Abstract
Background
Cirrhosis is increasingly common and morbid. Optimal utilisation of therapeutic strategies to prevent and control the complications of cirrhosis are central to improving clinical and patient‐reported outcomes.
Methods
We conducted a narrative review of the literature focusing on the most recent advances.
Results
We review the aetiology‐focused therapies that can prevent cirrhosis and its complications. These include anti‐viral therapies, psychopharmacological therapy for alcohol‐use disorder, and the current landscape of clinical trials for non‐alcoholic steatohepatitis. We review the current standard of care and latest developments in the management of hepatic encephalopathy (HE), ascites and hepatorenal syndrome. We evaluate the promise and drawbacks of chemopreventative therapies that have been examined in trials and observational studies which may reduce the risk of hepatocellular carcinoma and cirrhosis complications. Finally, we examine the therapies which address the non‐pain symptoms of cirrhosis including pruritis, muscle cramps, sexual dysfunction and fatigue.
Conclusion
The improvement of clinical and patient‐reported outcomes for patients with cirrhosis is possible by applying evidence‐based pharmacotherapeutic approaches to the prevention and treatment of cirrhosis complications.

1. INTRODUCTION
Cirrhosis is common and morbid. Its prevalence exceeds >1 million persons in the United States (US) alone, 1 rising by 50% in the past 20 years. 2 Hospitalisations for cirrhosis have increased by 90% and their rate now exceeds that for heart failure. 3 Cirrhosis mortality has risen by 65% since 2008. 4 Despite these poor indicators, there has been substantial progress in supportive care for cirrhosis with interventions that enrich quality of life and prevent the development of cirrhosis complications. Effort to optimise therapy for cirrhosis care and prevention will improve public health. Herein, we provide a narrative review of the state‐of‐the art for the pharmacotherapeutic management and prevention of cirrhosis complications.
2. AETIOLOGIC THERAPIES
The most effective strategies to reduce the burden of cirrhosis are those which address the underlying aetiology of cirrhosis. In this section, we review the evidence for multiple chronic liver diseases.
2.1. Viral hepatitis
Eradication of hepatitis C virus (HCV) is associated with a reduced risk of incident cirrhosis and its complications. 5 Even for patients with decompensated cirrhosis, HCV eradication is associated with marked improvement in liver function. HCV eradication may lead to clinical recompensation with the resolution of HE. 6 , 7 , 8 , 9 Combining the trials of the direct‐acting antivirals indicated for patients with decompensated cirrhosis—ledipasvir/sofosbuvir and velpatisvir/sofosbuvir—many patients returned to Child A, particularly those with single decompensations. 10 In general, antiviral therapy is indicated for all patients with HCV. However, treatment may be best deferred to after liver transplantation for waitlisted patients with model for end‐stage liver disease (MELD) scores >27 to avoid the risk of reduced MELD with persistent decompensation (MELD purgatory). 11 , 12 HCV eradication also reduces the risk of HCC, 13 , 14 but the risk of HCC can remain elevated, particularly among older persons with low platelets or albumin, high liver stiffness or fibrosis‐4 indices, and those who are actively drinking alcohol. 13 , 15
Similarly, control of hepatitis B is associated with substantial benefits. It is likely that antiviral therapy for hepatitis B prevents the development of cirrhosis. 16 Antiviral therapy is also indicated for patients with chronic hepatitis B and cirrhosis. 17 Therapy is associated with improved Child–Pugh scores 18 and reduced risk of HCC. 19 There is a small 5%–9% risk of increased serum creatinine >0.5 mg/dl, 20 one which is potentially limited by the use of Tenofovir alafenamide, 21 and, rarely, lactic acidosis. 17 The patients at lowest risk of complications including HCC appear to be those who achieve a functional cure or loss of surface antigen >1 year. 22 There are numerous therapies under investigation which aim to improve the rate of functional cure or complete cure (elimination of cccDNA or DNA integration). These include immunomodulators, viral entry inhibitors, core protein modulators, nucleic acid polymers, anti‐sense oligonucleotides, CRISPR‐Cas9, nucleases and interfering RNA. 23
2.2. Alcohol‐related liver disease
The most effective therapy for alcohol‐related liver disease (ALD) is abstinence and harm reduction. Psychotherapy is widely recommended and most effective when integrated with medical care. 24 When successfully linked to care, residential or outpatient psychotherapy and counselling are associated with a reduced risk of hospital readmission. 25 There are several pharmacotherapeutic options that are associated with reduced alcohol consumption and abstinence. Though none are specifically approved in the setting of cirrhosis, their use is associated with a reduced risk of decompensation and death. 26 Baclofen is notable for having been studied among patients with decompensated cirrhosis and successfully reducing alcohol consumption in a small study where patients were hospitalised for the initiation of therapy. 27 Most evidence, therefore, needs to be cautiously applied from data involving patients with alcohol use disorder without decompensated cirrhosis. The most effective therapies in alcohol use disorder are naltrexone (daily/oral or intramuscular/long‐acting) 28 , 29 and gabapentin. 30 , 31 Naltrexone has a label warning is associated with transiently elevated liver enzymes based on experience in a trial of 26 obese patients; however, no liver injury has been observed in trials enrolling patients with alcohol use disorder or cholestatic liver disease (where it has been used for pruritis). 32 , 33 Gabapentin is unique as an agent that is effective for persons with or at risk for alcohol withdrawal symptoms. 30 , 34
2.3. Non‐alcoholic steatohepatitis cirrhosis
2.3.1. Weight loss in obese/overweight
Weight loss through dietary and lifestyle modification is the current cornerstone of treatment for patients with non‐alcoholic steatohepatitis (NASH) among overweight and obese patients (not specific to cirrhosis). 35 , 36 , 37 A prospective study of 261 patients with biopsy‐proven NASH were encouraged to adopt recommended lifestyle changes, followed by a repeat liver biopsy. 38 The degree of NASH and fibrosis resolution correlated with the degree of weight loss, with the greatest improvements among patients who lost ≥10% of their body weight. However, patients with cirrhosis were excluded from this study, and it is unclear if the findings of this study can be generalised to patients with cirrhosis.
Bariatric surgery appears to be effective in improving NASH among patients with severe obesity especially those with significant comorbidities. 39 A prospective French study conducted among 180 severely obese patients with biopsy‐proven NASH who underwent bariatric surgery revealed that 84% of patients had resolution of NASH 5 years after surgery. 39 A retrospective U.S. study of 1158 patients with biopsy‐proven NASH compared 650 patients who underwent bariatric surgery with 508 patients who were managed non‐surgically and determined that bariatric surgery was associated with a lower risk of major adverse liver outcomes defined by diagnostic codes (adjusted absolute risk difference 12.4%). 40 Owing to the exclusion of large numbers of patients with cirrhosis, data are insufficient for bariatric surgery to be routinely recommended for patients with NASH cirrhosis and obesity. Additional data to define the population most likely to benefit are urgently needed.
2.3.2. Pharmacological therapies
To date, there are no approved pharmacological therapies for NASH, with regulatory approval hindered by challenges with clinical trials including slow disease progression, the requirement for repeat liver biopsies, and heterogenous patient populations. 36 Several clinical trials studying candidates specifically for the treatment of NASH cirrhosis are summarised in Table 1. A recent phase 3 placebo‐controlled trial of selonsertib, a selective inhibitor of apoptosis signal‐regulating kinase 1 (ASK1) and a phase 2b placebo‐controlled trial of simtuzumab, a humanised monoclonal antibody directed against lysyl oxidase‐like 2 (LOXL2) were conducted in patients with compensated NASH cirrhosis. 41 , 42 Both studies were halted at week 48 and week 96 respectively due to lack of efficacy. A randomised phase 2b trial of belapectin, an inhibitor of galectin 3, among patients with NASH cirrhosis failed to reduce portal pressure. 43 However, in a subgroup analysis of patients without varices, belapectin reduced hepatic venous pressure gradient and improved fibrosis. Therefore, another phase 2b/3 trial of belapectin specifically in patients with NASH cirrhosis and signs of portal hypertension but without baseline varices is ongoing (NCT04365868, Table 1). A placebo‐controlled trial of Emricasan, a pan‐caspase inhibitor, among 217 participants with decompensated NASH cirrhosis did not meet the primary endpoints (mortality, new decompensation event or rise in MELD‐NA score ≥ 4 points). 44 FALCON 2, a phase 2, randomised study of pegbelfermin, a PEGylated fibroblast growth factor 2 analogue, reported that the study failed to meet the primary endpoint of fibrosis improvement without worsening of NASH. 45 A phase 2b trial of combination therapies (selonsertib, cilofexor or firsocostat) among 392 participants with NASH (56% with cirrhosis) demonstrated that participants treated with cilofexor/firsocostat had improvements in NASH activity, although the primary endpoint (fibrosis improvement without worsening of NASH) of the study was not met. 46 Phase 2 trials of the glucagon‐like peptide 1 (GLP‐1) receptor agonists liraglutide and semaglutide (which did not include patients with cirrhosis) showed a higher resolution of NASH compared with placebo. 47 , 48 The PIVENs clinical trial demonstrated that Vitamin E improves liver histology in non‐diabetic patients with biopsy‐proven NASH. 49 However, patients with cirrhosis were excluded from the PIVENs study. 35 , 49 Additional ongoing trials are summarised in Table 1, Figure 1. and Figure 2
TABLE 1.
Selected studies of drugs for NASH cirrhosis in Phase II/III development
| Agent | Mechanism | Phase | ClinicalTrials.gov number | Progress/results | Date of completion or expected completion |
| Simtuzumab 42 | Monoclonal antibody against lysyl oxidase‐like 2 | IIb | NCT01672879 | Ineffective in decreasing hepatic venous pressure gradient | Jan 2017 |
| Selonsertib 41 | Selective inhibitor of ASK1 | III | NCT03053063 | Ineffective in improving fibrosis without worsening NASH | May 2019 |
| Emricasan 44 | Pan‐caspase inhibitor | II | NCT03205345 | No reduction in composite outcome of mortality, decompensation or rise in MELD‐NA score ≥4 points | Aug 2019 |
| Pegbelfermin 45 | PEGylated fibroblast growth factor 21 (FGF21) analogue | IIa | NCT03486912 | Ineffective in improving fibrosis without worsening NASH | Oct 2021 |
| Aldafermin | Fibroblast growth factor 19 analogue | II | NCT04210245 | Ongoing; primary outcome: ≥ 1‐stage improvement in fibrosis without worsening NASH | Sep 2022 |
| Belapectin | Inhibitor of galectin 3 | IIb/3 | NCT04365868 | Ongoing; primary outcome: proportion of patients who develop new oesophageal varices | Dec 2023 |
| Semaglutide ± Cilofexor/Firsocostat | Glucagon‐like peptide‐1 receptor agonist (semaglutide) | II | NCT04971785 | Ongoing; primary outcome: ≥1‐stage improvement in fibrosis without worsening NASH | Mar 2024 |
| Efruxifermin | Fc‐ fibroblast growth factor 21 fusion protein | IIb | NCT05039450 | Ongoing; primary outcome: change from baseline in fibrosis regression with no worsening steatohepatitis | Apr 2024 |
| BMS‐986263 | siRNA designed to degrade HSP47 mRNA | II | NCT04267393 | Ongoing; primary outcome: ≥ 1‐stage improvement in fibrosis without worsening NASH | Jul 2024 |
Abbreviations: MELD, model for end‐stage liver disease; NASH, non‐alcoholic steatohepatitis.
FIGURE 1.
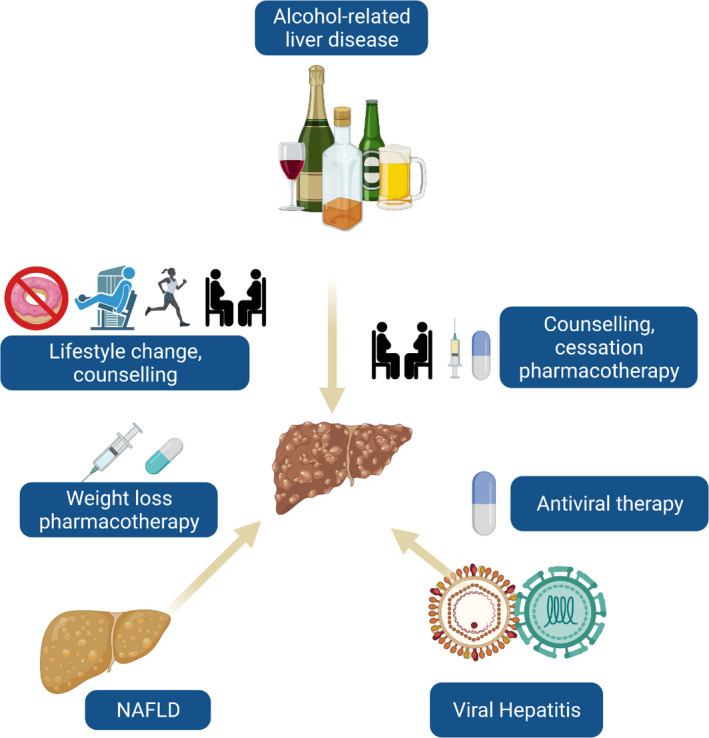
Aetiology directed therapies to prevent decompensations of cirrhosis. Patients with cirrhosis may have multiple disease aetiologies. Efforts to address alcohol use, lifestyle change and elimination or control of viral hepatitis are essential
FIGURE 2.
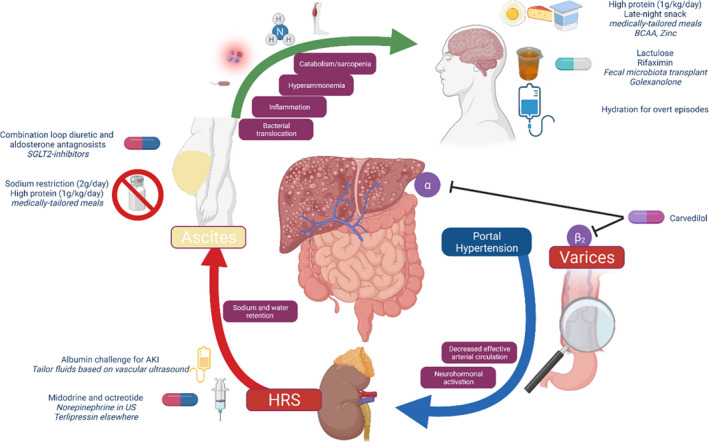
The physiological basis of therapies to prevent and manage cirrhosis complications. AKI, acute kidney injury; BCAA, branch‐chain amino acids; HRS, hepatorenal syndrome; US, United States
2.4. Complications of cirrhosis
2.4.1. Variceal haemorrhage
Based on guidance from Baveno VI to VII, patients with compensated cirrhosis may avoid a screening oesophagogastroduodenoscopy (EGD) if their liver stiffness on vibration controlled transient elastography is <20 kPa and the platelet count is >150,000. 50 However, recent data has emerged refining the thresholds for patients with NASH cirrhosis, 51 with a platelet count >110,000/mm 3 and LSM < 30 kPa for M probe, and platelet count >110,000/mm3 and LSM < 25 kPa for XL probe as the updated threshold beyond which a screening EGD may be avoided.
2.4.2. Hepatic encephalopathy
Current best‐practice for the management of HE includes nutritional support as well as therapeutic agents that mitigate the effects of factors in the causal pathway for the disease process. Given that muscle is critical for the metabolism of systemic ammonia, 52 all patients with HE are recommended to consume 1 g dietary protein per kilogram actual bodyweight. 53 As patients with cirrhosis often have inadequate hepatic gluconeogenesis, fasting should be avoided and all patients should consume high‐calorie/high‐protein night‐time or early‐morning snack. 54 Branch‐chain amino acids are specifically required and are frequently supplemented but may not be required in the setting of adequate protein supplementation. 53
Lactulose titrated to produce soft stools—specifically 2‐3 bowel movements with Bristol stool scale 55 —remains first‐line and is the only agent shown to improve quality of life and sleep quality among persons with covert HE. 56 Rifaximin has been approved for breakthrough episodes of HE on lactulose and its use is associated with reduced mortality and hospitalisations after incident HE. 57 , 58 Ammonia scavengers have had mixed results. Glycerol phenylbutyrate improves freedom from recurrent HE but is not available for this indication. Oral l‐ornthine/l‐aspartate (LOLA) is associated with improved cognitive function in small trials which do not compare to other therapies. 59 Intravenous LOLA was recently associated with hastened recovery from overt HE compared to lactulose and rifaximin. 60 While LOLA did not improve length of stay, it was associated with a reduction in 28‐day mortality from 42% to 16%, which raises important questions about the population studied. 61 Finally, the ammonia and glutamine scavenger, intravenous ornithine phenylacetate did not improve the resolution of overt HE compared to lactulose. 62 Adjunctive therapies such as faecal microbiota transplantation and golexanolone (a neurosteroid antagonist) have been evaluated but not in phase 3 trials. 63 , 64 Case series have suggested a potential role for the closure of spontaneous portosystemic shunts, however, recurrence rates remain high. 65
2.4.3. Ascites
Ascites is a relatively neglected area of investigation. The standard of care for ascites management includes sodium restriction and combination loop and aldosterone antagonist diuretics. Sodium restriction can be efficacious but is only effective in 14% of patients. Adherence is limited and is often associated with unintended protein/calorie restriction. 66 , 67 This is likely due to gaps in patient education. Tapper et al. recently conducted a pilot 40‐subject randomised trial of home‐delivered medically‐tailored sodium‐restricted meals and observed lower paracentesis requirement as well as quality of life. 68 Diuretics are often dosed in a 2:5 ratio starting with 40 mg of furosemide and 100 mg of spironolactone, escalating to a maximum of 400 mg spironolactone. Weight loss is gradual as ascites clearance occurs at a maximum of 1 L/day. 69 Gynecomastia may worsen on spironolactone for which a switch to eplerenone (50 mg roughly equivalent to 100 mg spironolactone) or amiloride (10 mg/100 mg spironolactone) could be considered. 70
Hyponatremia and renal injury frequently limit the effectiveness of diuretics owing to the exacerbation of vascular underfilling and maladaptive neurohormonal activation. Advanced therapies like transjugular portosystemic intrahepatic shunts are reserved for persons with preserved liver and cardiac function. There are emerging reports regarding the role of Sodium‐Glucose Cotransporter 2 (SGLT‐2) inhibitors wherein patients with diabetes and refractory ascites can experience improvements in volume status with add‐on SGLT‐2 inhibitors. 71 Controlled trials are needed.
Transjugular intrahepatic portosystemic shunts (TIPS) is a vital salvage therapy for ascites for appropriate candidates. In appropriate candidates, typically Child score <12 and bilirubin<5.8 g/dl with preserved cardiac function, TIPS increases the odds of freedom from paracentesis and even survival. 72 The risk of HE is increased after TIPS but is not a contraindication. It is crucial to not stop HE therapy (ie, lactulose) if the patient is taking prior to TIPS. 73 Beyond that, both under‐dilation the TIPS (6 mm vs 8 mm) and rifaximin prior to TIPS may improve the risk of HE. 73 , 74
Spontaneous bacterial peritonitis (SBP) is a frequent and morbid complication of ascites. Efforts to prevent SBP centre on identifying candidates for antibiotic prophylaxis. There are several unresolved issues. First, effectiveness is questionable. Trials in this space have had mixed results. 75 , 76 There are observational data suggesting that rifaximin may reduce the incidence of SBP, but these have not been confirmed in randomised trial. 77 Data from a large randomised trial (ASEPTIC) enrolling British patients in a trial of trimethroprim‐sulfamethoxazole or placebo is forthcoming. Second, there is increasing concern that long‐term antibiotics may drive resistance. 78 Third, it is unclear if the dominant method for patient selection (ascitic protein <1.5 g/dL) actually discriminates risk. 79 Finally, one unblinded controlled study evaluated the impact of enoxaparin on the risk of portal vein thrombosis in persons with ascites. 80 Anticoagulation was associated with decreased thrombosis and also SBP, likely by decreasing bacterial translocation.
Hepatorenal syndrome (HRS) is the most devastating complication of ascites. The standard of care varies with access to approved therapies. As HRS is defined by a lack of fluid responsiveness, first‐line therapy is an albumin infusion. However, the optimal dose and duration of albumin infusion are unknown. In a recent trial (CONFIRM), 81 there was clear evidence of harm (respiratory failure) attributed to high cumulative doses of albumin in the context of increased afterload caused by vasoconstrictor therapy. Elsewhere, it has been observed in trials of ultrasound‐guided estimations of intravascular volume that many patients meet criteria for HRS without volume depletion and, in fact, benefit from early vasoconstrictors or paracentesis (to addresses abdominal compartment pressures). 82 The preferred vasoconstrictor therapy in Europe is terlipressin 83 , 84 while octreotide and midodrine are typically used where terlipressin is unavailable. Terlipressin is superior compared to octreotide/midodrine and yields similar results in comparison to norepinephrine. 85 Unfortunately, although terlipressin often reverses HRS, it has not been associated with a survival benefit. 81 In fact, the outcomes after HRS have not changed over multiple decades. 84
2.4.4. Sarcopenia and frailty
Sarcopenia, loss of muscle bulk and frailty, diminished physiologic reserve, are well‐established sequelae of cirrhosis. 53 , 86 These conditions result from malnutrition, hyperammonemia, negative energy balance (particularly due to ascites or fasting), and are potent predictors of falls, HE and mortality. 87 , 88 , 89 The nutritional management of sarcopenia and frailty are summarised above and in recent AASLD guidance, focusing on late‐night snacks, avoiding fasting, high (1 g/kg/day) protein and calorie (~30 kcal/kg/day) consumption, and increasing physical activity. 53 Pharmacologic agents are limited. Patients with frailty owing to cognitive impairment may benefit from initiation or intensification of HE therapy. Beyond that, testosterone has been trialled and is a promising therapy for men with low testosterone without HCC or prostate cancer who have been counselled regarding cardiovascular risks. 90 A trial of an oral testosterone ester is planned to address sarcopenia in patients with cirrhosis (NCT04874350). Finally, the EMPOWER trial (NCT04816916) is investigating a proprietary nutritional therapy consisting of 8 amino acids for the improvement of HE (and frailty).
2.5. Chemoprevention
Given dismal outcomes following the decompensation of cirrhosis, there is intense interest in developing therapies to forestall the progression of disease (Table 2). Few of such approaches have been examined in randomised trials with the exception of statins and non‐selective beta‐blockers (NSBB). NSBB reduce portal pressure, reducing the risk of variceal haemorrhage. 91 The PREDESCI trial tested whether this reduction could translate to reduced risk of all‐cause decompensation, randomising 201 compensated patients with manometry‐confirmed portal hypertension to placebo or non‐selective beta‐blocker (propranolol or carvedilol). NSBB was associated with a reduced risk of the primary outcome, hazard ratio (HR) 0.51, 95% CI 0.26‐0.97, driven by a reduced incidence of ascites, HR 0.44, 95% CI 0.20‐0.97. As carvedilol is associated with a higher rate of portal pressure reduction 92 and can be dosed daily, it may be the preferred NSBB. PREDESCI enrolled patients after portal manometry with predominantly undertreated HCV and as such further study is needed to determine whether similar effects can be observed in patients with NASH, ALD and those with elastography‐based risk‐assessments. Observational data regarding the impact of NSBB are less clear. A recent observational study at risk for survivorship bias suggests improved survival for patients with varices taking carvedilol compared to band‐ligation (from 7.8 to 4.2 years). 93 Another recent study of administrative data at risk for immortal time bias and confounding by indication showed a dramatic reduction in the risk of HCC with NSBB. 94
TABLE 2.
Therapies associated with reduced risk of cirrhosis complications
| Study type | Outcome | Exemplar study | Threats to validity from study design | ||||
| Specific | General | ||||||
| Observational studies | Aspirin | ||||||
| Hepatocellular carcinoma | Simon 2020 | Implausible 50% absolute risk reduction, largely non‐cirrhotic cohort | Retrospective cohort | Administrative data | Confounding by indication | Immortal time bias | |
| Beta‐blockers | |||||||
| Hepatocellular carcinoma | Wijarnpreecha 94 | Implausible 39% relative risk reduction | |||||
| Statins | |||||||
| Variceal bleeding | Mohanty 99 | Low event rates (1%–2%) | |||||
| Ascites | Unreliable diagnostic codes | ||||||
| Hepatocellular carcinoma | Simon 100 | Implausible 70% absolute risk reduction | |||||
| Largely non‐cirrhotic cohort | |||||||
| Hepatic encephalopathy | Tapper 98 | Did not use new‐user design | |||||
| Randomised trials | Carvedilol | ||||||
| Variceal bleeding | Sinagra 2014 (meta‐analysis of RCTs) 163 | Optimal method of patient selection unknown | None | ||||
| Ascites | Villaneuva 2018 91 | Highly adherent patients; most with viral untreated hepatitis C | |||||
| Statins | |||||||
| Mortality | Abraldes 97 | Small sample (N = 158) powered to detect the difference in bleeding but not mortality | All patients were on Non‐selective Beta‐Blockers | ||||
Statins are the subject of multiple trials. Two small randomised studies have shown short‐term simvastatin reduces portal pressure. 95 , 96 An RCT of 158 patients found that simvastatin did not prevent variceal haemorrhage but did improve the underpowered outcome of overall survival. 97 Observational data regarding the impact of statins suggests users are at lower risk of decompensation including bleeding, ascites and HE. 98 , 99 These studies are at risk of multiple biases. Similarly, a study of statins purported an implausible 50% reduction in the absolute risk of HCC. 100 Four trials of statins are underway. First, LIVERHOPE is randomising 240 Europeans with decompensated cirrhosis to 20 mg simvastatin and rifaximin for the prevention of acute‐on‐chronic liver failure. 101 Second, the European STAT‐LIVER trial is randomising 162 patients with Child‐Pugh <13 and portal hypertension to receive 10‐20 mg of atorvastatin or placebo. 102 The primary endpoint is overall survival. Third, the SACRED trial is hoping to randomise 500 US Veterans with compensated cirrhosis and portal hypertension who lack conventional statin indications to either 40 mg of simvastatin or placebo. Fourth, the NIDDK Liver Cirrhosis Network is also planning a trial of statins.
Beyond statins and NSBB, most data regarding chemoprevention for cirrhosis complications are derived from observational studies. As observational data are susceptible to biases, caution must be applied. For example, aspirin and statins have been associated with biologically implausible risk reductions for incident hepatocellular carcinoma that implicate immortal time bias or misclassification errors. 100 , 103 Metformin has also been associated with both improved and worsened outcomes, with the largest suggesting no difference in the risk of HCC or decompensation. 104 , 105 Meta‐analyses suggest a large (~50%) reduction of HCC risk with metformin, an unreliable estimate owing to multiple biases, namely immortal time bias. 106 , 107 , 108 One large observational study has evaluated the potential benefits of oral anticoagulants, finding an association with a lower risk of mortality but not specific decompensations raising questions regarding the mechanism and the risk of residual confounding. 109
2.6. Symptom management in cirrhosis care
Patients with cirrhosis experience many physical and psychological symptoms that are often underrecognised, under assessed and undertreated by their clinicians. 110 , 111 Following is an evidence‐based review of pharmacotherapies for the management of common non‐pain symptoms encountered in cirrhosis care: pruritus, muscle cramps, sleep disturbances, sexual dysfunction and fatigue (Table 3).
TABLE 3.
Therapeutic agents for the control of symptoms common to patients with cirrhosis
| Symptom | Therapy | RCT | Dosing | Patient population | Duration of treatment | Outcome | Reported side effects |
|---|---|---|---|---|---|---|---|
| Pruritus | Cholestyramine | Di Padova 115 | Cholestyramine (3 mg TID) vs placebo | 10 patients with either intra‐ or extra‐ hepatic cholestasis | 4 weeks | Improvement in itching intensity in cholestyramine group | No reported major/minor adverse effects |
| Colesevelam | Kuiper 116 | Colesevelam (1875 mg twice daily) vs placebo | 35 patients with cholestatic pruritus (PBC: 14, PSC: 14, Other: 7) | 3 weeks | No difference in pruritus (VAS), quality of life (SF‐36 and LDSI), or itching severity between both groups | No reported major/minor adverse effects | |
| Gabapentin | Bergasa 117 | Gabapentin (300‐2400 mg daily) vs placebo | 16 women with chronic liver disease (PBC: 9, PSC: 1, HCV: 6) and chronic pruritus | 4 weeks | Increased perception of pruritus (VAS) in gabapentin group | Minor: Pruritus, fatigue, dizziness, worsening symptoms of carpal tunnel syndrome, vomiting | |
| Rifampin | Ghent 164 | Rifampin (300‐450 mg daily in divided doses) vs placebo | Nine patients with compensated PBC and chronic pruritus | 4 weeks (randomised crossover: 2 weeks rifampin, 2 weeks placebo with washout period) | Improved pruritus scores (VAS) during rifampin therapy | No reported major/minor adverse effects | |
| Podesta 165 | Rifampin (300 mg twice daily) vs placebo | 14 patients with PBC | 2 weeks (randomised crossover: 1 week rifampin, 1 week placebo with washout period) | Improved pruritus severity (VAS) during rifampin therapy | No reported major/minor adverse effects | ||
| Naltrexone | Wolfhagen 119 | Naltrexone (50 mg daily) vs placebo | 16 patients (PBC: 13, PSC: 2, Other: 1) and chronic pruritus | 4 weeks | Improved daytime and nighttime itching (VAS) in naltrexone group | Minor: Opioid withdrawal‐like phenomena, nausea, dizziness, flushing, drowsiness, headache, tremors, abdominal cramps | |
| Terg 33 | Naltrexone (50 mg daily) vs placebo | 20 patients (PBC: 15, PSC 1, Other: 4) | 1–3 months | Improved pruritus severity (VAS) in naltrexone group | |||
| Sertraline | Mayo 122 | Sertraline (75–100 mg daily) vs placebo | 12 patients with cholestatic liver disease (PBC: 9, PSC: 2, Other: 1; 4 with MELD >15) and chronic pruritus | 12 weeks (randomised crossover: 6 weeks sertraline, 6 weeks placebo with washout period) | Improved itch scores (VAS) in sertraline group | Minor: Dizziness, loose stools | |
| Bezafibrate | De Vries 123 | Bezafibrate (400 mg daily) vs placebo | 70 patients with cholestatic liver disease (PSC: 44, PBC 24, Other: 2) with itch intensity of ≥5 on VAS | 3 weeks | Higher rates of ≥50% reduction of moderate to severe pruritus (VAS) in bezafibrate group | Minor: Pain in the mouth, lower back pain, general malaise, intensified itch and jaundice after stop of treatment | |
| Muscle cramps | Taurine | Vidot 127 | Taurine (500–1000 mg twice daily) vs placebo | 30 patients (CTP A: 6, CTP B: 20, CTP C: 4, 43% with prior or present HE) experiencing ≥3 cramps/week | 8 weeks (randomised crossover: 4 weeks taurine, 4 weeks placebo) | Reduced frequency, duration and intensity of muscle cramps (patient self‐report, VAS) in taurine group | No reported major/minor adverse effects |
| Branched‐chain amino acids (BCAA) | Hidaka 128 | Daytime BCAA (one sachet after each meal) vs Nocturnal BCAA (one sachet after breakfast and two sachets before bedtime) | 37 patients with cirrhosis and serum albumin 3.1–3.5 g/dl (CTP A: 21, CTP B: 16) | 12 weeks | Decreased occurrence of muscle cramps (HRQOL survey) in nocturnal group | Minor: Bloating, itching | |
| Quinidine | Lee 130 | Quinidine sulphate (400 mg daily) vs placebo | 31 patients with cirrhosis (CTP A: 23, CTP B: 7, CTP C: 1) and muscle cramps | 4 weeks | Reduced frequency of cramps (patient self‐report) in quinidine group |
Minor: diarrhoea *Not approved for use in the United States due to side effect profile |
|
| Baclofen | Elfert 131 | Baclofen (10–30 mg total daily dose) vs placebo | 100 patients with cirrhosis (CTP score 5–9) without HE experiencing ≥3 cramps/week | 12 weeks | Decreased frequency, severity and duration of muscle cramps (patient self‐report) in baclofen group | Minor: Drowsiness, constipation, nausea | |
| Methocarbamol | Abd‐Elsalam 132 | Methocarbamol (500 mg twice daily) vs placebo | 100 patients with HCV cirrhosis (CTP A: 21, CTP B: 42, CTP C: 37) experiencing ≥3 cramps/week | 4 weeks | Decreased frequency and duration of muscle cramps (patient self‐report) in methocarbamol group | Minor: Dry mouth, drowsiness | |
| Orphenadrine | Abd‐Elsalam 133 | Orphenadrine (100 mg twice daily) vs placebo | 124 patients with cirrhosis (CTP A: 61, CTP B: 24, CTP C: 39) experiencing ≥3 cramps/week | 4 weeks | Decreased frequency and duration (patient self‐report) of muscle cramps in orphenadrine group | Minor: Dry mouth, drowsiness, nausea | |
| Sexual dysfunction | Tadalafil | Jagdish 136 | Tadalafil (10 mg daily) vs placebo | 140 men with cirrhosis (CTP < 10) and erectile dysfunction (erectile function score <25 on IIEF) | 12 weeks | Improved erectile function (>5‐point increase in erectile function score of IIEF) in the tadalafil group | Minor: Dizziness, congestion, mild headache, bloating, peri‐orbital swelling, itching |
| Sleep Disturbances | Melatonin | De Silva 144 | Melatonin (3 mg nightly) vs placebo | 60 patients with cirrhosis (CTP A or B) with sleep disturbances, without present or prior overt HE | 2 weeks | Improved sleep quality (PSQI) and daytime sleepiness (ESS) in the melatonin group | Minor: Abdominal pain, headache, dizziness |
| Zolpidem | Sharma 143 | Zolpidem (5 mg nightly) vs placebo | 52 patients with cirrhosis (CTP A or B) with PSQI >5 without present or prior overt HE | 4 weeks | Increased total sleep time, sleep efficiency, subjective sleep quality (PSQI) and improvement in parameters of sleep initiation and maintenance (polysomnography) in zolpidem group | Minor: Excessive daytime sleepiness, constipation, dry mouth | |
| Hydroxyzine | Spahr 145 | Hydroxyzine (25 mg nightly) vs placebo | 35 patients with cirrhosis with minimal HE, >3 months of sleep difficulties, and ESS score >10 | 10 days | Improved sleep behaviour (VAS, wrist actigraphy) in hydroxyzine group | Major: 1 patient developed acute overt hepatic encephalopathy while receiving hydroxyzine | |
| Fatigue | Fluvoxamine | ter Borg 149 | Fluvoxamine (75 mg twice daily) vs placebo | 33 patients with cholestatic liver disease (PBC: 22, PSC: 11; CTP < 6) with self‐reported fatigue | 6 weeks | No statistically significant beneficial effect of fluvoxamine on fatigue (VAS, FFSS, MFI) or quality of life (SF‐36) | Minor: Headache, nausea, insomnia, dizziness |
| Modafinil | Silveira (2017) 150 | Modafinil (100 mg daily) vs placebo | 33 patients with PBC receiving UDCA >6 months before study enrolment. Excluded: MELD >15, recurrent variceal bleeding, diuretic‐refractory ascites or spontaneous HE | 12 weeks | No significant improvement in fatigue severity (≥50% improvement in FFSS) in modafinil compared to placebo | Minor: Headaches, diarrhoea, rash |
Abbreviations: CTP, Child‐Turcotte‐Pugh; ESS, Epworth Sleepiness Scale; FFSS, Fick Fatigue Severity Scale; HAS, Hourly scratching activity; HE, Hepatic encephalopathy; HRQOL, Health‐related quality of life; IIEF, International Index of Erectile Function; LDSI, Liver Disease Symptom Index; MELD, Model for End‐stage Liver Disease; PBC, Primary biliary cholangitis; PSC, Primary sclerosing cholangitis; PSQI, Pittsburgh Sleep Quality Index; SF‐36, Short‐Form 36 Questionnaire; UDCA, Ursodeoxycholic acid; VAS, visual analogue scale.
2.6.1. Pruritus
Cholestasis‐associated pruritus is a common symptom experienced by patients with cirrhosis, with much of the evidence base for its management coming from trials conducted in patients with cholestatic liver disease. 112 , 113 , 114 Pharmacotherapeutic options for cholestatic pruritus that have been tested in clinical trials include cholestyramine, colesevelam, gabapentin, rifampin, naltrexone, sertraline and bezafibrate. Small randomised studies have established the effectiveness of cholestyramine, a bile acid sequestrant, as a first‐line therapeutic agent for the treatment of cholestatic pruritus. 115 While cholestyramine is generally well‐tolerated, adverse effects of cholestyramine include drug‐drug interactions and gastrointestinal side effects. Colesevelam is a newer bile sequestrant with improved GI tolerability, however, it failed to demonstrate effectiveness in the treatment of cholestatic pruritus in a randomised placebo‐controlled trial. 116 Gabapentin was associated with an increase in the perception of itching compared to placebo in a double‐blind, randomised, placebo‐controlled trial. 117 Rifampin, through its action as a pregnane X receptor agonist, is a second‐line therapy with proven effectiveness in treating cholestatic pruritus in multiple randomised controlled trials, however, its risk for hepatotoxicity limits its use in patients with decompensated cirrhosis. 114 , 118 Naltrexone, an opioid antagonist with established efficacy in treating cholestatic pruritus, can be used as third‐line therapy. 33 , 119 , 120 Naltrexone should be used with caution in individuals receiving opioid therapy, as it can reduce analgesia or potentiate opioid withdrawal‐like reactions. Bioavailability is altered in patients with severe hepatic dysfunction 121 ; efficacy and safety estimates may not generalise to multiply decompensated patients. Sertraline, a selective serotonin reuptake inhibitor, has been shown to be a safe and effective treatment for pruritus in a small randomised placebo‐controlled trial of 12 patients with chronic liver disease. 122 Lastly, the FITCH trial (fibrates for cholestatic ITCH) demonstrated the effectiveness of bezafibrate, a peroxisome proliferator‐activated receptor (PPAR) agonist, in improving moderate to severe pruritus in patients with primary sclerosing cholangitis and primary biliary cholangitis. 123 The effect of other fibrates (eg, fenofibrate) have been examined, albeit in smaller studies. 124
2.6.2. Muscle cramps
Muscle cramps are frequently experienced by patients with cirrhosis, affecting over 1 in 3 patients and contributing to poor health‐related quality of life. 110 , 125 , 126 Despite its high prevalence, there is a paucity of robust clinical trials for the treatment of muscle cramps in patients with cirrhosis, with many published trials limited by small sample sizes. Pharmacological treatments for muscle cramps in patients with cirrhosis include taurine, branched‐chain amino acids, quinidine, muscle relaxants (baclofen, methocarbamol and orphenadrine), vitamin E, zinc and l‐carnitine. A recently completed trial examined pickle juice sips as a therapy to abort muscle cramps (Clinicaltrials.gov identifier: NCT04650295).
Daily oral taurine supplementation in 30 patients with cirrhosis and muscle cramps led to a reduction in cramp severity, duration and frequency without any reported serious adverse effects in a crossover randomised controlled trial. 127 A multicentre randomised controlled trial of daytime vs nighttime administration of branched‐chain amino acids granules in 37 patients with compensated cirrhosis showed a significant reduction in muscle cramping in both groups. 128 A small placebo‐controlled randomised controlled trial showed that quinidine treatment improved muscle cramping in 31 patients with cirrhosis. However, the risk of severe hematologic adverse effects of quinidine has restricted its use in the United States. 129 , 130 Short‐term trials of baclofen, methocarbamol and orphenadrine for the treatment of muscle cramps have shown promising efficacy data in single‐centre randomised controlled studies, however, larger studies with longer‐term follow‐up data are needed. 131 , 132 , 133 Daily vitamin E supplementation did not improve muscle cramp symptoms compared to placebo in a pilot randomised controlled crossover trial of 9 patients with cirrhosis. Two open‐label studies of l‐carnitine supplementation and oral zinc sulphate therapy showed preliminary efficacy in reducing muscle cramps in patients with cirrhosis, however, these trials were limited by small sample sizes and uncontrolled, non‐randomised study design. 134 , 135
2.6.3. Sexual dysfunction
Sexual dysfunction is estimated to affect 53%‐93% of patients with cirrhosis, occurring in a higher frequency among those with more advanced disease. 110 A randomised controlled trial of a 12‐week course of tadalafil therapy for 140 men with cirrhosis (Child‐Pugh Turcotte score <10) and erectile dysfunction showed that it enhanced erectile function and improved depression, anxiety and quality of life compared to placebo. 136 Tadalafil was well‐tolerated by patients in the trial; there were no significant differences in reproductive hormone levels or body composition between the two groups. There have been no clinical studies of pharmacological treatments for female sexual dysfunction in patients with cirrhosis. 137
2.6.4. Sleep disturbances
Sleep disturbances, which include sleep‐wake inversion, excessive daytime sleepiness and insomnia, affect 50%–80% of patients with cirrhosis. 110 , 138 , 139 , 140 Treatment of HE may help to improve sleep disturbances in patients with cirrhosis—an observational study and randomised controlled trial showed the benefit of using lactulose to improve sleep quality in patients with minimal HE. 141 , 142 Short courses of melatonin, zolpidem and hydroxyzine have also been shown to improve sleep quality in randomised trials of patients with Child Pugh A and B cirrhosis. 143 , 144 , 145 No pharmacological therapies for sleep disturbances have been trialed in patients with decompensated cirrhosis, likely due to the increased risk of cognitive dysfunction and falls in patients with more advanced disease. 146 Similarly, benzodiazepines should be avoided for the management of sleep disturbances in this population given the risk of sedation and HE. 147 , 148
2.6.5. Fatigue
Pharmacological therapies for fatigue have primarily been tested in patients with cholestatic liver disease. Fluvoxamine, an antidepressant, showed no beneficial effect on fatigue among patients with cholestatic liver disease in a placebo‐controlled randomised clinical trial. 149 Similarly, modafinil, a dopamine reuptake inhibitor, showed no significant impact on fatigue among patients with PBC. 150 , 151
2.6.6. Future directions
Many pharmacological symptom management trials in cirrhosis care have been limited by small sample sizes, single‐centre recruitment, lack of long‐term follow‐up safety data, and non‐randomised designs. Minimal data exist for the management of common symptoms such as pain, depression and anxiety in this population. Furthermore, efforts to enrol patients with decompensated cirrhosis are necessary to ensure generalisability of safety and efficacy data. 152 There is also a role for expanding the evaluation of non‐pharmacological interventions. For example, evidence‐based behavioural therapies such as mindfulness‐based stress reduction have shown promising preliminary efficacy for the treatment of sleep disturbances in patients with cirrhosis. 153
In oncology, routine symptom monitoring has been shown in prospective randomised trials to improve symptom control, quality of life, healthcare utilisation and even survival. 154 , 155 , 156 Routine symptom assessment may lead to early identification of and intervention for untreated symptoms, which may in turn improve health outcomes for patients with cirrhosis. As symptom science continues to expand in cirrhosis care, concurrent attention should be paid to examining the potential role of routine symptom monitoring in clinical care, developing algorithmic approaches to symptom management, and establishing collaborations with supportive care services such as pharmacy, palliative care, psychology, psychiatry and social work.
2.7. Implementation
As the prevalence and complexity of cirrhosis have risen, 1 , 2 , 3 , 4 so has the standard of care, the tools available to improve clinical outcomes, and our awareness of gaps in patient‐centred outcomes. The needs of patients with cirrhosis may outstrip the capacity of the hepatology workforce to them alone. In this context, in order to optimise the uptake and delivery of the therapies/approaches discussed above, several care delivery strategies must be embraced. First, collaborative models of care are needed. It may not be reasonable to expect generalists to manage the full breadth of cirrhosis‐related concerns. Educational seminars and outreach may be helpful but their effect is limited and often fleeting. Instead, hepatology can increase its reach by training advance practice providers (APPs) to semi‐specialise or develop shared‐care models with hepatologists involved in diagnosis and clinical changes. 157 Second, efforts to prevent cirrhosis complications may require early intervention to treat underlying conditions and initiate prophylactic therapies. The earlier that liver disease is detected, the simpler its management and the more likely its care can be centred within primary care clinics. This requires case finding strategies such as those pioneered by the Veterans Administration (outreach for hepatitis C screening and treatment and linkage to care for cirrhosis), 158 , 159 screening at‐risk persons for hepatitis C, 160 and population‐based screening for advanced liver disease. 161 Third, clinics should embrace the use of electronic reporting and monitoring of symptoms. Our group has integrated the assessment of PROs including ascites burden and alcohol use into the electronic record, pushing surveys to patients prior to all visits to objectively track the symptoms and enable early intervention. 162 Others have begun pilots of remote monitoring to track symptoms and vital signs with the capacity to alert care teams when intervention is needed (eg, CirrhoCare: NCT05045924). Fourth, in order to optimise the outcomes of patients with unique needs, structural practice changes may be needed. As we are failing to meet the needs of patients with alcohol use disorder, 26 the solutions are multifold: improve the cross‐disciplinary training of hepatologists and co‐localise clinics with or streamline referral pathways to addiction specialists. Additionally, given the growing subset of ageing patients with extrahepatic comorbidities, new cross‐collaborations with geriatric medicine or palliative care are warranted.
3. CONCLUSION
Therapies for cirrhosis include efforts to reduce the incidence of cirrhosis and its complications as well as those which address the symptoms of cirrhosis. Both approaches are unified by the goal of reducing the public and personal burden posed by cirrhosis. This review highlights the current standard of care and the opportunities to improve it. Improvements in the quality and quantity of life experienced by patients with cirrhosis will require efforts towards the implementation of evidence‐based therapies and the execution of patient‐centred trials.
AUTHORSHIP
Guarantor of the article: Elliot Tapper.
Author contributions: Tapper, Loomba: Concept. Tapper, Loomba, Ufere, Huang: Writing.
ACKNOWLEDGEMENT
Declaration of personal interests: Tapper has consulted for Bausch, Mallinckrodt, Axcella, Novo Nordisk, Ambys, Lipocine, Kaleido, Takeda.
Tapper EB, Ufere NN, Huang DQ & Loomba R. Review article: current and emerging therapies for the management of cirrhosis and its complications. Aliment Pharmacol Ther. 2022;55:1099–1115. doi: 10.1111/apt.16831
The Handling Editor for this article was Professor Gideon Hirschfield, and this was commissioned review was accepted for publication after full peer‐review.
Funding informationElliot Tapper receives funding from the National Institutes of Health. NNU is supported by a Clinical, Translational and Outcomes Research Award from the American Association of the Study of Liver Diseases. R.L. receives funding support from NIAAA (U01AA029019), NIEHS (5P42ES010337), NCATS (5UL1TR001442), NIDDK (U01DK130190, U01DK061734, R01DK106419, P30DK120515, R01DK121378, R01DK124318), NHLBI (P01HL147835) and DOD PRCRP (W81XWH‐18‐2‐0026). RL serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol‐Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen Inc., Madrigal, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bio, Terns Pharmaceuticals and Viking Therapeutics. In addition, his institutions received research grants from Arrowhead Pharmaceuticals, Astrazeneca, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Merck, Pfizer, Sonic Incytes and Terns Pharmaceuticals. Co‐founder of LipoNexus Inc. D.Q.H. receives funding support from Singapore Ministry of Health’s National Medical Research Council under its NMRC Research Training Fellowship (MOH‐000595‐01). In addition, he has served as an advisory board member for Eisai.
DATA AVAILABILITY STATEMENT
Not applicable to a review
REFERENCES
- 1. Gines P, Quintero E, Arroyo V, et al. Compensated cirrhosis: natural history and prognostic factors. Hepatology. 1987;7:122‐128. [DOI] [PubMed] [Google Scholar]
- 2. Mellinger JL, Shedden K, Winder GS, et al. The high burden of alcoholic cirrhosis in privately insured persons in the United States. Hepatology. 2018;68:872‐882. [DOI] [PubMed] [Google Scholar]
- 3. Asrani SK, Kouznetsova M, Ogola G, et al. Increasing health care burden of chronic liver disease compared with other chronic diseases, 2004‐2013. Gastroenterology. 2018;155:719‐729. e4. [DOI] [PubMed] [Google Scholar]
- 4. Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999‐2016: observational study. BMJ. 2018;362:k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all‐cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584‐2593. [DOI] [PubMed] [Google Scholar]
- 6. El‐Sherif O, Jiang Z, Tapper E, et al. Baseline Factors associated with improvements in decompensated cirrhosis after direct‐acting antiviral therapy for HCV infection. Gastroenterology 2018. [DOI] [PubMed] [Google Scholar]
- 7. Curry MP, O’Leary JG, Bzowej N, et al. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med. 2015;373:2618‐2628. [DOI] [PubMed] [Google Scholar]
- 8. Charlton M, Everson GT, Flamm SL, et al. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology. 2015;149:649‐659. [DOI] [PubMed] [Google Scholar]
- 9. Tapper EB, Parikh ND, Green PK, et al. Reduced incidence of hepatic encephalopathy and higher odds of resolution associated with eradication of HCV infection. Clin Gastroenterol Hepatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. El‐Sherif O, Jiang ZG, Tapper EB, et al. Baseline factors associated with improvements in decompensated cirrhosis after direct‐acting antiviral therapy for hepatitis C virus infection. Gastroenterology 2018;154:2111‐2121. e8. [DOI] [PubMed] [Google Scholar]
- 11. Tapper EB, Hughes MS, Buti M, et al. The optimal timing of hepatitis C therapy in transplant eligible patients with Child B and C cirrhosis: a cost‐effectiveness analysis. Transplantation. 2017;101:987‐995. [DOI] [PubMed] [Google Scholar]
- 12. Chhatwal J, Samur S, Kues B, et al. Optimal timing of hepatitis C treatment for patients on the liver transplant waiting list. Hepatology. 2017;65:777‐788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ioannou GN, Beste LA, Green PK, et al. Increased risk for hepatocellular carcinoma persists up to 10 years after HCV eradication in patients with baseline cirrhosis or high FIB‐4 scores. Gastroenterology 2019;157:1264‐1278. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nahon P, Layese R, Bourcier V, et al. Incidence of hepatocellular carcinoma after direct antiviral therapy for HCV in patients with cirrhosis included in surveillance programs. Gastroenterology 2018;155:1436‐1450.e6. [DOI] [PubMed] [Google Scholar]
- 15. Semmler G, Meyer EL, Kozbial K, et al. HCC risk stratification after cure of hepatitis C in patients with compensated advanced chronic liver disease. J Hepatol. 2021. [DOI] [PubMed] [Google Scholar]
- 16. Lok AS, Perrillo R, Lalama CM, et al. Low incidence of adverse outcomes in adults with chronic Hepatitis B virus infection in the era of antiviral therapy. Hepatology. 2021;73:2124‐2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560‐1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singal A, Fontana R. Meta‐analysis: oral anti‐viral agents in adults with decompensated hepatitis B virus cirrhosis. Aliment Pharmacol Ther. 2012;35:674‐689. [DOI] [PubMed] [Google Scholar]
- 19. Lok AS, McMahon BJ, Brown RS Jr, et al. Antiviral therapy for chronic hepatitis B viral infection in adults: a systematic review and meta‐analysis. Hepatology. 2016;63:284‐306. [DOI] [PubMed] [Google Scholar]
- 20. Liaw YF, Sheen IS, Lee CM, et al. Tenofovir disoproxil fumarate (TDF), emtricitabine/TDF, and entecavir in patients with decompensated chronic hepatitis B liver disease. Hepatology. 2011;53:62‐72. [DOI] [PubMed] [Google Scholar]
- 21. Toyoda H, Leong J, Landis C, et al. Treatment and renal outcomes up to 96 weeks after tenofovir alafenamide switch from tenofovir disoproxil fumarate in routine practice. Hepatology. [DOI] [PubMed] [Google Scholar]
- 22. Vittal A, Sharma D, Hu A, et al. Systematic review with meta‐analysis: the impact of functional cure on clinical outcomes in patients with chronic hepatitis B. Aliment Pharmacol Ther. 2022;55:8‐25. [DOI] [PubMed] [Google Scholar]
- 23. Khan IW, Dad Ullah MU, Choudhry M, et al. Novel therapies of hepatitis B and D. Microorganisms. 2021;9:2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khan A, Tansel A, White DL, et al. Efficacy of psychosocial interventions in inducing and maintaining alcohol abstinence in patients with chronic liver disease: a systematic review. Clin Gastroenterol Hepatol 2016;14:191‐202.e1‐4; quiz e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peeraphatdit TB, Kamath PS, Karpyak VM, et al. Alcohol Rehabilitation Within 30 Days of Hospital Discharge Is Associated With Reduced Readmission, Relapse, and Death in Patients With Alcoholic Hepatitis. Clin Gastroenterol Hepatol 2020;18:477‐485.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rogal S, Youk A, Zhang H, et al. Impact of alcohol use disorder treatment on clinical outcomes among patients with cirrhosis. Hepatology. 2020;71:2080‐2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Addolorato G, Leggio L, Ferrulli A, et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol‐dependent patients with liver cirrhosis: randomised, double‐blind controlled study. Lancet. 2007;370:1915‐1922. [DOI] [PubMed] [Google Scholar]
- 28. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003‐2017. [DOI] [PubMed] [Google Scholar]
- 29. Garbutt JC, Kranzler HR, O’Malley SS, et al. Efficacy and tolerability of long‐acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293:1617‐1625. [DOI] [PubMed] [Google Scholar]
- 30. Anton RF, Latham P, Voronin K, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180:728‐736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174:70‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lucey MR, Silverman BL, Illeperuma A, et al. Hepatic safety of once‐monthly injectable extended‐release naltrexone administered to actively drinking alcoholics. Alcohol Clin Exp Res. 2008;32:498‐504. [DOI] [PubMed] [Google Scholar]
- 33. Terg R, Coronel E, Sordá J, et al. Efficacy and safety of oral naltrexone treatment for pruritus of cholestasis, a crossover, double blind, placebo‐controlled study. J Hepatol. 2002;37:717‐722. [DOI] [PubMed] [Google Scholar]
- 34. Myrick H, Malcolm R, Randall PK, et al. A double‐blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33:1582‐1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328‐357. [DOI] [PubMed] [Google Scholar]
- 36. Loomba R, Ratziu V, Harrison SA. Expert panel review to compare FDA and EMA guidance on drug development and endpoints in nonalcoholic steatohepatitis. Gastroenterology: 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dufour JF, Caussy C, Loomba R. Combination therapy for non‐alcoholic steatohepatitis: rationale, opportunities and challenges. Gut. 2020;69:1877‐1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vilar‐Gomez E, Martinez‐Perez Y, Calzadilla‐Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015;149:367‐78.e5; quiz e14‐5. [DOI] [PubMed] [Google Scholar]
- 39. Lassailly G, Caiazzo R, Ntandja‐Wandji LC, et al. Bariatric surgery provides long‐term resolution of nonalcoholic steatohepatitis and regression of fibrosis. Gastroenterology 2020;159:1290‐1301.e5. [DOI] [PubMed] [Google Scholar]
- 40. Aminian A, Al‐Kurd A, Wilson R, et al. Association of bariatric surgery with major adverse liver and cardiovascular outcomes in patients with biopsy‐proven nonalcoholic steatohepatitis. JAMA. 2021;326:2031‐2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Harrison SA, Wong VW, Okanoue T, et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: Results from randomized phase III STELLAR trials. J Hepatol. 2020;73:26‐39. [DOI] [PubMed] [Google Scholar]
- 42. Harrison SA, Abdelmalek MF, Caldwell S, et al. Simtuzumab is ineffective for patients with bridging fibrosis or compensated cirrhosis caused by nonalcoholic steatohepatitis. Gastroenterology. 2018;155:1140‐1153. [DOI] [PubMed] [Google Scholar]
- 43. Chalasani N, Abdelmalek MF, Garcia‐Tsao G, et al. Effects of belapectin, an inhibitor of galectin‐3, in patients with nonalcoholic steatohepatitis with cirrhosis and portal hypertension. Gastroenterology 2020;158:1334‐1345.e5. [DOI] [PubMed] [Google Scholar]
- 44. Frenette C, Kayali Z, Mena E, et al. Emricasan to prevent new decompensation in patients with NASH‐related decompensated cirrhosis. J Hepatol. 74:274‐282. [DOI] [PubMed] [Google Scholar]
- 45. Manal F Abdelmalek AJS, Nakajima Atsushi, Neuschwander‐Tetri Brent A, Goodman Zachary, Eric J., SAH Lawitz, Jacobson Ira M., Imajo Kento, Gunn Nadege, Marzio Dina Halegoua‐De, Takemi, Akahane BB , Yamaguchi Masayuki, Chatterjee Arkendu, Tirucherai Giridhar S, Shevell Diane E,, et al. Efficacy and safety of Pegbelfermin in patients with nonalcoholic steatohepatitis and compensated cirrhosis: results from the Phase 2b, randomized, double‐blind, placebo‐controlled FALCON 2 study. Hepatology 2021;74:1‐156. [Google Scholar]
- 46. Loomba R, Noureddin M, Kowdley KV, et al. Combination therapies including cilofexor and firsocostat for bridging fibrosis and cirrhosis attributable to NASH. Hepatology. 2021;73:625‐643. [DOI] [PubMed] [Google Scholar]
- 47. Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non‐alcoholic steatohepatitis (LEAN): a multicentre, double‐blind, randomised, placebo‐controlled phase 2 study. Lancet. 2016;387:679‐690. [DOI] [PubMed] [Google Scholar]
- 48. Newsome PN, Buchholtz K, Cusi K, et al. A placebo‐controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384:1113‐1124. [DOI] [PubMed] [Google Scholar]
- 49. Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675‐1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. de Franchis R. Expanding consensus in portal hypertension: report of the Baveno VI consensus workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743‐752. [DOI] [PubMed] [Google Scholar]
- 51. Petta S, Sebastiani G, Bugianesi E, et al. Non‐invasive prediction of esophageal varices by stiffness and platelet in non‐alcoholic fatty liver disease cirrhosis. J Hepatol. 2018;69:878‐885. [DOI] [PubMed] [Google Scholar]
- 52. Tapper EB, Jiang ZG, Patwardhan VR. Refining the ammonia hypothesis: a physiology‐driven approach to the treatment of hepatic encephalopathy. In Mayo Clinic Proceedings: Elsevier; 2015. [DOI] [PubMed] [Google Scholar]
- 53. Lai JC, Tandon P, Bernal W, et al. Malnutrition, frailty, and sarcopenia in patients with cirrhosis: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Plank LD, Gane EJ, Peng S, et al. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: a randomized 12‐month trial. Hepatology. 2008;48:557‐566. [DOI] [PubMed] [Google Scholar]
- 55. Duong NK, Shrestha S, Park D, et al. Bristol Stool Scale as a determinant of hepatic encephalopathy management in patients with cirrhosis. Am J Gastroenterol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dhiman RK, Thumburu KK, Verma N, et al. Comparative efficacy of treatment options for minimal hepatic encephalopathy: a systematic review and network meta‐analysis. Clin Gastroenterol Hepatol 2020;18:800‐812. e25. [DOI] [PubMed] [Google Scholar]
- 57. Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071‐1081. [DOI] [PubMed] [Google Scholar]
- 58. Tapper EB, Aberasturi D, Zhao Z, et al. Outcomes after hepatic encephalopathy in population‐based cohorts of patients with cirrhosis. Alimentary Pharmacology & Therapeutics 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Butterworth RF, Kircheis G, Hilger N, et al. Efficacy of l‐ornithine l‐aspartate for the treatment of hepatic encephalopathy and hyperammonemia in cirrhosis: systematic review and meta‐analysis of randomized controlled trials. J Clin Exp Hepatol. 2018;8:301‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jain A, Sharma BC, Mahajan B, et al. L‐ornithine L‐aspartate in acute treatment of severe hepatic encephalopathy: a double‐blind randomized controlled trial. Hepatology. 2021. [DOI] [PubMed] [Google Scholar]
- 61. Labenz C, Labenz J, Galle PR. Letter to the Editor: Evidence on the use of L‐ornithine L‐aspartate in overt hepatic encephalopathy ‐ but does it really improve prognosis? Hepatology. 2021. [DOI] [PubMed] [Google Scholar]
- 62. Rahimi RS, Safadi R, Thabut D, et al. Efficacy and safety of ornithine phenylacetate for treating overt hepatic encephalopathy in a randomized trial. Clin Gastroenterol Hepatol 2020. [DOI] [PubMed] [Google Scholar]
- 63. Montagnese S, Lauridsen M, Vilstrup H, et al. A pilot study of golexanolone, a new GABA‐A receptor‐modulating steroid antagonist, in patients with covert hepatic encephalopathy. J Hepatol. 2021;75:98‐107. [DOI] [PubMed] [Google Scholar]
- 64. Bloom P, Tapper EB, Young VB, et al. Microbiome therapeutics for hepatic encephalopathy. J Hepatol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Park HY, Tsauo J, Shin JH, et al. Percutaneous transparaumbilical embolization of spontaneous portosystemic shunts for the treatment of hepatic encephalopathy. J Vasc Interv Radiol. 2017;28:1563‐1568. [DOI] [PubMed] [Google Scholar]
- 66. Gatta A, Angeli P, Caregaro L, et al. A pathophysiological interpretation of unresponsiveness to spironolactone in a stepped‐care approach to the diuretic treatment of ascites in nonazotemic cirrhotic patients. Hepatology. 1991;14:231‐236. [PubMed] [Google Scholar]
- 67. Morando F, Rosi S, Gola E, et al. Adherence to a moderate sodium restriction diet in outpatients with cirrhosis and ascites: a real‐life cross‐sectional study. Liver Int. 2015;35:1508‐1515. [DOI] [PubMed] [Google Scholar]
- 68. Tapper EB, Baki J, Nikirk S, et al. Medically tailored meals for the management of symptomatic ascites: the SALTYFOOD pilot randomized clinical trial. Gastroenterol Rep. 2020;8:453‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shear L, Swartz C, Shinaberger JA, et al. Kinetics of peritoneal fluid absorption in adult man. N Engl J Med. 1965;272:123‐127. [DOI] [PubMed] [Google Scholar]
- 70. Biggins SW, Angeli P, Garcia‐Tsao G, et al. Diagnosis, evaluation, and management of ascites and hepatorenal syndrome. Hepatology. 2021. [DOI] [PubMed] [Google Scholar]
- 71. Montalvo‐Gordon I, Chi‐Cervera LA, García‐Tsao G. Sodium‐glucose cotransporter 2 inhibitors ameliorate ascites and peripheral edema in patients with cirrhosis and diabetes. Hepatology. 2020;72:1880‐1882. [DOI] [PubMed] [Google Scholar]
- 72. Bureau C, Thabut D, Oberti F, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant‐free survival of patients with cirrhosis and recurrent ascites. Gastroenterology. 2017;152:157‐163. [DOI] [PubMed] [Google Scholar]
- 73. Bureau C, Thabut D, Jezequel C, et al. The use of rifaximin in the prevention of overt hepatic encephalopathy after transjugular intrahepatic portosystemic shunt: a randomized controlled trial. Ann Intern Med. 2021;174:633‐640. [DOI] [PubMed] [Google Scholar]
- 74. Schepis F, Vizzutti F, Garcia‐Tsao G, et al. Under‐dilated TIPS associate with efficacy and reduced encephalopathy in a prospective, non‐randomized study of patients with cirrhosis. Clin Gastroenterol Hepatol 2018;16:1153‐1162.e7. [DOI] [PubMed] [Google Scholar]
- 75. Moreau R, Elkrief L, Bureau C, et al. Effects of long‐term norfloxacin therapy in patients with advanced cirrhosis. Gastroenterology 2018;155:1816‐1827. e9. [DOI] [PubMed] [Google Scholar]
- 76. Terg R, Fassio E, Guevara M, et al. Ciprofloxacin in primary prophylaxis of spontaneous bacterial peritonitis: a randomized, placebo‐controlled study. J Hepatol. 2008;48:774‐779. [DOI] [PubMed] [Google Scholar]
- 77. Salehi S, Tranah TH, Lim S, et al. Rifaximin reduces the incidence of spontaneous bacterial peritonitis, variceal bleeding and all‐cause admissions in patients on the liver transplant waiting list. Aliment Pharmacol Ther. 2019;50:435‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fernandez J, Acevedo J, Castro M, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology. 2012;55:1551‐1561. [DOI] [PubMed] [Google Scholar]
- 79. Bruns T, Lutz P, Stallmach A, et al. Low ascitic fluid protein does not indicate an increased risk for spontaneous bacterial peritonitis in current cohorts. J Hepatol. 2015;63:527‐528. [DOI] [PubMed] [Google Scholar]
- 80. Villa E, Cammà C, Marietta M, et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology 2012;143:1253‐1260. e4. [DOI] [PubMed] [Google Scholar]
- 81. Wong F, Pappas SC, Curry MP, et al. Terlipressin plus albumin for the treatment of type 1 hepatorenal syndrome. N Engl J Med. 2021;384:818‐828. [DOI] [PubMed] [Google Scholar]
- 82. Velez JCQ, Petkovich B, Karakala N, et al. Point‐of‐care echocardiography unveils misclassification of acute kidney injury as hepatorenal syndrome. Am J Nephrol. 2019;50:204‐211. [DOI] [PubMed] [Google Scholar]
- 83. Cavallin M, Piano S, Romano A, et al. Terlipressin given by continuous intravenous infusion versus intravenous boluses in the treatment of hepatorenal syndrome: a randomized controlled study. Hepatology. 2016;63:983‐992. [DOI] [PubMed] [Google Scholar]
- 84. Thomson MJ, Taylor A, Sharma P, et al. Limited progress in hepatorenal syndrome (HRS) reversal and survival 2002‐2018: a systematic review and meta‐analysis. Dig Dis Sci. 2020;65:1539‐1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Singh V, Ghosh S, Singh B, et al. Noradrenaline vs terlipressin in the treatment of hepatorenal syndrome: a randomized study J Hepat 2012;56:1293‐1298. [DOI] [PubMed] [Google Scholar]
- 86. Lai JC, Sonnenday CJ, Tapper EB, et al. Frailty in liver transplantation: An expert opinion statement from the American Society of Transplantation Liver and Intestinal Community of Practice. Am J Transplant. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lai JC, Feng S, Terrault NA, et al. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant. 2014;14:1870‐1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Tapper EB, Nikirk S, Parikh N, et al. Falls are common, morbid, and predictable in patients with cirrhosis. J Hepatol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tapper EB, Zhao L, Nikirk S, et al. Incidence and bedside predictors of the first episode of overt hepatic encephalopathy in patients with cirrhosis. Off J Am College Gastroenterol ACG 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sinclair M, Grossmann M, Hoermann R, et al. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: A randomised controlled trial. J Hepatol. 2016;65:906‐913. [DOI] [PubMed] [Google Scholar]
- 91. Villanueva C, Albillos A, Genescà J, et al. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double‐blind, placebo‐controlled, multicentre trial. Lancet. 2019;393:1597‐1608. [DOI] [PubMed] [Google Scholar]
- 92. Reiberger T, Ulbrich G, Ferlitsch A, et al. Carvedilol for primary prophylaxis of variceal bleeding in cirrhotic patients with haemodynamic non‐response to propranolol. Gut. 2013;62:1634‐1641. [DOI] [PubMed] [Google Scholar]
- 93. McDowell HR, Chuah CS, Tripathi D, et al. Carvedilol is associated with improved survival in patients with cirrhosis: a long‐term follow‐up study. Aliment Pharmacol Ther. 2021;53:531‐539. [DOI] [PubMed] [Google Scholar]
- 94. Wijarnpreecha K, Li F, Xiang Y, et al. Nonselective beta‐blockers are associated with a lower risk of hepatocellular carcinoma among cirrhotic patients in the United States. Aliment Pharmacol Ther. 2021;54:481‐492. [DOI] [PubMed] [Google Scholar]
- 95. Pollo‐Flores P, Soldan M, Santos UC, et al. Three months of simvastatin therapy vs. placebo for severe portal hypertension in cirrhosis: A randomized controlled trial. Dig Liver Dis. 2015;47:957‐963. [DOI] [PubMed] [Google Scholar]
- 96. Abraldes JG, Albillos A, Bañares R, et al. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: a randomized controlled trial. Gastroenterology. 2009;136:1651‐1658. [DOI] [PubMed] [Google Scholar]
- 97. Abraldes JG, Villanueva C, Aracil C, et al. Addition of simvastatin to standard therapy for the prevention of variceal rebleeding does not reduce rebleeding but increases survival in patients with cirrhosis. Gastroenterology 2016;150:1160‐1170.e3. [DOI] [PubMed] [Google Scholar]
- 98. Tapper EB, Parikh ND, Sengupta N, et al. A risk score to predict the development of hepatic encephalopathy in a population‐based cohort of patients with cirrhosis. Hepatology. 2018;68:1498‐1507. [DOI] [PubMed] [Google Scholar]
- 99. Mohanty A, Tate JP, Garcia‐Tsao G. Statins are associated with a decreased risk of decompensation and death in veterans with hepatitis C‐related compensated cirrhosis. Gastroenterology 2016;150:430‐440. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Simon TG, Duberg A‐S, Aleman S, et al. Lipophilic statins and risk for hepatocellular carcinoma and death in patients with chronic viral hepatitis: results from a nationwide Swedish population. Ann Intern Med. 2019;171:318‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Pose E, Napoleone L, Amin A, et al. Safety of two different doses of simvastatin plus rifaximin in decompensated cirrhosis (LIVERHOPE‐SAFETY): a randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet Gastroenterol Hepatol. 2020;5:31‐41. [DOI] [PubMed] [Google Scholar]
- 102. Kimer N, Grønbæk H, Fred RG, et al. Atorvastatin for prevention of disease progression and hospitalisation in liver cirrhosis: protocol for a randomised, double‐blind, placebo‐controlled trial. BMJ Open. 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Simon TG, Ma Y, Ludvigsson JF, et al. Association between aspirin use and risk of hepatocellular carcinoma. JAMA Oncol. 2018;4:1683‐1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kaplan DE, Serper M. John BV, et al. Clin Gastroenterol Hepatol: Effects of metformin exposure on survival in a large national cohort of patients with diabetes and cirrhosis; 2020. [DOI] [PubMed] [Google Scholar]
- 105. Tapper EB, Henderson JB. Parikh ND, et al. Hepatol Commun: Incidence of and risk factors for hepatic encephalopathy in a population‐based cohort of americans with cirrhosis; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Singh S, Singh PP, Singh AG, et al. Anti‐diabetic medications and the risk of hepatocellular cancer: a systematic review and meta‐analysis. Am J Gastroenterol 2013;108:881‐91; quiz 892. [DOI] [PubMed] [Google Scholar]
- 107. Ma S, Zheng Y, Xiao Y, et al. Meta‐analysis of studies using metformin as a reducer for liver cancer risk in diabetic patients. Medicine (Baltimore). 2017;96:e6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Huang DQ, El‐Serag HB, Loomba R. Global epidemiology of NAFLD‐related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Serper M, Weinberg EM, Cohen JB, et al. Mortality and hepatic decompensation in patients with cirrhosis and atrial fibrillation treated with anticoagulation. Hepatology. 2021;73:219‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Peng JK, Hepgul N, Higginson IJ, et al. Symptom prevalence and quality of life of patients with end‐stage liver disease: A systematic review and meta‐analysis. Palliat Med. 2019;33:24‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hansen L, Chang MF, Hiatt S, et al. Symptom frequency and distress underestimated in decompensated cirrhosis. Dig Dis Sci 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lindor KD, Bowlus CL, Boyer J, et al. Primary biliary cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology. 2019;69:394‐419. [DOI] [PubMed] [Google Scholar]
- 113. Hegade VS, Kendrick SF, Jones DE. Drug treatment of pruritus in liver diseases. Clin Med (Lond). 2015;15:351‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. easloffice@easloffice.eu EAftSotLEa, Liver EAftSot . EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145‐172. [DOI] [PubMed] [Google Scholar]
- 115. Di Padova C, Tritapepe R, Rovagnati P, et al. Double‐blind placebo‐controlled clinical trial of microporous cholestyramine in the treatment of intra‐ and extra‐hepatic cholestasis: relationship between itching and serum bile acids. Methods Find Exp Clin Pharmacol. 1984;6:773‐776. [PubMed] [Google Scholar]
- 116. Kuiper EM, van Erpecum KJ, Beuers U, et al. The potent bile acid sequestrant colesevelam is not effective in cholestatic pruritus: results of a double‐blind, randomized, placebo‐controlled trial. Hepatology. 2010;52:1334‐1340. [DOI] [PubMed] [Google Scholar]
- 117. Bergasa NV, McGee M, Ginsburg IH, et al. Gabapentin in patients with the pruritus of cholestasis: a double‐blind, randomized, placebo‐controlled trial. Hepatology. 2006;44:1317‐1323. [DOI] [PubMed] [Google Scholar]
- 118. Khurana S, Singh P. Rifampin is safe for treatment of pruritus due to chronic cholestasis: a meta‐analysis of prospective randomized‐controlled trials. Liver Int. 2006;26:943‐948. [DOI] [PubMed] [Google Scholar]
- 119. Wolfhagen FH, Sternieri E, Hop WC, et al. Oral naltrexone treatment for cholestatic pruritus: a double‐blind, placebo‐controlled study. Gastroenterology. 1997;113:1264‐1269. [DOI] [PubMed] [Google Scholar]
- 120. Mansour‐Ghanaei F, Taheri A, Froutan H, et al. Effect of oral naltrexone on pruritus in cholestatic patients. World J Gastroenterol. 2006;12:1125‐1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bertolotti M, Ferrari A, Vitale G, et al. Effect of liver cirrhosis on the systemic availability of naltrexone in humans. J Hepatol. 1997;27:505‐511. [DOI] [PubMed] [Google Scholar]
- 122. Mayo MJ, Handem I, Saldana S, et al. Sertraline as a first‐line treatment for cholestatic pruritus. Hepatology. 2007;45:666‐674. [DOI] [PubMed] [Google Scholar]
- 123. de Vries E, Bolier R, Goet J, et al. Fibrates for Itch (FITCH) in fibrosing cholangiopathies: a double‐blind, randomized, placebo‐controlled trial. Gastroenterology 2021;160:734‐743.e6. [DOI] [PubMed] [Google Scholar]
- 124. Shen N, Pan J, Miao H, et al. Fibrates for the treatment of pruritus in primary biliary cholangitis: a systematic review and meta‐analysis. Ann Palliat Med. 2021;10:7697‐7705. [DOI] [PubMed] [Google Scholar]
- 125. Marchesini G, Bianchi G, Amodio P, et al. Factors associated with poor health‐related quality of life of patients with cirrhosis. Gastroenterology. 2001;120:170‐178. [DOI] [PubMed] [Google Scholar]
- 126. Chatrath H, Liangpunsakul S, Ghabril M, et al. Prevalence and morbidity associated with muscle cramps in patients with cirrhosis. Am J Med. 2012;125:1019‐1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Vidot H, Cvejic E, Carey S, et al. Randomised clinical trial: oral taurine supplementation versus placebo reduces muscle cramps in patients with chronic liver disease. Aliment Pharmacol Ther. 2018;48:704‐712. [DOI] [PubMed] [Google Scholar]
- 128. Hidaka H, Nakazawa T, Kutsukake S, et al. The efficacy of nocturnal administration of branched‐chain amino acid granules to improve quality of life in patients with cirrhosis. J Gastroenterol. 2013;48:269‐276. [DOI] [PubMed] [Google Scholar]
- 129. Houstoun M, Reichman ME, Graham DJ, et al. Use of an active surveillance system by the FDA to observe patterns of quinine sulfate use and adverse hematologic outcomes in CMS Medicare data. Pharmacoepidemiol Drug Saf. 2014;23:911‐917. [DOI] [PubMed] [Google Scholar]
- 130. Lee FY, Lee SD, Tsai YT, et al. A randomized controlled trial of quinidine in the treatment of cirrhotic patients with muscle cramps. J Hepatol. 1991;12:236‐240. [DOI] [PubMed] [Google Scholar]
- 131. Elfert AA, Abo Ali L, Soliman S, et al. Randomized placebo‐controlled study of baclofen in the treatment of muscle cramps in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2016;28:1280‐1284. [DOI] [PubMed] [Google Scholar]
- 132. Abd‐Elsalam S, Arafa M, Elkadeem M, et al. Randomized‐controlled trial of methocarbamol as a novel treatment for muscle cramps in cirrhotic patients. Eur J Gastroenterol Hepatol. 2019;31:499‐502. [DOI] [PubMed] [Google Scholar]
- 133. Abd‐Elsalam S, Ebrahim S, Soliman S, et al. Orphenadrine in treatment of muscle cramps in cirrhotic patients: a randomized study. Eur J Gastroenterol Hepatol. 2020;32:1042‐1045. [DOI] [PubMed] [Google Scholar]
- 134. Nakanishi H, Kurosaki M, Tsuchiya K, et al. L‐carnitine reduces muscle cramps in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13:1540‐1543. [DOI] [PubMed] [Google Scholar]
- 135. Kugelmas M. Preliminary observation: oral zinc sulfate replacement is effective in treating muscle cramps in cirrhotic patients. J Am Coll Nutr. 2000;19:13‐15. [DOI] [PubMed] [Google Scholar]
- 136. Jagdish RK, Kamaal A, Shasthry SM, et al. Tadalafil improves erectile dysfunction and quality of life in men with cirrhosis: a randomized double blind placebo controlled trial. Hepatol Int. 2021. [DOI] [PubMed] [Google Scholar]
- 137. Nappi RE, Cucinella L. Advances in pharmacotherapy for treating female sexual dysfunction. Expert Opin Pharmacother. 2015;16:875‐887. [DOI] [PubMed] [Google Scholar]
- 138. Ghabril M, Jackson M, Gotur R, et al. Most individuals with advanced cirrhosis have sleep disturbances, which are associated with poor quality of life. Clin Gastroenterol Hepatol. 2017;15:1271‐1278. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Plotogea OM, Ilie M, Bungau S, et al. Comprehensive overview of sleep disorders in patients with chronic liver disease. Brain Sci. 2021;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Cordoba J, Cabrera J, Lataif L, et al. High prevalence of sleep disturbance in cirrhosis. Hepatology. 1998;27:339‐345. [DOI] [PubMed] [Google Scholar]
- 141. Prasad S, Dhiman RK, Duseja A, et al. Lactulose improves cognitive functions and health‐related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology. 2007;45:549‐559. [DOI] [PubMed] [Google Scholar]
- 142. Singh J, Sharma BC, Puri V, et al. Sleep disturbances in patients of liver cirrhosis with minimal hepatic encephalopathy before and after lactulose therapy. Metab Brain Dis. 2017;32:595‐605. [DOI] [PubMed] [Google Scholar]
- 143. Sharma MK, Kainth S, Kumar S, et al. Effects of zolpidem on sleep parameters in patients with cirrhosis and sleep disturbances: A randomized, placebo‐controlled trial. Clin Mol Hepatol. 2019;25:199‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. De Silva AP, Niriella MA, Ediriweera DS, et al. Low‐dose melatonin for sleep disturbances in early‐stage cirrhosis: A randomized, placebo‐controlled, cross‐over trial. JGH Open. 2020;4:749‐756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Spahr L, Coeytaux A, Giostra E, et al. Histamine H1 blocker hydroxyzine improves sleep in patients with cirrhosis and minimal hepatic encephalopathy: a randomized controlled pilot trial. Am J Gastroenterol. 2007;102:744‐753. [DOI] [PubMed] [Google Scholar]
- 146. Lexi‐Drugs. Riverwoods, IL: Lexicomp. Wolters Kluwer Health, Inc.; 2021. [Google Scholar]
- 147. Tapper EB, Risech‐Neyman Y, Sengupta N. Psychoactive medications increase the risk of falls and fall‐related injuries in hospitalized patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13:1670‐1675. [DOI] [PubMed] [Google Scholar]
- 148. Thomson MJ, Lok AS, Tapper EB. Appropriate and potentially inappropriate medication use in decompensated cirrhosis. Hepatology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. ter Borg PC, van Os E, van den Broek WW, et al. Fluvoxamine for fatigue in primary biliary cirrhosis and primary sclerosing cholangitis: a randomised controlled trial [ISRCTN88246634]. BMC Gastroenterol. 2004;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Silveira MG, Gossard AA, Stahler AC, et al. A randomized, placebo‐controlled clinical trial of efficacy and safety: modafinil in the treatment of fatigue in patients with primary biliary cirrhosis. Am J Ther. 2017;24:e167‐e176. [DOI] [PubMed] [Google Scholar]
- 151. Lee JY, Danford CJ, Trivedi HD, et al. Treatment of fatigue in primary biliary cholangitis: a systematic review and meta‐analysis. Dig Dis Sci. 2019;64:2338‐2350. [DOI] [PubMed] [Google Scholar]
- 152. Wilcock A, Charlesworth S, Prentice W, et al. Prescribing in chronic severe hepatic impairment. J Pain Symptom Manag. 2019;58:515‐537. [DOI] [PubMed] [Google Scholar]
- 153. Bajaj JS, Ellwood M, Ainger T, et al. Mindfulness‐based stress reduction therapy improves patient and caregiver‐reported outcomes in cirrhosis. Clin Transl Gastroenterol. 2017;8:e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient‐reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34:557‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient‐reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318:197‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Maguire R, McCann L, Kotronoulas G, et al. Real time remote symptom monitoring during chemotherapy for cancer: European multicentre randomised controlled trial (eSMART). BMJ. 2021;374:n1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Tapper EB, Hao S, Lin M, et al. The quality and outcomes of care provided to patients with cirrhosis by advanced practice providers. Hepatology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Lau M, Beste LA, Kryskalla J, et al. Hepatitis C clinical dashboards: improving Liver specialty care access and quality federal practitioner: for the health care professionals of the VA, DoD, and PHS. 2015;32:32S‐36S. [PMC free article] [PubMed] [Google Scholar]
- 159. Belperio PS, Chartier M, Ross DB, et al. Curing hepatitis C virus infection: best practices from the U.S. Department of Veterans Affairs. Ann Intern Med. 2017;167:499‐504. [DOI] [PubMed] [Google Scholar]
- 160. Byrne C, Radley A, Inglis SK, et al. Reaching mEthadone users Attending Community pHarmacies with HCV: an international cluster randomised controlled trial protocol (REACH HCV). BMJ Open 2020;10:e036501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Dillon JF, Miller MH, Robinson EM, et al. Intelligent liver function testing (iLFT): A trial of automated diagnosis and staging of liver disease in primary care. J Hepatol. 2019;71:699‐706. [DOI] [PubMed] [Google Scholar]
- 162. Tapper EB, Parikh ND. The future of quality improvement for cirrhosis. Liver Transpl. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Sinagra E, Perricone G, D'Amico M, et al. Systematic review with meta‐analysis: the haemodynamic effects of carvedilol compared with propranolol for portal hypertension in cirrhosis. Aliment Pharmacol Ther. 2014;39:557‐568. [DOI] [PubMed] [Google Scholar]
- 164. Ghent CN, Carruthers SG. Treatment of pruritus in primary biliary cirrhosis with rifampin. Results of a double‐blind, crossover, randomized trial. Gastroenterology. 1988;94:488‐493. [DOI] [PubMed] [Google Scholar]
- 165. Podesta A, Lopez P, Terg R, et al. Treatment of pruritus of primary biliary cirrhosis with rifampin. Dig Dis Sci. 1991;36:216‐220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable to a review


