Abstract
Cellular senescence is an irreversible state of cell cycle arrest occurring in response to stressful stimuli, such as telomere attrition, DNA damage, reactive oxygen species, and oncogenic proteins. Although beneficial and protective in several physiological processes, an excessive senescent cell burden has been involved in various pathological conditions including aging, tissue dysfunction and chronic diseases. Oxidative stress (OS) can drive senescence due to a loss of balance between pro-oxidant stimuli and antioxidant defences. Therefore, the identification and characterization of antioxidant compounds capable of preventing or counteracting the senescent phenotype is of major interest. However, despite the considerable number of studies, a comprehensive overview of the main antioxidant molecules capable of counteracting OS-induced senescence is still lacking. Here, besides a brief description of the molecular mechanisms implicated in OS-mediated aging, we review and discuss the role of enzymes, mitochondria-targeting compounds, vitamins, carotenoids, organosulfur compounds, nitrogen non-protein molecules, minerals, flavonoids, and non-flavonoids as antioxidant compounds with an anti-aging potential, therefore offering insights into innovative lifespan-extending approaches.
Keywords: senescence, aging, antioxidants, oxidative stress, reactive oxygen species, minerals, flavonoids, vitamins
1. Introduction
It was 1961 when Hayflick and Moorhead introduced for the first time the concept of senescence [1]. Since then, a plethora of studies have been performed on this process, identifying highly complex and multi-step mechanisms leading to an irreversible cell cycle arrest, which can be initiated by various intrinsic and extrinsic stimuli, and developmental signals [2,3].
Distinct biological functions can be performed by senescent cells: from those beneficial falling under acute senescence to those dangerous falling under chronic senescence [4]. Concerning the beneficial functions, senescent cells guide tissue regeneration and embryonic development, limit tissue damage by reducing excessive cell proliferation and promote wound healing. Moreover, they encourage tumour suppression via upregulation of p53, p16 and p21 cell cycle inhibitors, or through production of interleukin-6 (IL-6) and IL-8. Finally, they play an important homeostatic role that is extremely dependent on their elimination by the immune system [5,6]. The senescence-associated secretory phenotype (SASP), the primary mediator of acute senescence, has the main role to signal the presence of senescent cells to the immune system and encourage their elimination. However, when senescent cells persist, their SASP profile becomes damaging, and this can transform senescent fibroblasts into pro-inflammatory cells, thereby promoting tumour progression [2,3].
Different molecular mechanisms are known to induce senescence [7]. Nuclear DNA damage is one crucial senescence mechanism, whose signals converge in p53 activation, which in turn induces cell cycle arrest. When the DNA damage response (DDR) is prolonged, it promotes senescence [4]. Further known mechanisms underlying senescence are: (1) persistent DDR activation at telomeres, the ends of chromosomes, which is sufficient to activate replicative cell senescence [8]; (2) oncogene activation partly via reactive oxygen species (ROS) production, determining hyperproliferation and altered DNA replication profiles [4,8]; (3) cell cycle arrest by upregulation of p21 and p16 [9]; (4) mitochondrial abnormalities with an increase in ROS synthesis and impairment in biogenesis and mitophagy [10]; (5) induction to resistance to apoptosis by upregulation of the antiapoptotic proteins [10]; (6) metabolic changes determined by senescence-associated-β-galactosidase (SA-β gal) accumulation along with the increase in cellular lysosomal content [10]; (7) large-scale chromatin reorganization occurring with the generation of senescence-associated heterochromatin foci, which suppress transcription of pro-proliferation genes [10]; (8) secretion of pro-inflammatory cytokines, chemokines, proteases, and growth factors that influence the neighbouring cells (SASP profile); (9) morphological alterations including cellular flattening and enlargement [10]; (10) post-transcriptional regulatory pathways taking place at different levels: through the action of mRNA-binding proteins (RBPs) and noncoding RNAs [11,12,13,14]; through a dysregulated splicing factor expression [12,15]; and through N6-methyladenosine (m6A) processes with specific m6A-binding proteins [14].
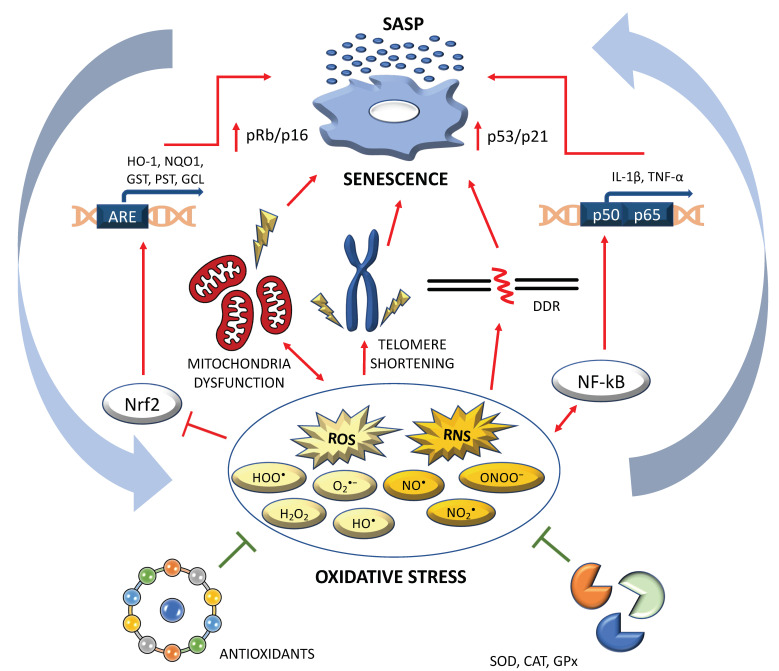
Increased oxidative stress (OS) is a further major driver of senescence [16,17,18,19,20,21]. The OS occurs when ROS/reactive nitrogen species (RNS) overproduction overwhelms the elimination ability of antioxidants. In a very recent exhaustive review [13], the authors summarized the major pathways inducing senescence through ROS/RNS deregulation. Specifically, a SASP profile can be promoted both by the failure of the antioxidant cascade due to defects in the well-known transcriptional factor Nrf2 (nuclear factor erythroid 2–related factor 2) [22], and by the activation of the redox-sensitive pathway influenced by another well-known transcriptional factor NF-κB [23]. A SASP profile can be further determined by the activation of molecular cascades linked to p53/p21 (due to persistent double strands breaks/telomere shortening), but also to p16/Rb (due to epigenetic modifications) [3,22,24,25,26]. Furthermore, an increase in ROS/RNS levels can be determined by mitochondrial dysfunctions, and this can contribute to telomere damage and epigenetic modifications [27]. Finally, alteration in the NAD+/sirtuin pathway can provoke senescence by the p53/p21 pathway, but it can also impact negatively on the specific functions of forkhead box O (FOXO) and peroxisome proliferator activated receptor γ coactivator 1α (PGC-1α), with consequent ROS increasing and mitochondrial dysfunctions (Figure 1).
Figure 1.
The interplay between oxidative stress (OS) and senescence. Excessive reactive oxygen species (ROS) and reactive nitrogen species (RNS) trigger senescence through different mechanisms: (i) via NF-kB stimulation, which induces the transcription of the main factors composing the senescence-associated secretory phenotype (SASP); (ii) through DNA double strand brakes, which trigger a sustained DDR response; (iii) via telomere shortening, which is directly linked to cellular senescence; (iv) through a double cross-talk between mitochondria dysfunction and ROS/RNS production and (v) via the inhibition of Nrf2, a crucial antioxidant transcription factor. Antioxidant molecules and antioxidant enzymes (i.e., superoxide dismutase, catalase and glutathione peroxidase) can counteract senescence through the inhibition of OS. Abbreviations: ARE: antioxidant responsive element; CAT: catalase; DDR: DNA damage response; GCL: glutamate cysteine ligase; GPx: glutathione peroxidase; GST: glutathione transferase; H2O2: hydrogen peroxide; HO-1: heme oxygenase-1; HO•: hydroxyl radical; HOO•: hydroperoxyl radical; IL-1β: interleukin 1β; NF-kB: nuclear factor kappa-light-chain-enhancer of activated B cells; NO•: nitric oxide radical; NO2•: nitrogen dioxide radical; NQO1: NAD(P)H quinone dehydrogenase 1; Nrf2: nuclear factor erythroid 2-related factor 2; O2•−: superoxide anion radical; ONOO−: peroxynitrite anion radical; PST: phenolsulfotransferase enzyme; SOD: superoxide dismutase; TNF-α: tumour necrosis factor α.
In this context, OS molecules could represent potential therapeutic targets to boost or delay cell senescence. Antioxidants compounds can be defined as senolytics, if they are able to selectively kill senescent cells, or as senomorphics, if they act by modulating the senescence phenotype [7,28]. Different mechanisms of action of senolytics have been reported in the literature: inhibition of the BCL-2 antiapoptotic family, negative modulation of the PI3K/Akt pathway and FOXO regulation [28]. On the other hand, senomorphics revert or slow down senescence by regulating the SASP [29].
Despite the considerable number of studies, a comprehensive overview of the main antioxidant molecules capable of counteracting OS-induced senescence is still lacking.
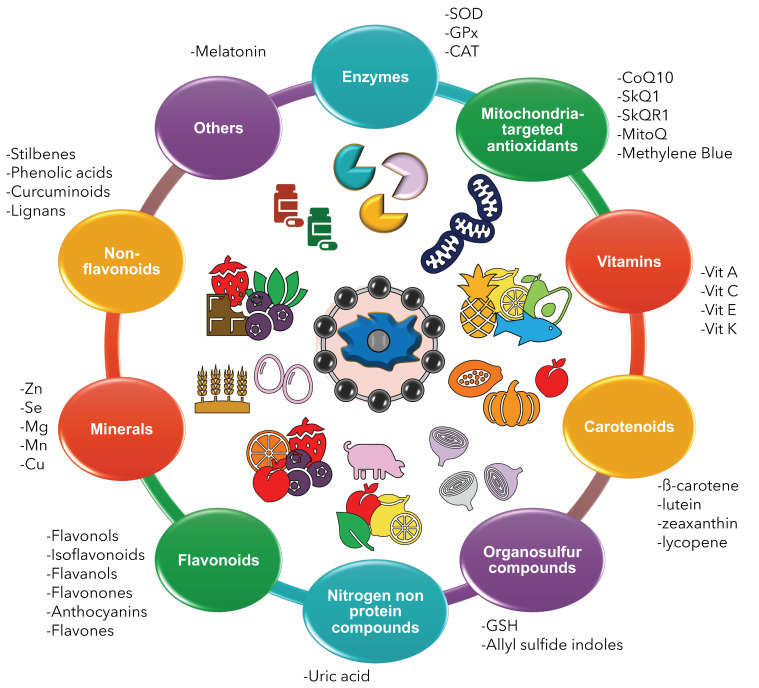
In this work, we describe the role of enzymes, mitochondria-targeting compounds, vitamins, carotenoids, organosulfur compounds, nitrogen non-protein molecules, minerals, flavonoids, and phenolic acids as antioxidant compounds with an anti-aging potential (Figure 2).
Figure 2.
Antioxidants: classification. The figure illustrates the main classes of antioxidants capable of counteracting oxidative stress-induced senescence: enzymes, mitochondria-targeted antioxidants, vitamins, carotenoids, organosulfur compounds, nitrogen non protein compounds, flavonoids, minerals, non-flavonoids, and others.
2. Results
2.1. Enzymatic Antioxidants
The term antioxidant refers to a wide class of molecules (bioactive substances and enzymatic complexes) that, present in small quantities (micronutrients) in the organism, can protect organic substrates, both natural (phospholipids, proteins, DNA) and synthetic (plastics, oils), from the attack of free radicals. All antioxidants inhibiting or reducing radical formation are acknowledged as preventive substances, as they work by preventing the formation of the so-called initiator radicals. In this group of molecules we can include: (a) chemical chelators, which are able, for example, to inhibit the Fenton reaction (Fe2+ + H2O2 → Fe3+ + OH– + •OH); (b) sulphur and sulphide groups, which are able to decompose hydroperoxides in a non-radical way (i.e., ROOH + RSR → ROH + RSOR); (c) the antioxidant enzymes superoxide dismutase (SOD, EC 1.15.1.1) and catalase (CAT, EC 1.11.1.6), which break down superoxide anion and hydrogen peroxide, respectively.
Aging is a complex process where most antioxidant enzymes, including peroxidases, undergo a marked change [30]. The main endogenous antioxidants are enzymes that reduce the danger of free radicals, i.e., SOD, glutathione peroxidase (GPx) and CAT. In order to carry out their functions, these enzymes need trace elements such as selenium, copper, manganese and zinc and, for this reason, a daily intake of them is necessary. Raw foods, or nutritional supplementation, provide exogenous antioxidants such as ascorbate, tocopherol, vitamin C, β-carotenoids, bioflavonoids, lipoic acid, coenzyme Q10, selenium and zinc. These micronutrients should enable our cells to face ROS excess by promoting the antioxidant cellular endowment.
The superoxide dismutases (SODs) represent a wide group of antioxidant enzymes with complex activities [31,32]. Their activity has a dynamic nature, as they can change metal specificity to fit the different requests from cells in different microenvironments and functional conditions [32]. The role of SODs in aging has been recently addressed [33]. Particularly for skin aging, an event characterized by impaired wound healing, atrophy, reduced tensile strength and wrinkle formation, a marked loss in skin structural integrity and in collagen and elastic fibres, with weakening in the fibre network, has been reported, due to dysfunctional fibroblasts [34,35]. These senescent fibroblasts rapidly develop a growth arrest, changes in morphology and function, increased ROS production with a marked up-regulation of SOD2 in terms of both transcripts and proteomics [36,37,38]. The upregulation of SOD2 is induced in the senescent phenotype also in a paracrine way by physical insults such as UV irradiation [39] or by the immune release of chemokines, soluble factors, and cytokines from keratinocytes [40]. Upregulation of SODs might, therefore, mirror an impaired regulation of the cell survival machinery, to the point of even increase mortality in elderly patients [41]. The recent contribution by Mao et al. reported that, in 858 deaths investigated throughout a period of 6 years, a strong effect of sex (female) in the association between SOD activity and mortality was observed [41]. Furthermore, a decrease in SOD plasma concentration, particularly the isozyme SOD3, which is highly expressed in the arterial walls, can be detected along with further biomarkers of OS, such as AOPP (advanced oxidation protein products) and 8-iso prostane. Interestingly, the T-allele of rs2284659 in the promoter region of SOD3 has been related to a safer plasma redox balance, leading to an improvement in the cardiovascular outcome in patients with type 2 diabetes [42]. The same complex relationship between SODs and senescence, usually characterized by a SASP, namely an irreversible process of cell cycle involution alongside with a pro-inflammatory phenotype, deals with another complex actor of aging, the mitochondria biology [43]. It is well known that a deficiency in SOD2 in connective tissue leads to a senescent phenotype in bones, muscles and skin [44], whereas deletion in the gene expression of SOD1 leads to the appearance of a SASP marker in the kidney [45], yet many of these results should be associated with the biology of activated mitochondria.
As cells and organisms increase their age, the respiratory chain tends to decrease, thus augmenting the release of electrons and reducing the generation of ATP. The theory of mitochondrial free radicals in senescence proposes that progressive mitochondrial dysfunction, which occurs with aging, results in an increased production of ROS, which in turn causes further mitochondrial and global cellular damage. This theory has been indeed reappraised in recent years [46].
When a switch from manganese (Mn2+) to iron (Fe2+) in SOD2 occurs, usually when due to a depletion Mn2+ is replaced by Fe2+, the new FeSOD2, which turns its function towards a pro-oxidant peroxidase, is a powerful causative factor of OS, mitochondria functional impairment and senescence [47]. A molecular cross-talk exists between Mn and Fe in mitochondria, able to switch SOD2 functionality [48,49]. This cross-talk may be impaired during aging, as, for instance, when fibroblasts accumulate iron during the development of a senescent phenotype [50], they may increase the Mn-Fe shift in SOD2, given that aging is also characterized by Mn and further micronutrients deficiency [51].
Catalase (CAT) is, most probably, a strong biomarker of senescence, due to the crucial role of H2O2 in modulating the OS response [52]. Further, peroxisomal OS is particularly crucial in the cell lifespan and survival ability and CAT plays an utmost role in this sense, so that CAT inactivation may lead, due to an impaired mitochondria-peroxisome cross-talk, to a condition of premature aging, also known as progeria phenotype [53]. Actually, hypocatalasemic fibroblasts show senescent-derived disorders [54,55].
2.2. Mitochondria-Targeted Antioxidants
The role of mitochondria in OS has long been established [56]. Due to the content of multiple electron carriers and an extensive antioxidant defence, they represent a key centre for ROS/antioxidant balance regulation [56]. Coenzyme Q10 (CoQ10), SkQ1 (a.k.a. visomitin), mitoquinone (MitoQ) and methylene blue are among the mitochondria targeted antioxidants exerting a role in counteracting OS-induced senescence, with CoQ10 being the most studied (Table 1 and Table 2) [57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82].
Table 1.
CoenzymeQ10 and ubiquinol in the prevention of OS-induced senescence.
| Ref. | Study Design | Treatment | Form | Results | Conclusion |
|---|---|---|---|---|---|
| Chen et al., 2019 [59] | HAEC chronically treated with NRTI | 5 μM CoQ10 continuously applied across passages | CoQ10 | ↓ NRTI-induced senescence ↓ ROS ↑ Mitochondrial respiration rate |
CoQ10 reduces cardiovascular side effects of NRTI treatment |
| Tarry-Adkins et al., 2013 [58] | Mouse model of low birth-weight and catch-up growth | Post-weaning dietary supplement | CoQ10 | ↓ Nitrosative and OS ↓ DNA damage ↓ Cellular senescence ↓Telomere shortening ↓ Apoptosis |
CoQ10 prevents cardiac aging and cardiovascular risk |
| Ma et al., 2014 [60] | PS-1-mutated AD fibroblasts | Medium with 50 μg/mL WS-CoQ10 | WS-CoQ10 | ↓ ROS ↑ Cell doubling ↓ SIPS ↑ PCNA expression ↓ MnSOD, p21, p16Ink4A, and Rb ↑ Autophagy |
WS-CoQ10 inhibits SIPS and improves autophagy |
| Xue et al., 2017 [61] | Mouse PSCs | Cell treatment with 1/10/100 μM CoQ10 for 24/48/72 h | CoQ10 | ↓ Apoptosis ↓ SA-β-Gal ↓ ROS and malondialdehyde after 72 h treatment |
CoQ10 may act as a target in PSC-related pathologies |
| Wu et al., 2020 [62] | ORX mice | CoQ10 50 mg/kg/day | CoQ10 | ↓ OS ↓ Cell senescence ↑ Osteoblastic bone formation |
CoQ10 is anti-osteoporosis and -senescence |
| Mine et al., 2021 [63] | H2O2-induced SIPS in human skin fibroblasts | 1 μM and 10 μM CoQ10 | CoQ10 | ↑ Cell viability ↓ OS ↓ SA-β-Gal ↓ SASP |
CoQ10 can contribute to increase lifespan |
| Zhang et al., 2015 [64] | D-galactose -induced aging in MSC | 1/10/100 mmol/L CoQ10 for 48 h | CoQ10 | ↓ p-AKT and p-mTOR ↓ MSC senescence |
CoQ10 inhibits MSC senescence and aging |
| Velichokovska et al., 2019 [65] | NPCs exposed to ART | NP-based delivery of CoQ10 to mitochondria | CoQ10 | ↓ ROS ↑ SIRT3 ↑ Cell proliferation ↓ SA-β-Gal ↑ Telomere length |
ART-induced senescence can be reversed by NP-CoQ10 |
| Marcheggiani et al., 2021 [66] | CoQ10-deprived HDF | 5, 10 or 15 μg/mL of either CoQ10 or CoQ10H2 for 24 h |
CoQ10 or CoQ10H2 | ↓ SA-β-Gal ↓ OS ↓ p21 ↑ Elastin, collagen type I |
CoQ10 or CoQ10H2 prevent skin aging and support skin vitality |
| Huo et al., 2018 [67] | HUVEC treated with H2O2 | 24 h in medium with 10 μM CoQ10H2 | CoQ10H2 | ↓ SA-β-Gal positive cells ↓ SASP ↓ ROS ↓ Apoptosis ↑ NO and eNOS ↑ Mitochondrial function |
CoQ10H2
delays vascular aging |
| Yan et al., 2006 [68] | SAMP1 mice | 250 mg/kg/day lifelong supplement | CoQ10H2 | ↓ Senescence grading scores ↑ Female body weight = Lifespan = Urinary 8-OHdG and acrolein-lysine adduct |
CoQ10H2 decreases cellular senescence in middle-aged SAMP1 mice |
| Olivieri et al., 2013 [69] | Senescent HUVECs in presence or absence of LPS | 10 µM CoQ10H2 for 24 h or 60 days | CoQ10H2 | ↓ LPS-induced NF-kB activation ↓ SASP |
CoQ10H2 may prevent aged-induced endothelial dysfunction |
| Maruoka et al., 2014 [70] | SAMP1 mice | 300 mg/kg (Group A) or 30 mg/kg CoQ10H2 (Group B) | CoQ10H2 | ↓ Senescence score at 10 months in Group A compared to B ↓ OS ↑ Antioxidant potential |
CoQ10H2 reduces senescence and OS in a dose-dependent manner |
| Schmelzer et al., 2010 [71] | Middle aged SAMP1 mice | 500 mg/kg/day of CoQ10H2 for 6 or 14 months | CoQ10H2 | ↓ Senescence grading score ↑ PPAR-alpha |
CoQ10H2 decelerates degeneration in SAMP1 mice |
| Cirilli et al., 2020 [72] | HUVEC treated with CSE for 24 h | 10 µM CoQ10H2 | CoQ10H2 and menaquinone 7 | ↓ OS ↓ Inflammation ↓ Apoptosis ↓ SA-β-Gal |
CoQ10H2 and menaquinone-7 counteract CSE-induced damage |
| Tian et al., 2014 [73] | SAMP1 mice | Dietary CoQ10H2 (0.3% w/w) | CoQ10H2 | ↑ PGC-1α, SOD2, IDH2, SIRT1, SIRT3 ↑ Mitochondrial complex I activity ↓ OS ↑ GSH/GSSG ratio |
CoQ10H2 protects against aging progression |
Abbreviations: AD: Alzheimer’s Disease; ART: antiretroviral therapy; CoQ10: coenzyme Q10; CoQ10H2: ubiquinol; CSE: cigarette smoke extract; eNOS: endothelial nitric oxide synthase; GSH: reduced glutathione; GSSG: oxidized glutathione; HAEC: human aortic endothelial cells; HDF: human dermal fibroblasts; HUVEC: human umbilical vein endothelial cells; IDH2: isocitrate dehydrogenase 2; LPS: lipopolysaccharide; MSC: mesenchymal stem cell; NF-kB: nuclear factor kappa-light-chain-enhancer of activated B cells; NO: nitric oxide; NP: nanoparticle; NPCs: neural progenitor cells; NRTI: nucleoside reverse transcriptase inhibitors; ORX: orchiectomized; OS: oxidative stress; PCNA: proliferating cell nuclear antigen; PGC-1α: peroxisome proliferator-activated receptor γ coactivator 1α; PPAR: peroxisome proliferator-activated receptor; PS-1: presenilin-1; PSCs: pancreatic stellate cells; ROS: reactive oxygen species; SA-β-Gal: senescence-associated β-galactosidase; SAMP1: one of the senescence accelerated mice (SAM) strains, which shows shortened life span and early signs of senescence; SASP: senescence-associated secretory phenotype; SIPS: stress induced premature senescence; SIRT: sirtuin; SOD2: superoxide dismutase 2; WS-CoQ10: water-soluble CoQ10; 8-OHdG: 8-hydroxy-2′-deoxyguanosine; ↑: increase; ↓: decrease.
Table 2.
SkQ1 and MB in the prevention of OS-induced senescence.
| Compound | Sample | Treatment | Results | Ref. |
|---|---|---|---|---|
| SkQ1 | Podospora anserina, Ceriodaphnia affinis, Drosophila melanogaster, and mouse | Nano- and subnanomolar concentrations of SkQ1 |
-Prolonged lifespan -Reduced senescence |
[74,75] |
| SkQ1 | Wistar and senescence-accelerated rats | 250 nmol per kg/day SkQ1 (starting from 19 months of age) | -Reduced and reversed age-related decline | [76] |
| SkQ1 | BALB/c and C57BL/6 mice | Lifelong administration of SkQ1 | -Decreased cardiomyopathy, fibrosis and cardiac hypertrophy | [77] |
| SkQ1 | Senescence-accelerated rats | 250 nmol/kg body weight, daily (from 1.5 to 23 months of age) | -Reduced Alzheimer’s disease pathology | [78] |
| MB | Human IMR90 fibroblasts | 10, 100 or 1000 nM of MB for 4 days | -Delayed senescence -Improved mitochondrial function |
[79] |
| MB | Human skin fibroblasts derived from progeria patients | 100 nM MB | -Effective ROS scavenging -Improved skin fibroblast proliferation -Delayed senescence |
[80] |
| MB | Human bone marrow-derived MSCs | 200 nM MB | -Improved expansion in vigorous MSCs -Improved differentiation in vigorous MSCs |
[81] |
| MB | Primary rat RGCs | 1 μM and 10 μM MB | -Stimulated mitochondrial function -Enhanced neuroprotection |
[82] |
Abbreviations: MB: methylene blue; MSCs: mesenchymal stem cells; RGCs: retinal ganglion cells.
CoQ10 is a lipid-soluble molecule involved in oxidative phosphorylation, metabolism, mitochondria permeability and antioxidant defence, either directly or indirectly [83]. A lack of CoQ10 has been related to several conditions, including aging and neurological disorders [83,84,85,86,87,88,89] (Table 1). A representative example is the increase in mitochondrial dysfunction, OS, apoptosis, and aging found in human dermal fibroblasts when CoQ10 production is pharmacologically inhibited [90]. Regarding senescence, CoQ10 deficiency has also been linked to increased p21 expression (a regulator of cell cycle progression), enhanced SASP production and downregulation of some extracellular matrix components (collagen type I and elastin) [66]. In vitro studies conducted on human skin fibroblasts exposed to H2O2 have shown that cell treatment with CoQ10 significantly reduced OS, decreased the amount of SA-β gal positive cells and restored collagen type I protein and the senescence-associated matrix metalloproteinase (MMP) production, therefore delaying skin aging [63]. Chronic treatment with nucleoside reverse transcriptase inhibitors (NRTI), which are clinically prescribed for the treatment of HIV, has been demonstrated to trigger oxidative damage, senescence, and endothelial toxicity. Recently, Chen et al. demonstrated that this phenotype could be reverted in vitro when human aortic endothelial cells are supplemented with CoQ10 [59], and similar findings were also reported concerning neural progenitor cells [65]. Stem cells are particularly sensitive to senescence induced by OS, as this condition may impact their self-renewal and repopulation capacity. In this respect, some in vitro studies indicate that CoQ10 can protect stem cells from OS-induced aging by influencing the Akt/mTOR signalling pathway, therefore preserving their proliferative balance [64].
In vivo administration of CoQ10 has long been known to improve immune functions by reducing immunological senescence that characterizes elderly mice [91]. More recently, studies conducted on mouse models of osteoporosis have demonstrated that CoQ10 supplementation (50 mg/kg/day) is sufficient to prevent osteoporosis by limiting ROS production and diminishing cellular senescence, both factors known to contribute to the disease development [62]. Moreover, CoQ10 may prevent cardiac aging, metabolic syndrome, and cardiovascular disorders when administered post-weaning to a rat model that mimics these conditions, and this improvement is mediated by the reduction of ROS and RNS, senescence, and apoptosis [58]. Similar beneficial effects have been obtained in cancer and Alzheimer’s disease (AD), in whose pathogenesis OS plays a predominant role [60,61]. Although beneficial, lifelong supplementation with CoQ10 may also be deleterious [92,93,94]. In this respect, results from a study designed to address CoQ10 administration only later in life showed that old mice subjected to a high CoQ10 diet displayed reduced OS in various tissues and were more efficient in performing the Morris water maze test compared to the untreated counterpart [95]. However, no improvements in other psychomotor or cognitive tests suggest that more research is needed to clarify the optimal timing of CoQ10 intake [95]. Nevertheless, the introduction of innovative delivery approaches to improve CoQ10 efficiency, such as the use of mitochondria-targeted nanoparticles, may represent a promising strategy to enhance CoQ10 antioxidant activity while limiting the possible side effects caused by high-doses administration [65].
CoQ10H2 (or ubiquinol), the reduced form of CoQ10, is even more efficient than CoQ10 itself in reverting senescence markers expression, both in vitro and in vivo [66,71]. The reason for this outperformance could be at least in part explained by a higher CoQ10H2 bioavailability at the same concentrations, therefore allowing a more efficient subcellular delivery [66,71]. For example, a study conducted by Huo et al. has shown that treatment with CoQ10H2 of H2O2-induced senescent human umbilical vein endothelial cells (HUVEC) is effective in reducing SA-β gal, SASP release and ROS production, but enhanced nitric oxide (NO) and endothelial NO synthase (eNOS) levels [67]. Diminished inflammation, OS-induced senescence and apoptosis have also been observed in the same cell line in other studies [69,72]. In vivo, experiments conducted on SAMP1 mice reported that ubiquinol administration at relatively high doses (250–300 mg/kg/day) for at least 10 months can reduce senescence grading scores and ROS production, while enhancing antioxidant defences [68,70]. However, no lifespan improvement was detected [68]. Upregulation of sirtuins 1 and 3 (SIRT1 and SIRT3), SOD2 and isocitrate dehydrogenase 2 (IDH2) enzymes, together with a higher reduced to oxidized glutathione (GSH/GSSG) ratio are also described upon dietary CoQ10H2 supplementation in an independent study, thus confirming the role of ubiquinol in protecting against cellular senescence progression and aging [73]. Finally, these improvements can be further enhanced by the combination of physical exercise and ubiquinol supplementation, as recently reported in in vivo studies carried out on SAMP8 mouse models [96].
The SkQs are a class of compounds made up by an antioxidant molecule (plastoquinone), a lipophilic cation and a linker moiety (decane or pentane). The family comprises SkQ1, SkQR1 and SkQ3, which belong to the mitochondria-targeted plastoquinone derivatives with antioxidant activity [97] (Table 2). In particular, SkQ1 and SkQR1 have been reported to reduce H2O2-induced senescence and apoptosis in vitro and to prevent senescence and tissue damage during aging [75]. Moreover, these benefits were achieved also in vivo in a wide range of age-related diseases and across species, even in the case of low doses administration later in life [74,76,97]. As for CoQ10, SkQ1 given to senescence prone rats at the concentration of 250 µmol/kg/day may be sufficient to prevent the physiological age-related deterioration of immunological defences [98]. Finally, AD-related cognitive decline, behavioural test scores and senescence-associated myocardial disease may improve in murine models upon long-term (lifelong) or limited (between 12 and 18 months of age) intake of SkQ1 [77,78,99,100,101]. Mechanistically, SkQ1 exerts its antioxidant properties by fatty acid co-mediated uncoupling, through interference with lipoperoxyl radicals and via regulating the electron flow at the level of mitochondria [102].
Methylene blue (MB) is a well-known mitochondria-targeted antioxidant that has shown promise in contrasting aging, especially skin aging [103] (Table 2). Methylene blue is reported to be particularly effective in delaying skin cellular senescence and extending fibroblasts lifespan in vitro, as well as in improving mitochondrial functions [79,80]. Although not yet fully understood, multiple mechanisms are thought to underlie its antioxidant function, including Keap/Nrf2 pathway upregulation, MB to MBH2 (the reduced form of MB) cycling in mitochondria, complex IV induction and increased production of collagen 2A1 and elastin, two components of the extracellular matrix fundamental for skin preservation [79,80,104]. Besides skin aging, MB may also prevent senescence and OS in other cell types, such as primary retinal ganglion cells and mesenchymal stem cells (MSCs), but its efficacy on stem cells remains limited to the cellular fraction characterized by a lower baseline level of OS [81,82].
Overall, despite promising evidence, results remain unclear. An important limitation is that studies are often performed on a specific cell line or murine model under certain conditions, which often prevent the results from being generalized and/or to be reproduced. In this respect, for example, data on extended fibroblast lifespan are debated, with some evidence showing that they can successfully decelerate aging and prevent senescence while other studies are inconclusive [105].
2.3. Vitamins
Vitamin A. Preformed vitamin A (all-trans-retinol and its esters) and pro-vitamin A (β-carotene) are essential dietary nutrients that provide a source of retinol, which regulate basic physiological processes [106,107]. Vitamin A and retinoic acid (a metabolite of all-trans-retinol) administration have been demonstrated to improve AD and age-related attenuation of memory/learning in mouse models, and this is probably due to their immunomodulatory effect and the reduction of pro-inflammatory cytokines and chemokines production by astrocytes and microglia, as well as to the promotion of differentiation of neural stem cells and regeneration of neural cells [108,109,110]. The role of vitamin A in the treatment of neurodegenerative diseases, such as amyotrophic lateral sclerosis (ALS) and schizophrenia, is currently under investigation [108,111]. Vitamin A has also been studied in association with quercetin, a well-known flavonol (see Section 2.7 Flavonoids) [112]. This combination has proven capable of reducing rapid senescence-like response induced by acute liver injury [113].
Vitamin C or ascorbic acid (AA) is a powerful antioxidant that can have beneficial effects on delaying the aging process and age-related diseases thorough its action on redox oxidative and mitochondrial pathways, on the immune system, on inflamm-aging, on endothelial integrity, and on lipoprotein metabolism [114,115,116,117,118,119,120,121]. Supplementation of AA also appears to prevent OS, immunosenescence, telomere attrition, disorganization of chromatin, and excessive secretion of inflammatory factors, and to prolong life [122]. For example, AA has been reported to extend replicative lifespan of human embryonic fibroblasts by restoring age-related decline of mitochondrial function and lowering cellular ROS, therefore reducing mitochondrial and DNA damages with decelerated telomere shortening [123,124]. Moreover, AA was found to have a protective effect also on human chondrocytes against OS by attenuating the increase of apoptosis, the loss of viability and the increase of senescence, and therefore hindering the development of osteoarthritis and aging of cartilage [125,126]. In the brain, AA has been increasingly found to promote several beneficial effects on neurodegeneration by direct neuroprotection against OS [116]. This vitamin has also been demonstrated to foster anti-senescence and anti-atherosclerotic effects via an improvement of lipoprotein parameters and microRNA expression through anti-oxidation and anti-glycation, especially in smokers [127,128,129]. Finally, a stable AA derivative, 2-O-α-glucopyranosyl-L-ascorbic acid (AA-2G), was also evaluated and compared with AA itself for its protective effect against cellular damage and senescence induced by hydrogen peroxide. The results suggest that the effect of AA-2G is longer-lasting compared to that of AA and this derivative might therefore be considered as a more stable form of vitamin C [130].
Vitamin E is a family of fat-soluble vitamins, which comprehends eight organic compounds with different degrees of antioxidant activity [131]. The impact of vitamin E on the prevention of chronic diseases is believed to be associated with OS and it has often been the subject of several studies. It has been recently observed that a higher consumption of antioxidants such as vitamin E is able to reduce ROS levels, leading to decreased telomere shortening, decelerating the cellular senescence, and potentially decreasing the risk of disease development [132,133].
Vitamin K compounds are a family of fat-soluble vitamins comprising structurally similar molecules including two main natural forms: phylloquinone (vitamin K1) and menaquinones (collectively known as vitamin K2). Besides being responsible for the activation of vitamin K-dependent proteins (VKDPs), which are involved in multiple functions such as bone and cardiovascular mineralization, vascular haemostasis, energy metabolism, immune response, brain metabolism, cellular growth, survival, and signalling [134,135,136,137], vitamin K appears to suppress the pro-inflammatory cytokines production through a non-carboxylative pathway, by modulating the gene expression of pro-inflammatory markers [138]. Accordingly, warfarin, a vitamin K antagonist, has been found to induce chronic low-grade inflammation in non-senescent vascular smooth muscle cells and enhance vascular aging and calcification, especially in young patients (<65 years old) [139,140].
2.4. Carotenoids
Carotenoids are naturally occurring lipophilic pigmented molecules found in fruits and vegetables with important antioxidant properties [141]. Chemically, their polyene structure, characterized by conjugated double carbon bonds, is at the basis of their ability to scavenge ROS and free radicals, therefore protecting from OS [141]. Although more than 750 carotenoids have been described [142], β-carotene, lycopene, lutein, and zeaxanthin remain the most examined for their implication in human health, with indications of their involvement in several age-related diseases [143,144,145,146,147,148,149,150]. There is evidence that carotenoids participate in the regulation of OS-induced senescence [151,152], and the same is true for parrodienes, which are structurally related to retinoids and carotenoids [153].
β-carotene is the precursor of retinoic acid [154,155]. Although it is generally considered an antioxidant, it can also function as a pro-oxidant compound depending on the circumstances, which are still not fully understood [156]. In vitro, keratinocytes treatment with β-carotene, prior to UVA exposure, prevents the upregulation of MMP-1, MMP-3 and MMP-10, therefore suggesting a protective role of β-carotene against OS-induced senescence [154].
Lutein and zeaxanthin are two macular pigment stereoisomers belonging to the xanthophyll group of dietary carotenoids [157]. Because of their unique ability to cross the blood-retina barrier, they accumulate in the macula and by virtue of their antioxidant, photoprotective and anti-inflammatory features are involved in the proper eye functioning [158,159,160]. A lack of lutein and zeaxanthin is generally associated to a poor cognitive performance in elderly [157,161]. Accordingly, improved cognitive functions were observed in elderly patients supplemented for one year with a mixture of lutein and zeaxanthin (12 mg/day), albeit not significant compared to the placebo group [162,163].
There is evidence that OS-induced senescence is involved in the pathogenesis of age-related macular degeneration (AMD), which represents the leading cause of blindness in aged individuals [158,164,165]. In this respect, Chae et al. documented that lutein treatment protects cells from H2O2-induced senescence by promoting the expression of antioxidant effectors such as nicotinamide adenine dinucleotide phosphate (NADPH) quinone dehydrogenase 1, heme oxygenase-1 (HO-1) and sirtuins (SIRT1 and SIRT3) [164]. Moreover, lutein and zeaxanthin intake, either as supplement or through xanthophyll-enriched foods, might delay AMD thanks to increased antioxidant protection [166]. Finally, data from Sen et al. show that a positive correlation exists between telomere length and xanthophyll carotenoids plasma levels, thus confirming the important role of lutein and zeaxanthin in the context of cellular senescence.
Lycopene is a lipophilic carotenoid naturally found in tomatoes and other red vegetables and fruits with potent cytoprotective and antioxidant properties [167,168]. During aging, lycopene protects from cognitive impairment, insulin resistance and cancer, among the others [169,170,171]. In a study involving 1973 participants, Weber et al. showed that plasma lycopene levels are significantly different between young and old women, thus suggesting that its antioxidant activity is crucial to prevent age-related diseases [161]. Similarly, studies conducted on MSCs demonstrated that cellular pretreatment with lycopene protects against H2O2-induced senescence, enhances antioxidant defences (i.e., improved MnSOD activity and reduced ROS production) and prevents apoptosis through the modulation of Bax and Bak proteins [172]. When used alone, lycopene is known to foster the increase in HO-1, which is detected in dermal fibroblasts after exposure to UVA, thus representing a cytoprotective mechanism [173,174]. Moreover, the combination of lycopene with the anti-aging compound nicotinamide mononucleotide (NMN) has proven effective in reducing OS both in vitro and in vivo by enhancing the activity of SOD, CAT, GPx enzymes [175]. This effect, combined with the activation of the Kaep1-Nrf2 antioxidant pathway, efficiently prevents cells to become senescent, therefore confirming the promising role of lycopene in improving the anti-aging effect of already established compounds [175]. These results indicate that multiple carotenoids might be responsible for the antioxidant effects reported in the literature, but more research is needed to clarify the optimal combination of these supplements.
2.5. Organosulphur Compounds
Glutathione is a natural tripeptide, that is γ-l-glutamyl-l-cysteinylglycine, harbouring a fundamental role in the regulation of redox homeostasis [176]. Glutathione can exist in two forms: reduced glutathione (GSH) and oxidized glutathione (GSSG), which are converted into each other by the enzymatic activity of GPx (that links two GSH in one GSSG through the formation of a disulphide bond) and glutathione reductase (that catalyses the reduction of one GSSG into two GSH to the expenses of NADPH) [177]. Being the main intracellular antioxidant buffer, both the levels of GSH and GSH/GSSH ratio are tightly controlled through a fine regulation of their synthesis, metabolism, transport, and degradation [176,177]. A GSH deficiency has been related to the onset and progression of several diseases, including cancer, immunodeficiencies, seizures, neurodegeneration, cardiovascular dysfunctions, and diabetes [178,179,180]. As GSH levels can be used as a biomarker for the oxidative status of the cell [176,181,182,183], a reduction in GSH and the GSH/GSSG ratio are often reported during normal aging and in cellular senescence, both conditions influenced by OS [183,184,185,186]. For example, inhibiting or reducing GSH synthesis is sufficient to induce premature senescence and OS-mediated telomere shortening in HUVEC, and this condition is not restored by telomerase activity [187]. Similarly, a decreased activity of the enzyme glutamate-cysteine ligase, which is involved in the synthesis of GSH, has been linked to senescence, ROS production and DNA damage in primary mouse fibroblasts [188]. Of note, these detrimental effects are reversed by N-acetylcysteine supplementation, which is known to increase intracellular GSH levels [188]. Further evidence demonstrates that GSH deficiency can also trigger senescence through a pathway involving ROS production and Erk/p38 regulation, in a mechanism independent from the canonical p53 activation [189]. Therapeutically, small extracellular vesicles enriched in the glutathione S-transferase Mu 2 (GSTM2) enzyme, which works in conjunction with GSH to reduce OS and detoxify the cell from harmful compounds, can relieve senescence in various tissues when injected intraperitoneally in old mice [190]. Although reproducible, these results are not always consistent. Contrary to expectations, Tong et al. reported no reduction in brain GSH levels when analysing human postmortem brain samples in elderly subjects compared to younger ones, albeit the lack of data in living tissues represents an important limit of this study [191]. Moreover, Barilani et al. recently showed that increased GSH levels accompany MSCs aging [192]. Nevertheless, this mechanism might be a protective strategy to counterbalance the age-related increase in ROS observed during cellular senescence [191,192].
At brain level, elderly people (>74 years old) are generally characterized by reduced glutathione-S-transferase activity accompanied by slightly lower cerebrospinal fluid (CSF) antioxidant defences compared to younger individuals [193]. These data are consistent with previous evidence reporting an age-related decline in GSH levels both in the brain and the liver of SAM mice, along with other antioxidant molecules [194]. In humans, this impaired glutathione homeostasis might be involved in the pathogenesis of brain disorders, including age-related neurodegeneration [195].
Because GSH is a crucial regulator of oxidative status, it might also represent a promising therapeutic target. Indeed, enrichment analysis research performed on the DrugAge database, a repository of compounds known to extend life, showed that GSH is among the most common targets of lifespan prolonging drugs [196]. In this respect, Rebrin et al. demonstrated that the benefits of diets enriched in vitamins and micronutrients should be ascribed to increased plasma levels of GSH and improved mitochondria redox homeostasis in a sex and tissue dependent manner [197]. Direct GSH delivery is another therapeutic option. However, the insufficient bioavailability of GSH remains a limit, and the use of prodrugs and precursors of GSH have been proposed as an alternative route [198]. Recent data from Kumar et al. showed that supplementation with glycine and N-acetylcysteine ensures the correct GSH balance and extends mice lifespan by 24% [199]. Similarly, the administration of glutathione precursors (i.e., glycine and cysteine) is sufficient to significantly increase GSH levels and reduce OS in aged individuals [200,201].
Overall, these data point to GSH as a key antioxidant regulator involved in OS-induced senescence. However, although promising, more research is needed to carefully address its potential role as biomarker and therapeutic compound in the context of aging and senescence.
Alliin, allicin, allyl sulphides, allylcysteines and other sulphur-containing compounds have long been known for their antioxidant properties [202]. Mainly contained in onion and garlic, they have shown to exert beneficial effects against cardiovascular diseases, cancer, aging, inflammation, OS, and infection, among the others [202,203]. Concerning aging, SAMP8 mice fed for 2 months with a diet containing 2% of aged garlic extract (AGE), which has been reported to have a higher antioxidant activity compared to fresh garlic extract [204], show improved lifespan and learning scores compared to the untreated counterpart [205,206,207]. The improvement in memory functions was then confirmed in vitro by a study conducted on primary hippocampal neurons derived from SAMP10 mice, whose dendrites are increased in length and number upon treatment with S-allylcysteine, the most abundant organosulphur compounds present in AGE [208]. In vivo, 12-week dietary supplementation with S-allylcysteine (0.05% or 0.2%) to 60-week-old wild type mice reduces senescence, improves mitochondrial functions, and ameliorates both aging and OS biomarkers [209]. At the molecular level, AGE reduces the production of ROS, increases glutathione levels, enhances the activity of the main antioxidant enzymes SOD, CAT and GPx, prevents lipid peroxidation and inhibits NF-kB (nuclear factor kappa-light-chain-enhancer of activated B cells) activity [203,210]. Despite encouraging results, discordant evidence emerged from some studies when the molecules contained in the AGE were tested individually. For example, while allicin shows senolytic activity when administered to breast cancer cells, alliin instead behaves as a pro-senolytic compound in the same conditions [211]. Still, when used as a whole, garlic extract exerts a strong NO scavenging function, reduces MMP-1 expression and ROS levels, inhibits SASP and improves SIRT1 activity, thus alleviating UVB-induced senescence in keratinocytes [212]. The combination of the beneficial effects exerted by the different AGE components may explain this discrepancy. For instance, recent evidence has shown that S-1-propenylcysteine, one of the AGE components, acts as an anti-inflammatory via stimulation of IL-10 expression and promotion of macrophage polarization towards an M2c status, which regulates the phagocytosis process of apoptotic cells [213]. According to these results, synergistic effects might be achieved by combining anti-inflammatory properties of S-1-propenylcysteine together with anti-aging and antioxidant activities reported for the other organosulfur compounds. Moreover, the administration dosage should be carefully evaluated because high concentrations of antioxidants may instead exacerbate OS. Overall, in line with the well-known beneficial effects of onion and garlic consumption, it is emerging that various organosulfur compounds commonly found in their extracts can prevent OS, thus supporting their usefulness in counteracting the aging process.
2.6. Nitrogen Non-Protein Compounds
Uric acid (UA) is a by-product of purine metabolism normally found in blood and urine. In the context of OS, although UA is classified as an important antioxidant molecule when circulating in the plasma, it exerts a potent pro-oxidant activity once inside the cell or in the form of extracellular crystals, probably due to different environmental interactions [214,215]. However, the molecular switch behind this dual role of UA, also defined as the “uric acid paradox”, remains largely unknown and controversial [216]. Accordingly, chronic serum hyperuricemia positively correlates with inflammation, DNA damage and OS, and has been implicated in the pathogenesis of several disorders, including renal, metabolic, and cardiovascular diseases [214,217,218]. Concerning senescence, several studies have demonstrated a link between UA levels, OS, and cell cycle arrest, both in vitro and in vivo, and improved aging-related functions have been observed following the administration of UA lowering agents [219]. For example, keratinocyte exposure to exogenous UA triggers cellular senescence and OS through a mechanism that is at the basis of the UV-induced damage [220], and a similar pattern has been reported for other cell lines [221]. Moreover, xanthine oxidoreductase, an enzyme involved in the production of UA, ROS and RNS, has been shown to promote aging and cellular senescence in vitro as well as in animal and clinical investigations [222]. Further, in vitro studies have reported an increased cellular senescence and enhanced ROS production in endothelial progenitor cells cultured in a medium containing UA at high concentrations (10 mg/dL) [223]. Of note, the same detrimental effects were shown in mice characterized by chronic hyperuricemia [223]. At the molecular level, there is evidence that UA triggers OS-induced senescence through the inhibition of the enzyme eNOS, which is essential to produce the scavenging molecule NO [215,223]. This condition triggers an OS imbalance, which promotes cellular senescence [215,223]. However, higher plasma UA levels in d-galactose rat models of accelerated aging were linked to decreased senescence and an increased SOD/(GPx + CAT) enzymatic ratio, which is indicative of antioxidant activity, thus confirming the beneficial role of UA when considered in the plasma [224].
In humans, results from a comparative study conducted on 26 elderly participants and 18 controls reported a 2-fold reduction in serum UA levels in aged individuals compared to controls, and this pattern was in line with diminished antioxidant defences [225].
Overall, these data show the existence of a correlation between UA and senescence. However, the dual role that UA may play in the context of OS should encourage further research to better clarify the befits and harms of UA-lowering agents.
2.7. Flavonoids
Flavonoids are a class of polyphenolic secondary metabolites found in plants and are routinely consumed by humans. Chemically, they are polyphenols with the structure of a 15-carbon skeleton (C6–C3–C6) formed by two aromatic rings and one pyran ring [226]. Tea, wine, and Chinese herbal plants are the primary sources of flavonoids, as well as leaf vegetables, onion, apples, cherries, berries, soybeans, and citrus fruit [227]. Flavonoid compounds are divided into six subclasses, flavones, flavonols, flavanones, isoflavones, flavanols, and anthocyanins [228]. Beside the antioxidant activity, flavonoids have anti-inflammatory, vasodilator, anticoagulant, cardioprotective, anti-diabetic, neuroprotective, and anti-obesity activities, which make them of great interest as anti-aging compounds (Table 3) [228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257].
Table 3.
Preclinical studies on flavonoids in aging.
| Type of Flavonoid | Effect | Reference |
|---|---|---|
| 4,4′-dimethoxychalcone |
|
|
| Naringenin |
|
|
| Nobiletin: Rutaceae family |
|
|
| Quercetin |
|
|
| Fisetin |
|
|
| Apigenin |
|
|
| Theaflavin |
|
|
| Myrecitin |
|
|
| Rutin |
|
|
| Luteonil |
|
|
| Kaempferol |
|
|
| Hesperidin |
|
|
| Dyhydromericetin |
|
|
| Epicatechin |
|
|
| Genistein |
|
Flavonols. Quercetin is a flavonol known for its antioxidant, anti-inflammatory, antitumor, and senolytic properties [258]. Results obtained on different cell lines show that treatment with quercetin significantly lowers the levels of ROS and inflammatory cytokines, reduces the expression of SA-β gal, p16 and p53 and markedly increases that of the antioxidant enzymes SOD and CAT, regardless of the type of oxidative trigger used to induce senescence [258,259,260,261,262]. In addition to promoting the expression of Nrf2 [263], the beneficial action of quercetin appears to be mediated by the microRNA-155-5p, which is involved in the regulation of SIRT1 and NF-kB [262,264]. Moreover, as aging is associated with an inefficient protein-degradation (which is required to protect against OS), the effect of quercetin and its derivatives on the restoration of proteasomal functioning is of interest as rejuvenating strategy [265]. In trials in patients with diabetic kidney disease [266] and idiopathic lung diseases, quercetin induced a reduction in the expression of the aging markers p16 and SA-β gal, suggesting an anti-aging effect on kidney cells [267]. When combined with dasatinib (a tyrosine kinase inhibitor used as an antitumoral drug), quercetin showed senolytic activity, improvement of physical function and increased lifespan in mice [268]. Interestingly, as quercetin plus dasatinib treatment reduces intestinal senescence and inflammation while altering specific microbiota signatures, this optimized senolytic regimen might improve health via reducing intestinal senescence, inflammation, and microbial dysbiosis in older subjects [269].
Another promising bioactive flavonol with antioxidant properties is fisetin [270]. In vitro cell treatment with fisetin has shown a reduction in senescence, ROS, and apoptosis [270,271]. In vivo, 6-week oral administration of fisetin drastically reduced senescence, ROS, lipid peroxidation and protein oxidation in a rat model of induced aging, and lifespan extension has been reported in mice [235,272]. This positive outcome is due both to a senolytic activity of fisetin but also to its function as caloric restriction mimetic, which is reported to prolong lifespan [273,274]. However, the timing of fisetin administration seems to be crucial for obtaining a biological benefit. If on the one hand fisetin is protective when administered in the presence of OS, if it is given chronically in physiological conditions, it may even cause telomere shortening, therefore promoting senescence [275]. For this reason, more studies are needed to better assess the optimal conditions of fisetin intake and its mechanism of action.
Isoflavonoids. Genistein is a phytoestrogen extracted by soya that is known for its antioxidant and anti-aging properties, although less potent than other flavonoids such as quercetin and kaempferol [264]. As for other antioxidants, the role of genistein is multiple: it can induce apoptosis acting as a cancer protective compound, but it can also reduce inflammation and OS acting as anti-aging and neurodegenerative protective agent [276]. Concerning senescence, genistein alleviates the genotoxicity and the cytotoxicity triggered by UVB exposure in human dermal fibroblasts [277]. Mechanistically, genistein reduces OS-induced senescence by mitigating the levels of mitochondrial ROS and of the DNA oxidation marker 8-OHdG, as well as by upregulating the SIRT1-FOXO3 axis, which is known to prevent aging [278].
Flavanols. There is consistency in the literature about the beneficial role of green tea on senescence-related mechanisms, thanks to its scavenging properties against ROS and RNS and its ability to stimulate autophagy [279,280,281,282]. These desirable effects derive from certain molecules known for their antioxidant role, mainly catechins [281,283]. Even if there are no conclusive results demonstrating the impact of green tea on the human diet, some studies investigated its effects on mice [284]. Catechins supplementation from green tea has been associated with a better memory performance and a protective role against DNA oxidative damage in SAM, independently from the age when the administration of green tea was started [285,286]. These antioxidants have a positive impact also on the brain structure, as murine models fed with green tea show an attenuated brain atrophy compared with SAM drinking pure water, thus suggesting an anti-aging property of these molecules [287]. (-)-Epigallocatechin-3-gallate (EGCG) is the most representative flavanol in green tea and its role in contrasting senescence is due to an activation of enzymatic and non-enzymatic antioxidative mechanisms (such as GPx and tocopherol), which are typically reduced in old age [288,289,290,291,292]. Interestingly, EGCG anti-senescence effects can also be observed at a macroscopic level as its supplementation reduces age-related sarcopenia in mice [293]. Nonetheless, an excessive amount of green tea has been associated with oxidative damage, underlying the need of further research to set the beneficial dose range [279,294].
Flavanones and flavones. Among flavanones, hesperidin is an antioxidant that can be typically found in citrus fruits [295]. Its properties impact positively on cardiomyocytes as it attenuates senescence-related oxidative damage, both independently and in combination with other molecules, through the induction of Nrf2 and of GST expression [247,296,297]. Citrus juice, which is rich in hesperidin and other flavanones as well as in flavones, anthocyanins, and other molecules, was reported to reduce ROS levels and reduce SA-β gal positive HUVEC [298]. Citrus fruit also contains another flavanone useful to counteract the effects of aging on myocardium, which is naringenin [299,300]. A recent study conducted in aging murine models suggests that the antioxidant properties of naringenin deriving from the activation of PI3K/Akt/Nrf2 pathway could greatly ameliorate both behavioural and neurological dysfunctions. The authors reported that naringenin administration markedly stimulated the activity of Nrf2 and improved the expression of the antioxidant enzymes HO-1 and NADPH-quinone oxidoreductase 1 [301].
Besides containing flavonones, citrus peels are also rich in flavones. One of these, nobiletin, was demonstrated to attenuate senescence-related cognitive deficits in SAMP8 mice by counteracting amyloid ß accumulation in the brain [302,303]. Flavones and flavonones are also significant components of bergamot juice and they confer anti-aging properties through the upregulation of SIRT1, Nrf2 and FOXO3 (that are involved in homeostasis, resistance to oxidative damage and overall health respectively), as it was demonstrated in models of senescent myocardiocytes and in vivo in mice [304].
Apigenin, also known as 5,7,4′-trihydroxyflavone, is a flavone typically found in parsley, oranges, and chamomile. Its ability to act as a metal chelator, free radical scavenger, and regulator of the main pathways involved in redox homeostasis [i.e., Nrf2, NF-kB, MAPK (mitogen-activated protein kinase) and Akt (a.k.a. protein kinase B] has increased its interest as an antioxidant molecule [305]. For example, creams rich in apigenin are used for their beneficial effects on skin aging prevention [306,307]. In vitro, human embryonic lung fibroblasts exposed to the pro-senescence stimuli H2O2 or doxorubicin, and subsequently treated with apigenin, show reduced SA-β gal activity, cell cycle promotion, increased levels of SIRT1, CAT and SOD and reduced expression of the senescence associated p21, p53 and p16 proteins compared to the untreated counterpart [308]. Similar results have been obtained in vivo following administration of apigenin daily for 8 weeks to a d-galactose-induced aging mouse model [309]. Moreover, thanks to its ability to inhibit the SASP and to interfere with the anti-apoptotic pathways, which are generally upregulated in senescent cancer cells, apigenin has been proposed as an adjuvant therapy for tumours, with promising results [237,310,311].
Anthocyanins. Bilberry and mulberry are considered promising nutrients for healthy aging because of their antioxidant properties related to anthocyanins that consist, among others, in the increase of SOD activity and AMPK (AMP-activated protein kinase)-mTOR autophagy pathway [312]. It has been reported that anthocyanins contrast senescence as they promote neural stem cells proliferation and diminish aging-related markers and cognitive impairment in mice [313]. The ability of anthocyanins to inhibit β amyloid aggregation is also of interest in therapeutic approaches aimed at slowing down cognitive decline [314]. In rats, the effect of mulberry extract was observed on the cardiovascular system, as it reduced the signs of senescence in endothelial cells [315,316].
Overall, a diet rich in these natural antioxidants may have a significant anti-aging effect. An indirect confirmation of this concept could be deduced by the fact that the Mediterranean Diet, which widely includes both flavanols, flavanones, flavons and anthocyanins, is characterized by well-known beneficial effects on health, including a healthy aging as it hinders the pathogenesis of many chronic diseases and extends life expectancy [317,318].
2.8. Non-Flavonoids
Non-flavonoid antioxidant substances, namely stilbenes (resveratrol), phenolic acids, curcuminoids (curcumin), and lignans [319] could be employed as anti-aging agents, acting against OS, inflammation, and cellular senescence (Table 4) [320,321,322,323,324,325,326,327,328,329,330,331,332].
Table 4.
Effects of non-flavonoids treatment in different experimental studies.
| Non-Flavonoid | Model | Effects | Reference |
|---|---|---|---|
| Resveratrol | HUVEC cells |
|
[320] |
| Senescence-accelerated mice |
|
[321] | |
| Old male mice |
|
[322] | |
| Gallic acid | Rat embryonic fibroblast cells |
|
[323] |
| UVB-irradiated human fibroblast cells |
|
[324] | |
| UVB-irradiated hairless mice |
|
||
| Ellagic acid | D-galactose-treated rats |
|
[325] |
| Ferulic acid | UVA-irradiated nHDF |
|
[326] |
| p-coumaric acid | Rat chondrocytes |
|
[327] |
| Mice fed with high-fat diet (HFD) |
|
[328] | |
| Curcumin | Senescence-accelerated mice |
|
[329] |
| Mice fed with HFD |
|
[330] | |
| Lignans | nPC12 cells |
|
[331] |
| D-galactose aging mice |
|
||
| Old HDFs |
|
[332] |
Abbreviations: AMPK: (AMP-activated protein kinase); CAT: (catalase); GPx: (glutathione peroxidase); GSH-Px: (plasma glutathione peroxidase); HO-1: (heme oxygenase 1); HUVEC: (Human umbilical vein endothelial cell); IL-1β: (interleukin 1 β); IL-6: (interleukin 6); MAPK: (mitogen-activated protein kinase); MCP-1: (monocyte chemoattractant protein-1); MDA: (malondialdehyde), COX-2 (cyclooxygenase 2); MMP-1: (matrix metalloproteinase 1); NF-kB: (nuclear factor kappa B); nHDF: (normal human dermal fibroblasts); nPC12: (neuronally differentiated phenchromocytoma cells); p-CaMKII: (p-calcium/calmodulin-dependent kinase II); p-NMDARI: (p-N-methyl-D-aspartate receptor subunit 1); p16: (cyclin-dependent kinase inhibitor 2A); p21: (cyclin-dependent kinase inhibitor 1); p27: (cyclin-dependent kinase inhibitor 1B); SOD: (superoxide dismutase), ROS (reactive oxygen species); SOD1: (superoxide dismutase 1); TNFα: (tumour necrosis factor); ↑: increase; ↓: decrease.
Stilbenes are a family of natural phenolic compounds found in many plant species capable of acting as antioxidants, anti-inflammatory, antibacterial, and anticancer agents [333,334]. The most important and well-known stilbene is resveratrol (RSV), a phytoalexin found in black grapes, peanuts, blackberries, red wine, and various herbal remedies [335,336,337]. It has many biological properties, including antioxidant, and anti-inflammatory effects [320,338]. As an antioxidant agent, RSV can scavenge free radicals and reduce ROS formation, by inhibiting the expression of NADPH and glycogen synthase kinase 3 beta proteins, and upregulating the expression of some antioxidant enzymes, such as SOD2, CAT, GPx, and thioredoxin [320,339,340]. It can also stimulate the production of HO-1 by activating Nrf2 [341]. Resveratrol treatment has also been shown to prevent or slow down the progression of cardiovascular, neurological, and metabolic disorders, as well as to be promising in the prevention of cancer, viral infection, and pathological inflammation [342]. The activation of the anti-aging protein SIRT1 by RSV is thought to be responsible for its antioxidant and anti-inflammatory properties, as well as for some of its protective effects [343,344,345]. Interestingly, this compound possesses anti-aging properties, modulating OS, inflammation, and cellular senescence [346]. It has been demonstrated that RSV can reduce oxidative damage in the brain of aged mice by increasing the levels of SOD and plasma GPx, decreasing malondialdehyde, and lowering the expression of several pro-inflammatory proteins (IL1β and tumour necrosis factor α) in old mice, as well as in patients with coronary artery disease [321,322]. Overall, these studies suggest that RSV can be a tool useful in preventing diseases and damages associated with aging. Furthermore, it can also be a valid strategy for counteracting bone fragility and skin aging [347,348].
Although RSV’s antioxidant properties have been widely demonstrated, some studies [349,350] have highlighted its ability to also act as a pro-oxidant molecule. This dual role depends upon cell type, used dosage, and exposure time [336]. Interestingly, RSV, which acts as a pro-oxidant agent at high doses, can be a cancer chemopreventive agent by promoting tumour cell senescence [351].
Phenolic acids are organic compounds commonly found in a variety of plant-based foods and beverages. They have numerous health properties (anti-inflammatory, anticarcinogenic, antibacterial), and their ability to act as antioxidants makes them an effective weapon against chronic diseases [352]. They are divided into two classes: hydroxybenzoic (including gallic and ellagic acids) and hydroxycinnamic acids (including ferulic and p-coumaric acids) [319].
Gallic acid (GA) is a natural substance found in berries, gallnuts, grapes, fruits, and wine [353]. Many studies have suggested the beneficial properties of this molecule [354,355,356,357]. Furthermore, thanks to its antioxidant activity, GA has numerous applications, especially in cosmetic and medical areas where it can be used as a UVB protective agent [358], by decreasing the production of MMP-1 and IL-6 and increasing the expression of elastin, type I procollagen and transforming growth factor β1 [324], and as a nutritional supplement to protect cells from oxidative damage [359]. Interestingly, in addition to these positive qualities, GA could be a protective anti-aging agent, able to counteract cellular senescence. Indeed, it has been shown that GA can reduce senescence markers in rat embryonic fibroblast cells, delay thymus involution in old mice, and protect cardiac cells from oxidative damage and senescence, enhancing GST expression [296,323,353]. Furthermore, as mentioned above, this acid is widely employed as a component of skincare products in the cosmetic branch. For example, the synergistic action of gallic, ellagic, and chebulinic acids confers to some cosmetic constituents, such as triphala (an ayurvedic herbal rasayana formula), antioxidant, anti-inflammatory, and anti-aging properties on human skin cells, increasing the mRNA expression of collagen-I, elastin, filaggrin, involucrin, as well as SOD2 and aquaporin-3, and decreasing the levels of tyrosinase [360].
Ellagic acid (EA), in addition to acting in combination with other phenolic acids, can perform numerous functions on its own. It is found in a variety of fruits and vegetables, including strawberries, walnuts, and grapes, and it has important antioxidant, anti-inflammatory, antiviral and anticarcinogenic properties [361,362]. Its beneficial antioxidant activity has been reported in numerous studies [363,364]. As an antioxidant agent, EA can activate cellular antioxidant enzymes, like SOD, CAT and GPx, protect DNA from ROS and chelate metal ions [365]. Additionally, EA could also act as an anti-aging agent [365]. Treatment with EA can reduce liver and brain damage in aged rats [325] and may display an anti-photoaging effect on the skin by restoring SOD and total GSH activity and increasing Nrf2 expression [361]. Interestingly, the consumption of walnuts, which contain EA and other neuroprotective compounds, has been shown to improve memory impairment and protect against AD [366].
Ferulic acid (FA) is an anti-inflammatory [367], anti-cancer [368], antithrombotic [369], antibacterial [370], and radioprotective agent [371] found in fruits (grapes), vegetables (spinach, rhubarb, carrots, eggplants), grain, and cereal seeds (rye, barley, and oats) [372]. Thanks to its antioxidants, anti-diabetic, and neuroprotective properties [373,374], it has been shown to prevent type 2 diabetes and AD [375,376] by regulating antioxidant enzymes and caspase activities [326]. Acting as an antioxidant, FA can inhibit the enzymes that lead to ROS formation, scavenge free radicals, and promote the antioxidant enzymes activity [372]. This makes FA a compound widely used in cosmetics and in food industry, especially as an anti-aging agent [377]. It has been demonstrated to protect skin from UV radiation through its capacity to reduce the activity of the stress-inducible protein Gadd45 α, the expression of MMP-1 and MMP-3 mRNAs, as well as enhancing the levels of the antioxidant enzymes SOD1 and CAT [326,378]. As a result of its anti-aging properties, it could be an excellent cosmetic component for face masks and antioxidant and protective creams. Moreover, it is used in skin-lightening lotions, inhibiting tyrosinase activity and melanocytic proliferation [372]. Peanuts also contain FA, which may partly explain their ability to prevent aging and cognitive decline [379].
p-coumaric acid (p-CA) is a dietary compound widely found in oranges, apples, grapes, kiwis, onions, potatoes, eggplant, beans, and grains [380,381], which is endowed with antibacterial [382], anti-diabetic [383], anti-cancer [384], and radioprotective properties [385]. Furthermore, p-CA has analgesic, antipyretic, and anxiolytic effects, as well as the ability to inhibit platelet aggregation [381]. Being an antioxidant agent, the treatment with this acid promotes the expression of Nrf2, with the consequent increased levels of some antioxidant enzymes, including HO-1, SOD, NAD(P)H quinone dehydrogenase 1, CAT, and GPx [328]. Coumaric acid can also slow down the aging processes, due to its antioxidant and anti-inflammatory effects. It has been shown that p-CA can have beneficial effects on skin aging by decreasing collagenase, elastase, and hyaluronidase activity [386], and by reducing the inflammatory response and chondrocytes senescence, inhibiting MAPK and NF-kB signalling pathways [327]. However, although some works have demonstrated the antioxidant property of p-CA, Pieńkowska et al. highlighted that this acid is unable to counteract the premature senescence of human fibroblasts [387]. Consequently, the presence of contradictory evidence in literature requires further research.
Curcuminoids are natural polyphenolic compounds used as spices and food additives thanks to their aromatic and colouring properties [388]. The numerous beneficial activities make them potential supportive therapeutics for cancer and inflammatory bowel diseases [389]. Curcumin (CUR), a yellow phenolic pigment, commonly used as a food spice and herbal remedy, is the most well-known curcuminoid [390]. This compound is known to have anti-cancer [391], anti-bacterial [392], anti-diabetic [393], and cytoprotective activities [394]. Further, CUR possesses antioxidant properties, through which it can prevent lipid peroxidation, stabilize Nrf2, with the consequent expression of HO-1, and increase the levels of antioxidant enzymes, such as SOD, GST, GSH and GPx [395]. It has been suggested that this natural compound possesses therapeutic features in several malfunctions, including neurological, cardiological, and metabolic disorders, as well as ulcers, arthritis, acne, and dyspepsia [396]. Curcumin is also thought to be a useful anti-aging agent [395]. Indeed, it has been shown to improve cognitive deficits, suppress vascular aging and inflammation in elderly mice, and attenuate neuronal aging both in vitro and in vivo by downregulating the expression of p16 and p21 and upregulating antioxidant enzymes, including SOD1, CAT and GPx [329,330,397]. Furthermore, CUR supplementation has positive benefits on age-related disorders [398,399,400]. Although an antioxidant action in aging has been widely demonstrated, conflicting studies are present in literature regarding its inability to counteract OS [401], therefore more research is needed to better understand its antioxidant role.
Lignans are found in many plant families and foods, including fruits, vegetables, nuts (sesame), grains, and seeds [402]. In addition to their numerous biological activities (antioxidant, anti-inflammatory, and antitumoral), as well as their ability to protect against the onset of chronic and metabolic diseases, lignans and their derivatives are also known to act as anti-aging agents [403]. In fact, they can suppress aging phenotypes in Drosophila adults, inhibit NADPH oxidase activity and upregulate antioxidant genes, such as SOD1, SOD2, catalase, and DNA repairing genes [221,404]. Further, they can reduce the levels of senescence in old human diploid fibroblasts, activating AMPK pathway [332]. Moreover, lignans molecules can protect tissues and organs against OS, inflammation, and senescence by acting as neuroprotective and radioprotective agents [405,406].
2.9. Minerals
Despite their tiny amount, micronutrients, i.e., vitamins and minerals, are essential for human health, exerting numerous functions, including antioxidant defence ranging from genome-related processes, such as DNA replication and repair, to metabolic processes and antioxidant defence [407,408]. Concerning the latter, the structural and functional roles of a few minerals such as zinc (Zn), selenium (Se), magnesium (Mg) copper (Cu) and manganese (Mn) is crucial (Table 5) [409,410,411,412,413,414,415,416,417,418,419,420].
Table 5.
Minerals as modulators of OS-induced senescence.
| Mineral | Sample | Treatment/Condition | Result | Ref. |
|---|---|---|---|---|
| Zinc | Colon cancer lines SW480 and SW620 | ↓ Zinc | ↑ Oxidative stress, cellular proliferation, stress signalling morphological changes, cell death | [409] |
| Zinc | Dermal fibroblast | ↑ Zinc | ↑ Oxidative stress and DNA damage | [410] |
| Zinc | HCAECs | ↑ Zinc | ↑ Senescence | [411] |
| Selenium | Bone marrow stromal cells | ↑ Selenium | ↓ Senescence | [412] |
| Selenium | Keratinocytes | ↑ Selenium | ↓ Senescence | [413] |
| Selenium | Human fibroblasts | ↑ Selenium | ↓ Senescence | [414] |
| Selenium | Mice | ↓ Selenium | ↑ Senescence | [415] |
| Magnesium | Endothelial cells | ↓ Magnesium | ↑ Oxidative stress and cell death | [416] |
| Magnesium | Endothelial cells | ↓ Magnesium | ↑ Pro-inflammatory molecules | [417] |
| Magnesium | Embryo-hepatocytes | ↓ Magnesium | ↑ Oxidative stress | [418] |
| Magnesium | Human fibroblasts | ↓ Magnesium | ↑ Telomere shortening | [419] |
| Magnesium | Rats | ↓ Magnesium | ↑ Age-related diseases | [420] |
Abbreviations: HCAECs: Human coronary artery endothelial cells; ↑: increase; ↓: decrease.
Zinc, the second most abundant trace mineral in the body after iron, is involved in a wide range of key biological functions exerted through its catalytic role in enzymes, structural function in proteins and other cellular components [421,422,423]. Importantly, Zn exerts antioxidant functions through its catalytic action in Zn- SOD, via the formation and stabilization of sulfhydryl groups in proteins, thus maintaining membrane integrity and protecting it from oxidation, and through regulation of Zn-binding protein metallothionein expression. In this respect, evidence showed that Zn deficiency causes destabilization of membrane structure and augments OS [424,425,426,427,428]. In addition, Zn suppresses anti-inflammatory responses that would otherwise promote OS [425]. In vitro studies have shown that Zn deficiency is associated with an increased production of ROS, oxidative damage to DNA, proteins and lipids, destabilization of membrane structure, dysregulation of Zn-binding protein metallothionein [426,429]. For instance, in a colon cancer cell line [409] and dermal fibroblasts [410], Zn dysregulation promoted cellular senescence activating stress response and pro-apoptosis pathways. Some Zn-finger proteins and Zn-dependent enzymes, such as PATZ1 [430], ZKSCAN3 [431], ZHX3 [432], KLF4 [433] or Zfp637 [434] might be responsible for this Zn-mediated cellular senescence inhibition, reducing ROS production, DNA damage and telomere shortening (Table 6) [430,431,432,433,434,435,436,437]. In support of this concept, there is evidence that the downregulation of the Zn-finger protein ZEB2 significantly promotes cell senescence in hepatic stellate cells and dermal fibroblasts, limiting the development of fibrosis [435,436]. Again, the Zn-finger protein 768 has been found to be overexpressed in cancer cells, contributing to cell proliferation and repressing senescence [437]. On the other hand, Zn overload can contribute to augment cellular OS and senescence through different mechanisms not yet well-defined, but possibly related to organelles dysfunction [411,427,438].
Table 6.
Interplay between zinc-finger proteins and senescence.
| Mineral | Sample | Zinc-Finger Proteins | Ref. |
|---|---|---|---|
| Zinc | Endothelial cells | PATZ1 is downregulated in senescence | [430] |
| Zinc | Mesenchymal stem cells | ZKSCAN3 upregulation contrast senescence | [431] |
| Zinc | Human diploid fibroblast | ZHX3 is downregulated in senescence | [432] |
| Zinc | Mouse embryonic fibroblasts | KLF4 reduces cellular senescence and DNA damage | [433] |
| Zinc | NIH3T3 and C2C12 cells | ZFP637 protects from oxidative stress | [434] |
| Zinc | Hepatic stellate cells | ZEB2 protects from oxidative stress and senescence | [435] |
| Zinc | Dermal fibroblasts | ZEB1 protects from oxidative stress and senescence | [436] |
| Zinc | Cell lines (A549, NCI-H441 and NCI-H460, 293T) | ZNF768 depletion induces senescence | [437] |
Abbreviations: KLF4: Kruppel-like factor 4; PATZ1: POZ/BTB and AT-hook-containing zinc finger protein 1; ZEB1: zinc finger E-box-binding homeobox 2; ZEB2: zinc finger E-box-binding homeobox 2; ZFP637: zinc finger protein 637; ZHX3: zinc fingers and homeoboxes 3; ZKSCAN3: zinc finger with KRAB and SCAN domains 3; ZNF768: zinc finger protein 768.
Selenium relevance in human body is primarily due to its structural and catalytic roles in selenoproteins involved in redox signalling and homeostasis. Most of the human selenoproteins are oxidoreductases containing the amino acid selenocysteine (SeCys) at their catalytic site [439]. In the antioxidant enzymes GPXs, SeCys residues catalyse the reduction of hydrogen peroxide and peroxide radicals using glutathione as a substrate, thus lowering free radicals and consequent DNA damage [440,441]. A second crucial family of proteins containing SeCys and involved in redox biology is thioredoxin reductases (TRs), which contribute to the regulation of gene expression of multiple transcription factors implicated in inflammatory and cell cycle pathways (e.g., NF-kB and p53) [442], as well as in the recycling of antioxidant molecules [441].
Several in vitro studies have shown that Se supplementation counteracts senescence processes. For instance, in bone marrow stromal cells [443], cultured human fibroblasts [412], and keratinocytes [413], Se supplementation reduces ROS levels, DNA damage, telomere shortening and senescence biomarkers. In support of this concept, cells deficient in Selenoprotein H (a nuclear protein) displayed high levels of OS, persistent DNA damage and a decreased content in antioxidant molecules (glutathione) [414]. Furthermore, Se deprivation in mice has been found to accelerate DNA damage, senescence, and aging-related processes [415].
Magnesium has structural roles in DNA, proteins and enzymes including telomerases. It promotes DNA replication and transcription, protein synthesis and mitochondrial functions [444,445]. Low Mg can favour cellular senescence, accelerate telomerase shortening and disturb DNA stability, protein synthesis and mitochondrial function. Cell culture studies have demonstrated that Mg shortage negatively impact antioxidant defence, cell cycle progression and cellular viability; in particular, its deficiency in endothelial cell cultures enhances free radicals production and cell apoptosis [416], and increases the release of pro-inflammatory molecules [417]. Further, an enhanced production of hydrogenase peroxide and oxidative damage were measured in Mg-deficient embryo-hepatocytes [418]. Additionally, human fibroblasts cultured in Mg-deficient conditions showed a rapid telomere shortening and a decreased replicative lifespan [419]. Accordingly, animal studies have confirmed that a long-term Mg-deficient diet disrupts the redox balance and homeostasis and increases inflammation, consequently exacerbating the development and progression of aging-related diseases such as cardiovascular diseases, hypertension, or diabetes [420,446].
Manganese and copper are further trace elements involved in antioxidant mechanisms through their structural role within SOD and other crucial enzymes useful for protecting cells from OS [447,448,449]. Despite some controversial data arise, other findings point out that Mn supplementation might improve antioxidant functions in the lungs and ameliorate asthma conditions, as reported in a recent review [449].
Taken together, evidence from human studies is still lacking, but it is undeniable that proper mineral levels are crucial for the maintenance of the redox balance. However, it should be emphasized that a narrow range exists between the therapeutic and the pro-oxidative effects of some metals, including Se, Mn, Mg, Cu and Zn; therefore, a cautious and rational supplement choice must be assessed by the experts in the field.
2.10. Others—Melatonin
Melatonin is a hormone produced and released by the pineal gland with immunomodulatory, oncostatic, anti-aging, and endocrine modulator functions [450,451]. Its antioxidant and anti-inflammatory activities are exerted through the suppression of cyclooxygenase 2, NLRP3 inflammasome, gasdermin D, TLR-4, NF-kB, and NO release, as well as the concomitant activation of SIRT1 and Nrf2 free radical scavenging network [452]. For example, inhibition of sodium nitroprusside-mediated NO release and increased production of transcripts coding for antioxidant enzymes (i.e., SOD1, GPx1 and CAT) have been reported upon melatonin treatment in neuroblastoma cells and HUVEC, respectively [451,453]. However, melatonin can also stimulate the release of pro-inflammatory factors depending on the concurrent conditions, although this response seems to be limited to early treatment stages and needs to be better investigated [454].
As a reduction in melatonin secretion has been observed during aging [452], several studies have explored the role of this hormone in counteracting cellular senescence [451]. In this respect, melatonin has been proven capable of reducing oxidative stress and replicative senescence by enhancing autophagy, activating AMPK/FOXO3 pathways and increasing mitochondrial membrane potential, both in vitro and in vivo [455,456,457]. Melatonin-induced decrease in p53, p21 and p16 proteins, together with enhanced SIRT1 activity, have also been reported in the context of H2O2-induced senescence [458,459]. Regarding MSCs, it is known that long term culture stimulates ROS generation, thus promoting oxidative stress-induced senescence [460]. In this context, decreased cellular senescence, preserved self-renewal and activation of antioxidant defence pathways have all been observed after melatonin supplementation [460,461]. In vivo, melatonin intake has been reported to diminish age-derived inflammation and apoptosis and to decrease lipid peroxidation, thiobarbituric acid reactive substances and protein carbonyls, thus counteracting hippocampal senescence and exerting an anti-aging and antioxidant effect on mice brains [462,463,464,465]. However, contrasting evidence remains about the effect of melatonin on the regulation of antioxidant enzymes [457,463,464,465]. Finally, brain oxidative stress amelioration and increased osteopontin and senescence marker protein-30 have been shown following melatonin treatment in the context of vascular demented rats [466]. Altogether, these findings suggest that, despite encouraging evidence, more research is needed to address the role of melatonin in vivo and identify possible side effects.
3. Discussion
The elderly population is growing exponentially in parallel with basic and clinical research discoveries and improvement of the quality of care for those who are sick. Aging is one of the risk factors for chronic diseases, atherosclerosis, cardiovascular diseases, stroke, kidney failure, chronic lung disease, cancers, diabetes, osteoporosis, arthritis, blindness, dementia, and neurodegenerative diseases [467,468]. Lifespans have increased dramatically over the last century in large part due to advances in medicine that have nearly eliminated certain deadly infectious diseases.
Nutrition is one of the factors that can influence aging. Interviews of older persons reveal that most of them have continuous physical activities, positive thinking, and eat healthy foods such as vegetables, fruits, fishes, and less meat. It was shown that less calories [469], good sleep [470], less stress [471], good relationships [472], no smoke and low alcohol drinking are environmental factors capable of delaying aging, while genetic factors play a role for in 25% to 30% of life expectancy [473]. In this context, finding anti-aging drugs that meet the safety and effectiveness of long-term use has always been an important strategy for intervention in the aging field.
Although cellular senescence is crucial for the proper functioning of several physiological processes, much scientific evidence has demonstrated that it also plays a leading role in the pathophysiology of aging and age-related diseases [3,474]. Because OS is an important senescence-triggering stimulus [13], the thorough investigation of existing antioxidants and the search for new ones are of major interest. In this narrative review, we have summarized the potential of the major classes of antioxidants in extending life and preventing senescence. However, most antioxidants are known to exert a dual effect (both pro-oxidant and antioxidant), especially based on the doses administered. Accordingly, it has been reported that high doses of antioxidants promote cellular senescence [156,475,476]. In this respect, more research is needed to define the optimal dosage of antioxidants, also considering the interaction of multiple compounds coming either from the diet or from supplements. Currently this remains a limit, as most of the studies investigated a single compound on a particular cell line or on a specific animal model, which often prevents generalizing the results in a broader context. Besides the dosage, a careful evaluation of the optimal administration window can be also crucial to achieve a clinical benefit as antioxidants efficacy might depend upon OS levels [81]. From a human perspective, not only dose assessment but also the time of initiation (childhood, adolescence, adulthood, old age) and the duration of the treatment (lifelong, at alternating intervals or for a defined period) might affect the clinical outcomes. There are still few studies on the synergy and interference of multiple antioxidants taken in combination—increasingly fashionable in the modern society—and the benefits and risks of this approach should be carefully evaluated. Similarly, when integrated as supplements, antioxidants may give rise to different effects depending upon the time of the day they are taken and individual differences such as physical activity and lifestyle, which may also greatly vary among subjects. Given these considerations, studies conducted in vitro under limited variable conditions should be reproduced and validated on large-cohorts clinical trials.
Current reports distinguish the existence of senolytic (able to remove senescent cells) or senomorphic (able to modulate senescent cells) substances, which can include some plant-derived antioxidants (e.g., quercein, fisetin) but usually are functional concepts, the description of which is outside our review remit, and which should encourage clinical research and nutraceutical application, more than in vitro investigations [266,477,478,479]. It is difficult, to date, to indicate if an anti-oxidant vitamin is senolytic or senomorphic, due to the huge complexity of biological phenomena.
4. Conclusions
Antioxidants are formidable substances, mostly derived from plants, which have been considered so far to be beneficial agents able to address many redox-mediated injuries. Aging is usually considered as a major playground of oxidative stress, but it should be highlighted that ROS are fundamentally signalling molecules and that most of the stress responding mechanisms are tuned by fine modulation of ROS as signalling agents. Therefore, a correct action to address senescence is to find approaches and methods to improve and promote this modulation. Wise people used to state that equilibria stand on the fine regulation of pro- and con- hallmarks of xenobiotics. This is also our wish and recommendation for the future.
Acknowledgments
We kindly thank Abdelouahab Bellou (Institute of Sciences in Emergency Med cine, Academy of Medical Sciences, Guangdong Provincial People’s Hospital, Guangzhou, China; Wayne State University School of Medicine, Emergency Department, Detroit, MI, USA) for his precious help in revising the manuscript prior to submission.
Author Contributions
Conceptualization, A.V. and A.P.; writing—original draft preparation, A.V., S.C., L.I.M.C., E.P., G.B.P., C.S., A.C. and C.B.; writing—review and editing, A.P. and A.V.; supervision, G.R. and A.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported by grants from the Italian Ministry of Health as Ricerca Corrente and as RF-2016-02363298.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hayflick L., Moorhead P.S. The Serial Cultivation of Human Diploid Cell Strains. Exp. Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 2.Calcinotto A., Kohli J., Zagato E., Pellegrini L., Demaria M., Alimonti A. Cellular Senescence: Aging, Cancer, and Injury. Physiol. Rev. 2019;99:1047–1078. doi: 10.1152/physrev.00020.2018. [DOI] [PubMed] [Google Scholar]
- 3.Gorgoulis V., Adams P.D., Alimonti A., Bennett D.C., Bischof O., Bishop C., Campisi J., Collado M., Evangelou K., Ferbeyre G., et al. Cellular Senescence: Defining a Path Forward. Cell. 2019;179:813–827. doi: 10.1016/j.cell.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 4.di Micco R., Krizhanovsky V., Baker D., d’Adda di Fagagna F. Cellular Senescence in Ageing: From Mechanisms to Therapeutic Opportunities. Nat. Rev. Mol. Cell Biol. 2021;22:75–95. doi: 10.1038/s41580-020-00314-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krizhanovsky V., Yon M., Dickins R.A., Hearn S., Simon J., Miething C., Yee H., Zender L., Lowe S.W. Senescence of Activated Stellate Cells Limits Liver Fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sagiv A., Krizhanovsky V. Immunosurveillance of Senescent Cells: The Bright Side of the Senescence Program. Biogerontology. 2013;14:617–628. doi: 10.1007/s10522-013-9473-0. [DOI] [PubMed] [Google Scholar]
- 7.Lozano-Torres B., Estepa-Fernández A., Rovira M., Orzáez M., Serrano M., Martínez-Máñez R., Sancenón F. The Chemistry of Senescence. Nat. Rev. Chem. 2019;3:426–441. doi: 10.1038/s41570-019-0108-0. [DOI] [Google Scholar]
- 8.Gao X., Yu X., Zhang C., Wang Y., Sun Y., Sun H., Zhang H., Shi Y., He X. Telomeres and Mitochondrial Metabolism: Implications for Cellular Senescence and Age-Related Diseases. Stem Cell Rev. Rep. 2022:1–13. doi: 10.1007/s12015-022-10370-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumari R., Jat P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021;9:645593. doi: 10.3389/fcell.2021.645593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roger L., Tomas F., Gire V. Mechanisms and Regulation of Cellular Senescence. Int. J. Mol. Sci. 2021;22:13173. doi: 10.3390/ijms222313173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casella G., Tsitsipatis D., Abdelmohsen K., Gorospe M. MRNA Methylation in Cell Senescence. WIREs RNA. 2019;10:e1547. doi: 10.1002/wrna.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crouch J., Shvedova M., Thanapaul R.J.R.S., Botchkarev V., Roh D. Epigenetic Regulation of Cellular Senescence. Cells. 2022;11:672. doi: 10.3390/cells11040672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lettieri-Barbato D., Aquilano K., Punziano C., Minopoli G., Faraonio R. MicroRNAs, Long Non-Coding RNAs, and Circular RNAs in the Redox Control of Cell Senescence. Antioxidants. 2022;11:480. doi: 10.3390/antiox11030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun J., Cheng B., Su Y., Li M., Ma S., Zhang Y., Zhang A., Cai S., Bao Q., Wang S., et al. The Potential Role of M6A RNA Methylation in the Aging Process and Aging-Associated Diseases. Front. Genet. 2022;13:869950. doi: 10.3389/fgene.2022.869950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harries L.W. Dysregulated RNA Processing and Metabolism: A New Hallmark of Ageing and Provocation for Cellular Senescence. FEBS J. 2022 doi: 10.1111/febs.16462. [DOI] [PubMed] [Google Scholar]
- 16.Gheitasi I., Azizi A., Omidifar N., Doustimotlagh A.H. Renoprotective Effects of Origanum Majorana Methanolic L and Carvacrol on Ischemia/Reperfusion-Induced Kidney Injury in Male Rats. Evid.-Based Complement. Altern. Med. 2020;2020:9785932. doi: 10.1155/2020/9785932. [DOI] [Google Scholar]
- 17.Gholami A., Emadi F., Amini A., Shokripour M., Chashmpoosh M., Omidifar N. Functionalization of Graphene Oxide Nanosheets Can Reduce Their Cytotoxicity to Dental Pulp Stem Cells. J. Nanomater. 2020;2020:6942707. doi: 10.1155/2020/6942707. [DOI] [Google Scholar]
- 18.Liguori I., Russo G., Curcio F., Bulli G., Aran L., Della-Morte D., Gargiulo G., Testa G., Cacciatore F., Bonaduce D., et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Omidifar N., moghadami M., Mousavi S.M., Hashemi S.A., Gholami A., Shokripour M., Sohrabi Z. Trends in Natural Nutrients for Oxidative Stress and Cell Senescence. Oxidative Med. Cell. Longev. 2021;2021:7501424. doi: 10.1155/2021/7501424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rangel-Zúñiga O.A., Corina A., Lucena-Porras B., Cruz-Teno C., Gómez-Delgado F., Jiménez-Lucena R., Alcalá-Díaz J.F., Haro-Mariscal C., Yubero-Serrano E.M., Delgado-Lista J., et al. TNFA Gene Variants Related to the Inflammatory Status and Its Association with Cellular Aging: From the CORDIOPREV Study. Exp. Gerontol. 2016;83:56–62. doi: 10.1016/j.exger.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Sriram S., Yuan C., Chakraborty S., Tay W., Park M., Shabbir A., Toh S.-A., Han W., Sugii S. Oxidative Stress Mediates Depot-Specific Functional Differences of Human Adipose-Derived Stem Cells. Stem Cell Res. Ther. 2019;10:141. doi: 10.1186/s13287-019-1240-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu C., Xiao J.-H. The Keap1-Nrf2 System: A Mediator between Oxidative Stress and Aging. Oxidative Med. Cell. Longev. 2021;2021:6635460. doi: 10.1155/2021/6635460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopes-Paciencia S., Saint-Germain E., Rowell M.-C., Ruiz A.F., Kalegari P., Ferbeyre G. The Senescence-Associated Secretory Phenotype and Its Regulation. Cytokine. 2019;117:15–22. doi: 10.1016/j.cyto.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Collado M., Serrano M. Senescence in Tumours: Evidence from Mice and Humans. Nat. Rev. Cancer. 2010;10:51–57. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narita M., Nuñez S., Heard E., Narita M., Lin A.W., Hearn S.A., Spector D.L., Hannon G.J., Lowe S.W. Rb-Mediated Heterochromatin Formation and Silencing of E2F Target Genes during Cellular Senescence. Cell. 2003;113:703–716. doi: 10.1016/S0092-8674(03)00401-X. [DOI] [PubMed] [Google Scholar]
- 26.Serrano M., Lin A.W., McCurrach M.E., Beach D., Lowe S.W. Oncogenic Ras Provokes Premature Cell Senescence Associated with Accumulation of P53 and P16INK4a. Cell. 1997;88:593–602. doi: 10.1016/S0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 27.Chapman J., Fielder E., Passos J.F. Mitochondrial Dysfunction and Cell Senescence: Deciphering a Complex Relationship. FEBS Lett. 2019;593:1566–1579. doi: 10.1002/1873-3468.13498. [DOI] [PubMed] [Google Scholar]
- 28.Zhu M., Meng P., Ling X., Zhou L. Advancements in Therapeutic Drugs Targeting of Senescence. Ther. Adv. Chronic Dis. 2020;11:204062232096412. doi: 10.1177/2040622320964125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elsallabi O., Patruno A., Pesce M., Cataldi A., Carradori S., Gallorini M. Fisetin as a Senotherapeutic Agent: Biopharmaceutical Properties and Crosstalk between Cell Senescence and Neuroprotection. Molecules. 2022;27:738. doi: 10.3390/molecules27030738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coling D., Chen S., Chi L.-H., Jamesdaniel S., Henderson D. Age-Related Changes in Antioxidant Enzymes Related to Hydrogen Peroxide Metabolism in Rat Inner Ear. Neurosci. Lett. 2009;464:22–25. doi: 10.1016/j.neulet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller A.-F. Superoxide Dismutases: Ancient Enzymes and New Insights. FEBS Lett. 2012;586:585–595. doi: 10.1016/j.febslet.2011.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frye K.A., Sendra K.M., Waldron K.J., Kehl-Fie T.E. Old Dogs, New Tricks: New Insights into the Iron/Manganese Superoxide Dismutase Family. J. Inorg. Biochem. 2022;230:111748. doi: 10.1016/j.jinorgbio.2022.111748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Treiber N., Maity P., Singh K., Ferchiu F., Wlaschek M., Scharffetter-Kochanek K. The Role of Manganese Superoxide Dismutase in Skin Aging. Derm.-Endocrinol. 2012;4:232–235. doi: 10.4161/derm.21819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisher G.J., Varani J., Voorhees J.J. Looking Older. Arch. Dermatol. 2008;144:666–672. doi: 10.1001/archderm.144.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quan T., Shao Y., He T., Voorhees J.J., Fisher G.J. Reduced Expression of Connective Tissue Growth Factor (CTGF/CCN2) Mediates Collagen Loss in Chronologically Aged Human Skin. J. Investig. Dermatol. 2010;130:415–424. doi: 10.1038/jid.2009.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen R.G., Tresini M., Keogh B.P., Doggett D.L., Cristofalo V.J. Differences in Electron Transport Potential, Antioxidant Defenses, and Oxidant Generation in Young and Senescent Fetal Lung Fibroblasts (WI-38) J. Cell Physiol. 1999;180:114–122. doi: 10.1002/(SICI)1097-4652(199907)180:1<114::AID-JCP13>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 37.Borlon C., Debacq-Chainiaux F., Hinrichs C., Scharffetter-Kochanek K., Toussaint O., Wlaschek M. The Gene Expression Profile of Psoralen plus UVA-Induced Premature Senescence in Skin Fibroblasts Resembles a Combined DNA-Damage and Stress-Induced Cellular Senescence Response Phenotype. Exp. Gerontol. 2007;42:911–923. doi: 10.1016/j.exger.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Lu C.-Y., Lee H.-C., Fahn H.-J., Wei Y.-H. Oxidative Damage Elicited by Imbalance of Free Radical Scavenging Enzymes Is Associated with Large-Scale MtDNA Deletions in Aging Human Skin. Mutat. Res./Fundam. Mol. Mech. Mutagenesis. 1999;423:11–21. doi: 10.1016/S0027-5107(98)00220-6. [DOI] [PubMed] [Google Scholar]
- 39.Meewes C., Brenneisen P., Wenk J., Kuhr L., Ma W., Alikoski J., Poswig A., Krieg T., Scharffetter-Kochanek K. Adaptive Antioxidant Response Protects Dermal Fibroblasts from UVA-Induced Phototoxicity. Free Radic. Biol. Med. 2001;30:238–247. doi: 10.1016/S0891-5849(00)00463-9. [DOI] [PubMed] [Google Scholar]
- 40.Naderi-Hachtroudi L., Peters T., Brenneisen P., Meewes C., Hommel C., Razi-Wolf Z., Schneider L.A., Schüller J., Wlaschek M., Scharffetter-Kochanek K. Induction of Manganese Superoxide Dismutase in Human Dermal Fibroblasts. Arch. Dermatol. 2002;138:1473–1479. doi: 10.1001/archderm.138.11.1473. [DOI] [PubMed] [Google Scholar]
- 41.Mao C., Yuan J.-Q., Lv Y.-B., Gao X., Yin Z.-X., Kraus V.B., Luo J.-S., Chei C.-L., Matchar D.B., Zeng Y., et al. Associations between Superoxide Dismutase, Malondialdehyde and All-Cause Mortality in Older Adults: A Community-Based Cohort Study. BMC Geriatr. 2019;19:104. doi: 10.1186/s12877-019-1109-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohammedi K., Bellili-Muñoz N., Marklund S.L., Driss F., le Nagard H., Patente T.A., Fumeron F., Roussel R., Hadjadj S., Marre M., et al. Plasma Extracellular Superoxide Dismutase Concentration, Allelic Variations in the SOD3 Gene and Risk of Myocardial Infarction and All-Cause Mortality in People with Type 1 and Type 2 Diabetes. Cardiovasc. Diabetol. 2015;14:845. doi: 10.1186/s12933-014-0163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martini H., Passos J.F. Cellular Senescence: All Roads Lead to Mitochondria. FEBS J. 2022 doi: 10.1111/febs.16361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Treiber N., Maity P., Singh K., Kohn M., Keist A.F., Ferchiu F., Sante L., Frese S., Bloch W., Kreppel F., et al. Accelerated Aging Phenotype in Mice with Conditional Deficiency for Mitochondrial Superoxide Dismutase in the Connective Tissue. Aging Cell. 2011;10:239–254. doi: 10.1111/j.1474-9726.2010.00658.x. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y., Unnikrishnan A., Deepa S.S., Liu Y., Li Y., Ikeno Y., Sosnowska D., van Remmen H., Richardson A. A New Role for Oxidative Stress in Aging: The Accelerated Aging Phenotype in Sod1− Mice Is Correlated to Increased Cellular Senescence. Redox Biol. 2017;11:30–37. doi: 10.1016/j.redox.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hajam Y.A., Rani R., Ganie S.Y., Sheikh T.A., Javaid D., Qadri S.S., Pramodh S., Alsulimani A., Alkhanani M.F., Harakeh S., et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells. 2022;11:552. doi: 10.3390/cells11030552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ganini D., Santos J.H., Bonini M.G., Mason R.P. Switch of Mitochondrial Superoxide Dismutase into a Prooxidant Peroxidase in Manganese-Deficient Cells and Mice. Cell Chem. Biol. 2018;25:413–425.e6. doi: 10.1016/j.chembiol.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naranuntarat A., Jensen L.T., Pazicni S., Penner-Hahn J.E., Culotta V.C. The Interaction of Mitochondrial Iron with Manganese Superoxide Dismutase. J. Biol. Chem. 2009;284:22633–22640. doi: 10.1074/jbc.M109.026773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang M., Cobine P.A., Molik S., Naranuntarat A., Lill R., Winge D.R., Culotta V.C. The Effects of Mitochondrial Iron Homeostasis on Cofactor Specificity of Superoxide Dismutase 2. EMBO J. 2006;25:1775–1783. doi: 10.1038/sj.emboj.7601064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Killilea D.W., Atamna H., Liao C., Ames B.N. Iron Accumulation During Cellular Senescence in Human Fibroblasts In Vitro. Antioxid. Redox Signal. 2003;5:507–516. doi: 10.1089/152308603770310158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Killilea D.W., Maier J.A.M. A Connection between Magnesium Deficiency and Aging: New Insights from Cellular Studies. Magnes Res. 2008;21:77–82. [PMC free article] [PubMed] [Google Scholar]
- 52.Cutler R.G. Oxidative Stress and Aging: Catalase Is a Longevity Determinant Enzyme. Rejuvenation Res. 2005;8:138–140. doi: 10.1089/rej.2005.8.138. [DOI] [PubMed] [Google Scholar]
- 53.Koepke J.I., Wood C.S., Terlecky L.J., Walton P.A., Terlecky S.R. Progeric Effects of Catalase Inactivation in Human Cells. Toxicol. Appl. Pharmacol. 2008;232:99–108. doi: 10.1016/j.taap.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 54.Koepke J.I., Nakrieko K.-A., Wood C.S., Boucher K.K., Terlecky L.J., Walton P.A., Terlecky S.R. Restoration of Peroxisomal Catalase Import in a Model of Human Cellular Aging. Traffic. 2007;8:1590–1600. doi: 10.1111/j.1600-0854.2007.00633.x. [DOI] [PubMed] [Google Scholar]
- 55.Wood C.S., Koepke J.I., Teng H., Boucher K.K., Katz S., Chang P., Terlecky L.J., Papanayotou I., Walton P.A., Terlecky S.R. Hypocatalasemic Fibroblasts Accumulate Hydrogen Peroxide and Display Age-Associated Pathologies. Traffic. 2006;7:97–107. doi: 10.1111/j.1600-0854.2005.00358.x. [DOI] [PubMed] [Google Scholar]
- 56.Lin M.T., Beal M.F. Mitochondrial Dysfunction and Oxidative Stress in Neurodegenerative Diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 57.Ahmed E., Donovan T., Yujiao L., Zhang Q. Mitochondrial Targeted Antioxidant in Cerebral Ischemia. J. Neurol. Neurosci. 2015;6:2. doi: 10.21767/2171-6625.100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tarry-Adkins J.L., Blackmore H.L., Martin-Gronert M.S., Fernandez-Twinn D.S., McConnell J.M., Hargreaves I.P., Giussani D.A., Ozanne S.E. Coenzyme Q10 Prevents Accelerated Cardiac Aging in a Rat Model of Poor Maternal Nutrition and Accelerated Postnatal Growth. Mol. Metab. 2013;2:480–490. doi: 10.1016/j.molmet.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Y.-F., Hebert V.Y., Stadler K., Xue S.Y., Slaybaugh K., Luttrell-Williams E., Glover M.C., Krzywanski D.M., Dugas T.R. Coenzyme Q10 Alleviates Chronic Nucleoside Reverse Transcriptase Inhibitor-Induced Premature Endothelial Senescence. Cardiovasc. Toxicol. 2019;19:500–509. doi: 10.1007/s12012-019-09520-1. [DOI] [PubMed] [Google Scholar]
- 60.Ma D., Stokes K., Mahngar K., Domazet-Damjanov D., Sikorska M., Pandey S. Inhibition of Stress Induced Premature Senescence in Presenilin-1 Mutated Cells with Water Soluble Coenzyme Q10. Mitochondrion. 2014;17:106–115. doi: 10.1016/j.mito.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 61.Xue R., Yang J., Wu J., Meng Q., Hao J. Coenzyme Q10 Inhibits the Activation of Pancreatic Stellate Cells through PI3K/AKT/MTOR Signaling Pathway. Oncotarget. 2017;8:92300–92311. doi: 10.18632/oncotarget.21247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu X., Liang S., Zhu X., Wu X., Dong Z. CoQ10 Suppression of Oxidative Stress and Cell Senescence Increases Bone Mass in Orchiectomized Mice. Am. J. Transl. Res. 2020;12:4314–4325. [PMC free article] [PubMed] [Google Scholar]
- 63.Mine Y., Takahashi T., Okamoto T. Protective Effects of Coenzyme Q10 on Cell Damage Induced by Hydrogen Peroxides in Cultured Skin Fibroblasts. J. Clin. Biochem. Nutr. 2021;69:20–185. doi: 10.3164/jcbn.20-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang D., Yan B., Yu S., Zhang C., Wang B., Wang Y., Wang J., Yuan Z., Zhang L., Pan J. Coenzyme Q10 Inhibits the Aging of Mesenchymal Stem Cells Induced by D-Galactose through Akt/MTOR Signaling. Oxidative Med. Cell. Longev. 2015;2015:867293. doi: 10.1155/2015/867293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Velichkovska M., Surnar B., Nair M., Dhar S., Toborek M. Targeted Mitochondrial COQ10 Delivery Attenuates Antiretroviral-Drug-Induced Senescence of Neural Progenitor Cells. Mol. Pharm. 2019;16:724–736. doi: 10.1021/acs.molpharmaceut.8b01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marcheggiani F., Kordes S., Cirilli I., Orlando P., Silvestri S., Vogelsang A., Möller N., Blatt T., Weise J.M., Damiani E., et al. Anti-Ageing Effects of Ubiquinone and Ubiquinol in a Senescence Model of Human Dermal Fibroblasts. Free Radic. Biol. Med. 2021;165:282–288. doi: 10.1016/j.freeradbiomed.2021.01.032. [DOI] [PubMed] [Google Scholar]
- 67.Huo J., Xu Z., Hosoe K., Kubo H., Miyahara H., Dai J., Mori M., Sawashita J., Higuchi K. Coenzyme Q10 Prevents Senescence and Dysfunction Caused by Oxidative Stress in Vascular Endothelial Cells. Oxidative Med. Cell. Longev. 2018;2018:3181759. doi: 10.1155/2018/3181759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yan J., Fujii K., Yao J., Kishida H., Hosoe K., Sawashita J., Takeda T., Mori M., Higuchi K. Reduced Coenzyme Q10 Supplementation Decelerates Senescence in SAMP1 Mice. Exp. Gerontol. 2006;41:130–140. doi: 10.1016/j.exger.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 69.Olivieri F., Lazzarini R., Babini L., Prattichizzo F., Rippo M.R., Tiano L., di Nuzzo S., Graciotti L., Festa R., Brugè F., et al. Anti-Inflammatory Effect of Ubiquinol-10 on Young and Senescent Endothelial Cells via MiR-146a Modulation. Free Radic. Biol. Med. 2013;63:410–420. doi: 10.1016/j.freeradbiomed.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 70.Maruoka H., Fujii K., Inoue K., Kido S. Long-Term Effect of Ubiquinol on Exercise Capacity and the Oxidative Stress Regulation System in SAMP1 Mice. J. Phys. Ther. Sci. 2014;26:367–371. doi: 10.1589/jpts.26.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmelzer C., Kubo H., Mori M., Sawashita J., Kitano M., Hosoe K., Boomgaarden I., Döring F., Higuchi K. Supplementation with the Reduced Form of Coenzyme Q10 Decelerates Phenotypic Characteristics of Senescence and Induces a Peroxisome Proliferator-Activated Receptor-α Gene Expression Signature in SAMP1 Mice. Mol. Nutr. Food Res. 2010;54:805–815. doi: 10.1002/mnfr.200900155. [DOI] [PubMed] [Google Scholar]
- 72.Cirilli I., Orlando P., Marcheggiani F., Dludla P.V., Silvestri S., Damiani E., Tiano L. The Protective Role of Bioactive Quinones in Stress-Induced Senescence Phenotype of Endothelial Cells Exposed to Cigarette Smoke Extract. Antioxidants. 2020;9:1008. doi: 10.3390/antiox9101008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tian G., Sawashita J., Kubo H., Nishio S., Hashimoto S., Suzuki N., Yoshimura H., Tsuruoka M., Wang Y., Liu Y., et al. Ubiquinol-10 Supplementation Activates Mitochondria Functions to Decelerate Senescence in Senescence-Accelerated Mice. Antioxid. Redox Signal. 2014;20:2606–2620. doi: 10.1089/ars.2013.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anisimov V.N., Bakeeva L.E., Egormin P.A., Filenko O.F., Isakova E.F., Manskikh V.N., Mikhelson V.M., Panteleeva A.A., Pasyukova E.G., Pilipenko D.I., et al. Mitochondria-Targeted Plastoquinone Derivatives as Tools to Interrupt Execution of the Aging Program. 5. SkQ1 Prolongs Lifespan and Prevents Development of Traits of Senescence. Biochemistry. 2008;73:1329–1342. doi: 10.1134/S0006297908120055. [DOI] [PubMed] [Google Scholar]
- 75.Skulachev M., Antonenko Y., Anisimov V., Chernyak B., Cherepanov D., Chistyakov V., Egorov M., Kolosova N., Korshunova G., Lyamzaev K., et al. Mitochondrial-Targeted Plastoquinone Derivatives. Effect on Senescence and Acute Age-Related Pathologies. Curr. Drug Targets. 2011;12:800–826. doi: 10.2174/138945011795528859. [DOI] [PubMed] [Google Scholar]
- 76.Kolosova N.G., Stefanova N.A., Muraleva N.A., Skulachev V.P. The Mitochondria-Targeted Antioxidant SkQ1 but Not N-Acetylcysteine Reverses Aging-Related Biomarkers in Rats. Aging. 2012;4:686–694. doi: 10.18632/aging.100493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Manskikh V.N., Gancharova O.S., Nikiforova A.I., Krasilshchikova M.S., Shabalina I.G., Egorov M.V., Karger E.M., Milanovsky G.E., Galkin I.I., Skulachev V.P., et al. Age-Associated Murine Cardiac Lesions Are Attenuated by the Mitochondria-Targeted Antioxidant SkQ1. Histol. Histopathol. 2015;30:353–360. doi: 10.14670/HH-30.353. [DOI] [PubMed] [Google Scholar]
- 78.Stefanova N.A., Muraleva N.A., Skulachev V.P., Kolosova N.G. Alzheimer’s Disease-Like Pathology in Senescence-Accelerated OXYS Rats Can Be Partially Retarded with Mitochondria-Targeted Antioxidant SkQ1. J. Alzheimer’s Dis. 2013;38:681–694. doi: 10.3233/JAD-131034. [DOI] [PubMed] [Google Scholar]
- 79.Atamna H., Nguyen A., Schultz C., Boyle K., Newberry J., Kato H., Ames B.N. Methylene Blue Delays Cellular Senescence and Enhances Key Mitochondrial Biochemical Pathways. FASEB J. 2008;22:703–712. doi: 10.1096/fj.07-9610com. [DOI] [PubMed] [Google Scholar]
- 80.Xiong Z.-M., O’Donovan M., Sun L., Choi J.Y., Ren M., Cao K. Anti-Aging Potentials of Methylene Blue for Human Skin Longevity. Sci. Rep. 2017;7:2475. doi: 10.1038/s41598-017-02419-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bertolo A., Capossela S., Fränkl G., Baur M., Pötzel T., Stoyanov J. Oxidative Status Predicts Quality in Human Mesenchymal Stem Cells. Stem Cell Res. Ther. 2017;8:3. doi: 10.1186/s13287-016-0452-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Daudt D.R., Mueller B., Park Y.H., Wen Y., Yorio T. Methylene Blue Protects Primary Rat Retinal Ganglion Cells from Cellular Senescence. Investig. Opthalmol. Vis. Sci. 2012;53:4657. doi: 10.1167/iovs.12-9734. [DOI] [PubMed] [Google Scholar]
- 83.Hargreaves I., Heaton R.A., Mantle D. Disorders of Human Coenzyme Q10 Metabolism: An Overview. Int. J. Mol. Sci. 2020;21:6695. doi: 10.3390/ijms21186695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yubero D., Montero R., Martín M.A., Montoya J., Ribes A., Grazina M., Trevisson E., Rodriguez-Aguilera J.C., Hargreaves I.P., Salviati L., et al. Secondary Coenzyme Q 10 Deficiencies in Oxidative Phosphorylation (OXPHOS) and Non-OXPHOS Disorders. Mitochondrion. 2016;30:51–58. doi: 10.1016/j.mito.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 85.Mantle D. Coenzyme Q10 Supplementation for Diabetes and Its Complications: An Overview. Br. J. Diabetes. 2017;17:145–148. doi: 10.15277/bjd.2017.149. [DOI] [Google Scholar]
- 86.Hargreaves I., Mantle D., Milford D. Chronic Kidney Disease and Coenzyme Q10 Supplementation. J. Kidney Care. 2019;4:82–90. doi: 10.12968/jokc.2019.4.2.82. [DOI] [Google Scholar]
- 87.Mantle D., Hargreaves I. Coenzyme Q10 and Degenerative Disorders Affecting Longevity: An Overview. Antioxidants. 2019;8:44. doi: 10.3390/antiox8020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Emmanuele V., López L.C., Berardo A., Naini A., Tadesse S., Wen B., D’Agostino E., Solomon M., DiMauro S., Quinzii C., et al. Heterogeneity of Coenzyme Q10 Deficiency: Patient study and literature review. Arch. Neurol. 2012;69:978–983. doi: 10.1001/archneurol.2012.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang S., Liu T., Li S., Zhang X., Ding Q., Que H., Yan X., Wei K., Liu S. Comparative Proteomic Analysis of Brains of Naturally Aging Mice. Neuroscience. 2008;154:1107–1120. doi: 10.1016/j.neuroscience.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 90.Marcheggiani F., Cirilli I., Orlando P., Silvestri S., Vogelsang A., Knott A., Blatt T., Weise J.M., Tiano L. Modulation of Coenzyme Q10 Content and Oxidative Status in Human Dermal Fibroblasts Using HMG-CoA Reductase Inhibitor over a Broad Range of Concentrations. From Mitohormesis to Mitochondrial Dysfunction and Accelerated Aging. Aging. 2019;11:2565–2582. doi: 10.18632/aging.101926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bliznakov E.G. Immunological Senescence in Mice and Its Reversal by Coenzyme Q10. Mech. Ageing Dev. 1978;7:189–197. doi: 10.1016/0047-6374(78)90065-9. [DOI] [PubMed] [Google Scholar]
- 92.Sohal R.S., Kamzalov S., Sumien N., Ferguson M., Rebrin I., Heinrich K.R., Forster M.J. Effect of Coenzyme Q10 Intake on Endogenous Coenzyme Q Content, Mitochondrial Electron Transport Chain, Antioxidative Defenses, and Life Span of Mice. Free Radic. Biol. Med. 2006;40:480–487. doi: 10.1016/j.freeradbiomed.2005.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sumien N., Heinrich K.R., Shetty R.A., Sohal R.S., Forster M.J. Prolonged Intake of Coenzyme Q10 Impairs Cognitive Functions in Mice. J. Nutr. 2009;139:1926–1932. doi: 10.3945/jn.109.110437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mcdonald S.R., Sohal R.S., Forster M.J. Concurrent Administration of Coenzyme Q10 and α-Tocopherol Improves Learning in Aged Mice. Free Radic. Biol. Med. 2005;38:729–736. doi: 10.1016/j.freeradbiomed.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 95.Shetty R.A., Forster M.J., Sumien N. Coenzyme Q10 Supplementation Reverses Age-Related Impairments in Spatial Learning and Lowers Protein Oxidation. Age. 2013;35:1821–1834. doi: 10.1007/s11357-012-9484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Andreani C., Bartolacci C., Guescini M., Battistelli M., Stocchi V., Orlando F., Provinciali M., Amici A., Marchini C., Tiano L., et al. Combination of Coenzyme Q10 Intake and Moderate Physical Activity Counteracts Mitochondrial Dysfunctions in a SAMP8 Mouse Model. Oxidative Med. Cell. Longev. 2018;2018:8936251. doi: 10.1155/2018/8936251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Skulachev V.P., Anisimov V.N., Antonenko Y.N., Bakeeva L.E., Chernyak B.V., Erichev V.P., Filenko O.F., Kalinina N.I., Kapelko V.I., Kolosova N.G., et al. An Attempt to Prevent Senescence: A Mitochondrial Approach. Biochim. Biophys. Acta (BBA)-Bioenerg. 2009;1787:437–461. doi: 10.1016/j.bbabio.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 98.Obukhova L.A., Skulachev V.P., Kolosova N.G. Mitochondria-Targeted Antioxidant SkQ1 Inhibits Age-Dependent Involution of the Thymus in Normal and Senescence-Prone Rats. Aging. 2009;1:389–401. doi: 10.18632/aging.100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stefanova N.A., Ershov N.I., Kolosova N.G. Suppression of Alzheimer’s Disease-Like Pathology Progression by Mitochondria-Targeted Antioxidant SkQ1: A Transcriptome Profiling Study. Oxidative Med. Cell. Longev. 2019;2019:3984906. doi: 10.1155/2019/3984906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Muraleva N.A., Stefanova N.A., Kolosova N.G. SkQ1 Suppresses the P38 MAPK Signaling Pathway Involved in Alzheimer’s Disease-Like Pathology in OXYS Rats. Antioxidants. 2020;9:676. doi: 10.3390/antiox9080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Loshchenova P.S., Sinitsyna O.I., Fedoseeva L.A., Stefanova N.A., Kolosova N.G. Influence of Antioxidant SkQ1 on Accumulation of Mitochondrial DNA Deletions in the Hippocampus of Senescence-Accelerated OXYS Rats. Biochemistry. 2015;80:596–603. doi: 10.1134/S0006297915050120. [DOI] [PubMed] [Google Scholar]
- 102.Ježek J., Engstová H., Ježek P. Antioxidant Mechanism of Mitochondria-Targeted Plastoquinone SkQ1 Is Suppressed in Aglycemic HepG2 Cells Dependent on Oxidative Phosphorylation. Biochim. Biophys. Acta (BBA)-Bioenerg. 2017;1858:750–762. doi: 10.1016/j.bbabio.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 103.Xue H., Thaivalappil A., Cao K. The Potentials of Methylene Blue as an Anti-Aging Drug. Cells. 2021;10:3379. doi: 10.3390/cells10123379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Atamna H., Atamna W., Al-Eyd G., Shanower G., Dhahbi J.M. Combined Activation of the Energy and Cellular-Defense Pathways May Explain the Potent Anti-Senescence Activity of Methylene Blue. Redox Biol. 2015;6:426–435. doi: 10.1016/j.redox.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sadowska-Bartosz I., Bartosz G. Effect of Antioxidants on the Fibroblast Replicative Lifespan In Vitro. Oxidative Med. Cell. Longev. 2020;2020:6423783. doi: 10.1155/2020/6423783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dawson M.I. The Importance of Vitamin A in Nutrition. Curr Pharm Des. 2000;6:311–325. doi: 10.2174/1381612003401190. [DOI] [PubMed] [Google Scholar]
- 107.Bar-El Dadon S., Reifen R. Vitamin A and the Epigenome. Crit. Rev. Food Sci. Nutr. 2017;57:2404–2411. doi: 10.1080/10408398.2015.1060940. [DOI] [PubMed] [Google Scholar]
- 108.Shudo K., Fukasawa H., Nakagomi M., Yamagata N. Towards Retinoid Therapy for Alzheimers Disease. Curr. Alzheimer Res. 2009;6:302–311. doi: 10.2174/156720509788486581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kitaoka K., Shimizu N., Ono K., Chikahisa S., Nakagomi M., Shudo K., Ishimura K., Séi H., Yoshizaki K. The Retinoic Acid Receptor Agonist Am80 Increases Hippocampal ADAM10 in Aged SAMP8 Mice. Neuropharmacology. 2013;72:58–65. doi: 10.1016/j.neuropharm.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 110.Fukasawa H., Nakagomi M., Yamagata N., Katsuki H., Kawahara K., Kitaoka K., Miki T., Shudo K. Tamibarotene: A Candidate Retinoid Drug for Alzheimer’s Disease. Biol. Pharm. Bull. 2012;35:1206–1212. doi: 10.1248/bpb.b12-00314. [DOI] [PubMed] [Google Scholar]
- 111.Malaspina A., Michael-Titus A.T. Is the Modulation of Retinoid and Retinoid-Associated Signaling a Future Therapeutic Strategy in Neurological Trauma and Neurodegeneration? J. Neurochem. 2008;104:584–595. doi: 10.1111/j.1471-4159.2007.05071.x. [DOI] [PubMed] [Google Scholar]
- 112.Li Y., Yao J., Han C., Yang J., Chaudhry M., Wang S., Liu H., Yin Y. Quercetin, Inflammation and Immunity. Nutrients. 2016;8:167. doi: 10.3390/nu8030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fang J., Liang W. ASCs-Derived Exosomes Loaded with Vitamin A and Quercetin Inhibit Rapid Senescence-like Response after Acute Liver Injury. Biochem. Biophys. Res. Commun. 2021;572:125–130. doi: 10.1016/j.bbrc.2021.07.059. [DOI] [PubMed] [Google Scholar]
- 114.Naidu K.A. Vitamin C in Human Health and Disease Is Still a Mystery? An Overview. Nutr. J. 2003;2:7. doi: 10.1186/1475-2891-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Padayatty S.J., Katz A., Wang Y., Eck P., Kwon O., Lee J.-H., Chen S., Corpe C., Dutta A., Dutta S.K., et al. Vitamin C as an Antioxidant: Evaluation of Its Role in Disease Prevention. J. Am. Coll. Nutr. 2003;22:18–35. doi: 10.1080/07315724.2003.10719272. [DOI] [PubMed] [Google Scholar]
- 116.Monacelli F., Acquarone E., Giannotti C., Borghi R., Nencioni A. Vitamin C, Aging and Alzheimer’s Disease. Nutrients. 2017;9:670. doi: 10.3390/nu9070670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ammar M.A., Ammar A.A., Condeni M.S., Bell C.M. Vitamin C for Sepsis and Septic Shock. Am. J. 2021;28:e649–e679. doi: 10.1097/MJT.0000000000001423. [DOI] [PubMed] [Google Scholar]
- 118.Kashiouris M.G., L’Heureux M., Cable C.A., Fisher B.J., Leichtle S.W., Fowler A.A. The Emerging Role of Vitamin C as a Treatment for Sepsis. Nutrients. 2020;12:292. doi: 10.3390/nu12020292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Teng J., Pourmand A., Mazer-Amirshahi M. Vitamin C: The next Step in Sepsis Management? J. Crit. Care. 2018;43:230–234. doi: 10.1016/j.jcrc.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 120.Fritz H., Flower G., Weeks L., Cooley K., Callachan M., McGowan J., Skidmore B., Kirchner L., Seely D. Intravenous Vitamin C and Cancer. Integr. Cancer Ther. 2014;13:280–300. doi: 10.1177/1534735414534463. [DOI] [PubMed] [Google Scholar]
- 121.van Gorkom G.N.Y., Lookermans E.L., van Elssen C.H.M.J., Bos G.M.J. The Effect of Vitamin C (Ascorbic Acid) in the Treatment of Patients with Cancer: A Systematic Review. Nutrients. 2019;11:977. doi: 10.3390/nu11050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Uchio R., Hirose Y., Murosaki S., Yamamoto Y., Ishigami A. High Dietary Intake of Vitamin C Suppresses Age-Related Thymic Atrophy and Contributes to the Maintenance of Immune Cells in Vitamin C-Deficient Senescence Marker Protein-30 Knockout Mice. Br. J. Nutr. 2015;113:603–609. doi: 10.1017/S0007114514003857. [DOI] [PubMed] [Google Scholar]
- 123.Hwang W.-S., Park S.-H., Kim H.-S., Kang H.-J., Kim M.-J., Oh S.-J., Park J.-B., Kim J., Kim S.C., Lee J.-Y. Ascorbic Acid Extends Replicative Life Span of Human Embryonic Fibroblast by Reducing DNA and Mitochondrial Damages. Nutr. Res. Pract. 2007;1:105–112. doi: 10.4162/nrp.2007.1.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Furumoto K., Inoue E., Nagao N., Hiyama E., Miwa N. Age-Dependent Telomere Shortening Is Slowed down by Enrichment of Intracellular Vitamin C via Suppression of Oxidative Stress. Life Sci. 1998;63:935–948. doi: 10.1016/S0024-3205(98)00351-8. [DOI] [PubMed] [Google Scholar]
- 125.Chang Z., Huo L., Li P., Wu Y., Zhang P. Ascorbic Acid Provides Protection for Human Chondrocytes against Oxidative Stress. Mol. Med. Rep. 2015;12:7086–7092. doi: 10.3892/mmr.2015.4231. [DOI] [PubMed] [Google Scholar]
- 126.Burger M., Steinitz A., Geurts J., Pippenger B., Schaefer D., Martin I., Barbero A., Pelttari K. Ascorbic Acid Attenuates Senescence of Human Osteoarthritic Osteoblasts. Int. J. Mol. Sci. 2017;18:2517. doi: 10.3390/ijms18122517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim S.-M., Lim S.-M., Yoo J.-A., Woo M.-J., Cho K.-H. Consumption of High-Dose Vitamin C (1250 Mg per Day) Enhances Functional and Structural Properties of Serum Lipoprotein to Improve Anti-Oxidant, Anti-Atherosclerotic, and Anti-Aging Effects via Regulation of Anti-Inflammatory MicroRNA. Food Funct. 2015;6:3604–3612. doi: 10.1039/C5FO00738K. [DOI] [PubMed] [Google Scholar]
- 128.Cho K.-H. Biomedicinal Implications of High-Density Lipoprotein: Its Composition, Structure, Functions, and Clinical Applications. BMB Rep. 2009;42:393–400. doi: 10.5483/BMBRep.2009.42.7.393. [DOI] [PubMed] [Google Scholar]
- 129.Ferretti G., Bacchetti T., Nègre-Salvayre A., Salvayre R., Dousset N., Curatola G. Structural Modifications of HDL and Functional Consequences. Atherosclerosis. 2006;184:1–7. doi: 10.1016/j.atherosclerosis.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 130.Taniguchi M., Arai N., Kohno K., Ushio S., Fukuda S. Anti-Oxidative and Anti-Aging Activities of 2-O-α-Glucopyranosyl-L-Ascorbic Acid on Human Dermal Fibroblasts. Eur. J. Pharm. 2012;674:126–131. doi: 10.1016/j.ejphar.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 131.Burton G.W., Traber M.G. Vitamin E: Antioxidant Activity, Biokinetics, and Bioavailability. Annu. Rev. Nutr. 1990;10:357–382. doi: 10.1146/annurev.nu.10.070190.002041. [DOI] [PubMed] [Google Scholar]
- 132.Shen J., Gammon M.D., Terry M.B., Wang Q., Bradshaw P., Teitelbaum S.L., Neugut A.I., Santella R.M. Telomere Length, Oxidative Damage, Antioxidants and Breast Cancer Risk. Int. J. Cancer. 2009;124:1637–1643. doi: 10.1002/ijc.24105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Corina A., Rangel-Zúñiga O.A., Jiménez-Lucena R., Alcalá-Díaz J.F., Quintana-Navarro G., Yubero-Serrano E.M., López-Moreno J., Delgado-Lista J., Tinahones F., Ordovás J.M., et al. Low Intake of Vitamin E Accelerates Cellular Aging in Patients With Established Cardiovascular Disease: The CORDIOPREV Study. J. Gerontol. Ser. A. 2019;74:770–777. doi: 10.1093/gerona/gly195. [DOI] [PubMed] [Google Scholar]
- 134.Shearer M.J., Newman P. Recent Trends in the Metabolism and Cell Biology of Vitamin K with Special Reference to Vitamin K Cycling and MK-4 Biosynthesis. J. Lipid Res. 2014;55:345–362. doi: 10.1194/jlr.R045559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kaisar M.A., Prasad S., Cucullo L. Protecting the BBB Endothelium against Cigarette Smoke-Induced Oxidative Stress Using Popular Antioxidants: Are They Really Beneficial? Brain Res. 2015;1627:90–100. doi: 10.1016/j.brainres.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Berendsen A., Santoro A., Pini E., Cevenini E., Ostan R., Pietruszka B., Rolf K., Cano N., Caille A., Lyon-Belgy N., et al. A Parallel Randomized Trial on the Effect of a Healthful Diet on Inflammageing and Its Consequences in European Elderly People: Design of the NU-AGE Dietary Intervention Study. Mech. Ageing Dev. 2013;134:523–530. doi: 10.1016/j.mad.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 137.Santoro A., Pini E., Scurti M., Palmas G., Berendsen A., Brzozowska A., Pietruszka B., Szczecinska A., Cano N., Meunier N., et al. Combating Inflammaging through a Mediterranean Whole Diet Approach: The NU-AGE Project’s Conceptual Framework and Design. Mech. Ageing Dev. 2014;136–137:3–13. doi: 10.1016/j.mad.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 138.Ohsaki Y., Shirakawa H., Miura A., Giriwono P.E., Sato S., Ohashi A., Iribe M., Goto T., Komai M. Vitamin K Suppresses the Lipopolysaccharide-Induced Expression of Inflammatory Cytokines in Cultured Macrophage-like Cells via the Inhibition of the Activation of Nuclear Factor ΚB through the Repression of IKKα/β Phosphorylation. J. Nutr. Biochem. 2010;21:1120–1126. doi: 10.1016/j.jnutbio.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 139.Wei N., Lu L., Zhang H., Gao M., Ghosh S., Liu Z., Qi J., Wang J., Chen J., Huang H. Warfarin Accelerates Aortic Calcification by Upregulating Senescence-Associated Secretory Phenotype Maker Expression. Oxidative Med. Cell. Longev. 2020;2020:2043762. doi: 10.1155/2020/2043762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nakano-Kurimoto R., Ikeda K., Uraoka M., Nakagawa Y., Yutaka K., Koide M., Takahashi T., Matoba S., Yamada H., Okigaki M., et al. Replicative Senescence of Vascular Smooth Muscle Cells Enhances the Calcification through Initiating the Osteoblastic Transition. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H1673-84. doi: 10.1152/ajpheart.00455.2009. [DOI] [PubMed] [Google Scholar]
- 141.Young A., Lowe G. Carotenoids—Antioxidant Properties. Antioxidants. 2018;7:28. doi: 10.3390/antiox7020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ross A.C., Caballero B.H., Cousins R.J., Tucker K.L., Ziegler T.R. Modern Nutrition in Health and Disease. 11th ed. Wolters Kluwer Health Adis (ESP); Alphen aan den Rijn, The Netherlands: 2012. [Google Scholar]
- 143.Mezzomo N., Ferreira S.R.S. Carotenoids Functionality, Sources, and Processing by Supercritical Technology: A Review. J. Chem. 2016;2016:3164312. doi: 10.1155/2016/3164312. [DOI] [Google Scholar]
- 144.Rafi M.M., Kanakasabai S., Gokarn S.V., Krueger E.G., Bright J.J. Dietary Lutein Modulates Growth and Survival Genes in Prostate Cancer Cells. J. Med. Food. 2015;18:173–181. doi: 10.1089/jmf.2014.0003. [DOI] [PubMed] [Google Scholar]
- 145.Cao W., Zeng F., Li B., Lin J., Liang Y., Chen Y. Higher Dietary Carotenoid Intake Associated with Lower Risk of Hip Fracture in Middle-Aged and Elderly Chinese: A Matched Case-Control Study. Bone. 2018;111:116–122. doi: 10.1016/j.bone.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 146.Akbaraly T.N., Fontbonne A., Favier A., Berr C. Plasma Carotenoids and Onset of Dysglycemia in an Elderly Population. Diabetes Care. 2008;31:1355–1359. doi: 10.2337/dc07-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Leermakers E.T., Darweesh S.K., Baena C.P., Moreira E.M., Melo van Lent D., Tielemans M.J., Muka T., Vitezova A., Chowdhury R., Bramer W.M., et al. The Effects of Lutein on Cardiometabolic Health across the Life Course: A Systematic Review and Meta-Analysis1,2. Am. J. Clin. Nutr. 2016;103:481–494. doi: 10.3945/ajcn.115.120931. [DOI] [PubMed] [Google Scholar]
- 148.Sandmann G. Carotenoids of Biotechnological Importance. Adv. Biochem. Eng. Biotechnol. 2014;148:449–467. doi: 10.1007/10_2014_277. [DOI] [PubMed] [Google Scholar]
- 149.Milani A., Basirnejad M., Shahbazi S., Bolhassani A. Carotenoids: Biochemistry, Pharmacology and Treatment. Br. J. Pharmacol. 2017;174:1290–1324. doi: 10.1111/bph.13625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ames B.N. Prolonging Healthy Aging: Longevity Vitamins and Proteins. Proc. Natl. Acad. Sci. USA. 2018;115:10836–10844. doi: 10.1073/pnas.1809045115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mária J., Ingrid Ž. Effects of Bioactive Compounds on Senescence and Components of Senescence Associated Secretory Phenotypes in Vitro. Food Funct. 2017;8:2394–2418. doi: 10.1039/C7FO00161D. [DOI] [PubMed] [Google Scholar]
- 152.Chisté R.C., Freitas M., Mercadante A.Z., Fernandes E. Carotenoids Inhibit Lipid Peroxidation and Hemoglobin Oxidation, but Not the Depletion of Glutathione Induced by ROS in Human Erythrocytes. Life Sci. 2014;99:52–60. doi: 10.1016/j.lfs.2014.01.059. [DOI] [PubMed] [Google Scholar]
- 153.Flori E., Mastrofrancesco A., Kovacs D., Ramot Y., Briganti S., Bellei B., Paus R., Picardo M. 2,4,6-Octatrienoic Acid Is a Novel Promoter of Melanogenesis and Antioxidant Defence in Normal Human Melanocytes via PPAR-γ Activation. Pigment Cell Melanoma Res. 2011;24:618–630. doi: 10.1111/j.1755-148X.2011.00887.x. [DOI] [PubMed] [Google Scholar]
- 154.Wertz K., Seifert N., Hunziker P.B., Riss G., Wyss A., Lankin C., Goralczyk R. β-Carotene Inhibits UVA-Induced Matrix Metalloprotease 1 and 10 Expression in Keratinocytes by a Singlet Oxygen-Dependent Mechanism. Free Radic. Biol. Med. 2004;37:654–670. doi: 10.1016/j.freeradbiomed.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 155.Fiedor J., Burda K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients. 2014;6:466–488. doi: 10.3390/nu6020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ribeiro D., Freitas M., Silva A.M.S., Carvalho F., Fernandes E. Antioxidant and Pro-Oxidant Activities of Carotenoids and Their Oxidation Products. Food Chem. Toxicol. 2018;120:681–699. doi: 10.1016/j.fct.2018.07.060. [DOI] [PubMed] [Google Scholar]
- 157.Feeney J., O’Leary N., Moran R., O’Halloran A.M., Nolan J.M., Beatty S., Young I.S., Kenny R.A. Plasma Lutein and Zeaxanthin Are Associated With Better Cognitive Function Across Multiple Domains in a Large Population-Based Sample of Older Adults: Findings from The Irish Longitudinal Study on Aging. J. Gerontol. Ser. A. 2017;72:1431–1436. doi: 10.1093/gerona/glw330. [DOI] [PubMed] [Google Scholar]
- 158.Li L.H., Lee J.C.-Y., Leung H.H., Lam W.C., Fu Z., Lo A.C.Y. Lutein Supplementation for Eye Diseases. Nutrients. 2020;12:1721. doi: 10.3390/nu12061721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jia Y.-P., Sun L., Yu H.-S., Liang L.-P., Li W., Ding H., Song X.-B., Zhang L.-J. The Pharmacological Effects of Lutein and Zeaxanthin on Visual Disorders and Cognition Diseases. Molecules. 2017;22:610. doi: 10.3390/molecules22040610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Stringham J.M., Johnson E.J., Hammond B.R. Lutein across the Lifespan: From Childhood Cognitive Performance to the Aging Eye and Brain. Curr. Dev. Nutr. 2019;3:nzz066. doi: 10.1093/cdn/nzz066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Weber D., Kochlik B., Demuth I., Steinhagen-Thiessen E., Grune T., Norman K. Plasma Carotenoids, Tocopherols and Retinol-Association with Age in the Berlin Aging Study II. Redox Biol. 2020;32:101461. doi: 10.1016/j.redox.2020.101461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lindbergh C.A., Renzi-Hammond L.M., Hammond B.R., Terry D.P., Mewborn C.M., Puente A.N., Miller L.S. Lutein and Zeaxanthin Influence Brain Function in Older Adults: A Randomized Controlled Trial. J. Int. Neuropsychol. Soc. 2018;24:77–90. doi: 10.1017/S1355617717000534. [DOI] [PubMed] [Google Scholar]
- 163.Mewborn C.M., Lindbergh C.A., Hammond B.R., Renzi-Hammond L.M., Miller L.S. The Effects of Lutein and Zeaxanthin Supplementation on Brain Morphology in Older Adults: A Randomized, Controlled Trial. J. Aging Res. 2019;2019:3709402. doi: 10.1155/2019/3709402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chae S., Park S., Park G. Lutein Protects Human Retinal Pigment Epithelial Cells from Oxidative Stress-induced Cellular Senescence. Mol. Med. Rep. 2018;18:5182–5190. doi: 10.3892/mmr.2018.9538. [DOI] [PubMed] [Google Scholar]
- 165.Sommerburg O., Keunen J.E.E., Bird A.C., van Kuijk F.J.G.M. Fruits and Vegetables That Are Sources for Lutein and Zeaxanthin: The Macular Pigment in Human Eyes. Br. J. Ophthalmol. 1998;82:907–910. doi: 10.1136/bjo.82.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Carpentier S., Knaus M., Suh M. Associations between Lutein, Zeaxanthin, and Age-Related Macular Degeneration: An Overview. Crit. Rev. Food Sci. Nutr. 2009;49:313–326. doi: 10.1080/10408390802066979. [DOI] [PubMed] [Google Scholar]
- 167.Paiva S.A.R., Russell R.M. β-Carotene and Other Carotenoids as Antioxidants. J. Am. Coll. Nutr. 1999;18:426–433. doi: 10.1080/07315724.1999.10718880. [DOI] [PubMed] [Google Scholar]
- 168.Leh H.E., Lee L.K. Lycopene: A Potent Antioxidant for the Amelioration of Type II Diabetes Mellitus. Molecules. 2022;27:2335. doi: 10.3390/molecules27072335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chen P., Zhang W., Wang X., Zhao K., Negi D.S., Zhuo L., Qi M., Wang X., Zhang X. Lycopene and Risk of Prostate Cancer. Medicine. 2015;94:e1260. doi: 10.1097/MD.0000000000001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Li J., Zhang Y., Zeng X., Cheng Y., Tang L., Hong D., Yang X. Lycopene Ameliorates Insulin Resistance and Increases Muscle Capillary Density in Aging via Activation of SIRT1. J. Nutr. Biochem. 2022;99:108862. doi: 10.1016/j.jnutbio.2021.108862. [DOI] [PubMed] [Google Scholar]
- 171.Chen D., Huang C., Chen Z. A Review for the Pharmacological Effect of Lycopene in Central Nervous System Disorders. Biomed. Pharmacother. 2019;111:791–801. doi: 10.1016/j.biopha.2018.12.151. [DOI] [PubMed] [Google Scholar]
- 172.Kim J.Y., Lee J.-S., Han Y.-S., Lee J.H., Bae I., Yoon Y.M., Kwon S.M., Lee S.H. Pretreatment with Lycopene Attenuates Oxidative Stress-Induced Apoptosis in Human Mesenchymal Stem Cells. Biomol. Ther. 2015;23:517–524. doi: 10.4062/biomolther.2015.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yeh S.-L., Hu M.-L., Huang C.-S. Lycopene Enhances UVA–Induced DNA Damage and Expression of Heme Oxygenase–1 in Cultured Mouse Embryo Fibroblasts. Eur. J. Nutr. 2005;44:365–370. doi: 10.1007/s00394-004-0536-5. [DOI] [PubMed] [Google Scholar]
- 174.Fernández-García E. Photoprotection of Human Dermal Fibroblasts against Ultraviolet Light by Antioxidant Combinations Present in Tomato. Food Funct. 2014;5:285–290. doi: 10.1039/C3FO60471C. [DOI] [PubMed] [Google Scholar]
- 175.Liu X., DILXAT T., Shi Q., Qiu T., Lin J. The Combination of Nicotinamide Mononucleotide and Lycopene Prevents Cognitive Impairment and Attenuates Oxidative Damage in D-Galactose Induced Aging Models via Keap1-Nrf2 Signaling. Gene. 2022;822:146348. doi: 10.1016/j.gene.2022.146348. [DOI] [PubMed] [Google Scholar]
- 176.Gaucher C., Boudier A., Bonetti J., Clarot I., Leroy P., Parent M. Glutathione: Antioxidant Properties Dedicated to Nanotechnologies. Antioxidants. 2018;7:62. doi: 10.3390/antiox7050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lv H., Zhen C., Liu J., Yang P., Hu L., Shang P. Unraveling the Potential Role of Glutathione in Multiple Forms of Cell Death in Cancer Therapy. Oxidative Med. Cell. Longev. 2019;2019:3150145. doi: 10.1155/2019/3150145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wu G., Fang Y.-Z., Yang S., Lupton J.R., Turner N.D. Glutathione Metabolism and Its Implications for Health. J. Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 179.Traverso N., Ricciarelli R., Nitti M., Marengo B., Furfaro A.L., Pronzato M.A., Marinari U.M., Domenicotti C. Role of Glutathione in Cancer Progression and Chemoresistance. Oxidative Med. Cell. Longev. 2013;2013:972913. doi: 10.1155/2013/972913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Staal F.J.T., Ela S.W., Roederer M., Anderson M.T., Herzenberg L.A., Herzenberg L.A. Glutathione Deficiency and Human Immunodeficiency Virus Infection. Lancet. 1992;339:909–912. doi: 10.1016/0140-6736(92)90939-Z. [DOI] [PubMed] [Google Scholar]
- 181.Waris S., Patel A., Ali A., Mahmood R. Acetaldehyde-Induced Oxidative Modifications and Morphological Changes in Isolated Human Erythrocytes: An in Vitro Study. Environ. Sci. Pollut. Res. 2020;27:16268–16281. doi: 10.1007/s11356-020-08044-4. [DOI] [PubMed] [Google Scholar]
- 182.Rusu M.E., Georgiu C., Pop A., Mocan A., Kiss B., Vostinaru O., Fizesan I., Stefan M.-G., Gheldiu A.-M., Mates L., et al. Antioxidant Effects of Walnut (Juglans regia L.) Kernel and Walnut Septum Extract in a D-Galactose-Induced Aging Model and in Naturally Aged Rats. Antioxidants. 2020;9:424. doi: 10.3390/antiox9050424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Morin D., Long R., Panel M., Laure L., Taranu A., Gueguen C., Pons S., Leoni V., Caccia C., Vatner S.F., et al. Hsp22 Overexpression Induces Myocardial Hypertrophy, Senescence and Reduced Life Span through Enhanced Oxidative Stress. Free Radic. Biol. Med. 2019;137:194–200. doi: 10.1016/j.freeradbiomed.2019.04.035. [DOI] [PubMed] [Google Scholar]
- 184.Armeni T., Ercolani L., Urbanelli L., Magini A., Magherini F., Pugnaloni A., Piva F., Modesti A., Emiliani C., Principato G. Cellular Redox Imbalance and Changes of Protein S-Glutathionylation Patterns Are Associated with Senescence Induced by Oncogenic H-Ras. PLoS ONE. 2012;7:e52151. doi: 10.1371/journal.pone.0052151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Redondo J., Sarkar P., Kemp K., Heesom K.J., Wilkins A., Scolding N.J., Rice C.M. Dysregulation of Mesenchymal Stromal Cell Antioxidant Responses in Progressive Multiple Sclerosis. Stem Cells Transl. Med. 2018;7:748–758. doi: 10.1002/sctm.18-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Huang C., Gao J., Wei T., Shen W. Angiotensin II-Induced Erythrocyte Senescence Contributes to Oxidative Stress. Rejuvenation Res. 2022;25:30–38. doi: 10.1089/rej.2021.0054. [DOI] [PubMed] [Google Scholar]
- 187.Kurz D.J., Decary S., Hong Y., Trivier E., Akhmedov A., Erusalimsky J.D. Chronic Oxidative Stress Compromises Telomere Integrity and Accelerates the Onset of Senescence in Human Endothelial Cells. J. Cell Sci. 2004;117:2417–2426. doi: 10.1242/jcs.01097. [DOI] [PubMed] [Google Scholar]
- 188.Chen Y., Johansson E., Fan Y., Shertzer H.G., Vasiliou V., Nebert D.W., Dalton T.P. Early Onset Senescence Occurs When Fibroblasts Lack the Glutamate–Cysteine Ligase Modifier Subunit. Free Radic. Biol. Med. 2009;47:410–418. doi: 10.1016/j.freeradbiomed.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Probin V., Wang Y., Zhou D. Busulfan-Induced Senescence Is Dependent on ROS Production Upstream of the MAPK Pathway. Free Radic. Biol. Med. 2007;42:1858–1865. doi: 10.1016/j.freeradbiomed.2007.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fafián-Labora J.A., Rodríguez-Navarro J.A., O’Loghlen A. Small Extracellular Vesicles Have GST Activity and Ameliorate Senescence-Related Tissue Damage. Cell Metab. 2020;32:71–86.e5. doi: 10.1016/j.cmet.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tong J., Fitzmaurice P.S., Moszczynska A., Mattina K., Ang L.-C., Boileau I., Furukawa Y., Sailasuta N., Kish S.J. Do Glutathione Levels Decline in Aging Human Brain? Free Radic. Biol. Med. 2016;93:110–117. doi: 10.1016/j.freeradbiomed.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 192.Barilani M., Lovejoy C., Piras R., Abramov A.Y., Lazzari L., Angelova P.R. Age-related Changes in the Energy of Human Mesenchymal Stem Cells. J. Cell. Physiol. 2022;237:1753–1767. doi: 10.1002/jcp.30638. [DOI] [PubMed] [Google Scholar]
- 193.Martin-de-Pablos A., Córdoba-Fernández A., Fernández-Espejo E. Analysis of Neurotrophic and Antioxidant Factors Related to Midbrain Dopamine Neuronal Loss and Brain Inflammation in the Cerebrospinal Fluid of the Elderly. Exp. Gerontol. 2018;110:54–60. doi: 10.1016/j.exger.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 194.Liu J., Mori A. Age-Associated Changes in Superoxide Dismutase Activity, Thiobarbituric Acid Reactivity and Reduced Glutathione Level in the Brain and Liver in Senescence Accelerated Mice (SAM): A Comparison with DdY Mice. Mech. Ageing Dev. 1993;71:23–30. doi: 10.1016/0047-6374(93)90032-M. [DOI] [PubMed] [Google Scholar]
- 195.Iskusnykh I.Y., Zakharova A.A., Pathak D. Glutathione in Brain Disorders and Aging. Molecules. 2022;27:324. doi: 10.3390/molecules27010324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Barardo D., Thornton D., Thoppil H., Walsh M., Sharifi S., Ferreira S., Anžič A., Fernandes M., Monteiro P., Grum T., et al. The DrugAge Database of Aging-Related Drugs. Aging Cell. 2017;16:594–597. doi: 10.1111/acel.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rebrin I., Zicker S., Wedekind K.J., Paetau-Robinson I., Packer L., Sohal R.S. Effect of Antioxidant-Enriched Diets on Glutathione Redox Status in Tissue Homogenates and Mitochondria of the Senescence-Accelerated Mouse. Free Radic. Biol. Med. 2005;39:549–557. doi: 10.1016/j.freeradbiomed.2005.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Homma T., Fujii J. Application of Glutathione as Anti-Oxidative and Anti-Aging Drugs. Curr. Drug Metab. 2015;16:560–571. doi: 10.2174/1389200216666151015114515. [DOI] [PubMed] [Google Scholar]
- 199.Kumar P., Osahon O.W., Sekhar R.V. GlyNAC (Glycine and N-Acetylcysteine) Supplementation in Mice Increases Length of Life by Correcting Glutathione Deficiency, Oxidative Stress, Mitochondrial Dysfunction, Abnormalities in Mitophagy and Nutrient Sensing, and Genomic Damage. Nutrients. 2022;14:1114. doi: 10.3390/nu14051114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sekhar R.V., Patel S.G., Guthikonda A.P., Reid M., Balasubramanyam A., Taffet G.E., Jahoor F. Deficient Synthesis of Glutathione Underlies Oxidative Stress in Aging and Can Be Corrected by Dietary Cysteine and Glycine Supplementation. Am. J. Clin. Nutr. 2011;94:847–853. doi: 10.3945/ajcn.110.003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sekhar R.V. GlyNAC Supplementation Improves Glutathione Deficiency, Oxidative Stress, Mitochondrial Dysfunction, Inflammation, Aging Hallmarks, Metabolic Defects, Muscle Strength, Cognitive Decline, and Body Composition: Implications for Healthy Aging. J. Nutr. 2021;151:3606–3616. doi: 10.1093/jn/nxab309. [DOI] [PubMed] [Google Scholar]
- 202.Ansary J., Forbes-Hernández T.Y., Gil E., Cianciosi D., Zhang J., Elexpuru-Zabaleta M., Simal-Gandara J., Giampieri F., Battino M. Potential Health Benefit of Garlic Based on Human Intervention Studies: A Brief Overview. Antioxidants. 2020;9:619. doi: 10.3390/antiox9070619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.El-Saber Batiha G., Magdy Beshbishy A., G. Wasef L., Elewa Y.H.A., A. Al-Sagan A., Abd El-Hack M.E., Taha A.E., M. Abd-Elhakim Y., Prasad Devkota H. Chemical Constituents and Pharmacological Activities of Garlic (Allium Sativum L.): A Review. Nutrients. 2020;12:872. doi: 10.3390/nu12030872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Elosta A., Slevin M., Rahman K., Ahmed N. Aged Garlic Has More Potent Antiglycation and Antioxidant Properties Compared to Fresh Garlic Extract in Vitro. Sci. Rep. 2017;7:39613. doi: 10.1038/srep39613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Moriguchi T., Saito H., Nishiyama N. Aged Garlic Extract Prolongs Longevity and Improves Spatial Memory Deficit in Senescence-Accelerated Mouse. Biol. Pharm. Bull. 1996;19:305–307. doi: 10.1248/bpb.19.305. [DOI] [PubMed] [Google Scholar]
- 206.Moriguchi T., Takashina K., Chu P., Saito H., Nishiyama N. Prolongation of Life Span and Improved Learning in the Senescence Accelerated Mouse Produced by Aged Garlic Extract. Biol. Pharm. Bull. 1994;17:1589–1594. doi: 10.1248/bpb.17.1589. [DOI] [PubMed] [Google Scholar]
- 207.Nishimura H., Higuchi O., Tateshita K., Tomobe K., Okuma Y., Nomura Y. Antioxidative Activity and Ameliorative Effects of Memory Impairment of Sulfur-Containing Compounds in Allium Species. BioFactors. 2006;26:135–146. doi: 10.1002/biof.5520260204. [DOI] [PubMed] [Google Scholar]
- 208.Hashimoto M., Nakai T., Masutani T., Unno K., Akao Y. Improvement of Learning and Memory in Senescence-Accelerated Mice by S-Allylcysteine in Mature Garlic Extract. Nutrients. 2020;12:1834. doi: 10.3390/nu12061834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chen P., Chang C., Lin W., Nagabhushanam K., Ho C., Pan M. S-Allylcysteine Ameliorates Aging Features via Regulating Mitochondrial Dynamics in Naturally Aged C57BL/6J Mice. Mol. Nutr. Food Res. 2022;66:2101077. doi: 10.1002/mnfr.202101077. [DOI] [PubMed] [Google Scholar]
- 210.Borek C. Antioxidant Health Effects of Aged Garlic Extract. J. Nutr. 2001;131:1010S–1015S. doi: 10.1093/jn/131.3.1010S. [DOI] [PubMed] [Google Scholar]
- 211.Deltcheva E., Chylinski K., Sharma C.M., Gonzales K., Chao Y., Pirzada Z.A., Eckert M.R., Vogel J., Charpentier E. CRISPR RNA Maturation by Trans-Encoded Small RNA and Host Factor RNase III. Nature. 2011;471:602–607. doi: 10.1038/nature09886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kim H. Protective Effect of Garlic on Cellular Senescence in UVB-Exposed HaCaT Human Keratinocytes. Nutrients. 2016;8:464. doi: 10.3390/nu8080464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Miki S., Suzuki J., Takashima M., Ishida M., Kokubo H., Yoshizumi M. S-1-Propenylcysteine Promotes IL-10-Induced M2c Macrophage Polarization through Prolonged Activation of IL-10R/STAT3 Signaling. Sci. Rep. 2021;11:22469. doi: 10.1038/s41598-021-01866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Roumeliotis S., Roumeliotis A., Dounousi E., Eleftheriadis T., Liakopoulos V. Dietary Antioxidant Supplements and Uric Acid in Chronic Kidney Disease: A Review. Nutrients. 2019;11:1911. doi: 10.3390/nu11081911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sánchez-Lozada L.G. The Pathophysiology of Uric Acid on Renal Diseases. Contrib. Neprhol. 2018;192:17–24. doi: 10.1159/000484274. [DOI] [PubMed] [Google Scholar]
- 216.Kang D.-H., Ha S.-K. Uric Acid Puzzle: Dual Role as Anti-Oxidantand Pro-Oxidant. Electrolytes Blood Press. 2014;12:1. doi: 10.5049/EBP.2014.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Vazirpanah N., Radstake T., Broen J. Inflamm-Ageing and Senescence in Gout: The Tale of an Old King’s Disease. Curr. Aging Sci. 2015;8:186–201. doi: 10.2174/1874609808666150727112434. [DOI] [PubMed] [Google Scholar]
- 218.Goldberg E.L., Dixit V.D. Drivers of Age-Related Inflammation and Strategies for Healthspan Extension. Immunol. Rev. 2015;265:63–74. doi: 10.1111/imr.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Fan Y., Xia J., Jia D., Zhang M., Zhang Y., Huang G., Wang Y. Mechanism of Ginsenoside Rg1 Renal Protection in a Mouse Model of D-Galactose-Induced Subacute Damage. Pharm. Biol. 2016;54:1815–1821. doi: 10.3109/13880209.2015.1129543. [DOI] [PubMed] [Google Scholar]
- 220.Cheong K., Lee A. Guanine Deaminase Stimulates Ultraviolet-Induced Keratinocyte Senescence in Seborrhoeic Keratosis via Guanine Metabolites. Acta Derm. Venereol. 2020;100:1–10. doi: 10.2340/00015555-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Yu M.-A., Sánchez-Lozada L.G., Johnson R.J., Kang D.-H. Oxidative Stress with an Activation of the Renin-Angiotensin System in Human Vascular Endothelial Cells as a Novel Mechanism of Uric Acid-Induced Endothelial Dysfunction. J. Hypertens. 2010;28:1234–1242. doi: 10.1097/HJH.0b013e328337da1d. [DOI] [PubMed] [Google Scholar]
- 222.Battelli M.G., Bortolotti M., Bolognesi A., Polito L. Pro-Aging Effects of Xanthine Oxidoreductase Products. Antioxidants. 2020;9:839. doi: 10.3390/antiox9090839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Chen I.-C., Kuo C.-S., Wu C.-C., Tsai H.-Y., Lin C.-P., Li S.-Y., Chou R.-H., Huang P.-H., Chen J.-W., Lin S.-J. Chronic Hyperuricemia Impairs Blood Flow Recovery in the Ischemic Hindlimb through Suppression of Endothelial Progenitor Cells. Oncotarget. 2018;9:9285–9298. doi: 10.18632/oncotarget.24290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mladenov M., Gokik M., Hadzi-Petrushev N., Gjorgoski I., Jankulovski N. The Relationship Between Antioxidant Enzymes and Lipid Peroxidation in Senescent Rat Erythrocytes. Physiol. Res. 2015;64:891. doi: 10.33549/physiolres.932890. [DOI] [PubMed] [Google Scholar]
- 225.Park K.H., Shin D.G., Kim J.R., Cho K.H. Senescence-Related Truncation and Multimerization of Apolipoprotein A-I in High-Density Lipoprotein With an Elevated Level of Advanced Glycated End Products and Cholesteryl Ester Transfer Activity. J. Gerontol. Ser. A: Biomed. Sci. Med. Sci. 2010;65:600–610. doi: 10.1093/gerona/glq034. [DOI] [PubMed] [Google Scholar]
- 226.Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016;5:e47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Khan H., Belwal T., Efferth T., Farooqi A.A., Sanches-Silva A., Vacca R.A., Nabavi S.F., Khan F., Prasad Devkota H., Barreca D., et al. Targeting Epigenetics in Cancer: Therapeutic Potential of Flavonoids. Crit. Rev. Food Sci. Nutr. 2021;61:1616–1639. doi: 10.1080/10408398.2020.1763910. [DOI] [PubMed] [Google Scholar]
- 228.Rufino A.T., Costa V.M., Carvalho F., Fernandes E. Flavonoids as Antiobesity Agents: A Review. Med. Res. Rev. 2021;41:556–585. doi: 10.1002/med.21740. [DOI] [PubMed] [Google Scholar]
- 229.Kim J.M., Lee E.K., Kim D.H., Yu B.P., Chung H.Y. Kaempferol Modulates Pro-Inflammatory NF-ΚB Activation by Suppressing Advanced Glycation Endproducts-Induced NADPH Oxidase. Age. 2010;32:197–208. doi: 10.1007/s11357-009-9124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hua Y.Q., Zeng Y., Xu J., Xu X. le Naringenin Alleviates Nonalcoholic Steatohepatitis in Middle-Aged Apoe−/−mice: Role of SIRT1. Phytomedicine. 2021;81:153412. doi: 10.1016/j.phymed.2020.153412. [DOI] [PubMed] [Google Scholar]
- 231.Chattopadhyay D., Sen S., Chatterjee R., Roy D., James J., Thirumurugan K. Context- and Dose-Dependent Modulatory Effects of Naringenin on Survival and Development of Drosophila Melanogaster. Biogerontology. 2016;17:383–393. doi: 10.1007/s10522-015-9624-6. [DOI] [PubMed] [Google Scholar]
- 232.Zhu Y., Tchkonia T., Pirtskhalava T., Gower A.C., Ding H., Giorgadze N., Palmer A.K., Ikeno Y., Hubbard G.B., Lenburg M., et al. The Achilles’ Heel of Senescent Cells: From Transcriptome to Senolytic Drugs. Aging Cell. 2015;14:644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ogrodnik M., Evans S.A., Fielder E., Victorelli S., Kruger P., Salmonowicz H., Weigand B.M., Patel A.D., Pirtskhalava T., Inman C.L., et al. Whole-body Senescent Cell Clearance Alleviates Age-related Brain Inflammation and Cognitive Impairment in Mice. Aging Cell. 2021;20:e13296. doi: 10.1111/acel.13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Dookun E., Passos J.F., Arthur H.M., Richardson G.D. Therapeutic Potential of Senolytics in Cardiovascular Disease. Cardiovasc. Drugs Ther. 2022;36:187–196. doi: 10.1007/s10557-020-07075-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Yousefzadeh M.J., Zhu Y., McGowan S.J., Angelini L., Fuhrmann-Stroissnigg H., Xu M., Ling Y.Y., Melos K.I., Pirtskhalava T., Inman C.L., et al. Fisetin Is a Senotherapeutic That Extends Health and Lifespan. EBioMedicine. 2018;36:18–28. doi: 10.1016/j.ebiom.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Chen T., Shen L., Yu J., Wan H., Guo A., Chen J., Long Y., Zhao J., Pei G. Rapamycin and Other Longevity-Promoting Compounds Enhance the Generation of Mouse Induced Pluripotent Stem Cells. Aging Cell. 2011;10:908–911. doi: 10.1111/j.1474-9726.2011.00722.x. [DOI] [PubMed] [Google Scholar]
- 237.Perrott K.M., Wiley C.D., Desprez P.-Y., Campisi J. Apigenin Suppresses the Senescence-Associated Secretory Phenotype and Paracrine Effects on Breast Cancer Cells. Geroscience. 2017;39:161–173. doi: 10.1007/s11357-017-9970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Cai Q., Ji S., Li M., Zheng S., Zhou X., Guo H., Deng S., Zhu J., Li D., Xie Z. Theaflavin-Regulated Imd Condensates Control Drosophila Intestinal Homeostasis and Aging. iScience. 2021;24:102150. doi: 10.1016/j.isci.2021.102150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Xiao Y.-Z., Yang M., Xiao Y., Guo Q., Huang Y., Li C.-J., Cai D., Luo X.-H. Reducing Hypothalamic Stem Cell Senescence Protects against Aging-Associated Physiological Decline. Cell Metab. 2020;31:534–548.e5. doi: 10.1016/j.cmet.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 240.Büchter C., Ackermann D., Havermann S., Honnen S., Chovolou Y., Fritz G., Kampkötter A., Wätjen W. Myricetin-Mediated Lifespan Extension in Caenorhabditis Elegans Is Modulated by DAF-16. Int. J. Mol. Sci. 2013;14:11895–11914. doi: 10.3390/ijms140611895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Jung H.-Y., Lee D., Ryu H.G., Choi B.-H., Go Y., Lee N., Lee D., Son H.G., Jeon J., Kim S.-H., et al. Myricetin Improves Endurance Capacity and Mitochondrial Density by Activating SIRT1 and PGC-1α. Sci. Rep. 2017;7:6237. doi: 10.1038/s41598-017-05303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Chattopadhyay D., Thirumurugan K. Longevity-Promoting Efficacies of Rutin in High Fat Diet Fed Drosophila Melanogaster. Biogerontology. 2020;21:653–668. doi: 10.1007/s10522-020-09882-y. [DOI] [PubMed] [Google Scholar]
- 243.Li T., Chen S., Feng T., Dong J., Li Y., Li H. Rutin Protects against Aging-Related Metabolic Dysfunction. Food Funct. 2016;7:1147–1154. doi: 10.1039/C5FO01036E. [DOI] [PubMed] [Google Scholar]
- 244.Yu X.-L., Li Y.-N., Zhang H., Su Y.-J., Zhou W.-W., Zhang Z.-P., Wang S.-W., Xu P.-X., Wang Y.-J., Liu R.-T. Rutin Inhibits Amylin-Induced Neurocytotoxicity and Oxidative Stress. Food Funct. 2015;6:3296–3306. doi: 10.1039/C5FO00500K. [DOI] [PubMed] [Google Scholar]
- 245.Burton M.D., Rytych J.L., Amin R., Johnson R.W. Dietary Luteolin Reduces Proinflammatory Microglia in the Brain of Senescent Mice. Rejuvenation Res. 2016;19:286–292. doi: 10.1089/rej.2015.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sun K., Xiang L., Ishihara S., Matsuura A., Sakagami Y., Qi J. Anti-Aging Effects of Hesperidin on Saccharomyces Cerevisiae via Inhibition of Reactive Oxygen Species and UTH1 Gene Expression. Biosci. Biotechnol. Biochem. 2012;76:640–645. doi: 10.1271/bbb.110535. [DOI] [PubMed] [Google Scholar]
- 247.Elavarasan J., Velusamy P., Ganesan T., Ramakrishnan S.K., Rajasekaran D., Periandavan K. Hesperidin-Mediated Expression of Nrf2 and Upregulation of Antioxidant Status in Senescent Rat Heart. J. Pharm. Pharmacol. 2012;64:1472–1482. doi: 10.1111/j.2042-7158.2012.01512.x. [DOI] [PubMed] [Google Scholar]
- 248.Fan X., Zeng Y., Fan Z., Cui L., Song W., Wu Q., Gao Y., Yang D., Mao X., Zeng B., et al. Dihydromyricetin Promotes Longevity and Activates the Transcription Factors FOXO and AOP in Drosophila. Aging. 2021;13:460–476. doi: 10.18632/aging.202156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Qian J., Wang X., Cao J., Zhang W., Lu C., Chen X. Dihydromyricetin Attenuates D-Galactose-Induced Brain Aging of Mice via Inhibiting Oxidative Stress and Neuroinflammation. Neurosci. Lett. 2021;756:135963. doi: 10.1016/j.neulet.2021.135963. [DOI] [PubMed] [Google Scholar]
- 250.Martínez-Coria H., Mendoza-Rojas M.X., Arrieta-Cruz I., López-Valdés H.E. Preclinical Research of Dihydromyricetin for Brain Aging and Neurodegenerative Diseases. Front. Pharmacol. 2019;10:1334. doi: 10.3389/fphar.2019.01334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Ramirez-Sanchez I., Mansour C., Navarrete-Yañez V., Ayala-Hernandez M., Guevara G., Castillo C., Loredo M., Bustamante M., Ceballos G., Villarreal F.J. (−)-Epicatechin Induced Reversal of Endothelial Cell Aging and Improved Vascular Function: Underlying Mechanisms. Food Funct. 2018;9:4802–4813. doi: 10.1039/C8FO00483H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Si H., Wang X., Zhang L., Parnell L.D., Ahmed B., LeRoith T., Ansah T.-A., Zhang L., Li J., Ordovás J.M., et al. Dietary Epicatechin Improves Survival and Delays Skeletal Muscle Degeneration in Aged Mice. FASEB J. 2019;33:965–977. doi: 10.1096/fj.201800554RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Navarrete-Yañez V., Garate-Carrillo A., Rodriguez A., Mendoza-Lorenzo P., Ceballos G., Calzada-Mendoza C., Hogan M.C., Villarreal F., Ramirez-Sanchez I. Effects of (−)-Epicatechin on Neuroinflammation and Hyperphosphorylation of Tau in the Hippocampus of Aged Mice. Food Funct. 2020;11:10351–10361. doi: 10.1039/D0FO02438D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Lee K.Y., Kim J.-R., Choi H.C. Genistein-Induced LKB1–AMPK Activation Inhibits Senescence of VSMC through Autophagy Induction. Vasc. Pharmacol. 2016;81:75–82. doi: 10.1016/j.vph.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 255.Kim J.M., Uehara Y., Choi Y.J., Ha Y.M., Ye B.H., Yu B.P., Chung H.Y. Mechanism of Attenuation of Pro-Inflammatory Ang II-Induced NF-ΚB Activation by Genistein in the Kidneys of Male Rats during Aging. Biogerontology. 2011;12:537–550. doi: 10.1007/s10522-011-9345-4. [DOI] [PubMed] [Google Scholar]
- 256.Bonet-Costa V., Herranz-Pérez V., Blanco-Gandía M., Mas-Bargues C., Inglés M., Garcia-Tarraga P., Rodriguez-Arias M., Miñarro J., Borras C., Garcia-Verdugo J.M., et al. Clearing Amyloid-β through PPARγ/ApoE Activation by Genistein Is a Treatment of Experimental Alzheimer’s Disease. J. Alzheimer’s Dis. 2016;51:701–711. doi: 10.3233/JAD-151020. [DOI] [PubMed] [Google Scholar]
- 257.Fan X., Fan Z., Yang Z., Huang T., Tong Y., Yang D., Mao X., Yang M. Flavonoids—Natural Gifts to Promote Health and Longevity. Int. J. Mol. Sci. 2022;23:2176. doi: 10.3390/ijms23042176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Zhang J., Hong Y., Liuyang Z., Li H., Jiang Z., Tao J., Liu H., Xie A., Feng Y., Dong X., et al. Quercetin Prevents Radiation-Induced Oral Mucositis by Upregulating BMI-1. Oxidative Med. Cell. Longev. 2021;2021:2231680. doi: 10.1155/2021/2231680. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 259.Smith M.J., Fowler M., Naftalin R.J., Siow R.C.M. UVA Irradiation Increases Ferrous Iron Release from Human Skin Fibroblast and Endothelial Cell Ferritin: Consequences for Cell Senescence and Aging. Free Radic. Biol. Med. 2020;155:49–57. doi: 10.1016/j.freeradbiomed.2020.04.024. [DOI] [PubMed] [Google Scholar]
- 260.Li S., Cao H., Shen D., Jia Q., Chen C., Xing S. Quercetin Protects against Ox-LDL-induced Injury via Regulation of ABCAl, LXR-α and PCSK9 in RAW264.7 Macrophages. Mol. Med. Rep. 2018;18:799–806. doi: 10.3892/mmr.2018.9048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Sohn E.-J., Kim J.M., Kang S.-H., Kwon J., An H.J., Sung J.-S., Cho K.A., Jang I.-S., Choi J.-S. Restoring Effects of Natural Anti-Oxidant Quercetin on Cellular Senescent Human Dermal Fibroblasts. Am. J. Chin. Med. 2018;46:853–873. doi: 10.1142/S0192415X18500453. [DOI] [PubMed] [Google Scholar]
- 262.Zoico E., Nori N., Darra E., Tebon M., Rizzatti V., Policastro G., de Caro A., Rossi A.P., Fantin F., Zamboni M. Senolytic Effects of Quercetin in an in Vitro Model of Pre-Adipocytes and Adipocytes Induced Senescence. Sci. Rep. 2021;11:23237. doi: 10.1038/s41598-021-02544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Wei Y., Fu J., Wu W., Ma P., Ren L., Yi Z., Wu J. Quercetin Prevents Oxidative Stress-Induced Injury of Periodontal Ligament Cells and Alveolar Bone Loss in Periodontitis. Drug Des. Dev. Ther. 2021;15:3509–3522. doi: 10.2147/DDDT.S315249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Zefzoufi M., Fdil R., Bouamama H., Gadhi C., Katakura Y., Mouzdahir A., Sraidi K. Effect of Extracts and Isolated Compounds Derived from Retama Monosperma (L.) Boiss. on Anti-Aging Gene Expression in Human Keratinocytes and Antioxidant Activity. J. Ethnopharmacol. 2021;280:114451. doi: 10.1016/j.jep.2021.114451. [DOI] [PubMed] [Google Scholar]
- 265.Chondrogianni N., Kapeta S., Chinou I., Vassilatou K., Papassideri I., Gonos E.S. Anti-Ageing and Rejuvenating Effects of Quercetin. Exp. Gerontol. 2010;45:763–771. doi: 10.1016/j.exger.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 266.Hickson L.J., Langhi Prata L.G.P., Bobart S.A., Evans T.K., Giorgadze N., Hashmi S.K., Herrmann S.M., Jensen M.D., Jia Q., Jordan K.L., et al. Corrigendum to ‘Senolytics Decrease Senescent Cells in Humans: Preliminary Report from a Clinical Trial of Dasatinib plus Quercetin in Individuals with Diabetic Kidney Disease’ EBioMedicine 47 (2019) 446–456. EBioMedicine. 2020;52:102595. doi: 10.1016/j.ebiom.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Abharzanjani F., Hemmati M. Protective Effects of Quercetin and Resveratrol on Aging Markers in Kidney under High Glucose Condition: In Vivo and in Vitro Analysis. Mol. Biol. Rep. 2021;48:5435–5442. doi: 10.1007/s11033-021-06550-3. [DOI] [PubMed] [Google Scholar]
- 268.Xu M., Pirtskhalava T., Farr J.N., Weigand B.M., Palmer A.K., Weivoda M.M., Inman C.L., Ogrodnik M.B., Hachfeld C.M., Fraser D.G., et al. Senolytics Improve Physical Function and Increase Lifespan in Old Age. Nat. Med. 2018;24:1246–1256. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Saccon T.D., Nagpal R., Yadav H., Cavalcante M.B., de Nunes A.D.C., Schneider A., Gesing A., Hughes B., Yousefzadeh M., Tchkonia T., et al. Senolytic Combination of Dasatinib and Quercetin Alleviates Intestinal Senescence and Inflammation and Modulates the Gut Microbiome in Aged Mice. J. Gerontol. Ser. A. 2021;76:1895–1905. doi: 10.1093/gerona/glab002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Molagoda I.M.N., Kavinda M.H.D., Choi Y.H., Lee H., Kang C.-H., Lee M.-H., Lee C.-M., Kim G.-Y. Fisetin Protects HaCaT Human Keratinocytes from Fine Particulate Matter (PM2.5)-Induced Oxidative Stress and Apoptosis by Inhibiting the Endoplasmic Reticulum Stress Response. Antioxidants. 2021;10:1492. doi: 10.3390/antiox10091492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Giri S., Takada A., Paudel D., Yoshida K., Furukawa M., Kuramitsu Y., Matsushita K., Abiko Y., Furuichi Y. An in Vitro Senescence Model of Gingival Epithelial Cell Induced by Hydrogen Peroxide Treatment. Odontology. 2022;110:44–53. doi: 10.1007/s10266-021-00630-3. [DOI] [PubMed] [Google Scholar]
- 272.Singh S., Garg G., Singh A.K., Bissoyi A., Rizvi S.I. Fisetin, a Potential Caloric Restriction Mimetic, Attenuates Senescence Biomarkers in Rat Erythrocytes. Biochem. Cell Biol. 2019;97:480–487. doi: 10.1139/bcb-2018-0159. [DOI] [PubMed] [Google Scholar]
- 273.Mitchell S.J., Bernier M., Mattison J.A., Aon M.A., Kaiser T.A., Anson R.M., Ikeno Y., Anderson R.M., Ingram D.K., de Cabo R. Daily Fasting Improves Health and Survival in Male Mice Independent of Diet Composition and Calories. Cell Metab. 2019;29:221–228.e3. doi: 10.1016/j.cmet.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Singh S., Garg G., Singh A.K., Tripathi S.S., Rizvi S.I. Fisetin, a Potential Caloric Restriction Mimetic, Modulates Ionic Homeostasis in Senescence Induced and Naturally Aged Rats. Arch. Physiol. Biochem. 2022;128:51–58. doi: 10.1080/13813455.2019.1662452. [DOI] [PubMed] [Google Scholar]
- 275.Boesten D.M.P.H.J., de Vos-Houben J.M.J., Timmermans L., den Hartog G.J.M., Bast A., Hageman G.J. Accelerated Aging during Chronic Oxidative Stress: A Role for PARP-1. Oxidative Med. Cell. Longev. 2013;2013:680414. doi: 10.1155/2013/680414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Mas-Bargues C., Borrás C., Viña J. Genistein, a Tool for Geroscience. Mech. Ageing Dev. 2022;204:111665. doi: 10.1016/j.mad.2022.111665. [DOI] [PubMed] [Google Scholar]
- 277.Wang Y.N., Wu W., Chen H.C., Fang H. Genistein Protects against UVB-Induced Senescence-like Characteristics in Human Dermal Fibroblast by P66Shc down-Regulation. J. Dermatol. Sci. 2010;58:19–27. doi: 10.1016/j.jdermsci.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 278.Zhang H., Pang X., Yu H., Zhou H. Genistein Suppresses Ox-LDL-elicited Oxidative Stress and Senescence in HUVECs through the SIRT1-p66shc-Foxo3a Pathways. J. Biochem. Mol. Toxicol. 2022;36:e22939. doi: 10.1002/jbt.22939. [DOI] [PubMed] [Google Scholar]
- 279.Prasanth M., Sivamaruthi B., Chaiyasut C., Tencomnao T. A Review of the Role of Green Tea (Camellia Sinensis) in Antiphotoaging, Stress Resistance, Neuroprotection, and Autophagy. Nutrients. 2019;11:474. doi: 10.3390/nu11020474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Kochman J., Jakubczyk K., Antoniewicz J., Mruk H., Janda K. Health Benefits and Chemical Composition of Matcha Green Tea: A Review. Molecules. 2020;26:85. doi: 10.3390/molecules26010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Cabrera C., Artacho R., Giménez R. Beneficial Effects of Green Tea—A Review. J. Am. Coll Nutr. 2006;25:79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- 282.Levine B., Kroemer G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell. 2019;176:11–42. doi: 10.1016/j.cell.2018.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Li F., Wang Y., Li D., Chen Y., Qiao X., Fardous R., Lewandowski A., Liu J., Chan T.-H., Dou Q.P. Perspectives on the Recent Developments with Green Tea Polyphenols in Drug Discovery. Expert Opin. Drug. Discov. 2018;13:643–660. doi: 10.1080/17460441.2018.1465923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Khan N., Mukhtar H. Tea Polyphenols in Promotion of Human Health. Nutrients. 2018;11:39. doi: 10.3390/nu11010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Unno K., Takabayashi F., Yoshida H., Choba D., Fukutomi R., Kikunaga N., Kishido T., Oku N., Hoshino M. Daily Consumption of Green Tea Catechin Delays Memory Regression in Aged Mice. Biogerontology. 2007;8:89–95. doi: 10.1007/s10522-006-9036-8. [DOI] [PubMed] [Google Scholar]
- 286.Unno K., Ishikawa Y., Takabayashi F., Sasaki T., Takamori N., Iguchi K., Hoshino M. Daily Ingestion of Green Tea Catechins from Adulthood Suppressed Brain Dysfunction in Aged Mice. Biofactors. 2008;34:263–271. doi: 10.1002/biof.5520340402. [DOI] [PubMed] [Google Scholar]
- 287.Unno K., Takabayashi F., Kishido T., Oku N. Suppressive Effect of Green Tea Catechins on Morphologic and Functional Regression of the Brain in Aged Mice with Accelerated Senescence (SAMP10) Exp. Gerontol. 2004;39:1027–1034. doi: 10.1016/j.exger.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 288.Hsu Y.-W., Chen W.-K., Tsai C.-F. Senescence-Mediated Redox Imbalance in Liver and Kidney: Antioxidant Rejuvenating Potential of Green Tea Extract. Int. J. Environ. Res. Public Health. 2021;19:260. doi: 10.3390/ijerph19010260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Kishido T., Unno K., Yoshida H., Choba D., Fukutomi R., Asahina S., Iguchi K., Oku N., Hoshino M. Decline in Glutathione Peroxidase Activity Is a Reason for Brain Senescence: Consumption of Green Tea Catechin Prevents the Decline in Its Activity and Protein Oxidative Damage in Ageing Mouse Brain. Biogerontology. 2007;8:423–430. doi: 10.1007/s10522-007-9085-7. [DOI] [PubMed] [Google Scholar]
- 290.Srividhya R., Jyothilakshmi V., Arulmathi K., Senthilkumaran V., Kalaiselvi P. Attenuation of Senescence-induced Oxidative Exacerbations in Aged Rat Brain by (−)-epigallocatechin-3-gallate. Int. J. Dev. Neurosci. 2008;26:217–223. doi: 10.1016/j.ijdevneu.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 291.Marrazzo P., Angeloni C., Freschi M., Lorenzini A., Prata C., Maraldi T., Hrelia S. Combination of Epigallocatechin Gallate and Sulforaphane Counteracts In Vitro Oxidative Stress and Delays Stemness Loss of Amniotic Fluid Stem Cells. Oxidative Med. Cell. Longev. 2018;2018:5263985. doi: 10.1155/2018/5263985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Shin J.-H., Jeon H.-J., Park J., Chang M.-S. Epigallocatechin-3-Gallate Prevents Oxidative Stress-Induced Cellular Senescence in Human Mesenchymal Stem Cells via Nrf2. Int. J. Mol. Med. 2016;38:1075–1082. doi: 10.3892/ijmm.2016.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Chang Y.-C., Liu H.-W., Chan Y.-C., Hu S.-H., Liu M.-Y., Chang S.-J. The Green Tea Polyphenol Epigallocatechin-3-Gallate Attenuates Age-Associated Muscle Loss via Regulation of MiR-486-5p and Myostatin. Arch. Biochem. Biophys. 2020;692:108511. doi: 10.1016/j.abb.2020.108511. [DOI] [PubMed] [Google Scholar]
- 294.Mao X., Gu C., Chen D., Yu B., He J. Oxidative Stress-Induced Diseases and Tea Polyphenols. Oncotarget. 2017;8:81649–81661. doi: 10.18632/oncotarget.20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Chanet A., Milenkovic D., Manach C., Mazur A., Morand C. Citrus Flavanones: What Is Their Role in Cardiovascular Protection? J. Agric. Food Chem. 2012;60:8809–8822. doi: 10.1021/jf300669s. [DOI] [PubMed] [Google Scholar]
- 296.Chularojmontri L., Gerdprasert O., Wattanapitayakul S.K. Pummelo Protects Doxorubicin-Induced Cardiac Cell Death by Reducing Oxidative Stress, Modifying Glutathione Transferase Expression, and Preventing Cellular Senescence. Evid.-Based Complement. Altern. Med. 2013;2013:254835. doi: 10.1155/2013/254835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Mulero J., Bernabé J., Cerdá B., García-Viguera C., Moreno D.A., Albaladejo M.D., Avilés F., Parra S., Abellán J., Zafrilla P. Variations on Cardiovascular Risk Factors in Metabolic Syndrome after Consume of a Citrus-Based Juice. Clin. Nutr. 2012;31:372–377. doi: 10.1016/j.clnu.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 298.Buachan P., Chularojmontri L., Wattanapitayakul S. Selected Activities of Citrus Maxima Merr. Fruits on Human Endothelial Cells: Enhancing Cell Migration and Delaying Cellular Aging. Nutrients. 2014;6:1618–1634. doi: 10.3390/nu6041618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Testai L., Piragine E., Piano I., Flori L., da Pozzo E., Miragliotta V., Pirone A., Citi V., di Cesare Mannelli L., Brogi S., et al. The Citrus Flavonoid Naringenin Protects the Myocardium from Ageing-Dependent Dysfunction: Potential Role of SIRT1. Oxidative Med. Cell. Longev. 2020;2020:4650207. doi: 10.1155/2020/4650207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.da Pozzo E., Costa B., Cavallini C., Testai L., Martelli A., Calderone V., Martini C. The Citrus Flavanone Naringenin Protects Myocardial Cells against Age-Associated Damage. Oxidative Med. Cell. Longev. 2017;2017:9536148. doi: 10.1155/2017/9536148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Zhang Y., Liu B., Chen X., Zhang N., Li G., Zhang L.-H., Tan L.-Y. Naringenin Ameliorates Behavioral Dysfunction and Neurological Deficits in a D-Galactose-Induced Aging Mouse Model Through Activation of PI3K/Akt/Nrf2 Pathway. Rejuvenation Res. 2017;20:462–472. doi: 10.1089/rej.2017.1960. [DOI] [PubMed] [Google Scholar]
- 302.Nakajima A., Aoyama Y., Nguyen T.-T.L., Shin E.-J., Kim H.-C., Yamada S., Nakai T., Nagai T., Yokosuka A., Mimaki Y., et al. Nobiletin, a Citrus Flavonoid, Ameliorates Cognitive Impairment, Oxidative Burden, and Hyperphosphorylation of Tau in Senescence-Accelerated Mouse. Behav. Brain Res. 2013;250:351–360. doi: 10.1016/j.bbr.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 303.Nakajima A., Aoyama Y., Shin E.-J., Nam Y., Kim H.-C., Nagai T., Yokosuka A., Mimaki Y., Yokoi T., Ohizumi Y., et al. Nobiletin, a Citrus Flavonoid, Improves Cognitive Impairment and Reduces Soluble Aβ Levels in a Triple Transgenic Mouse Model of Alzheimer’s Disease (3XTg-AD) Behav. Brain Res. 2015;289:69–77. doi: 10.1016/j.bbr.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 304.da Pozzo E., de Leo M., Faraone I., Milella L., Cavallini C., Piragine E., Testai L., Calderone V., Pistelli L., Braca A., et al. Antioxidant and Antisenescence Effects of Bergamot Juice. Oxidative Med. Cell. Longev. 2018;2018:9395804. doi: 10.1155/2018/9395804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Kashyap P., Shikha D., Thakur M., Aneja A. Functionality of Apigenin as a Potent Antioxidant with Emphasis on Bioavailability, Metabolism, Action Mechanism and in Vitro and in Vivo Studies: A Review. J. Food Biochem. 2022;46:e13950. doi: 10.1111/jfbc.13950. [DOI] [PubMed] [Google Scholar]
- 306.Choi S., Youn J., Kim K., Joo D.H., Shin S., Lee J., Lee H.K., An I.-S., Kwon S., Youn H.J., et al. Apigenin Inhibits UVA-Induced Cytotoxicity in Vitro and Prevents Signs of Skin Aging in Vivo. Int. J. Mol. Med. 2016;38:627–634. doi: 10.3892/ijmm.2016.2626. [DOI] [PubMed] [Google Scholar]
- 307.Salehi B., Venditti A., Sharifi-Rad M., Kręgiel D., Sharifi-Rad J., Durazzo A., Lucarini M., Santini A., Souto E., Novellino E., et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019;20:1305. doi: 10.3390/ijms20061305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Li B.S., Zhu R.Z., Lim S.-H., Seo J.H., Choi B.-M. Apigenin Alleviates Oxidative Stress-Induced Cellular Senescence via Modulation of the SIRT1-NAD+-CD38 Axis. Am. J. Chin. Med. 2021;49:1235–1250. doi: 10.1142/S0192415X21500592. [DOI] [PubMed] [Google Scholar]
- 309.Sang Y., Zhang F., Wang H., Yao J., Chen R., Zhou Z., Yang K., Xie Y., Wan T., Ding H. Apigenin Exhibits Protective Effects in a Mouse Model of D-Galactose-Induced Aging via Activating the Nrf2 Pathway. Food Funct. 2017;8:2331–2340. doi: 10.1039/C7FO00037E. [DOI] [PubMed] [Google Scholar]
- 310.Cháirez-Ramírez M.H., de la Cruz-López K.G., García-Carrancá A. Polyphenols as Antitumor Agents Targeting Key Players in Cancer-Driving Signaling Pathways. Front. Pharmacol. 2021;12:710304. doi: 10.3389/fphar.2021.710304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Sharma M., Hunter K.D., Fonseca F.P., Radhakrishnan R. Emerging Role of Cellular Senescence in the Pathogenesis of Oral Submucous Fibrosis and Its Malignant Transformation. Head Neck. 2021;43:3153–3164. doi: 10.1002/hed.26805. [DOI] [PubMed] [Google Scholar]
- 312.Li J., Zhao R., Zhao H., Chen G., Jiang Y., Lyu X., Wu T. Reduction of Aging-Induced Oxidative Stress and Activation of Autophagy by Bilberry Anthocyanin Supplementation via the AMPK–MTOR Signaling Pathway in Aged Female Rats. J. Agric. Food Chem. 2019;67:7832–7843. doi: 10.1021/acs.jafc.9b02567. [DOI] [PubMed] [Google Scholar]
- 313.Gao J., Wu Y., He D., Zhu X., Li H., Liu H., Liu H. Anti-Aging Effects of Ribes Meyeri Anthocyanins on Neural Stem Cells and Aging Mice. Aging. 2020;12:17738–17753. doi: 10.18632/aging.103955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Shih P.-H., Chan Y.-C., Liao J.-W., Wang M.-F., Yen G.-C. Antioxidant and Cognitive Promotion Effects of Anthocyanin-Rich Mulberry (Morus Atropurpurea L.) on Senescence-Accelerated Mice and Prevention of Alzheimer’s Disease. J. Nutr. Biochem. 2010;21:598–605. doi: 10.1016/j.jnutbio.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 315.Lee G., Hoang T., Jung E., Jung S., Han S., Chung M., Chae S., Chae H. Anthocyanins Attenuate Endothelial Dysfunction through Regulation of Uncoupling of Nitric Oxide Synthase in Aged Rats. Aging Cell. 2020;19:e13279. doi: 10.1111/acel.13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Parzonko A., Oświt A., Bazylko A., Naruszewicz M. Anthocyans-Rich Aronia Melanocarpa Extract Possesses Ability to Protect Endothelial Progenitor Cells against Angiotensin II Induced Dysfunction. Phytomedicine. 2015;22:1238–1246. doi: 10.1016/j.phymed.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 317.Tosti V., Bertozzi B., Fontana L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. Ser. A. 2018;73:318–326. doi: 10.1093/gerona/glx227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Gantenbein K.V., Kanaka-Gantenbein C. Mediterranean Diet as an Antioxidant: The Impact on Metabolic Health and Overall Wellbeing. Nutrients. 2021;13:1951. doi: 10.3390/nu13061951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Singla R.K., Dubey A.K., Garg A., Sharma R.K., Fiorino M., Ameen S.M., Haddad M.A., Al-Hiary M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019;102:1397–1400. doi: 10.5740/jaoacint.19-0133. [DOI] [PubMed] [Google Scholar]
- 320.Liu Y., Chen X., Li J. Resveratrol Protects against Oxidized Low-Density Lipoprotein-Induced Human Umbilical Vein Endothelial Cell Apoptosis via Inhibition of Mitochondrial-Derived Oxidative Stress. Mol. Med. Rep. 2017;15:2457–2464. doi: 10.3892/mmr.2017.6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Liu G.-S., Zhang Z.-S., Yang B., He W. Resveratrol Attenuates Oxidative Damage and Ameliorates Cognitive Impairment in the Brain of Senescence-Accelerated Mice. Life Sci. 2012;91:872–877. doi: 10.1016/j.lfs.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 322.Tung B.T., Rodríguez-Bies E., Talero E., Gamero-Estévez E., Motilva V., Navas P., López-Lluch G. Anti-Inflammatory Effect of Resveratrol in Old Mice Liver. Exp. Gerontol. 2015;64:1–7. doi: 10.1016/j.exger.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 323.Rahimifard M., Baeeri M., Bahadar H., Moini-Nodeh S., Khalid M., Haghi-Aminjan H., Mohammadian H., Abdollahi M. Therapeutic Effects of Gallic Acid in Regulating Senescence and Diabetes; an In Vitro Study. Molecules. 2020;25:5875. doi: 10.3390/molecules25245875. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 324.Hwang E., Park S.-Y., Lee H.J., Lee T.Y., Sun Z., Yi T.H. Gallic Acid Regulates Skin Photoaging in UVB-Exposed Fibroblast and Hairless Mice. Phytother. Res. 2014;28:1778–1788. doi: 10.1002/ptr.5198. [DOI] [PubMed] [Google Scholar]
- 325.Chen P., Chen F., Zhou B. Antioxidative, Anti-Inflammatory and Anti-Apoptotic Effects of Ellagic Acid in Liver and Brain of Rats Treated by D-Galactose. Sci. Rep. 2018;8:1465. doi: 10.1038/s41598-018-19732-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Hahn H.J., Kim K.B., Bae S., Choi B.G., An S., Ahn K.J., Kim S.Y. Pretreatment of Ferulic Acid Protects Human Dermal Fibroblasts against Ultraviolet A Irradiation. Ann. Dermatol. 2016;28:740. doi: 10.5021/ad.2016.28.6.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Huang X., You Y., Xi Y., Ni B., Chu X., Zhang R., You H. P-Coumaric Acid Attenuates IL-1β-Induced Inflammatory Responses and Cellular Senescence in Rat Chondrocytes. Inflammation. 2020;43:619–628. doi: 10.1007/s10753-019-01142-7. [DOI] [PubMed] [Google Scholar]
- 328.Shen Y., Song X., Li L., Sun J., Jaiswal Y., Huang J., Liu C., Yang W., Williams L., Zhang H., et al. Protective Effects of P-Coumaric Acid against Oxidant and Hyperlipidemia-an in Vitro and in Vivo Evaluation. Biomed. Pharmacother. 2019;111:579–587. doi: 10.1016/j.biopha.2018.12.074. [DOI] [PubMed] [Google Scholar]
- 329.Sun C.Y., Qi S.S., Zhou P., Cui H.R., Chen S.X., Dai K.Y., Tang M.L. Neurobiological and Pharmacological Validity of Curcumin in Ameliorating Memory Performance of Senescence-Accelerated Mice. Pharmacol. Biochem. Behav. 2013;105:76–82. doi: 10.1016/j.pbb.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 330.Takano K., Tatebe J., Washizawa N., Morita T. Curcumin Inhibits Age-Related Vascular Changes in Aged Mice Fed a High-Fat Diet. Nutrients. 2018;10:1476. doi: 10.3390/nu10101476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Yu S., Lin S., Yu Y., Chien M., Su K., Lin C., Way T., Yiang G., Lin C., Chan D., et al. Isochaihulactone Protects PC12 Cell against H2O2 Induced Oxidative Stress and Exerts the Potent Anti-Aging Effects in D-Galactose Aging Mouse Model. Acta Pharmacol. Sin. 2010;31:1532–1540. doi: 10.1038/aps.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Jang H.-J., Yang K.E., Oh W.K., Lee S.-I., Hwang I.-H., Ban K.-T., Yoo H.-S., Choi J.-S., Yeo E.-J., Jang I.-S. Nectandrin B-Mediated Activation of the AMPK Pathway Prevents Cellular Senescence in Human Diploid Fibroblasts by Reducing Intracellular ROS Levels. Aging. 2019;11:3731–3749. doi: 10.18632/aging.102013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Chong J., Poutaraud A., Hugueney P. Metabolism and Roles of Stilbenes in Plants. Plant Sci. 2009;177:143–155. doi: 10.1016/j.plantsci.2009.05.012. [DOI] [Google Scholar]
- 334.Flamini R., de Rosso M. High-Resolution Mass Spectrometry and Biological Properties of Grapevine and Wine Stilbenoids. Stud. Nat. Prod. Chem. 2019;61:175–210. [Google Scholar]
- 335.Griñán-Ferré C., Bellver-Sanchis A., Izquierdo V., Corpas R., Roig-Soriano J., Chillón M., Andres-Lacueva C., Somogyvári M., Sőti C., Sanfeliu C., et al. The Pleiotropic Neuroprotective Effects of Resveratrol in Cognitive Decline and Alzheimer’s Disease Pathology: From Antioxidant to Epigenetic Therapy. Ageing Res. Rev. 2021;67:101271. doi: 10.1016/j.arr.2021.101271. [DOI] [PubMed] [Google Scholar]
- 336.Li B., Hou D., Guo H., Zhou H., Zhang S., Xu X., Liu Q., Zhang X., Zou Y., Gong Y., et al. Resveratrol Sequentially Induces Replication and Oxidative Stresses to Drive P53-CXCR2 Mediated Cellular Senescence in Cancer Cells. Sci. Rep. 2017;7:208. doi: 10.1038/s41598-017-00315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Baur J.A., Sinclair D.A. Therapeutic Potential of Resveratrol: The in Vivo Evidence. Nat. Rev. Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 338.Lu X., Ma L., Ruan L., Kong Y., Mou H., Zhang Z., Wang Z., Wang J.M., Le Y. Resveratrol Differentially Modulates Inflammatory Responses of Microglia and Astrocytes. J. Neuroinflamm. 2010;7:46. doi: 10.1186/1742-2094-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Carrizzo A., Forte M., Damato A., Trimarco V., Salzano F., Bartolo M., Maciag A., Puca A.A., Vecchione C. Antioxidant Effects of Resveratrol in Cardiovascular, Cerebral and Metabolic Diseases. Food Chem. Toxicol. 2013;61:215–226. doi: 10.1016/j.fct.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 340.Simão F., Matté A., Pagnussat A.S., Netto C.A., Salbego C.G. Resveratrol Prevents CA1 Neurons against Ischemic Injury by Parallel Modulation of Both GSK-3β and CREB through PI3-K/Akt Pathways. Eur. J. Neurosci. 2012;36:2899–2905. doi: 10.1111/j.1460-9568.2012.08229.x. [DOI] [PubMed] [Google Scholar]
- 341.Chen C.-Y., Jang J.-H., Li M.-H., Surh Y.-J. Resveratrol Upregulates Heme Oxygenase-1 Expression via Activation of NF-E2-Related Factor 2 in PC12 Cells. Biochem. Biophys. Res. Commun. 2005;331:993–1000. doi: 10.1016/j.bbrc.2005.03.237. [DOI] [PubMed] [Google Scholar]
- 342.Singh A.P., Singh R., Verma S.S., Rai V., Kaschula C.H., Maiti P., Gupta S.C. Health Benefits of Resveratrol: Evidence from Clinical Studies. Med. Res. Rev. 2019;39:1851–1891. doi: 10.1002/med.21565. [DOI] [PubMed] [Google Scholar]
- 343.Cao W., Dou Y., Li A. Resveratrol Boosts Cognitive Function by Targeting SIRT1. Neurochem. Res. 2018;43:1705–1713. doi: 10.1007/s11064-018-2586-8. [DOI] [PubMed] [Google Scholar]
- 344.Ohtsu A., Shibutani Y., Seno K., Iwata H., Kuwayama T., Shirasuna K. Advanced Glycation End Products and Lipopolysaccharides Stimulate Interleukin-6 Secretion via the RAGE/TLR4-NF-κB-ROS Pathways and Resveratrol Attenuates These Inflammatory Responses in Mouse Macrophages. Exp. Ther. Med. 2017;14:4363–4370. doi: 10.3892/etm.2017.5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Zhang N., Li Z., Xu K., Wang Y., Wang Z. Resveratrol Protects against High-Fat Diet Induced Renal Pathological Damage and Cell Senescence by Activating SIRT1. Biol. Pharm. Bull. 2016;39:1448–1454. doi: 10.1248/bpb.b16-00085. [DOI] [PubMed] [Google Scholar]
- 346.Li Y.-R., Li S., Lin C.-C. Effect of Resveratrol and Pterostilbene on Aging and Longevity. BioFactors. 2018;44:69–82. doi: 10.1002/biof.1400. [DOI] [PubMed] [Google Scholar]
- 347.Ali D., Chen L., Kowal J.M., Okla M., Manikandan M., AlShehri M., AlMana Y., AlObaidan R., AlOtaibi N., Hamam R., et al. Resveratrol Inhibits Adipocyte Differentiation and Cellular Senescence of Human Bone Marrow Stromal Stem Cells. Bone. 2020;133:115252. doi: 10.1016/j.bone.2020.115252. [DOI] [PubMed] [Google Scholar]
- 348.Subedi L., Lee T.H., Wahedi H.M., Baek S.-H., Kim S.Y. Resveratrol-Enriched Rice Attenuates UVB-ROS-Induced Skin Aging via Downregulation of Inflammatory Cascades. Oxidative Med. Cell. Longev. 2017;2017:8379539. doi: 10.1155/2017/8379539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Kilic Eren M., Kilincli A., Eren Ö. Resveratrol Induced Premature Senescence Is Associated with DNA Damage Mediated SIRT1 and SIRT2 Down-Regulation. PLoS ONE. 2015;10:e0124837. doi: 10.1371/journal.pone.0124837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Martins L.A.M., Coelho B.P., Behr G., Pettenuzzo L.F., Souza I.C.C., Moreira J.C.F., Borojevic R., Gottfried C., Guma F.C.R. Resveratrol Induces Pro-Oxidant Effects and Time-Dependent Resistance to Cytotoxicity in Activated Hepatic Stellate Cells. Cell Biochem. Biophys. 2014;68:247–257. doi: 10.1007/s12013-013-9703-8. [DOI] [PubMed] [Google Scholar]
- 351.Heiss E.H., Schilder Y.D.C., Dirsch V.M. Chronic Treatment with Resveratrol Induces Redox Stress- and Ataxia Telangiectasia-Mutated (ATM)-Dependent Senescence in P53-Positive Cancer Cells. J. Biol. Chem. 2007;282:26759–26766. doi: 10.1074/jbc.M703229200. [DOI] [PubMed] [Google Scholar]
- 352.Chandrasekara A. Encyclopedia of Food Chemistry. Elsevier; Amsterdam, The Netherlands: 2019. Phenolic Acids; pp. 535–545. [Google Scholar]
- 353.Guo L., Cao J., Wei T., Li J., Feng Y., Wang L., Sun Y., Chai Y. Gallic Acid Attenuates Thymic Involution in the D-Galactose Induced Accelerated Aging Mice. Immunobiology. 2020;225:151870. doi: 10.1016/j.imbio.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 354.Liao C.-C., Chen S.-C., Huang H.-P., Wang C.-J. Gallic Acid Inhibits Bladder Cancer Cell Proliferation and Migration via Regulating Fatty Acid Synthase (FAS) J. Food Drug Anal. 2018;26:620–627. doi: 10.1016/j.jfda.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Punithavathi V.R., Stanely Mainzen Prince P., Kumar M.R., Selvakumari C.J. Protective Effects of Gallic Acid on Hepatic Lipid Peroxide Metabolism, Glycoprotein Components and Lipids in Streptozotocin-Induced Type II Diabetic Wistar Rats. J. Biochem. Mol. Toxicol. 2011;25:68–76. doi: 10.1002/jbt.20360. [DOI] [PubMed] [Google Scholar]
- 356.Szwajgier D., Borowiec K., Pustelniak K. The Neuroprotective Effects of Phenolic Acids: Molecular Mechanism of Action. Nutrients. 2017;9:477. doi: 10.3390/nu9050477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Moghtaderi H., Sepehri H., Delphi L., Attari F. Gallic Acid and Curcumin Induce Cytotoxicity and Apoptosis in Human Breast Cancer Cell MDA-MB-231. BioImpacts. 2018;8:185–194. doi: 10.15171/bi.2018.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Gao J., Hu J., Hu D., Yang X. A Role of Gallic Acid in Oxidative Damage Diseases: A Comprehensive Review. Nat. Prod. Commun. 2019;14:1934578X1987417. doi: 10.1177/1934578X19874174. [DOI] [Google Scholar]
- 359.Dludla P., Nkambule B., Jack B., Mkandla Z., Mutize T., Silvestri S., Orlando P., Tiano L., Louw J., Mazibuko-Mbeje S. Inflammation and Oxidative Stress in an Obese State and the Protective Effects of Gallic Acid. Nutrients. 2018;11:23. doi: 10.3390/nu11010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Varma S.R., Sivaprakasam T.O., Mishra A., Kumar L.M.S., Prakash N.S., Prabhu S., Ramakrishnan S. Protective Effects of Triphala on Dermal Fibroblasts and Human Keratinocytes. PLoS ONE. 2016;11:e0145921. doi: 10.1371/journal.pone.0145921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Baek B., Lee S.H., Kim K., Lim H.-W., Lim C.-J. Ellagic Acid Plays a Protective Role against UV-B-Induced Oxidative Stress by up-Regulating Antioxidant Components in Human Dermal Fibroblasts. Korean J. Physiol. Pharmacol. 2016;20:269. doi: 10.4196/kjpp.2016.20.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Vattem D.A., Shetty K. Biological Functionality of Ellagic Acid: A Review. J. Food Biochem. 2005;29:234–266. doi: 10.1111/j.1745-4514.2005.00031.x. [DOI] [Google Scholar]
- 363.Hwang J.M., Cho J.S., Kim T.H., Lee Y.I. Ellagic Acid Protects Hepatocytes from Damage by Inhibiting Mitochondrial Production of Reactive Oxygen Species. Biomed. Pharmacother. 2010;64:264–270. doi: 10.1016/j.biopha.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 364.Uzar E., Alp H., Cevik M.U., Fırat U., Evliyaoglu O., Tufek A., Altun Y. Ellagic Acid Attenuates Oxidative Stress on Brain and Sciatic Nerve and Improves Histopathology of Brain in Streptozotocin-Induced Diabetic Rats. Neurol. Sci. 2012;33:567–574. doi: 10.1007/s10072-011-0775-1. [DOI] [PubMed] [Google Scholar]
- 365.Baeeri M., Momtaz S., Navaei-Nigjeh M., Niaz K., Rahimifard M., Ghasemi-Niri S.F., Sanadgol N., Hodjat M., Sharifzadeh M., Abdollahi M. Molecular Evidence on the Protective Effect of Ellagic Acid on Phosalone-Induced Senescence in Rat Embryonic Fibroblast Cells. Food Chem. Toxicol. 2017;100:8–23. doi: 10.1016/j.fct.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 366.Muthaiyah B., Essa M.M., Lee M., Chauhan V., Kaur K., Chauhan A. Dietary Supplementation of Walnuts Improves Memory Deficits and Learning Skills in Transgenic Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2014;42:1397–1405. doi: 10.3233/JAD-140675. [DOI] [PubMed] [Google Scholar]
- 367.Ou L., Kong L.-Y., Zhang X.-M., Niwa M. Oxidation of Ferulic Acid by Momordica Charantia Peroxidase and Related Anti-Inflammation Activity Changes. Biol. Pharm. Bull. 2003;26:1511–1516. doi: 10.1248/bpb.26.1511. [DOI] [PubMed] [Google Scholar]
- 368.Zhang X., Lin D., Jiang R., Li H., Wan J., Li H. Ferulic Acid Exerts Antitumor Activity and Inhibits Metastasis in Breast Cancer Cells by Regulating Epithelial to Mesenchymal Transition. Oncol. Rep. 2016;36:271–278. doi: 10.3892/or.2016.4804. [DOI] [PubMed] [Google Scholar]
- 369.Hong Q., Ma Z.-C., Huang H., Wang Y.-G., Tan H.-L., Xiao C.-R., Liang Q.-D., Zhang H.-T., Gao Y. Antithrombotic Activities of Ferulic Acid via Intracellular Cyclic Nucleotide Signaling. Eur. J. Pharmacol. 2016;777:1–8. doi: 10.1016/j.ejphar.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 370.Borges A., Ferreira C., Saavedra M.J., Simões M. Antibacterial Activity and Mode of Action of Ferulic and Gallic Acids Against Pathogenic Bacteria. Microb. Drug Resist. 2013;19:256–265. doi: 10.1089/mdr.2012.0244. [DOI] [PubMed] [Google Scholar]
- 371.Wagle S., Sim H.-J., Bhattarai G., Choi K.-C., Kook S.-H., Lee J.-C., Jeon Y.-M. Supplemental Ferulic Acid Inhibits Total Body Irradiation-Mediated Bone Marrow Damage, Bone Mass Loss, Stem Cell Senescence, and Hematopoietic Defect in Mice by Enhancing Antioxidant Defense Systems. Antioxidants. 2021;10:1209. doi: 10.3390/antiox10081209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Zduńska K., Dana A., Kolodziejczak A., Rotsztejn H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Ski. Pharmacol. Physiol. 2018;31:332–336. doi: 10.1159/000491755. [DOI] [PubMed] [Google Scholar]
- 373.Balasubashini M.S., Rukkumani R., Viswanathan P., Menon V.P. Ferulic Acid Alleviates Lipid Peroxidation in Diabetic Rats. Phytother. Res. 2004;18:310–314. doi: 10.1002/ptr.1440. [DOI] [PubMed] [Google Scholar]
- 374.Ren Z., Zhang R., Li Y., Li Y., Yang Z., Yang H. Ferulic Acid Exerts Neuroprotective Effects against Cerebral Ischemia/Reperfusion-Induced Injury via Antioxidant and Anti-Apoptotic Mechanisms In Vitro and In Vivo. Int. J. Mol. Med. 2017;40:1444–1456. doi: 10.3892/ijmm.2017.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Meng G., Meng X., Ma X., Zhang G., Hu X., Jin A., Zhao Y., Liu X. Application of Ferulic Acid for Alzheimer’s Disease: Combination of Text Mining and Experimental Validation. Front. Neuroinform. 2018;12:31. doi: 10.3389/fninf.2018.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Narasimhan A., Chinnaiyan M., Karundevi B. Ferulic Acid Exerts Its Antidiabetic Effect by Modulating Insulin-Signalling Molecules in the Liver of High-Fat Diet and Fructose-Induced Type-2 Diabetic Adult Male Rat. Appl. Physiol. Nutr. Metab. 2015;40:769–781. doi: 10.1139/apnm-2015-0002. [DOI] [PubMed] [Google Scholar]
- 377.Stompor-Gorący M., Machaczka M. Recent Advances in Biological Activity, New Formulations and Prodrugs of Ferulic Acid. Int. J. Mol. Sci. 2021;22:12889. doi: 10.3390/ijms222312889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Kim S., Kim J., Lee Y.I., Jang S., Song S.Y., Lee W.J., Lee J.H. Particulate Matter-induced Atmospheric Skin Aging Is Aggravated by UVA and Inhibited by a Topical L-Ascorbic Acid Compound. Photodermatol. Photoimmunol. Photomed. 2022;38:123–131. doi: 10.1111/phpp.12725. [DOI] [PubMed] [Google Scholar]
- 379.Igarashi K., Kurata D. Effect of High-Oleic Peanut Intake on Aging and Its Hippocampal Markers in Senescence-Accelerated Mice (SAMP8) Nutrients. 2020;12:3461. doi: 10.3390/nu12113461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Manach C., Scalbert A., Morand C., Rémésy C., Jiménez L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 381.Pei K., Ou J., Huang J., Ou S. P-Coumaric Acid and Its Conjugates: Dietary Sources, Pharmacokinetic Properties and Biological Activities. J. Sci. Food Agric. 2016;96:2952–2962. doi: 10.1002/jsfa.7578. [DOI] [PubMed] [Google Scholar]
- 382.Lou Z., Wang H., Rao S., Sun J., Ma C., Li J. P-Coumaric Acid Kills Bacteria through Dual Damage Mechanisms. Food Control. 2012;25:550–554. doi: 10.1016/j.foodcont.2011.11.022. [DOI] [Google Scholar]
- 383.Amalan V., Vijayakumar N., Indumathi D., Ramakrishnan A. Antidiabetic and Antihyperlipidemic Activity of P-Coumaric Acid in Diabetic Rats, Role of Pancreatic GLUT 2: In Vivo Approach. Biomed. Pharmacother. 2016;84:230–236. doi: 10.1016/j.biopha.2016.09.039. [DOI] [PubMed] [Google Scholar]
- 384.Nasr Bouzaiene N., Kilani Jaziri S., Kovacic H., Chekir-Ghedira L., Ghedira K., Luis J. The Effects of Caffeic, Coumaric and Ferulic Acids on Proliferation, Superoxide Production, Adhesion and Migration of Human Tumor Cells in Vitro. Eur. J. Pharmacol. 2015;766:99–105. doi: 10.1016/j.ejphar.2015.09.044. [DOI] [PubMed] [Google Scholar]
- 385.Kook S.-H., Cheon S.-R., Kim J.-H., Choi K.-C., Kim M.-K., Lee J.-C. Dietary Hydroxycinnamates Prevent Oxidative Damages to Liver, Spleen, and Bone Marrow Cells in Irradiation-Exposed Mice. Food Sci. Biotechnol. 2017;26:279–285. doi: 10.1007/s10068-017-0037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Widowati W., Fauziah N., Herdiman H., Afni M., Afifah E., Kusuma H.S.W., Nufus H., Arumwardana S., Rihibiha D.D. Antioxidant and Anti Aging Assays of Oryza Sativa Extracts, Vanillin and Coumaric Acid. J. Nat. Remedies. 2016;16:88. doi: 10.18311/jnr/2016/7220. [DOI] [Google Scholar]
- 387.Pieńkowska N., Bartosz G., Pichla M., Grzesik-Pietrasiewicz M., Gruchala M., Sadowska-Bartosz I. Effect of Antioxidants on the H2O2-Induced Premature Senescence of Human Fibroblasts. Aging. 2020;12:1910–1927. doi: 10.18632/aging.102730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Morita H., Abe I., Noguchi H. Comprehensive Natural Products II. Elsevier; Amsterdam, The Netherlands: 2010. Plant Type III PKS; pp. 171–225. [Google Scholar]
- 389.Amalraj A., Pius A., Gopi S., Gopi S. Biological Activities of Curcuminoids, Other Biomolecules from Turmeric and Their Derivatives—A Review. J. Tradit. Complement. Med. 2017;7:205–233. doi: 10.1016/j.jtcme.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Tsuda T. Curcumin as a Functional Food-Derived Factor: Degradation Products, Metabolites, Bioactivity, and Future Perspectives. Food Funct. 2018;9:705–714. doi: 10.1039/C7FO01242J. [DOI] [PubMed] [Google Scholar]
- 391.Tomeh M., Hadianamrei R., Zhao X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019;20:1033. doi: 10.3390/ijms20051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Adamczak A., Ożarowski M., Karpiński T.M. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals. 2020;13:153. doi: 10.3390/ph13070153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Nabavi S., Thiagarajan R., Rastrelli L., Daglia M., Sobarzo-Sanchez E., Alinezhad H., Nabavi S. Curcumin: A Natural Product for Diabetes and Its Complications. Curr. Top. Med. Chem. 2015;15:2445–2455. doi: 10.2174/1568026615666150619142519. [DOI] [PubMed] [Google Scholar]
- 394.Donatus I.A., Sardjoko, Vermeulen N.P.E. Cytotoxic and Cytoprotective Activities of Curcumin. Biochem. Pharmacol. 1990;39:1869–1875. doi: 10.1016/0006-2952(90)90603-I. [DOI] [PubMed] [Google Scholar]
- 395.Zia A., Farkhondeh T., Pourbagher-Shahri A.M., Samarghandian S. The Role of Curcumin in Aging and Senescence: Molecular Mechanisms. Biomed. Pharmacother. 2021;134:111119. doi: 10.1016/j.biopha.2020.111119. [DOI] [PubMed] [Google Scholar]
- 396.Rahmani A., Alsahli M., Aly S., Khan M., Aldebasi Y. Role of Curcumin in Disease Prevention and Treatment. Adv. Biomed. Res. 2018;7:38. doi: 10.4103/abr.abr_147_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Lee J., Kim Y.S., Kim E., Kim Y., Kim Y. Curcumin and Hesperetin Attenuate D-Galactose-Induced Brain Senescence In Vitro and In Vivo. Nutr. Res. Pract. 2020;14:438. doi: 10.4162/nrp.2020.14.5.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Lee S.-J., Chandrasekran P., Mazucanti C.H., O’Connell J.F., Egan J.M., Kim Y. Dietary Curcumin Restores Insulin Homeostasis in Diet-Induced Obese Aged Mice. Aging. 2022;14:225–239. doi: 10.18632/aging.203821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Santos-Parker J.R., Lubieniecki K.L., Rossman M.J., van Ark H.J., Bassett C.J., Strahler T.R., Chonchol M.B., Justice J.N., Seals D.R. Curcumin Supplementation and Motor-Cognitive Function in Healthy Middle-Aged and Older Adults. Nutr. Healthy Aging. 2018;4:323–333. doi: 10.3233/NHA-170029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Tavakol S., Zare S., Hoveizi E., Tavakol B., Rezayat S.M. The Impact of the Particle Size of Curcumin Nanocarriers and the Ethanol on Beta_1-Integrin Overexpression in Fibroblasts: A Regenerative Pharmaceutical Approach in Skin Repair and Anti-Aging Formulations. DARU J. Pharm. Sci. 2019;27:159–168. doi: 10.1007/s40199-019-00258-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Schiborr C., P. Eckert G., Weissenberger J., E. Muller W., Schwamm D., Grune T., Rimbach G., Frank J. Cardiac Oxidative Stress and Inflammation Are Similar in SAMP8 and SAMR1 Mice and Unaltered by Curcumin and Ginkgo Biloba Extract Intake. Curr. Pharm. Biotechnol. 2010;11:861–867. doi: 10.2174/138920110793262006. [DOI] [PubMed] [Google Scholar]
- 402.Yoder S.C., Lancaster S.M., Hullar M.A.J., Lampe J.W. Diet-Microbe Interactions in the Gut. Elsevier; Amsterdam, The Netherlands: 2015. Gut Microbial Metabolism of Plant Lignans; pp. 103–117. [Google Scholar]
- 403.Adolphe J.L., Whiting S.J., Juurlink B.H.J., Thorpe L.U., Alcorn J. Health Effects with Consumption of the Flax Lignan Secoisolariciresinol Diglucoside. Br. J. Nutr. 2010;103:929–938. doi: 10.1017/S0007114509992753. [DOI] [PubMed] [Google Scholar]
- 404.Le T.D., Nakahara Y., Ueda M., Okumura K., Hirai J., Sato Y., Takemoto D., Tomimori N., Ono Y., Nakai M., et al. Sesamin Suppresses Aging Phenotypes in Adult Muscular and Nervous Systems and Intestines in a Drosophila Senescence-Accelerated Model. Eur. Rev. Med. Pharm. Sci. 2019;23:1826–1839. doi: 10.26355/eurrev_201902_17146. [DOI] [PubMed] [Google Scholar]
- 405.Sowndhararajan K., Deepa P., Kim M., Park S.J., Kim S. An Overview of Neuroprotective and Cognitive Enhancement Properties of Lignans from Schisandra Chinensis. Biomed. Pharmacother. 2018;97:958–968. doi: 10.1016/j.biopha.2017.10.145. [DOI] [PubMed] [Google Scholar]
- 406.Velalopoulou A., Chatterjee S., Pietrofesa R., Koziol-White C., Panettieri R., Lin L., Tuttle S., Berman A., Koumenis C., Christofidou-Solomidou M. Synthetic Secoisolariciresinol Diglucoside (LGM2605) Protects Human Lung in an Ex Vivo Model of Proton Radiation Damage. Int. J. Mol. Sci. 2017;18:2525. doi: 10.3390/ijms18122525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Shenkin A. Micronutrients in Health and Disease. Postgrad. Med. J. 2006;82:559–567. doi: 10.1136/pgmj.2006.047670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Mehri A. Trace Elements in Human Nutrition (II)—An Update. Int. J. Prev. Med. 2020;11:2. doi: 10.4103/ijpvm.IJPVM_48_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Rudolf E., Rudolf K. Low Zinc Environment Induces Stress Signaling, Senescence and Mixed Cell Death Modalities in Colon Cancer Cells. Apoptosis. 2015;20:1651–1665. doi: 10.1007/s10495-015-1182-5. [DOI] [PubMed] [Google Scholar]
- 410.Rudolf E., Cervinka M. Stress Responses of Human Dermal Fibroblasts Exposed to Zinc Pyrithione. Toxicol. Lett. 2011;204:164–173. doi: 10.1016/j.toxlet.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 411.Malavolta M., Costarelli L., Giacconi R., Basso A., Piacenza F., Pierpaoli E., Provinciali M., Ogo O.A., Ford D. Changes in Zn Homeostasis during Long Term Culture of Primary Endothelial Cells and Effects of Zn on Endothelial Cell Senescence. Exp. Gerontol. 2017;99:35–45. doi: 10.1016/j.exger.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 412.Legrain Y., Touat-Hamici Z., Chavatte L. Interplay between Selenium Levels, Selenoprotein Expression, and Replicative Senescence in WI-38 Human Fibroblasts. J. Biol. Chem. 2014;289:6299–6310. doi: 10.1074/jbc.M113.526863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Jobeili L., Rousselle P., Béal D., Blouin E., Roussel A.-M., Damour O., Rachidi W. Selenium Preserves Keratinocyte Stemness and Delays Senescence by Maintaining Epidermal Adhesion. Aging. 2017;9:2302–2315. doi: 10.18632/aging.101322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Wu R.T.Y., Cao L., Chen B.P.C., Cheng W.-H. Selenoprotein H Suppresses Cellular Senescence through Genome Maintenance and Redox Regulation. J. Biol. Chem. 2014;289:34378–34388. doi: 10.1074/jbc.M114.611970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Wu R.T., Cao L., Mattson E., Witwer K.W., Cao J., Zeng H., He X., Combs G.F., Cheng W. Opposing Impacts on Healthspan and Longevity by Limiting Dietary Selenium in Telomere Dysfunctional Mice. Aging Cell. 2017;16:125–135. doi: 10.1111/acel.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Dickens B.F., Weglicki W.B., Li Y.-S., Mak I.T. Magnesium Deficiency in Vitro Enhances Free Radical-Induced Intracellular Oxidation and Cytotoxicity in Endothelial Cells. FEBS Lett. 1992;311:187–191. doi: 10.1016/0014-5793(92)81098-7. [DOI] [PubMed] [Google Scholar]
- 417.Wolf F.I., Trapani V., Simonacci M., Ferré S., Maier J.A.M. Magnesium Deficiency and Endothelial Dysfunction: Is Oxidative Stress Involved? Magnes Res. 2008;21:58–64. [PubMed] [Google Scholar]
- 418.Yang Y., Wu Z., Chen Y., Qiao J., Gao M., Yuan J., Nie W., Guo Y. Magnesium Deficiency Enhances Hydrogen Peroxide Production and Oxidative Damage in Chick Embryo Hepatocyte In Vitro. BioMetals. 2006;19:71–81. doi: 10.1007/s10534-005-6898-1. [DOI] [PubMed] [Google Scholar]
- 419.Killilea D.W., Ames B.N. Magnesium Deficiency Accelerates Cellular Senescence in Cultured Human Fibroblasts. Proc. Natl. Acad. Sci. USA. 2008;105:5768–5773. doi: 10.1073/pnas.0712401105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Blache D., Devaux S., Joubert O., Loreau N., Schneider M., Durand P., Prost M., Gaume V., Adrian M., Laurant P., et al. Long-Term Moderate Magnesium-Deficient Diet Shows Relationships between Blood Pressure, Inflammation and Oxidant Stress Defense in Aging Rats. Free Radic. Biol. Med. 2006;41:277–284. doi: 10.1016/j.freeradbiomed.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 421.Chasapis C.T., Loutsidou A.C., Spiliopoulou C.A., Stefanidou M.E. Zinc and Human Health: An Update. Arch. Toxicol. 2012;86:521–534. doi: 10.1007/s00204-011-0775-1. [DOI] [PubMed] [Google Scholar]
- 422.Cousins R.J., Blanchard R.K., Moore J.B., Cui L., Green C.L., Liuzzi J.P., Cao J., Bobo J.A. Regulation of Zinc Metabolism and Genomic Outcomes. J. Nutr. 2003;133:1521S–1526S. doi: 10.1093/jn/133.5.1521S. [DOI] [PubMed] [Google Scholar]
- 423.Maret W. Zinc Biochemistry: From a Single Zinc Enzyme to a Key Element of Life. Adv. Nutr. 2013;4:82–91. doi: 10.3945/an.112.003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Choi S., Liu X., Pan Z. Zinc Deficiency and Cellular Oxidative Stress: Prognostic Implications in Cardiovascular Diseases. Acta Pharmacol. Sin. 2018;39:1120–1132. doi: 10.1038/aps.2018.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Lee S.R. Critical Role of Zinc as Either an Antioxidant or a Prooxidant in Cellular Systems. Oxidative Med. Cell. Longev. 2018;2018:9156285. doi: 10.1155/2018/9156285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Marreiro D., Cruz K., Morais J., Beserra J., Severo J., de Oliveira A. Zinc and Oxidative Stress: Current Mechanisms. Antioxidants. 2017;6:24. doi: 10.3390/antiox6020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Yu K.-N., Yoon T.-J., Minai-Tehrani A., Kim J.-E., Park S.J., Jeong M.S., Ha S.-W., Lee J.-K., Kim J.S., Cho M.-H. Zinc Oxide Nanoparticle Induced Autophagic Cell Death and Mitochondrial Damage via Reactive Oxygen Species Generation. Toxicol. Vitr. 2013;27:1187–1195. doi: 10.1016/j.tiv.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 428.O’Dell B.L. Role of Zinc in Plasma Membrane Function. J. Nutr. 2000;130:1432S–1436S. doi: 10.1093/jn/130.5.1432S. [DOI] [PubMed] [Google Scholar]
- 429.Eide D.J. The Oxidative Stress of Zinc Deficiency. Metallomics. 2011;3:1124. doi: 10.1039/c1mt00064k. [DOI] [PubMed] [Google Scholar]
- 430.Cho J.H., Kim M.J., Kim K.J., Kim J.-R. POZ/BTB and AT-Hook-Containing Zinc Finger Protein 1 (PATZ1) Inhibits Endothelial Cell Senescence through a P53 Dependent Pathway. Cell Death Differ. 2012;19:703–712. doi: 10.1038/cdd.2011.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Hu H., Ji Q., Song M., Ren J., Liu Z., Wang Z., Liu X., Yan K., Hu J., Jing Y., et al. ZKSCAN3 Counteracts Cellular Senescence by Stabilizing Heterochromatin. Nucleic Acids Res. 2020;48:6001–6018. doi: 10.1093/nar/gkaa425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Igata T., Tanaka H., Etoh K., Hong S., Tani N., Koga T., Nakao M. Loss of the Transcription Repressor ZHX3 Induces Senescence-Associated Gene Expression and Mitochondrial-Nucleolar Activation. PLoS ONE. 2022;17:e0262488. doi: 10.1371/journal.pone.0262488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Liu C., la Rosa S., Hagos E.G. Oxidative DNA Damage Causes Premature Senescence in Mouse Embryonic Fibroblasts Deficient for Krüppel-like Factor 4. Mol. Carcinog. 2015;54:889–899. doi: 10.1002/mc.22161. [DOI] [PubMed] [Google Scholar]
- 434.Gao B., Li K., Wei Y.-Y., Zhang J., Li J., Zhang L., Gao J.-P., Li Y.-Y., Huang L.-G., Lin P., et al. Zinc Finger Protein 637 Protects Cells against Oxidative Stress-Induced Premature Senescence by MTERT-Mediated Telomerase Activity and Telomere Maintenance. Cell Death Dis. 2014;5:e1334. doi: 10.1038/cddis.2014.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Yang J., Lu Y., Yang P., Chen Q., Wang Y., Ding Q., Xu T., Li X., Li C., Huang C., et al. MicroRNA-145 Induces the Senescence of Activated Hepatic Stellate Cells through the Activation of P53 Pathway by ZEB2. J. Cell. Physiol. 2019;234:7587–7599. doi: 10.1002/jcp.27521. [DOI] [PubMed] [Google Scholar]
- 436.Yi Y., Xie H., Xiao X., Wang B., Du R., Liu Y., Li Z., Wang J., Sun L., Deng Z., et al. Ultraviolet A Irradiation Induces Senescence in Human Dermal Fibroblasts by Down-Regulating DNMT1 via ZEB1. Aging. 2018;10:212–228. doi: 10.18632/aging.101383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Poirier A., Gagné A., Laflamme P., Marcoux M., Orain M., Plante S., Joubert D., Joubert P., Laplante M. ZNF768 Expression Associates with High Proliferative Clinicopathological Features in Lung Adenocarcinoma. Cancers. 2021;13:4136. doi: 10.3390/cancers13164136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Salazar G. NADPH Oxidases and Mitochondria in Vascular Senescence. Int. J. Mol. Sci. 2018;19:1327. doi: 10.3390/ijms19051327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Reich H.J., Hondal R.J. Why Nature Chose Selenium. ACS Chem. Biol. 2016;11:821–841. doi: 10.1021/acschembio.6b00031. [DOI] [PubMed] [Google Scholar]
- 440.Alehagen U., Opstad T.B., Alexander J., Larsson A., Aaseth J. Impact of Selenium on Biomarkers and Clinical Aspects Related to Ageing. A Review. Biomolecules. 2021;11:1478. doi: 10.3390/biom11101478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Hariharan S., Dharmaraj S. Selenium and Selenoproteins: It’s Role in Regulation of Inflammation. Inflammopharmacology. 2020;28:667–695. doi: 10.1007/s10787-020-00690-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Selenius M., Rundlöf A.-K., Olm E., Fernandes A.P., Björnstedt M. Selenium and the Selenoprotein Thioredoxin Reductase in the Prevention, Treatment and Diagnostics of Cancer. Antioxid. Redox Signal. 2010;12:867–880. doi: 10.1089/ars.2009.2884. [DOI] [PubMed] [Google Scholar]
- 443.Ebert R., Ulmer M., Zeck S., Meissner-Weigl J., Schneider D., Stopper H., Schupp N., Kassem M., Jakob F. Selenium Supplementation Restores the Antioxidative Capacity and Prevents Cell Damage in Bone Marrow Stromal Cells In Vitro. Stem cells. 2006;24:1226–1235. doi: 10.1634/stemcells.2005-0117. [DOI] [PubMed] [Google Scholar]
- 444.Hartwig A. Role of Magnesium in Genomic Stability. Mutat. Res./Fundam. Mol. Mech. Mutagenesis. 2001;475:113–121. doi: 10.1016/S0027-5107(01)00074-4. [DOI] [PubMed] [Google Scholar]
- 445.Maguire D., Neytchev O., Talwar D., McMillan D., Shiels P. Telomere Homeostasis: Interplay with Magnesium. Int. J. Mol. Sci. 2018;19:157. doi: 10.3390/ijms19010157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Mazur A., Maier J.A.M., Rock E., Gueux E., Nowacki W., Rayssiguier Y. Magnesium and the Inflammatory Response: Potential Physiopathological Implications. Arch. Biochem. Biophys. 2007;458:48–56. doi: 10.1016/j.abb.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 447.Altobelli G.G., van Noorden S., Balato A., Cimini V. Copper/Zinc Superoxide Dismutase in Human Skin: Current Knowledge. Front. Med. 2020;7:183. doi: 10.3389/fmed.2020.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Kinnula V.L., Crapo J.D. Superoxide Dismutases in the Lung and Human Lung Diseases. Am. J. Respir. Crit. Care Med. 2003;167:1600–1619. doi: 10.1164/rccm.200212-1479SO. [DOI] [PubMed] [Google Scholar]
- 449.Zajac D. Mineral Micronutrients in Asthma. Nutrients. 2021;13:4001. doi: 10.3390/nu13114001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Majidinia M., Reiter R.J., Shakouri S.K., Yousefi B. The Role of Melatonin, a Multitasking Molecule, in Retarding the Processes of Ageing. Ageing Res. Rev. 2018;47:198–213. doi: 10.1016/j.arr.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 451.Lahiri D.K., Ghosh C. Interactions between Melatonin, Reactive Oxygen Species, and Nitric Oxide. Ann. N. Y. Acad. Sci. 1999;893:325–330. doi: 10.1111/j.1749-6632.1999.tb07847.x. [DOI] [PubMed] [Google Scholar]
- 452.Hardeland R. Aging, Melatonin, and the Pro- and Anti-Inflammatory Networks. Int. J. Mol. Sci. 2019;20:1223. doi: 10.3390/ijms20051223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Huang H., Liu X., Chen D., Lu Y., Li J., Du F., Zhang C., Lu L. Melatonin Prevents Endothelial Dysfunction in SLE by Activating the Nuclear Receptor Retinoic Acid-Related Orphan Receptor-α. Int. Immunopharmacol. 2020;83:106365. doi: 10.1016/j.intimp.2020.106365. [DOI] [PubMed] [Google Scholar]
- 454.Radogna F., Diederich M., Ghibelli L. Melatonin: A Pleiotropic Molecule Regulating Inflammation. Biochem. Pharmacol. 2010;80:1844–1852. doi: 10.1016/j.bcp.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 455.Lee J.H., Yoon Y.M., Song K., Noh H., Lee S.H. Melatonin Suppresses Senescence-derived Mitochondrial Dysfunction in Mesenchymal Stem Cells via the HSPA1L–Mitophagy Pathway. Aging Cell. 2020;19:e13111. doi: 10.1111/acel.13111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Chen Z., Zhao C., Liu P., Huang H., Zhang S., Wang X. Anti-Apoptosis and Autophagy Effects of Melatonin Protect Rat Chondrocytes against Oxidative Stress via Regulation of AMPK/Foxo3 Pathways. CARTILAGE. 2021;13:1041S–1053S. doi: 10.1177/19476035211038748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Wu K.K. Control of Mesenchymal Stromal Cell Senescence by Tryptophan Metabolites. Int. J. Mol. Sci. 2021;22:697. doi: 10.3390/ijms22020697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Cai B., Ma W., Bi C., Yang F., Zhang L., Han Z., Huang Q., Ding F., Li Y., Yan G., et al. Long Noncoding RNA H19 Mediates Melatonin Inhibition of Premature Senescence of C-Kit+ Cardiac Progenitor Cells by Promoting MiR-675. J. Pineal Res. 2016;61:82–95. doi: 10.1111/jpi.12331. [DOI] [PubMed] [Google Scholar]
- 459.Zhou L., Chen X., Liu T., Gong Y., Chen S., Pan G., Cui W., Luo Z.-P., Pei M., Yang H., et al. Melatonin Reverses H2O2-Induced Premature Senescence in Mesenchymal Stem Cells via the SIRT1-Dependent Pathway. J. Pineal Res. 2015;59:190–205. doi: 10.1111/jpi.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Liao N., Shi Y., Zhang C., Zheng Y., Wang Y., Zhao B., Zeng Y., Liu X., Liu J. Antioxidants Inhibit Cell Senescence and Preserve Stemness of Adipose Tissue-Derived Stem Cells by Reducing ROS Generation during Long-Term In Vitro Expansion. Stem Cell Res. Ther. 2019;10:306. doi: 10.1186/s13287-019-1404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Shuai Y., Liao L., Su X., Yu Y., Shao B., Jing H., Zhang X., Deng Z., Jin Y. Melatonin Treatment Improves Mesenchymal Stem Cells Therapy by Preserving Stemness during Long-Term In Vitro Expansion. Theranostics. 2016;6:1899–1917. doi: 10.7150/thno.15412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Puig Á., Rancan L., Paredes S.D., Carrasco A., Escames G., Vara E., Tresguerres J.A.F. Melatonin Decreases the Expression of Inflammation and Apoptosis Markers in the Lung of a Senescence-Accelerated Mice Model. Exp. Gerontol. 2016;75:1–7. doi: 10.1016/j.exger.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 463.Parisotto E.B., Vidal V., García-Cerro S., Lantigua S., Wilhelm Filho D., Sanchez-Barceló E.J., Martínez-Cué C., Rueda N. Chronic Melatonin Administration Reduced Oxidative Damage and Cellular Senescence in the Hippocampus of a Mouse Model of Down Syndrome. Neurochem. Res. 2016;41:2904–2913. doi: 10.1007/s11064-016-2008-8. [DOI] [PubMed] [Google Scholar]
- 464.Okatani Y., Wakatsuki A., Reiter R.J., Miyahara Y. Melatonin Reduces Oxidative Damage of Neural Lipids and Proteins in Senescence-Accelerated Mouse. Neurobiol. Aging. 2002;23:639–644. doi: 10.1016/S0197-4580(02)00005-2. [DOI] [PubMed] [Google Scholar]
- 465.Sumsuzzman D.M., Khan Z.A., Choi J., Hong Y. Differential Role of Melatonin in Healthy Brain Aging: A Systematic Review and Meta-Analysis of the SAMP8 Model. Aging. 2021;13:9373–9397. doi: 10.18632/aging.202894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Bin-Jaliah I., Sakr H. Melatonin Ameliorates Brain Oxidative Stress and Upregulates Senescence Marker Protein-30 and Osteopontin in a Rat Model of Vascular Dementia. Physiol. Int. 2018;105:38–52. doi: 10.1556/2060.105.2018.1.1. [DOI] [PubMed] [Google Scholar]
- 467.Singh P.P., Demmitt B.A., Nath R.D., Brunet A. The Genetics of Aging: A Vertebrate Perspective. Cell. 2019;177:200–220. doi: 10.1016/j.cell.2019.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The Hallmarks of Aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Redman L.M., Smith S.R., Burton J.H., Martin C.K., Il’yasova D., Ravussin E. Metabolic Slowing and Reduced Oxidative Damage with Sustained Caloric Restriction Support the Rate of Living and Oxidative Damage Theories of Aging. Cell Metab. 2018;27:805–815.e4. doi: 10.1016/j.cmet.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Mazzotti D.R., Guindalini C., dos Moraes W.A.S., Andersen M.L., Cendoroglo M.S., Ramos L.R., Tufik S. Human Longevity Is Associated with Regular Sleep Patterns, Maintenance of Slow Wave Sleep, and Favorable Lipid Profile. Front. Aging Neurosci. 2014;6:134. doi: 10.3389/fnagi.2014.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Epel E.S., Lithgow G.J. Stress Biology and Aging Mechanisms: Toward Understanding the Deep Connection Between Adaptation to Stress and Longevity. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014;69:S10–S16. doi: 10.1093/gerona/glu055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Jenkinson C.E., Dickens A.P., Jones K., Thompson-Coon J., Taylor R.S., Rogers M., Bambra C.L., Lang I., Richards S.H. Is Volunteering a Public Health Intervention? A Systematic Review and Meta-Analysis of the Health and Survival of Volunteers. BMC Public Health. 2013;13:773. doi: 10.1186/1471-2458-13-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Young R.D. Validated Living Worldwide Supercentenarians, Living and Recently Deceased: February 2018. Rejuvenation Res. 2018;21:67–69. doi: 10.1089/rej.2018.2057. [DOI] [PubMed] [Google Scholar]
- 474.Hernandez-Segura A., Nehme J., Demaria M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018;28:436–453. doi: 10.1016/j.tcb.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 475.Lyublinskaya O., Kornienko J., Ivanova J., Pugovkina N., Alekseenko L., Lyublinskaya E., Tyuryaeva I., Smirnova I., Grinchuk T., Shorokhova M., et al. Induction of Premature Cell Senescence Stimulated by High Doses of Antioxidants Is Mediated by Endoplasmic Reticulum Stress. Int. J. Mol. Sci. 2021;22:11851. doi: 10.3390/ijms222111851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Kornienko J.S., Smirnova I.S., Pugovkina N.A., Ivanova J.S., Shilina M.A., Grinchuk T.M., Shatrova A.N., Aksenov N.D., Zenin V.V., Nikolsky N.N., et al. High Doses of Synthetic Antioxidants Induce Premature Senescence in Cultivated Mesenchymal Stem Cells. Sci. Rep. 2019;9:1296. doi: 10.1038/s41598-018-37972-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Lee B.P., Harries L.W. Senotherapeutic Drugs: A New Avenue for Skincare? Plast. Reconstr. Surg. 2021;148:21S–26S. doi: 10.1097/PRS.0000000000008782. [DOI] [PubMed] [Google Scholar]
- 478.Wissler Gerdes E.O., Zhu Y., Tchkonia T., Kirkland J.L. Discovery, Development, and Future Application of Senolytics: Theories and Predictions. FEBS J. 2020;287:2418–2427. doi: 10.1111/febs.15264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Kirkland J.L., Tchkonia T. Senolytic Drugs: From Discovery to Translation. J. Intern. Med. 2020;288:518–536. doi: 10.1111/joim.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.