Abstract
Background
This systematic review was conducted to evaluate any interventions to prevent incident delirium, or shorten the duration of prevalent delirium, in older adults presenting to the emergency department (ED).
Methods
Health sciences librarian designed electronic searches were conducted from database inception through September 2021. Two authors reviewed studies, and included studies that evaluated interventions for the prevention and/or treatment of delirium and excluded non‐ED studies. The risk of bias (ROB) was evaluated by the Cochrane ROB tool or the Newcastle‐Ottawa (NOS) scale. Meta‐analysis was conducted to estimate a pooled effect of multifactorial programs on delirium prevention.
Results
Our search strategy yielded 11,900 studies of which 10 met study inclusion criteria. Two RCTs evaluated pharmacologic interventions for delirium prevention; three non‐RCTs employed a multi‐factorial delirium prevention program; three non‐RCTs evaluated regional anesthesia for hip fractures; and one study evaluated the use of Foley catheter, medication exposure, and risk of delirium. Only four studies demonstrated a significant impact on delirium incidence or duration of delirium—one RCT of melatonin reduced the incidence of delirium (OR 0.19, 95% CI 0.06 to 0.62), one non‐RCT study on a multi‐factorial program decreased inpatient delirium prevalence (41% to 19%) and the other reduced incident delirium (RR 0.37, 95% CI 0.22 to 0.61). One case–control study on the use of ED Foley catheters in the ED increased the duration of delirium (proportional OR 3.1, 95% CI 1.3 to 7.4). A pooled odds ratio for three multifactorial programs on delirium prevention was 0.46 (95% CI 0.31–0.68, I2 = 0).
Conclusion
Few interventions initiated in the ED were found to consistently reduce the incidence or duration of delirium. Delirium prevention and treatment trials in the ED are still rare and should be prioritized for future research.
Keywords: delirium, intervention, prevention, systematic review
Key points
The use of melatonin in the selected indications, such as overnight stay in the ED, could be effective in reducing incident delirium, but further research is needed.
Multi‐factorial programs show the most promise in reducing delirium‐related outcomes, but a sustained, multi‐center, and likely international collaborative program of research is required to assess whether such interventions are feasible and effective in the ED.
Older adults with hip fracture appear to be a target population which investigators used both pharmacological and non‐pharmacological interventions; however, interventions studied to date have not demonstrated a significant impact.
Why does this paper matter?
Evaluation of the current status of prevention and intervention for delirium in the acute care setting will facilitate the design and development of future intervention programs for older adults who present to clinic, inpatient, and intensive care unit after the emergency department visits.
INTRODUCTION
Delirium is a disturbance of attention, awareness, and a change in baseline cognition. Delirium is common in the emergency department (ED), especially in older adults. Between 6% and 38% of older ED adults have incident delirium, defined as new development of delirium after arrival, or prevalent delirium defined as those who present to the ED with delirium. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 Importantly, ED delirium is associated with increases in the length of hospital stay and mortality, and decreases in independence and cognitive function. 3 , 7 , 9 , 10 , 11 , 12 Due to the prevalence of delirium in the ED setting and the associated morbidity and mortality, there is a growing support for ED‐based strategies to improve identification, management and prevention of delirium. Among accredited level 1 and level 2 geriatric EDs, 90% of level 1 and 40% of level 2 geriatric EDs reported protocols for delirium screening; in addition, many have implemented strategies to decrease delirium incidence. 13
The authors previously synthesized seven Cochrane systematic reviews that summarized delirium prevention or treatment trials across a variety of healthcare settings (Table S1). 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 Multifactorial interventions, including individualized care, an educational component, reorientation, and early mobilization, often effectively reduce the incidence of delirium in the inpatient setting. 19 , 22 Since 2012, four Cochrane reviews summarized pharmacologic and nonpharmacologic delirium interventions in both the hospital setting and long‐term care. 14 , 17 , 19 , 20 The generalizability of pharmacological and nonpharmacological interventions to the ED setting may be limited due to resource constraints and heterogeneous patient populations. 21 To date, no systematic review has been published assessing delirium prevention or intervention programs targeted at older adults in the ED.
Objective
The objective of this systematic review was to identify pharmacologic and nonpharmacologic interventions to prevent or treat delirium to reduce the incidence, severity, and duration of delirium in older adults presenting to the ED.
METHODS
Protocol and registration
The study follows the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA). 23 The study protocol was registered to PROSPERO (CRD42020169654). 21
Eligibility criteria
Study characteristics
To capture a broad scope of studies in the literature, we included studies where assessment started in the ED. We considered studies that evaluated interventions for the prevention or treatment of delirium in older adults admitted from the ED. We excluded studies that did not include ED or focused entirely on delirium tremens or emergence delirium, as these conditions are pathophysiologically distinct from the geriatric syndrome of delirium. 24
Types of studies
This review included randomized controlled trials (RCT), before and after studies, observational studies with statistical adjustment, and quality improvement studies. We did not exclude any study based on language.
Types of participants
For treatment studies, we included studies with participants 65 years of age or older who screened positive for delirium. For prevention studies, we included studies with participants 65 years of age or older who screened negative or did not get a diagnosis for delirium on ED presentation. We chose 65 as the relative cutoff as the risk of delirium is the highest in this age group. 25
Types of interventions
Any interventions that were quantitatively evaluated as delirium prevention or treatment were eligible. “Prevention” included any approach to reduce incident delirium during the ED episode of care or subsequent hospitalization for those admitted. “Treatment” included any method used to reduce the severity and/or duration of those diagnosed with delirium. The comparator group was the placebo, usual care, or pre‐ implementation baseline.
Types of outcome measures
For prevention studies, the primary outcome was delirium incidence or inpatient delirium prevalence, and delirium severity was the primary outcome for treatment studies. Secondary outcomes include delirium duration and mortality rates. Delirium identification required the use of valid delirium assessment tools or clinical diagnosis. This included brief screening tools and diagnosis codes. 26 , 27 , 28 , 29 , 30 Delirium duration was typically reported in consecutive days that a patient had a positive screen for delirium or met diagnostic criteria for delirium. The timing parameters spanned the initial arrival in the ED and the entire hospital stay.
Setting
The initial delirium assessment had to occur in the ED. We allowed the prevention or intervention to occur elsewhere in the hospital, including inpatient units. This was because we broadened the inclusion to include interventions initiated during the hospital stay to maximize the output of this review, while maintaining ED relevance by identifying potentially high‐yield future research directions to evaluate in the ED. We finalized the inclusion criteria before PROSPERO protocol was submitted.
Search strategy
A health sciences librarian (HH) trained in systematic reviews developed search strategies employing subject headings and keywords in conjunction with study investigators. The initial strategy was developed on Ovid MEDLINE and translated manually to the other databases. Another librarian peer‐reviewed the Ovid MEDLINE strategy according to the PRESS Peer Review of Electronic Search Strategies. The full search strategies are available in the Table S2. Search strategies were created for Ovid MEDLINE, Embase (Elsevier), Cochrane Library (Wiley), CINAHL (EBSCOhost), PsycINFO (EBSCOhost), and Dissertations and Theses Global (ProQuest). The searches were not limited by date or language. The searches were conducted on September 9 of 2021. Database records were de‐duplicated in EndNote. We also reviewed reference lists literature pertaining to delirium management in the ED, and gray literature from relevant conference abstracts.
Data management
The articles found in the database were transferred to Rayyan. 31 The data from the articles were extracted in Excel. Citations were stored in EndNote.
Selection process
Three authors (HC, SH, DM) completed the selection process. During the first phase of review, these authors independently assessed the title and abstract of each article to determine if the study met the eligibility criteria. Phase two of study selection was a full manuscript review of those studies that appeared relevant from the first phase. Two primary reviewers (HC, SH) independently reviewed the full manuscript for inclusion or exclusion and a third reviewer (DM) adjudicated discrepancies. A kappa statistic was calculated to measure reviewer interrater reliability. 32
Data collection
A standardized data collection Excel sheet was used by the reviewers to gather the relevant information. The form was developed from the Cochrane Collaboration: Data collection form for intervention review—RCTs and non‐RCTs. 33
Data items
The data collected from the studies include the year of publication, study design, and intervention for delirium prevention and/or treatment. The outcomes that we abstracted include incidence, duration, mortality, Intensive Care Unit (ICU) admission, discharge to skilled nursing facility, quality of life, and long‐term cognitive deficit.
Risk of bias assessment
We used the Cochrane risk of bias (ROB) assessment tool for RCTs, and the Newcastle‐Ottawa (NCO) scale for non‐RCTs. 34 , 35 Two independent reviewers (HC, SH) assessed the ROB, with a third reviewer (SL) resolving any discrepancies.
Summary measures
We reported the last author, study year, study type, study setting, type of intervention, effect size, and ROB. When possible, we estimate the number needed to treat (NNT), 36 using the online calculator, using the defined delirium prevalence in the ED from the GEAR review. 8 As for meta‐analysis, we pooled three studies that employed multifactorial programs using the random effect model and reported a pooled Odds ratio (OR).
RESULTS
Study selection
After initial screening, 29 studies were included in the full manuscript screening. Of the 29 manuscripts reviewed, there were 3 disagreements there were resolved by a third reviewer (DM). The interrater agreement for inclusion or exclusion was good with a kappa of 0.79 (95% CI:0.56–1.0). 32 We identified a total of 10 articles included in this review (Figure 1).
FIGURE 1.
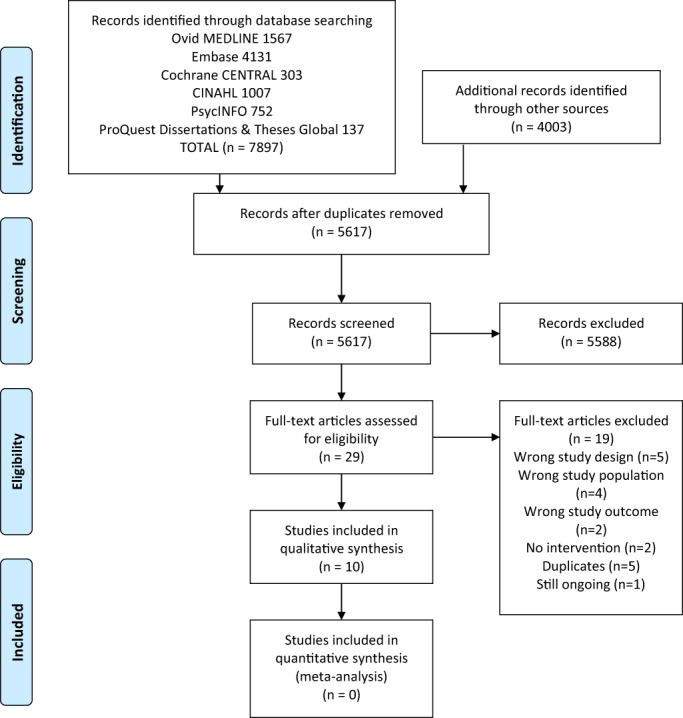
PRISMA flow diagram
Study characteristics
We summarize included studies in Tables 1 and S3. Five studies were only available as abstracts (Table 1). 39 , 40 , 41 , 45 , 46 Two studies were RCTs. 44 , 47 A total of six studies occurred across healthcare settings that included the ED. 38 , 41 , 42 , 44 , 45 , 47 Two RCTs evaluated pharmacologic interventions for delirium prevention, 44 , 47 three non‐RCTs employed a multi‐factorial delirium prevention program, 38 , 41 , 42 three non‐RCTs evaluated regional anesthesia for hip fractures, and one study evaluated the use of Foley catheter, medication exposure, and risk of delirium. 43
TABLE 1.
Intervention, outcomes, and effect size for included studies
| Author/Study year | Study design | Intervention | Preventive/Therapeutic | Delirium outcome | Effect size | Sample size | Delirium raters |
|---|---|---|---|---|---|---|---|
| Al‐Aama, T/2010 37 | RCT | Melatonin versus placebo | Preventative | Incidence defined using CAM and MDAS | OR 0.19 (95% CI 0.06, 0.62) | 122 | Nurses and Caregivers |
| Bjorkelund, K/2010 38 | Cohort | Multi‐factorial | Preventative | Incidence defined using OBS | RR 0.37 (95% CI 0.22, 0.61) | 263 | The researchers |
| Herrman, N/2020 a , 39 | Cohort | FNB | Preventative | Delirium diagnosis | 18.4% versus 15.1% (p = 0.56) | 308 | NA |
| LeBlanc, P/2016 a , 40 | Case control | Femoral nerve block (FNB) | Preventative | Incidence | 38.5% versus 23.1% (p = 0.4) | 29 | NA |
| Mok, W/2017 a , 41 | Cohort | Multi‐factorial | Preventative | Incidence defined using CAM | 10.1% versus “Reported Control” 48% | 494 | NA |
| Naughton, B/2005 42 | Cohort | Multi‐factorial | Preventative | Prevalence (delirium/total number of patients screened) defined using CAM. |
Baseline 45/110 4 months 35/154 9 months 21/110 |
374 | Nurses |
| Noel, C/2019 43 | Case Control | Foley catheter exposure | Therapeutic | Duration incidence defined using bCAM and CAM‐ICU | OR 3.1 (95%CI 1.3, 7.4) | 331 | Trained research assistants |
| Schrijver, E/2018 44 | RCT | Haloperidol versus Placebo | Preventative | Incidence defined DOSS and DRS‐R | OR 1.43 (95% CI 0.72, 2.78) | 242 | Research team members |
| Sun, H/2019 a , 45 | Cohort | Non‐pharmacological | Preventative | Delirium diagnosis | 40.1% pre‐ versus 34.3% post intervention (no raw number) | NA | Charge nurse |
| Yip, D/2019 a , 46 | Cohort | Fascia illiaca block (FIB) | Preventative | Drug induced delirium | 23% versus 26% (p = 0.58) | 282 | NA |
Abbreviations: bCAM, brief confusion assessment method; CAM, confusion assessment method; CAM‐ICU, CAM for the intensive care unit; DOSS, delirium outcome and severity scale; DRS‐R, delirium rating scale‐ revised; MDAS, memorial delirium assessment scale; OBS, organic brain syndrome scale; RCT, randomized controlled trial.
Abstract only.
Risk of bias in studies
ROB was evaluated using the Cochrane ROB tool for two RCTs, which showed the overall ROB as high and low (Figure 2). We evaluated the remainder of the studies with the NCO scale; six of them had high risk, and two had a low ROB (Figure 3).
FIGURE 2.
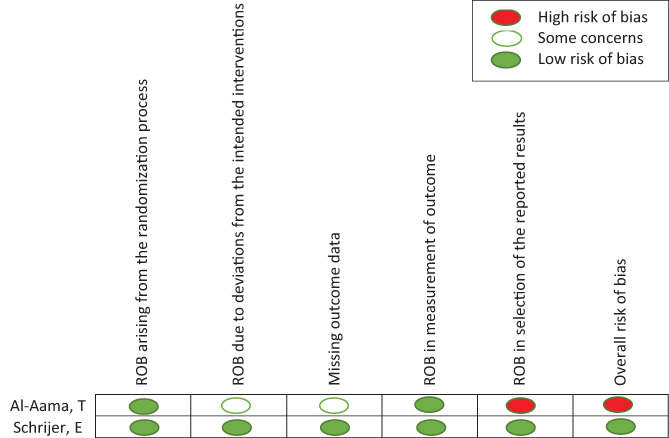
Risk of bias for randomized clinical trials
FIGURE 3.
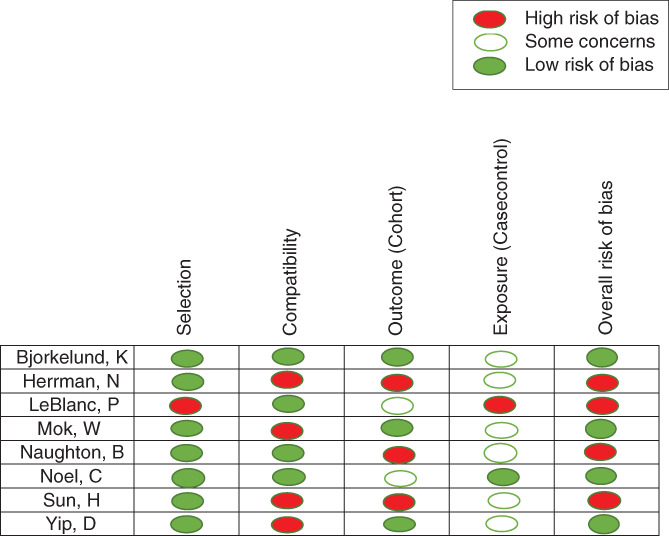
Risk of bias for non‐randomized clinical trials
Results of individual studies
Of the 10 studies included, only four studies demonstrated a significant impact on incident delirium or delirium duration—one RCT of melatonin, two non‐RCT studies on multi‐factorial programs, and one case–control study on the use of Foley catheter. The outcomes related to delirium, type of intervention, and effect size are listed in Table 1. We summarized the findings below.
Delirium prevention studies
Pharmacological
Positive study
Al‐alma et al. examined the impact of melatonin compared to placebo on the prevention of delirium among older adults admitted to the internal medicine unit from the ED. 47 They provided patients with 0.5 mg melatonin at night and prevented delirium with a Number Needed to Treat (NNT) of 6. Two patients (2/72 = 2.8%) who received melatonin reported side effects describing nightmares. However, the overall risk of bias was high (Figure 2).
Negative study
Schrijver et al. evaluated the used of haloperidol compared to placebo on the prevention of delirium among high‐risk older adults (age ≥ 70) hospitalized from the ED. 44 They identified a high‐risk population using the Veiligheidsmanagementsysteem (VMS) delirium risk assessment tool 48 and randomized them to 1 mg prophylactic Haloperidol or placebo. The study did not reach the target sample size, and the results did not show any significant reduction in the incident delirium.
Non‐pharmacological interventions
Positive studies
Naughton et al. evaluated a multifactorial intervention to prevent delirium in their acute geriatric unit (AGU) compared to routine clinical care before the AGU opened in the hospital. This intervention included avoidance of antipsychotics, family support, and treating medical factors. They assessed the prevalence of delirium on day 4 among older adults (age ≥ 75) admitted from the ED. 42 The prevalence of delirium in the unit at the baseline was compared to post‐implementation. The incident delirium on day 4 in AGU decreased from 41% to 19%.
Björkelund et al. investigated a multifactorial intervention to prevent delirium in older patients (age ≥ 65) with hip fractures compared to routine clinical care before the intervention started. 38 Interventions include the use of oxygen, intravenous fluids, increasing vital monitoring, pain relief, and daily screening for delirium. Their intervention decreased incident delirium (RR 0. 37, 95% CI 0.22–0.61).
Sun et al. evaluated the program called EmpowerRing Elder Novel Interventions (ERNI) comparing the effect of ERNI with routine clinical care before implementation of the program on incident delirium in older adults admitted to the ICU, ED observation unit, or inpatient service. 45 The ERNI was a non‐pharmacologic intervention to interact with the patient and provide cognitive stimulating activities. ERNI decreased incident delirium (40.1% pre‐ vs. 34.3% post intervention).
Mok et al. compared a multifactorial intervention with routine clinical care to reduce incident delirium in hip fracture patients admitted from the ED. 41 The intervention included delirium screening, and the proactive use of oxygen, fluid and electrolyte balance, continence management, pain treatment, nutrition, early mobilization, and physical therapy. Control group details were not reported, but the intervention decreased incident delirium (10.1% vs. “Reported Control” 48%).
Regional anesthesia
Negative studies
LeBlanc et al. evaluated the use of a femoral nerve block (FNB) with ultrasound guidance compared to standard pain control to reduce the incidence of delirium in hip fracture patients. 40 The total sample size was 26 for the intervention and control groups, and there was no statistically significant difference in incident delirium rates.
Herman et al. evaluated the use of ultrasound‐guided FNB compared to routine clinical care before implementation and its impact on multiple factors, including the frequency of incident delirium among hip fracture patients in the ED. 39 They looked at data 1 year prior and 1 year after the implementation of the nerve block, and found no significant difference in the incidence of delirium.
Yip et al. evaluated the use of ultrasound‐guided fascia‐illiaca block (FIB) compared to routine clinical care for hip fracture patients and its impact on medication induced delirium in the ED. 46 The study compared two groups of hip fracture patients; one group received the FIB and the other group received morphine. They demonstrated no significant difference in the incidence of medication induced delirium between the two groups.
Delirium treatment study
Non‐pharmacological intervention
Positive study
Noel et al. compared the use of Foley catheters and high‐risk medications on delirious and non‐delirious older patients in the ED. 43 They quantified the association between Foley catheter use and high‐risk medications with delirium duration measured. Foley catheter placement in the ED correlated with an increased duration of delirium (adjusted proportional OR 3.1, 95% CI 1.3–7.4, NNT 4). The mean difference in delirium duration was 1.6 days longer with insertion of ED Foley catheters after adjusting for high‐risk medications.
Meta‐analysis of multifactorial programs for delirium prevention
Next, we conducted a meta‐analysis of three studies (Naughton et al., 42 Björkelund et al., 38 and Sun et al. 45 ) which reported the 2 by 2 table to estimate the effect of delirium prevention. It was not feasible to include Mok et al. 41 as non‐exposure data were missing. Using the random effect model, a pooled OR was 0. 46(95% CI 0.31–0.68), I2 = 0% (Figure 4).
FIGURE 4.
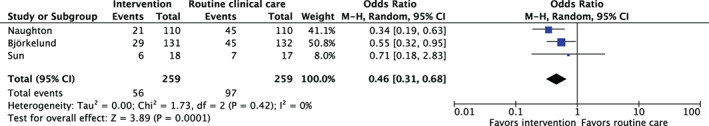
A pooled effect estimates for multifactorial programs to prevent delirium
DISCUSSION
Despite recommendations from the American Geriatrics Society and Society for Academic Emergency Medicine to prioritize delirium prevention and therapeutic research 15‐years ago, emergency medicine (EM) still has only consensus‐based recommendations upon which to formulate delirium treatment protocols. 37 , 49 Since 2012 seven Cochrane reviews have synthesized delirium interventions across a variety of patient populations and clinical settings, but none included study findings from the ED. 14 , 15 , 16 , 17 , 18 , 19 , 20 The Geriatric Emergency Care Applied Research (GEAR) Network reviewed a similar question, and did not identify any ED‐based research upon which to base prevention or intervention interventions. 8 Our systematic review adds to these prior publications by including interventions started in the ED, as opposed to interventions only taking place in the ED. Of the studies included, only four demonstrated any measurable impact on the incidence or duration of delirium. Effective delirium‐prevention interventions included one study using bedtime melatonin, another employing non‐pharmacological interventions, and the avoidance of Foley catheters. 38 , 42 , 43 , 47 Specifically, we identified just one actionable pharmacological finding based on a single study that may be implemented by EDs to decrease delirium frequency or duration: low dose, nighttime use of melatonin to decrease incident delirium with a small risk of nightmares and change in sensorium. In general, ED boarding should be avoided altogether to mitigate onset of delirium, but whether extrapolation of nocturnal melatonin to at‐risk populations stuck overnight in the ED is either viable or effective remains untested. 50 Multifactorial programs, which focus on modifying recognized deliriogenic factors such as high‐risk medications, pain, and immobilization in nonrandomized studies, were also associated with decreased incident delirium. Older adults with hip fractures appear to be a target for intervention. Interventions studied to date, such as regional anesthesia, have demonstrated a significant impact on pain reduction and opioid use, but failed to translate into delirium prevention. 51
Though it is recommended that pharmacologic management of delirium is reserved for those at immediate risk of harming themselves or others, antipsychotics medications and sedatives are commonly used to treat delirium in the ED setting. 52 Unfortunately, neither the potential benefits nor harms of these pharmacological strategies are rigorously based on any ED research. Haloperidol was found to be ineffective in preventing delirium in ED patients at high risk for delirium. 53 This is consistent with previous meta‐analyses that discourage the use of haloperidol for delirium treatment of prevention in non‐ED settings 15 , 16 , 19 , 53 and thus our systematic review does not support the safety or effectiveness of prophylactic haloperidol to prevent delirium.
Melatonin was the only other medication identified in our systematic review to prevent delirium in older ED patients. The finding that low‐dose melatonin could potentially prevent delirium is consistent with prior studies by Hatta et al. 54 and a meta‐analysis by Campbell et al. 55 Further research is needed to explore whether this would be helpful for ED patients arriving during daylight hours, or those boarding overnight in the ED. With these caveats, our review raises the question of using melatonin for patients at risk of delirium who have to board in the ED overnight. Compelling research from the inpatient and ICU settings suggest that multifactorial programs can prevent delirium. 56 , 57 , 58 , 59 Those include the Hospital Elder Life Program implemented in inpatient units,. 58 , 59 and the ABCDEF bundle in ICU. 57 We identified four non‐RCT studies on multi‐factorial programs and one case–control study on the use of a Foley catheter and medication exposures. 38 , 41 , 42 , 43 , 45 Our review did not find any RCTs using multi‐factorial programs. Because we found very limited evidence from ED‐based interventions, multi‐factorial programs await evaluation in the ED setting and should evaluate outcomes beyond the incidence of delirium, such as antipsychotic use, delirium severity, and delirium resolution. Also, many older adults with delirium or at increased risk of delirium may be unable to consent raising ethical conduct of research concerns. 60 One approach is to target delirium susceptibility factors that are common in the ED, such as impaired sleep and dehydration, or identifying a subset of older ED patients at highest risk of developing delirium from the environment and sensory stimuli in the ED, such as those with cognitive impairment and frailty. 8
Under‐treated pain is a common factor in delirium so older adults with hip fractures may be a target population for prevention and intervention 61 , 62 , 63 ; however, we did not identify oligoanalgesia interventions that reduced incident delirium. Ritcey et al. conducted a systematic review and concluded that those who received a FNB reported less pain and decreased opioid requirement, but the study was not powered to determine whether incident delirium decreased. 64 ED Ultrasonographic Regional Anesthesia to Prevent Incident Delirium in Hip Fracture Patients, (EDU‐RAPID), a large Canadian ED RCT), is currently underway. 65 There is not yet sufficient evidence to support or refute the use of regional anesthesia for hip fracture to reduce delirium duration or incident delirium. These findings should not discourage the use of regional anesthesia, as the primary outcome of current studies effectively reduced parenteral opioids and opioids‐related adverse events while safely providing superior analgesia.
Limitations
First, we were unable to conduct a meta‐analysis for any intervention or outcome as no two studies assessed an identical intervention, even when including those that used multi‐ interventions and the same outcome assessment. We believe this review still has value as our search strategy and selection were exhaustive and provide historical and contemporary context to often overlooked ED‐based delirium interventional research. Second, several abstracts failed to report statistical significance and effect size other than the p‐value. To mitigate this, we the contacted original study authors to clarify ambiguities, but received no additional details. 66 Third, seven studies used mixed study settings with the ED as only one of those settings, so the benefits or harms attributable to the ED portion of the intervention remain unclear. This limitation was anticipated as delirium care encompasses ED to inpatient.
Future recommendation for research
The multicomponent nature of both the causes and presentations of delirium make it a subject of scientific inquiry. 57 , 67 We highlight several possible solutions to address these difficulties. Investigations could initial aim to more carefully characterize the etiology and subtypes of delirium with more selective inclusion criteria to ensure that the interventions more precisely match the patient population's specific delirium risk factors (e.g., explanatory trials). 8 This would be well suited to an RCT, matched case control, and propensity matching. Alternatively, EM investigations could learn from non‐ED researchers by embracing the multifarious nature of delirium and implementing similarly multifactorial interventions. Use of trained volunteers similar to those employed in the Hospital Elder Life Program are one potential solution to resource constraints commonly seen in the EDs to implement such a program. 68 Multicomponent approaches, harmonized delirium assessment instruments, pragmatic study design, and implementation science will be important to address these theoretical constraints.
Delirium outcomes specific to EM have been limited to incidence, prevalence, and duration for 30 years. 2 , 4 , 7 Rose et al. published a study protocol for delirium core outcomes, which used systematic review and Delphi method to define these outcomes. 69 In addition, GEAR's highest priority research topics were ED delirium prevention and interventions that are not reliant on additional nurse or physician efforts and defining etiologic delirium phenotypes who should be targeted for prevention and intervention strategies. 8 As ED research focuses on delirium prevention and intervention, we foresee the incorporation of delirium severity, delirium subtypes and phenotypes, and a core set of outcomes as proposed by Oh et al 37 as essential components of impactful EM research in the future. Surprisingly, we did not find any experimental study testing the control of the length of stays in the ED and its effect on delirium. A recent movement on the age‐friendly health system might impact delirium‐related outcomes in the ED, and setting a policy for LOS may be an interesting intervention. Lastly, the consequences of delirium often extend beyond the ED into the hospital or ICU, so delirium management is by necessity a multidisciplinary task which will require coordination, transdisciplinary funding opportunities, and team science to accelerate the identification of effective and ultimately efficacious interventions. 60
CONCLUSION
Our systematic review found little evidence of trials that evaluated delirium prevention and treatment in the ED setting, as about half of our findings were from abstracts. The use of melatonin in the selected indications, such as overnight stay in the ED, could be tested. Multi‐factorial programs show the most promise in reducing delirium‐related outcomes. Still, sustained, multi‐center research is required to assess whether such interventions, whether the entire program or a part of it, are feasible and effective in the ED. Older adults with hip fracture appear to be a target population in which investigators used both pharmacological and non‐pharmacological interventions; however, interventions studied to date have not demonstrated a significant impact. The prevention and treatment of delirium is an opportunity for the improvement of patient care in the ED and requires further research.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHOR CONTRIBUTIONS
Sangil Lee designed the study, executed, and prepared manuscript. Daniel Miller, Hao Chen, Seikei Hibino conducted review, selection of articles, extracted data, and revised manuscript. Heather Healy conducted the literature search. Glenn Arendts, Jacques S. Lee, Jin Ho Han, and Maura Kennedy designed the study and revised manuscript. Christopher R Carpenter designed the study and revised the manuscript. All authors reviewed manuscript and approved.
SPONSOR'S ROLE
Not applicable.
Supporting information
Table S1. A list of Cochrane reviews examining delirium intervention outside of ED since 2012.
Table S2. Ovid MEDLINE strategy.
Table S3. Summary of selected articles.
Lee S, Chen H, Hibino S, et al. Can we improve delirium prevention and treatment in the emergency department? A systematic review. J Am Geriatr Soc. 2022;70(6):1838‐1849. doi: 10.1111/jgs.17740
REFERENCES
- 1. Hustey FM, Meldon SW, Smith MD, Lex CK. The effect of mental status screening on the care of elderly emergency department patients. Ann Emerg Med. 2003;41(5):678‐684. doi: 10.1067/mem.2003.152 [DOI] [PubMed] [Google Scholar]
- 2. Han JH, Zimmerman EE, Cutler N, et al. Delirium in older emergency department patients: recognition, risk factors, and psychomotor subtypes. Acad Emerg Med. 2009;16(3):193‐200. doi: 10.1111/j.1553-2712.2008.00339.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Han JH, Shintani A, Eden S, et al. Delirium in the emergency department: an independent predictor of death within 6 months. Ann Emerg Med. 2010;56(3):244‐252.e1. doi: 10.1016/j.annemergmed.2010.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lewis LM, Miller DK, Morley JE, Nork MJ, Lasater LC. Unrecognized delirium in ED geriatric patients. Am J Emerg Med. 1995;13(2):142‐145. doi: 10.1016/0735-6757(95)90080-2 [DOI] [PubMed] [Google Scholar]
- 5. Suffoletto B, Miller T, Frisch A, Callaway C. Emergency physician recognition of delirium. Postgrad Med J. 2013;89(1057):621‐625. doi: 10.1136/postgradmedj-2012-131608 [DOI] [PubMed] [Google Scholar]
- 6. Evensen S, Saltvedt I, Ranhoff AH, et al. Delirium and cognitive impairment among older patients in Norwegian emergency departments. Tidsskr Nor Laegeforen. 2019;139(6). doi: 10.4045/tidsskr.18.0578 [DOI] [PubMed] [Google Scholar]
- 7. Kennedy M, Enander RA, Tadiri SP, Wolfe RE, Shapiro NI, Marcantonio ER. Delirium risk prediction, healthcare use and mortality of elderly adults in the emergency department. J Am Geriatr Soc. 2014;62(3):462‐469. doi: 10.1111/jgs.12692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carpenter CR, Hammouda N, Linton EA, et al. Delirium prevention, detection, and treatment in emergency medicine settings: a geriatric emergency care applied research (GEAR) network scoping review and consensus statement. Acad Emerg Med. 2021;28(1):19‐35. doi: 10.1111/acem.14166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kakuma R, du Fort GG, Arsenault L, et al. Delirium in older emergency department patients discharged home: effect on survival. J Am Geriatr Soc. 2003;51(4):443‐450. doi: 10.1046/j.1532-5415.2003.51151.x [DOI] [PubMed] [Google Scholar]
- 10. Hsieh SJ, Madahar P, Hope AA, Zapata J, Gong MN. Clinical deterioration in older adults with delirium during early hospitalisation: a prospective cohort study. BMJ Open. 2015;5(9):e007496. doi: 10.1136/bmjopen-2014-007496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arendts G, Love J, Nagree Y, Bruce D, Hare M, Dey I. Rates of delirium diagnosis do not improve with emergency risk screening: results of the emergency department delirium initiative trial. J Am Geriatr Soc. 2017;65(8):1810‐1815. doi: 10.1111/jgs.14904 [DOI] [PubMed] [Google Scholar]
- 12. Giroux M, Émond M, Nadeau A, et al. Functional and cognitive decline in older delirious adults after an emergency department visit. Age Ageing. 2021;50(1):135‐140. doi: 10.1093/ageing/afaa128 [DOI] [PubMed] [Google Scholar]
- 13. Kennedy M, Lesser A, Israni J, et al. Reach and adoption of a geriatric emergency department accreditation program in the United States. Ann Emerg Med. 2021. doi: 10.1016/j.annemergmed.2021.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Woodhouse R, Burton JK, Rana N, Pang YL, Lister JE, Siddiqi N. Interventions for preventing delirium in older people in institutional long‐term care. Cochrane Database Syst Rev. 2019;4:CD009537. doi: 10.1002/14651858.CD009537.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalised non‐ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594. doi: 10.1002/14651858.CD005594.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herling SF, Greve IE, Vasilevskis EE, et al. Interventions for preventing intensive care unit delirium in adults. Cochrane Database Syst Rev. 2018;11:CD009783. doi: 10.1002/14651858.CD009783.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non‐ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494. doi: 10.1002/14651858.CD012494.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Punjasawadwong Y, Chau‐In W, Laopaiboon M, Punjasawadwong S, Pin‐On P. Processed electroencephalogram and evoked potential techniques for amelioration of postoperative delirium and cognitive dysfunction following non‐cardiac and non‐neurosurgical procedures in adults. Cochrane Database Syst Rev. 2018;5:CD011283. doi: 10.1002/14651858.CD011283.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Siddiqi N, Harrison JK, Clegg A, et al. Interventions for preventing delirium in hospitalised non‐ICU patients. Cochrane Database Syst Rev. 2016;3:CD005563. doi: 10.1002/14651858.CD005563.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Candy B, Jackson KC, Jones L, Leurent B, Tookman A, King M. Drug therapy for delirium in terminally ill adult patients. Cochrane Database Syst Rev. 2012;11:CD004770. doi: 10.1002/14651858.CD004770.pub2 [DOI] [PubMed] [Google Scholar]
- 21. Dahlstrom EB, Han JH, Healy H, et al. Delirium prevention and treatment in the emergency department (ED): a systematic review protocol. BMJ Open. 2020;10(10):e037915. doi: 10.1136/bmjopen-2020-037915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nikooie R, Neufeld KJ, Oh ES, et al. Antipsychotics for treating delirium in hospitalized adults: a systematic review. Ann Intern Med. 2019;171(7):485‐495. doi: 10.7326/M19-1860 [DOI] [PubMed] [Google Scholar]
- 23. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rahman A, Paul M. Delirium Tremens. StatPearls. StatPearls Publishing; 2021. [PubMed] [Google Scholar]
- 25. Lee S, Harland K, Mohr NM, et al. Evaluation of emergency department derived delirium prediction models using a hospital‐wide cohort. J Psychosom Res. 2019;127:109850. doi: 10.1016/j.jpsychores.2019.109850 [DOI] [PubMed] [Google Scholar]
- 26. Kim DH, Lee J, Kim CA, et al. Evaluation of algorithms to identify delirium in administrative claims and drug utilization database. Pharmacoepidemiol Drug Saf. 2017;26(8):945‐953. doi: 10.1002/pds.4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sands MB, Dantoc BP, Hartshorn A, Ryan CJ, Lujic S. Single question in delirium (SQiD): testing its efficacy against psychiatrist interview, the confusion assessment method and the memorial delirium assessment scale. Palliat Med. 2010;24(6):561‐565. doi: 10.1177/0269216310371556 [DOI] [PubMed] [Google Scholar]
- 28. Bellelli G, Morandi A, Davis DHJ, et al. Corrigendum to “validation of the 4AT, a new instrument for rapid delirium screening: a study in 234 hospitalised older people”. Age Ageing. 2015;44(1):175. doi: 10.1093/ageing/afu181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fick DM, Inouye SK, Guess J, et al. Preliminary development of an ultrabrief two‐item bedside test for delirium. J Hosp Med. 2015;10(10):645‐650. doi: 10.1002/jhm.2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Han JH, Wilson A, Vasilevskis EE, et al. Diagnosing delirium in older emergency department patients: validity and reliability of the delirium triage screen and the brief confusion assessment method. Ann Emerg Med. 2013;62(5):457‐465. doi: 10.1016/j.annemergmed.2013.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan‐a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Byrt T. How good is that agreement? Epidemiology. Published online September 7, 1996;(5):561. doi: 10.1097/00001648-199609000-00030 [DOI] [PubMed] [Google Scholar]
- 33. Cochrane Data collection form for intervention reviews, version 3. 2014. https://dplp.cochrane.org/data-extraction-forms
- 34. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wells GA, Shea B, O'connell D, et al. The Newcastle‐Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta‐Analyses. 2015. Accessed June 2, 2021. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 36. Gogtay NJ, Thatte UM. Number needed to treat. J Assoc Physicians India. 2017;65(8):90‐94. [PubMed] [Google Scholar]
- 37. Oh ES, Akeju O, Avidan MS, et al. A roadmap to advance delirium research: recommendations from the NIDUS scientific think tank. Alzheimers Dement. 2020;16(5):726‐733. doi: 10.1002/alz.12076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Björkelund KB, Hommel A, Thorngren KG, Gustafson L, Larsson S, Lundberg D. Reducing delirium in elderly patients with hip fracture: a multi‐factorial intervention study. Acta Anaesthesiol Scand. 2010;54(6):678‐688. doi: 10.1111/j.1399-6576.2010.02232.x [DOI] [PubMed] [Google Scholar]
- 39. Herrman N, Majkrzak A, Seleno N. Impact of a femoral nerve block guideline for hip fractures in a community emergency department. Acad Emerg Med. 2020;27(Suppl 1):S7‐S335. doi: 10.1111/acem.13961 [DOI] [PubMed] [Google Scholar]
- 40. LeBlanc P, Boucher V, Émond M, Courtemanche J, Ménassa M, Lee JS. P076: delirium prevention in the emergency department using regional anesthesia with ultrasound guidance in the elderly population with hip fracture: a pilot study. CJEM. 2016;18(S1):S103‐S104. doi: 10.1017/cem.2016.252 [DOI] [Google Scholar]
- 41. Mok WQ, Jagadish UM, Yiap PL, Yu LH, Lim SM, Ker SY. Implementation of an integrated delirium prevention system of care for elderly patients with hip fractures. Int J Integr Care. 2017;17(5):432. doi: 10.5334/ijic.3752 [DOI] [Google Scholar]
- 42. Naughton BJ, Saltzman S, Ramadan F, Chadha N, Priore R, Mylotte JM. A multifactorial intervention to reduce prevalence of delirium and shorten hospital length of stay. J Am Geriatr Soc. 2005;53(1):18‐23. doi: 10.1111/j.1532-5415.2005.53005.x [DOI] [PubMed] [Google Scholar]
- 43. Noel CB, Cirbus JR, Han JH. Emergency department interventions and their effect on delirium's natural course: the folly may be in the Foley. J Emerg Trauma Shock. 2019;12(4):280‐285. doi: 10.4103/JETS.JETS_137_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schrijver EJM, de Vries OJ, van de Ven PM, et al. Haloperidol versus placebo for delirium prevention in acutely hospitalised older at risk patients: a multi‐Centre double‐blind randomised controlled clinical trial. Age Ageing. 2018;47(1):48‐55. doi: 10.1093/ageing/afx124 [DOI] [PubMed] [Google Scholar]
- 45. Sun H, Zweig Y, Perskin M, Cunningham C, Sullivan R, Blachman N. Empowering elder novel interventions for delirium prevention. J Am Geriatr Soc. 2019;67(S1):S1‐S384. doi: 10.1111/jgs.15898 [DOI] [Google Scholar]
- 46. Yip D, Perry KJ, Fields JM. Association of Ultrasound‐Guided Fascia Iliaca Block on decreasing opiates for patients with hip fractures. Acad Emerg Med. 2019;26(Suppl 1):S9‐S318. doi: 10.1111/acem.13756 [DOI] [PubMed] [Google Scholar]
- 47. Al‐Aama T, Brymer C, Gutmanis I, Woolmore‐Goodwin SM, Esbaugh J, Dasgupta M. Melatonin decreases delirium in elderly patients: a randomized, placebo‐controlled trial. Int J Geriatr Psychiatry. 2011;26(7):687‐694. doi: 10.1002/gps.2582 [DOI] [PubMed] [Google Scholar]
- 48. Heim N, van Fenema EM, Weverling‐Rijnsburger AWE, et al. Optimal screening for increased risk for adverse outcomes in hospitalised older adults. Age Ageing. 2015;44(2):239‐244. doi: 10.1093/ageing/afu187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Carpenter CR, Shah MN, Hustey FM, Heard K, Gerson LW, Miller DK. High yield research opportunities in geriatric emergency medicine: prehospital care, delirium, adverse drug events, and falls. J Gerontol A Biol Sci Med Sci. 2011;66(7):775‐783. doi: 10.1093/gerona/glr040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bo M, Bonetto M, Bottignole G, et al. Length of stay in the emergency department and occurrence of delirium in older medical patients. J Am Geriatr Soc. 2016;64(5):1114‐1119. doi: 10.1111/jgs.14103 [DOI] [PubMed] [Google Scholar]
- 51. Lee JS, Bhandari T, Simard R, et al. Point‐of‐care ultrasound‐guided regional anaesthesia in older ED patients with hip fractures: a study to test the feasibility of a training programme and time needed to complete nerve blocks by ED physicians after training. BMJ Open. 2021;11(7):e047113. doi: 10.1136/bmjopen-2020-047113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kennedy M, Koehl J, Shenvi CL, et al. The agitated older adult in the emergency department: a narrative review of common causes and management strategies. J Am College Emergency Phys Open. 2020;1(5):812‐823. doi: 10.1002/emp2.12110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen Z, Chen R, Zheng D, et al. Efficacy and safety of haloperidol for delirium prevention in adult patients: an updated meta‐analysis with trial sequential analysis of randomized controlled trials. J Clin Anesth. 2020;61:109623. doi: 10.1016/j.jclinane.2019.09.017 [DOI] [PubMed] [Google Scholar]
- 54. Hatta K, Kishi Y, Wada K, et al. Preventive effects of ramelteon on delirium: a randomized placebo‐controlled trial. JAMA Psychiat. 2014;71(4):397‐403. doi: 10.1001/jamapsychiatry.2013.3320 [DOI] [PubMed] [Google Scholar]
- 55. Campbell AM, Axon DR, Martin JR, Slack MK, Mollon L, Lee JK. Melatonin for the prevention of postoperative delirium in older adults: a systematic review and meta‐analysis. BMC Geriatr. 2019;19(1):272. doi: 10.1186/s12877-019-1297-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Barnes‐Daly MA, Phillips G, Ely EW. Improving hospital survival and reducing brain dysfunction at seven California community hospitals: implementing PAD guidelines via the ABCDEF bundle in 6,064 patients. Crit Care Med. 2017;45(2):171‐178. doi: 10.1097/CCM.0000000000002149 [DOI] [PubMed] [Google Scholar]
- 57. Ely EW. The ABCDEF bundle: science and philosophy of how ICU liberation serves patients and families. Crit Care Med. 2017;45(2):321‐330. doi: 10.1097/CCM.0000000000002175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Inouye SK, Bogardus ST, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669‐676. doi: 10.1056/NEJM199903043400901 [DOI] [PubMed] [Google Scholar]
- 59. Hshieh TT, Yang T, Gartaganis SL, Yue J, Inouye SK. Hospital elder life program: systematic review and meta‐analysis of effectiveness. Am J Geriatr Psychiatry. 2018;26(10):1015‐1033. doi: 10.1016/j.jagp.2018.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Carpenter CR, McFarland F, Avidan M, et al. Impact of cognitive impairment across specialties: summary of a report from the U13 conference series. J Am Geriatr Soc. 2019;67(10):2011‐2017. doi: 10.1111/jgs.16093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58(1):76‐81. doi: 10.1093/gerona/58.1.m76 [DOI] [PubMed] [Google Scholar]
- 62. Sampson EL, West E, Fischer T. Pain and delirium: mechanisms, assessment, and management. Eur Geriatr Med. 2020;11(1):45‐52. doi: 10.1007/s41999-019-00281-2 [DOI] [PubMed] [Google Scholar]
- 63. Daoust R, Paquet J, Boucher V, Pelletier M, Gouin É, Émond M. Relationship between pain, opioid treatment, and delirium in older emergency department patients. Acad Emerg Med. 2020;27(8):708‐716. doi: 10.1111/acem.14033 [DOI] [PubMed] [Google Scholar]
- 64. Ritcey B, Pageau P, Woo MY, Perry JJ. Regional nerve blocks for hip and femoral neck fractures in the emergency department: a systematic review. CJEM. 2016;18(1):37‐47. doi: 10.1017/cem.2015.75 [DOI] [PubMed] [Google Scholar]
- 65. ED Ultrasonographic Regional Anesthesia to Prevent Incident Delirium in Hip Fracture Patients ‐ Full Text View ‐ ClinicalTrials.gov. Accessed May 27, 2021. https://clinicaltrials.gov/ct2/show/NCT02892968
- 66. Mullan RJ, Flynn DN, Carlberg B, et al. Systematic reviewers commonly contact study authors but do so with limited rigor. J Clin Epidemiol. 2009;62(2):138‐142. doi: 10.1016/j.jclinepi.2008.08.002 [DOI] [PubMed] [Google Scholar]
- 67. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456‐1466. doi: 10.1056/NEJMcp1605501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Inouye SK. Prevention of delirium in hospitalized older patients: risk factors and targeted intervention strategies. Ann Med. 2000;32(4):257‐263. doi: 10.3109/07853890009011770 [DOI] [PubMed] [Google Scholar]
- 69. Rose L, Agar M, Burry LD, et al. Development of core outcome sets for effectiveness trials of interventions to prevent and/or treat delirium (Del‐COrS): study protocol. BMJ Open. 2017;7(9):e016371. doi: 10.1136/bmjopen-2017-016371 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. A list of Cochrane reviews examining delirium intervention outside of ED since 2012.
Table S2. Ovid MEDLINE strategy.
Table S3. Summary of selected articles.


