Summary
The cancer research field is finally starting to unravel the mystery behind why males have a higher incidence and mortality rate than females for nearly all cancer types of the non-reproductive systems. Here, we explain how sex – specifically sex chromosomes and sex hormones – drives differential adaptive immunity across immune-related disease states including cancer, and why males are consequently more predisposed to tumor development. We highlight emerging data on the roles of cell-intrinsic androgen receptors in driving CD8+ T cell dysfunction or exhaustion in the tumor microenvironment and summarize ongoing clinical efforts to determine the impact of androgen blockade on cancer immunotherapy. Finally, we outline a framework for future research in cancer biology and immuno-oncology, underscoring the importance of a holistic research approach to understanding the mechanisms of sex dimorphisms in cancer, so sex will be considered as an imperative factor for guiding treatment decisions in the future.
Subject areas: Physiology, Immunology, Cancer
Graphical abstract
Physiology; Immunology; Cancer
Introduction
Sexual dimorphisms are most apparent in reproductive organ development and overall male versus female physical attributes, such as height, physical stature, and muscle mass. However, epidemiological and molecular studies demonstrate differences in sex can also be present through disease manifestation (Clocchiatti et al., 2016; Ober et al., 2008 ). Females exhibit a more responsive innate and adaptive immune response compared with males when faced with foreign pathogens (bacteria, viruses, parasites, and fungi) (Markle and Fish, 2014) and as such, generate an increased amount of inflammation, antibody production, T cell responses, and consequently, more effective pathogen clearance (Klein et al., 2010). Females also have a greater immune response to vaccines, and therefore, are less susceptible to infection – a prime example being the fewer hospitalizations and lower mortality as a result of SARS-CoV-2 infection compared with males (Bunders and Altfeld, 2020; Sette and Crotty, 2021). Whereas females have a more responsive immune system, they are also more susceptible to excessive inflammation and autoimmune diseases, such as lupus and rheumatoid arthritis (Klein and Flanagan, 2016).
Given the vital role of the immune system in monitoring healthy cell function and regulating tumorigenesis, a less effective immune system present in males also means therein lies a higher risk and worse prognosis for a large range of non-reproductive-related cancer cell types, such as cancers of the bladder, colon, esophagus, head and neck, skin, lung, and liver (Sung et al., 2021). A notable exception is thyroid cancer, where the incidence rate for females is substantially higher (Sung et al., 2021; Zhu et al., 2019 ). Whereas the increased risk for cancer in the male population was previously attributed to environmental or behavioral factors, such as a greater exposure to environmental carcinogens (Bertin et al., 2018) and/or a greater propensity for risk behaviors such as smoking or alcohol consumption (Zang and Wynder, 1996), recent advances in omics technologies and analytical methods have allowed for deeper, more molecular investigations into how human health and disease differ between males and females.
Several genetic postulates have been proposed to explain the sexual dimorphisms observed in cancer development, including the impact of evolution and heritable traits, biological effects from sex hormones and sex chromosomes, and the development of genetic insults from environmental carcinogens (Khramtsova et al., 2019). Whereas all models contribute to tumorigenesis, through either independent or compounded measures, the contribution of sex chromosomes and sex hormones (estrogens and androgens) to male versus female immune cell responses in driving tumor progression has not been well studied. Imperfect systems and inadequate experimental tools have also led to much confusion. Hence, clarifying the roles of sex bias in adaptive immunity against cancer is one of the main focuses of this review. Whereas estrogens (higher in females) have been shown to increase the production of survival cytokines, induce excretion of immunoglobulins, and modulate T cell activity, androgens (higher in males) stimulate anti-inflammatory cytokines, reduce antibody production, and decrease T cell proliferation (Ben-Batalla et al., 2020; Irelli et al., 2020). The primary biological actions of androgens involve binding to the androgen receptor (AR), a ligand-dependent nuclear transcription factor (TF), that is highly expressed in the male reproductive system, such as prostate tissue, but is also widely expressed in a variety of innate and adaptive immune cells including, but not limited to, neutrophils, monocytes, macrophages, immature B cells, and T cells (Ben-Batalla et al., 2020; Benten et al., 2002; Gubbels Bupp and Jorgensen, 2018; Viselli et al., 1997; Walecki et al., 2015).
Whereas mechanisms by which androgens mediate immune suppression are incompletely understood, targeting AR in combination with other immunotherapeutic approaches, such as immune checkpoint blockade (ICB), remains of great interest for treating prostate and non-prostate malignancies. This review aims to highlight the decades of evidence on how immune-related gene and protein expression between sexes translates into differential disease progression – and to emphasize the need to study how host adaptive immunity contributes to sex-biased cancer outcomes – for eventual translation of knowledge into better management of cancers for both sexes.
Overview of sex dimorphism in physiologic immune response
The immune system is a complex network of cells, tissues, and organs that function to distinguish pathogens and dysplastic cells from healthy tissue and to defend the body from disease development. The innate immune system is one of the first lines of defense against foreign pathogens initiated by pattern recognition receptors (PRRs) [e.g., Toll-like receptors (TLRs)], which generate rapid non-specific inflammatory responses to control the proliferation and spread of invading organisms. Whereas the innate and adaptive immune systems have separate, distinct roles in controlling the spread of disease, members of the innate immune system, particularly antigen-presenting cells (APCs), such as macrophages and dendritic cells (DCs), communicate with and instruct the adaptive immune system to amplify immune responses and initiate longer term memory toward a given pathogen. Before discussing contributing factors to differing immune profiles between sexes and how they relate to disease manifestation, we will first briefly review baseline differences in innate APCs and the adaptive immune system between healthy males and females.
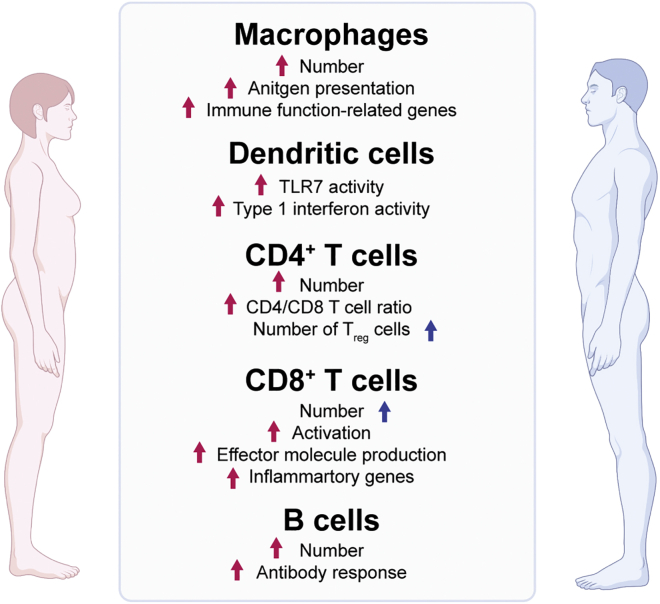
Most of the work on this topic in humans has been descriptive, focusing on understanding sex differences in the composition and function of peripheral blood mononuclear cells (PBMCs). In line with females having a more responsive immune system compared with males, growing evidence indicates that APCs in females are more functional in terms of proinflammation and antigen presentation, potentially owing to higher expression of MHC-II and cell surface co-stimulatory molecules (Figure 1) ( Gubbels Bupp, 2015; Gubbels Bupp et al., 2008; Togno-Peirce et al., 2013; Weinstein et al., 1984). Female PBMCs and DCs also produce 70% higher amounts of interferon-α (IFNα) upon TLR7 ligand stimulation, resulting in a stronger inflammatory immune response (Berghöfer et al., 2006; Griesbeck et al., 2015; Seillet et al., 2012). At baseline, healthy adult females have higher macrophage and CD4+ T cell counts, a higher CD4+/CD8+ ratio, and a lower number of regulatory T cells (Tregs) compared with males (Figure 1) (Abdullah et al., 2012; Ahnstedt et al., 2018; Lee et al., 1996; Scotland et al., 2011). Using mouse models, it is important to point out that very few differentially expressed genes are seen between male and female immune cells except in macrophages, especially after interferon stimulation (Gal-Oz et al., 2019). In general, CD8+ T cells from healthy females are more activated, produce higher levels of effector molecules, such as IFN-γ, TNF-α, and granzyme B, and express more inflammatory genes upon repeat stimulation ex vivo (Ahnstedt et al., 2018; Huang et al., 2021). As for humoral immunity, females are consistently found to possess a greater number of B cells (Abdullah et al., 2012), higher basal levels of immune globulin M (IgM) (Butterworth et al., 1967), and a stronger antibody response upon vaccination than males (Figure 1) (Furman et al., 2014; Huang et al., 2021). By examining 172 normal subjects longitudinally, males had a greater age-related decline of naive T and B cells (Márquez et al., 2020). Collectively, females demonstrate enhanced pro-inflammatory cytokine production, antigen presentation, T cell activation, and B cell response compared with males. The real questions that remain are – what contributes to varying immune cell profiles, how do they relate to differing disease pathologies, and how can we use this information to improve the clinical care of patients?
Figure 1.
Overview of sex bias in immunity
List of differential immune cell numbers and characteristics between males and females. Red and blue arrows indicate a female or male bias, respectively. Figure adapted from images created with BioRender.com.
Evidence of sex-biased immune regulation in human disease and vaccine response
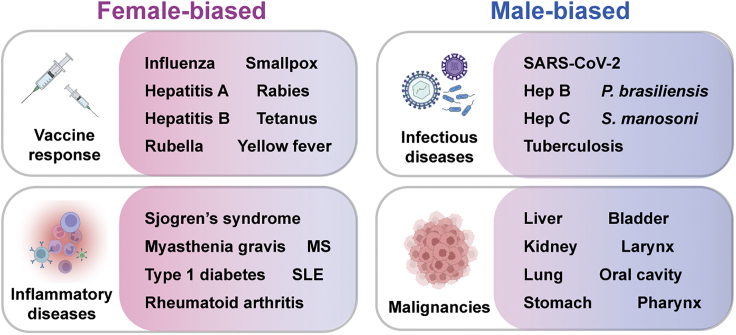
In terms of pathogenesis, prevalence, and severity of infection, substantial evidence details how males are more susceptible to infection by bacteria [e.g., Vibrio vulnificus (McClelland and Smith, 2011), Mycobacterium marinum (Yamamoto et al., 1991), and tuberculosis (TB) (Guerra-Silveira and Abad-Franch, 2013)], viruses [e.g., HIV (Sabra and Klein, 2015) and hepatitis C virus (HCV) (Grebely et al., 2014)], fungi (e.g., Cryptococcus neoformans and Paracoccidioides brasiliensis; McClelland and Smith, 2011), and parasites (e.g., Schistosoma manosoni, Plasmodium falciparum, Entamoeba histolytica, Wuchereria bancrofti, and Necator americanus;(Sabra and Klein, 2015) than females (Figure 2). Sex differences remain regardless of the route of transmission. Detailed further below, whereas females exhibit a greater degree of immunity against disease, they may also present with more severe symptoms, and these inflammatory side effects of fighting infection can in turn lead to increased rates of mortality. To emphasize differences in immunity between males and females and how these differences relate to cancer development and progression, we will provide an overview of immunologic differences between sexes for infectious and inflammatory diseases, as well as vaccine responsiveness.
Figure 2.
Sex-biased disease susceptibility and response to vaccines
Representative examples of female-biased (left) and male-biased (right) immune responses and disease acquisition. MS, multiple sclerosis. SLE, system lupus erythematosus. Hep, hepatitis. Figure adapted from images created with BioRender.com.
Bacterial infections
Several noteworthy studies found that altered levels of systemic sex hormones provide a potential mechanistic rationale for differences between sexes in susceptibility and mortality from bacterial infection. Using a rat model that recapitulates the male-biased (85%) V. vulnificus-mediated sepsis observed in humans, Merkel and colleagues demonstrated that oophorectomy resulted in lower estrogen levels, which correlated with decreased survival when subjected to V. vulnificus lipopolysaccharide (LPS). Further, estrogen replacement ameliorated disease severity and increased survival when given to both males and females after gonadectomy (Merkel et al., 2001). Another study evaluating M. marinum infection in male mice demonstrated how fertile males exhibited more severe disease pathologies compared with surgically castrated males, which could be reversed by continuous testosterone treatment. Similarly, the susceptibility of female mice to infection increased with exogenous testosterone (Yamamoto et al., 1991). Notably, a greater number of macrophages were found at the site of infection in females than males, and by performing T cell depletion and adoptive T cell transfer experiments, sex differences were found to be mediated by sex hormones as well as intrinsic sex-specific T cell function (Yamamoto et al., 1991).
Globally, TB, an infectious disease caused by Mycobacterium tuberculosis, is more common among males than females. A retrospective analysis of females who had undergone oophorectomy owing to salpingitis found the mortality rate increased to 7% compared with 0.7% in the general female population (Svanberg, 1981). In another study, conducted in 1969, males who had been castrated died from TB at a rate of 8.1% compared with normal males at 20.6% (Hamilton and Mestler, 1969). Overall, few studies have been conducted to evaluate sex hormones in the context of TB susceptibility. More recently, Hertz and colleagues discovered the presence of smaller B cell follicle formation in the lungs of male TB-infected mice compared with females (Hertz et al., 2020). Contrary to the above reports, Mycobacterium avium complex (MAC) pulmonary disease is an infection occurring at an increased frequency in females versus males. However, MAC tends to manifest in post-menopausal females where estrogen levels have substantially decreased (Han et al., 2005). After performing bilateral oophorectomy on female mice, the burden of MAC bacilli in the lungs increased, which was rescued to normal levels in the presence of exogenous estradiol treatment (Tsuyuguchi et al., 2001).
Viral infections
The prevalence of viral infections, such as SARS-CoV-2, HIV, HCV, and Hepatitis B Virus (HBV), is also higher in males than females, but disease outcomes vary between infection types. Whereas females tend to have decreased plasma viral loads, >40% less circulating HIV RNA, higher CD4+ T cell counts, and greater CD8+ T cell activation compared with males, they also have a higher risk of progressing to AIDS (Collazos et al., 2007; Sabra and Klein, 2015). As mentioned, persistent chronic inflammation in females can have adverse effects and in turn, damage the immune system and contribute to pathology. When females are exposed to HIV-1, TLR7 ligands in DCs become hyperactivated, high levels of TNF-α are produced, and stronger CD8+ T cell activation occurs compared with males – this inflammatory state is thought to account for the female-biased disease progression (Meier et al., 2009). In contrast, males have a higher frequency of serum HBV antigens and viral DNA and are more likely to develop hepatocellular carcinoma than females (vom Steeg and Klein, 2016). Similarly, females are more likely to spontaneously clear HCV, and males have a higher risk of developing cirrhosis after chronic HCV infection (Grebely et al., 2014; Rodríguez-Torres et al., 2006). Importantly, sex differences in cirrhosis and fibrotic progression are attenuated after menopause, and the severity of the disease can be ameliorated by estrogen hormone replacement therapy (Di Martino et al., 2004).
Vaccine response
Of note, the differential immune response between sexes also occurs after vaccination, with females reporting more severe local and systemic reactions (e.g., redness, muscle pain, headache, fever, and fatigue) to bacterial and viral vaccines (Cook, 2008). Whereas these observations could be attributed to reporting bias, corresponding antibody production in females also reflects differing responses to vaccination (Potluri et al., 2019; Cook, 2008). The elevated antibody response is primarily observed in younger reproductive females. Epidemiologic studies indicate that similar to the increase in non-responsiveness observed in males to HBV vaccination, females tend to lose much of their immune privilege after menopause (Potluri et al., 2019; Vermeiren et al., 2013). Estradiol has been shown to increase the efficacy of genital herpes simplex virus two vaccinations (Bhavanam et al., 2008; Pennock et al., 2009), and when combined with influenza vaccination, estradiol was able to rescue antibody titer levels in a post-menopausal mouse model (Nguyen et al., 2011). Giefing-Kroll and colleagues demonstrated that lower infection rates and higher antibody titers to vaccination for Hepatitis A, Hepatitis B, and Pneumococcus among females become equivalent to those of males in the elderly (post-menopause). Conversely, in male-biased vaccine responses, as seen with Tetanus and Diphtheria, antibody titers either remain higher in males or equivalent to females with age (Giefing-Kröll et al., 2015). Additional studies are needed to determine which contributing factors (e.g., environmental, genetic, or hormonal), or a combination thereof, result in the described differential infection and vaccine responses between sexes.
Autoimmune diseases
Given the apparent intensified immune system present in females, females present with a much higher frequency of inflammatory diseases and autoimmunity. Females account for nearly 80% of all autoimmune cases in the United States (Jacobson et al., 1997), presenting more commonly with Sjogren’s syndrome, system lupus erythematosus (SLE), rheumatoid arthritis, multiple sclerosis, and myasthenia gravis (Whitacre, 2001). Animal models that have helped to elucidate hormonal and immunological sex differences in disease progression include the non-obese diabetic (NOD) mouse model of spontaneous type 1 diabetes and the experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis. Several studies demonstrate that, in part, androgens exhibit protective effects. Both animal models have lower disease incidence and severity in male compared with female mice that increases after castration (Ahmed and Penhale, 1982; Fitzpatrick et al., 1991; Fox, 1992; Harbuz et al., 1995). Testosterone levels are lower during EAE relapse (Bebo et al., 1998), and if testosterone is given as a topical treatment for multiple sclerosis, brain atrophy is slowed and peripheral immune responses change: CD4+ T cell number and IL-2 production from PBMCs decrease, whereas NK cells and TGF-β production from PBMCs increase (Gold et al., 2008). Collectively, these results further demonstrate the immunosuppressive effects of endogenous androgens that also appear to play a role in tumor development and progression.
Malignancies
A well-known aspect of tumor biology is the interplay between tumor and immune cells, in conjunction with other cell types in the TME. Generally speaking, the immune system has an innate ability to defend the body from pathogens as well as to identify and destroy nascent malignant cells. It does so by providing protection against viral infections prone to inducing tumorigenesis, resolving inflammatory states that induce a tumorigenic environment, and recognizing tumor-specific antigens and cell stress-induced molecules (Swann and Smyth, 2007). In line with the differential responses to infection and other immune-related pathologies described above, the differing rates of tumor incidence and cancer-related deaths between sexes are due, in part, to differing immune cell numbers, phenotypes, and anti-tumor cytotoxicities.
With the primary exception of thyroid cancer, males have a greater risk of cancer development and mortality compared with females for the vast majority of non-reproductive tract-related cancers (Sung et al., 2021; Zhu et al., 2019). Cancers with the highest male-biased incidence rate ratios include urinary bladder, esophagus, larynx, pharynx, and liver/intrahepatic bile duct (Siegel et al., 2019; Sung et al., 2021; Zhu et al., 2019). Throughout this review, we will be highlighting studies using in vitro and in vivo bladder cancer models given that bladder cancer is also one of the leading causes of cancer-related deaths in males worldwide and has been studied extensively as a cancer type with an unmet medical need (Sung et al., 2021). As explained further below, male-biased disease etiologies still persist after accounting for established risk factors, such as carcinogenic environmental exposures and unhealthy behavioral practices. In the coming sections, we will detail the leading known contributing paradigms that provide mechanistic explanations for why males tend to have increased incidence and an overall worse prognosis for the majority of non-reproductive cancers.
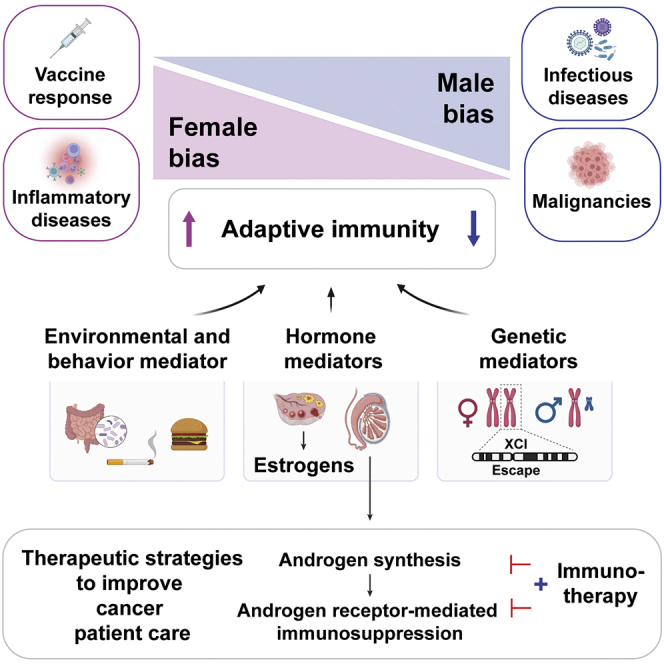
Mechanisms of sexual dimorphisms in immunity
To better understand differential immune regulation between sexes, we will explain several known sex-related environmental and behavioral factors, as well as genetic and hormonal components that contribute to differing adaptive immune cell regulation and cancer incidence between males and females.
Environmental and behavioral factors
Environmental and behavioral factors – such as the extent of chemical exposure, type of nutrition, and degree of healthcare seeking – are well known to differentially increase cancer risk between males and females (Figure 3). The use of tobacco products is a risk factor for the majority of cancers. A greater proportion of patients with cancer and a history of tobacco use are male, despite an overall decrease in prevalence over time (Higgins et al., 2015). Further, males reported they started smoking earlier in life and at an increased frequency than females (Zang and Wynder, 1996). Exposure to environmental toxins can have profound effects on the adaptive immune system. For example, smoking is one of the major causes of pulmonary inflammation (Laniado-Laborín, 2009; Lee et al., 2012), contributes to chronic obstructive pulmonary disease (COPD), and is associated with an increase of pro-inflammatory T helper type 1 (Th1) and Th17 cell subsets in both humans and mice (Harrison et al., 2008; Vargas-Rojas et al., 2011). In addition to tobacco use, males have historically experienced more occupational chemical exposures by working in factories and other industrial workplaces. Sex-based analyses using the National Toxicology Program database demonstrated a higher incidence of tumors in male rats, with 68 chemicals inducing cancer in males only versus 19 chemicals inducing tumors in females only (Kadekar et al., 2012).
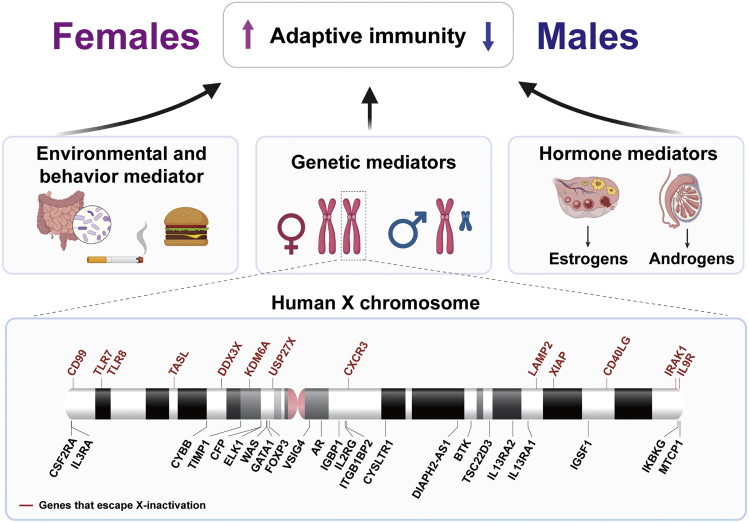
Figure 3.
Contributing factors including sex chromosomes to sex bias in immunity
Top, depiction of adaptive immune cell regulation between females and males. Red and blue arrows indicate a female or male bias, respectively. Middle, three major contributing factors to sex-biased immunity. Bottom, diagram of immune-related X-linked genes. Red lines represent X-linked genes that have the propensity to escape X chromosome inactivation. Figure adapted from images created with BioRender.com.
Partly owing to a longer life span and increased weight gain post-menopause, the overall percentage of females with obesity is higher than males (Heo et al., 2021; NCD Risk Factor Collaboration, 2016); however, males are reported as being less likely to select healthy food choices or exhibit an interest in self-care (Figure 3) (Mróz et al., 2011; Wardle et al., 2004 ). Diabetes mellitus is associated with a significantly increased risk for cancer development and is an overall poor prognostic marker for patients with cancer (Duan et al., 2014; Kautzky-Willer et al., 2016). Using mouse models of diabetes to evaluate the effects of hyperglycemia on immune cell function and cancer cell growth, Fainsod-Levi and colleagues demonstrated hyperglycemia impairs neutrophil mobilization to tumors and increases metastatic seeding (Fainsod-Levi et al., 2017). Other studies found hyperglycemia shifted macrophages into a tumor-promoting M2-like phenotype within the TME (Rodrigues Mantuano et al., 2020) and impaired antigen presentation of MHC class II-restricted antigens to T cells (Clement et al., 2021). Of note, the role of macrophages was implicated in lung metastases of bladder cancer – a process driven by the loss of the metastasis suppressor RhoGDI2 (Wu et al., 2009) and C-C motif chemokine ligand 2 (CCL2) signaling through the CCR2 receptor (Said et al., 2012). Importantly, inhibition of CCL2 in tumors was found to enhance response to immune checkpoint inhibitors in animal models of bladder and other cancers (Tu et al., 2019, 2020).
Sex is also a very important biological factor that influences, and is influenced by, the gut microbiome in both humans and mice (Figure 3) (Dominianni et al., 2015; Elderman et al., 2018; Org et al., 2016). Multiple groups have found that sex modifies the associations between diet, body mass index, and microbial diversity (Bolnick et al., 2014; Gao et al., 2018; Haro et al., 2016), which, in turn, plays an indispensable role in the development, homeostasis, and functional modulation of the immune system (Belkaid and Hand, 2014; Zheng et al., 2020). Imbalances here can contribute greatly to the induction and development of a number of immune-related diseases (Zheng et al., 2020). Studies have demonstrated that the gut microbiota can elevate testosterone levels, which in turn protects male mice from type 1 diabetes, an autoimmune disorder characterized by T cell-mediated destruction of pancreatic beta-cells. Further, the transfer of male microbiota to females provided robust protection against type 1 diabetes (Markle et al., 2013; Yurkovetskiy et al., 2013). Using 89 different inbred mice strains, Org et al. identified significant differences regarding the diversity and abundance of the microbiome between males and females in each strain (Org et al., 2016). In a comprehensive review, Taneja strongly suggests that sex hormones (predominantly estrogens) impact innate and adaptive immune cells and that gut microbiota may exert sex-specific effects on immune cell function owing to the ability of microbes to metabolize and/or produce estrogen and androgen metabolites (Taneja, 2021). Through either a direct effect on the tumor cells or an indirect effect on the immune system, it is well appreciated that the gut microbiome is capable of modulating host cancer progression and has tremendous effects on responsiveness to immunotherapy, as discussed further in the last section of this review.
In summary, environmental and behavioral factors clearly contribute to differential cancer risks between males and females – through either direct oncogenic effects or modulation of the immune system. However, the combined global incidence and mortality rates for all cancers in aggregation are 50 and 20% higher for males compared with females, respectively, which far exceeds contributions from environmental and behavior effects (Bray et al., 2018; Hartge et al., 1990). Thus, there is a critical need to understand the intrinsic biological factors that play a fundamental role in orchestrating the sexual dimorphism in cancer.
Sex chromosomes and genetics
Chromosomal, genetic, and epigenetic factors have been reported to significantly contribute to sexual dimorphisms in the immune response. Males and females vary in sex chromosome composition, with males carrying one paternally-inherited Y chromosome and one maternally-inherited X chromosome (XY), whereas females carry two X chromosomes (XX), one from each parent. The X and Y chromosomes evolved from a homologous autosome pair to become significantly different in size and gene number. In humans, the X chromosome is about 150 Mb in size with around 800 protein-coding genes, whereas the Y chromosome is 23 Mb in size and contains 78 known protein-coding genes (Bachtrog, 2013; Ross et al., 2005). One of the first steps in the sex chromosome evolution was the acquisition of SRY on the Y chromosome. Individuals that carry the SRY gene will develop testis and become gonadal males, whereas non-carriers will develop ovaries and become females (Berta et al., 1990; Gubbay et al., 1990; Sinclair et al., 1990). Of relevance, several immune-related genes are X-linked, such as cytokine receptors interleukin-two receptor-γ chain (IL2RG) and IL-13 receptor-α chain (IL13RA2), TLR7 and TLR8, and the TFs androgen receptor (AR) and forkhead box P3 (FOXP3) (Kawai and Akira, 2006; Klein et al., 2015; Lubahn et al., 1988; Su et al., 2009; Souyris et al., 2019; Zhao et al., 2020) (Figure 3), which when dysregulated have the potential to elicit dimorphic immune response between sexes.
To balance and regulate homogametic (XX) and heterogametic (XY) gene expression, one X chromosome in female cells is transcriptionally silenced primarily through the action of Xist (X-inactive specific transcript), a 17,000 nucleotide long non-coding RNA that physically coats the chromosome from which it is produced (Boumil and Lee, 2001; Heard et al., 2004 ). Despite chromosome-wide silencing, about 23% of X-linked genes escape X chromosome inactivation (XCI), resulting in sex-biased expression patterns (Tukiainen et al., 2017) (Figure 3). The sex-biased expression of many immune-related genes has been found to have an effect on the outcome of several immune-related diseases, including autoimmunity and cancer. For example, SLE, an autoimmune disease that predominantly affects females, can be partly explained by the expression of both copies of the TLR7 gene – including one that escaped XCI in certain immune cell subsets, such as monocytes, DCs, and B cells (Souyris et al., 2018). B cell-intrinsic TLR7 signaling is crucial for autoantibody production and systemic inflammation (Jackson et al., 2014), a deficiency of which protects mice against lupus-like diseases (Christensen et al., 2006). Similarly, overexpressing TLR7 was sufficient to induce acute systematic autoimmune disease in a non-lupus mouse model (Deane et al., 2007), overall suggesting additional copies of TLR7 may contribute to more functional adaptive immunity in females. Several XCI-escaped genes have also been suggested to have tumor-suppressing functions (Clocchiatti et al., 2016; Dunford et al., 2017). As an example, the biallelic expression of KDM6A, a sex-biasing tumor suppressor that escaped XCI in females, was found to partially explain the protection of females against bladder cancer (Dunford et al., 2017; Kaneko and Li, 2018; Ntziachristos et al., 2014). Additional immune-related XCI-escaped genes include CD99, TLR8, TASL, DDX3X, USP27X, CXCR3, LAMP2, XIAP, CD40LG, IRAK1, and IL9R (Figure 3) (Carrel and Willard, 2005; Mousavi et al., 2020; Oghumu et al., 2019; Vermeesch et al., 1997).
As mentioned, the SRY gene, located on the Y chromosome, is responsible for the formation of testes and therefore testosterone synthesis. The “four cores genotypes” (FCG) mouse model made use of the SRY gene to uncouple gonadal-dependent and -independent mechanisms and evaluate the impact of sex chromosomes versus gonadal type (Arnold and Chen, 2009). Gonadectomy of these mice helped to unmask multiple immune-related functions such as susceptibility to viral infection and autoimmune diseases (Robinson et al., 2011; Smith-Bouvier et al., 2008). By using this model, mice of the XX chromosome complement demonstrated greater susceptibility to both EAE and pristane-induced lupus, compared with XY mice with the same gonadal type (Smith-Bouvier et al., 2008). More recently, using this model, Kaneko and Li systematically investigated bladder cancer risk using the N-butyl-N-(4-hydroxybutyl) nitrosamine (BBN)-induced bladder cancer model (Kaneko and Li, 2018). The hazard ratio (HR) of tumor development in mice with testes versus ovaries was 4.714, and between mice with XY versus XX chromosomes was 2.549. Importantly, the combined effects of both sex chromosomes and hormones increased the HR to 12.390, signifying that sex chromosomes and sex hormones are both substantial modulators that impact the sexual dimorphisms seen in tumor development.
Sex steroid hormones
Sex hormones, such as estrogens, progesterone, and androgens, are steroid hormones that bind and signal through estrogen receptors (ER), progesterone receptors (PR), and the androgen receptor (AR), respectively. They have important roles in both the reproductive and non-reproductive systems and are well-known modulators of immune function and related disease onsets (Bereshchenko et al., 2018; Moulton, 2018; Taneja, 2018). By analyzing gene expression and sample-specific regulatory networks across 29 normal human tissues using the Geno-type-Tissue Expression (GTEx) project dataset, Lopes-Ramos and colleagues explain that the majority of genes differentially expressed between tissues were enriched for sex chromosome genes, including those known to escape XCI. When analyzed via gene set enrichment analyses (GSEA) and gene ontology (GO) terms, methylation and immune system-related processes dominated sex-related differences across tissue types (Lopes-Ramos et al., 2020). Of note, most TFs, including estrogen receptor genes, ESR1 and ESR2, and AR, were not differentially expressed between males and females; instead, gene target regulatory network analyses revealed strong differential targeting patterns by sex hormones existed in several tissues, including whole blood, and that they were associated with a divergent class of genes regulated by both sex hormone receptors and other various TFs (Lopes-Ramos et al., 2020).
A similar study evaluating sex-biased gene regulation using GTEx data, but in combination with genome-wide association studies (GWASs), found differential gene expression between sexes to be relatively widespread across the genome, albeit at low levels that were largely tissue-specific, with the largest differential expression stemming from X chromosome genes (Oliva et al., 2020). Here, the authors characterize TF-binding sites (TFBSs) across promoter regions of male- and female-biased genes and discovered enrichment of TFBSs for 92 TFs, including known hormone-related receptors [ESR1, AR, and glucocorticoid (NR3C1)], those that colocalize with hormone receptors, and additional TFBSs that have not been reported or have less of a hormone receptor association (Oliva et al., 2020). Given that these are RNA-based analyses, differential target gene regulation is likely owing to a combination of the following factors: differing TF protein abundance, epigenetic regulations, established TF cooperativity, and as discussed below, differing ligand (estrogen and androgen) levels that activate respective sex hormone activity.
Estrogen and estrogen receptor signaling on immunity
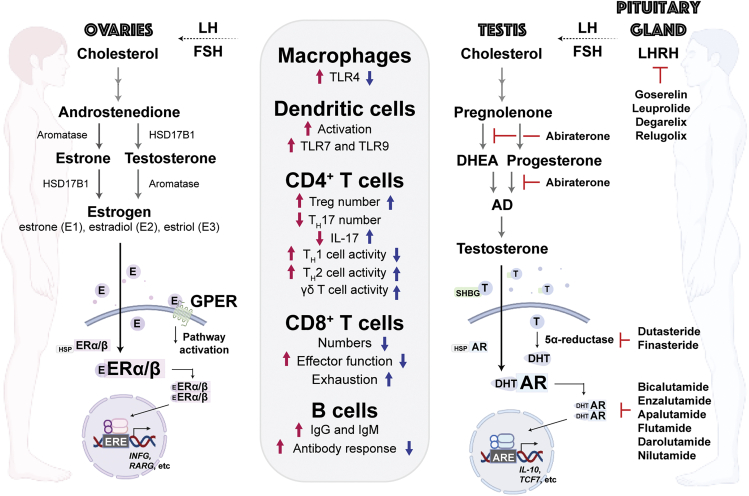
Estrogens are considered female sex hormones, given that they are primarily synthesized in female gonadal organs and are present at elevated levels in females compared with males. The lower quantities of estrogen found in males can be attributed to a smaller but significant amount of estrogen synthesized by non-gonadal organs, such as adipose tissue (Barakat et al., 2016; Nelson and Bulun, 2001). Even low levels of estrogen make important physiological contributions to tissue- and cell-specific function across sexes – as evidenced by the necessity of estrogen for normal gonadal development and spermatogenesis, as well as sperm maturation and motility (Schulster et al., 2016). The three main physiological forms of estrogen include estrone (E1), estradiol (E2 or 17β-estradiol), and estriol (E3), with E2 being the most potent and biologically relevant molecule, especially in premenopausal females (Figure 4) (Cui et al., 2013; Fuentes and Silveyra, 2019 ). Once released into circulation, estrogens travel to both reproductive and non-reproductive estrogen-responsive tissues. Estrogen synthesis and serum E2 levels are highest during reproductive years, especially during ovulation, and decline by 85–90% with menopause (Khosla et al., 1997). Estrogen primarily signals through two nuclear receptors, ERα (ESR1) and ERβ (ESR2), whose genes are located on separate chromosomes, as well as a membrane-bound G protein-coupled estrogen receptor (GPER), which we discuss further below. Whereas ERα and ERβ colocalize in many cell types, both genes are expressed in distinct tissue-specific patterns and distributions throughout the body that vary over time (Fuentes and Silveyra, 2019; Khan and Ansar Ahmed, 2015 ). Upon estrogen binding, ERα and ERβ either homo- or heterodimerize, translocate to the nucleus, and in conjunction with additional transcriptional regulators, bind to estrogen response elements (EREs) of tissue-specific ER target genes (Figure 4).
Figure 4.
Sex hormone pathways and their impacts on immunity
Canonical ER and AR signaling pathways that are more prevalent in females and males, respectively. Boxed list refers to changes in immune cell subsets by estrogen (magenta) or androgens (blue). Magenta and blue arrows denote positive and negative changes in response to estrogen or androgens, respectively. The lack of an arrow indicates there is either no change or it has not been evaluated. Right section includes compounds known to inhibit various nodes of the AR signaling pathway. LH, luteinizing hormone. FSH, follicle-stimulating hormone. LHRH, luteinizing hormone-releasing hormone. E, estrogen. GPER, G protein-coupled estrogen receptor. T, testosterone. DHT, dihydrotestosterone. HSP, heat shock protein. ERE, estrogen response element. ARE, androgen response element. DHEA, dehydroepiandrosterone. AD, androstenediol/androstenedione. SHBG, sex hormone binding globulin. Figure adapted from images created with BioRender.com.
Collectively, both ERα and ERβ are expressed in the majority of immune cells, with ERα being the predominant isoform expressed in macrophages (Calippe et al., 2010), DCs (Kovats and Carreras, 2008), CD4+ T cells, and B cells (Phiel et al., 2005). Interestingly, estrogen and ER activity appear to contribute to an increased immune response through specific mechanisms – essentially, through TH1/2 activation, CD8+ T cell effector function, and B cell-mediated antibody responses, as opposed to TH17-mediated activity (Figure 3). Whereas conflicting data exist, the majority of studies demonstrate estradiol signals through ERα to inhibit TH17 cell differentiation (Chen et al., 2015a; Khan et al., 2010; Lélu et al., 2011), – this occurs by forming a complex with a repressor of the ER activity (REA) and binding to EREs within the promoter region of the retinoic acid receptor (ROR)γT (Chen et al., 2015a). The absence of ERα results in increased IL-17 secretion and TH17 cell differentiation (Tyagi et al., 2012), and in post-menopausal females with decreased estrogen, there is an increase in plasma IL-17 levels (Molnár et al., 2014). Estrogen also potentiates suppressive effects by increasing CD25 and FOXP3 expression and enhancing Treg numbers and function, both in vitro and in vivo (Figure 4) (Polanczyk et al., 2004; Prieto and Rosenstein, 2006 ).
With regards to enhanced immunity, estrogen-induced ERα activation has been shown to induce DC development and stimulate DC-mediated pro-inflammatory effects of CD4+ T cells via CD40 and TLR9 stimulation (Douin-Echinard et al., 2008). Using the Flt3L-induced model of DC differentiation, ERα promotes conventional DC (cDC) and plasmacytoid DC (pDC) development with an increased propensity to invoke pro-inflammatory cytokine production after TLR stimulation (Seillet et al., 2013). Similar results have been observed in vivo in response to ER-mediated TLR-driven production of type 1 IFNs (Seillet et al., 2012). Estrogen also promoted TLR4-mediated pro-inflammatory factors via ERα in macrophages (Calippe et al., 2010) and increased IFN-γ mRNA levels in Jurkat cells using an IFN-γ promoter-driven reporter assay that contains consensus ERE binding motifs (Fox et al., 1991). When estrogen was administered to ovariectomized mice immunized with exogenous antigens, CD4+ T cells clonally expanded into antigen-specific, IFN-γ producing TH1 cells (Maret et al., 2003). Estrogen is well known to influence B cell differentiation, proliferation, and survival, whereas also enhancing immunoglobulin production (Figure 4) (Kanda and Tamaki, 1999; Sthoeger et al., 1988; Medina et al., 2000). Whereas CD8+ T cells have low expression of both ERα and ERβ and proliferation is not altered with estrogen exposure (Klein and Flanagan, 2016; Phiel et al., 2005), estrogen can increase the CD8+ T cell response (Robinson et al., 2014), likely through the activation of upstream mediators such as neutrophils and the immune cell subsets described above.
The rapid non-genomic activation of GPER is primarily induced by estradiol and results in the upregulation of epidermal growth factor receptor (EGFR), mitogen-activated protein kinase (MAPK), protein kinase A (PKA), and phosphoinositide 3-kinase (PI3K) pathway signaling (Hsu et al., 2019). GPER is expressed in several types of cancer, including pancreatic, breast, endometrial, lung, ovarian, and hepatocellular cancers, and plays an important role in cancer cell invasion, tumor expansion, and modulation of the TME (Notas et al., 2020). Interestingly, androgen has been shown to inhibit GPER expression and is highly expressed in castration-resistant prostate cancer but not in androgen-responsive prostate cancer (Lam et al., 2014). GPER regulates the life span of multiple immune cell types, including neutrophils, monocytes/macrophages, T lymphocytes, and B cells, and is overactive in immune-mediated diseases (such as MS and SLE) (Notas et al., 2020). GPER has been shown to play an important role in the regulation of cytokines and cytokine receptor expression (Notas et al., 2020). For example, in primary human and mouse macrophages, the use of G-1, a potent and selective GPER agonist, inhibited LPS-induced TNF-α and IL-6 secretion (Blasko et al., 2009). G-1 treatment of CD4+ T cells under Th17-polarizing conditions resulted in an increase of IL-10 (Brunsing and Prossnitz, 2011). In summary, GPER appears to be an important mediator of estrogenic action in tumor formation, progression, and metastasis and should also be considered when investigating hormone-related differences in immuno-oncology.
Progesterone and progesterone receptor signaling on immunity
Progesterone (P4) is an endogenous steroid sex hormone with increased secretion by the ovaries during menstruation and pregnancy (DeMayo et al., 2002). P4 is primarily known to regulate female reproductive functions by stimulating the growth of blood vessels that supply the endometrium, preparing the uterine lining for implantation, and sustaining the endometrium during pregnancy (Raghupathy and Szekeres-Bartho, 2022). Whereas males and females have no substantial quantitative difference in progesterone levels at baseline, P4 does have an important role in several male biological processes, including spermiogenesis (Oettel and Mukhopadhyay, 2004). P4 is also an intermediate metabolite for testosterone and neurosteriod biosynthesis and can affect the cardiovascular, respiratory, and immune systems (Oettel and Mukhopadhyay, 2004) – discussed below. P4 actions are mediated by two nuclear receptors, progesterone receptor A (PR-A) and progesterone receptor B (PR-B), which are transcribed from a single gene in response to estrogen (Li and O'Malley, 2003). In female mice, PR-A has been shown to regulate uterine functions, such as implantation and decidualization, whereas PR-B is important for mammary gland development (Mulac-Jericevic et al., 2000, 2003). PR is used as a biomarker for ER-α activity and breast cancer prognosis. ER-α induces PR expression, and in turn, PR modulates ER-α-associated activity (Mohammed et al., 2015). In the presence of an agonist, PR associates with ER-α to direct its chromatin binding, resulting in a gene expression profile associated with good clinical outcomes for patients with lumina A breast cancer (Mohammed et al., 2015).
Female-dominant autoimmune disease symptoms tend to decrease during menstruation and pregnancy, and increase after menopause (Hughes, 2012; Hughes and Choubey, 2014). PR is expressed by several immune cell types and is well known for its ability to suppress T cell activation during pregnancy (Arruvito et al., 2008; Shah et al., 2019; Szekeres-Bartho et al., 2001). Further, P4 has been shown to inhibit the activation of mouse DCs (Jones et al., 2010), macrophages (Menzies et al., 2011), and NK cells (Schumacher et al., 2014). The administration of P4 to pregnant mice infected with Brucella abortus resulted in reduced inflammatory cytokines by trophoblast cells, reduced placental inflammation, and increased viability of embryos (Ren et al., 2021). In addition to inhibiting the production of cytokines (Butts et al., 2007; Jones et al., 2008), P4 also suppresses the production of chemokines, such as macrophage inflammatory protein-1α, macrophage inflammatory protein-1β, and RANTES by CD8+ T cells (Vassiliadou et al., 1999). Collectively, progesterone is considered an anti-inflammatory hormone that controls the tolerance of the immune system during pregnancy. As such, the administration of P4 to healthy pregnant women has been shown to reduce markers of inflammation within the maternal blood stream (Shah et al., 2019). Whether a meaningful interplay between estrogen/ER and P4/PR exists in immune cells of the TME is yet to be determined.
Androgen and androgen receptor signaling on immunity
As previously described, the observed effects from AR pathway signaling have been immuno-suppressive and necessary for immune tolerance. It remains to be determined how AR signaling in innate APCs and adaptive immune cells contributes to tumor development and progression, and how we can use this knowledge to activate anti-tumor immunity and improve current immunotherapeutic strategies.
Androgens are male sex hormones that are primarily synthesized in male gonadal organs and are present at elevated levels in males compared with females. Androgen secretion is first initiated through the pulsatile release of luteinizing hormone-releasing hormones (LHRHs) from the hypothalamus, which bind and stimulate the release of luteinizing (LH) and follicle-stimulating (FSH) hormones from the anterior pituitary gland. Through a series of enzymatic reactions, cholesterol-derived testosterone synthesis occurs when LH binds to LH receptors on Leydig cells in the testes (Figure 4) (Crawford et al., 2019). Testosterone then circulates in the blood stream, either free or bound to serum albumin or sex hormone-binding globulin (SHBG), until it reaches its target tissue (Figure 4). Testosterone is then converted into the more active metabolite dihydrotestosterone (DHT) by 5α-reductase. The binding of androgens to AR results in a conformational change, dissociation from chaperone proteins, exposure of the nuclear localization signal (NLS), dimerization, and translocation to the nucleus, where it binds to androgen response elements (AREs) in complex with chromatin remodelers and other transcriptional coregulators (Figure 4) (Davey and Grossmann, 2016).
In addition to male reproductive organs, AR is expressed in a diverse range of tissue types, including adipose tissue, muscle, and bone, as well as cells of the cardiovascular, nervous, and immune systems (Davey and Grossmann, 2016; Hu et al., 2004; Walters et al., 2007; Yeh et al., 2003). Rare X-linked recessive AR loss-of-function mutations that result in decreased or a complete loss of AR expression, termed androgen insensitivity syndromes (AIS), have allowed us to delineate AR function in humans. Given that male cells only contain a single X chromosome and are more reliant on AR activity for the development of male gender characteristics, males have a greater propensity to develop phenotypic changes if they are an AR mutation carrier. Depending on the type and location of the mutation, phenotypes can range from mild (change in body hair patterns, body size, and/or impaired spermatogenesis) to more severe (impaired male genitalia development) physiological disorders (Quigley et al., 1995). Unfortunately, whereas lymphocytes can be analyzed to detect the AIS-causing AR mutations (Melo et al., 2011), few functional studies have been conducted to determine the impact of these mutations on lymphocyte biology. We summarize below the emerging roles of AR in regulating APC, T cell, and B cell function.
APCs
AR expression has been reported and functionally validated across numerous immune cell types, including neutrophils, monocytes, macrophages, CD4+ T cells, CD8+ T cells, and B cell progenitors. Through the use of androgen treatments and by disrupting the AR signaling pathway, investigators have begun to understand how AR-expressing immune cells are hormonally regulated. Macrophages are considered essential for mediating immune responses. Through the use of a macrophage cell line, cultured primary macrophages, and in vivo studies, testosterone has been shown to suppress the expression of TLR4, TNF-α, and IL-1β (Figure 4) (Corcoran et al., 2010; Rettew et al., 2008). Consistent with the notion that androgens suppress macrophage inflammatory responses, males have a greater risk of succumbing to sepsis than females. In a hemorrhaged mouse model, administration of the anti-androgen flutamide both increased cytokine release by splenic macrophages and significantly decreased mortality (Angele et al., 2014). On a separate but similar note, castration of male mice significantly increased susceptibility to inflammation-induced endotoxic shock from a systemic Gram-negative bacterial infection; exogenous testosterone was able to reverse the effect (Rettew et al., 2008). As mentioned above, ER appears to be the driving hormonal regulator in the development of several DC lineages (Douin-Echinard et al., 2008; Paharkova-Vatchkova et al., 2004; Seillet et al., 2013). Whereas TLR-mediated inflammatory responses in male DCs are lower than in females, and DCs in hypogonadal males are more immunologically responsive (Corrales et al., 2012), it remains unclear whether these observations are owing to direct or indirect effects of androgens.
T cells
It is well known that the thymus enlarges in response to decreased androgen signaling, such as with castration or as a result of AR deficiency (Henderson, 1904; Olsen et al., 1998). Decreased androgens also increase the thymic egression of T cells (Olsen and Kovacs, 2011). The elevated thymic output of T cells can be observed in healthy versus hypogonadal males before and after testosterone replacement therapy (Olsen and Kovacs, 2011). Thymic hypertrophy is reversed with the administration of androgens to castrated mice – a process that involves rapid apoptotic involution of the thymus that thereby affects the size, cellular composition, and degree of T cell proliferation (McMurray et al., 2001; Olsen et al., 1998 ). As such, increased egression and peripheral T cell numbers are also reversed upon androgen replacement (Olsen and Kovacs, 2011). Whereas mice carrying the AIS testicular feminization AR mutation (Tfm) also showed thymic enlargement, androgen treatment did not reverse the thymus phenotype caused by defective AR function (Olsen et al., 1998). Of note, androgen-related effects on thymic size were primarily observed when AR was knocked out of thymic epithelial cells (TEC) as opposed to thymocytes (Lai et al., 2013). Consistent with these findings, incorporation of the dysfunctional AR-Tfm gene specifically in TECs also caused thymus enlargement and increased T cell number (Olsen et al., 1998). Whereas it is unclear whether the effects on T cells are direct or stem from indirect contributions from TECs during T cell development, additional studies demonstrated T cells isolated from surgically castrated mice had more proliferative activity in response to antigen-specific activation and upon T cell receptor (TCR)- and CD28-mediated co-stimulation (Roden et al., 2004), and T cells isolated from medically castrated males had increased mitogen-induced CD8+ T cell IFNγ expression (Page et al., 2006), further demonstrating how androgens suppress CD8+ T cell proliferation and activity (Figure 4). As discussed later, using multiple strategies, including a single-cell omics platform, we recently discovered a key role for T cell-intrinsic AR in orchestrating the CD8+ T cell exhaustion program in the TME, and the contribution of such to sex bias in cancer (Kwon et al., 2022).
The distribution and activity of CD4+ T cell subsets are also regulated by sex hormones. However, compared with CD8+ T cells, the sex disparity of CD4+ T cell phenotypes is less clear. Mouse (Elderman et al., 2016; Roberts et al., 2001) and human (Girón-González et al., 2000; Zhang et al., 2012) studies report inconsistent data regarding TH1 or TH2 CD4+ T cell populations in males versus females. As low versus high levels of estrogen can promote TH1 versus TH2 CD4+ T cell differentiation, respectively, differences in TH1/2 populations between sexes may be dependent on the menstrual cycle, age, and/or experimental methods used for assessment (Girón-González et al., 2000; Straub, 2007 ). However, in the presence of androgen, both in vitro and in vivo experiments demonstrate androgens mount an overall suppressive effect on Th1 CD4+ T cell differentiation and corresponding IL-12 signaling, but induce a CD4+ Th2 cell response characterized by enhanced production of the immunosuppressive cytokine IL-10 (Kissick et al., 2014; Liva and Voskuhl, 2001). Importantly, androgen deprivation was able to enhance TH1 responses and IFN signaling (Kissick et al., 2014). As previously mentioned, males also have an increased proportion of IL-17-producing CD4+ T cells compared with females. A recent study demonstrated a vital role of IL-17-producing γδ T cells on local tissue immune surveillance of the testis. γδ T cells seeded the testis of naive mice, expanded at puberty, and decreased mortality from infectious pathogens such as Listeria monocytogenes (Figure 4) (Wilharm et al., 2021).
Depending on the site of origin and pathology involved, the ratio of Tregs between sexes differs among mouse studies, whereas an increased number of Tregs are more consistently reported in healthy adult males compared with females in human studies (Afshan et al., 2012). When males were treated with an LHRH antagonist, thereby decreasing androgen levels, peripheral blood Tregs cell counts were less than both placebo-treated males. Treatments did not affect overall CD4+/CD8+ T cell ratios (Page et al., 2006). In vitro stimulation of naive T cells with testosterone has also been shown to increase Treg cell expansion with immunosuppressive activity (Fijak et al., 2011). Consistent with these results, androgen administration to treat autoimmune disorders [e.g., experimental autoimmune orchitis (EAO) and SLE] resulted in increased Tregs (Fijak et al., 2011; Rutkowski et al., 2014). Of significance, AR has been shown to directly bind to AR binding sites upstream of FOXP3, a master regulator of Treg differentiation, and could be the reason for the increase in Tregs after androgen treatments (Walecki et al., 2015).
B cells
As previously mentioned, males have fewer B cells and less antibody production compared with females. First, AR is expressed only in B cell progenitors. Earlier studies evaluating the effects of androgens on B cell development and function discovered that the proliferative effects on B cells after male castration was owing to increased B cell expansion from the bone marrow (Olsen et al., 2001; Viselli et al., 1997). Supplementation with DHT was able to restore B cell counts back to normal levels but not in a global AR knockout model, indicating that maturation of B cells is AR dependent (Figure 4) (Altuwaijri et al., 2009). Further, the suppressive effects of androgens were only observed in the presence of marrow stromal cells or supernatant collected from androgen-treated stromal cells (Olsen et al., 2001). DHT treatments increased stromal cell production of TGF-β and TGF-β neutralization reversed the suppressive effects on B cells (Olsen et al., 2001). These results suggest that the effects of androgens on B cell development were, at least in part, owing to androgen-sensitive stromal cell-derived TGF-β-mediated effects. An additional indirect consequence of androgens on B cells is the less efficient positioning of male B cells in germinal centers (GCs) of secondary lymphoid organs compared with female B cells owing to GPR174-mediated migration toward CCL21. Male B cells are therefore less likely to proliferate, mature, and elicit a strong humoral immune response than female cells. Castration as well as deletion of GPR174 resulted in more efficient positioning within the follicular center, which with testosterone administration returned to less efficient male B cell migratory patterns (Zhao et al., 2020).
Sexual dimorphisms in anti-cancer immunity
Whereas numerous studies demonstrate females are better at defending the body from infectious diseases, few studies have evaluated sex differences in cancer biology and how we could harness differential immunity between males and females to improve cancer therapeutics. One of the most comprehensive cancer-focused studies in this context was recently published in 2016. Yuan and colleagues evaluated 13 cancer cell types from The Cancer Genome Atlas (TCGA) to determine the molecular differences between male and female patients (Yuan et al., 2016). Genomic assessments revealed 53% of clinically actionable genes had a sex-biased molecular pattern, and not surprisingly, differential gene expression analyses demonstrated the vast majority of differentially expressed genes were sex-chromosome-specific. Of note, one of the sex-affected pathways highlighted in the study was a group of genes related to immune responses, including IL2 and STAT5 signaling, JAK, STAT3 signaling, IL6, inflammatory responses, IFNα and IFNγ responses, and TNF-α signaling and complement (Yuan et al., 2016). Given the elevated frequency of bladder cancer incidence in males versus females, Miyamoto and colleagues investigated the involvement of androgens and AR in bladder cancer development. Of significance, knocking out AR in mice before treatment with the carcinogen BBN completely prevented tumors from occurring in both male and female AR null mice, and castration before BBN exposure resulted in a 50% reduction in bladder cancer incidence (Miyamoto et al., 2007). Whereas the results are quite promising, conclusions to whether fewer tumors developed owing to the loss of AR in bladder epithelial cells or immune cell populations remain unclear.
In a more recent immune-focused cancer study using mouse and human cancers of various cell origins, including the BBN-induced bladder cancer mouse model, our group established that a male bias exists in the intratumoral frequency of CD8+TCF1+ progenitor exhausted T cells with poor effector function, which required T cell-intrinsic AR signaling. By performing surgical castrations and by disrupting AR signaling through both AR chemical inhibition and gene deletion, we demonstrated that AR contributes to CD8+ T cell dysfunction, positively regulates Tcf7 gene (encoding TCF1) expression, and when AR signaling is perturbed, results in decreased tumor growth (Kwon et al., 2022). Further, we identified a novel sex-specific regulon in progenitor exhausted CD8+ T cells that encompasses several genes with AR-chromatin immunoprecipitation (ChIP)-verified AREs (Chen et al., 2015b). Consistent with our findings, another group investigating resistance to anti-PD-1 immunotherapy found elevated AR signaling in metastatic castration-resistant prostate cancer (mCRPC) from patients with a poor response to pembrolizumab, a monoclonal antibody that targets and blocks PD-1 activity (Guan et al., 2022). Here, AR was suggested to exhibit direct negative regulation of Ifng (IFNγ) expression. In both studies, inhibition of AR signaling rewired the TME to favor effector T cell differentiation and potentiated the efficacy of anti-PD-1 immunotherapy. Collectively, these findings demonstrate a role for T cell-intrinsic AR in driving CD8+ T cell dysfunction and imply additional therapeutic strategies to treat tumors regardless of the AR status of the tumors. A greater mechanistic understanding of how AR regulates the effector vs exhausted T cell programs will be an active area of investigation moving forward.
Interestingly, CD24 and CD44, two of the most important markers for cancer stem cells and tumor progression, have been found to be regulated by AR. Using BBN-induced invasive and metastatic bladder cancer, investigators showed that Cd24a-deficient male mice developed fewer bladder tumors and less metastases than control mice (Overdevest et al., 2012). Knockdown and overexpression studies demonstrated an important role for CD24 in urothelial tumorigenesis and metastasis. Further, evaluation of these findings in human tumors showed that outcomes of males, but not females, were stratified by CD24 (Overdevest et al., 2012). Whereas AR is important in the development of both experimental and human bladder cancer, its role in the progression is less clear, with literature indicating that more advanced stage and grade of disease is associated with reduced AR expression. By performing AR ChIP-seq and complementary transcriptomic approaches on AR-expressing human bladder cancer cells grown in vitro, CD44 was found to be significantly associated with androgen stimulation. CRISPR-based mutagenesis of putative AREs identified a novel silencer element leading to direct AR-mediated transcriptional repression of CD44 (Sottnik et al., 2021). AR activity in bladder cancer cells described here as well as progenitor exhausted T cells described above both reveal novel mechanisms that explain, in part, the relationship between AR and bladder cancer tumor progression.
Conforti and colleagues analyzed transcriptomic data from 2,575 early-stage non-small cell lung cancer (NSCLC) samples to determine s. ex-based differences in molecular mechanisms behind anti-tumor immune response and evasion (Conforti et al., 2021a). Use of xCell to estimate the abundance of 64 cell types in the TME of each tumor indicated female tumors were more inflamed with increased expression of inhibitory immune checkpoint molecules, a greater abundance of immune-suppressive cells [myeloid-derived suppressor cells (MDSCs), cancer-associated fibroblasts, and Tregs], and a higher T cell dysfunction status compared with males (Conforti et al., 2021a). In contrast, males showed a significant enrichment for a T cell exclusion phenotype, likely owing to a presumed impairment of neoantigen presentation, given the smaller TCR clonality repertoire observed. No sex differences were found in the activation status of TGF-β or WNT/β-catenin pathways, and the TME of male tumors was characterized by a higher degree of hypoxia and VEGF-A expression (Conforti et al., 2021a). Histological evaluations to confirm T cell exclusion from male samples and studies detailing the mechanisms driving differences in immune cell abundances warrant further investigation.
As previously mentioned, AR is highly expressed in prostate tissue and functions as an oncogene to drive prostate cancer cell growth as well as metastatic progression, through both canonical and non-canonical mechanisms. In a recent study evaluating mechanisms that promote prostate cancer migration and invasion, Cioni and colleagues turned their eye from AR expression in prostate epithelial cells to AR expression in macrophages within the TME (Cioni et al., 2020). Through the use of a monocyte cell line and ChIP-sequencing, the authors conclude that AR signaling in monocytes contributes to macrophage differentiation and induces the expression of TREM-1 (Cioni et al., 2020), a cell surface receptor that amplifies inflammatory processes and is known to promote tumorigenesis and support tumor cell growth of various tissue types (Saurer et al., 2017). The authors conclude that whereas AR inhibitors are meant to block AR-mediated proliferation of prostate tumor cells, inhibition of AR signaling in macrophages is likely a beneficial “off-target” effect that can synergize to reduce prostate cancer progression.
On a similar note, the TME has been shown to remodel in response to androgen deprivation therapy (ADT). By performing RNA-sequencing on locally advanced prostate cancer pre- and post-ADT alongside paracancerous benign tissue, Long and colleagues found immune-related pathways were enriched post-ADT (Long et al., 2020). ESTIMATE (Estimation of STromal and Immune cells in MAlignant Tumor tissues using Expression data) analyses demonstrated that immune and stromal scores were also both significantly elevated, expression of antigen presentation, IFN-γ signaling, and immune checkpoint genes were elevated, and tumor cell purity had decreased (Long et al., 2020). By using weighted gene co-expression network analysis (WGCNA), the authors found five genes central to the remodeling process, three of which (SOCS3, ZFP36, and JUNB) were associated with increased regression-free survival and a favorable prognosis for patients (Long et al., 2020). Whereas ADT often leads to increased T cell infiltration, mechanisms of resistance are starting to emerge, including the accompaniment of adaptive Tregs (Obradovic et al., 2020). A better understanding of how ADT impacts tumor immune regulation has the potential to lead to novel therapeutic approaches for cancer patients.
Androgen deprivation and cancer immunotherapy
How androgens affect adaptive immunity in humans may be gleaned from studies involving patients who received ADT for prostate cancer. The use of medical or surgical castration for the treatment of prostate cancer was first described in 1941 by Huggins and Hodges, ultimately leading to a Nobel Prize in Medicine in 1966 (Charles Huggins, 1941). Over time, more refined medical approaches have been developed to modulate the hypothalamic-pituitary-gonadal axis. Surgical castration with bilateral orchiectomy has been largely supplanted by the use of LHRH agonists and antagonists, AR antagonists, and CYP17A1 inhibitors. Figure 4 depicts the normal androgen synthesis pathways and sites of action of hormone therapy drug classes. Currently, the mainstay of ADT for prostate cancer involves targeting LHRH, also known as gonadotropin-releasing hormone (GnRH), which, as described above, is normally released from the hypothalamus to initiate the synthesis of testosterone in the testes. Disruption of LHRH release by either an LHRH agonist or antagonist will result in decreased LH and FSH, and, in turn, lower testosterone levels. Current clinical ADT regimens use an LHRH agonist (leuprolide, goserelin) or antagonist (degarelix, relugolix) as the backbone of therapy, to which an anti-androgen (bicalutamide, flutamide, nilutamide, enzalutamide, apalutamide, darolutamide) or CYP17A1 inhibitor (abiraterone acetate) may be added (Figure 4).
Observational clinical studies have looked at the effect of medical and surgical castration on adaptive immunity in patients with cancer (Table 1). Most of the studies included patients with prostate cancer and, reflective of clinical practice patterns, used an LHRH agonist alone or in combination with an AR antagonist. Predominantly, studies used flow cytometry to quantify changes in immune cell subsets in peripheral blood. The findings are somewhat difficult to generalize owing to differences in study design, ADT regimen, and immune cell populations analyzed. The most consistent finding was an increase in T cells (Giltay et al., 2000; Johnke et al., 2005; Madan et al., 2021; Oliver et al., 1995; Sutherland et al., 2005, 2008), though there was some disagreement, particularly on the effect on T cell subtypes (Ma et al., 2020; Page et al., 2006; Vuk-Pavlović et al., 2010). The number of circulating B cells appears to be unaffected by ADT (Giltay et al., 2000; Johnke et al., 2005; Sutherland et al., 2005; Vuk-Pavlović et al., 2010), and there are limited studies and conflicting reports on how ADT effects the prevalence of MDSCs (Madan et al., 2021; Pal et al., 2019). Another significant subset of studies looked at the effect of ADT on tumor-infiltrating immune cells, mostly in prostate cancer tissue, using immunohistochemistry (IHC). In tissue, increased infiltration of T cell subtypes is also consistently observed (Calagua et al., 2017; Gannon et al., 2009; Guinan et al., 1997; Long et al., 2020; Mercader et al., 2001; Obradovic et al., 2020; Rubinow et al., 2017). A couple of studies also noted increased CD68+ tissue macrophages (Gannon et al., 2009; Mercader et al., 2001). Overall, studies to date evaluating the effect of ADT on adaptive immunity in humans are heterogeneous with only small to moderate sample sizes. Definitive conclusions cannot be drawn from them. Future work should focus on larger, well-designed, randomized studies employing modern multiplex immunophenotyping, next-generation sequencing, and spatial techniques. Ongoing randomized and observational studies registered at ClinicalTrials.gov are listed in Table 2, which will further elucidate the effect of ADT on adaptive immunity.
Table 1.
Completed studies in humans describing the effects of ADT on immunity
| ADT Regimen | N | Compartment | Patient population or disease | Time on ADT | Method | Findings | Study |
|---|---|---|---|---|---|---|---|
| LHRH agonist | 12 | Peripheral blood | Metastatic prostate cancer | 28 days | Flow | ↑: lymphocytes | Oliver et al. (1995) |
| LHRH agonist | 16 | Peripheral blood | Node-positive localized prostate cancer initiation ADT prior to radiation | 4 months | Flow, RT-PCR | ↑: total lymphocytes, total T cells, CD4+ T cells, naive CD4+ T cells, naive CD8+ T cells, memory CD8+ T cells, NK cells, naive CD4+TREC + cells. Naive: memory CD4+ T cells Unchanged: B cells, Ki-67 CD4+ or CD8+ T cells | Sutherland et al. (2005) |
| LHRH agonist with melanoma vaccine | 33 | Peripheral blood | Stage IIb-IV melanoma | 6 months | Flow, RT-PCR, ELISA | Unchanged: vaccine peptide-specific T cells, TREC, cytokines, or T regulatory cells | Vence et al. (2013) |
| LHRH agonist with allogeneic or autologous hematopoietic stem cell transplant (HSCT) | 40 | Peripheral blood | Hematologic malignancies undergoing allogeneic or autologous HSCT, investigating the effect of ADT on engraftment | 4 months | Flow, RT-PCR, ELISA, TCR PCR | ↑: neutrophils, lymphocytes, total and naive CD4+ T cells, TREC+CD4+ T cells, T cell receptor repertoire, peripheral T cell function Unchanged: CD8+ T cells, regulatory T cells, NK cells, gamma-delta T cells, cytokine production | Sutherland et al. (2008) |
| LHRH antagonist | 4 | Peripheral blood | Healthy males age 35–55 | 28 days | Flow | ↑: NK cells Unchanged: total lymphocytes, CD4+ or CD8+ T cells, CD4+:CD8+ ratio, expression of NKG2D or CXCR1 ↓: CD4+CD25+ T cells, CD8+ T cell IFN-γ expression | Page et al. (2006) |
| AR antagonist +/− orchiectomy | 61 | Peripheral blood | Localized and metastatic prostate cancer | N/A | NK activity assay | Unchanged: NK cell activity | Kastelan et al. (1992) |
| AR antagonist +/− prostate cancer vaccine | 38 | Peripheral blood | Non-metastatic (M0) prostate cancer with biochemical failure after definitive therapy | 3 months | Flow, RT-PCR, ELISA | ↑: NK cells, mature NK cells, Tim3+ NK cells, naive T cells, TRECs Unchanged: VEGF ↓: MDSCs | Madan et al. (2021) |
| AR antagonist with estrogen | 10 | Peripheral blood | Transgender male to female | 4 months | Flow, ELISA | ↑: leukocytes, TH1-associated chemokine receptors CCR1, CXCR3, CCR5 Unchanged: CD4+ T cells, CD8+ T cells, CD4:CD8 ratio, total lymphocytes, B cells, Ig levels ↓: NK cells | Giltay et al. (2000) |
| AR antagonist or CYP17A1 inhibitor | 44 | Peripheral blood | Metastatic castration resistant prostate cancer | 3 months | Flow, Luminex | Unchanged: polymorphonuclear myeloid-derived suppressor cells | Pal et al. (2019) |
| LHRH agonist + AR antagonist + radiation | 19 | Peripheral blood | Localized prostate cancer | 6 weeks | Flow |
↑: CD4+ and CD8+ T cells Unchanged: B cells, NK cells |
Johnke et al. (2005) |
| LHRH agonist plus AR antagonist or dexamethasone | 22 | Peripheral blood | Localized prostate cancer on adjuvant ADT | N/A | Flow | ↑: CD14+HLA-DRlow/- monocytes, CD4+CD25+CD127low/- Tregs Unchanged: B cells, CD4 T cells, or CD8 T cells | Vuk-Pavlović et al. (2010) |
| Bilateral orchiectomy | 57 | Peripheral blood | Locally advanced or metastatic prostate cancer | 1 month | Flow | ↑: CD8+ T cells ↓: CD4+ cells and CD4+:CD8+ ratio | Ma et al. (2020) |
| LHRH antagonist | 15 | Adipose tissue | Healthy males | 1 month | Flow | ↑: CD3+, CD4+, and CD8+ T cells, CD11c+ macrophages | Rubinow et al. (2017) |
| LHRH antagonist | 29 | Prostate cancer tissue | Localized prostate cancer | 2 weeks | IHC, Nanostring | ↑: CD8+ T cells and Tregs | Obradovic et al. (2020) |
| CYP17A1 inhibitor | 44 | Prostate cancer tissue | Localized prostate cancer | 6 months | IHC | ↓: CD8+ T cells | Calagua et al. (2017) |
| LHRH agonist + AR antagonist | 26 | Prostate cancer tissue | Localized prostate cancer | 28 days | IHC, TCR PCR | ↑: CD3 T cell, CD4 T cell, CD8 T cell, CD4:CD8, CD68 macrophage, CD83 dendritic cell | Mercader et al. (2001) |
| LHRH agonist + AR antagonist | 6 | Prostate cancer tissue | Localized prostate cancer | 2 months | RNAseq, IHC |
↑: CD8 T cells. Based on gene expression, increased immune cells infiltration and activity. Activation of antigen presentation, immune checkpoint, and IFN-γ signaling pathways Unchanged: gene fusions |
Long et al. (2020) |
| LHRH agonist + AR antagonist | 14 | Prostate cancer tissue | Localized prostate cancer | 3 months | H&E | ↑: lymphocytes | Guinan et al. (1997) |
| LHRH agonist + AR antagonist or AR antagonist alone | 35 | Prostate cancer tissue | Localized prostate cancer | 3 months | IHC |
↑: CD3+ and CD8+ T cells, CD68+ macrophages Unchanged: CD20+ B cells, Foxp3+ lymphocytes, CD56+ NK cells |
Gannon et al. (2009) |
Table 2.
Ongoing clinical trials to elucidate the effect of androgens and androgen blockade on immunity
| Trial ID | Title | Comment |
|---|---|---|
| NCT04624828 | Pilot Study of Immune Response Evaluation in Oligorecurrent and Oligoprogressive Prostate Cancer Patients Treated With Metastases-directed Stereotactic Body Radiation Therapy (SBRT) With and Without Concomitant Androgen Deprivation Therapy (IOSCAR) | Will monitor the dynamics of innate (monocytes, neutrophils, NK cells) and adaptive (T cells, B cells) immune cell subsets in the peripheral blood with flow cytometry before and after SBRT +/− ADT |
| NCT03654638 | The Effect of a Soy Bread Diet Intervention on Immune Function in Men with Prostate Cancer | Will compare effect of soy bread vs wheat bread on immune function when starting ADT for prostate cancer. Peripheral blood MDSCs, cytokines, and T cell proliferation will be measured |
| NCT03344211 | Immune Activation and Cellular Response from Enzalutamide Alone or With Radium-223 in Men with Metastatic Castration-Resistant Prostate Cancer | One of the primary objectives is to evaluate the immune activation of enzalutamide +/− radium-223 |
| NCT03649841 | Radiation Enhancement of Local and Systemic Anti-Prostate Cancer Immune Responses | A phase II trial of ADT + abiraterone +/− neutron radiation therapy. The primary outcome is the change in peripheral blood effector T cells from pre- to post-treatment |
| NCT04384835 | Analysis of Sexual Bias in Type 2 Innate Lymphoid Cells (ILC2) in Asthmatic Patients: Role of Androgens | Will compare the proportion of ILC2 in blood between males and females with asthma. Plan to expand ILC2 in vitro and expose to AR antagonist or agonist |
Whereas immune checkpoint blockers (ICBs), such as anti-CTLA-4 and anti-PD-L1, have created a paradigm shift in cancer treatment, only a minority of patients currently benefit from a life-altering durable survival. As a whole, the field of cancer immunotherapy faces many outstanding questions in the pursuit of a more predictable as well as higher therapeutic response. Given the previously discussed sexual dimorphism in immunity, there exists great interest in determining how and why the effects of ICB differ between males and females. Conforti and colleagues provided the first evidence of sex bias in ICB efficacy from their meta-analysis of pooled overall survival data from eligible randomized clinical trials (Conforti et al., 2018b). Out of the 20 phase II/III trials, seven examined melanoma and six involved non-small cell lung cancer (NSCLC). Authors identified a greater magnitude of efficacy in males over females, even in sub-group analyses based on the cancer type and type of ICB treatments received (Conforti et al., 2018b). A large scale, individual-level analysis of >1,000 patients with various cancer types resulted in a similar conclusion on the prognostic role of sex on the ICB response (Litchfield et al., 2021). Further, in a sub-group analysis of patients with PD-L1-high NSCLC, males were found to have prolonged overall survival after treatment with anti-PD-L1 as opposed to those treated with chemotherapy in the control arm (Conforti et al., 2021b). In females, the benefit of anti-PD-L1 was not statistically significant. Interestingly, females with NSCLC appeared to benefit more from combining chemotherapy with anti-PD-L1 alone compared with males (Conforti et al., 2019).
Sex bias in ICB efficacy remains uncertain as interpretations and generalizability of the conclusions from meta-analyses are challenging, especially in the context of anti-PD-L1 and anti-CTLA4 therapies that result in highly heterogeneous efficacy (Carrera et al., 2018; Claggett et al., 2018; Conforti et al., 2018a; Kwon et al., 2018; Liang, 2018; McQuade et al., 2018; Zhang et al., 2018). In fact, other large-scale meta analyses have failed to identify significant sex-associated differences in ICB efficacy (Botticelli et al., 2017; Kim et al., 2020; Lai et al., 2021; Wallis et al., 2019). These conflicting results are in part owing to inclusion criteria that differ between trial data meta-analyses. Upon investigating all 27 clinical trials used by Conforti et al. (2018b) and Wallis et al. (2019), Ye and colleagues failed to identify a significant pooled HR using the random-effects model (Ye et al., 2020). Interestingly, whereas six out of seven clinical trials on melanoma indicated a male bias in improved overall survival, those on NSCLC were inconsistent, with six out of 11 clinical trials suggesting a male bias and the rest a female bias (Ye et al., 2020). Other meta-analyses have similarly shown that male bias in ICB efficacy appears more evident in patients with melanoma versus NSCLC (Grassadonia et al., 2018; Wu et al., 2018a). Interpretations of NSCLC trials with anti-PD-L1 may be further complicated by conflicting conclusions on sex-based differences, with a female-biased benefit in terms of overall survival as opposed to a male bias in terms of progression-free survival (Wang et al., 2019).
Given this enormous heterogeneity, meta-analyses that pool multiple cancer types cannot accurately assess the interaction between sex and ICB efficacy. Furthermore, meta-analyses are often limited by the usage of published sub-group HRs and not on individual patient-level data. Finally, proposed sex differences may be associated instead with differences in lifestyle factors (e.g., smoking), disease comorbidities, and clinicopathological subtypes between males and females. The effects of sex on lesser used cancer immunotherapies, such as cancer vaccines or adoptive cell transfer (ACT), are not yet known. Prospective clinical studies of therapeutic cancer vaccines for patients with melanoma have not detected any sex-based differences in survival outcomes (Hsueh et al., 2002; Morton et al., 2002; Ramirez et al., 2015), and whereas a preclinical study by Jenq et al. suggested that ACT of female CD4+ T cells have higher anti-tumor activity in the TRAMP-C2 prostate cancer model than male CD4+ T cells (Jenq et al., 2012), clinical data describe a lower response rate to ACT for females with melanoma compared with males (Besser et al., 2013). In another similar study, there was no sex difference in response (Besser et al., 2013).
As mentioned, the clinical benefit of androgen signaling blockade in the treatment of locally advanced and metastatic prostate cancer has partly been attributed to its role in remodeling the tumor immune microenvironment in addition to its well-established direct anti-tumor effects (Ardiani et al., 2014; Long et al., 2020). Furthermore, whereas ICB monotherapy historically showed limited efficacy in patients with metastatic prostate cancer without mismatch repair deficiency or biallelic loss of CDK12 (Abida et al., 2019; Le et al., 2017; Wu et al., 2018b), several clinical studies suggest that it may synergize with androgen signaling blockade. Only a few studies on early-stage prostate cancer have investigated the utility of this combination strategy with anti-CTLA4 therapy. In a phase I trial of tremelimumab and high-dose bicalutamide in patients with recurrent, non-metastatic prostate cancer, limited efficacy was observed in three out of 11 patients, demonstrating prolonged doubling time in prostate-specific antigen (PSA) during the surveillance period (McNeel et al., 2012). In a separate phase II trial involving patients with locally advanced prostate cancer, those on ipilimumab and ADT were more likely to result in undetectable PSA (55 vs 38%) compared with ADT alone (Tollefson et al., 2010). Further study of anti-CTLA4 and androgen signaling blockade is required with a larger sample size and with advanced-stage prostate cancer.
In contrast, the utility of anti-PD-1 therapy with androgen therapy has been mostly studied in patients with metastatic castration-resistant prostate cancer (mCRPC) who progressed on enzalutamide. In an early single-arm phase II study (Graff et al., 2016, 2020b), 5 of 28 patients on pembrolizumab and enzalutamide showed a PSA decline of over 50%, with improved median overall survival of 41.7 months as opposed to 21.9 months in all patients. These encouraging results led to further investigation of KEYNOTE-199 (NCT02787005; cohorts 4 and 5) and KEYNOTE-365 (NCT02861573; cohort C). In KEYNOTE-199, chemotherapy-naïve mCRPC patients with measurable disease per RECIST v1.1 (cohort 4) or bone-predominant disease (cohort 5) who progressed on a standard-of-care dose of enzalutamide were given pembrolizumab in addition to enzalutamide (Graff et al., 2020a). The combination strategy resulted in modest therapeutic efficacy, with a 12% objective response rate for cohort 4 and a 51% disease control rate for both cohorts (Graff et al., 2020a). In KEYNOTE-365, chemotherapy-naïve patients with mCRPC who progressed or were intolerant of abiraterone acetate were given pembrolizumab and enzalutamide, which similarly resulted in a 12% objective response rate (Mourey et al., 2020). Currently, a multicenter phase III KEYNOTE-641 study (NCT03834493) is evaluating the efficacy of pembrolizumab and enzalutamide versus enzalutamide and placebo in patients with mCRPC.
Of note, current evaluations on changes in immune components of the TME in response to ADT have been primarily confined to studies evaluating its effects on prostate cancer, a disease intrinsically characterized by the same protein (AR) we hope to inhibit and properly regulate in surrounding immune cells. Whereas future studies evaluating AR signaling in immune cell types should be conducted in non-prostate tissue, existing data will likely pave the way for more informed experimental approaches. For example, Koh and colleagues found that DCs were only able to fully induce primary and secondary T cell responses when ADT was applied after immunotherapy (Koh et al., 2009). Importantly, the recent two landmark studies showed conclusively that ADT can impede the CD8+ T cell exhaustion program in the TME and improve the effectiveness of anti-PD-1 ICB, which rekindles the hope of using ADT to routinely enhance immunotherapy (Guan et al., 2022; Kwon et al., 2022). Overall, more studies are needed to identify the optimal agent for androgen signaling blockade in concurrent use with ICB, their dose selection, treatment sequence, and, finally, the utility of this combination strategy in other cancers. Analysis of the tumor immune microenvironment, particularly with regards to mechanisms by which androgen signaling blockade may potentiate T cell immunity, will be key to addressing these questions.
Whereas evidence presented in this review would suggest that cancer patients would benefit of estrogen administration owing to increased adaptive immune responses, the only cancer-related clinical modulation of ER pathway signaling has been to treat ER + breast cancer through pathway inhibition. ER + breast cancer, the most common subtype of breast cancer, is characterized by estrogen-dependent ER-mediated tumor cell growth and proliferation (Hanker et al., 2020). The subtype is known for its relatively low TIL infiltration, PD-L1 expression, and mutational burden (Goldberg et al., 2021). As such, patients with ER + breast cancer do not respond well to ICB. However, clinical investigations using ER pathway inhibitors with ICB are currently underway to determine how best to turn this immunologically cold tumor into a more responsive tumor type. It is yet to be determined whether the combination of estrogens and ICB would be advantageous to treat non-ER + tumors.
Another rapidly emerging field with great promise in improving cancer patient care involves the understanding and potential modulation of the microbiome. As accumulating evidence suggests intrinsic sex-based differences in anti-tumor immunity and different response profiles to ICB between males and females, it is important to keep in mind how commensal microbiota varies between males and females. As previously mentioned, symbioses between sex and distinct microbiota are linked to immune cell function as well as dysfunction. Of significance, growing evidence shows that the gut microbiome has an effect on ICB efficacy. Commensal microbiota, Bifidobacterium and Bacteroidales fragilis, improved tumor control when combined with anti-PD-L1 and anti-CTLA-4, respectively (Vétizou et al., 2015; Sivan et al., 2015), and in melanoma patients, responders to anti-PD-1 treatment had significantly higher abundance of Ruminococcaceae and Faecalibacterium, whereas non-responders had a higher abundance of Bacteroidales (Gopalakrishnan et al., 2018). Therefore, in future studies, it will also be essential to consider the extent to which commensal microbiota are associated with sex hormone-dependent differences in anti-tumor immunity.
Concluding remarks and future perspective
The immunological role of sex in cancer development and progression is poorly understood and severely understudied, despite decades of evidence demonstrating females generally have a heightened innate and adaptive immune system compared with males. The mission of the National Institutes of Health (NIH), the single largest funder of biomedical research, is to seek fundamental knowledge about the nature and behavior of living systems and to apply that knowledge to enhance health, lengthen life, and reduce illness and disability. In recognition that sex influences both health and disease across medical disciplines, they implemented a policy to consider sex as a biological variable (SABV) in NIH-funded research (NIH, 2015a). Starting in 1993, the NIH initiated the Revitalization Act that required clinical studies to include the appropriate participation of women and under-represented minorities. Since 2015, the NIH expects all applicants proposing studies in vertebrate animals and humans to factor SABV into research designs, analyses, and reporting – or to provide a strong rationale to investigate only one sex. (National Institutes of Health, 2022a, NIH, 2015b) To further enhance transparency and accountability, the 21st Century Cures Act, an amendment of the Revitalization Act, requires valid analyses by sex/gender, ethnicity, and race to be reported for every Phase III clinical trial investigating biologics, therapeutics, or devices regulated by the FDA (NIH, 2017). To advance sex-focused analyses in preclinical research studies, the NIH also launched a Sex/Gender Administrative Supplement program to study the influence of sex and gender within the context of existing research awards.
To help researchers implement these policies and others, the NIH Office of Research on Women’s Health (ORWH), the FDA, and the National Institutes of General Medical Sciences (NIGMS) are collaborating on many fronts to provide educational resources (National Institutes of Health, 2022b). Four interactive modules were designed to educate the next generation of biomedical researchers on how to incorporate SABV into their research designs and prepare NIH grant applications accordingly. The course, Bench-to-Bedside: Integrating Sex and Gender to Improve Human Health, was released in 2020 to inform bench researchers and clinicians across various disciplines, such as cardiovascular medicine, pulmonology, and immunology, of the influences of sex on health and disease and how to apply SABV when conducting research or interpreting results during clinical practice. And, in partnership with the NIH Institutes and Centers (ICs), the ORWH co-funded the Specialized Center of Research Excellence (SCORE) on Sex Differences program. The centers for excellence contain a disease-agnostic program designed to model how SABV could be applied to interdisciplinary medical efforts in order to bridge basic and clinical research on the topic of sex. Of note, several journals have adopted the international standard, Sex and Gender Equity in Research (SAGER) guidelines, to help authors and editors determine whether appropriate considerations have been given to sex and gender, as well as to promote its application to biomedical research disciplines (Heidari et al., 2016). In short, not only do we emphasize the consideration of sex in the planning, execution, and analyses of preclinical and clinical immuno-oncology (IO) research, but it is now a key component of future NIH-funded research applications and the publication process.
Whereas the incorporation of sex into most preclinical biomedical research studies can be as simple as including both sexes into research designs and associated analyses, identifying the root causes of gender disparities in diseases is anything but simple – given that sex-biasing factors are often cofounding variables. Innovative technologies, such as the FCG mouse model, NEDD4 knockout mice, and Foxl2 null mice, are needed to pinpoint the independent as well as the interactive rules of each factor (Arnold and Chen, 2009; Uda et al., 2004; Windley et al., 2022). An integrated approach to define “sexomics,” i.e., the impact of sex on transcriptomics, epigenomics, metabolomics, microbiome, etc. needs to be done to move the field forward in a significant way. The investigation of how sex contributes to immunological differences in cancer etiology also comes with several unique challenges. First, the TME is composed of a complicated network of structures and cells, including blood vessels, lymphatics, fibroblasts, various cells of the immune system, as well as cancer cells (Giraldo et al., 2019). These highly complex and heterogeneous ecosystems are maintained and operated through autocrine, paracrine, and endocrine signaling networks; protein and ion concentration gradients; and numerous dynamic cell-to-cell interactions – and are highly plastic depending on countless factors, such as the age, size of the tumor, stage of the disease, type of microbiota present, and exposure to prior treatments. The vast majority of solid tumor IO research involves the mechanical and/or enzymatic disruption of tumors to isolate and evaluate cells of the TME, thereby disrupting these fragile ecosystems. Furthermore, to keep cells alive during processing, they are often placed into serums from foreign animal species that introduce exogenous growth factors and sex hormones or mimetics. Thus, investigators must be thoughtful in experimental design and reagent selection, to mitigate the introduction of confounding factors related to sex hormone signaling.
The methods used to analyze distinct cell subsets from the TME pose additional challenges and limitations in sex-focused IO research because these populations are generally small in number and inherently heterogeneous in terms of biology and stage of differentiation. The leading methods to analyze immune cell repertoires include single-cell sequencing technologies, such as single-cell RNA sequencing (scRNA-seq), cellular indexing of transcriptomes and epitopes seq (scCITE-seq), T cell repertoire seq (scTCR-seq), assay for transposase-accessible chromatin seq (scATAC-seq), and simultaneously profiling of gene expression and open chromatin from the same cell (chromium single-cell multiome ATAC + gene expression). Whereas these technologies have inherent limitations in terms of sequencing depth, gene expression dropout, and data integration from multiple modalities, information gained from these assays has greatly accelerated our overall understanding of tumor-immune cell biology (Lähnemann et al., 2020). As discussed in the “Sex steroid hormones” section, sex hormone receptors ESR1, ESR2, and AR are not differentially expressed in normal human tissue according to analyses using bulk RNA sequencing GTEx datasets (Lopes-Ramos et al., 2020). However, strong gene targeting patterns by sex hormone receptors contributed to differential regulatory networks, indicating there are different cofactors and target genes between sexes. Therefore, analyses to evaluate associated binding sites in individual immune cell subsets, perhaps through the use of scRNA-seq and scATAC-seq coupled with micro-ChIP, are needed to further delineate differing adaptive immune responses to cancer between sexes.
It is also critical to evaluate single-cell sex-biased tumor biology and IO without disruption of tissue architecture. Up-and-coming platforms currently leading the IO field involve the spatial assessment of immune cell transcripts and protein expression across tumors. Spatial transcriptomics was named the 2021 Method of the Year by Nature Methods as it allows for the evaluation of RNA gene expression in association with cellular location within a given tissue (Marx, 2021). This in situ approach allows researchers to study the TME without disrupting the tumor biology or exposing single cells to exogenous stimulants. Spatial transcriptomics can be coupled with additional spatial techniques, such as multispectral immunofluorescence (mIF) imaging, fluorescence in situ hybridization (FISH), and co-detection by indexing (CODEX), allowing investigators to gain additional insights and a more comprehensive understanding of gene amplification, transcript levels, and protein expression across tumor sections. A final challenge in the described IO research includes the consideration of how differing TMEs from different metastatic sites can impact therapeutic response to immunotherapies. Not only are the cancer cells that colonize these organs differently but so are the cells that infiltrate them (Nicholson et al., 2004; Smith et al., 2009).
In conclusion, the immune system plays a key role in monitoring healthy cell function and inhibiting tumorigenesis. Given that estrogens appear to further activate adaptive immune responses and androgens dampening them, males and females are prone to induce/resolve inflammation at differing rates and are therefore predisposed to handle pathogens and development diseases differently. One key objective in writing this review is to drive the point that global differences in immune response and regulation between males and females is a substantial factor that contributes to sex differences in tumor incidence and progression. Thus, it is of great importance that research in the IO field focuses on the impact and underlying mechanisms of sex on outcomes. This line of investigation has bearing in fundamental biology as well as in translational medicine, which is exemplified by recent findings that T cell-intrinsic AR promotes CD8+ T cell exhaustion in the TME (Kwon et al., 2022). Regarding the translational significance of these findings, the prevalence of readily available AR inhibitors originally designed to target AR-driven prostate cancer can be repurposed. Combining AR inhibitors with immunotherapy, such as ICB, cell therapy, and cytokine therapeutics, is just one potential therapeutic strategy to reinvigorate T cell effector function against tumor cells. An improved mechanistic understanding of how differential sex chromosomes and sex hormones regulate immune cell biology, and how these differences contribute to tumor control between sexes, has great potential to guide novel approaches to improve clinical care for both male and female patients with cancer.
Acknowledgments
This work was supported by National Institutes of Health grants: R01 CA213290, R01 AI077283, R01 CA255334, R01 CA255334, and R01 CA262069 (to Z.L.). J.M.S. was supported by the Ohio State University Comprehensive Cancer Center’s Tumor Immunology T32 (2T32CA09223-16A1) post-doctoral fellowship award. All figures were created with BioRender.com.
Author contributions
Conceptual development was done by J.M.S. and Z.L. Figures were created by J.M.S. and T.X. Tables were created by K.C. Literature review was performed by J.M.S, T.X., H.K., K.C., Y.C., H.A-H., D.S., D.T., X.L., and Z.L. All authors contributed to writing and final editing of the manuscript.
Declaration of interests
Z.L. serves as a member of the scientific advisory board for Heat Biologics, Alphamab, Hengenix, and Ikonisys. Other authors declare no competing interests.
References
- Abdullah M., Chai P.S., Chong M.Y., Tohit E.R.M., Ramasamy R., Pei C.P., Vidyadaran S. Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cell. Immunol. 2012;272:214–219. doi: 10.1016/j.cellimm.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Abida W., Cheng M.L., Armenia J., Middha S., Autio K.A., Vargas H.A., Rathkopf D., Morris M.J., Danila D.C., Slovin S.F., et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 2019;5:471–478. doi: 10.1001/jamaoncol.2018.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afshan G., Afzal N., Qureshi S. CD4+CD25(hi) regulatory T cells in healthy males and females mediate gender difference in the prevalence of autoimmune diseases. Clin. Lab. 2012;58:567–571. [PubMed] [Google Scholar]
- Ahmed S.A., Penhale W.J. The influence of testosterone on the development of autoimmune thyroiditis in thymectomized and irradiated rats. Clin. Exp. Immunol. 1982;48:367–374. [PMC free article] [PubMed] [Google Scholar]
- Ahnstedt H., Roy-O'Reilly M., Spychala M.S., Mobley A.S., Bravo-Alegria J., Chauhan A., Aronowski J., Marrelli S.P., McCullough L.D. Sex differences in adipose tissue CD8(+) T cells and regulatory T cells in middle-aged mice. Front. Immunol. 2018;9:659. doi: 10.3389/fimmu.2018.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altuwaijri S., Chuang K.H., Lai K.P., Lai J.J., Lin H.Y., Young F.M., Bottaro A., Tsai M.Y., Zeng W.P., Chang H.C., et al. Susceptibility to autoimmunity and B cell resistance to apoptosis in mice lacking androgen receptor in B cells. Mol. Endocrinol. 2009;23:444–453. doi: 10.1210/me.2008-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angele M.K., Pratschke S., Hubbard W.J., Chaudry I.H. Gender differences in sepsis: cardiovascular and immunological aspects. Virulence. 2014;5:12–19. doi: 10.4161/viru.26982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardiani A., Gameiro S.R., Kwilas A.R., Donahue R.N., Hodge J.W. Androgen deprivation therapy sensitizes prostate cancer cells to T-cell killing through androgen receptor dependent modulation of the apoptotic pathway. Oncotarget. 2014;5:9335–9348. doi: 10.18632/oncotarget.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold A.P., Chen X. What does the "four core genotypes" mouse model tell us about sex differences in the brain and other tissues? Front. Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruvito L., Giulianelli S., Flores A.C., Paladino N., Barboza M., Lanari C., Fainboim L. NK cells expressing a progesterone receptor are susceptible to progesterone-induced apoptosis. J. Immunol. 2008;180:5746–5753. doi: 10.4049/jimmunol.180.8.5746. [DOI] [PubMed] [Google Scholar]
- Bachtrog D. Y-chromosome evolution: emerging insights into processes of Y-chromosome degeneration. Nat. Rev. Genet. 2013;14:113–124. doi: 10.1038/nrg3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat R., Oakley O., Kim H., Jin J., Ko C.J. Extra-gonadal sites of estrogen biosynthesis and function. BMB Rep. 2016;49:488–496. doi: 10.5483/BMBRep.2016.49.9.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebo B.F., Jr., Zelinka-Vincent E., Adamus G., Amundson D., Vandenbark A.A., Offner H. Gonadal hormones influence the immune response to PLP 139-151 and the clinical course of relapsing experimental autoimmune encephalomyelitis. J. Neuroimmunol. 1998;84:122–130. doi: 10.1016/s0165-5728(97)00214-2. [DOI] [PubMed] [Google Scholar]
- Belkaid Y., Hand T.W. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Batalla I., Vargas-Delgado M.E., von Amsberg G., Janning M., Loges S. Influence of androgens on immunity to self and foreign: effects on immunity and cancer. Front. Immunol. 2020;11:1184. doi: 10.3389/fimmu.2020.01184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benten W.P.M., Becker A., Schmitt-Wrede H.P., Wunderlich F. Developmental regulation of intracellular and surface androgen receptors in T cells. Steroids. 2002;67:925–931. doi: 10.1016/s0039-128x(02)00055-7. [DOI] [PubMed] [Google Scholar]
- Bereshchenko O., Bruscoli S., Riccardi C. Glucocorticoids, sex hormones, and immunity. Front. Immunol. 2018;9:1332. doi: 10.3389/fimmu.2018.01332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghöfer B., Frommer T., Haley G., Fink L., Bein G., Hackstein H. TLR7 ligands induce higher IFN-alpha production in females. J. Immunol. 2006;177:2088–2096. doi: 10.4049/jimmunol.177.4.2088. [DOI] [PubMed] [Google Scholar]
- Berta P., Hawkins J.R., Sinclair A.H., Taylor A., Griffiths B.L., Goodfellow P.N., Fellous M. Genetic evidence equating SRY and the testis-determining factor. Nature. 1990;348:448–450. doi: 10.1038/348448A0. [DOI] [PubMed] [Google Scholar]
- Bertin M., Thébaud-Mony A., Counil E., Giscop93 study group Do women and men have the same patterns of multiple occupational carcinogenic exposures? Results from a cohort of cancer patients. Ann. Work Expo. Health. 2018;62:450–464. doi: 10.1093/annweh/wxx116. [DOI] [PubMed] [Google Scholar]
- Besser M.J., Shapira-Frommer R., Itzhaki O., Treves A.J., Zippel D.B., Levy D., Kubi A., Shoshani N., Zikich D., Ohayon Y., et al. Adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma: intent-to-treat analysis and efficacy after failure to prior immunotherapies. Clin. Cancer Res. 2013;19:4792–4800. doi: 10.1158/1078-0432.CCR-13-0380. [DOI] [PubMed] [Google Scholar]
- Bhavanam S., Snider D.P., Kaushic C. Intranasal and subcutaneous immunization under the effect of estradiol leads to better protection against genital HSV-2 challenge compared to progesterone. Vaccine. 2008;26:6165–6172. doi: 10.1016/j.vaccine.2008.08.045. [DOI] [PubMed] [Google Scholar]
- Blasko E., Haskell C.A., Leung S., Gualtieri G., Halks-Miller M., Mahmoudi M., Dennis M.K., Prossnitz E.R., Karpus W.J., Horuk R. Beneficial role of the GPR30 agonist G-1 in an animal model of multiple sclerosis. J. Neuroimmunol. 2009;214:67–77. doi: 10.1016/j.jneuroim.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolnick D.I., Snowberg L.K., Hirsch P.E., Lauber C.L., Org E., Parks B., Lusis A.J., Knight R., Caporaso J.G., Svanbäck R. Individual diet has sex-dependent effects on vertebrate gut microbiota. Nat. Commun. 2014;5:4500. doi: 10.1038/ncomms5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botticelli A., Onesti C.E., Zizzari I., Cerbelli B., Sciattella P., Occhipinti M., Roberto M., Di Pietro F., Bonifacino A., Ghidini M., et al. The sexist behaviour of immune checkpoint inhibitors in cancer therapy? Oncotarget. 2017;8:99336–99346. doi: 10.18632/oncotarget.22242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumil R.M., Lee J.T. Forty years of decoding the silence in X-chromosome inactivation. Hum. Mol. Genet. 2001;10:2225–2232. doi: 10.1093/hmg/10.20.2225. [DOI] [PubMed] [Google Scholar]
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Brunsing R.L., Prossnitz E.R. Induction of interleukin-10 in the T helper type 17 effector population by the G protein coupled estrogen receptor (GPER) agonist G-1. Immunology. 2011;134:93–106. doi: 10.1111/j.1365-2567.2011.03471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunders M.J., Altfeld M. Implications of sex differences in immunity for SARS-CoV-2 pathogenesis and design of therapeutic interventions. Immunity. 2020;53:487–495. doi: 10.1016/j.immuni.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth M., McClellan B., Allansmith M. Influence of sex in immunoglobulin levels. Nature. 1967;214:1224–1225. doi: 10.1038/2141224a0. [DOI] [PubMed] [Google Scholar]
- Butts C.L., Shukair S.A., Duncan K.M., Bowers E., Horn C., Belyavskaya E., Tonelli L., Sternberg E.M. Progesterone inhibits mature rat dendritic cells in a receptor-mediated fashion. Int. Immunol. 2007;19:287–296. doi: 10.1093/intimm/dxl145. [DOI] [PubMed] [Google Scholar]
- Calagua C., Russo J., Sun Y., Schaefer R., Lis R., Zhang Z., Mahoney K., Bubley G.J., Loda M., Taplin M.E., et al. Expression of PD-L1 in hormone-naïve and treated prostate cancer patients receiving neoadjuvant abiraterone acetate plus prednisone and leuprolide. Clin. Cancer Res. 2017;23:6812–6822. doi: 10.1158/1078-0432.CCR-17-0807. [DOI] [PubMed] [Google Scholar]
- Calippe B., Douin-Echinard V., Delpy L., Laffargue M., Lélu K., Krust A., Pipy B., Bayard F., Arnal J.F., Guéry J.C., et al. 17Beta-estradiol promotes TLR4-triggered proinflammatory mediator production through direct estrogen receptor alpha signaling in macrophages in vivo. J. Immunol. 2010;185:1169–1176. doi: 10.4049/jimmunol.0902383. [DOI] [PubMed] [Google Scholar]
- Carrel L., Willard H.F. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- Carrera C., Potrony M., Puig S. Sex as a predictor of response to cancer immunotherapy. Lancet Oncol. 2018;19:e375. doi: 10.1016/S1470-2045(18)30443-1. [DOI] [PubMed] [Google Scholar]
- Charles Huggins C.H. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–297. [Google Scholar]
- Chen R.Y., Fan Y.M., Zhang Q., Liu S., Li Q., Ke G.L., Li C., You Z. Estradiol inhibits Th17 cell differentiation through inhibition of RORγT transcription by recruiting the ERα/REA complex to estrogen response elements of the RORγT promoter. J. Immunol. 2015;194:4019–4028. doi: 10.4049/jimmunol.1400806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Lan X., Thomas-Ahner J.M., Wu D., Liu X., Ye Z., Wang L., Sunkel B., Grenade C., Chen J., et al. Agonist and antagonist switch DNA motifs recognized by human androgen receptor in prostate cancer. EMBO J. 2015;34:502–516. doi: 10.15252/embj.201490306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen S.R., Shupe J., Nickerson K., Kashgarian M., Flavell R.A., Shlomchik M.J. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Cioni B., Zaalberg A., van Beijnum J.R., Melis M.H.M., van Burgsteden J., Muraro M.J., Hooijberg E., Peters D., Hofland I., Lubeck Y., et al. Androgen receptor signalling in macrophages promotes TREM-1-mediated prostate cancer cell line migration and invasion. Nat. Commun. 2020;11:4498. doi: 10.1038/s41467-020-18313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claggett B., Tian L., McCaw Z.R., Takeuchi M., Wei L.-J. Sex as a predictor of response to cancer immunotherapy. Lancet Oncol. 2018;19:e377. doi: 10.1016/S1470-2045(18)30517-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement C.C., Nanaware P.P., Yamazaki T., Negroni M.P., Ramesh K., Morozova K., Thangaswamy S., Graves A., Kim H.J., Li T.W., et al. Pleiotropic consequences of metabolic stress for the major histocompatibility complex class II molecule antigen processing and presentation machinery. Immunity. 2021;54:721–736.e10. doi: 10.1016/j.immuni.2021.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clocchiatti A., Cora E., Zhang Y., Dotto G.P. Sexual dimorphism in cancer. Nat. Rev. Cancer. 2016;16:330–339. doi: 10.1038/nrc.2016.30. [DOI] [PubMed] [Google Scholar]
- Collazos J., Asensi V., Cartón J.A. Sex differences in the clinical, immunological and virological parameters of HIV-infected patients treated with HAART. AIDS. 2007;21:835–843. doi: 10.1097/QAD.0b013e3280b0774a. [DOI] [PubMed] [Google Scholar]
- Conforti F., Pala L., Bagnardi V., De Pas T., Marco M., Viale G., Gelber R., Goldhirsch A. Sex as a predictor of response to cancer immunotherapy – authors' reply. Lancet Oncol. 2018;19:e380–e381. doi: 10.1016/S1470-2045(18)30535-7. [DOI] [PubMed] [Google Scholar]
- Conforti F., Pala L., Bagnardi V., De Pas T., Martinetti M., Viale G., Gelber R.D., Goldhirsch A. Cancer immunotherapy efficacy and patients' sex: a systematic review and meta-analysis. Lancet Oncol. 2018;19:737–746. doi: 10.1016/S1470-2045(18)30261-4. [DOI] [PubMed] [Google Scholar]
- Conforti F., Pala L., Bagnardi V., Viale G., De Pas T., Pagan E., Pennacchioli E., Cocorocchio E., Ferrucci P.F., De Marinis F., et al. Sex-based heterogeneity in response to lung cancer immunotherapy: a systematic review and meta-analysis. J. Natl. Cancer Inst. 2019;111:772–781. doi: 10.1093/jnci/djz094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti F., Pala L., Pagan E., Bagnardi V., De Pas T., Queirolo P., Pennacchioli E., Catania C., Cocorocchio E., Ferrucci P.F., et al. Sex-based dimorphism of anticancer immune response and molecular mechanisms of immune evasion. Clin. Cancer Res. 2021;27:4311–4324. doi: 10.1158/1078-0432.CCR-21-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti F., Pala L., Pagan E., Corti C., Bagnardi V., Queirolo P., Catania C., De Pas T., Giaccone G. Sex-based differences in response to anti-PD-1 or PD-L1 treatment in patients with non-small-cell lung cancer expressing high PD-L1 levels. A systematic review and meta-analysis of randomized clinical trials. ESMO Open. 2021;6:100251. doi: 10.1016/j.esmoop.2021.100251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook I.F. Sexual dimorphism of humoral immunity with human vaccines. Vaccine. 2008;26:3551–3555. doi: 10.1016/j.vaccine.2008.04.054. [DOI] [PubMed] [Google Scholar]
- Corcoran M.P., Meydani M., Lichtenstein A.H., Schaefer E.J., Dillard A., Lamon-Fava S. Sex hormone modulation of proinflammatory cytokine and C-reactive protein expression in macrophages from older men and postmenopausal women. J. Endocrinol. 2010;206:217–224. doi: 10.1677/JOE-10-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales J.J., Almeida M., Cordero M., Martín-Martín L., Méndez C., Miralles J.M., Orfao A. Enhanced immunological response by dendritic cells in male hypogonadism. Eur. J. Clin. Invest. 2012;42:1205–1212. doi: 10.1111/j.1365-2362.2012.02712.x. [DOI] [PubMed] [Google Scholar]
- Crawford E.D., Heidenreich A., Lawrentschuk N., Tombal B., Pompeo A.C.L., Mendoza-Valdes A., Miller K., Debruyne F.M.J., Klotz L. Androgen-targeted therapy in men with prostate cancer: evolving practice and future considerations. Prostate Cancer Prostatic Dis. 2019;22:24–38. doi: 10.1038/s41391-018-0079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Shen Y., Li R. Estrogen synthesis and signaling pathways during aging: from periphery to brain. Trends Mol. Med. 2013;19:197–209. doi: 10.1016/j.molmed.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey R.A., Grossmann M. Androgen receptor structure, function and biology: from bench to bedside. Clin. Biochem. Rev. 2016;37:3–15. [PMC free article] [PubMed] [Google Scholar]
- Deane J.A., Pisitkun P., Barrett R.S., Feigenbaum L., Town T., Ward J.M., Flavell R.A., Bolland S. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27:801–810. doi: 10.1016/j.immuni.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMayo F.J., Zhao B., Takamoto N., Tsai S.Y. Mechanisms of action of estrogen and progesterone. Ann. N. Y. Acad. Sci. 2002;955:48–59. doi: 10.1111/j.1749-6632.2002.tb02765.x. discussion 86-8, 396-406. [DOI] [PubMed] [Google Scholar]
- Di Martino V., Lebray P., Myers R.P., Pannier E., Paradis V., Charlotte F., Moussalli J., Thabut D., Buffet C., Poynard T. Progression of liver fibrosis in women infected with hepatitis C: long-term benefit of estrogen exposure. Hepatology. 2004;40:1426–1433. doi: 10.1002/hep.20463. [DOI] [PubMed] [Google Scholar]
- Dominianni C., Sinha R., Goedert J.J., Pei Z., Yang L., Hayes R.B., Ahn J. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS One. 2015;10:e0124599. doi: 10.1371/journal.pone.0124599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douin-Echinard V., Laffont S., Seillet C., Delpy L., Krust A., Chambon P., Gourdy P., Arnal J.F., Guéry J.C. Estrogen receptor alpha, but not beta, is required for optimal dendritic cell differentiation and [corrected] CD40-induced cytokine production. J. Immunol. 2008;180:3661–3669. doi: 10.4049/jimmunol.180.6.3661. [DOI] [PubMed] [Google Scholar]
- Duan W., Shen X., Lei J., Xu Q., Yu Y., Li R., Wu E., Ma Q. Hyperglycemia, a neglected factor during cancer progression. Biomed. Res. Int. 2014;2014:461917. doi: 10.1155/2014/461917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunford A., Weinstock D.M., Savova V., Schumacher S.E., Cleary J.P., Yoda A., Sullivan T.J., Hess J.M., Gimelbrant A.A., Beroukhim R., et al. Tumor-suppressor genes that escape from X-inactivation contribute to cancer sex bias. Nat. Genet. 2017;49:10–16. doi: 10.1038/ng.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elderman M., Hugenholtz F., Belzer C., Boekschoten M., van Beek A., de Haan B., Savelkoul H., de Vos P., Faas M. Sex and strain dependent differences in mucosal immunology and microbiota composition in mice. Biol. Sex Differ. 2018;9:26. doi: 10.1186/s13293-018-0186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elderman M., van Beek A., Brandsma E., de Haan B., Savelkoul H., de Vos P., Faas M. Sex impacts Th1 cells, Tregs, and DCs in both intestinal and systemic immunity in a mouse strain and location-dependent manner. Biol. Sex Differ. 2016;7:21. doi: 10.1186/s13293-016-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fainsod-Levi T., Gershkovitz M., Völs S., Kumar S., Khawaled S., Sagiv J.Y., Sionov R.V., Grunewald M., Keshet E., Granot Z. Hyperglycemia impairs neutrophil mobilization leading to enhanced metastatic seeding. Cell Rep. 2017;21:2384–2392. doi: 10.1016/j.celrep.2017.11.010. [DOI] [PubMed] [Google Scholar]
- Fijak M., Schneider E., Klug J., Bhushan S., Hackstein H., Schuler G., Wygrecka M., Gromoll J., Meinhardt A. Testosterone replacement effectively inhibits the development of experimental autoimmune orchitis in rats: evidence for a direct role of testosterone on regulatory T cell expansion. J. Immunol. 2011;186:5162–5172. doi: 10.4049/jimmunol.1001958. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick F., Lepault F., Homo-Delarche F., Bach J.F., Dardenne M. Influence of castration, alone or combined with thymectomy, on the development of diabetes in the nonobese diabetic mouse. Endocrinology. 1991;129:1382–1390. doi: 10.1210/endo-129-3-1382. [DOI] [PubMed] [Google Scholar]
- Fox H.S. Androgen treatment prevents diabetes in nonobese diabetic mice. J. Exp. Med. 1992;175:1409–1412. doi: 10.1084/jem.175.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox H.S., Bond B.L., Parslow T.G. Estrogen regulates the IFN-gamma promoter. J. Immunol. 1991;146:4362–4367. [PubMed] [Google Scholar]
- Fuentes N., Silveyra P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019;116:135–170. doi: 10.1016/bs.apcsb.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman D., Hejblum B.P., Simon N., Jojic V., Dekker C.L., Thiébaut R., Tibshirani R.J., Davis M.M. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc. Natl. Acad. Sci. USA. 2014;111:869–874. doi: 10.1073/pnas.1321060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal-Oz S.T., Maier B., Yoshida H., Seddu K., Elbaz N., Czysz C., Zuk O., Stranger B.E., Ner-Gaon H., Shay T. ImmGen report: sexual dimorphism in the immune system transcriptome. Nat. Commun. 2019;10:4295. doi: 10.1038/s41467-019-12348-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon P.O., Poisson A.O., Delvoye N., Lapointe R., Mes-Masson A.M., Saad F. Characterization of the intra-prostatic immune cell infiltration in androgen-deprived prostate cancer patients. J. Immunol. Methods. 2009;348:9–17. doi: 10.1016/j.jim.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Gao X., Zhang M., Xue J., Huang J., Zhuang R., Zhou X., Zhang H., Fu Q., Hao Y. Body mass index differences in the gut microbiota are gender specific. Front. Microbiol. 2018;9:1250. doi: 10.3389/fmicb.2018.01250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giefing-Kröll C., Berger P., Lepperdinger G., Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015;14:309–321. doi: 10.1111/acel.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giltay E.J., Fonk J.C., von Blomberg B.M., Drexhage H.A., Schalkwijk C., Gooren L.J. In vivo effects of sex steroids on lymphocyte responsiveness and immunoglobulin levels in humans. J. Clin. Endocrinol. Metab. 2000;85:1648–1657. doi: 10.1210/jcem.85.4.6562. [DOI] [PubMed] [Google Scholar]
- Giraldo N.A., Sanchez-Salas R., Peske J.D., Vano Y., Becht E., Petitprez F., Validire P., Ingels A., Cathelineau X., Fridman W.H., et al. The clinical role of the TME in solid cancer. Br. J. Cancer. 2019;120:45–53. doi: 10.1038/s41416-018-0327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girón-González J.A., Moral F.J., Elvira J., García-Gil D., Guerrero F., Gavilán I., Escobar L. Consistent production of a higher TH1:TH2 cytokine ratio by stimulated T cells in men compared with women. Eur. J. Endocrinol. 2000;143:31–36. doi: 10.1530/eje.0.1430031. [DOI] [PubMed] [Google Scholar]
- Gold S.M., Chalifoux S., Giesser B.S., Voskuhl R.R. Immune modulation and increased neurotrophic factor production in multiple sclerosis patients treated with testosterone. J. Neuroinflammation. 2008;5:32. doi: 10.1186/1742-2094-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J., Pastorello R.G., Vallius T., Davis J., Cui Y.X., Agudo J., Waks A.G., Keenan T., McAllister S.S., Tolaney S.M., et al. The immunology of hormone receptor positive breast cancer. Front. Immunol. 2021;12:674192. doi: 10.3389/fimmu.2021.674192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan V., Spencer C.N., Nezi L., Reuben A., Andrews M.C., Karpinets T.V., Prieto P.A., Vicente D., Hoffman K., Wei S.C., et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff J.N., Alumkal J.J., Drake C.G., Thomas G.V., Redmond W.L., Farhad M., Cetnar J.P., Ey F.S., Bergan R.C., Slottke R., et al. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget. 2016;7:52810–52817. doi: 10.18632/oncotarget.10547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff J.N., Antonarakis E.S., Hoimes C.J., Tagawa S.T., Hwang C., Kilari D., Ten Tije A., Omlin A.G., McDermott R.S., Vaishampayan U.N., et al. Pembrolizumab (pembro) plus enzalutamide (enza) for enza-resistant metastatic castration-resistant prostate cancer (mCRPC): KEYNOTE-199 cohorts 4-5. J. Clin. Oncol. 2020;38:15. [Google Scholar]
- Graff J.N., Beer T.M., Alumkal J.J., Slottke R.E., Redmond W.L., Thomas G.V., Thompson R.F., Wood M.A., Koguchi Y., Chen Y., et al. A phase II single-arm study of pembrolizumab with enzalutamide in men with metastatic castration-resistant prostate cancer progressing on enzalutamide alone. J. Immunother. Cancer. 2020;8:e000642. doi: 10.1136/jitc-2020-000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassadonia A., Sperduti I., Vici P., Iezzi L., Brocco D., Gamucci T., Pizzuti L., Maugeri-Saccà M., Marchetti P., Cognetti G., et al. Effect of gender on the outcome of patients receiving immune checkpoint inhibitors for advanced cancer: a systematic review and meta-analysis of phase III randomized clinical trials. J. Clin. Med. 2018;7:E542. doi: 10.3390/jcm7120542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebely J., Page K., Sacks-Davis R., van der Loeff M.S., Rice T.M., Bruneau J., Morris M.D., Hajarizadeh B., Amin J., Cox A.L., et al. The effects of female sex, viral genotype, and IL28B genotype on spontaneous clearance of acute hepatitis C virus infection. Hepatology. 2014;59:109–120. doi: 10.1002/hep.26639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesbeck M., Ziegler S., Laffont S., Smith N., Chauveau L., Tomezsko P., Sharei A., Kourjian G., Porichis F., Hart M., et al. Sex differences in plasmacytoid dendritic cell levels of IRF5 drive higher IFN-α production in women. J. Immunol. 2015;195:5327–5336. doi: 10.4049/jimmunol.1501684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X., Polesso F., Wang C., Sehrawat A., Hawkins R.M., Murray S.E., Thomas G.V., Caruso B., Thompson R.F., Wood M.A., et al. Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature. 2022;606:791–796. doi: 10.1038/s41586-022-04522-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbay J., Collignon J., Koopman P., Capel B., Economou A., Münsterberg A., Vivian N., Goodfellow P., Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990;346:245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- Gubbels Bupp M.R., Jorgensen T.N. Androgen-induced immunosuppression. Front. Immunol. 2018;9:794. doi: 10.3389/fimmu.2018.00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbels Bupp M.R. Sex, the aging immune system, and chronic disease. Cell. Immunol. 2015;294:102–110. doi: 10.1016/j.cellimm.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Gubbels Bupp M.R., Jørgensen T.N., Kotzin B.L. Identification of candidate genes that influence sex hormone-dependent disease phenotypes in mouse lupus. Genes Immun. 2008;9:47–56. doi: 10.1038/sj.gene.6364447. [DOI] [PubMed] [Google Scholar]
- Guerra-Silveira F., Abad-Franch F. Sex bias in infectious disease epidemiology: patterns and processes. PLoS One. 2013;8:e62390. doi: 10.1371/journal.pone.0062390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan P., Didomenico D., Brown J., Shaw M., Sharifi R., Ray V., Shott S., Rubenstein M. The effect of androgen deprivation on malignant and benign prostate tissue. Med. Oncol. 1997;14:145–152. doi: 10.1007/BF02989642. [DOI] [PubMed] [Google Scholar]
- Hamilton J.B., Mestler G.E. Mortality and survival: comparison of eunuchs with intact men and women in a mentally retarded population. J. Gerontol. 1969;24:395–411. doi: 10.1093/geronj/24.4.395. [DOI] [PubMed] [Google Scholar]
- Han X.Y., Tarrand J.J., Infante R., Jacobson K.L., Truong M. Clinical significance and epidemiologic analyses of Mycobacterium avium and Mycobacterium intracellulare among patients without AIDS. J. Clin. Microbiol. 2005;43:4407–4412. doi: 10.1128/JCM.43.9.4407-4412.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanker A.B., Sudhan D.R., Arteaga C.L. Overcoming endocrine resistance in breast cancer. Cancer Cell. 2020;37:496–513. doi: 10.1016/j.ccell.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbuz M.S., Perveen-Gill Z., Lightman S.L., Jessop D.S. A protective role for testosterone in adjuvant-induced arthritis. Br. J. Rheumatol. 1995;34:1117–1122. doi: 10.1093/rheumatology/34.12.1117. [DOI] [PubMed] [Google Scholar]
- Haro C., Rangel-Zúñiga O.A., Alcalá-Díaz J.F., Gómez-Delgado F., Pérez-Martínez P., Delgado-Lista J., Quintana-Navarro G.M., Landa B.B., Navas-Cortés J.A., Tena-Sempere M., et al. Intestinal microbiota is influenced by gender and body mass index. PLoS One. 2016;11:e0154090. doi: 10.1371/journal.pone.0154090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison O.J., Foley J., Bolognese B.J., Long E., 3rd, Podolin P.L., Walsh P.T. Airway infiltration of CD4+ CCR6+ Th17 type cells associated with chronic cigarette smoke induced airspace enlargement. Immunol. Lett. 2008;121:13–21. doi: 10.1016/j.imlet.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Hartge P., Harvey E.B., Linehan W.M., Silverman D.T., Sullivan J.W., Hoover R.N., Fraumeni J.F., Jr. Unexplained excess risk of bladder cancer in men. J. Natl. Cancer Inst. 1990;82:1636–1640. doi: 10.1093/jnci/82.20.1636. [DOI] [PubMed] [Google Scholar]
- Heard E., Chaumeil J., Masui O., Okamoto I. Mammalian X-chromosome inactivation: an epigenetics paradigm. Cold Spring Harb. Symp. Quant. Biol. 2004;69:89–102. doi: 10.1101/sqb.2004.69.89. [DOI] [PubMed] [Google Scholar]
- Heidari S., Babor T.F., De Castro P., Tort S., Curno M. Sex and Gender Equity in Research: rationale for the SAGER guidelines and recommended use. Res. Integr. Peer Rev. 2016;1:2. doi: 10.1186/s41073-016-0007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson J. On the relationship of the thymus to the sexual organs: I. The influence of castration on the thymus. J. Physiol. 1904;31:222–229. doi: 10.1113/jphysiol.1904.sp001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J.W., Kim S.E., Sung M.K. Sex differences in the incidence of obesity-related gastrointestinal cancer. Int. J. Mol. Sci. 2021;22:1253. doi: 10.3390/ijms22031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz D., Dibbern J., Eggers L., von Borstel L., Schneider B.E. Increased male susceptibility to Mycobacterium tuberculosis infection is associated with smaller B cell follicles in the lungs. Sci. Rep. 2020;10:5142. doi: 10.1038/s41598-020-61503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins S.T., Kurti A.N., Redner R., White T.J., Gaalema D.E., Roberts M.E., Doogan N.J., Tidey J.W., Miller M.E., Stanton C.A., et al. A literature review on prevalence of gender differences and intersections with other vulnerabilities to tobacco use in the United States, 2004-2014. Prev. Med. 2015;80:89–100. doi: 10.1016/j.ypmed.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu L.H., Chu N.M., Lin Y.F., Kao S.H. G-protein coupled estrogen receptor in breast cancer. Int. J. Mol. Sci. 2019;20:E306. doi: 10.3390/ijms20020306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh E.C., Essner R., Foshag L.J., Ollila D.W., Gammon G., O'Day S.J., Boasberg P.D., Stern S.L., Ye X., Morton D.L. Prolonged survival after complete resection of disseminated melanoma and active immunotherapy with a therapeutic cancer vaccine. J. Clin. Oncol. 2002;20:4549–4554. doi: 10.1200/JCO.2002.01.151. [DOI] [PubMed] [Google Scholar]
- Hu Y.C., Wang P.H., Yeh S., Wang R.S., Xie C., Xu Q., Zhou X., Chao H.T., Tsai M.Y., Chang C. Subfertility and defective folliculogenesis in female mice lacking androgen receptor. Proc. Natl. Acad. Sci. USA. 2004;101:11209–11214. doi: 10.1073/pnas.0404372101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Chen B., Liu X., Li H., Xie L., Gao Y., Duan R., Li Z., Zhang J., Zheng Y., et al. Effects of sex and aging on the immune cell landscape as assessed by single-cell transcriptomic analysis. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2023216118. e2023216118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes G.C., Choubey D. Modulation of autoimmune rheumatic diseases by oestrogen and progesterone. Nat. Rev. Rheumatol. 2014;10:740–751. doi: 10.1038/nrrheum.2014.144. [DOI] [PubMed] [Google Scholar]
- Hughes G.C. Progesterone and autoimmune disease. Autoimmun. Rev. 2012;11:A502–A514. doi: 10.1016/j.autrev.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irelli A., Sirufo M.M., D'Ugo C., Ginaldi L., De Martinis M. Sex and gender influences on cancer immunotherapy response. Biomedicines. 2020;8:E232. doi: 10.3390/biomedicines8070232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S.W., Scharping N.E., Kolhatkar N.S., Khim S., Schwartz M.A., Li Q.Z., Hudkins K.L., Alpers C.E., Liggitt D., Rawlings D.J. Opposing impact of B cell-intrinsic TLR7 and TLR9 signals on autoantibody repertoire and systemic inflammation. J. Immunol. 2014;192:4525–4532. doi: 10.4049/jimmunol.1400098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson D.L., Gange S.J., Rose N.R., Graham N.M. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin. Immunol. Immunopathol. 1997;84:223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- Jenq R.R., Curran M.A., Goldberg G.L., Liu C., Allison J.P., van den Brink M.R.M. Repertoire enhancement with adoptively transferred female lymphocytes controls the growth of pre-implanted murine prostate cancer. PLoS One. 2012;7:e35222. doi: 10.1371/journal.pone.0035222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnke R.M., Edwards J.M., Kovacs C.J., Evans M.J., Daly B.M., Karlsson U.L., Lee T.K., Allison R.R., Arastu H.H., Cariveau M.J., O'Brien K.F. Response of T lymphocyte populations in prostate cancer patients undergoing radiotherapy: influence of neoajuvant total androgen suppression. Anticancer Res. 2005;25:3159–3166. [PubMed] [Google Scholar]
- Jones L.A., Anthony J.P., Henriquez F.L., Lyons R.E., Nickdel M.B., Carter K.C., Alexander J., Roberts C.W. Toll-like receptor-4-mediated macrophage activation is differentially regulated by progesterone via the glucocorticoid and progesterone receptors. Immunology. 2008;125:59–69. doi: 10.1111/j.1365-2567.2008.02820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L.A., Kreem S., Shweash M., Paul A., Alexander J., Roberts C.W. Differential modulation of TLR3- and TLR4-mediated dendritic cell maturation and function by progesterone. J. Immunol. 2010;185:4525–4534. doi: 10.4049/jimmunol.0901155. [DOI] [PubMed] [Google Scholar]
- Kadekar S., Peddada S., Silins I., French J.E., Högberg J., Stenius U. Gender differences in chemical carcinogenesis in National Toxicology Program 2-year bioassays. Toxicol. Pathol. 2012;40:1160–1168. doi: 10.1177/0192623312446527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda N., Tamaki K. Estrogen enhances immunoglobulin production by human PBMCs. J. Allergy Clin. Immunol. 1999;103:282–288. doi: 10.1016/s0091-6749(99)70503-8. [DOI] [PubMed] [Google Scholar]
- Kaneko S., Li X. X chromosome protects against bladder cancer in females via a KDM6A-dependent epigenetic mechanism. Sci. Adv. 2018;4:eaar5598. doi: 10.1126/sciadv.aar5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastelan M., Kraljić I., Tarle M. NK cell activity in treated prostate cancer patients as a probe for circulating tumor cells: hormone regulatory effects in vivo. Prostate. 1992;21:111–120. doi: 10.1002/pros.2990210204. [DOI] [PubMed] [Google Scholar]
- Kautzky-Willer A., Harreiter J., Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr. Rev. 2016;37:278–316. doi: 10.1210/er.2015-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T., Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- Khan D., Ansar Ahmed S. The immune system is a natural target for estrogen action: opposing effects of estrogen in two prototypical autoimmune diseases. Front. Immunol. 2015;6:635. doi: 10.3389/fimmu.2015.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan D., Dai R., Karpuzoglu E., Ahmed S.A. Estrogen increases, whereas IL-27 and IFN-gamma decrease, splenocyte IL-17 production in WT mice. Eur. J. Immunol. 2010;40:2549–2556. doi: 10.1002/eji.201040303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S., Atkinson E.J., Melton L.J., 3rd, Riggs B.L. Effects of age and estrogen status on serum parathyroid hormone levels and biochemical markers of bone turnover in women: a population-based study. J. Clin. Endocrinol. Metab. 1997;82:1522–1527. doi: 10.1210/jcem.82.5.3946. [DOI] [PubMed] [Google Scholar]
- Khramtsova E.A., Davis L.K., Stranger B.E. The role of sex in the genomics of human complex traits. Nat. Rev. Genet. 2019;20:173–190. doi: 10.1038/s41576-018-0083-1. [DOI] [PubMed] [Google Scholar]
- Kim Y.J., Oremus M., Chen H.H., McFarlane T., Shah D., Horton S. Real-world effectiveness of nivolumab in patients with non-small-cell lung cancer: a systematic review and meta-analysis. Future Oncol. 2020;16:2045–2058. doi: 10.2217/fon-2020-0248. [DOI] [PubMed] [Google Scholar]
- Kissick H.T., Sanda M.G., Dunn L.K., Pellegrini K.L., On S.T., Noel J.K., Arredouani M.S. Androgens alter T-cell immunity by inhibiting T-helper 1 differentiation. Proc. Natl. Acad. Sci. USA. 2014;111:9887–9892. doi: 10.1073/pnas.1402468111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- Klein S.L., Jedlicka A., Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis. 2010;10:338–349. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S.L., Marriott I., Fish E.N. Sex-based differences in immune function and responses to vaccination. Trans. R. Soc. Trop. Med. Hyg. 2015;109:9–15. doi: 10.1093/trstmh/tru167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh Y.T., Gray A., Higgins S.A., Hubby B., Kast W.M. Androgen ablation augments prostate cancer vaccine immunogenicity only when applied after immunization. Prostate. 2009;69:571–584. doi: 10.1002/pros.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovats S., Carreras E. Regulation of dendritic cell differentiation and function by estrogen receptor ligands. Cell. Immunol. 2008;252:81–90. doi: 10.1016/j.cellimm.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H., Lin C.Y., Chung D., Li X., Li Z. Sex as a predictor of response to cancer immunotherapy. Lancet Oncol. 2018;19:e379. doi: 10.1016/S1470-2045(18)30445-5. [DOI] [PubMed] [Google Scholar]
- Kwon H., Schafer J.M., Song N.J., Kaneko S., Li A., Xiao T., Ma A., Allen C., Das K., Zhou L., et al. Androgen conspires with the CD8(+) T cell exhaustion program and contributes to sex bias in cancer. Sci. Immunol. 2022 doi: 10.1126/sciimmunol.abq2630. https://doi: 10.1126/sciimmunol.abq2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lähnemann D., Köster J., Szczurek E., McCarthy D.J., Hicks S.C., Robinson M.D., Vallejos C.A., Campbell K.R., Beerenwinkel N., Mahfouz A., et al. Eleven grand challenges in single-cell data science. Genome Biol. 2020;21:31. doi: 10.1186/s13059-020-1926-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai K.P., Lai J.J., Chang P., Altuwaijri S., Hsu J.W., Chuang K.H., Shyr C.R., Yeh S., Chang C. Targeting thymic epithelia AR enhances T-cell reconstitution and bone marrow transplant grafting efficacy. Mol. Endocrinol. 2013;27:25–37. doi: 10.1210/me.2012-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai L.-T., Gu W.-G., Hu M.-B., Wang W.-J., Wang S.-S., Huai Y.-J., Mei J.-H., Wang C.-L. Sex-related differences in the efficacy of immune checkpoint inhibitors in malignancy: a systematic review and meta-analysis. Aging. 2021;13:15413–15432. doi: 10.18632/aging.203100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam H.M., Ouyang B., Chen J., Ying J., Wang J., Wu C.L., Jia L., Medvedovic M., Vessella R.L., Ho S.M. Targeting GPR30 with G-1: a new therapeutic target for castration-resistant prostate cancer. Endocr. Relat. Cancer. 2014;21:903–914. doi: 10.1530/ERC-14-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laniado-Laborín R. Smoking and chronic obstructive pulmonary disease (COPD). Parallel epidemics of the 21 century. Int. J. Environ. Res. Public Health. 2009;6:209–224. doi: 10.3390/ijerph6010209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le D.T., Durham J.N., Smith K.N., Wang H., Bartlett B.R., Aulakh L.K., Lu S., Kemberling H., Wilt C., Luber B.S., et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B.W., Yap H.K., Chew F.T., Quah T.C., Prabhakaran K., Chan G.S., Wong S.C., Seah C.C. Age- and sex-related changes in lymphocyte subpopulations of healthy Asian subjects: from birth to adulthood. Cytometry. 1996;26:8–15. doi: 10.1002/(SICI)1097-0320(19960315)26:1<8::AID-CYTO2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Lee J., Taneja V., Vassallo R. Cigarette smoking and inflammation: cellular and molecular mechanisms. J. Dent. Res. 2012;91:142–149. doi: 10.1177/0022034511421200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lélu K., Laffont S., Delpy L., Paulet P.E., Périnat T., Tschanz S.A., Pelletier L., Engelhardt B., Guéry J.C. Estrogen receptor α signaling in T lymphocytes is required for estradiol-mediated inhibition of Th1 and Th17 cell differentiation and protection against experimental autoimmune encephalomyelitis. J. Immunol. 2011;187:2386–2393. doi: 10.4049/jimmunol.1101578. [DOI] [PubMed] [Google Scholar]
- Li X., O'Malley B.W. Unfolding the action of progesterone receptors. J. Biol. Chem. 2003;278:39261–39264. doi: 10.1074/jbc.R300024200. [DOI] [PubMed] [Google Scholar]
- Liang F. Sex as a predictor of response to cancer immunotherapy. Lancet Oncol. 2018;19:e378. doi: 10.1016/S1470-2045(18)30531-X. [DOI] [PubMed] [Google Scholar]
- Litchfield K., Reading J.L., Puttick C., Thakkar K., Abbosh C., Bentham R., Watkins T.B.K., Rosenthal R., Biswas D., Rowan A., et al. Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition. Cell. 2021;184:596–614.e14. doi: 10.1016/j.cell.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liva S.M., Voskuhl R.R. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J. Immunol. 2001;167:2060–2067. doi: 10.4049/jimmunol.167.4.2060. [DOI] [PubMed] [Google Scholar]
- Long X., Hou H., Wang X., Liu S., Diao T., Lai S., Hu M., Zhang S., Liu M., Zhang H. Immune signature driven by ADT-induced immune microenvironment remodeling in prostate cancer is correlated with recurrence-free survival and immune infiltration. Cell Death Dis. 2020;11:779. doi: 10.1038/s41419-020-02973-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes-Ramos C.M., Chen C.Y., Kuijjer M.L., Paulson J.N., Sonawane A.R., Fagny M., Platig J., Glass K., Quackenbush J., DeMeo D.L. Sex differences in gene expression and regulatory networks across 29 human tissues. Cell Rep. 2020;31:107795. doi: 10.1016/j.celrep.2020.107795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubahn D.B., Joseph D.R., Sullivan P.M., Willard H.F., French F.S., Wilson E.M. Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science. 1988;240:327–330. doi: 10.1126/science.3353727. [DOI] [PubMed] [Google Scholar]
- Ma Z., Jiang M., Chen J., Shen G., Zhou Y., Li G. Castration aggravates insulin resistance, reduces immune function and improves quality of life of prostate cancer patients. J. BUON. 2020;25:1148–1154. [PubMed] [Google Scholar]
- Madan R.A., Karzai F., Donahue R.N., Al-Harthy M., Bilusic M., Rosner I.I., Singh H., Arlen P.M., Theoret M.R., Marté J.L., et al. Clinical and immunologic impact of short-course enzalutamide alone and with immunotherapy in non-metastatic castration sensitive prostate cancer. J. Immunother. Cancer. 2021;9:e001556. doi: 10.1136/jitc-2020-001556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maret A., Coudert J.D., Garidou L., Foucras G., Gourdy P., Krust A., Dupont S., Chambon P., Druet P., Bayard F., et al. Estradiol enhances primary antigen-specific CD4 T cell responses and Th1 development in vivo. Essential role of estrogen receptor alpha expression in hematopoietic cells. Eur. J. Immunol. 2003;33:512–521. doi: 10.1002/immu.200310027. [DOI] [PubMed] [Google Scholar]
- Markle J.G., Fish E.N. SeXX matters in immunity. Trends Immunol. 2014;35:97–104. doi: 10.1016/j.it.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Markle J.G.M., Frank D.N., Mortin-Toth S., Robertson C.E., Feazel L.M., Rolle-Kampczyk U., von Bergen M., McCoy K.D., Macpherson A.J., Danska J.S. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- Márquez E.J., Chung C.H., Marches R., Rossi R.J., Nehar-Belaid D., Eroglu A., Mellert D.J., Kuchel G.A., Banchereau J., Ucar D. Sexual-dimorphism in human immune system aging. Nat. Commun. 2020;11:751. doi: 10.1038/s41467-020-14396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx V. Method of the Year: spatially resolved transcriptomics. Nat. Methods. 2021;18:9–14. doi: 10.1038/s41592-020-01033-y. [DOI] [PubMed] [Google Scholar]
- McClelland E.E., Smith J.M. Gender specific differences in the immune response to infection. Arch. Immunol. Ther. Exp. 2011;59:203–213. doi: 10.1007/s00005-011-0124-3. [DOI] [PubMed] [Google Scholar]
- McMurray R.W., Suwannaroj S., Ndebele K., Jenkins J.K. Differential effects of sex steroids on T and B cells: modulation of cell cycle phase distribution, apoptosis and bcl-2 protein levels. Pathobiology. 2001;69:44–58. doi: 10.1159/000048757. [DOI] [PubMed] [Google Scholar]
- McNeel D.G., Smith H.A., Eickhoff J.C., Lang J.M., Staab M.J., Wilding G., Liu G. Phase I trial of tremelimumab in combination with short-term androgen deprivation in patients with PSA-recurrent prostate cancer. Cancer Immunol. Immunother. 2012;61:1137–1147. doi: 10.1007/s00262-011-1193-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuade J.L., Daniel C.R., Hess K.R., Davies M.A. Sex as a predictor of response to cancer immunotherapy. Lancet Oncol. 2018;19:e376. doi: 10.1016/S1470-2045(18)30483-2. [DOI] [PubMed] [Google Scholar]
- Medina K.L., Strasser A., Kincade P.W. Estrogen influences the differentiation, proliferation, and survival of early B-lineage precursors. Blood. 2000;95:2059–2067. [PubMed] [Google Scholar]
- Meier A., Chang J.J., Chan E.S., Pollard R.B., Sidhu H.K., Kulkarni S., Wen T.F., Lindsay R.J., Orellana L., Mildvan D., et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat. Med. 2009;15:955–959. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo C.O., Silva D.M., da Cruz A.D. Challenges in clinical and laboratory diagnosis of androgen insensitivity syndrome: a case report. J. Med. Case Rep. 2011;5:446. doi: 10.1186/1752-1947-5-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies F.M., Henriquez F.L., Alexander J., Roberts C.W. Selective inhibition and augmentation of alternative macrophage activation by progesterone. Immunology. 2011;134:281–291. doi: 10.1111/j.1365-2567.2011.03488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercader M., Bodner B.K., Moser M.T., Kwon P.S., Park E.S., Manecke R.G., Ellis T.M., Wojcik E.M., Yang D., Flanigan R.C., et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc. Natl. Acad. Sci. USA. 2001;98:14565–14570. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel S.M., Alexander S., Zufall E., Oliver J.D., Huet-Hudson Y.M. Essential role for estrogen in protection against Vibrio vulnificus-induced endotoxic shock. Infect. Immun. 2001;69:6119–6122. doi: 10.1128/IAI.69.10.6119-6122.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto H., Yang Z., Chen Y.T., Ishiguro H., Uemura H., Kubota Y., Nagashima Y., Chang Y.J., Hu Y.C., Tsai M.Y., et al. Promotion of bladder cancer development and progression by androgen receptor signals. J. Natl. Cancer Inst. 2007;99:558–568. doi: 10.1093/jnci/djk113. [DOI] [PubMed] [Google Scholar]
- Mohammed H., Russell I.A., Stark R., Rueda O.M., Hickey T.E., Tarulli G.A., Serandour A.A., Serandour A.A.A., Birrell S.N., Bruna A., et al. Progesterone receptor modulates ERalpha action in breast cancer. Nature. 2015;523:313–317. doi: 10.1038/nature14583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnár I., Bohaty I., Somogyiné-Vári É. High prevalence of increased interleukin-17A serum levels in postmenopausal estrogen deficiency. Menopause. 2014;21:749–752. doi: 10.1097/GME.0000000000000125. [DOI] [PubMed] [Google Scholar]
- Morton D.L., Hsueh E.C., Essner R., Foshag L.J., O'Day S.J., Bilchik A., Gupta R.K., Hoon D.S.B., Ravindranath M., Nizze J.A., et al. Prolonged survival of patients receiving active immunotherapy with canvaxin therapeutic polyvalent vaccine after complete resection of melanoma metastatic to regional lymph nodes. Ann. Surg. 2002;236:438–448. doi: 10.1097/00000658-200210000-00006. discussion 448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton V.R. Sex hormones in acquired immunity and autoimmune disease. Front. Immunol. 2018;9:2279. doi: 10.3389/fimmu.2018.02279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourey L., Conter H.J., Shore N., Berry W.R., Fong P.C., Piulats J.M., Appleman L.J., Todenhöfer T., Gravis G., Laguerre B., et al. 625P Pembrolizumab (pembro) plus enzalutamide (enza) in patients with abiraterone acetate (abi)-pretreated metastatic castration-resistant prostate cancer (mCRPC): KEYNOTE-365 Cohort C update. Ann. Oncol. 2020;31:S516–S517. [Google Scholar]
- Mousavi M.J., Mahmoudi M., Ghotloo S. Escape from X chromosome inactivation and female bias of autoimmune diseases. Mol. Med. 2020;26:127. doi: 10.1186/s10020-020-00256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mróz L.W., Chapman G.E., Oliffe J.L., Bottorff J.L. Men, food, and prostate cancer: gender influences on men's diets. Am. J. Men's Health. 2011;5:177–187. doi: 10.1177/1557988310379152. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B., Lydon J.P., DeMayo F.J., Conneely O.M. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc. Natl. Acad. Sci. USA. 2003;100:9744–9749. doi: 10.1073/pnas.1732707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulac-Jericevic B., Mullinax R.A., DeMayo F.J., Lydon J.P., Conneely O.M. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289:1751–1754. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health . 2015. NOT-OD-15-102. Consideration of Sex as a Biological Variable in NIH-Funded Research.https://grants.nih.gov/grants/guide/notice-files/not-od-15-102.html (Accessed 15 Feb 2022) [Google Scholar]
- National Institutes of Health . 2015. NOT-OD-15-102. Consideration of Sex as a Biological Variable in NIH-funded Research. Guidance PDF.https://orwh.od.nih.gov/sites/orwh/files/docs/NOT-OD-15-102_Guidance.pdf (Accessed 15 Feb 2022) [Google Scholar]
- National Institutes of Health . 2017. NOT-OD-18-014. Amendment: NIH Policy and Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research.https://grants.nih.gov/grants/guide/notice-files/NOT-OD-18-014.html (Accessed 15 Feb 2022) [Google Scholar]
- National Institutes of Health . Administrative Supplements for Research on Sex/Gender Differences. Women’s Health Research; 2022. https://orwh.od.nih.gov/research/funded-research-and-programs/administrative-supplements (Accessed 15 Feb 2022) [Google Scholar]
- National Institutes of Health Career Development & Interprofessional Education. E-Learning. 2022 https://orwh.od.nih.gov/career-development-education/e-learning (Accessed 15 Feb 2022) [Google Scholar]
- NCD Risk Factor Collaboration Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387:1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson L.R., Bulun S.E. Estrogen production and action. J. Am. Acad. Dermatol. 2001;45:S116–S124. doi: 10.1067/mjd.2001.117432. [DOI] [PubMed] [Google Scholar]
- Nguyen D.C., Masseoud F., Lu X., Scinicariello F., Sambhara S., Attanasio R. 17β-Estradiol restores antibody responses to an influenza vaccine in a postmenopausal mouse model. Vaccine. 2011;29:2515–2518. doi: 10.1016/j.vaccine.2011.01.080. [DOI] [PubMed] [Google Scholar]
- Nicholson B.E., Frierson H.F., Conaway M.R., Seraj J.M., Harding M.A., Hampton G.M., Theodorescu D. Profiling the evolution of human metastatic bladder cancer. Cancer Res. 2004;64:7813–7821. doi: 10.1158/0008-5472.CAN-04-0826. [DOI] [PubMed] [Google Scholar]
- Notas G., Kampa M., Castanas E. G protein-coupled estrogen receptor in immune cells and its role in immune-related diseases. Front. Endocrinol. 2020;11:579420. doi: 10.3389/fendo.2020.579420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntziachristos P., Tsirigos A., Welstead G.G., Trimarchi T., Bakogianni S., Xu L., Loizou E., Holmfeldt L., Strikoudis A., King B., et al. Contrasting roles of histone 3 lysine 27 demethylases in acute lymphoblastic leukaemia. Nature. 2014;514:513–517. doi: 10.1038/nature13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober C., Loisel D.A., Gilad Y. Sex-specific genetic architecture of human disease. Nat. Rev. Genet. 2008;9:911–922. doi: 10.1038/nrg2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obradovic A.Z., Dallos M.C., Zahurak M.L., Partin A.W., Schaeffer E.M., Ross A.E., Allaf M.E., Nirschl T.R., Liu D., Chapman C.G., et al. T-cell infiltration and adaptive Treg resistance in response to androgen deprivation with or without vaccination in localized prostate cancer. Clin. Cancer Res. 2020;26:3182–3192. doi: 10.1158/1078-0432.CCR-19-3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettel M., Mukhopadhyay A.K. Progesterone: the forgotten hormone in men? Aging Male. 2004;7:236–257. doi: 10.1080/13685530400004199. [DOI] [PubMed] [Google Scholar]
- Oghumu S., Varikuti S., Stock J.C., Volpedo G., Saljoughian N., Terrazas C.A., Satoskar A.R. Cutting edge: CXCR3 escapes X chromosome inactivation in T cells during infection: potential implications for sex differences in immune responses. J. Immunol. 2019;203:789–794. doi: 10.4049/jimmunol.1800931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva M., Muñoz-Aguirre M., Kim-Hellmuth S., Wucher V., Gewirtz A.D.H., Cotter D.J., Parsana P., Kasela S., Balliu B., Viñuela A., et al. The impact of sex on gene expression across human tissues. Science. 2020;369:eaba3066. doi: 10.1126/science.aba3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver R.T., Joseph J.V., Gallagher C.J. Castration-induced lymphocytosis in prostate cancer: possible evidence for gonad/thymus endocrine interaction in man. Urol. Int. 1995;54:226–229. doi: 10.1159/000282729. [DOI] [PubMed] [Google Scholar]
- Olsen N.J., Kovacs W.J. Evidence that androgens modulate human thymic T cell output. J. Investig. Med. 2011;59:32–35. doi: 10.2310/jim.0b013e318200dc98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen N.J., Gu X., Kovacs W.J. Bone marrow stromal cells mediate androgenic suppression of B lymphocyte development. J. Clin. Invest. 2001;108:1697–1704. doi: 10.1172/JCI13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen N.J., Viselli S.M., Fan J., Kovacs W.J. Androgens accelerate thymocyte apoptosis. Endocrinology. 1998;139:748–752. doi: 10.1210/endo.139.2.5729. [DOI] [PubMed] [Google Scholar]
- Org E., Mehrabian M., Parks B.W., Shipkova P., Liu X., Drake T.A., Lusis A.J. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microb. 2016;7:313–322. doi: 10.1080/19490976.2016.1203502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overdevest J.B., Knubel K.H., Duex J.E., Thomas S., Nitz M.D., Harding M.A., Smith S.C., Frierson H.F., Conaway M., Theodorescu D. CD24 expression is important in male urothelial tumorigenesis and metastasis in mice and is androgen regulated. Proc. Natl. Acad. Sci. USA. 2012;109:E3588–E3596. doi: 10.1073/pnas.1113960109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page S.T., Plymate S.R., Bremner W.J., Matsumoto A.M., Hess D.L., Lin D.W., Amory J.K., Nelson P.S., Wu J.D. Effect of medical castration on CD4+ CD25+ T cells, CD8+ T cell IFN-gamma expression, and NK cells: a physiological role for testosterone and/or its metabolites. Am. J. Physiol. Endocrinol. Metab. 2006;290:E856–E863. doi: 10.1152/ajpendo.00484.2005. [DOI] [PubMed] [Google Scholar]
- Paharkova-Vatchkova V., Maldonado R., Kovats S. Estrogen preferentially promotes the differentiation of CD11c+ CD11b(intermediate) dendritic cells from bone marrow precursors. J. Immunol. 2004;172:1426–1436. doi: 10.4049/jimmunol.172.3.1426. [DOI] [PubMed] [Google Scholar]
- Pal S.K., Moreira D., Won H., White S.W., Duttagupta P., Lucia M., Jones J., Hsu J., Kortylewski M. Reduced T-cell numbers and elevated levels of immunomodulatory cytokines in metastatic prostate cancer patients De novo resistant to abiraterone and/or enzalutamide therapy. Int. J. Mol. Sci. 2019;20:E1831. doi: 10.3390/ijms20081831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennock J.W., Stegall R., Bell B., Vargas G., Motamedi M., Milligan G., Bourne N. Estradiol improves genital herpes vaccine efficacy in mice. Vaccine. 2009;27:5830–5836. doi: 10.1016/j.vaccine.2009.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phiel K.L., Henderson R.A., Adelman S.J., Elloso M.M. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol. Lett. 2005;97:107–113. doi: 10.1016/j.imlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Polanczyk M.J., Carson B.D., Subramanian S., Afentoulis M., Vandenbark A.A., Ziegler S.F., Offner H. Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J. Immunol. 2004;173:2227–2230. doi: 10.4049/jimmunol.173.4.2227. [DOI] [PubMed] [Google Scholar]
- Potluri T., Fink A.L., Sylvia K.E., Dhakal S., Vermillion M.S., Vom Steeg L., Deshpande S., Narasimhan H., Klein S.L. Age-associated changes in the impact of sex steroids on influenza vaccine responses in males and females. NPJ Vaccines. 2019;4:29. doi: 10.1038/s41541-019-0124-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto G.A., Rosenstein Y. Oestradiol potentiates the suppressive function of human CD4 CD25 regulatory T cells by promoting their proliferation. Immunology. 2006;118:58–65. doi: 10.1111/j.1365-2567.2006.02339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley C.A., De Bellis A., Marschke K.B., el-Awady M.K., Wilson E.M., French F.S. Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr. Rev. 1995;16:271–321. doi: 10.1210/edrv-16-3-271. [DOI] [PubMed] [Google Scholar]
- Raghupathy R., Szekeres-Bartho J. Progesterone: a unique hormone with immunomodulatory roles in pregnancy. Int. J. Mol. Sci. 2022;23:1333. doi: 10.3390/ijms23031333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez A.G., Wages N.A., Hu Y., Smolkin M.E., Slingluff C.L., Jr. Defining the effects of age and gender on immune response and outcomes to melanoma vaccination: a retrospective analysis of a single-institution clinical trials' experience. Cancer Immunol. Immunother. 2015;64:1531–1539. doi: 10.1007/s00262-015-1758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J., Hou H., Zhao W., Wang J., Peng Q. Administration of exogenous progesterone protects against Brucella abortus infection-induced inflammation in pregnant mice. J. Infect. Dis. 2021;224:532–543. doi: 10.1093/infdis/jiaa722. [DOI] [PubMed] [Google Scholar]
- Rettew J.A., Huet-Hudson Y.M., Marriott I. Testosterone reduces macrophage expression in the mouse of toll-like receptor 4, a trigger for inflammation and innate immunity. Biol. Reprod. 2008;78:432–437. doi: 10.1095/biolreprod.107.063545. [DOI] [PubMed] [Google Scholar]
- Roberts C.W., Walker W., Alexander J. Sex-associated hormones and immunity to protozoan parasites. Clin. Microbiol. Rev. 2001;14:476–488. doi: 10.1128/CMR.14.3.476-488.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D.P., Hall O.J., Nilles T.L., Bream J.H., Klein S.L. 17β-estradiol protects females against influenza by recruiting neutrophils and increasing virus-specific CD8 T cell responses in the lungs. J. Virol. 2014;88:4711–4720. doi: 10.1128/JVI.02081-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D.P., Huber S.A., Moussawi M., Roberts B., Teuscher C., Watkins R., Arnold A.P., Klein S.L. Sex chromosome complement contributes to sex differences in coxsackievirus B3 but not influenza A virus pathogenesis. Biol. Sex Differ. 2011;2:8. doi: 10.1186/2042-6410-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden A.C., Moser M.T., Tri S.D., Mercader M., Kuntz S.M., Dong H., Hurwitz A.A., McKean D.J., Celis E., Leibovich B.C., et al. Augmentation of T cell levels and responses induced by androgen deprivation. J. Immunol. 2004;173:6098–6108. doi: 10.4049/jimmunol.173.10.6098. [DOI] [PubMed] [Google Scholar]
- Rodrigues Mantuano N., Stanczak M.A., Oliveira I.d.A., Kirchhammer N., Filardy A.A., Monaco G., Santos R.C., Fonseca A.C., Fontes M., Bastos C.d.S., Jr., et al. Hyperglycemia enhances cancer immune evasion by inducing alternative macrophage polarization through increased O-GlcNAcylation. Cancer Immunol. Res. 2020;8:1262–1272. doi: 10.1158/2326-6066.CIR-19-0904. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Torres M., Ríos-Bedoya C.F., Rodríguez-Orengo J., Fernández-Carbia A., Marxuach-Cuétara A.M., López-Torres A., Salgado-Mercado R., Bräu N. Progression to cirrhosis in Latinos with chronic hepatitis C: differences in Puerto Ricans with and without human immunodeficiency virus coinfection and along gender. J. Clin. Gastroenterol. 2006;40:358–366. doi: 10.1097/01.mcg.0000210105.66994.dc. [DOI] [PubMed] [Google Scholar]
- Ross M.T., Grafham D.V., Coffey A.J., Scherer S., McLay K., Muzny D., Platzer M., Howell G.R., Burrows C., Bird C.P., et al. The DNA sequence of the human X chromosome. Nature. 2005;434:325–337. doi: 10.1038/nature03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinow K.B., Chao J.H., Hagman D., Kratz M., Van Yserloo B., Gaikwad N.W., Amory J.K., Page S.T. Circulating sex steroids coregulate adipose tissue immune cell populations in healthy men. Am. J. Physiol. Endocrinol. Metab. 2017;313:528–539. doi: 10.1152/ajpendo.00075.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski K., Sowa P., Rutkowska-Talipska J., Kuryliszyn-Moskal A., Rutkowski R. Dehydroepiandrosterone (DHEA): hypes and hopes. Drugs. 2014;74:1195–1207. doi: 10.1007/s40265-014-0259-8. [DOI] [PubMed] [Google Scholar]
- Sabra L., Klein C.W.R. Springer International Publishing; 2015. Sex and Gender Differences in Infection and Treatments for Infectious Diseases. [Google Scholar]
- Said N., Sanchez-Carbayo M., Smith S.C., Theodorescu D. RhoGDI2 suppresses lung metastasis in mice by reducing tumor versican expression and macrophage infiltration. J. Clin. Invest. 2012;122:1503–1518. doi: 10.1172/JCI61392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurer L., Zysset D., Rihs S., Mager L., Gusberti M., Simillion C., Lugli A., Zlobec I., Krebs P., Mueller C. TREM-1 promotes intestinal tumorigenesis. Sci. Rep. 2017;7:14870. doi: 10.1038/s41598-017-14516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulster M., Bernie A.M., Ramasamy R. The role of estradiol in male reproductive function. Asian J. Androl. 2016;18:435–440. doi: 10.4103/1008-682X.173932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher A., Costa S.D., Zenclussen A.C. Endocrine factors modulating immune responses in pregnancy. Front. Immunol. 2014;5:196. doi: 10.3389/fimmu.2014.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotland R.S., Stables M.J., Madalli S., Watson P., Gilroy D.W. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood. 2011;118:5918–5927. doi: 10.1182/blood-2011-03-340281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seillet C., Laffont S., Trémollières F., Rouquié N., Ribot C., Arnal J.F., Douin-Echinard V., Gourdy P., Guéry J.C. The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor α signaling. Blood. 2012;119:454–464. doi: 10.1182/blood-2011-08-371831. [DOI] [PubMed] [Google Scholar]
- Seillet C., Rouquié N., Foulon E., Douin-Echinard V., Krust A., Chambon P., Arnal J.F., Guéry J.C., Laffont S. Estradiol promotes functional responses in inflammatory and steady-state dendritic cells through differential requirement for activation function-1 of estrogen receptor α. J. Immunol. 2013;190:5459–5470. doi: 10.4049/jimmunol.1203312. [DOI] [PubMed] [Google Scholar]
- Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184:861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah N.M., Lai P.F., Imami N., Johnson M.R. Progesterone-related immune modulation of pregnancy and labor. Front. Endocrinol. 2019;10:198. doi: 10.3389/fendo.2019.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- Sinclair A.H., Berta P., Palmer M.S., Hawkins J.R., Griffiths B.L., Smith M.J., Foster J.W., Frischauf A.M., Lovell-Badge R., Goodfellow P.N. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990;346:240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- Sivan A., Corrales L., Hubert N., Williams J.B., Aquino-Michaels K., Earley Z.M., Benyamin F.W., Lei Y.M., Jabri B., Alegre M.L., et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.C., Nicholson B., Nitz M., Frierson H.F., Jr., Smolkin M., Hampton G., El-Rifai W., Theodorescu D. Profiling bladder cancer organ site-specific metastasis identifies LAMC2 as a novel biomarker of hematogenous dissemination. Am. J. Pathol. 2009;174:371–379. doi: 10.2353/ajpath.2009.080538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Bouvier D.L., Divekar A.A., Sasidhar M., Du S., Tiwari-Woodruff S.K., King J.K., Arnold A.P., Singh R.R., Voskuhl R.R. A role for sex chromosome complement in the female bias in autoimmune disease. J. Exp. Med. 2008;205:1099–1108. doi: 10.1084/jem.20070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottnik J.L., Vanderlinden L., Joshi M., Chauca-Diaz A., Owens C., Hansel D.E., Sempeck C., Ghosh D., Theodorescu D. Androgen receptor regulates CD44 expression in bladder cancer. Cancer Res. 2021;81:2833–2846. doi: 10.1158/0008-5472.CAN-20-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souyris M., Cenac C., Azar P., Daviaud D., Canivet A., Grunenwald S., Pienkowski C., Chaumeil J., Mejía J.E., Guéry J.C. TLR7 escapes X chromosome inactivation in immune cells. Sci. Immunol. 2018;3:eaap8855. doi: 10.1126/sciimmunol.aap8855. [DOI] [PubMed] [Google Scholar]
- Souyris M., Mejía J.E., Chaumeil J., Guéry J.C. Female predisposition to TLR7-driven autoimmunity: gene dosage and the escape from X chromosome inactivation. Semin. Immunopathol. 2019;41:153–164. doi: 10.1007/s00281-018-0712-y. [DOI] [PubMed] [Google Scholar]
- Sthoeger Z.M., Chiorazzi N., Lahita R.G. Regulation of the immune response by sex hormones. I. In vitro effects of estradiol and testosterone on pokeweed mitogen-induced human B cell differentiation. J. Immunol. 1988;141:91–98. [PubMed] [Google Scholar]
- Straub R.H. The complex role of estrogens in inflammation. Endocr. Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- Su M.A., Stenerson M., Liu W., Putnam A., Conte F., Bluestone J.A., Anderson M.S. The role of X-linked FOXP3 in the autoimmune susceptibility of Turner Syndrome patients. Clin. Immunol. 2009;131:139–144. doi: 10.1016/j.clim.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Sutherland J.S., Goldberg G.L., Hammett M.V., Uldrich A.P., Berzins S.P., Heng T.S., Blazar B.R., Millar J.L., Malin M.A., Chidgey A.P., et al. Activation of thymic regeneration in mice and humans following androgen blockade. J. Immunol. 2005;175:2741–2753. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- Sutherland J.S., Spyroglou L., Muirhead J.L., Heng T.S., Prieto-Hinojosa A., Prince H.M., Chidgey A.P., Schwarer A.P., Boyd R.L. Enhanced immune system regeneration in humans following allogeneic or autologous hemopoietic stem cell transplantation by temporary sex steroid blockade. Clin. Cancer Res. 2008;14:1138–1149. doi: 10.1158/1078-0432.CCR-07-1784. [DOI] [PubMed] [Google Scholar]
- Svanberg L. Effects of estrogen deficiency in women castrated when young. Acta Obstet. Gynecol. Scand. Suppl. 1981;106:11–15. doi: 10.3109/00016348209155324. [DOI] [PubMed] [Google Scholar]
- Swann J.B., Smyth M.J. Immune surveillance of tumors. J. Clin. Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekeres-Bartho J., Barakonyi A., Miko E., Polgar B., Palkovics T. The role of gamma/delta T cells in the feto-maternal relationship. Semin. Immunol. 2001;13:229–233. doi: 10.1006/smim.2000.0318. [DOI] [PubMed] [Google Scholar]
- Taneja V. Sex hormones determine immune response. Front. Immunol. 2018;9:1931. doi: 10.3389/fimmu.2018.01931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneja V. Sexual dimorphism, aging and immunity. Vitam. Horm. 2021;115:367–399. doi: 10.1016/bs.vh.2020.12.015. [DOI] [PubMed] [Google Scholar]
- Togno-Peirce C., Nava-Castro K., Terrazas L.I., Morales-Montor J. Sex-associated expression of co-stimulatory molecules CD80, CD86, and accessory molecules, PDL-1, PDL-2 and MHC-II, in F480+ macrophages during murine cysticercosis. Biomed. Res. Int. 2013;2013:570158. doi: 10.1155/2013/570158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefson M., Karnes R.J., Thompson R.H., Granberg C., Hillman D., Breau R., Allison J., Kwon E., Blute M. 668 A randomized phase II study of ipilimumab with androgen ablation compared with androgen ablation alone in patients with advanced prostate cancer. J. Urol. 2010;183:e261. [Google Scholar]
- Tsuyuguchi K., Suzuki K., Matsumoto H., Tanaka E., Amitani R., Kuze F. Effect of oestrogen on Mycobacterium avium complex pulmonary infection in mice. Clin. Exp. Immunol. 2001;123:428–434. doi: 10.1046/j.1365-2249.2001.01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu M.M., Abdel-Hafiz H.A., Jones R.T., Jean A., Hoff K.J., Duex J.E., Chauca-Diaz A., Costello J.C., Dancik G.M., Tamburini B.A.J., et al. Inhibition of the CCL2 receptor, CCR2, enhances tumor response to immune checkpoint therapy. Commun. Biol. 2020;3:720. doi: 10.1038/s42003-020-01441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu M.M., Lee F.Y.F., Jones R.T., Kimball A.K., Saravia E., Graziano R.F., Coleman B., Menard K., Yan J., Michaud E., et al. Targeting DDR2 enhances tumor response to anti-PD-1 immunotherapy. Sci. Adv. 2019;5:eaav2437. doi: 10.1126/sciadv.aav2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukiainen T., Villani A.C., Yen A., Rivas M.A., Marshall J.L., Satija R., Aguirre M., Gauthier L., Fleharty M., Kirby A., et al. Landscape of X chromosome inactivation across human tissues. Nature. 2017;550:244–248. doi: 10.1038/nature24265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi A.M., Srivastava K., Mansoori M.N., Trivedi R., Chattopadhyay N., Singh D. Estrogen deficiency induces the differentiation of IL-17 secreting Th17 cells: a new candidate in the pathogenesis of osteoporosis. PLoS One. 2012;7:e44552. doi: 10.1371/journal.pone.0044552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uda M., Ottolenghi C., Crisponi L., Garcia J.E., Deiana M., Kimber W., Forabosco A., Cao A., Schlessinger D., Pilia G. Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum. Mol. Genet. 2004;13:1171–1181. doi: 10.1093/hmg/ddh124. [DOI] [PubMed] [Google Scholar]
- Vargas-Rojas M.I., Ramírez-Venegas A., Limón-Camacho L., Ochoa L., Hernández-Zenteno R., Sansores R.H. Increase of Th17 cells in peripheral blood of patients with chronic obstructive pulmonary disease. Respir. Med. 2011;105:1648–1654. doi: 10.1016/j.rmed.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Vassiliadou N., Tucker L., Anderson D.J. Progesterone-induced inhibition of chemokine receptor expression on peripheral blood mononuclear cells correlates with reduced HIV-1 infectability in vitro. J. Immunol. 1999;162:7510–7518. [PubMed] [Google Scholar]
- Vence L.M., Wang C., Pappu H., Anson R.E., Patel T.A., Miller P., Bassett R., Lizee G., Overwijk W.W., Komanduri K., et al. Chemical castration of melanoma patients does not increase the frequency of tumor-specific CD4 and CD8 T cells after peptide vaccination. J. Immunother. 2013;36:276–286. doi: 10.1097/CJI.0b013e31829419f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeesch J.R., Petit P., Kermouni A., Renauld J.C., Van Den Berghe H., Marynen P. The IL-9 receptor gene, located in the Xq/Yq pseudoautosomal region, has an autosomal origin, escapes X inactivation and is expressed from the Y. Hum. Mol. Genet. 1997;6:1–8. doi: 10.1093/hmg/6.1.1. [DOI] [PubMed] [Google Scholar]
- Vermeiren A.P.A., Hoebe C.J.P.A., Dukers-Muijrers N.H.T.M. High non-responsiveness of males and the elderly to standard hepatitis B vaccination among a large cohort of healthy employees. J. Clin. Virol. 2013;58:262–264. doi: 10.1016/j.jcv.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Vétizou M., Pitt J.M., Daillère R., Lepage P., Waldschmitt N., Flament C., Rusakiewicz S., Routy B., Roberti M.P., Duong C.P.M., et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viselli S.M., Reese K.R., Fan J., Kovacs W.J., Olsen N.J. Androgens alter B cell development in normal male mice. Cell. Immunol. 1997;182:99–104. doi: 10.1006/cimm.1997.1227. [DOI] [PubMed] [Google Scholar]
- vom Steeg L.G., Klein S.L. SeXX matters in infectious disease pathogenesis. PLoS Pathog. 2016;12:e1005374. doi: 10.1371/journal.ppat.1005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuk-Pavlović S., Bulur P.A., Lin Y., Qin R., Szumlanski C.L., Zhao X., Dietz A.B. Immunosuppressive CD14+HLA-DRlow/- monocytes in prostate cancer. Prostate. 2010;70:443–455. doi: 10.1002/pros.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walecki M., Eisel F., Klug J., Baal N., Paradowska-Dogan A., Wahle E., Hackstein H., Meinhardt A., Fijak M. Androgen receptor modulates Foxp3 expression in CD4+CD25+Foxp3+ regulatory T-cells. Mol. Biol. Cell. 2015;26:2845–2857. doi: 10.1091/mbc.E14-08-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis C.J.D., Butaney M., Satkunasivam R., Freedland S.J., Patel S.P., Hamid O., Pal S.K., Klaassen Z. Association of patient sex with efficacy of immune checkpoint inhibitors and overall survival in advanced cancers: a systematic review and meta-analysis. JAMA Oncol. 2019;5:529–536. doi: 10.1001/jamaoncol.2018.5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters K.A., Allan C.M., Jimenez M., Lim P.R., Davey R.A., Zajac J.D., Illingworth P., Handelsman D.J. Female mice haploinsufficient for an inactivated androgen receptor (AR) exhibit age-dependent defects that resemble the AR null phenotype of dysfunctional late follicle development, ovulation, and fertility. Endocrinology. 2007;148:3674–3684. doi: 10.1210/en.2007-0248. [DOI] [PubMed] [Google Scholar]
- Wang C., Qiao W., Jiang Y., Zhu M., Shao J., Ren P., Liu D., Li W. Effect of sex on the efficacy of patients receiving immune checkpoint inhibitors in advanced non-small cell lung cancer. Cancer Med. 2019;8:4023–4031. doi: 10.1002/cam4.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle J., Haase A.M., Steptoe A., Nillapun M., Jonwutiwes K., Bellisle F. Gender differences in food choice: the contribution of health beliefs and dieting. Ann. Behav. Med. 2004;27:107–116. doi: 10.1207/s15324796abm2702_5. [DOI] [PubMed] [Google Scholar]
- Weinstein Y., Ran S., Segal S. Sex-associated differences in the regulation of immune responses controlled by the MHC of the mouse. J. Immunol. 1984;132:656–661. [PubMed] [Google Scholar]
- Whitacre C.C. Sex differences in autoimmune disease. Nat. Immunol. 2001;2:777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- Wilharm A., Brigas H.C., Sandrock I., Ribeiro M., Amado T., Reinhardt A., Demera A., Hoenicke L., Strowig T., Carvalho T., et al. Microbiota-dependent expansion of testicular IL-17-producing Vγ6(+) γδ T cells upon puberty promotes local tissue immune surveillance. Mucosal Immunol. 2021;14:242–252. doi: 10.1038/s41385-020-0330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windley S.P., Mayère C., McGovern A.E., Harvey N.L., Nef S., Schwarz Q., Kumar S., Wilhelm D. Loss of NEDD4 causes complete XY gonadal sex reversal in mice. Cell Death Dis. 2022;13:75. doi: 10.1038/s41419-022-04519-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.M., Cieślik M., Lonigro R.J., Vats P., Reimers M.A., Cao X., Ning Y., Wang L., Kunju L.P., de Sarkar N., et al. Inactivation of CDK12 delineates a distinct immunogenic class of advanced prostate cancer. Cell. 2018;173:1770–1782.e14. doi: 10.1016/j.cell.2018.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Ju Q., Jia K., Yu J., Shi H., Wu H., Jiang M. Correlation between sex and efficacy of immune checkpoint inhibitors (PD-1 and CTLA-4 inhibitors) Int. J. Cancer. 2018;143:45–51. doi: 10.1002/ijc.31301. [DOI] [PubMed] [Google Scholar]
- Wu Y., Moissoglu K., Wang H., Wang X., Frierson H.F., Schwartz M.A., Theodorescu D. Src phosphorylation of RhoGDI2 regulates its metastasis suppressor function. Proc. Natl. Acad. Sci. USA. 2009;106:5807–5812. doi: 10.1073/pnas.0810094106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Saito H., Setogawa T., Tomioka H. Sex differences in host resistance to Mycobacterium marinum infection in mice. Infect. Immun. 1991;59:4089–4096. doi: 10.1128/iai.59.11.4089-4096.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Jing Y., Li L., Mills G.B., Diao L., Liu H., Han L. Sex-associated molecular differences for cancer immunotherapy. Nat. Commun. 2020;11:1779. doi: 10.1038/s41467-020-15679-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh S., Hu Y.C., Wang P.H., Xie C., Xu Q., Tsai M.Y., Dong Z., Wang R.S., Lee T.H., Chang C. Abnormal mammary gland development and growth retardation in female mice and MCF7 breast cancer cells lacking androgen receptor. J. Exp. Med. 2003;198:1899–1908. doi: 10.1084/jem.20031233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y., Liu L., Chen H., Wang Y., Xu Y., Mao H., Li J., Mills G.B., Shu Y., Li L., et al. Comprehensive characterization of molecular differences in cancer between male and female patients. Cancer Cell. 2016;29:711–722. doi: 10.1016/j.ccell.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurkovetskiy L., Burrows M., Khan A.A., Graham L., Volchkov P., Becker L., Antonopoulos D., Umesaki Y., Chervonsky A.V. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39:400–412. doi: 10.1016/j.immuni.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang E.A., Wynder E.L. Differences in lung cancer risk between men and women: examination of the evidence. J. Natl. Cancer Inst. 1996;88:183–192. doi: 10.1093/jnci/88.3-4.183. [DOI] [PubMed] [Google Scholar]
- Zhang M.A., Rego D., Moshkova M., Kebir H., Chruscinski A., Nguyen H., Akkermann R., Stanczyk F.Z., Prat A., Steinman L., et al. Peroxisome proliferator-activated receptor (PPAR)α and -γ regulate IFNγ and IL-17A production by human T cells in a sex-specific way. Proc. Natl. Acad. Sci. USA. 2012;109:9505–9510. doi: 10.1073/pnas.1118458109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Jia H., Wu Z. Sex as a predictor of response to cancer immunotherapy. Lancet Oncol. 2018;19:e374. doi: 10.1016/S1470-2045(18)30446-7. [DOI] [PubMed] [Google Scholar]
- Zhao R., Chen X., Ma W., Zhang J., Guo J., Zhong X., Yao J., Sun J., Rubinfien J., Zhou X., et al. A GPR174-CCL21 module imparts sexual dimorphism to humoral immunity. Nature. 2020;577:416–420. doi: 10.1038/s41586-019-1873-0. [DOI] [PubMed] [Google Scholar]
- Zheng D., Liwinski T., Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Shao X., Wang X., Liu L., Liang H. Sex disparities in cancer. Cancer Lett. 2019;466:35–38. doi: 10.1016/j.canlet.2019.08.017. [DOI] [PubMed] [Google Scholar]