Abstract
Lactic acid bacteria are food-grade microorganisms that are potentially good candidates for production of heterologous proteins of therapeutical or technological interest. We developed a model for heterologous protein secretion in Lactococcus lactis using the staphylococcal nuclease (Nuc). The effects on protein secretion of alterations in either (i) signal peptide or (ii) propeptide sequences were examined. (i) Replacement of the native Nuc signal peptide (SPNuc) by that of L. lactis protein Usp45 (SPUsp) resulted in greatly improved secretion efficiency (SE). Pulse-chase experiments showed that Nuc secretion kinetics was better when directed by SPUsp than when directed by SPNuc. This SPUsp effect on Nuc secretion is not due to a better antifolding activity, since SPUsp:Nuc precursor proteins display enzymatic activity in vitro, while SPNuc:Nuc precursor proteins do not. (ii) Deletion of the native Nuc propeptide dramatically reduces Nuc SE, regardless of which SP is used. We previously reported that a synthetic propeptide, LEISSTCDA, could efficiently replace the native Nuc propeptide to promote heterologous protein secretion in L. lactis (Y. Le Loir, A. Gruss, S. D. Ehrlich, and P. Langella, J. Bacteriol. 180:1895–1903, 1998). To determine whether the LEISSTCDA effect is due to its acidic residues, specific substitutions were introduced, resulting in neutral or basic propeptides. Effects of these two new propeptides and of a different acidic synthetic propeptide were tested. Acidic and neutral propeptides were equally effective in enhancing Nuc SE and also increased Nuc yields. In contrast, the basic propeptide strongly reduced both SE and the quantity of secreted Nuc. We have shown that the combination of the native SPUsp and a neutral or acidic synthetic propeptide leads to a significant improvement in SE and in the quantity of synthesized Nuc. These observations will be valuable in the production of heterologous proteins in L. lactis.
Gram-positive lactic acid bacteria (LAB) are widely used in food industries for the production and preservation of fermented products. They are considered safe and even beneficial organisms. The potential of using LAB for new applications such as in production of heterologous proteins for biotechnology, in fermented food products, or in the digestive tract of humans or animals is currently under active study (3, 11, 13, 17, 19, 22, 30, 49, 54).
We are focused on optimizing heterologous protein secretion and export in Lactococcus lactis (30, 32), a well-characterized LAB for which genetic tools and the genome sequence are available (5, 11). To date, heterologous proteins such as bovine plasmin (3), bovine beta-lactoglobulin (BLG [6a], bovine rotavirus nonstructural protein 4 (NSP4 [13a]), murine interleukin-2 (IL-2) and IL-6 (54), or Listeria monocytogenes bacteriophage lysin (17) have been fused to lactococcal signal peptides (SPs) to direct their secretion in the medium. However, secretion efficiency (SE) has been rarely evaluated, and comparison of SE using native or heterologous SP has not been performed. The extent to which these and other features can be refined or improved to optimize protein secretion in L. lactis is the subject of this study.
In bacteria, most proteins that are secreted via the Sec pathway are synthesized as precursors containing the mature protein and an N-terminal SP (61) that is essential for precursor secretion. Although the primary sequences are poorly conserved, all SPs display a common tripartite structure including a positively charged N terminus, a hydrophobic core, and a neutral or negatively charged C terminus containing the SP cleavage site (61). Nevertheless, SPs of gram-positive bacteria are longer than those of gram-negative bacteria (61). Therefore, a gram-negative SP may be unable to direct secretion of a protein in a gram-positive host (8). Moreover, in a given species, the SE of a protein can vary with the SP chosen to direct its secretion (40, 47).
Even with the appropriate SP, secretion may be inefficient, and some heterologous proteins remain poorly or not at all secreted, even when fused to a homologous SP (6a, 13a, 46, 47). Notably, the N terminus of the mature moiety may greatly affect the translocation efficiency across the cytoplasmic membrane (2, 32). In Escherichia coli, the charge balance between the N termini of the SP and of the mature moiety may be critical for SE (26, 60). Although this charge balance rule was clearly demonstrated for gram-negative bacterial precursors, it may not apply to all gram-positive bacterial and eukaryotic precursors (25, 26). Until now, no detailed investigation was performed on charge balance in protein secretion in LAB.
Some precursors are synthesized as preproproteins, in which the SP is followed by a propeptide that is cleaved after translocation, giving rise to the mature protein (for a review, see reference 50). The propeptides can reportedly influence protein activities as well as SE. The antifolding activity and the role of the long class I propeptides (e.g., propeptides of proteases) in SE have been clearly demonstrated, whereas that of the short class II propeptides, e.g., the Staphylococcus aureus nuclease (Nuc), the Bacillus amyloliquefaciens barnase, or the Bacillus subtilis amylase, is less studied (39, 57). In S. aureus, the Nuc protein containing the propeptide (NucB) is localized in the cell wall, whereas the cleaved mature protein (NucA) is in the medium (9). Nevertheless, this localization is not observed in other hosts such as L. lactis or Corynebacterium glutamicum (32, 34). Results for E. coli demonstrated that the Nuc propeptide slows precursor folding, plays a positive role in SE of a fusion using an E. coli SP, and alleviates SecA dependency of precursor secretion in E. coli (55).
The secretion capacity of L. lactis was previously investigated using Nuc as a secretion reporter (32). Nuc, a small and stable secreted protein, is genetically and biochemically well characterized (51). Its enzymatic activity is readily detectable on petri plates as well as in zymograms (31, 34). Translational fusions to the N terminus and/or the C terminus of the mature protein are enzymatically active (30, 32, 41). SPNuc is atypical, as it is unusually long (60 residues) and contains two hydrophobic stretches that may form a hairpin in the cytoplasmic membrane during translocation (27). In L. lactis, as in E. coli, the native Nuc propeptide greatly affects SE. Furthermore, replacement of the native Nuc propeptide by a synthetic one can restore or even enhance SE (32).
Here, we examine the effects of changing the SP and/or propeptide on the secretion and enzymatic activity of the Nuc reporter. We found that the use of the homologous Usp45 SP (SPUsp [58,59]) significantly improves SE. Furthermore, the Nuc propeptide is required for an optimal Nuc SE but can be replaced by synthetic propeptides that are acidic or neutral. The activities and role of charge balance in the enhancement capacity of these propeptides are discussed. The combination of SPUsp and a synthetic propeptide resulted in significant enhancement in SE and also in overall production yields of Nuc. These observations will be valuable in the production of heterologous proteins in L. lactis.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and growth conditions.
E. coli strain TG1 (20) and L. lactis strains MG1363 (18) and NZ9000 (28) were used as hosts. Plasmids used are described in Fig. 1 and listed in Table 1. E. coli was grown on Luria-Bertani medium (48) and incubated at 37°C. L. lactis was grown on M17 medium (56) in which lactose was replaced by 0.5% glucose (M17-glu; Difco) and on brain heart infusion (Difco) and incubated at 30°C. SA medium was used to grow L. lactis for pulse-chase experiments (24). Antibiotics were added at the given concentrations: erythromycin, 5 μg/ml for L. lactis or 150 μg/ml for E. coli; chloramphenicol, 5 μg/ml for L. lactis and E. coli; and ampicillin, 100 μg/ml for E. coli. Induction of the nisin promoter was carried out as follows: an overnight culture was diluted 1:250 into fresh medium and incubated at 30°C until the optical density at 600 nm reached ∼0.5. The culture was then divided into two equal volumes, and 1 ng of nisin/ml was added in one tube. The other tube was kept as the noninduced culture control. Cultures were further incubated, and protein samples were prepared after 1 h of induction.
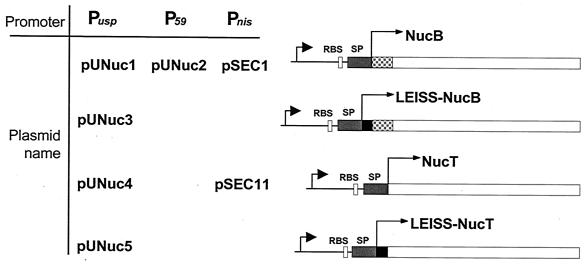
FIG. 1.
Expression cassettes for Nuc production and export using the host SPUsp. Schematic structures of fusion proteins (right panel) expressed under the indicated promoters and carried by the indicated plasmids (left panel) are shown. For details of plasmid construction, see the text and Table 1. Black arrowheads indicate L. lactis promoter sequences of either the usp45 gene (Pusp), the strong promoter (P59), or the nisin-inducible promoter (Pnis). RBS, ribosome binding site of the usp45 gene; gray bar (SP), Usp45 SP coding region; gray checkered bar, native propeptide coding region; black bar, LEISSTCDA synthetic propeptide coding region; open bar, NucB or NucT coding sequence (not to scale).
TABLE 1.
Plasmids used in this work
| Plasmid | Replicon | Plasmid characteristic(s); cloned nuc or usp45 characteristic(s) | Reference or source |
|---|---|---|---|
| pBSa | ColE1 | Apr | Stratagene |
| pVE3556 | pAMβ1 | Emr, derivative of high-copy-number plasmid pIL253 | 31 |
| pNZ1011 | pUC19 | Apr, usp45 gene | 58 |
| pBS:Nuc1b | ColE1 | Apr; nuc gene expressed from native staphylococcal promoter, Pstaf | 31 |
| pBS:Nuc2b | ColE | Apr; gene, expressed from Pstaf, encodes SPNuc:LEISSTCDA-NucB precursor | 31 |
| pBS:Nuc3 | ColE1 | Apr; promoterless nuc gene | 32 |
| pBS:Nuc4 | ColE1 | Apr; gene, expressed from P59, encodes SPNuc:LEISSTCDA-NucB precursor | 32 |
| pBS:Nuc5 | ColE1 | Apr; DNA encodes SPNuc:NucT (not expressed) | 32 |
| pBS:Nuc6 | ColE1 | Apr; gene, expressed from P59, encodes SPNuc:NucB precursor | 32 |
| pBS:Nuc7 | ColE1 | Apr /Emr; gene, expressed from Pstaf, encodes SPNuc:LGISSTCNA-NucB precursor | This work |
| pBS:Nuc8 | ColE1 | Apr /Emr; gene, expressed from Pstaf, encodes SPNuc:LQVDDIPSA-NucB precursor | This work |
| pBS:Nuc9 | ColE1 | Apr /Emr; gene, expressed from Pstaf, encodes SPNuc:LKISSTCHA-NucB precursor | This work |
| pNuc6 | pVE3556 | Emr; gene, expressed from P59, encodes SPNuc:NucB precursor | 32 |
| pNuc9 | pVE3556 | Emr; gene, expressed from P59, encodes SPNuc:NucT precursor | 32 |
| pNuc13 | ColE1:pVE3556 | Apr/Emr; gene, expressed from Pstaf, encodes SPNuc:NucB precursor | This work |
| pNuc14 | ColE1:pVE3556 | Apr/Emr; gene, expressed from Pstaf, encodes SPNuc:LEISSTCDA-NucB precursor | This work |
| pNuc15 | ColE1:pVE3556 | Apr/Emr; gene, expressed from Pstaf, encodes SPNuc:LGISSTCNA-NucB precursor | This work |
| pNuc16 | ColE1:pVE3556 | Apr/Emr; gene, expressed from Pstaf, encodes SPNuc:LQVDDIPSA-NucB precursor | This work |
| pNuc17 | ColE1:pVE3556 | Apr/Emr; gene, expressed from Pstaf, encodes SPNuc:LKISSTCHA-NucB precursor | This work |
| pBS:U1 | ColE1 | Apr; PCR fragment encoding Pusp promoter and SPUsp; amplified with oligonucleotides 5 and 6 | This work |
| pBS:UNuc1 | ColE1 | Apr; PCR fragment encoding SPUsp and nuc mature form; amplified with oligonucleotides 7 and 8 | This work |
| pBS:UNuc2 | ColE1 | Apr; gene, expressed from P59 encodes SPUsp:NucB precursor | This work |
| pBS:UNuc3 | ColE1 | Apr; gene, expressed from Pusp encodes SPUsp:NucB precursor | This work |
| pBS:UNuc4 | ColE1 | Apr; gene, expressed from Pusp encodes SPUsp:NucT precursor | This work |
| pBS:UNuc5 | ColE1 | Apr; gene, expressed from Pusp encodes SPUsp:LEISSTCDA-NucB precursor | This work |
| pBS:UNuc6 | ColE1 | Apr; gene, expressed from Pusp encodes SPUsp:LEISSTCDA-NucT precursor | This work |
| pUNuc1 | pVE3556 | Emr; gene, expressed from Pusp, encodes SPUsp:NucB precursor | This work |
| pUNuc2 | ColE1:pVE3556 | Apr/Emr; gene, expressed from P59, encodes SPUsp:NucB precursor | This work |
| pUNuc3 | pVE3556 | Emr; gene, expressed from Pusp, encodes SPUsp:LEISSTCDA-NucB precursor | This work |
| pUNuc4 | pVE3556 | Emr; gene, expressed from Pusp, encodes SPUsp:NucT precursor | This work |
| pUNuc5 | pVE3556 | Emr; gene, expressed from Pusp, encodes SPUsp:LEISSTCDA-NucT precursor | This work |
| pVE3655 | pWV01 | Cmr; carries the nisin-inducible promoter PnisA | P. Langella |
| pSEC1c | pWV01 | Cmr; gene, expressed under PnisA, encodes SPUsp:NucB precursor | 6a |
| pSEC11 | pWV01 | Cmr; gene, expressed under PnisA, encodes SPUsp:NucT precursor | This work |
DNA manipulation.
Whole-cell lysates were prepared as described previously (44), except that proteinase K was added after lysozyme treatment. This additional proteolytic step eliminates mature Nuc forms associated with protoplasts prior to cell lysis.
Plasmid DNA was isolated essentially as described elsewhere (4), except that, for L. lactis, TES buffer (sucrose, 25%; EDTA, 1 mM; Tris-HCl, 50 mM [pH 8]) containing 10 mg of lysozyme/ml was used for 10 min at 37°C to prepare protoplasts. Enzymes were used as recommended by the suppliers. General procedures for DNA manipulations were performed as described elsewhere (32). Electroporation of L. lactis was performed as described elsewhere (29), and transformants were plated on M17-glu agar or brain heart infusion agar plates containing the required antibiotic.
Design of synthetic propeptides.
PCRs were performed with a Perkin-Elmer Cetus (Norwalk, Conn.) apparatus using Thermophilus aquaticus DNA polymerase (Promega) as recommended by the manufacturer. Oligonucleotides were synthesized by MWG Biotech (see Table 2).
TABLE 2.
Oligonucleotides used in this study
| Oligonucleotide no. | Sequencea | Description | Reference |
|---|---|---|---|
| 1 | 5′ CTC GGA ATA TCG TCG ACT TGT AAT GCA 3′ | Neutral propeptide coding strand | This work |
| L G I S S T C N A | |||
| 2 | 5′ TT ACA AGT CGA TAT TCC GAG TGC A 3′ | Neutral propeptide complementary strand | This work |
| 3 | 5′ CTC AAA ATA TCG TCG ACT TGT CAT GCA 3′ | Basic propeptide coding strand | This work |
| L K I S S T C H A | |||
| 4 | 5′ TG ACA AGT CGA CGA TAT TTT GAG TGC A 3′ | Basic propeptide complementary strand | This work |
| 5 | 5′ TCT AGA GCG CCT ACA CTT TTG CTC 3′ | Pusp:SPusp amplification, forward primer | This work |
| 6 | 5′ GTA TGC ATA AAC ACC TGA CAA CGG GG 3′ | Pusp:SPusp amplification, reverse primer | This work |
| NsiI | |||
| 7 | 5′ GTCTAGAACCGAACTTAATGGGAG 3′ | SPusp:nuc amplification, forward primer | This work |
| 8 | 5′ GGAATTCCGATCTAAAAATTATAAAAGT 3′ | SPusp:nuc amplification, reverse primer | 31 |
The sequence shown below the oligonucleotide sequence is the peptide sequence.
To modify the LEISSTCDA synthetic propeptide sequence, a set of oligonucleotides were designed in which acidic residues (glutamate and aspartate) were replaced by neutral (glycine and asparagine, in oligonucleotides 1 and 2, respectively) or basic (lysine and histidine, in oligonucleotides 3 and 4, respectively) residues (see Table 2). The modified oligonucleotides were inserted in NsiI-cut pBS:Nuc1 (31). Both orientations were obtained for each oligonucleotide. When cloned in the noncoding orientation, oligonucleotides 3 and 4 encoded a stop codon and oligonucleotides 1 and 2 encoded a nine-residue propeptide, the sequence of which was LQVDDIPSA. This latter propeptide contained two acidic amino acid residues and was also used to test the effect of negatively charged residues at positions 4 and 5 (instead of 2 and 8). The resulting plasmids are pBS:Nuc7, pBS:Nuc8, and pBS:Nuc9 (listed in Table 1). All constructions were confirmed by DNA sequencing.
To replace SPNuc by the Usp45 signal peptide (SPUsp), a 291-bp fragment was PCR amplified from pNZ1011 matrix (58) (see Table 1) with oligonucleotides 5 and 6 (see sequences in Table 2). This PCR fragment also contains the usp45 promoter region (Pusp) including 121 bp upstream of the −35 sequence. The reverse primer (oligonucleotide 6) was designed such that an NsiI site was introduced in the last two codons of the fragment. Insertion of an NsiI site allows cloning of fragments encoding the mature Nuc without changing the −2 and −1 residues of SPUsp. This DNA fragment was then cloned on pBluescript (pBS) vector in E. coli TG1, resulting in pBS:U1.
An SPUsp:NucB fusion was obtained by joining the NsiI-SpeI fragment of pBS:Nuc3 containing the nuc mature moiety to NsiI-SpeI-cut pBS:U1, resulting in pBS:UNuc3. In pBS:UNuc3, the production of SPUsp:NucB is controlled by the Pusp promoter. To test the effect of different promoters on SPUsp:NucB secretion, Pusp was deleted from pBS:UNuc3 by PCR amplification using oligonucleotides 7 and 8 (see sequences in Table 2). A 695-bp DNA fragment was generated (SPusp:nucB) and cloned into SmaI-cut pBS vector in E. coli TG1, resulting in pBS:UNuc1.
Derivatives of SPusp:nuc fusions.
To generate a Nuc derivative devoid of its propeptide, a DNA fragment containing nucT (32) was isolated from NsiI-XbaI-cut pBS:Nuc5 and cloned into an NsiI-XbaI-cut pBS:UNuc3 backbone, resulting in pBS:UNuc5. The NucT mature form contains three positive charges in its first 10 residues (32). A synthetic oligonucleotide encoding LEISSTCDA (32) was inserted into NsiI-cut pBS:UNuc3, resulting in pBS:UNuc5. To generate an SPUsp:LEISSTCDA:NucT fusion, an NsiI-XbaI-cut nucT fragment was cloned into an NsiI-XbaI-cut pBS:UNuc5 backbone, resulting in pBS:UNuc6. These SPusp:nuc derivative cassettes were introduced in L. lactis MG1363 as SacI-XhoI or SacI-EcoRI fragments cloned into a SacI-XhoI- or SacI-EcoRI-digested pVE3556 backbone vector resulting in plasmids pUNuc1 and pUNuc3 (SacI-XhoI cloning) or pUNuc4 and pUNuc5 (SacI-EcoRI cloning), respectively (Table 1).
High constitutive expression of the SPUsp:NucB precursor was obtained from plasmid pUNuc2, which was constructed as follows. First, the fragment encoding native SPNuc:NucB on pBS:Nuc6 was replaced by an SPusp:nucB cassette isolated from pBS:UNuc1, resulting in pBS:UNuc2. Plasmid pUNuc2 was obtained as a cointegrate of pBS:UNuc2 and pVE3556 joined at the SacI site.
Inducible expression.
Nisin-controlled expression is a tightly controlled expression system with high levels of induction (10). Abundant precursor is accumulated using this system, which allowed us to examine the enzymatic activities of the two SPUsp:NucB and SPUsp:NucT precursor forms. The corresponding encoding cassettes were placed under the transcriptional control of the nisin-inducible promoter (PnisA), resulting in plasmids pSEC1 (6a) and pSEC11. For each pSEC plasmid, a XhoI-BamHI SPuspnucB cassette was cloned into a XhoI-BamHI-cut pVE3655 backbone vector, which contains PnisA, followed by a multicloning site. The latter plasmid is a derivative of pNZ8010 (10) (kindly provided by Oscar Kuipers), from which the gus gene, expressed from PnisA, was deleted by XbaI digestion. Constructions were obtained in E. coli TG1 and then established in L. lactis NZ9000 (kindly provided by O. Kuipers [28]), a derivative of L. lactis MG1363 that carries the nisRK regulatory genes.
Expression of novel propeptide fusions to Nuc in L. lactis.
To test the effect of the different synthetic propeptides on secretion efficiency (the proportion of total protein present in mature secreted form, SE) in L. lactis, plasmids pNuc13 to pNuc17 were established in strain MG1363 as cointegrates between XbaI-cut pVE3556 and XbaI-cut pBS:Nuc1, pBS:Nuc2, pBS:Nuc7, pBS:Nuc8, and pBS:Nuc9. The orientation of the resulting cointegrates was determined by restriction analysis, and plasmids harboring the same backbone structure were selected for further experiments.
Preparation of protein extracts and detection of Nuc fusions by immunoblotting.
Protein samples from L. lactis cultures were prepared as described previously (32). Briefly, for cell fractionation, 2 ml of L. lactis exponential-phase cultures was harvested after a 5-min centrifugation at 6,000 × g at 4°C. Cell and supernatant fractions were treated separately. Supernatants were filtered on 0.2-μm-pore-size filters (Millipore, Bedford, Mass.) and precipitated with trichloroacetic acid (15% final concentration). Cell pellets were washed and resuspended in TES, prior to trichloroacetic acid precipitation (10% final concentration). Cell pellets were then washed once with 1 ml of cold acetone, dried, and resuspended in TES containing lysozyme (1 mg/ml; 30 min at 37°C). Cells were lysed with 20% sodium dodecyl sulfate (SDS). Equal volumes of 2× loading buffer were added to all samples. Both supernatant and cell fractions were denatured (5 min at 95°C) prior to SDS-polyacrylamide gel electrophoresis (PAGE).
SE was determined by scanning different nonsaturated film exposures and using the ImageQuant program to get average values. SDS-PAGE, electroblotting on polyvinylidene difluoride membranes (Millipore), and immunoblotting were performed as described elsewhere (32) or according to the manufacturer's recommendations. Rabbit anti-Nuc antibodies were kindly provided by J. R. Miller. Immunodetection was performed with protein G horseradish peroxidase conjugate (Bio-Rad) and an enhanced chemiluminescence kit (Dupont-NEN) as recommended by the suppliers. To evaluate Nuc distribution or to quantitate Nuc SE, several (three to six, depending on construction) independent samples were prepared. Samples to be compared were prepared at the same time and loaded on the same gel. After enhanced chemiluminescence detection, different nonsaturated film exposures were scanned by a Scanjet II (Hewlett-Packard) using Deskscan II and ImageQuant programs and average values were determined. For quantification, signals were compared to those of known amounts of a commercial NucA sample. Both B and A forms of Nuc were included in these estimations.
Pulse-chase conditions.
Pulse-chase experiments were performed essentially as described previously (32). An overnight culture of the appropriate L. lactis strain grown on SA medium (24) was used to inoculate, at 2%, 20 ml of SA medium with 33.5 μM methionine (Met). Cells were grown at 30°C to an optical density at 600 nm of 0.5; 10 ml of culture was then harvested and washed in SA medium without Met. Cells were resuspended in 2 ml of SA medium without Met and incubated at 30°C for 2 min. Cultures were pulse-labeled for 1 min by the addition of 10 μl of [35S]Met (10 mCi/ml). Seven hundred microliters of Met (5%; 2,500,000-fold excess) was added (chase), and 250-μl samples were taken at given time intervals. Samples were prepared and immunoprecipitated as previously described (32, 37).
Nuc plate activity assays and zymogram.
Nuc plate assays were performed as described previously (31). Nuc enzyme activity was evaluated on zymograms. After SDS-PAGE, protein samples were renatured as described elsewhere (34). A 2-mm toluidine blue-DNA agar (TBD-agar) layer was poured in the vertical support used for the polyacrylamide gel (PAG) (Protean II; Bio-Rad). After polymerization, the TBD-agar layer is kept on a single glass plate and dried at 55°C for 30 min. The renatured PAG is then placed on the TBD-agar layer covered with plastic film and incubated at 37°C for 1 to 5 h, depending on the amount of Nuc protein that was loaded in the PAG. After the appearance of bands corresponding to Nuc activity, the zymogram is photographed. Note that the SDS-PAG can be stained with Coomassie blue prior to renaturation treatment. The staining remains after renaturation and does not prevent visualization of Nuc activity (data not shown).
RESULTS
Replacement of SPNuc by the lactococcal SPUsp enhances Nuc secretion in L. lactis.
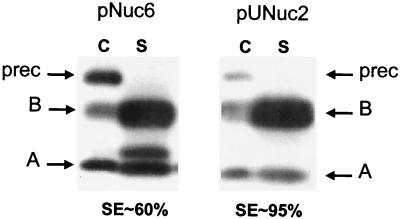
Nuc secretion in L. lactis driven by the SPNuc signal is inefficient (32). We therefore tested the effect of replacing SPNuc by SPUsp, the SP of the major L. lactis secreted protein Usp45 (58). SPUsp comprises 27 residues and is typical of gram-positive bacterial SPs (59). The SPUsp:NucB fusion was constructed and expressed from the Pusp promoter (plasmid pUNuc1) (Table 1 and Fig. 1). Western blotting was performed to compare secretion of SPUsp:NucB to that of SPNuc:NucB (the native Nuc protein is encoded by pNuc6) (data not shown). SE was around 95% with SPUsp, compared to 60% with the native SPNuc. However, in these experiments, expression was driven by different-strength promoters (Pusp on pUNuc1 is weaker than P59 on pNuc6); Northern blotting confirmed that Nuc expression from pUNuc1 was about eightfold lower than that observed from pNuc6 (data not shown). To test whether improved SE of SPUsp:NucB was due to lower-level Nuc expression on pUNuc1, expression was ensured by the P59 promoter (on plasmid pUNuc2) (Table 1 and Fig. 1). Nuc secretion was also very efficient with this construction compared to that of SPNuc:NucB (Fig. 2). Note that, in Western experiments, some mature NucA is found associated with the cell fraction (in the cell wall), as already shown and discussed by Dieye et al. (12) and Liebl et al. (34), in L. lactis and C. glutamicum, respectively. Immunodetection of L. lactis Usp45 protein on these samples showed no accumulation of intracellular precursor, indicating that Usp45 secretion was not altered by high-level secretion of Nuc driven by a common SP (data not shown). These results show that replacement of SPNuc by SPUsp leads to efficient secretion of Nuc even at high expression levels.
FIG. 2.
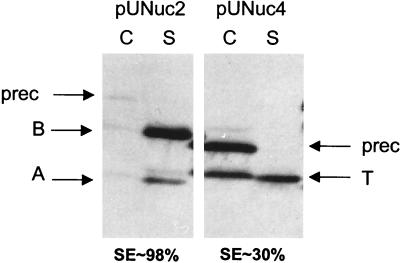
Replacement of SPNuc by SPUsp improves Nuc SE. Nuc SE was estimated by Western blot analysis on exponential-phase cultures of lactococcal strains containing pNuc6 (encoding SPNuc:NucB) and pUNuc2 (encoding SPUsp:NucB). Migration positions of precursor forms (prec) or mature forms of both NucA (A) and NucB (B) are indicated by arrows. C, cell lysates; S, supernatant fraction; SE, the proportion of total protein present in mature secreted form.
SPUsp:NucB is more efficiently processed than SPNuc:NucB.
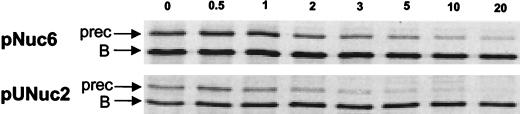
Processing of the precursors SPUsp:NucB and SPNuc:NucB was analyzed by pulse-chase labeling experiments using [35S]Met (Fig. 3). The effect of SP replacement on SE in SA, the medium used for pulse-chase labeling, was found to be comparable with that observed in rich medium (data not shown). The proportions of precursor and mature NucB in pulse-labeled SPNuc:NucB and SPUsp:NucB expressed from pNuc6 and pUNuc2, respectively, were comparable at time zero and 1 min after the chase. SPNuc:NucB was still present but at decreasing concentrations at 5, 10, and 20 min. In contrast, SPUsp:NucB was detected in only trace amounts at 5 min and was absent at 10 and 20 min. These results are consistent with the conclusion that SPUsp:NucB is more efficiently processed in L. lactis than is SPNuc:NucB.
FIG. 3.
Comparison of kinetics of SPNuc:NucB and SPUsp:NucB precursor processing by pulse-chase experiments. MG1363 containing pNuc6 (encoding SPNuc:NucB precursor) (upper panel) or pUNuc 2 (encoding SPUsp:NucB precursor) (lower panel) was grown in SA medium (24) and pulse-labeled with [35S]Met for 1 min. Samples were taken at different times after the pulse as indicated (in minutes). Time zero corresponds to a sample taken just at the end of the pulse. Migration positions of the different Nuc species are indicated by arrows, prec, precursor; B, NucB.
The antifolding activity of SPUsp is not better than that of SPNuc.
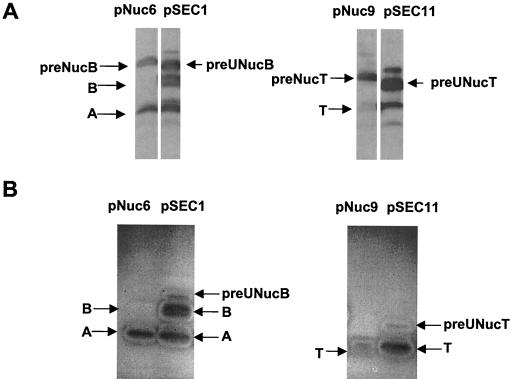
The more efficient processing of SPUsp:NucB could be due to an antifolding activity of SPUsp higher than that of SPNuc (intramolecular feature). The antifolding activities of the SPs were compared in vitro by means of a Nuc activity assay (zymogram). Constructs in which expression is driven by a nisin-inducible promoter were used to achieve high-level accumulation of the SPUsp:Nuc(B or T) precursor forms (Table 1). Using this system, SPNuc:Nuc(B or T) and SPUsp:Nuc(B or T) forms were detected in cell extracts by Western blotting (Fig. 4A). Enzymatically active forms were examined by zymograms on samples run on an SDS-PAG. No activity was detected for the precursors SPNuc:NucB and SPNuc:NucT (Fig. 4B) even after long exposure of zymograms, thus suggesting that precursor SPNuc:Nuc(B or T) is inactive in vitro. Similar results were already obtained with SPNuc:NucB produced from pNuc6 (32). In contrast, Nuc activity bands were detected for both SPUsp:NucB and SPUsp:NucT (Fig. 4B). Although amounts of precursor SPUsp:Nuc(B or T) and SPNuc:Nuc(B or T) in cell fractions are comparable as revealed by the Western blot, only precursors comprising SPUsp are active in zymograms. Nevertheless, the precursor forms show very weak activity compared to that of mature Nuc forms, suggesting that SPUsp impairs enzyme activity in vitro.
FIG. 4.
Detection of enzymatic Nuc activity in precursor depends on the nature of the SP used to drive Nuc secretion. Protein samples were prepared from overnight cultures of lactococcal strains containing pNuc6 or pNuc9 (carrying cassette P59SPNuc:NucB or P59SPNuc:NucT, respectively) or nisin-induced cultures of lactococcal strains containing pSEC1 or pSEC11 (carrying cassette PnisASPUsp:NucB or PnisASPUsp:NucT, respectively). Strains containing pNuc6 and pNuc9 accumulate precursor forms in cell fraction. To achieve such accumulation with SPUsp, strains containing pSEC1 and pSEC11 were strongly induced with 10 ng of nisin/ml for 1 h. (A) Western blot analysis of cell fractions of lactococcal strains producing Nuc. A faint band is visible upon the precursor band for pSEC1 or pSEC11. This band corresponds probably to precursor aggregation due to overproduction of SPUsp:NucB and SPUsp:NucT. (B) Zymogram for detection of enzyme activity performed with the same protein samples after SDS-PAGE and gel renaturation. The positions of SPUsp:NucB (preUNucB), SPUsp:NucT (preUNucT), SPNuc:NucB (preNucB), and SPNuc:NucT (preNucT) precursor forms and of NucB (B), NucT (T), and NucA (A) mature forms are indicated by arrows.
To test for SPUsp:Nuc activity in vivo, whole-cell lysates were prepared on cultures producing SPNuc:NucB or SPUsp:NucB and compared with a nonproducing strain. Whole-cell lysates were analyzed by agarose gel electrophoresis, and total genomic DNA was visualized by ethidium bromide staining. No DNA hydrolysis was detected in any sample (data not shown). We conclude from these results and in keeping with the good growth of strains producing SPUsp:NucB that this precursor is active in vitro but not in vivo. This is consistent with the data of Poquet et al., who demonstrated that a mature form of Nuc produced in the cytoplasm is enzymatically active in the zymogram but inactive in vivo (42).
Altogether, these results suggest that the antifolding activity of SPUsp is not better than that of SPNuc and thus suggest that the secretion enhancement is due to a better interaction between the precursor bearing the homologous SPUsp and the host secretion chaperones (intermolecular feature).
Deletion of the natural propeptide severely reduces Nuc SE in L. lactis.
The native Nuc propeptide is necessary for efficient secretion of Nuc driven by its native SP in L. lactis (32). The putative positive effects of the Nuc propeptide were also evaluated with the SPUsp in place of the native SPNuc. A transcriptional and translational fusion between usp45 expression and secretion signals and a fragment encoding NucT (devoid of its natural propeptide [32]) was constructed to produce SPUsp:NucT (encoded by pUNuc4) (Table 1 and Fig. 1). SPUsp:NucB (pUNuc1) and SPUsp:NucT (pUNuc4) secretion levels were compared by Western blotting on protein samples prepared on exponential cultures of the corresponding L. lactis strains (Fig. 5). The total amounts of detected Nuc forms are comparable for the two strains. However, SE of SPUsp:NucT is only 30%, compared to around 95% for SPUsp:NucB. Some mature NucT is also found associated with the cell fraction; this was previously observed for native NucA and/or NucT forms in L. lactis and C. glutamicum (12, 32, 34). It could be due to electrostatic interactions between negatively charged cell wall and charged residues in the N terminus of NucA and NucT (12). These results confirm that the Nuc propeptide is needed for efficient Nuc secretion in L. lactis and that this effect is independent of the SP used.
FIG. 5.
Deletion of the natural propeptide strongly decreases Nuc SE. Nuc SE was estimated by Western blot analysis on exponential-phase cultures of lactococcal strains containing pUNuc1 (encoding SPUsp:NucB) and pUNuc4 (encoding SPUsp:NucT). Migration positions of precursor forms (prec) or mature forms of NucA (A), NucB (B), and NucT (T) are indicated by arrows. C, cell lysates; S, supernatant fraction; SE, the proportion of total protein which is present in mature secreted form.
The synthetic propeptide LEISSTCDA improves both the SE and yields of NucB and NucT secreted via SPUsp.
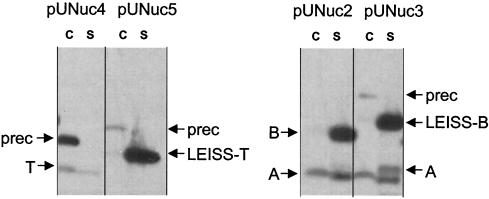
The synthetic propeptide LEISSTCDA exerts a positive effect when acting in combination with the native SPNuc (32). To test its effects when combined with a nonnative SP, we constructed fusions SPUsp:NucT (encoded by pUNuc4) and SPUsp:LEISSTCDA-NucT (encoded by pUNuc5) (Table 1 and Fig. 1) and compared secretion profiles by Western blotting (Fig. 6). SPUsp:LEISSTCDA-NucT processing was significantly more efficient (above 95%) than that of SPUsp:NucT (30%). In addition, SPUsp:LEISSTCDA-NucT also displayed a three- to fourfold-greater overall Nuc yield than did SPUsp:NucT (Fig. 6).
FIG. 6.
The synthetic propeptide enhances Nuc SE when used in combination with SPUsp. Nuc SE was estimated by Western blot analysis on exponential-phase cultures of four lactococcal Nuc-producing strains. Right panel, MG1363 containing pUNuc4 (encoding SPUsp:NucT) and pUNuc5 (encoding SPUsp:LEISSTCDA:NucT). Left panel, MG1363 containing pUNuc1 (encoding SPUsp:NucB) and pUNuc3 (encoding SPUsp:LEISSTCDA:NucB). Migration positions of precursor forms (prec) or mature forms of NucA (A), NucB (B), NucT (T), LEISSTCDA:NucT (LEISS-T), and LEISSTCDA:NucB (LEISS-B) are indicated by arrows. C, cell lysates; S, supernatant fraction.
These results suggest that the LEISSTCDA propeptide may positively affect protein yield and/or the SE. To separate these potential effects, we examined the effects of LEISSTCDA propeptide on a protein that has a high SE, SPUsp:NucB. Nuc secretion was analyzed in L. lactis strains producing SPUsp:NucB (from pUNuc1) and SPUsp:LEISSTCDA-NucB (from pUNuc3) (Fig. 6). As shown above, the SE of SPUsp:NucB is already optimal (above 95%). Nevertheless, LEISSTCDA confers a significant increase (three- to fourfold) in the total Nuc yield. Analysis of plasmid DNA and Northern blotting confirmed that this increase was not due to differences in plasmid copy number or amounts of mRNA (data not shown). Furthermore, similar increases of detected Nuc forms were also observed when SPUsp:LEISSTCDA-Nuc fusions were expressed from other promoters (data not shown). These results show that the LEISSTCDA synthetic propeptide may have positive effects on both SE (e.g., for SPUsp:LEISSTCDA-NucT) and yield (e.g., for SPUsp:LEISSTCDA-NucB).
The combination of these results shows that the enhancing effect of the synthetic propeptide on SE and yield does not depend on the nature of the SP used to direct Nuc secretion. Similar results were obtained when the LEISSTCDA propeptide preceded other heterologous secreted proteins (L. A. Ribeiro, V. Azevedo, Y. Le Loir, S. C. Oliveira, Y. Dieye, J. C. Piard, A. Gruss, and P. Langella, submitted for publication).
The SE of Nuc in L. lactis depends on the net global charge of the N terminus of the mature moiety.
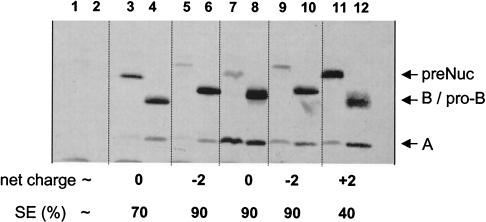
LEISSTCDA is characterized by its net global negative charge (−2), conferred by acidic amino acid residues at positions +2 and +8. To test whether these two acidic residues are necessary for secretion enhancement by LEISSTCDA, two new propeptides were designed such that acidic amino acids were replaced by neutral or basic residues: LGISSTCNA (neutral global net charge) and LKISSTCHA (positive global net charge of +2). A third propeptide, LQVDDIPSA, was also obtained; it has a different primary structure but contains two acidic residues at positions +4 and +5 (see Materials and Methods) (Table 2 and Fig. 7). The effects of the different propeptides were evaluated on SPNuc:NucB. L. lactis MG1363 strains secreting native NucB and LEISSTCDA-NucB were used as controls. Exponential-phase L. lactis cultures containing the different Nuc fusions were processed and examined by Western blotting. In each case, the three expected Nuc forms (precursor and mature NucB and NucA) were detected (Fig. 7). The presence of basic residues (on LKISSTCHA) drastically reduced Nuc SE, to around 40% (Fig. 7, lanes 11 and 12), compared to 70% obtained with native Nuc (Fig. 7, lanes 3 and 4) and 90% obtained with SPNuc:LEISSTCDA-Nuc (Fig. 7, lanes 5 and 6). The LKISSTCHA-Nuc fusion was found mainly in precursor form. This effect is consistent with our previous finding that NucT (containing three positive charges in the first 10 residues of the mature moiety) has an SE of 30% (32). In contrast, introduction of two different negatively charged propeptides (Fig. 7, lanes 5, 6, 7, and 8) leads to a higher SE and an increased yield compared to that of native Nuc (Fig. 7, lanes 3 and 4). Insertion of a neutral propeptide also increases Nuc SE (Fig. 7, lanes 7 and 8) as well as the quantity of secreted Nuc. A maximum increase in secreted Nuc yield was approximately fourfold compared to the control (Fig. 7, lanes 3 and 4). These results show that (i) a mature protein with a positively charged N-terminal end is poorly secreted in L. lactis and (ii) both negatively charged and neutral propeptides enhance Nuc secretion in L. lactis and exhibit a dual effect of improving SE and increasing protein yield. Taken together, these findings suggest that a synthetic propeptide (devoid of basic residues) may act as a spacer that separates the globular Nuc protein from the region involved in Nuc maturation by the signal peptidase and thus facilitates precursor processing in L. lactis.
FIG. 7.
Secretion profiles of Nuc with or without synthetic propeptide derivatives. Nuc SE was estimated by Western blot analysis on cell and supernatant fractions extracted from exponential-phase L. lactis cultures. Supernatant and cell fractions were prepared separately, and immunodetection of the different Nuc forms was performed after SDS-PAGE. SE and the net charge of the first 10 residues of the mature moiety (net charge) are given below each lane. Strains contain the cloning vector alone (lanes 1 and 2); pNuc13 encoding SPNuc:Nuc (lanes 3 and 4); pNuc14 encoding the fusion protein containing the original synthetic propeptide SPNuc:LEISSTCDA-Nuc (lanes 5 and 6); and the fusion proteins containing the mutated propeptides SPNuc:LGISSTCNA-Nuc (lanes 7 and 8), SPNuc:LQVDDIPSA-Nuc (lanes 9 and 10), and SPNuc:LKISSTCHA-Nuc (lanes 11 and 12). We noted heterogeneity in the apparent sizes of precursors and secreted proproteins on SDS-PAGE, possibly due to charge modifications introduced on the synthetic propeptides. preNuc, native Nuc precursor form; B, NucB; A, NucA; pro-B, mature forms of propeptides fused to NucB.
DISCUSSION
SP effects on Nuc secretion.
SPs, although poorly conserved in their primary structure, are characterized by a conserved tripartite secondary structure (61). In comparison, SPNuc has an atypical structure (37). It is 60 residues long (the mean size of gram-positive bacterial SP is 28 residues [57]) and contains two highly hydrophobic stretches of approximately the same length separated by a hydrophilic region containing three basic residues. Miller et al. (37) proposed a model for insertion of native SPNuc:Nuc precursor in the bacterial membrane where SPNuc forms a hairpin. This atypical structure may be poorly recognized by the lactococcal secretion machinery, thereby explaining why Nuc precursor accumulates in L. lactis.
The Usp45 signal peptide (SPUsp) has a more consensual structure. The N-terminal region of Usp45 (including SPUsp and, in some cases, several amino acids of the mature protein) has already been used to drive secretion of heterologous proteins in L. lactis, e.g., α-amylase (59), bovine plasmin (3), IL-2 and IL-6 (54), Nuc (12, 42), BLG (6a), NSP4 (13a), and lipase (13). When estimated, SE of these different fusions was heterogeneous. For instance, α-amylase fusion to SPUsp resulted in 80% of precursor accumulation in the cell fraction (59). Precursor accumulation was also observed for lipase fusions (13), whereas fusion of Nuc to SPUsp plus the first 16 residues of the Usp45 mature moiety resulted in a good SE (12, 42).
We compared here the Nuc SE driven by its native SP with the Nuc SE driven by the homologous SPUsp. Western blotting and pulse-chase experiments revealed a significant increase of Nuc SE when SPUsp was used. Altogether, these results show that the use of a homologous SP may be necessary, but not sufficient, to guarantee efficient protein secretion. When homologous SP does not improve the SE of a given protein, some alternative tools such as synthetic propeptides may be useful as mentioned below.
How does the homologous SPUsp enhance Nuc SE?
The SP reportedly acts as an intramolecular chaperone to retard protein folding. The SP thus facilitates interactions with chaperones dedicated to secretion and participates in maintaining the precursor in a conformation compatible with translocation (14, 45, 57). In vitro studies demonstrate that interaction between SP and the mature protein moiety can greatly retard the kinetics of protein folding (35). Nevertheless, in the absence of an external chaperone, although folding is retarded, it often occurs, resulting in precursor activity (as shown elsewhere for several enzymes [6, 15, 23, 53]). However, in vivo, cytoplasmic activity of a secreted protein could be lethal for the cell; precursor activity may be prevented through interactions with the secretion machinery or a dedicated inhibitor (1, 7, 21). Here, we confirmed that SPNuc:Nuc precursors are inactive in vitro (32), suggesting a strong intramolecular chaperone activity for SPNuc. In contrast, SPUsp:Nuc precursors have some enzymatic activity in zymogram tests. This result shows that the two SPs have different antifolding capacities. The lower antifolding capacity of SPUsp suggests that its intramolecular chaperone activity is not better than that of SPNuc. SPUsp may then improve Nuc SE by allowing a better recognition of SPUsp:Nuc precursor by the lactococcal secretion machinery (intermolecular interaction).
Effects of native and synthetic propeptides on SE of Nuc.
Long propeptides that are present, for example, in proproteases have intramolecular chaperone activities and are involved in protein folding, protein secretion, and inhibition of enzyme activity (52). However, little is known about the role of short propeptides (e.g., those present in barnase, some amylases, and Nuc). Our studies rule out a role of Nuc propeptide in Nuc enzymatic activity, in keeping with previous reports (9, 32). In L. lactis, we observed a positive effect of the natural Nuc propeptide that is independent of the SP that precedes it. A synthetic propeptide, LEISSTCDA, was previously described as a secretion enhancer that can mimic the positive role of native Nuc propeptide in the SE and yield of both NucB and NucT (32).
In addition to LEISSTCDA propeptide, LQVDDIPSA and LGISSTCNA have similar effects in improving NucB secretion. All these peptides are devoid of basic residues. These results suggest that acidic and neutral residues are equally efficient in enhancing Nuc secretion in L. lactis. It is notable that other SPUsp fusions that include an N terminus having a global net charge of −2 also appeared to be efficiently secreted (12, 42). In contrast, a fusion to the basic propeptide LKISSTCHA is very poorly secreted in L. lactis. The behavior of these fusions suggests that, at least for the gram-positive L. lactis, proteins designed for membrane translocation have similar charge requirements as in E. coli. In both cases, basic residues at the mature N terminus may drastically reduce SE (33, 36), while the presence of an acidic or neutral spacer improves SE.
Effect of synthetic propeptide on protein yield.
The combination of the host SPUsp with synthetic propeptide LEISSTCDA led to protein yields slightly higher (around 25 mg/liter) than those observed with pNuc7 (described in reference 32, combining SPNuc and LEISSTCDA). Possibly, in the absence of any synthetic propeptide, the precursor SPUsp:Nuc is subject to a partial intracellular degradation; in this case, the real SE would actually be lower than the apparent SE. In the case of NucB, this may result in an apparent optimal SE. The insertion of a synthetic propeptide could affect the charge balance in the area of the SP cleavage site and/or the conformation of the precursor. The resulting effect could render the precursor less sensitive to intracellular degradation and/or could help it to escape the intracellular degradation thanks to a better SE. Degradation of hybrid precursor has already been observed in B. subtilis due to a poor SE of this precursor (38). Hybrid precursor could be a target for degradation by intracellular or membrane proteases such as ClpP (16) or HtrA (43). We are currently addressing this question by comparing yields and SEs of protein fusions in the different mutant backgrounds.
Combination of homologous SP and synthetic propeptide for the design of new secretion tools.
The SPUsp:LEISSTCDA combination can be used to direct secretion of other heterologous proteins in L. lactis (Ribeiro et al., submitted). In some cases, protein production is increased when LEISSTCDA propeptide is inserted between SPUsp and the mature moiety of the hybrid precursor (Ribeiro et al., submitted). In those studies, the synthetic propeptide insertion did not interfere with antigenic properties or with activity of the heterologous protein. These results indicate that the synthetic propeptide can improve secretion of heterologous proteins other than Nuc. Recently, we successfully used, in L. lactis, the combination of SPUsp and LEISSTCDA to improve the secretion of L7/L12, the Brucella abortus immunodominant antigen (Ribeiro et al., submitted). The host range of this combination is being currently evaluated. We propose that the combination of SPUsp and a properly designed synthetic propeptide such as the nonapeptides reported here could be a valuable tool for enhancement of secretion of heterologous proteins in gram-positive bacteria, including various LAB species such as Streptococcus thermophilus, Lactobacillus casei, and Lactobacillus sakei.
ACKNOWLEDGMENTS
We thank James R. Miller and Willem M. de Vos for their generous gifts of antisera against Nuc and Usp45, respectively. We thank Oscar P. Kuipers for the L. lactis strain NZ9000 and for the plasmid pNZ8020. We are grateful to Yakhya Dieye, Jean-Christophe Piard, Isabelle Poquet, and Luciana Ribeiro for helpful discussions during the course of this work.
REFERENCES
- 1.Ahrenholtz I, Lorenz M G, Wackernagel W. A conditional suicide system in Escherichia coli based on the intracellular degradation of DNA. Appl Environ Microbiol. 1994;60:3746–3751. doi: 10.1128/aem.60.10.3746-3751.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson H, von Heijne G. A 30-residue-long “export initiation domain” adjacent to the signal sequence is critical for protein translocation across the inner membrane of Escherichia coli. Proc Natl Acad Sci USA. 1991;88:9751–9754. doi: 10.1073/pnas.88.21.9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnau J, Hjerl-Hansen E, Israelsen H. Heterologous gene expression of bovine plasmin in Lactococcus lactis. Appl Environ Microbiol. 1997;48:331–338. doi: 10.1007/s002530051058. [DOI] [PubMed] [Google Scholar]
- 4.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolotin A, Wincker P, Mauger S, Jaillon O, Malarme K, Weissenbach J, Ehrlich S D, Sorokin A. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 2001;11:731–753. doi: 10.1101/gr.169701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buist G, Kok J, Leenhouts K J, Dabrowska M, Venema G, Haandrikman A J. Molecular cloning and nucleotide sequence of the gene encoding the major peptidoglycan hydrolase of Lactococcus lactis, a muramidase needed for cell separation. J Bacteriol. 1995;177:1554–1563. doi: 10.1128/jb.177.6.1554-1563.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Chatel J M, Langella P, Adel-Patient K, Commissaire J, Wal J M, Corthier G. Induction of mucosal immune response after intranasal or oral inoculation of mice with Lactococcus lactis producing bovine beta-lactoglobulin. Clin Diagn Lab Immunol. 2001;8:545–551. doi: 10.1128/CDLI.8.3.545-551.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen M W, Nagarajan V. The roles of signal peptide and mature protein in RNase (barnase) export from Bacillus subtilis. Mol Gen Genet. 1993;239:409–415. doi: 10.1007/BF00276939. [DOI] [PubMed] [Google Scholar]
- 8.Collier D N. Escherichia coli signal peptides direct inefficient secretion of an outer membrane protein (OmpA) and periplasmic proteins (maltose-binding protein, ribose-binding protein, and alkaline phosphatase) in Bacillus subtilis. J Bacteriol. 1994;176:3013–3020. doi: 10.1128/jb.176.10.3013-3020.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis A, Moore I B, Parker D S, Taniuchi H. Nuclease B: a possible precursor of nuclease A, an extracellular nuclease of Staphylococcus aureus. J Biol Chem. 1977;252:6544–6553. [PubMed] [Google Scholar]
- 10.de Ruyter P G, Kuipers O P, de Vos W M. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl Environ Microbiol. 1996;62:3662–3667. doi: 10.1128/aem.62.10.3662-3667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Vos W M. Gene expression systems for lactic acid bacteria. Curr Opin Microbiol. 1999;2:289–295. doi: 10.1016/S1369-5274(99)80050-2. [DOI] [PubMed] [Google Scholar]
- 12.Dieye Y, Usai S, Clier F, Gruss A, Piard J C. Design of a protein-targeting system for lactic acid bacteria. J Bacteriol. 2001;183:4157–4166. doi: 10.1128/JB.183.14.4157-4166.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drouault S, Corthier G, Ehrlich S D, Renault P. Expression of the Staphylococcus hyicus lipase in Lactococcus lactis. Appl Environ Microbiol. 2000;66:588–598. doi: 10.1128/aem.66.2.588-598.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Enouf V, Langella P, Commissaire J, Cohen J, Corthier G. Bovine rotavirus nonstructural protein 4 produced by Lactococcus lactis is antigenic and immunogenic. Appl Environ Microbiol. 2001;67:1423–1428. doi: 10.1128/AEM.67.4.1423-1428.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fekkes P, Driessen A J. Protein targeting to the bacterial cytoplasmic membrane. Microbiol Mol Biol Rev. 1999;63:161–173. doi: 10.1128/mmbr.63.1.161-173.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferenci T, Randall L L. Precursor of maltose-binding protein is active in binding substrate. J Biol Chem. 1979;254:9979–9981. [PubMed] [Google Scholar]
- 16.Frees D, Ingmer H. ClpP participates in the degradation of misfolded protein in Lactococcus lactis. Mol Microbiol. 1999;31:79–87. doi: 10.1046/j.1365-2958.1999.01149.x. [DOI] [PubMed] [Google Scholar]
- 17.Gaeng S, Scherer S, Neve H, Loessner M J. Gene cloning and expression of Listeria monocytogenes bacteriophage-lytic enzymes in Lactococcus lactis. Appl Environ Microbiol. 2000;66:2951–2958. doi: 10.1128/aem.66.7.2951-2958.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gasson M J. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic acid streptococci after protoplast-induced curing. J Bacteriol. 1983;154:1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geoffroy M C, Guyard C, Quatannens B, Pavan S, Lange M, Mercenier A. Use of green fluorescent protein to tag lactic acid bacterium strains under development as live vaccine vectors. Appl Environ Microbiol. 2000;66:383–391. doi: 10.1128/aem.66.1.383-391.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibson T J. Ph.D. thesis. Cambridge, England: University of Cambridge; 1984. [Google Scholar]
- 21.Hartley R W. Barnase and Barstar. Expression of its cloned inhibitor permits expression of a cloned ribonuclease. J Mol Biol. 1988;202:913–915. doi: 10.1016/0022-2836(88)90568-2. [DOI] [PubMed] [Google Scholar]
- 22.Hols P, Slos P, Dutot P, Reymund J, Chabot P, Delplace B, Delcour J, Mercenier A. Efficient secretion of the model antigen M6-gp41E in Lactobacillus plantarum NCIMB 8826. Microbiology. 1997;143:2733–2741. doi: 10.1099/00221287-143-8-2733. [DOI] [PubMed] [Google Scholar]
- 23.Inouye M, Beckwith J. Synthesis and processing of an Escherichia coli alkaline phosphatase in vitro. Proc Natl Acad Sci USA. 1977;74:1440–1444. doi: 10.1073/pnas.74.4.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen P R, Hammer K. Minimal requirements for exponential growth of Lactococcus lactis. Appl Environ Microbiol. 1993;59:4363–4366. doi: 10.1128/aem.59.12.4363-4366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johansson M, Nilsson I, von Heijne G. Positively charged amino acids placed next to the signal sequence block protein translocation more efficiently in Escherichia coli than in mammalian microsomes. Mol Gen Genet. 1993;239:251–256. doi: 10.1007/BF00281625. [DOI] [PubMed] [Google Scholar]
- 26.Kajava A V, Zolov S N, Kalinin A E, Nesmeyanova M A. The net charge of the first 18 residues of the mature sequence affects protein translocation across the cytoplasmic membrane of gram-negative bacteria. J Bacteriol. 2000;182:2163–2169. doi: 10.1128/jb.182.8.2163-2169.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovacevic S, Veal L E, Hsiung H M, Miller J R. Secretion of staphylococcal nuclease by Bacillus subtilis. J Bacteriol. 1985;162:521–528. doi: 10.1128/jb.162.2.521-528.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuipers O P, de Ruyter P G, Kleerebezen M, de Vos W M. Quorum sensing-controlled gene expression in lactic acid bacteria. J Biotechnol. 1998;64:15–21. [Google Scholar]
- 29.Langella P, Le Loir Y, Ehrlich S D, Gruss A. Efficient plasmid mobilization by pIP501 in Lactococcus lactis subsp. lactis. J Bacteriol. 1993;175:5806–5813. doi: 10.1128/jb.175.18.5806-5813.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langella P, Le Loir Y. Heterologous protein secretion in Lactococcus lactis: a novel antigen delivery system. Braz J Med Biol Res. 1999;32:191–198. doi: 10.1590/s0100-879x1999000200007. [DOI] [PubMed] [Google Scholar]
- 31.Le Loir Y, Gruss A, Ehrlich S D, Langella P. Direct screening of recombinants in gram-positive bacteria using the secreted staphylococcal nuclease as a reporter. J Bacteriol. 1994;176:5135–5139. doi: 10.1128/jb.176.16.5135-5139.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Loir Y, Gruss A, Ehrlich S D, Langella P. A nine-residue synthetic propeptide enhances secretion efficiency of heterologous proteins in Lactococcus lactis. J Bacteriol. 1998;180:1895–1903. doi: 10.1128/jb.180.7.1895-1903.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li P J, Beckwith J, Inouye H. Alteration of the amino terminus of the mature sequence of a periplasmic protein can severely affect protein export in Escherichia coli. Proc Natl Acad Sci USA. 1988;85:7685–7689. doi: 10.1073/pnas.85.20.7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liebl W, Sinskey A J, Schleifer K H. Expression, secretion, and processing of staphylococcal nuclease by Corynebacterium glutamicum. J Bacteriol. 1992;174:1854–1861. doi: 10.1128/jb.174.6.1854-1861.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu G, Topping T B, Randall L L. Physiological role during export for the retardation of folding by the leader peptide of maltose-binding protein. Proc Natl Acad Sci USA. 1989;86:9213–9217. doi: 10.1073/pnas.86.23.9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacIntyre S, Eschbach M L, Mutschler B. Export incompatibility of N-terminal basic residues in a mature polypeptide of Escherichia coli can be alleviated by optimising the signal peptide. Mol Gen Genet. 1990;221:466–474. doi: 10.1007/BF00259413. [DOI] [PubMed] [Google Scholar]
- 37.Miller J R, Kovacevic S, Veal L E. Secretion and processing of staphylococcal nuclease by Bacillus subtilis. J Bacteriol. 1987;169:3508–3514. doi: 10.1128/jb.169.8.3508-3514.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagarajan V, Borchert T V. Levansucrase: a tool to study protein secretion in Bacillus subtilis. Res Microbiol. 1991;142:787–792. doi: 10.1016/0923-2508(91)90056-g. [DOI] [PubMed] [Google Scholar]
- 39.Nagarajan V. Protein secretion. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria. Washington, D.C.: American Society for Microbiology; 1993. pp. 713–726. [Google Scholar]
- 40.Page N, Kluepfel D, Shareck F, Morosoli R. Effect of signal peptide alterations and replacement on export of xylanase A in Streptomyces lividans. Appl Environ Microbiol. 1996;62:109–114. doi: 10.1128/aem.62.1.109-114.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piard J C, Jimenez-Diaz R, Ehrlich S D, Fischetti V A, Gruss A. The M6 protein of Streptococcus pyogenes and its potential as a tool to anchor biologically active molecules at the surface of lactic acid bacteria. Adv Exp Med Biol. 1997;418:545–550. doi: 10.1007/978-1-4899-1825-3_126. [DOI] [PubMed] [Google Scholar]
- 42.Poquet I, Ehrlich S D, Gruss A. An export-specific reporter designed for gram-positive bacteria: application to Lactococcus lactis. J Bacteriol. 1998;180:1904–1912. doi: 10.1128/jb.180.7.1904-1912.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poquet I, Saint V, Seznec E, Simoes N, Bolotin A, Gruss A. HtrA is the unique surface housekeeping protease in Lactococcus lactis and is required for natural protein processing. Mol Microbiol. 2000;35:1042–1051. doi: 10.1046/j.1365-2958.2000.01757.x. [DOI] [PubMed] [Google Scholar]
- 44.Projan S, Carlton S, Novick R. Determination of plasmid copy number by fluorescence densitometry. Plasmid. 1983;9:182–190. doi: 10.1016/0147-619x(83)90019-7. [DOI] [PubMed] [Google Scholar]
- 45.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puohiniemi R, Simonen M, Muttilainen S, Himanen J P, Sarvas M. Secretion of Escherichia coli outer membrane proteins OmpA and OmpF in Bacillus subtilis is blocked at an early intracellular step. Mol Microbiol. 1992;6:981–990. doi: 10.1111/j.1365-2958.1992.tb02164.x. [DOI] [PubMed] [Google Scholar]
- 47.Ravn P, Arnau J, Madsen S M, Wrang A, Israelsen H. The development of TnNuc and its use for the isolation of novel secretion signals in Lactococcus lactis. Gene. 2000;242:347–356. doi: 10.1016/s0378-1119(99)00530-2. [DOI] [PubMed] [Google Scholar]
- 48.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 49.Savijoki K, Kahala M, Palva A. High-level heterologous protein production in Lactococcus and Lactobacillus using a new secretion system based on the Lactobacillus brevis S-layer signals. Gene. 1997;186:255–262. doi: 10.1016/s0378-1119(96)00717-2. [DOI] [PubMed] [Google Scholar]
- 50.Shinde U, Inouye M. Intramolecular chaperones: polypeptide extensions that modulate protein folding. Semin Cell Dev Biol. 2000;11:35–44. doi: 10.1006/scdb.1999.0349. [DOI] [PubMed] [Google Scholar]
- 51.Shortle D. A genetic system analysis of staphylococcal nuclease. Gene. 1983;22:181–189. doi: 10.1016/0378-1119(83)90102-6. [DOI] [PubMed] [Google Scholar]
- 52.Simonen M, Palva I. Protein secretion in Bacillus species. Microbiol Rev. 1993;57:109–137. doi: 10.1128/mr.57.1.109-137.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith H, de Jong A, Bron S, Venema G. Characterization of signal-sequence-encoding regions selected from Bacillus subtilis chromosome. Gene. 1988;70:351–361. doi: 10.1016/0378-1119(88)90207-7. [DOI] [PubMed] [Google Scholar]
- 54.Steidler L, Robinson K, Chamberlain L, Schofield K M, Remaut E, Le Page R W, Wells J M. Mucosal delivery of murine interleukin-2 (IL-2) and IL-6 by recombinant strains of Lactococcus lactis coexpressing antigen and cytokine. Infect Immun. 1998;66:3183–3189. doi: 10.1128/iai.66.7.3183-3189.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suciu D, Inouye M. The 19-residue propeptide of staphylococcal nuclease has a profound secretion-enhancing ability in Escherichia coli. Mol Microbiol. 1996;21:181–195. doi: 10.1046/j.1365-2958.1996.6211341.x. [DOI] [PubMed] [Google Scholar]
- 56.Terzaghi B E, Sandine W E. Improved medium for lactic streptococci and their bacteriophages. Appl Environ Microbiol. 1975;29:807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tjalsma H, Bolhuis A, Jongbloed J D H, Bron S, van Dijl J M. Signal peptide-dependent protein transport in Bacillus subtilis: a genome-based survey of the secretome. Microbiol Mol Biol Rev. 2000;64:515–547. doi: 10.1128/mmbr.64.3.515-547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Asseldonk M, Rutten G, Oteman M, Siezen R J, de Vos W M, Simons G. Cloning, expression in Escherichia coli and characterization of usp45, a gene encoding a highly secreted protein from Lactococcus lactis MG1363. Gene. 1990;95:155–160. doi: 10.1016/0378-1119(90)90428-t. [DOI] [PubMed] [Google Scholar]
- 59.van Asseldonk M, de Vos W M, Simons G. Functional analysis of the Lactococcus lactis usp45 secretion signal in the secretion of a homologous proteinase and a heterologous α-amylase. Mol Gen Genet. 1993;240:428–434. doi: 10.1007/BF00280397. [DOI] [PubMed] [Google Scholar]
- 60.von Heijne G. Net N-C charge imbalance may be important for signal sequence function in bacteria. J Mol Biol. 1986;192:287–290. doi: 10.1016/0022-2836(86)90365-7. [DOI] [PubMed] [Google Scholar]
- 61.von Heijne G. The signal peptide. J Membr Biol. 1990;115:195–201. doi: 10.1007/BF01868635. [DOI] [PubMed] [Google Scholar]