Abstract
CTP biosynthesis is carried out by two pathways: salvage and de novo. CTPsyn catalyzes the latter. The study of CTPsyn activity in mammalian cells began in the 1970s, and various fascinating discoveries were made regarding the role of CTPsyn in cancer and development. However, its ability to fit into a cellular serpent-like structure, termed ‘cytoophidia,’ was only discovered a decade ago by three independent groups of scientists. Although the self-assembly of CTPsyn into a filamentous structure is evolutionarily conserved, the enzyme activity upon this self-assembly varies in different species. CTPsyn is required for cellular development and homeostasis. Changes in the expression of CTPsyn cause developmental changes in Drosophila melanogaster. A high level of CTPsyn activity and formation of cytoophidia are often observed in rapidly proliferating cells such as in stem and cancer cells. Meanwhile, the deficiency of CTPsyn causes severe immunodeficiency leading to immunocompromised diseases caused by bacteria, viruses, and parasites, making CTPsyn an attractive therapeutic target. Here, we provide an overview of the role of CTPsyn in cellular and disease perspectives along with its potential as a drug target.
Keywords: CTP synthetase, CTPsyn, CTPS, Cytoophidium, Filamentation, Rods and rings, Drosophila melanogaster
Introduction
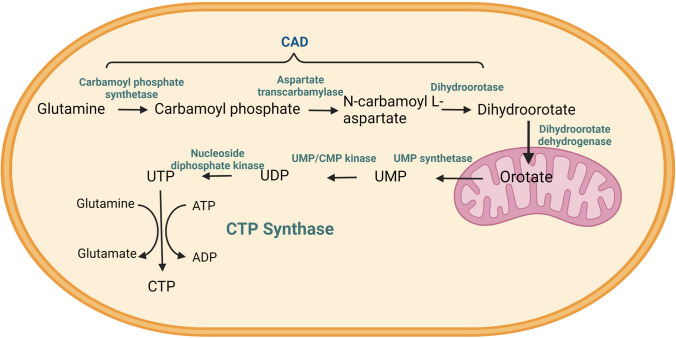
The cytidine triphosphate (CTP) is a pyrimidine nucleotide, which is an essential building block of DNA and RNA. It is also important for phospholipid biosynthesis and protein sialylation; hence, it plays a critical role in cell proliferation and cellular expansion. CTP can be synthesised via two pathways: (1) the salvage pathway and (2) the de novo biosynthesis pathway. CTP biosynthesis is a tightly regulated process as among all the nucleotides, CTP has the lowest presentation at cellular concentration levels (Higgins et al. 2007). Cytidine triphosphate synthase/synthetase (CTPsyn) catalyzes the rate-limiting step of the de novo CTP biosynthesis pathway by converting the uridine triphosphate (UTP) to CTP. The conversion proceeds through three reactions: a kinase reaction to phosphorylate uracil O4 atom in an Mg2+-ATP-dependent manner, a glutaminase reaction to generate ammonia from glutamine hydrolysis, and a ligase reaction to displace the uracil O4 phosphate with ammonia (Fig. 1) (Bhagavan and Ha 2011; Endrizzi et al. 2004; Kent and Carman 1999).
Fig. 1.
The de novo cytidine triphosphate (CTP) synthesis pathway. Schematic diagram of the de novo CTP (pyrimidine) synthesis pathway. CTP synthesis enzymes CAD carbamoyl-phosphate synthetase 2, aspartate transcarbamylase, and dihydroorotase; UMP synthetase uridine monophosphate synthetase, UMP/CMP kinase cytidylate monophosphate kinase, CTP synthetase cytidine triphosphate synthetase. Created with BioRender.com
CTPsyn can create intracellular structure referred cytoophidium in bacteria, yeast, Drosophila, and mammalian cells, implying that filamentation is conserved through evolution (Ingerson-Mahar et al. 2010; Liu 2010; Noree et al. 2010). Large-scale polymers play a vital role in cellular organization and in maintaining cellular homeostasis. It is notable that apart from the widely studied model organism, Drosophila melanogaster (the common fruit fly), cytoophidia were also found in different species of the fruit flies such as in the Drosophila virilis and Drosophila pseudoobscura. Interestingly, in Drosophila melanogaster, cytoophidia are formed in different organs and tissues including the testis, ovary, gut, brain accessory gland, salivary gland, and lymph gland (Liu 2010). In Escherichia coli bacteria, CTPsyn enzyme activity is inactivated in the presence of these polymeric cytoophidia. As the nucleotide CTP is the product synthesised through the catalysis of CTPsyn, abundance of the CTP in the cellular microenvironment causes the activity of CTPsyn through the formation of inactive CTPsyn polymers, thus preventing the accumulation of nucleotides, which could lead to uncontrolled growth and cell division. Meanwhile, the abundance of the substrates UTP and ATP in the cellular microenvironment disassembles this reversible polymeric CTPsyn structure into their enzymatically active tetrameric form, thus making this CTPsyn polymerization and enzyme activity inversely related (Barry et al. 2014).
However, for human CTPsyn, polymerization increases catalytic activity, thus defying the assumption that the CTPsyn filaments only store inactive enzymes. Surprisingly, in the presence of substrates (UTP and ATP), human CTPsyn polymerizes into CTPsyn filaments with increased catalytic activity (Lynch et al. 2017). Correspondingly, during oogenesis in Drosophila ovarian germline cells, CTPsyn forms catalytically active CTPsyn filaments in order to temporally control the synthesis of CTP nucleotides, which are required in abundance during the period of exquisite genomic endoreplication and stockpiling of ribosomal RNA (Strochlic et al. 2014). To dispute the statement that the bacterial filaments are stabilized by inactive product-bound CTPsyn polymers, meanwhile, the eukaryotic filaments are stabilized by active substrate-bound CTPsyn tetramers; another interesting study has speculated that both the prokaryotic and eukaryotic filaments could be formed by both substrate-bound and product-bound forms, albeit the variation in filament forming ability could differ among species, thus providing insights that these filaments could switch from active to inactive form or vice-versa in the same cell to regulate cellular metabolic activities (Zhou et al. 2019).
The mechanism behind the compartmentalizing property of CTPsyn is being studied extensively for its contributions in nucleotide metabolism and its interaction with other biological pathways. CTPsyn activity along with CTP nucleotide levels is often elevated in rapidly proliferating cells, especially in various types of cancers and immune-related diseases (Carcamo et al. 2011). As CTPsyn is a metabolic enzyme that is required for basic nucleotide biosynthesis, its potential role in cell and developmental biology is being actively explored because it is also an attractive drug discovery target for various disorders. Here, we discuss the role of CTPsyn and cytoophidia in developmental and disease biology along with its potential as a drug target for infectious diseases.
CTPsyn and cytoophidia—a developmental perspective
Brain and optic lobe
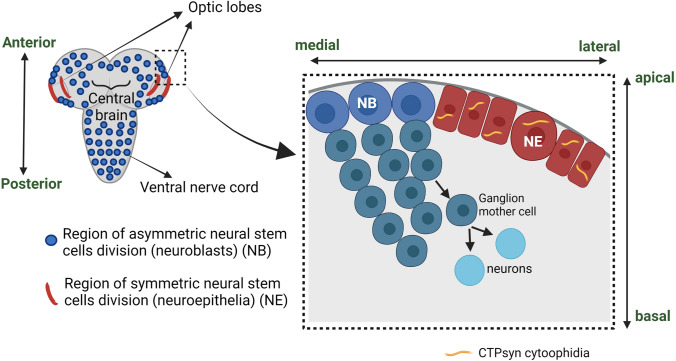
Presence of prominent CTPsyn activity in rat brain is well known, and this activity decreases with the aging process (Genchev and Mandel 1976). However, the role of CTPsyn in Drosophila optic lobe development was only characterized years ago. The Drosophila brain visual processing system is controlled by the optic lobe, which encompasses neural stem cells called neuroblasts that are tightly regulated by developmental cues that are parallel to the development of the mammalian cerebral cortex. Drosophila larval optic lobe development is well characterized. In short, the neuroepithelial (NE) stem cell of the optic lobe divides symmetrically to create a population of NEs that then differentiates into neuroblasts (NBs), and finally these NBs asymmetrically divide to give rise to medulla neurons (Fig. 2) (Egger et al. 2007).
Fig. 2.
A Drosophila larval nervous system comprising ventral nerve cord, central brain, and optic lobes. At optic lobes, sequential transition of neuroepithelial (NE) stem cells to neuroblasts (NBs) occurs (in box). NE stem cells undergo symmetric cell division to expand the pool of cells and subsequently give rise to NBs that divide asymmetrically to produce ganglion mother cells, which undergo mitotic division to produce neuronal daughter cells. CTPsyn forms cytoophidia at the NE stem cell region of the optic lobe. Created with BioRender.com
CTPsyn cytoophidia was observed in abundance in the Drosophila post-embryonic neuroblasts and epithelial stem cells of the larval central nervous system (CNS) when the flies were fed with 6-diazo-5-oxo-l-norleucine (DON), a glutamine analogue that inhibits CTPsyn activity, implicating a higher CTPsyn activity in third-instar larval neuroblasts (Chen et al. 2011). This finding encouraged researchers to further investigate the roles of CTPsyn and cytoophidia during CNS development. Since some early findings suggest that incorporation of CTPsyn into cytoophidia in Drosophila inhibits CTPsyn activity as these filaments are composed of inactive CTPsyn dimers (Chen et al. 2011; Liu 2011), when CTPsyn cytoophidia was observed to be highly prevalent in quiescent and starved neuroblasts at early larval CNS, and upon a re-entry into the cell cycle during neuroblast cell proliferation, these cytoophidia might be disassembling for active CTPsyn in order to supply the nucleotide required for cell division (Aughey et al. 2014). CTPsyn plays a critical role in the development of the optic lobe in the larval central nervous system (CNS) (Tastan and Liu 2015). It forms cytoophidia in the NE stem cells, and the cytoophidia disassembles as the NE stem cell transitions into NBs. Any changes in the expression of CTPsyn leads to developmental defects in the optic lobes. Overexpression of CTPsyn impairs the development of the brain, with a shrinkage in the lamina region and an expansion of NE region. However, the expansion of NE region could be an indication of lacking differentiation of NE cells, whereas, in CTPsyn mutant flies, the optic lobes lack a medulla region, and no obvious lamina region was detected (Tastan and Liu 2015), suggesting that CTPsyn maintains the optic lobe homeostasis. Specifically, it could be important for the maintenance and differentiation of NE stem cells.
Overexpression of CTPsyn in embryonic mouse brain also induces cytoophidia formation in developing cortical neurons and impedes the migration of neurons. Furthermore, it also hastens neuronal differentiation while inhibiting the proliferation of neural progenitor cells and impairing mitotic activity. Interestingly, at G1 phase of mitosis, the cytoophidia dispersed into cytoplasm because of the high cellular demand for nucleotides at G1 phase, and high CTPsyn activity (Li et al. 2018).
Intestine
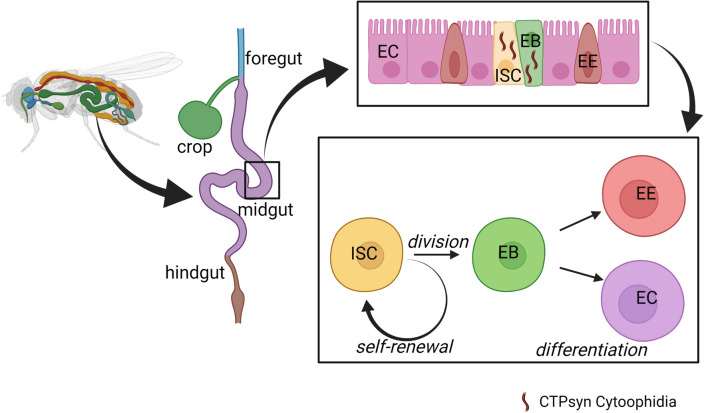
The Drosophila intestinal tissues are similar to the human intestine in terms of anatomy and physiology (Apidianakis & Rahme 2011). The adult fly intestinal stem cells (ISCs) signaling and cell differentiation are also similar to the mammalian intestinal stem cells, thus making Drosophila a powerful model to study human intestinal development, infection, and pathology. Both mammalian and fly gut consists of enterocytes for nutrient absorption and enteroendocrine cells for hormone production and secretion (Ohlstein and Spradling 2006). Despite all these similarities, transient amplifying (TA) cells are absent in Drosophila midgut at normal homeostatic conditions, but such cells have been observed during pathogenic infections. The midgut lineage in Drosophila is quite simple; the ISCs undergo asymmetric divisions to produce enteroblasts (EBs) that remain close to the ISCs until they differentiate into enterocytes (ECs) or enteroendocrine (EEs) cells (Jiang et al. 2016) (Fig. 3).
Fig. 3.
The Drosophila intestinal stem cell. The intestinal stem cell niche houses the ISCs, which divide and give rise to committed progenitor EB cells. Then, the EB cells differentiate into EEs and ECs. It is made up of simple monolayer epithelium cells, and unlike mammalian TA cells, the EBs rarely divide. The midgut homeostasis is maintained by the replenishment of aged or damaged gut cells with the new ones (Jiang and Edgar 2011). The presence of CTPsyn cytoophidia was observed in ISCs and EBs. Created with BioRender.com
A recent study has found that CTPsyn forms cytoophidia in the ISCs and EBs of female Drosophila. They also found that CTPsyn is necessary for the proliferation of ISCs. At the instance of CTPsyn knockdown, the number of EBs reduced significantly in the posterior midgut. Furthermore, the CTPsyn filamentation is required for ISC proliferation. This study showed the role of CTPsyn and its filamentation in cell division and development (Zhou et al. 2021).
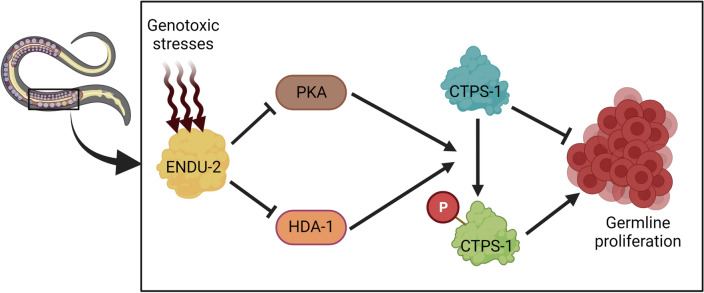
Meanwhile, in Caenorhabditis elegans, the germ cell proliferation collapses in response to pyrimidine nucleotide deprivation. A healthy germ cell proliferation requires rapid DNA replication and RNA synthesis with a stable nucleotide pool (Chi et al. 2016). ENDU-2 nuclease has been found to be the key factor that regulates nucleotide metabolism and germline proliferation in response to genotoxic stresses and nucleotide imbalance. Fascinatingly, ENDU-2 interacts with CTPsyn in the process of regulating nucleotide metabolism via controlling the phosphorylation of CTPS-1. Under nucleotide depletion or genotoxic stress, ENDU-2 activity is elevated, and it positively regulates CTPS-1 by blocking its phosphorylation through the protein kinase A (PKA) pathway; thus, the germline proliferation in the intestines of Caenorhabditis elegans is halted (Fig. 4) (Jia et al. 2020).
Fig. 4.
Proposed role of ENDU-2 and its interaction with CTPS-1 in regulating nucleotide metabolism and germ cell proliferation. In response to nucleotide deprivation and genotoxic stress in the intestine of Caenorhabditis elegans, ENDU-2 shuts down germline proliferation via inhibiting the phosphorylation of CTPS-1 by repressing the protein kinase A (PKA) pathway and histone deacetylase-1 (HDA-1) (Jia et al. 2020). Created with BioRender.com
Gonads
Ovaries
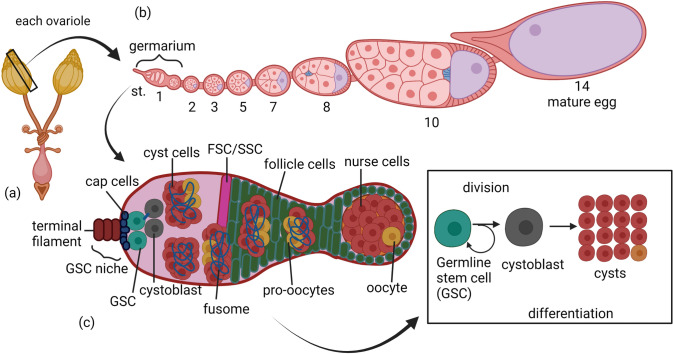
The Drosophila ovariole has germarium that houses 2–3 germline stem cells (GSC) and somatic stem cells (SSC) or follicular stem cells (FSC). GSCs undergo multiple divisions and differentiations to develop into oocytes. Interestingly, the number of ring canals determines which cyst is fated to be an oocyte and which are to be nurse cells (Fig. 5). Upon the exit of mitoses of cyst cells, the nurse cells undergo series of 10–12 endocycles to polyploidize their genome to supply mRNA and proteins for the enrichment of the oocyte. The oocyte develops well with the consumption of yolk protein synthesized in the follicle cells and fat and occupies half the egg chamber by the time it reaches stage 10. As series of 3 endocycle replication also occurs in follicle cells to produce eggshell protein that is required for the vitelline membrane formation (Wu et al. 2008; Hudson and Cooley 2014). At stage 14 the mature egg exits after maturation. Various cell signaling pathways are involved in tightly regulating the oogenesis.
Fig. 5.
Oogenesis in Drosophila. a Schematic diagram of the two ovaries of Drosophila that is made up of approximately 18 ovarioles. b Each of this ovarioles undergo 14 stages of development to from mature egg (st. 14) from germarium (st. 1). c The germarium contains SSCs and GSCs at the anterior end of ovariole. These GSCs divide asymmetrically to self-renew and to produce a daughter cell, cystoblast which then undergoes four rounds of asymmetric and incomplete mitoses to form germline cysts (16 cells) interconnected by ring canals. Only one from these cysts become oocyte which then develop into mature egg while the other 15 differentiate to become nurse cells. Meanwhile, the SSCs give rise to precursor follicle cells, and about 16 out of these precursor follicle cells, then become polar cells and stalk cells. The rest of the precursor follicle cells encysts cyst cells and become egg chamber. Created with BioRender.com
Interestingly, proto-oncogene Casitas B-lineage lymphoma (Cbl) promote endocycle during oogenesis by regulating CTPsyn activity. Mutation of Cbl resulted in defect in S-phase endocycle endoreplication and overexpression of CTPsyn is sufficient to reverse this effect. Furthermore, absence of Cbl, disrupted the cytoophidia formation. This study suggests that to meet the nucleotide demand during endocycle of oogenesis, cytoophidia is required to allow CTPsyn activity (Wang et al. 2015). To support this further, a reduction in CTPsyn expression causes defects in oogenesis, emphasizing that cytoophidia formation is to promote CTP biosynthesis to support endoreplication. Drosophila homologue of mammalian activated cdc42-associated kinase 1 (DAck) localizes to CTPsyn cytoophidia and is required for endocycle replication as well. Morphological defects with reduced size of nuclei of nurse cells that correlate to compromised fertility were observed when DAck is deficient during oogenesis, further supporting the statement that CTPsyn cytoophidia is required for adequate CTP biosynthesis during endoreplication. Fascinatingly, the active human CTPsyn could assemble into filaments and also was able to rescue the membrane integrity defect and sterility of the heteroallelic CTPsyn mutant fly, indicating that CTPsyn could be catalytically active within the filaments. Besides, lacking DAck and CTPsyn leads to lower RNA levels, implicating the close relationship between these two colocalizing enzymes in regulating the CTP nucleotide pool (Strochlic et al. 2014).
The compartmentalization of CTPsyn into cytoophidia in Drosophila was first observed in ovaries (Liu 2010). A recent finding states that the egg production in Drosophila strongly depends on this polymerization of CTPsyn into cytoophidia. Disrupting the cytoophidia formation at early-stage egg chambers dramatically lowers the egg production. Feeding with DON to inhibit CTPsyn activity aggravates this condition further, suggesting that egg production strongly supported by assembly of CTPsyn filaments (Simonet et al. 2020). Although formation of cytoophidia is naturally abundant in ovaries, glutamine and glucose deprivation induces elongated cytoophidia formation. Starvation has induced the reversible elongation of cytoophidia and apoptosis during mid-oogenesis in Drosophila ovaries. Interestingly, this elongated cytoophidia travels through ring canals from nurse cells to the oocyte. This could be a stress response strategy to store CTPsyn until the environment is conducive for the organism to grow and the reversibility could be the release of CTPsyn when the environment improves (Wu and Liu 2019).
Testis
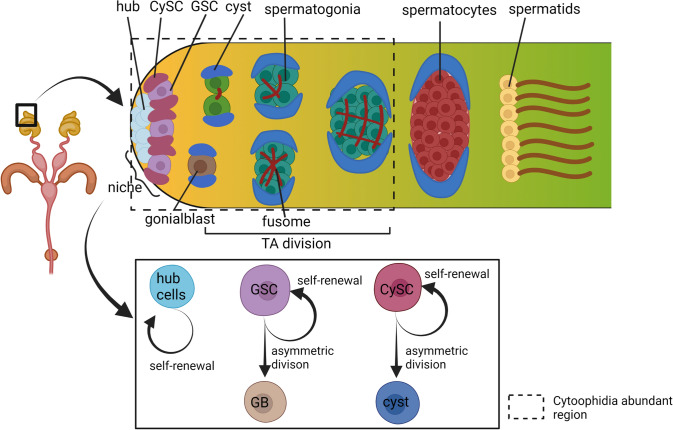
The testis of Drosophila is an interesting model to study stem cell division and maintenance for it houses germline stem cells (GSCs) and cyst stem cells (CySCs) in 1:2 ratio adhered to hub cells via cell adhesion molecules (Fig. 6). This testicular niche is tightly regulated by JAK/STAT signaling pathway (Singh et al. 2016). It is important to note that, in testis, CTPsyn forms cytoophidia naturally only at the apical region where a higher rate of cell division occurs, showing the requirement for CTP to meet the cellular nucleotide demand during active cell division (Fig. 6). However, during overexpression studies of D. melanogaster, CTP synthetase isoform C (CTPsynIsoC), which is the only isoform that forms cytoophidia (Azzam and Liu 2013), causes the elongation of cytoophidia in the entirety of Drosophila testicular body, and around 34% of these males were observed to have testicular apical tip bulging phenotype due to the over-proliferation of germline cells and spermatocytes (Woo et al. 2019). This suggests that germline cell proliferation, which is a hallmark of a few types of tumorigenesis and cancer formation, could be due to the overexpression of CTPsyn in the rapidly proliferating cells. Besides, interestingly the overexpression of CTPsyn interacting micro-RNA, miR-975 in Drosophila testis, also showed a similar bulging phenotype in the apical tip, and the cytoophidia were also observed to be lengthened. qPCR assay for both the CTPsynIsoC and miR-975 overexpressions provide evidence for the differential expression of several cancer-related genes, Ack, Brat, IMPDH, Myc, and CTPS, thus reaffirming that CTPsyn upregulation could harness tumor-like testicular overgrowth due to over-proliferation of germline cells (Woo et al. 2019).
Fig. 6.
Spermatogenesis in Drosophila. The stem cell niche is maintained by 12 non-dividing somatic cells, called ‘hub,’ anchored with GSCs and CySCs. The stem cell niche's maintenance is tightly regulated by a cascade of genes and pathways. Each GSC was encysted by two CySCs. Upon asymmetric division, each GSC self-renews and gives rise to gonialblast (GB), and each CySC self-renews and gives rise to cyst cells (early cysts and late cysts) that encyst the germ cells throughout the spermatogenesis until they develop into spermatids. The GB undergoes four rounds of transit amplifying (TA) mitotic divisions to produce 16 interconnected (by ring canals) spermatogonial cells that differentiate into early spermatocytes, and these spermatocytes undergo meiosis to produce 64 haploid spermatids. They then undergo elongation and coiling to become mature spermatids. The box represents the region where cytoophidia is normally abundant. Created with BioRender.com
CTPsyn and its assembly into cytoophidia are required for the proper maintenance of cellular homeostasis in Drosophila and in other organisms. Various pathways regulate stem cell maintenance in Drosophila for proper tissue development, such as the JAK/STAT, Notch, Egfr/Ras/MAPK, insulin/TGF-β, Wnt, and hippo signaling pathways. However, the signaling pathways that CTPsyn involved for maintaining stem cell development is remaining elusive.
CTPsyn activity and its filamentation from a disease perspective
CTPsyn and cancer
Proper development of a tissue and organ essentially requires precise cell growth and division, which is attained via controlled regulation of cellular developmental signaling. Few key genes and metabolic pathways have been correlated with tumor progression and cancer formation. A study that has been published in 1978 reported that the highest CTPsyn activity was observed in rapidly growing tumors. It was observed that the CTPsyn activity varies proportionally to the tumor growth rate, where higher enzyme activity was observed in the rapidly proliferating liver tumor than that in tumors with a slow and medium growth rate (Kizaki et al. 1980; Williams et al. 1978). This could be due to the CTP biosynthesis mechanism, which allows the cancerous cells to adjust the availability of CTP for RNA synthesis and formation of 2’-deoxy CTP for DNA synthesis (Williams et al. 1978). Interestingly, in another study, CTPsyn activity was observed to be elevated in all examined hepatomas, and the increase in activity correlated positively with the growth rates of tumor (Tzeng et al. 1981). Furthermore, a few studies have detected a significantly higher CTPsyn enzyme activity in acute lymphocytic leukemia cells compared to that in healthy lymphocyte controls (Ford et al. 1991). In another study, the CTPsyn activity was observed to be increased in MOLT-3 cells, which are human T-lymphoid cell lines derived from leukemic cells of a patient with acute lymphoblastic leukemia compared to the proliferating normal human T lymphocytes (Berg et al. 1993).
CTPsyn forms cytoophidia in various human cancers and in some non-cancerous tissues (Kizaki et al. 1980). The formation of cytoophidia in human Huh7 cells, which is an HCC cell line, is reversible, where in the cytoophidia that have formed in the absence of glutamine disintegrate in the presence of glutamine. Interestingly, the expression of heat shock protein 90 (HSP90), which is linked to aggressive tumor growth and poor prognosis in a few types of cancers, was significantly higher in the HCC samples with the massive amount of cytoophidia than in those without cytoophidia, thus suggesting that this formation of cytoophidia could be due to the high cellular stress tolerance in tumorigenic cells. Furthermore, some features of the HCC tumor, such as the proliferation rate, metabolic preference of cancer cells, and restricted blood supply in certain tissue regions, could be a potential reason for the presence of cytoophidia in HCC cells but not in normal hepatocytes. However, their finding also further suggests that the formation of cytoophidia may potentially reflect the impaired glutamine metabolism in cells (Chang et al. 2017). CTPsyn cytoophidia is proclaimed to be a natural occurrence and accounts for some HCC tumor features (Chang et al. 2017; Sun and Liu 2019a, b).
Formation of cytoophidia in cancer cell line requires post-translational modifications such as ubiquitination and methylation when glutamine is deprived; histidine is required for this starvation-induced filament formation via GCN2/ATF4/MTHFD2 stress response pathway, suggesting that cancer cell homeostasis at stress conditions is maintained through histidine-mediated methylation to promote cellular growth (Pai et al. 2016; Lin et al. 2018). Furthermore, synaptosome-associated protein 29 (SNAP29) interacts with the cytokeratin network to form CTPS filaments along the network upon glutamine starvation, and this could shed light on the role of metabolic intermediate filaments in vesicle trafficking for growth and proliferation (Chakraborty et al. 2020).
Ras oncogene mutation is prevalent in > 30% of human cancers. A previous study conducted by Friedman et al. (2011) shows that CTPsyn enhances the expression of Ras in Drosophila wing vein phenotype (Friedman et al. 2011). To discover a rapid and economical novel bioavailable anti-tumor drug, an in vivo Ras-driven Drosophila tumor model, larvae with tumorous overgrowth were fed with drug and the glutamine analogue, acivicin, was observed to hamper the tumorous overgrowth in Drosophila. Further analyzing the acivicin-mediated inhibition using RNAi knockdown of nine glutamine-dependent amidotransferases reveals that CTPsyn could be a possible target of acivicin, thus shedding light on the glutamine dependency and the vitality of CTPsyn in Drosophila tumors, as well as some of the human cancers. The glutamine analogue’s anti-tumorigenic activity has been widely studied over the past decades. Fascinatingly, this study provides further evidence of the close relationship between Ras-driven tumor overgrowth and CTPsyn. Knocking down CTPsyn using CTPsynRNAi in Ras85D overexpressed flies resulted in significant reduction in tumor growth, suggesting that CTPsyn is the key target of acivicin-mediated anti-tumorigenic activity in Ras-driven tumor growth in Drosophila (Willoughby et al. 2013).
CTPsyn activity was also observed to be high in triple-negative breast cancer (TNBC) tissues and cell lines (Lin et al. 2022). A dramatic inhibition of proliferation, migration, invasion, and apoptotic induction was observed upon silencing of CTPS1 in cell lines. Furthermore, knockdown of CTPS1 is sufficient to reduce tumor growth in mice. The transcription factor Y-box binding protein 1 (YBX1) binds to CTPS1 to promote its transcription for TNBC tumor progression. This study suggests that CTPS1 could be used as a target for prevention and treatment of TNBC in the future (Lin et al. 2022).
The Myc oncogene expression is deregulated in at least 70% of all human cancers, and these oncoproteins are “super-transcription factors” that is estimated to regulate the transcription of at least 15% human genome (Dong et al. 2020). Furthermore, Myc also plays a role as a “super-competitor” during cellular expansion to promote a biased cell proliferation at the expense of the neighboring cells for the aberrant growth of primary tumors (de la Cova et al. 2004; Moreno et al. 2004). Interestingly, Myc has been identified to regulate a few genes that are involved in de novo nucleotide synthesis; IMPDH2, CTPS1, and CAD. Furthermore, it also regulates glutamine metabolism, which is a precursor for de novo nucleotide synthesis. In mantle cell lymphoma (MCL), the oncogenic Myc was observed to be upregulated along with a significant upregulation of IMPDH2, CTPS1, and CAD, demonstrating the substantial role of the “undruggable” Myc oncogene on nucleotide synthesis. Treatment with DON to inhibit CTPS1 activity, however, induced apoptosis and dramatically supressed cell proliferation via a remarkable reduction in a pyrimidine nucleotide pool, making de novo nucleotide synthesis the potential targeted therapy to treat MCL to combat its resistance to the available therapies (Yao et al. 2018). In a recent study to selectively kill Myc elevated cancer cells, inhibition of CTPS1 along with ataxia telangiectasia and Rad3-related protein (ATR) inhibitor resulted in selective cell death and decreased tumor growth, suggesting that this approach could be favorable to selectively combat Myc-overexpressing cancer progression. Inhibition of CTPS1 limits the CTP nucleotide pool for DNA replication and induces replication stress; hence, with the combination of ATR inhibitor, a selective apoptosis takes place in Myc-driven cancer cells (Sun et al. 2022). This study is significant because it has reshaped our initial understanding about CTPS1 deficiency, which was once thought to induce immunodeficiency only. However, with this study, a unique role of CTPS1 in Myc overexpressed cancer was discovered. Although the reason behind this is not completely understood, perhaps it could be an embarkment to unveil the role of CTPS1 in oncogene driven cancer metabolism in depth.
Pro-tumorigenic genes that regulate cytoophidia assembly
Inosine monophosphate dehydrogenase (IMPDH)
Inosine monophosphate dehydrogenase (IMPDH) is another metabolic enzyme which catalyses the rate-limiting de novo synthesis of purine nucleotides. It catalyses the conversion of inosine monophosphate (IMP) into xanthosine-5-monophosphate (XMP) to synthesize guanosine triphosphate (GTP) nucleotide. A study has found that mycophenolic acid (MPA), an uncompetitive inhibitor of IMPDH, induces the filament formation reversibly in human cell lines (Ji et al. 2006). Despite their individuality in forming cytoophidia, both of these enzymes, IMPDH and CTPsyn, were observed to co-localize together in a single cytoophidium (Keppeke et al. 2015; Chang et al. 2018). Furthermore, glutamine starvation induces the formation of IMPDH2/CTPS1 mixed RR structures in the hypoxic region of various cancer or tumor cell lines (Calise et al. 2014). It was speculated that the presence of IMPDH2/CTPS1 mixed RR structures in the mouse embryonic stem cells (ESCs) could be due to the strong GTP/CTP nucleotide requirement during active cell proliferation (Carcamo et al. 2011). An increase in CTP pool also triggers the assembly of this IMPDH cytoophidia in mouse liver cells, implying an activation of de novo purine synthesis and deactivation of de novo pyrimidine synthesis as both require phosphoribosyl pyrophosphate (PRPP) as a common substrate for the nucleotide biosynthesis (Chang et al. 2015).
Target of rapamycin (TOR)
Target of rapamycin (TOR) positively regulates cell growth and proliferation; biosynthesis of nucleotides, proteins, lipids, and organelles; autophagy; and finally, mitochondrial metabolism and biogenesis. It is often deregulated in several human diseases including cancers, immune deficiency, obesity, diabetes, neurological disorders, aging-related diseases, and developmental disorders (Bjornsti and Houghton 2004). mTOR pathway inhibition disrupts the cytoophidium assembly in both Drosophila and mammalian cells. Supremely, the cytoophidia assembly is chiefly in the grasp of mTOR1/S6K1 signal axis, because its knockdown of downstream target S6K1 inhibits cytoophidia formation, while its overexpression reverses the cytoophidia disassembly caused by mTOR’s knockdown (Sun and Liu 2019a, b). Defective TOR also inhibits Cts1 (Schizosaccharomyces pombe CTPsyn orthologue) cytoophidia formation. The downstream effector kinases of both TORC1 and TORC2 complexes are also required for proper cytoophidium assembly (Andreadis et al. 2019). It is well known that mTOR-S6K1 pathway regulates the de novo pyrimidine nucleotide synthesis via S6K1-mediated phosphorylation of CAD, the first step of de novo pyrimidine nucleotide synthesis (Ben-Sahra et al. 2013). Inhibition of IMPDH that is involved in de novo purine nucleotide synthesis halts mTORC1-dependent cell proliferation via upregulation of tuberous sclerosis complex 2 (TSC2), highlighting the essential role of mTORC1 in TSC-deficient tumor cells and the interaction of TOR in de novo nucleotide synthesis pathway (Valvezan et al. 2017). Given that the purpose of cytoophidia formation could be to increase CTPsyn enzymatic activity to supply the demand for nucleotide at the time of dependencies as polymerization is faster than transcription, TOR, which is an important pathway in cancer metabolism, influences the formation of CTPsyn cytoophidia (Sun and Liu 2019a, b).
Δ1-Pyrroline-5-carboxylate synthase (P5CS)
Δ1-Pyrroline-5-carboxylate synthase (P5CS) is an enzyme that carries out both the glutamate kinase and γ-glutamyl phosphate reductase activities simultaneously in order to catalyse the biosynthesis of proline, a non-essential amino acid from glutamate through two subsequent mechanisms, which are reduction of glutamate to pyrroline-5-carboxylate, followed by conversion of these Δ1-pyrroline-5-carboxylate intermediates to proline by P5C reductase (P5CR). Similar to CTPsyn, P5CS controls the rate-limiting step in proline biosynthesis and is also negatively regulated by proline concentration in a cellular microenvironment (Hong et al. 2000). Malfunctioning P5CS enzyme has been linked to many types of diseases in both humans and plants, including various types of human cancers as cancer cells have high dependency on proline supplementation (Sahu et al. 2016). P5CS proteins form a filamentous structure in D. melanogaster cells along with CTPsyn cytoophidium. This coordinated filamentation between CTPsyn and P5CS could be due to metabolic channeling as glutamate, which is the product of CTPsyn, serves as a substrate for P5CS, thus regulating the interdependent filament formation (Zhang et al. 2020).
Asparagine synthetase (ASNS)
Asparagine synthetase (ASNS) utilizes glutamine alike the CTPsyn as a precursor, but to convert aspartate into asparagine, a non-essential amino acid. Saccharomyces cerevisiae has two isoforms of ASNS, ASN1 and ASN2: both form cytoophidia during exponential and stationary phases. Carbon source starvation induces a reversible assembly of ASNS cytoophidia. ASNS also forms cytoophidia in both cytoplasm and nucleus as CTPsyn cytoophidia. However, they do not co-localize; instead, the ASNS cytoophidia localize adjacent to the CTPsyn cytoophidia, indicating that both these enzymes coordinate the cellular metabolic homeostasis in the cytoplasm and nucleus. The disruption of ASNS impacts the length of the CTPS cytoophidia, but the deletion of CTPS has no significant effect on the ASNS cytoophidia (Zhang et al. 2018, 2021). Notably, the increase in cytosolic aspartate, a precursor of CAD, which is enriched in cancers, facilitates the synthesis of pyrimidine nucleotides, hence promoting cancer cell proliferation (Rabinovich et al. 2015).
Myc
One of the well-studied proto-oncogenes and a key cellular growth regulator, Myc was identified to be necessary and sufficient for the assembly of CTPsyn containing cytoophidia; conversely, CTPsyn is required for Myc-mediated cell growth. Knocking down Myc expression in Drosophila results in loss of cytoophidia and formation of diminutive nuclei in the follicle cells during oogenesis. Alternatively, overexpression of Myc induces the formation of elongated cytoophidia and increases the cell size. This interplay between Myc and CTPsyn suggests that the regulation of cellular growth and metabolism through Myc-mediated coordination could be constrained by transcriptional control of the expression of nucleotide biosynthetic enzymes (Aughey et al. 2016). Myc targets nucleotide synthesis pathway to ramp up nucleotide supplementation for the cell proliferation. Along with CTPsyn, Myc also targets CAD, IMPDH, and phosphoribosyl pyrophosphate synthetase (PRPS), which is also able to form cytoophidia (Begovich et al. 2020; Huang et al. 2021) in order to manipulate nucleotide synthesis for its advantage (McMahon 2008).
Activated cdc42-associated kinase (Ack)
The Drosophila non-receptor tyrosine kinase, DAck is the homologue of mammalian-activated cdc42-associated kinase 1 (Ack1) that regulates key developmental processes. Ack1 is uniquely able to shuttle extracellular signals to the cytoplasm and nucleus of a cell (Mahajan and Mahajan 2015). DAck colocalizes with CTPsyn cytoophidia in the germline cells of Drosophila egg chamber. Furthermore, it is also critically required for the assembly of cytoophidia. DAck loss of function studies revealed that Drosophila egg chambers deficient in DAck or lack DAck catalytic activity display distorted CTPsyn cytoophidia (Strochlic et al. 2014). DAck also promotes tissue growth and could have an anti-apoptotic function, and it would be interesting if these functional roles are also explored for CTPsyn to understand its role in cancer metabolism (Hu et al. 2016).
Casitas B-lineage lymphoma (Cbl)
Casitas B-lineage lymphoma (Cbl), an E3 ubiquitin ligase which is also a proto-oncogene, is required during the endocycle in the polyploid cells such as follicle cells and salivary glands for the maintenance of nucleotide pool balance for rapid endoreplication, and it controls the CTPsyn cytoophidia formation during endoreplication, without affecting the CTPsyn protein level, suggesting that the CTPsyn filament could be composed of inactive enzymes. In the absence of Cbl, the CTPsyn cytoophidia disassembled, leading to defective S-phase of the cell cycle; however, this was rescued with an overexpression of wild-type CTPsyn, indicating the role of Cbl in regulating nucleotide metabolism through CTPsyn for endoreplication in the follicle cells. Furthermore, the ubiquitination signal from Cbl induces the polymerization of CTPsyn, hence making this the first study to report the role of ubiquitination in polymeric structure assembly (Wang et al. 2015).
Ras
Ras is one of the well-known and well-studied oncogenes that contributes to many human cancers. An aberrant activation of Ras induces several cellular proliferative signals to induce tumorigenesis. Ras family proteins, which include KRAS (splice variants KRAS4A and KRAS4B), NRAS, and HRAS in humans, are among the most deregulated oncogenes in a wide variety of human cancers (Gimple and Wang 2019). In a Drosophila tumor model, EGFR/Ras/MAPK pathway induces ISC proliferation in the gut (Jiang et al. 2011). A recent finding has identified that Ras interacts with CTPsyn when an ectopic overexpression of Ras (RasV12) induces CTPsyn cytoophidia in Drosophila ISCs and EBs. Furthermore, CTPsyn is required for the Ras-dependent cell proliferation in the gut. Knocking down CTPsyn in the background of Ras overexpressing flies reduced cell proliferation significantly (Zhou et al. 2022), supporting a previous finding that suggests blocking CTPsyn activity could be a therapeutic target to treat cancer to inhibit cell proliferation (Sun et al. 2022). Fascinatingly, blocking the cytoophidia-forming ability of CTPsyn in an active Ras overexpressing environment is sufficient to reduce Ras-dependent cell proliferation (Zhou et al. 2022), suggesting that cytoophidia formation most likely could be an indication of rapid cell proliferation (Woo et al. 2019). It is important to note that Ras is also involved in nucleotide synthesis pathway via activation of Myc (Santana-Codina et al. 2018).
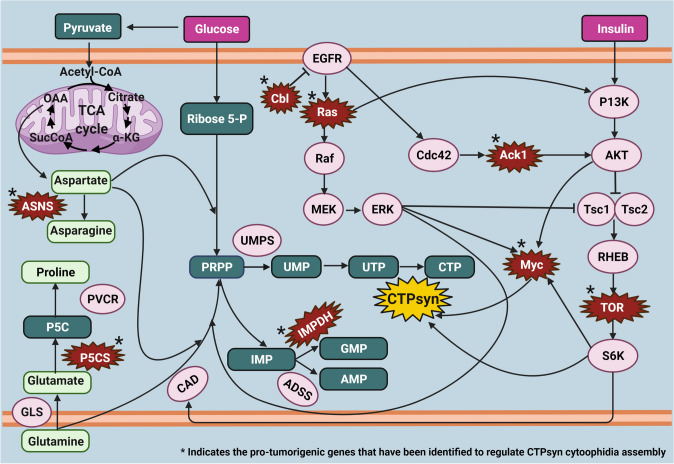
De novo nucleotide synthesis is activated during proliferation to feed the cellular replicational demand for nucleotides in order to synthesize DNA and RNA. Recent findings focusing on oncogenes and tumor suppressor genes have suggested that these genes and the pathways that they regulate are directly interacting with the de novo nucleotide synthesis pathways for the proliferation of cancer cells (Santana-Codina et al. 2018; Li et al. 2021; Yao et al. 2018). Furthermore, amino acid synthesis pathways are also reprogrammed to supply nucleotides for the cancer metabolism. Recent reports have supported the role of one-carbon metabolism in cancel cell survival, growth, and proliferation (Bhingarkar et al. 2021; Krall et al. 2016; Lieu et al 2020; Sahu et al. 2016). Since CTPsyn is involved in the de novo pyrimidine synthesis pathway, Fig. 7 depicts the pathways that CTPsyn could be regulating in modulating cancer metabolism based on the previous reports. This illustration describes the ability of cancer cells to hijack and redistribute available cellular metabolic pathways for its advantage to progress and proliferate.
Fig. 7.
Model illustrating the putative pathways that CTPsyn is involved and the crosstalk between these signaling pathways in cancer metabolism. The pro-tumorigenic genes (depicted with *) regulates the assembly of CTPsyn cytoophidia. The de novo nucleotide synthesis is acutely modulated in cancer cells. Glycolysis, tricarboxylic acid (TCA) cycle, amino acid biosynthesis pathways, epidermal growth factor receptor (EGFR), Ras, insulin, and TOR signaling pathways interact with de novo purine and pyrimidine nucleotide synthesis pathways along with the activation of Myc oncogene to increase nucleotide synthesis to cater to the nucleotide dependency of uncontrolled cell proliferation and tissue growth in cancer cells. Created with BioRender.com
CTPsyn and immunodeficiency
A part of the immune system is made up of lymphocytes, a type of white blood cells which houses the T-cells, B-cells, and natural killer (NK) cells. Purine and pyrimidine’s collective productions are vital for lipid and nucleic acid synthesis, henceforth contributing to the cell cycle progression for activated T-lymphocytes (Quéméneur et al. 2003); when T-lymphocytes are activated, there was an increased pool of purine and pyrimidine (Fairbanks et al. 1995). Two isoforms of CTPsyn, CTPS1 and CTPS2, are required for the de novo synthesis of CTP in humans (Yamauchi et al. 1990), and they play a crucial role in DNA synthesis for proliferating cells.
CTPS1 is a key regulator of the immune system as it is required for the proliferation of activated lymphocytes during immune response. Loss of function mutation of CTPS1 caused impaired T- and B-cell proliferation upon antigen-receptor-mediated activation of immune response, resulting in a life-threatening immunodeficiency. Whereas in resting T-cells the CTPS1 expression was low, upon T-cell receptor (TCR) stimulation the expression level of CTPS1 was upregulated. The similar situation was also observed when B-cells were activated. The T-cell proliferation is severely compromised upon CTPS1 deficiency because of the damage in cell-cycle progression, which arrests the majority of the T-cells at G1 phase, indicating the important role of nucleotide synthesis in regulating the proliferation of immune cells (Martin et al. 2014).
Deficiency of CTPS1 causes severe immunodeficiency, and this could lead to death if infected with Epstein Barr virus (EBV) and varicella zoster virus (VZV), and viral EBV infections would especially result in lymphoproliferation (LP) and non-Hodgkin B-cell lymphoma (Sharma et al. 2022). Hematopoietic stem cell transplantation (HSCT) could be the curative method to restore CTPS1-deficient immunodeficiency (Nademi et al. 2019). This immunodeficiency phenotype includes a low number of invariant natural killer T (iNKT), mucosal-associated invariant T (MAIT), NK cells, and memory B-cells. CTPS1 is highly expressed in iNKT and MAIT cells, highlighting the importance of this enzyme for the division, differentiation, or maintenance of these cells (Martin et al. 2020).
CTPsyn and other diseases—a note in advance
The commonly observed cytoophidia in the eukaryotic and bacterial cytoplasm were also discovered to be present in the nucleus of the eukaryotic cells (N-cytoophidia) upon glutamine starvation, proving that CTPsyn in the nucleus also could form cytoophidia; this phenomenon is cell population dependent. The finding suggests cell population-specific N-cytoophidia formation could be due to various factors such as the cellular metabolic status of the cell, cell-cycle phase of the cell, CTPsyn concentration in the nucleus, and so on (Gou et al. 2014). This finding has opened new possibilities to understand the role of CTPsyn in the nucleus. Oculopharyngeal muscular dystrophy (OPMD) is a rare genetic disorder, which affects the muscles and causes myopathy (Kroon et al. 2021). The pathological hallmark of this disease is the formation of intranuclear inclusion bodies, which are made up of tubular filaments in the skeletal muscle fibers (Tome and Fardeau 1980). Poly(A)-binding protein nuclear 1 (PABPN1) mutation by the extension of GCG trinucleotide repeat at first exon causes this disease. The aggregate of this mutant protein is composed of mRNA and other proteins. Researchers are still finding proteins are interacting with this PABPN1 to induce the nuclear inclusion bodies in the OPMD (Abu-Baker and Rouleau 2007). However, whether CTPsyn interacts with PABPN1 to form this intranuclear filamentous structure is still unknown, and it will be interesting if this could be discovered in order to understand the role of CTPsyn in OPMD (Liu 2010).
CTPsyn inhibitors as a drug target for diseases
CTPsyn is a key metabolic enzyme that is required for nucleic acid synthesis, phospholipid biosynthesis, and protein sialyation. Furthermore, in bacteria polymerization inhibits CTPsyn activity, which is contrary to the situation in humans (Lynch et al. 2017). Investigating the molecular insights of the structure and allostery has encouraged the therapeutic drug target development focusing on CTPsyn (Table 1).
Fig. 8.
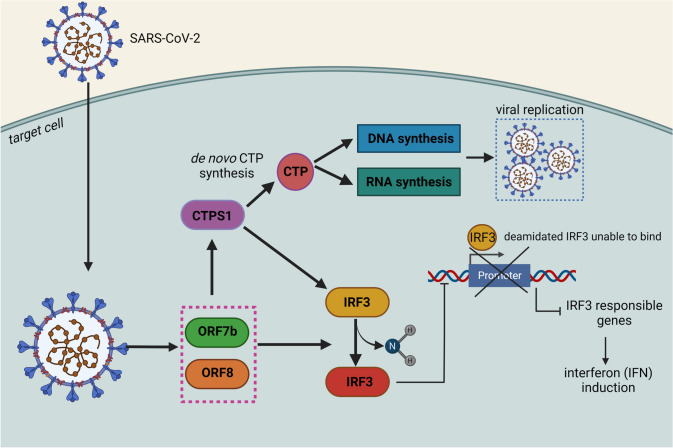
Mechanism of SARS-CoV-2 exploiting CTPS1 for immune evasion, survival, and replication. SARS-CoV-2 suppresses IFN induction upon infection by exploiting host CTPS1 via the deamination of IRF3. SARS-CoV-2 also hijacks the human de novo CTP synthesis for its replication by expressing ORF7b and ORF8 (viral accessory proteins also inhibits IFN induction) that interact and activate CTPS1 (Rao et al. 2021). Created with BioRender.com
Table 1.
CTPsyn as a drug target for diseases caused by viruses, bacteria, and parasites
| Disease | Role of CTPsyn | Reference |
|---|---|---|
| Toxoplasmosis (caused by Toxoplasma gondii) | TgCTPS is essential for the parasite T. gondii survival, and it forms stage-dependent foci like structure, where extracellular parasite forms more pronounced foci than the intracellular parasite as the former requires low CTPS activity; thus, filaments were used to store the enzyme. Meanwhile, upon infection into host cell, the parasite requires high CTPS activity, thus releasing the stored enzymes from the filaments. T. gondii have restricted capacity to salvage CTP synthesis; hence, its dependency on de novo CTP biosynthesis with the aid of CTPsyn could be used as a potential anti-parasitic target | (Narvaez-Ortiz et al. 2018) |
| African sleeping sickness (caused by Trypanosoma brucei) | T. brucei has a low level of CTP due to its limitation in the de novo CTP synthesis and lacking ability to salvage CTP. The parasite CTP level was severely reduced with DON and acivicin, inhibiting the proliferation of the parasite in the host. Therefore, a CTPsyn inhibitor could be a potential drug target for treatment of sleeping sickness | (Hofer et al. 2001; Fijolek et al. 2007) |
| Tuberculosis (caused by Mycobacterium tuberculosis) | Drug-resistant M. tuberculosis is identified to be resistant to two different compounds. Both compounds were activated by EthA monooxygenase, and its metabolite was found to target CTPyn, PyrG. Therefore, this validates that targeting CTPsyn PyrG could be a potential drug target to treat tuberculosis (Mori et al. 2015). 4-(Pyridin-2-yl) thiazole derivatives were found to target PyrG activity, but low activity towards human CTPsyn, hence making it an attractive target to combat tuberculosis | (Esposito et al. 2017) |
| Covid-19 (caused by SARS-CoV-2) | CTPS1 is hijacked by SARS-CoV-2 to mute IFN induction to shut down downstream immune responses and to promote virus replication in the host cell. CTPS1 inhibitor molecules could restore the IFN induction and shortage of CTP impedes virus replication in infected cells. This makes CTPS1 as a potential antiviral therapy target that could resist SARS-CoV-2 variants via restoring innate immune response upon infection (Fig. 8) | (Rao et al. 2021) |
| Infectious mononucleosis and cancers (caused by Epstein-Barr virus (EBV)) | Patients with CTPS1 deficiency often have high EBV viral loads due to impaired T-cell surveillance. CTPS1/2 could be a potential therapeutic target for lymphoproliferative diseases caused by EBV | (Hislop et al. 2007; Liang et al. 2021) |
| Pox virus infections | Carbodine [cyclopentyl cytosine (C-Cyd)] and cyclopentenyl cytosine (Ce-Cyd) are antiviral agents that inhibit CTPsyn activity. These inhibitors suppress RNA synthesis and induce cytotoxicity in proliferating cells | (Marquez et al. 1988) |
| Antibiotic resistance (by Bacillus subtilis) | To develop anti-infectives, PyrG, CTPsyn is targeted by isoquinoline compounds, which have a higher antibiotic effect on B. subtilis mutant lacking all four class A penicillin-binding proteins (PBPs). Since the effect of polymerization of CTPsyn in human and bacteria are completely opposite, it could be a therapeutic target to achieve desired inhibition specificity | (Emami et al. 2020) |
| Respiratory tract infection (RTI) (caused by Streptococcus pneumoniae, Staphylococcus aureus, Haemophilus influenzae) | 2-(3-[3-oxo-1,2-Benzisothiazol-2(3H)-yl]phenylsulfonylamino) benzoic acid (compound G1) could be the potential anti-microbial agent that targets PyrG, CTPsyn. Compound G1 could compete with ATP and/or UTP to bind with PyrG in order to inhibit CTPsyn activity. It was able to have anti-microbial property against different RTI causing bacteria | (Yoshida et al. 2012) |
| Human immunodeficiency virus (HIV) | Lamivudine (3TC), a deoxycytidine analogue, is used to treat HIV and its combination with 3-deazauridine or acivicin (both are CTPsyn inhibitors) increases anti-HIV activity significantly and halts HIV replication | (Dereuddre-Bosquet et al. 2004) |
Concluding remarks and future perspectives
Filament forming enzymes, including CTPsyn and IMPDH, have been identified to be closely related to various types of human cancers and other diseases. CTPsyn cytoophidia have been previously observed in human cancerous cells, and it is also observed in immune-deficient cells. Some studies also have reported incidence of testicular bulging in Drosophila with CTPsyn overexpression. This could be due to the immune compromised microenvironment that is common in vast number of tumor development prognosis. Further studies must be done to study the communication between CTPsyn-induced tumor formation and immunodeficiency. Besides, few metabolic enzymes and miRNAs have been observed to co-regulate CTPsyn activities. Studying these proteins and novel miRNAs could shed light on the discovery of new biological pathways that CTPsyn is directly or indirectly involved in triggering these human diseases and/or biological responses. The CTPsyn regulatory role in responding to environmental and cellular cues to maintain intact cell and developmental homeostasis could be a new perspective to be explored further to decipher cellular signaling and mechanistic pathways CTPsyn is involved.
Outstanding questions
Silencing of CTPS1 induces apoptosis in TNBC cell lines. Does this mean CTPsyn plays a role in blocking cell death via apoptosis and/or autophagy to induce cell proliferation in cancer cell lines? What are the mechanisms/pathways behind this?
Since CTPsyn is required for cell proliferation and it has interaction with Myc, does this have anything to do with cell competition that provides winner-loser status to cells to encourage the proliferation of the advantageous cells?
What could be the possible reasons behind the asymmetric inheritance of CTPsyn cytoophidia in S. pombe? Does CTPsyn play a role in cell fitness, aging, and stress coping mechanisms (as starvation-induced autophagy also induces cytoophidia formation)?
CTPS1 could co-immunoprecipitate with Filamin A (FLNA) (Higgins et al. 2008), which is required for spindle orientation and asymmetric division during oocyte meiosis. Could this mean that CTPsyn interacts with FLNA to maintain asymmetric stem cell divisions and cell polarity during cell migration?
CTPsyn is required to maintain cellular homeostasis and development; therefore, cytoophidia were often observed in actively dividing stem cells. Could this be due to the role of CTPsyn in stem cell signaling and maintenance of a stem cell niche for a proper development?
Upon glutamine starvation, CTPsyn filaments form along with the cytokeratin networks in cells. Do these CTPsyn filaments aid in membrane trafficking to hijack nutrients (or other forms of cargo) for cancer cell growth and survival during rapid cell proliferation when glutamine is scarce?
Acknowledgements
All the CTPsyn-related works in G.A. laboratory is supported by Fundamental Research Grant Scheme (203/PBIOLOGI/6711778).
Author contributions
GA: conceptualized and provided idea for the manuscript. ST: drafted the manuscript. MB and SM: performed the literature search and prepared the figures and tables. GA and NMK: critically revised the work.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abu-Baker A, Rouleau GA. Oculopharyngeal muscular dystrophy: recent advances in the understanding of the molecular pathogenic mechanisms and treatment strategies. Biochim Biophys Acta. 2007;1772(2):173–185. doi: 10.1016/j.bbadis.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Andreadis C, Hulme L, Wensley K, Liu JL. The TOR pathway modulates cytoophidium formation in Schizosaccharomyces pombe. J Biol Chem. 2019;294(40):14686–14703. doi: 10.1074/jbc.RA119.009913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apidianakis Y, Rahme LG. Drosophila melanogaster as a model for human intestinal infection and pathology. Dis Model Mech. 2011;4(1):21–30. doi: 10.1242/dmm.003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aughey GN, Grice SJ, Shen QJ, Xu Y, Chang CC, Azzam G, Wang PY, Freeman-Mills L, Pai LM, Sung LY, Yan J, Liu JL. Nucleotide synthesis is regulated by cytoophidium formation during neurodevelopment and adaptive metabolism. Biology Open. 2014;3(11):1045–1056. doi: 10.1242/bio.201410165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aughey GN, Grice SJ, Liu JL. The interplay between Myc and CTP synthase in Drosophila. PLoS Genet. 2016;12(2):e1005867. doi: 10.1371/journal.pgen.1005867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzam G, Liu JL. Only one isoform of Drosophila melanogaster CTP synthase forms the cytoophidium. PLoS Genet. 2013 doi: 10.1371/journal.pgen.1003256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry RM, Bitbol AF, Lorestani A, Charles EJ, Habrian CH, Hansen JM, Li HJ, Baldwin EP, Wingreen NS, Kollman JM, Gitai Z. Large-scale filament formation inhibits the activity of CTP synthetase. Elife. 2014;3(July2014):1–19. doi: 10.7554/eLife.03638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begovich K, Yelon D, Wilhelm JE. PRPS polymerization influences lens fiber organization in zebrafish. Dev Dyn. 2020;249(8):1018–1031. doi: 10.1002/dvdy.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AAVD, Lenthe HV, Busch S, Korte DD, Roos D, Kuilenburg ABV, Gennip AHV. Evidence for transformation-related increase in CTP synthetase activity in situ in human lymphoblastic leukemia. Eur J Biochem. 1993;216:161–167. doi: 10.1111/j.1432-1033.1993.tb18128.x. [DOI] [PubMed] [Google Scholar]
- Ben-Sahra I, Howell JJ, Asara JM, Manning BD. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science. 2013;339(6125):1323–1328. doi: 10.1126/science.1228792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagavan NV, Ha CE. Essentials of medical biochemistry : with clinical cases. Amsterdam: Elsevier/Academic Press; 2011. [Google Scholar]
- Bhingarkar A, Vangapandu HV, Rathod S, Hoshitsuki K, Fernandez CA. Amino acid metabolic vulnerabilities in acute and chronic myeloid leukemias. Front Oncol. 2021 doi: 10.3389/fonc.2021.694526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsti MA, Houghton PJ. The tor pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4(5):335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- Calise SJ, Carcamo WC, Krueger C, Yin JD, Purich DL, Chan EKL. Glutamine deprivation initiates reversible assembly of mammalian rods and rings. Cell Mol Life Sci. 2014;71(15):2963–2973. doi: 10.1007/s00018-014-1567-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcamo WC, Satoh M, Kasahara H, Terada N, Hamazaki T, Chan JYF, Yao B, Tamayo S, Covini G, von Mühlen CA, Chan EKL. Induction of cytoplasmic rods and rings structures by inhibition of the CTP and GTP synthetic pathway in mammalian cells. PLoS ONE. 2011 doi: 10.1371/journal.pone.0029690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty A, Lin WC, Lin YT, Huang KJ, Wang PY, Chang IY, Wang HI, Ma KT, Wang CY, Huang XR, Lee YH, Chen BC, Hsieh YJ, Chien KY, Lin TY, Liu JL, Sung LY, Yu JS, Chang YS, Pai LM. SNAP29 mediates the assembly of histidine-induced CTP synthase filaments in proximity to the cytokeratin network. J Cell Sci. 2020;133(9):jcs240200. doi: 10.1242/jcs.240200. [DOI] [PubMed] [Google Scholar]
- Chang CC, Jeng YM, Peng M, Keppeke GD, Sung LY, Liu JL. CTP synthase forms the cytoophidium in human hepatocellular carcinoma. Exp Cell Res. 2017;361(2):292–299. doi: 10.1016/J.YEXCR.2017.10.030. [DOI] [PubMed] [Google Scholar]
- Chang CC, Keppeke GD, Sung LY, Liu JL. Interfilament interaction between IMPDH and CTPS cytoophidia. FEBS J. 2018;285(20):3753–3768. doi: 10.1111/febs.14624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Lin WC, Pai LM, Lee HS, Wu SC, Ding ST, Liu JL, Sung LY. Cytoophidium assembly reflects upregulation of IMPDH activity. J Cell Sci. 2015;128(19):3550–3555. doi: 10.1242/jcs.175265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Zhang J, Tastan ÖY, Deussen ZA, Siswick MYY, Liu JL. Glutamine analogs promote cytoophidium assembly in human and Drosophila cells. J Genet Genom. 2011;38(9):391–402. doi: 10.1016/j.jgg.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Chi C, Ronai D, Than MT, Walker CJ, Sewell AK, Han M. Nucleotide levels regulate germline proliferation through modulating GLP-1/Notch signaling in C. elegans. Genes Dev. 2016;30(3):307–320. doi: 10.1101/gad.275107.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cova C, Abril M, Bellosta P, Gallant P, Johnston LA. Drosophila Myc regulates organ size by inducing cell competition. Cell. 2004;117(1):107–116. doi: 10.1016/S0092-8674(04)00214-4. [DOI] [PubMed] [Google Scholar]
- Dereuddre-Bosquet N, Roy B, Routledge K, Clayette P, Foucault G, Lepoivre M. Inhibitors of CTP biosynthesis potentiate the anti-human immunodeficiency virus type 1 activity of 3TC in activated peripheral blood mononuclear cells. Antivir Res. 2004;61(1):67–70. doi: 10.1016/j.antiviral.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Dong Y, Tu R, Liu H, Qing G. Regulation of cancer cell metabolism: oncogenic MYC in the driver’s seat. Signal Transduct Target Ther. 2020;5(1):124. doi: 10.1038/s41392-020-00235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger B, Boone JQ, Stevens NR, Brand AH, Doe CQ. Regulation of spindle orientation and neural stem cell fate in the Drosophila optic lobe. Neural Dev. 2007;2(1):1. doi: 10.1186/1749-8104-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami K, Wu LJ, Errington J. A small molecule inhibitor of CTP synthetase identified by differential activity on a Bacillus Subtilis mutant deficient in class a penicillin-binding proteins. Front Microbiol. 2020;11:2001. doi: 10.3389/fmicb.2020.02001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endrizzi JA, Kim H, Anderson PM, Baldwin EP. Crystal structure of Escherichia coli cytidine triphosphate synthetase, a nucleotide-regulated glutamine amidotransferase/ATP-dependent amidoligase fusion protein and homologue of anticancer and antiparasitic drug targets. Biochemistry. 2004;43(21):6447–6463. doi: 10.1021/bi0496945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M, Szadocka S, Degiacomi G, Orena BS, Mori G, Piano V, Boldrin F, Zemanová J, Huszár S, Barros D, Ekins S, Lelièvre J, Manganelli R, Mattevi A, Pasca MR, Riccardi G, Ballell L, Mikušová K, Chiarelli LR. A phenotypic based target screening approach delivers new antitubercular CTP synthetase inhibitors. ACS Infect Dis. 2017;3(6):428–437. doi: 10.1021/acsinfecdis.7b00006. [DOI] [PubMed] [Google Scholar]
- Fairbanks LD, Bofill M, Ruckemann K, Simmonds HA. Importance of ribonucleotide availability to proliferating T-lymphocytes from healthy humans disproportionate expansion of pyrimidine pools and contrasting effects of de novo synthesis inhibitors. J Biol Chem. 1995;270(50):29682–29689. doi: 10.1074/jbc.270.50.29682. [DOI] [PubMed] [Google Scholar]
- Fijolek A, Hofer A, Thelander L. Expression, purification, characterization, and in vivo targeting of trypanosome CTP synthetase for treatment of African sleeping sickness. J Biol Chem. 2007;282(16):11858–11865. doi: 10.1074/jbc.M611580200. [DOI] [PubMed] [Google Scholar]
- Ford H, Cooney DA, Ahluwalia GS, Hao Z, Rommel ME, Hicks L, Dobyns KA, Tomaszewski JE, Johns DG. Cellular pharmacology of cyclopentenyl cytosine in molt-4 lymphoblasts. Can Res. 1991;51:3733–3740. [PubMed] [Google Scholar]
- Friedman AA, Tucker G, Singh R, Yan D, Vinayagam A, Hu Y, Binari R, Hong P, Sun X, Porto M, Pacifico S, Murali T, Finley RL, Asara JM, Berger B, Perrimon N. Proteomic and functional genomic landscape of receptor tyrosine kinase and Ras to extracellular signal-regulated kinase signaling. Science Signaling. 2011;4(196):1–15. doi: 10.1126/scisignal.2002029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genchev DD, Mandel P. Changes of CTP synthetase activity during postnatal rat brain development. J Neurosci Res. 1976;2(5–6):413–418. doi: 10.1002/jnr.490020509. [DOI] [PubMed] [Google Scholar]
- Gimple RC, Wang X. RAS: striking at the core of the oncogenic circuitry. Front Oncol. 2019 doi: 10.3389/fonc.2019.00965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou KM, Chang CC, Shen QJ, Sung LY, Liu JL. CTP synthase forms cytoophidia in the cytoplasm and nucleus. Exp Cell Res. 2014;323(1):242–253. doi: 10.1016/j.yexcr.2014.01.029. [DOI] [PubMed] [Google Scholar]
- Higgins MJ, Graves PR, Graves LM. Regulation of human cytidine triphosphate synthetase 1 by glycogen synthase kinase 3 *. J Biol Chem. 2007;282(40):29493–29503. doi: 10.1074/jbc.M703948200. [DOI] [PubMed] [Google Scholar]
- Higgins MJ, Loiselle D, Haystead TA, Graves LM. Human cytidine triphosphate synthetase 1 interacting proteins. Nucleosides, Nucleotides Nucleic Acids. 2008;27(6–7):850–857. doi: 10.1080/15257770802146502. [DOI] [PubMed] [Google Scholar]
- Hislop AD, Taylor GS, Sauce D, Rickinson AB. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu Rev Immunol. 2007;25(1):587–617. doi: 10.1146/annurev.immunol.25.022106.141553. [DOI] [PubMed] [Google Scholar]
- Hofer A, Steverding D, Chabes A, Brun R, Thelander L. Trypanosoma brucei CTP synthetase: a target for the treatment of African sleeping sickness. Proc Natl Acad Sci USA. 2001;98(11):6412–6416. doi: 10.1073/pnas.111139498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z, Lakkineni K, Zhang Z, Verma DPS. Removal of feedback inhibition of Δ1-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol. 2000;122(4):1129–1136. doi: 10.1104/pp.122.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Xu J, Yin MX, Zhang L, Lu Y, Wu W, Xue Z, Ho MS, Gao G, Zhao Y, Zhang L. Ack promotes tissue growth via phosphorylation and suppression of the Hippo pathway component expanded. Cell Discovery. 2016;2(1):15047. doi: 10.1038/celldisc.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Huffman KE, Wang Z, Wang X, Li K, Cai F, Yang C, Cai L, Shih TS, Zacharias LG, Chung A, Yang Q, Chalishazar MD, Ireland AS, Stewart CA, Cargill K, Girard L, Liu Y, Ni M, Xu J, Wu X, Zhu H, Drapkin B, Byers LA, Oliver TJ, Gazdar AF, Minna JD, DeBerardinis RJ. Guanosine triphosphate links MYC-dependent metabolic and ribosome programs in small-cell lung cancer. J Clin Investig. 2021 doi: 10.1172/JCI139929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson AM, Cooley L. Methods for studying oogenesis. Methods. 2014;68(1):207–217. doi: 10.1016/j.ymeth.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingerson-Mahar M, Briegel A, Werner JN, Jensen GJ, Gitai Z. The metabolic enzyme CTP synthase forms cytoskeletal filaments. Nat Cell Biol. 2010;12(8):739–746. doi: 10.1038/ncb2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Gu J, Makhov AM, Griffith JD, Mitchell BS. Regulation of the interaction of inosine monophosphate dehydrogenase with mycophenolic acid by GTP *. J Biol Chem. 2006;281(1):206–212. doi: 10.1074/jbc.M507056200. [DOI] [PubMed] [Google Scholar]
- Jia F, Chi C, Han M. Regulation of nucleotide metabolism and germline proliferation in response to nucleotide imbalance and genotoxic stresses by endoU nuclease. Cell Rep. 2020;30(6):1848–1861.e5. doi: 10.1016/j.celrep.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Edgar BA. Intestinal stem cells in the adult Drosophila midgut. Exp Cell Res. 2011;317(19):2780–2788. doi: 10.1016/j.yexcr.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Grenley MO, Bravo MJ, Blumhagen RZ, Edgar BA. EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell. 2011;8(1):84–95. doi: 10.1016/j.stem.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Tian A, Jiang J. Intestinal stem cell response to injury: lessons from Drosophila. Cell Mol Life Sci. 2016;73(17):3337–3349. doi: 10.1007/s00018-016-2235-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent C, Carman GM. Interactions among pathways for phosphatidylcholine metabolism, CTP synthesis and secretion through the Golgi apparatus. Trends Biochem Sci. 1999;24(4):146–150. doi: 10.1016/S0968-0004(99)01365-1. [DOI] [PubMed] [Google Scholar]
- Keppeke GD, Calise SJ, Chan EKL, Andrade LEC. Assembly of IMPDH2-based, CTPS-based, and mixed rod/ring structures is dependent on cell type and conditions of induction. J Genet Genomics. 2015;42(6):287–299. doi: 10.1016/j.jgg.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Kizaki H, Williams JC, Morris HP, Weber G. Increased cytidine 5′-triphosphate synthetase activity in rat and human tumors. Can Res. 1980;40(11):3921–3927. [PubMed] [Google Scholar]
- Krall AS, Xu S, Graeber TG, Braas D, Christofk HR. Asparagine promotes cancer cell proliferation through use as an amino acid exchange factor. Nat Commun. 2016;7(1):11457. doi: 10.1038/ncomms11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon RHMJM, Kalf JG, de Swart BJM, van der Sluijs BM, Glennon JC, Raz V, van Engelen BG, Horlings CGC. Longitudinal assessment of strength, functional capacity, oropharyngeal function, and quality of life in oculopharyngeal muscular dystrophy. Neurology. 2021;97(15):e1475–e1483. doi: 10.1212/WNL.0000000000012640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, He Y, Li Y, Li J, Zhao H, Song G, Miyagishi M, Wu S, Kasim V. NeuroD1 promotes tumor cell proliferation and tumorigenesis by directly activating the pentose phosphate pathway in colorectal carcinoma. Oncogene. 2021;40(50):6736–6747. doi: 10.1038/s41388-021-02063-2. [DOI] [PubMed] [Google Scholar]
- Li X, Xie J, Hei M, Tang J, Wang Y, Förster E, Zhao S. High level of CTP synthase induces formation of cytoophidia in cortical neurons and impairs corticogenesis. Histochem Cell Biol. 2018;149(1):61–73. doi: 10.1007/s00418-017-1612-2. [DOI] [PubMed] [Google Scholar]
- Liang JH, Wang C, Yiu S, Zhao B, Guo R, Gewurz BE. Epstein-Barr virus induced cytidine metabolism roles in transformed B-cell growth and survival. Mbio. 2021;12(4):0153021. doi: 10.1128/mBio.01530-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu EL, Nguyen T, Rhyne S, Kim J. Amino acids in cancer. Exp Mol Med. 2020;52(1):15–30. doi: 10.1038/s12276-020-0375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WC, Chakraborty A, Huang SC, Wang PY, Hsieh YJ, Chien KY, Lee YH, Chang CC, Tang HY, Lin YT, Tung CS, Luo JD, Chen TW, Lin TY, Cheng ML, Chen YT, Yeh CT, Liu JL, Sung LY, Shiao MS, Yu JS, Chang YS, Pai LM. Histidine-dependent protein methylation is required for compartmentalization of CTP synthase. Cell Rep. 2018;24(10):2733–2745.e7. doi: 10.1016/j.celrep.2018.08.007. [DOI] [PubMed] [Google Scholar]
- Lin Y, Zhang J, Li Y, Guo W, Chen L, Chen M, Chen X, Zhang W, Jin X, Jiang M, Xiao H, Wang C, Song C, Fu F. CTPS1 promotes malignant progression of triple-negative breast cancer with transcriptional activation by YBX1. J Transl Med. 2022;20(1):17. doi: 10.1186/s12967-021-03206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JL. Intracellular compartmentation of CTP synthase in Drosophila. J Genet Genom. 2010;37(5):281–296. doi: 10.1016/S1673-8527(09)60046-1. [DOI] [PubMed] [Google Scholar]
- Liu JL. The enigmatic cytoophidium: compartmentation of CTP synthase via filament formation. BioEssays. 2011;33(3):159–164. doi: 10.1002/bies.201000129. [DOI] [PubMed] [Google Scholar]
- Lynch EM, Hicks DR, Shepherd M, Endrizzi JA, Maker A, Hansen JM, Barry RM, Gitai Z, Baldwin EP, Kollman JM. Human CTP synthase filament structure reveals the active enzyme conformation. Nat Struct Mol Biol. 2017;24(6):507–514. doi: 10.1038/nsmb.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan K, Mahajan NP. ACK1/TNK2 tyrosine kinase: molecular signaling and evolving role in cancers. Oncogene. 2015;34(32):4162–4167. doi: 10.1038/onc.2014.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez VE, Lim MI, Treanor SP, Plowman J, Priest MA, Markovac A, Khan MS, Kaskar B, Driscoll JS. Cyclopentenylcytosine A carbocyclic nucleoside with antitumor and antiviral properties. J Med Chem. 1988;31(9):1687–1694. doi: 10.1021/jm00117a004. [DOI] [PubMed] [Google Scholar]
- Martin E, Minet N, Boschat AC, Sanquer S, Sobrino S, Lenoir C, de Villartay JP, Leite-de-Moraes M, Picard C, Soudais C, Bourne T, Hambleton S, Hughes SM, Wynn RF, Briggs TA, Patel S, Lawrence MG, Fischer A, Arkwright PD, Latour S, Genomics England Research Consortium Impaired lymphocyte function and differentiation in CTPS1-deficient patients result from a hypomorphic homozygous mutation. JCI Insight. 2020;5(5):e133880. doi: 10.1172/jci.insight.133880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E, Palmic N, Sanquer S, Lenoir C, Hauck F, Mongellaz C, Fabrega S, Nitschké P, Esposti MD, Schwartzentruber J, Taylor N, Majewski J, Jabado N, Wynn RF, Picard C, Fischer A, Arkwright PD, Latour S. CTP synthase 1 deficiency in humans reveals its central role in lymphocyte proliferation. Nature. 2014;510(7504):288–292. doi: 10.1038/nature13386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SB. Control of nucleotide biosynthesis by the MYC oncoprotein. Cell Cycle. 2008;7(15):2275–2276. doi: 10.4161/cc.6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E, Basler K. dMyc transforms cells into super-competitors. Cell. 2004;117(1):117–129. doi: 10.1016/S0092-8674(04)00262-4. [DOI] [PubMed] [Google Scholar]
- Mori G, Chiarelli LR, Esposito M, Makarov V, Bellinzoni M, Hartkoorn RC, Degiacomi G, Boldrin F, Ekins S, de Jesus Lopes Ribeiro AL, Marino LB, Centárová I, Svetlíková Z, Blaško J, Kazakova E, Lepioshkin A, Barilone N, Zanoni G, Porta A, Fondi M, Fani R, Baulard AR, Mikušová K, Alzari PM, Manganelli R, de Carvalho LPS, Riccardi G, Cole ST, Pasca MR. Thiophenecarboxamide derivatives activated by EthA kill Mycobacterium tuberculosis by inhibiting the CTP synthetase PyrG. Chem Biol. 2015;22(7):917–927. doi: 10.1016/j.chembiol.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nademi Z, Wynn RF, Slatter M, Hughes SM, Bonney D, Qasim W, Latour S, Trück J, Patel S, Abinun M, Flood T, Hambleton S, Cant AJ, Gennery AR, Arkwright PD. Hematopoietic stem cell transplantation for cytidine triphosphate synthase 1 (CTPS1) deficiency. Bone Marrow Transplant. 2019;54(1):130–133. doi: 10.1038/s41409-018-0246-x. [DOI] [PubMed] [Google Scholar]
- Narvaez-Ortiz HY, Lopez AJ, Gupta N, Zimmermann BH. A CTP synthase undergoing stage-specific spatial expression is essential for the survival of the intracellular parasite Toxoplasma gondii. Front Cell Infect Microbiol. 2018;8:83. doi: 10.3389/fcimb.2018.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noree C, Sato BK, Broyer RM, Wilhelm JE. Identification of novel filament-forming proteins in Saccharomyces cerevisiae and Drosophila melanogaster. J Cell Biol. 2010;190(4):541–551. doi: 10.1083/jcb.201003001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Pai LM, Wang PY, Lin WC, Chakraborty A, Yeh CT, Lin YH. Ubiquitination and filamentous structure of cytidine triphosphate synthase. Fly. 2016;10(3):108–114. doi: 10.1080/19336934.2016.1182268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quéméneur L, Gerland LM, Flacher M, Ffrench M, Revillard JP, Genestier L. Differential control of cell cycle, proliferation, and survival of primary T lymphocytes by purine and pyrimidine nucleotides. J Immunol. 2003;170(10):4986–4995. doi: 10.4049/jimmunol.170.10.4986. [DOI] [PubMed] [Google Scholar]
- Rabinovich S, Adler L, Yizhak K, Sarver A, Silberman A, Agron S, Stettner N, Sun Q, Brandis A, Helbling D, Korman S, Itzkovitz S, Dimmock D, Ulitsky I, Nagamani SCS, Ruppin E, Erez A. Diversion of aspartate in ASS1-deficient tumours fosters de novo pyrimidine synthesis. Nature. 2015;527(7578):379–383. doi: 10.1038/nature15529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao Y, Wang TY, Qin C, Espinosa B, Liu Q, Ekanayake A, Zhao J, Savas AC, Zhang S, Zarinfar M, Liu Y, Zhu W, Graham N, Jiang T, Zhang C, Feng P. Targeting CTP synthetase 1 to restore interferon induction and impede nucleotide synthesis in SARS-CoV-2 infection. BioRxiv. 2021 doi: 10.1101/2021.02.05.429959. [DOI] [Google Scholar]
- Sahu N, Cruz DD, Gao M, Sandoval W, Haverty PM, Liu J, Stephan JP, Haley B, Classon M, Hatzivassiliou G, Settleman J. Proline starvation induces unresolved ER stress and hinders mTORC1-dependent tumorigenesis. Cell Metab. 2016;24(5):753–761. doi: 10.1016/j.cmet.2016.08.008. [DOI] [PubMed] [Google Scholar]
- Santana-Codina N, Roeth AA, Zhang Y, Yang A, Mashadova O, Asara JM, Wang X, Bronson RT, Lyssiotis CA, Ying H, Kimmelman AC. Oncogenic KRAS supports pancreatic cancer through regulation of nucleotide synthesis. Nat Commun. 2018;9(1):4945. doi: 10.1038/s41467-018-07472-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Pilania RK, Anjani G, Sudhakar M, Arora K, Tyagi R, Dhaliwal M, Vignesh P, Rawat A, Singh S. Lymphoproliferation in inborn errors of immunity: the eye does not see what the mind does not know. Front Immunol. 2022 doi: 10.3389/fimmu.2022.856601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet JC, Foster MJ, Lynch EM, Kollman JM, Nicholas E, O’Reilly AM, Peterson JR. CTP synthase polymerization in germline cells of the developing Drosophila egg supports egg production. Biol Open. 2020 doi: 10.1242/BIO.05032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SR, Liu Y, Zhao J, Zeng X, Hou SX. The novel tumour suppressor Madm regulates stem cell competition in the Drosophila testis. Nat Commun. 2016;7:10473. doi: 10.1038/ncomms10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strochlic TI, Stavrides KP, Thomas SV, Nicolas E, O’Reilly AM, Peterson JR. Ack kinase regulates CTP synthase filaments during Drosophila oogenesis. EMBO Rep. 2014;15(11):1184–1191. doi: 10.15252/embr.201438688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Liu JL. Forming cytoophidia prolongs the half-life of CTP synthase. Cell Discov. 2019;5(1):1–5. doi: 10.1038/s41421-019-0098-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Liu JL. mTOR-S6K1 pathway mediates cytoophidium assembly. J Genet Genom. 2019;46(2):65–74. doi: 10.1016/j.jgg.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Zhang Z, Wang QQ, Liu JL. Combined inactivation of CTPS1 and ATR is synthetically lethal to MYC-overexpressing cancer cells. Can Res. 2022;82(6):1013–1024. doi: 10.1158/0008-5472.CAN-21-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tastan ÖY, Liu JL. CTP synthase is required for optic lobe homeostasis in Drosophila. J Genet Genom. 2015;42(5):261–274. doi: 10.1016/j.jgg.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tome FM, Fardeau M. Nuclear inclusions in oculopharyngeal dystrophy. Acta Neuropathol. 1980;49:85–87. doi: 10.1007/BF00692226. [DOI] [PubMed] [Google Scholar]
- Tzeng D, Sakiyama S, Kizaki H, Weber G. Increased concentration of CTP synthetase in hepatoma 3924A: immunological evidence. Life Sci. 1981;28(22):2537–2543. doi: 10.1016/0024-3205(81)90596-8. [DOI] [PubMed] [Google Scholar]
- Valvezan AJ, Turner M, Belaid A, Lam HC, Miller SK, McNamara MC, Baglini C, Housden BE, Perrimon N, Kwiatkowski DJ, Asara JM, Henske EP, Manning BD. mTORC1 couples nucleotide synthesis to nucleotide demand resulting in a targetable metabolic vulnerability. Cancer Cell. 2017;32(5):624–638.e5. doi: 10.1016/j.ccell.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PY, Lin WC, Tsai YC, Cheng ML, Lin YH, Tseng SH, Chakraborty A, Pai LM. Regulation of CTP synthase filament formation during DNA endoreplication in Drosophila. Genetics. 2015;201(4):1511–1523. doi: 10.1534/genetics.115.180737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JC, Kizaki H, Weber G, Morris HP. Increased CTP synthetase activity in cancer cells. Nature. 1978;271(5640):71–73. doi: 10.1038/271071a0. [DOI] [PubMed] [Google Scholar]
- Willoughby LF, Schlosser T, Manning SA, Parisot JP, Street IP, Richardson HE, Humbert PO, Brumby AM. An in vivo large-scale chemical screening platform using Drosophila for anti-cancer drug discovery. Dis Model Mech. 2013;6(2):521–529. doi: 10.1242/dmm.009985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo WK, Dzaki N, Thangadurai S, Azzam G. Ectopic miR-975 induces CTP synthase directed cell proliferation and differentiation in Drosophila melanogaster. Sci Rep. 2019;9(6096):1–14. doi: 10.1038/s41598-019-42369-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Liu JL. Cytoophidia respond to nutrient stress in Drosophila. Exp Cell Res. 2019;376(2):159–167. doi: 10.1016/j.yexcr.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Tanwar PS, Raftery LA. Drosophila follicle cells: morphogenesis in an eggshell. Semin Cell Dev Biol. 2008;19(3):271–282. doi: 10.1016/j.semcdb.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi M, Yamauchi N, Meuth M. Molecular cloning of the human CTP synthetase gene by functional complementation with purified human metaphase chromosomes. EMBO J. 1990;9(7):2095–2099. doi: 10.1002/j.1460-2075.1990.tb07377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Liu Y, Guo H, Ahmed M, Bhuiyan S, Nomie K, Zhang L, Wang M. Metabolic profiling identifies de novo nucleotide synthesis as a potential metabolic vulnerability for targeted therapy against mantle cell lymphoma. Blood. 2018;132(1):2945. doi: 10.1182/blood-2018-99-112192. [DOI] [Google Scholar]
- Yoshida T, Nasu H, Namba E, Ubukata O, Yamashita M. Discovery of a compound that acts as a bacterial PyrG (CTP synthase) inhibitor. J Med Microb. 2012;61(9):1280–1285. doi: 10.1099/jmm.0.046052-0. [DOI] [PubMed] [Google Scholar]
- Zhang S, Ding K, Shen QJ, Zhao S, Liu JL. Filamentation of asparagine synthetase in Saccharomyces cerevisiae. PLoS Genet. 2018;14(10):e1007737. doi: 10.1371/journal.pgen.1007737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Feng HC, Liu JL. ASNS disruption shortens CTPS cytoophidia in Saccharomyces cerevisiae. G3 Genes Genom Genet. 2021;11(1):060. doi: 10.1093/g3journal/jkaa060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Tastan ÖY, Zhou X, Guo CJ, Liu X, Thind A, Hu HH, Zhao S, Liu JL. The proline synthesis enzyme P5CS forms cytoophidia in Drosophila. J Genet Genom. 2020 doi: 10.1016/j.jgg.2020.02.005. [DOI] [PubMed] [Google Scholar]
- Zhou X, Guo CJ, Hu HH, Zhong J, Sun Q, Liu D, Zhou S, Chang CC, Liu JL. Drosophila CTP synthase can form distinct substrate- and product-bound filaments. J Genet Genom. 2019;46(11):537–545. doi: 10.1016/j.jgg.2019.11.006. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liu J, Liu JL. Connecting Ras and CTP synthase in Drosophila. Exp Cell Res. 2022;416(1):113155. doi: 10.1016/j.yexcr.2022.113155. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liu J, Zhang Y, Liu JL. Drosophila intestinal homeostasis requires CTP synthase. Exp Cell Res. 2021;408(1):112838. doi: 10.1016/j.yexcr.2021.112838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.