Abstract
Buprenorphine possesses many unique attributes that make it a practical agent for adults and adolescents with opioid use disorder (OUD) and/or acute or chronic pain. Sublingual buprenorphine has been the standard of care for treating OUD, but its use in pain management is not as clearly defined. Current practice guidelines recommend a period of mild‐to‐moderate withdrawal from opioids before transitioning to buprenorphine due to its ability to displace full agonists from the μ‐opioid receptor. However, this strategy can lead to negative physical and psychological outcomes for patients. Novel initiation strategies suggest that concomitant administration of small doses of buprenorphine with opioids can avoid the unwanted withdrawal associated with buprenorphine initiation. We aim to systematically review the buprenorphine initiation strategies that have emerged in the last decade. Embase, PubMed, and Cochrane Databases were searched for relevant literature. Studies were included if they were published in the English language and described the transition to buprenorphine from opioids. Data were collected from each study and synthesized using descriptive statistics. This review included 7 observational studies, 1 feasibility study, and 39 case reports/series which included 924 patients. The strategies utilized between the literature included traditional initiation (47.9%), microdosing with various buprenorphine formulations (16%), and miscellaneous methods (36.1%). Traditional initiation and microdosing initiation were compared in the data synthesis and analysis; miscellaneous methods were omitted given the high variability between methods. Overall, 95.6% of patients in the traditional initiation group and 96% of patients in the microdosing group successfully rotated to sublingual buprenorphine. Initiation regimens can vary widely depending on patient‐specific factors and buprenorphine formulation. A variety of buprenorphine transition strategies are published in the literature, many of which were effective for patients with OUD, pain, or both.
Keywords: buprenorphine, chronic pain, initiation, opioid use disorder
1. INTRODUCTION
Buprenorphine, a semi‐synthetic opioid, was developed in the 1960s and is derived from the thebaine alkaloid extracted from the poppy plant. 1 In 2002, the sublingual (SL) formulations, Subutex® and Suboxone®, were approved by the United States Food and Drug Administration (FDA) for opioid use disorder (OUD) and have since been the standard of care in treatment guidelines. 2 , 3 , 4 For acute and chronic pain indications, the FDA approved injectable buprenorphine in 1981, the transdermal (TD) system in 2010, and buccal film in 2015. 5 Although SL buprenorphine is not FDA indicated for pain, off‐label use has become popular among prescribers partially due to the difficulty in managing pain for patients with opioid misuse or OUD and its advantageous safety profile. 6 Unlike other opioids, buprenorphine is a partial μ‐opioid receptor agonist, κ‐opioid receptor antagonist, δ‐opioid receptor agonist, and orphan‐like receptor 1 (ORL‐1) agonist. 7 , 8 The partial agonism activity at the μ‐opioid receptor and the antagonism at the κ‐opioid receptor give rise to unique mechanistic differences compared to its full agonist counterparts.
Chronic pain is a pervasive condition, affecting over 100 million adults in the United States, with low back pain in particular being one of the top ten leading contributors to global decreases in disability‐adjusted life years from 1990 to 2019. 9 , 10 Simultaneously, harms from OUD are on the rise, with 2020 being the worst year yet for fatal opioid overdoses in the United States and Canada. 10 These overlapping concerns have led clinicians and other stakeholders to improve treatment strategies for patients with chronic pain, OUD, or both. 9 , 10 , 11 , 12 , 13 Due to its unique pharmacologic properties, buprenorphine is a suitable agent for patients with OUD and/or chronic pain. Buprenorphine possesses stronger affinity for the μ‐opioid receptor compared with full opioid agonists. A study comparing the binding affinity (Ki) of different opioids for the µ‐opioid receptor showed that buprenorphine had the second highest binding affinity with a Ki of 0.2157 nM. It demonstrated 120 times higher affinity compared to oxycodone, 15.6 times higher than methadone, 6.2 times higher than fentanyl, 5.4 times higher than morphine, and 1.7 times higher than hydromorphone. 14 Buprenorphine's high affinity for the μ‐receptor causes full agonist receptor displacement when given concomitantly and then is not displaced once bound. 15 The abrupt displacement of full agonists from the receptor can precipitate opioid withdrawal, which is the basis for patients to traditionally be in mild withdrawal prior to initiating buprenorphine therapy.
Another unique feature of buprenorphine is its ability to bind to a specific truncated subtype of the μ‐opioid receptor, the arylepoxamide receptor, which plays a role in its analgesic potential. 8 Although classified as a partial μ‐opioid receptor agonist, buprenorphine exhibited full analgesic efficacy for acute and chronic pain in rodent models. 16 These rodent models indicated that mice who lacked the arylepoxamide receptor did not experience pain relief with buprenorphine administration. 8 , 16
Traditional mu agonists have their place in pain management; however, their use is limited by opioid‐induced hyperalgesia, adverse events, and tolerance. Opioid‐induced hyperalgesia occurs due to multiple mechanisms. During opioid administration, dynorphin upregulation and binding to the kappa receptor produces an increased sensitivity and response to pain. 8 , 17 The antagonist activity of buprenorphine at the kappa receptor opposes the hyperalgesia effect produced by opioids. 17 Buprenorphine exhibits biased signaling of the μ‐opioid receptor thus only causing G‐protein‐dependent signaling. It does not recruit β‐arrestin to the receptor, which is associated with adverse effects, such as respiratory depression, constipation, and tolerance, seen with traditional opioids. 8 Buprenorphine, therefore, is a safer option, particularly for those at greater risk of opioid‐related adverse events (e.g., comorbid respiratory disease, co‐prescribed benzodiazepines). Given these actions, buprenorphine may have a niche role in the treatment of pain, particularly in patients with opioid‐induced hyperalgesia or individuals at an increased risk of opioid‐related adverse events, tolerance, and/or dependence. Buprenorphine is also an option for patients with comorbid OUD and pain, or those with uncontrolled pain despite escalating doses of opioids; however, given its pharmacologic profile, it can be difficult to transition patients to buprenorphine.
The traditional initiation regimen of buprenorphine for OUD considers the patient's current opioid regimen, timing of administration, and the pharmacology of buprenorphine. Guidelines recommend initiating buprenorphine once the patient is experiencing mild‐to‐moderate withdrawal symptoms indicated by a Clinical Opiate Withdrawal Scale (COWS) score of 11 to 12 or more after tapering or cessation of full opioid agonists. 2 The Subjective Opiate Withdrawal Scale (SOWS) is another assessment tool that can be used to determine whether a patient is experiencing withdrawal symptoms. The SOWS scores slightly differ from the COWS assessment, and mild‐to‐moderate withdrawal is defined as a score of 1 to 20. Buprenorphine initiation should begin approximately 6–12 h after short‐acting opioids and 24–72 h after long‐acting opioids. This traditional dosing regimen has proven to be challenging for patients with OUD due to the uncomfortable physical and psychological effects from opioid withdrawal (e.g., diaphoresis, muscle aches, agitation, and anxiety) leading to treatment failure, relapse, and potentially overdose. 18 , 19 , 20 The psychological effects of experiencing withdrawal prior to and during buprenorphine initiation can cause hesitancy and opposition when completing the initiation schedule and impacts patients' decisions to even attempt therapy again in the future. 19 Likewise, this approach can be problematic in patients with uncontrolled pain as interruption of opioid analgesics may exacerbate the pain, in addition to causing unpleasant withdrawal symptoms.
More recently, novel initiation approaches, such as buprenorphine microdosing, have been trialed to eliminate the need for anticipated opioid withdrawal associated with the traditional initiation method. Microdosing differs from traditional initiation by bypassing the requirement for acute withdrawal by overlapping smaller doses of buprenorphine with the full opioid agonist. With this method, small, repeated doses of buprenorphine slowly accumulate at the receptor causing a gradual displacement of full opioid agonists. The slow accumulation of buprenorphine at the receptor evades the precipitated withdrawal that is seen with larger doses, therefore eliminating the need for opioid discontinuation or tapering prior to buprenorphine initiation. The body of literature detailing the different buprenorphine initiation strategies that deviate from the traditional initiation regimen is growing. However, the majority of this literature involves case reports and case series. There is a lack of randomized controlled trials (RCTs) and prospective studies directly comparing the clinical outcomes between traditional initiation and microdosing approaches in patients with OUD and/or pain. Notably, the current buprenorphine medication labels and American Society of Addiction Medicine guidelines do not mention the microdosing approach. 2 , 4 , 21
This review will evaluate the available literature on buprenorphine initiation strategies for patients with OUD and/or pain. Traditional initiation regimens were defined as those regimens that included an opioid‐free period prior to buprenorphine initiation. Microdosing initiation regimens were defined as those that contained a period of concomitant buprenorphine and full‐agonist opioid administration. Other regimens that fell outside of these definitions were categorized as miscellaneous and are described separately. The goal of this paper was to synthesize the various buprenorphine initiation methods that have emerged and provide a beneficial reference for clinicians attempting these conversions.
2. METHODS
This review was conducted following the 2020 Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) checklist. 22
2.1. Eligibility criteria
To be eligible for inclusion in this review, studies needed to be published in the English language and describe the transition from prescribed or illicit opioids to SL buprenorphine and initiation outcomes for adult or adolescent patients with OUD and/or pain. Given the limited data on buprenorphine microdosing initiation regimens, included studies could be retrospective or prospective and include RCTs, observational studies, case reports, and case series. Systematic reviews were not included; however, the reference sections of relevant reviews were evaluated for independent studies that met inclusion criteria. Both inpatient and outpatient studies were included. Grey literature and animal studies were excluded from this review. The search years were not limited.
2.2. Information sources, search strategy
Embase, PubMed, and Cochrane Database were independently searched by one reviewer for published studies through November 26, 2021. MESH terms and search terms included the following: “buprenorphine,” “belbuca,” “buprenex,” “butrans,” “probuphine,” “sublocade,” “subutex,” “prefin,” “buprex,” “temgesic,” “microdosing,” “micro dosing,” “microdose,” “micro dose," "micro induction," "micro inductions," “rapid induction," "low dose," "low doses," “Bernese method,” “chronic pain,” “pain,” “dose‐response relationship,” “buprenorphine initiation,” “buprenorphine induction,” and “buprenorphine rotation.”
2.3. Selection process
After the initial database searches, duplicates were removed, and titles and abstracts were screened for inclusion. The studies deemed eligible for inclusion then underwent a full manuscript review. The relevant systematic reviews that populated in the initial search were also screened for additional individual studies.
2.4. Data collection process and data items
One author (LS) extracted data from all studies and another author (ED) conducted an audit to ensure data validity. For case reports and case series, the extracted data included the following: title, author, year of publication, study type, number of patients, age of patients, gender, initiation setting, buprenorphine indication (either OUD, pain, or both), previous illicit opioid use, previous OUD or pain treatments, pre‐initiation opioid regimen defined as the immediate regimen used prior to initiation, transition plans if hospitalized, current opioid agonist at time of initiation and oral morphine equivalents (OME), buprenorphine initiation regimen, duration of buprenorphine initiation, COWS/SOWS score range during initiation, highest COWS/SOWS score during initiation, initiation outcome (successful versus unsuccessful), status after initiation (relapsed [return to previous misuse], abstinent, or stable), and withdrawal symptoms during initiation. The initiation outcome was determined to be successful if the patient completed the full initiation schedule as described in the manuscripts.
For the cohort studies, the information extracted included the following: title, author, year of publication, study type, sample size, baseline characteristics, buprenorphine indication, initiation setting, reasons for buprenorphine microdosing initiation, buprenorphine initiation regimen, duration of initiation, withdrawal symptoms, and outcomes.
Information collected from the feasibility study included the following: title, author, year of publication, study type, sample size, baseline characteristics, indication, interventions, buprenorphine initiation regimen, duration of initiation, and outcomes.
2.5. Study risk of bias assessment
Each study was independently assessed for risk of bias using the Joanna Briggs Institute (JBI) critical appraisal tools by two authors (LS, ED). 23 , 24 , 25 Three separate tools were used depending on the study type. If there was a difference of opinion between the reviewers, the study was reviewed again and a joint decision on the risk of bias of the study was made. After assessing all studies, a mutual decision was made to exclude #4 in the JBI critical appraisal tool for case reports as this did not apply to our specific population.
2.6. Effect measures
Descriptive statistics were used to assess the range, mean, and median of data points in the synthesis of the case reports.
2.7. Synthesis methods
Included case studies were separated by indication which was comprised of pain, OUD, or both, prior to data synthesis. Studies were then further divided depending on the type of buprenorphine initiation regimen: (1) traditional initiation, (2) microdosing, and (3) miscellaneous. Microdosing initiation was further subdivided into: SL buprenorphine, TD buprenorphine, intravenous (IV) buprenorphine, and buccal buprenorphine. Any initiation that was outside the definitions of traditional or microdosing were included as miscellaneous. Data were reported as a number and percent or a range with the mean and/or median depending on the data. The median was collected for data that did not have a normal distribution, such as a significantly long duration of initiation or significantly high OME before initiation compared to other studies.
2.8. Reporting bias assessment
If data were missing for any case studies, it was collected as “not reported” during data collection. Likewise, during data synthesis an asterisk or other denotations were used to represent that not every case study reported information for that specific data point. The authors reached out to obtain more information from the included authors in this review when necessary.
2.9. Certainty assessment
In the microdosing studies, withdrawal symptoms were positive if the patient had any documented signs of mild withdrawal, indicated by the lowest threshold of a COWS score ≥5 or a SOWS score ≥1. In traditional initiation studies, withdrawal was expected prior to initiation, and it was distinguished from precipitated withdrawal in the microdosing cases in the data analysis.
3. RESULTS
3.1. Study selection
After the initial database search, 1436 records resulted. A total of 1151 records remained after duplicate removal, and the titles and abstracts were screened for further review. After the initial screening, a complete manuscript review of 70 records was performed. Records were excluded for the following reasons: poster abstracts (n = 8), lacked a specific dosing regimen (n = 6), were clinical reviews or letters to the editor (n = 5), only included opioid dependence diagnosis (n = 3), were low quality based on meeting only one JBI criterion (n = 2), evaluated an unrelated medication (n = 1), described buprenorphine maintenance rather than initiation (n = 1), did not transition from opioids (n = 1), included only the protocol (n = 1), or published in another language other than English (n = 1). (Figure 1). After reviewing the relevant systematic reviews, seven more studies were evaluated and included in the review. A total of 7 observational studies, 1 feasibility study, and 39 case reports and case series were included, totaling 48 studies (Figure 1).
FIGURE 1.
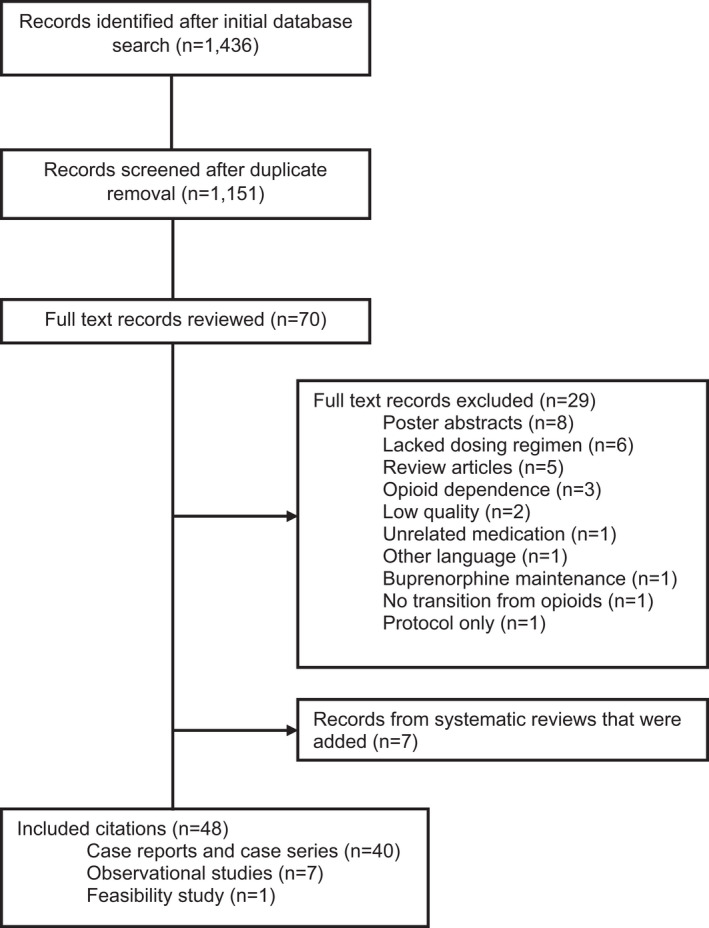
Search strategy and study inclusion
3.2. Study characteristics
One thousand one hundred and ten initiations were included between the observational/feasibility studies (n = 982) and the case reports/series (n = 128). The majority of patients were male patient (60.9%), with a diagnosis of OUD (63% vs. 29.3% with pain and 7.7% with both), who completed a transition to buprenorphine in the inpatient setting (69%). Traditional initiation was completed in 47.9% of initiations, while microdosing was utilized in 16% of initiations. The remaining 36.1% of patients were transitioned to buprenorphine using a miscellaneous method. These characteristics are summarized in Table 1. Individual study characteristics are presented in Tables 2, 3, 4, 5, 6 (observational/feasibility) and Tables 7 and 8 (case reports/case series). The following sections provide more detailed information on patients rotated to buprenorphine using traditional initiation (Tables 2, 5, and 7) and microdosing initiation (Tables 3, 6, and 7). Miscellaneous initiation strategies are included in Tables 4 and 7. Within each table, those that included patients with OUD are listed first, followed by pain, then both diagnoses.
TABLE 1.
Patient and buprenorphine initiation characteristics
| Patient characteristic | N (%) |
|---|---|
| Age, range | 16–84 |
| Gender | |
| Male | 563 (60.9) |
| Female | 359 (38.9) |
| Unknown | 2 (0.2) |
| Total patients | 924 |
| Buprenorphine indication | |
| OUD | 700 (63.0) |
| Pain | 325 (29.3) |
| Both | 85 (7.7) |
| Setting | |
| Inpatient | 766 (69.0) |
| Outpatient | 344 (31.0) |
| Buprenorphine initiation strategy | |
| Traditional initiation | 532 (47.9) |
| Microdosing | 177 (16.0) |
| Using SL BUP | 82 (7.4) |
| Using the BUP patch | 91 (8.2) |
| Using IV BUP | 3 (0.3) |
| Using the BUP buccal film | 1 (0.1) |
| Miscellaneous | 401 (36.1) |
| Total initiations | 1110 |
Abbreviations: BUP, buprenorphine; IV, intravenous; OUD, opioid use disorder; SL, sublingual.
TABLE 2.
Observational studies that described traditional buprenorphine initiation for patients with OUD
| Author, year | Sample size | Study type | BUP indication (%) | Inpatient, n (%) | Intervention | Starting BUP dose (mg)/day, (mean) | Duration of initiation (days), range (mean) | Withdrawal symptoms, n (%) | Completed induction, n (%) | Post initiation outcome | Funding |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 28 Jones HE, 2006 | 4 | Open‐label exploratory study | OUD (100) | 0 (0) | SL BUP | 12–16 mg (14 mg) | 9–13 days | 4 (100); SOWS score ranged from 1–24 | 0 (0) | 100% returned to methadone use after initiation | Govt |
| 27 Moe J, 2020 a | 21 | Feasibility study | OUD (100) | 0 (0) | SL BUP | 1 mg | 6 days | NR | NR | 23.8% remained on OAT at the 30‐day follow‐up | NR |
| 74 Herring AA, 2021 a | 213 | Retrospective record review | OUD (100) | 213 (100) | SL BUP | NA | 1 day | 4 (1.9) experienced precipitated withdrawal | 213 (100) | Traditional initiation was safe and tolerated | Govt, Edu |
Abbreviations: BUP, buprenorphine; Edu, educational institution; Govt, government; NR, not reported; OAT, opioid agonist therapy; OUD, opioid use disorder; SL, sublingual; SOWS, subjective opiate withdrawal scale.
TABLE 3.
Observational studies that described microdosing buprenorphine initiation for patients with OUD
| Author, year | Sample size | Study type | BUP indication (%) | Inpatient, n (%) | Intervention | Starting BUP dose (mg)/day | Duration of initiation (days) | Withdrawal symptoms | Completed induction, n (%) | Post initiation outcome | Funding |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 27 Moe J, 2020 a | 25 | Feasibility study | OUD (100) | 0 (0) | SL BUP | 1 mg | 6 days | NR | NR | 32% of patients in the microdosing group remained on OAT at the 30‐day follow‐up | NR |
Abbreviations: BUP, buprenorphine; NR, not reported; OAT, opioid agonist therapy; OUD, opioid use disorder.
Studies that compared traditional initiation to microdosing initiation. The traditional initiation method can be found in Table 2.
TABLE 4.
Observational studies that described miscellaneous initiation for patients with OUD
| Author, year | Sample size | Study type | BUP indication (%) | Inpatient, n (%) | Intervention, n (dose) | Duration of initiation, days | Withdrawal symptoms, n (%) | Completed induction, n (%) | Post initiation outcome | Funding |
|---|---|---|---|---|---|---|---|---|---|---|
| 74 Herring AA, 2021 a | 366 | Retrospective record review | OUD (100) | 391 (100) | High‐dose SL BUP (>12 mg/day) | 1 day | 1 (0.3) experienced precipitated withdrawal | 366 (100) | Patients treated with a high‐dose buprenorphine initiation did not experience toxicity | Govt, Edu |
Abbreviations: BUP, buprenorphine; Edu, educational institution; Govt, government; NR, not reported; OUD, opioid use disorder; SL, sublingual.
Studies that compared traditional initiation to miscellaneous initiation. The traditional initiation method can be found in Table 2.
TABLE 5.
Observational studies that described traditional buprenorphine initiation for patients with pain
| Author, year | Sample size | Study type | BUP indication (%) | Inpatient, n (%) | Intervention | Starting BUP dose (mg)/day range (mean) | Duration of initiation (days) | Withdrawal symptoms | Completed induction, n (%) | Post initiation outcome | Funding |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 52 Daitch J, 2012 | 104 | Observational study | Pain (100) | 0 (0) | SL BUP | 8–16 mg/day | 7 days | NR | NR | The difference in pain scores at baseline and after conversion to SL BUP were statistically and clinically significant. | NR |
| 53 Rosenblum A, 2012 | 12 | Observational study | Pain (100) | 0 (0) | SL BUP | 2–8 mg (4 mg) | NR | COWS: 1–23; SOWS: 4–56 after the first dose | 4 (33.3) | Average pain for all patients significantly declined from baseline (mean = 6.6) to after baseline (mean = 3.4), | Govt |
| 51 Malinoff HL, 2005 | 95 | Cohort study | Pain (100) | 0 (0) | SL BUP | 1–2 mg | 1 day | NR | 89 (93.7) | Pain reports were improved in 86% of patients | NR |
| 41 Berland DW, 2013 | 76 | Cohort study | Pain (100) | 76 (100) | IM then SL BUP | 2–8 mg/day | 1–6 (median 2) | Reported no provoked withdrawal or severe withdrawal symptoms | 76 (100) | 20% returned to full agonist use and 33% reported no improvement in pain | NR |
Abbreviations: BUP, buprenorphine; COWS, clinical opiate withdrawal scale; Govt, government; NR, not reported; SL, sublingual; SOWS, subjective opiate withdrawal scale.
TABLE 6.
Observational studies that described microdosing buprenorphine initiation for patients with OUD and pain
| Author, year | Sample size | Study type | BUP indication, n | Inpatient, n (%) | Intervention | Starting BUP dose (mg)/day | Duration of initiation, days (mean) | Withdrawal symptoms | Completed induction, n (%) | Post initiation outcome | Funding |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 45 Button D, 2021 | 66 | Cohort study | OUD and pain | NR | BUP TD patch then SL BUP | NR | 1–15 (6) | NR | NR for individual indications; overall 50 initiations were successful rate was 50 (69.4) | NR | Govt, Edu |
Abbreviations: BUP, buprenorphine; Edu, educational institution; Govt, government; NR, not reported; OUD, opioid use disorder; SL, sublingual; TD, transdermal.
TABLE 7.
Case studies
| Author, year | No. of patients | Indication(s) | OME before initiation | Strategy | Duration | Success Rate | Funding |
|---|---|---|---|---|---|---|---|
| 29 Agapoff JR, 2019 | 1 | OUD | Unable to calculate a | Traditional initiation | 1 day | 100% | NR |
| 26 Mariani JJ, 2020 | 5 | OUD | Unable to calculate a | Traditional initiation | 2–3 days | 100% | Govt |
| 34 Azar P, 2020 | 1 | OUD | 125,000–250,000 | Microdosing with SL BUP | 4 days | 100% | Govt |
| 36 Brar R, 2020 | 7 | OUD | 150–250,000 | Microdosing with SL BUP | 8 days | 100% | F, Govt |
| 32 Caulfield MDG, 2020 | 1 | OUD | 8700 | Microdosing with SL BUP | 24 days | 100% | NR |
| 37 DeWeese JP, 2021 | 1 | OUD | 1418 | Microdosing with SL BUP | 10 days | 100% | Ind |
| 30 Hammig R, 2016 | 2 | OUD | 1120 a | Microdosing with SL BUP | 9–33 days | 100% | NR |
| 42 Jafari S, 2021 | 1 | OUD | 2400 | Microdosing with SL BUP | 120 days | 100% | NR |
| 33 Payler DK, 2016 | 6 | OUD | 80‐200 a | Microdosing with SL BUP | 2–11 days b | 83% | NR |
| 31 Rozylo J, 2020 | 1 | OUD | 600 | Microdosing with SL BUP | 7 days | 100% | NR |
| 44 Singh G, 2021 | 2 | OUD | 420–500 | Microdosing with SL BUP | 6–7 days | 100% | NR |
| 38 Vogel M, 2019 | 1 | OUD | 1340 | Microdosing with SL BUP | >250 days | 100% | NR |
| 48 De Aquino JP, 2020 | 1 | OUD | 900 | Microdosing with BUP TD patch | 12 days | 100% | Govt |
| 50 Crane K, 2020 | 1 | OUD | 500 | Microdosing with IV BUP | 6 days | 100% | NR |
| 75 Hess M, 2011 | 11 | OUD | 600–1200 | Miscellaneous | 4 days | 91% | NR |
| 76 Azar P, 2018 | 1 | OUD | 60 | Miscellaneous | 1 day | 100% | NR |
| 77 Tang VM, 2020 | 23 |
OUD Pain |
152.2–325.7 | Miscellaneous | 2–6 days | 96% | NR |
| 39 Vytialingam RC, 2021 | 2 |
OUD Pain |
900–2500 | Microdosing with SL BUP | 8–13 days | 100% | NR |
| 35 Robbins JL, 2021 | 8 |
OUD Pain |
75–240 | Microdosing with SL BUP | 6 days | 100% | NR |
| 56 Becker WC, 2020 | 6 | Pain | 105–390 | Microdosing with SL BUP | 5 days | 100% | NR |
| 54 Buchheit BM, 2020 | 2 | Pain | 106– 270 | Microdosing with SL BUP | 7–8 days | 100% | NR |
| 58 Crum IT, 2020 | 1 | Pain | 1655 | Microdosing with SL BUP | 6 days | 100% | NR |
| 57 Irwin M, 2021 | 1 | Pain | 109 | Microdosing with SL BUP | 3 days | 100% | NR |
| 78 Irwin M, 2021 | 1 | Pain | 155 | Microdosing with SL BUP | 9 days | 100% | NR |
| 55 Lee DS, 2020 | 1 | Pain | 177 | Microdosing with SL BUP | 5 days | 100% | Govt |
| 59 Tara A, 2021 | 1 | Pain | Unable to calculate a | Microdosing with SL BUP | 19 days | 100% | NR |
| 60 Kornfeld H, 2015 | 3 | Pain | 40–320 | Microdosing with BUP TD patch | 5 days b | 100% | NR |
| 61 Weimer MB, 2021 | 1 | Pain | 750–1282 | Microdosing with BUP buccal film | 7 days | 100% | NR |
| 40 Ward HB, 2019 | 1 | OUD/Pain | 800 | Traditional initiation | 1 day | 100% | NR |
| 66 Hamata B, 2020 | 1 | OUD/Pain | Unable to calculate a | Microdosing with SL BUP | 4 days | 100% | NR |
| 62 Klaire S, 2019 | 2 | OUD/Pain | Unable to calculate a | Microdosing with SL BUP | 3–5 days | 100% | NR |
| 64 Martin L, 2019 | 2 | OUD/Pain | Unable to calculate a | Microdosing with SL BUP | 14–16 days | 100% | NR |
| 63 Mortaji P, 2021 | 1 | OUD/Pain | 86 | Microdosing with SL BUP | 7 days | 100% | NR |
| 65 Sandhu, 2019 | 1 | OUD/Pain | 145 | Microdosing with SL BUP | 7 days | 100% | NR |
| 67 Stanciu CN, 2021 | 1 | OUD/Pain | Microdosing with SL BUP | 4 days | 100% | NR | |
| 43 Terasaki D, 2019 | 3 | OUD/Pain OUD | 320–1230 | Microdosing with SL BUP | 8 days | 100% | NR |
| 47 Raheemullah A, 2019 | 15 |
OUD/Pain OUD Pain |
30–341 | Microdosing with BUP TD patch | 4 days | 100% | NR |
| 46 Saal D, 2020 | 5 |
OUD/Pain OUD Pain |
45‐640 a | Microdosing with BUP TD patch | 5–7 days | 100% | NR |
| 68 Thakrar AP, 2021 | 2 | OUD/Pain | 320 a | Microdosing with IV BUP | 3–4 days | 100% | NR |
Abbreviations: BUP, buprenorphine; Edu, educational institution; F, foundation; Govt, government; Ind, industry; IV, intravenous; NR, not reported; OME, oral morphine equivalents; OUD, opioid use disorder; SL, sublingual; TD, transdermal.
Unable to calculate in some cases
Not reported in some cases
TABLE 8.
Case reports data synthesis
| BUP initiation strategy | Number of patients, n (%) | Age, range (mean) | Male, n (%) | Inpatient setting, n (%) | Previous heroin use, n (%) | OME prior to initiation, range (mean, median) | BUP starting dose, mg/day, range (mean, SD) | BUP ending dose (mg/day), range (mean, SD) | Duration of full opioid agonist overlap in days, range (mean) | Duration of initiation in days, range (mean, median) | Highest COWS/SOWS score reported during initiation | Experienced withdrawal, n (%) | Successful initiation, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OUD Indication (n = 45) | |||||||||||||
| Traditional initiation | 6 (13.3) | 28–55 (40) | 6 (100) | 0 (0) | 5 (83.3) | Unable to calculate | 2–24 (18, 9.63) | 8–16 (14.7, 3.27) | NA | 1–3 (2.3) | 16 (COWS) a | 5 (83.3) a | 6 (100) |
| Microdosing with SL BUP | 28 (62.3) | 19–67 (40.7) | 17 (60.7) | 5 (17.9) | 14 (50) a | 80–250,000 (18406, 550) a | 0.2–2 (0.7,0.64) a | 8–32 (16.7, 7.89) a | 2–28 (7.9) a | 2‐ >250 (21.8, 8) a | 9 (COWS), 11 (SOWS) a | 6 (21.4) a | 27 (96.4) |
| Microdosing with BUP patch | 10 (22.2) | 21–65 (43.4) | 8 (80) | 8 (80) | 7 (70) a | 30–1680 (359.6, 106.5) a | 5–35 μg/h patch (16.5, 5.79) | 7–24 (12.6, 5.25) | 1–10 (2.7) | 2–12 (4.9) | 16 a | 6 (60) a | 10 (100) |
| Microdosing with IV BUP | 1 (2.2) | 62 | 1 (100) | 1 (100) | 1 (100) | 500 | 0.1 b | 10 | 4 | 6 | 10 | 1 (100) | 1 (100) |
| Pain indication (n = 29) | |||||||||||||
| Microdosing with SL BUP | 20 (69) | 11–76 (53.8) | 11 (55) | 6 (30) | NA | 65–2500 (375.6, 155) | 0.5–2 (0.67, 0.46) a | 0–18 (9.6, 5.72) | 2–18 (6.9) a | 3–19 (7.4) a | 12 a | 2 (0.1) a | 20 (100) |
| Microdosing with BUP patch | 8 (27.6) | 38–72 (55.3) | 6 (75) | 3 (37.5) | NA | 32–320 (118.3, 60) a | 10–20 μg/h patch (16.25, 5.18) | 0.75–32 (13.9, 12.23) | 0–4 (1.8) a | 4–6 (4.7) a | 3 a | 0 (0) a | 8 (100) |
| Microdosing with BUP buccal film | 1 (3.4) | 59 | 0 (0) | 1 (100) | NA | 750–1282 | 225 μg film | 16 | 6 | 7 | 3 | 0 (0) | 1 (100) |
| OUD and pain indication (n = 19) | |||||||||||||
| Traditional initiation | 1 (5.3) | 38 | 0 (0) | 1 (100) | 1 (100) | 800 | 26 | 26 | NA | 1 | 17 | 1 (100) | 1 (100) |
| Microdosing with SL BUP | 9 (47.4) | 29–63 (40.3) | 1 (11.1) a | 9 (100) | 7 (77.8) a | 86–1230 (369.3, 120) a | 0.25–8 (1.8, 2.44) | 10–24 (15.7, 4.18) | 1–16 (6.6) | 3–16 (7.6) | 2 a | 0 (0) | 9 (100) |
| Microdosing with BUP patch | 7 (36.8) | 21–67 (48) | 4 (57.1) | 6 (85.7) | 4 (57.1) a | 75–640 (262.4, 230) | 10–20 μg/h patch (18.6, 3.78) | 4–16 (11.4, 4.28) | 3–6 (3.6) | 4–7 (4.9) | 5 a | 2 (28.6) a | 7 (100) |
| Microdosing with IV BUP | 2 (10.5) | 60–65 (62.5) | 0 (0) | 2 (100) | NR | 320 a | 0.6 b | 16–28 (22, 8.49) | 3–4 (3.5) | 3–4 (3.5) | NR | 0 (0) a | 2 (100) |
Abbreviations: BUP, buprenorphine; IV, intravenous; NR, not reported; OME, oral morphine equivalents; OUD, opioid use disorder; SL, sublingual; TD, transdermal.
Not reported in some cases.
IV dose.
3.3. Overall success rates
In total, the success rates between traditional initiation versus microdosing initiation were comparable, with 95.6% and 96% successful, respectively.
3.4. Initiation outcomes of patients with OUD
3.4.1. Traditional initiation
Two hundred and forty‐four traditional initiations were utilized for patients with a diagnosis of OUD. 26 , 27 , 28 , 29 Pre‐initiation drug use included heroin, fentanyl, oxycodone, and methadone. The mean pre‐initiation OME was 770 mg; however, the pre‐initiation dosages were not reported in three of the four studies. Traditional initiation methods utilized the SL formulation of buprenorphine. The mean daily starting dose of SL buprenorphine was 16.4 mg, and the mean daily ending dose was 15.2 mg. The duration of initiation varied from 1 day to 13 days for all patients. The success rate for all patients in this group was 98.2%; however, neither success nor completion rate was reported in the study performed by Moe and colleagues. 27 When reported, a total of six patients either relapsed or returned to their pre‐initiation drug use. 26 , 28
3.4.2. Microdosing initiation
Seventy microdosing initiations were utilized for patients with a diagnosis of OUD. The overall success rate among the three different buprenorphine formulations was 98.6%.
3.4.3. Microdosing initiation with SL buprenorphine
Fifty‐three initiations utilized SL buprenorphine for patients with OUD. Pre‐initiation drug use included heroin, 30 , 31 , 32 , 33 fentanyl, 34 , 35 , 36 morphine, 35 , 36 , 37 hydrocodone, 35 oxycodone, 35 hydromorphone, 35 diacetyl morphine, 30 , 38 and methadone. 20 , 33 , 35 , 37 , 39 , 40 The mean OME was 18,045 mg. The mean starting buprenorphine daily dose for all patients was 0.84 mg, and the mean ending dose was 20.2 mg. Initiation success/completion rates and relapse rates were not reported in one study. 41 For the remaining patients, 96.4% were successfully transitioned to buprenorphine and 16.3% relapsed post‐initiation (n = 8). 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 42 , 43 , 44 , 45
3.4.4. Microdosing initiation with buprenorphine TD patch
Ten initiations utilized buprenorphine TD patches to transition to SL buprenorphine for patients with OUD. Pre‐initiation drug use included heroin 46 , 47 and methadone, 46 , 48 , 49 and the mean OME prior to initiation was 359.6 mg. The patch was discontinued anywhere from the second day of initiation to the fifth day, and SL buprenorphine was initiated on either the second day or the fourth day. The patch was initiated at a mean daily dose of 16.5 μg/h with a mean ending SL buprenorphine dose of 12.6 mg. The mean duration of full opioid agonist therapy overlap with the buprenorphine patch was 2.7 days and the mean duration of initiation was 4.9 days. Six patients experienced withdrawal, but all patients had a successful initiation. There were no reports of relapse post‐initiation. 46 , 47 , 48 , 49
3.4.5. Microdosing initiation with IV buprenorphine
One initiation utilized IV buprenorphine to transition to SL buprenorphine for a patient with OUD. This patient had a history of previous heroin use, on chronic methadone, and the total OME prior to initiation was 500 mg. Intravenous buprenorphine was initiated at 0.1 mg and was titrated up to 1.6 mg per day with methadone 50 mg daily. The methadone was not tapered during the regimen and was discontinued on day 5 when SL buprenorphine was added. The SL buprenorphine total daily dose at the end of the initiation on day 6 was 10 mg. Although the patient experienced some withdrawal symptoms, they were transitioned successfully to buprenorphine and remained abstinent at the 4‐week follow‐up. 50
3.5. Initiation outcomes of patients with pain
3.5.1. Traditional initiation
Two hundred and eighty‐seven traditional initiations were utilized for patients with a diagnosis of pain. 41 , 51 , 52 , 53 The reported pre‐initiation opioids included oxycodone, 41 , 52 , 53 fentanyl, 41 , 52 , 53 hydrocodone, 52 methadone, 41 , 52 , 53 oxymorphone, 52 codeine, 53 and morphine. 41 , 52 , 53 The OME was not reported in each study, but it ranged from 15 mg to 450 mg. Sublingual buprenorphine was initiated at doses ranging from 1 mg to 16 mg. Duration of initiation lasted anywhere from 1 day to 7 days. Most patients had improvement in pain scores after initiation. The success rate for all patients who underwent traditional initiation was 92.3%, but Daitch and colleagues did not report completion or success rates. 52 When reported, 5.6% of patients returned to full opioid agonist use after initiation. 41
3.5.2. Microdosing initiation
Twenty‐nine microdosing initiations were utilized for patients with a diagnosis of pain. The overall success rate among the three different buprenorphine formulations was 100%.
3.5.3. Microdosing initiation with sublingual buprenorphine
Twenty initiations utilized SL buprenorphine for patients with pain. Previous drug use included methadone, 35 , 44 , 54 , 55 , 56 oxycodone, 35 , 54 , 55 , 56 fentanyl, 35 hydrocodone‐acetaminophen, 35 morphine, 35 , 56 , 57 and hydromorphone. 35 , 58 The mean OME was 375.6 mg prior to buprenorphine administration. The mean starting and ending daily doses of SL buprenorphine were 0.73 mg and 9.6 mg, respectively. The mean duration of initiation was 6.9 days. All 20 patients successfully completed the initiation, but 20% returned to full opioid agonist use after the initiation. 35 , 39 , 44 , 54 , 55 , 56 , 57 , 58 , 59
3.5.4. Microdosing initiation with buprenorphine TD patch
Eight initiations utilized buprenorphine TD patches to transition to SL buprenorphine for patients with pain. Patients had previously tried oxycodone, 46 , 60 tapentadol, 46 hydromorphone, 46 , 60 and hydrocodone‐acetaminophen 46 , 60 for pain management. The mean OME prior to initiation was 118.3 mg. The buprenorphine patch was started at a mean dose of 16.25 μg/h in addition to continuing full opioid agonists. In some cases, the buprenorphine patch was overlapped with SL buprenorphine. The mean ending SL buprenorphine daily dose was 13.9 mg. The mean duration of initiation was 4.7 days. All eight patients were successfully transitioned to buprenorphine and there were no reports of patients transitioning back to full opioid agonists. 46 , 47 , 60
3.5.5. Microdosing initiation with buprenorphine buccal film
One initiation utilized the buprenorphine buccal film to transition to SL buprenorphine for one patient with pain. The buccal formulation was started on the first day of initiation at 225 μg in addition to a morphine patient‐controlled analgesia (PCA) pump with an OME range of 750–1282 mg. The buccal film was subsequently increased to 450 μg by day 3. On day 4, the buccal film was substituted for 2 mg of SL buprenorphine twice daily. The morphine PCA was discontinued after 6 days, and the initiation was successfully completed on the seventh day. The ending SL buprenorphine dose was 16 mg, and the patient successfully completed the initiation. At the 3‐ and 6‐month follow‐ups, the patient was stable on buprenorphine and did not require full opioid agonist use for pain management. 61
3.6. Initiation outcomes of patients with OUD and pain
3.6.1. Traditional initiation
One traditional initiation was utilized for one patient with a diagnosis of OUD and pain. The patient had a previous history of heroin use and, the daily pre‐initiation regimen included methadone and oxycodone, with an OME of 800 mg. The patient was given naltrexone to induce withdrawal prior to starting SL buprenorphine. Sublingual buprenorphine was given as 2 mg shortly after the patient was in withdrawal, followed by 4 mg an hour later, and finally 8 mg 4 h after the previous dose, totaling 26 mg altogether for the 1‐day initiation. The patient completed the initiation; however, the patient relapsed shortly after. 40
3.6.2. Microdosing initiation
Eighty‐four initiations utilized SL buprenorphine for patients with a diagnosis of OUD and pain. The overall success rate among the three different buprenorphine formulations was 100%.
3.6.3. Microdosing initiation with SL buprenorphine
Nine initiations utilized SL buprenorphine for patients with OUD and pain. Seventy‐eight percent of patients had prior heroin use, 62 and the current opioid agonists at the time of initiation included hydromorphone, 62 , 63 , 64 , 65 fentanyl, 66 oxycodone, 43 and methadone. 43 , 62 The mean OME prior to starting buprenorphine was 369.3 mg. The microdosing regimen started with a mean SL buprenorphine daily dose of 1.8 mg and was continued for a mean of 8 days. Buprenorphine and the full opioid agonist were continued for a mean duration of 6.6 days, and the mean buprenorphine dose at the end of the initiation was 15.7 mg. All nine patients successfully completed the initiation and no patient relapsed. 43 , 62 , 63 , 64 , 65 , 66 , 67
3.6.4. Microdosing initiation with buprenorphine TD patch
Seventy‐three initiations utilized the buprenorphine TD system followed by SL buprenorphine for patients with OUD and pain. Fifty‐seven percent had a history of previous heroin 46 , 47 use and the current opioid agonists at the time of the transition were hydromorphone 46 and fentanyl, 46 but it was only reported in two patients. The mean OME prior to initiation was 230.2 mg between the case reports and the observational study. The transdermal system was initiated on the first day at doses ranging from 10 to 20 μg/hour while the patient transitioned onto SL buprenorphine. On the last day of initiation, the SL buprenorphine daily doses ranged from 4 to 16 mg, and the duration of initiation ranged from 4 to 10 days. Seven initiations were successful; however, the 66 initiations described by Button and colleagues did not include success or completion rates for individual diagnoses and were therefore excluded from this calculation. 45 There were no reports of patients relapsing or transitioning back to full opioid agonist use. 45 , 46 , 47
3.6.5. Microdosing initiation with IV buprenorphine
Two initiations utilized IV buprenorphine to transition to SL buprenorphine for patients with OUD and pain. Before buprenorphine initiation, one patient was taking methadone with a total daily OME of 320 mg and the other was using an illicit opioid. Both patients were started on IV buprenorphine 0.15 mg every 6 h in addition to a full opioid agonist which was continued in tandem for a mean of 3.5 days. In both cases, SL buprenorphine was initiated on the last day, with a mean ending daily dose of 22 mg. Both patients completed the regimen successfully. The first patient was lost to follow‐up, but the second patient remained in remission for OUD and her pain was controlled at her 6‐week follow‐up. 68
4. DISCUSSION
4.1. Summary of findings
This systematic review aimed to evaluate the reported methods of buprenorphine initiation for patients with diagnoses of OUD, pain, or both. In total, the vast majority of initiations were successful. From the 1110 initiations included across the observational studies and case reports, 709 were initiated with a traditional method or microdosing method and were therefore included in the synthesis and analysis. The patients who were initiated using miscellaneous methods were not included in the data synthesis or final analysis due to the high variability between methods, but the individual characteristics can be found in the preceding tables. Omitting the miscellaneous methods, 44.3% were initiated on buprenorphine for OUD, 44.6% for pain, and 10.7% for both diagnoses.
4.2. Overall outcomes of patients with OUD
The success rate for patients initiated on buprenorphine for OUD was 98.3%. From these patients, 7.5% (n = 22) experienced withdrawal. Nine of these patients were initiated using the traditional initiation method where withdrawal was expected. 26 , 28 The remaining 13 patients were initiated using the microdosing method with SL buprenorphine (n = 6), 30 , 32 , 34 , 38 , 39 , 44 TD buprenorphine (n = 6), 47 , 49 or IV buprenorphine (n = 1). 50 A total of two patients experienced precipitated withdrawal during the induction, one patient in the SL microdosing group 36 and one patient in the TD microdosing group. 49 Mild‐to‐moderate withdrawal symptoms were reported among the other patients and included headache, anxiety, diaphoresis, tachycardia, hypertension, nausea, yawning, and general discomfort. The relapse rate for patients initiated on buprenorphine for OUD was 13.9%, and the methods utilized in these cases were traditional initiation (n = 6) 26 , 28 and microdosing with SL buprenorphine (n = 8). 30 , 31 , 34 , 36 , 43
4.3. Overall outcomes of patients with pain
The success rate for patients initiated on buprenorphine for pain was 95.6%. From these patients, 0.6% (n = 2) experienced mild withdrawal. The method utilized for both patients was microdosing with SL buprenorphine (n = 2), and the withdrawal symptoms included anxiety, pain, and restlessness. 39 , 55 The rate of patients who transitioned back to full agonist use was 6%, and the regimen utilized was microdosing with SL buprenorphine (n = 19). 35 , 41 , 56 , 57
4.4. Overall outcomes of patients with OUD and pain
The success rate for patients initiated on buprenorphine for both indications was 100%; however, this percentage most likely does not represent the true success rate due to the outcomes reported in the observational study by Button et al. 45 A reported total of 69.4% of patients completed the initiation in the hospital, but this was for all initiations and was not broken down by indication. The remaining patients were scheduled to complete the initiation in the outpatient setting or discontinued initiation during the hospitalization due to adverse effects. 45 The number of patients who completed the initiation as outpatients was not reported, and therefore, this study could not be included in the calculation of the success rate.
The mild‐to‐moderate withdrawal rate for patients initiated on buprenorphine for both indications was 3.5%. From these patients, the regimens utilized were traditional initiation (n = 1) and microdosing with TD buprenorphine (n = 2). Withdrawal symptoms included restlessness, joint aches, diarrhea, vomiting, tremor, yawning, and anxiety. 40 , 47 Only one patient relapsed post‐traditional initiation. 40
Overall, 95.6% of patients in the traditional initiation group and 96% of patients in the microdosing group successfully rotated to SL buprenorphine. It is clear from these data that switching to buprenorphine is both well‐tolerated and effective for OUD, pain, and dual indications, although direct comparisons are limited. The success rates for each indication were relatively comparable with the lowest success rate occurring in the pain indication group. This could be explained by the complicated hospitalizations that some patients experienced.
Systematic reviews have been performed that evaluate the efficacy and tolerability of buprenorphine microdosing. The systematic review conducted by Moe and colleagues assessed the buprenorphine regimens for OUD from 20 studies that included 57 patients. 69 All patients completed the microdosing initiation, but 38.5% experienced withdrawal symptoms during the transition as assessed by the authors. 69
A systematic review performed by Adams and colleagues evaluated different buprenorphine initiation regimens in 24 patients. There were 10 patients (41.7%) that trialed buprenorphine for OUD and for the combined indication of OUD and pain management. Buprenorphine was used for analgesia in the remaining four patients. They described SL microdosing, microdosing using a buprenorphine patch, and bridging with a fentanyl patch among others. The authors reported a 92% completion rate among the different dosing protocols. 70
Ahmed and colleagues completed a systematic review in 2021 that also analyzed the different buprenorphine microdosing strategies in the literature. Their review described regimens from 18 studies and included a total of 63 patients. The same microdosing formulations were described in this review, and the authors reported a 100% completion rate. According to the authors, a total of 58.3% of patients experienced some type of withdrawal symptoms during the initiation. 71
To our knowledge, this is the first review comparing traditional initiation to microdosing initiation, as well other types of initiation such as high‐dose initiation. A direct comparison between traditional buprenorphine initiation and microdosing was conducted in the feasibility study by Moe and colleagues in 2020. 27 More patients in the microdosing group had better outcomes at the 30‐day follow‐up compared to traditional initiation. This is currently the only available direct comparator study of both types of initiation regimens that was found. Randomized controlled trials are being performed comparing traditional buprenorphine initiation against microdosing initiation strategies for OUD. The results from the RCTs will hopefully further guide clinical practice with non‐traditional initiation regimens. Buprenorphine microdosing initiation is an enticing strategy to transition patients off traditional opioid agonists both in the context of chronic pain and opioid misuse. Avoidance of an opioid‐free period and mild withdrawal is a common reason for using microdosing initiations in patients who are dependent on opioids for analgesia. 45 , 72 A history of experiencing or witnessing precipitated withdrawal or anxiety about withdrawal can make patients or clinicians wary of the transition to buprenorphine, making microdosing initiation attractive in this population as well. 45 Furthermore, the increasing use of illicit fentanyl and resultant pharmacologic challenges can make the “opioid washout” necessary for traditional inductions difficult in clinical practice. 73 The results of this review make clear that both traditional and non‐traditional initiations are usually successful in transitioning patients to buprenorphine; however, microdosing initiations may become more commonplace as buprenorphine use for chronic pain becomes more commonplace and traditional initiations in the setting of opioid misuse become more fraught.
4.5. Limitations
Due to the limited available literature on this topic, the records examined and included in this review consisted primarily of retrospective observational research. Our data predominantly came from observational studies (n = 796). 27 , 28 , 41 , 45 , 51 , 52 , 53 , 74 Therefore, the data gathered from the included literature were not as robust as data from prospective studies and could be representative of only positive outcomes and not inclusive of all transitions.
Because there was no standardized method of reporting individual cases or observational data, data collection was limited to what was reported by the authors. Information about full opioid agonist use, initiation strategy, and the presence or the absence of withdrawal symptoms was insufficient in some cases. Our methods attempted to mitigate this limitation by collecting all relevant information from each study, recording when data points were absent, arranging the information based on indication, and further organizing that data according to initiation strategy.
5. CONCLUSION
Initiation regimens can vary widely depending on the buprenorphine formulation, decision to overlap with full agonists, and starting and ending doses. A variety of initiation strategies were presented in this review, and we found that many patients effectively transitioned from opioids to buprenorphine regardless of strategy. Based on the data presented in the review, clinicians should individualize buprenorphine initiation for each patient depending on prior illicit drug use or opioid use, treatment setting, indication, timeframe, and goals of care. For patients with previous experience with intolerable withdrawal symptoms or for those wishing to avoid withdrawal symptoms altogether, a microdosing approach is reasonable. For patients where there is a more immediate need to transition to buprenorphine, a traditional initiation may be preferred. Both strategies can be completed in or out of the hospital depending on the patient; however, more prudent monitoring is often warranted. Future studies should be conducted that directly compare traditional and microdosing initiation strategies.
CONFLICT OF INTEREST
The authors declare no conflicts of interests.
Spreen LA, Dittmar EN, Quirk KC, Smith MA. Buprenorphine initiation strategies for opioid use disorder and pain management: A systematic review. Pharmacotherapy. 2022;42:411–427. doi: 10.1002/phar.2676
REFERENCES
- 1. Pathan H, Williams J. Basic opioid pharmacology: an update. Br J Pain. 2012;6(1):11‐16. doi: 10.1177/2049463712438493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cunningham C, Edlund MJ, Fishman M. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. Am Soc Addict Med. 2020;14(2S):1‐91. doi: 10.1097/ADM.0000000000000633 [DOI] [PubMed] [Google Scholar]
- 3. U.S Food and Drug Administration . Drug approval package. 2004. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/20732_20733_subutex.cfm#:%7E:text=Approval%20Date:%2010/08/2002. Accessed March 11, 2022.
- 4. Subutex [package insert]. North Chesterfield, VA; Indivior Inc. 2002. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/020732s018lbl.pdf. Accessed March 11, 2022.
- 5. Webster L, Gudin J, Raffa RB, et al. Understanding buprenorphine for use in chronic pain: expert opinion. Pain Med. 2020;21(4):714‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen KY, Chen L, Mao J. Buprenorphine‐naloxone therapy in pain management. Anesthesiology. 2014;120(5):1262‐1274. doi: 10.1097/ALN.0000000000000170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kumar R, Viswanath O, Saadabadi A. Buprenorphine. In: StatPearls [Internet]. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK459126. Accessed March 11, 2022. [Google Scholar]
- 8. Davis MP, Pasternak G, Behm B. Treating chronic pain: an overview of clinical studies centered on the buprenorphine option. Drugs. 2018;78(12):1211‐1228. doi: 10.1007/s40265-018-0953-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burgess DJ, Vallone D, Bair MJ, Matthias MS, Taylor BC, Taylor SL. Shifting the national consciousness about pain treatment: the critical need for a national public education campaign. J Pain. 2021;22(10):1129‐1133. doi: 10.1016/j.jpain.2021.03.156 [DOI] [PubMed] [Google Scholar]
- 10. Humphreys K, Shover CL, Andrews CM, et al. Responding to the opioid crisis in North America and beyond: recommendations of the Stanford‐Lancet Commission. Lancet. 2022;399(10324):555‐604. doi: 10.1016/S0140-6736(21)02252-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dowell D, Haegerich T, Chou R. No shortcuts to safer opioid prescribing. N Engl J Med. 2019;380(24):2285‐2287. doi: 10.1056/NEJMp1904190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Powell VD, Rosenberg JM, Yaganti A, et al. Evaluation of buprenorphine rotation in patients receiving long‐term opioids for chronic pain: a systematic review. JAMA Netw Open. 2021;4(9):e2124152. doi: 10.1001/jamanetworkopen.2021.24152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Juurlink DN. Rethinking "doing well" on chronic opioid therapy. CMAJ. 2017;189(39):E1222‐E1223. doi: 10.1503/cmaj.170628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Volpe DA, McMahon Tobin GA, Mellon RD, et al. Uniform assessment and ranking of opioid μ receptor binding constants for selected opioid drugs. Regul Toxicol Pharmacol. 2011;59(3):385‐390. doi: 10.1016/j.yrtph.2010.12.007 [DOI] [PubMed] [Google Scholar]
- 15. Coe MA, Lofwall MR, Walsh SL. Buprenorphine pharmacology review: update on transmucosal and long‐acting formulations. J Addict Med. 2019;13(2):93‐103. doi: 10.1097/ADM.0000000000000457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Christoph T, Kögel B, Schiene K, Méen M, De Vry J, Friderichs E. Broad analgesic profile of buprenorphine in rodent models of acute and chronic pain. Eur J Pharmacol. 2005;10(507):87‐98. doi: 10.1016/j.ejphar.2004.11.052 [DOI] [PubMed] [Google Scholar]
- 17. Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Phys. 2009;12(3):679‐684. [PubMed] [Google Scholar]
- 18. Hser YI, Saxon AJ, Huang D, et al. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi‐site trial. Addiction. 2014;109(1):79‐87. doi: 10.1111/add.12333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Teruya C, Schwartz RP, Mitchell SG, et al. Patient perspectives on buprenorphine/naloxone: a qualitative study of retention during the starting treatment with agonist replacement therapies (START) study. J Psychoactive Drugs. 2014;46(5):412‐426. doi: 10.1080/02791072.2014.921743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kelty E, Hulse G. Fatal and non‐fatal opioid overdose in opioid dependent patients treated with methadone, buprenorphine or implant naltrexone. Int J Drug Policy. 2017;46:54‐60. doi: 10.1016/j.drugpo.2017.05.039 [DOI] [PubMed] [Google Scholar]
- 21. Suboxone [package insert]. North Chesterfield, VA; Indivior Inc. 2002. https://www.suboxone.com/pdfs/prescribing‐information.pdf. Accessed March 11, 2022.
- 22. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Joanna Briggs Institute . Checklist for Case Reports; 2020. [Google Scholar]
- 24. Joanna Briggs Institute . Checklist for Case Series; 2020. [Google Scholar]
- 25. Joanna Briggs Institute . Checklist for Cohort Studies; 2020. [Google Scholar]
- 26. Mariani JJ, Mahony A, Iqbal MN, Luo SX, Naqvi NH, Levin FR. Case Series: Rapid induction onto long acting buprenorphine injection for high potency synthetic opioid users. Am J Addict. 2020;29(4):345‐348. doi: 10.1111/ajad.13018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moe J, Badke K, Pratt M, et al. Microdosing and standard‐dosing take‐home buprenorphine from the emergency department: a feasibility study. J Am Coll Emerg Physicians Open. 2020;1(6):1712‐1722. doi: 10.1002/emp2.12289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jones HE, Suess P, Jasinski DR, Johnson RE. Transferring methadone‐stabilized pregnant patients to buprenorphine using an immediate release morphine transition: an open‐label exploratory study. Am J Addict. 2006;15(1):61‐70. doi: 10.1080/10550490500419094 [DOI] [PubMed] [Google Scholar]
- 29. Agapoff JR, Kilaru U. Outpatient buprenorphine induction and maintenance treatment for kratom dependence: a case study. J Subst Use. 2019;24(6):575‐577. doi: 10.1080/14659891.2019.1638459 [DOI] [Google Scholar]
- 30. Hammig R, Kemter A, Strasser J, et al. Use of microdoses for induction of buprenorphine treatment with overlapping full opioid agonist use: the Bernese method. Subst Abuse Rehabil. 2016;7:99‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rozylo J, Mitchell K, Nikoo M, et al. Case Report: Successful induction of buprenorphine/naloxone using a microdosing schedule and assertive outreach. Addict Sci Clin Pract. 2020;15(1):2. doi: 10.1186/s13722-020-0177-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Caulfield MDG, Brar R, Sutherland C, Nolan S. Transitioning a patient from injectable opioid agonist therapy to sublingual buprenorphine/naloxone for the treatment of opioid use disorder using a microdosing approach. BMJ Case Rep. 2020;13(3):e233715. doi: 10.1136/bcr-2019-233715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Payler DK. Substitution of heroin and methadone with buprenorphine using an overlap method without needing to wait for withdrawal. Drugs Alcohol Today. 2016;16:259‐266. [Google Scholar]
- 34. Azar P, Wong JSH, Jassemi S, et al. A case report: rapid micro‐induction of buprenorphine/naloxone to administer buprenorphine extended‐release in an adolescent with severe opioid use disorder. Am J Addict. 2020;29(6):531‐535. doi: 10.1111/ajad.13050 [DOI] [PubMed] [Google Scholar]
- 35. Robbins JL, Englander H, Gregg J. Buprenorphine microdose induction for the management of prescription opioid dependence. J Am Board Fam Med. 2021;34:S141‐S146. doi: 10.3122/jabfm.2021.S1.200236 [DOI] [PubMed] [Google Scholar]
- 36. Brar R, Fairbairn N, Sutherland C, Nolan S. Use of a novel prescribing approach for the treatment of opioid use disorder: Buprenorphine/naloxone micro‐dosing – a case series. Drug Alcohol Rev. 2020;39(5):588‐594. doi: 10.1111/dar.13113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. DeWeese JP, Krenz JR, Wakeman SE, Peckham AM. Rapid buprenorphine microdosing for opioid use disorder in a hospitalized patient receiving very high doses of full agonist opioids for acute pain management: titration, implementation barriers, and strategies to overcomes. Subst Abus. 2021;4:1‐6. doi: 10.1080/08897077.2021.1915914 [DOI] [PubMed] [Google Scholar]
- 38. Vogel M, Köck P, Strasser J, Wiesbeck G, Walter M, Dürsteler KM. Chronic high‐dose buprenorphine does not block subjective high from diacetylmorphine in a patient in heroin‐assisted treatment. J Psychoactive Drugs. 2019;51(4):377‐382. doi: 10.1080/02791072.2019.1610200 [DOI] [PubMed] [Google Scholar]
- 39. Vytialingam RC, Schug SA, O'Regan R. Successful rotation from long‐acting full agonist opioids to sublingual buprenorphine/naloxone using a microdosing approach. J Opioid Manag. 2021;17(7):159‐166. doi: 10.5055/jom.2021.0653 [DOI] [PubMed] [Google Scholar]
- 40. Ward HB, Barnett BS, Suzuki J. Rapid transition from methadone to buprenorphine using naltrexone‐induced withdrawal: a case report. Subst Abus. 2019;40(2):140‐145. doi: 10.1080/08897077.2019.1573776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Berland DW, Malinoff HL, Weiner MA, Przybylski R. When opioids fail in chronic pain management: the role for buprenorphine and hospitalization. Am J Ther. 2013;20(4):316‐321. doi: 10.1097/MJT.0b013e31827ab599 [DOI] [PubMed] [Google Scholar]
- 42. Jafari S, Rafizadeh R. Successful transition from high‐dose methadone to buprenorphine via microdosing in the outpatient setting: a case report. Can J Hosp Pharm. 2021;74(1):83‐85. [PMC free article] [PubMed] [Google Scholar]
- 43. Terasaki D, Smith C, Calcaterra SL. Transitioning hospitalized patients with opioid use disorder from methadone to buprenorphine without a period of opioid abstinence using a microdosing protocol. Pharmacotherapy. 2019;39(10):1023‐1029. doi: 10.1002/phar.2313 [DOI] [PubMed] [Google Scholar]
- 44. Singh G, Sri Konakanchi J, Betsch B, Thapa A, Sethi R. Rapid microinduction of sublingual buprenorphine from methadone in an outpatient setting: "A case series". J Opioid Manag. 2021;17(7):167‐170. doi: 10.5055/jom.2021.0654 [DOI] [PubMed] [Google Scholar]
- 45. Button D, Hartley J, Robbins J, Levander XA, Smith NJ, Englander H. Low‐dose buprenorphine initiation in hospitalized adults with opioid use disorder: a retrospective cohort analysis. J Addict Med. 2022;16(2):e105‐e111. doi: 10.1097/ADM.0000000000000864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Saal D, Lee F. Rapid induction therapy for opioid‐use disorder using buprenorphine transdermal patch: a case series. Perm J. 2020;24:19.124. doi: 10.7812/TPP/19.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Raheemullah A, Lembke A. Buprenorphine induction without opioid withdrawal: a case series of 15 opioid‐dependent inpatients induced on buprenorphine using microdoses of transdermal buprenorphine. Am J Ther. 2021;28(4):e504‐e508. doi: 10.1097/MJT.0000000000001108 [DOI] [PubMed] [Google Scholar]
- 48. De Aquino JP, Fairgrieve C, Klaire S, Garcia‐Vassallo G. Rapid transition from methadone to buprenorphine utilizing a micro‐dosing protocol in the outpatient veteran affairs setting. J Addict Med. 2020;14(5):e271‐e273. doi: 10.1097/ADM.0000000000000618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cortina S, Mihic T, Fennemore M, McLean M. Case report: high‐dose methadone transition to buprenorphine/naloxone in an inpatient with a prolonged QT interval. Addict Med. 2017;8(1):25‐28. doi: 10.1097/02024458-201706000-00006 [DOI] [Google Scholar]
- 50. Crane K, Snead J, Stanley R, Avery J, Ghosh SM, Mints G. Intravenous buprenorphine micro‐dosing induction in a patient on methadone treatment: a case report. J Acad Consult Liaison Psychiatry. 2021;62(2):243‐247. doi: 10.1016/j.psym.2020.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Malinoff HL, Wilson G, Barkin RL. Sublingual buprenorphine is effective in the treatment of chronic pain syndrome. Am J Ther. 2005;12(5):379‐384. [DOI] [PubMed] [Google Scholar]
- 52. Daitch J, Frey ME, Silver D, Mitnick C, Daitch D, Pergolizzi J Jr. Conversion of chronic pain patients from full‐opioid agonists to sublingual buprenorphine. Pain Phys. 2012;15(3 Suppl):ES59‐ES66. [PubMed] [Google Scholar]
- 53. Rosenblum A, Cruciani RA, Strain EC, et al. Sublingual buprenorphine/naloxone for chronic pain in at‐risk patients: development and pilot test of a clinical protocol. J Opioid Manag. 2012;8(6):369‐382. doi: 10.5055/jom.2012.0137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Buchheit BM, Joslin T, Turner HN, Wong TE. Ambulatory microdose induction of buprenorphine‐naloxone in two adolescent patients with sickle cell disease. Pediatr Blood Cancer. 2021;68(1):e28766. doi: 10.1002/pbc.28766 [DOI] [PubMed] [Google Scholar]
- 55. Lee DS, Hann JE, Klaire SS, Nikoo M, Negraeff MD, Rezazadeh‐Azar P. Rapid induction of buprenorphine/naloxone for chronic pain using a microdosing regimen: a case report. A A Pract. 2020;14(2):44‐47. doi: 10.1213/XAA.0000000000001138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Becker WC, Frank JW, Edens EL. Switching from high‐dose, long‐term opioids to buprenorphine: a case series. Ann Intern Med. 2020;173(1):70‐71. doi: 10.7326/L19-0725 [DOI] [PubMed] [Google Scholar]
- 57. Irwin M, Petersen KS, Smith MA. Rapid buprenorphine induction for cancer pain in pregnancy. J Palliat Med. 2021;24(8):1257‐1262. doi: 10.1089/jpm.2020.0524 [DOI] [PubMed] [Google Scholar]
- 58. Crum IT, Meyer Karre VM, Balasanova AA. Transitioning from intrathecal hydromorphone to sublingual buprenorphine‐naloxone through microdosing: a case report. A A Pract. 2020;14(11):e01316. doi: 10.1213/XAA.0000000000001316 [DOI] [PubMed] [Google Scholar]
- 59. Tara A, Acampora G, Wang J, De Sousa K, Zhang Y. Facilitating discontinuation of intravenous opioids by ‐concurrent use of sublingual buprenorphine with rapid microdosing ‐induction: a pain management case study. J Opioid Manag. 2021;17(7):153‐158. doi: 10.5055/jom.2021.0652 [DOI] [PubMed] [Google Scholar]
- 60. Kornfeld H, Reetz H. Transdermal buprenorphine, opioid rotation to sublingual buprenorphine, and the avoidance of precipitated withdrawal: a review of the literature and demonstration in three chronic pain patients treated with butrans. Am J Ther. 2015;22(3):199‐205. doi: 10.1097/MJT.0b013e31828bfb6e [DOI] [PubMed] [Google Scholar]
- 61. Weimer MB, Guerra M, Morrow G, Adams K. Hospital‐based buprenorphine micro‐dose initiation. J Addict Med. 2021;15(3):255‐257. doi: 10.1097/ADM.0000000000000745 [DOI] [PubMed] [Google Scholar]
- 62. Klaire S, Zivanovic R, Barbic SP, Sandhu R, Mathew N, Azar P. Rapid micro‐induction of buprenorphine/naloxone for opioid use disorder in an inpatient setting: a case series. Am J Addict. 2019;28(4):262‐265. doi: 10.1111/ajad.12869 [DOI] [PubMed] [Google Scholar]
- 63. Mortaji P, Terasaki D, Moo‐Young J. Advanced inpatient management of opioid use disorder in a patient requiring serial surgeries. J Gen Intern Med. 2021;36(8):2448‐2451. doi: 10.1007/s11606-021-06739-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Martin L, Lennox R, Regenstreif L, O’Shea T. Case report: “striving to skip the withdrawal” using buprenorphine‐naloxone microdosing for hospitalized patients. Can J Addict. 2019;10:35‐40. doi: 10.1097/CXA.0000000000000048 [DOI] [Google Scholar]
- 65. Sandhu R, Zivanovic R, Klaire S, Nikoo M, Rozylo J, Azar P. Buprenorphine/naloxone induction for treatment of acute on chronic pain using a micro‐dosing regimen: a case report. Can J Pain. 2019;3(1):79‐84. doi: 10.1080/24740527.2019.1599279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hamata B, Griesdale D, Hann J, Rezazadeh‐Azar P. Rapid micro‐induction of buprenorphine/naloxone for opioid use disorder in a critically ill intubated patient: a case report. J Addict Med. 2020;14(6):514‐517. doi: 10.1097/ADM.0000000000000675 [DOI] [PubMed] [Google Scholar]
- 67. Stanciu CN, Gibson S, Teja N, Healey CJ. An efficient and smooth methadone‐to‐buprenorphine transition protocol utilizing a transdermal fentanyl bridge and a pharmacokinetic inducer: the Stanciu method. Cureus. 2020;27(12):e8310. doi: 10.7759/cureus.8310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Thakrar AP, Jablonski L, Ratner J, Rastegar DA. Micro‐dosing intravenous buprenorphine to rapidly transition from full opioid agonists. J Addict Med. 2022;16(1):122‐124. doi: 10.1097/ADM.0000000000000838 [DOI] [PubMed] [Google Scholar]
- 69. Moe J, O'Sullivan F, Hohl CM, et al. Short Communication: Systematic review on effectiveness of micro‐induction approaches to buprenorphine initiation. Addict Behav. 2021;114:106740. doi: 10.1016/j.addbeh.2020.106740 [DOI] [PubMed] [Google Scholar]
- 70. Adams KK, Machnicz M, Sobieraj DM. Initiating buprenorphine to treat opioid use disorder without prerequisite withdrawal: a systematic review. Addict Sci Clin Pract. 2021;16(1):36. doi: 10.1186/s13722-021-00244-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ahmed S, Bhivandkar S, Lonergan BB, Suzuki J. Microinduction of buprenorphine/naloxone: a review of the literature. Am J Addict. 2021;30(4):305‐315. doi: 10.1111/ajad.13135 [DOI] [PubMed] [Google Scholar]
- 72. Quirk K, Stevenson M. Buprenorphine microdosing for the pain and palliative care clinician. J Palliat Med. 2022;25(1):145‐154. doi: 10.1089/jpm.2021.0378 [DOI] [PubMed] [Google Scholar]
- 73. Randhawa PA, Brar R, Nolan S. Buprenorphine‐naloxone, "microdosing": an alternative induction approach for the treatment of opioid use disorder in the wake of North America's increasingly potent illicit drug market. CMAJ. 2020;192(3):E73. doi: 10.1503/cmaj.74018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Herring AA, Vosooghi AA, Luftig J, et al. High‐dose buprenorphine induction in the emergency department for treatment of opioid use disorder. JAMA Netw Open. 2021;4(7):e2117128. doi: 10.1001/jamanetworkopen.2021.17128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hess M, Boesch L, Leisinger R, Stohler R. Transdermal buprenorphine to switch patients from higher dose methadone to buprenorphine without severe withdrawal symptoms. Am J Addict. 2011;20(5):480‐481. doi: 10.1111/j.1521-0391.2011.00159.x [DOI] [PubMed] [Google Scholar]
- 76. Azar P, Nikoo M, Miles I. Methadone to buprenorphine/naloxone induction without withdrawal utilizing transdermal fentanyl bridge in an inpatient setting‐Azar method. Am J Addict. 2018;27(8):601‐604. doi: 10.1111/ajad.12809 [DOI] [PubMed] [Google Scholar]
- 77. Tang VM, Lam‐Shang‐Leen J, Brothers TD, et al. Case series: limited opioid withdrawal with use of transdermal buprenorphine to bridge to sublingual buprenorphine in hospitalized patients. Am J Addict. 2020;29(1):73‐76. doi: 10.1111/ajad.12964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Irwin M, Gunther W, Keefer P, et al. Buprenorphine for chronic pain in a pediatric patient with sickle‐cell disease. J Pain Symptom Manage. 2021;62(5):1086‐1091. doi: 10.1016/j.jpainsymman.2021.04.007 [DOI] [PubMed] [Google Scholar]


