Abstract
Background:
In patients with intracerebral hemorrhage (ICH) and prevalent atrial fibrillation (AF), the optimal stroke prevention strategy is unclear. We sought to estimate the risk of cerebrovascular events among ICH survivors with AF.
Methods:
We used the Danish Stroke Registry to identify patients with incident ICH and prevalent AF between 2003 and 2018. Key inclusion/exclusion criteria of the PRESTIGE-AF (Prevention of Stroke in Intracerebral hemorrhage Survivors With Atrial Fibrillation) trial were applied. Cumulative incidence of recurrent ICH, cerebrovascular ischemic event, and all-cause death were investigated after one year.
Results:
A total of 1885 patients (median age 80.0 years; 47.6% females) were included in the study. We observed 191 cerebrovascular events and 650 all-cause deaths, and more cerebrovascular ischemic events (N=63) than recurrent ICH events (N=40). Risks of recurrent ICH, cerebrovascular ischemic event, and all-cause death were 1.5%, 3.2%, and 30.3%, respectively, among patients not exposed to OAC during follow-up. The cumulative incidences were 2.8% for recurrent ICH, 3.2% for cerebrovascular ischemic events, and 22.0% for all-cause death among patients initiating/resuming OAC during follow-up.
Conclusions:
We observed a high risk of cerebrovascular ischemic events and a very high risk of all-cause death at one year after the incident ICH. The results of ongoing clinical trials are warranted to determine optimal stroke prevention treatment among ICH survivors with concomitant AF.
Keywords: anticoagulants, atrial fibrillation, epidemiology, hemorrhagic stroke, ischemic stroke
Atrial fibrillation (AF) is the most common sustained arrhythmia and substantially increases the risk of ischemic stroke.1 Oral anticoagulant therapy (OAC) has a class Ia guideline recommendation in patients with AF with a CHA2DS2-VASc score ≥2 in males and CHA2DS2-VASc score ≥3 in females to prevent thromboembolic events.2,3 However, in patients with AF who have suffered an intracerebral hemorrhage (ICH), the optimal stroke prevention strategy is debatable.2,3
ICH is a devastating vascular event and the risk of recurrent ICH is high,4,5 but in patients with AF surviving an ICH, the risk of ischemic stroke is also high.6 A previous study found a higher risk of cerebrovascular ischemic events among patients with AF surviving an ICH than among those without a prior ICH.6 No clear recommendations on the decision to initiate or resume antithrombotic treatment after an ICH in patients with AF exist.2,3 Consequently, clinicians around the globe have widely different preferences.7,8
Several randomized controlled trials are currently investigating antithrombotic treatment options for stroke prevention in ICH survivors with concomitant AF.9–14 An ongoing European multinational randomized controlled trial, the PRESTIGE-AF (Prevention of Stroke in Intracerebral Haemorrhage Survivors With Atrial Fibrillation) trial is investigating stroke prevention strategies in patients with AF surviving an ICH.13 Patients are randomized to either a nonvitamin K antagonist oral anticoagulant (NOAC) or no anticoagulation. The results of the PRESTIGE-AF trial will provide important information about the optimal stroke prevention strategy in patients with AF and recent ICH.
To provide data from routine clinical practice on the risk of cerebrovascular events, we examined risk estimates for ischemic and hemorrhagic cerebrovascular events in a cohort of ICH survivors with AF mirroring the PRESTIGE-AF trial population. The cohort was stratified by levels of CHA2DS2-VASc score to reveal potential differential risk according to a widely used clinical risk scoring system in AF patients and supplemented with analyses accounting for stroke prevention treatment.
Methods
This study was an observational cohort study describing the prognosis for patients with AF presenting with ICH at specialized stroke units in Danish hospitals from March 2003 through October 2018. The reporting of this study followed the STROBE checklist (see Supplemental Material).15
Data Availability
Data not published within this article cannot be shared because of Danish laws of data protection.
Data Sources
Data from 4 Danish nationwide registries was used: (1) The Danish Stroke Registry, which holds detailed quality data on stroke patients since 2003 including data on type of stroke, stroke severity, smoking status, and alcohol consumption,16 (2) The Danish Civil Registration System, which holds information on sex, date of birth, vital, and emigration status of all persons living in Denmark (3) the National Prescription Registry which contains data on all prescriptions dispensed from Danish pharmacies, coded according to the Anatomic Therapeutic Chemical Classification System, and (4) the Danish National Patient Registry which has registered dates of hospital admissions and discharges, outpatient and emergency room contacts, and discharge diagnoses classified according to the 10th revision of the International Classification of Diseases for >99% of hospital admissions in Denmark. Data from the abovementioned registries were linked via a unique personal identification number, which is used across all Danish national registries.
Study Population and Design
The Danish Stroke Registry was used to identify a study population of patients presenting with incident ICH and prevalent AF between March 2003 to June 2018. The discharge date of the ICH event was defined as baseline (index date). Patients with a previous diagnosis of ICH from before the Danish Stroke Registry was established, and patients registered with thrombolysis treatment during admission were excluded. To resemble the expected trial population of the PRESTIGE-AF trial, key inclusion and exclusion criteria of the PRESTIGE-AF trial were applied. First, we identified patients with prevalent AF, or patients who received an AF diagnosis within 30 days after the inclusion event. This approach was selected to include patients with previously undiagnosed AF. To identify a population with a clear indication for OAC, patients with a CHA2DS2-VASc score ≥2 for males and CHA2DS2-VASc score ≥3 for females were considered for inclusion, as per the PRESTIGE-AF trial inclusion criteria. The CHA2DS2-VASc score was calculated based on points according to prevalent congestive heart failure (1 point), hypertension (1 point), diabetes (1 point), prior ischemic stroke/systemic embolism/transient ischemic attack (2 points), vascular disease (1 point), age 65 to 74 years (1 point), age ≥75 years (2 points), and female sex (1 point).17 Patients were excluded on the basis of age <18 years, the index ICH event occurred as a result of trauma, no brain imaging following the index ICH was available, had another indication for long-term anticoagulation (a diagnosis of venous thromboembolism within the last year or several diagnoses at earlier times), strong indication for antiplatelet therapy at baseline (acute myocardial infarction or any percutaneous coronary intervention within 12 months), prior left atrial appendage occlusion, or severe renal dysfunction requiring dialysis. Information on additional baseline comorbidities were obtained from the Danish Stroke Registry, the Danish National Patient Registry, and the Danish National Prescription Registry. Comorbidity was based on hospital discharge data using any primary or secondary record available up to and including baseline, excluding emergency room codes. Baseline medication use was ascertained from prescription claims within 180 days before the index event. Detailed information about the definition of inclusion and exclusion criteria, comorbidities, and medical therapies are provided in Table S1.
Exposure to Oral Anticoagulant Treatment
At the incidence of an ICH event, cessation of OAC treatment is clinically mandated. However, patients may initiate/resume OAC during follow-up because of the risk of ischemic stroke associated with AF. Redemption of a prescription of an oral anticoagulant drug (warfarin or a NOAC agent) was used to identify exposure groups of patients who initiate/resume OAC during follow-up. In the analytic strategy to describe risk of outcomes by treatment exposure groups, time at risk was split according to the initiation/resumption of OAC during follow-up. The timepoint for a change in status was defined by the date of a redemption of a prescription of an oral anticoagulant drug during follow-up. The risk of outcomes was subsequently calculated separately in patients followed from the incident ICH until the initiation/resumption of OAC, and in patients followed from the initiation/resumption of OAC and up to one year after the incident ICH.
Outcomes and Follow-Up
Information on outcomes was obtained from the Danish Stroke Registry. The outcomes of interest were recurrent ICH, cerebrovascular ischemic events (ischemic stroke, unspecified stroke, or transient ischemic attack), and all-cause death. The coding validity of these outcomes has previously been validated in a Danish setting with a positive predictive value of 90% in the Danish Stroke Registry.18 International Classification of Diseases codes used to define each outcome are provided in Table S1.
Statistical Methods
Baseline characteristics were described using means/medians and SD/interquartile range (IQR) for continuous variables and proportions for categorical variables. Patients were categorized according to their CHA2DS2-VASc score at baseline and stratified into 3 score categories (score 2–3, score 4–6, score >6).
Time-to-event analyses were used to investigate the risk of outcomes for the full study population and according to the CHA2DS2-VASc score categories. At-risk time was measured from the discharge date related to the index event (incident ICH) until the outcome of interest, emigration, death, end of follow-up (one year after index date), or end of study period (December 1, 2018), whichever came first. Risk estimates were calculated separately for each outcome of interest; thus, patients were followed until each outcome of interest separately in each analysis.
To present the risk of outcomes over time, we constructed cumulative incidence curves according to the CHA2DS2-VASc score categories, with death as a competing risk.19 In detail, we calculated absolute risk of outcomes after one year of follow-up using the Aalen-Johansen estimator to take competing risk of death into consideration.20 To allow for additional clinical evaluation on the risk of cerebrovascular outcomes, we estimated the risk difference between an ischemic cerebrovascular event versus the risk of recurrent ICH. The risk differences within levels of the CHA2DS2-VASc were also calculated using the pseudo-value method to take into account competing risks of death.21
In a sensitivity analysis, the follow-up period for patients initiating/resuming OAC during follow-up were extended to one year after the initiation/resumption of OAC, and the analyses of the absolute risks of outcomes were repeated.
Statistical analyses were performed using SAS 9.3 software (SAS Institute, Cary, NC) and Stata version 16 software (StataCorp LP, College Station, TX).
Ethical Considerations
The Danish Health Data Agency provided the data for the study. The study was conducted in compliance with General Data Protection Regulation Article 30, recorded at Aalborg University Hospital and Aalborg University (project no. 2017-40). No ethical approval or patient consent are required for studies based on data from administrative Danish registries according to Danish laws.
Results
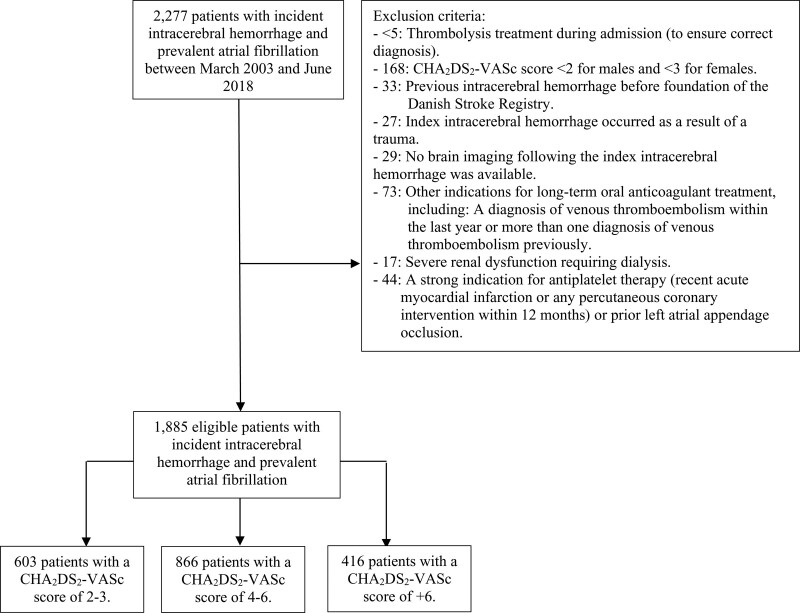
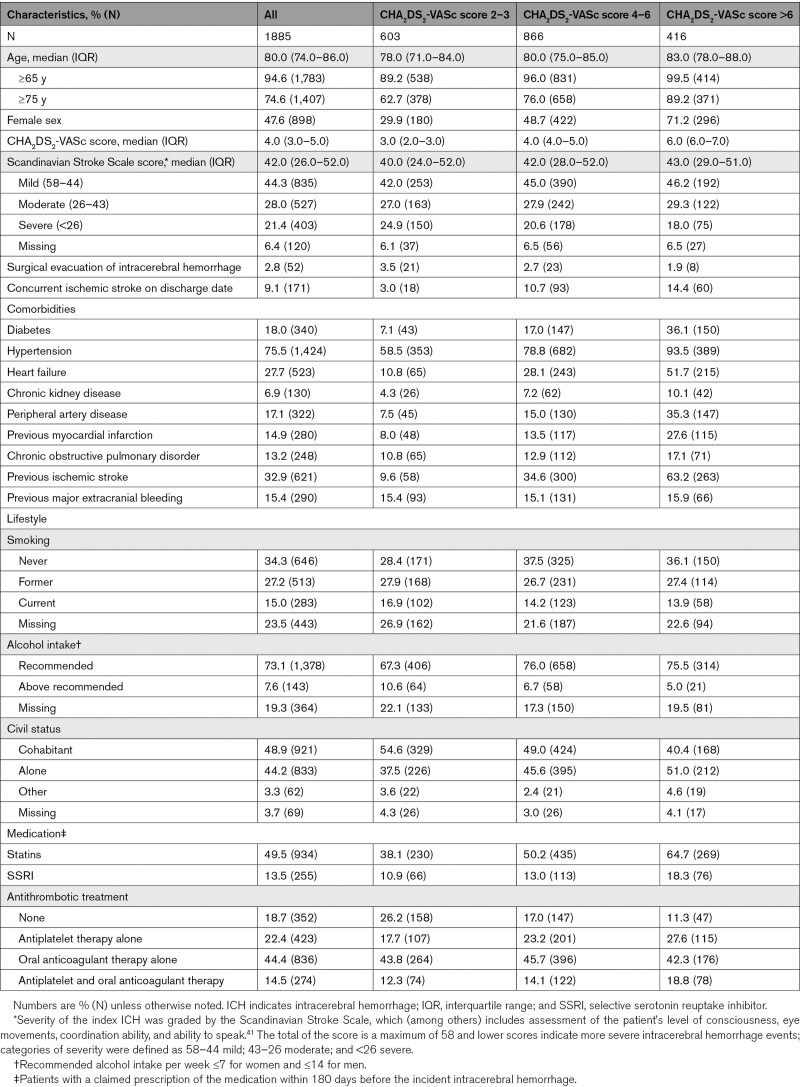
Using the Danish Stroke Registry, we identified 2277 patients with incident ICH and concomitant AF between January 2003 and October 2018. After mirroring the expected PRESTIGE-AF trial population, a total of 1885 patients were included in our study (Figure 1). A total of 603 patients had a CHA2DS2-VASc score of 2 to 3, 866 patients had a CHA2DS2-VASc score of 4 to 6, and 416 patients had a CHA2DS2-VASc score of >6. Baseline patient characteristics for the full study population and according to the CHA2DS2-VASc score categories are summarized in Table 1. Patients were generally elderly (median age ranged between 78 and 83 years), and the percentage of females ranged between 29.9% and 71.2% in the 3 CHA2DS2-VASc score categories. The median Scandinavian Stroke Scale score was similar in the 3 CHA2DS2-VASc score categories, but the prevalence of cardiovascular risk factors was highest in the group of patients with a CHA2DS2-VASc score of >6. The most prevalent cardiovascular risk factors of the CHA2DS2-VASc score were hypertension and prior stroke/heart failure in the 3 CHA2DS2-VASc score categories.
Figure 1.
Flowchart of the study population. All patients were aged ≥18 y.
Table 1.
Baseline Patient Characteristics of Intracerebral Hemorrhage Survivors With Atrial Fibrillation
Risk of Cerebrovascular Outcomes and All-Cause Death
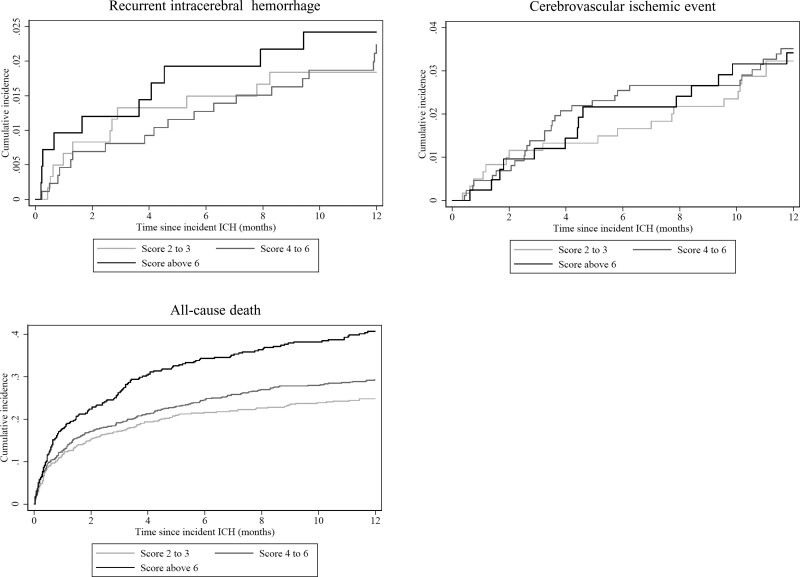
At one year after the incident ICH, the overall number of outcome events were 841 in the study population; 40 recurrent ICH, 63 cerebrovascular ischemic events, and 650 all-cause deaths. Risks of cerebrovascular outcomes after one year were 2.2% for recurrent ICH and 3.4% for cerebrovascular ischemic events, and all-cause death was 34.8%. For the full study population, we observed a trend towards higher risk of all-cause death and recurrent ICH with increasing CHA2DS2-VASc score category at one year after the incident ICH, but for the outcomes of cerebrovascular ischemic events, the risks were largely similar across the categories (Figure 2). For the outcomes of all-cause death and recurrent ICH, the cumulative incidence curves demonstrated an apparent high risk immediately following the incident ICH event.
Figure 2.
Cumulative incidence of recurrent intracerebral hemorrhage (ICH), cerebrovascular ischemic event, and all-cause death after 1 y of follow-up according to the baseline CHA2DS2-VASc score category.
Stratified Analyses Based on OAC Initiation/Resumption
When analyzing outcomes during the follow-up period according to time-dependent status of initiation/resumption of OAC, 526 patients initiated/resumed OAC during the first year after ICH where 184 redeemed a warfarin prescription and 342 patients redeemed a NOAC prescription. The median time to initiation/resumption of OAC after the incident ICH was 5.3 months (IQR, 1.1–12.0).
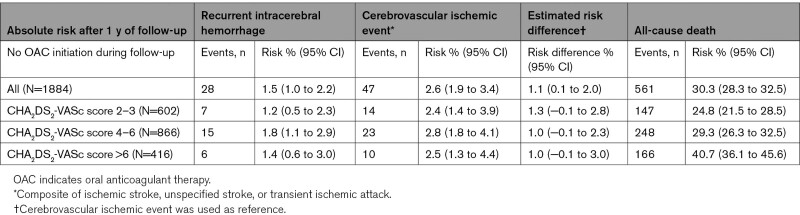
For the subpopulation of patients not initiating/resuming OAC during follow-up, the median follow-up time after the incident ICH was 4.8 months (IQR, 1.1–12.0). We observed a 2.4% to 2.8% risk of cerebrovascular ischemic events, a 1.2% to 1.8% risk of recurrent ICH, and a 24.8% to 40.7% risk of all-cause death at one year after the incident ICH in this subpopulation (Table 2). The risk difference revealed a higher risk of cerebrovascular ischemic events, and most pronounced in the lowest score levels, risk difference 1.3 (−0.1 to 2.8), which indicated a higher risk for ischemic events than for recurrent ICH.
Table 2.
Absolute Risk of Cerebrovascular Events and All-Cause Death After 1 Year of Follow-Up in the Subpopulation Not Initiating/Resuming Oral Anticoagulant Therapy During Follow-Up
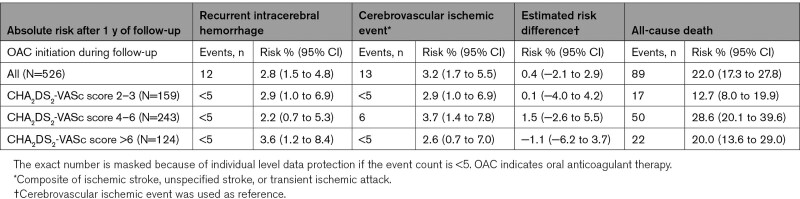
For the subpopulation of patients initiating/resuming OAC during follow-up, the median follow-up time was 9.2 months (IQR, 6.0–10.9) after initiation/resumption of OAC. A low number of cerebrovascular events was observed in the follow-up period of this subpopulation: the risk of cerebrovascular ischemic events was 2.6% to 3.7%, and 2.2% to 3.6% risk of recurrent ICH, and a 12.7% to 28.6% risk of all-cause death at one year after the incident ICH in this subpopulation (Table 3). The risk difference among patients with a score level of 4 to 6 was 1.5 (−2.6 to 5.5), while the risk difference in the highest score group (>6 points) displayed higher risk for recurrent ICH, risk difference −1.2 (−6.2 to 3.7). However, these risk differences were based on few numbers of events and with wide CIs reflecting the uncertainty of the estimated risk difference.
Table 3.
Absolute Risk of Cerebrovascular Events and All-Cause Death After 1 Year of Follow-Up in the Subpopulation Initiating/Resuming Oral Anticoagulant Therapy During Follow-Up
Sensitivity Analyses
Baseline patient characteristics for the patients initiating/resuming OAC during follow-up are summarized in Table S2. In this subpopulation, median age was 78.0 years (IQR, 73.0–84.0), 63.3% were using OAC before the incident ICH, the median Scandinavian Stroke Scale score was 47.0 (IQR, 34.0–54.0), 34.4% had experienced a prior ischemic stroke, and 17.7% had experienced a prior extracranial major bleeding. In the sensitivity analysis, where the follow-up period for patients initiating/resuming OAC during follow-up was extended to one year after the initiation/resumption of OAC, the median follow-up time was 12 months (IQR, 7.7–12.0). We observed marginally more events than in the main analysis of this subpopulation, as expected with longer follow-up time, yet most events occurred during the first months after OAC initiation/resumption (Table S3).
Discussion
In this observational cohort study including a population of 1885 ICH survivors with concomitant AF mirroring the expected PRESTIGE-AF trial population, our main findings were (1) one year after the incident ICH, we observed a high risk of cerebrovascular events and a very high risk of all-cause death; (2) we observed more cerebrovascular ischemic events than recurrent ICH events; (3) for the full study population, we observed a trend towards higher risk of all-cause death and recurrent ICH with increasing CHA2DS2-VASc score category, but a similar risk across the categories for the other outcomes; (4) cerebrovascular outcomes were highest among those initiating/resuming OAC during follow-up, but with lower risk of all-cause death.
Several randomized controlled trials are currently investigating the optimal antithrombotic treatment strategy for stroke prevention in ICH survivors with concomitant AF,9–14 and 2 of these trials have now been concluded and reported.22,23 Both trials were inconclusive and likely underpowered to detect a signal of benefit or harm from OAC treatment, and the trial investigators call for additional trial data to provide more robust evidence. Awaiting these results of ongoing trials become available, observational data such as the present on the risk of both ischemic and hemorrhagic cerebrovascular events in patient with AF surviving an ICH are important to provide additional scientific basis for the multidisciplinary expert consensus-decision approach on antithrombotic treatment that is presently recommended by guidelines.
Patients who experience an ICH and have concomitant AF are considered a very high-risk population, with a substantial risk of particularly death but also of both cerebrovascular ischemic events and recurrent ICH.4,6,24–28 In our large study population comprising high-quality stroke data,16 we observed a numerically higher number of cerebrovascular ischemic events than recurrent ICH events. In general, we observed a high overall risk of cerebrovascular events and a very high risk of all-cause death at one year after the incident ICH, which confirm previous findings.4,6,24–28 The absolute risk of a cerebrovascular ischemic event in the subpopulation not initiating/resuming OAC during follow-up exceeded the commonly accepted threshold for OAC to yield a net clinical benefit in the overall AF population.29,30 However, we also observed a high risk of recurrent ICH in the subpopulation of patients initiating/resuming OAC during follow-up, which is the most feared adverse event of OAC, and likely the main reason why OAC is not initiated/resumed in patients with AF surviving an ICH. Additionally, we estimated the risk difference between cerebrovascular ischemic events and recurrent ICH to be marginal, with largest risk difference amounting to 1.3%. However, we emphasize that our aim of the study was solely to describe the risk of cerebrovascular events in patients with AF surviving an ICH, and the risks observed in the 2 subpopulations, therefore, cannot be interpreted as reflecting the effect of anticoagulation. The subpopulation of patients initiating/resuming OAC during follow-up (184 patients redeemed a prescription for warfarin and 342 patients redeemed a prescription a NOAC) is unlikely to be a random subset of the study population. These patients were slightly younger, but more often used OAC before the incident ICH, more often had a higher Scandinavian Stroke Scale score (eg, less severe ICH), and, generally, had more comorbidities including more often a history of ischemic stroke and extracranial major bleeding than the full study population. When estimating the risk difference between cerebrovascular ischemic events and recurrent ICH, we observed a risk difference of −1.2 (−6.2 to 3.7), which indicated that the risk of recurrent ICH exceeded that of cerebrovascular events. Importantly, this subpopulation included patients who have survived until the initiation/resumption of OAC, which is very likely to impact the observed risks as it constitutes a selected group of patients. Indeed, physicians may have been more inclined to provide OAC treatment after NOACs became available given the appealing safety profile as a class effect with low risk of ICH in comparison with warfarin.31
Few observational studies specifically designed to investigate the comparative effectiveness of initiation/resumption of OAC versus no OAC in patients with AF surviving an ICH exist.24–28 A meta-analysis of 7 observational studies including patients with AF surviving an ICH showed that use of OAC was associated with a lower risk of ischemic stroke and did not increase the rate of recurrent ICH.32 Additionally, the meta-analysis demonstrated that in patients not taking any antithrombotic agent, the rates of ischemic stroke were higher than the rates of recurrent ICH.32 However, the observational studies are inherently prone to confounding by indication, a notoriously stubborn bias,33 and thus, have very limited capacity for guiding clinical practice on efficacy and safety on the use of OAC among ICH survivors with concomitant AF. In our descriptive study, we also observed a higher risk of cerebrovascular ischemic events than recurrent ICH in the subpopulation of patients not initiating/resuming OAC, but we also observed a high risk of both recurrent ICH and cerebrovascular ischemic events in the subpopulation of patients initiating/resuming OAC during follow-up.
Since no randomized data exist, individual risk assessment by a multidisciplinary team is currently recommended to guide the decision of initiating/resuming OAC in AF patients surviving an ICH.3 Yet, the CHA2DS2-VASc score is the guideline-recommended tool to be used in the decision-making for prescribing life-long OAC in patients with AF. However, a previous study found a poor discriminative performance of the CHA2DS2-VASc score for cerebrovascular ischemic events in patients with AF surviving an ICH,34 and another study did not find the CHA2DS2-VASc score predictive of recurrent ICH among patients with AF surviving an ICH.4 In contrast, another study observed a trend towards higher risk of ischemic stroke with increasing CHA2DS2-VASc score in patients with AF surviving an ICH.35 We observed a trend towards higher risk of all-cause death and recurrent ICH with increasing CHA2DS2-VASc score category in the full study population but a similar risk across the categories for the outcome of cerebrovascular ischemic events. Thus, other risk stratification methods for guiding initiation/resumption of OAC in patients with AF surviving an ICH may be necessary.
Strengths and Limitations
We assessed the presence of the risk components of the CHA2DS2-VASc score at baseline, yet the stroke risk profile is not static. Given the advanced age and multiple comorbidities, the study population may change their risk profile during follow-up.36 However, we chose a relatively short follow-up period to minimize the impact of this limitation.
Although antiplatelets do not provide optimal stroke prophylaxis in patients with AF, some evidence of benefit in terms of lower thromboembolic risk compared with no treatment may still persist.37 Thus, use of antiplatelets could have affected the estimated risks in our study.
In the subpopulation of patients initiating/resuming OAC, we have no clear evidence of accurate timing of initiation or resumption of OAC, as we relied on the date for pharmacy dispensed medication. Our risk estimates were merely descriptive in these stratified analyses and cannot be compared with guide risk of the outcomes according to initiate/resume OAC treatment versus no treatment. Additionally, we did not have information on cerebral imaging features (given the association of ischemic stroke and re-bleeding with some imaging features38), and did not have information on the international normalized ratio values or time in therapeutic range, and therefore, did not have information about the quality of anticoagulation with a vitamin K antagonist. However, NOACs have gradually become the preferred oral anticoagulant drug in patients with AF given the appealing safety profile.39,40
Lastly, for the outcome of all-cause death, some deaths may be from unrecorded fatal strokes or ICH because not all outcomes are adjudicated and postmortem examinations are not mandated, hereby potentially underestimating the incidence of cerebrovascular events.
Conclusions
In this observational cohort study, we used the Danish Stroke Registry to identify a population of ICH survivors with concomitant AF mirroring the expected PRESTIGE-AF trial population. One year after the incident ICH, we observed a high risk of cerebrovascular ischemic events and a very high risk of all-cause death. We observed more cerebrovascular ischemic events than recurrent ICH events, with lower mortality among those restarting OAC. Prospective randomized trials such as the PRESTIGE-AF trial are warranted to determine optimal stroke prevention treatment among ICH survivors with concomitant AF.
Article Information
Sources of Funding
This study is part of the PRESTIGE-AF project (Prevention of Stroke in Intracerebral Hemorrhage Survivors With Atrial Fibrillation) which received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 754517. The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and decision to submit the article for publication.
Disclosures
Dr Lip is a consultant and speaker for BMS/Pfizer, Boehringer Ingelheim and Daiichi-Sankyo. No fees are received personally. Dr Larsen is a investigator for Janssen Scientific Affairs, LLC, and Boehringer Ingelheim. Speaker for Bayer, BMS/Pfizer, Janssen Pharmaceuticals, Takeda, Roche Diagnostics, and Boehringer Ingelheim. No fees are received personally. Dr Nielsen received Speaking fees Daiichi-Sankyo, consulting fees from Bayer and Daiichi-Sankyo, and grant support from BMS/Pfizer and Daiichi-Sankyo. The other authors report no conflicts.
Supplemental Material
Tables S1–S3
PRESTIGE-AF Consortium Author List
STROBE Statement
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AF
- atrial fibrillation
- ICH
- intracerebral hemorrhage
- IQR
- interquartile range
- NOAC
- nonvitamin K antagonist oral anticoagulant
- OAC
- oral anticoagulant therapy
- PRESTIGE-AF
- Prevention of Stroke in Intracerebral Haemorrhage Survivors With Atrial Fibrillation
A list of all PRESTIGE-AF consortium members is given in the Supplemental Material.
This article was sent to Mayank Goyal, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.121.038331.
For Sources of Funding and Disclosures, see page 2566.
Contributor Information
Line Melgaard, Email: linemelgaard@hotmail.com.
Thure Filskov Overvad, Email: t.overvad@rn.dk.
Martin Jensen, Email: mjen@rn.dk.
Torben Bjerregaard Larsen, Email: tobl@rn.dk.
Gregory Y.H. Lip, Email: gregory.lip@liverpool.ac.uk.
References
- 1.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983 [DOI] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Calkins H, Field ME, Chen LY, Furie KL, Cigarroa JE, Heidenreich PA, Cleveland JC, Murray KT, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation. J Am Coll Cardiol. 2019;140:e125–e151. doi: 10.1016/j.jacc.2019.01.011 [Google Scholar]
- 3.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan G-A, Dilaveris PE, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2020;42:373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 4.Overvad TF, Andersen SD, Larsen TB, Lip GYH, Søgaard M, Skjøth F, Nielsen PB. Incidence and prognostic factors for recurrence of intracerebral hemorrhage in patients with and without atrial fibrillation: a cohort study. Thromb Res. 2020;191:1–8. doi: 10.1016/j.thromres.2020.03.024 [DOI] [PubMed] [Google Scholar]
- 5.Huhtakangas J, Löppönen P, Tetri S, Juvela S, Saloheimo P, Bode MK, Hillbom M. Predictors for recurrent primary intracerebral hemorrhage: a retrospective population-based study. Stroke. 2013;44:585–590. doi: 10.1161/STROKEAHA.112.671230 [DOI] [PubMed] [Google Scholar]
- 6.Brønnum Nielsen P, Larsen TB, Gorst-Rasmussen A, Skjøth F, Rasmussen LH, Lip GYH. Intracranial hemorrhage and subsequent ischemic stroke in patients with atrial fibrillation: a nationwide cohort study. Chest. 2015;147:1651–1658. doi: 10.1378/chest.14-2099 [DOI] [PubMed] [Google Scholar]
- 7.Xu Y, Shoamanesh A, Schulman S, Dowlatshahi D, Al-Shahi Salman R, Moldovan ID, Wells PS, AlKherayf F. Oral anticoagulant re-initiation following intracerebral hemorrhage in non-valvular atrial fibrillation: Global survey of the practices of neurologists, neurosurgeons and thrombosis experts. PLoS One. 2018;13:e0191137. doi: 10.1371/journal.pone.0191137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sembill JA, Wieser CY, Sprügel MI, Gerner ST, Giede-Jeppe A, Reindl C, Eyüpoglu IY, Hoelter P, Lücking H, Kuramatsu JB, et al. Initiating anticoagulant therapy after ICH is associated with patient characteristics and treatment recommendations. J Neurol. 2018;265:2404–2414. doi: 10.1007/s00415-018-9009-2 [DOI] [PubMed] [Google Scholar]
- 9.Larsen KT, Forfang E, Pennlert J, Glader EL, Kruuse C, Wester P, Ihle-Hansen H, Carlsson M, Berge E, Al-Shahi Salman R, et al. STudy of antithrombotic treatment after intracerebral haemorrhage: protocol for a randomised controlled trial. Eur Stroke J. 2020;5:414–422. doi: 10.1177/2396987320954671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Nieuwenhuizen KM, van der Worp HB, Algra A, Kappelle LJ, Rinkel GJ, van Gelder IC, Schutgens RE, Klijn CJ; APACHE-AF Investigators. Apixaban versus Antiplatelet drugs or no antithrombotic drugs after anticoagulation-associated intraCerebral HaEmorrhage in patients with Atrial Fibrillation (APACHE-AF): study protocol for a randomised controlled trial. Trials. 2015;16:393. doi: 10.1186/s13063-015-0898-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Shahi Salman R, Keerie C, Stephen J, Lewis S, Norrie J, Dennis MS, Newby DE, Wardlaw JM, Lip GYH, Parry-Jones A, et al. Effects of oral anticoagulation for atrial fibrillation after spontaneous intracranial haemorrhage in the UK: a randomised, open-label, assessor-masked, pilot-phase, non-inferiority trial. Lancet Neurol. 2021;20:842–853. doi: 10.1016/S1474-4422(21)00264-7 [DOI] [PubMed] [Google Scholar]
- 12.ClinicalTrials.gov. Anticoagulation in ICH Survivors for Stroke Prevention and Recovery (ASPIRE) [Internet]. 2022. Accessed March 28, 2022. https://clinicaltrials.gov/ct2/show/NCT03243175.
- 13.ClinicalTrials.gov. PREvention of STroke in Intracerebral haemorrhaGE Survivors With Atrial Fibrillation [Internet]. 2022. Accessed March 28, 2022. https://clinicaltrials.gov/ct2/show/NCT03996772.
- 14.ClinicalTrials.gov. Avoiding Anticoagulation After IntraCerebral Haemorrhage (A3ICH) [Internet]. 2022. Accessed March 28, 2022. https://clinicaltrials.gov/ct2/show/NCT03243175.
- 15.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–577. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 16.Johnsen SP, Ingeman A, Hundborg HH, Schaarup SZ, Gyllenborg J. The danish stroke registry. Clin Epidemiol. 2016;8:697–702. doi: 10.2147/CLEP.S103662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 18.Wildenschild C, Mehnert F, Thomsen RW, Iversen HK, Vestergaard K, Ingeman A, Johnsen SP. Registration of acute stroke: validity in the Danish Stroke Registry and the Danish National Registry of Patients. Clin Epidemiol. 2014;6:27–36. doi: 10.2147/CLEP.S50449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coviello V, Boggess M. Cumulative incidence estimation in the presence of competing risks. Stata J. 2004;4:103–112. doi: 10.1016/j.jclinepi.2017.09.011 [Google Scholar]
- 20.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o [DOI] [PubMed] [Google Scholar]
- 21.Klein JP, Andersen PK. Regression modeling of competing risks data based on pseudovalues of the cumulative incidence function. Biometrics. 2005;61:223–229. doi: 10.1111/j.0006-341X.2005.031209.x [DOI] [PubMed] [Google Scholar]
- 22.Schreuder FHBM, van Nieuwenhuizen KM, Hofmeijer J, Vermeer SE, Kerkhoff H, Zock E, Luijckx GJ, Messchendorp GP, van Tuijl J, Bienfait HP, et al. ; APACHE-AF Trial Investigators. Apixaban versus no anticoagulation after anticoagulation-associated intracerebral haemorrhage in patients with atrial fibrillation in the Netherlands (APACHE-AF): a randomised, open-label, phase 2 trial. Lancet Neurol. 2021;20:907–916. doi: 10.1016/S1474-4422(21)00298-2 [DOI] [PubMed] [Google Scholar]
- 23.Al-Shahi Salman R, Keerie C, Stephen J, Lewis S, Norrie J, Dennis MS, Newby DE, Wardlaw JM, Lip GYH, Parry-Jones A, et al. Effects of oral anticoagulation for atrial fibrillation after spontaneous intracranial haemorrhage in the UK: a randomised, open-label, assessor-masked, pilot-phase, non-inferiority trial. Lancet Neurol. 2021;20:842–853. doi: 10.1016/S1474-4422(21)00264-7 [DOI] [PubMed] [Google Scholar]
- 24.Kuramatsu JB, Gerner ST, Schellinger PD, Glahn J, Endres M, Sobesky J, Flechsenhar J, Neugebauer H, Jüttler E, Grau A, et al. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA. 2015;313:824–836. doi: 10.1001/jama.2015.0846 [DOI] [PubMed] [Google Scholar]
- 25.Nielsen PB, Larsen TB, Skjøth F, Gorst-Rasmussen A, Rasmussen LH, Lip GY. Restarting anticoagulant treatment after intracranial hemorrhage in patients with atrial fibrillation and the impact on recurrent stroke, mortality, and bleeding: a Nationwide Cohort Study. Circulation. 2015;132:517–525. doi: 10.1161/CIRCULATIONAHA.115.015735 [DOI] [PubMed] [Google Scholar]
- 26.Gathier CS, Algra A, Rinkel GJ, van der Worp HB. Long-term outcome after anticoagulation-associated intracerebral haemorrhage with or without restarting antithrombotic therapy. Cerebrovasc Dis. 2013;36:33–37. doi: 10.1159/000351151 [DOI] [PubMed] [Google Scholar]
- 27.Majeed A, Kim YK, Roberts RS, Holmström M, Schulman S. Optimal timing of resumption of warfarin after intracranial hemorrhage. Stroke. 2010;41:2860–2866. doi: 10.1161/STROKEAHA.110.593087 [DOI] [PubMed] [Google Scholar]
- 28.Vidal-Jordana A, Barroeta-Espar I, Pelayo MS, Mateo J, Delgado-Mederos R, Martí-Fàbregas J. Intracerebral haemorrhage in anticoagulated patients: what do we do afterwards. Neurología. 2012;27:136–142. doi: 10.1016/j.nrl.2011.04.020 [DOI] [PubMed] [Google Scholar]
- 29.Sulzgruber P, Wassmann S, Semb AG, Doehner W, Widimsky P, Gremmel T, Kaski JC, Savarese G, Rosano GMC, Borghi C, et al. Oral anticoagulation in patients with non-valvular atrial fibrillation and a CHA2DS2-VASc score of 1: a current opinion of the European Society of Cardiology Working Group on Cardiovascular Pharmacotherapy and European Society of Cardiology Council on Str. Eur Hear J Cardiovasc Pharmacother. 2019;5:171–180. doi: 10.1093/ehjcvp/pvz016 [DOI] [PubMed] [Google Scholar]
- 30.Eckman MH, Singer DE, Rosand J, Greenberg SM. Moving the Tipping Point. Circ Cardiovasc Qual Outcomes. 2011;4:14–21. doi: 10.1161/CIRCOUTCOMES.110.958108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0 [DOI] [PubMed] [Google Scholar]
- 32.Korompoki E, Filippidis FT, Nielsen PB, Del Giudice A, Lip GYH, Kuramatsu JB, Huttner HB, Fang J, Schulman S, Martí-Fàbregas J, et al. Long-term antithrombotic treatment in intracranial hemorrhage survivors with atrial fibrillation. Neurology. 2017;89:687–696. doi: 10.1212/WNL.0000000000004235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J, Bosco F, Geiger AM, Lash TL. A most stubborn bias: confounding by indication in observational studies. J Clin Epidemiol. 2011;63:64–74. doi: 10.1016/j.jclinepi.2009.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nielsen PB, Overvad TF, Andersen SD, Larsen TB, Skjøth F, Søgaard M, Lip GYH. Risk stratification for ischemic cerebrovascular events and mortality among intracerebral hemorrhage patients with and without atrial fibrillation: a Nationwide Cohort Study. Cerebrovasc Dis. 2019;48:236–243. doi: 10.1159/000504926 [DOI] [PubMed] [Google Scholar]
- 35.Lerario MP, Gialdini G, Lapidus DM, Shaw MM, Navi BB, Merkler AE, Lip GY, Healey JS, Kamel H. Risk of ischemic stroke after intracranial hemorrhage in patients with atrial fibrillation. PLoS One. 2015;10:e0145579. doi: 10.1371/journal.pone.0145579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoon M, Yang PS, Jang E, Yu HT, Kim TH, Uhm JS, Kim JY, Pak HN, Lee MH, Lip GYH, et al. Dynamic changes of CHA2DS2-VASc score and the risk of ischaemic stroke in Asian patients with atrial fibrillation: a Nationwide Cohort Study. Thromb Haemost. 2018;118:1296–1304. doi: 10.1055/s-0038-1651482 [DOI] [PubMed] [Google Scholar]
- 37.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007 [DOI] [PubMed] [Google Scholar]
- 38.Best JG, Ambler G, Wilson D, Lee KJ, Lim JS, Shiozawa M, Koga M, Li L, Lovelock C, Chabriat H, et al. ; Microbleeds International Collaborative Network. Development of imaging-based risk scores for prediction of intracranial haemorrhage and ischaemic stroke in patients taking antithrombotic therapy after ischaemic stroke or transient ischaemic attack: a pooled analysis of individual patient data from cohort studies. Lancet Neurol. 2021;20:294–303. doi: 10.1016/S1474-4422(21)00024-7 [DOI] [PubMed] [Google Scholar]
- 39.Gadsbøll K, Staerk L, Fosbøl EL, Sindet-Pedersen C, Gundlund A, Lip GYH, Gislason GH, Olesen JB. Increased use of oral anticoagulants in patients with atrial fibrillation: temporal trends from 2005 to 2015 in Denmark. Eur Heart J. 2017;38:899–906. doi: 10.1093/eurheartj/ehw658 [DOI] [PubMed] [Google Scholar]
- 40.Maura G, Billionnet C, Drouin J, Weill A, Neumann A, Pariente A. Oral anticoagulation therapy use in patients with atrial fibrillation after the introduction of non-vitamin K antagonist oral anticoagulants: findings from the French healthcare databases, 2011-2016. BMJ Open. 2019;9:e026645. doi: 10.1136/bmjopen-2018-026645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindenstrøm E, Boysen G, Waage Christiansen L, à Rogvi Hansen B, Würtzen Nielsen P. Reliability of scandinavian neurological stroke scale. Cerebrovasc Dis. 1991;1:103–107. doi: 10.1159/000108825 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data not published within this article cannot be shared because of Danish laws of data protection.