Abstract
Bipolar androgen therapy (BAT) is a new treatment concept for men whose prostate cancer has become resistant to standard hormone‐blocking therapy. Over the past decade, we have performed a series of clinical studies testing BAT in asymptomatic men with castration‐resistant prostate cancer. The key findings from these clinical studies are that BAT (a) can be safely administered to asymptomatic patients with metastatic castrate‐resistant prostate cancer; (b) does not produce symptomatic disease progression; (c) produces sustained prostate‐specific antigen and objective responses in 30%–40% of patients; and (d) can resensitize and prolong response to subsequent antiandrogen therapy. The concept of BAT has generated significant interest from men with prostate cancer, their families, and their physicians. Here we provide a “Patient's Guide” that answers questions about BAT in a form that is accessible to patients, their families, and physicians. Our goal is to provide information to help patients make the most informed decisions they can regarding their prostate cancer treatment.
Keywords: androgen deprivation, antiandrogen, bipolar androgen therapy, castration resistant
1. INTRODUCTION
Bipolar androgen therapy (BAT) is a new treatment concept for men whose prostate cancer has become resistant to standard hormone‐blocking therapy. 1 , 10 At first blush, the concept of using high doses of the male hormone testosterone as a treatment for men with advanced prostate cancer seems crazy. However, over the past decade, our team at Johns Hopkins has performed a series of clinical studies testing BAT in asymptomatic men with castration‐resistant prostate cancer. These studies showed that BAT was safe, could block prostate cancer growth and progression, and could improve quality of life (QOL) in some men. The concept of BAT has generated significant interest from men with prostate cancer, their families, and their physicians. Here we provide a “Patient's Guide” that answers questions about BAT in a form that is accessible to patients, their families, and physicians. Our goal is to provide information to help patients make the most informed decisions they can regarding their prostate cancer treatment.
2. WHAT IS HORMONE THERAPY FOR ADVANCED PROSTATE CANCER?
In 1941, a urologist at the University of Chicago named Dr. Charles Huggins published the first paper showing the almost miraculous effects of surgical removal of the testicles, a procedure also known as surgical castration or orchiectomy, to relieve pain in men with end‐stage metastatic prostate cancer. Since that time, therapies aimed at depriving prostate cancer cells of the male hormone testosterone have remained the mainstay of treatment. This testosterone deprivation can be achieved through surgical removal of the testicles, which produce most of the testosterone in a man. Since most men prefer not to part with their testicles, drugs have been developed that can also turn off testicular production of testosterone. These drugs interfere with normal production of another hormone called luteinizing hormone‐releasing hormone that is made by the brain and controls the amount of testosterone in the blood. This treatment is also referred to as medical castration. Drugs in this class come in long‐acting injectable forms that include Lupron, Eligard, Zoladex, Trelstar, and Firmagon and a new oral form called Orgovyx.
2.1. Hormone therapy
“Hormone therapy” is a broad term used to describe any type of therapy that blocks testosterone production or action to treat prostate cancer. Although it is actually a misnomer because it is technically “anti‐hormonal therapy,” the former term has stuck. Male hormones, including testosterone, are called androgens, and treatment to shut down male hormones is known as androgen deprivation therapy, or ADT. Antiandrogens are drugs that block the action of androgens in the prostate cancer cell, and these include Xtandi, Erleada, and Nubeqa. These are known as “second‐generation” or “next‐generation” antiandrogens to distinguish them from older, less effective drugs. Zytiga is included in this group, although it works by blocking the enzyme required by the body to make testosterone (which can be found in the testes but also in the adrenal glands). Finally, there's the term, “castration‐resistant prostate cancer” (CRPC). This is used to describe the situation when prostate cancer cells, which have become resistant to ADT, can still respond favorably to other types of hormone therapy like antiandrogens. Conversely, “castration‐sensitive” or “hormone‐sensitive” are terms used to indicate prostate cancer that has not yet developed resistance to androgen deprivation.
2.2. How does hormone therapy work?
Androgens control the growth, survival, and function of the normal prostate gland and prostate cancer cells. Androgens achieve this control by binding to a protein within the cells called the androgen receptor (also known as AR). How does this work? It may help to think about baseball, with androgen as the ball, and the AR as the glove. The baseball (androgen) needs to fit into the pocket of the glove (AR) to function properly (Figure 1A). Once bound to androgen, the AR can turn on genes that control the production of prostate‐specific proteins like prostate‐specific antigen (PSA), as well as factors that affect the growth and survival of the cancer cell. ADT works by blocking the production of testosterone (the baseball), to keep the glove empty (Figure 1B). Now, taking this analogy further, think of antiandrogens as oranges; they are kind of ball shaped. These oranges (antiandrogens) can get into the glove and block androgen from binding (Figure 1C). Prostate cancer cells need to have androgen bound to the AR—the ball in the glove—to survive. ADT has clinical benefit because it lowers the amount of androgen‐bound receptor below a critical level, which triggers the death of the prostate cell.
Figure 1.
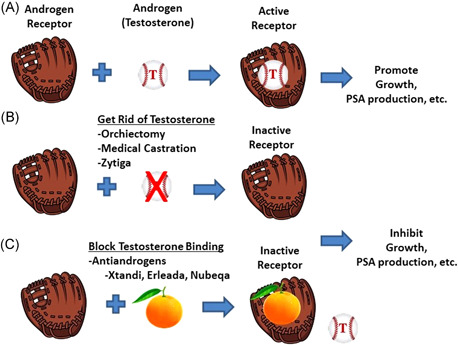
(A) Androgen receptor (AR) “glove” and androgen “ball” come together in the prostate cancer cell to function. (B) Androgen deprivation involves getting rid of the androgen ball by shutting off the production of testosterone. Testosterone can also be blocked by chemicals called antiandrogens that are ball‐shaped “oranges” that prevent testosterone from binding in the pocket of the AR glove. Antiandrogens include bicalutamide (Casodex), nilutamide (Nilandron), enzalutamide (Xtandi), apalutamide (Erleada), and darolutamide (Nubeqa). PSA, prostate‐specific antigen. [Color figure can be viewed at wileyonlinelibrary.com]
2.3. Why does prostate cancer become castration resistant?
Almost all men initially respond, sometimes dramatically, to ADT. In fact, with more than 90% of patients showing good response, often for many years, ADT can be considered the most effective single therapy for any cancer. Pain improves, PSA levels plummet and disease often melts away on scans. However, hormone therapy almost never cures prostate cancer. After this initial beneficial response, there is a period of dormancy where PSA levels are low and disease is not growing. At some point, usually within 1–2 years after starting therapy prostate cancer cells begin to grow again. Why?
Given enough time, prostate cancer cells can adapt to the changing environment. Those cells unable to adapt will die. Prostate cancer cells respond to the low testosterone conditions by markedly increasing the level of the AR glove to catch any testosterone that remains after therapy (Figure 2). Levels of the receptor can increase 100‐fold or more. These cells fine‐tune the level of the AR until they find the “sweet‐spot” that allows them to grow again despite the low level of testosterone in the body. As ever more intensive hormone therapy is given, the prostate cancer cells further adapt. Castration resistance is not unexpected, it is the inevitable outcome of chronic, prolonged exposure to AR blockade.
Figure 2.
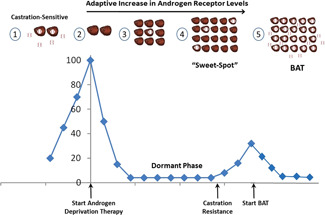
Prostate cancer is initially highly sensitive to ADT. (1) AR levels in castration‐sensitive prostate cancer cells are relatively low. Androgen receptor “glove” is bound to androgen “ball.” (2) Androgen deprivation removes androgen, leaving receptor empty. This produces an initial beneficial effect: PSA levels drop, symptoms improve, and the tumor shrinks. (3) The surviving prostate cancer cells then enter a dormant phase. Then they begin to increase production of levels of the androgen receptor “glove.” (4) Eventually, these cells reach a “sweet spot” where they have made enough glove to start growing again despite low testosterone levels in the blood. At this point prostate cancer cells are considered castration‐resistant. How can we shake up the cancer? (5) BAT floods the system with androgen “balls” resulting in too much AR bound to androgen, moving the prostate cancer cell away from the “sweet‐spot” and inducing growth inhibition and prostate cancer cell death. ADT, androgen deprivation therapy; AR, androgen receptor; BAT, bipolar androgen therapy; PSA, prostate‐specific antigen. [Color figure can be viewed at wileyonlinelibrary.com]
2.4. What is BAT?
BAT is a new treatment approach for asymptomatic men with castration‐resistant prostate cancer. BAT involves administration of sufficient amounts of testosterone to rapidly achieve a higher than normal level (i.e., supraphysiologic) of testosterone in the blood (Figure 3). The normal level of testosterone in the blood of a 70‐year‐old‐man is 300–400 ng/dl. The testosterone level in a man on ADT is <50 ng/dl. BAT is achieved by injecting a long‐lasting (depot) generic form of testosterone known as testosterone cypionate into the muscle of the buttocks every 28 days. The dose of testosterone cypionate is 400 mg. This is considered a high dose of testosterone but this dosage is within the FDA‐approved dose range of this drug. The term “bipolar androgen therapy” was coined to reflect the fact that over a 28‐day treatment cycle, the blood levels of testosterone oscillate between the polar extremes of supraphysiologic (1000–3000 ng/dl) to nearly castrate (100–200 ng/dl). In order for this to occur, all patients should continue to receive concurrent ADT throughout treatment with BAT, and ADT should not be stopped when BAT is administered.
Figure 3.
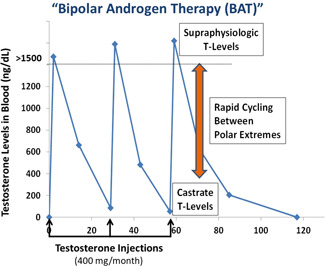
Bipolar androgen therapy involves rapid oscillation of blood testosterone levels between the polar extremes of supraphysiologic (>1000 ng/dl) to near castrate (~100 ng/dl) levels over a 28‐day cycle. [Color figure can be viewed at wileyonlinelibrary.com]
An injectable form of testosterone was selected for BAT because it is inexpensive and readily achieves supraphysiologic levels of testosterone in the blood. Testosterone is a controlled substance that can also be directly applied to the skin as a gel or as a patch (transdermal), absorbed through the lip (transbuccal), or sniffed (intranasal). Recently, the FDA has approved oral formulations of testosterone that are designed to avoid metabolism in the liver. However, all of these forms of testosterone are expensive and are not covered by health insurance for prostate cancer. Reaching supraphysiologic levels of testosterone with these preparations would be difficult; it would also likely require using a dose of each preparation that is not approved by the FDA. Unregulated androgens known as anabolic steroids, used by weight lifters, bodybuilders, and other athletes, are readily available over the internet. These agents are selected based on strong anabolic effects to build up muscle but minimal androgenic effects on sex tissues like the prostate. In addition to being illegal, they would not be appropriate to achieve BAT.
2.5. How was BAT discovered?
While the concept of using high‐dose testosterone for prostate cancer is a new treatment approach, it is an old idea. Dr. Huggins, in his 1966 Nobel Prize acceptance speech, outlined two opposite sorts of changes to induce regression of hormone‐dependent cancers: deprivation of essential hormones, and the opposite, hormone interference with large amounts of hormones. Further work by Dr. Shutsung Liao first demonstrated that prostate cancer cells that became castrate‐resistant by prolonged exposure to low testosterone levels could be killed or growth inhibited by doses of testosterone that were above the normal physiologic range (i.e., were supraphysiologic). Subsequently, multiple research labs reported a similar finding that prostate cancer cell growth could paradoxically be blocked by supraphysiologic levels of androgens like testosterone. Key points from these studies were that the treatment only worked in prostate cancer cells that had been made resistant to low testosterone and had high levels of the AR. Prostate cancer cells with low or normal levels of AR were not growth inhibited, but instead were stimulated by testosterone. Also, supraphysiologic levels of testosterone were required to achieve growth inhibition in prostate cancer cells growing in a dish and in mice. Two small clinical studies have been completed in which men with CRPC were given testosterone replacement therapy that produced normal, “physiologic” blood levels of testosterone. No therapeutic effect was observed for testosterone in those studies. Thus, many doctors and prostate cancer researchers almost gave up on the idea of using testosterone to treat prostate cancer.
Based on the extensive laboratory studies supporting the BAT concept, we performed the first BAT pilot study in 2010. This study was supported by the One‐in‐Six Foundation in Akron, OH started by a former patient with prostate cancer. The results of this pilot study allowed us to successfully obtain grants from the National Institutes of Health (NIH), the Department of Defense (DoD) Prostate Cancer Research Program, and the Prostate Cancer Foundation (PCF) to support the larger studies that followed (called the RESTORE, TRANSFORMER, and COMBAT studies) and which are described below.
2.6. How does BAT work?
BAT has been shown to disrupt the normal progression through the cell growth cycle. BAT can produce breaks in DNA strands, can turn off important growth‐promoting genes, can activate cell stress pathways, and it can stimulate production of immune‐activating proteins.
One of the key things to emphasize is that BAT was developed to work against CRPC cells. These cells have adaptively fine‐tuned the AR to high levels in response to low testosterone produced by androgen deprivation. This high level of AR, paradoxically, makes the prostate cancer cell vulnerable to sudden exposure to high amounts of testosterone. In this case, “too much of a bad thing can be a good thing.” Flooding the prostate cancer cell with testosterone creates a problem for the cell. Now it has to suddenly deal with too much androgen bound to AR. This high level “gums up the works” so to speak. It disrupts the ability of the prostate cancer cell to divide as part of the growth cycle. In response, the prostate cancer cell either stops growing or dies.
Time is a critical component of hormone therapy. Androgen deprivation works initially because prostate cancer cells undergo a “hormone shock” when they are suddenly deprived of testosterone. The majority of the prostate cancer cells cannot survive this shock and die, resulting in a large drop in PSA level, disappearance of cancer sites on imaging scans, and symptomatic improvement. However, given sufficient time, the prostate cancer cells that survive this initial shock adapt to living in a new low testosterone environment and begin to grow again. BAT is a similar type of “hormone shock” but just in the opposite direction. However, if the testosterone is maintained at a steady high level, the prostate cancer cells can also adapt again to this new high testosterone environment. They become resistant to BAT and begin to grow again. A key feature of BAT is the rapid change from a very high to low testosterone level over a single course of therapy. The high levels of testosterone produced initially by BAT are effective against CRPC cells that are making very high levels of AR. Prostate cancer that has lowered levels of the AR will not be killed by high levels of androgen. However, these low AR cells become vulnerable to cell death when testosterone levels drop suddenly over a cycle of BAT. Thus, BAT is designed to repeatedly shock the prostate cancer cells, by alternating between these polar extremes of high and low testosterone levels. The rapid change in testosterone levels does not give prostate cancer cells sufficient time to adapt to the underlying environment because it is always changing. This method keeps the prostate cancer cells constantly guessing.
2.7. Who should not get BAT?
ADT is highly effective initial treatment for prostate cancer but produces significant side effects. Loss of sexual function, loss of muscle mass, and fatigue are the side effects that have the biggest impact on QOL. Across the clinical studies with BAT we have observed restoration of sexual function in men with impotence due to androgen deprivation. We have measured an increase in muscle mass and seen significant improvement in fatigue scores. Based on this potential improvement in QOL, many men have asked if BAT could be used as initial therapy for prostate cancer instead of androgen deprivation. Others have asked if BAT could be used as primary therapy instead of radical prostatectomy or radiation.
The answer to both questions is “No.” As described above, BAT was designed and primarily tested as a new therapy for men with CRPC based on its production of high amounts of the AR. To date, we have treated about 350 men with the castrate‐resistant disease across four clinical studies. We have sufficient evidence to support the use of BAT in men with castration‐resistant prostate cancer whose disease is worsening on androgen deprivation or on other hormone agents like Xtandi or Zytiga. However, outside of a well‐controlled clinical study, BAT should not be given to men with castration‐sensitive locally advanced or metastatic disease, as it has the potential to worsen disease rather than treat it.
Previous studies and our own experience have shown that BAT has a potential to worsen pain significantly in men with symptomatic bone pain from metastatic prostate cancer. This pain escalation occurs within hours of BAT and resolves as testosterone blood levels decline over the first cycle of BAT. The worsening pain is probably not due to rapid growth of prostate cancer. Instead, it is more likely due to testosterone‐stimulated release of inflammatory factors that can make bone pain worse in men who already have pain. Thus, a key eligibility requirement across all of our BAT studies was that men had to be asymptomatic with no pain from prostate cancer to enroll in the studies. Another potential concern with BAT is in patients with bulky prostate glands or large pelvic lymph nodes, in whom BAT might cause blockage of the bladder or upper urinary tract. So we do not recommend using BAT in patients who are at risk of bladder or urinary tract obstruction.
2.8. How safe is BAT?
At the outset, the major concern for use of BAT in men with CRPC was the potential to stimulate prostate cancer cells to grow. The worry was that this could cause worsening symptoms, especially bone pain. So, across all BAT studies we have only allowed men without bone pain due to prostate cancer to enroll. We also have not allowed men with worrisome lesions (i.e., pending spinal cord compression, concern for bone fracture) that could cause severe symptoms in the event of tumor progression. We also excluded patients with urinary obstruction requiring catheterization due to enlarged prostate secondary to prostate cancer or benign prostatic hypertrophy, as discussed above.
With those caveats, to date we have observed that BAT is remarkably safe in men with asymptomatic CRPC. In the RESTORE study of 90 patients, side effects to BAT were primarily mild with the most common being generalized achiness, and lower leg swelling. Sexual side effects were common and included breast tenderness, breast tissue enlargement, and hot flashes. Even though men go from very high to low testosterone levels over a 28‐day period, BAT did not cause mood swings or any other behavioral changes. BAT also did not cause significant urinary symptoms, although we did not treat with BAT who were at risk of bladder or urinary tract obstruction. Serious side effects occurred in individual patients and were not thought to be due to BAT, with exception of high‐grade hypertension that occurred in three patients.
The TRANSFORMER clinical study compared BAT to the antiandrogen Xtandi in 195 patients who had developed progressive disease despite prior Zytiga. Once again, the majority of side effects to BAT were mild. The number of patients having a side effect was generally similar in the two groups. Notable exceptions included fatigue with about half of patients on Xtandi having mild and 7% having more severe fatigue, compared with 30% of BAT patients experiencing only mild fatigue. More patients on Xtandi had constitutional symptoms such as lack of appetite, depression, anxiety, insomnia, headache, and generalized muscle weakness as well as gastrointestinal complaints (diarrhea, constipation, abdominal pain, and flatulence) compared to BAT. BAT was associated with increased sexual side effects (hot flashes, breast tenderness, and breast enlargement), swelling in the lower legs and generalized musculoskeletal pain and achiness. More severe side effects to BAT were seen in a few patients and were primarily due to worsening pain in the back or extremity. More severe side effects for Xtandi were worse bone pain, fatigue, and hypertension. One patient on BAT had a mild stroke and two patients on Xtandi had blood clots. One death occurred on the study in a patient on Xtandi.
In summary, we have treated about 350 men with BAT to date. Most men received an average of six 28‐day treatment cycles of BAT. Across the studies, BAT had mild to moderate side effects, with most common being hot flashes, breast tenderness, generalized muscle aches, and swelling in the lower legs. Since testosterone is known to cause fluid retention, caution should be taken when using BAT in men with underlying congestive heart failure. Adjustments to antihypertensive (blood pressure) regimens may also be required during BAT therapy in some patients. Notably, the drug label for testosterone cypionate lists cardiovascular events as potential severe side effects. While we have observed isolated incidents of heart attack and stroke in a few men across the BAT studies, in each case it was not clear if the event was due to BAT. We have also seen cardiovascular events from Xtandi. However, given the potential concern, caution should be taken using BAT in patients with known underlying heart disease.
2.9. What are effects of BAT on QOL in men with CRPC?
In the initial study of BAT, we were encouraged that many of the patients reported improvement in overall QOL, particularly in the areas of physical function, fatigue, and sexual activity. In the RESTORE study, patients completed quality of life questionnaires. Significant improvement was observed in the areas of physical function, emotional well‐being, and fatigue. The TRANSFORMER study (discussed above) compared QOL for patients on BAT versus Xtandi. Patients on BAT again showed significant improvement compared to Xtandi in the areas of physical function, emotional well‐being, and fatigue. Across studies, the most significant effect of BAT in QOL was in the area of sexual function, with most men reporting improvement in sexual desire and sexual satisfaction. BAT restored the ability to have an erection suitable for intercourse in many men who had maintained the ability to have erections after surgery or radiation for prostate cancer but became impotent with androgen deprivation.
In a recent study designed to assess the effect of BAT on body composition, we analyzed images from computed tomography (CT) scans before and after three cycles of BAT. Overall, we observed about a 9% decrease in abdominal fat and a 12% increase in muscle mass. However, change in body composition did not correlate with improvements in the fatigue scales. Three cycles of BAT also produced a 12% decrease in low‐density lipoprotein “bad” cholesterol and a 27% decrease in triglycerides, but produced a 9% decrease in high‐density lipoprotein “good” cholesterol.
In summary, BAT has proven to be safe and well‐tolerated by most men with CRPC. However, BAT should not be given to men with uncontrolled bone pain due to prostate cancer or to those at risk of urinary tract obstruction. BAT has the potential to improve fatigue, physical activity, muscle strength, and sexual function in some men.
2.10. How do you measure therapeutic effectiveness of BAT?
There are multiple ways to measure whether a new therapy is working in men with prostate cancer. These include looking for improvement in symptoms such as bone pain, measuring PSA levels, and looking for tumors to shrink on imaging CT scans or bone scans. The Axumin and recently FDA‐approved PSMA‐PET scans are both new ways to image and may allow for detection of prostate cancer at an earlier stage. However, these new PET scans are expensive and may not be reimbursed by health insurance companies. They will likely not be used routinely to follow response to BAT.
Since we have strongly recommending against using BAT in men with pain due to prostate cancer, one cannot use improvement in bone pain as an indicator of response to BAT. However, BAT can improve other symptoms such as fatigue, muscle strength and sexual function that are due to androgen deprivation. Thus, many men experience clinical benefit from BAT, even if PSA does not go down or if tumors do not shrink. We often continue to use BAT in those men experiencing such clinical benefit despite an increase in PSA as long as they have “stable disease (no new cancer‐related symptoms)” and no evidence of disease worsening on CT scan or bone scan.
Using PSA to assess response to BAT is very tricky. PSA production is controlled by testosterone. When prostate cancer cells are exposed to testosterone they are stimulated to make PSA, even if their growth is blocked by testosterone. This can be seen in the laboratory using castration‐resistant human prostate cancer cells, Figure 4A. Testosterone blocks the growth of these cells by 50% while Xtandi has no effect on growth compared to no treatment at all. Although there are 50% less cancer cells following testosterone treatment, there is a big increase in PSA. In contrast, although Xtandi has no effect on growth, it almost completely blocks the prostate cancer cells ability to make PSA. Thus, we cannot rely on PSA response alone to assess the effectiveness of BAT in prostate cancer patients, although when PSA goes down it is usually a good thing.
Figure 4.
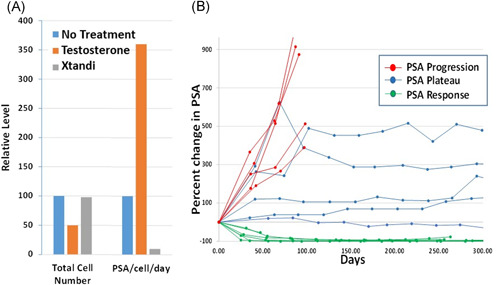
(A) Human prostate cancer cells growing in the lab are growth inhibited by 50% when exposed to testosterone (orange bar) while Xtandi (gray bar) has no effect on growth compared to no treatment (blue bar). Despite a 50% decrease in cell number, testosterone‐treated cells increase production of PSA by 3.5‐fold (orange bar) compared to untreated cells (blue bar). While Xtandi has no effect on growth of cells it reduces PSA production by >90% (gray bar). (B) Three patterns of PSA response in representative patients treated with BAT. These three patterns include those with “PSA response,” those with stable disease (“PSA plateau”), and those with “PSA progression.” BAT, bipolar androgen therapy; PSA, prostate‐specific antigen. [Color figure can be viewed at wileyonlinelibrary.com]
In patients treated with BAT, three general types of PSA changes are observed asdepicted in Figure 4B. In the first type (red line) patients show no response to BAT with PSA levels that continues to rise over the course of therapy. These patients often also show signs of worsening prostate cancer on initial scans and usually come off BAT quickly, usually within 3 months. In the second type (green line) PSA drops very quickly often >50% when BAT is started. Response to BAT can last for many months in this scenario. These patients frequently also show decreasing tumor size on CT scans. They remain on BAT until PSA begins to rise above the starting level or until bone or CT scans begin to show renewed growth of the cancer. The third type (blue lines) are the most difficult patients to manage on BAT. These patients typically have a big increase in PSA after the first or second cycle of BAT. The PSA then often plateaus as a stable level or rises very gradually over time. These patients do not show decrease in tumor size on CT scans. Instead, they have prolonged stabilization of disease, in some cases for several years. Even though PSA is not going down, we usually continue these patients on BAT if they are having a clinical benefit and their disease remains otherwise stable on imaging studies and without progression of clinical symptoms.
CT scans are used to follow soft tissue response to BAT, Figure 5. Responses are observed in lymph nodes and visceral metastases of the liver, lung, and adrenal glands. The use of the bone scan to follow response to BAT is also complicated. BAT can induce an initial “flare response” on the bone scan, as do many other effective prostate cancer therapies. Spots on the bone scan can appear darker, and sometimes new spots that weren't seen before can appear. Therefore, careful attention must be paid by the treating physician to closely following the recommendation of the Prostate Cancer Working Group 3 (PCWG3) in analyzing initial response to BAT in the bone. Patients who appear to be benefitting from BAT but show an initial worse bone scan can be continued on BAT with repeat bone scan in 2‐3 months to help determine if further treatment is warranted. The effects of BAT on the Axumin and PSMA‐based PET scans are not known and require further study. This may be particularly important for PSMA scans since PSMA expression can be negatively regulated by androgens (when androgens go down, PSMA can go up, and vice versa).
Figure 5.
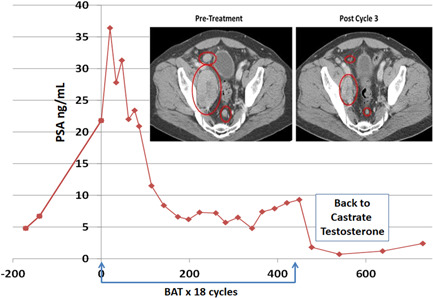
PSA and CT scan (inset) response in lymph nodes in patient receiving 18 cycles of BAT on the pilot study. BAT, bipolar androgen therapy; CT, computed tomography; PSA, prostate‐specific antigen. [Color figure can be viewed at wileyonlinelibrary.com]
2.11. How effective is BAT for metastatic CRPC?
BAT has been tested in four clinical trials at Johns Hopkins, primarily in men with CRPC who have also received treatment with Xtandi or Zytiga, or both. To provide perspective, the current standard treatment sequence is to give ADT either alone or in combination with one of the newer hormone drugs like Xtandi or Zytiga. For patients who just get ADT, these newer drugs are added when the patient's prostate cancer progresses. The numbers we typically quote to our patients are that 95% of patients have a good PSA response to the initial androgen deprivation and 65% have a good PSA response to the adding of second hormone therapy. What to do after that is less clear. A series of small studies show that the PSA response to adding another hormone therapy (i.e., Xtandi after Zytiga or vice versa) is only about 25%. The number of patients who show tumor shrinkage on scans (i.e., objective response) is less than 10%. The duration of the response to this third hormone is usually about 6 months. The optimal sequencing of Xtandi and Zytiga and other hormone‐blocking drugs like Erleada and Nubeqa is not clear.
In patients with CRPC progressing on either Xtandi or Zytiga, about 33% of patients have a PSA or objective response after three cycles of BAT. The duration of this response is about 6 months. In the randomized TRANSFORMER study, BAT was compared directly to Xtandi in men with CRPC progressing on Zytiga. Although BAT (androgen) and Xtandi (antiandrogen) are directly opposite therapies, the response to treatment was remarkably similar. A 50% decrease in PSA level (i.e., a PSA50 response) was seen in about 25% of men on either treatment. The duration of response was about 6 months for both treatments. A higher percentage of men on BAT had objective response compared to Xtandi (24% vs. 4%, respectively). BAT was found to be significantly more effective than Xtandi in the subset of men who had a short (<6 months) response to treatment with Zytiga. BAT also showed significant improvement in QOL, particularly in areas of fatigue, physical, and sexual function compared to Xtandi.
In ongoing studies, we are trying to understand the reasons why some patients respond well to BAT while others do not. In the recently completed COMBAT study, we treated a group of incredible men who agreed to have tumor biopsies before and after three cycles of BAT. These valuable samples are being extensively studied to look for specific markers and mutations that might help predict who will respond. We are also studying the effects of BAT on immune function and metabolism to help identify other drugs that might be combined with BAT to improve response. In studies conducted so far, it appears that patients with mutations in the genes TP53, BRCA2, or other DNA repair enzymes may be more likely to have a significant response to BAT.
2.12. Can BAT restore sensitivity to hormone‐blocking therapy?
As described above, prostate cancer cells adapt to low testosterone conditions by increasing the levels of the AR “glove” to compensate for low levels of the androgen “ball.” Conversely, when exposed to high levels of androgen, the surviving prostate cancer cells adaptively decrease the level of the AR to a new “sweet spot” level. At this point, these prostate cancer cells should once again be sensitive to androgen blocking therapies like Xtandi.
This concept was first tested in the RESTORE study. In this study, patients with CRPC progressing on either Xtandi or Zytiga were treated with BAT. Once BAT stopped working, the patients were retreated with the same drug Xtandi or Zytiga that initially was not working before BAT. Remarkably, for the patients who received Xtandi then BAT and the Xtandi again, 70% had a PSA response to rechallenge with Xtandi. The response duration was about 6 months. This renewed response to Xtandi was not dependent on response to BAT. Patients who had no response to BAT after three cycles still responded to Xtandi. For patients receiving Zytiga then BAT then Zytiga again, the PSA response was much lower at 17% and the response duration was only 4 months.
In the TRANSFORMER study, patients were given the option to cross‐over to the opposite therapy. Thus, patients on BAT could go on to get Xtandi and vice versa. For those patients who received Xtandi directly after Zytiga, the PSA response was 25% and time to PSA progression was about 4 months. For those patients who received BAT first, then received Xtandi, the PSA response was almost 80% with a time to PSA progression of about 11 months. The overall survival for patients who received BAT then Xtandi was about 37 months compared to about 29 months for those receiving Xtandi alone.
Finally, in one arm of the RESTORE study, patients with CRPC who had never received Zytiga or Xtandi were treated with BAT. The PSA response to BAT was low in this group at about 15%. However, some patients in this study went on to receive Xtandi or Zytiga after BAT. In these patients, the PSA response was 95%. Remarkably, 85% of the patients had more than a 90% decrease in PSA and 50% had PSA levels that became undetectable. The duration of response to Xtandi or Zytiga after BAT in this small study was about 25 months. As a comparison, in the studies that led to the FDA approval of Xtandi or Zytiga as first‐line therapy for men with castration‐resistant prostate cancer before chemotherapy, the PSA response was 78% and 62%, respectively. The duration of PSA response in both studies was 11 months. While the patient groups were not entirely similar, the results point to the potential for BAT to improve response to subsequent hormonal therapies once the patient becomes castrate resistant.
2.13. what does BAT cost?
Testosterone cypionate is a generic form of testosterone that is relatively inexpensive, costing less than $50 per month. However, it is not FDA‐approved for prostate cancer. Thus, it is possible, even likely, that it will not be covered by insurance and will require payment out‐of‐pocket. BAT also requires an injection into the gluteal muscle (i.e., buttock) every 28 days. The injection is usually given by a healthcare provider in a clinic setting, and this will result in additional costs depending on the area and the provider. In contrast, current antiandrogen drugs for prostate cancer such as Xtandi, Erleada, and Nubeqa can cost $10,000 or more per month but are usually covered by insurance with co‐payments required that depend on individual insurance plans. These oral drugs can be administered at home and do not require frequent visits to the clinic.
2.14. What are the future studies planned for BAT?
These combined results, reflecting a decade of clinical and laboratory research, establish the meaningful clinical activity and safety of BAT. The results suggest that additional studies are needed to determine the best way to integrate BAT as treatment for castration‐resistant prostate cancer. The combined results from the RESTORE and TRANSFORMER studies also show that BAT is highly effective at restoring sensitivity and disrupting resistance to antiandrogens like Xtandi. These results support further consideration of sequential BAT → Xtandi as one single therapy continuum. A larger randomized study comparing overall patient survival following treatment with sequential BAT → Xtandi versus enzalutamide or chemotherapy alone will be required to obtain FDA approval of this approach. Funding such a large expensive trial will be difficult given that BAT is a generic therapy with no pharmaceutical sponsor supporting its development.
Thus far we have only evaluated the effects of one cycle of BAT → Xtandi. Currently, we are conducting the “Sequential Testosterone and Enzalutamide Prevents Unfavorable Progression (STEP‐UP) trial (NCT04363164) at Johns Hopkins and several other sites around the United States. This three‐arm study will determine the safety and effectiveness of repeat cycles of BAT → Xtandi in men with CRPC progressing on Zytiga (Figure 6). The study is sponsored by a grant from the DoD Prostate Cancer Research Program and funding from Astellas, the manufacturer of Xtandi.
Figure 6.
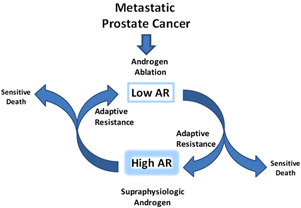
Repeat cycling between high and low levels of androgen receptor (AR) activity by either blocking androgens or giving supraphysiologic testosterone (BAT) may be a new therapeutic approach that takes advantage of prostate cancer cell ability to adapt to changing testosterone levels in the environment. [Color figure can be viewed at wileyonlinelibrary.com]
Although the initial results have been promising, with some men responding to BAT alone for several years, the median duration of response to BAT across these studies is approximately 6 months. Thus, we and other groups around the world are also studying rational combinatorial approaches that could further enhance and prolong the effect of BAT. These include BAT in combination with the immunotherapy Nivolumab (Opdivo) (NCT03554317), the DNA‐repair inhibitor olaparib (Lynparza) (NCT03516812), the bone targeted radiation therapy 223Radium (Xofigo) (NCT04704505), and the DNA‐damaging drug carboplatin (NCT03522064).
3. CONCLUSIONS
The key findings from these clinical studies are that BAT (a) can be safely administered to asymptomatic patients with metastatic CRPC; (b) does not produce symptomatic disease progression; (c) produces sustained PSA and objective responses in 30‐40% of patients and (d) can resensitize and prolong response to subsequent antiandrogen therapy. Patients need to remember that BAT is not FDA‐approved therapy, does not work for everyone, and is not without risk. It is important for physicians not to overpromise results from BAT on quality of life and sexual function.
4. WHAT ARE OUR RECOMMENDATIONS FOR BAT?
There is still much to be learned about BAT in prostate cancer and we encourage patients to seek out and participate in clinical trials when possible. For those without access to trials, there are two clinical settings in which we would recommend a patient consider BAT. First, for asymptomatic patients who have initially progressed on ADT, we would consider BAT before starting second‐line therapy with Xtandi or Zytiga due to its ability to markedly enhance response to these agents. Second, in men with CRPC progressing on Zytiga, we would recommend considering BAT as part of sequential therapy with Xtandi (i.e., Zytiga then BAT then Enzalutamide) based on results of patients treated with BAT then Xtandi on the randomized TRANSFORMER study. Please remember that BAT is always given in conjunction with ongoing ADT, and ADT should not be stopped when delivering BAT.
BAT should be given in these settings for an initial minimal period of three cycles. At that point, based on the combination of symptoms, clinical benefit, PSA levels, and radiographic response, a decision can be made as to whether to continue BAT or switch to antiandrogen therapy. For those patients with PSA and/or objective response we would recommend continuing BAT with imaging every 3–4 cycles. For those patients receiving symptomatic clinical benefit but with PSA elevation, we would continue BAT if imaging studies are stable. For those patients who develop worsening clinical symptoms, or radiographic disease progression, we would recommend stopping BAT and proceeding to antiandrogen therapy.
Finally, in those few patients who experienced pain flares, we have observed that the flare usually occurs within 24–48 h post‐BAT injection. It can be treated with anti‐inflammatory medications but may be severe enough to require narcotics. It typically resolves after about 1‐week post‐BAT, when the testosterone level begins to fall. For those patients who do develop a pain flare on the first dose of BAT that resolves before the next dose of BAT, we would consider continuing BAT for a second cycle. We have seen pain improve and not return in such patients with subsequent cycles of BAT.
5. BAT CLINICAL PEARLS
-
−
BAT is given as an intramuscular injection of testosterone cypionate every 28 days.
-
−
BAT should only be given to patients with castrate‐resistant (NOT hormone‐sensitive) prostate cancer.
-
−
BAT should NOT be given to prostate cancer patients with cancer‐related bone pain.
-
−
BAT should not be given to patients with urinary obstruction due to enlarged prostate or prostate cancer.
-
−
BAT should be given together with ongoing ADT or surgical castration.
-
−
BAT may be continued despite PSA elevation, if there is clinical benefit and stable scans showing no progression.
-
−
BAT should not be combined with Zytiga, Xtandi, Erleada, or Nubeqa (or with taxane chemotherapy).
-
−
BAT may render CRPC patients sensitive to Zytiga or Xtandi after ADT or after prior progression on these drugs
-
−
BAT should preferentially be administered in the context of a clinical trial, especially when being used in conjunction with other experimental therapies.
ACKNOWLEDGMENTS
We acknowledge all of the brave patients who agreed to take the leap of faith with us and participate in our BAT clinical trials. We also wish to acknowledge the dedicated work of our entire prostate cancer research team of oncologists, scientists, research nurses, study coordinators and administrators at Johns Hopkins who worked so diligently to complete the BAT clinical studies.
Denmeade S, Antonarakis ES, Markowski MC. Bipolar androgen therapy (BAT): a patient's guide. The Prostate. 2022;82:753‐762. 10.1002/pros.24328
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this review article as no new data were created or analyzed in this study
REFERENCES
- 1. Huggins C. The effects of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43:209. [Google Scholar]
- 2. Chuu CP, Hiipakka RA, Fukuchi J, Kokontis JM, Liao S. Androgen causes growth suppression and reversion of androgen‐independent prostate cancer xenografts to an androgen‐stimulated phenotype in athymic mice. Cancer Res. 2005;65:2082‐2084. [DOI] [PubMed] [Google Scholar]
- 3. Denmeade SR, Isaacs JT. Bipolar androgen therapy: the rationale for rapid cycling of supraphysiologic androgen/ablation in men with castration resistant prostate cancer. Prostate. 2010;70:1600‐1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Isaacs JT, D'Antonio JM, Chen S, et al. Adaptive auto‐regulation of androgen receptor provides a paradigm shifting rationale for bipolar androgen therapy (BAT) for castrate resistant human prostate cancer. Prostate. 2012;72:1491‐1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schweizer MT, Antonarakis ES, Wang H, et al. Effect of bipolar androgen therapy for asymptomatic men with castration‐resistant prostate cancer: results from a pilot clinical study. Sci Transl Med. 2015;7:269ra2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Teply BA, Wang H, Luber B, et al. Bipolar androgen therapy in men with metastatic castration‐resistant prostate cancer after progression on enzalutamide: an open‐label, phase 2, multicohort study. Lancet Oncol. 2018;19:76‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Markowski MC, Wang H, Sullivan R, et al. A multicohort open‐label Phase II Trial of bipolar androgen therapy in men with metastatic castration‐resistant prostate cancer (RESTORE): a comparison of post‐abiraterone versus post‐enzalutamide cohorts. Eur Urol. 2021;79:692‐699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sena LA, Wang H, Lim ScM SJ, et al. Bipolar androgen therapy sensitizes castration‐resistant prostate cancer to subsequent androgen receptor ablative therapy. Eur J Cancer. 2021;144:302‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Denmeade SR, Wang H, Agarwal N, et al. TRANSFORMER: a randomized phase II study comparing bipolar androgen therapy versus enzalutamide in asymptomatic men with castration‐resistant metastatic prostate cancer. J Clin Oncol. 2021;39:1371‐1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marshall CH, Tunacao J, Danda V, et al. Reversing the effects of androgen‐deprivation therapy in men with metastatic castration‐resistant prostate cancer. BJU Int. 2021;128:366‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this review article as no new data were created or analyzed in this study


