Abstract
Background
Local progression of intracranial tumors can be the consequence of insufficient radiation dose delivered. Dose increases in the brain must be made carefully so as not to risk debilitating adverse effects such as radiation necrosis.
Hypothesis
A new protocol with 10 × 4 Gy + 11% physical dose increase limited to the macroscopic tumor volume results in a clinically better outcome compared to a 10 × 4 Gy protocol.
Animals
Fifty‐seven client‐owned dogs with primary intracranial neoplasia.
Methods
Randomized controlled trial. Twenty‐eight dogs were assigned to the control protocol (10 × 4 Gy) and 29 to the simultaneous integrated boost (SIB) protocol with 4.45 Gy dose increase. Treatment groups were compared for outcome and signs of toxicity.
Results
Mild, transient acute or early‐delayed adverse radiation effects were observed in 5 dogs. Severe late adverse effects were not seen. Between the protocols, no significant differences were found for outcome (intention‐to‐treat analysis): overall time to progression (TTP) was 708 days (95% confidence interval (95% CI) [545,872]), in the control group it was 828 days (95% CI [401,1256]), and in the SIB group 627 days (95% CI [282,973]; P = .07). Median overall survival (OS) was 684 days (95% CI [516,853]), in the control group it was 724 days (95% CI [623,826]), and in the SIB group 557 days (95% CI [95,1020]; P = .47). None of the tested variables was prognostic in terms of outcome.
Conclusion and Clinical Importance
The dose escalation used with an 11% physical dose increase did not result in better outcome.
Keywords: brain tumor, cancer, canine, glioma, meningioma, radiation therapy, simultaneous integrated boost
Abbreviations
- 95% CI
95% confidence interval
- CBCT
cone beam computed tomography
- CT
computed tomography
- CTV
clinical target volume
- DSS
disease‐specific survival
- FLAIR
fluid‐attenuated inversion recovery
- GTV
gross tumor volume
- (IG‐) IMRT
(image‐guided) intensity‐modulated radiation therapy
- ITT
intention to treat
- MRI
magnetic resonance imaging
- OAR
organs at risk
- OS
overall survival
- PP
per protocol
- PTV
planning target volume
- RT
radiation therapy
- SIB
simultaneous integrated boost
- T2w
T2‐weighted
- TTP
time to progression
1. INTRODUCTION
Intracranial neoplasia may go unnoticed until signs of neurologic disease change an animal's behavior. Return to normal behavior, neurologic status, or decrease in seizure frequency often is observed soon after the start of radiation therapy (RT). 1 , 2 , 3 , 4 , 5 , 6
In recent years, differently fractionated protocols for treatment of intracranial tumors in symptomatic dogs have yielded promising results, offering the benefit of fewer treatments while maintaining comparable tumor control. 3 , 7 In more finely fractionated protocols, median overall survival (OS) times ranged between 7.5 and 27.0 months 1 , 3 , 4 , 8 , 9 , 10 before local disease progression, indicating an insufficient dose of radiation.
The efficacy of radiation depends not only on the total dose, but also on the fractionation schedule, with larger fraction sizes being relatively more potent. Larger fraction sizes, however, increase the risk of complications in the normal tissue surrounding the tumor. 11 , 12 Adverse effects (eg, radiation necrosis, vasculopathy) can impair functionality to a similar degree as tumor progression. 13
Different strategies aimed at dose escalation have been investigated in human medicine, such as a simultaneous integrated boost (SIB). This approach incorporates a higher dose level to the gross tumor volume (GTV) within each fraction. Hence, the GTV receives an increased dose in addition to the regular treatment dose of the planning target volume (PTV). In humans, SIB protocols have been investigated for different tumor types and anatomical regions, including brain tumors. 14 , 15 , 16 , 17 , 18 , 19
In dogs, SIB protocols have focused mainly on sinonasal tumors. 20 , 21 , 22 , 23 , 24 , 25 In the 1990s, a SIB approach for treating sinonasal tumors was discarded because of poor survival and unacceptable adverse effects. 20 Twenty years later, advances such as (image‐guided) intensity‐modulated RT ([IG‐] IMRT) have improved tolerability. 23 These improvements are based predominantly on refined technology, and investigating its use on brain tumors is warranted. 23 , 24 , 25
In a previous study, we explored the feasibility of a shorter protocol than the former 20 × 2.5 Gy protocol, with acceptable risk of late complications and the same tumor control probability. 7 The calculations suggested that small‐ to intermediate‐sized tumors in regions other than the optic chiasm or brainstem can be treated safely with 10 × 4.35 Gy.
Our first attempt to clinically apply this protocol was conservative: 40 Gy in 10 fractions was given with the same risk of toxicity as the 20‐fraction protocol to dogs with brain tumors, accepting possible inferior tumor control. Surprisingly, this protocol led to a similar outcome compared to the 20‐fraction standard protocol. 3 Because the calculated spectrum of acceptable risk had not been fully exhausted, we implemented the SIB approach described here to increase the dose to the GTV while limiting the dose to the surrounding tissues. The GTV was boosted with an additional 11% physical dose.
In this prospective randomized clinical trial, we compare our institution's conventional, definitive‐intent protocol (10 × 4 Gy) to a novel protocol adding an 11% boost to the macroscopic part of the tumor (ie, GTV). We hypothesized the new protocol would result in a clinically detectable better outcome for time to progression (TTP; primary analysis) and survival time.
2. MATERIAL AND METHODS
2.1. Study design, power analysis
Ours was a single‐center, prospective, block‐randomized (equal 1:1 allocation ratio), controlled, parallel‐group study conducted at the Division of Radiation Oncology of the Vetsuisse Faculty, University of Zurich, Zurich, Switzerland.
A power analysis was performed based on our own results and outcomes (percentage of dogs free of progression at 2 years) of dogs treated using the conventional protocol (10 × 4 Gy). We estimated an increase in TTP from 33% to 75% at 2 years as hypothesized by the theoretical planning study with a SIB of 11%. 3 , 7 Calculations were made with a power of 80%, and significance level (alpha) set at 5% for a 2‐sided log‐rank test using a previously described formula. 26 Given a drop‐out rate of 5%, the sample size needed was 52 dogs.
2.2. Inclusion criteria
Dogs with signs of neurologic disease and a radiological diagnosis of intracranial neoplasia 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 were included. All breeds, ages, and sexes were included if they had a meningioma, glioma, pituitary tumor, or choroid plexus tumor that had not been pre‐treated with surgery, radiation, or chemotherapy, and if there was no evidence of metastases. The study required adequate clinical condition (ie, American Society of Anesthesiologists Classification III or less anesthetic risk, and a Veterinary Cooperative Oncology Group performance status level ≤ grade 3). 35
After the owners' written consent, dogs were allocated to 1 treatment group by computer‐generated block randomization. Dogs were treated under approval by the Animal Ethics Council of the Canton of Zurich, Switzerland (Permit Number: ZH075/17).
2.3. Contouring of organs at risk and target volumes
Target volumes and organs at risk (OAR) were contoured in a facility‐internal standardized manner as previously described. 7 In brief, the GTV was delineated using co‐registered contrast‐enhanced computed tomography (CT) images or CT and magnetic resonance (MR) images. In tumors with no contrast uptake, T2‐weighted (T2w) sequences were used for delineation. In dogs treated using a SIB, the GTV equaled the boost subvolume. The clinical target volume (CTV), accounting for subclinical microscopic disease extension, was defined to be 2 mm for meningeal and pituitary tumors and 3‐5 mm for glial tumors, respecting anatomical boundaries such as bone. The CTV margin then was extended 3‐dimensionally by 2 mm to define the PTV, accounting for setup uncertainties in daily image‐guided photon treatment. Doses to target volumes were reported as recommended. 36 , 37
2.4. Normal tissue complication probability
Normal tissue complication probability (NTCP) was calculated for the brain as previously described. 7
2.5. Treatment planning and delivery
For dynamic IMRT treatment planning, an external beam planning system (Eclipse Planning system, version 15.1 Varian Oncology Systems, Palo Alto, California) was used, with an anisotropic analytical algorithm and heterogeneity correction. The SIB design intended to boost the GTV with a per‐day additional dose corresponding to an increase of 15% in biologically effective dose (BED). Given our fractionation scheme with 10‐fractions and using a typical model parameter of α/β = 10 for tumor tissue, this protocol then amounted to an 11% increase in physical dose to 4.45 Gy to the GTV above the daily 4.0 Gy to the PTV. 7 Hence, the plan included 2 dose levels: 44.5 Gy to the GTV and 40.0 Gy to the PTV. For the treatment plans, the dose was prescribed to D50%, and D98% (= Dnear‐min) and D2% (= Dnear‐max) were reported. For adequate PTV coverage (or GTV boost in the SIB plans), D98/95 also had to be fulfilled (ie, 98% of the target volume had to be covered by 95% of the prescribed dose). The minimal target coverage D98% (= Dnear‐min) was 95% of the prescribed dose: 38.0 Gy in the PTV and 42.3 Gy in the GTV boost. The maximum dose D2% (= Dnear‐max) was 107% of the GTV boost dose (47.6 Gy).
Computer‐based inverse planning using a dynamic 120‐leaf multileaf collimator was carried out with the primary goal to optimize target coverage and with a secondary goal to spare OAR. Doses to the OAR were kept as low as possible without specific dose constraints. Dose specification adhered to the International Commission on Radiation Units and Measurements (ICRU Report 83) and the version adapted for veterinary medicine. 23 , 38 Each treatment plan was dosimetrically verified before treatment using an Octavius‐Phantom (PTW Freiburg, Germany) and approved by a medical physicist.
The definitive‐intent photon treatment of 10 × 4.0 Gy + 11% SIB (total dose 40 Gy/SIB 44.5 Gy) was applied daily (workdays) over 2 consecutive weeks to anesthetized and routinely positioned dogs. Treatment was delivered by a 6‐MV linear accelerator (Clinac iX, Varian, Palo Alto, California).
Daily image‐guidance (IGRT) was used for treatment verification, using kilovolt (kV)‐kV orthogonal radiographs, kV cone‐beam CT (CBCT), or both. Therapy was delivered in a dynamic IMRT mode with isocentrically planned beams arranged in a coplanar manner. Quality assurance of the linear accelerator and on‐board imager was performed daily, weekly, monthly, and annually as required by institutional and federal guidelines. 39 , 40
2.6. Medical treatment
If indicated, the dogs received corticosteroids (prednisolone) after diagnosis. Treatment depended on the presence and degree of perilesional T2w and fluid‐attenuated inversion recovery (FLAIR) hyperintensity compatible with edema (if MRI was available), and of the neurologic status (eg, abnormal mental state, seizures). Until the 3‐week re‐evaluation, the dosage was not decreased below 0.5 mg/kg PO q24h. Dogs symptomatic with epileptic seizures received antiepileptic drugs. Drug selection, monitoring, and dose adjustments were made in cooperation with neurologists and consistent with published guidelines. 41 Dosage of prednisolone at the beginning and end of RT and dosage of antiepileptic drugs were recorded.
2.7. Clinical evaluation and follow‐up
Before treatment, a resident or diplomate of the European College of Veterinary Neurology examined all the dogs, scoring neurologic signs using a published neurodisability scoring scheme. 42
The dogs were classified depending on the severity of the neurologic deficits according to an adapted scheme 9 (no neurologic sign; mild = mild neurologic deficits [e.g., head tilt or proprioceptive deficits only, mild lethargy, with the animal otherwise being alert and ambulatory]; moderate = overt clinical neurologic dysfunction present in an ambulatory animal without impaired consciousness [e.g., paresis, vestibular or cerebellar ataxia, hypermetria, several cranial nerve deficits, nystagmus]; and severe = debilitating neurologic deficits [e.g., ataxia with leaning or falling, non‐ambulatory paresis, blindness, functional dysphagia, circling, decreased mentation]). Epileptic seizures were recorded separately.
The follow‐up time points were at the end of RT, 3 and 12 weeks after completed RT, then every 3 months for the first year, and semi‐annually thereafter. They included clinical and neurologic examinations. The radiation oncologists were aware of the randomized treatment group; other clinicians (eg, neurologists, radiologists) were not informed.
To assess tumor response, follow‐up imaging (preferably MRI) 6 and 12 months after completed RT was encouraged. In the event of clinical deterioration non‐responsive to transient administration of corticosteroids, MRI was recommended.
Re‐evaluation MR/CT scans were imported into the Eclipse External beam planning software and any tumor remnant was contoured on the co‐registered images of the initial scan. A volumetric measurement was performed. The relative change in size compared to the initial GTV was calculated. The change in relative tumor volumes over time was plotted using Microsoft Excel for Mac (version 16.54).
Progressive disease and adverse radiation effects were considered if neurologic signs worsened. The time elapsed since completed RT and response to corticosteroids provided first evidence that adverse effects might be responsible for neurologic signs. Acute (during, or days to weeks after RT) and early‐delayed (up to 6 months after RT) adverse effects are usually transient, self‐limiting, and corticosteroid‐responsive. 43 , 44 Late (≥ 6 months after RT) adverse effects are irreversible and can be debilitating and difficult to distinguish from tumor progression. 43 , 44 , 45
When the owners were not available in person, follow‐up was by telephone or email. If dogs were lost to follow‐up, every attempt was made to obtain clinical information and imaging data from the practicing veterinarian.
2.8. Statistical analysis
Descriptive statistics were used in the analysis of dogs and tumor characteristics. When appropriate, data were tested for normality using the Shapiro‐Wilk normality test. Values were expressed as mean ± SD in case of normal distribution or as median and range in case of non‐normal distribution.
Follow‐up time was defined as the time from the first radiation treatment until death, loss to follow‐up or time of data analysis.
The primary analysis (TTP) was based on the intention‐to‐treat (ITT) principle (ie, patients entered the analysis according to the treatment randomly assigned). In addition, a per‐protocol (PP) analysis was performed, including only those dogs in the respective group that completed the treatment as randomly assigned.
As secondary analyses, OS and disease‐specific survival (DSS) were calculated (ITT and PP).
The TTP was defined as the interval between start of RT and discovery of new or progressive signs of neurologic disease or evidence of disease progression based on MRI (whichever occurred first). 46 Dogs dying without clinical or imaging‐based evidence of disease progression were censored for TTP analysis. The OS was defined as the interval between start of RT until death of any cause.
When calculating DSS, dogs that died of non‐tumor‐related causes were censored at the time of death. Dogs still alive at the time of data evaluation or lost to follow‐up were censored.
Time to progression, OS, and DSS were analyzed using the Kaplan‐Meier product‐limit estimator, accompanied by log‐rank or Breslow‐Gehan‐Wilcoxon tests. Survival estimates and median survival time are reported with the corresponding 95% confidence intervals (95% CI).
Cox's regression analysis was used to determine whether co‐variates had an influence on TTP, OS, or DSS. The following variables were investigated for prognostic significance: sex, age, weight, tumor type (meningioma, glioma, pituitary tumor, choroid plexus tumor), meningioma vs. non‐meningioma, intra‐ vs. extra‐axial, NTCP, and GTV/brain volume ratio (relative tumor volume).
A 2‐sample t‐test was conducted to compare serum phenobarbital concentrations between the dogs with and without uncontrolled seizures.
Data were analyzed using SPSS (IBM SPSS Statistics, Version 26, IBM Corp., Armonk, New York) and R version 4.1.1. 47 Kaplan‐Meier graphs were created using the “survminer” package. 48
Results of statistical tests with P < .05 were considered significant.
3. RESULTS
3.1. Dog and tumor characteristics, signs of neurologic disease
Figure 1 provides an overview of the animals that were screened, included, and analyzed for the study. In total, 57 dogs were randomly assigned to a treatment group (ITT population). Three dogs (control group: n = 1, SIB group: n = 2) deviated from the assigned protocol. The 57 dogs completed treatment between September 2017 and October 2020 and were followed‐up until March 11, 2022.
FIGURE 1.
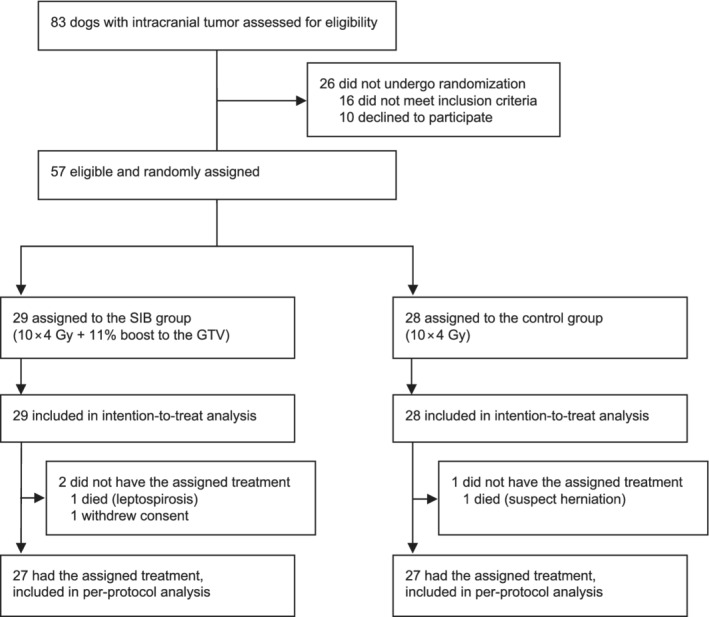
Overview of dogs eligible for the study and distribution between the 2 different treatment groups. GTV, gross tumor volume; SIB, simultaneous integrated boost.
Table 1 summarizes details concerning the dogs, tumors, and signs of neurologic disease. Purebred dogs (n = 44) represented 77% of the study population, with Boxers, French Bulldogs, and Labrador Retrievers (n = 5 each) being the most common breeds. Sixteen (28%) dogs were brachycephalic.
TABLE 1.
Signalment, tumor characteristics, and signs of neurologic disease in the ITT population; comparison of the study population and the 2 treatment groups
| Total (n = 57) | Control [10 × 4 Gy] (n = 28) | SIB [10 × 4 Gy + 11% boost to the GTV] (n = 29) | |
|---|---|---|---|
| Age (years) a | 9.6 (3.4‐14.5) | 9.5 (3.4‐14.5) | 9.6 (5.8‐12.8) |
| Weight (kg) a | 17.4 (2.5‐40.8) | 15.6 (3.3‐40.8) | 23.0 (2.5‐40.0) |
| Sex | |||
| Female, intact | 3 (5%) | 2 (7%) | 1 (3%) |
| Female, spayed | 25 (44%) | 12 (43%) | 13 (45%) |
| Male, intact | 10 (18%) | 6 (21%) | 4 (14%) |
| Male, castrated | 19 (33%) | 8 (29%) | 11 (38%) |
| Breed and head conformance | |||
| Purebred | 44 (77%) | 20 (71%) | 24 (83%) |
| Mixed breed | 13 (23%) | 8 (29%) | 5 (17%) |
| Brachycephalic | 16 (28%) | 6 (21%) | 10 (34%) |
| Non‐brachycephalic | 41 (72%) | 22 (79%) | 19 (66%) |
| Tumor type and neuraxis | |||
| Extra‐axial | |||
| Meningioma | 28 (49%) | 13 (46%) | 15 (52%) |
| Pituitary tumor | 9 (16%) | 6 (21%) | 3 (10%) |
| Choroid plexus tumor | 4 (7%) | 1 (4%) | 3 (10%) |
| Intra‐axial | |||
| Glioma | 16 (28%) | 8 (29%) | 8 (28%) |
| Tumor location | |||
| Forebrain | 36 (63%) | 15 (54%) | 21 (72%) |
| Cerebellum | 3 (5%) | 1 (4%) | 2 (7%) |
| Brainstem | 9 (16%) | 6 (21%) | 3 (10%) |
| Pituitary | 9 (16%) | 6 (21%) | 3 (10%) |
| Rostral cranial fossa | 12 (21%) | 6 (21%) | 6 (21%) |
| Middle cranial fossa | 16 (28%) | 6 (21%) | 10 (34%) |
| Caudal cranial fossa | 12 (21%) | 7 (25%) | 5 (17%) |
| Overlapping | 17 (30%) | 9 (32%) | 8 (28%) |
| Sign of neurologic disease—severity before RT | |||
| No sign | 1 (2%) | 1 (4%) | 0 (0%) |
| Mild | 12 (21%) | 4 (14%) | 8 (28%) |
| Moderate | 8 (14%) | 5 (18%) | 3 (10%) |
| Severe | 14 (25%) | 9 (32%) | 5 (17%) |
| Seizures only | 22 (39%) | 9 (32%) | 13 (45%) |
| Sign of neurologic disease—category | |||
| Cranial nerve deficit(s) | 24 (42%) | 11 (39%) | 13 (45%) |
| Seizures | 31 (54%) | 14 (50%) | 17 (59%) |
| Isolated seizures | 14 (25%) | 7 (25%) | 7 (24%) |
| Cluster seizures | 16 (28%) | 6 (21%) | 10 (34%) |
| Status epilepticus | 1 (2%) | 1 (4%) | 0 (0%) |
| Ataxia | 23 (40%) | 16 (57%) | 7 (24%) |
| Paresis | 15 (26%) | 12 (43%) | 3 (10%) |
| GTV (cm3) a | 2.6 (0.2‐11.8) | 2.6 (0.2‐10.8) | 2.6 (0.3‐11.8) |
| Brain volume (cm3) a | 85.2 (46.9‐133.0) | 82.2 (57.0‐106.7) | 86.1 (46.9‐133.0) |
| GTV/brain volume (%) a | 3.2 (0.2‐10.8) | 3.3 (0.2‐10.6) | 3.2 (0.4‐10.8) |
| NTCP (%) a | 3.9 (0.1‐20.1) | 2.7 (0.1‐19.8) | 5.6 (0.8‐20.1) |
Values expressed as median and range.
Abbreviations: GTV, gross tumor volume; NTCP, normal tissue complication probability; RT, radiation therapy.
The most common tumor type was meningioma (49%), followed by glioma (28%), pituitary tumor (16%), and choroid plexus tumor (7%). The forebrain was most commonly (63%) affected.
Seizures were the most common sign of neurologic disease (54%), 39% presented with seizures only.
Forty‐nine (86%) dogs had an MRI before treatment. In the other 8 cases (1 pituitary tumor, 7 meningiomas), the diagnosis was made with CT.
In the control group, significantly more dogs showed paresis at initial presentation (P = .01). In the SIB group, the NTCP was significantly higher compared to the conventionally treated dogs (P = .003). Otherwise, the 2 groups were balanced with respect to baseline variables (Table 1).
3.2. Radiation therapy and supportive treatment
Twenty‐eight (49%) dogs were assigned to the 10 × 4 Gy, and 29 (51%) dogs to the 10 × 4 Gy + 11% boost protocol (ITT). Twenty‐seven dogs in each group completed RT as assigned (PP). Volume and radiation dose reporting with mean and SD of the target volumes and near‐maximum dose (D2%), median dose (D50%), and near‐minimum dose (D98%) are listed for each group in Table S1 (ITT)/Table S2 (PP).
Table 2 summarizes the medical supportive treatment of dogs that were treated with corticosteroids (prednisolone) and, in case of epileptic seizures, antiepileptic drugs, or a combination thereof.
TABLE 2.
Perorally administered corticosteroids and antiepileptic drugs during treatment, ITT population
| Total (n = 57) | Control [10 × 4 Gy] (n = 28) | SIB [10 × 4 Gy + 11% boost to the GTV] (n = 29) | |
|---|---|---|---|
| Prednisolone at the start of RT (n, %) | 53 (93%) | 25 (89%) | 28 (97%) |
| Dosage (mg/kg) a q24h | 0.60 (0.14‐2.00) | 0.60 (0.19‐2.00) | 0.59 (0.14‐1.34) |
| Prednisolone at the end of RT (n, %) | 53 (93%) | 25 (89%) | 28 (97%) |
| Dosage (mg/kg) a q24h | 0.54 (0.14‐2.00) | 0.52 (0.19‐2.00) | 0.55 (0.14‐1.50) |
| Antiepileptic drugs at the end of RT (n, %) | 30 (53%) | 14 (50%) | 16 (55%) |
| Phenobarbital (n, %); dosage (mg/kg) a q12h | 23 (40%); 2.50 (1.59‐3.78) | 11 (39%); 2.50 (1.70‐3.75) | 12 (41%); 2.62 (1.59‐3.78) |
| Levetiracetam (n, %), dosage (mg/kg) a q8h | 16 (28%); 17.95 (9.10‐28.40) | 7 (25%); 17.20 (13.10‐25.90) | 9 (31%); 18.70 (9.10‐28.40) |
| Imepitoin (n, %), dosage (mg/kg) a q12h | 3 (5%); 13.30 (11.90‐32.00) | 0 (0%); − | 3 (10%); 13.30 (11.90‐32.00) |
| Monotherapy (n, %) | 18 (32%) | 10 (36%) | 8 (28%) |
| >1 antiepileptic drug (n, %) | 12 (21%) | 4 (14%) | 8 (28%) |
Values expressed as median and range; q × h, every × hours.
In 1 dog (SIB group) with obstructive hydrocephalus secondary to a choroid plexus tumor, a ventriculoperitoneal shunt was placed 4 days before RT. Because of a concurrent mast cell tumor with suspected metastases, another dog (SIB group) received chemotherapy (8 doses of vinblastine IV; median dosage 2.29 mg/m2, starting 11 days after RT) and prednisolone PO.
3.3. Follow‐up, outcome, and prognostic variables
Median follow‐up time for all dogs was 642 days (range, 1‐1412), and for the dogs still alive (n = 20) 831 days (range, 507‐1412). No dog was lost to follow‐up.
At the end of RT, 54 (95%) of 57 dogs were re‐evaluated by a neurologist. One dog of each group had died, 1 dog (control group) improved in neurologic status and was not available for examination. The neurologic score improved in 41 (76%) of 54, was stable in 11 (20%) of 54, and deteriorated in 2 (4%) dogs. Neurologic examination results were within normal limits in 27 (50%) of 54 dogs at the end of RT.
In the first 24 months after treatment, 36 dogs (63%; control group: n = 16, SIB group: n = 20) had at least 1 follow‐up imaging procedure (range, 1‐4) between the end of RT and the end of follow‐up, relapse treatment, or death, respectively. Figure 2 illustrates individual changes in relative tumor sizes after treatment.
FIGURE 2.
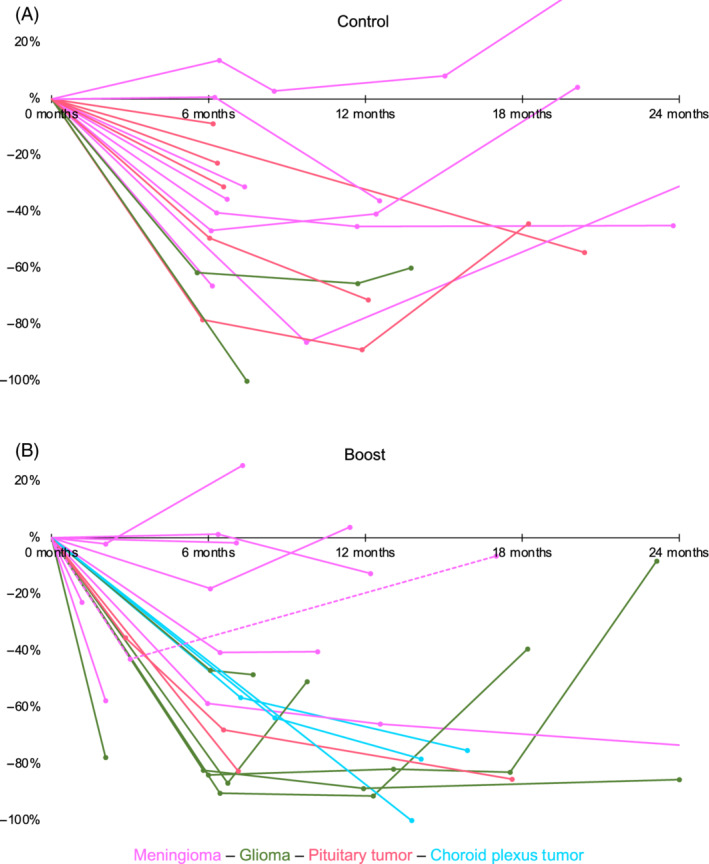
Changes in relative tumor volumes in the first 2 years after treatment, split by group (A, control group; B, SIB group), ITT population. Each dot represents an imaging time point, and each line corresponds to an individual dog with follow‐up imaging, the initial GTV representing the baseline value (ie, before treatment). In the first 24 months after treatment, 36 dogs (control group: n = 16, SIB group: n = 20) had at least one follow‐up imaging. The connecting lines are for better visualization and do not reflect a linear volume change between scan time points. Changes in the relative tumor volume after treatment of relapses are not shown. The dashed line represents the single case that was randomized to the SIB group but was treated conventionally after withdrawal of owner consent. Because this dog is analyzed as part of the SIB group according to the ITT approach, it also has been included in this graph
Three dogs (control group: n = 1, SIB group: n = 2) underwent additional imaging: the dog in the control group after 28 months, 1 of the SIB treated dogs after 38 months (= 29 months after relapse surgery), and the second of the SIB treated dogs at 29 and 36 months (= 7 and 14 months after a second course of RT) after RT. Progressive disease was diagnosed in the first dog (control group), an approximately 42% smaller volume than before RT in the second, and an approximately 79% smaller volume at both time points in the third dog.
Table 3 summarizes the treatment results of the 2 protocols, with TTP representing the primary analysis; OS and DSS are secondary analyses. At the end of data collection, in the ITT analysis, 25 of 57 dogs (44%; control group: n = 10, SIB group: n = 15), and in the PP analysis, 24 of 54 dogs (44%; control group: n = 10, SIB group: n = 14) were considered to have progressive disease.
TABLE 3.
Overall treatment results and comparison of the 2 protocols
| Total (ITT: n = 57, PP: n = 54) | Control [10 × 4 Gy] (ITT: n = 28, PP: n = 27) | SIB [10 × 4 Gy + 11% boost to the GTV] (ITT: n = 29, PP: n = 27) | P value | |
|---|---|---|---|---|
| TTP (days) a | ||||
| ITT | 708 (range, 46‐1121; 95% CI [545,872]) | 828 (range, 146‐1121; 95% CI [401,1256]) | 627 (range, 46‐953; 95% CI [282,973]) | .07, n.s. |
| PP | 708 (range, 46‐1121; 95% CI [523,894]) | 828 (range, 146‐1121; 95% CI [401,1256]) | 707 (range, 46‐953; 95% CI [481,934]) | .09, n.s. |
| OS (days) a | ||||
| ITT | 684 (range, 1‐1180; 95% CI [516,853]) | 724 (range, 1‐1180; 95% CI [623,826]) | 557 (range, 13‐981; 95% CI [95,1020]) | .47, n.s. |
| PP | 709 (range, 45‐1180; 95% CI [545,874]) | 724 (range, 49‐1180; 95% CI [452,997]) | 557 (range, 45‐883; 95% CI [114,1001]) | .38, n.s. |
| DSS (days) a | ||||
| ITT | 873 (range, 1‐1180; 95% CI [614,1133]) | 1025 (range, 1‐1180; 95% CI [628,1423]) | 873 (range, 46‐981; 95% CI [487,1260]) | .58, n.s. |
| PP | 873 (range, 46‐1180; 95% CI [583,1164]) | 1025 (range, 49‐1180; 95% CI [631,1420]) | 873 (range, 46‐883; 95% CI [309,1438]) | .35, n.s. |
Values expressed as median, range, and 95% confidence interval.
Abbreviations: DSS, disease‐specific survival; GTV, gross tumor volume; ITT, intention to treat; n.s., statistically non‐significant; OS, overall survival; PP, per protocol; SIB, simultaneous integrated boost; TTP, time to progression.
In the ITT analysis, median TTP for all dogs was 708 days (95% CI [545,872]), in the control group 828 days (95% CI [401,1256]), and in the SIB group 627 days (95% CI [282,973]; Figure 3). The difference was not significant (P = .07).
FIGURE 3.
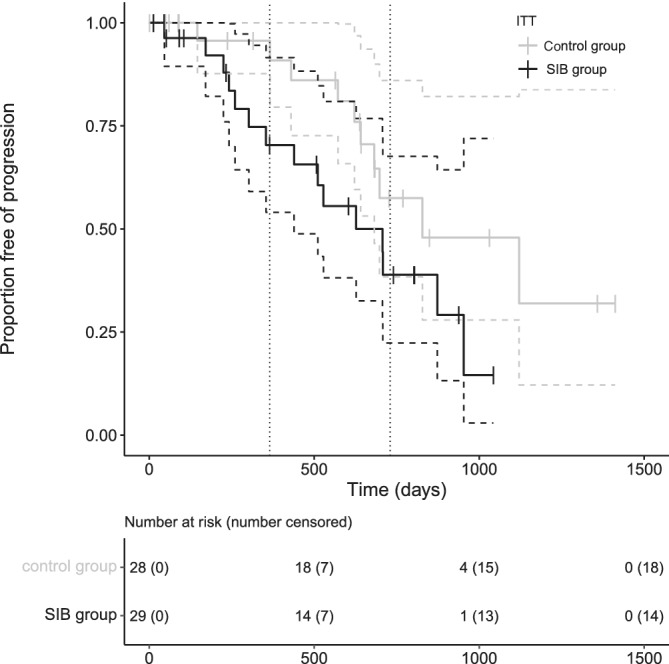
Time to progression Kaplan‐Meier curves of the dogs (ITT population) split by protocol: the black line represents the SIB protocol with median TTP of 627 days (95% CI [282,973]), the gray line the control protocol with median TTP of 828 days (95% CI [401,1256]). There was no significant difference between protocols (P = .07). The tick marks represent censored cases, the vertical dotted lines mark 1 and 2 years
In the PP analysis, median TTP for all dogs was 708 days (95% CI [523,894]), in the control group 828 days (95% CI [401,1256]), and in the SIB group 707 days (95% CI [481,934]). The difference was not significant (P = .09).
Four dogs had waxing and waning signs over several months, but a specific time point of clinical progression could not be determined.
Of the 25 dogs with suspected tumor progression, imaging was performed in 15 cases. Of these 15 cases, routine re‐evaluation imaging identified progressive disease in 5 asymptomatic dogs.
In 10 (67%) of 15 dogs, tumors progressed locally. One (6%) tumor progressed both locally and metastasized to the ventricular system, the subarachnoid space, and to the cervical medulla (“drop metastasis”). In 4 dogs (27%), the primary tumor was controlled well, but drop metastases were detected. Three of the 5 tumors forming metastases were presumed gliomas (1 confirmed at necropsy) and in these 3 cases, drop metastases were identified outside of the brain. The other 2 metastatic tumors were suspected choroid plexus carcinomas.
In the ITT analysis, 37 of 57 dogs (65%; control group: n = 18, SIB group: n = 19), and in the PP analysis, 34 of 54 dogs (63%; control group: n = 17, SIB group: n = 17) were dead at the time of data analysis.
In the ITT analysis, median OS for all dogs was 684 days (95% CI [516,853]), in the control group 724 days (95% CI [623,826]), and in the SIB group 557 days (95% CI [95,1020]; Figure 4). This difference was not significant (P = .47).
FIGURE 4.
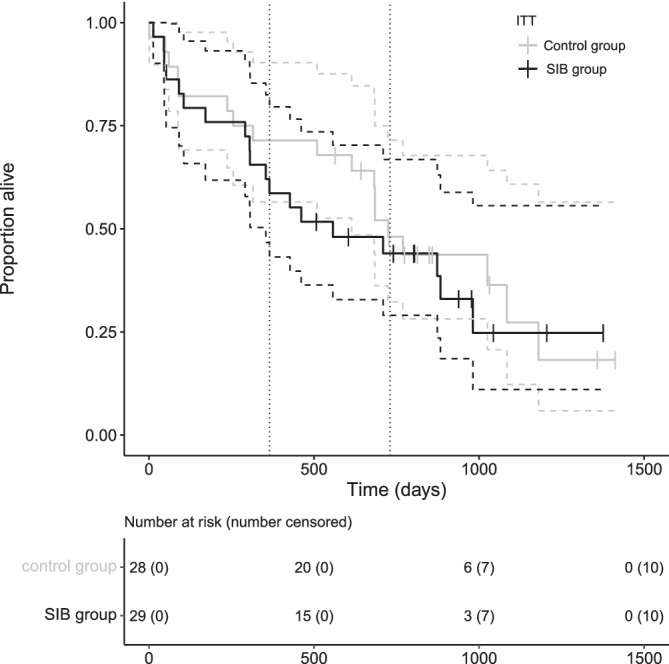
Overall survival Kaplan‐Meier curves of the dogs (ITT population) split by protocol: the black line represents the SIB protocol with median OS of 557 days (95% CI [95,1020]), the gray line the control protocol with median OS of 724 days (95% CI [623,826]). There was no significant difference between protocols (P = .47). The tick marks represent censored cases, the vertical dotted lines mark 1 and 2 years
In the PP analysis, median OS for all dogs was 709 days (95% CI [545,874]), in the control group 724 days (95% CI [452,997]), and in the SIB group 557 days (95% CI [114,1001]). This difference was not significant (P = .38).
In the ITT analysis, 28 of 57 dogs (49%; control group: n = 13, SIB group: n = 15), and in the PP analysis, 26 of 54 dogs (48%; control group: n = 12, SIB group: n = 14) were considered to have died because of the brain tumor (disease‐specific death).
In the ITT analysis, median DSS for all dogs was 873 days (95% CI [614,1133]), in the control group 1025 days (95% CI [628,1423]), and in the SIB group 873 days (95% CI [487,1260]). This difference was not significant (P = .58).
In the PP analysis, median DSS for all dogs was 873 days (95% CI [583,1164]), in the control group 1025 days (95% CI [631,1420]), and in the SIB group 873 days (95% CI [309,1438]). This difference was not significant (P = .35).
One‐ and 2‐year DSS (ITT) for the control group was 75% (95% CI [56.7,88.2]) and 55% (95% CI [34.1,76.1]), and for the SIB group 69% (95% CI [51.4,87.4]) and 52% (95% CI [31.8,72.4]), respectively.
Even after adjusting for the mentioned group imbalance (paresis at initial presentation and NTCP) between the 2 protocols, no change regarding treatment effect was seen. Furthermore, neither in the ITT nor in the PP analyses were any of the tested dog‐, clinical sign‐, or tumor‐related variables prognostic in terms of TTP, OS, or DSS.
3.4. Uncontrolled epileptic seizures and presumed adverse radiation effects
Six dogs (10.5%; 4/6 in the SIB group) with presumed meningioma were euthanized because of uncontrolled seizures 46, 52, 60, 90, 105, and 171 days, respectively, after the start of treatment. The 1 dog surviving 52 days (SIB group) underwent MRI 4 days before euthanasia that identified a tumor volume reduction by 23%, but also more extensive perilesional hyperintensity on T2w and FLAIR‐weighted images compared to before treatment.
Another dog (confirmed meningioma; SIB group) temporarily deteriorated neurologically 61 days after treatment. Magnetic resonance imaging indicated stable tumor size, but progressive perilesional T2w and FLAIR hyperintensity. This dog responded well to prednisolone treatment, suggesting early‐delayed adverse radiation effects.
Four more dogs (2 of each group) were suspected to suffer from milder, transient acute (18, 26, and 31 days), and early‐delayed adverse radiation effects (115 days after completed treatment), respectively. All of these episodes were corticosteroid‐responsive.
One dog with a pituitary macroadenoma (confirmed at necropsy; control group) was presented because of clinical signs consistent with progressive disease 20 months after RT. Magnetic resonance imaging indicated pituitary apoplexy, but the tumor volume was still 54% smaller than before treatment. Besides this potential case, no late adverse radiation effects were diagnosed.
3.5. Serum phenobarbital concentrations
The last measured mean serum phenobarbital concentration was 23.4 μg/mL (SD = 7.6) for all dogs. Data were normally distributed according to Shapiro‐Wilk‐test (P = .08). In 5 of 6 dogs with uncontrolled seizures, serum phenobarbital concentrations were available, with a mean of 16.4 μg/mL (SD = 2.6). They were significantly lower than in the 18 dogs with controlled seizures (mean, 25.4 μg/mL; SD = 7.4; t (21)=2.6; P = .02).
3.6. Histopathology and necropsy
The dog that deteriorated neurologically 61 days after treatment underwent surgical debulking because of progression 8 months after RT. Histopathology confirmed the initial imaging diagnosis (meningioma).
Six dogs underwent necropsy after euthanasia. In 4 of 6 dogs, the imaging diagnosis was confirmed (2 gliomas, 1 meningioma, 1 pituitary tumor), whereas in the other 2 dogs, no remaining tumor was identified. In these 2, the initial MRI findings were consistent with glial tumors.
4. DISCUSSION
The new treatment protocol with 10 × 4 Gy + 11% dose limited to the GTV did not confer longer TTP or survival in our study population. Also, neither dog‐ nor tumor‐related variables were found to influence outcome. Despite the calculated significantly higher NTCP (for late adverse effects) in the SIB group, no clinically detectable late adverse radiation effects were identified in this group.
The present study was a sequel to 2 prior studies. 3 , 7 In the first study, an irradiation protocol was developed with the same tumor control probability as the previously established 20 × 2.5 Gy protocol (total dose 50 Gy). This protocol resulted in 10 × 4.35 Gy (total dose 43.5 Gy), which was assumed to be isoeffective and to have an acceptable, low‐risk estimate of late toxicity, provided the treated tumors were not located in the region of the optic chiasm or brainstem. 7 In the second study, the new moderately hypofractionated protocol was applied clinically, albeit using a more conservative approach of 10 × 4 Gy. The aim of this conservative approach was to keep the incidence of adverse effects constant and very low. The 2 protocols examined, however, did not differ significantly in terms of outcome. 3 Those results and the theoretically calculated scope of further dose increases were the basis of the present study. If dogs succumb to their intracranial tumor after treatment, it is usually because of local progression. Thus, tumor cells might have been treated with an insufficient dose. However, considering the susceptibility of neural tissue to late radiation injury, especially in the case of large fraction sizes, dose cannot be escalated arbitrarily.
The dose increase in the form of a SIB was limited to the macroscopic tumor volume (ie, the volume that can readily be seen on imaging, sometimes only after administration of contrast agent). This approach is a sensible compromise between delivering a higher dose to the tumor and protecting peritumoral normal tissue from debilitating adverse effects. The SIB used in our study did not show any benefit to the overall study population in terms of TTP or OS. It remains unclear why the higher dose did not confer a survival benefit because a higher total dose should (mathematically) lead to a higher tumor control probability. The chosen dose escalation still may have been too conservative to show clinical benefit. However, it is equally conceivable that the effect is the opposite. Although TTP and OS did not differ significantly, it remains possible that a dose increase negatively affects outcome. If tumor progression is presumed in the case of clinical deterioration without imaging examination or necropsy, then clinical deterioration also could be the consequence of adverse radiation effects or a combination of tumor progression and adverse effects.
Figure 2 shows a stronger volume reduction in the tumors treated with the boosted protocol. Because the timing of the follow‐up imaging was not the same for all dogs and the tumors were of different cellular origin, we decided not to statistically interpret this finding. Moreover, if this stronger volume reduction does not translate into longer TTP or OS, it might not be worth the additional, albeit not clinically observed, risk of normal tissue complication.
Our groups included all types of intracranial tumors. Apart from our own findings in a small group, 3 no published reports indicate that glial tumors have a worse prognosis compared to meningiomas. Two other studies indicate that glial tumors treated with RT do not differ significantly in outcome compared to meningiomas. 4 , 10 Median survival times after RT range from 226 to 698 days. 1 , 3 , 4 , 5 , 10 , 49 , 50 This result is consistent with the results of our current study. However, the course of progression might differ with different tumor types. In glial tumors, drop metastasis within the nervous system can occur, limiting survival times, even if the tumor is well‐controlled locally. 5 , 50 , 51
Meningiomas grow slowly and require weeks to months to decrease in size after irradiation. Six dogs (4 in the SIB group) with presumed meningioma were euthanized because of uncontrollable seizures 46, 52, 60, 90, 105, and 171 days after the start of treatment. These seizures could have been caused by unusually early progression (excluded in 1 dog by imaging), by acute or early‐delayed adverse effects, insufficient anti‐epileptic drug treatment, or a combination thereof. We cannot exclude that the escalated dose caused vasculopathy contributing to peritumoral edema and subsequent seizures. In human medicine, post‐treatment edema is a known complication, especially after treatment with high fraction sizes. 52
Overall, the observed adverse effects were limited to transient, corticosteroid‐responsive acute or early‐delayed toxicity in few dogs.
In the 1 dog scanned shortly before euthanasia, extensive white matter edema was seen together with bilateral symmetrical hyperintensity in the cingulate gyrus and mesial temporal cortex. Although white matter edema is generally compatible with post‐treatment edema, the gray matter changes mimic reported post‐ictal changes in dogs. 53 This dog had been in status epilepticus for several hours before imaging and it could not be determined whether the edema caused seizures or vice versa. Epileptic seizures remain a risk in the post‐treatment phase, especially because serum concentrations of antiepileptic drugs might not yet have been finely adjusted. For dogs with epileptogenic tumors, adequate treatment with antiepileptic drugs is of particular importance. Aiming for the upper therapeutic serum phenobarbital concentration (30‐35 μg/mL), 41 with or without additional levetiracetam, most probably provides a safe measure for preventing seizures in these patients. Although this recommendation originally targeted dogs with idiopathic epilepsy, 41 in our experience it also proves effective in dogs with intracranial neoplasia. In 5 dogs with uncontrolled seizures, the serum phenobarbital concentrations were significantly lower compared to the 18 dogs with controlled seizures, and with a mean of 16.4 μg/mL (SD = 2.6), clearly below the upper therapeutic concentration. Although administering an initial high dose of phenobarbital to these already compromised dogs might seem excessive, the dogs usually adapted within days to weeks. The high serum phenobarbital concentration might decrease the risk of seizures and subsequent euthanasia because of uncontrollable seizures or falsely presumed tumor progression.
In 1 dog, a late adverse effect could not be completely excluded. The dog with a non‐functional pituitary macroadenoma was diagnosed with pituitary apoplexy 20 months after treatment. Apoplexy has been reported to occur in non‐irradiated adenomatous and non‐adenomatous pituitary glands. 54 , 55 Radiation therapy as a risk factor has been described in dogs, most likely because vascular damage can occur in the high‐dose area. 56
No dog suffered from late adverse radiation effects, such as radiation necrosis. However, especially many months after treatment, when tumor progression becomes increasingly likely, potential adverse radiation effects are difficult to distinguish clinically. Advanced brain tumor imaging (eg, diffusion‐ or perfusion‐weighted imaging, MR spectroscopy) might provide further indications, but definitive confirmation is still only possible by histopathology. 57
A study limitation was that dogs were included and treated based on an imaging diagnosis. Although there are documented imaging features correlated to histopathologic diagnoses making a specific tumor type probable, 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 histopathology remains the gold standard. This limitation similarly concerns tumor grade and the differentiation of tumor progression from (late) adverse radiation effects. 32 , 58 Misclassification could have confounded outcome.
One advantage of our study is the close clinical monitoring and high number of follow‐up scans, which allows visualization of the longitudinal course of tumor volume change. Volume only, however, might not be sufficiently representative of tumor activity or durability of treatment response, because other factors (eg, presence and proportion of solid vs. cystic components), signal intensities, and patterns of contrast enhancement (ie, not only quantitative, but also qualitative) could indicate progression before it is reflected by an increase in size.
Another advantage was the randomized nature of the trial that determines the role of an SIB protocol for the treatment of intracranial tumors in dogs. The effect of potential confounders was minimized by the randomization procedure. Because certain glial tumors feature a different clinical progression with central nervous system metastasis, 50 , 51 potentially profiting from additional chemotherapy, stratification into histological tumor types would be ideal.
In conclusion, the SIB approach used in our study with 11% physical dose increase did not result in better outcomes.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Dogs were treated under approval by the Animal Ethics Council of the Canton of Zurich, Switzerland (Permit Number: ZH075/17).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Table S1. Mean volumes and doses for target volumes for the 2 different treatment protocols (ITT).
Table S2. Mean volumes and doses for target volumes for the 2 different treatment protocols (PP).
ACKNOWLEDGMENT
Funding provided by the Swiss National Science Foundation (SNSF), grant number: 320030‐182490; primary investigator: Carla Rohrer Bley. Part of the data was presented as an oral abstract at the 2020 American College of Veterinary Radiology (ACVR) Virtual Conference: Tolerability of a high‐dose 10‐fraction radiation protocol in dogs with brain tumors, Staudinger C, Meier V, Beckmann K, Körner M, Günther C, Rohrer Bley C. The authors acknowledge the collaboration with the Clinic for Diagnostic Imaging and the Division of Neurology, Vetsuisse Faculty, University of Zurich, Zurich, Switzerland, with referring veterinarians and dog owners. The authors thank Richard Evans for his support in statistical analysis and for his input on scientific writing.
Staudinger C, Meier V, Beckmann K, Körner M, Rohrer Bley C. Treatment of intracranial neoplasia in dogs using higher doses: A randomized controlled trial comparing a boosted to a conventional radiation protocol. J Vet Intern Med. 2022;36(4):1353‐1364. doi: 10.1111/jvim.16472
Funding information Swiss National Science Foundation, Grant/Award Number: 320030‐182490
REFERENCES
- 1. Bley CR, Sumova A, Roos M, Kaser‐Hotz B. Irradiation of brain tumors in dogs with neurologic disease. J Vet Intern Med. 2005;19:849‐854. [DOI] [PubMed] [Google Scholar]
- 2. Monforte Monteiro SR, Rossmeisl JH, Russell J, et al. Effect of radiotherapy on freedom from seizures in dogs with brain tumors. J Vet Intern Med. 2020;34:821‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schwarz P, Meier V, Soukup A, et al. Comparative evaluation of a novel, moderately hypofractionated radiation protocol in 56 dogs with symptomatic intracranial neoplasia. J Vet Intern Med. 2018;32:2013‐2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Debreuque M, De Fornel P, David I, et al. Definitive‐intent uniform megavoltage fractioned radiotherapy protocol for presumed canine intracranial gliomas: retrospective analysis of survival and prognostic factors in 38 cases (2013‐2019). BMC Vet Res. 2020;16:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dolera M, Malfassi L, Bianchi C, et al. Frameless stereotactic radiotherapy alone and combined with temozolomide for presumed canine gliomas. Vet Comp Oncol. 2018;16:90‐101. [DOI] [PubMed] [Google Scholar]
- 6. Hansen KS, Zwingenberger AL, Théon AP, Kent MS. Long‐term survival with stereotactic radiotherapy for imaging‐diagnosed pituitary tumors in dogs. Vet Radiol Ultrasound. 2019;60:219‐232. [DOI] [PubMed] [Google Scholar]
- 7. Rohrer Bley C, Meier V, Schwarz P, Roos M, Besserer J. A complication probability planning study to predict the safety of a new protocol for intracranial tumour radiotherapy in dogs. Vet Comp Oncol. 2017;15:1295‐1308. [DOI] [PubMed] [Google Scholar]
- 8. Keyerleber MA, McEntee MC, Farrelly J, et al. Three‐dimensional conformal radiation therapy alone or in combination with surgery for treatment of canine intracranial meningiomas. Vet Comp Oncol. 2015;13:385‐397. [DOI] [PubMed] [Google Scholar]
- 9. Treggiari E, Maddox TW, Goncalves R, et al. Retrospective comparison of three‐dimensional conformal radiation therapy vs. prednisolone alone in 30 cases of canine infratentorial brain tumors. Vet Radiol Ultrasound. 2017;58:106‐116. [DOI] [PubMed] [Google Scholar]
- 10. Magalhães TR, Benoît J, Néčová S, et al. Outcome after radiation therapy in canine intracranial meningiomas or gliomas. In Vivo. 2021;35:1117‐1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lawrence YR, Li XA, el Naqa I, et al. Radiation dose‐volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010;76:S20‐S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hall EJ, Giaccia AJ. Time, dose, and fractionation in radiotherapy. Radiobiology for the Radiologist. 8th ed. Philadelphia, PA: Wolters Kluwer; 2019:417‐436. [Google Scholar]
- 13. Kelsey KL, Gieger TL, Nolan MW. Single fraction stereotactic radiation therapy (stereotactic radiosurgery) is a feasible method for treating intracranial meningiomas in dogs. Vet Radiol Ultrasound. 2018;59:632‐638. [DOI] [PubMed] [Google Scholar]
- 14. Farzin M, Molls M, Astner S, Rondak IC, Oechsner M. Simultaneous integrated vs. sequential boost in VMAT radiotherapy of high‐grade gliomas. Strahlenther Onkol. 2015;191:945‐952. [DOI] [PubMed] [Google Scholar]
- 15. De Felice F, Bonomo P, Sanguineti G, et al. Moderately accelerated intensity‐modulated radiation therapy using simultaneous integrated boost: practical reasons or evidence‐based choice? A critical appraisal of literature. Head Neck. 2020;42:3405‐3414. [DOI] [PubMed] [Google Scholar]
- 16. Mohan R, Wu Q, Manning M, Schmidt‐Ullrich R. Radiobiological considerations in the design of fractionation strategies for intensity‐modulated radiation therapy of head and neck cancers. Int J Radiat Oncol Biol Phys. 2000;46:619‐630. [DOI] [PubMed] [Google Scholar]
- 17. Nitsche M, Dunst J, Carl UM, Hermann RM. Emerging role of hypofractionated radiotherapy with simultaneous integrated boost in modern radiotherapy of breast cancer. Breast Care (Basel). 2015;10:320‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baisden JM, Sheehan J, Reish AG, et al. Helical tomotherapy simultaneous integrated boost provides a dosimetric advantage in the treatment of primary intracranial tumors. Neurol Res. 2011;33:820‐824. [DOI] [PubMed] [Google Scholar]
- 19. Dogan N, King S, Emami B, et al. Assessment of different IMRT boost delivery methods on target coverage and normal‐tissue sparing. Int J Radiat Oncol Biol Phys. 2003;57:1480‐1491. [DOI] [PubMed] [Google Scholar]
- 20. Thrall DE, Mcentee MC, Novotney C, et al. A boost technique for irradiation of malignant canine nasal tumors. Vet Radiol Ultrasound. 1993;34:295‐300. [Google Scholar]
- 21. Vaudaux C, Schneider U, Kaser‐Hotz B. Potential for intensity‐modulated radiation therapy to permit dose escalation for canine nasal cancer. Vet Radiol Ultrasound. 2007;48:475‐481. [DOI] [PubMed] [Google Scholar]
- 22. Gutierrez AN, Deveau M, Forrest LJ, et al. Radiobiological and treatment planning study of a simultaneously integrated boost for canine nasal tumors using helical tomotherapy. Vet Radiol Ultrasound. 2007;48:594‐602. [DOI] [PubMed] [Google Scholar]
- 23. Soukup A, Meier V, Pot S, Voelter K, Rohrer Bley C. A prospective pilot study on early toxicity from a simultaneously integrated boost technique for canine sinonasal tumours using image‐guided intensity‐modulated radiation therapy. Vet Comp Oncol. 2018;16:441‐449. [DOI] [PubMed] [Google Scholar]
- 24. Meier V, Besserer J, Rohrer BC. Using biologically based objectives to optimize boost intensity‐modulated radiation therapy planning for brainstem tumors in dogs. Vet Radiol Ultrasound. 2020;61:77‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meier V, Staudinger C, Körner M, Soukup A, Rohrer Bley C. Dose‐escalated simultaneously integrated boost radiation protocol fails to result in a survival advantage for sinonasal tumors in dogs. Vet Radiol Ultrasound. 2022;13086. htts://doi.org/10.1111/vru.13086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schoenfeld DA. Sample‐size formula for the proportional‐hazards regression model. Biometrics. 1983;39:499‐503. [PubMed] [Google Scholar]
- 27. Ródenas S, Pumarola M, Gaitero L, Zamora À, Añor S. Magnetic resonance imaging findings in 40 dogs with histologically confirmed intracranial tumours. Vet J. 2011;187:85‐91. [DOI] [PubMed] [Google Scholar]
- 28. Kraft SL, Gavin PR, DeHaan C, Moore M, Wendling LR, Leathers CW. Retrospective review of 50 canine intracranial tumors evaluated by magnetic resonance imaging. J Vet Intern Med. 1997;11:218‐225. [DOI] [PubMed] [Google Scholar]
- 29. Wisner ER, Dickinson PJ, Higgins RJ. Magnetic resonance imaging features of canine intracranial neoplasia. Vet Radiol Ultrasound. 2011;52:S52‐S61. [DOI] [PubMed] [Google Scholar]
- 30. Bentley RT. Magnetic resonance imaging diagnosis of brain tumors in dogs. Vet J. 2015;205:204‐216. [DOI] [PubMed] [Google Scholar]
- 31. Cherubini GB, Mantis P, Martinez TA, Lamb CR, Cappello R. Utility of magnetic resonance imaging for distinguishing neoplastic from non‐neoplastic brain lesions in dogs and cats. Vet Radiol Ultrasound. 2005;46:384‐387. [DOI] [PubMed] [Google Scholar]
- 32. Sturges BK, Dickinson PJ, Bollen AW, et al. Magnetic resonance imaging and histological classification of intracranial meningiomas in 112 dogs. J Vet Intern Med. 2008;22:586‐595. [DOI] [PubMed] [Google Scholar]
- 33. Wolff CA, Holmes SP, Young BD, et al. Magnetic resonance imaging for the differentiation of neoplastic, inflammatory, and cerebrovascular brain disease in dogs. J Vet Intern Med. 2012;26:589‐597. [DOI] [PubMed] [Google Scholar]
- 34. Young BD, Fosgate GT, Holmes SP, et al. Evaluation of standard magnetic resonance characteristics used to differentiate neoplastic, inflammatory, and vascular brain lesions in dogs. Vet Radiol Ultrasound. 2014;55:399‐406. [DOI] [PubMed] [Google Scholar]
- 35. Veterinary Cooperative Oncology Group . Common terminology criteria for adverse events (VCOG‐CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Vet Comp Oncol. 2016;14:417‐446. [DOI] [PubMed] [Google Scholar]
- 36. Rohrer Bley C, Meier VS, Besserer J, Schneider U. Intensity‐modulated radiation therapy dose prescription and reporting: sum and substance of the international commission on radiation units and measurements report 83 for veterinary medicine. Vet Radiol Ultrasound. 2019;60:255‐264. [DOI] [PubMed] [Google Scholar]
- 37. Keyerleber MA, McEntee MC, Farrelly J, et al. Completeness of reporting of radiation therapy planning, dose, and delivery in veterinary radiation oncology manuscripts from 2005 to 2010. Vet Radiol Ultrasound. 2012;53:221‐230. [DOI] [PubMed] [Google Scholar]
- 38. International Commission on Radiation Units and Measurements . Prescribing, Recording, and Reporting Photon‐Beam Intensity‐Modulated Radiation Therapy (IMRT) (Report 83). Oxford: Oxford University Press; 2010. [Google Scholar]
- 39. Quality assurance of gantry‐mounted image‐guided radiotherapy systems. In http://www.ssrpm.ch/old/recrep-m.htm#rec: Swiss Society of Radiobiology and Medical Physics: 2010.
- 40. Quality control for intensity‐modulated radiation therapy. In. http://www.ssrpm.ch/old/recrep-m.htm#rec: Swiss Society for Radiobiology and Medical Physics: 2007.
- 41. Bhatti SFM, De Risio L, Muñana K, et al. International veterinary epilepsy task force consensus proposal: medical treatment of canine epilepsy in Europe. BMC Vet Res. 2015;11:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smith PM, Stalin CE, Shaw D, Granger N, Jeffery ND. Comparison of two regimens for the treatment of meningoencephalomyelitis of unknown etiology. J Vet Intern Med. 2009;23:520‐526. [DOI] [PubMed] [Google Scholar]
- 43. Schultheiss TE, Kun LE, Ang KK, Stephens LC. Radiation response of the central nervous system. Int J Radiat Oncol Biol Phys. 1995;31:1093‐1112. [DOI] [PubMed] [Google Scholar]
- 44. Béhin A, Delattre JY. Complications of radiation therapy on the brain and spinal cord. Semin Neurol. 2004;24:405‐417. [DOI] [PubMed] [Google Scholar]
- 45. Keime‐Guibert F, Napolitano M, Delattre JY. Neurological complications of radiotherapy and chemotherapy. J Neurol. 1998;245:695‐708. [DOI] [PubMed] [Google Scholar]
- 46. Nguyen SM, Thamm DH, Vail DM, London CA. Response evaluation criteria for solid tumours in dogs (v1.0): a veterinary cooperative oncology group (VCOG) consensus document. Vet Comp Oncol. 2015;13:176‐183. [DOI] [PubMed] [Google Scholar]
- 47. R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/; 2021. [Google Scholar]
- 48. Kassambara A, Kosinski M, Biecek P. survminer: Drawing Survival Curves using 'ggplot2'. R package version 0.4.9. https://CRAN.R-project.org/package=survminer; 2021.
- 49. Brearley MJ, Jeffery ND, Phillips SM, et al. Hypofractionated radiation therapy of brain masses in dogs: a retrospective analysis of survival of 83 cases (1991‐1996). J Vet Intern Med. 1999;13:408‐412. [DOI] [PubMed] [Google Scholar]
- 50. Rohrer Bley C, Staudinger C, Bley T, et al. Canine presumed glial brain tumours treated with radiotherapy: is there an inferior outcome in tumours contacting the subventricular zone? Vet Comp Oncol. 2021;20:29. [DOI] [PubMed] [Google Scholar]
- 51. Vigeral M, Bentley RT, Rancilio NJ, Miller MA, Heng HG. Imaging diagnosis ‐ antemortem detection of oligodendroglioma "cerebrospinal fluid drop metastases" in a dog by serial magnetic resonance imaging. Vet Radiol Ultrasound. 2018;59:E32‐e37. [DOI] [PubMed] [Google Scholar]
- 52. Conti A, Pontoriero A, Siddi F, et al. Post‐treatment edema after meningioma radiosurgery is a predictable complication. Cureus. 2016;8:e605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nagendran A, McConnell JF, De Risio L, et al. Peri‐ictal magnetic resonance imaging characteristics in dogs with suspected idiopathic epilepsy. J Vet Intern Med. 2021;35:1008‐1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bertolini G, Rossetti E, Caldin M. Pituitary apoplexy‐like disease in 4 dogs. J Vet Intern Med. 2007;21:1251‐1257. [DOI] [PubMed] [Google Scholar]
- 55. Long SN, Michieletto A, Anderson TJ, Williams A, Knottenbelt CM. Suspected pituitary apoplexy in a German shorthaired pointer. J Small Anim Pract. 2003;44:497‐502. [DOI] [PubMed] [Google Scholar]
- 56. Sawada H, Mori A, Lee P, Sugihara S, Oda H, Sako T. Pituitary size alteration and adverse effects of radiation therapy performed in 9 dogs with pituitary‐dependent hypercortisolism. Res Vet Sci. 2018;118:19‐26. [DOI] [PubMed] [Google Scholar]
- 57. Ali FS, Arevalo O, Zorofchian S, et al. Cerebral radiation necrosis: incidence, pathogenesis, diagnostic challenges, and future opportunities. Curr Oncol Rep. 2019;21:66. [DOI] [PubMed] [Google Scholar]
- 58. Siu A, Wind JJ, Iorgulescu JB, Chan TA, Yamada Y, Sherman JH. Radiation necrosis following treatment of high grade glioma ‐ a review of the literature and current understanding. Acta Neurochir. 2012;154:191‐201. discussion 201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Mean volumes and doses for target volumes for the 2 different treatment protocols (ITT).
Table S2. Mean volumes and doses for target volumes for the 2 different treatment protocols (PP).


