Abstract
Objectives:
Arginine is an amino-acid supplement and precursor for nitric-oxide synthesis, which affects various biologic processes. The objective of this study was to determine the effects of arginine supplementation on growth hormone (GH) and metabolic parameters.
Methods:
Thirty physically active, healthy men (age 18–39 y; body mass index: 18.5–25 kg/m2) were randomized in a double-blind, placebo-controlled, crossover trial. Arginine (10 g) and placebo (0 g) beverages were consumed after an overnight fast. Blood samples were collected at baseline and 1.5, 3.0, and 24 h after supplementation. The primary outcomes were serum GH and metabolomics. Also, amino acids, glucose, insulin, triacylglycerols, thyroid hormones, testosterone, cortisol, dehydroepiandrosterone, and mood state were assessed. Individuals with detectable increases in GH were analyzed separately (responders: n = 16; < 0.05 ng/mL at 1.5 h). Repeated-measure analyses of variance estimated the treatment effects at each timepoint.
Results:
Arginine levels increased at 1.5 h (146%) and 3.0 h (95%; P ≤ 0.001) and GH (193%) and thyroid-stimulating hormone (TSH; 10%) levels at 24 h (P < 0.05) after arginine versus placebo consumption. Arginine versus placebo increased glucose levels at 1.5 h (5%) and 3.0 h (3%; P ≤ 0.001). Arginine versus placebo did not affect other dependent measures, including mood state (P > 0.05), but changes in the urea, glutamate, and citric-acid pathways were observed. Among responders, arginine versus placebo increased GH at 1.5 h (37%), glucose at 1.5 h (4%) and 3.0 h (4%), and TSH at 24 h (9%; P < 0.05). Responders had higher levels of benzoate metabolites at baseline and 1.5 h, and an unknown compound (X-16124) at baseline, 1.5 h, and 24 h that corresponds to a class of gut microbes (P < 0.05).
Conclusions:
Arginine supplementation modestly increased GH, glucose, and TSH levels in younger men. Responders had higher benzoate metabolites and an unknown analyte attributed to the gut microbiome. Future studies should examine whether the increased prevalence of these gut microorganisms corresponds with GH response after arginine supplementation.
Keywords: Protein, Amino acids, Growth hormone Benzoate, Vascular function, Urea cycle
Introduction
More than half of U.S. adults and nearly 90% of young male athletes take a dietary supplement [1–3]. One specific amino acid found in protein foods that is supplemented in individuals attempting to improve their physical function is arginine, which is a precursor to nitric oxide (NO) and regulates peripheral blood flow [4,5]. Arginine is a conditionally essential amino acid, and can be synthesized from citrulline.
The no observed adverse event level for arginine is 30 g/d [6], and the average dietary intake is approximately 4.4 g/d [7]. For protein, the acceptable macronutrient distribution range is 10% to 35% of calories, and the recommended dietary allowance is 0.80 g of protein per kg of body weight. However, this level of protein is not sufficient during exposure to various types of metabolic stressors, and a greater amount of protein is required during these conditions [8,9]. Most research has focused on the health outcomes of protein intake, but limited data are available on the metabolic effects of individual amino acids. Thus, this study focused on the effects of an acute dose of the amino acid arginine on metabolism, because its endocrine effects could alter physical and mental functions [10].
One potentially positive effect of arginine is the enhancing release of NO, which may regulate vascular tone and cardiovascular homeostasis [4]. Arginine may function as an ergogenic aid by modifying a variety of functions, including protein synthesis, glucose homeostasis, and muscle function [11]. Additionally, oral arginine may acutely increase plasma growth hormone (GH) levels [12–14], but has been used as a test for healthy levels of GH secretion when administered via intravenous infusion [15]. GH can increase secretion of insulin-like growth factor-1 [16], which has positive effects on muscle mass in adults [17]. Thus, increasing levels of arginine intake may potentially be beneficial to preserve muscle mass and quality of life [18].
Oral arginine of 5 g and 9 g has been reported to elicit significant increases in GH levels in healthy young men [19]. A higher area under the curve for GH was observed after the 9 g dose of arginine in that study, and supports the use of higher arginine doses. The objective of the current investigation was to determine the effects of acute arginine supplementation on GH levels, endothelial function, appetite, and mood in younger, healthy men. Additionally, this study examined the effects of arginine on the serum metabolomic profile, and provides new information regarding the endocrine and cardiovascular responses to this amino acid in younger, healthy men. New information will help elucidate the mechanism by which arginine has previously been shown to increase GH levels. The hypothesis was that arginine supplementation increases GH levels compared with placebo.
Methods
This study was conducted according to the guidelines of the Declaration of Helsinki. All participants were given verbal and written explanations about the study, provided signed informed consent, and received a monetary stipend. The study was approved by both the Pennington Biomedical institutional review board and U.S. Army Medical Research and Development Command, Human Research Protection Office. The study was registered at ClinicalTrials.gov (NCT 03409380).
Participants
Participants were healthy men, age 18 to 39 y, with a normal body mass index (18.5–25 kg/m2). All participants were physically active for at least 2 d/wk, and willing to refrain from alcohol and dietary supplement use for the duration of the study. The exclusion criteria included human immunodeficiency virus/acquired immunodeficiency syndrome infection, uncontrolled cardiovascular disease, type I or II diabetes, eating disorders, non-normal sleeping patterns (i.e., nightshift worker), use of nicotine or tobacco products, heavy caffeine use (≥350 mg caffeine/d), or protein wasting disease. Participants could not be taking medication that affected fluid balance, metabolism, or body weight.
Study design
This study was a double-blind, randomized, controlled, crossover trial examining acute arginine versus placebo supplementation performed at the Pennington Biomedical Research Center in Baton Rouge, Louisiana. A biostatistician (RAB) used a block randomization of size 4 and random number procedure in SAS, version 9.4, to order the two sets of treatments. Participants initially visited the center for a screening visit. If they volunteered to participate and met the inclusion/exclusion criteria, the participants underwent a washout period (≥14 d) without alcohol and nutritional supplement use, if necessary. Then, participants returned to the center for 2 d of food provision and the first supplement, followed by a washout period (≥7 d). Finally, participants returned for 2 d of food provision and the second supplement.
Screening visit
All participants underwent a screening visit to determine eligibility. At the screening visit, medical history information was collected. Medications, including supplement use and caffeine intake, were assessed. Anthropometrics, including height, weight, waist and hip circumference, and blood pressure, were obtained. For screening purposes, a fasting blood sample was obtained to measure glucose, triacylglycerol, high density lipoprotein cholesterol, and total cholesterol levels, as well as standard clinical indicators of liver and kidney function and a complete blood count.
Supplements
The arginine supplement contained 10 g of arginine, as well as 2.8 g of cranberry-pomegranate–flavored powder, 6.6 g of lemonade–flavored powder, 3.5 g of citric acid, and 0.6 g of lemon-lime–flavored powder. The placebo had 0 g of arginine, 2.5 g of cranberry-pomegranate–flavored powder, 5.5 g of lemonade–flavored powder, 0.5 g of citric acid, and 0.3 g of lemon-lime–flavored powder. Both powdered supplements were mixed with approximately 355 mL of water and chilled. Pilot testing confirmed that the cranberry and lemonade beverages could not be reliably distinguished. Participants had 5 min to consume the beverage from the time of the first sip.
Study visits
At the study visits, participants arrived after a 10 h to 12 h fast, and were well rested. Fasting body weight was obtained, and baseline blood sampling was initiated between 07:30 h and 08:30 h. For each participant, each supplement (or period) blood sampling started at the same time. Blood samples were collected at baseline (fasting), 1.5 h, 3.0 h, and 24 h after supplementation. Blood was collected in vacutainers for serum, sodium heparinized plasma, and ethylenediaminetetra-acetic-acid-treated plasma. After the baseline (fasting) sample was drawn, the supplement was consumed. Peripheral arterial tonometry (PAT) was performed after the 1.5 h blood draw and questionnaire administration.
Blood parameters
Serum GH, prolactin, insulin, thyroid-stimulating hormone (TSH), triiodothyronine, thyroxine, sex hormone binding globulin, testosterone and dehydroepiandrosterone, and cortisol were measured on a Siemens Immulite 2000 using immunoassay with chemiluminescent detection. Serum glucose and triacylglycerols were measured on a Beckman Coulter DXC600 using an oxygen electrode and blanked timed endpoint, respectively. Amino acids, including citrulline, were measured using heparinized plasma on an Agilent Technologies HPLC 1100 using HPLC with fluorescent detection. Frozen serum was sent to Metabolon, Inc. (Durham, NC) for analysis. A metabolomic analysis was conducted using standardized procedures [20].
Diet
Isocaloric diets were provided to participants for consumption for 3 d for each supplementation period. The first 2 d were for consumption preceding each supplement and day 3 was for postsupplement consumption (but before 24 h timepoint). Day 3 was isocaloric to other food provision days, but consumed in a shorter time span. Energy needs were determined by estimated resting energy expenditure × 1.7 physical activity level [21]. The menu provided 12.5% protein, 30% fat, and 57.5% carbohydrate. Arginine intake was 3 to 5 g/d during the feeding periods. The first meal (breakfast the first 2 d and lunch day 3) was consumed on site, but the remaining meals were packaged and eaten at home.
Psychological assessments
Several questionnaires were administered at baseline, as well as 1.5 h, 3.0 h,6.0 h, and 24 h after supplementation.
Profile of mood states
The profile of mood states (POMS) is a 65-item inventory of self-reported mood states that is sensitive to a wide variety of nutritional manipulations, including undernutrition and environmental factors (e.g., sleep loss and subclinical doses of various drugs) [22–25]. Participants rated each of the 65 mood-related adjectives on a five-point scale in response to the question “how are you feeling right now?” The adjectives factor into six mood subscales (tension/anxiety, depression/ dejection, anger/hostility, vigor/activity, fatigue/inertia, and confusion/bewilderment). The POMS has six subscales and 65 questions: Tension (score range, 0–36), depression (score range, 0–60), anger (score range, 0v32), confusion (score range, 0–28), vigor (score range, 0–32), fatigue (score range, 0v28), and a total mood disturbance score (score range, –32 to 200) [26].
Visual analogue scale for self-reported ratings of appetite
The visual analogue scale assessed average ratings of satiety that participants experienced. Its validity and reliability to assess cognitive states related to energy intake have been demonstrated [27].
Peripheral arterial tonometry
Endothelial function was assessed after the 1.5 h blood sample using an Endo-PAT 2000 device (Itamar Medical Ltd., Caesarea, Israel) to examine endothelial function in response to NO release generated by arterial occlusion [28]. The measurement has been previously described [29]. During the measurement, participants were in the supine position, with the hands at the level of the heart and fingers hanging freely. Fingertip probes were placed on both index fingers, and the amplitude of pulse waves was detected and recorded. After an approximate 10-min baseline measurement, arterial flow was occluded using a cuff on the non-dominant arm. The cuff was inflated to 60 mmHg above systolic pressure (but not <200 mmHg), and increased as needed to occlude blood flow. After 5 min of occlusion, the cuff was rapidly deflated to allow reactive hyperemia to occur. Pulse wave amplitudes were recorded again for at least 5 min.
Endo-PAT software compared the arterial pressure ratio in the two fingers before and after occlusion. The reactive hyperemia index (RHI), a ratio of the average pulse wave amplitude measured over 60 s starting 1 min after cuff deflation to the average pulse wave amplitude measured at baseline, was also calculated. The arm that was not occluded served as a control, and the ratio was corrected for changes in the systemic vascular tone. The RHI is the post-to-preocclusion PAT signal in the occluded arm relative to the same ratio in the control arm, corrected for baseline vascular tone of the occluded arm. The augmentation index (AI), a measure of arterial stiffness calculated based on a pulse wave analysis of the signal measured by the EndoPAT device, was calculated. Also, AI.75 is the augmentation index (%) normalized to heart rate (75 bpm).
Metabolomics
Blood samples collected at 0 h, 1.5 h, and 24 h were used for the metabolomic analysis. Samples were processed immediately, and plasma was isolated and stored at −80°C until shipped to Metabolon, Inc. for analysis. The untargeted metabolomic analysis procedures were conducted as described previously [30–32]. Briefly, a reverse phase/ultraphase liquid chromatography–tandem mass spectrometry with positive and negative ion electrospray ionization and hydrophilic interaction liquid chromatography/ultraperformance liquid chromatography-tandem mass spectrometry with negative ion electrospray ionization were conducted for the metabolomic analyses. A Thermo Scientific Q-Exactive high resolution/accurate mass spectrometer interfaced with a heated electrospray ionization source, and an Orbitrap mass analyzer operated at 35 000 mass resolution. Dynamic exclusion was used during the mass spectrometry analysis, and the scan range covered 70 to 1000 mass-to-charge ratio.
Raw data were extracted, peak-identified, and quality-control processed. Peaks were quantified using the area under the curve. Compounds were identified by comparison with library entries of purified standards or recurrent unknown entities. Biochemical identifications were based on the retention index within a narrow retention-index window of the proposed identification, accurate mass match with the library ±10 ppm, and the tandem mass spectrometry forward and reverse scores between the experimental data and authentic standards. Samples were quality controlled and curated using software developed at Metabolon, Inc. to ensure high-quality data for the statistical analysis. A pathway enrichment analysis was conducted using MetaboAnalyst (version 5.0) to determine the metabolic pathways involved from significant time × treatment interactions.
Statistical analysis
Power calculation
Based on a previous study [19], 30 participants were needed to be powered at 80% with α = 0.05 to detect a difference of 98 min μg/L of growth hormone between arginine and placebo treatments. Also, the Metabolon, Inc. recommended power was approximately 25 to 40 samples per group. However, fewer samples may be needed if taking repeated samples from the same individual.
Data analyses
Descriptive statistics were performed to characterize participants. Continuous variables were summarized using means and standard deviations. Categorical variables were summarized by counts/frequencies. Data were checked for normality. A mixed effect model was used to compare the differences between the two supplements (treatments for statistical purposes) at 1.5 h, 3.0 h, and 24 h, and accounted for the crossover study design. For variables that have nondetectable values, a Wilcoxon test was used to compare the two groups accordingly, while taking the crossover design into account [33]. Carryover effects were tested before the main comparisons were performed. The intent-to-treat principle was applied in all analyses.
A subgroup analysis for responders was performed. The responder analysis was based on whether there were detectable levels of GH in the immunoassay (with ≤ 0.05 ng/mL considered as nondetected) at the 1.5-h timepoint after only the arginine supplement. Responders had GH levels < 0.05 ng/mL at the 1.5-h timepoint of the arginine supplement. This determined the split for this analysis throughout the results, and examined these 16 responders versus the same participants in the placebo supplement. Tukey’s honestly significant difference test was used to compare the two groups at each timepoint. P ≤ 0.05 was considered significant.
Similar models were used for all secondary outcomes, and all model assumptions were met for the statistical analyses. Multiple testing adjustment was not performed. The proc mixed procedure of the SAS software (version 9.4; SAS Institute, Cary, NC) and the base package of the R software (version 3.3.2; R Core Team, Vienna, Austria) were used for the statistical analysis and data representation. Metabolomic data were analyzed by Metabolon, Inc. A linear mixed model was used to identify significant (P < 0.05; q < 0.05) metabolomic interactions between the supplements (arginine vs. placebo) and time (0 h, 1.5 h, 24 h). Q was adjusted for the false discovery rate. Significant interactions were followed with pairwise contrasts to determine metabolomic differences (P < 0.05) between the supplements. An exploratory analysis of variance (P < 0.05; q < 0.20) with pairwise analysis-of-variance contrasts was conducted to determine metabolomic differences between responders and nonresponders.
Results
Participants
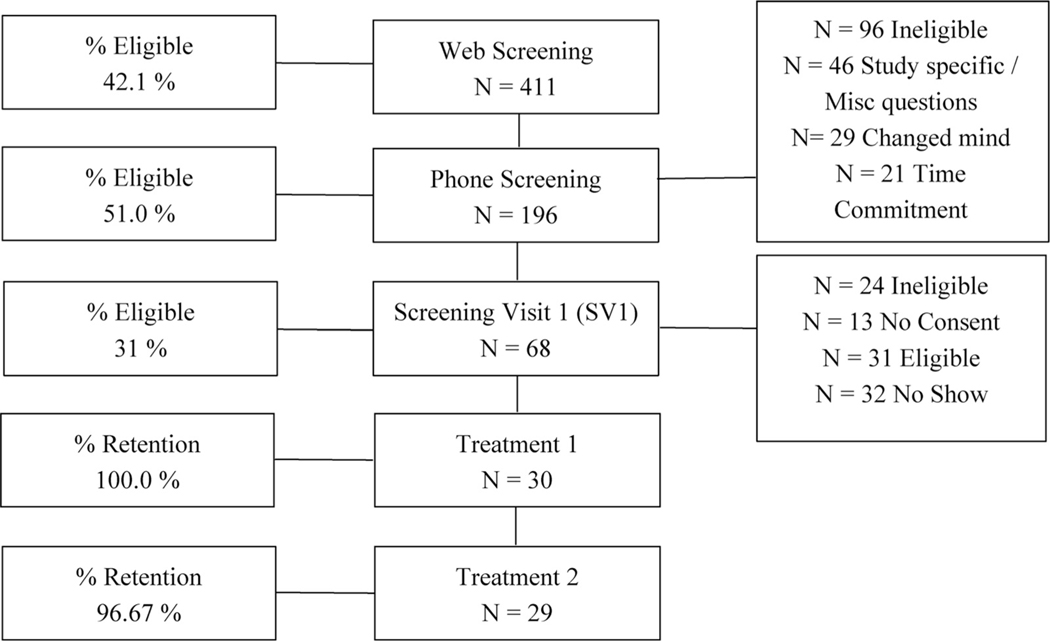
Thirty participants enrolled in the study, and 29 participants completed both study periods (Fig. 1). Participant characteristics are shown in Table 1. No differences were seen in baseline demographic characteristics and anthropometrics in responders versus nonresponders (data not shown; P > 0.05). Also, body weight did not differ between visits.
Fig. 1.
CONsolidated Standards of Reporting Trials (CONSORT) diagram. Not all persons performed Web Screening. N = 173 eligible Web Screenings phone screened. N = 23 just performed Phone Screening. Study was performed from February 2018 till November 2018 in Baton Rouge, Louisiana.
Table 1.
Subject characteristics
| Age, y | 26.1 ± 5.8 |
| Race, n | |
| Caucasian | 21 |
| Other | 9 |
| Body mass index, kg/m2 | 22.6 ± 1.6 |
| Waist circumference, cm | 78.7 ± 6.3 |
| Hip circumference, cm | 91.1 ± 6.0 |
| Waist-to-hip ratio | 0.85 ± 0.05 |
| Systolic blood pressure, mmHg | 115 ± 9 |
| Diastolic blood pressure, mmHg | 71 ± 6 |
| Resting heart rate, bpm | 62 ± 7 |
Data are mean ± standard deviation, except for race.
Arginine versus placebo analysis
GH was significantly higher at 24 h after arginine compared with placebo supplementation (0.41 ± 0.85 vs. 0.14 ± 0.28 ng/mL; P < 0.05). Glucose was higher at 1.5 h and 3.0 h after arginine versus placebo supplementation (Table 2). TSH was higher at 24 h after arginine supplementation compared with the placebo supplement. Other neuroendocrine parameters and timepoints were not different (data not shown; P > 0.05).
Table 2.
Effects of arginine and placebo on biomarkers in young, healthy men after acute supplementation (N = 29)
| Biomarkers | Time × treatment Interaction P-value | Time, h | Arginine |
Placebo |
|
|---|---|---|---|---|---|
| Estimate (95% CI) | Estimate (95% CI) | P-value | |||
|
| |||||
| Glucose, mg/dL | < 0.001 | 0 | 86.4 (84.3–88.4) | 85.4 (83.3–87.5) | 0.238 |
| 1.5 | 89.2 (87.2–91.1) | 85.2 (83.3–86.9) | 0.001 | ||
| 3.0 | 88.4 (86.4–90.4) | 85.48 (83.8–87.2) | 0.001 | ||
| 24 | 88.9 (86.8–90.9) | 89.87 (87.8–91.9) | 0.228 | ||
| Thyroid-stimulating hormone, μIU/mL | 0.043 | 0 | 2.24 (1.93–2.55) | 2.07 (1.76–2.38) | 0.079 |
| 1.5 | 1.64 (1.33–1.95) | 1.57 (1.26–1.88) | 0.480 | ||
| 3.0 | 1.61 (1.30–1.92) | 1.49 (1.18–1.80) | 0.221 | ||
| 24 | 2.19 (1.83–2.55) | 1.99 (1.67–2.32) | 0.035 | ||
| Isoleucine, μmol/L | < 0.001 | 0 | 77.5 (72.7–82.3) | 75.7 (70.9–80.5) | 0.434 |
| 1.5 | 60.9 (56.1–65.7) | 69.2 (64.4–74.1) | 0.001 | ||
| 3.0 | 58.3 (53.5–63.1) | 65.2 (60.4–70.0) | 0.003 | ||
| 24 | 88.8 (84.0–93.7) | 85.78 (81.0–90.6) | 0.177 | ||
| Leucine, μmol/L | 0.002 | 0 | 144.0 (135.7–152.3) | 144.6 (136.4–152.9) | 0.875 |
| 1.5 | 121.0 (112.7–129.3) | 135.9 (127.7–144.2) | 0.001 | ||
| 3.0 | 120.0 (111.7–128.3) | 133.4 (125.1 −141.7) | 0.001 | ||
| 24 | 152.4 (144.1–160.7) | 148.4 (140.1–156.7) | 0.321 | ||
| Phenylalanine, μmol/L | 0.025 | 0 | 58.0 (54.56–61.5) | 58.8 (55.3–62.3) | 0.658 |
| 1.5 | 50.1 (46.7–53.6) | 56.9 (53.4–60.4) | 0.001 | ||
| 3.0 | 49.9 (46.4–53.4) | 55.00 (51.5–58.5) | 0.005 | ||
| 24 | 60.6 (57.1–64.0) | 60.90 (57.4–64.4) | 0.849 | ||
| Valine, μmol/L | 0.026 | 0 | 285.4 (269.9–301.0) | 285.8 (270.3–301.4) | 0.961 |
| 1.5 | 253.5 (237.9–269.1) | 274.9 (259.3–290.5) | 0.010 | ||
| 3.0 | 247.4 (231.8–263.0) | 264.9 (249.3–280.5) | 0.035 | ||
| 24 | 307.2 (291.6–322.7) | 297.1 (281.6–312.7) | 0.225 | ||
| Glutamic acid, μmol/L | 0.018 | 0 | 53.2 (47.8–58.7) | 45.16 (39.7–50.6) | 0.038 |
| 1.5 | 59.4 (54.0–64.9) | 42.81 (37.4–48.3) | 0.001 | ||
| 3.0 | 44.3 (38.9–49.8) | 41.62 (36.2–47.1) | 0.484 | ||
| 24 | 39.4 (33.9–44.8) | 38.90 (33.4–44.4) | 0.900 | ||
| Arginine, μmol/L | < 0.001 | 0 | 111.2 (100.7–121.7) | 110.7 (100.2–121.2) | 0.937 |
| 1.5 | 271.6 (261.1–282.1) | 110.6 (100.1–121.2) | 0.001 | ||
| 3.0 | 209.3 (198.7–219.8) | 107.1 (96.54–117.6) | 0.001 | ||
| 24 | 130.7 (120.2–141.2) | 119.1 (108.6–129.6) | 0.061 | ||
CI, confidence interval
Data are presented as estimate and 95% CI. A mixed effect model was used to model the distributions of the biomarkers, taking into account correlations among the measurements. Biomarkers that do not have significant treatment-by-time interaction were not presented. Tukey’s honestly significant difference test was performed to compare the two groups at individual timepoints. Treatment is supplement.
Circulating levels of arginine increased with the arginine versus placebo supplement at 1.5 h and 3 h (P < 0.001). Citrulline did not have a significant time-by-treatment interaction. Leucine, isoleucine, and valine levels decreased at 1.5 h and 3.0 h in the arginine versus placebo periods (P < 0.05). Histidine, cysteine, methionine, threonine, tyrosine, alanine, glutamine, lysine, glycine, and asparagine levels were not different at any timepoint for arginine versus placebo supplements.
PAT outcomes are shown in Table 3. RHI and AI were not different between the arginine and placebo supplements.
Table 3.
Effects of acute arginine versus placebo supplementation on peripheral arterial tonometry in young, healthy men (N = 29)
| Arginine | Placebo | P-value | |
|---|---|---|---|
|
| |||
| Reactive hyperemia index | 22.5 (1.99−2.51) | 2.26 (2.07−2.45) | 0.922 |
| Augmentation index | −10.01 (−14.72 to −5.30) | −12.76 (−19.99 to −5.23) | 0.118 |
| Augmentation index.75* | −20.15 (−24.72 to −15.58) | −22.55 (−29.24 to −15.86) | 0.098 |
Data are presented as estimate and 95% confidence interval.
Augmentation index percentage normalized to heart rate (75 bpm)
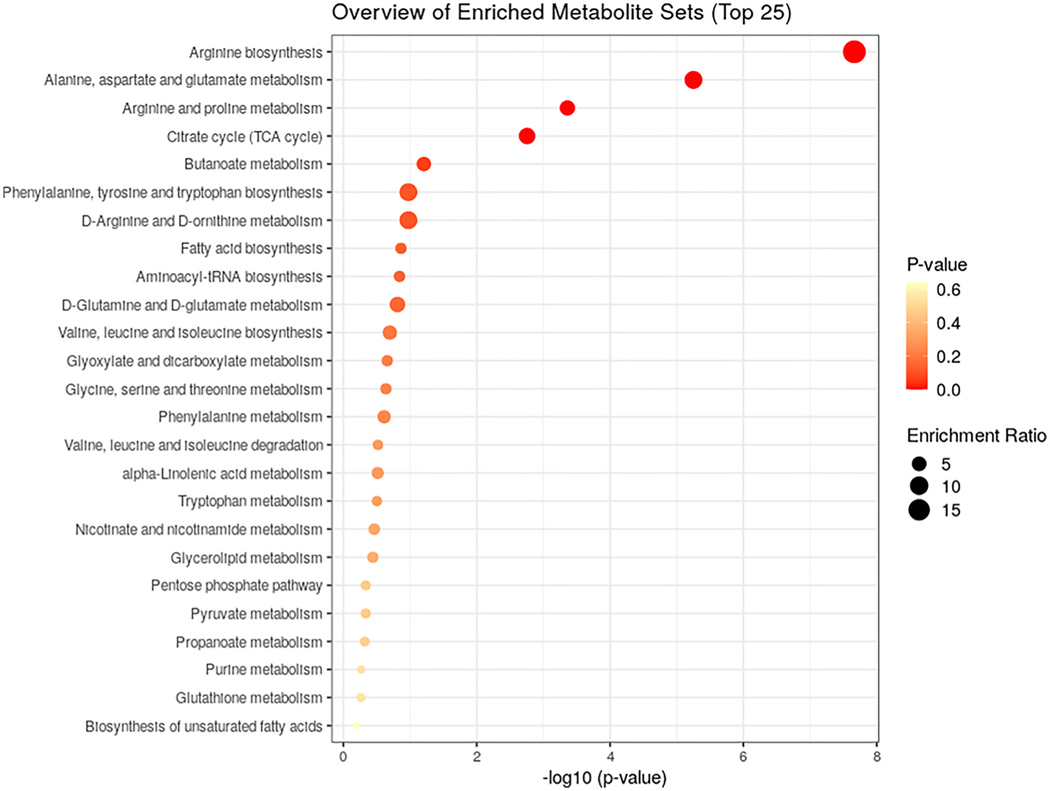
Arginine supplementation resulted in a shift in metabolomic profiles identified by significant interactions (treatment × time). Of the 1286 metabolytes, 93 were altered after arginine supplementation (q < 0.05; Table 4). The metabolomic effects of arginine were predominately found at 1.5 h, with a few differences found at 24 h, and are consistent with the pattern of plasma arginine observed as arginine levels peaked at 1.5 h. Metabolomics demonstrated that many analytes in the urea cycle, arginine, and proline subpathways (i.e., arginine, argininosuccinate, and ornithine) were elevated after arginine supplementation (Table 4). Additionally, arginine supplementation decreased the levels of some amino acids (i.e., isoleucine and leucine), medium- (caprate [10:0], laurate [12:0]) and long-chain fatty acids (myristoleate [14:1ώ5], myristate [14:0], and stearidonate), and increased several tricarboxylic acid cycle intermediates (α-ketoglutarate, citrate, and succinate). We also conducted a pathway enrichment analysis to determine the pathways enriched from the time × treatment interactions (Fig. 2). These findings suggest significant enrichment in arginine biosynthesis; the alanine, aspartate, and glutamate metabolism; and arginine and proline metabolism.
Table 4.
Metabolomics differences after acute arginine versus placebo supplementation in young, healthy men (N = 29)
| Subpathway | Biochemical name | Treatment:time interaction |
0 hr P-value | 1.5 hr P-value | 24 hr P-value | Arginine placebo |
|||
|---|---|---|---|---|---|---|---|---|---|
| P-value | q-value | 0 hr | 1.5 hr | 24 hr | |||||
|
| |||||||||
| Ascorbate and aldarate metabolism | Ascorbic acid 3-sulfate* | ≤ 0.001 | 0.000 | ≤ 0.823 | ≤ 0.001 | ≤ 0.191 | 1.01 | 1.54 | 1.09 |
| Oxalate (ethanedioate) | ≤ 0.001 | 0.000 | ≤ 0.586 | ≤0.001 | ≤ 0.170 | 1.03 | 1.29 | 1.08 | |
| Threonate | ≤ 0.001 | 0.000 | ≤ 0.656 | ≤ 0.001 | ≤ 0.168 | 1.02 | 1.31 | 1.09 | |
| Bacterial/fungal | Lactobacillic acid | ≤ 0.004 | 0.049 | ≤ 0.759 | ≤ 0.016 | ≤ 0.322 | 1.03 | 0.78 | 1.12 |
| Chemical | O-sulfo-L-tyrosine | ≤0.001 | 0.000 | ≤ 0.593 | ≤ 0.001 | ≤ 0.672 | 0.98 | 0.80 | 0.98 |
| 6-hydroxyindole sulfate | ≤ 0.004 | 0.049 | ≤ 0.808 | ≤ 0.548 | ≤ 0.218 | 0.96 | 1.13 | 0.86 | |
| Creatine metabolism | Guanidinoacetate | ≤ 0.001 | 0.000 | ≤ 0.925 | ≤ 0.001 | ≤ 0.615 | 1.00 | 1.80 | 1.02 |
| Fatty-acid metabolism (also branched-chain amino-acid metabolism) | Methylmalonate | ≤ 0.001 | 0.008 | ≤ 0.660 | ≤ 0.192 | ≤ 0.728 | 0.97 | 1.13 | 0.95 |
| Fatty acid, branched | Pristanate | ≤ 0.001 | 0.019 | ≤ 0.555 | ≤ 0.046 | ≤ 0.004 | 1.15 | 0.79 | 1.54 |
| Fatty acid, monohydroxy | 3-hydroxymyristate | ≤ 0.001 | 0.006 | ≤ 0.880 | ≤ 0.064 | ≤ 0.079 | 1.07 | 0.82 | 1.22 |
| 3-hydroxylaurate | ≤ 0.001 | 0.014 | ≤ 0.786 | ≤ 0.053 | ≤ 0.125 | 1.11 | 0.73 | 1.26 | |
| 3-hydroxydecanoate | ≤ 0.003 | 0.038 | ≤ 0.910 | ≤ 0.127 | ≤ 0.217 | 1.09 | 0.81 | 1.16 | |
| Fibrinogen cleavage peptide | Fibrinopeptide B (1–12) | ≤ 0.001 | 0.000 | ≤ 0.959 | ≤ 0.005 | ≤ 0.743 | 1.01 | 1.31 | 1.03 |
| Food component/plant | Sucralose | ≤ 0.001 | 0.001 | ≤ 0.630 | ≤ 0.001 | ≤ 0.240 | 0.85 | 0.53 | 0.80 |
| 3-formylindole | ≤ 0.004 | 0.049 | ≤ 0.986 | ≤ 0.043 | ≤ 0.560 | 0.99 | 0.86 | 0.94 | |
| Glutamate metabolism | S-1-pyrroline-5-carboxylate | ≤ 0.001 | 0.000 | ≤ 0.394 | ≤ 0.001 | ≤ 0.525 | 0.97 | 1.59 | 0.94 |
| Gamma-glutamylglutamate | ≤ 0.001 | 0.001 | ≤ 0.422 | ≤ 0.002 | ≤ 0.876 | 0.93 | 1.22 | 1.00 | |
| Beta-citrylglutamate | ≤ 0.001 | 0.001 | ≤ 0.159 | ≤ 0.035 | ≤ 0.686 | 0.86 | 1.31 | 0.96 | |
| Alpha-ketoglutaramate* | ≤ 0.004 | 0.048 | ≤ 0.410 | ≤ 0.401 | ≤ 0.360 | 0.97 | 1.03 | 0.96 | |
| Glycerolipid metabolism | Glycerophosphoglycerol | ≤ 0.001 | 0.006 | ≤ 0.092 | ≤ 0.001 | ≤ 0.676 | 1.30 | 2.09 | 1.09 |
| Glycolysis, gluconeogenesis, and pyruvate metabolism | Glycerate | ≤ 0.001 | 0.014 | ≤ 0.848 | ≤ 0.002 | ≤ 0.249 | 0.99 | 1.13 | 1.04 |
| Guanidino and acetamido metabolism | 4-guanidinobutanoate | ≤ 0.001 | 0.000 | ≤ 0.682 | ≤ 0.001 | ≤ 0.174 | 0.96 | 2.35 | 1.10 |
| Glutamate metabolism | Isoleucine | ≤ 0.001 | 0.000 | ≤ 0.639 | ≤ 0.001 | ≤ 0.945 | 1.02 | 0.85 | 1.00 |
| Leucine | ≤ 0.001 | 0.001 | ≤ 0.747 | ≤ 0.014 | ≤ 0.913 | 0.99 | 0.93 | 1.00 | |
| Malonate | ≤ 0.001 | 0.005 | ≤ 0.899 | ≤ 0.001 | ≤ 0.801 | 1.04 | 1.36 | 1.02 | |
| Long-chain monounsaturated fatty acid | Myristoleate (14:1ω5) | ≤ 0.002 | 0.035 | ≤ 0.358 | ≤ 0.004 | ≤ 0.486 | 0.98 | 0.75 | 1.17 |
| Long-chain polyunsaturated fatty acid | Stearidonate (18:4ω3) | ≤ 0.001 | 0.005 | ≤ 0.267 | ≤ 0.001 | ≤ 0.247 | 0.93 | 0.71 | 1.10 |
| (n3 and n6) | Linolenate (alpha or gamma; 18:3ω3 or 6) | ≤ 0.002 | 0.027 | ≤ 0.288 | ≤ 0.003 | ≤ 0.376 | 0.93 | 0.76 | 1.12 |
| Hexadecadienoate (16:2ω6) | ≤ 0.003 | 0.039 | ≤ 0.132 | ≤ 0.001 | ≤ 0.607 | 0.89 | 0.72 | 1.06 | |
| Long-chain saturated fatty acid | Myristate (14:0) | ≤ 0.003 | 0.039 | ≤ 0.298 | ≤ 0.005 | ≤ 0.488 | 0.97 | 0.80 | 1.12 |
| Lysine metabolism | N6,N6-dimethyllysine | ≤ 0.001 | 0.000 | ≤ 0.978 | ≤ 0.001 | ≤ 0.959 | 1.00 | 0.74 | 1.00 |
| N6-methyllysine | ≤ 0.001 | 0.003 | ≤ 0.905 | ≤ 0.813 | ≤ 0.955 | 1.07 | 0.97 | 0.98 | |
| Medium-chain fatty acid | Caprate (10:0) | ≤ 0.001 | 0.006 | ≤ 0.781 | ≤ 0.030 | ≤ 0.041 | 1.06 | 0.84 | 1.15 |
| Laurate (12:0) | ≤ 0.002 | 0.028 | ≤ 0.419 | ≤ 0.013 | ≤ 0.368 | 0.98 | 0.79 | 1.14 | |
| (2 or3)-decenoate (10:1 ω7 or n8) | ≤ 0.003 | 0.037 | ≤ 0.556 | ≤ 0.174 | ≤ 0.107 | 1.01 | 0.83 | 1.32 | |
| Cis-4-decenoate (10:1 ω6)* | ≤ 0.003 | 0.038 | ≤ 0.446 | ≤ 0.066 | ≤ 0.489 | 0.94 | 0.82 | 1.11 | |
| 5-dodecenoate (12:1ω7) | ≤ 0.003 | 0.038 | ≤ 0.505 | ≤ 0.020 | ≤ 0.303 | 1.02 | 0.82 | 1.19 | |
| Methionine, cysteine, S-adenosylmethionine, and taurine metabolism | Methionine sulfone | ≤ 0.001 | 0.006 | ≤ 0.963 | ≤ 0.128 | ≤ 0.755 | 1.02 | 1.13 | 0.97 |
| Monoacylglycerol | 1-linolenoylglycerol (18:3) | ≤ 0.004 | 0.049 | ≤ 0.401 | ≤ 0.091 | ≤ 0.210 | 0.85 | 0.82 | 1.20 |
| Nicotinate and nicotinamide metabolism | Nicotinamide | ≤ 0.001 | 0.006 | ≤ 0.141 | ≤ 0.001 | ≤ 0.499 | 0.82 | 0.56 | 1.13 |
| Partially characterized molecules | Glucuronide of C10H18O2 (6)1 | ≤ 0.001 | 0.000 | ≤ 1.000 | ≤ 0.001 | ≤ 0.625 | 1.00 | 2.16 | 1.06 |
| Branched-chain, straight-chain, or cyclopropyl 12:1 fatty acid* | ≤ 0.002 | 0.026 | ≤ 0.661 | ≤ 0.045 | ≤ 0.210 | 1.08 | 0.77 | 1.27 | |
| Glycine conjugate of C10H14O2 (1)* Phenylpyruvate |
≤ 0.003 | 0.039 | ≤ 0.282 | ≤ 0.023 | ≤ 0.602 | 0.89 | 0.81 | 1.05 | |
| Phenylalanine metabolism | ≤ 0.003 | 0.036 | ≤ 0.602 | ≤ 0.003 | ≤ 0.940 | 1.03 | 0.84 | 1.00 | |
| Purine metabolism, (hypo)xanthine/inosine containing | Urate | ≤ 0.003 | 0.036 | ≤ 0.564 | ≤ 0.574 | ≤ 0.524 | 1.02 | 1.01 | 0.98 |
| Tricarboxylic acid cycle | Aconitate (cis or trans) | ≤ 0.001 | 0.000 | ≤ 0.488 | ≤ 0.001 | ≤ 0.849 | 0.98 | 1.57 | 0.99 |
| Alpha-ketoglutarate | ≤ 0.001 | 0.000 | ≤ 0.459 | ≤ 0.001 | ≤ 0.842 | 0.97 | 1.51 | 1.02 | |
| Succinate | ≤ 0.001 | 0.000 | ≤ 0.001 | ≤ 0.023 | ≤ 0.873 | 0.88 | 1.12 | 1.00 | |
| Citrate | ≤ 0.001 | 0.000 | ≤ 0.472 | ≤ 0.002 | ≤ 0.809 | 0.98 | 1.26 | 0.99 | |
| Fumarate | ≤ 0.001 | 0.001 | ≤ 0.473 | ≤ 0.001 | ≤ 0.544 | 0.97 | 1.24 | 1.03 | |
| Malate | ≤ 0.001 | 0.002 | ≤ 0.059 | ≤ 0.069 | ≤ 0.974 | 0.90 | 1.15 | 0.99 | |
| Tryptophan metabolism | 8-methoxykynurenate | ≤ 0.001 | 0.023 | ≤ 0.901 | ≤ 0.021 | ≤ 0.969 | 1.00 | 0.72 | 1.01 |
| Indolepropionylglycine | ≤ 0.002 | 0.031 | ≤ 0.982 | ≤ 0.855 | ≤ 0.060 | 1.01 | 0.99 | 0.60 | |
| 3-indoxyl sulfate | ≤ 0.002 | 0.034 | ≤ 0.941 | ≤ 0.341 | ≤ 0.273 | 1.01 | 1.17 | 0.90 | |
| Kynurenate | ≤ 0.001 | 0.001 | ≤ 0.490 | ≤ 0.001 | ≤ 0.807 | 0.95 | 0.79 | 0.98 | |
| Indolepropionate | ≤ 0.001 | 0.001 | ≤ 0.902 | ≤ 0.963 | ≤ 0.077 | 0.99 | 1.05 | 0.66 | |
| Kynurenine | ≤ 0.001 | 0.004 | ≤ 0.339 | ≤ 0.001 | ≤ 0.192 | 0.95 | 0.82 | 0.94 | |
| Tyrosine metabolism | Phenol sulfate | ≤ 0.002 | 0.035 | ≤ 0.233 | ≤ 0.520 | ≤ 0.433 | 0.85 | 0.91 | 1.08 |
| Arginine | ≤ 0.001 | 0.000 | ≤ 0.864 | ≤ 0.001 | ≤ 0.315 | 1.00 | 1.96 | 1.04 | |
| Urea cycle; arginine, and proline | Argininosuccinate | ≤ 0.001 | 0.000 | ≤ 0.818 | ≤ 0.001 | ≤ 0.376 | 1.06 | 3.24 | 1.14 |
| metabolism | Ornithine | ≤ 0.001 | 0.000 | ≤ 0.806 | ≤ 0.001 | ≤ 0.005 | 0.98 | 2.08 | 1.13 |
| 3-amino-2-piperidone | ≤ 0.001 | 0.000 | ≤ 0.249 | ≤ 0.001 | ≤ 0.001 | 0.91 | 1.64 | 2.11 | |
| 2-oxoarginine1 | ≤ 0.001 | 0.000 | ≤ 0.855 | ≤ 0.001 | ≤ 0.492 | 1.04 | 1.95 | 1.05 | |
| N-acetylarginine | ≤ 0.001 | 0.000 | ≤ 0.734 | ≤ 0.001 | ≤ 0.558 | 1.03 | 2.01 | 1.05 | |
| Dimethylarginine (asymmetric + dimethylarginine) | ≤ 0.001 | 0.000 | ≤ 0.346 | ≤ 0.001 | ≤ 0.867 | 0.98 | 1.14 | 1.00 | |
| Argininate* | ≤ 0.001 | 0.000 | ≤ 0.487 | ≤ 0.005 | ≤ 0.143 | 0.96 | 1.26 | 1.12 | |
| N-alpha-acetylornithine | ≤ 0.001 | 0.003 | ≤ 0.749 | ≤ 0.001 | ≤ 0.947 | 0.97 | 1.54 | 1.04 | |
| N-acetylproline | ≤ 0.002 | 0.029 | ≤ 0.456 | ≤ 0.001 | ≤ 0.910 | 1.05 | 1.24 | 0.96 | |
| Proline | ≤ 0.003 | 0.037 | ≤ 0.420 | ≤ 0.020 | ≤ 0.483 | 1.03 | 1.07 | 1.02 | |
| Homoarginine | ≤ 0.004 | 0.046 | ≤ 0.866 | ≤ 0.523 | ≤ 0.926 | 0.99 | 1.06 | 1.01 | |
| Urea | ≤ 0.004 | 0.048 | ≤ 0.414 | ≤ 0.488 | ≤ 0.223 | 0.97 | 1.03 | 1.06 | |
| Not applicable | X-13507 | ≤ 0.001 | 0.000 | ≤ 0.756 | ≤ 0.001 | ≤ 0.001 | 1.02 | 3.13 | 1.81 |
| X-24425 | ≤ 0.001 | 0.000 | ≤ 0.549 | ≤ 0.001 | ≤ 0.249 | 1.04 | 5.76 | 1.26 | |
| X-12680 | ≤ 0.001 | 0.000 | ≤ 0.103 | ≤ 0.001 | ≤ 0.738 | 0.90 | 1.46 | 1.03 | |
| X-16576 | ≤ 0.001 | 0.000 | ≤ 0.939 | ≤ 0.001 | ≤ 0.396 | 1.01 | 1.37 | 0.93 | |
| X-21821 | ≤ 0.001 | 0.001 | ≤ 0.954 | ≤ 0.683 | ≤ 0.033 | 1.07 | 1.01 | 0.61 | |
| X-17351 | ≤ 0.001 | 0.001 | ≤ 0.922 | ≤ 0.807 | ≤ 0.016 | 1.05 | 0.92 | 0.62 | |
| X-11787 | ≤ 0.001 | 0.003 | ≤ 0.741 | ≤ 0.204 | ≤ 0.815 | 1.01 | 1.05 | 0.99 | |
| X-12283 | ≤ 0.001 | 0.005 | ≤ 0.808 | ≤ 0.268 | ≤ 0.017 | 1.14 | 0.85 | 0.63 | |
| X-17685 | ≤ 0.001 | 0.005 | ≤ 0.537 | ≤ 0.418 | ≤ 0.124 | 1.10 | 1.12 | 0.64 | |
| X-25617 | ≤ 0.001 | 0.006 | ≤ 0.648 | ≤ 0.342 | ≤ 0.658 | 1.16 | 1.36 | 0.90 | |
| X-21353 | ≤ 0.001 | 0.010 | ≤ 0.735 | ≤ 0.061 | ≤ 0.259 | 1.05 | 0.79 | 1.16 | |
| X-24411 | ≤ 0.001 | 0.010 | ≤ 0.098 | ≤ 0.411 | ≤ 0.001 | 0.79 | 1.08 | 1.51 | |
| X-15666 | ≤ 0.001 | 0.014 | ≤ 0.135 | ≤ 0.001 | ≤ 0.385 | 0.81 | 0.41 | 0.91 | |
| X-15245 | ≤ 0.001 | 0.014 | ≤ 0.543 | ≤ 0.003 | ≤ 0.228 | 1.15 | 1.48 | 0.86 | |
| X-12740 | ≤ 0.001 | 0.014 | ≤ 0.671 | ≤ 0.001 | ≤ 0.487 | 1.14 | 5.68 | 1.29 | |
| X-25432 | ≤ 0.001 | 0.022 | ≤ 0.486 | ≤ 0.003 | ≤ 0.105 | 0.96 | 0.74 | 0.86 | |
| X-16570 | ≤ 0.002 | 0.028 | ≤ 0.170 | ≤ 0.003 | ≤ 0.757 | 0.91 | 0.80 | 1.01 | |
| X-17686 | ≤ 0.002 | 0.030 | ≤ 0.702 | ≤ 0.010 | ≤ 0.211 | 1.01 | 1.45 | 0.75 | |
| X-15461 | ≤ 0.003 | 0.039 | ≤ 0.136 | ≤ 0.630 | ≤ 0.061 | 0.85 | 0.96 | 1.28 | |
| X-21310 | ≤ 0.003 | 0.039 | ≤ 0.979 | ≤ 0.425 | ≤ 0.222 | 1.00 | 1.13 | 0.89 | |
| X-17328 | ≤ 0.004 | 0.047 | ≤ 0.374 | ≤ 0.007 | ≤0.599 | 0.83 | 1.47 | 1.17 | |
| X-16946 | ≤ 0.004 | 0.049 | ≤ 0.575 | ≤ 0.006 | ≤ 0.637 | 0.82 | 0.60 | 1.02 | |
Data represent significance values (P-value) and false discover rates (q-values) for the analysis-of-variance response (arginine vs. placebo) by time (0, 1.5, and 24 h) interaction. Bold values indicate significant (interaction q < 0.05, contrast P < 0.05) between arginine and placebo conditions.
Partially characterized molecules
Fig. 2.
KEGG pathway enrichment analysis. The overview of the enriched metabolite sets following arginine vs. placebo supplementation.
Arginine consumption did not affect mood state (P > 0.05). Arginine supplementation versus placebo decreased reports of “like to eat something sweet” at the 1.5 h and 6.0 h timepoints (42.5 ± 23.9 vs. 48.3 ± 25.7; 47.6 ± 25.5 vs. 52.3 ± 2 5.7; P > 0.05), but other timepoint and variables were not different.
Responder analysis in arginine supplement group
GH levels increased in arginine responders at 1.5 h compared with placebo (placebo 1.53 ± 3.3 vs. arginine 2.1 ± 3.2 ng/mL; P < 0.05). Arginine responders also had elevated glucose levels after 1.5 h and 3.0 h compared with the placebo supplement (P ≤ 0.01). TSH was higher in arginine versus placebo responders at 24 h (P < 0.05). The other assays were not different between the supplements in the arginine responders versus the placebo supplements.
Arginine supplementation increased arginine versus placebo after 1.5 h and 3.0 h in responders (P < 0.001; Table 5). Leucine and isoleucine levels decreased in the arginine versus placebo supplement groups at 1.5 h, and glutamic acid levels decreased in the arginine versus placebo supplement groups at 1.5 h (P < 0.05). No differences were found at 24 h. No differences were seen between responders and nonresponders with RHI, AI, or AI.75 (data not shown; P > 0.05).
Table 5.
Effects of arginine and placebo on biomarkers in young, healthy, male responders after acute supplementation (n = 16)
| Amino acid | Time × treatment interaction P-value | Time, h | Arginine |
Placebo |
|
|---|---|---|---|---|---|
| Estimate (95% CI) | Estimate (95% CI) | P-value | |||
|
| |||||
| Glucose, mg/dL | 0.001 | 0 | 85.6 (83.3–88.0) | 84.7 (82.3–87.0) | 0.368 |
| 1.5 | 88.7 (86.2–91.3) | 85.2 (83.0–87.5) | 0.010 | ||
| 3.0 | 88.3 (85.8–90.8) | 85.1 (82.9–87.4) | 0.006 | ||
| 24 | 87.7 (85.3–90.0) | 89.8 (87.5–92.1) | 0.150 | ||
| Thyroid-stimulating | 0.049 | 0 | 2.39 (1.89–2.89) | 2.21 (1.71–2.71) | 0.193 |
| hormone, μIU/mL | |||||
| 1.5 | 1.75 (1.25–2.25) | 1.69 (1.19–2.19) | 0.918 | ||
| 3.0 | 1.70 (1.20–2.20) | 1.56 (1.06–2.05) | 0.408 | ||
| 24 | 2.30 (1.81–2.79) | 2.11 (1.61–2.59) | 0.031 | ||
| Isoleucine, μmol/L | 0.025 | 0 | 78.7 (72.0–85.4) | 75.0 (68.2–81.7) | 0.230 |
| 1.5 | 62.3 (55.6–69.0) | 70.5 (63.8–77.2) | 0.010 | ||
| 3.0 | 58.9 (52.2–65.6) | 64.2 (57.5–70.9) | 0.089 | ||
| 24 | 86.5 (79.8–93.2) | 84.9 (78.2–91.7) | 0.611 | ||
| Glutamic acid, μmol/L | 0.045 | 0 | 50.2 (43.8–56.7) | 43.66 (37.2–50.1) | 0.181 |
| 1.5 | 56.6 (50.2–63.1) | 42.7 (36.3–49.2) | 0.006 | ||
| 3.0 | 39.6 (33.1–46.0) | 43.39 (36.9–49.8) | 0.433 | ||
| 24 | 34.9 (28.4–41.3) | 36.88 (30.4–43.3) | 0.683 | ||
| Arginine, μmol/L | < 0.001 | 0 | 114.1 (99.8–128.5) | 111.0 (96.6–125.3) | 0.709 |
| 1.5 | 267.3 (253.0–281.7) | 112.9 (98.5–127.3) | 0.001 | ||
| 3.0 | 204.5 (190.2–218.9) | 105.2 (90.9–119.6) | 0.001 | ||
| 24 | 127.8 (113.4–142.2) | 118.9 (104.5–133.3) | 0.292 | ||
CI, confidence interval
Data are presented as estimate and 95% CI. A mixed effect model was used to model the distributions of the biomarkers, taking into account correlations among the measurements. Biomarkers that do not have significant treatment-by-time interaction were not presented. Tukey’s honestly significant difference test was performed to compare the two groups at individual timepoints. Responders had growth hormones levels >0.05 ng/ml at the 1.5-hr timepoint of the arginine treatment. Treatment is supplement.
The metabolomics analysis revealed seven analytes that were different between GH arginine responders and nonresponders (P < 0.001; q < 0.20). Of the seven analytes, methyl-4-hydroxybenzoate sulfate, X-13729, and X-16124 were different at 0 h and 1.5 h. Both X-13729 and X-16124 have been found to be associated with the presence of certain gut microorganisms in humans [34]. Differences were also found in amino- and fatty-acid profiles, but were less consistent.
Arginine consumption did not affect sleep or mood state in responders versus nonresponders (P > 0.05). In addition, most feelings of appetite questions were unaffected, but responses to “how strong is your desire to eat”, “would you like to eat something fatty”, and “would you like to eat something sweet” were decreased in the arginine versus placebo supplement groups at the 1.5 h, 1.5 h, and 6.0 h timepoints, respectively (57.9 ± 15.2 vs. 66.2 ± 21.3; 51.7 ± 14.4 vs. 61.6 ± 11.6; 42.3 ± 20.6 vs. 48.4 ± 18.3; P < 0.05), but other timepoints and variables were not different.
Discussion
This study examined neuroendocrine, cardiovascular, and metabolic effects after an acute high dose of arginine. Acute oral arginine supplementation had limited physiological effects compared with placebo in healthy men. GH levels were elevated 24 h after arginine supplementation compared with placebo. Arginine supplementation increased plasma arginine levels and associated subpathway metabolites, which likely led to changes in branched chain amino acids. Arginine had no effect on endothelium-mediated changes in vascular tone (PAT). Responders, defined as individuals with detectable GH values 1.5 h after arginine supplementation, had elevated glucose and TSH levels, as well as altered amino acid profiles. Additionally, the metabolomic analysis revealed higher levels of dicarboxylic fatty acids, benzoate metabolites, and unknown compounds after arginine supplementation in responders compared with nonresponders. To our knowledge, this is the first well-powered study to comprehensively investigate the effects of arginine in healthy participants, using a dose that is approximately twice the average daily intake of arginine, on neuroendocrine, cardiovascular, and metabolic endpoints, including an untargeted metabolomic analysis.
When arginine is administered intravenously, the elevation of GH and other endocrine parameters appears to be a greater and more rapid [35,36]. Oral ingestion is an inherently slower process than intravenous administration, so a delayed response is to be expected. This suggests that method of administration is an important variable when conducting and comparing arginine studies. Also, arginine appears to have dose-dependent effects regardless of the type of administration [19,36].
Reports of the effects of arginine on glucose have had mixed results [37]. One study reported increased glucose concentrations, a maximum 124 mg/dL after 30 min, after participants consumed 1 mmol arginine/kg of lean mass plus 25 g of glucose [38]. However, the arginine-only supplementation resulted in no differences to the glucose area under the curve compared with water. Furthermore, no difference was seen in levels of insulin with arginine only. In this study with 30 participants, an increase in glucose was noted. In three studies of healthy adults at rest, acute supplementation of arginine at various doses did not have a significant effect on insulin concentrations as shown in the present study [38–40]. Thus, arginine may modestly increase glucose levels.
This study found that arginine supplementation had no effect on endothelial function in younger, healthy men. Arginine is a precursor of NO, which is a key factor in vascular function [41]. As previously shown, acute or chronic ingestion of oral arginine does not appear to have a significant effect on endothelial function in healthy men at rest or after resistance exercise [42–46]. However, a meta-analysis of placebo-controlled, randomized clinical trials suggests that short-term oral arginine supplementation improves flow-mediated dilation when baseline flow-mediated dilation is low [47]. In support of this hypothesis, acute ingestion of arginine improved markers of endothelial function in healthy young smokers [48]. Thus, arginine may improve function in a less healthy population, but has limited beneficial effects in a healthy, younger, adult population.
To the best of our knowledge, no prior study has investigated comprehensive metabolomic response to arginine supplementation. The metabolomic analysis confirmed increased arginine and urea cycle activity (e.g., arginosuccinate and ornithine) after arginine supplementation in all participants. Similarly, acetylated (e.g., n-acetylarginine) and methylated (e.g., dimethylarginine) arginine derivatives also increased. Dimethylarginine may inhibit NO synthase synthesis, which may explain the null PAT findings observed in this and other studies [49]. The changes observed 24 h after supplementation suggest that excess arginine is preferentially redirected toward ornithine biosynthesis, and these effects of arginine supplementation were at least 24 h in duration. Under healthy conditions, mammals can tolerate excess ornithine levels by a reversible enzymatic reaction between ornithine and glutamate semialdehyde via the ornithine aminotransferase [50,51]. However, in conditions of ornithine-aminotransferase deficiency, low arginine diets are prescribed to prevent hyperornithinemia [52]. Although speculative, this pathway may explain the elevated glutamate (glutamic acid) levels we observed 1.5 h after arginine ingestion. Besides ornithine, glutamate can form proline; thus, glutamate may increase proline levels through glutamate semialdehyde after arginine supplementation.
The synthesis of glutamate from excess ornithine may have also increased Krebs cycle activity. Glutamate dehydrogenase acts enzymatically on glutamate to form α-ketoglutarate, a Krebs-cycle constituent that was elevated in this study. Other Krebs-cycle analytes were also elevated. Acetyl-CoA is necessary for the formation of citrate (Krebs cycle intermediate), and can be synthesized from leucine and isoleucine. This study found that both leucine and isoleucine concentrations were lower after arginine supplementation (Table 2). To the authors knowledge, previous research has not observed a decrease in branched chain amino acids with arginineonly supplementation. However, arginine-plus-glucose supplementation in healthy participants has also resulted in decreased branched chain amino acids [38]. Research with rodents showed that arginine had limited effects on branch chain amino acids; however, this was not in response to an arginine bolus as performed in this study, but the chronic administration of high amounts of arginine in food [53]. The increased glutamate pool likely resulted in the decreased levels of branched chain amino acids, possibly due to decreased transamination. Although speculative, supplemented and dietary amino acids may need to be coordinated to ensure balanced amino-acid intake to prevent the pooling of specific amino acids.
GH increased in 16 volunteers tested in this study after arginine consumption (i.e., responders). This finding suggests that in some, but not all, healthy individuals, arginine intake may have a possible endocrine benefit. In the present study, approximately 55% of participants were responders, and approximately 75% were responders in the study by Collier et al. [19]. GH is increased by suppressing somatostatin. GH secretion is also associated with increased lipolysis of triacylglycerol in adipose tissue and elevated fatty acid uptake by skeletal muscles to promote growth [54]. Interestingly, 3-methyladipate was elevated in responders 1.5 h after arginine supplementation, and may represent the mobilization of fatty acids for skeletal muscle uptake. The skeletal muscle uptake of fatty acids may also be responsible for the decreased levels of (R)-3-hydroxybutyrylcarnitine at 24 h in responders.
Responders had higher levels of methyl-4-hydroxybenzoate sulfate and X-16124 at 0 h and 1.5 h in both the arginine and placebo supplement groups. The elevations of both X-13729 and X-16124 indicate that the microbiome could be catabolizing benzoate and driving the GH response. X-16124 is an unidentified metabolite that recently has been linked to the Eggerthellaceae family of bacteria in the microbiome, and multiple gut microbiome species are capable of catabolizing benzoate [34,55]. Additionally, bacteria of the phylum Firmicutes family are phylogenetically associated with benzoate catabolism and X-13729, the latter of which was elevated in responders at 0 h and 1.5 h during placebo supplementation. Although our analysis is exploratory, the upregulation in benzoate analytes suggests that the microbiome and gut permeability may be critical in determining responder versus nonresponder status after arginine supplementation. To confirm this link, future investigations should examine whether the GH response to arginine corresponds with the presence of certain gut microbes, gut permeability, and their corresponding serum analytes.
This study was a double-blind, placebo-controlled, randomized, crossover trail that provided 2 d of controlled feeding before testing, assessed numerous blood parameters, and performed untargeted global metabolomics, which are all study strengths. However, this study also had limitations. A younger, healthy, male population was studied, so the findings may not be applicable to other populations. Women, unhealthy individuals, and older adults could additionally be studied. In addition, only a single dose of 10 g of arginine was administered. Only blood samples were analyzed, so although markers of gut microorganisms were found, gut microbiota were not measured. Lastly, chronic supplementation may have differential effects. Some [56,57], but not all [58–63], articles suggest that L-arginine supplementation may cause harm. Additionally, chronic or long-term supplementation should be investigated in additional, well-powered, separate studies.
Conclusions
Arginine ingestion was associated with an increase in arginine levels and its derivatives, changes in glutamate metabolism, and increases in tricarboxylic acid cycle intermediates, but had limited effects on most neuroendocrine parameters, as assessed in younger, healthy men. However, glucose was increased at 1.5 h and 3 h, but GH was not until 24 h. Arginine supplements also decreased branched chain amino acids and altered the fatty-acid metabolism. In addition, responders had higher levels of benzoate- and microbiome-associated metabolites. This finding suggests the metabolites of the microbiome found in serum may mediate the GH response after arginine supplementation. Follow-up studies are needed to confirm the potential role of specific gut microbes in serum that correspond to GH responses after arginine supplementation.
Acknowledgments
The authors thank the study volunteers, as well as the Pennington Biomedical Research Center cores and their support staff. Also, the authors thank Danielle Anderson of the Combat Feeding Directorate, Combat Capabilities Development Command Soldier Center in Natick, Massachusetts for assistance with the arginine supplement formulation used in this study and Victor L. Fulgoni III for assistance with the study design.
Funding for this research was provided by the Defense Health Program and U.S. Army Medical Research and Development Command (award number W81 XWH-141–0335 and Battelle), as well as supported in part by U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center and by a National Obesity Research Center grant (# P30 DK072476) entitled “Nutrition and Metabolic Health Through the Lifespan” sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors, and does not necessarily represent the official views of the National Institutes of Health. Also, the views expressed in this paper are those of the authors, and do not reflect the official policy of the Department of the Army, Department of Defense, or U.S. Government. Any citations of commercial organizations and trade names in this report do not constitute an official Department of the Army endorsement or approval of the products or services of these organizations.
Footnotes
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.nut.2022.111658.
References
- [1].Froiland K, Koszewski W, Hingst J, Kopecky L. Nutritional supplement use among college athletes and their sources of information. Int J Sport Nutr Exerc Metab 2004;14:104–20. [DOI] [PubMed] [Google Scholar]
- [2].Burns RD, Schiller MR, Merrick MA, Wolf KN. Intercollegiate student athlete use of nutritional supplements and the role of athletic trainers and dietitians in nutrition counseling. J Am Diet Assoc 2004;104:246–9. [DOI] [PubMed] [Google Scholar]
- [3].Cowan AE, Jun S, Gahche JJ, Tooze JA, Dwyer JT, Eicher-Miller HA, et al. Dietary supplement use differs by socioeconomic and health-related characteristics among U.S. adults, NHANES 2011–2014.Nutrients 2018;10:1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Luiking YC, Ten Have GA, Wolfe RR, Deutz NE. Arginine de novo and nitric oxide production in disease states. Am J Physiol Endocrinol Metab 2012;303: E1177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Vallance P, Collier J, Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet 1989;2:997–1000. [DOI] [PubMed] [Google Scholar]
- [6].Cynober L, Bier DM, Kadowaki M, Morris SM Jr, Elango R, Smriga M. Proposals for upper limits of safe intake for arginine and tryptophan in young adults and an upper limit of safe intake for leucine in the elderly. J Nutr 2016;146:2652S–4. [DOI] [PubMed] [Google Scholar]
- [7].Mirmiran P, Bahadoran Z, Ghasemi A, Azizi F. The association of dietary l-arginine intake and serum nitric oxide metabolites in adults: A population-based study. Nutrients 2016;8:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Phillips SM, Paddon-Jones D, Layman DK. Optimizing adult protein intake during catabolic health conditions. Adv Nutr 2020;11:S1058–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Carbone JW, Pasiakos SM. Dietary protein and muscle mass: Translating science to application and health benefit. Nutrients 2019;11:1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Viribay A, Burgos J, Fernandez-Landa J, Seco-Calvo J, Mielgo-Ayuso J. Effects of arginine supplementation on athletic performance based on energy metabolism: A systematic review and meta-analysis. Nutrients 2020;12:1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Alvares TS, Meirelles CM, Bhambhani YN, Paschoalin VM, Gomes PS. L-arginine as a potential ergogenic aid in healthy subjects. Sports Med 2011;41:233–48. [DOI] [PubMed] [Google Scholar]
- [12].Blum A, Cannon RO 3rd, Costello R, Schenke WH, Csako G. Endocrine and lipid effects of oral L-arginine treatment in healthy postmenopausal women. J Lab Clin Med 2000;135:231–7. [DOI] [PubMed] [Google Scholar]
- [13].Isidori A, Lo Monaco A, Cappa M. A study of growth hormone release in man after oral administration of amino acids. Curr Med Res Opin 1981;7:475–81. [DOI] [PubMed] [Google Scholar]
- [14].Campbell BI, La Bounty PM, Roberts M. The ergogenic potential of arginine. J Int Soc Sports Nutr 2004;1:35–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tritos NA, Biller BMK. Current concepts of the diagnosis of adult growth hormone deficiency. Rev Endocr Metab Disord 2021;22:109–16. [DOI] [PubMed] [Google Scholar]
- [16].Nicholls AR, Holt RI. Growth hormone and insulin-like growth factor-1. Front Horm Res 2016;47:101–14. [DOI] [PubMed] [Google Scholar]
- [17].Velloso CP. Regulation of muscle mass by growth hormone and IGF-I. Br J Pharmacol 2008;154:557–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Coman D, Yaplito-Lee J, Boneh A. New indications and controversies in arginine therapy. Clin Nutr 2008;27:489–96. [DOI] [PubMed] [Google Scholar]
- [19].Collier SR, Casey DP, Kanaley JA. Growth hormone responses to varying doses of oral arginine. Growth Horm IGF Res 2005;15:136–9. [DOI] [PubMed] [Google Scholar]
- [20].Evans AM, Bridgewater BR, Liu Q, Mitchell MW, Robinson RJ, Dai H, et al. High resolution mass spectrometry improves data quantity and quality as compared to unit mass resolution mass spectrometry in high-throughput profiling metabolomics. Metabolomics 2014;4:1–7. [Google Scholar]
- [21].Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 1990;51:241–7. [DOI] [PubMed] [Google Scholar]
- [22].Banderet LE, Lieberman HR. Treatment with tyrosine, a neurotransmitter precursor,reducesenvironmentalstressinhumans.BrainResBull1989;22:759–62. [DOI] [PubMed] [Google Scholar]
- [23].Lieberman HR, Tharion WJ, Shukitt-Hale B, Speckman KL, Tulley R. Effects of caffeine, sleep loss, and stress on cognitive performance and mood during U.S. Navy SEAL training. Sea-Air-Land. Psychopharmacology (Berl) 2002;164: 250–61. [DOI] [PubMed] [Google Scholar]
- [24].Lieberman HR, Bathalon GP, Falco CM, Kramer FM, Morgan CA 3rd, Niro P. Severe decrements in cognition function and mood induced by sleep loss, heat, dehydration, and undernutrition during simulated combat. Biol Psychiatry 2005;57:422–9. [DOI] [PubMed] [Google Scholar]
- [25].Shukitt-Hale B, Askew EW, Lieberman HR. Effects of 30 days of undernutrition on reaction time, moods, and symptoms. Physiol Behav 1997;62: 783–9. [DOI] [PubMed] [Google Scholar]
- [26].McNair DM, Lorr M, Droppleman LE. The profile of mood states. San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- [27].Flint A, Raben A, Blundell JE, Reproducibility Astrup A. power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord 2000;24:38–48. [DOI] [PubMed] [Google Scholar]
- [28].Hambrecht R, Hilbrich L, Erbs S, Gielen S, Fiehn E, Schoene N, et al. Correction of endothelial dysfunction in chronic heart failure: Additional effects of exercise training and oral L-arginine supplementation. J Am Coll Cardiol 2000;35:706–13. [DOI] [PubMed] [Google Scholar]
- [29].Gupta AK, Ravussin E, Johannsen DL, Stull AJ, Cefalu WT, Johnson WD. Endothelial dysfunction: An early cardiovascular risk marker in asymptomatic obese individuals with prediabetes. Br J Med Med Res 2012;2:413–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Karl JP, Margolis LM, Murphy NE, Carrigan CT, Castellani JW, Madslien EH, et al. Military training elicits marked increases in plasma metabolomic signatures of energy metabolism, lipolysis, fatty acid oxidation, and ketogenesis. Physiol Rep 2017;5:e13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dehaven CD, Evans AM, Dai H, Lawton KA. Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J Cheminform 2010;2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem 2009;81:6656–67. [DOI] [PubMed] [Google Scholar]
- [33].Tudor G, Koch GG. Review of nonparametric methods for the analysis of crossover studies. Stat Methods Med Res 1994;3:345–81. [DOI] [PubMed] [Google Scholar]
- [34].Bar N, Korem T, Weissbrod O, Zeevi D, Rothschild D, Leviatan S, et al. A reference map of potential determinants for the human serum metabolome. Nature 2020;588:135–40. [DOI] [PubMed] [Google Scholar]
- [35].Tangphao O, Grossmann M, Chalon S, Hoffman BB, Blaschke TF. Pharmacokinetics of intravenous and oral L-arginine in normal volunteers. Br J Clin Pharmacol 1999;47:261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bode-Boger SM, Boger RH, Galland A, Tsikas D, Frolich JC. L-arginine-induced vasodilation in healthy humans: Pharmacokinetic-pharmacodynamic relationship. Br J Clin Pharmacol 1998;46:489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hage M, Kamenicky P, Chanson P. Growth hormone response to oral glucose load: From normal to pathological conditions. Neuroendocrinology 2019;108:244–55. [DOI] [PubMed] [Google Scholar]
- [38].Gannon MC, Nuttall JA, Nuttall FQ. Oral arginine does not stimulate an increase in insulin concentration but delays glucose disposal. Am J Clin Nutr 2002;76:1016–22. [DOI] [PubMed] [Google Scholar]
- [39].Forbes SC, Bell GJ. The acute effects of a low and high dose of oral L-arginine supplementation in young active males at rest. Appl Physiol Nutr Metab 2011;36:405–11. [DOI] [PubMed] [Google Scholar]
- [40].Robinson TM, Sewell D, Greenhaff PL. L-arginine ingestion after rest and exercise: Effects on glucose disposal. Med Sci Sports Exerc 2003;35:1309–15. [DOI] [PubMed] [Google Scholar]
- [41].Luiking YC, Deutz NE. Biomarkers of arginine and lysine excess. J Nutr 2007;137:1662S–8. [DOI] [PubMed] [Google Scholar]
- [42].Ast J, Cieslewicz AR, Korzeniowska K, Bogdanski P, Kazmierczak E, Olszewski J, et al. Supplementation with L-arginine does not influence arterial blood pressure in healthy people: A randomized, double blind, trial. Eur Rev Med Pharmacol Sci 2011;15:1375–84. [PubMed] [Google Scholar]
- [43].Fahs CA, Heffernan KS, Fernhall B. Hemodynamic and vascular response to resistance exercise with L-arginine. Med Sci Sports Exerc 2009;41:773–9. [DOI] [PubMed] [Google Scholar]
- [44].Adams MR, Forsyth CJ, Jessup W, Robinson J, Celermajer DS. Oral L-arginine inhibits platelet aggregation but does not enhance endothelium-dependent dilation in healthy young men. J Am Coll Cardiol 1995;26:1054–61. [DOI] [PubMed] [Google Scholar]
- [45].Chin-Dusting JP, Kaye DM, Lefkovits J, Wong J, Bergin P, Jennings GL. Dietary supplementation with L-arginine fails to restore endothelial function in forearm resistance arteries of patients with severe heart failure. J Am Coll Cardiol 1996;27:1207–13. [DOI] [PubMed] [Google Scholar]
- [46].Hind JM, Doodson AC. Oral L-arginine supplementation has no effect on cardiovascular responses to lower body negative pressure in man. Clin Auton Res 1994;4:293–7. [DOI] [PubMed] [Google Scholar]
- [47].Bai Y, Sun L, Yang T, Sun K, Chen J, Hui R. Increase in fasting vascular endothelial function after short-term oral L-arginine is effective when baseline flow-mediated dilation is low: A meta-analysis of randomized controlled trials. Am J Clin Nutr 2009;89:77–84. [DOI] [PubMed] [Google Scholar]
- [48].Siasos G, Tousoulis D, Vlachopoulos C, Antoniades C, Stefanadi E, Ioakeimidis N, et al. The impact of oral L-arginine supplementation on acute smoking-induced endothelial injury and arterial performance. Am J Hypertens 2009;22:586–92. [DOI] [PubMed] [Google Scholar]
- [49].Boger RH. Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, explains the “L-arginine paradox” and acts as a novel cardiovascular risk factor. J Nutr 2004;134:2842S–7. discussion 53S. [DOI] [PubMed] [Google Scholar]
- [50].Strecker HJ. Purification and properties of rat liver ornithine delta-transaminase. J Biol Chem 1965;240:1225–30. [PubMed] [Google Scholar]
- [51].Smith AD, Benziman M, Strecker HJ. The formation of ornithine from proline in animal tissues. Biochem J 1967;104:557–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Valle D, Walser M, Brusilow SW, Kaiser-Kupfer M. Gyrate atrophy of the choroid and retina: amino acid metabolism and correction of hyperornithinemia with an arginine-deficient diet. J Clin Invest 1980;65:371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Holecek M, Sispera L. Effects of arginine supplementation on amino acid profiles in blood and tissues in fed and overnight-fasted rats. Nutrients 2016;8:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kopchick JJ, Berryman DE, Puri V, Lee KY, Jorgensen JOL. The effects of growth hormone on adipose tissue: Old observations, new mechanisms. Nat Rev Endocrinol 2020;16:135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Yadav M, Lomash A, Kapoor S, Pandey R, Chauhan NS. Mapping of the benzoate metabolism by human gut microbiome indicates food-derived metagenome evolution. Sci Rep 2021;11:5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Boger RH. L-Arginine therapy in cardiovascular pathologies: Beneficial or dangerous? Curr Opin Clin Nutr Metab Care 2008;11:55–61. [DOI] [PubMed] [Google Scholar]
- [57].Wilson AM, Harada R, Nair N, Balasubramanian N, Cooke JP. L-arginine supplementation in peripheral arterial disease: No benefit and possible harm. Circulation 2007;116:188–95. [DOI] [PubMed] [Google Scholar]
- [58].Shiraseb F, Asbaghi O, Bagheri R, Wong A, Figueroa A, Mirzaei K. The effect of L-arginine supplementation on blood pressure in adults: A systematic review and dose-response meta-analysis of randomized clinical trials. Adv Nutr 2021. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Smeets E, Mensink RP, Joris PJ. Effects of L-citrulline supplementation and watermelon consumption on longer-term and postprandial vascular function and cardiometabolic risk markers: A meta-analysis of randomized controlled trials in adults. Br J Nutr 2021:1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Costa G, Shushanof M, Bouskela E, Bottino D. Oral L-arginine (5 g/day) for 14 days improves microcirculatory function in healthy young women and healthy and yype 2 diabetes mellitus elderly women. J Vasc Res 2022;59: 24–33. [DOI] [PubMed] [Google Scholar]
- [61].Mariotti F Arginine supplementation and cardiometabolic risk. Curr Opin Clin Nutr Metab Care 2020;23:29–34. [DOI] [PubMed] [Google Scholar]
- [62].McNeal CJ, Meininger CJ, Reddy D, Wilborn CD, Wu G. Safety and effectiveness of arginine in adults. J Nutr 2016;146. 2587S93. [DOI] [PubMed] [Google Scholar]
- [63].McKnight JR, Satterfield MC, Jobgen WS, Smith SB, Spencer TE, Meininger CJ, et al. Beneficial effects of L-arginine on reducing obesity: Potential mechanisms and important implications for human health. Amino Acids 2010;39:349–57. [DOI] [PubMed] [Google Scholar]