Abstract
One of the most severe human health problems is gastric ulceration. The main aim of our study is to explore the gastroprotective effect of the Psidium guajava seeds extract (PGE). Metabolic profiling based on LC-HRMS for the extract led to the dereplication of 23 compounds (1–23). We carried out a gastric ulcer model induced by indomethacin in male albino rats in vivo and the extract of PGE was investigated at a dose of 300 mg/kg in comparison to cimetidine (100 mg/kg). Furthermore, the assessment of gastric mucosal lesions and histopathology investigation of gastric tissue was done. It has been proved that Psidium guajava seeds significantly decreased the ulcer index and protected the mucosa from lesions. The antiulcer effect of Psidium guajava seed extract, which has the power of reducing the ensuing inflammatory reactions, can counteract the inflammation induced by indomethacin by the downregulation of relative genes expression (IL-1β, IL-6, and TNF-α). Moreover, PGE significantly downregulated the increased COX-2, TGF-β, and IGF-1 relative genes expression, confirming its beneficial effect in ulcer healing. Moreover, the possible PGE antioxidant potential was determined by in vitro assays using hydrogen peroxide and superoxide radical scavenging and revealed high antioxidant potential. Additionally, on the putatively annotated metabolites, an in silico study was conducted, which emphasized the extract’s antiulcer properties might be attributed to several sterols such as stigmasterol and campesterol. The present study provided evidence of Psidium guajava seeds considered as a potential natural gastroprotective agent.
Keywords: Psidium guajava, metabolic profiling, gastro protective, docking
1. Introduction
Psidium guajava (Family: Myrtaceae) is a tropical edible fruiting plant. They are evergreen trees indigenous to subtropical and tropical climatic regions, it originated from Latin America and was cultivated in many locations of Asia, Africa, and Europe [1]. The plant is an economically important crop cultivated for fruit production such as food and raw material in food industry [2,3]. Moreover, P. guajava has a wide range of medicinal applications [4]. P. guajava is a plentiful source of diverse secondary metabolites including phenolic acids (chlorogenic, ferulic and caffeic acids) [5], flavonoids (quercetin, kaempferol, and morin glycosides) [6], tannins (guavins A, B, C, D) [7], ellagitannins (casuarinin, flavonotannins; psidinins A, B, C) [8], Benzophenone glycosides (guavinoside B) [9,10]. Guava leaves and bark were assigned to treat gastrointestinal disorders; particularly diarrhea [11]. In the last decades, several studies were performed using the decocted or infused extracts of leaves, fruits, stems, and flower buds to study their different biological activities [12,13,14,15,16,17,18,19]. The anti-inflammatory, antihypertensive, blood glucose lowering effect, antiviral and cough sedative activities are the key factors which labelled the guava fruit to be a perfect candidate to protect against COVID-19 infection especially for chronic diseased individuals [20]. Guava seeds (PGE) as a waste product of the commercially important fruit was evaluated as an inhibitor for the proliferation of the human erythroleukemic cells with low toxicity [21], the seed oil contains unsaturated fatty acids especially, linoleic acid and possessed a high antioxidant activity [22]. In addition to seed fatty acids, the seeds were found to be rich with proteopolysaccharides with potent anti-inflammatory and immunomodulatory effects [23], peptides from guava seeds were reported to perform antimicrobial effects against gram-negative bacteria [24], the phenolic content of the seeds extracted by supercritical fluid extraction performed a high antioxidant activity [25]. The anticorrosion potential of Psidium guajava seeds on carbon steel in an acid medium was reported as an ecofriendly application [26]. Guava seeds are considered a rich reservoir of variable potent phytochemical constitutes, so we investigated for the first time the gastroprotective and antioxidant of the Psidium Guajava seeds as well as their anti-inflammatory potential. Moreover, we carried out LC-MS analysis to reveal the chemical entities of Psidium guajava seeds. Finally, a docking study was carried within three different active sites, which includes the gastric proton pump, muscarinic receptors as well as H2 receptors.
2. Materials and Methods
2.1. Plants Material
The seeds of Psidium guajava were collected in September 2021 from Minia area, Egypt. Mr. Abdallah Salem authenticated the plant (at Minia University, Minia, Egypt). A voucher specimen (PS 2-2021) was conserved at Deraya University.
2.2. Extraction of Psidium guajava Seeds
One kilogram of dried Psidium guajava seeds were extracted three times by maceration in methanol at an ambient temperature. The alcoholic extract was vacuum-dried to produce a dense residue (100 g).
2.3. Metabolic Profiling
LCMS was performed by using a Synapt G2 HDMS quadrupole time-of-flight hybrid mass spectrometer (Waters, Milford, CT, USA). One mg of the sample was injected into the BEH C18 column, at 40 °C, and connected to the guard column. A gradient elution was used, starting from no solvent A and 0.1% formic acid in water to 100% acetonitrile as solvent B. MZmine 2.12 San Diego, USA was used to investigate MS data using mzML format converted with ProteoWizard Palo Alto, CA, USA [27].
2.4. Docking Study
Investigated compounds 3D structures were downloaded from PubChem and prepared as reported earlier [28]. Compounds were docked against a gastric proton pump (PDB 5YLU), M3 muscarinic receptor (PDB 5ZHP) obtained from http://www.rcsb.org/ (accessed on 23 March 2022) and against a H2 receptor as obtained from GPCR data base. Docking was done in the active sites of target protein as recognized by co-crystalized ligands. Docking was done using Autodock Vina [29] using cubic grid box with 25 Å side and exhaustiveness of 16. Similarly docking was also done using the same procedure against other targets involved in inflammation including COX-1 (PDB 1EQH), COX-2 (PDB 3LN1), TNF-α (PDB 2AZ5), TGF-β (PDB 5E8S), EGFR (PDB 1M17) and IGFR (PDB 5XFS).
2.5. Animal Model
The experiment was conducted on 150–200 g Albino rats (Wistar) kept under normal conditions (room temperature 24–27 °C, moisture 60–65%) with a 12-h light-dark cycle. Rats were fasted for 24 h before the test to guarantee an empty stomach. The animals were housed in standard cages with free access to water and food. The animals were acclimatized for seven days prior to the experiment start day, and all circumstances were designed to eliminate animal suffering. To eliminate differences owing to diurnal rhythms as possible regulators of stomach functions, all rats were used in the experiment at the same time of day [30,31]. The rats were separated into four groups: A, B, C and D, each of which had 6 rats. The normal control group, Group A received only the vehicle (0.5 percent CMC solution). Group B (positive control) was given the vehicle (0.5 percent CMC solution). Group C (market comparable reference group) was administered oral cimetidine (100 mg/kg p.o) (oral administration), whereas Group D was given guava seed extracts of 300 mg/kg p.o. (dissolved in a 0.5% CMC solution). After 1 h, groups B, C, and D received a single acute dosage of indomethacin (IND) (40 mg/kg) orally to induce gastric ulcers. All animals of the four groups were killed four hours after starting the experiment [32,33]. The stomach was cut and the larger curvature of the stomach was sliced apart. The stomachs were placed flat on a cork board after being washed under a steady stream of water. The ulcer index was obtained by counting the number of ulcer spots in the glandular region of the stomach in both control and drug-treated animals [30,34].
2.6. Gastric Mucosal Lesions Evaluation
The ulcer index (UI) was determined using a 0–3 scoring system and an inspection of the stomachs using an eyepiece. The severity factor was determined as follows: level 0 = no lesions, level 1 = lesions, level 2 = lesions, level 3 = lesions. Lesions with a length of less than 1 mm were classified as level 1, lesions with a length of 2–4 mm were classified as level 2, and lesions with a length of more than 4 mm were classified as level 3. Each rat’s lesion score was determined by multiplying the number of lesions by their respective factor. The drug’s preventative index (PI) was determined by the use the following formula:
PI = (UI of indomethacin group − treated group) × 100\(UI of indomethacin group) [35].
2.7. Histopathological Examination
The stomach tissue was preserved in a 10% ethanol formalin solution and treated with graded ethanol, paraffin wax, and xylene before being cut into slices using a microtome. The slices were viewed under a microscope after being stained with Eosin stain and Hematoxylin. The various histological indicators were examined [36].
2.8. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Quantitative Real-Time Polymerase Chain Reaction is discussed in detail in the Supplementary Materials, as mentioned in Table S1.
2.9. In Vitro Antioxidant Activity
The H2O2 and SOD scavenging activities of Psidium Guajava seed crude extract are thoroughly discussed in the Supplementary Materials.
2.10. Statistical Analysis
All of the data were set as a mean ± SD. A one-way analysis of variance (ANOVA) was used to evaluate the data of all groups, followed by the Dunnett’s test with statistical significance at p < 0.05.
3. Results and Discussion
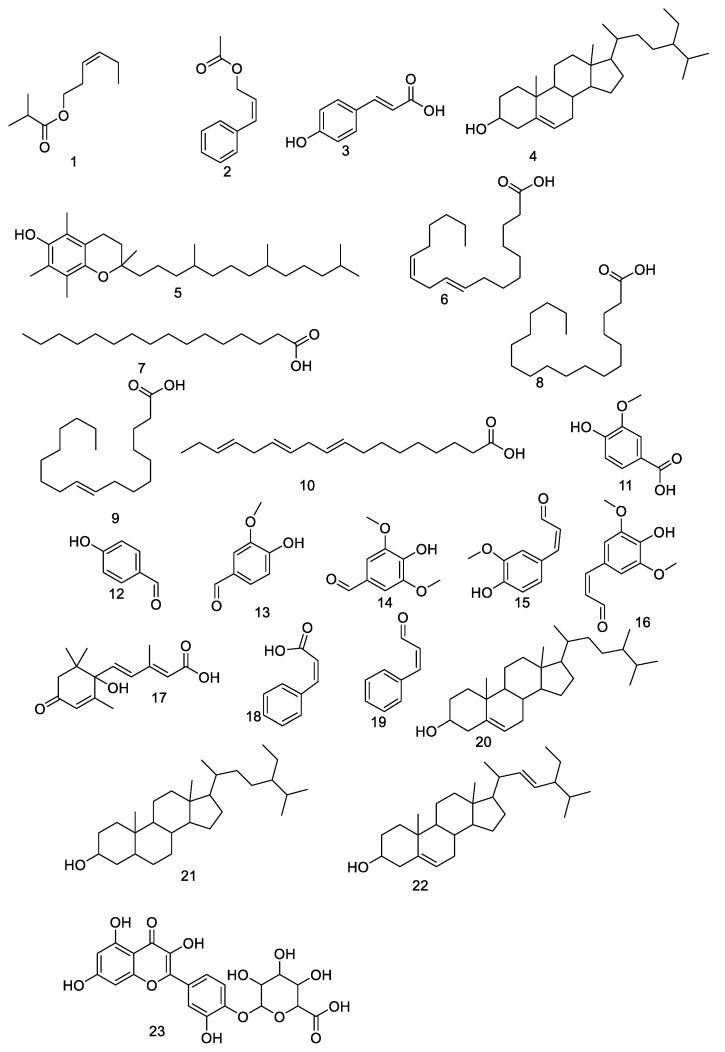
Metabolic profiling using HR-LCMS was performed on the crude extract of Psidium guajava seeds to investigate the chemical constituents that might be responsible for the protective role against ulcer formation. The identified compounds as shown in Figure 1 were assorted into different chemical classes. Identification of the compounds was achieved based on HR-ESIMS data and comparison with literature data as follows: Cis-3-Hexenyl isobutyrate (1) [37], cinnamyl acetate (2) [38], coumaric acid (3) [39], β-sitosterol (4) [39], α-tocopherol (5) [39], linoleic acid (6) [39], palmitic acid (7) [39], stearic acid (8) [39], oleic acid (9) [39], linolenic acid (10) [39], vanillic acid (11) [39], 4-Hydroxybenzaldehyde (12) [39], vanillin (13) [39], syringaldehyde (14) [39], coniferylaldehyde (15) [39], sinapaldehyde (16) [39], abscisic acid (17) [39], cinnamic acid (18) [39], cinnamaldehyde (19) [39], campesterol (20) [39], stigmastanol (21) [39], stigmasterol (22) [39] and quercetin 4′-glucuronide (23) [40]. Guava seed extract provided substantial protection against the ulcerogenic effects of indomethacin in a dose-dependent manner (Table 1). We carried out the study as follows: group A was a normal group that didn’t have any ulcers, while group B (indomethacin) showed lesions that were hemorrhagic and shaped in a linear or dotted pattern. Furthermore, group C (cimetidine) showed mild lesions. Finally, group D (guava seed extract) had a similar number of ulcers to the normal group (Figure 2). Our results showed that the indomethacin group (group B) had a lesion index of (117.32 ± 23.3), while the cimetidine treated group (group C) had a lesion index of (2.0 ± 1.01) and preventive index of 98.2%. On the other hand, group D (IND+ Psidium Guajava seeds extract) had the lowest ulcer index (1.33 ± 0.33) and a high preventive index (98.6%). These results proved the powerful gastroprotective potential of guava seed extract, which showed a higher preventive index (98.6%) comparable to the cimetidine group. Moreover, our extract exerts a powerful gastroprotective effect, more than many natural extracts such as Ocimum forskolei, which has a preventive index 91.17% [41], and Elaeocarpus grandis, which has a preventive index of 92.9% [42].
Figure 1.
Putative compounds identified from Psidium guajava seed.
Table 1.
Antiulcer activity of guava seed extract.
| Groups | Level 1 | Level 2 | Level 3 | Ulcer Index | PI% |
|---|---|---|---|---|---|
| Group A | - | - | - | - | - |
| Group B | 22 ± 3.02 | 26.66 ± 2.17 | 14 ± 5.26 | 117.32 ± 23.3 | - |
| Group C | 1.31 ± 0.32 | 0.3 ± 0.31 | 0 | 2.0 ± 1.01 *** | 98.2% |
| Group D | 1.33 ± 0.57 | 0 | 0 | 1.33 ± 0.33 *** | 98.6% |
Data are represented as mean ± SD. One-way ANOVA followed by Dunnett’s multiple comparison tests software (accessed on 15 April 2022) were used for statistical analysis. Results were regarded significant as follows: *** p < 0.001.
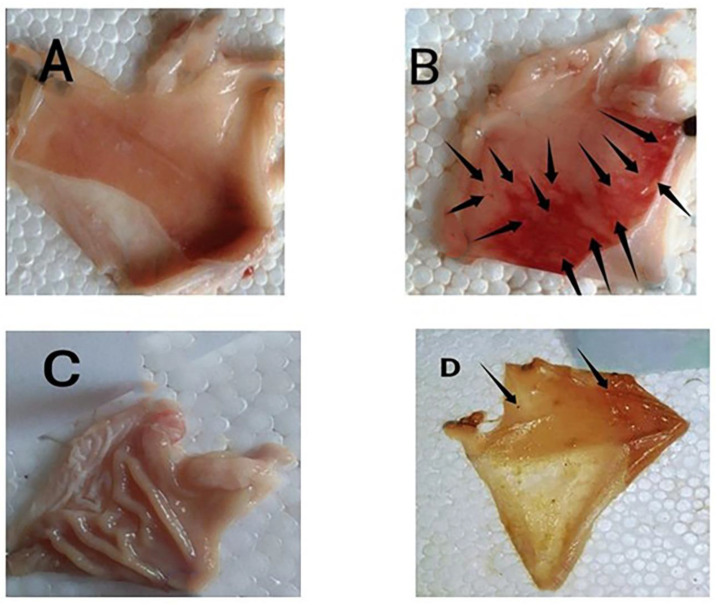
Figure 2.
(A–D) Photos of rat stomachs. (A) (normal group), (B) (positive control group), (C) (cimetidine group), (D) (guava seed extract group). Effect of Guava seed extract on the gastric lesion severity (gross examination). (A) Control: intact gastric mucosa tissues; (B) indomethacin (ulcer): severe lesions are seen with extensive visible hemorrhagic necrosis of gastric mucosa; (C) cimetidine-treated rats: mild lesions of the gastric mucosa are observed compared to the lesions in indomethacin-induced ulcer rats; (D) guava seed extract-treated rats: nearly normal gastric mucosa tissues.
3.1. Histopathological Studies
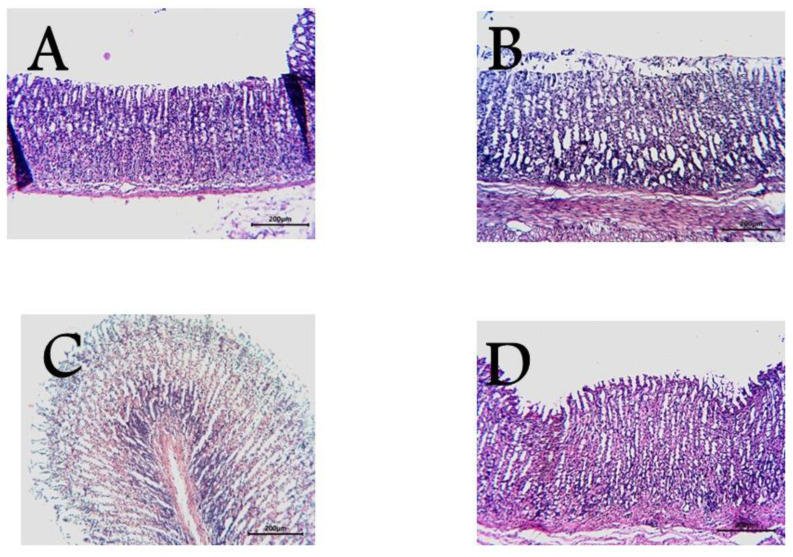
Moreover, the pharmacological and biochemical findings are compatible with histological investigations of stomach tissue. The normal stomach mucosa is shown in Figure 3A. The negative control group (Figure 3B) showed ulceration and bleeding on the mucosa. The positive control group (Figure 3C) had cimetidine. Guava seed extract pretreatment of rats resulted in a lack of mucosal ulcers and bleeding, as shown in Figure 3D.
Figure 3.
Light micrographs showing the effect of guava seed extract on Indomethacin-induced gastric ulcers of rats. (A) Group A (normal mucosa), (B) Group B (Indomethacin-induced gastric mucosal lesions), (C) Group C (cimetidine treated group show low mucosal lesions), (D) Group D (pretreatment of rats with guava seed extract) show no mucosal alterations.
3.2. Docking Study
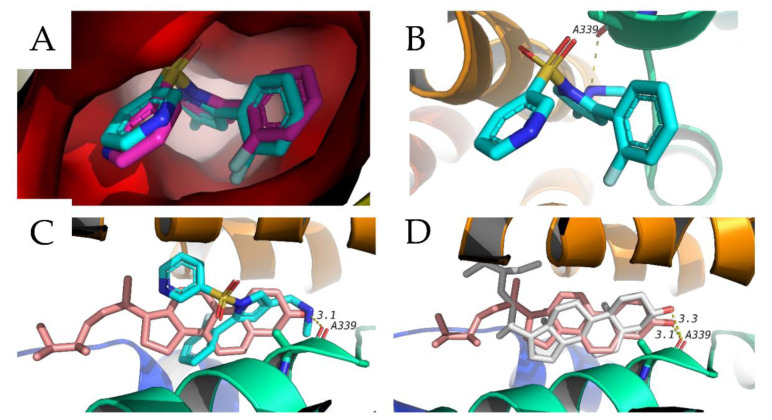
In this study, we used a molecular docking technique to predict potential mechanisms of action of the identified compounds acting as an antiulcer. Twenty-three compounds were docked against three antiulcer targets including the gastric proton pump, muscarinic receptor and H2 receptor. Two of these targets are available on the protein date bank, which are the proton pump and muscarinic receptor under 5YLU and 5ZHP, respectively. The structure of the H2 receptor was obtained in homology model form in the GPCR data base. Validation of docking procedure was done by redocking of the co-crystalized ligand and calculation of RMSD between docked and crystalized poses. An RMSD of less than 2 Å is considered acceptable (Figure 4A shows and example of the validation of the gastric proton pump). Docking results are shown in Table 2, and show that several sterol compounds have docking scores better than the co-crystalized ligand vonoprazan in cases with the gastric proton pump. These compounds include β-Sitosterol, campesterol, stigmastanol and stigmasterol. The scores of these compounds with gastric proton pump is better than their scores with the other two targets. In addition to these sterols, Quercetin-4′-glucuronide showed a docking score with the same target, the gastric proton pump, that is very close to the co-crystalized ligand. The docked sterols were able to maintain the same hydrogen bond that is formed by vonoprazone with A339 of the gastric proton pump (Figure 4B). Among those sterols, campesterol showed the best docking score (−9.9 kcal/mol) with proton pump protein. Its docking pose overlapped with the docking position of vonoperazone, forming the same hydrogen bonds as well as hydrophobic interactions, as shown in Figure 4C. Another example is the next sterol, stigmasterol (−9.7 kcal/mol), that showed a similar docking pose to campesterol, as shown in Figure 4D. These results suggest that these sterols might be responsible for the antiulcer effect through the inhibition of the gastric proton pump, which might require further follow-up studies to investigate this effect. In addition, the same twenty-three identified compounds were also docked in the active sites of several inflammatory targets and mediators which include COX-1, COX-2, TNF-α, TGF-β, EGFR, and IGFR. This was conducted to investigate whether the antiulcer effect of these compounds could be mediated through the modification of any of these inflammatory mediators. The results of this docking study are also shown in Table 2. Several sterols were able to hit some of these inflammatory targets; for example, stigmasterol had good docking scores with TGF-β (−9.6 kcal/mol) and EGFR (−9.4 kcal/mol). Another example of a nonsteroidal compound that showed good results with one of these inflammatory mediators is quercetin-4′-glucuronide, which showed its best docking score with TGF-β (−9.5 kcal/mol). These results suggest that other mechanisms could also be involved in the antiulcer effect of the study extracts and open the door for further investigation in this important area.
Figure 4.
Docking of identified compounds against gastric proton pump (PDB 5YLU). (A) Validation of docking procedure with target protein. (B) Interactions of vonoperazone (blue) with the active site of protein. (C) Docking pose of campesterol (pink) overlapped with co-crystalized ligand (blue). (D) Docking pose of stigmasterol (white) and campesterol (pink).
Table 2.
Docking results of identified compounds against antiulcer targets.
| Docking Score (Kcal/Mol) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Anti-Ulcer Targets | Anti-Inflammatory Targets | ||||||||
| Ligand | Proton Pump (5YLU) | M3 Receptor (5ZHP) | H2-Receptor Model | COX-1 (1EQH) | COX-2 (3LN1) |
TNF-α (2AZ5) | TGF-β (5E8S) | EGFR (1M17) | IGFR (5XFS) |
| cis-3-Hexenyl-isobutyrate | −5.6 | −6.0 | −5.8 | −6.1 | −6.0 | −5.1 | −5.4 | −5.2 | −4.7 |
| Cinnamyl-acetate | −6.3 | −6.9 | −6.6 | −6.9 | −7.2 | −5.7 | −6.5 | -6.1 | −5.8 |
| Coumaric-acid | −6.3 | −6.7 | −5.8 | −6.3 | −6.9 | −5.7 | −6.2 | −5.7 | −6.1 |
| β-SITOSTEROL | −9.6 | −8.9 | −5.8 | −7.7 | −7.0 | −8.5 | −8.7 | −8.5 | −8.1 |
| α-Tocopherol | −8.4 | −9.2 | −6.8 | −8.7 | −8.2 | −7.5 | −8.0 | −7.6 | −7.3 |
| linoleic acid | −6.5 | −7.0 | −6.8 | −6.7 | −7.3 | −5.9 | −6.0 | −5.3 | −5.6 |
| Palmitic acid | −6.1 | −6.8 | −6.4 | −6.2 | −7.0 | −5.2 | −5.7 | −5.0 | −5.2 |
| Stearic acid | −6.1 | −6.4 | −6.4 | −6.2 | −7.0 | −5.0 | −5.8 | −5.2 | −5.2 |
| Oleic acid | −6.1 | −7.1 | −6.5 | −7.2 | −7.2 | −5.7 | −5.8 | −5.6 | −5.5 |
| Linolenic acid | −6.6 | −7.2 | −7.1 | −6.8 | −7.5 | −5.7 | −6.6 | −5.7 | −5.8 |
| Vanillic acid | −6.0 | −6.3 | −5.6 | −6.3 | −6.3 | −5.4 | −6.6 | −6.2 | −5.5 |
| p-Hydroxybenzaldehyde | −5.1 | −5.8 | −5.0 | −5.9 | −5.8 | −4.9 | −5.8 | −5.5 | −4.9 |
| Vanillin | −5.4 | −5.9 | −5.4 | −6.0 | −5.8 | −5.3 | −6.2 | −5.5 | −5.2 |
| Syringaldehyde | −5.4 | −6.0 | −5.6 | −6.1 | −6.0 | −5.2 | −5.3 | −5.2 | −5.2 |
| Coniferylaldehyde | −6.1 | −6.6 | −5.5 | −6.6 | −6.8 | −5.6 | −6.0 | −5.6 | −6.0 |
| Sinapaldehyde | −6.0 | −6.3 | −5.6 | −5.8 | −6.7 | −5.4 | −6.2 | −5.9 | −5.8 |
| Abscisic acid | −7.9 | −8.6 | −6.8 | −8.0 | −8.1 | −7.3 | −7.0 | −6.8 | −6.8 |
| Cinnamic acid | −6.3 | −6.7 | −6.1 | −6.2 | −6.9 | −5.9 | −6.0 | −5.6 | −5.9 |
| Cinnamaldehyde | −5.6 | −6.0 | −5.5 | −6.0 | −6.1 | −5.6 | −5.4 | −5.0 | −5.3 |
| Campesterol | −9.9 | −8.7 | −5.8 | −7.6 | −6.9 | −8.8 | −8.9 | −9.1 | −8.3 |
| Stigmastanol | −9.6 | −8.5 | −6.2 | −7.5 | −7.4 | −8.6 | −8.8 | −8.3 | −8 |
| Stigmasterol | −9.7 | −8.5 | −6.3 | −7.9 | −7.5 | −8.9 | −9.6 | −9.4 | −8.5 |
| Quercetin-4′-glucuronide | −9.3 | −8.6 | −7.8 | −7.6 | −9.0 | −8.3 | −9.5 | −8.5 | −8.4 |
| Co-crystalized Ligand |
−9.5 | −11.2 | - | −9.6 | −13.2 | −9.2 | - | −7.1 | −10.0 |
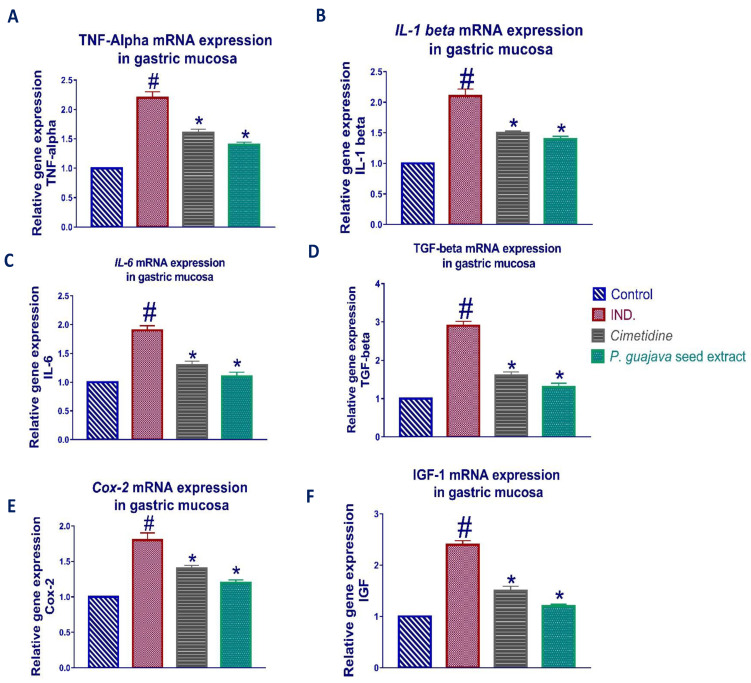
3.3. Effect of Psidium guajava Seed Extract on Expression of TNF-α, IL-1β, IL-6, TGF-β, COX-2, and IGF-1
After mucosal damage, the healing of a stomach ulcer is a complex physiological process including tissue auto-repair, reperfusion, and regeneration. A stomach ulcer is a lesion comprised of connective and granular tissues with fibroblasts and macrophages, as well as the generation of proliferating microvessels at the ulcer base and ulcer edge via angiogenesis. This lesion is surrounded by non-necrotic mucosa, which creates the ulcer edge that heals too quickly, resulting in the formation of an ulcer scar. There are four stages to the ulcer healing process: Indomethacin-induced inflammation, ulcer formation as evidenced by inflammatory cell infiltration, tissue necrosis, and lastly, the creation of granulation tissue and ulcer margin [43].
Upon activation of immune cells, a variety of proinflammatory cytokines such as interleukin-1beta (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) are secreted from macrophages. A proinflammatory cytokine, IL1β, regulates a variety of genes involved in the inflammatory reaction and related tissue damage, such as compromising the function of entero-chromaffin-like cells. In addition, IL-1β inhibits the proliferation of gastric epithelial cells [44], an effect that involves tyrosine kinase, protein kinase C, and multiple mitogen-activated protein kinases. The inflammation triggered by IL-1β, Il-6 and TNF-α can be antagonized by the antiulcer action of Psidium guajava seed extract, which attains the capability of suppressing the resultant inflammatory responses, as shown in Figure 5.
Figure 5.
TNF-α (A), IL-1β (B), IL-6 (C), TGF-β (D), COX-2 (E), and IGF-1 (F) relative gene expression in gastric mucosa tissue of different groups of rats was assessed using quantitative RT-PCR. After normalization to GAPDH, the data reflect a fold difference in expression compared to the normal control group. The bars show the mean ± standard deviation. To see if there is a significant difference between groups, a one-way ANOVA test is performed, with # p < 0.05 compared to the normal control group and * p < 0.05 compared to the indomethacin induced group.
The induction of COX-2 in fibroblasts, epithelial, parietal, and mononuclear cells of the mucosa, and in response to IL-1β elevation, is related with indomethacin-induced gastritis. The results showed a marked reduction of the elevated COX-2 relative gene expression upon treatment with Psidium guajava seed extract, which further confirms its protective role in ulcer healing, as shown in Figure 5.
IL-1β contributes to the initial upregulation of TGF-β, and IGF-1 in the base of indomethacin-evoked ulcers in a rat stomach [45]. Although TGF-β inhibits cell proliferation in a variety of cells, it induces angiogenesis, cell migration, and increases extracellular matrix (ECM) production, all of which are required for gastrointestinal ulcer healing at the intermediate and late stages of the healing process. It also acts as an anti-inflammatory cytokine [46]. Macrophages are activated following indomethacin-induced inflammation, which increases the release of active TGF-β with increasing plasmin activation [47].
Furthermore, IGF-1 expression can contribute to the acceleration of ulcer healing in both the early and late phases of the healing process [46]. IGF-1, a major mediator of soft tissue regeneration, is released from platelet granules shortly after damage and plays a crucial role in angiogenesis and epithelialization in gastric ulceration [48]. Accordingly, we found that the IGF-1 mRNA level was significantly increased in the stomach mucosa following ulceration in normal rats. Finally, after treatment with Psidium guajava seed extract, the results showed marked downregulation of the elevated TGF-β, and IGF-1 relative gene expression, as shown in Figure 5.
3.4. In Vitro Antioxidant Activity of Psidium guajava Seed Extract
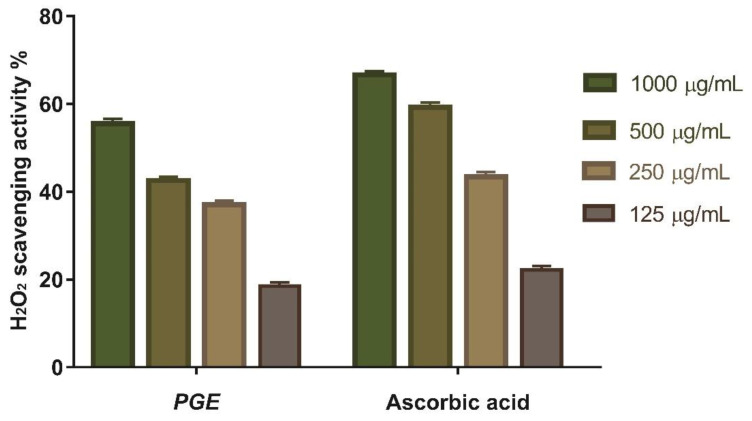
3.4.1. Hydrogen Peroxide Scavenging Activity
This study looked at the antioxidant activity of Psidium Guajava seed extract as a scavenger potential against (H2O2). According to the findings, the maximum H2O2 radical scavenging activity of PGE was 55.55% at a 1000 µg/mL concentration. When compared to conventional ascorbic acid (IC50 = 182.7 µg/mL), Psidium guajava seed extract suppressed the formation of H2O2 in a dose-dependent manner (Figure 6).
Figure 6.
H2O2 radical scavenging activity of PGE at different concentration (1000 µg/mL, 500 µg/mL, 250 µg/mL, and 125 µg/mL). Bars represent mean ± standard deviation (SD). Significant difference between groups is analyzed by a two-way ANOVA test.
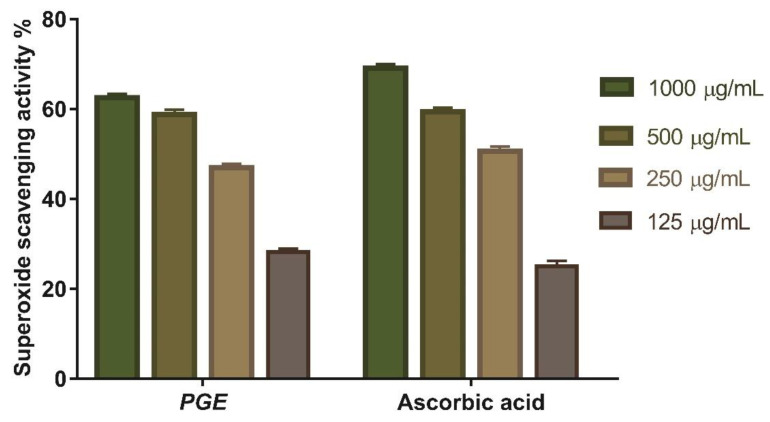
3.4.2. Superoxide Radical Scavenging Activity
The superoxide scavenging (SOD) activity of Psidium guajava seed extract was determined. The scavenging potential of PGE and the standard increase are concentration dependent (Figure 7), with Psidium guajava seed extract showing the maximum superoxide radical scavenging activity. Psidium guajava seed extract had 62.5% superoxide scavenging efficacy at a 1000 μg/mL concentration. The concentration of Psidium guajava seed extract needed for 50% inhibition (IC50) was found to be 147.1 µg/mL compared to (IC50) 158.8 μg/mL for ascorbic acid.
Figure 7.
Superoxide radical scavenging activity of Psidium guajava seed extract at different concentration (1000 µg/mL, 500 µg/mL, 250 µg/mL, and 125 µg/mL). Bars represent mean ± SD (standard deviation). Significant difference between groups is analyzed by a two-way ANOVA test.
4. Conclusions
The present research examined the possible gastroprotective effect of Psidium Guajava seed extract against NSAID-induced gastric ulcers, as well as its antioxidant activity (hydrogen peroxide scavenging activity and superoxide radical scavenging activity). Metabolomic profiling via LC-HRMS-based chemical profiling of Psidium guajava revealed the abundance of many putative active compounds. Psidium guajava seed extracts significantly decreased the ulcer index and protected the mucosa from lesions. The antiulcer effect of Psidium guajava seed extract downregulated the cytokines expression (IL-1β, IL-6, and TNF-α). Moreover, PGE significantly downregulated the increased COX-2, TGF-β, and IGF-1 relative gene expressions, confirming its beneficial effect in ulcer healing. Moreover, the identified compounds were also docked in the active sites of several inflammatory targets and mediators, which include COX-1, COX-2, TNF-α, TGF-β, EGFR, and IGFR. The gastroprotective effect could be potentially attributed to the effect of several sterols such as stigmasterol and campesterol, as suggested by docking studies. Our experiment proved the possible use of Psidium guajava seeds as a protective agent against ulceration, and a possible alternative to antiulcer agents. These results might need further investigation to support this potential effect, but they highlight the mechanism of the gastro protective and antioxidant effect of Psidium guajava seeds.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox11071230/s1, Table S1: Primer sequence of the genes included in the study [35,49,50,51,52].
Author Contributions
Conceptualization: U.R.A., A.A. and N.H.S.; Methodology: N.H.S., A.A., A.M.S. and U.R.A. Data analysis: N.H.S., H.S.A., S.A.M., M.A.E. and U.R.A.; Data validation: S.A.M., H.F.O., F.A.M. and Writing—original draft: N.H.S., A.A., H.S.A., S.A.M., F.A.M. and U.R.A.; Supervision: M.A.E. and U.R.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Extreme care was taken and all procedures on rabbits were adopted according to Deraya laboratory guidelines. The approval code is 9/2021.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was funded by the Deanship of Scientific Research at Umm Al-Qura University by Grant Code: (22UQU4331174DSR13).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Caballero B., Finglas P., Toldrá F. Encyclopedia of Food and Health. Academic Press; Cambridge, MA, USA: 2015. [Google Scholar]
- 2.Angulo-López J.E., Flores-Gallegos A.C., Torres-León C., Ramírez-Guzmán K.N., Martínez G.A., Aguilar C.N. Guava (Psidium guajava L.) Fruit and Valorization of Industrialization By-Products. Processes. 2021;9:1075. doi: 10.3390/pr9061075. [DOI] [Google Scholar]
- 3.Lantzouraki D.Z., Sinanoglou V.J., Tsiaka T., Proestos C., Zoumpoulakis P. Total phenolic content, antioxidant capacity and phytochemical profiling of grape and pomegranate wines. RSC Adv. 2015;5:101683–101692. doi: 10.1039/C5RA20064D. [DOI] [Google Scholar]
- 4.Smith N.J., Williams J.T., Plucknett D.L., Talbot J.P. Tropical Forests and Their Crops. Cornell University Press; Ithaca, NY, USA: 2018. [Google Scholar]
- 5.Gutiérrez R.M.P., Mitchell S., Solis R.V. Psidium guajava: A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2008;117:1–27. doi: 10.1016/j.jep.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 6.Tachakittirungrod S., Ikegami F., Okonogi S. Antioxidant active principles isolated from Psidium guajava grown in Thailand. Sci. Pharm. 2007;75:179–193. doi: 10.3797/scipharm.2007.75.179. [DOI] [Google Scholar]
- 7.Okuda T., Yoshida T., Hatano T., Yazaki K., Ikegami Y., Shingu T. Guavins A, C and D, complex tannins from Psidium guajava. Chem. Pharm. Bull. 1987;35:443–446. doi: 10.1248/cpb.35.443. [DOI] [Google Scholar]
- 8.TANAKA T., ISHIDA N., ISHIMATSU M., NONAKA G.-i., NISHIOKA I. Tannins and related compounds. CXVI. Six new complex tannins, guajavins, psidinins and psiguavin from the bark of Psidium guajava L. Chem. Pharm. Bull. 1992;40:2092–2098. doi: 10.1248/cpb.40.2092. [DOI] [Google Scholar]
- 9.Li Y., Xu J., Li D., Ma H., Mu Y., Huang X., Li L. Guavinoside B from Psidium guajava alleviates acetaminophen-induced liver injury via regulating the Nrf2 and JNK signaling pathways. Food Funct. 2020;11:8297–8308. doi: 10.1039/D0FO01338B. [DOI] [PubMed] [Google Scholar]
- 10.Qaralleh H., Al-Limoun M., Khlaifat A., Khleifat K., Al-Tawarah N., Alsharafa K., Abu-Harirah H. Antibacterial and antibiofilm activities of a traditional herbal formula against respiratory infection causing bacteria. arXiv. 20212102.04301 [Google Scholar]
- 11.Heinrich M. Plants as antidiarrhoeals in medicine and diet; Proceedings of the joint conference of the Society for Economic Botany and the International Society for Ethnopharmacology; London, UK. 1–6 July 1996. [Google Scholar]
- 12.Huang Z., Luo Y., Xia X., Wu A., Wu Z. Bioaccessibility, safety, and antidiabetic effect of phenolic-rich extract from fermented Psidium guajava Linn. leaves. J. Funct. Foods. 2021;86:104723. doi: 10.1016/j.jff.2021.104723. [DOI] [Google Scholar]
- 13.Wang H.-J., Chiang B.-H. Anti-diabetic effect of a traditional Chinese medicine formula. Food Funct. 2012;3:1161–1169. doi: 10.1039/c2fo30139c. [DOI] [PubMed] [Google Scholar]
- 14.Ademiluyi A.O., Oboh G., Ogunsuyi O.B., Oloruntoba F.M. A comparative study on antihypertensive and antioxidant properties of phenolic extracts from fruit and leaf of some guava (Psidium guajava L.) varieties. Comp. Clin. Pathol. 2016;25:363–374. doi: 10.1007/s00580-015-2192-y. [DOI] [Google Scholar]
- 15.Feng X.-h., Wang Z.-h., Meng D.-l., Li X. Cytotoxic and antioxidant constituents from the leaves of Psidium guajava. Bioorg. Med. Chem. Lett. 2015;25:2193–2198. doi: 10.1016/j.bmcl.2015.03.058. [DOI] [PubMed] [Google Scholar]
- 16.Teixeira R.d.O., Camparoto M.L., Mantovani M.S., Vicentini V.E.P. Assessment of two medicinal plants, Psidium guajava L. and Achillea millefolium L., in in vitro and in vivo assays. Genet. Mol. Biol. 2003;26:551–555. doi: 10.1590/S1415-47572003000400021. [DOI] [Google Scholar]
- 17.Vasconcelos A.G., das GN Amorim A., Dos Santos R.C., Souza J.M.T., de Souza L.K.M., de SL Araújo T., Nicolau L.A.D., de Lima Carvalho L., de Aquino P.E.A., da Silva Martins C., et al. Lycopene rich extract from red guava (Psidium guajava L.) displays anti-inflammatory and antioxidant profile by reducing suggestive hallmarks of acute inflammatory response in mice. Food Res. Int. 2017;99:959–968. doi: 10.1016/j.foodres.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Zhu X., Ouyang W., Pan C., Gao Z., Han Y., Song M., Feng K., Xiao H., Cao Y. Identification of a new benzophenone from Psidium guajava L. leaves and its antineoplastic effects on human colon cancer cells. Food Funct. 2019;10:4189–4198. doi: 10.1039/C9FO00569B. [DOI] [PubMed] [Google Scholar]
- 19.Sriwilaijaroen N., Fukumoto S., Kumagai K., Hiramatsu H., Odagiri T., Tashiro M., Suzuki Y. Antiviral effects of Psidium guajava Linn.(guava) tea on the growth of clinical isolated H1N1 viruses: Its role in viral hemagglutination and neuraminidase inhibition. Antivir. Res. 2012;94:139–146. doi: 10.1016/j.antiviral.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Oladele J.O., Ajayi E.I., Oyeleke O.M., Oladele O.T., Olowookere B.D., Adeniyi B.M., Oyewole O.I., Oladiji A.T. A systematic review on COVID-19 pandemic with special emphasis on curative potentials of Nigeria based medicinal plants. Heliyon. 2020;6:e04897. doi: 10.1016/j.heliyon.2020.e04897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prommaban A., Utama-ang N., Chaikitwattana A., Uthaipibull C., Srichairatanakool S. Linoleic acid-rich guava seed oil: Safety and bioactivity. Phytother. Res. 2019;33:2749–2764. doi: 10.1002/ptr.6449. [DOI] [PubMed] [Google Scholar]
- 22.Malacrida C., Jorge N. Fatty acids and some antioxidant compounds of Psidium guajava seed oil. Acta Aliment. 2013;42:371–378. doi: 10.1556/AAlim.2012.0010. [DOI] [Google Scholar]
- 23.Lin H.-C., Lin J.-Y. Characterization of guava (Psidium guajava Linn) seed polysaccharides with an immunomodulatory activity. Int. J. Biol. Macromol. 2020;154:511–520. doi: 10.1016/j.ijbiomac.2020.03.137. [DOI] [PubMed] [Google Scholar]
- 24.Pelegrini P.B., Murad A.M., Silva L.P., Dos Santos R.C., Costa F.T., Tagliari P.D., Bloch Jr C., Noronha E.F., Miller R.N., Franco O.L., et al. Identification of a novel storage glycine-rich peptide from guava (Psidium guajava) seeds with activity against Gram-negative bacteria. Peptides. 2008;29:1271–1279. doi: 10.1016/j.peptides.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Castro-Vargas H.I., Rodríguez-Varela L.I., Ferreira S.R., Parada-Alfonso F. Extraction of phenolic fraction from guava seeds (Psidium guajava L.) using supercritical carbon dioxide and co-solvents. J. Supercrit. Fluids. 2010;51:319–324. doi: 10.1016/j.supflu.2009.10.012. [DOI] [Google Scholar]
- 26.Kumar K.V., Pillai M.S.N., Thusnavis G.R. Seed extract of Psidium guajava as ecofriendly corrosion inhibitor for carbon steel in hydrochloric acid medium. J. Mater. Sci. Technol. 2011;27:1143–1149. doi: 10.1016/S1005-0302(12)60010-3. [DOI] [Google Scholar]
- 27.Osama N., Bakeer W., Raslan M., Soliman H.A., Abdelmohsen U.R., Sebak M. Anti-cancer and antimicrobial potential of five soil Streptomycetes: A metabolomics-based study. R. Soc. Open Sci. 2022;9:211509. doi: 10.1098/rsos.211509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ebada S.S., Al-Jawabri N.A., Youssef F.S., Albohy A., Aldalaien S.e.M., Disi A.M., Proksch P. In vivo antiulcer activity, phytochemical exploration, and molecular modelling of the polyphenolic-rich fraction of Crepis sancta extract. Inflammopharmacology. 2020;28:321–331. doi: 10.1007/s10787-019-00637-x. [DOI] [PubMed] [Google Scholar]
- 29.Trott O., Olson A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deshpande S., Shah G., Parmar N. Antiulcer activity of Tephrosia purpurea in rats. Indian J. Pharmacol. 2003;35:168–172. [Google Scholar]
- 31.El-Dien R.T.M., Maher S.A., Abdelmohsen U.R., AboulMagd A.M., Fouad M.A., Kamel M.S. Antiulcer secondary metabolites from Elaeocarpus grandis, family Elaeocarpaceae, supported by in silico studies. RSC Adv. 2020;10:34788–34799. doi: 10.1039/D0RA06104B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kota B.P., Teoh A.W., Roufogalis B.D. Pharmacology of traditional herbal medicines and their active principles used in the treatment of peptic ulcer, diarrhoea and inflammatory bowel disease. New Adv. Basic Clin. Gastroenterol. 2012;14:297–310. [Google Scholar]
- 33.Khan H.A. Computer-assisted visualization and quantitation of experimental gastric lesions in rats. J. Pharmacol. Toxicol. Methods. 2004;49:89–95. doi: 10.1016/j.vascn.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Dos Santos M.M., Olaleye M.T., Ineu R.P., Boligon A.A., Athayde M.L., Barbosa N.B., Rocha J.B.T. Antioxidant and antiulcer potential of aqueous leaf extract of Kigelia africana against ethanol-induced ulcer in rats. EXCLI J. 2014;13:323. [PMC free article] [PubMed] [Google Scholar]
- 35.Musa A., Shady N.H., Ahmed S.R., Alnusaire T.S., Sayed A.M., Alowaiesh B.F., Sabouni I., Al-Sanea M.M., Mostafa E.M., Youssif K.A., et al. Antiulcer Potential of Olea europea L. cv. Arbequina Leaf Extract Supported by Metabolic Profiling and Molecular Docking. Antioxidants. 2021;10:644. doi: 10.3390/antiox10050644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Howiriny T., Alsheikh A., Alqasoumi S., Al-Yahya M., ElTahir K., Rafatullah S. Gastric antiulcer, antisecretory and cytoprotective properties of celery (Apium graveolens) in rats. Pharm. Biol. 2010;48:786–793. doi: 10.3109/13880200903280026. [DOI] [PubMed] [Google Scholar]
- 37.Üçüncüoğlu D., Sivri-Özay D. Geographical origin impact on volatile composition and some quality parameters of virgin olive oils extracted from the “Ayvalık” variety. Heliyon. 2020;6:e04919. doi: 10.1016/j.heliyon.2020.e04919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Secondini O., Secondini O. Handbook of Perfumes and Flavors. Chemical Publishing Company; Revere, MA, USA: 1990. [Google Scholar]
- 39.Narváez-Cuenca C.-E., Inampues-Charfuelan M.-L., Hurtado-Benavides A.-M., Parada-Alfonso F., Vincken J.-P. The phenolic compounds, tocopherols, and phytosterols in the edible oil of guava (Psidium guava) seeds obtained by supercritical CO2 extraction. J. Food Compos. Anal. 2020;89:103467. doi: 10.1016/j.jfca.2020.103467. [DOI] [Google Scholar]
- 40.Molla T. Ph.D. Thesis. Addis Ababa University; Addis Ababa, Ethiopia: 2011. A Systemic Review on Antioxidant and Hepatoprotective Effect of Psidium Guajava Leaf and Fruit Extract. [Google Scholar]
- 41.Zahran E.M., Abdelmohsen U.R., Ayoub A.T., Salem M.A., Khalil H.E., Desoukey S.Y., Fouad M.A., Kamel M.S. Metabolic profiling, histopathological anti-ulcer study, molecular docking and molecular dynamics of ursolic acid isolated from Ocimum forskolei Benth. (family Lamiaceae) S. Afr. J. Bot. 2020;131:311–319. doi: 10.1016/j.sajb.2020.03.004. [DOI] [Google Scholar]
- 42.Zahran E.M., Abdelmohsen U.R., Hussein A.S., Salem M.A., Khalil H.E., Yehia Desoukey S., Kamel M.S. Antiulcer potential and molecular docking of flavonoids from Ocimum forskolei Benth.; family Lamiaceae. Nat. Prod. Res. 2021;35:1933–1937. doi: 10.1080/14786419.2019.1645662. [DOI] [PubMed] [Google Scholar]
- 43.Srikanta B. Ph.D. Thesis. University of Mysore; Mysore, India: 2010. Mechanism of Action of Multi–Potent Ulcer Blockers in In Vitro and In Vivo Models. [Google Scholar]
- 44.Choi S.S., Lee H.J., Lim I., Satoh J.-i., Kim S.U. Human astrocytes: Secretome profiles of cytokines and chemokines. PLoS ONE. 2014;9:e92325. doi: 10.1371/journal.pone.0092325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Playford R.J., Macdonald C.E., Johnson W.S. Colostrum and milk-derived peptide growth factors for the treatment of gastrointestinal disorders. Am. J. Clin. Nutr. 2000;72:5–14. doi: 10.1093/ajcn/72.1.5. [DOI] [PubMed] [Google Scholar]
- 46.Magierowska K., Bakalarz D., Wójcik D., Chmura A., Hubalewska-Mazgaj M., Licholai S., Korbut E., Kwiecien S., Sliwowski Z., Ginter G. Time-dependent course of gastric ulcer healing and molecular markers profile modulated by increased gastric mucosal content of carbon monoxide released from its pharmacological donor. Biochem. Pharmacol. 2019;163:71–83. doi: 10.1016/j.bcp.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y., Alexander P.B., Wang X.-F. TGF-β family signaling in the control of cell proliferation and survival. Old Spring Harb. Perspect. Biol. 2017;9:a022145. doi: 10.1101/cshperspect.a022145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kato S., Tanaka A., Ogawa Y., Kanatsu K., Seto K., Yoneda T., Takeuchi K., Takeuchi K.J.M.S.M. Effect of polaprezinc on impaired healing of chronic gastric ulcers in adjuvant-induced arthritic rats-role of insulin-like growth factors (IGF)-1. Med. Sci. Monit. 2001;7:20–25. [PubMed] [Google Scholar]
- 49.Hassan H., Abdel-Aziz A.J.F., Toxicology C. Evaluation of free radical-scavenging and anti-oxidant properties of black berry against fluoride toxicity in rats. Food Chem. Toxicol. 2010;48:1999–2004. doi: 10.1016/j.fct.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 50.Shady N.H., Soltane R., Maher S.A., Saber E.A., Elrehany M.A., Mostafa Y.A., Sayed A.M., Abdelmohsen U.R. Wound Healing and Antioxidant Capabilities of Zizyphus mauritiana Fruits: In-Vitro, In-Vivo, and Molecular Modeling Study. Plants. 2022;11:1392. doi: 10.3390/plants11111392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sonboli A., Mojarrad M., Ebrahimi S.N., Enayat S.J.I.J.o.P.R.I. Free radical scavenging activity and total phenolic content of methanolic extracts from male inflorescence of Salix aegyptiaca grown in Iran. Iran. J. Pharm. Res. 2010;9:293. [PMC free article] [PubMed] [Google Scholar]
- 52.Alsenani F., Ashour A.M., Alzubaidi M.A., Azmy A.F., Hetta M.H., Abu-Baih D.H., Elrehany M.A., Zayed A., Sayed A.M., Abdelmohsen U.R., et al. Wound Healing Metabolites from Peters’ Elephant-Nose Fish Oil: An In Vivo Investigation Supported by In Vitro and In Silico Studies. Mar. Drugs. 2021;19:605. doi: 10.3390/md19110605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article and supplementary material.