Abstract
Inflammasomes are intracellular signaling complexes of the innate immune system, which is part of the response to exogenous pathogens or physiological aberration. The multiprotein complexes mainly consist of sensor proteins, adaptors, and pro-caspase-1. The assembly of the inflammasome upon extracellular and intracellular cues drives the activation of caspase-1, which processes pro-inflammatory cytokines IL-1β and IL-18 to maturation and gasdermin-D for pore formation, leading to pyroptosis and cytokine release. Inflammasome signaling functions in numerous infectious or sterile inflammatory diseases, including inherited autoinflammatory diseases, metabolic disorders, cardiovascular diseases, cancers, neurodegenerative disorders, and COVID-19. In this review, we summarized current ideas on the organization and activation of inflammasomes, with details on the molecular mechanisms, regulations, and interventions. The recent developments of pharmacological strategies targeting inflammasomes as disease therapeutics were also covered.
Keywords: inflammasome, NLRP3, AIM2, caspase, IL-1β, GSDMD, inflammation, targeting, disease, inhibitor
1. Introduction
Innate immunity provides the most rapid and conserved defense against cellular damage caused by pathogenic infections, injuries, and cellular stresses. This response depends on the monitoring of pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) through sensors called pattern recognition receptors (PRRs), located in both immune cells and non-immune cells [1,2]. Distinct families of PRRs have been identified, including Toll-like receptors (TLRs) [3], NOD-like receptors (NLRs) [4], C-type lectin receptors (CLRs) [5], absent in melanoma 2 (AIM2)-like receptors (ALRs) [6], retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs) [7], cyclic GMP-AMP synthase (cGAS) [8], and pyrin [9]. Among them, most NLRs and ALRs can form a large protein complex called an inflammasome, which serves as a platform for caspase-1 activation, pro-interleukin-1β (IL-1β) and pro-IL-18 maturation, as well as gasdermin D (GSDMD)-mediated pore formation, or pyroptotic cell death [10,11,12,13]. This review summarizes the recent advances in understanding the mechanisms of inflammasome organization and activation, and the pharmacological approaches that target the inflammasome for therapeutic purposes.
2. Discovery and Overview of Inflammasomes
The concept of the inflammasome was first defined by Dr. Jurg Tschopp in 2002 [14], upon identifying a caspase-activation complex consisting of caspase-1, caspase-5, Pycard/ASC, and NALP1 (now known as NLRP1, the nucleotide-binding oligomerization (NOD)−, leucine-rich repeat (LRR)−, and pyrin domain-containing 1), which is responsible for IL-1β maturation. Two years later, Dr. Jurg Tschopp and co-workers discovered the NLRP3 inflammasome and highlighted its essential role in autoinflammatory disorders [15]. Currently, the well-defined inflammasome sensors include the NLR family members NLRP1, NLRP3, and NLRC4 (the nucleotide-binding oligomerization (NOD)−, leucine-rich repeat (LRR)−, and CARD-containing 4), as well as AIM2, and pyrin. The inflammasomes organized by these five sensors can directly engage and activate caspase-1, thus, termed canonical inflammasomes [16]. The canonical inflammasome sensor proteins are widely expressed in many tissues, especially elevated in the bone marrow and lymphoid tissues, and enriched in immune cells such as monocytes, macrophages and neutrophils. Moreover, the NLRP3 inflammasome is reported to be activated in a wide range of endothelial, epithelial, and mesenchymal cells, thus, related to various inflammatory diseases in different organs, such as the skin, brain, heart, and liver [17]. Additionally, the NLRP1 is regarded as the principal inflammasome sensor in human keratinocytes and skin [18]. A variety of inflammasome sensors were identified thereafter, such as CARD8 [19], NLRP2 [20,21,22,23], NLRP6 [24], NLRP7 [25], NLRP12 [26], and interferon gamma-inducible protein 16 (IFI16) [27,28,29], whose mechanisms for inflammasome activation need further exploration.
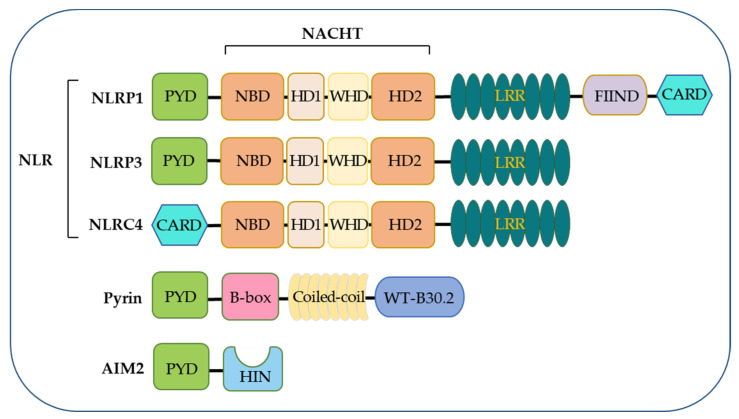
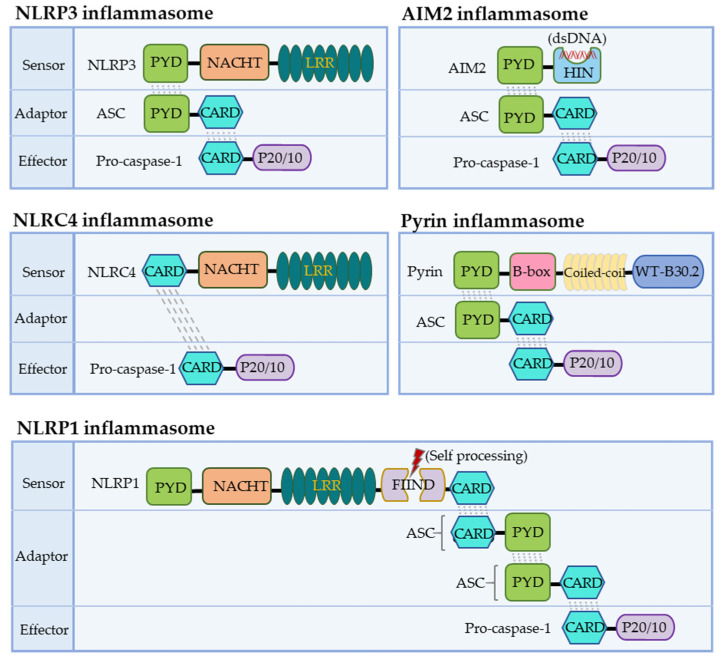
NLRs function as the primary sensors for inflammasome formation. Structurally, these family members are composed of an N-terminal pyrin domain (PYD), a central nucleotide-binding and oligomerization domain (NACHT), and C-terminal leucine-rich repeats (LRRs). While the NACHT and LRR domains are present in all NLR proteins (except NLRP10), the PYD determining the interaction partners is the most variable (Figure 1). Upon activation, NLR recruits an adaptor protein ASC (apoptosis-associated speck-like protein containing a CARD) through its PYD domain, leading to NLR oligomerization [30]. ASC comprises an N-terminal PYD and a C-terminal CARD domain, which keeps an autoinhibited conformation at the resting state. This pyrin–pyrin interaction allows ASC to release its C-terminal CARD domain, thus, bridging the sensor protein to pro-caspase-1 to assemble a canonical inflammasome via a CARD–CARD interaction [31,32]. Most inflammasomes use ASC to initiate their assembly, while in some particular cases, pro-caspase-1 can be recruited by directly interacting with NLRs through the CARD domain. Pro-caspase-1 is an inactive precursor protein in the resting state. Upon inflammasome assembly, this precursor engages the platform through a CARD–CARD interaction, and the high local concentration of pro-caspase-1 drives its heterodimerization, self-cleavage, and activation [16,33]. Activated caspase-1 converts pro-IL-1β and pro-IL-18 to their mature forms, unleashed into the extracellular and surrounding environment through GSDMD-formed transmembrane pores. These pro-inflammatory cytokines then trigger the activation of NF-κB signaling via transmembrane receptors IL-1R and IL-18R to induce or promote inflammation, locally or distantly [34,35]. The domain organization of NLRP3 and other inflammasomes is illustrated in Figure 2.
Figure 1.
Domain architecture of canonical inflammasome sensors. Overview and alignment of the distinct domains for sensors that initiate canonical inflammasome signaling.
Figure 2.
Assembly of canonical inflammasomes. An organization summary for the assembly of canonical inflammasomes, comprising sensors, adaptors/ASC, and effectors/caspase-1.
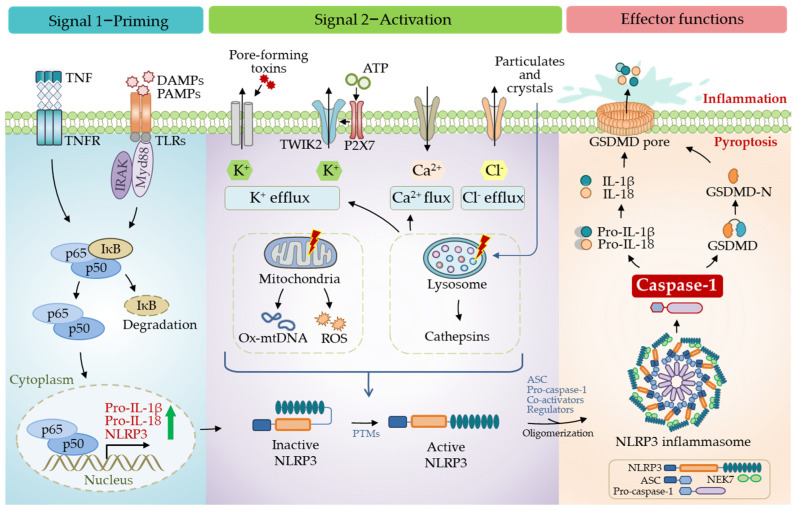
Another critical and direct substrate of caspase-1 is GSDMD, the primary executor of pyroptosis. Caspase-1 processes cytosolic GSDMD to release its N-terminal domain from an autoinhibited conformation [36,37], a high affinity for the plasma membrane, where they insert and form a pore structure on the membrane [38,39]. This particular structure allows the release of IL-1β and IL-18 into the extracellular space [34,35]. Besides releasing pro-inflammatory cytokines and other contents, plasma membrane pores also cause pathological ion fluxes, ultimately resulting in cell death, known as pyroptosis (Figure 3). A recent study revealed that gasdermin pores are not permanently open, but display phosphoinositide-dependent dynamics, thus, allowing the regulation of cellular pyroptosis [40]. Unlike the canonical inflammasomes that employ caspase-1 as an effector, non-canonical inflammasomes activate caspase-11 in mice and caspase-4/5 in humans [41,42].
Figure 3.
Activation of the NLRP3 inflammasome. Activation of the NLRP3 inflammasome undergoes two sequential steps. The priming process (signal 1) includes the recognition of PAMPs/DAMPs through TLRs and/or sensing of TNFα through TNFR, which induces the expression of NLRP3, pro-IL-1β, and pro-IL-18 proteins via NF-κB signaling. The activation process requires a signal (signal 2) that multiple pathogenic and sterile inflammatory cues can provide. Oligomerization and activation of NLRP3 inflammasome lead to the processing of pro-caspase-1 into the mature form, which in turn processes pro-IL-1β, pro-IL-18, and GSDMD. The released N-terminal domain of GSDMD enters the membrane, forming a pore structure for releasing matured IL-1β and IL-18 into the extracellular space, inducing pyroptosis and causing inflammation.
This review summarized the recent advances in understanding the organization and activation mechanisms of the inflammasomes, the diseases relevant to their dysfunctions, and the natural and designed inhibitors targeting the inflammasome signaling for a therapeutic purpose.
3. Organization of the Canonical Inflammasomes
3.1. The NLRP1 Inflammasome
The NLRP1 inflammasome was the first one identified, initially described as a caspase-activating complex comprising caspase-1/5, Pycard/ASC, and NALP1 [14]. Human NLRP1 consists of a PYD, a NOD, a short leucine-rich repeat domain (LRR), a function-to-find domain (FIIND), and a CARD domain (Figure 1). The FIIND domain undergoes a self-processing event before NLRP1 responds to diverse stimuli, a process considered necessary for the complete maturation of NLRP1 [43,44]. Upon activation, human NLRP1 associates with ASC via its C-terminal CARD domain rather than its N-terminal PYD domain (Figure 2). Although NLRP1 contains a CARD domain, the CARD domain of ASC is required and adequate for recruiting pro-caspase-1 via the CARD–CARD interaction [43,44]. Of note, the PYD domain is missing in mouse NLRP1, and the function of human PYD is still elusive. Dipeptidyl peptidases 8 and 9 (DPP8/DPP9) are known as critical inhibitors of NLRP1 [45,46,47], which interact with NLRP1 and, via an unclear mechanism, prevent the proteasomal degradation of its N-terminal fragment [46,48,49]. A recent cryo-EM analysis of the NLRP1–DPP9 complex revealed that the C-terminus of NLRP1 inserts into the tunnel of the DPP9 dimer, thus, advancing the mechanism insight of NLRP1 inflammasome activation [50,51]. Several stimuli have been reported to activate NLRP1 inflammasomes in a protease-dependent manner. Muramyl dipeptide (MDP), a peptidoglycan fragment from both Gram-positive and negative bacteria, directly binds to human NLRP1 and triggers a structural change that promotes NLRP1 oligomerization and assembly [52]. 3C proteases from enteroviruses, such as human rhinovirus (HRV), can cleave human NLRP1 between the E130 and G131 residues to relieve its C-terminal fragment for inflammasome assembly [53]. ORF45 from Kaposi’s sarcoma-associated herpesvirus (KSHV) also facilitates the protease-independent activation of the hNLRP1 inflammasome, which was conserved in primates instead of rodents [54]. Three paralogues of human NLRP1 are found in mice, including NLRP1a, NLRP1b, and NLRP1c. The Bacillus anthracis lethal toxin [55], or a reduction in cellular ATP production, results in NLRP1b-dependent inflammasome formation in mice [56]. In contrast to a well-defined NLRP3, the study of NLRP1 inflammasomes is preliminary, both in the activating mechanism and biological relevance.
3.2. The NLRP3 Inflammasome
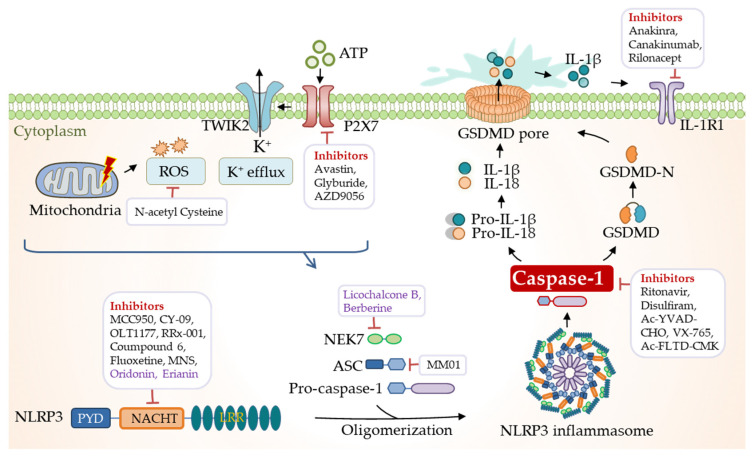
The NLRP3 inflammasome is the most extensively studied. Human NLRP3 comprises a PYD, a NACHT, and an LRR domain [57]. The NACHT domain, comprising of the nucleotide-binding domain (NBD), helical domains HD1 and HD2, and a middle-winged helix domain (WHD) between HD1 and HD2, is vital for NLRP3 self-association and function (Figure 1). Under resting conditions, the LRR domain folds back onto the NACHT domain and keeps NLRP3 in an ADP-bound, auto-repressed conformation [58]. Once activated, NLRP3 directly binds to mitotic Ser/Thr kinase NEK7 (NIMA-related kinase 7), a recently identified co-activator of NLRP3 [57], and undergoes a conformational transition by hydrolyzing ATP into ADP [59,60]. ATP hydrolysis further triggers the activation of NLRP3, allowing the assembly and recruitment of adaptor protein ASC and pro-caspase-1, thus, enabling caspase-1 self-cleavage and activation (Figure 2 and Figure 3). The assembly of the NLRP3 inflammasome is indicated to occur in mitochondria [61,62,63], as well as in the dispersed trans-Golgi network (dTGN) [64]. Moreover, it has newly been identified to occur at the microtubule-organizing center (MTOC) in an HDAC6-dependent manner, and a similar mechanism has also been observed during pyrin inflammasome activation [65]. The assembly of non-canonical NLRP3 inflammasomes is initiated by caspase-11 in mice, and caspase-4/5 in humans, in response to intracellular LPS, which induces pyroptosis instead of pro-IL-1β and pro-IL-18 maturation. The K+ efflux caused by pyroptosis can further trigger the assembly of the NLRP3 inflammasome and promote NLRP3-caspase-1-dependent cytokine maturation [42,66,67]. The NLRP3 inflammasome responds to a surprisingly diverse set of stimuli, the cellular mechanism of which is discussed below (in Section 4. Cellular Mechanisms Driving NLRP3 Inflammasome Activation).
3.3. The NLRC4 Inflammasome
NLRC4 was initially identified as a pro-apoptotic protein named the ICE-protease-activating factor (IPAF) based on its similarity to apoptotic protease-activating factor 1 (APAF1) [68]. Besides the NACHT domain and C-terminal LRRs, NLRC4 contains an additional N-terminal CARD domain, enabling the direct recruitment of pro-caspases-1 via a CARD–CARD interaction (Figure 1 and Figure 2). The NLRC4 inflammasome responds to bacterial flagellin and components of the bacterial type III secretory system (T3SS), a process requiring the NLR family of apoptosis inhibitory proteins (NAIPs) as direct upstream receptors [69]. A variety of NAIPs in mice can initiate NLRC4 inflammasome assembly, including NAIP1, NAIP2, and NAIP5/6, which recognize the needle proteins, rod proteins, and flagellin, respectively. However, only one NAIP in humans detects all components [70,71,72]. NLRC4 keeps an auto-inhibited conformation achieved through the inter-domain interaction that covers the nucleotide-binding site [73]. Upon an NAIP recognizing this specific bacterial ligand, the interaction between NAIP and the NACHT domain of NLRC4 triggers the assembly of the NLRC4 inflammasome, followed by the maturation of caspase-1 and the release of inflammatory cytokines and pyroptosis [74,75]. Notably, a NAIP-independent non-canonical NLRC4 inflammasome has recently been identified [76].
3.4. The AIM2 Inflammasome
Nucleic acids are predominant PAMPs and ubiquitous in living organisms. In addition to cytosolic DNA sensors such as cGAS and the critical roles of cGAS-STING signaling in cellular physiology [77,78,79,80], AIM2, thus far, is the sole sensor that detects cytosolic double-stranded DNA (dsDNA) among canonical inflammasome sensors [81]. Several independent groups identified AIM2 as a DNA-binding protein while searching for new DNA sensors, and found that it can activate caspase-1 and caspase-3 [82]. The interaction between cytosolic DNA and AIM2 triggers the oligomerization and, ultimately, the formation of the AIM2 inflammasome [82,83]. AIM2 belongs to the AIM2-like receptors (ALRs), structurally defined by two main domains, the HIN domain and the pyrin domain (PYD) (Figure 1). In detecting cytosolic dsDNA, the HIN domain directly binds to dsDNA, thus, relieving the auto-inhibited conformation and releasing the PYD domain, allowing for the recruitment of the adaptor protein ASC and the subsequent AIM2 oligomerization around the DNA molecule. ASC bridges AIM2 to pro-caspase-1 via the CARD–CARD interaction and, in turn, activates caspase-1 and the cleavage of IL-1β and IL-18 (Figure 2). AIM2 is crucial for protecting the host from DNA viruses and other pathogens and the aberrant accumulation of self-DNA in the cytosol. Due to its ability to recognize host DNA, AIM2 involves the pathogenesis of systemic lupus erythematosus (SLE) and tumorigenesis [84]. Intriguingly, recent reports revealed a role of the AIM2 inflammasome in normal brain development through the surveillance of DNA damage during neurodevelopment [85].
3.5. The Pyrin Inflammasome
Pyrin (also known as marenostrin and TRIM20) is encoded by the gene MEFV. In 1997, several groups disclosed that the mutations of the MEFV gene in humans are responsible for the monogenic autoinflammatory disease familial Mediterranean fever (FMF) [86]. Later, it proved that the mutations on the C-terminal of human pyrin led to the constitutive activation of the pyrin inflammasome and subsequent caspase-1 activation and IL-1β release [87,88]. Pyrin contains PYD, B-box, and coiled-coil domains (Figure 1). While the PYD domain binds to the ASC through the PYD–PYD interaction, its CARD–CARD interaction with pro-caspase-1 is critical for the recruitment and maturation of caspase-1. The B-box and coiled-coil domain may be involved in the oligomerization of pyrin and modulation of its activation (Figure 2). Unlike mouse pyrin, human pyrin has an additional C-terminal B30.2 domain (also known as a SPRY/PRY), which contains the highest frequency of mutations that lead to FMF. The pyrin inflammasome responds to bacterial toxins and is modified by Rho GTPases. For instance, Clostridium difficile TcdB, a major virulence factor of Clostridium difficile, covalently modifies RhoA and inactivates these GTPases, which triggers the assembly of pyrin inflammasomes in an ASC-dependent manner [89]. However, pyrin does not directly detect modified RhoA, although many bacterial toxins trigger the pyrin inflammasome through the modification of RhoA [89,90]. Nevertheless, the mechanism by which the pyrin inflammasome is triggered needs further investigation.
4. Cellular Mechanisms Driving NLRP3 Inflammasome Activation
4.1. The Canonical Pathway
4.1.1. The Role of Priming
Inflammasome activation is generally considered a two-step process, a priming step (signal one) and an activation step (signal two). Initially, the priming step is characterized by the transcriptional induction of NLRP3, pro-IL-1β, and pro-IL-18 through an inflammatory stimulus, including TLR ligands and cytokines such as TNFα [91]. The enhanced expression of these effectors is thought to ensure a rapid and appropriate activation of inflammasomes toward signal two (Figure 3). For instance, LPS is a commonly used ligand to induce the transcription and expression of pro-IL-1β, pro-IL-18, and NLRP3 by activating the transcription factor NF-κB [91]. However, subsequent studies indicated that transcription is dispensable in priming, supported by the time-course observations between the activation of the NLRP3 inflammasome and the upregulation of NLRP3 expression [92,93]. Moreover, although downstream TLR signaling is essential for the rapid NLRP3 activation, this process appears to be independent of the transcriptional function of TLR signaling, but rather depends on adaptor protein MyD88 and the IL-1 receptor-associated kinases IRAK-1 and IRAK-4 [94,95]. Further studies also suggest that post-translational modifications (PTMs) of NLRP3 in the priming process play an essential role in NLRP3 inflammasome assembly, which is discussed later. Collectively, the priming process mainly functions for two purposes: to upregulate the inflammasome components that are ready for a sufficient response to signal two and to order post-translational modifications (PTMs) of NLRP3, which are essential for NLRP3 activation.
4.1.2. Activation of NLRP3 Inflammasome
Multi-faceted pathogenic and sterile inflammatory signals can activate NLRP3, such as foreign PAMPs from fungi, bacteria, and viruses, and host-derived molecules such as reactive oxygen species (ROS), extracellular ATP, and crystalline and particulate matters (uric acid crystals, silica, asbestos, alum, etc.). Additionally, perturbing intracellular homeostasis, including lysosomal destabilization, mitochondrial dysfunction, and ion fluxes (K+ efflux, Ca2+ signaling, Na+ influx, and Cl− efflux), can also activate the NLRP3 inflammasome [96] (Figure 3). The reasons why the NLRP3 inflammasome can respond to such a surprising variety of stimuli are still less known, and, recently, an intriguing report suggests a role of membrane domain disturbance [64].
Ion Fluxes
The K+ efflux is a joint event during NLRP3 activation, with few exceptions. A decrease in intracellular K+ can be induced by ATP and other DAMPs [97,98]. For instance, the purinergic P2X7 receptor (P2X7R) senses the accumulation of extracellular ATP and cooperates with tandem pore domains in weak inward rectifying K+ channel 2 (TWIK2), leading to a K+ efflux and subsequent NLRP3 activation [99]. Nigericin, a K+/H+ ionophore, is commonly used as an NLRP3 inflammasome agonist via the K+ efflux [97]. Notably, a low extracellular concentration of K+ is sufficient to activate the NLRP3 inflammasome, while the high extracellular concentration prevents it [100,101]. A plethora of stimuli, such as LPS, toxins, and particulate matter induce the K+ efflux to trigger the NLRP3 inflammasome activation [101]. Despite the vital role of the K+ efflux in NLRP3 activation, the exact nature underlying it is still not fully understood.
Calcium-sensing receptor (CaSR) signaling is able to promote NLRP3 inflammasome activation by decreasing the intracellular cyclic AMP (cAMP) level [102,103], as exemplified by platelets that trigger CaSRs signaling and boost NLRP3 inflammasome activation [104]. Additionally, the formation of fetuin-A-based calcium and phosphate-containing matter that functions to prevent extraosseous calcification in vivo increases extracellular Ca2+, thus, inducing CaSRs signaling and leading to NLRP3 inflammasome activation in rheumatoid arthritis [105]. ADP released from injured colonic tissue can also activate the NLRP3 inflammasome through P2Y1 receptor-mediated calcium signaling, thereby regulating inflammatory bowel disease [106].
Distinct opinions regarding how the Cl− efflux facilitates NLRP3 inflammasome activation have been presented. While some studies indicated that the Cl− efflux is responsible for the upstream signaling for NLRP3 activation, some groups showed that the Cl− efflux is required for ASC oligomerization [107,108]. The decreased extracellular Cl− level has previously been shown to promote IL-1β secretion [109]. Later, it was found that inflammasome activation led to the translocation of the chloride intracellular channel 1 (CLIC1) and CLIC4 to the plasma membrane, where they mediated the Cl− efflux [107].
Additionally, ion fluxes have frequently been found coordinated in NLRP3 activation. For instance, ATP activates P2X7R, which promotes the Ca2+ and Na+ influxes and mediates the K+ efflux through TWIK2 [99]. The Ca2+ flux, regulated by the K+ efflux, is presumed to be critical in NLRP3 inflammasome activation [110], although other controversial reports have been presented [111,112]. Therefore, the precise crosstalk among ion fluxes that regulate inflammasome formation remains to be elucidated.
Organelle Damage
Studies have shown that particulate stimuli such as alum and cholesterol crystals activate the NLRP3 inflammasome by causing lysosomal rupture and the release of the particulates into the cytoplasm. This notion is supported by dipeptide Leu-Leu-OMe, a soluble lysosomotropic agent, which is sufficient to activate the NLRP3 inflammasome [113]. Similarly, beta-2-microglobulin (beta2m) accumulation in the lysosomes of myeloma-associated macrophages (MAMs) resulted in its disruption, which acted as a driver to initiate NLRP3 inflammation [114]. Additionally, nicotine and carbon-based nanomaterials, such as multi-walled carbon nanotubes (MWCNT), have enhanced NLRP3 inflammasome activation by inducing the lysosomal membrane permeability and releasing cathepsin B [115,116]. Intriguingly, a recent study suggested the direct interaction between cathepsin B and NLRP3 at the endoplasmic reticulum (ER) that facilitates caspase-1 activation [117]. Lysosomal damage is typically accompanied by the K+ efflux and Ca2+ influx [118], emphasizing its critical role in inflammasome modulation. Lysophosphatidylcholine (LPC), a major lipid component of the plasma membrane, was shown to activate the NLRP3 inflammasome through lysosomal damage and the K+ efflux in human monocytes [119].
Additionally, NLRP3 stimuli can promote the disassembly of the trans-Golgi network into the dTGN, which serves as a scaffold for NLRP3 aggregation and activation [64]. Another study also suggested that NLRP3 is translocated to the Golgi apparatus adjacent to a mitochondrial cluster in a complex with sterol regulatory element-binding protein 2 (SREBP2) and SREBP cleavage activation protein (SCAP) to reach optimal assembly and activation [120]. Nevertheless, most current evidence relies heavily on pharmacological approaches that induce ion fluxes and organelle damage simultaneously. Therefore, the exact nature of these events contributing to NLRP3 inflammasome activation needs further investigation.
Mitochondria
Besides lysosomes, mitochondria are considered central organelles in regulating the NLRP3 inflammasome activation. Mitochondria can serve as the docking platform for the NLRP3 inflammasome assembly. Upon activation, NLRP3 translocates from the cytosol and ER to the mitochondria and mitochondria-associated membrane, and associates with the adaptor protein ASC [61,62,63]. Several mitochondrial proteins, including cardiolipin, mitofusin 2 (MFN2) [121], mitochondrial uncoupling protein 1 (UCP1) [122], and the adaptor protein mitochondrial anti-viral signaling protein (MAVS), have been reported to interact with NLRP3 and regulate its activation [63].
Small molecules, such as imiquimod and CL097, can drive NLRP3 inflammasome activation by targeting mitochondria and inducing mtROS production [112]. mtROS, released by mitochondria dysfunction, functions as the key upstream signaling in NLRP3 activation. Notably, mtROS-contributed inflammation participates considerably in the progression of Parkinson’s disease [123], cardiovascular risk [124], and human respiratory syncytial virus (RSV) infection [125]. In addition to mtROS, cytosolic mtDNA also mediates NLRP3 activation. A variety of NLRP3 activators can induce mtDNA release, and the synthesis of oxidized mtDNA, following TLR signaling, triggers NLRP3 inflammasome assembly [126]. Moreover, oxidized mtDNA also directly interacts with NLRP3 and functions as a critical component of the NLRP3 inflammasome [127]. During severe fever upon thrombocytopenia syndrome virus (SFTSV) infection, mtDNA is released into the cytosol through BAK/BAX signaling, which activates the NLRP3 inflammasome [128]. Moreover, the mitochondrial electron transport chain (ETC) is essential for NLRP3 inflammasome activation through the phosphocreatine-dependent generation of ATP [129].
Co-Activators of NLRP3
Several co-activators of NLRP3 have been identified, including dsDNA-binding protein PKR [130], guanylate-binding protein GBP5 [131], receptor for activated C kinase RACK1 [132], Bruton tyrosine kinase BTK [133,134,135], actin-bundling protein L-plastin [136], stress granule protein DDX3X [137], NLRP11 [138], and NIMA-related kinase NEK7 [139,140,141]. Among them, NEK7 is an essential component of the NLRP3 inflammasome, but not NLRC4 or AIM2 inflammasomes, and the assembly of NEK7 and NLRP3 requires the K+ efflux [139]. Recently, the cryo-EM structure of NLRP3-NEK7 was revealed, elucidating an intriguing role of NEK7 by bridging adjacent NLRP3 proteins through the LRR domains of NLRP3, functioning as a licensing step for NLRP3 inflammasome assembly [57]. However, the binding of NEK7 on NLRP3 itself is insufficient to activate the NLRP3 inflammasome. The key factor, which integrates all the signals and directly drives NLRP3 activation, has not been uncovered.
4.2. Post-Translational Modifications of NLRP3
4.2.1. Ubiquitination
Emerging evidence in recent years has suggested a critical role of NLRP3 PTMs in inflammasome priming and activation. Ubiquitination-mediated protein degradation controls NLRP3 inflammasome activation by regulating the availability of NLRP3. The lifespan of NLRP3 is coordinated by F-box/LRR-repeat protein 2 (FBXL2) and F-box-only protein (FBXO3) [142]. FBXL2 ubiquitinates NLRP3 and marks its proteasomal degradation, while FBXO3 targets and degrades FBXL2, thus, stabilizing NLRP3 proteins. Besides FBXL2, several other E3 ligases have been identified to mediate the degradation of NLRP3, including tripartite motif-containing protein 31 (TRIM31) [143], Ariadne homolog 2 (ARIH2) [144], casitas-B-lineage lymphoma protein-b (Cbl-b) [145], and membrane-associated RING finger protein 7 (MARCH7) [146]. On the other hand, E3 ligase Cullin1 inactivates NLRP3 through an unknown mechanism, without mediating its degradation [147].
Intriguingly, ubiquitination has a dual role in NLRP3 inflammation activation. The deubiquitination of NLRP3 contributes to inflammasome activation. For instance, the BRCA2-containing complex subunit 3 (BRCC3) deubiquitinase complex interacts with NLRP3 and removes the K63-linked ubiquitin chain from the LRR domain of NLRP3, allowing its oligomerization and activation [148]. By contrast, the inhibition of deubiquitinating enzymes (DUBs), such as ubiquitin-specific peptidase 7 (USP7) and USP47, can completely block NLRP3 activation [149]. Additionally, the stimulator of interferon genes (STING), which acts as an essential adaptor in the DNA-sensing pathway, can promote the localization of NLRP3 in the ER and attenuate its K48- and K63-linked polyubiquitination, thereby enhancing inflammasome activation [150]. By contrast, the E3 ligase Pellino2 and RNF125 [145] positively regulate NLRP3 activation by facilitating the K63-linked ubiquitination of NLRP3 during the LPS-induced priming process. The HUWE1-mediated K27-linked polyubiquitination of NLRP3, NLRC4, and AIM2 promotes their inflammasome assembly [151]. The diverse effects of ubiquitination on the NLRP3 inflammasome probably depend on the modification motifs and cellular localization of NLRP3 and are context-dependent.
4.2.2. Phosphorylation and Other Modifications
Phosphorylation events control NLRP3 inflammasome assembly and activation, as exemplified by several cases. During the priming, c-Jun N-terminal kinase 1 (JNK1) directly phosphorylates human NLRP3 at Ser198 (mouse Ser194), which is critical for NLRP3 inflammasome assembly and function [152]. The phosphorylation of Thr659 by p21-activated kinases 1 and 2 (Pak1/2) during bacteremia is necessary for the NLRP3–Nek7 interaction, inflammasome activation, and IL-1β maturation [153]. The BTK-mediated tyrosine phosphorylation of the NLRP3 PYD-NACHT linker region promotes inflammasome assembly and IL-1β maturation [154]. Ser295 in human NLRP3 (mouse Ser 293), phosphorylated by protein kinase D (PKD), is essential for inflammasome assembly [155]. However, another group reported that protein kinase A (PKA) could directly phosphorylate the same residue that inhibited the ATPase function of the NACHT domain and, thus, dampened the oligomerization of NLRP3 [156].
Phosphorylation also plays a negative role in regulating NLRP3 activation. For instance, the phosphorylation of human NLRP3 at Ser5 (mouse Ser3) by AKT inhibits NLRP3 inflammasome activation by disrupting the PYD–PYD interaction [157], while protein phosphatase 2A (PP2A) dephosphorylates it [158]. TANK-binding kinase 1 (TBK1) and its homolog, I-kappa-B kinase epsilon (IKKɛ), can also switch off NLRP3 inflammasome activation, although the exact residue(s) modified by these two kinases remains undetermined [159]. Apart from Ser/Thr phosphorylation, the tyrosine phosphorylation of NLRP3 at Tyr918 by Lyn suppresses NLRP3 inflammasome activity [160], while Tyr861 phosphorylation prevents its activation. By contrast, the dephosphorylation of Tyr861 mediated by protein tyrosine phosphatase non-receptor type 22 (PTPN22) relieves this inhibition [161], while the elimination of Tyr32 phosphorylation by PTEN is essential for NLRP3 activation [162].
In recent years, besides ubiquitination and phosphorylation, PTMs have also been shown to emerge in regulating the NLRP3 inflammasome. These NLRP3 modifications include SUMOylation [163,164,165], nitrosylation [166,167,168], alkylation [169,170], acetylation [171], and dicarboxypropylation, which is a rare modification mediated by 4-octyl itaconate, a derivative of immunomodulatory metabolite itaconate [172,173]. Collectively, phosphorylation and various PTMs are critical modulations on the NLRP3 inflammasome, indicating the importance of fine tuning NLRP3 priming and activation.
4.3. Non-Canonical Pathway
In addition to the canonical pathway, the NLRP3 inflammasome can be activated via the direct sensing of cytosolic LPS by human caspase-4/5 or mouse caspase-11, the process of which is defined as the non-canonical pathway [41,42,174]. The underlying mechanism of this non-canonical activation is not fully elucidated. Nevertheless, the caspase-11–GSDMD-mediated cellular perturbations may provide a hypothesis in a cell-intrinsic manner. Briefly, intracellular LPS, released by Gram-negative bacteria, can be directly monitored by the CARD domain of caspase-4/5/11, leading to their oligomerization and enzymatic activation through auto-cleavage [42,175]. Subsequently, these activated caspases cleave GSDMD and release their N-terminal cell death domain, which oligomerizes and enters the plasma membrane to form membrane pores and induce pyroptosis [36,37]. The K+ efflux caused by GSDMD pores activates NLRP3 and initiates caspase1-dependent IL-1β and IL-18 maturation. Galectin-3 can augment caspase-4/11 oligomerization and promote non-canonical inflammasome activation through LPS binding [176]. Notably, Moretti et al. unveiled another mechanism by which the simultaneous detection of bacterial RNA by NLRP3 and the binding of LPS by pro-caspase-11 promoted a pro-caspase-11–NLRP3 interaction that facilitated their interdependent activation [177]. Besides the intracellular signaling, extracellular LPS activates TLR4 signaling and induces a type I interferon response that upregulates the expression of caspase-11, guanylate-binding proteins (GBPs), and IFN-inducible protein IRGB10 [178,179,180]. GBPs and IRGB10 can lyse intracellular bacteria and release LPS into the cytosol. By contrast, some studies also suggest that extracellular LPS sensing is dispensable for non-canonical NLRP3 inflammasome activation [174,181].
5. Roles of Inflammasomes in Diseases
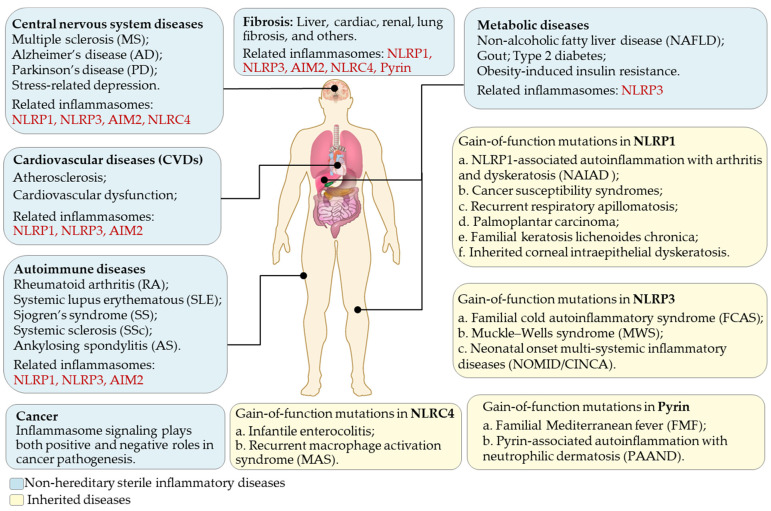
Acute inflammation is beneficial for pathogen clearance and tissue repair, while chronic inflammation is a pathological condition leading to tissue damage. A gain-of-function mutations study on the NLRP3 gene linked its functions to some inherited autoinflammatory diseases [182]. Mutations induce the constitutive activation of the NLRP3 inflammasome and trigger sterile inflammatory diseases, including familial cold autoinflammatory syndrome (FCAS) [182], Muckle–Wells syndrome (MWS) [182], and neonatal-onset multi-systemic inflammatory diseases/chronic infantile neurological cutaneous articular syndrome (NOMID/CINCA) [183]. These three closely related diseases, alternatively called cryopyrin-associated periodic syndromes (CAPS), are characterized by intermittent episodes of a rash, arthralgia, fever, and inflammation in the central nervous system [183]. The increased expression of IL-1β and IL-18 was found in monocytes and macrophages isolated from CAPS patients without any external stimuli [15]. Mechanistically, CAPS-associated NLRP3 mutants form cryo-sensitive aggregates that trigger NLRP3 inflammasome assembly distinct from canonical NLRP3 inflammasome activation [184].
Besides monocytes and macrophages, the NLRP3 inflammasome can also be activated in a wide range of endothelial, epithelial, and mesenchymal cells, thus, related to various inflammatory diseases in different organs such as the skin, brain, and liver. Thus, the NLRP3 inflammasome has been implicated in central nervous system (CNS) diseases, such as multiple sclerosis (MS), Alzheimer’s disease (AD), and Parkinson’s disease (PD) [185,186], metabolic diseases including non-alcoholic fatty liver disease (NAFLD), gout, type 2 diabetes, and obesity-induced insulin resistance [187], cardiovascular diseases (CVDs) [17,188], and rheumatoid arthritis (RA) [189] (Figure 4). Moreover, the increased IL-1β and ASC expression can be detected in the colon of patients with inflammatory bowel diseases (IBDs) [190], while systemic lupus erythematosus (SLE) patients exhibit an increased level of NLRP3 proteins in macrophages and tissues [191,192]. Evidence also suggests that the NLRP3 inflammasome participates in stress-related depression [193,194], and depressive symptoms can be curbed with anti-inflammatory agents [195]. Recent advances also suggest a role of the NLRP3 inflammasome in ovarian aging and female fertility. An elevated level of NLRP3 proteins was observed in the ovary of female mice during reproductive aging and in granulosa cells from patients with ovarian insufficiency, while the genomic ablation or pharmacological inhibition of NLRP3 ameliorated fertility [196]. Chronic inflammation induced by inflammasomes also contributes to tumorigenesis [197]. By contrast, inflammasome signaling inhibits tumor growth in animal models of colon cancer by maintaining intestinal barrier integrity [198]. Recent studies on Coronavirus Diseases 2019 (COVID-19) showed that the NLRP3 inflammasome contributes to developing respiratory, cardiovascular, and neurological symptoms in COVID-19 patients [199]. Besides the NLRP3 inflammasome, other inflammasomes are also involved in multiple sterile inflammatory diseases (Figure 4). Collectively, inflammasome-mediated inflammation is considerably implicated in a wide variety of human diseases, while manipulating its activation state can be beneficial for treating various diseases.
Figure 4.
Inflammasome-related human diseases. Canonical inflammasomes are involved in multiple inherited diseases (yellow-shaded boxes) and non-hereditary sterile inflammatory diseases (blue-shaded boxes). The highly relevant inflammasomes responsible for sterile inflammatory diseases are labeled in red.
6. Pharmacological Regulation of Inflammasome
6.1. Targeting Inflammasome Assembly
NLRP3 is a drug target of significant interest in the pharmaceutical industry, with the potential for treating various inflammatory diseases. Small molecules directly targeting NLRP3 are developing rapidly (Figure 5). The most well-studied one is the diarylsulfonylurea-containing compound, MCC950 (also known as CP-456773) [200], which directly targets the NACHT domain of NLRP3, thus, maintaining NLRP3 in the inactive state [201,202]. MCC950 has been employed as a tool in a wide range of studies on NLRP3-related diseases. For instance, MCC950 was tested in phase II clinical trials for rheumatoid arthritis, but was found to cause liver injuries by elevating the serum liver enzyme levels. Considering the pharmacokinetic and toxic properties, the high doses of the administration of MCC950 were limited in the clinic, and most studies in the field were pre-clinical [203]. MCC950-related compounds, including IZD334 and inzomelid, have completed phase I clinical trials [204]. RRx-001, an anti-cancer agent in phase III clinical trials, is currently identified as a highly selective NLRP3 inhibitor that covalently binds to cysteine 409 of NLRP3 and, therefore, blocks the assembly of the inflammasome [205]. Similarly, fluoxetine, an FDA-approved drug for treating clinical depression, has recently been identified as a direct NLRP3 inhibitor that prevents the NLRP3-ASC assembly [206]. Moreover, MM01 was identified as an inhibitor of ASC that interferes with ASC speck formation [207]. A novel humanized antibody targeting ASC, IC100, was validated to suppress disease in the experimental autoimmune encephalomyelitis (EAE) model [208]. These pharmacological findings provide a new sight into NLRP3-related disease treatment with ensured safety of drugs (Table 1).
Figure 5.
Pharmacological targeting of the NLRP3 inflammasome mechanisms. A wide variety of small synthetic molecules, natural compounds, and antibodies have been developed to target the canonical NLRP3 inflammasome signaling components as research tools and medicines in trials and clinics. Natural, but not synthetic, products are labeled in purple.
Table 1.
Small molecule inhibitors targeting NLRP3 inflammasome signaling.
| Targets | Agents | Description |
|---|---|---|
| NLRP3 | Direct | |
| MCC950 (CP-456773) | Binds to walker B motif of NACHT domain and locks inactive conformation | |
| RRx-001 | Covalently binds to cysteine 409 of NLRP3 | |
| CY-09 | Binds to walker A motif of NACHT domain and inhibits ATPase activity | |
| Compound 6 | Directly binds to the NACHT domain of NLRP3 to inhibit NLRP3 ATPase activity | |
| OLT1177 | Covalently modifies NACHT domain to inhibits its ATPase activity | |
| MNS | Directly binds to NLRP3, inhibits ATPase activity and prevents NLRP3-ASC interaction | |
| BOT-4-one | Leads to NLRP3 alkylation, inhibits ATPase activity of NLRP3 | |
| INF39 | Inhibits ATPase activity of NLRP3; inhibits priming | |
| Fluoxetine | Inhibits activation of the NLRP3-ASC inflammasome | |
| Indirect | ||
| Thiolutin (THL) | Inhibits NLRP3 deubiquitination and activation | |
| dihydrotanshinone I (DHT) | Inhibits ASC oligomerization induced by NLRP3 agonists | |
| methyl gallate | Blocks the ROS over-generation and oligomerization of NLRP3 | |
| IL-1β | Canakinumab | Neutralization IL-1β antibody |
| Anakinra | IL-1β receptor antagonist | |
| Rilonacept | Neutralizes circulating IL-1β and IL-1α | |
| P2X7 | AZ10606120 | P2X7R antagonist |
| Avastin | P2X7R inhibitor | |
| AZD9056 | P2X7R inhibitor | |
| Glyburide | Broad-spectrum inhibitor of P2X7R | |
| Caspase-1 | Ritonavir | Specific caspase-1 inhibitor |
| Disulfiram | Specific caspase-1 inhibitor | |
| Ac-YVAD-CHO | Caspase-1 inhibitory peptide | |
| VX-740/765 | Caspase-1 inhibitor | |
| Pralnacasan (VX-740) | Caspase-1 inhibitor | |
| Ac-FLTD-CMK | Inflammatory caspases inhibitor, targets caspases-1, -4, -5, and -11 | |
| Tetracycline | Unknown | |
| ASC | MM01 | Prevents ASC speck formation |
| IC100 | Humanized antibody targeting ASC | |
| ROS | N-acetyl Cysteine (NAC) | ROS generation inhibitor |
| NF-κB | BAY-11-7082 | NF-κB inihibitor |
| GSDMD | dimethyl fumarate (DMF) | Reacts with GSDMD and prevents its capacity to induce cell death |
6.2. Targeting the ATPase Activity of NLRP3
Besides disrupting the NLRP3 inflammasome assembly, many inhibitors are developed to target its ATPase activity (Figure 5). Recently, a series of tetrahydroquinoline inhibitors of NLRP3 inflammasome were discovered, including compound 6 that specifically inhibits NLRP3 activation through directly binding to the NACHT domain and inhibiting its ATPase activity [209], and CY-09 that targets the Walker A motif of the NACHT domain to disrupt ATPase activity [210]. OLT1177 is suggested to covalently modify the NACHT domain to block its ATPase activity [211] and ameliorate cognitive impairment in a mouse model of Alzheimer’s disease [212]. The ATPase activity of NLRP3 can also be interrupted by the direct binding of 3, 4-methylenedioxy-β-nitrostyrene (MNS) [213], and several other compounds, including BOT-4-one [214] and INF39 [215] (Table 1). Nevertheless, the efficacies of these agents in treating NLRP3-driven human diseases need further investigation.
6.3. Targeting Upstream and Downstream Signaling
Other alternative strategies have also been attempted to inhibit the NLRP3 inflammasome, such as the blockade of ATP receptor P2X7, NF-κB, caspase-1, IL-1β/IL-1R, and IL-18 (Figure 5). Avastin is a specific inhibitor of the P2X7 receptors (P2X7R), which blocks the ATP-induced activation of the NLRP3 inflammasome [216]. Caspase-1 inhibitors include ritonavir, disulfiram, and VX740/765 [217,218,219], which can inhibit the maturation of IL-1β. Moreover, a GSDMD-derived inhibitor, N-acetyl-Phe-Leu-Thr-Asp-chloromethylketone (Ac-FLTD-CMK), was designed to target inflammatory caspase, such as caspase-1, -4, -5, and -11, but not the apoptotic caspases such as caspase-3. It was shown to inhibit GSDMD cleavage and suppress the pyroptosis downstream of both canonical and non-canonical inflammasomes [220]. Notably, the most promising results were found in anti-IL-1β therapy, which is clinically effective in ameliorating inflammasome-related diseases. The FDA has approved three biologics, including the IL-1β receptor antagonist anakinra [221] and the neutralization IL-1β antibodies canakinumab [222] and rilonacept [223]. However, IL-1β signaling is merely one downstream pathway activated by NLRP3, and the complete inhibition of IL-1β signaling may increase the risk of fatal infection in clinical treatment. Intriguingly, the current study indicated that dimethyl fumarate (DMF) could react with GSDMD at its critical cysteine residues to form S-(2-succinyl)-cysteine, therefore, dampening GSDMD-induced cell death [224] (Table 1). These studies provide new strategies to target the elements involved in inflammasome formation and progression.
Other inflammasome sensors, such as NLRP1 and AIM2, are also implicated in diseases (Figure 4). Val-boroPro (VbP, also known as talabostat or PT-100) is an inhibitor of DPP8/DPP9, and has been validated to activate all functional NLRP1 homologs in humans and rodents [45,48,225,226]. Nevertheless, no specific small molecule has, thus far, directly targeted NLRP1 or AIM2 to regulate inflammasomes. The discovery of specific molecule tools for other types of inflammasomes would expand our frontiers in inflammasome biology.
6.4. Natural Inflammasome Inhibitors
In addition to synthetic compounds, herb-based medicine shows excellent potential for inflammatory disease treatment (Table 2). An alkaloid from Piper longum L., piperlongumine (PL), was shown to disrupt the assembly of NLRP3 and NEK7 and subsequent NLRP3 oligomerization [227]. Licochalcone B (LicoB), a primary component of the traditional medicinal herb licorice, directly binds to NEK7 and inhibits the interaction between NLRP3 and NEK7 [228]. Andrographolide, a bioactive chemical from Andrographis paniculate, is characterized to prevent NLRP3 inflammasome formation [229,230]. Other herb-based medicines identified recently include brevilin A (BA) [231], rpistimerin (Pri) [232], pterostilbene derivatives [233], and berberine [234], which all exhibit protective effects against NLRP3 inflammasome activation (Figure 5). These natural compounds provide potential alternatives in treating inflammatory diseases.
Table 2.
Natural inhibitors targeting NLRP3 inflammasome signaling.
| Targets | Agents | Description |
|---|---|---|
| NLRP3 | Erianin | Directly interacts with NLRP3 and inhibits NLRP3 inflammasome assembly |
| Piperlongumine | Inhibits the NLRP3-NEK7 interaction and NLRP3 oligomerization | |
| Oridonin | Targets Cysteine 279 of NLRP3 | |
| Pristimerin (Pri) | Blocks the assembly of NEK7-NLRP3 | |
| compound 47 (pterostilbene derivatives) | Affects the assembly of the NLRP3 inflammasomes by targeting NLRP3 | |
| Andrographolide | Inhibits the activation of NLRP3 inflammasome | |
| Tranilast | Binds to NACHT domain and blocks NLRP3-NLRP3 interaction | |
| β-hydroxybutyrate | Prevents K+ efflux and reduces ASC oligomerization and speck formation | |
| 1,2,4-trimethoxybenzene (1,2,4-TTB) | Inhibits the interaction of NLRP3 with ASC | |
| Parthenolide | ATPase inhibitor and caspase-1 inhibitor | |
| NLRs | Brevilin A (BA) | Inhibits NLRs inflammasomes, blocks ASC oligomerization |
| NEK7 | Berberine | Directly targets the NEK7 protein to block NEK7-NLRP3 assembly |
| Licochalcone B (LicoB) | Directly binds to NEK7 and inhibits the interaction between NLRP3 and NEK7 |
7. Concluding Remarks and Perspectives
Inflammasomes are dynamic protein complexes that multiple pathogenic and sterile inflammatory signals can trigger and induce infectious and non-infectious insults. Unlike other innate immune signalosomes that are generally membrane-anchored and less systemic in structure, inflammasomes appear unique. Given the large number of mutually unrelated upstream signals documented, inflammasome sensors seem to respond to general homeostasis stress, but are not a direct agonist. Characterizing the molecular mechanisms underlying inflammasome organization and cellular compartmentation would also be intriguing and critical to understanding this exquisite molecular machinery. Is there a key factor that integrates all the signals? How do the inflammasomes communicate with metabolism and nutrients and form special cellular compartments? Evidently, inhibitors targeting the inflammasome signaling components, such as those developed by studying cGAS-STING signaling [79], provide some attractive potential in understanding the fundamental rationale of this innate immune system and treating various inflammasome-related diseases.
Author Contributions
P.X. designed the review. C.C. and P.X. wrote the review. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Key Research and Development Program of China (2021YFA1301401 to P.X.) and the NSFC Projects (31725017 and 31830052 to P.X.).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 3.Lind N.A., Rael V.E., Pestal K., Liu B., Barton G.M. Regulation of the nucleic acid-sensing toll-like receptors. Nat. Rev. Immunol. 2022;22:224–235. doi: 10.1038/s41577-021-00577-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis B.K., Wen H., Ting J.P. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu. Rev. Immunol. 2011;29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown G.D., Willment J.A., Whitehead L. C-type lectins in immunity and homeostasis. Nat. Rev. Immunol. 2018;18:374–389. doi: 10.1038/s41577-018-0004-8. [DOI] [PubMed] [Google Scholar]
- 6.Sharma B.R., Karki R., Kanneganti T.D. Role of AIM2 inflammasome in inflammatory diseases, cancer and infection. Eur. J. Immunol. 2019;49:1998–2011. doi: 10.1002/eji.201848070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loo Y.M., Gale M., Jr. Immune signaling by RIG-I-like receptors. Immunity. 2011;34:680–692. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun L., Wu J., Du F., Chen X., Chen Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heilig R., Broz P. Function and mechanism of the pyrin inflammasome. Eur. J. Immunol. 2018;48:230–238. doi: 10.1002/eji.201746947. [DOI] [PubMed] [Google Scholar]
- 10.Lamkanfi M., Dixit V.M. Inflammasomes and their roles in health and disease. Annu. Rev. Cell Dev. Biol. 2012;28:137–161. doi: 10.1146/annurev-cellbio-101011-155745. [DOI] [PubMed] [Google Scholar]
- 11.Strowig T., Henao-Mejia J., Elinav E., Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 12.Pandey A., Shen C., Feng S., Man S.M. Cell biology of inflammasome activation. Trends Cell Biol. 2021;31:924–939. doi: 10.1016/j.tcb.2021.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Sharma D., Kanneganti T.D. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J. Cell Biol. 2016;213:617–629. doi: 10.1083/jcb.201602089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinon F., Burns K., Tschopp J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell. 2002;10:417–426. doi: 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 15.Agostini L., Martinon F., Burns K., McDermott M.F., Hawkins P.N., Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/S1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 16.Yang X., Chang H.Y., Baltimore D. Autoproteolytic activation of pro-caspases by oligomerization. Mol. Cell. 1998;1:319–325. doi: 10.1016/S1097-2765(00)80032-5. [DOI] [PubMed] [Google Scholar]
- 17.Li Y., Huang H., Liu B., Zhang Y., Pan X., Yu X.Y., Shen Z., Song Y.H. Inflammasomes as therapeutic targets in human diseases. Signal Transduct. Target Ther. 2021;6:247. doi: 10.1038/s41392-021-00650-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fenini G., Karakaya T., Hennig P., Di Filippo M., Beer H.D. The NLRP1 Inflammasome in Human Skin and Beyond. Int. J. Mol. Sci. 2020;21:4788. doi: 10.3390/ijms21134788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taabazuing C.Y., Griswold A.R., Bachovchin D.A. The NLRP1 and CARD8 inflammasomes. Immunol. Rev. 2020;297:13–25. doi: 10.1111/imr.12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minkiewicz J., de Rivero Vaccari J.P., Keane R.W. Human astrocytes express a novel NLRP2 inflammasome. Glia. 2013;61:1113–1121. doi: 10.1002/glia.22499. [DOI] [PubMed] [Google Scholar]
- 21.Bruey J.M., Bruey-Sedano N., Newman R., Chandler S., Stehlik C., Reed J.C. PAN1/NALP2/PYPAF2, an inducible inflammatory mediator that regulates NF-kappaB and caspase-1 activation in macrophages. J. Biol. Chem. 2004;279:51897–51907. doi: 10.1074/jbc.M406741200. [DOI] [PubMed] [Google Scholar]
- 22.Conti B.J., Davis B.K., Zhang J., O’Connor W., Jr., Williams K.L., Ting J.P. CATERPILLER 16.2 (CLR16.2), a novel NBD/LRR family member that negatively regulates T cell function. J. Biol. Chem. 2005;280:18375–18385. doi: 10.1074/jbc.M413169200. [DOI] [PubMed] [Google Scholar]
- 23.Lich J.D., Ting J.P. Monarch-1/PYPAF7 and other CATERPILLER (CLR, NOD, NLR) proteins with negative regulatory functions. Microbes Infect. 2007;9:672–676. doi: 10.1016/j.micinf.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng D., Kern L., Elinav E. The NLRP6 inflammasome. Immunology. 2021;162:281–289. doi: 10.1111/imm.13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carriere J., Dorfleutner A., Stehlik C. NLRP7: From inflammasome regulation to human disease. Immunology. 2021;163:363–376. doi: 10.1111/imm.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tuladhar S., Kanneganti T.D. NLRP12 in innate immunity and inflammation. Mol. Asp. Med. 2020;76:100887. doi: 10.1016/j.mam.2020.100887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ansari M.A., Singh V.V., Dutta S., Veettil M.V., Dutta D., Chikoti L., Lu J., Everly D., Chandran B. Constitutive interferon-inducible protein 16-inflammasome activation during Epstein-Barr virus latency I, II, and III in B and epithelial cells. J. Virol. 2013;87:8606–8623. doi: 10.1128/JVI.00805-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh V.V., Kerur N., Bottero V., Dutta S., Chakraborty S., Ansari M.A., Paudel N., Chikoti L., Chandran B. Kaposi’s sarcoma-associated herpesvirus latency in endothelial and B cells activates gamma interferon-inducible protein 16-mediated inflammasomes. J. Virol. 2013;87:4417–4431. doi: 10.1128/JVI.03282-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerur N., Veettil M.V., Sharma-Walia N., Bottero V., Sadagopan S., Otageri P., Chandran B. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection. Cell Host Microbe. 2011;9:363–375. doi: 10.1016/j.chom.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai X., Chen J., Xu H., Liu S., Jiang Q.X., Halfmann R., Chen Z.J. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell. 2014;156:1207–1222. doi: 10.1016/j.cell.2014.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu A., Magupalli V.G., Ruan J., Yin Q., Atianand M.K., Vos M.R., Schroder G.F., Fitzgerald K.A., Wu H., Egelman E.H. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell. 2014;156:1193–1206. doi: 10.1016/j.cell.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu A., Li Y., Schmidt F.I., Yin Q., Chen S., Fu T.M., Tong A.B., Ploegh H.L., Mao Y., Wu H. Molecular basis of caspase-1 polymerization and its inhibition by a new capping mechanism. Nat. Struct. Mol. Biol. 2016;23:416–425. doi: 10.1038/nsmb.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ayala J.M., Yamin T.T., Egger L.A., Chin J., Kostura M.J., Miller D.K. IL-1 beta-converting enzyme is present in monocytic cells as an inactive 45-kDa precursor. J. Immunol. 1994;153:2592–2599. [PubMed] [Google Scholar]
- 34.Evavold C.L., Ruan J., Tan Y., Xia S., Wu H., Kagan J.C. The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages. Immunity. 2018;48:35–44.e36. doi: 10.1016/j.immuni.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heilig R., Dick M.S., Sborgi L., Meunier E., Hiller S., Broz P. The Gasdermin-D pore acts as a conduit for IL-1beta secretion in mice. Eur. J. Immunol. 2018;48:584–592. doi: 10.1002/eji.201747404. [DOI] [PubMed] [Google Scholar]
- 36.Kayagaki N., Stowe I.B., Lee B.L., O’Rourke K., Anderson K., Warming S., Cuellar T., Haley B., Roose-Girma M., Phung Q.T., et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 37.Shi J., Zhao Y., Wang K., Shi X., Wang Y., Huang H., Zhuang Y., Cai T., Wang F., Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 38.Ding J., Wang K., Liu W., She Y., Sun Q., Shi J., Sun H., Wang D.C., Shao F. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 39.Liu X., Zhang Z., Ruan J., Pan Y., Magupalli V.G., Wu H., Lieberman J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santa Cruz Garcia A.B., Schnur K.P., Malik A.B., Mo G.C.H. Gasdermin D pores are dynamically regulated by local phosphoinositide circuitry. Nat. Commun. 2022;13:52. doi: 10.1038/s41467-021-27692-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kayagaki N., Warming S., Lamkanfi M., Vande Walle L., Louie S., Dong J., Newton K., Qu Y., Liu J., Heldens S., et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 42.Shi J., Zhao Y., Wang Y., Gao W., Ding J., Li P., Hu L., Shao F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 43.Finger J.N., Lich J.D., Dare L.C., Cook M.N., Brown K.K., Duraiswami C., Bertin J., Gough P.J. Autolytic proteolysis within the function to find domain (FIIND) is required for NLRP1 inflammasome activity. J. Biol. Chem. 2012;287:25030–25037. doi: 10.1074/jbc.M112.378323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frew B.C., Joag V.R., Mogridge J. Proteolytic processing of Nlrp1b is required for inflammasome activity. PLoS Pathog. 2012;8:e1002659. doi: 10.1371/journal.ppat.1002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okondo M.C., Rao S.D., Taabazuing C.Y., Chui A.J., Poplawski S.E., Johnson D.C., Bachovchin D.A. Inhibition of Dpp8/9 Activates the Nlrp1b Inflammasome. Cell Chem. Biol. 2018;25:262–267.e265. doi: 10.1016/j.chembiol.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhong F.L., Robinson K., Teo D.E.T., Tan K.Y., Lim C., Harapas C.R., Yu C.H., Xie W.H., Sobota R.M., Au V.B., et al. Human DPP9 represses NLRP1 inflammasome and protects against autoinflammatory diseases via both peptidase activity and FIIND domain binding. J. Biol. Chem. 2018;293:18864–18878. doi: 10.1074/jbc.RA118.004350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Vasconcelos N.M., Vliegen G., Goncalves A., De Hert E., Martin-Perez R., Van Opdenbosch N., Jallapally A., Geiss-Friedlander R., Lambeir A.M., Augustyns K., et al. DPP8/DPP9 inhibition elicits canonical Nlrp1b inflammasome hallmarks in murine macrophages. Life Sci. Alliance. 2019;2:e201900313. doi: 10.26508/lsa.201900313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chui A.J., Okondo M.C., Rao S.D., Gai K., Griswold A.R., Johnson D.C., Ball D.P., Taabazuing C.Y., Orth E.L., Vittimberga B.A., et al. N-terminal degradation activates the NLRP1B inflammasome. Science. 2019;364:82–85. doi: 10.1126/science.aau1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Griswold A.R., Ball D.P., Bhattacharjee A., Chui A.J., Rao S.D., Taabazuing C.Y., Bachovchin D.A. DPP9’s Enzymatic Activity and Not Its Binding to CARD8 Inhibits Inflammasome Activation. ACS Chem. Biol. 2019;14:2424–2429. doi: 10.1021/acschembio.9b00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hollingsworth L.R., Sharif H., Griswold A.R., Fontana P., Mintseris J., Dagbay K.B., Paulo J.A., Gygi S.P., Bachovchin D.A., Wu H. DPP9 sequesters the C terminus of NLRP1 to repress inflammasome activation. Nature. 2021;592:778–783. doi: 10.1038/s41586-021-03350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang M., Zhang X., Toh G.A., Gong Q., Wang J., Han Z., Wu B., Zhong F., Chai J. Structural and biochemical mechanisms of NLRP1 inhibition by DPP9. Nature. 2021;592:773–777. doi: 10.1038/s41586-021-03320-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Faustin B., Lartigue L., Bruey J.M., Luciano F., Sergienko E., Bailly-Maitre B., Volkmann N., Hanein D., Rouiller I., Reed J.C. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol. Cell. 2007;25:713–724. doi: 10.1016/j.molcel.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 53.Robinson K.S., Teo D.E.T., Tan K.S., Toh G.A., Ong H.H., Lim C.K., Lay K., Au B.V., Lew T.S., Chu J.J.H., et al. Enteroviral 3C protease activates the human NLRP1 inflammasome in airway epithelia. Science. 2020;370:eaay2002. doi: 10.1126/science.aay2002. [DOI] [PubMed] [Google Scholar]
- 54.Yang X., Zhou J., Liu C., Qu Y., Wang W., Xiao M.Z.X., Zhu F., Liu Z., Liang Q. KSHV-encoded ORF45 activates human NLRP1 inflammasome. Nat. Immunol. 2022;23:916–926. doi: 10.1038/s41590-022-01199-x. [DOI] [PubMed] [Google Scholar]
- 55.Boyden E.D., Dietrich W.F. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat. Genet. 2006;38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- 56.Liao K.C., Mogridge J. Activation of the Nlrp1b inflammasome by reduction of cytosolic ATP. Infect. Immun. 2013;81:570–579. doi: 10.1128/IAI.01003-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharif H., Wang L., Wang W.L., Magupalli V.G., Andreeva L., Qiao Q., Hauenstein A.V., Wu Z., Nunez G., Mao Y., et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature. 2019;570:338–343. doi: 10.1038/s41586-019-1295-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hafner-Bratkovic I., Susjan P., Lainscek D., Tapia-Abellan A., Cerovic K., Kadunc L., Angosto-Bazarra D., Pelegrin P., Jerala R. NLRP3 lacking the leucine-rich repeat domain can be fully activated via the canonical inflammasome pathway. Nat. Commun. 2018;9:5182. doi: 10.1038/s41467-018-07573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maharana J., Panda D., De S. Deciphering the ATP-binding mechanism(s) in NLRP-NACHT 3D models using structural bioinformatics approaches. PLoS ONE. 2018;13:e0209420. doi: 10.1371/journal.pone.0209420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duncan J.A., Bergstralh D.T., Wang Y., Willingham S.B., Ye Z., Zimmermann A.G., Ting J.P. Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proc. Natl. Acad. Sci. USA. 2007;104:8041–8046. doi: 10.1073/pnas.0611496104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iyer S.S., He Q., Janczy J.R., Elliott E.I., Zhong Z., Olivier A.K., Sadler J.J., Knepper-Adrian V., Han R., Qiao L., et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity. 2013;39:311–323. doi: 10.1016/j.immuni.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou R., Yazdi A.S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 63.Subramanian N., Natarajan K., Clatworthy M.R., Wang Z., Germain R.N. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell. 2013;153:348–361. doi: 10.1016/j.cell.2013.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen J., Chen Z.J. PtdIns4P on dispersed trans-Golgi network mediates NLRP3 inflammasome activation. Nature. 2018;564:71–76. doi: 10.1038/s41586-018-0761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Magupalli V.G., Negro R., Tian Y., Hauenstein A.V., Di Caprio G., Skillern W., Deng Q., Orning P., Alam H.B., Maliga Z., et al. HDAC6 mediates an aggresome-like mechanism for NLRP3 and pyrin inflammasome activation. Science. 2020;369:eaas8995. doi: 10.1126/science.aas8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmid-Burgk J.L., Gaidt M.M., Schmidt T., Ebert T.S., Bartok E., Hornung V. Caspase-4 mediates non-canonical activation of the NLRP3 inflammasome in human myeloid cells. Eur. J. Immunol. 2015;45:2911–2917. doi: 10.1002/eji.201545523. [DOI] [PubMed] [Google Scholar]
- 67.Ruhl S., Broz P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. Eur. J. Immunol. 2015;45:2927–2936. doi: 10.1002/eji.201545772. [DOI] [PubMed] [Google Scholar]
- 68.Poyet J.L., Srinivasula S.M., Tnani M., Razmara M., Fernandes-Alnemri T., Alnemri E.S. Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J. Biol. Chem. 2001;276:28309–28313. doi: 10.1074/jbc.C100250200. [DOI] [PubMed] [Google Scholar]
- 69.Miao E.A., Mao D.P., Yudkovsky N., Bonneau R., Lorang C.G., Warren S.E., Leaf I.A., Aderem A. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc. Natl. Acad. Sci. USA. 2010;107:3076–3080. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kofoed E.M., Vance R.E. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 2011;477:592–595. doi: 10.1038/nature10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao Y., Yang J., Shi J., Gong Y.N., Lu Q., Xu H., Liu L., Shao F. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477:596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 72.Duncan J.A., Canna S.W. The NLRC4 Inflammasome. Immunol. Rev. 2018;281:115–123. doi: 10.1111/imr.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hu Z., Yan C., Liu P., Huang Z., Ma R., Zhang C., Wang R., Zhang Y., Martinon F., Miao D., et al. Crystal structure of NLRC4 reveals its autoinhibition mechanism. Science. 2013;341:172–175. doi: 10.1126/science.1236381. [DOI] [PubMed] [Google Scholar]
- 74.Zhang L., Chen S., Ruan J., Wu J., Tong A.B., Yin Q., Li Y., David L., Lu A., Wang W.L., et al. Cryo-EM structure of the activated NAIP2-NLRC4 inflammasome reveals nucleated polymerization. Science. 2015;350:404–409. doi: 10.1126/science.aac5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Diebolder C.A., Halff E.F., Koster A.J., Huizinga E.G., Koning R.I. Cryoelectron Tomography of the NAIP5/NLRC4 Inflammasome: Implications for NLR Activation. Structure. 2015;23:2349–2357. doi: 10.1016/j.str.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 76.Wang S.B., Narendran S., Hirahara S., Varshney A., Pereira F., Apicella I., Ambati M., Ambati V.L., Yerramothu P., Ambati K., et al. DDX17 is an essential mediator of sterile NLRC4 inflammasome activation by retrotransposon RNAs. Sci. Immunol. 2021;6:eabi4493. doi: 10.1126/sciimmunol.abi4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meng F., Yu Z., Zhang D., Chen S., Guan H., Zhou R., Wu Q., Zhang Q., Liu S., Venkat Ramani M.K., et al. Induced phase separation of mutant NF2 imprisons the cGAS-STING machinery to abrogate antitumor immunity. Mol. Cell. 2021;81:4147–4164.e4147. doi: 10.1016/j.molcel.2021.07.040. [DOI] [PubMed] [Google Scholar]
- 78.Zhang D., Liu Y., Zhu Y., Zhang Q., Guan H., Liu S., Chen S., Mei C., Chen C., Liao Z., et al. A non-canonical cGAS-STING-PERK pathway facilitates the translational program critical for senescence and organ fibrosis. Nat. Cell Biol. 2022;24:766–782. doi: 10.1038/s41556-022-00894-z. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Q., Chen C., Xia B., Xu P. Chemical regulation of the cGAS-STING pathway. Curr. Opin. Chem. Biol. 2022;69:102170. doi: 10.1016/j.cbpa.2022.102170. [DOI] [PubMed] [Google Scholar]
- 80.Gui X., Yang H., Li T., Tan X., Shi P., Li M., Du F., Chen Z.J. Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature. 2019;567:262–266. doi: 10.1038/s41586-019-1006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burckstummer T., Baumann C., Bluml S., Dixit E., Durnberger G., Jahn H., Planyavsky M., Bilban M., Colinge J., Bennett K.L., et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat. Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 82.Roberts T.L., Idris A., Dunn J.A., Kelly G.M., Burnton C.M., Hodgson S., Hardy L.L., Garceau V., Sweet M.J., Ross I.L., et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science. 2009;323:1057–1060. doi: 10.1126/science.1169841. [DOI] [PubMed] [Google Scholar]
- 83.Hornung V., Ablasser A., Charrel-Dennis M., Bauernfeind F., Horvath G., Caffrey D.R., Latz E., Fitzgerald K.A. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kumari P., Russo A.J., Shivcharan S., Rathinam V.A. AIM2 in health and disease: Inflammasome and beyond. Immunol. Rev. 2020;297:83–95. doi: 10.1111/imr.12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lammert C.R., Frost E.L., Bellinger C.E., Bolte A.C., McKee C.A., Hurt M.E., Paysour M.J., Ennerfelt H.E., Lukens J.R. AIM2 inflammasome surveillance of DNA damage shapes neurodevelopment. Nature. 2020;580:647–652. doi: 10.1038/s41586-020-2174-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.French F.M.F.C. A candidate gene for familial Mediterranean fever. Nat. Genet. 1997;17:25–31. doi: 10.1038/ng0997-25. [DOI] [PubMed] [Google Scholar]
- 87.Manthiram K., Zhou Q., Aksentijevich I., Kastner D.L. The monogenic autoinflammatory diseases define new pathways in human innate immunity and inflammation. Nat. Immunol. 2017;18:832–842. doi: 10.1038/ni.3777. [DOI] [PubMed] [Google Scholar]
- 88.Schnappauf O., Chae J.J., Kastner D.L., Aksentijevich I. The Pyrin Inflammasome in Health and Disease. Front. Immunol. 2019;10:1745. doi: 10.3389/fimmu.2019.01745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu H., Yang J., Gao W., Li L., Li P., Zhang L., Gong Y.N., Peng X., Xi J.J., Chen S., et al. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature. 2014;513:237–241. doi: 10.1038/nature13449. [DOI] [PubMed] [Google Scholar]
- 90.Loeven N.A., Medici N.P., Bliska J.B. The pyrin inflammasome in host-microbe interactions. Curr. Opin. Microbiol. 2020;54:77–86. doi: 10.1016/j.mib.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bauernfeind F.G., Horvath G., Stutz A., Alnemri E.S., MacDonald K., Speert D., Fernandes-Alnemri T., Wu J., Monks B.G., Fitzgerald K.A., et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schroder K., Sagulenko V., Zamoshnikova A., Richards A.A., Cridland J.A., Irvine K.M., Stacey K.J., Sweet M.J. Acute lipopolysaccharide priming boosts inflammasome activation independently of inflammasome sensor induction. Immunobiology. 2012;217:1325–1329. doi: 10.1016/j.imbio.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 93.Juliana C., Fernandes-Alnemri T., Kang S., Farias A., Qin F., Alnemri E.S. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J. Biol. Chem. 2012;287:36617–36622. doi: 10.1074/jbc.M112.407130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin K.M., Hu W., Troutman T.D., Jennings M., Brewer T., Li X., Nanda S., Cohen P., Thomas J.A., Pasare C. IRAK-1 bypasses priming and directly links TLRs to rapid NLRP3 inflammasome activation. Proc. Natl. Acad. Sci. USA. 2014;111:775–780. doi: 10.1073/pnas.1320294111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fernandes-Alnemri T., Kang S., Anderson C., Sagara J., Fitzgerald K.A., Alnemri E.S. Cutting edge: TLR signaling licenses IRAK1 for rapid activation of the NLRP3 inflammasome. J. Immunol. 2013;191:3995–3999. doi: 10.4049/jimmunol.1301681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Swanson K.V., Deng M., Ting J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019;19:477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Perregaux D., Gabel C.A. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J. Biol. Chem. 1994;269:15195–15203. doi: 10.1016/S0021-9258(17)36591-2. [DOI] [PubMed] [Google Scholar]
- 98.Walev I., Reske K., Palmer M., Valeva A., Bhakdi S. Potassium-inhibited processing of IL-1 beta in human monocytes. EMBO J. 1995;14:1607–1614. doi: 10.1002/j.1460-2075.1995.tb07149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Di A., Xiong S., Ye Z., Malireddi R.K.S., Kometani S., Zhong M., Mittal M., Hong Z., Kanneganti T.D., Rehman J., et al. The TWIK2 Potassium Efflux Channel in Macrophages Mediates NLRP3 Inflammasome-Induced Inflammation. Immunity. 2018;49:56–65.e54. doi: 10.1016/j.immuni.2018.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Petrilli V., Papin S., Dostert C., Mayor A., Martinon F., Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 101.Munoz-Planillo R., Kuffa P., Martinez-Colon G., Smith B.L., Rajendiran T.M., Nunez G. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Murakami T., Ockinger J., Yu J., Byles V., McColl A., Hofer A.M., Horng T. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc. Natl. Acad. Sci. USA. 2012;109:11282–11287. doi: 10.1073/pnas.1117765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee G.S., Subramanian N., Kim A.I., Aksentijevich I., Goldbach-Mansky R., Sacks D.B., Germain R.N., Kastner D.L., Chae J.J. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492:123–127. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rolfes V., Ribeiro L.S., Hawwari I., Bottcher L., Rosero N., Maasewerd S., Santos M.L.S., Prochnicki T., Silva C.M.S., Wanderley C.W.S., et al. Platelets Fuel the Inflammasome Activation of Innate Immune Cells. Cell Rep. 2020;31:107615. doi: 10.1016/j.celrep.2020.107615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jager E., Murthy S., Schmidt C., Hahn M., Strobel S., Peters A., Staubert C., Sungur P., Venus T., Geisler M., et al. Calcium-sensing receptor-mediated NLRP3 inflammasome response to calciprotein particles drives inflammation in rheumatoid arthritis. Nat. Commun. 2020;11:4243. doi: 10.1038/s41467-020-17749-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang C., Qin J., Zhang S., Zhang N., Tan B., Siwko S., Zhang Y., Wang Q., Chen J., Qian M., et al. ADP/P2Y1 aggravates inflammatory bowel disease through ERK5-mediated NLRP3 inflammasome activation. Mucosal Immunol. 2020;13:931–945. doi: 10.1038/s41385-020-0307-5. [DOI] [PubMed] [Google Scholar]
- 107.Tang T., Lang X., Xu C., Wang X., Gong T., Yang Y., Cui J., Bai L., Wang J., Jiang W., et al. CLICs-dependent chloride efflux is an essential and proximal upstream event for NLRP3 inflammasome activation. Nat. Commun. 2017;8:202. doi: 10.1038/s41467-017-00227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Green J.P., Yu S., Martin-Sanchez F., Pelegrin P., Lopez-Castejon G., Lawrence C.B., Brough D. Chloride regulates dynamic NLRP3-dependent ASC oligomerization and inflammasome priming. Proc. Natl. Acad. Sci. USA. 2018;115:E9371–E9380. doi: 10.1073/pnas.1812744115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Domingo-Fernandez R., Coll R.C., Kearney J., Breit S., O’Neill L.A.J. The intracellular chloride channel proteins CLIC1 and CLIC4 induce IL-1beta transcription and activate the NLRP3 inflammasome. J. Biol. Chem. 2017;292:12077–12087. doi: 10.1074/jbc.M117.797126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yaron J.R., Gangaraju S., Rao M.Y., Kong X., Zhang L., Su F., Tian Y., Glenn H.L., Meldrum D.R. K(+) regulates Ca(2+) to drive inflammasome signaling: Dynamic visualization of ion flux in live cells. Cell Death Dis. 2015;6:e1954. doi: 10.1038/cddis.2015.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Katsnelson M.A., Rucker L.G., Russo H.M., Dubyak G.R. K+ efflux agonists induce NLRP3 inflammasome activation independently of Ca2+ signaling. J. Immunol. 2015;194:3937–3952. doi: 10.4049/jimmunol.1402658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang Y., Rong H., Zhang F.X., Wu K., Mu L., Meng J., Xiao B., Zamponi G.W., Shi Y. A Membrane Potential- and Calpain-Dependent Reversal of Caspase-1 Inhibition Regulates Canonical NLRP3 Inflammasome. Cell Rep. 2018;24:2356–2369.e2355. doi: 10.1016/j.celrep.2018.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hornung V., Bauernfeind F., Halle A., Samstad E.O., Kono H., Rock K.L., Fitzgerald K.A., Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hofbauer D., Mougiakakos D., Broggini L., Zaiss M., Buttner-Herold M., Bach C., Spriewald B., Neumann F., Bisht S., Nolting J., et al. beta2-microglobulin triggers NLRP3 inflammasome activation in tumor-associated macrophages to promote multiple myeloma progression. Immunity. 2021;54:1772–1787.e1779. doi: 10.1016/j.immuni.2021.07.002. [DOI] [PubMed] [Google Scholar]
- 115.Svadlakova T., Hubatka F., Turanek Knotigova P., Kulich P., Masek J., Kotoucek J., Macak J., Motola M., Kalbac M., Kolackova M., et al. Proinflammatory Effect of Carbon-Based Nanomaterials: In Vitro Study on Stimulation of Inflammasome NLRP3 via Destabilisation of Lysosomes. Nanomaterials. 2020;10:418. doi: 10.3390/nano10030418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jessop F., Hamilton R.F., Jr., Rhoderick J.F., Fletcher P., Holian A. Phagolysosome acidification is required for silica and engineered nanoparticle-induced lysosome membrane permeabilization and resultant NLRP3 inflammasome activity. Toxicol. Appl. Pharmacol. 2017;318:58–68. doi: 10.1016/j.taap.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chevriaux A., Pilot T., Derangere V., Simonin H., Martine P., Chalmin F., Ghiringhelli F., Rebe C. Cathepsin B Is Required for NLRP3 Inflammasome Activation in Macrophages, Through NLRP3 Interaction. Front. Cell Dev. Biol. 2020;8:167. doi: 10.3389/fcell.2020.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Katsnelson M.A., Lozada-Soto K.M., Russo H.M., Miller B.A., Dubyak G.R. NLRP3 inflammasome signaling is activated by low-level lysosome disruption but inhibited by extensive lysosome disruption: Roles for K+ efflux and Ca2+ influx. Am. J. Physiol. Cell Physiol. 2016;311:C83–C100. doi: 10.1152/ajpcell.00298.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Correa R., Silva L.F.F., Ribeiro D.J.S., Almeida R.D.N., Santos I.O., Correa L.H., de Sant’Ana L.P., Assuncao L.S., Bozza P.T., Magalhaes K.G. Lysophosphatidylcholine Induces NLRP3 Inflammasome-Mediated Foam Cell Formation and Pyroptosis in Human Monocytes and Endothelial Cells. Front. Immunol. 2019;10:2927. doi: 10.3389/fimmu.2019.02927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Guo C., Chi Z., Jiang D., Xu T., Yu W., Wang Z., Chen S., Zhang L., Liu Q., Guo X., et al. Cholesterol Homeostatic Regulator SCAP-SREBP2 Integrates NLRP3 Inflammasome Activation and Cholesterol Biosynthetic Signaling in Macrophages. Immunity. 2018;49:842–856.e847. doi: 10.1016/j.immuni.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 121.Xu F., Qi H., Li J., Sun L., Gong J., Chen Y., Shen A., Li W. Mycobacterium tuberculosis infection up-regulates MFN2 expression to promote NLRP3 inflammasome formation. J. Biol. Chem. 2020;295:17684–17697. doi: 10.1074/jbc.RA120.014077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gu P., Hui X., Zheng Q., Gao Y., Jin L., Jiang W., Zhou C., Liu T., Huang Y., Liu Q., et al. Mitochondrial uncoupling protein 1 antagonizes atherosclerosis by blocking NLRP3 inflammasome-dependent interleukin-1beta production. Sci. Adv. 2021;7:eabl4024. doi: 10.1126/sciadv.abl4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Panicker N., Sarkar S., Harischandra D.S., Neal M., Kam T.I., Jin H., Saminathan H., Langley M., Charli A., Samidurai M., et al. Fyn kinase regulates misfolded alpha-synuclein uptake and NLRP3 inflammasome activation in microglia. J. Exp. Med. 2019;216:1411–1430. doi: 10.1084/jem.20182191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Alves J.V., da Costa R.M., Pereira C.A., Fedoce A.G., Silva C.A.A., Carneiro F.S., Lobato N.S., Tostes R.C. Supraphysiological Levels of Testosterone Induce Vascular Dysfunction via Activation of the NLRP3 Inflammasome. Front. Immunol. 2020;11:1647. doi: 10.3389/fimmu.2020.01647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bedient L., Pokharel S.M., Chiok K.R., Mohanty I., Beach S.S., Miura T.A., Bose S. Lytic Cell Death Mechanisms in Human Respiratory Syncytial Virus-Infected Macrophages: Roles of Pyroptosis and Necroptosis. Viruses. 2020;12:932. doi: 10.3390/v12090932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhong Z., Liang S., Sanchez-Lopez E., He F., Shalapour S., Lin X.J., Wong J., Ding S., Seki E., Schnabl B., et al. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature. 2018;560:198–203. doi: 10.1038/s41586-018-0372-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shimada K., Crother T.R., Karlin J., Dagvadorj J., Chiba N., Chen S., Ramanujan V.K., Wolf A.J., Vergnes L., Ojcius D.M., et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li S., Li H., Zhang Y.L., Xin Q.L., Guan Z.Q., Chen X., Zhang X.A., Li X.K., Xiao G.F., Lozach P.Y., et al. SFTSV Infection Induces BAK/BAX-Dependent Mitochondrial DNA Release to Trigger NLRP3 Inflammasome Activation. Cell Rep. 2020;30:4370–4385.e4377. doi: 10.1016/j.celrep.2020.02.105. [DOI] [PubMed] [Google Scholar]
- 129.Billingham L.K., Stoolman J.S., Vasan K., Rodriguez A.E., Poor T.A., Szibor M., Jacobs H.T., Reczek C.R., Rashidi A., Zhang P., et al. Mitochondrial electron transport chain is necessary for NLRP3 inflammasome activation. Nat. Immunol. 2022;23:692–704. doi: 10.1038/s41590-022-01185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lu B., Nakamura T., Inouye K., Li J., Tang Y., Lundback P., Valdes-Ferrer S.I., Olofsson P.S., Kalb T., Roth J., et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488:670–674. doi: 10.1038/nature11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shenoy A.R., Wellington D.A., Kumar P., Kassa H., Booth C.J., Cresswell P., MacMicking J.D. GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science. 2012;336:481–485. doi: 10.1126/science.1217141. [DOI] [PubMed] [Google Scholar]
- 132.Duan Y., Zhang L., Angosto-Bazarra D., Pelegrin P., Nunez G., He Y. RACK1 Mediates NLRP3 Inflammasome Activation by Promoting NLRP3 Active Conformation and Inflammasome Assembly. Cell Rep. 2020;33:108405. doi: 10.1016/j.celrep.2020.108405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ito M., Shichita T., Okada M., Komine R., Noguchi Y., Yoshimura A., Morita R. Bruton’s tyrosine kinase is essential for NLRP3 inflammasome activation and contributes to ischaemic brain injury. Nat. Commun. 2015;6:7360. doi: 10.1038/ncomms8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu X., Pichulik T., Wolz O.O., Dang T.M., Stutz A., Dillen C., Delmiro Garcia M., Kraus H., Dickhofer S., Daiber E., et al. Human NACHT, LRR, and PYD domain-containing protein 3 (NLRP3) inflammasome activity is regulated by and potentially targetable through Bruton tyrosine kinase. J. Allergy Clin. Immunol. 2017;140:1054–1067.e1010. doi: 10.1016/j.jaci.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 135.Mao L., Kitani A., Hiejima E., Montgomery-Recht K., Zhou W., Fuss I., Wiestner A., Strober W. Bruton tyrosine kinase deficiency augments NLRP3 inflammasome activation and causes IL-1beta-mediated colitis. J. Clin. Investig. 2020;130:1793–1807. doi: 10.1172/JCI128322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Joshi H., Almgren-Bell A., Anaya E.P., Todd E.M., Van Dyken S.J., Seth A., McIntire K.M., Singamaneni S., Sutterwala F., Morley S.C. L-plastin enhances NLRP3 inflammasome assembly and bleomycin-induced lung fibrosis. Cell Rep. 2022;38:110507. doi: 10.1016/j.celrep.2022.110507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Samir P., Kesavardhana S., Patmore D.M., Gingras S., Malireddi R.K.S., Karki R., Guy C.S., Briard B., Place D.E., Bhattacharya A., et al. DDX3X acts as a live-or-die checkpoint in stressed cells by regulating NLRP3 inflammasome. Nature. 2019;573:590–594. doi: 10.1038/s41586-019-1551-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gangopadhyay A., Devi S., Tenguria S., Carriere J., Nguyen H., Jager E., Khatri H., Chu L.H., Ratsimandresy R.A., Dorfleutner A., et al. NLRP3 licenses NLRP11 for inflammasome activation in human macrophages. Nat. Immunol. 2022;23:892–903. doi: 10.1038/s41590-022-01220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.He Y., Zeng M.Y., Yang D., Motro B., Nunez G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016;530:354–357. doi: 10.1038/nature16959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shi H., Wang Y., Li X., Zhan X., Tang M., Fina M., Su L., Pratt D., Bu C.H., Hildebrand S., et al. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat. Immunol. 2016;17:250–258. doi: 10.1038/ni.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schmid-Burgk J.L., Chauhan D., Schmidt T., Ebert T.S., Reinhardt J., Endl E., Hornung V. A Genome-wide CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) Screen Identifies NEK7 as an Essential Component of NLRP3 Inflammasome Activation. J. Biol. Chem. 2016;291:103–109. doi: 10.1074/jbc.C115.700492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Han S., Lear T.B., Jerome J.A., Rajbhandari S., Snavely C.A., Gulick D.L., Gibson K.F., Zou C., Chen B.B., Mallampalli R.K. Lipopolysaccharide Primes the NALP3 Inflammasome by Inhibiting Its Ubiquitination and Degradation Mediated by the SCFFBXL2 E3 Ligase. J. Biol. Chem. 2015;290:18124–18133. doi: 10.1074/jbc.M115.645549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Song H., Liu B., Huai W., Yu Z., Wang W., Zhao J., Han L., Jiang G., Zhang L., Gao C., et al. The E3 ubiquitin ligase TRIM31 attenuates NLRP3 inflammasome activation by promoting proteasomal degradation of NLRP3. Nat. Commun. 2016;7:13727. doi: 10.1038/ncomms13727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kawashima A., Karasawa T., Tago K., Kimura H., Kamata R., Usui-Kawanishi F., Watanabe S., Ohta S., Funakoshi-Tago M., Yanagisawa K., et al. ARIH2 Ubiquitinates NLRP3 and Negatively Regulates NLRP3 Inflammasome Activation in Macrophages. J. Immunol. 2017;199:3614–3622. doi: 10.4049/jimmunol.1700184. [DOI] [PubMed] [Google Scholar]
- 145.Tang J., Tu S., Lin G., Guo H., Yan C., Liu Q., Huang L., Tang N., Xiao Y., Pope R.M., et al. Sequential ubiquitination of NLRP3 by RNF125 and Cbl-b limits inflammasome activation and endotoxemia. J. Exp. Med. 2020;217:e20182091. doi: 10.1084/jem.20182091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yan Y., Jiang W., Liu L., Wang X., Ding C., Tian Z., Zhou R. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell. 2015;160:62–73. doi: 10.1016/j.cell.2014.11.047. [DOI] [PubMed] [Google Scholar]
- 147.Wan P., Zhang Q., Liu W., Jia Y., Ai S., Wang T., Wang W., Pan P., Yang G., Xiang Q., et al. Cullin1 binds and promotes NLRP3 ubiquitination to repress systematic inflammasome activation. FASEB J. 2019;33:5793–5807. doi: 10.1096/fj.201801681R. [DOI] [PubMed] [Google Scholar]
- 148.Ren G., Zhang X., Xiao Y., Zhang W., Wang Y., Ma W., Wang X., Song P., Lai L., Chen H., et al. ABRO1 promotes NLRP3 inflammasome activation through regulation of NLRP3 deubiquitination. EMBO J. 2019;38:e100376. doi: 10.15252/embj.2018100376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Palazon-Riquelme P., Worboys J.D., Green J., Valera A., Martin-Sanchez F., Pellegrini C., Brough D., Lopez-Castejon G. USP7 and USP47 deubiquitinases regulate NLRP3 inflammasome activation. EMBO Rep. 2018;19:e44766. doi: 10.15252/embr.201744766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang W., Hu D., Wu C., Feng Y., Li A., Liu W., Wang Y., Chen K., Tian M., Xiao F., et al. STING promotes NLRP3 localization in ER and facilitates NLRP3 deubiquitination to activate the inflammasome upon HSV-1 infection. PLoS Pathog. 2020;16:e1008335. doi: 10.1371/journal.ppat.1008335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Guo Y., Li L., Xu T., Guo X., Wang C., Li Y., Yang Y., Yang D., Sun B., Zhao X., et al. HUWE1 mediates inflammasome activation and promotes host defense against bacterial infection. J. Clin. Investig. 2020;130:6301–6316. doi: 10.1172/JCI138234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Song N., Liu Z.S., Xue W., Bai Z.F., Wang Q.Y., Dai J., Liu X., Huang Y.J., Cai H., Zhan X.Y., et al. NLRP3 Phosphorylation Is an Essential Priming Event for Inflammasome Activation. Mol. Cell. 2017;68:185–197.e186. doi: 10.1016/j.molcel.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 153.Dufies O., Doye A., Courjon J., Torre C., Michel G., Loubatier C., Jacquel A., Chaintreuil P., Majoor A., Guinamard R.R., et al. Escherichia coli Rho GTPase-activating toxin CNF1 mediates NLRP3 inflammasome activation via p21-activated kinases-1/2 during bacteraemia in mice. Nat. Microbiol. 2021;6:401–412. doi: 10.1038/s41564-020-00832-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bittner Z.A., Liu X., Mateo Tortola M., Tapia-Abellan A., Shankar S., Andreeva L., Mangan M., Spalinger M., Kalbacher H., Duwell P., et al. BTK operates a phospho-tyrosine switch to regulate NLRP3 inflammasome activity. J. Exp. Med. 2021;218:e20201656. doi: 10.1084/jem.20201656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang Z., Meszaros G., He W.T., Xu Y., de Fatima Magliarelli H., Mailly L., Mihlan M., Liu Y., Puig Gamez M., Goginashvili A., et al. Protein kinase D at the Golgi controls NLRP3 inflammasome activation. J. Exp. Med. 2017;214:2671–2693. doi: 10.1084/jem.20162040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Guo C., Xie S., Chi Z., Zhang J., Liu Y., Zhang L., Zheng M., Zhang X., Xia D., Ke Y., et al. Bile Acids Control Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome. Immunity. 2016;45:802–816. doi: 10.1016/j.immuni.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 157.Zhao W., Shi C.S., Harrison K., Hwang I.Y., Nabar N.R., Wang M., Kehrl J.H. AKT Regulates NLRP3 Inflammasome Activation by Phosphorylating NLRP3 Serine 5. J. Immunol. 2020;205:2255–2264. doi: 10.4049/jimmunol.2000649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Stutz A., Kolbe C.C., Stahl R., Horvath G.L., Franklin B.S., van Ray O., Brinkschulte R., Geyer M., Meissner F., Latz E. NLRP3 inflammasome assembly is regulated by phosphorylation of the pyrin domain. J. Exp. Med. 2017;214:1725–1736. doi: 10.1084/jem.20160933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fischer F.A., Mies L.F.M., Nizami S., Pantazi E., Danielli S., Demarco B., Ohlmeyer M., Lee M.S.J., Coban C., Kagan J.C., et al. TBK1 and IKKepsilon act like an OFF switch to limit NLRP3 inflammasome pathway activation. Proc. Natl. Acad. Sci. USA. 2021;118:e2009309118. doi: 10.1073/pnas.2009309118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tang J., Xiao Y., Lin G., Guo H., Deng H.X., Tu S., Langdon W.Y., Yang H., Tao L., Li Y., et al. Tyrosine phosphorylation of NLRP3 by the Src family kinase Lyn suppresses the activity of the NLRP3 inflammasome. Sci. Signal. 2021;14:eabe3410. doi: 10.1126/scisignal.abe3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Spalinger M.R., Kasper S., Gottier C., Lang S., Atrott K., Vavricka S.R., Scharl S., Raselli T., Frey-Wagner I., Gutte P.M., et al. NLRP3 tyrosine phosphorylation is controlled by protein tyrosine phosphatase PTPN22. J. Clin. Investig. 2016;126:1783–1800. doi: 10.1172/JCI83669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Huang Y., Wang H., Hao Y., Lin H., Dong M., Ye J., Song L., Wang Y., Li Q., Shan B., et al. Myeloid PTEN promotes chemotherapy-induced NLRP3-inflammasome activation and antitumour immunity. Nat. Cell Biol. 2020;22:716–727. doi: 10.1038/s41556-020-0510-3. [DOI] [PubMed] [Google Scholar]
- 163.Qin Y., Li Q., Liang W., Yan R., Tong L., Jia M., Zhao C., Zhao W. TRIM28 SUMOylates and stabilizes NLRP3 to facilitate inflammasome activation. Nat. Commun. 2021;12:4794. doi: 10.1038/s41467-021-25033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shao L., Liu Y., Wang W., Li A., Wan P., Liu W., Shereen M.A., Liu F., Zhang W., Tan Q., et al. SUMO1 SUMOylates and SENP3 deSUMOylates NLRP3 to orchestrate the inflammasome activation. FASEB J. 2020;34:1497–1515. doi: 10.1096/fj.201901653R. [DOI] [PubMed] [Google Scholar]
- 165.Barry R., John S.W., Liccardi G., Tenev T., Jaco I., Chen C.H., Choi J., Kasperkiewicz P., Fernandes-Alnemri T., Alnemri E., et al. SUMO-mediated regulation of NLRP3 modulates inflammasome activity. Nat. Commun. 2018;9:3001. doi: 10.1038/s41467-018-05321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Park Y.J., Yoon S.J., Suh H.W., Kim D.O., Park J.R., Jung H., Kim T.D., Yoon S.R., Min J.K., Na H.J., et al. TXNIP deficiency exacerbates endotoxic shock via the induction of excessive nitric oxide synthesis. PLoS Pathog. 2013;9:e1003646. doi: 10.1371/journal.ppat.1003646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mishra B.B., Rathinam V.A., Martens G.W., Martinot A.J., Kornfeld H., Fitzgerald K.A., Sassetti C.M. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1beta. Nat. Immunol. 2013;14:52–60. doi: 10.1038/ni.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hernandez-Cuellar E., Tsuchiya K., Hara H., Fang R., Sakai S., Kawamura I., Akira S., Mitsuyama M. Cutting edge: Nitric oxide inhibits the NLRP3 inflammasome. J. Immunol. 2012;189:5113–5117. doi: 10.4049/jimmunol.1202479. [DOI] [PubMed] [Google Scholar]
- 169.Juliana C., Fernandes-Alnemri T., Wu J., Datta P., Solorzano L., Yu J.W., Meng R., Quong A.A., Latz E., Scott C.P., et al. Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J. Biol. Chem. 2010;285:9792–9802. doi: 10.1074/jbc.M109.082305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cocco M., Miglio G., Giorgis M., Garella D., Marini E., Costale A., Regazzoni L., Vistoli G., Orioli M., Massulaha-Ahmed R., et al. Design, Synthesis, and Evaluation of Acrylamide Derivatives as Direct NLRP3 Inflammasome Inhibitors. ChemMedChem. 2016;11:1790–1803. doi: 10.1002/cmdc.201600055. [DOI] [PubMed] [Google Scholar]
- 171.He M., Chiang H.H., Luo H., Zheng Z., Qiao Q., Wang L., Tan M., Ohkubo R., Mu W.C., Zhao S., et al. An Acetylation Switch of the NLRP3 Inflammasome Regulates Aging-Associated Chronic Inflammation and Insulin Resistance. Cell Metab. 2020;31:580–591.e585. doi: 10.1016/j.cmet.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bambouskova M., Potuckova L., Paulenda T., Kerndl M., Mogilenko D.A., Lizotte K., Swain A., Hayes S., Sheldon R.D., Kim H., et al. Itaconate confers tolerance to late NLRP3 inflammasome activation. Cell Rep. 2021;34:108756. doi: 10.1016/j.celrep.2021.108756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hooftman A., Angiari S., Hester S., Corcoran S.E., Runtsch M.C., Ling C., Ruzek M.C., Slivka P.F., McGettrick A.F., Banahan K., et al. The Immunomodulatory Metabolite Itaconate Modifies NLRP3 and Inhibits Inflammasome Activation. Cell Metab. 2020;32:468–478.e467. doi: 10.1016/j.cmet.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kayagaki N., Wong M.T., Stowe I.B., Ramani S.R., Gonzalez L.C., Akashi-Takamura S., Miyake K., Zhang J., Lee W.P., Muszynski A., et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 175.Lee B.L., Stowe I.B., Gupta A., Kornfeld O.S., Roose-Girma M., Anderson K., Warming S., Zhang J., Lee W.P., Kayagaki N. Caspase-11 auto-proteolysis is crucial for noncanonical inflammasome activation. J. Exp. Med. 2018;215:2279–2288. doi: 10.1084/jem.20180589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lo T.H., Chen H.L., Yao C.I., Weng I.C., Li C.S., Huang C.C., Chen N.J., Lin C.H., Liu F.T. Galectin-3 promotes noncanonical inflammasome activation through intracellular binding to lipopolysaccharide glycans. Proc. Natl. Acad. Sci. USA. 2021;118:e2026246118. doi: 10.1073/pnas.2026246118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Moretti J., Jia B., Hutchins Z., Roy S., Yip H., Wu J., Shan M., Jaffrey S.R., Coers J., Blander J.M. Caspase-11 interaction with NLRP3 potentiates the noncanonical activation of the NLRP3 inflammasome. Nat. Immunol. 2022;23:705–717. doi: 10.1038/s41590-022-01192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Napier B.A., Brubaker S.W., Sweeney T.E., Monette P., Rothmeier G.H., Gertsvolf N.A., Puschnik A., Carette J.E., Khatri P., Monack D.M. Complement pathway amplifies caspase-11-dependent cell death and endotoxin-induced sepsis severity. J. Exp. Med. 2016;213:2365–2382. doi: 10.1084/jem.20160027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Man S.M., Karki R., Sasai M., Place D.E., Kesavardhana S., Temirov J., Frase S., Zhu Q., Malireddi R.K.S., Kuriakose T., et al. IRGB10 Liberates Bacterial Ligands for Sensing by the AIM2 and Caspase-11-NLRP3 Inflammasomes. Cell. 2016;167:382–396.e317. doi: 10.1016/j.cell.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Meunier E., Dick M.S., Dreier R.F., Schurmann N., Kenzelmann Broz D., Warming S., Roose-Girma M., Bumann D., Kayagaki N., Takeda K., et al. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature. 2014;509:366–370. doi: 10.1038/nature13157. [DOI] [PubMed] [Google Scholar]
- 181.Hagar J.A., Powell D.A., Aachoui Y., Ernst R.K., Miao E.A. Cytoplasmic LPS activates caspase-11: Implications in TLR4-independent endotoxic shock. Science. 2013;341:1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hoffman H.M., Mueller J.L., Broide D.H., Wanderer A.A., Kolodner R.D. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat. Genet. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Aksentijevich I., Nowak M., Mallah M., Chae J.J., Watford W.T., Hofmann S.R., Stein L., Russo R., Goldsmith D., Dent P., et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): A new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 2002;46:3340–3348. doi: 10.1002/art.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Karasawa T., Komada T., Yamada N., Aizawa E., Mizushina Y., Watanabe S., Baatarjav C., Matsumura T., Takahashi M. Cryo-sensitive aggregation triggers NLRP3 inflammasome assembly in cryopyrin-associated periodic syndrome. Elife. 2022;11:e75166. doi: 10.7554/eLife.75166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhang Y., Zhao Y., Zhang J., Yang G. Mechanisms of NLRP3 Inflammasome Activation: Its Role in the Treatment of Alzheimer’s Disease. Neurochem. Res. 2020;45:2560–2572. doi: 10.1007/s11064-020-03121-z. [DOI] [PubMed] [Google Scholar]
- 186.Lunemann J.D., Malhotra S., Shinohara M.L., Montalban X., Comabella M. Targeting Inflammasomes to Treat Neurological Diseases. Ann. Neurol. 2021;90:177–188. doi: 10.1002/ana.26158. [DOI] [PubMed] [Google Scholar]
- 187.Sharma B.R., Kanneganti T.D. NLRP3 inflammasome in cancer and metabolic diseases. Nat. Immunol. 2021;22:550–559. doi: 10.1038/s41590-021-00886-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Liao Y., Liu K., Zhu L. Emerging Roles of Inflammasomes in Cardiovascular Diseases. Front. Immunol. 2022;13:834289. doi: 10.3389/fimmu.2022.834289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Spel L., Martinon F. Inflammasomes contributing to inflammation in arthritis. Immunol. Rev. 2020;294:48–62. doi: 10.1111/imr.12839. [DOI] [PubMed] [Google Scholar]
- 190.Liu L., Dong Y., Ye M., Jin S., Yang J., Joosse M.E., Sun Y., Zhang J., Lazarev M., Brant S.R., et al. The Pathogenic Role of NLRP3 Inflammasome Activation in Inflammatory Bowel Diseases of Both Mice and Humans. J. Crohns Colitis. 2017;11:737–750. doi: 10.1093/ecco-jcc/jjw219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kahlenberg J.M., Kaplan M.J. The inflammasome and lupus: Another innate immune mechanism contributing to disease pathogenesis? Curr. Opin. Rheumatol. 2014;26:475–481. doi: 10.1097/BOR.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yang C.A., Huang S.T., Chiang B.L. Sex-dependent differential activation of NLRP3 and AIM2 inflammasomes in SLE macrophages. Rheumatology. 2015;54:324–331. doi: 10.1093/rheumatology/keu318. [DOI] [PubMed] [Google Scholar]
- 193.Wang D., Wang H., Gao H., Zhang H., Zhang H., Wang Q., Sun Z. P2X7 receptor mediates NLRP3 inflammasome activation in depression and diabetes. Cell Biosci. 2020;10:28. doi: 10.1186/s13578-020-00388-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Alcocer-Gomez E., Ulecia-Moron C., Marin-Aguilar F., Rybkina T., Casas-Barquero N., Ruiz-Cabello J., Ryffel B., Apetoh L., Ghiringhelli F., Bullon P., et al. Stress-Induced Depressive Behaviors Require a Functional NLRP3 Inflammasome. Mol. Neurobiol. 2016;53:4874–4882. doi: 10.1007/s12035-015-9408-7. [DOI] [PubMed] [Google Scholar]
- 195.Zhai Y., Meng X., Ye T., Xie W., Sun G., Sun X. Inhibiting the NLRP3 Inflammasome Activation with MCC950 Ameliorates Diabetic Encephalopathy in db/db Mice. Molecules. 2018;23:522. doi: 10.3390/molecules23030522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Navarro-Pando J.M., Alcocer-Gomez E., Castejon-Vega B., Navarro-Villaran E., Condes-Hervas M., Mundi-Roldan M., Muntane J., Perez-Pulido A.J., Bullon P., Wang C., et al. Inhibition of the NLRP3 inflammasome prevents ovarian aging. Sci. Adv. 2021;7:eabc7409. doi: 10.1126/sciadv.abc7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Karki R., Kanneganti T.D. Diverging inflammasome signals in tumorigenesis and potential targeting. Nat. Rev. Cancer. 2019;19:197–214. doi: 10.1038/s41568-019-0123-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Terlizzi M., Casolaro V., Pinto A., Sorrentino R. Inflammasome: Cancer’s friend or foe? Pharmacol. Ther. 2014;143:24–33. doi: 10.1016/j.pharmthera.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 199.Vora S.M., Lieberman J., Wu H. Inflammasome activation at the crux of severe COVID-19. Nat. Rev. Immunol. 2021;21:694–703. doi: 10.1038/s41577-021-00588-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Coll R.C., Robertson A.A., Chae J.J., Higgins S.C., Munoz-Planillo R., Inserra M.C., Vetter I., Dungan L.S., Monks B.G., Stutz A., et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 2015;21:248–255. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Coll R.C., Hill J.R., Day C.J., Zamoshnikova A., Boucher D., Massey N.L., Chitty J.L., Fraser J.A., Jennings M.P., Robertson A.A.B., et al. MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat. Chem. Biol. 2019;15:556–559. doi: 10.1038/s41589-019-0277-7. [DOI] [PubMed] [Google Scholar]
- 202.Tapia-Abellan A., Angosto-Bazarra D., Martinez-Banaclocha H., de Torre-Minguela C., Ceron-Carrasco J.P., Perez-Sanchez H., Arostegui J.I., Pelegrin P. MCC950 closes the active conformation of NLRP3 to an inactive state. Nat. Chem. Biol. 2019;15:560–564. doi: 10.1038/s41589-019-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mangan M.S.J., Olhava E.J., Roush W.R., Seidel H.M., Glick G.D., Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 2018;17:588–606. doi: 10.1038/nrd.2018.97. [DOI] [PubMed] [Google Scholar]
- 204.Mullard A. NLRP3 inhibitors stoke anti-inflammatory ambitions. Nat. Rev. Drug Discov. 2019;18:405–407. doi: 10.1038/d41573-019-00086-9. [DOI] [PubMed] [Google Scholar]
- 205.Chen Y., He H., Lin B., Chen Y., Deng X., Jiang W., Zhou R. RRx-001 ameliorates inflammatory diseases by acting as a potent covalent NLRP3 inhibitor. Cell Mol. Immunol. 2021;18:1425–1436. doi: 10.1038/s41423-021-00683-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ambati M., Apicella I., Wang S.B., Narendran S., Leung H., Pereira F., Nagasaka Y., Huang P., Varshney A., Baker K.L., et al. Identification of fluoxetine as a direct NLRP3 inhibitor to treat atrophic macular degeneration. Proc. Natl. Acad. Sci. USA. 2021;118:e2102975118. doi: 10.1073/pnas.2102975118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Soriano-Teruel P.M., Garcia-Lainez G., Marco-Salvador M., Pardo J., Arias M., DeFord C., Merfort I., Vicent M.J., Pelegrin P., Sancho M., et al. Identification of an ASC oligomerization inhibitor for the treatment of inflammatory diseases. Cell Death Dis. 2021;12:1155. doi: 10.1038/s41419-021-04420-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Desu H.L., Plastini M., Illiano P., Bramlett H.M., Dietrich W.D., de Rivero Vaccari J.P., Brambilla R., Keane R.W. IC100: A novel anti-ASC monoclonal antibody improves functional outcomes in an animal model of multiple sclerosis. J. Neuroinflamm. 2020;17:143. doi: 10.1186/s12974-020-01826-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Dai Z., Chen X.Y., An L.Y., Li C.C., Zhao N., Yang F., You S.T., Hou C.Z., Li K., Jiang C., et al. Development of Novel Tetrahydroquinoline Inhibitors of NLRP3 Inflammasome for Potential Treatment of DSS-Induced Mouse Colitis. J. Med. Chem. 2021;64:871–889. doi: 10.1021/acs.jmedchem.0c01924. [DOI] [PubMed] [Google Scholar]
- 210.Jiang H., He H., Chen Y., Huang W., Cheng J., Ye J., Wang A., Tao J., Wang C., Liu Q., et al. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J. Exp. Med. 2017;214:3219–3238. doi: 10.1084/jem.20171419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Marchetti C., Swartzwelter B., Gamboni F., Neff C.P., Richter K., Azam T., Carta S., Tengesdal I., Nemkov T., D’Alessandro A., et al. OLT1177, a beta-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc. Natl. Acad. Sci. USA. 2018;115:E1530–E1539. doi: 10.1073/pnas.1716095115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lonnemann N., Hosseini S., Marchetti C., Skouras D.B., Stefanoni D., D’Alessandro A., Dinarello C.A., Korte M. The NLRP3 inflammasome inhibitor OLT1177 rescues cognitive impairment in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2020;117:32145–32154. doi: 10.1073/pnas.2009680117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.He Y., Varadarajan S., Munoz-Planillo R., Burberry A., Nakamura Y., Nunez G. 3,4-methylenedioxy-beta-nitrostyrene inhibits NLRP3 inflammasome activation by blocking assembly of the inflammasome. J. Biol. Chem. 2014;289:1142–1150. doi: 10.1074/jbc.M113.515080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Shim D.W., Shin W.Y., Yu S.H., Kim B.H., Ye S.K., Koppula S., Won H.S., Kang T.B., Lee K.H. BOT-4-one attenuates NLRP3 inflammasome activation: NLRP3 alkylation leading to the regulation of its ATPase activity and ubiquitination. Sci. Rep. 2017;7:15020. doi: 10.1038/s41598-017-15314-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cocco M., Pellegrini C., Martinez-Banaclocha H., Giorgis M., Marini E., Costale A., Miglio G., Fornai M., Antonioli L., Lopez-Castejon G., et al. Development of an Acrylate Derivative Targeting the NLRP3 Inflammasome for the Treatment of Inflammatory Bowel Disease. J. Med. Chem. 2017;60:3656–3671. doi: 10.1021/acs.jmedchem.6b01624. [DOI] [PubMed] [Google Scholar]
- 216.Adinolfi E., Raffaghello L., Giuliani A.L., Cavazzini L., Capece M., Chiozzi P., Bianchi G., Kroemer G., Pistoia V., Di Virgilio F. Expression of P2X7 receptor increases in vivo tumor growth. Cancer Res. 2012;72:2957–2969. doi: 10.1158/0008-5472.CAN-11-1947. [DOI] [PubMed] [Google Scholar]
- 217.Wannamaker W., Davies R., Namchuk M., Pollard J., Ford P., Ku G., Decker C., Charifson P., Weber P., Germann U.A., et al. (S)-1-((S)-2-{[1-(4-amino-3-chloro-phenyl)-methanoyl]-amino}-3,3-dimethyl-butanoy l)-pyrrolidine-2-carboxylic acid ((2R,3S)-2-ethoxy-5-oxo-tetrahydro-furan-3-yl)-amide (VX-765), an orally available selective interleukin (IL)-converting enzyme/caspase-1 inhibitor, exhibits potent anti-inflammatory activities by inhibiting the release of IL-1beta and IL-18. J. Pharmacol. Exp. Ther. 2007;321:509–516. doi: 10.1124/jpet.106.111344. [DOI] [PubMed] [Google Scholar]
- 218.Foley K., Kast R.E., Altschuler E.L. Ritonavir and disulfiram have potential to inhibit caspase-1 mediated inflammation and reduce neurological sequelae after minor blast exposure. Med. Hypotheses. 2009;72:150–152. doi: 10.1016/j.mehy.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 219.Dinarello C.A., Simon A., van der Meer J.W. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 2012;11:633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yang J., Liu Z., Wang C., Yang R., Rathkey J.K., Pinkard O.W., Shi W., Chen Y., Dubyak G.R., Abbott D.W., et al. Mechanism of gasdermin D recognition by inflammatory caspases and their inhibition by a gasdermin D-derived peptide inhibitor. Proc. Natl. Acad. Sci. USA. 2018;115:6792–6797. doi: 10.1073/pnas.1800562115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hawkins P.N., Lachmann H.J., McDermott M.F. Interleukin-1-receptor antagonist in the Muckle-Wells syndrome. N. Engl. J. Med. 2003;348:2583–2584. doi: 10.1056/NEJM200306193482523. [DOI] [PubMed] [Google Scholar]
- 222.Lachmann H.J., Kone-Paut I., Kuemmerle-Deschner J.B., Leslie K.S., Hachulla E., Quartier P., Gitton X., Widmer A., Patel N., Hawkins P.N., et al. Use of canakinumab in the cryopyrin-associated periodic syndrome. N. Engl. J. Med. 2009;360:2416–2425. doi: 10.1056/NEJMoa0810787. [DOI] [PubMed] [Google Scholar]
- 223.Hoffman H.M., Throne M.L., Amar N.J., Sebai M., Kivitz A.J., Kavanaugh A., Weinstein S.P., Belomestnov P., Yancopoulos G.D., Stahl N., et al. Efficacy and safety of rilonacept (interleukin-1 Trap) in patients with cryopyrin-associated periodic syndromes: Results from two sequential placebo-controlled studies. Arthritis Rheum. 2008;58:2443–2452. doi: 10.1002/art.23687. [DOI] [PubMed] [Google Scholar]
- 224.Humphries F., Shmuel-Galia L., Ketelut-Carneiro N., Li S., Wang B., Nemmara V.V., Wilson R., Jiang Z., Khalighinejad F., Muneeruddin K., et al. Succination inactivates gasdermin D and blocks pyroptosis. Science. 2020;369:1633–1637. doi: 10.1126/science.abb9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Okondo M.C., Johnson D.C., Sridharan R., Go E.B., Chui A.J., Wang M.S., Poplawski S.E., Wu W., Liu Y., Lai J.H., et al. DPP8 and DPP9 inhibition induces pro-caspase-1-dependent monocyte and macrophage pyroptosis. Nat. Chem. Biol. 2017;13:46–53. doi: 10.1038/nchembio.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Gai K., Okondo M.C., Rao S.D., Chui A.J., Ball D.P., Johnson D.C., Bachovchin D.A. DPP8/9 inhibitors are universal activators of functional NLRP1 alleles. Cell Death Dis. 2019;10:587. doi: 10.1038/s41419-019-1817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Shi J., Xia Y., Wang H., Yi Z., Zhang R., Zhang X. Piperlongumine Is an NLRP3 Inhibitor with Anti-inflammatory Activity. Front. Pharmacol. 2021;12:818326. doi: 10.3389/fphar.2021.818326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Li Q., Feng H., Wang H., Wang Y., Mou W., Xu G., Zhang P., Li R., Shi W., Wang Z., et al. Licochalcone B specifically inhibits the NLRP3 inflammasome by disrupting NEK7-NLRP3 interaction. EMBO Rep. 2022;23:e53499. doi: 10.15252/embr.202153499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ahmed S., Kwatra M., Ranjan Panda S., Murty U.S.N., Naidu V.G.M. Andrographolide suppresses NLRP3 inflammasome activation in microglia through induction of parkin-mediated mitophagy in in-vitro and in-vivo models of Parkinson disease. Brain Behav. Immun. 2021;91:142–158. doi: 10.1016/j.bbi.2020.09.017. [DOI] [PubMed] [Google Scholar]
- 230.Yan W., Yu H., Liu B., Jiang Z., Jin H., Li Z., Li L., Zou D., Jiang H. Andrographolide suppresses osteoarthritis progression by regulating circ_Rapgef1/miR-383-3p/NLRP3 signaling axis. Transpl. Immunol. 2022;71:101548. doi: 10.1016/j.trim.2022.101548. [DOI] [PubMed] [Google Scholar]
- 231.Qin Q., Xu G., Zhan X., Wang Z., Wang Y., Liu H., Hou X., Shi W., Ma J., Bai Z., et al. Brevilin A inhibits NLRP3 inflammasome activation in vivo and in vitro by acting on the upstream of NLRP3-induced ASC oligomerization. Mol. Immunol. 2021;135:116–126. doi: 10.1016/j.molimm.2021.03.025. [DOI] [PubMed] [Google Scholar]
- 232.Zhao Q., Bi Y., Guo J., Liu Y.X., Zhong J., Pan L.R., Tan Y., Yu X.J. Pristimerin protects against inflammation and metabolic disorder in mice through inhibition of NLRP3 inflammasome activation. Acta Pharmacol. Sin. 2021;42:975–986. doi: 10.1038/s41401-020-00527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Chen L.Z., Zhang X.X., Liu M.M., Wu J., Ma D., Diao L.Z., Li Q., Huang Y.S., Zhang R., Ruan B.F., et al. Discovery of Novel Pterostilbene-Based Derivatives as Potent and Orally Active NLRP3 Inflammasome Inhibitors with Inflammatory Activity for Colitis. J. Med. Chem. 2021;64:13633–13657. doi: 10.1021/acs.jmedchem.1c01007. [DOI] [PubMed] [Google Scholar]
- 234.Zeng Q., Deng H., Li Y., Fan T., Liu Y., Tang S., Wei W., Liu X., Guo X., Jiang J., et al. Berberine Directly Targets the NEK7 Protein to Block the NEK7-NLRP3 Interaction and Exert Anti-inflammatory Activity. J. Med. Chem. 2021;64:768–781. doi: 10.1021/acs.jmedchem.0c01743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable.