore, age has been identified as a major risk factor for developing cancer.8
The failure to achieve durable responses in elderly patients is multifactorial, and one of the barriers to success is the lack of aging-related research and clinical trials that include aged patients.9,10 For instance, between 2007 and 2018, an average of 40% of patients 65 and older were enrolled in clinical trials, despite constituting 60% of all patients with cancer.11,12 Furthermore, efficacy and safety information was only reported in 46% of these studies.11 The inconsistent recruitment of elderly patients in clinical trials has led to the development of treatments mainly in younger, healthier patients who typically have different biological and physiological responses.11
In addition to poor enrollment in clinical trials, the lack of preclinical studies conducted in aged animal models have further hampered the development of efficacious and safe drugs for aged patients.13,14 In the limited studies that have been conducted using aged mice and humans, it is consistently documented that aging alters the pharmacodynamics and pharmacokinetics of chemotherapies.15,16 A common manifestation of altered drug metabolism in aged patients is increased drug-induced toxicity, which often limits the dosage of chemotherapies that can be safely administered to elderly patients.17–19 Due to the reduced efficacy of chemotherapies in aged patients, there is increased interest in identifying novel therapies that can be used to effectively treat aging-associated cancers without accompanying toxicities.
Cellular and antibody-based immune-based therapies (immunotherapies) have revolutionized our ability to successfully treat patients with intractable diseases such as melanoma, non-small cell lung cancer (NSCLC), and relapsed/refractory B-cell malignancies.20–23 Unfortunately, the efficacy of immunotherapies has not been thoroughly investigated in patients with compromised immune systems. Given the extensive immunological decline associated with aging and the associated onset of chronic inflammation (inflamm-aging),24,25 in this review we discuss how aging-associated immunosenescence impacts the efficacy of immunotherapies. We will also discuss potential treatment strategies that could be combined with immunotherapies to optimize responses and mitigate adverse events (AEs) in this rapidly growing patient demographic.
2 |. THE IMPACT OF AGING ON IMMUNITY
2.1 |. Immunity and aging
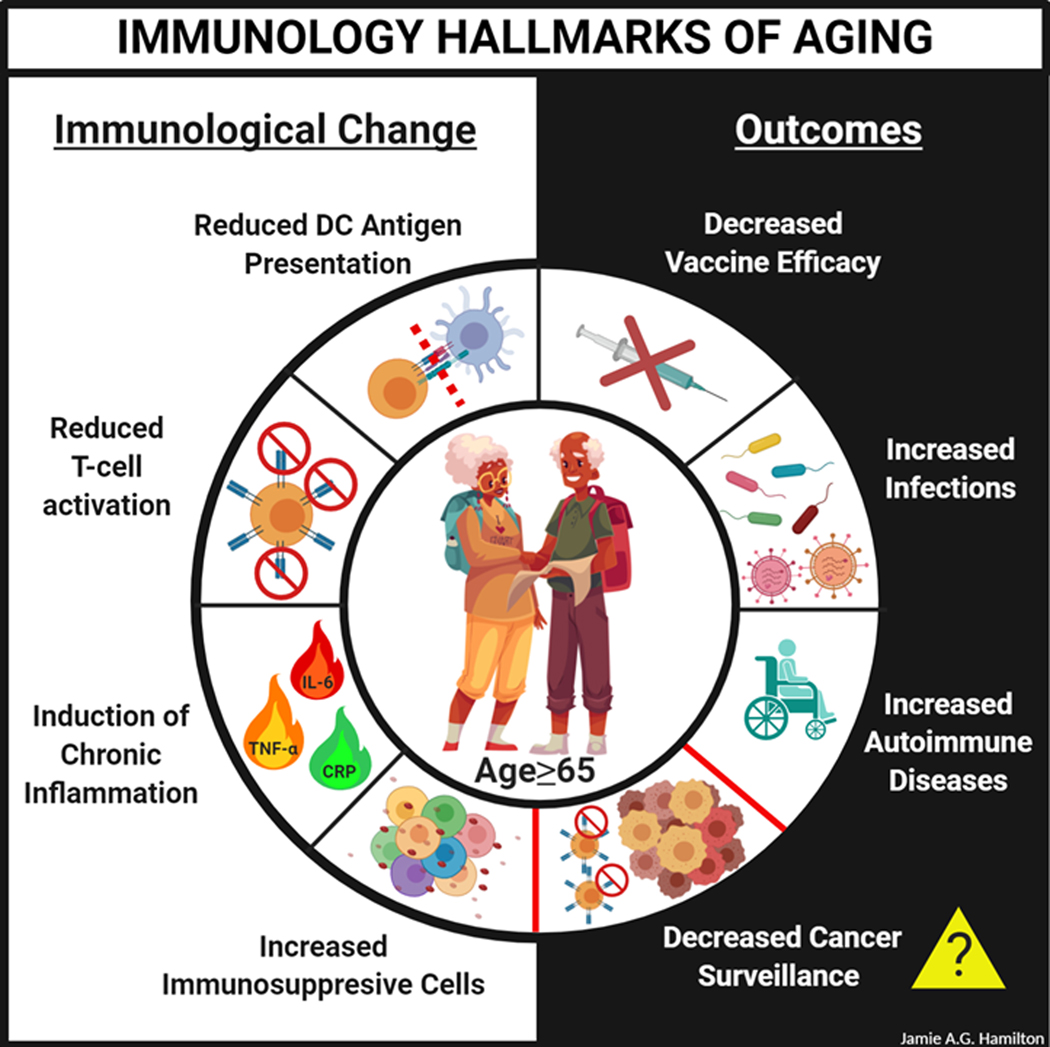
One hallmark of aging is immunological decline, known as immunosenescence, which has been documented in most vertebrates including mice and humans (Figure 1). This change in the immunological landscape is pleiotropic, marked by attenuated immune responses in T cells (CD4+ T-helper cells and CD8+ cytotoxic T cells), B cells, and innate immune cells such as dendritic cells (DCs), macrophages, and neutrophils.24 The decline in immunological integrity in elderly individuals increases their risk of infection, attenuates vaccine responses, and compromises tumor surveillance mechanisms, which increases their chances of developing cancer.24,26–28
FIGURE 1.
Aging is associated with a loss of immune homeostasis that contributes to various aging-associated pathologies
2.1.1 |. Drivers of immunosenescence
The underlying causes of aging-associated immunosenescence are still under investigation with aging-associated chronic inflammation, known as inflamm-aging, postulated to be a major driver of compromised immunity in aged mice and humans.29 The onset of this state in both species is characterized by elevated circulating levels of pro-inflammatory cytokines such as IL-1β, IL-6, IL-8, CRP, IFN-γ, and TNF-α29 and shows a strong association with aging-associated multimorbidities (co-occurring diseases) including frailty,30 heart disease,31 and cognitive impairment.32 Emerging studies in mice have demonstrated a causal role of deregulated inflammation in perturbing immunity by altering homeostasis of hematopoietic cells.33 Aging in mice is characterized by a loss of stemness in hematopoietic stem cells (HSCs) and a shift toward myelopoiesis (the production of myeloid cells).34 The latter is due, in part, to a population shift toward myeloid-biased HSCs and fewer lymphoid-biased HSCs contributing to hematopoiesis34; however, emerging studies highlighting the role of inflammation are challenging this model.35 Pro-inflammatory cytokines such as IFN-α, IFN-β, IFN-γ, TGF-β, and TNF-α act directly on HSCs to regulate the production of hematopoietic progenitor cells.36–44 A major driver of myelopoiesis is TNF-α,43,44 which is commonly found at higher systemic levels in aged mice and humans.45,46 Recently, it has been demonstrated that reducing TNF-α, IL-1β, and IL-6 levels in aged mice using the anti-inflammatory mediators alpha-1-antitrypsin (AAT) and interleukin-37 (IL-37) restores fitness parameters (e.g., key mediators of cell replication) of B-progenitor cells to almost youthful levels, whereas the aging-associated decline in the numbers of B-progenitor cells was not restored.47 Indeed, reducing inflammation in aged mice increased IL-7 signaling and the expression of nucleotide synthesis genes in B-progenitor cells to near youthful levels.47 Aging-associated alterations in hematopoiesis have also been documented in humans.33,34,48,49 When cord blood and adult bone marrow samples from young and old individuals is compared, the frequency of immunophenotypically defined hematopoietic stem and progenitor cells (HSPCs) was reduced in aged donors and gene expression analyses of these cells revealed a myeloid-megakaryocyte-erythroid bias and reduced expression of genes involved in lymphopoiesis.49 Furthermore, the aging-associated changes in human HSCs resulted in reduced proliferation, clonogenic potential, and attenuated productiton of lymphoid cells.24,48
The source of inflamm-aging is under investigation and dysbiosis has been identified as a major contributor to this phenomenon.50–53 In mice, lipopolysaccharide (LPS) from gut microbiota can accelerate inflammaging54; however, mice lacking the receptor for LPS (Toll-like receptor 4) have significantly lower levels of circulating pro-inflammatory cytokines.55 Cytokines play a key role in aging-associated increases in intestinal permeability, with TNF-α identified as a major driver of dysbiosis and compromised gut integrity.53 Additionally, in germ-free mice, the transfer of gut microbiota from aged, but not young, recipients was sufficient to induce systemic inflammation and increase the percentage of immunosuppressive T-regulatory cells (T-regs) in the spleen.56 Additionally, the accumulation of both visceral adipose tissue57,58 and senescent cells59,60 are thought to contribute to inflamm-aging. In addition to cell extrinsic factors promoting aging-associated immunosenescence, cell autonomous changes including increased DNA damage,61 mitochondrial dysfunction,62 and oxidative stress63,64 are thought to compromise the function of aged immune cells. These results suggest that treatments that reduce chronic inflammation, maintain microbial homeostasis, and mitigate mitochondrial dysfunction may improve hematopoiesis and immunity in aged hosts.
3 |. IMMUNOTHERAPIES AND NEW HORIZONS FOR ELDERLY PATIENTS
Definition of immunotherapy:
Immune-based therapies or immunotherapies are classes of treatments that prevent or target diseases with substances that regulate the immune system. Notable treatment options include recombinant cytokines (e.g., IFN-α, IL-2, and IL-12), antibodies that target immune checkpoints (e.g., PD-1, PD-L1, and CTLA-4), chimeric antigen receptor (CAR) T cells, bispecific T-cell engagers (BiTEs), and oncolytic viruses.
3.1 |. A brief history lesson on immunotherapies
The birth of immunotherapies originated from the field of bacteriology. Over 150 years ago, two German physicians, W. Busch and F. Fehleisen, documented that cancers significantly regressed in patients who accidentally developed erysipelas (a bacterial infection of the skin).65,66 W. Busch followed up this observation in 1868 by purposefully inducing erysipelas in cancer patients and again observed tumor shrinkage.65,66 In 1882, Fehleisen identified the causative agent of erysipelas as Streptococcus pyogenes.65,66 Despite these initial reports, the “Father of Immunotherapy” is widely considered to be an American surgeon named William Bradley Coley.65,66 In 1891, W.B. Coley treated bone and soft tissue sarcoma patients with Streptococcus.65,66 In his study, 66% of patients (n = 3) treated with live bacteria died, prompting him to administered heat-killed bacteria instead (which was also combined with heat-killed Serratia marcescens).65,66 This combinatorial approach was dubbed “Coley’s Toxin” and he reported high success rates in patients.65,66 However, his work was not highly appreciated or recognized at the time as it was considered anecdotal, poorly reproducible, and highly controversial.65,66
The results of Coley’s work would gain momentum in the 1960s, with the identification of T cells and their critical role in immunity being documented in 1967 by the French scientist Jacques Francis Albert Pierre Miller.65,66 The multitude of significant discoveries preceding and following the identification of T cells eventually resulted in FDA approval of immunotherapies to treat various solid tumors and hematological malignancies (Table 1).
TABLE 1.
A brief history of immunotherapies
| Immunotherapy | Year approved | Target cancer(s) |
|---|---|---|
| Interleukin-2 (IL-2) | 1991 | Metastatic kidney cancer |
| Rituximab (CD20 targeting monoclonal antibody) | 1997 | B-cell leukemia and lymphoma |
| Interleukin-2 (IL-2) | 1998 | Metastatic melanoma |
| Sipuleucel-T (activated autologous PBMCs combined with recombinant fusion protein PA2024) | 2010 | Castration-resistant prostate cancer |
| Ipilimumab (CTLA-4 targeting monoclonal antibody) | 2011 | Melanoma |
| Blinatumomab (bispecific T-cell engager [BiTE] targeting CD19 on B cells and CD3 on T cells) | 2014 | B-cell precursor acute lymphoblastic leukemia |
| Nivolumab (PD-1 targeting monoclonal antibody) | 2014, 2015 | Melanoma, non-small cell lung cancer |
| Talimogene laherparepvec (T-VEC), (first oncolytic virus) | 2015 | Metastatic melanoma |
| Elotuzmab (SLAMF7-targeting monoclonal antibody) | 2015 | Multiple myeloma |
| Pembrolizumab (PD-1 targeting monoclonal antibody) | 2017 | Urothelial cancer |
| Axicabtagene ciloleucel (CD19-directed CAR T cells) | 2017 | Large B-cell lymphoma |
| Gemtuzumab ozogamicin | 2017 | Acute myeloid leukemia |
| Tisagenlecleucel (CD19-directed CAR T-cells) | 2017, 2018 | B-cell acute lymphoblastic leukemia, diffuse large B-cell lymphoma |
| Atezolizumab (PD-L1 targeting monoclonal antibody) | 2017, 2019 | Urothelial cancer, triple-negative breast cancer |
| Nivolumab + ipilimumab + chemotherapy | 2020 | Metastatic non-small cell lung cancer |
| Gemtuzumab ozogamicin | 2020 | Acute myeloid leukemia |
This list is not exhaustive.
The revolutionary potential of immunotherapies was recently recognized by the scientific community when Drs. James Allison and Tasuku Honjo were awarded the 2018 Nobel Prize for their groundbreaking work on checkpoint molecules as potential therapeutic targets. Based on the number of active clinical trials determining the efficacy of immunotherapies for the treatment of solid and hematological malignancies, what is clear is that the burgeoning field of immunotherapy will continue to expand and additional research will be needed to understand how immune-based drugs behave in various patient demographics, particularly those with compromised immune systems.
3.2 |. Preclinical and clinical studies on aging and the efficacy of immunotherapies
Despite the increasing use of immunotherapies as treatments for patients with relapsed and refractory disease, and the growing number being tested as frontline options in clinical trials, preclinical studies using aged model systems to test the efficacy of immunotherapies and clinical trials enrolling aged patients are noticeably sparse.9,13,67,68
The scope of this section is to evaluate immunotherapy studies that used aged model systems and discuss results from clinical trials that reported AEs and treatment outcomes in aged study participants. Despite our desire to provide preclinical and clinical data summaries for each immunotherapy highlighted in Table 1, we were often hampered by the lack of reports containing one or both research modalities. Therefore, we have chosen to provide an assessment of select studies that delineate the impact of aging on the efficacy of immunotherapies in multiple disease settings. Furthermore, we attempted to summarize outcomes from clinical trials where age was independently evaluated, or the patient demographic had a median age of ≥65 years old.
3.2.1 |. αPD-1 antibody studies
The efficacy of immune checkpoint inhibitors (ICI) is partially dependent on the functional capacity of endogenous T-cells and the composition of the T-cell pool. In mice, the surface expression of PD-1 increases with age on CD4+ and CD8+ T cells resulting from a change in frequency of the T-cell repertoire from naïve to memory T cells.69 In addition to frequency changes, aged naïve CD4+ and CD8+ T cells exhibited significantly lower surface levels of CD127, CD25, and CD28 compared to naïve T cells isolated from young mice,69 which supports documented proliferative and survival defects in aged murine T cells.24 In addition to quantitative and qualitative changes in the aged T cells, similar alterations are observed in DCs. The frequency of CD8α− DCs increases in the spleen, lung, and Peyer’s patches of aged mice, whereas a larger representation of myeloid DCs is found in the lymph node and lungs of aged relative to young mice.69 In addition to this aging-associated shift in DC frequency, aged DC subsets from multiple organs expressed higher levels of PD-L1 and PD-L2.69 In functional studies, the addition of αPD-1, αPD-L1, or αPD-L2 antibody treatment with αCD3 T-cell activating antibody did not rescue proliferative defects in aged CD4+ or CD8+ T cells.69 In addition to not rescuing proliferative defects, cytokine production (IFN-γ) was only modestly increased in aged T cells treated with αPD-1, αPD-L1, or αPD-L2 antibody and augmented function was mainly observed in aged T cells not expressing PD-1.69
Despite functional defects in aged T cells and higher surface PD-1 expression, a recent study demonstrates that the efficacy of αPD-1 antibody treatment increases in aged mice and patients with melanoma.70 Aged mice (≥10 months of age) transplanted with murine melanoma cells exhibited a significant decrease in tumor burden by 2 weeks posttreatment.70 Furthermore, there was a significant increase in the frequency of IFN-γ and TNF-α producing CD8+ T cells in tumor-bearing mice receiving αPD-1 antibody treatment compared to responses observed in young (6–10 weeks of age) mice.70 In addition to functional changes in effector T-cells, the ratio of CD8+ T-cells to T-regs was significantly higher in aged mice receiving αPD-1 antibody treatment for melanoma.70 The importance of T-regs in the response to αPD-1 antibody treatment was highlighted by the observation that depleting T-regs from young tumor-bearing mice receiving αPD-1 antibody treatment led to highest degree of suppression of tumor burden compared to single-agent treatment alone.70
In humans, αPD-1 antibody treatment for melanoma led to similar outcomes (progressive disease, stable disease, and complete responses) in young (<62 years of age; n = 238), older (≥62 and ≤75 years of age; n = 300), and elderly (>80 years old; n = 62) patients.70,71 The outcomes of these studies support similar trials of this scope enrolling older patients with melanoma (clinicaltrials.gov). Similar outcomes are observed in older patients receiving αPD-1 antibody treatment for NSCLC, where overall survival (OS) rates are equivalent in young and older patients (with a stratifying age of 70).72 Furthermore, toxicity profiles are similar between patients younger and older than 75 years of age.73
In all, these results suggest that despite the declining function and increased expression of PD-1 on aged T cells, the efficacy of αPD-1 antibody treatment is effective in older patients with various malignancies. Furthermore, the clinical studies suggest that safety profiles may not be impacted by age.
3.2.2 |. αCTLA-4 antibody studies
In murine studies, αCTLA-4 antibody treatment was ineffective at prolonging the survival of aged (>12 months old) mice challenged with triple negative breast cancer (TNBC) cells, whereas young (8–10 weeks) mice exhibited a significant extension in survival.74 The lack of protection with αCTLA-4 antibody treatment in aged mice was observed in two mouse strains (Balb/c and FVB) using two TNBC cell lines (4T1 and Met1).74 In clinical studies, seven trials have initiated (with two completed), which aim to determine the impact of Ipilimumab on TNBC outcomes. At this time, no results have been reported. These results suggest that the aged microenvironment compromises the efficacy of αCTLA-4 antibody treatment for TNBC. However, reports from clinical trials will be instrumental in determining the validity of this conclusion, which is currently hampered by limited preclinical and clinical studies.
In clinical studies of melanoma, 188 aged patients (>70 years of age) were evaluated for their tumor response at baseline, at the end of induction therapy, and throughout the 3-week treatment period for AEs, including those induced by immune cells.75 The responses in patients between 70 and 80 years of age (n = 118) and those ≤70 years of age (n = 645) with melanoma appeared equivalent after αCTLA-4 antibody treatment. Furthermore, immune-related best overall response rates, immune-related partial response rates, immune-related stable disease, immune-related progressive disease, and immune-related disease control rates profiles were similar between young and older patients.75 However, in patients >80 years of age (n = 26), the immune-related best overall response rate significantly declined.75 Despite changes in the response rates, which appeared to decline in the oldest cohort of patients in this study, the progression-free survival (PFS), OS, and treatment-related AEs were not statistically different between patients under and over 70 years of age receiving αCTLA-4 antibody treatment.75 Given the small number of patients over 80 years of age included in this study, PFS and OS were not determined for this population. Therefore, additional studies are needed to determine the safety and efficacy of αCTLA-4 antibody treatment in geriatric patients with melanoma.
Overall, these results demonstrate that the efficacy of αCTLA-4 antibody treatment in older patients (<80 years) with melanoma is comparable to younger patients; however, clinical results will be useful for ascertaining this information for older patients with TNBC. Regarding safety, treatment-related AEs in aged patients receiving αCTLA-4 antibody treatment for melanoma are relatively tolerable with an average of 60% of older patients (mean age of 59) reporting serious AEs in the clinical trials analyzed in this review (Table 2).
TABLE 2.
A list of selective clinical trials registered in ClinicalTrials.gov that included ipilimumab treatment for melanoma
| Drug | Clinical trial-official title | PMID | Study id | Cancer/trial phase | Patients age Mean/ Median = X (range or SD) | Total No. of patients | Serious AE: affected/total at risk (%) |
|---|---|---|---|---|---|---|---|
| Ipilimumab | Addition of ipilimumab (MDX-010) to isolated limb infusion (ILI) with standard melphalan and dactinomycin in the treatment of advanced unresectable melanoma of the extremity | — | NCT01323517 | Mel/Phase I | Median = 64 (37–80) | 26 | 10/26 (38.46%) |
| Ipilimumab | A Phase Ib study of Yervoy with Sylatron for patients with unresectable stages IIIB/C/IV melanoma | 28031816 | NCT01496807 | Mel/Phase I | Median = 65 (38–83) | 31 | 14/31 (45.16%) |
| Ipilimumab | Pilot ipilimumab in Stage IV melanoma receiving palliative radiation therapy | 27681753 | NCT01449279 | Mel/Phase II | Mean = 59 (18–89) | 22 | 11/22 (50.00%) |
| Ipilimumab | Phase 2 study of ipilimumab plus dacarbazine in Japanese patients with advanced melanoma | 26407818 | NCT01681212 | Mel/Phase II | Mean = 55 (12.66) | 15 | 14/15 (93.33%) |
| Ipilimumab | Phase 2 study of ipilimumab in Japanese advanced melanoma patients | 26410424 | NCT01990859 | Mel/Phase II | Median = 62.5 (29–76) | 20 | 11/20 (55.00%) |
| Ipilimumab | Study of nivolumab given sequentially with ipilimumab in subjects with advanced or metastatic melanoma (CheckMate 064) | 27269740 | NCT01783938 | Mel/Phase II | Mean = 59.8 (14.61) | 138 | 51/70 (72.86%)b |
| Ipilimumab | Phase 3 trial in subjects with metastatic melanoma comparing 3 mg/kg ipilimumab versus 10 mg/kg ipilimumab | 28359784 | NCT01515189 | Mel/Phase III | Mean = 59.7 (13.92) | 726 | 3 mg/kg, 194/362 (53.59%) 10 mg/kg, 245/364 (67.31%) |
| Ipilimumab | a comparative study in Chinese subjects with chemotherapy naïve stage IV melanoma receiving ipilimumab (3 mg/kg) versus dacarbazine | — | NCT02545075 | Mel/Phase III | Mean = 53.8 (13.56) | 182 | 34/122 (27.87%)a |
| Ipilimumab | Efficacy study of ipilimumab versus placebo to prevent recurrence after complete resection of high-risk Stage III melanoma | 25840693 | NCT00636168 | Mel/Phase III | Mean = 51.1 (12.86) | 951 | 257/471 (54.56%)c |
| Ipilimumab | Ipilimumab + temozolomide in metastatic melanoma | — | NCT01119508 | Mel/Phase II | Median = 62 (33–75) | 64 | 59/64 (92.19%) |
Ipilimumab arm of trial.
Ipilimumab followed by nivolumab.
Ipilimumab arm of trial.
3.2.3 |. αCD40 and IL-2 studies
The impact of αCD40 and IL-2 combination treatment was determined in young (4 months) and old (22 months) mice.76 Unlike in young mice, old mice treated with high-dose combination therapy rapidly succumbed to a lethal cytokine release syndrome (CRS) characterized by high systemic levels of IL-6, IFN-γ, TNF-α, and severe gut, liver, and lung pathology.76 The multiorgan pathology observed in aged mice treated with immunotherapy was not impacted by the depletion of T or NK cells; however, the depletion of macrophages completely ameliorated the treatment-related toxicity observed in aged mice.76 Furthermore, depleting macrophages abrogated the lethal effects of high-dose αCD40 and IL-2 combination therapy in aged mice and this effect was also phenocopied with the TNF-α inhibitor etanercept.76 Of note, TNF-α secretion from LPS-stimulated human macrophages increased in an age-dependent manner with significant increases observed in cells from donors between 63 and 95 years of age relative to younger donors (28–59 years of age).76 In addition to protecting aged mice from the lethal effects of high-dose αCD40 and IL-2 treatment, the combination therapy of αCD40/IL-2/etanercept led to a significant extension in the survival of aged mice transplanted with Lewis lung carcinoma cells relative to those treated with αCD40/IL-2 or rIgG/PBS.76
In all, these data demonstrate that aging-associated changes in immune hemostasis can be lethal in aged mice treated with αCD40 and IL-2 combination immunotherapy. However, responses to this treatment regimen can be fined-tuned to reduce toxicity and optimize efficacy when macrophages are depleted or pro-inflammatory cytokines are directly targeted (etanercept). Recent studies have corroborated the therapeutic benefits of repolarizing77 or depleting macrophages78 as strategies to optimize αCD40 and IL-2 immunotherapy treatment in aged mice.
3.2.4 |. BiTE studies
The impact of age was assessed in two Phase 2 clinical trial reports determining the efficacy of single-agent blinatumomab (a BiTE which targets CD19 on B cells and CD3 on T cells) treatment in patients with relapsed/refractory B-precursor acute lymphoblastic leukemia (BP-ALL).79 In these studies, 225 patients were <65 years of age (median age = 34) and 36 patients were ≥65 years of age (median age = 70).79 After receiving two cycles of treatment, complete remission (CR) was achieved in 46% of younger patients and 56% of older patients,79 and survival differences did not differ between the two cohorts.79
Two clinically significant AEs known to occur with blinatumomab treatment are neurological events and CRS. Despite similar CR and OS rates, significantly more grade 3–4 neurological events occurred in older patients receiving blinatumomab treatment.79 Furthermore, the number of patients presenting with CRS was significantly higher in patients ≥65 years of age (10% for younger adults and 19% for older adults); however, no fatal treatment-related AEs were reported in this study.79
Results from this study demonstrate that blinatumomab treatment is effective in older patients (≥65 years of age) with relapsed/refractory BP-ALL; however, treatment-related AEs (particularly neurological complications and CRS) are more common in older patients.
3.3 |. In the pipeline
In this section, we review clinical trial results reported in ClinicalTrials.gov, which specifically provide data on immunotherapy outcomes in aged patients. Of the 30 trials reviewed, only 11 reported data that was stratified by age group including those over 65 years of age (Table 3). Furthermore, many of the identified studies that fit our criteria were Phase I or Phase II clinical trials, highlighting the lack of clinical data available reporting on the impact of aging on the efficacy of immunotherapies. Therefore, we focused our analysis on the presentation of AEs in aged study participants. From this analysis, serious AEs (Grade 3 or 4) were reported in 45% of older patients treated with atezolizumab (n = 4 studies), 40% of older patients treated with ipilimumab (n = 4 studies), 36% of older patients treated with pembrolizumab (n = 2 studies), and 24% of older patients treated with sipuleucel-T (n = 1 study). Although limited for reasons stated above, our analysis suggests that close to 40% of older patients receiving ICI or sipuleuclel-T therapy will experience serious AEs during treatment, which is higher than toxicity rates observed in younger patients. Moving forward, it will be important to define efficacy and safety profiles for aged patients and to stratify the data by immunotherapy class and cancer type.
TABLE 3.
A list of selective clinical trials registered in ClinicalTrials.gov that enrolled and provided data specifically on aged (>65) patients with cancer
| Drug | Clinical trial-official title | PMID | Study id | Cancer/tria phase | Patients age Mean/ Median = X (range or SD) | (Review again) Total No. of patients | Serious AE: affected/total at risk (%) |
|---|---|---|---|---|---|---|---|
| Atezolizumab | A study of atezolizumab compared with chemotherapy in participants with locally advanced or metastatic urothelial bladder cancer [IMvigor211] | 29268948 | NCT02302807 (2014–2019) | BLC/ Phase III | Mean = 65.9 | 459 | 192/459 (41.83%)a |
| Atezolizumab | A study of atezolizumab as first-line monotherapy for advanced or metastatic non-small cell lung cancer (B-F1RST) | — | NCT02848651 (2016–2020) | NSCLC/ Phase II | Mean = 68.7 | 152 | 81/152 (52.29%) |
| Atezolizumab | A study of atezolizumab in participants with programmed death-ligand 1 (PD-L1) positive locally advanced or metastatic non-small cell lung cancer (BIRCH) | 28609226 | NCT02031458 (2014–2020) | NSCLC/ Phase II | Median = 66.8 | 138 | 47/138 (34.06%)b |
| Atezolizumab | A study of atezolizumab in participants with programmed death-ligand 1 (PD-L1) positive locally advanced or metastatic non-small cell lung cancer (NSCLC) [FIR] | 29775807 | NCT01846416 (2013–2019) | NSCLC/ Phase II | Mean = 65.7 | 137 | 69/137 (50.36)c |
| Ipilimumab | A phase Ib study of Yervoy with sylatron for patients with unresectable stages IIIB/C/IV Melanoma | 28031816 | NCT01496807 (2011–2017) | Mel/Phase I | Median = 65 | 31 | 14/31 (45.16%) |
| Ipilimumab | Evaluation of circulating T cells and tumor infiltrating lymphocytes with specificities against tumor associated antigens during and after neoadjuvant chemotherapy and phased ipilimumab in non-small cell lung cancer | 29258674 | NCT01820754 (2013–2018) | NSCLC/ Phase II | Mean = 65.3 | 24 | 6/24 (25.00%) |
| Ipilimumab | A phase 2, randomized, double-blind study of ipilimumab administered at 3 mg/kg versus 10 mg/kg in adult subjects with metastatic chemotherapy-naïve castration resistant prostate cancer who are asymptomatic or minimally symptomatic | — | NCT02279862 (2014–2018) | PC/ Phase II | Mean = 66 | 51 | 21/51 (41.17%)c |
| Ipilimumab | Phase II study of combined ionizing radiation and ipilimumab in metastatic non-small cell lung cancer (NSCLC) | 30397353 | NCT02221739 (2014–2020) | NSCLC/ Phase II | Median = 68 | 39 | 19/39 (43.59%) |
| Ipilimumab | A phase I/II, open-label, dose-escalation study of MDX-010 administered every 3 weeks for four doses in patients with metastatic hormone-refractory prostate cancer | 23535954 | NCT00323882 (2006–2014) | PC/ Phase I & II | Mean = 65.7 | 71 | 35/71 (49.29%)c |
| Pembrolizumab | Randomized phase 2 trial of ACP-196 and pembrolizumab immunotherapy dual CHECKpoint inhibition in platinum resistant metastatic urothelial carcinoma (RAPID CHECK study) | — | NCT02351739 (2015–2019) | UC/ Phase II | Mean = 65.8 | 35 | 15/35 (42.86%)d |
| Pembrolizumab | Phase 2B single-site, open-label, nonrandomized study evaluating the efficacy of neoadjuvant pembrolizumab for unresectable stage III and unresectable stage IV melanoma | — | NCT02306850 (2014–2019) | MEL/ Phase II | Mean = 66.3 | 10 | 3/10 (30.00%) |
| Sipuleucel-T | A randomized, double blind, placebo controlled phase 3 trial of immunotherapy with autologous antigen presenting cells loading with PA2024 (Provenge(R), APC8015) in men with metastatic androgen independent prostatic adenocarcinoma d treatment. y. lotherapy, arm 1 of study. study. | 20818862 | NCT00065442 (2003–2010) | PC/ Phase III | Median = 71.1 | 341 | 82/338 (24.26%)e |
Patients who received treatment.
Cohort-1 in this study.
All cohorts in study.
Pembrolizumab monotherapy, arm 1 of study.
Sipuleucel-T arm of study.
4 |. CONCLUSIONS
4.1 |. Current landscape
Immunotherapies have revolutionized the medical field by providing effective treatment options for patients with relapsed and refractory diseases such as melanoma, TNBC, NSCLC, prostate cancer, B-cell acute lymphoblastic leukemia, and diffuse large B-cell lymphoma.80 Furthermore, the development of novel immunotherapies continues to rise, and these classes of drugs are more frequently being tested in clinical trials as frontline therapies in combination with standard of care protocols.80
Despite numerous success stories, many patients will relapse within 3 years of treatment initiation, and in many cases, patients will fail to respond to frontline treatment.81–84 In those that do respond to treatment, serious AEs, including the onset of CRS, can reduce therapeutic efficacy due to the early termination of the treatment protocol.85–89 Given the staggering prices associated with immunotherapy treatment (≥$100,000-$500,000 in many cases90,91) and the potential of developing treatment-related toxicities, it is imperative to identify which patients will effectively respond to immunotherapy treatment.
4.1.1 |. Efficacy
There is a growing concern that altered immunological homeostasis will negatively impact the efficacy and safety of immunotherapy treatments in aged and elderly individuals.92,93 This belief stems from the well-established immunological decline documented in aged mice and humans.24 To this end, we sought to determine whether aging adversely impacts the efficacy of various classes of immunotherapy by reviewing studies conducted in aged murine models and clinical trials enrolling aged study participants. In our assessment of preclinical literature and reviews of clinical trial data, it appears that aging does not significantly impair the efficacy αPD-1 and αCTLA-4 immunotherapy in murine models and in patients <80 years of age. In fact, it appears that older patients respond better to ICI therapies compared to younger patients. The age-dependent response differences may reflect aging-associated increases in PD-1 and CTLA-4 surface expression on T cells,69,94–96 thus the “release” from immune suppression in aged patients receiving ICI therapy may be more apparent than responses observed in young patients.
In addition to ICI therapy, the efficacy of blinatumomab was similar between young (a mean of 34 years of age; n = 225) and aged (a mean of 70 years of age; n = 36) patients with relapse/refractory BP-ALL.79 These observations suggest that aging-associated declines in T-cell function do not limit the efficacy of blinatumomab, αPD-1 antibody therapy, and αCTLA-4 antibody therapy in patients <80 years old. However, in patients >80 years old receiving αCTLA-4 antibody therapy (n = 2675) or αPD-1 antibody therapy (n = 6271) to treat melanoma, overall response rates and OS decline. These results suggest that in very old patients receiving ICI therapy, the loss of immune homeostasis maybe a major barrier to treatment success. Overall, these findings suggest that the efficacy of immunotherapies may differ in patients less than and older than 80 years of age. Before definitive conclusions can be made, future studies should include larger samples sizes (particularly those >80 years of age) and testing should be performed on more classes of immunotherapies.
4.1.2 |. Safety
Serious AEs were more frequently reported in aged mice receiving immunotherapy treatment. In preclinical models, high-dose αCD40/IL-2 combination treatment was lethal in aged but not young mice,76 which was mitigated by the TNF-α inhibitor etanercept. Furthermore, in the studies reviewed, a significant portion of aged patients developed serious AEs when receiving immunotherapy treatment, which is supported by results from ongoing clinical trials (Table 3). These observations may reflect the impact of deregulated inflammation in aged mice and humans, which may predispose older patients to serious AEs, including the manifestation of CRS after receiving immunotherapy treatment.
4.2 |. Moving forward
The usage of immunotherapies as treatments for cancer is still in its infancy. Refinements in the design of preclinical and clinical trials are necessary to determine how aging impacts the efficacy and safety of each class of immunotherapy. Specific areas of improvements should be considered moving forward.
4.2.1 |. Preclinical models
Increasing the number of immunotherapy studies conducted in aged model systems.97
Using humanized mice (reconstituted with aged hematopoietic and immune cells) to determine efficacy and safety profiles for various classes of immunotherapies and cancer types.
Standardizing what is considered “middle-aged” and “old” in murine studies. Given emerging reports that the efficacy of certain classes of immunotherapies may decline in patients >80 years of age, stratifying murine-based studies into “middle-aged; 10–14 months of age” and “old; >18 months of age” may be useful in determining the impact of advanced age on immunotherapy efficacy and safety.98–100
4.2.2 |. Clinical trials
Recruit and enroll more patients ≥65 years of age in immunotherapy-focused clinical trials: In the United States, people ≥65 years of age account for 61% of all new cancer cases and 70% of all cancer deaths; however, their enrollment in oncology-focused clinical trials active between 1993 and 1996 was only 25%.101
Report age-group specific information on AEs and survival outcomes: As reported in this review and stated by others, specific data regarding AEs and outcomes (PFS and OS) are not routinely reported for older patients enrolled in clinical trials. The ability to glean these data from current and future clinical trials will be critical in establishing safety and efficacy profiles for specific classes of immunotherapies in aged patients.
Change federal laws to require ≥50% of study participants enrolling in oncology-focused clinical trials to be ≥65 years of age when the immunotherapy being tested targets aging-associated diseases. Federal laws require that cancer trials enroll representative samples of women and members of underrepresented groups; however, these laws do not exist for elderly patients. A review of elderly patients’ enrollment in cancer drug registrations by U.S. Food and Drug Administration (USFDA) found statistically significant underrepresentation of the elderly study participants.102
4.3 |. New horizons
Immunotherapies are showing significant clinical promise in older patients with intractable diseases in the few studies conducted to date in this patient demographic. We are truly living in exciting times where older patients have more therapeutic opportunities to explore than ever before. Due to the tremendous success documented in young patients, and emerging data demonstrating efficacy in older patients, novel immune-based therapies are being generated and tested at a fast pace that will further increase treatment options moving forward.103,104
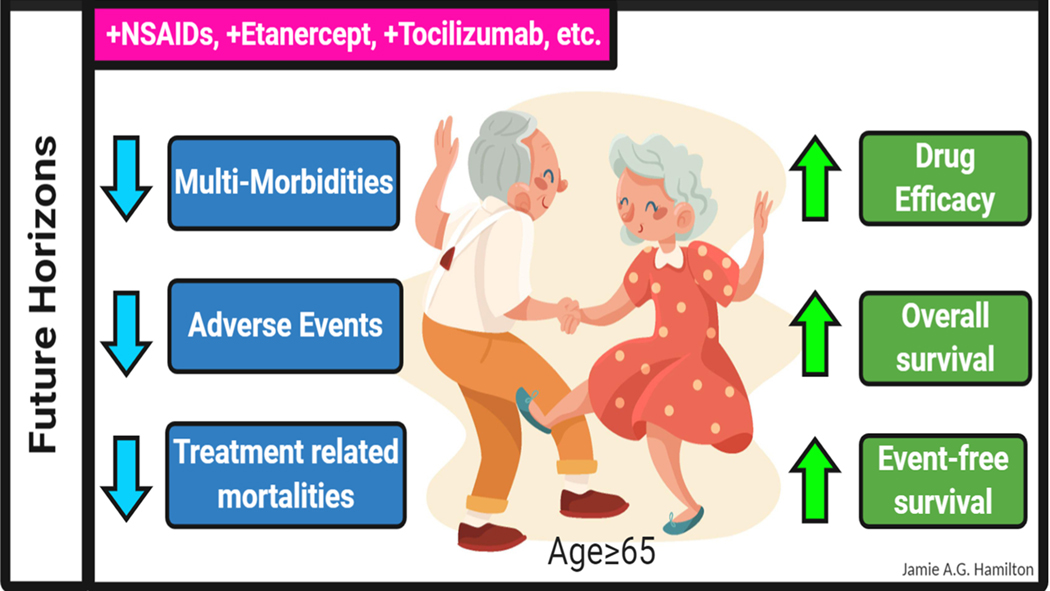
As highlighted at several points in this review, serious AEs appear to be a common manifestation in aged patients receiving immunotherapies; however, the frequency and severity of these events may be disease and treatment specific. In pediatric patients receiving CAR T-cell treatment for relapsed and refractory B-cell malignancies, CRS is a common serious AE, which is successfully mitigated by treatment with the IL-6 inhibitor tocilizumab.89,105,106 Given this observation, the inclusion of anti-inflammatory drugs in immunotherapy treatment backbones should also be explored as a therapeutic option for treating CRS in aged patients. Aging in mice and humans is accompanied by the onset of chronic inflammation (also referred to as “inflamm-aging107”), and the impact of this state on the safety and efficacy of immunotherapies in aged patients should be considered in future studies. Given the multitude of anti-inflammatory drugs that are currently approved for use in humans (Table 4), rational combinatorial treatment strategies could be easily explored in preclinical models and clinical settings.108–111 Targeting deregulated inflammation represents an attractive approach with documented preclinical and clinical success, and thus, the future of immunotherapy treatment in aged populations looks even brighter (Figure 2).
TABLE 4.
Anti-inflammatory drugs which may improve the efficacy and safety of immunotherapies in aged patients with cancer
| Drug | Current understanding of MOA | Used in patients ≥65 years old | AGS 2019 BEERS recommendation (PMID: 30693946) | Cancer-specific clinical trial ID, patients ≥65 years old |
|---|---|---|---|---|
| Diclofenac | Non-selective COX-1/COX-2 inhibition | Yes | Avoid chronic use | NCT00601640 |
| Diflunisal | Prostaglandin-synthase inhibitor | Yes | Avoid chronic use | — |
| Etanercept | Tumor necrosis factor (TNF) inhibitor | Yes | N/A | NCT00201838 |
| Etodolac | COX-2 preferential inhibitor, can inhibit COX-1 | Yes | Avoid chronic use | NCT00527319 |
| Fenoprofen | Prostaglandin-synthase inhibitor | Yes | Avoid chronic use | — |
| Flurbiprofen | Non-selective COX-1/COX-2 inhibition | Yes | Avoid chronic use | — |
| Ibuprofen | Non-selective COX-1/COX-2 inhibition | Yes | Avoid chronic use | — |
| Indomethacin | Non-selective COX-1/COX-2 inhibition | No | Avoid use | NCT00210470 |
| Ketoprofen | COX-2 preferential, but can inhibit COX-1, may inhibit bradykinin | Yes | Avoid chronic use | — |
| Ketorolac | Non-selective COX-1/COX-2 inhibition | Yes | Avoid use | NCT01806259 |
| Mefenamic acid | Non-selective COX-1/COX-2 inhibition | Yes | Avoid chronic use | — |
| Meloxicam | COX-2 preferential, can inhibit COX-1 | Yes | Avoid chronic use | — |
| Nabumetone | COX-2 preferential, can inhibit COX-1 | Yes | Avoid chronic use | — |
| Naproxen | Non-selective COX-1/COX-2 inhibition | Yes | Avoid chronic use | NCT01712009 |
| Oxaprozin | Non-selective COX-1/COX-2 inhibition | Yes | Avoid chronic use | — |
| Piroxicam | COX-1 inhibitor | Yes | Avoid chronic use | — |
| Sulindac | Non-selective COX-1/COX-2 inhibition | Yes | Avoid chronic use | NCT00368927 |
| Tocilizumab | Inhibits the binding of IL-6 to the IL-6R | Yes | N/A | NCT00883753 |
| Tolmetin | Prostaglandin-synthase inhibitor | Yes | Avoid chronic use | — |
FIGURE 2.
Combining anti-inflammatory agents with immunotherapies represents an attractive strategy that should be explored in future preclinical and clinical settings
ACKNOWLEDGMENTS
This review was supported by grants from the NIH (K01 CA160798, T32 AG000279), Emory University School of Medicine Bridge Funding (Grant No. 00098174), the UNCF/Merck Science Initiative (Grant No. 2510259), and the Winship Invest$ Pilot Grant (Project ID: 00099018) to C.J. Henry, as well as, the ASH Minority Hematology Graduate Award (Grant No. 0000055928) to J. A. G. Hamilton. The virtual abstract and Figures 1 and 2 were created using BioRender.com (user: Jamie A.G. Hamilton). We would like to thank Mrs. Adeiye A. Henry for her careful review of the manuscript.
Funding information
The Winship Invest$ Pilot Grant; Emory University School of Medicine, Grant/Award Number: 00098174;The UNCF/Merck Science Initiative; National Institutes of Health, Grant/Award Numbers: K01CA160798, T32AG000279; American Society of Hematology, Grant/Award Number: 0000055928
Footnotes
DATA AVAILABILITY STATEMENT
Data sharing not applicable and no new data are generated.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Kanasi E, Ayilavarapu S, Jones J. The aging population: demographics and the biology of aging. Periodontol 2000. 2016;2(1):13–18. [DOI] [PubMed] [Google Scholar]
- 2.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “Silver Tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rappuoli R, Pizza M, Del Giudice G, De Gregorio E. Vaccines, new opportunities for a new society. Proc Natl Acad Sci USA. 2014;111(34):12288–12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T. 2015;40(4):277–283. [PMC free article] [PubMed] [Google Scholar]
- 5.Bae J, Kim YY, Lee JS. Factors associated with subjective life expectancy: comparison With actuarial life expectancy. J Prev Med Public Health. 2017;50(4):240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lunenfeld B, Stratton P. The clinical consequences of an ageing world and preventive strategies. Best Pract Res Clin Obstet Gynaecol. 2013;27(5):643–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikolich-Zugich J, Goldman DP, Cohen PR, et al. Preparing for an aging world: engaging biogerontologists, geriatricians, and the society. J Gerontol A Biol Sci Med Sci. 2016;71(4):435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DePinho RA. The age of cancer. Nature. 2000;408(6809):248–254. [DOI] [PubMed] [Google Scholar]
- 9.Herrera AP, Snipes SA, King DW, et al. Disparate inclusion of older adults in clinical trials: priorities and opportunities for policy and practice change. Am J Public Health. 2010;100(Suppl 1):S105–S112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aapro MS, Köhne C-H, Cohen HJ, Extermann M. Never too old? Age should not be a barrier to enrollment in cancer clinical trials. Oncologist. 2005;10(3):198–204. [DOI] [PubMed] [Google Scholar]
- 11.Ruiter R, Burggraaf J, Rissmann R. Under-representation of elderly in clinical trials: an analysis of the initial approval documents in the Food and Drug Administration database. Br J Clin Pharmacol. 2019;85(4):838–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scher KS, Hurria A. Under-representation of older adults in cancer registration trials: known problem, little progress. J Clin Oncol. 2012;30(17):2036–2038. [DOI] [PubMed] [Google Scholar]
- 13.Burd CE, Gill MS, Niedernhofer LJ, et al. Barriers to the preclinical development of therapeutics that target aging mechanisms. J Gerontol A Biol Sci Med Sci. 2016;71(11):1388–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fane M, Weeraratna AT. How the ageing microenvironment influences tumour progression. Nat Rev Cancer. 2020;20(2):89106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huizer-Pajkos A, Kane AE, Howlett SE, et al. Adverse geriatric outcomes secondary to polypharmacy in a mouse model: the influence of aging. J Gerontol A Biol Sci Med Sci. 2016;71(5):571577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amlacher R, Baumgart J, Härtl A, et al. Influence of age on antileukemic action, subacute toxicity and tissue distribution of ambazone in B6D2F1 mice. Arch Geschwulstforsch. 1990;60(1):11–18. [PubMed] [Google Scholar]
- 18.von Moltke LL, Greenblatt DJ, Romach MK, Sellers EM. Cognitive toxicity of drugs used in the elderly. Dialogues Clin Neurosci. 2001;3(3):181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritz P, Vellas B. Pharmacokinetics and drug toxicity in elderly patients: a case for geriatric core data in clinical trials. J Nutr Health Aging. 2007;11(3):261–264. [PubMed] [Google Scholar]
- 20.Ghione P, Moskowitz AJ, De Paola NEK, Horwitz SM, Ruella M. Novel immunotherapies for T cell lymphoma and leukemia. Curr Hematol Malig Rep. 2018;13(6):494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maia MC, Hansen AR. A comprehensive review of immunotherapies in prostate cancer. Crit Rev Oncol Hematol. 2017;113:292–303. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez-Cerdeira C, Gregorio MC, López-Barcenas AL. Advances in immunotherapy for melanoma: a comprehensive review. Mediators Inflamm. 2017;2017:3264217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rolfo C, Caglevic C, Santarpia M, et al. Immunotherapy in NSCLC: a promising and revolutionary weapon. Adv Exp Med Biol. 2017;995:97–125. [DOI] [PubMed] [Google Scholar]
- 24.Henry CJ, Marusyk A, DeGregori J. Aging-associated changes in hematopoiesis and leukemogenesis: what’s the connection? Aging (Albany NY). 2011;3(6):643–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung HY, Kim DH, Lee EK, et al. Redefining chronic inflammation in aging and age-related diseases: proposal of the senoinflammation concept. Aging Dis. 2019;10(2):367–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinberger B. Vaccines for the elderly: current use and future challenges. Immun Ageing. 2018;15:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lord JM. The effect of ageing of the immune system on vaccination responses. Hum Vaccin Immunother. 2013;9(6):1364–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fulop T, Larbi A, Kotb R, de Angelis F, Pawelec G. Aging, immunity, and cancer. Discov Med. 2011;11(61):537–550. [PubMed] [Google Scholar]
- 29.Franceschi C, Capri M, Monti D, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128(1):92–105. [DOI] [PubMed] [Google Scholar]
- 30.Cesari M, Penninx BWJH, Pahor M, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59(3):242–248. [DOI] [PubMed] [Google Scholar]
- 31.Partridge L, Deelen J, Slagboom PE. Facing up to the global challenges of ageing. Nature. 2018;561(7721):45–56. [DOI] [PubMed] [Google Scholar]
- 32.Yaffe K, Lindquist K, Kluse M, et al. Telomere length and cognitive function in community-dwelling elders: findings from the Health ABC Study. Neurobiol Aging. 2011;32(11):2055–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovtonyuk LV, Fritsch K, Feng X, Manz MG. Inflamm-aging of hematopoiesis, hematopoietic stem cells, and the bone marrow microenvironment. Front Immunol. 2016;7:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Groarke EM, Young NS. Aging and hematopoiesis. Clin Geriatr Med. 2019;35(3):285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montecino-Rodriguez E, Kong Y, Casero D, et al. Lymphoid-biased hematopoietic stem cells are maintained with age and efficiently generate lymphoid progeny. Stem Cell Rep. 2019;12(3):584–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Bruin AM, Demirel O, Hooibrink B, et al. Interferon-gamma impairs proliferation of hematopoietic stem cells in mice. Blood. 2013;121(18):3578–3585. [DOI] [PubMed] [Google Scholar]
- 37.Sawamiphak S, Kontarakis Z, Stainier DY. Interferon gamma signaling positively regulates hematopoietic stem cell emergence. Dev Cell. 2014;31(5):640–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qin Y, Fang K, Lu N, Hu Y, Tian Z, Zhang C. Interferon gamma inhibits the differentiation of mouse adult liver and bone marrow hematopoietic stem cells by inhibiting the activation of notch signaling. Stem Cell Res Ther. 2019;10(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith JN, Kanwar VS, MacNamara KC. Hematopoietic stem cell regulation by type I and II interferons in the pathogenesis of acquired aplastic anemia. Front Immunol. 2016;7:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Passegue E, Ernst P. IFN-alpha wakes up sleeping hematopoietic stem cells. Nat Med. 2009;15(6):612–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blank U, Karlsson S. TGF-beta signaling in the control of hematopoietic stem cells. Blood. 2015;125(23):3542–3550. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Dong F, Zhang S, et al. TGF-beta1 negatively regulates the number and function of hematopoietic stem cells. Stem Cell Rep. 2018;11(1):274–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamashita M, Passegue E. TNF-alpha coordinates hematopoietic stem cell survival and myeloid regeneration. Cell Stem Cell. 2019;25(3):357–372 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rezzoug F, Huang Y, Tanner MK, et al. TNF-alpha is critical to facilitate hemopoietic stem cell engraftment and function. J Immunol. 2008;180(1):49–57. [DOI] [PubMed] [Google Scholar]
- 45.Bruunsgaard H, Skinhøj P, Pedersen AN, Schroll M, Pedersen BK. Ageing, tumour necrosis factor-alpha (TNF-alpha) and atherosclerosis. Clin Exp Immunol. 2000;121(2):255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giovannini S, Onder G, Liperoti R, et al. Interleukin-6, C-reactive protein, and tumor necrosis factor-alpha as predictors of mortality in frail, community-living elderly individuals. J Am Geriatr Soc. 2011;59(9):1679–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henry CJ, Casás-Selves M, Kim J, et al. Aging-associated inflammation promotes selection for adaptive oncogenic events in B cell progenitors. J Clin Invest. 2015;125(12):4666–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee J, Yoon SR, Choi I, Jung H. Causes and mechanisms of hematopoietic stem cell aging. Int J Mol Sci. 2019;20(6). 10.3390/ijms20061272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pang WW, Price EA, Sahoo D, et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci U S A. 2011;108(50):20012–20017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buford TW. (Dis)Trust your gut: the gut microbiome in age-related inflammation, health, and disease. Microbiome. 2017;5(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aleman FDD, Valenzano DR. Microbiome evolution during host aging. PLoS Pathog. 2019;15(7):e1007727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thevaranjan N, Puchta A, Schulz C, et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe. 2017;21(4):455–466 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tran L, Greenwood-Van Meerveld B. Age-associated remodeling of the intestinal epithelial barrier. J Gerontol A Biol Sci Med Sci. 2013;68(9):1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim KA, Jeong J-J, Yoo S-Y, Kim D-H. Gut microbiota lipopolysaccharide accelerates inflamm-aging in mice. BMC Microbiol. 2016;16:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghosh AK, O’Brien M, Mau T, Yung R. Toll-like receptor 4 (TLR4) deficient mice are protected from adipose tissue inflammation in aging. Aging (Albany NY). 2017;9(9):1971–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fransen F, van Beek AA, Borghuis T, et al. Aged gut microbiota contributes to systemical inflammaging after transfer to germfree mice. Front Immunol. 2017;8:1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trim W, Turner JE, Thompson D. Parallels in immunometabolic adipose tissue dysfunction with ageing and obesity. Front Immunol. 2018;9:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen G, Yung R. Meta-inflammaging at the crossroad of geroscience. Aging Med (Milton). 2019;2(3):157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olivieri F, Prattichizzo F, Grillari J, Balistreri CR. Cellular senescence and inflammaging in age-related diseases. Mediators Inflamm. 2018;2018:9076485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rea IM, Gibson DS, McGilligan V, et al. Age and age-related diseases: role of inflammation triggers and cytokines. Front Immunol. 2018;9:586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ioannidou A, Goulielmaki E, Garinis GA. DNA damage: from chronic inflammation to age-related deterioration. Front Genet. 2016;7:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Picca A, Lezza AMS, Leeuwenburgh C, et al. Fueling inflammaging through mitochondrial dysfunction: mechanisms and molecular targets. Int J Mol Sci. 2017;18(5):933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zuo L, Prather ER, Stetskiv M, et al. Inflammaging and oxidative stress in human diseases: from molecular mechanisms to novel treatments. Int J Mol Sci. 2019;20(18):4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bullone M, Lavoie JP. The contribution of oxidative stress and inflamm-aging in human and equine asthma. Int J Mol Sci. 2017;18(12). 10.3390/ijms18122612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dobosz P, Dzieciatkowski T. The intriguing history of cancer immunotherapy. Front Immunol. 2019;10:2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Decker WK, da Silva RF, Sanabria MH, et al. Cancer immunotherapy: historical perspective of a clinical revolution and emerging preclinical animal models. Front Immunol. 2017;8:829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kazmierska J. Do we protect or discriminate? Representation of senior adults in clinical trials. Rep Pract Oncol Radiother. 2012;18(1):6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Santulli G, Borras C, Bousquet J, et al. Models for preclinical studies in aging-related disorders: one is not for all. Transl Med UniSa. 2015;13:4–12. [PMC free article] [PubMed] [Google Scholar]
- 69.Lages CS, Lewkowich I, Sproles A, Wills-Karp M, Chougnet C. Partial restoration of T-cell function in aged mice by in vitro blockade of the PD-1/PD-L1 pathway. Aging Cell. 2010;9(5):785798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kugel CH 3rd, Douglass SM, Webster MR, et al. Age correlates with response to anti-PD1, reflecting age-related differences in intratumoral effector and regulatory T-cell populations. Clin Cancer Res. 2018;24(21):5347–5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ben-Betzalel G, Steinberg-Silman Y, Stoff R, et al. Immunotherapy comes of age in octagenarian and nonagenarian metastatic melanoma patients. Eur J Cancer. 2019;108:61–68. [DOI] [PubMed] [Google Scholar]
- 72.Caterino JM, Valasek T, Werman HA. Identification of an age cutoff for increased mortality in patients with elderly trauma. Am J Emerg Med. 2010;28(2):151–158. [DOI] [PubMed] [Google Scholar]
- 73.Nosaki K, Saka H, Hosomi Y, et al. Safety and efficacy of pembrolizumab monotherapy in elderly patients with PD-L1-positive advanced non-small-cell lung cancer: pooled analysis from the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 studies. Lung Cancer. 2019;135:188–195. [DOI] [PubMed] [Google Scholar]
- 74.Sceneay J, Goreczny GJ, Wilson K, et al. Interferon signaling is diminished with age and is associated with immune checkpoint blockade efficacy in triple-negative breast cancer. Cancer Discov. 2019;9(9):1208–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chiarion Sileni V, Pigozzo J, Ascierto PA, et al. Efficacy and safety of ipilimumab in elderly patients with pretreated advanced melanoma treated at Italian centres through the expanded access programme. J Exp Clin Cancer Res. 2014;33:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bouchlaka MN, Sckisel GD, Chen M, et al. Aging predisposes to acute inflammatory induced pathology after tumor immunotherapy. J Exp Med. 2013;210(11):2223–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jackaman C, Dye DE, Nelson DJ. IL-2/CD40-activated macrophages rescue age and tumor-induced T cell dysfunction in elderly mice. Age (Dordr). 2014;36(3):9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duong L, Radley-Crabb HG, Gardner JK, et al. Macrophage depletion in elderly mice improves response to tumor immunotherapy, increases anti-tumor T cell activity and reduces treatment-induced cachexia. Front Genet. 2018;9:526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kantarjian HM, Stein AS, Bargou RC, et al. Blinatumomab treatment of older adults with relapsed/refractory B-precursor acute lymphoblastic leukemia: results from 2 phase 2 studies. Cancer. 2016;122(14):2178–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18(3):197–218. [DOI] [PubMed] [Google Scholar]
- 81.Lee DS, White DE, Hurst R, Rosenberg SA, Yang JC. Patterns of relapse and response to retreatment in patients with metastatic melanoma or renal cell carcinoma who responded to interleukin-2-based immunotherapy. Cancer J Sci Am. 1998;4(2):86–93. [PubMed] [Google Scholar]
- 82.Nixon NA, Blais N, Ernst S, et al. Current landscape of immunotherapy in the treatment of solid tumours, with future opportunities and challenges. Curr Oncol. 2018;25(5):e373–e384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu X, Sun Q, Liang X, et al. Mechanisms of relapse after CD19 CAR T-cell therapy for acute lymphoblastic leukemia and its prevention and treatment strategies. Front Immunol. 2019;10:2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer. 2018;118(1):9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abdel-Wahab N, Alshawa A, Suarez-Almazor ME. Adverse events in cancer immunotherapy. Adv Exp Med Biol. 2017;995:155–174. [DOI] [PubMed] [Google Scholar]
- 86.Puzanov I, Abdallah K, Bingham CO, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer(SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arnaud-Coffin P, Maillet D, Gan HK, et al. A systematic review of adverse events in randomized trials assessing immune checkpoint inhibitors. Int J Cancer. 2019;145(3):639–648. [DOI] [PubMed] [Google Scholar]
- 88.Kroschinsky F, Stölzel F, von Bonin S, et al. New drugs, new toxicities: severe side effects of modern targeted and immunotherapy of cancer and their management. Crit Care. 2017;21(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Siddiqui M, Rajkumar SV. The high cost of cancer drugs and what we can do about it. Mayo Clin Proc. 2012;87(10):935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dusetzina SB. Drug pricing trends for orally administered anticancer medications reimbursed by commercial health plans, 2000–2014. JAMA Oncol. 2016;2(7):960–961. [DOI] [PubMed] [Google Scholar]
- 92.van Holstein Y, Kapiteijn E, Bastiaannet E, et al. Efficacy and adverse events of immunotherapy with checkpoint inhibitors in older patients with cancer. Drugs Aging. 2019;36(10):927–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hurez V, Daniel BJ, Sun L, et al. Mitigating age-related immune dysfunction heightens the efficacy of tumor immunotherapy in aged mice. Cancer Res. 2012;72(8):2089–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leng Q, Bentwich Z, Borkow G. CTLA-4 upregulation during aging. Mech Ageing Dev. 2002;123(10):1419–1421. [DOI] [PubMed] [Google Scholar]
- 95.Canaday DH, Parker KE, Aung H, et al. Age-dependent changes in the expression of regulatory cell surface ligands in activated human T-cells. BMC Immunol. 2013;14:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McClanahan F, Riches JC, Miller S, et al. Mechanisms of PD-L1/PD-1-mediated CD8 T-cell dysfunction in the context of aging-related immune defects in the Emicro-TCL1 CLL mouse model. Blood. 2015;126(2):212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mitchell SJ, Scheibye-Knudsen M, Longo DL, et al. Animal models of aging research: implications for human aging and age-related diseases. Annu Rev Anim Biosci. 2015;3:283–303. [DOI] [PubMed] [Google Scholar]
- 98.Pinchuk LM, Filipov NM. Differential effects of age on circulating and splenic leukocyte populations in C57BL/6 and BALB/c male mice. Immun Ageing. 2008;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jackson SJ, Andrews N, Ball D, et al. Does age matter? The impact of rodent age on study outcomes. Lab Anim. 2017;51(2):160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pieren DKJ, Smits NAM, van de Garde MDB, et al. Response kinetics reveal novel features of ageing in murine T cells. Sci Rep. 2019;9(1):5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341(27):2061–2067. [DOI] [PubMed] [Google Scholar]
- 102.Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22(22):4626–4631. [DOI] [PubMed] [Google Scholar]
- 103.Paczesny S, Pavletic SZ, Bollard CM. Introduction to a review series on emerging immunotherapies for hematologic diseases. Blood. 2018;131(24):2617–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015;125(9):3335–3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kotch C, Barrett D, Teachey DT. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert Rev Clin Immunol. 2019;15(8):813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maude SL, Barrett D, Teachey DT, Grupp SA, et al. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014;20(2):119–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Valiathan R, Ashman M, Asthana D. Effects of ageing on the immune system: infants to elderly. Scand J Immunol. 2016;83(4):255–266. [DOI] [PubMed] [Google Scholar]
- 108.Ali N, Haematology F. Chimeric antigen T cell receptor treatment in hematological malignancies. Blood Res. 2019;54(2):81–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yanez L, Sanchez-Escamilla M, Perales MA. CAR T cell toxicity: current management and future directions. Hemasphere. 2019;3(2):e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127(26):3321–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dholaria BR, Bachmeier CA, Locke F. Mechanisms and management of chimeric antigen receptor T-cell therapy-related toxicities. BioDrugs. 2019;33(1):45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]