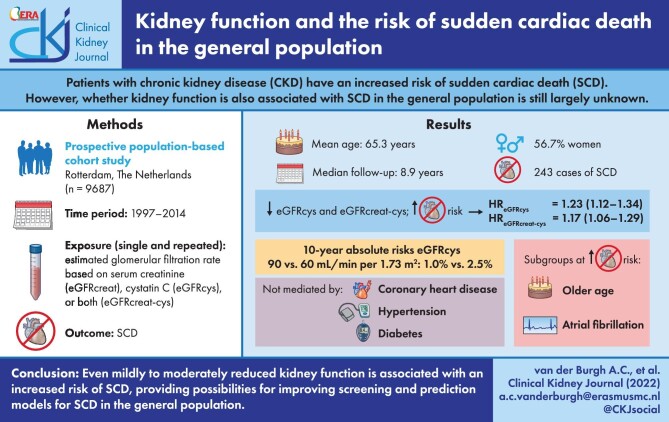
ABSTRACT
Background
Chronic kidney disease increases sudden cardiac death (SCD) risk, but the association between kidney function and SCD in a general population is largely unknown. Therefore, we investigated the association between kidney function and SCD in a general middle-aged and elderly population.
Methods
We included individuals aged ≥45 years from a prospective population-based cohort study. The association between kidney function assessments [estimated glomerular filtration rate based on serum creatinine (eGFRcreat), cystatin C (eGFRcys) or both (eGFRcreat-cys)] and SCD was investigated using Cox proportional-hazards and joint models. Absolute 10-year risks were computed using competing risk analyses. Mediation analyses were performed using a four-way decomposition method.
Results
We included 9687 participants (median follow-up 8.9 years; mean age 65.3 years; 56.7% women; 243 SCD cases). Lower eGFRcys and eGFRcreat-cys were associated with increased SCD risk [hazard ratio (HR) 1.23, 95% confidence interval (CI) 1.12–1.34 and HR 1.17, 95% CI 1.06–1.29, per 10 mL/min/1.73 m2 eGFR decrease]. A significant trend (P = 0.001) across eGFRcys categories was found, with an HR of 2.11 (95% CI 1.19–3.74) for eGFRcys <60 compared with eGFRcys >90 mL/min/1.73 m2. Comparing eGFRcys of 90 to 60 mL/min/1.73 m2, absolute 10-year risk increased from 1.0% to 2.5%. Identified subgroups at increased risk included older participants and participants with atrial fibrillation. The associations were not mediated by coronary heart disease, hypertension or diabetes.
Conclusions
Reduced kidney function is associated with increased SCD risk in the general population, especially with eGFRcys. eGFRcys could be added to prediction models and screening programmes for SCD prevention.
Keywords: creatinine, cystatin C, estimated glomerular filtration rate, kidney function, sudden cardiac death
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Cardiovascular diseases (CVD) are responsible for approximately 31% of all deaths worldwide. Half of this cardiovascular mortality can be attributed to sudden cardiac death (SCD) [1], with 4–5 million new cases annually worldwide [2]. Underlying coronary heart disease (CHD) and cardiomyopathies account for 90% of all SCDs [3]. However, in almost half of the cases, SCD is the first manifestation of the underlying cardiac disease [4]. This highlights the importance of identifying risk factors for SCD in order to improve the prediction and prevention of SCD in the general population [5].
Some subgroups within the general population are at higher risk of developing SCD, including patients with chronic kidney disease (CKD). In patients with CKD, SCD accounts for 16% of mortality [6] and several studies have suggested an increased risk of SCD in patients with reduced kidney function [7–13]. However, it is still largely unknown whether kidney function is also associated with SCD in the general population. The Atherosclerosis Risk in Communities (ARIC) Study [8], investigating community-dwelling participants up to 64 years of age from 1990 to 2001, reported that a lower estimated glomerular filtration rate (eGFR) was associated with increased SCD risk. However, there is currently inadequate evidence regarding the association between kidney function and SCD in middle-aged and elderly individuals from the general population, in which the SCD incidence is the highest, highlighting the need for prediction and prevention strategies in especially these individuals. Also, in the previous decades, primary prevention of CVD may have improved, especially in individuals up to the age of 65 years [14]. Furthermore, no study to date has investigated the possible mechanistic role of putative mediators such as CHD and hypertension. Identifying persons at risk and unravelling potential pathophysiological mechanisms is crucial for potential prevention efforts and treatment indications, including more stringent primary prevention efforts in subgroups at risk.
The use of serum creatinine to determine the eGFR has limitations as it can be affected by several factors that are unrelated to kidney function, most importantly lean muscle mass [15, 16]. An alternative marker for determining the eGFR is cystatin C, which is independent of lean muscle mass [17] and which has been proposed to be a better marker of kidney function in the elderly population than creatinine, as serum cystatin C is probably less affected by ageing and loss of muscle mass [18, 19]. Previous studies have suggested that serum cystatin C or combining serum cystatin C with serum creatinine could result in a more accurate prediction of the risk of CVD and kidney failure [15, 16, 20–23].
Hence, we aimed to investigate the association between kidney function, defined by eGFR based on serum creatinine and/or cystatin C levels and the risk of SCD in middle-aged and elderly individuals participating in a prospective population-based cohort study. In addition, we aimed to investigate the potential mediating role of cardiovascular risk factors within this association. Finally, we also investigated whether the association between kidney function and SCD differs depending on whether a single assessment or multiple assessments of eGFR are used.
MATERIALS AND METHODS
A more detailed description of the methods can be found in the Supplementary data.
Study design and population
This study was embedded within the Rotterdam Study, an ongoing prospective, population-based cohort study. Details regarding the design and rationale of the Rotterdam Study have been described elsewhere [24]. Participants were eligible for the current study if they had measurements of serum creatinine and serum cystatin C available at baseline and if information on incident SCD was available. All participants were followed from their date of baseline laboratory blood measurement until the date of SCD, the date of death from other causes or the end of data collection on 1 January 2014, whichever came first. The Rotterdam Study complies with the declaration of Helsinki and has been approved by the Medical Ethics Committee of the Erasmus MC (registration number MEC 02.1015) and by the Dutch Ministry of Health, Welfare and Sport (Population Screening Act WBO, license number 1 071 272–159521-PG). All participants provided written informed consent to participate in the study and to have their information obtained from treating physicians. Participants were not involved in the design, conduct, reporting or dissemination of this research.
Assessment of kidney function and other covariables
Serum creatinine (µmol/L) and serum cystatin C (mg/L) were determined at baseline and serum creatinine measurements from the Rotterdam Study were supplemented with those from the Star-MDC database, which is a database from a centre for medical diagnostics for outpatients in the city of Rotterdam [25]. eGFR was calculated based on serum creatinine (eGFRcreat), serum cystatin C (eGFRcys) or both (eGFRcreat-cys) according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula [26]. In addition, we categorized eGFRcreat using the ‘Kidney Disease: Improving Global Outcomes’ (KDIGO) 2013 guideline [27] into into three categories: eGFRcreat ≥90 mL/min/1.73 m2, GFRcreat 60–90 mL/min/1.73 m2 and eGFRcreat <60 mL/min/1.73 m2. Categorization of eGFRcys was performed using the same cut-offs. Information on other covariables was collected at baseline as well; more information about the data collection of other covariables can be found in the Supplementary data.
Assessment of SCD
The definition and validation of SCD cases have been described previously [28, 29]. Briefly, the medical records of participants selected on relevant International Classification of Diseases, 10th Revision codes were reviewed by two research physicians to assess if the diagnosis of SCD should be assigned and confirmed by an experienced cardiologist. Witnessed SCD was defined according to the Myerburg definition [30] which is endorsed by the European Society of Cardiology [31], as ‘a natural death due to cardiac causes, heralded by abrupt loss of consciousness within 1 h from onset of acute symptoms; preexisting heart disease may have been known to be present, but the time and mode of death are unexpected’. Death was classified as unwitnessed SCD if the person was in a stable medical condition 24 h before he or she was found dead and if there was no evidence of a non-cardiac cause of death. Furthermore, in case of limited information, cases were labelled as SCD when treating physicians labelled the death of the person as sudden or unexpected.
Statistical analysis
Multivariable Cox proportional-hazards hazards models were used to assess the association between baseline continuous and categorized eGFRcreat, eGFRcys and eGFRcreat-cys with the risk of SCD. All analyses were performed using two final models. In the first model, analyses were adjusted for age, sex and Rotterdam Study cohort. In the second model, we further adjusted for heart rate, body mass index (BMI), smoking, alcohol, serum cholesterol, history of CHD, diabetes and hypertension. Hazard ratios (HR) with 95% confidence intervals (CI) were given per 10 mL/min/1.73 m2 decrease in eGFR. Absolute 10-year risks were estimated for the total population and for age and sex strata separately, using the Fine and Gray model [32] and taking into account the competing risk of death of other causes than SCD. These analyses were adjusted for all covariates present in Model 2. Pre-defined stratification by Rotterdam Study cohort, age, sex, diabetes, hypertension, heart failure, CHD and atrial fibrillation (AF) categories were performed. A P for interaction <0.10 was considered to be statistically significant. Several sensitivity analyses were performed to study the robustness of our findings. A joint modelling approach was used to assess the association between repeated assessments of eGFRcreat and SCD. Mediation analyses based on the four-way decomposition technique described by VanderWeele [33] were performed to assess potential mediating effects of the history of CHD, hypertension and diabetes, on the association between eGFR and the risk of SCD, overall and stratified by age. Statistical analyses were performed using R statistical software [R-project, R Foundation for Statistical Computing (2020), 3.6.3].
RESULTS
The total study population consisted of 9687 participants with a median follow-up time of 8.9 years (interquartile range 6.2–13.6 years) (Supplementary data, Figure S1). During follow-up, 2764 individuals died of any cause, of which 808 were attributed to cardiovascular- and cerebrovascular-related events. In total, 243 SCD events (30% of all cardiovascular- and cerebrovascular-related events) occurred during follow-up, with an incidence rate of 2.6 per 1000 person-years. The mean age of the study participants was 65.3 years and 56.7% were women (Table 1). Mean values of eGFRcreat, eGFRcys and eGFRcreat-cys were 79, 77 and 79 mL/min/1.73 m2, respectively.
Table 1.
Baseline characteristics of the total population (n = 9687)
| Variable | Total population (n = 9687) |
|---|---|
| Age, years (n = 9687) | 65.3 ± 9.9 |
| Sex, women, n (%) (n = 9687) | 5496 (56.7) |
| Body mass index, kg/m2 (n = 9547) | 27.3 ± 4.2 |
| Systolic blood pressure, mmHg (n = 9630) | 140 ± 21 |
| Diastolic blood pressure, mmHg (n = 9630) | 79 ± 11 |
| Hypertension, n (valid %) (n = 9333) | 5964 (62.8) |
| Heart rate, beats/minute (n = 8888) | 69.1 ± 11.5 |
| QTc interval, ms (n = 8888) | 422.3 ± 21.6 |
| Diabetes, n (%) (n = 9687) | 1174 (12.1) |
| History of CHD, n (valid %) (n = 9635) | 570 (5.9) |
| History of atrial fibrillation, n (%) (n = 9687) | 408 (4.2) |
| History of heart failure, n (%) (n = 9687) | 30 (0.3) |
| Smoking (n = 9582) | |
| Current smoking, n (valid %) | 1847 (19.3) |
| Past smoking, n (valid %) | 4598 (48.0) |
| Never smoking, n (valid %) | 3137 (32.7) |
| Alcohol use, g/day (n = 7868) | 5.7 (0.5–14.4 |
| eGFRcreat mL/min/1.73 m2 (n = 9687) | 79 ± 15 |
| eGFRcys, mL/min/1.73 m2 (n = 9687) | 77 ± 19 |
| eGFRcreat-cys, mL/min/1.73 m2 (n = 9687) | 78 ± 16 |
| Serum cholesterol, mmol/L (n = 9629) | 5.7 ± 1.0 |
| Serum sodium, mmol/L (n = 7993) | 142 ± 2 |
| Serum potassium, mmol/L (n = 7994) | 4.4 ± 0.3 |
| Serum magnesium, mmol/L (n = 9679) | 0.84 ± 0.06 |
| Serum calcium, mmol/L (n = 9678) | 2.43 ± 0.10 |
| Serum phosphate, mmol/L (n = 9675) | 1.11 ± 0.16 |
Data are presented as number (%), number (valid %), mean ± standard deviation or median (interquartile range). Values are shown for non-imputed data. For variables with missing data, valid % is given.
CHD, coronary heart disease; eGFRcreat, estimated glomerular filtration rate (eGFR) determined by serum creatinine; eGFRcreat-cys, eGFR determined by serum creatinine and serum cystatin C; eGFRcys,eGFR determined by serum cystatin C; n, number. QTc interval, corrected QT interval.
eGFR and risk of SCD
Lower levels of eGFRcys and eGFRcreat-cys (per 10 mL/min/1.73 m2 decrease in eGFR) were significantly associated with an increased risk of SCD, with a larger HR reported for eGFRcys (HR 1.23, 95% CI 1.12–1.34) than for eGFRcreat-cys (HR 1.17, 95% CI 1.06–1.29, Table 2). Lower levels of eGFRcreat, however, were not significantly associated with an increased risk of SCD (HR 1.05, 95% CI 0.96–1.16). Additionally adjusting all analyses for atrial fibrillation, heart failure, C-reactive protein (CRP), thyroid-stimulating hormone (TSH), glucocorticoid use, physical activity, serum electrolytes and corrected QT interval (QTc) interval did not meaningfully change the results (Supplementary data, Table S1). Sensitivity analyses did not modify the risk estimates substantially (Table 3).
Table 2.
Association between eGFRcys, eGFRcreat and eGFRcreat-cys at baseline and the risk of sudden cardiac death
| eGFR | SCD events/total N (% SCD events) | HR (95% CI), Model 1 | HR (95% CI), Model 2 |
|---|---|---|---|
| Continuous, baseline assessment | |||
| eGFRcys, mL/min/1.73 m2 | 243/9687 (2.51%) | 1.27 (1.16–1.39) | 1.23 (1.12–1.34) |
| eGFRcreat, mL/min/1.73 m2 | 243/9687 (2.51%) | 1.06 (0.96–1.17) | 1.05 (0.96–1.16) |
| eGFRcreat-cys, mL/min/1.73 m2 | 243/9687 (2.51%) | 1.21 (1.09–1.33) | 1.17 (1.06–1.29) |
| Categorical, baseline assessment | |||
| eGFRcys categories | |||
| eGFRcys ≥90 mL/min/1.73 m2 | 18/2536 (0.71%) | Reference | Reference |
| eGFRcys 60–90 mL/min/1.73 m2 | 116/5280 (2.20%) | 1.42 (0.84–2.39) | 1.36 (0.81–2.28) |
| eGFRcys <60 mL/min/1.73 m2 | 109/1871 (5.83%) | 2.40 (1.35–4.25) | 2.11 (1.19–3.74) |
| P for trend | <0.001 | 0.001 | |
| eGFRcreat categories | |||
| eGFRcreat ≥90 mL/min/1.73 m2 | 30/2546 (1.18%) | Reference | Reference |
| eGFRcreat 60–90 mL/min/1.73 m2 | 157/6172 (2.54%) | 0.77 (0.50–1.17) | 0.83 (0.54–1.28) |
| eGFRcreat <60 mL/min/1.73 m2 | 56/969 (5.78%) | 1.08 (0.65–1.81) | 1.07 (0.64–1.80) |
| P for trend | 0.36 | 0.48 | |
| eGFRcreat-cys categories | |||
| eGFRcreat-cys ≥90 mL/min/1.73 m2 | 25/2479 (1.01%) | Reference | Reference |
| eGFRcreat-cys 60–90 mL/min/1.73 m2 | 141/5969 (2.36%) | 0.89 (0.56;1.41) | 0.88 (0.55;1.39) |
| eGFRcreat-cys <60 mL/min/1.73 m2 | 77/1239 (6.21%) | 1.42 (0.83;2.43) | 1.28 (0.75;2.20) |
| P for trend | 0.03 | 0.09 | |
Model 1 is adjusted for age, sex and Rotterdam Study cohort; Model 2 is additionally adjusted for heart rate, body mass index, smoking, alcohol, serum cholesterol, history of CHD, diabetes and hypertension.
Hazard ratios given per 10 mL/min/1.73 m2 decrease in eGFR.
CHD, coronary heart disease; CI, confidence interval; eGFRcreat, estimated glomerular filtration rate (eGFR) determined by serum creatinine; eGFRcreat-cys, eGFR determined by serum creatinine and serum cystatin C; eGFRcys, eGFR determined by serum cystatin C; HR, hazard ratio; n, number; SCD, sudden cardiac death.
Table 3.
Sensitivity analyses for the association between eGFRcys, eGFRcreat and eGFRcreat-cys at baseline and the risk of sudden cardiac death
| Sensitivity analysis | SCD events/total N | HR (95% CI), Model 1 | HR (95% CI), Model 2 |
|---|---|---|---|
| Taking the competing risk of death of other causes into account | |||
| eGFRcys, mL/min/1.73 m2 | 243/9679 | 1.27 (1.19–1.36) | 1.23 (1.15–1.31) |
| eGFRcreat, mL/min/1.73 m2 | 243/9679 | 1.06 (0.99–1.14) | 1.05 (0.98–1.13) |
| eGFRcreat-cys, mL/min/1.73 m2 | 243/9679 | 1.21 (1.13–1.29) | 1.17 (1.09–1.26) |
| Restricting to participants with eGFRcreat below 120 mL/min/1.73 m2 | |||
| eGFRcys, mL/min/1.73 m2 | 243/9679 | 1.27 (1.16–1.39) | 1.23 (1.12–1.34) |
| eGFRcreat, mL/min/1.73 m2 | 243/9679 | 1.06 (0.96–1.17) | 1.05 (0.96–1.16) |
| eGFRcreat-cys, mL/min/1.73 m2 | 243/9679 | 1.21 (1.09–1.33) | 1.17 (1.06–1.29) |
| Restricting to participants with eGFRcreat above 60 mL/min/1.73 m2 | |||
| eGFRcys, mL/min/1.73 m2 | 187/8718 | 1.28 (1.13–1.44) | 1.23 (1.09–1.38) |
| eGFRcreat, mL/min/1.73 m2 | 187/8718 | 0.91 (0.79–1.06) | 0.96 (0.82–1.11) |
| eGFRcreat-cys, mL/min/1.73 m2 | 187/8718 | 1.19 (1.03–1.38) | 1.16 (1.01–1.35) |
| Censoring at time of initiation of kidney replacement therapy | |||
| eGFRcys, mL/min/1.73 m2 | 243/9687 | 1.27 (1.16–1.39) | 1.23 (1.12–1.34) |
| eGFRcreat, mL/min/1.73 m2 | 243/9687 | 1.06 (0.96–1.17) | 1.06 (0.96–1.16) |
| eGFRcreat-cys, mL/min/1.73 m2 | 243/9687 | 1.21 (1.09–1.33) | 1.17 (1.06–1.29) |
| Restricting to witnessed or probable SCDs | |||
| eGFRcys, mL/min/1.73 m2 | 212/9687 | 1.26 (1.14–1.39) | 1.21 (1.10–1.34) |
| eGFRcreat, mL/min/1.73 m2 | 212/9687 | 1.03 (0.93–1.15) | 1.03 (0.93–1.15) |
| eGFRcreat-cys, mL/min/1.73 m2 | 212/9687 | 1.18 (1.06–1.31) | 1.15 (1.04–1.28) |
| Excluding participants with heart failure and CHD at baselinea | |||
| eGFRcys, mL/min/1.73 m2 | 185/8863 | 1.24 (1.12–1.39) | 1.20 (1.07–1.33) |
| eGFRcreat, mL/min/1.73 m2 | 185/8863 | 0.98 (0.87–1.11) | 0.99 (0.88–1.12) |
| eGFRcreat-cys, mL/min/1.73 m2 | 185/8863 | 1.15 (1.02–1.30) | 1.13 (1.00–1.27) |
| Censoring at time of incident atrial fibrillationb | |||
| eGFRcys, mL/min/1.73 m2 | 206/9279 | 1.25 (1.13–1.38) | 1.20 (1.08–1.33) |
| eGFRcreat, mL/min/1.73 m2 | 206/9279 | 1.02 (0.91–1.13) | 1.02 (0.92–1.14) |
| eGFRcreat-cys, mL/min/1.73 m2 | 206/9279 | 1.17 (1.05–1.30) | 1.14 (1.02–1.27) |
Model 1 is adjusted for age, sex and Rotterdam Study cohort; Model 2 is additionally adjusted for heart rate, body mass index, smoking, alcohol, serum cholesterol, history of CHD, diabetes and hypertension.
Hazard ratios given per 10 mL/min/1.73 m2 decrease in eGFR.
aModel 2 was not corrected for history of CHD. The non-imputed data is used to exclude the participants with heart failure and CHD at baseline.
bPrevalent cases of atrial fibrillation are excluded for this analysis.
CHD, coronary heart disease; CI, confidence interval; eGFRcreat, estimated glomerular filtration rate (eGFR) determined by serum creatinine; eGFRcreat-cys, eGFR determined by serum creatinine and serum cystatin C; eGFRcys, eGFR determined by serum cystatin C; HR, hazard ratio; N, number; SCD, sudden cardiac death.
eGFR category and risk of SCD
When categorizing eGFRcys, 7151 participants had eGFRcys <90 mL/min/1.73 m2 of which 1871 had eGFRcys <60 mL/min/1.73 m2. When categorizing eGFRcreat and eGFRcreat-cys, 7141 and 7208 participants had eGFR <90 mL/min/1.73 m2 of which 969 and 1239 had eGFR <60 mL/min/1.73 m2, respectively. In the analysis of eGFRcys categories, eGFRcys 60–90 mL/min/1.73 m2 was non-significantly associated with an increased risk of SCD (HR 1.36, 95% CI 0.81–2.28, Model 2), while eGFRcys <60 mL/min/1.73 m2 was significantly associated with an increased risk of SCD, with an HR of 2.11 (95% CI 1.19–3.74), both compared with eGFRcys ≥90 mL/min/1.73 m2. The P for trend across eGFRcys categories was 0.001. In the analysis of eGFRcreat and eGFRcreat-cys categories, eGFR 60–90 and <60 mL/min per 1.73 m2 were not significantly associated with the risk of SCD (eGFRcreat: HR 0.83, 95% CI 0.54–1.28 and HR 1.07, 95% CI 0.64–1.80; eGFRcreat-cys HR 0.88, 95% CI 0.55–1.39 and HR 1.28, 95% CI 0.75–2.20, Model 2) when compared with eGFR ≥90 mL/min/1.73 m2. The P for trend across eGFRcreat and eGFRcreat-cys categories was 0.48 and 0.09, respectively (Table 2). Similar results were reported when 45 mL/min/1.73 m2 was used as cut-off instead of 60 mL/min/1.73 m2, although higher HRs were found with eGFR <45 mL/min/1.73 m2 compared with eGFR <60 mL/min/1.73 m2 (Supplementary data, Table S2).
Absolute 10-year risks of SCD by eGFR
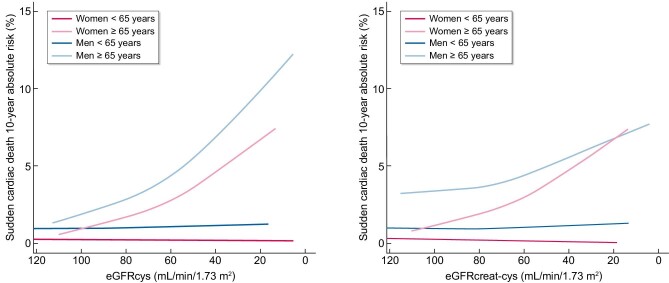
Absolute 10-year risks of SCD were comparable for eGFRcys and eGFRcreat-cys (Figure 1). The absolute 10-year SCD risk for eGFRcys increased from 1.0% up to 2.5% when comparing eGFRcys of 90 versus 60 mL/min/1.73 m2. Similarly, SCD risk for eGFRcreat-cys increased from 1.0% up to 2.8% when comparing eGFRcreat-cys of 90 versus 60 mL/min/1.73 m2. Comparing the same eGFR values, absolute 10-year SCD risk of men older than 65 years increased from 2.3% to 4.3% for eGFRcys, while the absolute 10-year SCD risk of men younger than 65 years increased from 1.0% to 1.1% (Figure 2, Supplementary data, Table S3). Absolute 10-year SCD risk for women older than 65 years increased from 1.3% to 2.7%, comparing eGFRcys of 90 versus 60 mL/min/1.73 m2.
FIGURE 1:
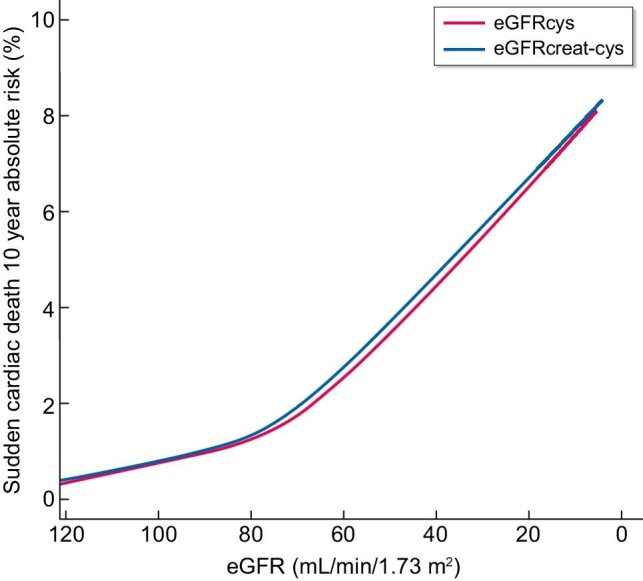
Absolute 10-year risks of sudden cardiac death by eGFRcys and eGFRcreat-cys at baseline. Absolute 10-year risks of sudden cardiac death were calculated taking the competing risk of death of other causes into account. Adjusted for age, sex, Rotterdam Study cohort, heart rate, body mass index, smoking, alcohol, serum cholesterol, history of CHD, diabetes and hypertension. CHD, coronary heart disease; eGFRcreat-cys, estimated glomerular filtration rate (eGFR) determined by serum creatinine and serum cystatin C; eGFRcys, eGFR determined by serum cystatin C.
FIGURE 2:
Absolute 10-year risks of sudden cardiac death by eGFRcys and eGFRcreat-cys at baseline, separately for age and sex strata. Absolute 10-year risks of sudden cardiac death were calculated taking the competing risk of death of other causes into account. Adjusted for Rotterdam Study cohort, heart rate, body mass index, smoking, alcohol, serum cholesterol, history of CHD, diabetes and hypertension. CHD, coronary heart disease, eGFRcreat-cys, estimated glomerular filtration rate (eGFR) determined by serum creatinine and serum cystatin C; eGFRcys, eGFR determined by serum cystatin C.
Stratified analyses
Stratification by Rotterdam Study cohort did not show any differences in the association between all three GFR estimates and SCD (data not shown). When stratifying by age, lower levels of eGFRcys, eGFRcreat and eGFRcreat-cys were associated with an increased risk of SCD only in participants aged above 65 years (in whom most SCD events occurred), with HRs of 1.29 (95% CI 1.16–1.44), 1.07 (95% CI 0.96–1.19) and 1.22 (95% CI 1.09–1.36) per 10 mL/min/1.73 m2 decrease in eGFR, respectively (P for interaction for all analyses <0.10) (Table 4, Supplementary data Table S4). There seemed to be a differential risk of SCD in atrial fibrillation subgroups for all three GFR estimates, although the P for interaction was borderline non-significant for eGFRcys (P for interaction 0.11, Model 2) (Table 4, Supplementary data, Table S4). Stratification by prevalent atrial fibrillation showed similar results with higher HRs for all three GFR estimates in participants with atrial fibrillation (Table 4). No differential risks were found for sex, diabetes, hypertension, heart failure and history of CHD (P for interaction >0.10) (Supplementary data, Tables S4 and S5).
Table 4.
Stratified analyses for the association between eGFRcys, eGFRcreat and eGFRcreat-cys at baseline and the risk of sudden cardiac death based on baseline age and atrial fibrillation
| Stratification variable | SCD events/total N | eGFR | HR (95% CI), Model 1 | HR (95% CI), Model 2 |
|---|---|---|---|---|
| Age | ||||
| <65 years | 44/5312 | eGFRcys, mL/min/1.73 m2 | 1.02 (0.83–1.26) | 0.97 (0.79–1.21) |
| eGFRcreat, mL/min/1.73 m2 | 0.88 (0.68–1.15) | 0.92 (0.71–1.19) | ||
| eGFRcreat-cys, mL/min/1.73 m2 | 0.95 (0.74–1.22) | 0.93 (0.73–1.19) | ||
| ≥65 years | 199/4375 | eGFRcys, mL/min/1.73 m2 | 1.35 (1.22–1.50) | 1.29 (1.16–1.44) |
| eGFRcreat, mL/min/1.73 m2 | 1.09 (0.99–1.22) | 1.07 (0.96–1.19) | ||
| eGFRcreat-cys, mL/min/1.73 m2 | 1.27 (1.14–1.41) | 1.22 (1.09–1.36) | ||
| Atrial fibrillation | ||||
| No | 222/9279 | eGFRcys, mL/min/1.73 m2 | 1.24 (1.12–1.36) | 1.18 (1.07–1.30) |
| eGFRcreat, mL/min/1.73 m2 | 1.01 (0.90–1.12) | 1.00 (0.90–1.11) | ||
| eGFRcreat-cys, mL/min/1.73 m2 | 1.16 (1.04–1.29) | 1.12 (1.01–1.24) | ||
| Yes | 21/408 | eGFRcys, mL/min/1.73 m2 | 1.61 (1.21–2.13) | 1.69 (1.24–2.32) |
| eGFRcreat, mL/min/1.73 m2 | 1.52 (1.19–1.94) | 1.51 (1.15–1.99) | ||
| eGFRcreat-cys, mL/min/1.73 m2 | 1.62 (1.25–2.10) | 1.67 (1.24–2.25) |
Model 1 is adjusted for age, sex and Rotterdam Study cohort; Model 2 is additionally adjusted for heart rate, body mass index, smoking, alcohol, serum cholesterol, history of CHD, diabetes and hypertension.
Hazard ratios given per 10 mL/min/1.73 m2 decrease in eGFR.
CHD, coronary heart disease; CI, confidence interval; eGFRcreat, eGFR determined by serum creatinine; eGFRcreat-cys, estimated glomerular filtration rate (eGFR) determined by serum creatinine and serum cystatin C; eGFRcys, eGFR determined by serum cystatin C; HR, hazard ratio; N, number; SCD, sudden cardiac death.
Repeated assessments of eGFRcreat and the risk of SCD
The median number of repeated assessments of eGFRcreat was 5 (interquartile range 3–8 assessments). A 10 mL/min/1.73 m2 decrease in eGFRcreat had minimal impact on the risk of SCD (HR 1.04, 95% CI 0.94–1.16, Model 2, Table 5). This result was similar to the HR found for the association between baseline eGFRcreat and the risk of SCD (HR 1.05, 95% CI 0.96–1.16, Table 2).
Table 5.
Association between repeated assessments of eGFRcreat and the risk of sudden cardiac death
| SCD events/total N | HR (95% CI), Model 1 | HR (95% CI), Model 2 | |
|---|---|---|---|
| eGFRcreat, mL/min/1.73 m2 | 243/9687 | 1.06 (0.96–1.18) | 1.04 (0.94–1.16) |
Model 1 is adjusted for age, sex and Rotterdam Study cohort; Model 2 is additionally adjusted for heart rate, body mass index, smoking, alcohol, serum cholesterol, history of CHD, diabetes and hypertension.
Hazard ratios given per 10 mL/min/1.73 m2 decrease in eGFR.
CHD, coronary heart disease; CI, confidence interval; eGFRcreat, estimated glomerular filtration rate (eGFR) determined by serum creatinine; HR, hazard ratio; N, number; SCD, sudden cardiac death.
Mediation analyses
In men, the association of eGFRcys and eGFRcreat-cys with SCD was not mediated by a history of CHD, hypertension or diabetes (Supplementary data, Table S6). Stratification by age revealed similar results, as no signs of a mediating role of a history of CHD, hypertension or diabetes were found in participants younger or older than 65 years (Supplementary data, Table S7). Similar results were found in women (Supplementary data, Tables S8 and S9).
DISCUSSION
In this prospective cohort study, lower levels of eGFRcys and eGFRcreat-cys were associated with an increased risk of SCD irrespective of age, sex and other potentially important confounders. We also identified an increased risk of SCD in participants with eGFRcys <60 mL/min/1.73 m2. In addition, a significant trend between the different eGFRcys categories and SCD was shown. Conversely, we did not find a significant association between eGFRcreat and SCD. The results were comparable when using either a single assessment or multiple assessments of eGFRcreat. The discrepancy in findings between eGFRcreat and eGFRcys may be explained by the non-eGFR determinants of serum creatinine, including muscle mass, diet and physical activity [15]. However, adjusting for these determinants did not change the results, which suggests that the association between kidney function and SCD is independent of those determinants. In the absence of non-eGFR determinants, other mechanisms such as the shrunken pore syndrome, a novel syndrome characterized by a decreased diameter of glomerular pores impairing the filtration of larger molecules more than the filtration of smaller molecules [34, 35] might also explain our findings, although more research into this topic is first needed. Another explanation for the eGFRcreat and eGFRcys discrepancy could be the power of our analyses, as an almost twice as high proportion of the participants was classified as having eGFR <60 mL/min/1.73 m2 with eGFRcys when compared with eGFRcreat. However, the percentage of SCD events was similar in participants with eGFRcreat and eGFRcys <60 mL/min/1.73 m2 and still, only a significant and strong association with SCD was found in participants with eGFRcys <60 mL/min/1.73 m2. This was also confirmed by the continuous analyses in which power differences are not playing a role and where a significant association with SCD was only reported with lower levels of eGFRcys and eGFRcreat-cys.
Several previous studies suggested an increased risk of SCD in patients with reduced kidney function [7–13]. However, it is largely unknown whether kidney function is also associated with SCD in a general population. In the current study, we investigated the association of kidney function and SCD in middle-aged and elderly individuals from a single community, where most SCD events occurred in individuals aged 65 years and older. A previous population-based study including the ARIC cohort [8] showed that low baseline eGFRcreat, eGFRcys and eGFRcreat-cys were associated with increased SCD risk. We also show that lower levels of eGFRcys and eGFRcreat-cys were associated with increased SCD risk, but we did not find a significant association between eGFRcreat and SCD. Several differences between this previous study and our study may explain the differences in results. First, the maximum age of the population in the ARIC study was only 64 years, so elderly participants were not included, and the follow-up for SCD was completed until 2001, after which substantial changes in primary and secondary prevention of CVD and SCD have occurred. The younger participants included in our study may have already benefited from this improved prevention, which might explain why we found no significant association of all three GFR estimates with SCD risk and no increase in the absolute 10-year risks of SCD for all three GFR estimates in participants younger than 65 years. To our knowledge, no prior studies have investigated the association of kidney function with SCD in the setting of current prevention guidelines. Second, the previous study analysed eGFR in categories only, while continuous analyses allow for potential non-linearity of the predictor and takes all available information within the predictor into account [36]. Third, the investigators defined SCD as ventricular arrhythmias, while this is not the only cause of SCD. In the current study, a definition of SCD was used that includes all possible SCD causes and is in accordance with the Myerburg definition [30], which is endorsed by the European Society of Cardiology [31].
Several possible mechanisms explaining the association between lower kidney function and SCD have been proposed. Although individuals with lower kidney function are at an increased risk of CVD [37–41], our data suggest that the association between eGFR and SCD was not mediated by a history of CHD, hypertension or diabetes. We could not perform mediation analyses with heart failure due to insufficient power, but excluding participants with both heart failure and CHD did not change our main results. Reduced kidney function is also associated with adverse cardiac remodelling, including left ventricular hypertrophy and cardiac fibrosis [42], which both increase the risk of ventricular arrhythmias and SCD partly due to prolongation of the QTc interval [43–45]. However, adjusting for the QTc interval did not meaningfully change our results. Furthermore, reduced kidney function is associated with a higher prevalence of hypokalaemia and hyperkalaemia [46, 47], although adjusting for serum electrolytes did not meaningfully change our results as well. Therefore, it is possible that other mechanisms explain the association between lower kidney function and SCD, including the tendency towards atherosclerosis with lower GFR (mediated by a pro-inflammatory state, hyperphosphatemia and vascular calcification) [48–54], sympathetic overactivity [48] and impaired arterial baroreflex [48, 55].
This study has several strengths. The major strength is the large number of participants from a population-based cohort study with middle-aged and elderly individuals, which comprises a population at high risk for SCD. Also, the prospective population-based design together with the high participation rate limits the chance of selection bias and makes the results generalizable to the general population. All data were collected irrespective of the current research question, which reduced the chance of information bias. The Rotterdam Study provides extensive data on SCD and on a wide variety of confounders. We are the first to use multiple eGFR assessments over time to investigate the association between kidney function and SCD in the general population, which reduces bias introduced by random variation and transient declines in eGFR, and increases statistical power. To our knowledge, we are also the first to perform mediation analyses to provide more insight into the potential mechanisms underlying the association between kidney function and SCD. Several limitations should also be acknowledged. First, we only had one assessment of eGFRcys and eGFRcreat-cys available, and therefore repeated measurement analyses were not possible for these two eGFR assessments. However, low variability of eGFR in our study participants was shown when comparing single and multiple assessments of eGFRcreat, resulting in a low probability of misclassification of kidney function when using only one single assessment. Second, insufficient data on the urine albumin-to-creatinine ratio was available to generate sufficient power to study the association between albuminuria and SCD. Third, due to the observational design of the study, we cannot fully exclude the possibility that the included mediators CHD, hypertension and diabetes are also confounders of the association between kidney function and SCD. Finally, residual confounding cannot be excluded even though we adjusted for various confounders and as our study mainly included Caucasians aged above 45 years, the generalizability of our results to other populations might be limited.
The high incidence of SCD worldwide together with the devastating consequences and the sudden aspect of this event shows the importance of identifying modifiable risk factors for SCD, such as kidney function and identifying subgroups at risk. Our study suggests an increased SCD risk with lower levels of eGFR in middle-aged and elderly individuals from the general population, even without CKD, only when using serum cystatin C measurements. This association was independent of non-eGFR determinants of serum cystatin C. This suggests that even mildly to moderately reduced kidney function could be a risk factor for SCD. Our findings could be clinically relevant, as they open up new avenues for future research. This includes further investigation of the pathophysiological mechanisms underlying the association between kidney function and SCD. Unravelling these unknown mechanisms could identify potential therapeutic targets, which is crucial as SCD is often the first manifestation of underlying CVD. This also highlights the need for well-performing prediction models for SCD and our findings suggest the relevance of adding eGFRcys, but not eGFRcreat, to these prediction models. In addition, future research should determine whether eGFRcys can be utilized in screening programmes in the general population for underlying but as yet undiagnosed CVD.
Supplementary Material
ACKNOWLEDGEMENTS
We gratefully acknowledge the dedication, commitment and contribution of the study participants, the staff of the Rotterdam Study and the participating general practitioners and pharmacists.
Contributor Information
Anna C van der Burgh, Department of Internal Medicine, Erasmus Medical Center, University Medical Center Rotterdam, Rotterdam, The Netherlands; Department of Epidemiology, Erasmus Medical Center, University Medical Center Rotterdam, Rotterdam, The Netherlands.
Bruno H Stricker, Department of Internal Medicine, Erasmus Medical Center, University Medical Center Rotterdam, Rotterdam, The Netherlands; Department of Epidemiology, Erasmus Medical Center, University Medical Center Rotterdam, Rotterdam, The Netherlands.
Dimitris Rizopoulos, Department of Biostatistics, Erasmus Medical Center, University Medical Center Rotterdam, Rotterdam, The Netherlands.
M Arfan Ikram, Department of Epidemiology, Erasmus Medical Center, University Medical Center Rotterdam, Rotterdam, The Netherlands.
Ewout J Hoorn, Department of Internal Medicine, Erasmus Medical Center, University Medical Center Rotterdam, Rotterdam, The Netherlands.
Layal Chaker, Department of Internal Medicine, Erasmus Medical Center, University Medical Center Rotterdam, Rotterdam, The Netherlands; Department of Epidemiology, Erasmus Medical Center, University Medical Center Rotterdam, Rotterdam, The Netherlands.
CONFLICT OF INTEREST STATEMENT
The authors declare that there is no conflict of interest.
FUNDING
The Rotterdam Study is funded by the Erasmus Medical Centre and Erasmus University, Rotterdam, Netherlands Organization for the Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the European Commission (DG XII) and the Municipality of Rotterdam. None of the funders had any role in design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review, or approval of the manuscript.
AUTHORS’ CONTRIBUTIONS
A.C.v.d.B., E.J.H. and L.C. designed the study. A.C.v.d.B. performed data analysis. A.C.v.d.B., E.J.H and L.C. contributed to the interpretation of the results. A.C.v.d.B. drafted the manuscript. Each author contributed important intellectual content during the writing and revision of the manuscript. All authors approved the final version of the manuscript.
DATA AVAILABILITY STATEMENT
The datasets analysed during the current study are not publicly available due to legal and ethical restraints. Data are available from the corresponding author on reasonable request.
REFERENCES
- 1. Morin DP, Homoud MK, Estes NAM III. Prediction and prevention of sudden cardiac death. Card Electrophysiol Clin 2017; 9: 631–638 [DOI] [PubMed] [Google Scholar]
- 2. Chugh SS, Reinier K, Teodorescu Cet al. Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis 2008; 51: 213–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Srinivasan NT, Schilling RJ. Sudden cardiac death and arrhythmias. Arrhythm Electrophysiol Rev 2018; 7: 111–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wellens HJ, Schwartz PJ, Lindemans FWet al. Risk stratification for sudden cardiac death: current status and challenges for the future. Eur Heart J 2014; 35: 1642–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fishman GI, Chugh SS, Dimarco JPet al. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation 2010; 122: 2335–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Caravaca F, Chavez E, Alvarado Ret al. Sudden cardiac death in non-dialysis chronic kidney disease patients. Nefrologia 2016; 36: 404–409 [DOI] [PubMed] [Google Scholar]
- 7. Pun PH, Smarz TR, Honeycutt EFet al. Chronic kidney disease is associated with increased risk of sudden cardiac death among patients with coronary artery disease. Kidney Int 2009; 76: 652–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Suzuki T, Agarwal SK, Deo Ret al. Kidney function and sudden cardiac death in the community: The Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 2016; 180: 46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saxon LA, Bristow MR, Boehmer Jet al. Predictors of sudden cardiac death and appropriate shock in the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Trial. Circulation 2006; 114: 2766–2772 [DOI] [PubMed] [Google Scholar]
- 10. Goldenberg I, Moss AJ, McNitt Set al. Relations among renal function, risk of sudden cardiac death, and benefit of the implanted cardiac defibrillator in patients with ischemic left ventricular dysfunction. Am J Cardiol 2006; 98: 485–490 [DOI] [PubMed] [Google Scholar]
- 11. Deo R, Sotoodehnia N, Katz Ret al. Cystatin C and sudden cardiac death risk in the elderly. Circ Cardiovasc Qual Outcomes 2010; 3: 159–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deo R, Lin F, Vittinghoff Eet al. Kidney dysfunction and sudden cardiac death among women with coronary heart disease. Hypertension 2008; 51: 1578–1582 [DOI] [PubMed] [Google Scholar]
- 13. Dalal D, de Jong JS, Tjong FVet al. Mild-to-moderate kidney dysfunction and the risk of sudden cardiac death in the setting of acute myocardial infarction. Heart Rhythm 2012; 9: 540–545 [DOI] [PubMed] [Google Scholar]
- 14. Conroy RM, Pyorala K, Fitzgerald APet al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 2003; 24: 987–1003 [DOI] [PubMed] [Google Scholar]
- 15. Shlipak MG, Matsushita K, Arnlov Jet al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med 2013; 369: 932–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shlipak MG, Sarnak MJ, Katz Ret al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 2005; 352: 2049–2060 [DOI] [PubMed] [Google Scholar]
- 17. Wang CH, Rubinsky AD, Minichiello Tet al. Creatinine versus cystatin C: differing estimates of renal function in hospitalized veterans receiving anticoagulants. J Gen Intern Med 2018; 33: 1299–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis 2002; 40: 221–226 [DOI] [PubMed] [Google Scholar]
- 19. Akoudad S, Sedaghat S, Hofman Aet al. Kidney function and cerebral small vessel disease in the general population. Int J Stroke 2015; 10: 603–608 [DOI] [PubMed] [Google Scholar]
- 20. Peralta CA, Katz R, Sarnak MJet al. Cystatin C identifies chronic kidney disease patients at higher risk for complications. J Am Soc Nephrol 2011; 22: 147–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peralta CA, Shlipak MG, Judd Set al. Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA 2011; 305: 1545–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shlipak MG, Praught ML, Sarnak MJ. Update on cystatin C: new insights into the importance of mild kidney dysfunction. Curr Opin Nephrol Hypertens 2006; 15: 270–275 [DOI] [PubMed] [Google Scholar]
- 23. Jernberg T, Lindahl B, James Set al. Cystatin C: a novel predictor of outcome in suspected or confirmed non-ST-elevation acute coronary syndrome. Circulation 2004; 110: 2342–2348 [DOI] [PubMed] [Google Scholar]
- 24. Ikram MA, Brusselle G, Ghanbari Met al. Objectives, design and main findings until 2020 from the Rotterdam Study. Eur J Epidemiol 2020; 35: 483–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van der Burgh AC, Rizopoulos D, Ikram MAet al. Determinants of the evolution of kidney function with age. Kidney Int Rep 2021; 6: 3054–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Inker LA, Schmid CH, Tighiouart Het al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012; 367: 20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int 2013; 3: 1–150 [DOI] [PubMed] [Google Scholar]
- 28. Niemeijer MN, van den Berg ME, Leening MJet al. Declining incidence of sudden cardiac death from 1990-2010 in a general middle-aged and elderly population: The Rotterdam Study. Heart Rhythm 2015; 12: 123–129 [DOI] [PubMed] [Google Scholar]
- 29. Straus SM, Kors JA, De Bruin MLet al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol 2006; 47: 362–367 [DOI] [PubMed] [Google Scholar]
- 30. Myerburg RJ, Interian A Jr, Mitrani RMet al. Frequency of sudden cardiac death and profiles of risk. Am J Cardiol 1997; 80: 10F–19F [DOI] [PubMed] [Google Scholar]
- 31. Priori SG, Aliot E, Blomstrom-Lundqvist Cet al. Task force on sudden cardiac death of the European Society of Cardiology. Eur Heart J 2001; 22: 1374–1450 [DOI] [PubMed] [Google Scholar]
- 32. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 496–509 [Google Scholar]
- 33. VanderWeele TJ. A unification of mediation and interaction: a 4-way decomposition. Epidemiology 2014; 25: 749–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rippe B, Haraldsson B. Transport of macromolecules across microvascular walls: the two-pore theory. Physiol Rev 1994; 74: 163–219 [DOI] [PubMed] [Google Scholar]
- 35. Grubb A, Lindstrom V, Jonsson Met al. Reduction in glomerular pore size is not restricted to pregnant women. Evidence for a new syndrome: ‘Shrunken pore syndrome’. Scand J Clin Lab Invest 2015; 75: 333–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schmidt CO, Ittermann T, Schulz Aet al. Linear, nonlinear or categorical: how to treat complex associations in regression analyses? Polynomial transformations and fractional polynomials. Int J Public Health 2013; 58: 157–160 [DOI] [PubMed] [Google Scholar]
- 37. Chae CU, Albert CM, Glynn RJet al. Mild renal insufficiency and risk of congestive heart failure in men and women >or = 70 years of age. Am J Cardiol 2003; 92: 682–686 [DOI] [PubMed] [Google Scholar]
- 38. Fried LF, Shlipak MG, Crump Cet al. Renal insufficiency as a predictor of cardiovascular outcomes and mortality in elderly individuals. J Am Coll Cardiol 2003; 41: 1364–1372 [DOI] [PubMed] [Google Scholar]
- 39. Weiner DE, Tighiouart H, Griffith JLet al. Kidney disease, Framingham risk scores, and cardiac and mortality outcomes. Am J Med 2007; 120: 552 e1–138. [DOI] [PubMed] [Google Scholar]
- 40. Yu Z, Coresh J, Qi Get al. A bidirectional Mendelian randomization study supports causal effects of kidney function on blood pressure. Kidney Int 2020; 98: 708–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lin CY, Hsieh MC, Kor CTet al. Association and risk factors of chronic kidney disease and incident diabetes: a nationwide population-based cohort study. Diabetologia 2019; 62: 438–447 [DOI] [PubMed] [Google Scholar]
- 42. Whitman IR, Feldman HI, Deo R. CKD and sudden cardiac death: epidemiology, mechanisms, and therapeutic approaches. J Am Soc Nephrol 2012; 23: 1929–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roes SD, Borleffs CJ, van der Geest RJet al. Infarct tissue heterogeneity assessed with contrast-enhanced MRI predicts spontaneous ventricular arrhythmia in patients with ischemic cardiomyopathy and implantable cardioverter-defibrillator. Circ Cardiovasc Imaging 2009; 2: 183–190 [DOI] [PubMed] [Google Scholar]
- 44. Reinier K, Dervan C, Singh Tet al. Increased left ventricular mass and decreased left ventricular systolic function have independent pathways to ventricular arrhythmogenesis in coronary artery disease. Heart Rhythm 2011; 8: 1177–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Haider AW, Larson MG, Benjamin EJet al. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol 1998; 32: 1454–1459 [DOI] [PubMed] [Google Scholar]
- 46. Kovesdy CP, Matsushita K, Sang Yet al. Serum potassium and adverse outcomes across the range of kidney function: a CKD prognosis consortium meta-analysis. Eur Heart J 2018; 39: 1535–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pun PH, Goldstein BA, Gallis JAet al. Serum potassium levels and risk of sudden cardiac death among patients with chronic kidney disease and significant coronary artery disease. Kidney Int Rep 2017; 2: 1122–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shamseddin MK, Parfrey PS. Sudden cardiac death in chronic kidney disease: epidemiology and prevention. Nat Rev Nephrol 2011; 7: 145–154 [DOI] [PubMed] [Google Scholar]
- 49. Pai AS, Giachelli CM. Matrix remodeling in vascular calcification associated with chronic kidney disease. J Am Soc Nephrol 2010; 21: 1637–1640 [DOI] [PubMed] [Google Scholar]
- 50. Hu MC, Shi M, Zhang Jet al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 2011; 22: 124–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Silverstein DM. Inflammation in chronic kidney disease: role in the progression of renal and cardiovascular disease. Pediatr Nephrol 2009; 24: 1445–1452 [DOI] [PubMed] [Google Scholar]
- 52. Parekh RS, Plantinga LC, Kao WHet al. The association of sudden cardiac death with inflammation and other traditional risk factors. Kidney Int 2008; 74: 1335–1342 [DOI] [PubMed] [Google Scholar]
- 53. Jono S, Shioi A, Ikari Yet al. Vascular calcification in chronic kidney disease. J Bone Miner Metab 2006; 24: 176–181 [DOI] [PubMed] [Google Scholar]
- 54. Schwarz U, Buzello M, Ritz Eet al. Morphology of coronary atherosclerotic lesions in patients with end-stage renal failure. Nephrol Dial Transplant 2000; 15: 218–223 [DOI] [PubMed] [Google Scholar]
- 55. Johansson M, Gao SA, Friberg Pet al. Reduced baroreflex effectiveness index in hypertensive patients with chronic renal failure. Am J Hypertens 2005; 18: 995–1000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analysed during the current study are not publicly available due to legal and ethical restraints. Data are available from the corresponding author on reasonable request.