Abstract
Carotenoids are a family of pigment compounds, a subset of which are precursors for vitamin A biosynthesis. These pigments are derived from isopentenyl pyrophosphate (IPP), with geranylgeranyl diphosphate being the first metabolite unique to carotenoid biosynthesis in plants, algae, fungi, some bacteria, and arthropods. This chapter highlights the metal-dependent enzymes involved in synthesizing carotenoids in plants and the current state of knowledge of their cofactors and mechanisms. Emphasis is given to spectroscopic methods used to characterize metal centers. The recently discovered heme-dependent isomerase Z-ISO is presented as a case study in how to interrogate a metalloenzyme. Use of UV–vis, electron paramagnetic resonance, and magnetic circular dichroism spectroscopies of a metal center at various oxidation states and with external small molecule probes (CN−, CO, and •NO) can provide information about the nature of the metal center, the identity of its ligands, and its mechanism of action. Z-ISO is a histidine/cysteine ligated heme-dependent enzyme that is only active in the ferrous state and possesses redox-linked ligand switching. The choice and design of experiments are discussed as well as the conclusions that can be drawn.
1. Introduction: Metalloenzymes discovered in carotenoid biosynthesis in plants
1.1. Synthesis of IPP from glucose
Isopentenyl diphosphate (IPP) is the universal precursor for carotenoids (Moise, Al-Babili, & Wurtzel, 2014; Wurtzel, 2019). It is biosynthesized from glucose by two routes, the plastidial MEP pathway and the cytosolic MVA pathway. The MEP pathway takes place in the plastids of plants and uses the metal-dependent enzymes discussed below, Fig. 1 (Schwender, Seemann, Lichtenthaler, & Rohmer, 1996). The MVA pathway is mainly used to synthesize cytosolic terpenoids, but some archaea and fungi use it for synthesizing carotenoids. Due to limited metabolite exchange between the cytosol and plastids, a small percentage of carotenoids may be generated via the MVA pathway in plants (Romanowski, Bonanno, & Burley, 2002). Transition metal-dependent enzymes involved in carotenoid biosynthesis are summarized in Table 1 and discussed in detail below.
Fig. 1.
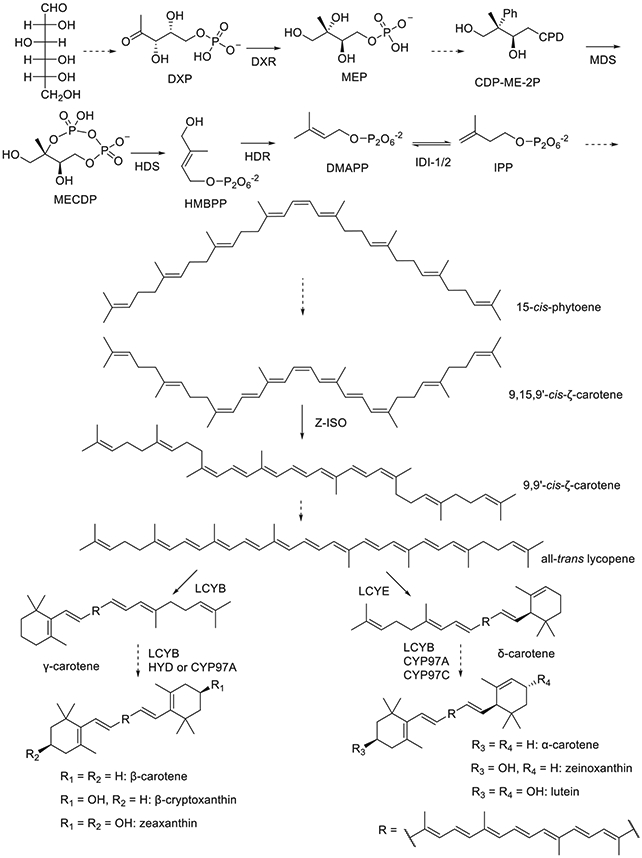
Metalloenzymes in the carotenoid biosynthetic pathway of higher plants. Dashed lines represent multiple steps.
Table 1.
Summary of transition metal dependent enzymes in the biosynthesis of carotenoids.
| Enzyme | Substrate | Product | Chemistry | Metal cofactor | Proposed role of the metal |
|---|---|---|---|---|---|
| 1-Deoxy-d-xylulose-5-phosphate reductoisomerase (DXR) | 1-Deoxy-d-xylulose-5-phosphate | 2-C-methyl-d-erythritol-4-phosphate | Aldol rearrangement and reduction | Mn>Co> Mg | Substrate binding and activation |
| MECDP synthase (MDS) | 4-(cytidine 5′-diphospho)-2-C-methyl-d-erythritol-2-phosphate | 2-C-methyl-d-erythritol-2,4-cyclodiphosphate | Intramolecular transphosphorylation | Zn and Mn or Mg | Substrate binding and transition state stabilization |
| HMBPP synthase (HDS) | 2-C-methyl-d-erythritol-2,4-cyclodiphosphate | 1-Hydroxyl-2-methyl-2-(E)-butenyl-4-diphosphate | Reduction of carbon | [4Fe-4S] | Electron transfer |
| HMBPP reductase (HDR) | 1-Hydroxyl-2-methyl-2-(E)-butenyl-4-diphosphate | Isopentenyl diphosphate or dimethylallyl diphosphate | Reduction of carbon | [4Fe-4S] | Substrate binding and electron transfer |
| IPP-DMAPP isomerase type 1 (IDI-1) | Isopentenyl diphosphate or dimethylallyl diphosphate | Isopentenyl diphosphate or dimethylallyl diphosphate | Isomerization | Zn or Mn and Mg | Structural and diphosphate stabilization |
| IPP-DMAPP isomerase type 2 (IDI-2) | Isopentenyl diphosphate or dimethylallyl diphosphate | Isopentenyl diphosphate or dimethylallyl diphosphate | Isomerization | Mg/FMN | Diphosphate stabilization |
| 15-cis-ζ-carotene isomerase (Z-ISO) | 9,15,9′-tri-cis-ζ-carotene | 9,9′-di-cis-ζ-carotene | Isomerization | Heme | Delocalizing the π electrons of the substrate |
| Ferrodoxin-dependent nonheme diiron enzymes (HYD) | β-Carotene β-Cryptoxanthin |
β-Cryptoxanthin Zeaxanthin |
Hydroxylation | Non-heme diiron | Oxygen activation and insertion |
| P450 carotenoid beta-ring hydroxylase (CYP97A) | β-Carotene α-Carotene β-Cryptoxanthin |
β-Cryptoxanthin Zeinoxanthin Zeaxanthin |
Hydroxylation | Heme | Oxygen activation and insertion |
| P450 carotenoid epsilon-ring hydroxylase (CYP97C) | Zeinoxanthin α-Cryptoxanthin |
Lutein | Hydroxylation | Heme | Oxygen activation and insertion |
1-Deoxy-d-xylulose-5-phosphate (DXP) reductoisomerase (DXR) synthesizes 2-C-methyl-d-erythritol-4-phosphate (MEP) from DXP. DXR requires a divalent metal cation for activity. If bovine serum albumin (BSA) is not included during an assay, Mn2+ is the most effective metal, with Co2+ and Mg2+ being two- and threefold slower, respectively. Other divalent cations do not promote catalysis (Takahashi, Kuzuyama, Watanabe, & Seto, 1998). The specificity constants (kcat/KM) for all three are similar, with Co2+ being two- and fourfold larger than Mn2+ and Mg2+, respectively (Kuzuyama, Takahashi, Takagi, & Seto, 2000). However, if BSA is included in the assay mixture to more closely mimic the plastid environment, those three metals show similar catalytic efficiency (Koppisch, Fox, Blagg, & Poulter, 2002). The reaction mechanism is shown to proceed via a retroaldol/aldol rearrangement (Munos, Pu, Mansoorabadi, Kim, & Liu, 2009), which is followed by reduction using NADPH (Proteau, Woo, Williamson, & Phaosiri, 1999; Radykewicz et al., 2000). The metal ion is coordinated by three carboxylates, one aspartate, and two glutamates, provided by the protein in a facial triad with three additional ligands provided by the solvent to form a regular octahedral geometry. The binding of DXP and the aldol intermediate to the metal have been proposed based on an inhibitor-bound structure (Steinbacher et al., 2003).
2-C-methyl-d-erythritol-2,4-cyclodiphosphate (MECDP) is formed from 4-(cytidine 5′-diphospho)-2-C-methyl-d-erythritol-2-phosphate (CDP-ME-2P) via an intramolecular transphosphorylation catalyzed by MECDP synthase (MDS), which requires divalent cations for activity (Herz et al., 2000). The active assembly of MDS is a homotrimer, with an active site being formed at each of the three interaction interfaces. A Zn2+ ion is found in the active site in a tetrahedral geometry with one aspartate and two histidines contributed by the enzyme and the fourth ligand is phosphate oxygen from CDP. Additionally, a Mn2+ is observed in octahedral geometry with one protein-derived glutamate, two phosphate oxygens from CDP, and three water molecules (Kemp, Bond, & Hunter, 2002; Richard et al., 2002). Plant MDS also uses a Zn2+ ion; however, the substrate-binding cavity is sufficiently different from the bacterial versions that it may not share the same binding mode or catalytic mechanism (Calisto et al., 2007).
MECDP is converted to 1-hydroxyl-2-methyl-2-(E)-butenyl-4-diphosphate (HMBPP) by HMBPP synthase (HDS) (Hecht et al., 2001). The overall reaction is reductive dehydration. UV–vis spectroscopy shows that HDS has features consistent with a [4Fe-4S] cluster after reconstitution with exogenous iron and sulfide and that reconstitution was required for activity (Kollas et al., 2002; Seemann et al., 2002). Mössbauer spectroscopy has been used to characterize the [4Fe-4S]2+ cluster of HDS from a bacterial and plant source as being bound to HDS by three cysteine residues, and it was found that the plant version could not use the same reductase system as its bacterial counterpart (Seemann et al., 2005). Mechanistic studies using rapid freeze-quench EPR, Mössbauer, and ENDOR spectroscopies suggest that the substrate coordinates to the [4Fe-4S]2+ cluster, and the reaction proceeds via sequential electron transfer (Xu et al., 2010). This mechanism is further supported by the characterization of a reaction intermediate as a ferraoxetane (Wang et al., 2011; Wang & Oldfield, 2014).
HMBPP is further reduced by HMBPP reductase (HDR) to dimethylallyl diphosphate (DMAPP) or IPP, isomers that can be interconverted by isopentenyl diphosphate isomerase type 1 or type 2 (IDI-1 and IDI-2, respectively) (Altincicek et al., 2002; Guevara-García et al., 2005). UV–vis and EPR spectroscopies were used to show that HDR requires an oxygen-sensitive [4Fe-4S]2+ cluster as well as a reducing system for activity (Wolff et al., 2003). Mössbauer spectroscopy showed that the [4Fe-4S]2+ cluster is bound to HDR by three cysteine residues with one hexacoordinate Fe ion (Seemann et al., 2009). The mechanism begins with the C1 hydroxide of HMBPP coordinating the unique iron atom followed by one-electron reduction of the substrate, generating water and a substrate radical (Gräwert et al., 2010). A second electron transfer from the [4Fe-4S] cluster and proton transfer give the final product, DMAPP or IPP, depending on which carbon is protonated (Span, Gräwert, Bacher, Eisenreich, & Groll, 2012).
Two separately evolved enzymes can interconvert IPP and DMAPP, IDI-1 and IDI-2 (Berthelot, Estevez, Deffieux, & Peruch, 2012). IDI-1 catalyzes the isomerization by protonating the carbon-carbon double bond, stabilizing a carbocation intermediate, and deprotonating to give the product (Toteva & Richard, 1997). The enzyme requires a structural hexacoordinate Zn2+ ion to organize the active site for catalysis, and an Mg2+ ion is also necessary to stabilize the diphosphate group of the substrate and product (Carrigan & Poulter, 2003; Durbecq et al., 2001; Lee & Poulter, 2006; Zhang et al., 2007). IDI-2 uses reduced FMN as an acid/base catalyst and only requires Mg2+ to stabilize the diphosphate group of substrate and product (Kaneda, Kuzuyama, Takagi, Hayakawa, & Seto, 2001; Thibodeaux, Chang, & Liu, 2010).
1.2. Lycopene synthesis and modification
Regardless of the source, IPP is then condensed, desaturated, and isomerized to yield lycopene. During lycopene biosynthesis, isomerization of the 15-cis precursor, 9,15,9-cis-ζ-carotene, is catalyzed by the heme-dependent enzyme, 15-cis-ζ-carotene isomerase (Z-ISO). Light can also mediate this isomerization in light exposed tissues, although the reaction is less efficient such that carotenoid biosynthesis is dependent on induction of the gene encoding the Z-ISO enzyme (Beltrán et al., 2015; Chen, Li, & Wurtzel, 2010; Li, Murillo, & Wurtzel, 2007; Sugiyama et al., 2020). Z-ISO is a transmembrane enzyme that embeds in chloroplasts (Beltrán et al., 2015). The enzyme is found to be active at the ferrous but not ferric oxidation state (Beltrán et al., 2015). After its formation, the ends of lycopene are cyclized by lycopene cyclase (LYC) LYCB and/or LYCE to generate the various versions of carotene (Porter & Lincoln, 1950; Sugiyama & Takaichi, 2020; Zhao, Liu, & Mao, 2020). Carotene can then be hydroxylated by non-heme diiron hydroxylases (HYD) or the cytochrome P450 enzymes, CYP97A and CYP97C (Quinlan, Jaradat, & Wurtzel, 2007), to form several derivatives. Carotenoids can also be further modified by hydroxylation, ketonization, or epoxidation of their β- or ε-rings to produce a variety of natural products (Mann, Harker, Pecker, & Hirschberg, 2000; Martin, Gudina, & Barredo, 2008).
1.3. Heme-dependent enzymes involved in carotenoid biosynthesis
Of the enzymes involved in carotenoid biosynthesis, only two types of heme-dependent enzymes have been found, Z-ISO, which will be discussed in detail below, and members of the CYP97 superfamily, which were initially found in the lut operon for lutein biosynthesis from α-carotene in Arabidopsis (Fiore, Dall’osto, Fraser, Bassi, & Giuliano, 2006; Pogson, McDonald, Truong, Britton, & DellaPenna, 1996; Quinlan et al., 2007; Tian, Musetti, Kim, Magallanes-Lundback, & DellaPenna, 2004). LUT1 (CYP97C1) catalyzes the final step of lutein biosynthesis by hydroxylating the ε-ring of zeinoxanthin (Quinlan et al., 2007). The major activity of LUT5 (CYP97A3) is to hydroxylate the β-ring of α-carotene with minor activity hydroxylating β-carotene and its derivatives (Kim & DellaPenna, 2006; Quinlan et al., 2007). Phylogenetic analysis shows that CYP97 enzymes used for lutein biosynthesis arose from an ancient gene duplication event before green algae and higher plants diverged, and CYP97 activity is confirmed in green algae (Cui et al., 2013; Kim, Smith, Tian, & Dellapenna, 2009). The function of CYP97A and CYP97C as carotene hydroxylases has also been experimentally verified in citrus fruits, rice, maize, and tomato (Chang et al., 2015; Lv et al., 2012; Ma et al., 2016; Quinlan et al., 2007; Stigliani, Giorio, & D’Ambrosio, 2011). In addition to CYP97A and CYP97C, some algae also use CYP97B and H in their carotenoid biosynthetic pathways (Liang, Xie, Chen, Liang, & Jiang, 2020; Tamaki, Kato, Shinomura, Ishikawa, & Imaishi, 2019). It has been shown that CYP97A and CYP97C must interact in order to mediate lutein biosynthesis. Also, while CYP97A and the nonheme diiron enzymes (HYD) can both mediate carotene β-ring hydroxylation, these structurally distinct enzymes are not interchangeable in mediating lutein biosynthesis because HYD and CYP97C cannot bind to each other. Little is known about the multienzyme organization of the carotenoid pathway, despite extensive evidence suggesting this idea (Quinlan et al., 2007).
2. Z-ISO: A case study
During the synthesis of lycopene from DMAPP and IPP, Z-ISO catalyzes the isomerization of the cis double bond at the 15 position of 9,15,9′-tri-cis-ζ-carotene, forming 9,9′-di-cis-ζ-carotene, Fig. 2 (Chen et al., 2010; Li et al., 2007). A mechanistic study of Z-ISO has recently been pursued, and its findings are elaborated here (Beltrán et al., 2015). The isomerization activity can only be observed in vitro if Z-ISO is treated with dithionite prior to assays. Analysis of the primary sequence of Z-ISO predicted and fluorescence microscopy confirm that Z-ISO is an integral membrane protein embedded in chloroplasts. Homology modeling suggested that Z-ISO may contain a heme or other iron cofactor and a suite of UV–vis, electron paramagnetic resonance (EPR), and magnetic circular dichroism (MCD) spectroscopic experiments were performed to determine the nature of the metal cofactor in Z-ISO.
Fig. 2.
Isomerization reaction catalyzed by Z-ISO.
Inductively coupled plasma optical emission spectrometry shows that purified Z-ISO contains iron but no other divalent or transition metals, and staining of SDS-PAGE samples confirm the presence of heme. Pyridine hemochrome assays are used to show that the heme of Z-ISO is type b. The as-isolated, ferric heme of Z-ISO has a Soret band centered at 415 nm with α/β-bands at 531 and 558 nm. Upon reduction with dithionite to yield ferrous heme, the Soret band narrows and red-shifts to 418 nm and the α/β-bands become more pronounced and slightly shift to 529 and 559 nm, respectively. CO and CN− were then used as small molecule probes to assess whether Z-ISO binds its heme with one or two axial ligands in the ferrous and ferric states, respectively. CO was found to bind ferrous Z-ISO stoichiometrically, whereas CN− binding to ferric Z-ISO was weak and substoichiometric. Together, these data support that reduced, Fe(II)-Z-ISO may have one proximal ligand with no distal ligand or a weakly bound distal ligand and that upon oxidation, another protein-derived ligand binds to the Fe(III) heme at the distal position or the weak distal ligand binds more strongly.
Further details about the heme of Z-ISO were pursued with EPR spectroscopy, which is an experimental technique that directly measures unpaired electrons by applying a magnetic field to cause a difference in energy between spin up and spin down states and using microwave radiation to flip the spin when resonance conditions are met (i.e., a principle similar to that of nuclear magnetic resonance, NMR). The EPR spectrum of as-isolated, ferric Z-ISO indicates several heme species and minor adventitious iron, Fig. 3A. Ferric heme contains five d-electrons and typically gives rise to either a high-spin (S = 5/2) or low-spin (S = 1/2) EPR signal depending on the number and strength of the axial ligands, Fig. 4. In the case of a weak ligand field containing fewer and/or weaker ligands, a high-spin species is expected. However, if the heme is coordinationally saturated with moderate or strong ligands, a low-spin species should be observed. Of the heme-based signals of as-isolated, ferric Z-ISO, one arises from a high-spin (S = 5/2) heme with axial symmetry, shows a g∥ of 5.8, and is expected to be 5-coordinate with no axial ligand at the distal position or 6-coordinate with a weakly-associated sixth ligand at the distal position. These features are more common for histidyl- rather than thiolate-ligated heme proteins. The low-spin species (S = 1/2) were more heterogeneous. Based on their characteristic g-values and anisotropy, the resonances were proposed to arise from 6-coordinate, bis-histidine or histidine-cysteine ligation. The EPR spectra have a relatively low signal-to-noise ratio due to poor heme occupancy. However, recently the expression system for Z-ISO has been further optimized to give five-fold better expression and double the heme occupancy, which should facilitate further spectroscopic characterizations and mechanistic studies (Wurtzel & Beltrán, 2020).
Fig. 3.

EPR spectra of Z-ISO in the as-isolated, ferric state (A) and after reduction with dithionite and treatment with •NO (B).
Fig. 4.

Orbital diagram for heme and its nitrosyl adduct.
Upon reacting as isolated, ferric Z-ISO with dithionite, the heme is reduced by one electron to the ferrous state, which contains six d-electrons, and no EPR signal is observed. When in the ferrous state, iron may be diamagnetic (S = 0) and thus EPR silent in heme systems, or contain an integer spin (S = 1, 2, or 3) in non-heme systems. In the case of integer spin species, electron–electron interactions cause the ground state to have differences in energy among various potential spin states, causing zero-field splitting. As such, integer spin systems may not give rise to EPR signals, even though they are paramagnetic. One method to circumvent the problem of ferrous samples not giving EPR signals is to react with an exogenous radical-bearing molecule, such as nitric oxide (•NO). Upon ligation to the ferrous heme, the heme iron can be considered as an {FeNO}7 species as described by the Enemark and Feltham notation, where the number “7” represents six d-electrons from the Fe(II) ion plus one unpaired electron contributed from •NO (Enemark & Feltham, 1974). In this state, the {FeNO}7 heme species has a non-integer number of electrons and adopts a low-spin (S = 1/2) electronic state (Fig. 4), where the g-values and nitrogen hyperfine structures of the EPR signals are diagnostic of the heme environment (Goodrich, Paulat, Praneeth, & Lehnert, 2010). Non-heme {FeNO}7 species typically adopt an S = 3/2, high-spin state (Brown et al., 2002). In the case of Z-ISO, reduction and treatment with •NO generate an EPR active, low-spin, ferrous-nitrosyl adduct, Fig. 3B, which is consistent with the CO-binding observed by UV–vis spectroscopy. The {FeNO}7 signal of Fe(II)-Z-ISO is most similar to histidine-ligated heme proteins. This finding suggests that the thiol/thiolate ligand observed in ferric Z-ISO is not retained or is weakly associated upon reduction from the Fe(III) to Fe(II) state.
The finding of a heterogeneous heme environment with variable ligands was further probed with MCD spectroscopy. First, the high-spin component in as-isolated Fe(III)-Z-ISO was estimated to be less than 20% in the solution state by comparison with cytochrome b5 (low spin) and myoglobin (high spin). Fe(III)-Z-ISO UV–vis and MCD spectra are recapitulated fairly well as a 1:1 mixture of bis-histidine (cytb5) and histidinecystine (imidazole-bound P450cam) ferric heme proteins. However, upon reduction, the UV–vis and MCD spectra of Z-ISO are nearly identical to a single bis-histidine heme species (imidazole-bound H93G myoglobin). One of the histidine ligands of Fe(II)-Z-ISO is expected to be relatively weak since it can be readily displaced by CO or •NO.
The observation of ligand switching along with Z-ISO only being active in the reduced, Fe(II) state raises the question of how the heme iron and/or the switching ligands catalyze the cis/trans isomerization. This question was addressed by identifying conserved histidine and cysteine residues and mutating each of them, respectively, to alanine. As a result, only two histidine residues were found to be necessary for catalysis, and mutating either to alanine caused a dramatic decrease in heme incorporation. Deletion of the sole cysteine of Z-ISO, which is strictly conserved, did not have a statistically significant effect on the isomerization activity. However, it did reduce heme occupancy to less than half of wild-type. These data suggest that the ligand switching in Z-ISO is likely to be a regulatory mechanism or a means to tune some property of the heme center of Z-ISO, perhaps the redox potential, and the Fe(II) ion, not the cysteine residue, is responsible for catalysis.
Taking the data together, the activity of Z-ISO is proposed to be gated by a redox-dependent ligand switch. The heme center is locked; however, upon reduction, some structural rearrangement should occur, with the distal histidine ligand only loosely binding the ferrous heme. Ferrous Z-ISO is then proposed to bind its substrate and facilitate isomerization through interactions between the 15-cis double bond and an empty d-orbital of the heme iron. The π electrons of the substrate would act as a Lewis base, coordinating the Fe(II) ion. Delocalizing the π electrons of the double bond into an empty orbital of the heme iron would allow facile isomerization to the trans conformation and disassociation affords the product.
3. Concluding remarks
Metalloenzymes are critical for carotenoid biosynthesis in plants. Such enzymes employ a wide array of metal cofactors, which are needed to accomplish the complex chemistry required for this biosynthetic process. Spectroscopic methods, such as EPR, MCD, resonance Raman, and Mössbauer are useful tools to characterize the metal cofactors, especially those containing heme or non-heme iron at their active sites. Judicious experimental design can allow one to decipher the catalytic mechanisms of these metalloenzymes. The diverse catalytic strategies employed by these plant proteins have broadened our understandings in mechanistic enzymology and provide opportunities to discover novel chemistry.
Acknowledgment
This work was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number R01GM108988. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Altincicek B, et al. (2002). LytB protein catalyzes the terminal step of the 2-C-methyl-D-erythritol-4-phosphate pathway of isoprenoid biosynthesis. FEBS Letters, 532(3), 437–440. [DOI] [PubMed] [Google Scholar]
- Beltràn J, et al. (2015). Control of carotenoid biosynthesis through a heme-based cis-trans isomerase. Nature Chemical Biology, 11(8), 598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthelot K, Estevez Y, Deffieux A, & Peruch F (2012). Isopentenyl diphosphate isomerase: A checkpoint to isoprenoid biosynthesis. Biochimie, 94(8), 1621–1634. [DOI] [PubMed] [Google Scholar]
- Brown CA, et al. (2002). Spectroscopic and theoretical description of the electronic structure of S = 3/2 iron-nitrosyl complexes and their relation to O2 activation by non-heme iron enzyme active sites. Journal of the American Chemical Society, 117(2), 715–732. [Google Scholar]
- Calisto BM, Perez-Gil J, Bergua M, Querol-Audi J, Fita I, & Imperial S (2007). Biosynthesis of isoprenoids in plants: Structure of the 2C-methyl-D-erithrytol 2,4-cyclodiphosphate synthase from Arabidopsis thaliana. Comparison with the bacterial enzymes. Protein Science: A Publication of the Protein Society, 16(9), 2082–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrigan CN, & Poulter CD (2003). Zinc is an essential cofactor for type I isopentenyl diphosphate: Dimethylallyl diphosphate isomerase. Journal of the American Chemical Society, 125(30), 9008–9009. [DOI] [PubMed] [Google Scholar]
- Chang S, et al. (2015). Cloning and functional characterization of the maize (Zea mays L.) carotenoid epsilon hydroxylase gene. PLoS One, 10(6), e0128758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Li F, & Wurtzel ET (2010). Isolation and characterization of the Z-ISO gene encoding a missing component of carotenoid biosynthesis in plants. Plant Physiology, 153(1), 66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, et al. (2013). Evolutionary origins, molecular cloning and expression of carotenoid hydroxylases in eukaryotic photosynthetic algae. BMC Genomics, 14, 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbecq V, et al. (2001). Crystal structure of isopentenyl diphosphate:Dimethylallyl diphosphate isomerase. The EMBO Journal, 20(7), 1530–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enemark JH, & Feltham RD (1974). Principles of structure, bonding, and reactivity for metal nitrosyl complexes. Coordination Chemistry Reviews, 13(4), 339–406. [Google Scholar]
- Fiore A, Dall’osto L, Fraser PD, Bassi R, & Giuliano G (2006). Elucidation of the β-carotene hydroxylation pathway in Arabidopsis thaliana. FEBS Letters, 580(19), 4718–4722. [DOI] [PubMed] [Google Scholar]
- Goodrich LE, Paulat F, Praneeth VKK, & Lehnert N (2010). Electronic structure of heme-nitrosyls and its significance for nitric oxide reactivity, sensing, transport, and toxicity in biological systems. Inorganic Chemistry, 49(14), 6293–6316. [DOI] [PubMed] [Google Scholar]
- Gräwert T, et al. (2010). Probing the reaction mechanism of IspH protein by X-ray structure analysis. Proceedings of the National Academy of Sciences of the United States of America, 107(3), 1077–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara-García A, San Román C, Arroyo A, Cortés ME, de la Luz Gutiérrez-Nava, M., & León P. (2005). Characterization of the Arabidopsis clb6 mutant illustrates the importance of posttranscriptional regulation of the methyl-D-erythritol 4-phosphate pathway. The Plant Cell, 17(2), 628–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht S, et al. (2001). Studies on the non-mevalonate pathway to terpenes: The role of the GcpE (IspG) protein. Proceedings of the National Academy of Sciences of the United States of America, 98(26), 14837–14842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz S, et al. (2000). Biosynthesis of terpenoids: YgbB protein converts 4-diphosphocytidyl-2C-methyl-D-erythritol 2-phosphate to 2C-methyl-D-erythritol 2,4-cyclodiphosphate. Proceedings of the National Academy of Sciences of the United States of America, 97(6), 2486–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda K, Kuzuyama T, Takagi M, Hayakawa Y, & Seto H (2001). An unusual isopentenyl diphosphate isomerase found in the mevalonate pathway gene cluster from streptomyces sp. strain CL190. Proceedings of the National Academy of Sciences of the United States of America, 98(3), 932–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp LE, Bond CS, & Hunter WN (2002). Structure of 2C-methyl-D-erythritol 2,4-cyclodiphosphate synthase: An essential enzyme for isoprenoid biosynthesis and target for antimicrobial drug development. Proceedings of the National Academy of Sciences of the United States of America, 99(10), 6591–6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, & DellaPenna D (2006). Defining the primary route for lutein synthesis in plants: The role of Arabidopsis carotenoid beta-ring hydroxylase CYP97A3. Proceedings of the National Academy of Sciences of the United States of America, 103(9), 3474–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Smith JJ, Tian L, & Dellapenna D (2009). The evolution and function of carotenoid hydroxylases in Arabidopsis. Plant & Cell Physiology, 50(3), 463–479. [DOI] [PubMed] [Google Scholar]
- Kollas AK, et al. (2002). Functional characterization of GcpE, an essential enzyme of the non-mevalonate pathway of isoprenoid biosynthesis. FEBS Letters, 532(3), 432–436. [DOI] [PubMed] [Google Scholar]
- Koppisch AT, Fox DT, Blagg BS, & Poulter CD (2002). E. coli MEP synthase: Steady-state kinetic analysis and substrate binding. Biochemistry, 41(1), 236–243. [DOI] [PubMed] [Google Scholar]
- Kuzuyama T, Takahashi S, Takagi M, & Seto H (2000). Characterization of 1-deoxy-D-xylulose 5-phosphate reductoisomerase, an enzyme involved in isopentenyl diphosphate biosynthesis, and identification of its catalytic amino acid residues. The Journal of Biological Chemistry, 275(26), 19928–19932. [DOI] [PubMed] [Google Scholar]
- Lee S, & Poulter CD (2006). Escherichia coli type I isopentenyl diphosphate isomerase: Structural and catalytic roles for divalent metals. Journal of the American Chemical Society, 128(35), 11545–11550. [DOI] [PubMed] [Google Scholar]
- Li F, Murillo C, & Wurtzel ET (2007). Maize Y9 encodes a product essential for 15-cis-ζ-carotene isomerization. Plant Physiology, 144(2), 1181–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang MH, Xie H, Chen HH, Liang ZC, & Jiang JG (2020). Functional identification of two types of carotene hydroxylases from the green alga Dunaliella bardawil rich in lutein. ACS Synthetic Biology, 9(6), 1246–1253. [DOI] [PubMed] [Google Scholar]
- Lv MZ, et al. (2012). Rice carotenoid β-ring hydroxylase CYP97A4 is involved in lutein biosynthesis. Plant & Cell Physiology, 53(6), 987–1002. [DOI] [PubMed] [Google Scholar]
- Ma G, et al. (2016). Expression and functional analysis of citrus carotene hydroxylases: Unravelling the xanthophyll biosynthesis in citrus fruits. BMC Plant Biology, 16(1), 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann V, Harker M, Pecker I, & Hirschberg J (2000). Metabolic engineering of astaxanthin production in tobacco flowers. Nature Biotechnology, 18(8), 888–892. [DOI] [PubMed] [Google Scholar]
- Martin JF, Gudina E, & Barredo JL (2008). Conversion of β-carotene into astaxanthin: Two separate enzymes or a bifunctional hydroxylase-ketolase protein? Microbial Cell Factories, 7, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moise AR, Al-Babili S, & Wurtzel ET (2014). Mechanistic aspects of carotenoid biosynthesis. Chemical Reviews, 114(1), 164–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munos JW, Pu X, Mansoorabadi SO, Kim HJ, & Liu HW (2009). A secondary kinetic isotope effect study of the 1-deoxy-D-xylulose-5-phosphate reductoisomerase-catalyzed reaction: Evidence for a retroaldol-aldol rearrangement. Journal of the American Chemical Society, 131(6), 2048–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogson B, McDonald KA, Truong M, Britton G, & DellaPenna D (1996). Arabidopsis carotenoid mutants demonstrate that lutein is not essential for photosynthesis in higher plants. The Plant Cell, 8(9), 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JW, & Lincoln RE (1950). Lycopersicon selections containing a high content of carotenes and colorless polyenes; the mechanism of carotene biosynthesis. Archives of Biochemistry, 27(2), 390–403. [PubMed] [Google Scholar]
- Proteau PJ, Woo YH, Williamson RT, & Phaosiri C (1999). Stereochemistry of the reduction step mediated by recombinant 1-deoxy-D-xylulose 5-phosphate isomeroreductase. Organic Letters, 1(6), 921–923. [DOI] [PubMed] [Google Scholar]
- Quinlan RF, Jaradat TT, & Wurtzel ET (2007). Escherichia coli as a platform for functional expression of plant P450 carotene hydroxylases. Archives of Biochemistry and Biophysics, 458(2), 146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radykewicz T, et al. (2000). Biosynthesis of terpenoids: 1-deoxy-D-xylulose-5-phosphate reductoisomerase from Escherichia coli is a class B dehydrogenase. FEBS Letters, 465(2–3), 157–160. [DOI] [PubMed] [Google Scholar]
- Richard SB, et al. (2002). Structure and mechanism of 2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase. An enzyme in the mevalonate-independent isoprenoid biosynthetic pathway. The Journal of Biological Chemistry, 277(10), 8667–8672. [DOI] [PubMed] [Google Scholar]
- Romanowski MJ, Bonanno JB, & Burley SK (2002). Crystal structure of the Streptococcus pneumoniae phosphomevalonate kinase, a member of the GHMP kinase superfamily. Proteins, 47(4), 568–571. [DOI] [PubMed] [Google Scholar]
- Schwender J, Seemann M, Lichtenthaler HK, & Rohmer M (1996). Biosynthesis of isoprenoids (carotenoids, sterols, prenyl side-chains of chlorophylls and plastoquinone) via a novel pyruvate/glyceraldehyde 3-phosphate non-mevalonate pathway in the green alga Scenedesmus obliquus. The Biochemical Journal, 316(Pt 1), 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann M, et al. (2002). Isoprenoid biosynthesis through the methylerythritol phosphate pathway: The (E)-4-hydroxy-3-methylbut-2-enyl diphosphate synthase (GcpE) is a [4Fe-4S] protein. Angewandte Chemie International Edition, 41(22), 4337–4339. [DOI] [PubMed] [Google Scholar]
- Seemann M, et al. (2005). Isoprenoid biosynthesis in chloroplasts via the methylerythritol phosphate pathway: The (E)-4-hydroxy-3-methylbut-2-enyl diphosphate synthase (GcpE) from Arabidopsis thaliana is a [4Fe-4S] protein. Journal of Biological Inorganic Chemistry, 10(2), 131–137. [DOI] [PubMed] [Google Scholar]
- Seemann M, et al. (2009). Isoprenoid biosynthesis via the MEP pathway: In vivo Mössbauer spectroscopy identifies a [4Fe-4S] 2+ center with unusual coordination sphere in the LytB protein. Journal of the American Chemical Society, 131(37), 13184–13185. [DOI] [PubMed] [Google Scholar]
- Span I, Gräwert T., Bacher A, Eisenreich W, & Groll M. (2012). Crystal structures of mutant IspH proteins reveal a rotation of the substrate’s hydroxymethyl group during catalysis. Journal of Molecular Biology, 416(1), 1–9. [DOI] [PubMed] [Google Scholar]
- Steinbacher S, Kaiser J, Eisenreich W, Huber R, Bacher A, & Rohdich F (2003). Structural basis of fosmidomycin action revealed by the complex with 2-C-methyl-D-erythritol 4-phosphate synthase (IspC). Implications for the catalytic mechanism and anti-malaria drug development. The Journal of Biological Chemistry, 278(20), 18401–18407. [DOI] [PubMed] [Google Scholar]
- Stigliani AL, Giorio G, & D’Ambrosio C (2011). Characterization of P450 carotenoid β- and ε-hydroxylases of tomato and transcriptional regulation of xanthophyll biosynthesis in root, leaf, petal and fruit. Plant & Cell Physiology, 52(5), 851–865. [DOI] [PubMed] [Google Scholar]
- Sugiyama K, & Takaichi S (2020). Carotenogenesis in cyanobacteria: CruA/CruP-type and CrtL-type lycopene cyclases. The Journal of General and Applied Microbiology, 66(2), 53–58. [DOI] [PubMed] [Google Scholar]
- Sugiyama K, et al. (2020). Oxygenic phototrophs need zeta-carotene isomerase (Z-ISO) for carotene synthesis: Functional analysis in Arthrospira and euglena. Plant & Cell Physiology, 61(2), 276–282. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Kuzuyama T, Watanabe H, & Seto H (1998). A 1-deoxy-D-xylulose 5-phosphate reductoisomerase catalyzing the formation of 2-C-methyl-D-erythritol 4-phosphate in an alternative non-mevalonate pathway for terpenoid biosynthesis. Proceedings of the National Academy of Sciences of the United States of America, 95(17), 9879–9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S, Kato S, Shinomura T, Ishikawa T, & Imaishi H (2019). Physiological role of β-carotene monohydroxylase (CYP97H1) in carotenoid biosynthesis in Euglena gracilis. Plant Science: An International Journal of Experimental Plant Biology, 278, 80–87. [DOI] [PubMed] [Google Scholar]
- Thibodeaux CJ, Chang WC, & Liu HW (2010). Linear free energy relationships demonstrate a catalytic role for the flavin mononucleotide coenzyme of the type II isopentenyl diphosphate:Dimethylallyl diphosphate isomerase. Journal of the American Chemical Society, 132(29), 9994–9996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Musetti V, Kim J, Magallanes-Lundback M, & DellaPenna D (2004). The Arabidopsis LUT1 locus encodes a member of the cytochrome P450 family that is required for carotenoid ε-ring hydroxylation activity. Proceedings of the National Academy of Sciences of the United States of America, 101(1), 402–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toteva MM, & Richard JP (1997). Mechanistic imperatives for the reaction catalyzed by isopentenyl pyrophosphate isomerase: Free energy profile for stepwise isomerization in water through a tertiary carbocation intermediate. Bioorganic Chemistry, 25(4), 239–245. [Google Scholar]
- Wang W, & Oldfield E (2014). Bioorganometallic chemistry with IspG and IspH: Structure, function, and inhibition of the [Fe(4)S(4)] proteins involved in isoprenoid biosynthesis. Angewandte Chemie International Edition, 53(17), 4294–4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Wang K, Li J, Nellutla S, Smirnova TI, & Oldfield E (2011). An ENDOR and HYSCORE investigation of a reaction intermediate in IspG (GcpE) catalysis. Journal of the American Chemical Society, 133(22), 8400–8403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff M, et al. (2003). Isoprenoid biosynthesis via the methylerythritol phosphate pathway: The (E)-4-hydroxy-3-methylbut-2-enyl diphosphate reductase (LytB/IspH) from Escherichia coli is a [4Fe-4S] protein. FEBS Letters, 541(1–3), 115–120. [DOI] [PubMed] [Google Scholar]
- Wurtzel ET (2019). Changing form and function through carotenoids and synthetic biology. Plant Physiology, 179(3), 830–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzel ET, & Beltrán J (2020). Improved expression and purification of the carotenoid biosynthetic enzyme Z-ISO. Methods in Molecular Biology, 2083, 53–61. [DOI] [PubMed] [Google Scholar]
- Xu W, et al. (2010). Paramagnetic intermediates of (E)-4-hydroxy-3-methylbut-2-enyl diphosphate synthase (GcpE/IspG) under steady-state and pre-steady-state conditions. Journal of the American Chemical Society, 132(41), 14509–14520. [DOI] [PubMed] [Google Scholar]
- Zhang C, et al. (2007). Crystal structures of human IPP isomerase: New insights into the catalytic mechanism. Journal of Molecular Biology, 366(5), 1437–1446. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Liu Z, & Mao X (2020). Biotechnological advances in lycopene beta-Cyclases. Journal of Agricultural and Food Chemistry, 68(43), 11895–11907. [DOI] [PubMed] [Google Scholar]



