Abstract
Introduction
Orthopedic procedures have been associated with increased pain, making perioperative analgesia a major clinical concern. We assessed the efficacy and safety of intravenous parecoxib administration during the perioperative period for postoperative pain relief after orthopedic surgery in adults.
Methods
PubMed, Cochrane Library, EMBASE, and clinicaltrial.gov were searched from inception to 23 August 2021 without language restrictions. Randomized controlled trials comparing intravenous parecoxib with placebo or another active treatment for acute postoperative pain in adults after orthopedic surgery were included. The primary outcomes were the pain scores and cumulative morphine consumption. The secondary outcomes included the proportion of patients requiring rescue analgesics and the incidence of adverse events. The meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines and was registered on the International Prospective Register of Systematic Reviews Registration (PROSPERO).
Results
Twenty-seven trials (n = 2840) from more than 20 countries involving six types of orthopedic surgery met the inclusion criteria. Compared with placebo, intravenous parecoxib administration led to reductions in postoperative resting pain scores at 6, 12, 24, and 48 h [mean difference (MD) −0.87, 95% confidence interval [CI] −1.71 to −0.03; MD −0.86, 95% CI −1.26 to −0.46; MD −0.57, 95% CI −0.84 to −0.31; MD −0.40, 95% CI −0.69 to −0.11, respectively], postoperative movement pain scores at 24 and 48 h (MD −0.66, 95% CI −1.14 to −0.19; MD −0.78, 95% CI −1.16 to −0.39, respectively), cumulative morphine consumption (MD −11.30 mg, 95% CI −14.79 to −7.81 mg), and the proportion of patients requiring rescue analgesia (relative risk 0.83, 95% CI 0.77–0.89). There was no difference in the incidence of adverse events between groups.
Conclusion
Low to moderate evidence indicates that parecoxib might be an effective and safe analgesic in perioperative orthopedic settings. It relieves postoperative orthopedic pain while sparing opioid analgesic consumption without increasing the incidence of adverse events.
PROSPERO Registration
CRD42021274939.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40122-022-00400-1.
Keywords: Morphine consumption, Orthopedic surgery, Pain score, Parecoxib, Perioperative analgesia
Key Summary Points
| This review and meta-analysis assessed the efficacy and safety of intravenous parecoxib administration during the perioperative period for postoperative pain relief following orthopedic surgery in adults. |
| We included 27 randomized controlled trials comparing intravenous parecoxib with placebo or another active treatment. |
| Parecoxib relieves postoperative orthopedic pain while sparing opioid analgesic consumption without increasing the incidence of adverse events. |
| Parecoxib might be an efficacious and safe analgesic in perioperative orthopedic settings. |
Introduction
Perioperative analgesia is often a major clinical concern for orthopedic surgeons. Increased pain has been related to orthopedic procedures such as lumbar spine surgery and joint replacement [1, 2]. Quality of life, function, and recovery time may be affected by suboptimal perioperative pain relief [3]. Thus, multimodal analgesia and enhanced recovery are gaining attention, and their clinical applications are becoming more widespread [4]. Nonsteroidal anti-inflammatory drugs (NSAIDs) are important components of multimodal analgesia [5]. Guidelines for the management of acute pain recommend multimodal analgesia, routinely including the administration of both an opioid and one or more non-opioid, and, frequently, an NSAID [6, 7].
In addition to relieving pain, perioperative NSAID administration has been shown to reduce patients’ requirements for opioids without significant side effects [8, 9]. Selective cyclooxygenase (COX)-2 inhibitors administered via the enteral route (e.g., celecoxib) are effective in relieving mild to moderate pain. However, they are ineffective in cases of postoperative nausea and vomiting or when the oral route of administration is inaccessible. Therefore, appropriate NSAID use is of great importance, especially when patients are likely to be administered intravenous NSAIDs during the perioperative period. Parecoxib was the first parenterally administered COX-2 inhibitor approved in Europe and Asia for the treatment of postoperative pain [10]. According to a Cochrane systematic review of randomized controlled trials (RCTs) on postoperative pain control, a single dose of parecoxib provided effective analgesia compared with a placebo [11]. However, the aforementioned study did not differentiate between various types of surgery. While some studies have reported that parecoxib reduces opioid consumption and relieves pain [12, 13], in other trials, parecoxib administration did not reduce postoperative pain compared with placebo after knee and hip arthroplasty surgery [14, 15]. Although more studies have been published, the analgesic efficacy and safety of parecoxib use in orthopedic surgery remain unclear. Therefore, this systematic review and meta-analysis aimed to evaluate the benefits and risks of parecoxib use in orthopedic surgery.
Methods
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [16] and Assessing the Methodological Quality of Systematic Reviews Guidelines [17]. The study protocol was registered in the International Prospective Register of Systematic Reviews (CRD42021274939). This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Eligibility Criteria and Outcome Definitions
Studies were selected on the basis of the following inclusion criteria: (1) RCTs comparing parecoxib injection with a placebo or other analgesics for pain relief; (2) having enrolled adults > 18 years of age undergoing orthopedic surgery; and (3) reporting data on postoperative pain score, cumulative analgesic consumption, proportion of patients requiring rescue analgesics, and incidence of adverse events. The exclusion criteria were as follows: (1) trials with no placebo or treatment group; (2) trials including participants with active gastrointestinal bleeding, ulceration, or severe liver dysfunction; and (3) abstracts, letters, editorials, conference articles, or duplicated studies. The primary outcomes included pain scores at different timepoints and cumulative morphine consumption (mg) over the first 24 h. Secondary outcomes included the proportion of patients requiring rescue analgesics and the incidence of adverse events.
We assessed clinical outcomes on the basis of the following predetermined definitions: (1) pain score reported using the visual analog scale (VAS: 0–10 cm, 0 = no pain and 10 = maximum pain; VAS: 0–100 mm, 0 = no pain and 100 = maximum pain) or verbal numerical rating scale (VNRS: 0–10, 0 = no pain and 10 = maximum pain); (2) cumulative morphine consumption (mg) in groups at 24 h postoperatively was recorded for each group to determine the effect of opioid-sparing, and other opioids were converted to intravenous morphine equivalents; (3) adverse events were recorded regardless of treatment or potential causal relationship with parecoxib.
Information Sources and Search Strategy
PubMed, Embase, Cochrane Library, and clinicaltrial.gov were searched from inception to 23 August 2021. There were no restrictions on specific language or year of publication. The following keywords were searched: “parecoxib,” “orthopedic procedures,” “perioperative period,” “pain,” “analgesia,” “randomized controlled trial,” and other related Medical Subject Headings terms or expressions. Additional searches were conducted by reviewing the reference lists of published articles. Details regarding the search strategy are presented in Table S1 (see the electronic supplementary material for details).
Study Selection and Data Extraction
After removing duplicates, screening for eligible articles (abstract and full text) was performed independently by two reviewers. Discrepancies were resolved by a third reviewer. Information on the first author, publication year, type of surgery, number of patients, interventions and controls, supplemental analgesics, and outcomes of interest was retrieved independently by two reviewers using a predesigned form. The agreement level of selections between two investigators was calculated by the kappa scores. A κ value of 0.81–1.00 indicated almost perfect agreement; 0.61–0.80 indicated substantial agreement; 0.21–0.60 indicated moderate agreement; and 0.20 indicated lower or slight agreement. Disagreement were resolved through discussion or by a third coordinator. The article authors were contacted for missing or incomplete data when necessary.
Quality Assessment
According to the Cochrane Risk of Bias tool, the risk of bias of each RCT was graded as low, high, or unclear on the basis of the following seven domains: (1) random sequence generation; (2) allocation concealment; (3) blinding of participants and personnel; (4) blinding of outcome assessment; (5) incomplete outcome data; (6) selective reporting; (7) other bias [18].
Data Synthesis and Analysis
Meta-analyses were performed using Review Manager version 5.4. (The Cochrane Collaboration, London, England). Opioids were converted to intravenous morphine equivalents according to Table S2 (see the electronic supplementary material for details). VAS 0–100 and VNRS 0–10, were converted to VAS 0–10. Median and interquartile range/range were converted to mean and standard deviation according to the Cochrane Handbook. Some trials had more than one intervention group. In these cases, we split the control group, according to the Cochrane Handbook.
Continuous variables are expressed as mean difference (MD) with 95% confidence intervals (CIs), and relative risk (RR) with 95% CI was calculated for dichotomous variables. Forest plots were used to summarize estimates and provide a comprehensive evaluation of the available evidence and pooled effect sizes from the meta-analyses. Heterogeneity across studies was evaluated using the chi-square (χ2) test and I2 index (P > 0.1 and I2 < 50% indicated acceptable heterogeneity) [19, 20]. A random-effect model was used for the meta-analyses [21]. Sensitivity analysis was performed by excluding high-risk bias studies or trials with different characteristics. Subgroup analyses were performed on the basis of orthopedic procedures. Funnel plots were constructed and tested for publication bias of outcome indicators when more than ten studies were included [22].
Grading the Evidence Strength
Two reviewers independently rated the certainty of the evidence for each outcome using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach, which considers five factors: reporting bias, inconsistency, indirectness of evidence, imprecision, and other considerations such as publication bias [23]. Disagreements during the evaluation process were resolved by discussion or consultation with a third reviewer.
Results
Search Results
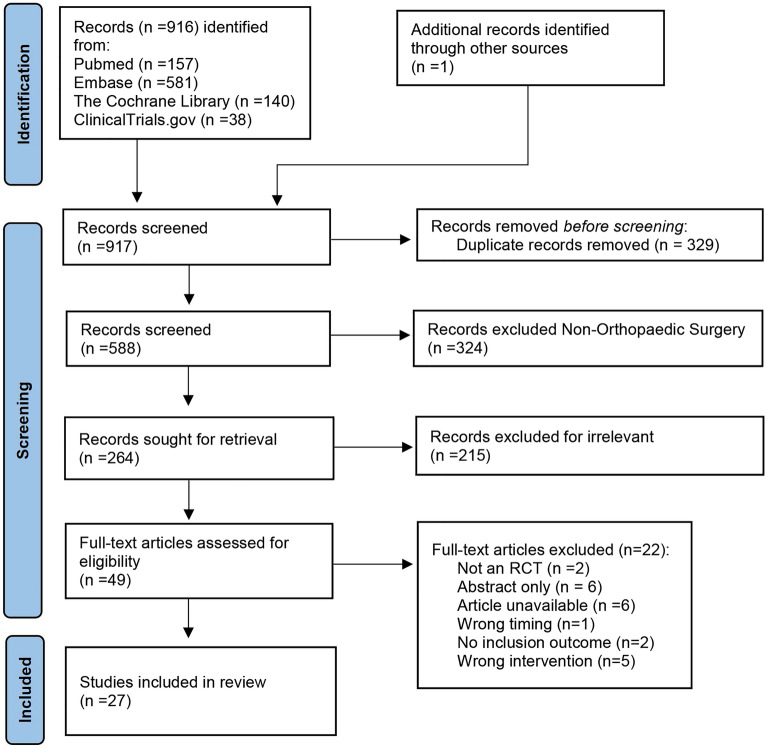
The PRISMA flowchart is presented in Fig. 1. Database searches yielded a total of 916 citations, and a reference search identified one additional relevant abstract. A total of 329 duplicates were removed, and 264 abstracts were screened for orthopedic surgery. Finally, on the basis of the abstract, 49 were selected for full-text assessment, and 27 of these were included according to the inclusion and exclusion criteria. Perfect agreement was detected between the two reviewers during all steps of the review process and data extraction (κ = 0.81–1.00).
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram
Characteristics of Included Studies
The characteristics of the included studies are presented in Table S3 (see the electronic supplementary material for details). A total of 27 RCTs with 2840 participants were included [14, 15, 24–48], with between 15 and 310 participants receiving intravenous parecoxib per study. There were 15 RCTs on knee and hip arthroplasty [14, 15, 24–27, 29–31, 33, 36, 42, 46–48], 5 on lumbar spine surgery [32, 38, 40, 41, 44], 2 on anterior cruciate ligament reconstruction [34, 37], 2 on bunionectomy [39, 45], and 3 on other orthopedic surgery [28, 35, 43]. The control groups included placebo (23 trials), ketorolac (3 trials), paracetamol (3 trials), and celecoxib (1 trial). The included trials originated from China (7/27, 25.9%), the USA (5/27, 18.5%), Thailand (4/27, 14.8%), Germany (3/27, 11.1%), and other countries (Korea, Belgium, France, Germany, Sweden, Egypt, Czech Republic, and Cuba).
Risk of Bias Assessment
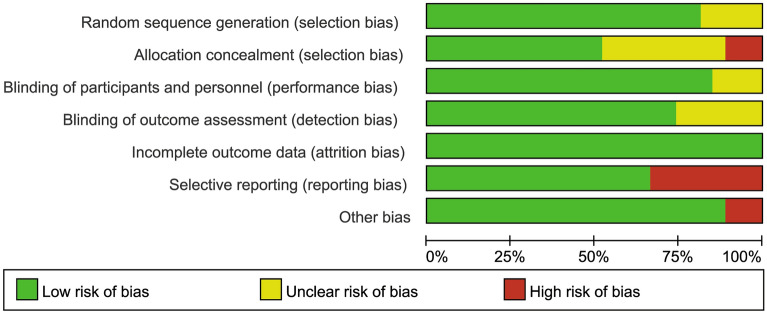
The risk of bias assessment for RCTs is summarized in Figs. 2 and 3. All studies were randomized; 23 trials were double-blind placebo-controlled, 22 described adequate random sequence generation processes, and 14 described allocation concealment methods. Therefore, 7 trials were evaluated as having low risk of bias, 11 trials were identified as having unclear risk of bias, and 9 trials as having high risk of bias.
Fig. 2.
Risk of bias graph
Fig. 3.
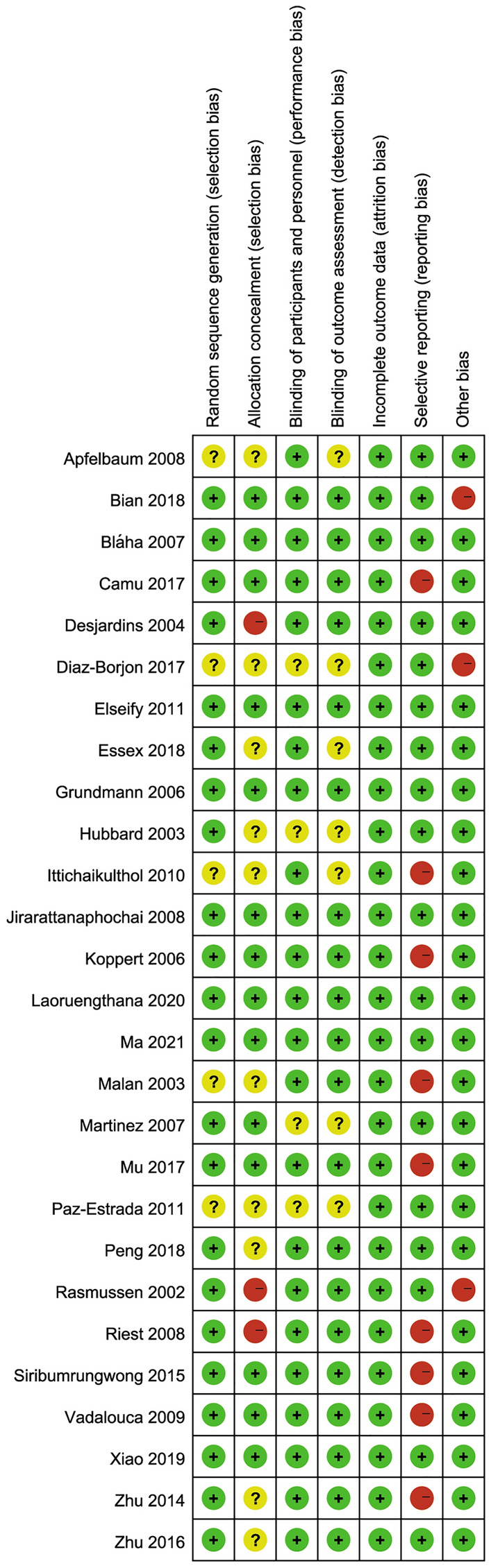
Risk of bias summary
Postoperative Pain Scores
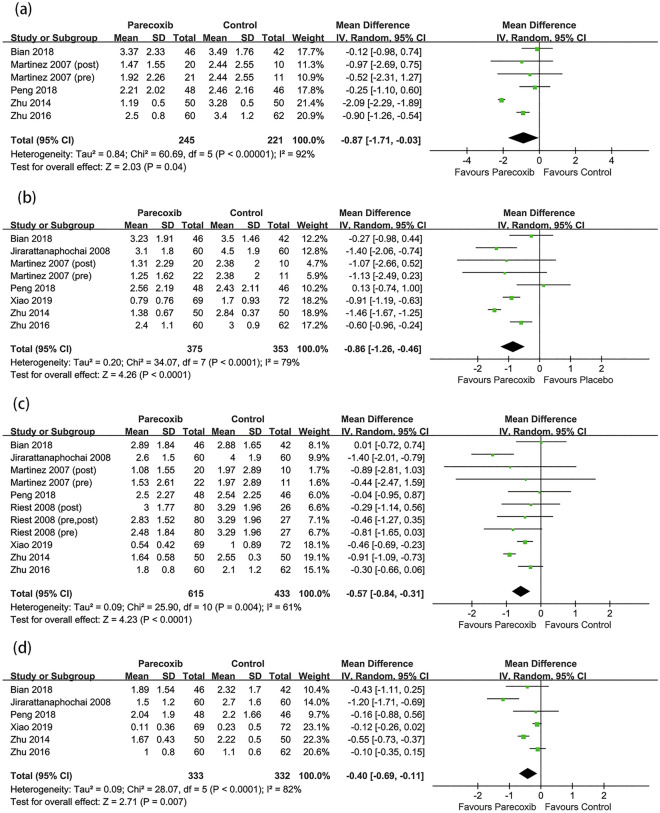
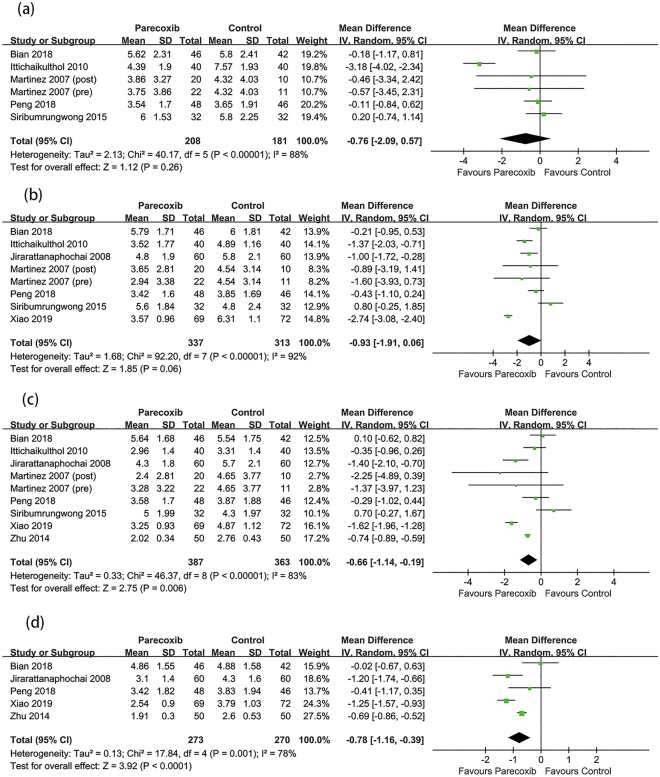
The postoperative pain scores at different timepoints at rest and during movement are shown in Figs. 4 and 5. The results demonstrated that parecoxib could reduce the postoperative pain scores at rest compared with placebo at 6, 12, 24, and 48 h (MD −0.87, 95% CI −1.71 to −0.03, P = 0.04; MD −0.86, 95% CI −1.26 to −0.46, P < 0.0001; MD −0.57, 95% CI −0.84 to −0.31, P < 0.0001; MD −0.40, 95% CI −0.69 to −0.11, P = 0.007; respectively). Parecoxib did not reduce the postoperative pain scores during movement at 6 and 12 h but could lower the postoperative movement pain scores at 24 and 48 h (MD −0.66, 95% CI −1.14 to −0.19, P = 0.006; MD −0.78, 95% CI −1.16 to −0.39, P < 0.0001; respectively). In the subgroup analysis, compared with placebo, parecoxib was shown to reduce the resting pain scores at 24 h in lumbar spine surgery and hip arthroplasty (MD −0.79, 95% CI −1.32 to −0.26, P = 0.003; MD −0.44, 95% CI −0.66 to −0.22, P < 0.0001; respectively), although no difference in knee arthroplasty was detected (MD −0.47; 95% CI −1.02 to 0.08; P = 0.10; see Fig. S1 in the electronic supplementary material for details).
Fig. 4.
Forest plot of pain scores at rest between the parecoxib and placebo groups at a 6 h, b 12 h, c 24 h, d 48 h after surgery
Fig. 5.
Forest plot of pain scores during movement between the parecoxib and placebo groups at a 6 h, b 12 h, c 24 h, d 48 h after surgery
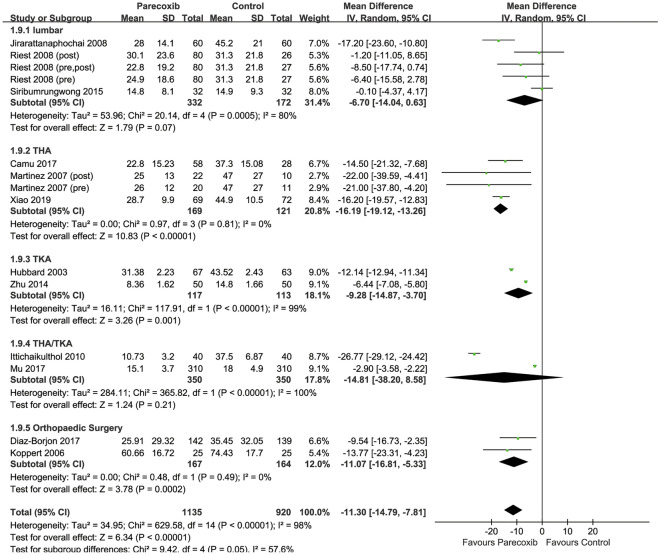
Cumulative Morphine Consumption over 24 h
Twelve RCTs were included [26, 28–30, 32, 33, 36, 38, 40, 42, 43, 46], with 1135 patients in the parecoxib group and 920 patients in the placebo group. The results showed that, compared with placebo, parecoxib could reduce cumulative morphine consumption over 24 h (MD −11.30 mg, 95% CI −14.79 to −7.81; P < 0.00001). Subgroup analyses revealed significant reductions in cumulative morphine consumption with parecoxib in knee arthroplasty, hip arthroplasty, and other orthopedic procedures (MD −16.19 mg, 95% CI −19.12 to −13.26, P < 0.00001; MD −9.28 mg, 95% CI −14.87 to −3.70, P = 0.001; MD −11.07 mg, 95% CI −16.81 to −5.33, P = 0.0002; Fig. 6), but there was no difference between comparators in lumbar surgery.
Fig. 6.
Forest plot of postoperative morphine consumption over 24 h, comparing parecoxib and control groups
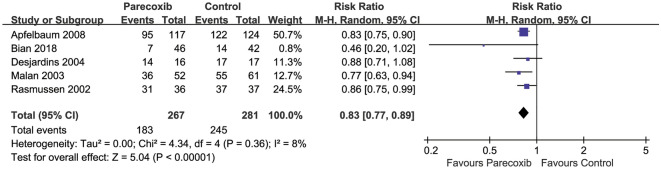
Proportion of Patients Requiring Rescue Analgesia
Pooled data from five RCTs (548 participants) [14, 39, 45, 47, 48] showed that parecoxib use resulted in significant reduction in the proportion of patients requiring rescue analgesia compared with placebo (RR 0.83, 95% CI 0.77–0.89, P < 0.00001, Fig. 7).
Fig. 7.
Forest plot of the proportion of patients requiring rescue analgesia between the parecoxib and placebo groups
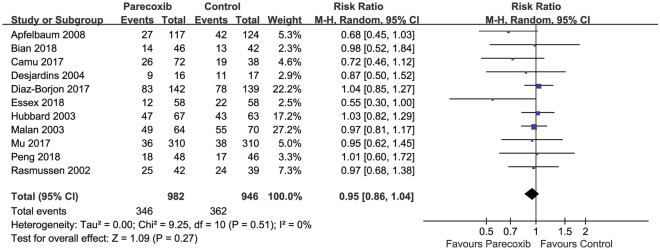
Incidence of Adverse Events
Pooled results from 11 RCTs [14, 15, 27–30, 39, 45–48] with 982 and 946 patients in the parecoxib and placebo groups, respectively, showed no significant difference in the incidence of adverse events (RR 0.95, 95% CI 0.86–1.04, P = 0.27, Fig. 8). All events were minor, including nausea and vomiting, headache and dizziness, pruritus or skin rash, and constipation. No gastrointestinal bleeding or cardiovascular events occurred.
Fig. 8.
Forest plot of AEs in the parecoxib group compared with the placebo group
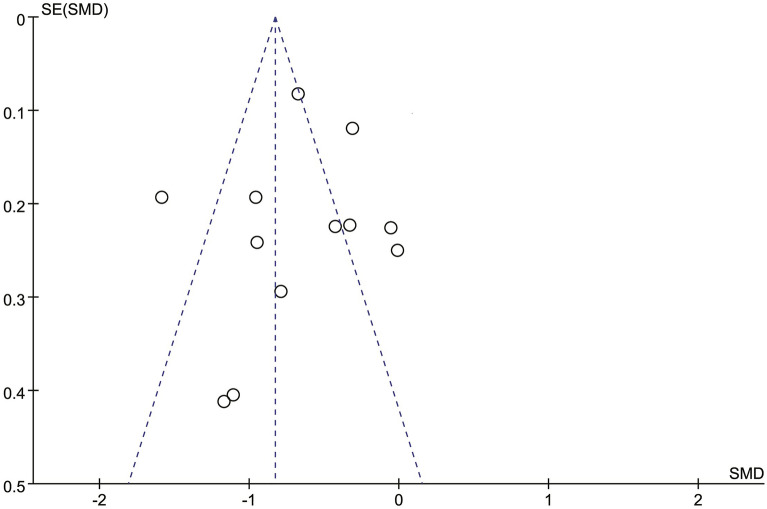
Publication Bias of Included Studies
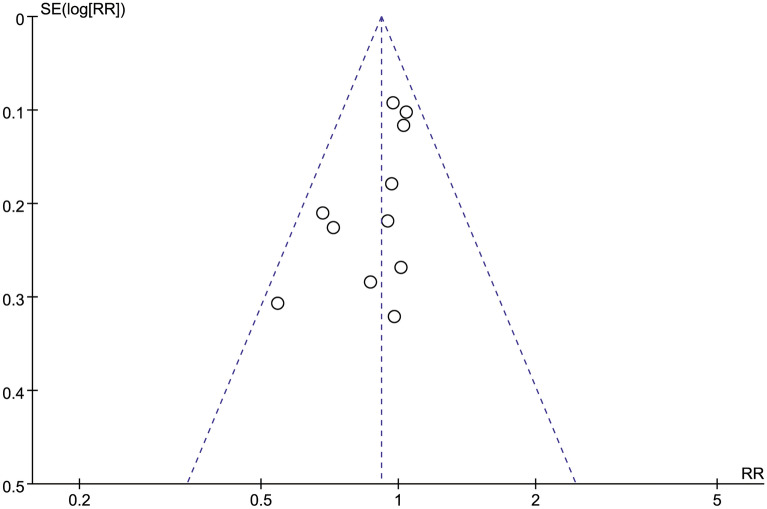
The analyses produced asymmetric funnel plots for postoperative 24 h morphine consumption, and Egger’s test revealed no publication bias for the included studies (P = 0.253). The funnel plot for the incidence of adverse events was symmetrical, indicating no significant publication bias (Figs. 9, 10).
Fig. 9.
Funnel plots of postoperative morphine consumption over 24 h
Fig. 10.
Funnel plots of the incidence of adverse events
Studies Not Included in the Quantitative Synthesis
Ten trials [24, 25, 30, 32, 34–36, 43, 44, 48] compared parecoxib with other active therapies. Parecoxib was compared with ketorolac in three studies [25, 32, 48] and paracetamol in another three studies [34, 43, 44]. The results showed that parecoxib had comparable analgesic effects to those of ketorolac, which was not significantly different from those of paracetamol. Other NSAIDs (diclofenac and celecoxib) were used in two studies [35, 36].
Sensitivity Analysis
Substantial heterogeneity, as demonstrated by I2 > 50%, occurred in most of primary outcomes. Sensitivity analyses were performed, showing robust results and revealing reduced heterogeneity for postoperative resting pain scores at 6, 12, and 24 h and postoperative movement pain scores at 6 h. However, heterogeneity remained high in other outcomes (see Table S4 in the electronic supplementary material for details).
GRADE Assessment
The GRADE assessment demonstrated an overall moderate level of evidence for each of the following outcomes: postoperative resting pain score at 6, 12, 24, and 48 h; postoperative movement pain score at 48 h; and proportion of patients requiring rescue analgesia. The level of evidence for the following outcomes was considered low: postoperative movement pain score at 6, 12, and 24 h and incidence of adverse events (see Table S5 in the electronic supplementary material for details).
Discussion
The findings of this systematic review and meta-analysis demonstrated that parecoxib could relieve pain in most types of orthopedic surgery and spare cumulative morphine consumption without increasing the incidence of adverse events. The strength of this review over previous studies [11, 12, 49] was its focus on orthopedic surgeries and producing the evidence according to the most up-to-date RCTs available. Movement pain is more severe than resting pain [50, 51] and is significantly associated with a patient’s ability to perform postoperative recovery activities. In our review, pain was differentiated depending on whether the patient was at rest or in movement, and the analgesic effect of parecoxib in various orthopedic procedures was evaluated at different timepoints. This study highlights that, for researchers, different orthopedic procedures and pain states may influence the outcome of analgesic drug assessments, and therefore this is a vital influence that needs to be taken into account in study design and clinical observations. Furthermore, this study facilitates a more comprehensive and objective understanding of parecoxib by orthopedic surgeons and patients and helps to improve the efficiency of decision-making in perioperative drug selection.
NSAIDs exert analgesic effects through multiple mechanisms in the postoperative period and have become an irreplaceable component of multimodal analgesia regimens. The primary effect of NSAIDs is COX inhibition [52]. COX-1 is considered to be involved in physiological functions, such as gastric protection and hemostasis [53], while COX-2 is linked to pathophysiologic processes, such as inflammation, pain, and fever [54]. As a selective COX-2 inhibitor, parecoxib provides analgesic effect by blocking the arachidonic acid cascade and production of prostaglandins [55, 56], reducing spinal prostaglandin release [57], and preventing peripheral and central sensitization while maintaining COX-1 physiological function with minimal side effects [58, 59]. In addition, parecoxib sodium is a water-soluble drug that can be rapidly hydrolyzed to valdecoxib after intravenous injection [10], has a long half-life, and can quickly penetrate the blood–brain barrier [60].
In terms of pain relief, the main measurement methods were the VAS and VNRS. Owing to the subjectivity of the pain measurements, this outcome may show high heterogeneity. Unlike previous studies [12, 49], when categorizing pain scores into resting and movement states, we discovered that parecoxib relieved postoperative resting pain but not postoperative movement pain at 6 and 12 h. Furthermore, we reckoned that the different timing, frequency, and duration of parecoxib administration in the included studies contributed to the differences in pain relief outcomes. However, it should not be overlooked that the mean differences in pain scores are very small and may not be clinically significant. Previous trials have also reached inconsistent conclusions regarding this issue. Some trials reported that parecoxib administration for 2 or 3 days reduced the postoperative pain scores both at rest and during movement [26, 33], whereas another trial found that pre-incisional administration of a single dose of parecoxib did not significantly reduce the postoperative VAS scores either at rest or during movement compared with placebo [15]. As a result, 2–3 days of administration provided better postoperative analgesia than a single parecoxib dose. Parecoxib use < 3 days for effective analgesia is also suggested in the drug prospect and considered to benefit patient recovery and avoid influencing platelet function as occurs during long-term use [58]. Additionally, the proportion of patients requiring rescue analgesia was significantly lower in the parecoxib group than in the placebo group in this review, similar to previous findings [11].
Although opioids remain the most commonly used analgesics for postoperative pain control, their use in clinical postoperative pain is limited by significant side effects such as nausea, vomiting [61], and even respiratory depression [62], as well as dose-dependent adverse events that reduce patient comfort and delay recovery. Therefore, multimodal analgesia regimens have been used to reduce the need for opioids in clinical practice. Our study found that parecoxib reduced cumulative morphine consumption over 24 h by approximately 11.30 mg. The results of the subgroup analysis showed that parecoxib reduced opioid analgesic consumption in both hip and knee replacements as well as in other orthopedic surgeries. The outcome of cumulative morphine consumption in 24 h was included in a total of 12 trials, 11 of which used morphine as a rescue analgesic and one of which used piritramide or tramadol. Although one trial used piritramide or tramadol, the authors converted it to a morphine equivalent dose in the outcome report. Therefore, we considered that the difference in morphine equivalents was not clinically relevant in this study. According to our findings, parecoxib did not significantly increase the adverse event risk. A pooled analysis of 28 RCTs and of > 10 years of retrospective safety data of parecoxib revealed infrequent gastrointestinal events and other prespecified safety events, highlighting its satisfactory safety and tolerance in patients with postoperative pain [63].
When assessing the certainty of findings using GRADE, we ranked certainty as ranging from low to moderate for efficacy and safety outcomes. “Low certainty” means that our confidence in the effect estimate is limited, and the true effect may be substantially different from the effect estimate. Some individual studies had an unclear risk of bias for issues such as allocation concealment and blinding, and a high risk of reporting bias. For the outcomes for which we were able to perform pooled analysis, we further downgraded the certainty of evidence owing to issues with imprecision or important inconsistency.
This study had several limitations. First, most of the included RCTs compared the treatments against placebo, which limited comparative effectiveness inferences. Therefore, further head-to-head trials of active therapies are warranted. Second, we combined orthopedic surgery of various types. The fact that different types of surgery are performed with varying degrees of intensity and characteristics may have an impact on the results’ validity. Third, since baseline pain scores were missing in some included trials, we only extracted and synthesized endpoint pain score values. In addition, considering the differences in pain relief measurement (VAS or VNRS), drug selection and dosage, and timing of drug administration among studies, heterogeneity was unavoidable. We selected a random-effect model, instead of a fixed-effect model, to synthesize the outcome measures; however, the reliability of the related outcomes was evaluated using the GRADE approach.
To the best of our knowledge, whether parecoxib should be administered prior to surgery to provide preemptive analgesia has not been clarified. Further research is required to determine whether there are differences in analgesic effects between pre- and postoperative administration and single-dose and multiple administrations. In addition, more studies comparing parecoxib with other NSAIDs are required.
Conclusion
The certainty of the evidence for intravenous parecoxib as a treatment for orthopedic surgical pain varies in efficacy and safety outcomes, from low to moderate. The available evidence indicates that parecoxib might relieve postoperative orthopedic pain and reduce opioid analgesic consumption. Additional data comparing parecoxib with other NSAIDs in a perioperative setting are required to confirm the present findings.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study and the Rapid Service Fee were funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
XL and PZ were the main author drafting the manuscript, reviewing all articles, extracting data, and conducting the statistical analyses for further analyses. HT helped with data interpretation, and assisted in drafting the manuscript. ZL contributed to the study design and coordination, helped with data interpretation, and assisted in drafting the manuscript. SZ was the main supervisor of the study design and coordination, reviewed all articles, conducted data extraction, interpreted data, and assisted in the manuscript drafting. All authors participated in the discussion and decision on the PICOs and the methodology of this systematic review. All authors contributed to and approved the protocol (CRD42021274939) as well as the final manuscript.
Disclosures
Xiaofei Li, Pengxiang Zhou, Zhengqian Li, Huilin Tange, and Suodi Zhai have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
This is a secondary study; hence all data used in this study are available in the literature.
Footnotes
Xiaofei Li and Pengxiang Zhou contributed equally to this work.
References
- 1.Treatment Guideline for Lumbar Spine Surgery. Washington State Department of Labor & Industries. 2021. https://www.lni.wa.gov/patient-care/advisory-committees.
- 2.Joint replacement (primary): hip, knee and shoulder. NICE guideline. 2020. https://www.nice.org.uk/guidance/ng157. [PubMed]
- 3.Debono B, Wainwright TW, Wang MY, Sigmundsson FG, Yang MMH, Smid-Nanninga H, et al. Consensus statement for perioperative care in lumbar spinal fusion: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Spine J. 2021;21(5):729–752. doi: 10.1016/j.spinee.2021.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Jildeh TR, Abbas MJ, Hasan L, Moutzouros V, Okoroha KR. Multimodal nonopioid pain protocol provides better or equivalent pain control compared to opioid analgesia following arthroscopic rotator cuff surgery: a prospective randomized controlled trial. Arthroscopy. 2022;38(4):1077–1085. doi: 10.1016/j.arthro.2021.11.028. [DOI] [PubMed] [Google Scholar]
- 5.Wick EC, Grant MC, Wu CL. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg. 2017;152(7):691–697. doi: 10.1001/jamasurg.2017.0898. [DOI] [PubMed] [Google Scholar]
- 6.Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, et al. Management of postoperative pain: a Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131–157. doi: 10.1016/j.jpain.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Acute pain management: scientific evidence (5th Edition). EvidenceUpdates. 2020. https://www.anzca.edu.au/news/top-news/acute-pain-management-scientific-evidence-5th-edit.
- 8.Moore RA, Derry S, Aldington D, Wiffen PJ. Single dose oral analgesics for acute postoperative pain in adults—an overview of Cochrane reviews. Cochrane Database Syst Rev. 2015;9:D8659. doi: 10.1002/14651858.CD008659.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derry S, Moore RA. Single dose oral celecoxib for acute postoperative pain in adults. Cochrane Database Syst Rev. 2013;10:D4233. doi: 10.1002/14651858.CD004233.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheer SM, Goa K. Parecoxib (parecoxib sodium) Drugs. 2001;61(8):1133–1141. doi: 10.2165/00003495-200161080-00010. [DOI] [PubMed] [Google Scholar]
- 11.Lloyd R, Derry S, Moore RA, Mcquay HJ. Intravenous or intramuscular parecoxib for acute postoperative pain in adults. Cochrane Database Syst Rev. 2009;2:D4771. doi: 10.1002/14651858.CD004771.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du X, Gu J. The efficacy and safety of parecoxib for reducing pain and opioid consumption following total knee arthroplasty: a meta-analysis of randomized controlled trials. Int J Surg. 2018;59:67–74. doi: 10.1016/j.ijsu.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Villasís-Keever MA, Rendón-Macías ME, Escamilla-Núñez A. Systematic review to assess the effectiveness and safety of parecoxib. Acta Ortop Mex. 2009;23(6):342–350. [PubMed] [Google Scholar]
- 14.Bian YY, Wang LC, Qian WW, et al. Role of parecoxib sodium in the multimodal analgesia after total knee arthroplasty: a randomized double-blinded controlled trial. Orthop Surg. 2018;10(4):321–327. doi: 10.1111/os.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng HM, Wang LC, Wang W, et al. Preemptive analgesia with parecoxib in total hip arthroplasty: a randomized, double-blind, placebo-controlled trial. Pain Physician. 2018;21(5):483–488. [PubMed] [Google Scholar]
- 16.Page MJ, Mckenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 17.Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dickersin K, Berlin JA. Meta-analysis: state-of-the-science. Epidemiol Rev. 1992;14:154–176. doi: 10.1093/oxfordjournals.epirev.a036084. [DOI] [PubMed] [Google Scholar]
- 21.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 22.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma HH, Chou TFA, Wang HY, et al. An opioid-sparing protocol with intravenous parecoxib can effectively reduce morphine consumption after simultaneous bilateral total knee arthroplasty. Sci Rep-UK. 2021;11(1):7362. doi: 10.1038/s41598-021-86826-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laoruengthana A, Rattanaprichavej P, Reosanguanwong K, Chinwatanawongwan B, Chompoonutprapa P, Pongpirul K. A randomized controlled trial comparing the efficacies of ketorolac and parecoxib for early pain management after total knee arthroplasty. Knee. 2020;27(6):1708–1714. doi: 10.1016/j.knee.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Xiao K, Yu L, Xiao W, et al. Pain management using perioperative administration of parecoxib for total hip arthroplasty: a randomized, double-blind, placebo-controlled trial. Pain Physician. 2019;22(6):575–582. [PubMed] [Google Scholar]
- 27.Essex MN, Choi HY, Brown PB, Cheung R. A randomized study of the efficacy and safety of parecoxib for the treatment of pain following total knee arthroplasty in Korean patients. J Pain Res. 2018;11:427–433. doi: 10.2147/JPR.S147481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diaz-Borjon E, Torres-Gomez A, Essex MN, et al. Parecoxib provides analgesic and opioid-sparing effects following major orthopedic surgery: a subset analysis of a randomized, placebo-controlled clinical trial. Pain Therapy. 2017;6(1):61–72. doi: 10.1007/s40122-017-0066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mu DL, Zhang DZ, Wang DX, et al. Parecoxib supplementation to morphine analgesia decreases incidence of delirium in elderly patients after hip or knee replacement surgery: a randomized controlled trial. Anesth Analg. 2017;124(6):1992–2000. doi: 10.1213/ANE.0000000000002095. [DOI] [PubMed] [Google Scholar]
- 30.Camu F, Borgeat A, Heylen RJ, Viel EJ, Boye ME, Cheung RY. Parecoxib, propacetamol, and their combination for analgesia after total hip arthroplasty: a randomized non-inferiority trial. Acta Anaesthesiol Scand. 2017;61(1):99–110. doi: 10.1111/aas.12841. [DOI] [PubMed] [Google Scholar]
- 31.Zhu YZ, Yao R, Zhang Z, Xu H, Wang LW. Parecoxib prevents early postoperative cognitive dysfunction in elderly patients undergoing total knee arthroplasty: a double-blind, randomized clinical consort study. Medicine (Baltimore) 2016;95(28):e4082. doi: 10.1097/MD.0000000000004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siribumrungwong K, Cheewakidakarn J, Tangtrakulwanich B, Nimmaanrat S. Comparing parecoxib and ketorolac as preemptive analgesia in patients undergoing posterior lumbar spinal fusion: a prospective randomized double-blinded placebo-controlled trial. BMC Musculoskelet Disord. 2015;16:59. doi: 10.1186/s12891-015-0522-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu Y, Wang S, Wu H, Wu Y. Effect of perioperative parecoxib on postoperative pain and local inflammation factors PGE2 and IL-6 for total knee arthroplasty: a randomized, double-blind, placebo-controlled study. Eur J Orthop Surg Traumatol. 2014;24(3):395–401. doi: 10.1007/s00590-013-1203-4. [DOI] [PubMed] [Google Scholar]
- 34.Elseify ZA, El-Khattab SO, Khattab AM, Atta EM, Ajjoub LF. Combined parecoxib and I.V. paracetamol provides additional analgesic effect with better postoperative satisfaction in patients undergoing anterior cruciate ligament reconstruction. Saudi J Anaesth. 2011;5(1):45–49. doi: 10.4103/1658-354X.76510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paz-Estrada C, Vilaplana-Santalo CA, Perdomo-Gutiérrez RE. Preemptive analgesia with diclofenac and parecoxib in orthopedic surgery. Revista Mexicana de Anestesiologia. 2011;34(3):159–163. [Google Scholar]
- 36.Ittichaikulthol W, Prachanpanich N, Kositchaiwat C, Intapan T. The post-operative analgesic efficacy of celecoxib compared with placebo and parecoxib after total hip or knee arthroplasty. J Med Assoc Thai. 2010;93(8):937–942. [PubMed] [Google Scholar]
- 37.Vadalouca A, Moka E, Chatzidimitriou A, Siafaka I, Sikioti P, Argyra E. A randomized, double-blind, placebo-controlled study of preemptively administered intravenous parecoxib: effect on anxiety levels and procedural pain during epidural catheter placement for surgical operations or for chronic pain therapy. Pain Pract. 2009;9(3):181–194. doi: 10.1111/j.1533-2500.2009.00271.x. [DOI] [PubMed] [Google Scholar]
- 38.Jirarattanaphochai K, Thienthong S, Sriraj W, et al. Effect of parecoxib on postoperative pain after lumbar spine surgery: a bicenter, randomized, double-blinded, placebo-controlled trial. Spine (Phila Pa 1976) 2008;33(2):132–139. doi: 10.1097/BRS.0b013e3181604529. [DOI] [PubMed] [Google Scholar]
- 39.Apfelbaum JL, Desjardins PJ, Brown MT, Verburg KM. Multiple-day efficacy of parecoxib sodium treatment in postoperative bunionectomy pain. Clin J Pain. 2008;24(9):784–792. doi: 10.1097/AJP.0b013e31817a717c. [DOI] [PubMed] [Google Scholar]
- 40.Riest G, Peters J, Weiss M, et al. Preventive effects of perioperative parecoxib on post-discectomy pain. Br J Anaesth. 2008;100(2):256–262. doi: 10.1093/bja/aem345. [DOI] [PubMed] [Google Scholar]
- 41.Bláha M, Kukla R, Kozák J, Tichý M. Parecoxib in the treatment of postoperative pain after lumbar discectomy—a prospective randomized double blind trial. Bolest. 2007;10(3):156–159. [Google Scholar]
- 42.Martinez V, Belbachir A, Jaber A, et al. The influence of timing of administration on the analgesic efficacy of parecoxib in orthopedic surgery. Anesth Analg. 2007;104(6):1521–1527. doi: 10.1213/01.ane.0000262039.69513.9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koppert W, Frotsch K, Huzurudin N, et al. The effects of paracetamol and parecoxib on kidney function in elderly patients undergoing orthopedic surgery. Anesth Analg. 2006;103(5):1170–1176. doi: 10.1213/01.ane.0000244324.87947.29. [DOI] [PubMed] [Google Scholar]
- 44.Grundmann U, Wörnle C, Biedler A, Kreuer S, Wrobel M, Wilhelm W. The efficacy of the non-opioid analgesics parecoxib, paracetamol and metamizol for postoperative pain relief after lumbar microdiscectomy. Anesth Analg. 2006;103(1):217–222. doi: 10.1213/01.ane.0000221438.08990.06. [DOI] [PubMed] [Google Scholar]
- 45.Desjardins PJ, Traylor L, Hubbard RC. Analgesic efficacy of preoperative parecoxib sodium in an orthopedic pain model. J Am Podiatr Med Assoc. 2004;94(3):305–314. doi: 10.7547/0940305. [DOI] [PubMed] [Google Scholar]
- 46.Hubbard RC, Naumann TM, Traylor L, Dhadda S. Parecoxib sodium has opioid-sparing effects in patients undergoing total knee arthroplasty under spinal anaesthesia. Br J Anaesth. 2003;90(2):166–172. doi: 10.1093/bja/aeg038. [DOI] [PubMed] [Google Scholar]
- 47.Malan TJ, Marsh G, Hakki SI, Grossman E, Traylor L, Hubbard RC. Parecoxib sodium, a parenteral cyclooxygenase 2 selective inhibitor, improves morphine analgesia and is opioid-sparing following total hip arthroplasty. Anesthesiology. 2003;98(4):950–956. doi: 10.1097/00000542-200304000-00023. [DOI] [PubMed] [Google Scholar]
- 48.Rasmussen GL, Steckner K, Hogue C, Torri S, Hubbard RC. Intravenous parecoxib sodium for acute pain after orthopedic knee surgery. Am J Orthop (Belle Mead NJ) 2002;31(6):336–343. [PubMed] [Google Scholar]
- 49.Zhang Z, Xu H, Zhang Y, et al. Nonsteroidal anti-inflammatory drugs for postoperative pain control after lumbar spine surgery: a meta-analysis of randomized controlled trials. J Clin Anesth. 2017;43:84–89. doi: 10.1016/j.jclinane.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 50.Trost Z. All pain is not created equal: differentiating between pain during movement versus pain at rest following total knee arthroplasty. Pain. 2012;153(11):2161–2162. doi: 10.1016/j.pain.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 51.Rakel BA, Blodgett NP, Zimmerman BM, et al. Predictors of postoperative movement and resting pain following total knee replacement. Pain. 2012;153(11):2192–2203. doi: 10.1016/j.pain.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vane JR, Botting RM. Mechanism of action of nonsteroidal anti-inflammatory drugs. Am. J. Med. 1998; 104 (3A):2S-8S, 21S-22S. [DOI] [PubMed]
- 53.Meade EA, Smith WL, Dewitt DL. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1993;268(9):6610–6614. doi: 10.1016/S0021-9258(18)53294-4. [DOI] [PubMed] [Google Scholar]
- 54.Dewitt DL, Meade EA, Smith WL. PGH synthase isoenzyme selectivity: the potential for safer nonsteroidal antiinflammatory drugs. Am J Med. 1993;95(2A):40S–44S. doi: 10.1016/0002-9343(93)90396-7. [DOI] [PubMed] [Google Scholar]
- 55.FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2. N Engl J Med. 2001;345(6):433–442. doi: 10.1056/NEJM200108093450607. [DOI] [PubMed] [Google Scholar]
- 56.Padi SS, Jain NK, Singh S, Kulkarni SK. Pharmacological profile of parecoxib: a novel, potent injectable selective cyclooxygenase-2 inhibitor. Eur J Pharmacol. 2004;491(1):69–76. doi: 10.1016/j.ejphar.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 57.Yaksh TL, Dirig DM, Conway CM, Svensson C, Luo ZD, Isakson PC. The acute antihyperalgesic action of nonsteroidal, anti-inflammatory drugs and release of spinal prostaglandin E2 is mediated by the inhibition of constitutive spinal cyclooxygenase-2 (COX-2) but not COX-1. Neurosci. 2001;21(16):5847–5853. doi: 10.1523/JNEUROSCI.21-16-05847.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teerawattananon C, Tantayakom P, Suwanawiboon B, Katchamart W. Risk of perioperative bleeding related to highly selective cyclooxygenase-2 inhibitors: a systematic review and meta-analysis. Semin Arthritis Rheum. 2017;46(4):520–528. doi: 10.1016/j.semarthrit.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 59.Schug SA, Joshi GP, Camu F, Pan S, Cheung R. Cardiovascular safety of the cyclooxygenase-2 selective inhibitors parecoxib and valdecoxib in the postoperative setting: an analysis of integrated data. Anesth Analg. 2009;108(1):299–307. doi: 10.1213/ane.0b013e31818ca3ac. [DOI] [PubMed] [Google Scholar]
- 60.Mehta V, Johnston A, Cheung R, Bello A, Langford RM. Intravenous parecoxib rapidly leads to COX-2 inhibitory concentration of valdecoxib in the central nervous system. Clin Pharmacol Ther. 2008;83(3):430–435. doi: 10.1038/sj.clpt.6100304. [DOI] [PubMed] [Google Scholar]
- 61.Gan TJ, Diemunsch P, Habib AS, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118(1):85–113. doi: 10.1213/ANE.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 62.Algera MH, Kamp J, Schrier R, et al. Opioid-induced respiratory depression in humans: a review of pharmacokinetic-pharmacodynamic modelling of reversal. Br J Anaesth. 2019;122(6):e168–e179. doi: 10.1016/j.bja.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 63.Schug SA, Parsons B, Li C, Xia F. The safety profile of parecoxib for the treatment of postoperative pain: a pooled analysis of 28 randomized, double-blind, placebo-controlled clinical trials and a review of over 10 years of postauthorization data. J Pain Res. 2017;10:2451–2459. doi: 10.2147/JPR.S136052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This is a secondary study; hence all data used in this study are available in the literature.