Abstract
Zirconium-89 (89Zr) has been explored for molecularly targeted positron emission tomography (PET) imaging of various diseases. We synthesized and evaluated a novel chelator (DA-18C6-BHA) for 89Zr. The new chelator is structured on a macrocyclic backbone (1,10-diaza-18-crown-6) and contains hydroxamates as acyclic donor groups. The new chelator ((DA-18C6-BHA) was rapidly labeled with 89Zr under mild conditions. The 89Zr-labeled DA-18C6-BHA complex remained stable in human serum and apotransferrin for 7 days. When challenged with excess EDTA solution, 89Zr-labeled DA-18C6-BHA was shown to hold 89Zr without losing considerable radioactivity to EDTA. The 89Zr-labeled DA-18C6-BHA complex displayed high complex stability in normal mice as evidenced by low bone uptake.
Keywords: Zr-89, Chelator, PET Imaging
Positron-emitting radionuclides used for positron emission tomography (PET) imaging include 18F (t1/2 = 1.83 h, Eβ+ = 0.63 MeV), 64Cu (t1/2 = 12.7 h, Eβ+ = 0.66 MeV, Eγ = 1.35 MeV), 68Ga (t1/2 = 1.12 h, Eβ+ = 1.88 MeV, Eγ = 0.51 MeV), and 89Zr (t1/2 = 78.4 h, Eβ+ = 0.90 MeV, Eγ = 0.91 MeV).1–5 Research efforts have been made on discovery of PET imaging agents with high binding affinity and selectivity to specific biomarkers on tumor cells.1–3 A number of target-specific biomolecules including peptides and antibodies have been labeled with radionuclides for preclinical and clinical evaluations of targeted PET imaging of cancers.5–7
Among the positron-emitting radionuclides in clinical use, a long-lived 89Zr is well suited for PET imaging of molecular targets using an antibody with a long biological half-life.1,5–8 In particular, 89Zr-based PET tracers have a clinical value in pre- and post-treatment imaging and monitoring tumor response in antibody therapy.5–6 89Zr(IV) is a highly charged cation with strong binding with anionic oxygens such as hydroxyl group in bone mineral hydroxylapatite (HA).1,5,9
DFO (desferrioxamine) is a hydroxamate-based chelator in clinical use for the treatment of iron overload diseases including β-thalassemia.10 Given the high binding avidity of the hydroxamates for a hard oxophilic cation, DFO has been also extensively explored for labeling of various biomolecules with 89Zr.8,11 DFO was shown to rapidly form a 6-coordinate complex with Zr(IV), and 89Zr-DFO complex remained intact in human serum.12,13 However, DFO-antibody conjugate labeled with 89Zr has a limited in vivo stability in mice and displayed high bone uptake.12,13 The elevated bone uptake of 89Zr-DFO can be rationalized by high affinity of 89Zr(IV) released from the complex for bone mineral, hydroxylapatite (HA). While research efforts have been made to develop improved chelation chemistry that can sequester the bone-seeking 89Zr with high in vivo stability, DFO remains the standard chelator for clinical use.1,12
In this paper, we report synthesis and evaluation of a novel chelating agent (DA-18C6-BHA) that is built on a macrocyclic backbone (1,10-diaza-18-crown-6) tethered with hydroxamate as an acyclic donor group. The new chelator and DFO were comparatively studied for radiolabeling with 89Zr. The 89Zr-radiolabeled new chelator (89Zr-DA-18C6-BHA) and 89Zr-DFO were comparatively evaluated for complex stability in human serum and apotransferrin and excess EDTA solution and biodistribution profile in normal mice.
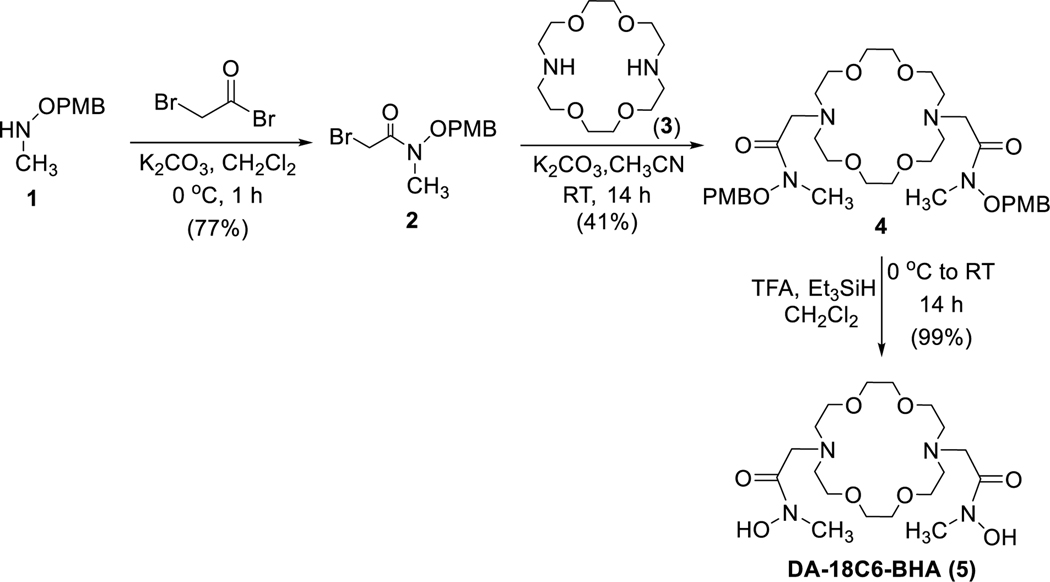
The novel chelator (DA-18C6-BHA) contains diaza-18-crown-6 as a macrocyclic backbone and hydroxamates as tetradentate binding moieties. The four oxygens in the macrocyclic backbone are expected to participate in complexation with 89Zr(IV) and contribute to the formation of an eight-coordinate Zr complex. Synthesis of the new chelator (DA-18C6-BHA) includes reaction of compound 214 as a key precursor molecule with 1,10-diaza-18-crown-6 (3, Scheme 1). Compound 2 was prepared from O-paramethoxybenzyl (PMB)-protected N-methyl hydroxylamine (1)15 by a modified procedure of the reported method.14 Compound 1 was reacted with bromoacetyl bromide in CH3CN at 0 oC to afford compound 2 in 77% isolated yield. Compound 2 was further reacted with 1,10-diaza-18-crown-6 (3) to produce O-PMB protected chelating agent 4 in 41% isolated yield. Deprotection of PMB in 4 was accomplished by reaction of 4 with TFA and triethylsilane to produce the desired chelating agent 5 in nearly quantitative yield.
Scheme 1.
Synthesis of chelator DA-18C6-BHA (5) for Zr(IV)-89
The new chelator (DA-18C6-BHA) and DFO were comparatively evaluated for radiolabeling with 89Zr at room temperature (Supporting Information). The new chelator (DA-18C6-BHA) and DFO rapidly bound to 89Zr and were shown to be effective in binding 89Zr at pH 7 and room temperature. Radiolabeling of DA-18C6-BHA or DFO with 89Zr was nearly complete in 10 min (>98% radiolabeling efficiency). Stability of 89Zr-DA-18C6-BHA and 89Zr-DFO in human serum was determined (Table 1 and Supporting Information). Dissociation of 89Zr from 89Zr-DA-18C6-BHA or 89Zr-DFO in human serum (pH 7.0 and 37 oC) was measured over 7 days. The serum stability data suggest that DA-18C6-BHA and DFO remained inert in serum for 7 days. A minimal amount of 89Zr from 89Zr-DA-18C6-BHA or 89Zr-DFO was released to serum (<1% at 168 h time point).
Table 1.
Complex Stability of 89Zr-DA-18C6-BHA and 89Zr-DFO in human serum (pH 7.0, 37°C, ITLC, duplicate).
|
89Zr-Chelator Complex (%) |
||
|---|---|---|
| Time | DA-18C6-BHA | DFO |
|
| ||
| 0 h | 99.4 ± 0.1 | 99.5 ± 0.5 |
| 24 h | 99.1 ± 0.0 | 99.2 ± 0.1 |
| 48 h | 98.8 ± 0.7 | 99.3 ± 0.3 |
| 72 h | 98.7 ± 0.2 | 98.7 ± 0.8 |
| 168 h | 99.0 ± 0.3 | 99.0 ± 0.5 |
89Zr-DA-18C6-BHA and 89Zr-DFO were evaluated for complex stability in the presence of EDTA (Table 2 and Supporting Information). 89Zr-DA-18C6-BHA or 89Zr-DFO was incubated with a 100-fold molar excess of EDTA. 89Zr-DA-18C6-BHA was shown to have high complex stability in EDTA solution, and only a trace amount of 89Zr (<1%) was transchelated to EDTA. In contrast, a significant amount of 89Zr was dissociated from 89Zr-DFO complex at 168 h-post incubation (>12%).
Table 2.
Complex Stability of 89Zr-DA-18C6-BHA and 89Zr-DFO in EDTA solution (pH 7.0, 37°C, ITLC, duplicate).
|
89Zr-Chelator Complex (%) |
||
|---|---|---|
| Time | DA-18C6-BHA | DFO |
|
| ||
| 0 h | 99.3 ± 0.1 | 99.4 ± 0.1 |
| 1 h | 99.6 ± 0.1 | 98.1 ± 0.3 |
| 24 h | 99.0 ± 0.2 | 92.5 ± 0.4 |
| 48 h | 99.0 ± 0.2 | 89.1 ± 0.2 |
| 168 h | 99.1 ± 0.3 | 87.3 ±1.8 |
89Zr-DA-18C6-BHA and 89Zr-DFO were further evaluated for complex stability in the presence of apotransferrin (Table 3 and Supporting Information). 89Zr-DA-18C6-BHA or 89Zr-DFO was incubated with a 5-fold molar excess of apotransferrin. Both 89Zr-DA-18C6-BHA and 89Zr-DFO complex remained stable in apotransferrin solution (PBS, pH 7.0), and no considerable amount of 89Zr was released to the apotransferrin solution over 7 days.
Table 3.
Complex Stability of 89Zr-DA-18C6-BHA and 89Zr-DFO in apotransferrin solution (pH 7.0, 37°C, ITLC, duplicate).
|
89Zr-Chelator Complex (%) |
||
|---|---|---|
| Time | DA-18C6-BHA | DFO |
|
| ||
| 0 h | 98.8 ± 0.5 | 99.0 ± 0.1 |
| 24 h | 98.3 ± 0.4 | 98.8 ± 0.2 |
| 48 h | 98.0 ± 0.2 | 97.8 ± 0.6 |
| 72 h | 97.9 ± 0.4 | 98.0 ± 0.3 |
| 168 h | 98.8 ± 0.3 | 98.4 ± 0.4 |
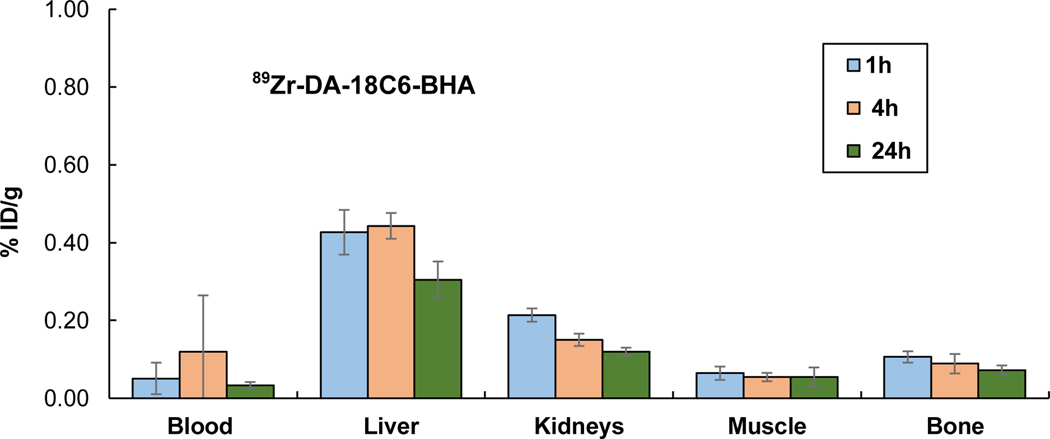
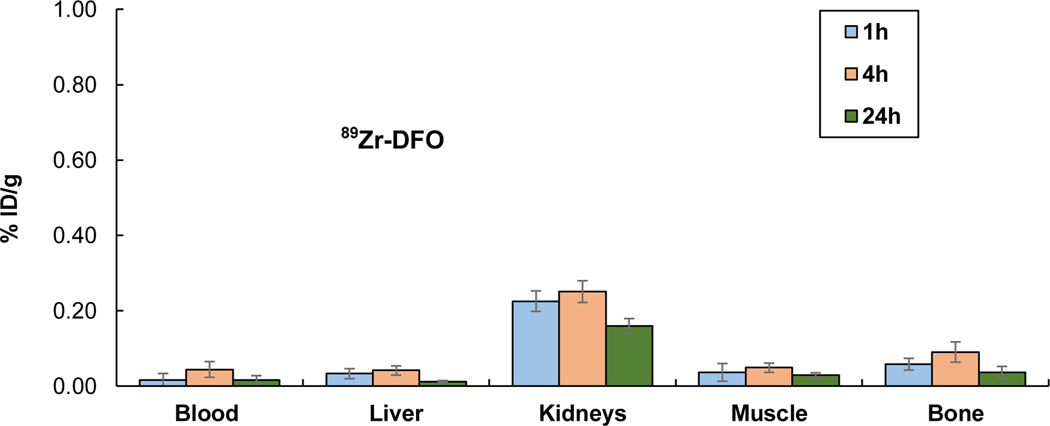
We performed biodistribution studies in CD-1 normal mice (intravenous injection, n = 4) to evaluate in vivo stability of 89Zr-DFO and 89Zr-DA-18C6-BHA. The mice were euthanized at 1 h, 4 h, and 24 h. The selected organs (liver, kidney, muscle, bone) and the blood were harvested, wet weighed, and the radioactivity was measured in a γ-counter (Figures 1 and 2 and Supporting Information). Both 89Zr-DA-18C6-BHA (Figure 1) and 89Zr-DFO (Figure 2) showed rapid blood clearance and low uptake in normal organs (<0.44% ID/g) over 24 hours. 89Zr-DA-18C6-BHA exhibited a negligible level of radioactivity in blood at all time points (≤0.04 %ID/g). The bone and muscle uptake of 89Zr- DA-18C6-BHA remained minimal at all time points (≤0.11% ID/g). 89Zr-DA-18C6-BHA exhibited the highest retention in liver (0.44 %ID/g at 4 h). Renal uptake of 89Zr-DA-18C6-BHA was decreased from 0.21 %ID/g (1 h) to 0.12 %ID/g (24 h). 89Zr-DFO showed a minimal uptake in blood, liver, and muscle at 24 hours (0.02 %ID/g, 0.01 %ID/g, and 0.03 %ID/g, respectively). 89Zr-DFO showed relatively higher accumulation of radioactivity in the kidney at all time points (≤0.25 %ID/g) when compared to other organs. 89Zr-DFO displayed minimal bone uptake which was decreased from 0.09 %ID/g (4 h) to 0.04 %ID/g (24 h). The in vivo data suggest that both 89Zr-DFO and 89Zr-DA-18C6-BHA presented an excellent biodistribution profile in normal mice as evidenced by low uptake in blood and normal organs including bone.
Figure 1.
In Vivo Biodistribution of 89Zr-DA-18C6-BHA in CD-1 mice (n = 4, intravenous injection)
Figure 2.
In Vivo Biodistribution of 89Zr-DFO in CD-1 mice (n = 4, intravenous injection)
In summary, the new chelator (DA-18C6-BHA) built on diaza-18-crown-6 containing two hydroxamate donor groups was synthesized and evaluated. The new chelator rapidly bound to 89Zr, and the corresponding 89Zr-labeled DA-18C6-BHA was shown to display high complex stability in human serum and apotransferrin for 7 days. When challenged by excess EDTA solution, 89Zr-DA-18C6-BHA remained inert and was favorably compared to 89Zr-DFO for complex stability. Both 89Zr–DA-18C6-BHA and 89Zr–DFO were shown to be stable in mice and have favorable biodistribution profiles with low bone uptake. The radiolabeling and in vitro and in vivo complex stability data clearly demonstrate that DA-18C6-BHA is an efficient chelator for 89Zr radiolabeling.
Supplementary Material
Acknowledgement.
We acknowledge the financial support from the National Institutes of Health (R01CA112503 and R01EB029800 to Hyun-Soon Chong). Molecular graphics image was produced using the UCSF Chimera package from the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco (supported by NIH P41 RR-01081).
Footnotes
Supporting material. Materials and methods and copies of NMR spectra and TLC chromatograms for assessment of radiolabeling reaction kinetics and serum stability and EDTA challenge and in vivo biodistribution data. This material is available free of charge online.
References
- (1).Heskamp S; Raavé R; Boerman O; Rijpkema M; Goncalves V; Denat F.89Zr-immuno-positron emission tomography in oncology: state-of-the-art 89Zr radiochemistry. Bioconjugate Chem. 2017, 28, 2211–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Wadas TJ; Wong EH; Weisman GR; Anderson CJ Coordinating radiometals of copper, gallium, indium, yttrium, and zirconium for PET and SPECT imaging of disease. J. Chem. Rev 2010, 110, 2858–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Fletcher JW; Djulbegovic B; Soares HP; Siegel BA; Lowe VJ; Lyman GH; Coleman RE; Wahl R; Paschold JC; Avril N; Einhorn LH; Suh WW; Samson D; Delbeke D; Gorman M; Shields AF Recommendations on the use of 18F-FDG PET in oncology. J. Nucl. Med 2008, 49, 480–508. [DOI] [PubMed] [Google Scholar]
- (4).Koselnik TI; Orvig C.Radioactive main group and rare earth metals for imaging and therapy. Chem. Rev 2019, 119, 902–956. [DOI] [PubMed] [Google Scholar]
- (5).Nayak TK; Brechbiel MW Radioimmunoimaging with longer-lived positron-emitting radionuclides: Potentials and challenges. Bioconjugate Chem. 2009, 20, 825–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).McKnight Brooke N; Viola-Villegas Nerissa T.89Zr-ImmunoPET companion diagnostics and their impact in clinical drug development. J. Labelled Comp Radiopharm 2018, 61, 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Fay R; Holland JP The impact of emerging bioconjugation chemistries on radiopharmaceuticals. J. Nucl. Med 2019, 60, 587–591. [DOI] [PubMed] [Google Scholar]
- (8).Vosjan MJ; Perk LR; Visser GW; Budde M; Jurek P; Kiefer GE; van Dongen GA Conjugation and radiolabeling of monoclonal antibodies with zirconium-89 for PET imaging using the bifunctional chelate p-isothiocyanatobenzyl-desferrioxamine. Nat Protocol. 2010, 5, 739–743. [DOI] [PubMed] [Google Scholar]
- (9).Abou DS; Ku T; Smith-Jones PM In vivo biodistribution and accumulation of 89Zr in mice. Nucl Med. Biol 2011, 38, 675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Kalinowski Danuta S. and Des Richardson R. The evolution of iron chelators for the treatment of iron overload disease and cancer. Pharmacol. Rev 2005, 57, 547–583. [DOI] [PubMed] [Google Scholar]
- (11).Meijs WE; Herscheid JDM; Haisma HJ; Pinedo HM Evaluation of desferal as a bifunctional chelating agent for labeling antibodies with Zr-89. Int. J. Appl. Instrum A 1992, 43, 1443–1447. [DOI] [PubMed] [Google Scholar]
- (12).Raavé R; Sandker G; Adumeau P; Jacobsen CB; Mangin F; Meyer M; Moreau M; Bernhard C; Da Costa L; Dubois A; Goncalves V; Gustafsson M; Rijpkema M; Boerman O; Chambron J-C; Heskamp S; Denat F.Direct comparison of the in vitro and in vivo stability of DFO, DFO* and DFOcyclo* for 89Zr-immunoPET. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1966–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Deri MA, Ponnala S; Zeglis BM; Pohl G; Dannenberg JJ; Lewis JS; Francesconi LC Alternative chelator for 89Zr radiopharmaceuticals: radiolabeling and evaluation of 3,4,3-(LI-1,2-HOPO). J. Med. Chem 2014, 57, 4849–4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Ait-Mohand S; Denis C; Tremblay G; Paquette M; Guérin B.Development of bifunctional chelates bearing hydroxamate arms for highly efficient 64Cu radiolabeling. Org. Lett, 2014, 16, 4512–4515. [DOI] [PubMed] [Google Scholar]
- (15).Wencewicz TA; Yang B; Rudloff JR; Oliver AG; Miller MJ N−O Chemistry for Antibiotics: Discovery of N-Alkyl-N-(Pyridin-2-yl)hydroxylamine scaffolds as selective antibacterial agents using nitroso Diels-Alder and Ene chemistry. J. Med. Chem 2011, 54, 6843–6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.