Abstract
Cytotoxic CD8+ T cells are a key element of the adaptative immune system to protect the organism against infections and malignant cells. During their activation and response, T cells undergo different metabolic pathways to support their energetic needs according to their localization and function. However, it has also been recently appreciated that this metabolic reprogramming also directly supports T‐cell lineage differentiation. Accordingly, metabolic deficiencies and prolonged stress exposure can impact T‐cell differentiation and skew them into an exhausted state. Here, we review how metabolism defines CD8+ T‐cell differentiation and function. Moreover, we cover the principal metabolic dysregulation that promotes the exhausted phenotype under tumor or chronic virus conditions. Finally, we summarize recent strategies to reprogram impaired metabolic pathways to promote CD8+ T‐cell effector function and survival.
Keywords: T cells, metabolism, T‐cell differentiation, T‐cell exhaustion, infection
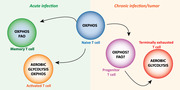
Introduction
CD8+ T cells are one of the most crucial components of the adaptive immune system, and play a key role in response to pathogens and tumors. Upon antigen stimulation, naive CD8+ T cells get activated and differentiate into effector cells and a small subset of memory cells can form after antigen clearance. Emerging evidences support that metabolic reprogramming not only provides energy and biomolecules to support pathogen clearance, but is also tightly linked to T‐cell differentiation [1, 2]. The dynamic metabolic profiles and the exposure to pathological microenvironmental impact CD8+ T‐cell functions and differentiation program. Metabolic programming is, thus, considered to be one of driving players to regulate T‐cell biology and to ultimately tailor the adaptive immune protection. In this review, we summarize the metabolic profiles in CD8+ T cells and highlight the metabolic switch between different differentiation states. In addition, we cover current knowledge about metabolic dysregulation that supports the establishment of the exhaustion state in response to chronic antigen exposure.
Metabolic reprogramming during CD8+ T‐cell activation and differentiation
Overview of metabolic profiles in differentiation states
Naive CD8+ T cells are activated in response to the coordination of three signals, including TCR (signal 1), costimulation (signal 2), and inflammatory cytokines (signal 3) [3]. After activation, those pathogen‐specific CD8+ T cells undergo massive expansion and differentiation into effector T cells, which contribute directly to pathogen clearance. Then, the majority of CD8+ T cells undergo contraction phase and die by apoptosis. Eventually, a small percentage of T cells survive and form different memory subsets, which can provide long‐term protection [4]. Interestingly, emerging evidence reveals that different differentiation states of CD8+ T cells require distinct metabolic supplies to support their bioenergetic demands and epigenetic programming. In general, naive CD8+ T cells are maintained in a quiescent phenotype, which is characterized by low metabolic activities and catabolic metabolism [5]. Since naive CD8+ T cells have limited demands for biomolecular production, they predominantly rely on mitochondrial OXPHOS to produce ATP and sustain homeostasis [6, 7]. The long‐term survival and metabolic regulation of naive CD8+ T cells depend on signals induced by self‐ligands/TCR and IL‐7 [8, 9, 10]. To prevent the atrophy of quiescent T cells, IL7‐IL7R signaling promotes basal glucose catabolism by regulating the expression of glucose transporter 1 (GLUT1) through activation of AKT, which plays a central role in glucose uptake [11, 12]. Glucose then acts as the crucial nutrient to fuel oxidative phosphorylation (OXPHOS) (Fig. 1).
Figure 1.
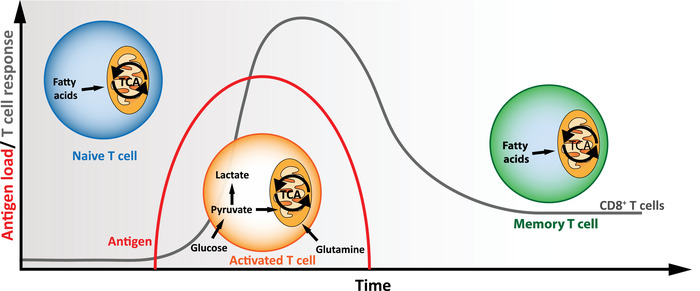
Metabolic profiles of CD8+ T cells during an immune response. Upon acute infection, the activation of naive CD8+ T cells triggers massive expansion and differentiation into effector T cells, which contribute directly to pathogen clearance. TCR signaling stimulates aerobic glycolysis to support the intense cell proliferation. Following the elimination of the antigen, the majority of CD8+ T cells undergoes the contraction phase. However, a small percentage of T cells survive and form memory subsets, which can provide long‐term protection. These cells rely on fatty‐acid oxidation and oxidative phosphorylation for their maintenance.
Upon acute infection, pathogen‐specific CD8+ T cells undergo activation and expansion with metabolic transition from catabolism to anabolism, where extracellular nutrients are utilized to promote biosynthesis and ATP production for proliferation and effector function [13, 14]. These metabolic features are characterized by the increase of aerobic glycolysis, glutaminolysis, as well as mitochondrial biogenesis [15] (Fig. 1). During this process, glucose is utilized for aerobic glycolysis and pentose phosphate pathway to facilitate synthesis of amino acid, NADPH, nucleotide, and ribosome. Moreover, the metabolites from glycolysis and glutaminolysis enter tricarboxylic acid cycle, which supports mitochondrial metabolism and OXPHOS, and mitochondrial dynamics also instruct T‐cell differentiation program [16]. Of note, emerging works reveal that metabolic programs intertwine with intracellular signaling to tailor the functions of effector CD8 + T cells [14, 17] (Fig. 2). TCR engagement combined with CD28 costimulation triggers PI3K‐AKT signaling, which in turn, facilitates GLUT1‐controlled glucose uptake [18, 19]. The expression of GLUT1 is also upregulated by transcription factors such as Nuclear Factor of activated T‐cells 1 (NFAT1) [20]. AKT can further induces the activity of mammalian Target of Rapamycin (mTOR), the central driver of anabolism, to promote the glycolytic flux by its downstream transcription factors [17, 21]. Diverse glycolytic enzymes, including hexokinase 2 and pyruvate dehydrogenase, are also tuned following activation [20, 22]. Amino acids promote T cell activation and effector features through multiple mechanisms. First, increased glutamine uptake and glutaminolysis catabolize the glutamine to support essential biosynthesis [23]. Among the glutamine transporters, Slc32a1 and Slc32a2 are upregulated via transcription factor Myc in an AKT‐mTOR dependent manner. Second, system L transporter Slc7a5 (also known as LAT1) controls the uptake of large neutral amino acid, such as leucine uptake, and supports protein synthesis in T cells. The loss of Slc7a5 and deprivation of leucine impair the activation of mTOR and expression of c‐Myc, which results in impairment of effector T‐cell differentiation [23]. Third, methionine uptake is essential to sustain S‐adenosylmethionine synthesis, which provide substrate for DNA and histone methylations and subsequently impact on epigenetic program during T‐cell differentiation [24].
Figure 2.
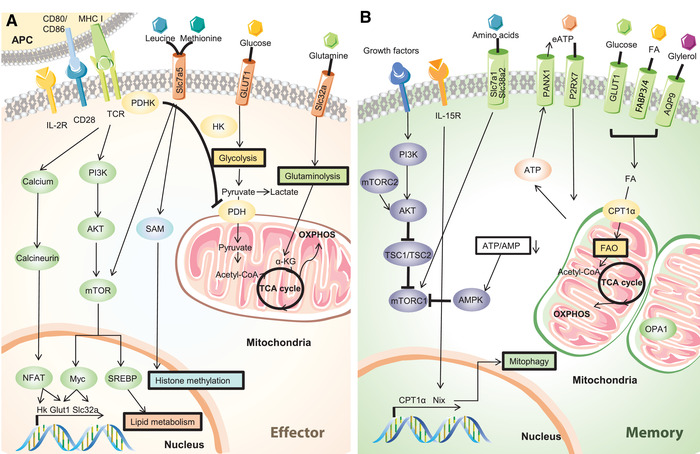
Metabolic reprogramming in CD8+ T‐cell activation and memory formation. (A) Metabolic changes in CD8+ T cells upon activation. Upon antigen recognition, stimulatory signals T‐cell receptor (TCR) and costimulation CD28 induce the activation of PI3K‐AKT pathway, mTOR, and the calcium release in the cytosol. This pathway result in the upregulation of the transcription factors NFAT and Myc that promote glycolysis and glutaminolysis. (B) Metabolic regulation during memory CD8+ T‐cell formation. After antigen clearance, mTORC1 is inhibited which leads to the downregulation of aerobic glycolysis. Fatty acids are used as main substrates for energy production: fatty acids enter the mitochondria through CPT1‐α and are transformed in acetyl‐CoA by fatty‐acid oxidation. Acetyl‐CoA enters then the tricarboxylic acid cycle to support the oxidative phosphorylation and energy production. Mitochondrial fitness is also closely regulated by proteins involved in mitophagy like Nix and also by inner mitochondrial membrane protein Optic atrophy 1 (OPA1) that promotes mitochondrial fusion.
During the contraction phase, some antigen‐specific CD8 + T cells differentiate into memory phenotype and enter a more quiescent state, which displays catabolic metabolism. However, memory CD8 + T cells are metabolically different from naive CD8 + T cell, which has been speculated to allow different metabolic niche and support a stronger reaction after re‐encountering the same antigen [15, 25, 26]. The survival of memory cells is maintained by IL‐7 and IL‐15, as well as mitochondrial metabolism [27, 28, 29] (Fig. 2). Previous studies in mouse models showed that memory cells present elevated spare respiratory capacity, which is the extra amount of ATP that can be produced by OXPHOS upon high energetic demand, increased mitochondrial content, and altering cristae that favor the proximity of electron transport chain proteins in memory CD8 + T cells [16, 27]. Additionally, IL‐15 signaling facilitates the expression of carnitine palmitoyltransferase Ia (CPT1a), which is a rate‐limiting enzyme that transports fatty acid into mitochondria and promotes fatty‐acid oxidation [27]. Of note, the contribution of CPT1a in supporting memory T‐cell survival remains to be confirmed due to the potential off‐target effect caused by etomoxir [30]. Meanwhile, arginine metabolism plays an important role in regulating memory T‐cell differentiation [31]. T cells cultured with increased l‐arginine exhibit reduced glycolysis, increased OXPHOS, and central memory phenotype, which are mediated in part by arginine sensors in the nucleus [31].
Since mitochondria controls numerous metabolic programs that can impact T‐cell activation and differentiation, it is reasonable to imagine that regulatory circuit controlling mitochondrial dynamics has to be fined tuned for supporting T‐cell activation and differentiation. In support of this, dynamin‐related protein 1 (DRP1) that controls mitochondrial fission has been shown to enhance anabolic metabolism, whereas loss of DRP1 leads to a more quiescent state in CD8+ T cells [16]. Besides, absence of mitochondrial fission protein DRP1 promotes memory formation by dictating mitochondrial fusion [16]. In line with this observation, the deletion of Optic atrophy 1 (Opa1), an inner mitochondrial membrane fusion protein, results in a strong impairment in the development of central memory CD8 + T cells [16, 32]. Moreover, the impairment of mitophagy, a process that degrades dysfunctional mitochondria [33], leads to in impaired memory T‐cell formation. In addition, the increased expression of Nix, which is a key mitophagy molecule, promotes effector memory formation, but not central memory formation [33].
Metabolic program intertwines with differentiation program of T‐cell exhaustion
Differentiation program of T‐cell exhaustion
In the context of tumors and chronic viral infection, such as HBV, HCV, and HIV, the immune system fails to control the inflammation and to clear the antigen efficiently, which leads to a prolonged and persistent antigen exposure in the organism. The continued stimulation in CD8+ T cells is believed to skew the T cells into a particular differentiated state known as “exhaustion” [34, 35, 36]. This state is characterized by the loss of effector cytokine production, impaired proliferation, upregulation of inhibitory receptors including programmed cell death protein‐1 (PD‐1), T‐cell immunoglobulin and mucin‐domain containing‐3 (TIM‐3), and lymphocyte activating‐3, and altered transcriptional and epigenetics profiles [34, 37, 38]. The establishment of the exhausted phenotype has been described as a progressive differentiation process, starting from the precursors of memory cells [39]. The consequence of this process is a heterogenic pool of exhausted T cells [40]. Tremendous efforts have been made in the past few years to unveil this heterogeneity with the hope to better understand various responses to immune checkpoint blockade [41, 42]. A CD8+ T‐cell lineage, characterized by the expression of the T‐cell factor‐1 (TCF‐1) and named as “progenitor exhausted T cells” or “memory‐like T cells,” displays stem‐like properties including self‐renewal and the ability to differentiate into terminally exhausted cells (Tex) [40, 43, 44, 45] (Fig. 3). Interestingly, progenitor exhausted T cells (Tpex) have been reported to increase their proliferation, expansion, and further differentiation in effector and terminally exhausted T cells upon PD‐1 blockade treatment, which make them a particularly attractive therapeutic target in treating cancer and chronic viral infections [40, 43, 45, 46]. Recent work in mice further refined the current understanding of this differentiation process by introducing four subsets of progenitor, intermediate and terminally exhausted T cells linked in a hierarchical development pathway [47]. Those subsets are defined by their CD69 and Ly108 markers and by the expression of TCF‐1, TOX, and T‐bet which coordinate the development of this lineage. Of note, it has also been shown that only the TCF‐1+ Tpex population, but not the terminally exhausted T cells, is able to replenish T cells upon transfer in another infected mouse, suggesting that the presence of Tpex population supports a long‐term protection in the diseases where pathogens and tumor cells cannot be eliminated [40]. However, a cooperation between progenitor and exhausted T cells has been shown to be crucial for the long‐term control of the viral load and tumors [48]. These observations suggest that maintaining a balance in this lineage is critical to keep the infection and tumor in check.
Figure 3.
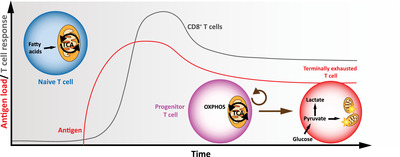
Metabolic deficiency in exhausted CD8+ T cells in chronic environment. Upon antigen persistence, T cells form an intermediate progenitor subset that presents memory‐like characteristics like self‐renewal and tcf‐1 expression. This progenitor subset irreversibly gives rise to the short‐lived terminally exhausted subset, which is associated with a glycolytic gene signature as well as mitochondrial damage.
Mitochondrial dysfunction in exhausted T cells
Given the high interest toward the therapeutic potential of targeting progenitor T cells [40, 43, 45, 46], understanding the metabolic profile and how metabolic program orchestrate T‐cell differentiation could provide opportunities to promote formation of progenitor T cells and potentially enhance immune checkpoint blockade via metabolic interventions. In the early phase of chronic lymphocytic choriomeningitis virus infection, virus‐specific CD8+ T cells have been shown to present depolarized mitochondria, causing bioenergetic defects and loss of effector function [38]. Interestingly, overexpression of the peroxisome proliferator‐activated receptor coactivator‐1α (PGC1‐α) could lead to lower depolarized mitochondrial content and rescue effector function, suggesting that metabolic manipulation could present a great therapeutic opportunity. These findings are supported by human studies showing that exhausted T cells extracted from HBV‐infected patients, presented a strong decreased expression of genes involved in the mitochondrial biogenesis and fitness [49]. Moreover, these HBV‐specific CD8+ T cells also accumulated depolarized mitochondria. This accumulation leads to strong production of mitochondrial reactive oxygen species, which is known to modulate T‐cell effector functions [50, 51]. PD‐1 signaling also contributes to the impaired bioenergetic profile by repressing aerobic glycolysis, promoting fatty acid oxidation, and compromising mitochondrial fitness by repressing the expression of PGC1‐α [38, 52]. Furthermore, PD‐1 signaling was shown to reduce the length and number of mitochondrial cristae, in human CD8+ T cells, which also promotes the apparition of dysfunctional mitochondria [53]. This suggests that PD‐1 blockade could be used to reinvigorate exhausted T cells by promoting glucose uptake and mitochondrial fitness [38, 54].
Competitive and immunosuppressive microenvironment
Similar to virus‐specific CD8+ T cells in chronic viral infections, tumor‐infiltrating lymphocytes also become exhausted in the tumor microenvironment because of persistent antigen stimulation, the exposure to nutrient deficiency, and various stress signals [55, 56] and the accumulation of dysfunctional mitochondria [57]. Activated T cells require high amounts of nutrients as source of energy and building blocks for their proliferation and effector function. Nutrients competition and deprivation is known to impact human T‐cell differentiation, metabolic profile, and shift the survival balance [58, 59]. Notably, nutrient shortage has been shown to promote apoptosis through the Bcl‐2 family members, Puma and Noxa [58]. Furthermore, the tumor microenvironment contains immunosuppressive molecules that can also impair T‐cell metabolism and promote exhaustion. For example, cholesterol present in the mouse tumoral microenvironment is taken up by the cells which increase ER stress and XPB1 pathway, resulting in declined antitumor activity [60]. Reprogramming of the methionine recycling machinery by tumor cells, in hepatocellular carcinoma of mice, increases the methionine metabolites S‐adenosylmethionine and 5‐methylthioadenosine levels in the environment, leading to T‐cell dysfunction [61]. Other environmental cues can drive T‐cell exhaustion like persistent IL‐2 stimulation in the tumoral microenvironment. It was reported that continuous IL‐2 presence triggered persistent activation of STAT‐5 which leads to metabolic changes in murine T cells by overexpressing the tryptophan hydroxylase 1. This enzyme catalyzes the conversion of tryptophan to 5‐hydroxytryptophan, which activates AhR nuclear translocation and then an overexpression of inhibitory receptors and an inhibition of cytokine production in T cells [62].
Recent work reports that ER stress, in human ovarian cancer, engages the IRE1‐α‐XBP1 pathway in T cells which decreased mitochondrial respiration and effector functions [63]. Mechanistically, the activation of the IRE1‐α‐XBP1 signaling limited glutamine uptake by repressing the abundance of glutamine carriers and, thus, limiting fuel necessary to engage mitochondrial metabolism in a glucose‐deficient tumoral microenvironment [63]. The stimulator of IFN genes pathway (STING) is another stress‐response pathway and has the ability to sense foreign or self‐DNA aberrantly localized in cytosol and respond by the induction of type I IFNs [64, 65]. Moreover, STING expression has been reported to be largely decreased in CD8+ T cells from cancer patients compared with healthy donors. In the same study, the authors further unveiled that the STING cascade enhances TCF‐1 expression by inhibiting Akt activity and the maintenance of progenitor CD8+ T cells in a type I IFN‐dependent manner [66]. These findings are consistent with previous results demonstrating that Akt signaling promotes CD8+ T‐cell effector differentiation by facilitating aerobic glycolysis, while inhibiting memory‐precursor formation [67, 68]. In that regard, it has been shown that enhanced aerobic glycolysis was a characteristic of terminally exhausted T cells and a glycolytic gene signature was increased in Tex compared to Tpex derived from human melanomas [69]. Since mitochondrial impairment can also promote dependency on aerobic glycolysis to meet cellular metabolic demands, it is possible that Tex cells display a higher glycolytic score as a result of mitochondrial defects. In line with this postulate, several groups recently uncovered that CD8+ TILs accumulating depolarized and dysfunctional mitochondria displayed characteristics of terminally exhausted T cells such as diminished TCF‐1 expression, increased expression of PD‐1 and TIM‐3, and a loss of cytokine production [70, 71]. Moreover, forcing the accumulation of depolarized mitochondria is sufficient to trigger the apparition of an exhausted phenotype in vitro [70].
Taken together, these recent findings point out the metabolic differences in different T‐cell differentiation states during exhaustion. These observations are suggesting that exhausted CD8+ T cells from different stages of exhaustion present distinct metabolic profiles to support metabolic demands of each differentiation state. The metabolic preference and mitochondrial fitness seem to be crucial for their function and long‐term persistence. We could speculate that the immune system, in response to overwhelming challenges, instructs the formation of this particular T‐cell lineage to control as best as possible the infection load and to adapt the metabolic features supporting this new organization structure. The progenitor exhausted subset does not engage into heavy proliferation and cytokine production, but maintain a basal self‐renewal capacity and differentiation capacity to become effector and terminally exhausted T cells. Thus, those progenitors may have metabolic needs similar to memory T cells to cope with their long‐lived ability. These distinct metabolic demands in Tpex and Tex could spare metabolic niche to simultaneously support the survival and functions of both subsets, a critical step for fighting the persisting infection and tumors. Another related question is how can the TCF‐1+ Tpex population be maintained in tumors where T cells receiving persistent stimulation and the environmental stress. Different groups tried to follow the differentiation and the localization of this lineage and reported pools of Tpex in niches within tumor draining LNs. They found a stable reservoir of tumor‐specific TCF‐1+ CD8+ T cells in dLN and those migrated toward the tumor [72, 73]. In presence of the tumoral microenvironment, these cells progressively became terminally differentiated. These findings indicate that nutritional changes happening in LNs or as a result of the metabolic crosstalk between T cells and APCs could guide T‐cell differentiation in the context of prolonged antigen exposure.
Metabolic reprogramming
Emerging strategies have been focusing on reprogramming the metabolic profile and flexibility of virus‐ and tumor‐specific T cells to improve their function and persistence in response to metabolic stress. Targeting the mitochondrial fitness and biogenesis is one of the most promising strategies (Fig. 4). In that regard, using the mitophagy process could be beneficial for exhausted cells. Mitophagy is the selective removal of damaged mitochondria to maintain mitochondrial health and turnover [74, 75]. Yu et al., showed that stimulating mitophagy by providing the NAD precursor nicotinamide riboside [76] reduced the accumulation of depolarized mitochondria in a drp‐1‐dependent manner and decreased mitochondrial reactive oxygen species levels [70]. Furthermore, nicotinamide riboside treatment on melanoma‐bearing mice boosted the effector cytokines production ability of TILs and improved tumor control [70]. Inhibiting mitochondrial regulators, such as REGNASE‐1, has been shown to promote the mitochondrial fitness, in a BATF‐dependent manner, and the formation of long‐lived effector CD8+ T cells and result in better tumor control [77]. In addition, preserving the mitochondrial quality by N‐acetylcysteine, an antioxidant, is able to protect T cells from mitochondrial ROS‐induced mitochondrial damage. As a result of this action, N‐acetylcysteine treatment could restore T‐cell proliferation and effector function and elicit a memory‐cell associated transcriptome [69]. Mitochondrial activity of terminally exhausted T cells can also be rescued by 4‐1BB stimulation, which is a costimulatory molecule expressed on exhausted T cells. The 4‐1BB stimulation supported mitochondrial fusion and biogenesis by activating p38‐MAPK and PGC1‐α in mice [78]. 4‐1BB induced mitochondrial fitness enhancement could improve tumor control in an adoptive cell therapy model and synergized with anti‐PD‐1 treatment. Given the promising results of 4‐1BB stimulation, this strategy has been further used for CAR‐T‐cell generation. Incorporation of the intracellular domain of 4‐1BB showed enhanced central memory phenotype associated with increased respiratory capacity, fatty acid oxidation, mitochondrial biogenesis, and partially preventing exhausted phenotype [79, 80]. Our recent work also reported that treating tumor‐bearing mice with an IL‐10‐Fc fusion protein could reprogram the metabolism of terminally exhausted TILs by promoting the mitochondrial pyruvate carrier‐dependent oxidative phosphorylation [81]. This resulted in the enhancement of the expansion and the effector function of terminally exhausted cells and contributed to better tumor control in a progenitor T cell‐independent manner. Interestingly, this therapeutic mode of action is independent progenitor cells, unlike currently used therapies that rely heavily on progenitor subset stimulation like anti‐PD‐1 therapy and subsequently could be used in a complementary manner to boost at the same time progenitor and terminally exhausted T cells.
Figure 4.
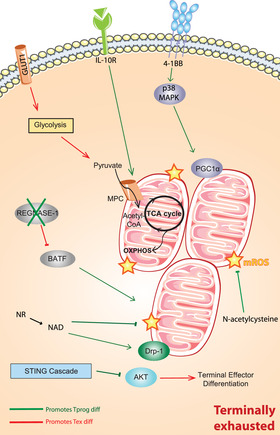
Metabolic reprogramming of terminally exhausted T cells. Different strategies could be used to specifically target the metabolism and the mitochondrial fitness of terminally exhausted T cells. The deletion of mitochondrial regulator REGNASE‐1 can increase mitochondrial quality in a BATF‐dependent manner. Preserving mitochondrial health against the accumulation of mitochondrial ROS by N‐acetylcysteine can also restore T‐cell effector function and memory‐like phenotype. Mitochondrial fusion and biogenesis can be rescued through p38‐MAPK and PGC1‐α activation by 4‐1BB stimulation. Treatment with IL‐10‐Fc fusion protein was able reprogram the metabolism of terminally exhausted TILs by promoting oxidative phosphorylation by the mitochondrial pyruvate carrier MPC.
Conclusions
Delineating how the metabolic profile in CD8+ T cells evolves during the course of immune response against infection and tumors has been a major interest in the immunology field. However, the main metabolic regulators that trigger those transitions remain unclear, which hampers our progress to optimize long‐term immunization against pathogens by harnessing immunometabolic regulations. Moreover, the dysregulated metabolic activities have been shown to disarm CD8+ T‐cell responses and orchestrate the exhaustion program in response to chronic infection or cancers. Recent studies gave a glimpse on how targeting metabolic program in CD8+ T cells can improve CD8+ T‐cell function in chronic viral infections and tumor control. However, given the diversity of metabolic states in different tissues and the impacts caused by metabolic status of subjects, it remains challenging to exploit our knowledge in immunometabolism of CD8+ T cells to customize treatments. One could also expect metabolic discrepancies in T cells between chronic infection and tumors since the tumor microenvironment exhibits more complicated metabolic stress and communications. We postulate that environmental cues further disrupt the metabolic profile of T cells in tumors compared to chronic infection, potentially causing faster and stronger terminal differentiation in tumor site resulting in accumulation of depolarized mitochondria and alter lactate metabolism. This could be the reason why we can observe niches of TCF1+ Tpex in draining LNs. In that regard, metabolic reprogramming designs should take into consideration nutrients access, localization, and environmental cues that CD8+ T cells will undergo. In addition, most studies about T‐cell metabolism are conducted by using mouse models and no direct comparison has been made between the metabolic profile in murine and human T cells which may lead to translational issues. In conclusion, a full consideration on the interplay between intrinsic and extrinsic metabolic programs and a better understanding of the different specific metabolic status in tumor and infection, and in human and mouse, could pave a critical step for us to tailor desirable CD8+ T‐cell immune responses.
Conflict of Interest
P.‐C.H. serves as scientific advisors for Elixiron Immunotherapeutics and Novartis. The rest of the authors confirm no commercial or financial conflict of interest.
Abbreviations
- CPT1a
carnitine palmitoyltransferase Ia
- DRP1
dynamin‐related protein 1
- mTOR
mammalian Target of Rapamycin
- Opa1
Optic atrophy 1
- PD‐1
programmed cell death protein‐1
- TIM‐3
T‐cell immunoglobulin and mucin‐domain containing‐3
- TCF‐1
T‐cell factor‐1
- Tex
terminally exhausted cells
- Tpex
progenitor exhausted T cells
- PGC1‐α
proliferator‐activated receptor coactivator‐1α
- STING
stimulator of IFN genes pathway
Acknowledgements
P.‐C.H. was supported by the SNSF project grants (31003A_182470), the European Research Council Staring Grant (802773‐MitoGuide), and EMBO Young Investigator award. Z. L. was supported by the State Scholarship Fund from China Scholarship Council (Grant No: 202006270216). Open access funding provided by Universite de Lausanne.
Open Access Funding provided by Universite de Lausanne.
[Correction added on May 10th 2022, after first online publication: CSAL funding statement has been added.]
Contributor Information
Zhiyu Li, Email: lizhiyu@whu.edu.cn.
Ping‐Chih Ho, Email: ping-chih.ho@unil.ch.
References
- 1. Yerinde, C. , Siegmund, B. , Glauben, R. and Weidinger, C. , Metabolic control of epigenetics and its role in CD8+ T cell differentiation and function. Front. Immunol. 2019. 10: 2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Almeida, L. , Lochner, M. , Berod, L. and Sparwasser, T. , Metabolic pathways in T cell activation and lineage differentiation. Semin. Immunol. 2016. 28: 514–524. [DOI] [PubMed] [Google Scholar]
- 3. Chang, J. T. , Wherry, E. J. and Goldrath, A. W. , Molecular regulation of effector and memory T cell differentiation. Nat. Immunol. 2014. 15: 1104–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaech, S. M. and Cui, W. , Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 2012. 12: 749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van der Windt, G. J. W. and Pearce, E. L. , Metabolic switching and fuel choice during T‐cell differentiation and memory development. Immunol. Rev. 2012. 249: 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tarasenko, T. N. , Pacheco, S. E. , Koenig, M. K. , Gomez‐Rodriguez, J. , Kapnick, S. M. , Diaz, F. , Zerfas, P. M. et al., Cytochrome c oxidase activity is a metabolic checkpoint that regulates cell fate decisions during T cell activation and differentiation. Cell Metab. 2017. 25: 1254–1268.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Krauss, S. , Brand, M. D. and Buttgereit, F. , Signaling takes a breath: new quantitative perspectives on bioenergetics and signal transduction. Immunity. 2001. 15: 497–502. [DOI] [PubMed] [Google Scholar]
- 8. Voss, K. , Larsen, S. E. and Snow, A. L. , Metabolic reprogramming and apoptosis sensitivity: defining the contours of a T cell response. Cancer Lett. 2017. 408: 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sprent, J. and Surh, C. D. , Normal T cell homeostasis: the conversion of naive cells into memory‐phenotype cells. Nat. Immunol. 2011. 12: 478–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mendoza, A. , Fang, V. , Chen, C. , Serasinghe, M. , Verma, A. , Muller, J. , Chaluvadi, V. S. et al., Lymphatic endothelial S1P promotes mitochondrial function and survival in naive T cells. Nature. 2017. 546: 158–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rathmell, J. C. , Farkash, E. A. , Gao, W. and Thompson, C. B. , IL‐7 enhances the survival and maintains the size of naive T cells. J. Immunol. 2001. 167: 6869–6876. [DOI] [PubMed] [Google Scholar]
- 12. Wofford, J. A. , Wieman, H. L. , Jacobs, S. R. , Zhao, Y. and Rathmell, J. C. , IL‐7 promotes Glut1 trafficking and glucose uptake via STAT5‐mediated activation of Akt to support T‐cell survival. Blood. 2008. 111: 2101–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Geltink, R. I. K. , Kyle, R. L. and Pearce, E. L. , Unraveling the complex interplay between T cell metabolism and function. Annu. Rev. Immunol. 2018. 36: 461–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang, R. , Dillon, C. P. , Shi, L. Z. , Milasta, S. , Carter, R. , Finkelstein, D. , McCormick, L. L. et al., The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011. 35: 871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Der Windt, G. J. W. , O'Sullivan, D. , Everts, B. , Huang, S. C. C. , Buck, M. D. , Curtis, J. D. , Chang, C. H. et al., CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. Proc. Natl. Acad. Sci. U. S. A. 2013. 110: 14336–14341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buck, M. D. D. , O'Sullivan, D. , Klein Geltink, R. I. I. , Curtis, J. D. D. , Chang, C. H. , Sanin, D. E. E. , Qiu, J. et al., Mitochondrial dynamics controls T cell fate through metabolic programming. Cell. 2016. 166: 63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tan, H. , Yang, K. , Li, Y. , Shaw, T. I. , Wang, Y. , Blanco, D. B. , Wang, X. et al., Integrative proteomics and phosphoproteomics profiling reveals dynamic signaling networks and bioenergetics pathways underlying T cell activation. Immunity. 2017. 46: 488–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rathmell, J. C. , Heiden, M. G. V. , Harris, M. H. , Frauwirth, K. A. and Thompson, C. B. , In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Mol. Cell. 2000. 6: 683–692. [DOI] [PubMed] [Google Scholar]
- 19. Frauwirth, K. A. , Riley, J. L. , Harris, M. H. , Parry, R V. , Rathmell, J. C. , Plas, D. R. , Elstrom, R. L. et al., The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002. 16: 769–777. [DOI] [PubMed] [Google Scholar]
- 20. Klein‐Hessling, S. , Muhammad, K. , Klein, M. , Pusch, T. , Rudolf, R. , Flöter, J. , Qureischi, M. et al., NFATc1 controls the cytotoxicity of CD8+ T cells. Nat. Commun. 2017. 8: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang, R. , Dillon, C. P. , Shi, L. Z. , Milasta, S. , Carter, R. , Finkelstein, D. , McCormick, L. L. et al., The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011. 35: 871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Menk, A V. , Scharping, N. E. , Moreci, R. S. , Zeng, X. , Guy, C. , Salvatore, S. , Bae, H. et al., Early TCR signaling induces rapid aerobic glycolysis enabling distinct acute T cell effector functions. Cell Rep. 2018. 22: 1509–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sinclair, L V. , Rolf, J. , Emslie, E. , Shi, Y. B. , Taylor, P. M. and Cantrell, D. A. , Control of amino‐acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat. Immunol. 2013. 14: 500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gubser, P. M. and Kallies, A. , Methio “mine”! Cancer cells steal methionine and impair CD8 T‐cell function. Immunol. Cell Biol. 2020. 98: 623–625. [DOI] [PubMed] [Google Scholar]
- 25. Gubser, P. M. , Bantug, G. R. , Razik, L. , Fischer, M. , Dimeloe, S. , Hoenger, G. , Durovic, B. et al., Rapid effector function of memory CD8+ T cells requires an immediate‐early glycolytic switch. Nat. Immunol. 2013. 14: 1064–1072. [DOI] [PubMed] [Google Scholar]
- 26. Sukumar, M. , Liu, J. , Ji, Y. , Subramanian, M. , Crompton, J. G. , Yu, Z. , Roychoudhuri, R. et al., Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J. Clin. Invest. 2013. 123: 4479–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van der Windt, G. J. W. , Everts, B. , Chang, C. H. , Curtis, J. D. , Freitas, T. C. , Amiel, E. , Pearce, E. J. et al., Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012. 36: 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kieper, W. C. , Tan, J. T. , Bondi‐Boyd, B. , Gapin, L. , Sprent, J. , Ceredig, R. and Surh, C. D. , Overexpression of interleukin (IL)‐7 leads to IL‐15‐independent generation of memory phenotype CD8+ T cells. J. Exp. Med. 2002. 195: 1533–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ku, C. C. , Murakami, M. , Sakamoto, A. , Kappler, J. and Marrack, P. , Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science (80‐. ). 2000. 288: 675–678. [DOI] [PubMed] [Google Scholar]
- 30. Raud, B. , Roy, D. G. , Divakaruni, A. S. , Tarasenko, T. N. , Franke, R. , Ma, E. H. , Samborska, B. et al., Etomoxir actions on regulatory and memory T cells are independent of cpt1a‐mediated fatty acid oxidation. Cell Metab. 2018. 28: 504–515.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Geiger, R. , Rieckmann, J. C. , Wolf, T. , Basso, C. , Feng, Y. , Fuhrer, T. , Kogadeeva, M. et al., L‐arginine modulates T cell metabolism and enhances survival and anti‐tumor activity. Cell. 2016. 167: 829–842.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Song, Z. , Chen, H. , Fiket, M. , Alexander, C. and Chan, D. C. , OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J. Cell Biol. 2007. 178: 749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gupta, S. S. , Sharp, R. , Hofferek, C. , Kuai, L. , Dorn, G. W. , Wang, J. and Chen, M. , NIX‐mediated mitophagy promotes effector memory formation in antigen‐specific CD8+ T cells. Cell Rep. 2019. 29: 1862–1877.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McLane, L. M. , Abdel‐Hakeem, M. S. and Wherry, E. J. , CD8 T cell exhaustion during chronic viral infection and cancer. Annu. Rev. Immunol. 2019. 37: 457–495. [DOI] [PubMed] [Google Scholar]
- 35. Mueller, S. N. and Ahmed, R. , High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. U. S. A. 2009. 106: 8623–8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Franco, F. , Jaccard, A. , Romero, P. , Yu, Y. R. and Ho, P. C. , Metabolic and epigenetic regulation of T‐cell exhaustion. Nat. Metab. 2020. 2: 1001–1012. [DOI] [PubMed] [Google Scholar]
- 37. Wherry, E. J. and Kurachi, M. , Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015. 15: 486–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bengsch, B. , Johnson, A. L. , Kurachi, M. , Odorizzi, P. M. , Pauken, K. E. , Attanasio, J. , Stelekati, E. et al., Bioenergetic insufficiencies due to metabolic alterations regulated by the inhibitory receptor PD‐1 are an early driver of CD8(+) T cell exhaustion. Immunity. 2016. 45: 358–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Angelosanto, J. M. , Blackburn, S. D. , Crawford, A. and Wherry, E. J. , Progressive loss of memory T cell potential and commitment to exhaustion during chronic viral infection. J. Virol. 2012. 86: 8161–8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Utzschneider, D. T. , Charmoy, M. , Chennupati, V. , Pousse, L. , Ferreira, D. P. , Calderon‐Copete, S. , Danilo, M. et al., T cell factor 1‐expressing memory‐like CD8+ T cells sustain the immune response to chronic viral infections. Immunity. 2016. 45: 415–427. [DOI] [PubMed] [Google Scholar]
- 41. Daud, A. I. , Loo, K. , Pauli, M. L. , Sanchez‐Rodriguez, R. , Sandoval, P. M. , Taravati, K. , Tsai, K. et al., Tumor immune profiling predicts response to anti‐PD‐1 therapy in human melanoma. J. Clin. Invest. 2016. 126: 3447–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sade‐Feldman, M. , Yizhak, K. , Bjorgaard, S. L. , Ray, J. P. , de Boer, C. G. , Jenkins, R. W. , Lieb, D. J. et al., Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell. 2018. 175: 998–1013.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Siddiqui, I. , Schaeuble, K. , Chennupati, V. , Fuertes Marraco, S. A. , Calderon‐Copete, S. , Pais Ferreira, D. , Carmona, S. J. et al., Intratumoral Tcf1 + PD‐1 + CD8 + T cells with stem‐like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity. 2019. 50: 195–211.e10. [DOI] [PubMed] [Google Scholar]
- 44. Miller, B. C. , Sen, D. R. , Al Abosy, R. , Bi, K. , Virkud, Y. V. , LaFleur, M. W. , Yates, K. B. et al., Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 2019. 20: 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Im, S. J. , Hashimoto, M. , Gerner, M. Y. , Lee, J. , Kissick, H. T. , Burger, M. C. , Shan, Q. et al., Defining CD8+ T cells that provide the proliferative burst after PD‐1 therapy. Nature. 2016. 537: 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Blackburn, S. D. , Shin, H. , Freeman, G. J. and Wherry, E. J. , Selective expansion of a subset of exhausted CD8 T cells by αPD‐L1 blockade. Proc. Natl. Acad. Sci. U. S. A. 2008. 105: 15016–15021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Beltra, J. C. , Manne, S. , MS, A. ‐. H. , Kurachi, M. , Giles, J. R. , Chen, Z. , Casella, V. et al., Developmental relationships of four exhausted CD8+ T cell subsets reveals underlying transcriptional and epigenetic landscape control mechanisms. Immunity. 2020. 52: 825–841.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Paley, M. A. , Kroy, D. C. , Odorizzi, P. M. , Johnnidis, J. B. , Dolfi, D V. , Barnett, B. E. , Bikoff, E. K. et al., Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science (80‐. ). 2012. 338: 1220–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fisicaro, P. , Barili, V. , Montanini, B. , Acerbi, G. , Ferracin, M. , Guerrieri, F. , Salerno, D. et al., Targeting mitochondrial dysfunction can restore antiviral activity of exhausted HBV‐specific CD8 T cells in chronic hepatitis B. Nat. Med. 2017. 23: 327–336. [DOI] [PubMed] [Google Scholar]
- 50. Franchina, D. G. , Dostert, C. and Brenner, D. , Reactive oxygen species: involvement in T cell signaling and metabolism. Trends Immunol. 2018. 39: 489–502. [DOI] [PubMed] [Google Scholar]
- 51. Sena, L. A. , Li, S. , Jairaman, A. , Prakriya, M. , Ezponda, T. , Hildeman, D. A. , Wang, C. R. et al., Mitochondria are required for antigen‐specific T cell activation through reactive oxygen species signaling. Immunity. 2013. 38: 225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Patsoukis, N. , Bardhan, K. , Chatterjee, P. , Sari, D. , Liu, B. , Bell, L. N. , Karoly, E. D. et al., PD‐1 alters T‐cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat. Commun. 2015. 6: 6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ogando, J. , Saéz, M. E. , Santos, J. , Nuevo‐Tapioles, C. , Gut, M. , Esteve‐Codina, A. , Heath, S. et al., PD‐1 signaling affects cristae morphology and leads to mitochondrial dysfunction in human CD8+ T lymphocytes. J. Immunother. Cancer. 2019. 7: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gubin, M. M. , Zhang, X. , Schuster, H. , Caron, E. , Ward, J. P. , Noguchi, T. , Ivanova, Y. et al., Checkpoint blockade cancer immunotherapy targets tumour‐specific mutant antigens. Nature. 2014. 515: 577–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ho, P. ‐. C. , Bihuniak, J. D. , Macintyre, A. N. , Staron, M. , Liu, X. , Amezquita, R. , Tsui, Y. ‐. C. et al., Phosphoenolpyruvate is a metabolic checkpoint of anti‐tumor T cell responses. Cell. 2015. 162: 1217–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chang, C. ‐. H. , Qiu, J. , O'Sullivan, D. , Buck, M. D. , Noguchi, T. , Curtis, J. D. , Chen, Q. et al., Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015. 162: 1229–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. NE, S. , AV, M. , RS, M. , RD, W. , RE, D. , SC, W. , RL, F. et al., The tumor microenvironment represses T cell mitochondrial biogenesis to drive intratumoral T cell metabolic insufficiency and dysfunction. Immunity. 2016. 45: 374–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Alves, N. L. , van Lier, R. A. W. and Eldering, E. , Withdrawal symptoms on display: Bcl‐2 members under investigation. Trends Immunol. 2007. 28: 26–32. [DOI] [PubMed] [Google Scholar]
- 59. Wensveen, F. M. , van Gisbergen, K. and Eldering, E. , The fourth dimension in immunological space: how the struggle for nutrients selects high‐affinity lymphocytes. Immunol. Rev. 2012. 249: 84–103. [DOI] [PubMed] [Google Scholar]
- 60. Ma, X. , Bi, E. , Lu, Y. , Su, P. , Huang, C. , Liu, L. , Wang, Q. et al., Cholesterol induces CD8+ T cell exhaustion in the tumor microenvironment. Cell Metab. 2019. 30: 143–156.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hung, M. H. , Lee, J. S. , Ma, C. , Diggs, L. P. , Heinrich, S. , Chang, C. W. , Ma, L. et al., Tumor methionine metabolism drives T‐cell exhaustion in hepatocellular carcinoma. Nat. Commun. 2021. 12: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Liu, Y. , Zhou, N. , Zhou, L. , Wang, J. , Zhou, Y. , Zhang, T. , Fang, Y. et al., IL‐2 regulates tumor‐reactive CD8+ T cell exhaustion by activating the aryl hydrocarbon receptor. Nat. Immunol. 2021. 22: 358–369. [DOI] [PubMed] [Google Scholar]
- 63. Song, M. , Sandoval, T. A. , Chae, C. S. , Chopra, S. , Tan, C. , Rutkowski, M. R. , Raundhal, M. et al., IRE1α–XBP1 controls T cell function in ovarian cancer by regulating mitochondrial activity. Nature. 2018. 562: 423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ablasser, A. and Chen, Z. J. , CGAS in action: Expanding roles in immunity and inflammation. Science (80‐. ). 2019; 363: eaat8657. [DOI] [PubMed] [Google Scholar]
- 65. Bai, J. , Cervantes, C. , He, S. , He, J. , Plasko, G. R. , Wen, J. , Li, Z. et al., Mitochondrial stress‐activated cGAS‐STING pathway inhibits thermogenic program and contributes to overnutrition‐induced obesity in mice. Commun. Biol. 2020. 3: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li, W. , Lu, L. , Lu, J. , Wang, X. , Yang, C. , Jin, J. , Wu, L. et al., cGAS‐STING‐mediated DNA sensing maintains CD8+ T cell stemness and promotes antitumor T cell therapy. Sci. Transl. Med. 2020; 12: eaay9013. [DOI] [PubMed] [Google Scholar]
- 67. Rao, R. R. , Li, Q. , Odunsi, K. and Shrikant, P. A. , The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T‐bet and eomesodermin. Immunity. 2010. 32: 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Crompton, J. G. , Sukumar, M. , Roychoudhuri, R. , Clever, D. , Gros, A. , Eil, R. L. , Tran, E. et al., Akt inhibition enhances expansion of potent tumor‐specific lymphocytes with memory cell characteristics. Cancer Res. 2015. 75: 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vardhana, S. A. , Hwee, M. A. , Berisa, M. , Wells, D. K. , Yost, K. E. , King, B. , Smith, M. et al., Impaired mitochondrial oxidative phosphorylation limits the self‐renewal of T cells exposed to persistent antigen. Nat. Immunol. 2020. 21: 1022–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yu, Y. R. , Imrichova, H. , Wang, H. , Chao, T. , Xiao, Z. , Gao, M. , Rincon‐Restrepo, M. et al., Disturbed mitochondrial dynamics in CD8+ TILs reinforce T cell exhaustion. Nat. Immunol. 2020. 21: 1540–1551. [DOI] [PubMed] [Google Scholar]
- 71. Scharping, N. E. , Rivadeneira, D. B. , Menk, A V. , Vignali, P. D. A. , Ford, B. R. , Rittenhouse, N. L. , Peralta, R. et al., Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nat. Immunol. 2021. 22: 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Connolly, K. A. , Kuchroo, M. , Venkat, A. , Khatun, A. , Wang, J. , William, I. , Hornick, N. I. et al., A reservoir of stem‐like CD8+ T cells in the tumor‐draining lymph node preserves the ongoing antitumor immune response. Sci. Immunol. 2021;6:7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Schenkel, J. M. , Herbst, R. H. , Canner, D. , Li, A. , Hillman, M. , Shanahan, S. L. , Gibbons, G. et al., Conventional type I dendritic cells maintain a reservoir of proliferative tumor‐antigen specific TCF‐1+ CD8+ T cells in tumor‐draining lymph nodes. Immunity. 2021. 54: 2338–2353.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Beaulaton, J. and Lockshin, R. A. , Ultrastructural study of the normal degeneration of the intersegmental muscles of Antheraea polyphemus and Manduca sexta (Insecta, lepidoptera) with particular reference to cellular autophagy. J. Morphol. 1977. 154: 39–57. [DOI] [PubMed] [Google Scholar]
- 75. Song, Y. , Zhou, Y. and Zhou, X. , The role of mitophagy in innate immune responses triggered by mitochondrial stress. Cell Commun. Signal. 2020. 18: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jang, S. Y. , Kang, H. T. and Hwang, E. S. , Nicotinamide‐induced mitophagy: Event mediated by high NAD+/NADH ratio and SIRT1 protein activation. J. Biol. Chem. 2012. 287: 19304–19314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wei, J. , Long, L. , Zheng, W. , Dhungana, Y. , Lim, S. A. , Guy, C. , Wang, Y. et al., Targeting REGNASE‐1 programs long‐lived effector T cells for cancer therapy. Nature. 2019. 576: 471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Menk, A V. , Scharping, N. E. , Rivadeneira, D. B. , Calderon, M. J. , Watson, M. J. , Dunstane, D. , Watkins, S. C. et al., 4‐1BB costimulation induces T cell mitochondrial function and biogenesis enabling cancer immunotherapeutic responses. J. Exp. Med. 2018. 215: 1091–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kawalekar, O. U. , O'Connor, R. S. , Fraietta, J. A. , Guo, L. , McGettigan, S. E. , Posey, A. D. , Patel, P. R. et al., Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity. 2016. 44: 380–390. [DOI] [PubMed] [Google Scholar]
- 80. Long, A. H. , Haso, W. M. , Shern, J. F. , Wanhainen, K. M. , Murgai, M. , Ingaramo, M. , Smith, J. P. et al., 4‐1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 2015. 21: 581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Guo, Y. , Xie, Y. Q. , Gao, M. , Zhao, Y. , Franco, F. , Wenes, M. , Siddiqui, I. et al., Metabolic reprogramming of terminally exhausted CD8+ T cells by IL‐10 enhances anti‐tumor immunity. Nat. Immunol. 2021. 22: 746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]


