Abstract
The ability to sense the environment is essential to survival and the primary purpose of the somatosensory nervous system. However, despite its highly conserved nature, the sensation of itch has been historically overlooked and its importance in medicine underappreciated. Herein, we highlight how fundamental discoveries, coupled to rapid successes of new therapeutics, have placed itch biology at the forefront of a translational revolution in the field of somatosensation and beyond.
Summary
In ‘The Youngest Science’, Lewis Thomas describes living through an era in which scientific innovation transformed medicine from an art of diagnosis to a discipline of treatment leading to unprecedented extension of life. Even during this era of science-based treatment, sensory disorders such as itch were cast aside. However, recent translational successes in the neuroimmunology of itch points to a future of patient care in which we can improve not only longevity, but also quality, of life.
In Brief
In this Perspective by Kim, the historically overlooked neurobiology and medicine of itch are reviewed to show how unexpected breakthroughs have put forth new paradigms of sensation, neuroimmunology, and therapy extending beyond itch.
Introduction
“I base most of my fashion sense on what doesn’t itch.” – Gilda Radner (comedian)
One of the earliest formal definitions of itch was coined by the German physician Samuel Haffenreffer in 1660, where he described itch as ‘an uncomfortable sensation that provokes a desire to scratch.’ When itch lasts for longer than six weeks it is defined as chronic, and it is estimated that about 15% of the population suffers from chronic pruritus (Leader et al., 2015). Studies have demonstrated that chronic itch and chronic pain negatively impact quality of life to similar degrees (Kini et al., 2011). Yet, in contrast to pain, there are currently no FDA-approved medications specifically indicated to treat chronic pruritus. Indeed, even in conditions where chronic itch is a defining hallmark of the disease, such as atopic dermatitis (AD) and chronic spontaneous urticaria (CSU), itch has not been properly assessed as a primary endpoint in clinical trials.
This viewpoint highlights how historically itch may be one of the most overlooked sensations in terms of scientific advancements and medical recognition. However, due to recent seminal discoveries in basic itch biology and clinical trials, chronic pruritus is now emerging as a paradigmatic example of how translational success can address major unmet needs in medicine.
Why Was Itch Overlooked?
In 1922, Max Von Frey observed that stimulation of the skin with spicules, while initially evoking pain, resulted in subsequent itch sensations. These and other observations led to the development of the intensity hypothesis, which postulated that itch is triggered by milder stimulation with substances that also cause pain. Although other theories were proposed, for decades, itch was largely viewed as a mild variant of pain rather than as a distinct sensation that could be specifically targeted. Thus, while significant resources have been devoted to the study of basic mechanisms of chronic pain, chronic itch has been relatively overlooked.
There are additional biases that further explain why itch is a frequently dismissed symptom. Modern Western medicine values objective measurements of disease (labs, imaging, etc.) over subjective symptoms such as itch, tinnitus, and vertigo, which have few if any FDA-labeled drugs. While even pain has been shown to evoke empathic consolation behavior (Langford et al., 2006; Smith et al., 2021), itch, in contrast, is associated with tremendous social stigma (Silverberg et al., 2018) and is linked to lack of personal hygiene, psychiatric dysfunction, and communicable diseases (e.g., body louse, scabies, etc.) (Weisshaar et al., 2015). Taken together, numerous factors from well-intentioned but precarious scientific assumptions to medical apathy and social biases have led to patients with chronic pruritus being cast aside. However, new hope has been generated by a confluence of discoveries in basic immunology, neuroscience, and the newer interdisciplinary field of peripheral neuroimmunology.
The Emergence of Itch
Although many studies had isolated polymodal C fibers as the key mediators of itch, whether itch was a truly distinct sensation remained a topic of controversy for decades (Handwerker, 2014). Indeed, even the classical itch-inducing molecule (i.e. pruritogen) histamine was known to cause both itch and pain (Rosenthal, 1977). Then, in a seminal study in 1997, Schemlz et al. employed application of histamine by iontophoresis to the skin of humans, and coupled this with single fiber microneurography, to show that histamine activates a previously unrecognized subset of C fibers, and not traditional mechanoreceptors or heat nociceptors (Schmelz et al., 1997). A few years later, Andrew and Craig identified second-order spinothalamic neurons that also exhibit similar functional specificity and uniquely thalamic projections (Andrew and Craig, 2001). Taken together, in the context of histaminergic itch, a paradigm was emerging that suggested the possibility of an itch-specific neural pathway extending from the periphery to the spinal cord and brain (Figure 1). However, whether truly itch-specific receptors exist remained an open question.
Figure 1. A schematic of basic neuroimmune itch circuitry.
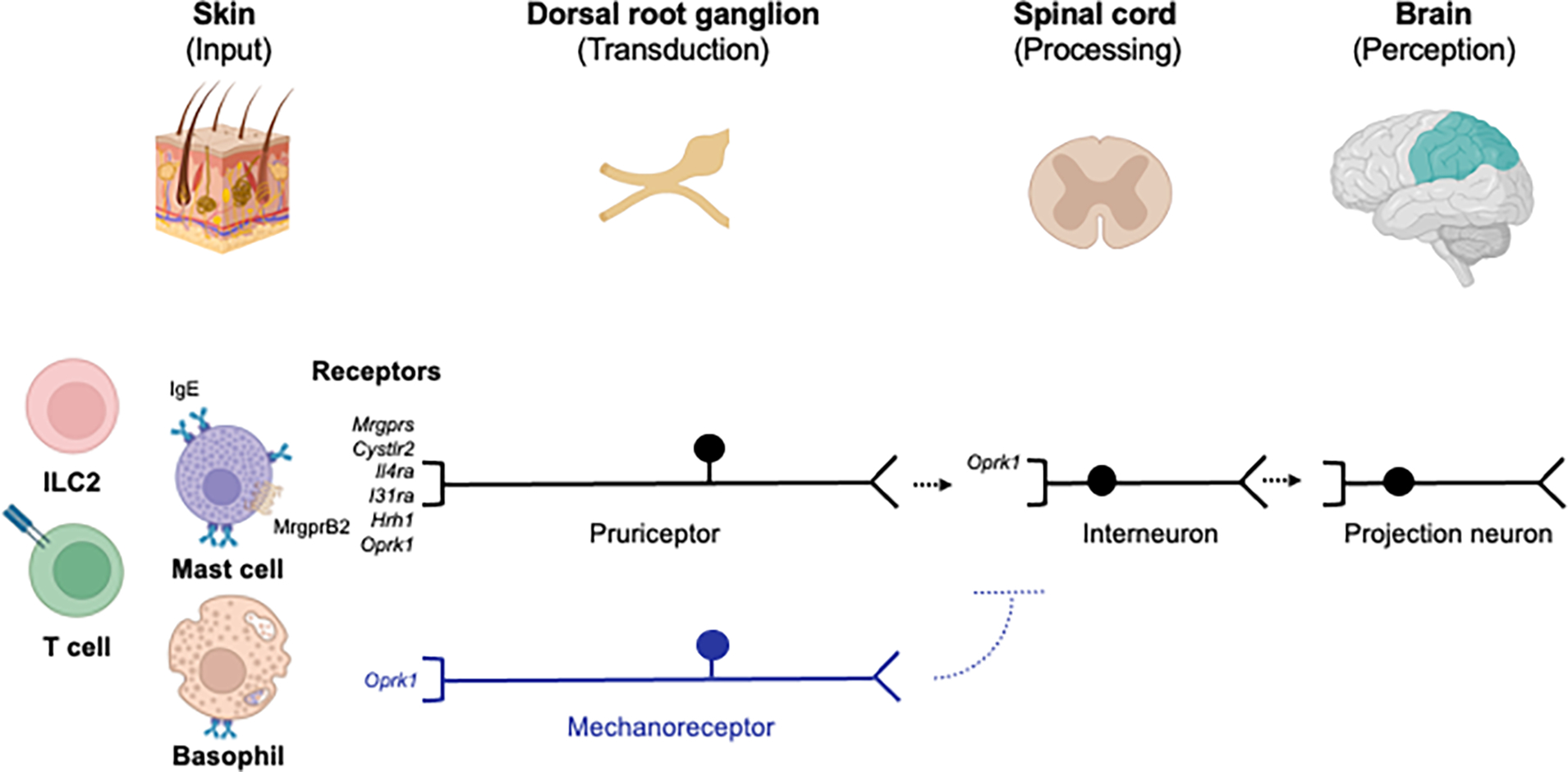
Tissue-resident immune cells in the skin can be activated by a variety of signals including cytokines and/or antigen. Mast cells can be activated by endogenous or exogenous factors via MRGPRX2 and/or IgE and release various pruritogens. These include molecules such as neurotransmitters, neuropeptides, leukotrienes, and cytokines that can activate receptors on pruriceptors encoded by Mrgprs, Cysltr2, Il4ra, Il31ra, or Hrh1 in the skin. These signals stimulate peripheral neuronal transmission to the spinal cord and onto the brain. Other receptors function to suppress itch such as the kappa opioid receptor (KOR) encoded by Oprk1 which is expressed on C fibers, mechanoreceptors, and interneurons. MrgprB2 - Mas-related G protein-coupled receptor (MRGPR) B2; IgE – immunoglobulin E; *Cysltr2 – cysteinyl leukotriene receptor 2; *Il4ra – interleukin 4 receptor; *Il31ra – interleukin 31 receptor A; Hrh1 – histamine H1 receptor; ILC2 – group 2 innate lymphoid cell. *Receptors more specifically expressed on pruriceptors. Created with Biorender.com.
In 2007 Sun and Chen discovered gastrin-releasing peptide receptor (GRPR) as an itch-specific receptor in the spinal cord that was entirely independent of the histamine pathway (Sun and Chen, 2007). They further demonstrated that GRPR+ neurons critically mediate itch, but not other sensations (Sun et al., 2009), supporting the ‘labeled line’ hypothesis that itch-specific neurons exist within the spinal cord as previously suggested by Andrew and Craig with regard to histamine (Andrew and Craig, 2001; Handwerker and Schmelz, 2009). Although the itch-specificity of GRPR and the labeled line theory remains debated, these conceptual advances established a paradigm in which even nonhistaminergic itch could be targeted in a specific manner, apart from other sensations such as pain, for therapeutic relief. Additionally, these studies further supported the the longstanding hypothesis that there are yet likely itch-specific receptors and neurons in the periphery as well.
Mas-related G protein-coupled receptors (Mrgprs) were discovered in 2001 (Dong et al., 2001), although it was not until 2009, that the itch-specific roles of this family were revealed. For example, similar to GRPR+ neurons in the spinal cord, MrgprA3+ neurons in the dorsal root ganglia (DRG) were found to be critically important in transmitting a variety of different itch signals, independently of histamine and pain (Figure 1) (Liu et al., 2009). In 2013, brain natriuretic peptide (BNP) was identified as a key neuropeptide that is released from primary peripheral itch-sensory neurons to stimulate itch circuits within the spinal cord (i.e. GRPR+ neurons) (Mishra and Hoon, 2013). Indeed, pharmacologic blockade of the receptor for BNP demonstrates potent anti-itch efficacy in mice (Jürgen et al., 2019). Collectively, these studies advanced the concept that itch could be triggered in the periphery via dedicated receptors and neurons (i.e. pruriceptors) as observed in the spinal cord itself. Indeed, investigating the role of various Mrgprs in itch and even visceral sensory processes is a major field of inquiry (Inclan-Rico et al., 2021). Thus, the development of antagonists for these pathways is a new area of pharmaceutical development not only for itch but a variety of sensory disorders across multiple barrier surfaces.
Mast cells have long been appreciated to reside in close approximation to sensory neurons across numerous tissues (Forsythe and Bienenstock, 2012). Therefore, the functional mast cell-nerve unit represents what can be considered the classical neuroimmune axis in the periphery. Although originally thought to be involved in pain, the functional relevance of Mrgprs on sensory neurons was not truly appreciated until their discovery as itch receptors (Liu et al., 2009). Strikingly, one of the Mrgprs, MrggprB2 in mice, has emerged as a mast cell-specific receptor (McNeil et al., 2015) that mediates nonhistaminergic itch (Meixiong et al., 2019). Thus, while other Mrgprs such as MrgprA3 and Mrgprc11 reside on pruriceptors, MrgprB2, and its human ortholog MRGPRX2, have emerged as key receptors on mast cells that can rapidly trigger itch responses (Figure 1). Recent cryo-EM studies have successfully solved the structure of human mast cell-expressed MRGPRX2, as well as sensory neuron-expressed MRGPRX4, which has been implicated in cholestatic itch (Cao et al., 2021; Yang et al., 2021). These studies will likely accelerate rational drug design for the development of antagonists that may be used to treat mast cell-associated itch in conditions like CSU and beyond. Notwithstnading this, the biology of Mrgprs further supports the mast cell-nerve unit as a central neuroimmune axis in itch and likely other physiologic processes.
The Translational Success of Neuroimmune Therapeutics in Itch
In recent years, a series of single cell RNA-sequencing (scRNA-seq) studies in mice codified DRG neurons at the transcriptional level. Notably, it has now been shown that there is convergent expression of pruritogen receptors on the same neuronal subsets such as the receptors for the immune cell-derived pruritogens histamine (Hrh1), leukotriene C4 (Cysltr2), IL-4 (Il4ra), IL-13 (Il13ra1), and IL-31 (Il31ra) (Figure 1) (Li et al., 2016; Usoskin et al., 2015; Zeisel et al., 2018). Subsequently, expression of Janus kinase 1 (Jak1), a key downstream mediator of a number of cytokines (e.g. IL-4, IL-13, and IL-31) was also identified within these same pruriceptors (Oetjen et al., 2017). A series of scRNA-seq and functional studies studies in human DRG have confirmed that distinct pruriceptors likely exist within humans as well. For example, expression of IL31RA and its co-receptor OSMR as well as JAK1 has been detected within putative human pruriceptors (Diana et al., 2022; Nguyen et al., 2021) and type 2 cytokines have been shown to activate human DRG neurons (Oetjen et al., 2017). However, there are notable differences as well. For example, OSMR-expressing neurons appear to subdivide into two distinct populations, in contrast to a single population in mice (Nguyen et al., 2021). Further, human pruriceptors appear to co-express receptors associated with mechanosensation, suggesting more polymodality in humans (Diana et al., 2022; Klein et al., 2021; Nguyen et al., 2021). Notwithstanding these differences, the early findings in mice provoked the hypothesis that disrupting these key neuroimmune circuits may represent a new therapeutic paradigm for chronic pruritus.
In the context of AD, a classical inflammatory itch disorder, a number of agents directed at these neuron-associated pathways have demonstrated unprecedented anti-itch efficacy in clinical trials. The first example of this was the approval in 2017 of dupilumab, a monoclonal antibody (mAb) directed at the receptor (IL-4Rα) for both IL-4 and IL-13. Thus, it is widely speculated that the anti-itch efficacy of dupilumab is derived, in part, from its ability to limit signaling of these cytokines directly on sensory neurons (Oetjen et al., 2017; Silverberg et al., 2020). Indeed, it is believed that the investigational anti-IL-13 mAbs lebrikizumab and tralokinumab also derive their anti-itch efficacy, in part, via similar disruption at the neuroimmune interface (Miron et al., 2022; Simpson et al., 2018; Wollenberg et al., 2019). Strikingly, dupilumab may represent a broader anti-itch strategy due to its success in alleviating itch in conditions beyond AD. Indeed, recent pivotal phase 3 clinical trials in CSU and prurigo nodularis (PN) met all primary endpoints including for itch (NCT04180488, NCT04183335), paving the way for FDA approval. The broad success of dupilumab across a number of chronic itch conditions strongly suggests its ability to disrupt itch through its direct effect on pruriceptors.
The development of nemolizumab, a mAb against the IL-31RA receptor subunit, is a unique example of what is now considered a primary anti-itch agent. Consistent with its identification as the original cytokine pruritogen (Cevikbas et al., 2014; Dillon et al., 2004), both phase 2 and phase 3 clinical trials in AD have exhibited more pronounced effects on itch than even skin inflammation (Kabashima et al., 2020; Ruzicka et al., 2017). Further, this agent has already demonstrated efficacy as an anti-pruritic therapeutic in PN as well (Ständer et al., 2020). In further support of the IL-31 pathway’s central role in itch, targeting oncostatin M (OSM) receptor beta (OSMRβ), the co-receptor for IL-31RA, with the mAb vixarelimab has also demonstrated efficacy in phase 2 clinical trials for PN (NCT03816891). Recent studies have shown that OSM itself can sensitize itch neurons to inflammatory pruritogenic stimuli (Pang-Yen and A., 2022). Taken together, blockade of the IL-31/OSM pathway represents one of the clearest examples of how disruption of key neuroimmune itch circuits is a promising therapeutic approach.
Given that signaling of numerous cytokines in immune cells rely on JAK1, and pruriceptors also express this molecule, it was hypothesized that JAK1-selective inhibitors would demonstrate rapid and potent anti-itch efficacy. In murine studies, conditional deletion of JAK1 within sensory neurons resulted in rapid and broad attenuation of itch across multiple itch models (Oetjen et al., 2017). Consistent with these findings, recent phase 3 clinical trials with the JAK1-selective inhibitors abrocitinib and upadacitinib have demonstrated superior anti-itch efficacy in direct comparison to dupilumab in AD (Bieber et al., 2021; Blauvelt et al., 2021). Whether JAK1-selective inhibitors are broadly efficacious, and demonstrate improvements in itch across a variety of chronic pruritic disorders, remains to be determined in future randomized trials.
Beyond simply blocking pruritogens and their signaling, an emerging and potentially broader anti-pruritic strategy utilizes activation of itch-suppressive circuits. Stimulation of the kappa opioid receptor (KOR) results in activation of non-pruriceptive neurons both in the periphery and the spinal cord to suppress itch signals (Figure 1) (Kardon et al., 2014; Munanairi et al., 2018; Snyder et al., 2018). In contrast, stimulation of the mu opioid receptor (MOR) has traditionally been associated with the provocation of itch both centrally and in the periphery (Ko et al., 2004; Melo et al., 2018). Clinical trials are underway in a number of chronic pruritic disorders including AD, PN, uremic/kidney disease-associated pruritus, and cholestatic/liver disease-associated pruritus with the KOR agonist difelikefalin and the KOR agonist/MOR antagonist nalbuphine. Indeed, nalbuphine reported positive data in phase 2/3 clinical trials and difelikefalin FDA-approved in 2021 for uremic pruritus in dialysis patients (Fishbane et al., 2019; Mathur et al., 2017). Further, nalbuphine has demonstrated efficacy in PN (NCT02174419), while difelikefalin exhibited improvement of itch in a subset of patients with mild-to-moderate AD in phase 2 studies (NCT04018027).
The identification of multiple itch-specific molecular targets and their rapid conversion into successful clinical trials serves as a testament to the translational success of itch neuroimmunology. There is increasing appreciation that many of these neuroimmune mechanisms are likely conserved across different organs. Thus, whether similar translational success will be seen in the future for other proinflammatory or neuropathic conditions that trigger neurosensory dysfunction remains an exciting area of future inquiry.
Itch as a Broader Paradigm of Neuroimmunological Disorders
One can appreciate that itch is exemplary of a poorly understood and overlooked sensory disorder with unmet clinical need. However, can breakthroughs in itch biology and successes in clinical trials for chronic pruritus lend insight into other sensory maladies such as cough, dysphagia, and functional bowel disorders? There may indeed be considerable shared and synergistic biology across different sensory paradigms.
Although we present a paradigm in which itch-specific pathways have been identified in mice, and therapeutics in humans exhibit itch-selective properties, there is likely still polymodality of itch neurons. For example, MrgprD, although originally considered an itch receptor, has demonstrated pain-mediating properties in both the skin and gut and has reported mixed itch and pain responses in humans (Klein et al., 2021; Tereza et al., 2018; Wang et al., 2019). Further, transient receptor potential (TRP) channels influence both itch and pain responses (Esancy et al., 2018; Moore et al., 2018). As noted above, scRNA-seq studies in human DRG confirm more polymodality of pruriceptors in humans compared to mice, with more mechanoreceptive genes identified on human neurons (Diana et al., 2022; Klein et al., 2021; Nguyen et al., 2021). These findings may explain, in part, why humans are so susceptible to mechanical itch or alloknesis. In a sense, the original identification of a restricted repertoire of 8 Mrgpr genes in humans, compared to 50 in mice, foreshadowed the likelihood of more polymodality of itch receptors abnd/or neurons in humans (Dong et al., 2001).
Our understanding of sensation is mostly derived from neurons that communicate information about the external environment to the brain (e.g. somatosensory nerves that innervate the skin). Indeed, the Nobel Prize in Medicine was awarded to David Julius and Ardem Patapoutian for their discoveries of how we sense temperature and touch. However, how we sense our internal organs like the lung and gut is less well understood and is largely mediated by the visceral sensory nervous system (Mazzone and Undem, 2016). There is increasing evidence that studying itch biology may provide insight into the key molecular mechanisms of visceral sensation and its dysfunction. The vagus nerve conveys a wide array of internal information from the host and the cell bodies of vagal afferents reside within the jugular and nodose ganglia. Although these nerves have been classically considered distinct from the afferent nerves located in the DRG, new scRNA-seq studies suggest that subsets of jugular/nodose neurons closely resemble itch-sensory neurons (Kupari et al., 2019). Indeed, Mrgprs, originally associated with pruriceptors, appear to have important functions on afferent nerves that innervate the lung and gut as well (Inclan-Rico et al., 2021). Thus, discoveries that are central to itch in the skin may inform diseases beyond itch and even lead to novel therapeutic treatments of pulmonary or gastrointestinal diseases.
One such example is the purinergic P2X3 receptor which, although associated with pain responses, which has been shown to be expressed across multiple pruriceptor subsets (Usoskin et al., 2015). Notably, P2X3 is also widely expressed within jugular and nodose ganglia neurons that innervate the lung (Kollarik et al., 2019). Indeed, in addition to P2X3 antagonists being developed for itch, they are among the first agents to demonstrate improvement of chronic cough symptoms in clinical trials and are currently advancing towards FDA approval (Muccino et al., 2020). Another example of how anti-itch therapeutics can lend insight into other sensory disorders is the expansion of dupilumab from itch in AD to smell in chronic rhinosinusitis with nasal polyps (CRSwNP). Remarkably, in addition to meeting all primary endpoints, the improvement in smell was one of the most robust results in phase 3 clinical trials, with virtually no effect seen in the placebo group (Bachert et al., 2019). Taken together, these studies highlight how itch serves as a model sensation by which we may begin to probe other neuroimmune-based therapies across multiple organs.
Table 1.
Anti-Itch Therapeutics.
| Target | Medication | Disease | Stage |
|---|---|---|---|
| IL4R | Dupilumab | AD | Approved |
| PN, CSU | Phase 3 | ||
| CBP-201 | Phase 2 | ||
|
| |||
| IL13 | Lebrikizumab | AD | Phase 3 |
| Tralokinumab | AD | Approved | |
|
| |||
| IL13RA1 | ASLAN004 | AD | Phase 2 |
|
| |||
| IL31RA | Nemolizumab | AD, PN | Phase 3 |
|
| |||
| OSMR | Vixarelimab | PN | Phase 2 |
|
| |||
| JAK | Abrocitinib | AD | Approved |
| Baricitinib | AD | Pending approval | |
| Delgocitinib | Hand dermatitis | Phase 3 | |
| Ruxolitinib | AD | Approved | |
| Upadacitinib | AD | Approved | |
|
| |||
| OPRK1 | Difelikefalin | AD | Phase 2 |
| Uremic pruritus on dialysis | Approved | ||
| Notalgia paresthetica | Phase 2 | ||
| Nalbuphine | PN | Phase 2/3 | |
| Uremic pruritus | Phase 2 | ||
| Chronic liver disease pruritus | Phase 1 | ||
A list of therapeutics targeting pathways discussed in this article for itch relief. AD – atopic dermatitis, CSU – chronic spontaneous urticaria, PN – prurigo nodularis.
Funding
This work was funded by the National Institutes of Health, National Institutes of Arthritis Musculoskeletal and Skin Disease (NIAMS) R01AR070116 and R01AR077007, National Institute of Allergy and Infectious Disease (NIAID) R21AI167047.
Footnotes
Declaration of Interests
B.S.K. has served as a consultant for AbbVie Inc., Almirall S.A., Amagma, Argenx, AstraZeneca, Bellus Health, Blueprint Medicines, Boehringer Ingelheim Corporation, Bristol-Myers Squibb, Cara Therapeutics, Daewoong Pharmaceutical, Eli Lilly and Company, Guidepoint Global, Janssen Pharmaceuticals, Inc., Incyte Corporation, Kiniksa Pharmaceuticals, LectureLinx, LEO Pharma, Maruho, Novartis, OM Pharma, Pfizer, Sanofi Genzyme, Shaperon, Third Rock Ventures, and Trevi Therapeutics; is a stockholder of Recens Medical and Locus Biosciences; and serves on the scientific advisory boards for Abrax Japan, Granular Therapeutics, Recens Medical, National Eczema Association, Cell Reports Medicine, and Journal of Allergy and Clinical Immunology; holds a patent for the use of JAK inhibitors for chronic pruritus.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrew D, and Craig AD (2001). Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nat. Neurosci. 4, 72–77. [DOI] [PubMed] [Google Scholar]
- Bachert C, Han JK, Desrosiers M, Hellings PW, Amin N, Lee SE, Mullol J, Greos LS, Bosso JV, Laidlaw TM, et al. (2019). Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 394, 1638–1650. [DOI] [PubMed] [Google Scholar]
- Bieber T, Simpson EL, Silverberg JI, Thaçi D, Paul C, Pink AE, Kataoka Y, Chu C-Y, DiBonaventura M, Rojo R, et al. (2021). Abrocitinib versus Placebo or Dupilumab for Atopic Dermatitis. N. Engl. J. Med. 384, 1101–1112. [DOI] [PubMed] [Google Scholar]
- Blauvelt A, Teixeira HD, Simpson EL, Costanzo A, De Bruin-Weller M, Barbarot S, Prajapati VH, Lio P, Hu X, Wu T, et al. (2021). Efficacy and Safety of Upadacitinib vs Dupilumab in Adults With Moderate-to-Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C, Kang HJ, Singh I, Chen H, Zhang C, Ye W, Hayes BW, Liu J, Gumpper RH, Bender BJ, et al. (2021). Structure, function and pharmacology of human itch GPCRs. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal A, Kukova G, Buhl T, Ikoma A, Buddenkotte J, et al. (2014). A sensory neuron–expressed IL-31 receptor mediates T helper cell–dependent itch: Involvement of TRPV1 and TRPA1. J. Allergy Clin. Immunol. 133, 448–460.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana T-F, Stephanie S, R.P. R, Andi W, Vivekanand J, Ishwarya S,M, C. CA, Alexander RJ, A. C, C.B., et al. (2022). Spatial transcriptomics of dorsal root ganglia identifies molecular signatures of human nociceptors. Sci. Transl. Med. 14, eabj8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, Presnell SR, Haugen HS, Maurer M, Harder B, Johnston J, et al. (2004). Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat. Immunol. 5, 752–760. [DOI] [PubMed] [Google Scholar]
- Dong X, Han S. kyou, Zylka MJ, Simon MI, and Anderson DJ (2001). A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. [DOI] [PubMed] [Google Scholar]
- Esancy K, Condon L, Feng J, Kimball C, Curtright A, and Dhaka A (2018). A zebrafish and mouse model for selective pruritus via direct activation of TRPA1. Elife 7, e32036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbane S, Jamal A, Munera C, Wen W, and Menzaghi F (2019). A Phase 3 Trial of Difelikefalin in Hemodialysis Patients with Pruritus. N. Engl. J. Med. 382, 222–232. [DOI] [PubMed] [Google Scholar]
- Forsythe P, and Bienenstock J (2012). The Mast Cell-Nerve Functional Unit: A Key Component of Physiologic and Pathophysiologic Responses. In Chemical Immunology and Allergy, pp. 196–221. [DOI] [PubMed] [Google Scholar]
- Handwerker HO (2014). Itch Hypotheses - From pattern to specificity and to population coding. Itch Mech. Treat. [PubMed] [Google Scholar]
- Handwerker HO, and Schmelz M (2009). Itch without pain—a labeled line for itch sensation? Nat. Rev. Neurol. 5, 640–641. [DOI] [PubMed] [Google Scholar]
- Inclan-Rico JM, Kim BS, and Abdus-Saboor I (2021). Beyond somatosensation: Mrgprs in mucosal tissues. Neurosci. Lett. 748, 135689. [DOI] [PubMed] [Google Scholar]
- Jürgen SH, Patricia D, Erin O, Xinglong G, E.T. W, John B, James I, and H.M. A (2019). Inhibition of natriuretic peptide receptor 1 reduces itch in mice. Sci. Transl. Med. 11, eaav5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabashima K, Matsumura T, Komazaki H, and Kawashima M (2020). Trial of Nemolizumab and Topical Agents for Atopic Dermatitis with Pruritus. N. Engl. J. Med. 383, 141–150. [DOI] [PubMed] [Google Scholar]
- Kardon AP, Polgár E, Hachisuka J, Snyder LM, Cameron D, Savage S, Cai X, Karnup S, Fan CR, Hemenway GM, et al. (2014). Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron 82, 573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kini SP, DeLong LK, Veledar E, McKenzie-Brown AM, Schaufele M, and Chen SC (2011). The impact of pruritus on quality of life: The skin equivalent of pain. Arch. Dermatol. [DOI] [PubMed] [Google Scholar]
- Klein A, Solinski HJ, Malewicz NM, Ieong HF-H, Sypek EI, Shimada SG, Hartke TV, Wooten M, Wu G, Dong X, et al. (2021). Pruriception and neuronal coding in nociceptor subtypes in human and nonhuman primates. Elife 10, e64506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MCH, Song MS, Edwards T, Lee H, and Naughton NN (2004). The Role of Central μ Opioid Receptors in Opioid-Induced Itch in Primates. J. Pharmacol. Exp. Ther. 310, 169 LP–176. [DOI] [PubMed] [Google Scholar]
- Kollarik M, Ru F, and Undem BJ (2019). Phenotypic distinctions between the nodose and jugular TRPV1-positive vagal sensory neurons in the cynomolgus monkey. Neuroreport 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupari J, Häring M, Agirre E, Castelo-Branco G, and Ernfors P (2019). An Atlas of Vagal Sensory Neurons and Their Molecular Specialization. Cell Rep. 27, 2508–2523.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford DJ, Crager SE, Shehzad Z, Smith SB, Sotocinal SG, Levenstadt JS, Chanda ML, Levitin DJ, and Mogil JS (2006). Social Modulation of Pain as Evidence for Empathy in Mice. Science (80-. ). 312, 1967 LP–1970. [DOI] [PubMed] [Google Scholar]
- Leader B, Carr CW, and Chen SC (2015). Pruritus Epidemiology and Quality of Life BT - Pharmacology of Itch. Cowan A, and Yosipovitch G, eds. (Berlin, Heidelberg: Springer Berlin Heidelberg; ), pp. 15–38. [DOI] [PubMed] [Google Scholar]
- Li C-L, Li K-C, Wu D, Chen Y, Luo H, Zhao J-R, Wang S-S, Sun M-M, Lu Y-J, Zhong Y-Q, et al. (2016). Somatosensory neuron types identified by high-coverage single-cell RNA-sequencing and functional heterogeneity. Cell Res. 26, 83–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, et al. (2009). Sensory Neuron-Specific GPCR Mrgprs Are Itch Receptors Mediating Chloroquine-Induced Pruritus. Cell 139, 1353–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur VS, Kumar J, Crawford PW, Hait H, and Sciascia T (2017). A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial of Nalbuphine ER Tablets for Uremic Pruritus. Am. J. Nephrol. 46, 450–458. [DOI] [PubMed] [Google Scholar]
- Mazzone SB, and Undem BJ (2016). Vagal Afferent Innervation of the Airways in Health and Disease. Physiol. Rev. 96, 975–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, and Dong X (2015). Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meixiong J, Anderson M, Limjunyawong N, Sabbagh MF, Hu E, Mack MR, Oetjen LK, Wang F, Kim BS, and Dong X (2019). Activation of Mast-Cell-Expressed Mas-Related G-Protein-Coupled Receptors Drives Non-histaminergic Itch. Immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo H, Basso L, Iftinca M, MacNaughton WK, Hollenberg MD, McKay DM, and Altier C (2018). Itch induced by peripheral mu opioid receptors is dependent on TRPV1-expressing neurons and alleviated by channel activation. Sci. Rep. 8, 15551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron Y, Miller PE, Hughes C, Indersmitten T, Lerner EA, and Cevikbas F (2022). Mechanistic Insights into the Anti-Pruritic Effects of Lebrikizumab, an Anti-IL-13 Monoclonal Antibody. J. Allergy Clin. Immunol. [DOI] [PubMed] [Google Scholar]
- Mishra SK, and Hoon MA (2013). The cells and circuitry for itch responses in mice. Science (80-. ). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C, Gupta R, Jordt S-E, Chen Y, and Liedtke WB (2018). Regulation of Pain and Itch by TRP Channels. Neurosci. Bull. 34, 120–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muccino DR, Morice AH, Birring SS, Dicpinigaitis PV, Pavord ID, Assaid C, Kleijn HJ, Hussain A, La Rosa C, McGarvey L, et al. (2020). Design and rationale of two phase 3 randomised controlled trials (COUGH-1 and COUGH-2) of gefapixant, a P2X3 receptor antagonist, in refractory or unexplained chronic cough. ERJ Open Res. 6, 284–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munanairi A, Liu X-Y, Barry DM, Yang Q, Yin J-B, Jin H, Li H, Meng Q-T, Peng J-H, Wu Z-Y, et al. (2018). Non-canonical Opioid Signaling Inhibits Itch Transmission in the Spinal Cord of Mice. Cell Rep. 23, 866–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MQ, von Buchholtz LJ, Reker AN, Ryba NJ, and Davidson S (2021). Single-nucleus transcriptomic analysis of human dorsal root ganglion neurons. Elife 10, e71752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oetjen LKLK, Mack MRMRMR, Feng J, Whelan TM, Niu H, Guo CJCJCJ, Chen S, Trier AMAM, Xu AZAZAZ, Tripathi SVSV, et al. (2017). Sensory Neurons Co-opt Classical Immune Signaling Pathways to Mediate Chronic Itch. Cell 171, 217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang-Yen T, and H.M. A (2022). Oncostatin M can sensitize sensory neurons in inflammatory pruritus. Sci. Transl. Med. 13, eabe3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal SR (1977). HISTAMINE AS THE CHEMICAL MEDIATOR FOR CUTANEOUS PAIN. J. Invest. Dermatol. 69, 98–105. [DOI] [PubMed] [Google Scholar]
- Ruzicka T, Hanifin JM, Furue M, Pulka G, Mlynarczyk I, Wollenberg A, Galus R, Etoh T, Mihara R, Yoshida H, et al. (2017). Anti–Interleukin-31 Receptor A Antibody for Atopic Dermatitis. N. Engl. J. Med. 376, 826–835. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Bickel A, Handwerker HO, and Torebjörk HE (1997). Specific C-Receptors for Itch in Human Skin. J. Neurosci. 17, 8003 LP–8008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg JI, Kantor RW, Dalal P, Hickey C, Shaunfield S, Kaiser K, Lai J-S, and Cella D (2018). A Comprehensive Conceptual Model of the Experience of Chronic Itch in Adults. Am. J. Clin. Dermatol. 19, 759–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg JI, Yosipovitch G, Simpson EL, Kim BS, Wu JJ, Eckert L, Guillemin I, Chen Z, Ardeleanu M, Bansal A, et al. (2020). Dupilumab treatment results in early and sustained improvements in itch in adolescents and adults with moderate to severe atopic dermatitis: Analysis of the randomized phase 3 studies SOLO 1 and SOLO 2, AD ADOL, and CHRONOS. J. Am. Acad. Dermatol. [DOI] [PubMed] [Google Scholar]
- Simpson EL, Flohr C, Eichenfield LF, Bieber T, Sofen H, Taïeb A, Owen R, Putnam W, Castro M, DeBusk K, et al. (2018). Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: A randomized, placebo-controlled phase II trial (TREBLE). J. Am. Acad. Dermatol. 78, 863–871.e11. [DOI] [PubMed] [Google Scholar]
- Smith ML, Asada N, and Malenka RC (2021). Anterior cingulate inputs to nucleus accumbens control the social transfer of pain and analgesia. Science (80-. ). 371, 153 LP–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder LM, Chiang MC, Loeza-Alcocer E, Omori Y, Hachisuka J, Sheahan TD, Gale JR, Adelman PC, Sypek EI, Fulton SA, et al. (2018). Kappa Opioid Receptor Distribution and Function in Primary Afferents. Neuron 99, 1274–1288.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ständer S, Yosipovitch G, Legat FJ, Lacour J-P, Paul C, Narbutt J, Bieber T, Misery L, Wollenberg A, Reich A, et al. (2020). Trial of Nemolizumab in Moderate-to-Severe Prurigo Nodularis. N. Engl. J. Med. 382, 706–716. [DOI] [PubMed] [Google Scholar]
- Sun Y-GG, and Chen Z-FF (2007). A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 448, 700–703. [DOI] [PubMed] [Google Scholar]
- Sun Y-G, Zhao Z-Q, Meng X-L, Yin J, Liu X-Y, and Chen Z-F (2009). Cellular Basis of Itch Sensation. Science (80-. ). 3 25, 1531 LP–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tereza B, H.J.R. F, Teresa P-B, Julien P, T.M. M, Cleo D, Raffaella BM, Lilian B, Pauline LF, Corinne R, et al. (2018). 5-oxoETE triggers nociception in constipation-predominant irritable bowel syndrome through MAS-related G protein–coupled receptor D. Sci. Signal. 11, eaal2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usoskin D, Furlan A, Islam S, Abdo H, Lönnerberg P, Lou D, Hjerling-Leffler J, Haeggström J, Kharchenko O, Kharchenko PV, et al. (2015). Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 18, 145–153. [DOI] [PubMed] [Google Scholar]
- Wang C, Gu L, Ruan Y, Geng X, Xu M, Yang N, Yu L, Jiang Y, Zhu C, Yang Y, et al. (2019). Facilitation of MrgprD by TRP-A1 promotes neuropathic pain. FASEB J. 33, 1360–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisshaar E, Eckart WU, and Bernhard JD (2015). Historical Background of Itch BT - Pharmacology of Itch. Cowan A, and Yosipovitch G, eds. (Berlin, Heidelberg: Springer Berlin Heidelberg; ), pp. 1–14. [DOI] [PubMed] [Google Scholar]
- Wollenberg A, Howell MD, Guttman-Yassky E, Silverberg JI, Kell C, Ranade K, Moate R, and van der Merwe R (2019). Treatment of atopic dermatitis with tralokinumab, an anti–IL-13 mAb. J. Allergy Clin. Immunol. 143, 135–141. [DOI] [PubMed] [Google Scholar]
- Yang F, Guo L, Li Y, Wang G, Wang J, Zhang C, Fang G-X, Chen X, Liu L, Yan X, et al. (2021). Structure, function and pharmacology of human itch receptor complexes. Nature. [DOI] [PubMed] [Google Scholar]
- Zeisel A, Hochgerner H, Ernfors P, Lo P, Marklund U, Linnarsson S, Johnsson A, Memic F, and Zwan J. Van Der (2018). Molecular Architecture of the Mouse Nervous System. Cell 174, 999–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]


