Abstract
Objective
Combination therapy has become the hallmark of lung cancer treatment, as it reduces the dosage intensity of individual drugs while increasing their efficacy. In the current study, we analyzed the combinatorial effect of decitabine and aspirin on non-small cell lung cancer (NSCLC) cell growth.
Methods
In this study, we investigated the combinatorial effect of decitabine and aspirin by MTT, colony formation, and Transwell assays. We also explored the underlying molecular mechanism via a series of in vitro and in vivo experiments.
Results
The combination of decitabine and aspirin regulated cell viability and migration in vitro. Moreover, the combination therapy suppressed tumor cell growth by inhibiting the β-catenin/STAT3 signaling pathway. Our study also found that the regimen increased the phosphorylation of β-catenin and decreased the expression of STAT3 and β-catenin.
Conclusion
The combined administration of decitabine and aspirin significantly reduced tumor growth compared with single-agent treatment and the control in vivo. The study results indicated that decitabine and aspirin could suppress NSCLC cell growth and metastasis via the β-catenin/STAT3 signaling pathway.
Keywords: Decitabine, aspirin, cell proliferation, metastasis, non-small cell lung cancer, β-catenin, STAT3
Introduction
Non-small cell lung cancer (NSCLC), one of the most common causes of cancer mortality worldwide, comprises approximately 80% of all lung cancer cases. 1 Despite recent advances in the diagnosis and treatment of lung cancer, the prognosis of patients with NSCLC remains poor, and the 5-year overall survival rate is approximately 15%. 2
Decitabine (5-aza-2′-deoxycytidine), a DNA methyltransferases inhibitor, has a wide range of anti-metabolic and anti-cancer activities. Prior research indicated that decitabine could induce cell apoptosis and cell cycle arrest via DNA hypomethylation. 3 Decitabine combined with other therapies (cytotoxic drugs, molecular targeted agents and other epigenetic agents, or immunotherapy) produced better outcomes than decitabine alone. Decitabine enhances the sensitivity of tumor cells to other drugs, inhibits the growth of cancer cells, and activates the immune response. 4 In addition, decitabine has been demonstrated to have significant cytotoxic and anti-neoplastic activities in many tumors.5–7 In preclinical studies, decitabine inhibited lung cancer cell proliferation and induced their metastasis. 8
Aspirin, a non-steroidal anti-inflammatory drug, is extensively used in clinical to treat rheumatism and prevent cardiovascular disease. It has been reported as a chemopreventive drug that decreases the risk of several human cancers, including lung cancer.9,10 The targets of aspirin include cyclooxygenase (COX), cyclin-dependent kinases, and AMP-activated protein kinase. 9 In addition, aspirin can induce COX1 and COX2 acetylation at S529 and S516, respectively, thereby inhibiting arachidonic acid binding and preventing arachidonic acid transfer to thromboxane A2.11,12 Aspirin can suppress inflammation, immune escape, epithelial cell growth, and cancer metastasis by inhibiting platelet activation. Moreover, aspirin can inhibit the NF-κB and Wnt/β-catenin signaling pathways, which are tightly involved in tumorigenesis. 9 In addition, aspirin has been reported to enhance the sensitivity of colon cancer cells to cisplatin by abrogating the binding of NF-κB to the COX-2 promoter. 13
Increasing evidence indicates that WNT signaling plays an important part in lung carcinogenesis. Multiple studies examined the role of WNT signaling, primarily focusing on canonical or β-catenin–dependent WNT signaling. In the canonical pathway, Disheveled becomes phosphorylated, and it inhibits β-catenin phosphorylation. β-catenin subsequently accumulates in the cytoplasm. β-catenin binds with the LEF/TCF4 complex to activate Wnt signaling. 14 The Wnt/β-catenin pathway is frequently activated in lung cancer, and it promotes tumor growth and metastasis. 15 Interestingly, WNT signaling increases STAT3 activation during tumorigenesis. 16 However, the mechanism by which this pathway is aberrantly activated in lung cancer is unclear.
These reported actions suggest possible chemopreventive benefits of the combination of decitabine and aspirin. However, this interesting possibility has not been investigated in lung cancer. In the present study, we evaluated the effects of decitabine in combination with aspirin on the growth and metastasis of A549 and H1299 cells. We hypothesized that the combination would exert synergistic anti-tumor effects via the regulation of Wnt/β-catenin signaling and inactivation of STAT3. A better understanding of the mechanistic activities of decitabine and aspirin will provide insights into rational use of these agents as therapies for patients with solid tumors, including potential uses as combination, adjuvant, and maintenance therapies.
Materials and methods
Reagents and antibodies
Decitabine was purchased from Johnson & Johnson (New Brunswick, NJ, USA) and dissolved in phosphate-buffered saline (PBS). Aspirin was purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in dimethyl sulfoxide (DMSO) immediately before use. The final concentration of the drug vehicle (DMSO) in cell cultures was <0.1%. Antibodies specific for STAT3 and p-β-catenin (Ser675) were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibodies specific for β-catenin and β-actin were purchased from Proteintech (Rosemont, IL, USA). Horseradish peroxidase-conjugated goat anti-rabbit (ZB-2301) and anti-mouse (ZB-2305) secondary antibodies were obtained from ZSGB-BIO (Beijing, China).
Cell culture and treatment
NSCLC cell lines (A549 and H1299) were purchased from the American Type Culture Collection (Manassas, VA, USA). A549 and H1299 cells were cultured in RPMI 1640 medium (Thermo Fisher Scientific, Waltham, MA, USA), supplemented with 10% fetal bovine serum (Thermo Fisher Scientific). Cells were incubated at 37°C in a humidified incubator containing 5% CO2. Cells were treated with decitabine and/or aspirin (2 mM).
Cell migration and invasion assay
To investigate the effects of decitabine and aspirin on NSCLC cells, A549 and H1299 cells were treated with decitabine and/or aspirin for 24 hours. In the wound-healing assay, equal numbers of NSCLC cells were plated in each well of a six-well plate. At the bottom of each well, a horizontal line was drawn when the plates were covered. The wounded monolayers were washed twice with PBS to remove non-adherent cells. After adding serum-free medium, cells were incubated in a 37°C, 5% CO2 incubator. Cells were photographed at different time points (magnification, ×40) using a BX43 microscope (Olympus, Japan).
In the Transwell assay, cells were seeded in 24-well Transwell inserts (Corning, NY, USA) at a density of 2 × 104 cells, and the bottom of the inserts was filled with 200 µL of serum-free medium. The lower chambers were filled with 600 µL of complete medium. For the Transwell invasion assay, the filter in the insert was precoated with Matrigel (BD Biosciences, San Jose, CA, USA). After 12 hours of incubation at 37°C, cells remaining on the top surface of the filter were removed using a cotton swab, whereas cells that had migrated to or invaded the bottom surface were washed with PBS, fixed with methanol, and then stained with crystal violet. The stained cells were subsequently photographed (magnification, ×100) using a microscope.
Western blot analysis
Cells were harvested and lysed with mammalian protein extraction buffer (CWBIO, Boston, MA, USA) supplemented with 1 mM phenylmethylsulfonyl fluoride (Sigma-Aldrich) and 1 mM phosphatase inhibitor cocktail (Sigma-Aldrich). The concentration of protein samples was determined using a BCA Protein Assay Kit (KeyGen, Nanjing, China). The same amount of protein was loaded into each lane of 10% SDS-PAGE gels, and following electrophoresis, protein was transferred onto nitrocellulose membranes (Millipore, Billerica, MA, USA). The membranes were blocked with 5% non-fat milk and then incubated at 4°C overnight with primary antibodies (1:1000). Next, these membranes were washed three times with PBST and incubated with secondary antibodies (1:2000), and the protein bands were visualized using a Western bright ECL kit (Advansta, Menlo Park, CA, USA).
MTT assay
NSCLC cells were seeded into 96-well culture plates at a density of 1 × 104 cells/well. The cells were treated with different concentrations of decitabine and/or aspirin (2 mM) for 24 hours, and then 20 µL of MTT reagent (5 mg/mL) were added into each well, followed by incubation for 4 hours at 37°C. After the incubation, the supernatant was removed, and 150 µL of DMSO were added to dissolve the formazan crystals for 10 minutes at room temperature. After that, the optical density of each well was detected at a wavelength of 570 nm using an EnSpire™ 2300 Multilabel Reader (PerkinElmer, Waltham, MA, USA).
Colony formation assay
NSCLC cells were seeded in six-well culture dishes (1000 cells/well) for 24 hours and then treated with decitabine (10 mM) and/or aspirin (2 mM) for 24 hours. Subsequently, the medium was replaced with fresh medium every 3 days. After 10 days of culture, colonies were fixed in methanol for 15 minutes and stained with crystal violet for 15 minutes at room temperature for visualization and counting.
Animal studies
The protocol was approved by the Animal Care and Ethics Committee of Jiaxing University. Male BALB/c nude mice (4 weeks old) were obtained from the Laboratory Animal Research Center of Jiaxing University. The animals were maintained in a specific pathogen-free environment in an animal facility of Jiaxing University. A549 cells (1 × 106 cells) were inoculated subcutaneously into the flank of each mouse. When the tumor diameters reached 4 to 5 mm, mice were divided into four groups: control, decitabine, aspirin, and combination groups. Decitabine was administered via intraperitoneal injection (2.5 mg/kg per day) for 10 consecutive days, whereas aspirin was administered intragastrically at 100 mg/kg daily for 2 weeks. Tumor volumes were calculated as V = 1/2 (width2 × length).
Statistical analysis
All experiments were performed at least three times. Data are presented as the mean ± standard error of the mean. Student’s t-test or one-way analysis of variance was used to compare differences among the groups. All statistical analyses were performed using SPSS 23.0 (IBM Corp, Armonk, NY, USA) or GraphPad Prism 7.0 software (GraphPad Software, La Jolla, CA, USA). P < 0.05 was considered statistically significant.
Results
Responses of lung cancer cells to decitabine and aspirin as single agents and in combination
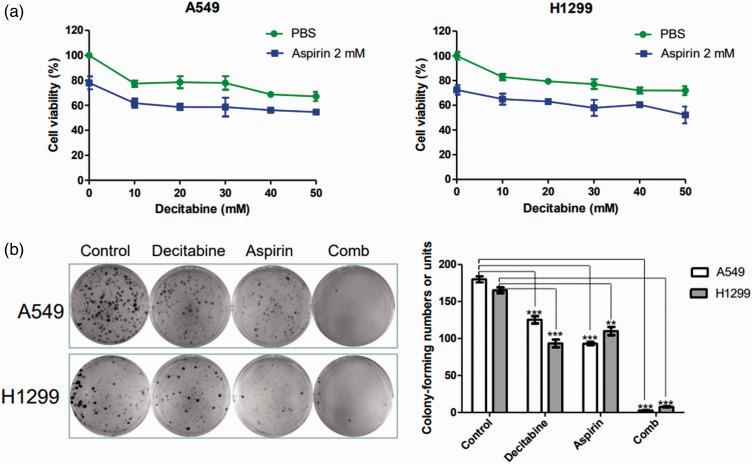
Decitabine and aspirin have anti-tumor effects on several cancers. Accumulating evidence indicates that aspirin can suppress lung cancer cell viability.17,18 To investigate the effects of decitabine and aspirin on NSCLC cells, the cells were treated with different concentration of decitabine and/or aspirin (2 mM) for 24 hours. MTT assay and colony formation assays were used to examine the anti-proliferative effects of the drugs. Figure 1a illustrates that decitabine inhibited NSCLC cell proliferation in a concentration-dependent manner. The 20% inhibitory concentration of decitabine was 10 mM, and it was selected for the subsequent experiments. In addition, decitabine in combination with aspirin robustly inhibited NSCLC cell proliferation (P < 0.05, Figure 1b).
Figure 1.
Responses of lung cancer cells to decitabine and aspirin as single agents and in combination. (a) A549 and H1299 cells were treated with decitabine (0–50 mM) and/or aspirin (2 mM) for 24 hours, and cell viability was analyzed using the MTT assay and (b) A549 and H1299 cells were treated with decitabine (10 mM) and/or aspirin (2 mM). Then, cells were analyzed using the colony formation assay. All results are expressed as the mean ± standard error of the mean (*P < 0.05, **P < 0.01, and ***P < 0.001) of at least three independent experiments.
Effects of decitabine and aspirin as single agents and in combination on the migration and invasion of lung cancer cells
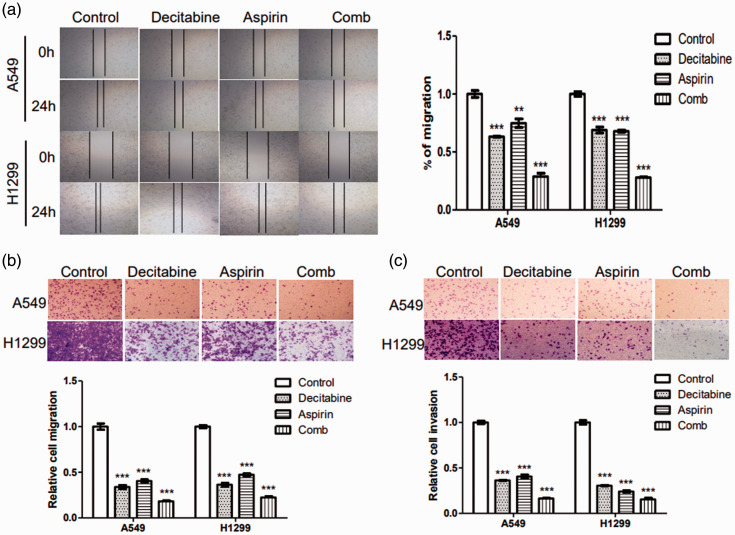
To further determine whether the combination of decitabine and aspirin had better inhibitory effects on tumor cell migration and invasion than each agent alone, wound-healing and Transwell assays were performed. Data illustrated that decitabine or aspirin alone decreased the invasiveness and migration of NSCLC cells, and the combination regimen significantly enhanced these effects (P < 0.05, Figure 2). Collectively, these results indicated that decitabine and aspirin potently inhibit NSCLC cell migration and invasion.
Figure 2.
Effects of decitabine and aspirin as single agents and in combination on the migration and invasion of lung cancer cells. A549 and H1299 cells were treated with or without decitabine (10 mM) and/or aspirin (2 mM). (a) Wound healing in the control, decitabine group, aspirin, and combination groups (magnification, ×40). (b) Migration in the control, decitabine, aspirin, and combination groups (magnification, ×100) and (c) Invasion in the control, decitabine, aspirin, and combination groups (magnification, ×100). All results are representative of at least three independent experiments. All results are expressed as the mean ± standard error of the mean (*P < 0.05, **P < 0.01, and ***P < 0.001) of at least three independent experiments.
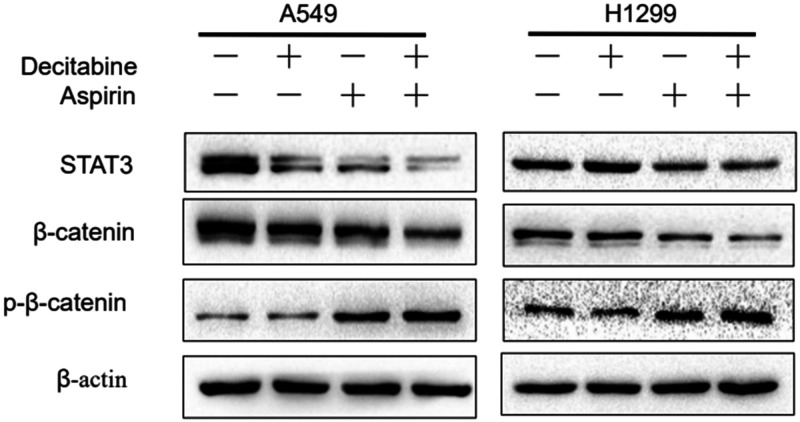
Decitabine and aspirin decrease β-catenin/STAT3 signaling activation in lung cancer cells
Various studies indicated that β-catenin/STAT3 signaling was significantly correlated with higher tumor metastasis rates and poor prognosis.19–21 However, the effects of decitabine and aspirin on β-catenin/STAT3 signaling remains unknown. The effects of decitabine and aspirin on β-catenin/STAT3 signal transduction pathways in NSCLC cells were evaluated. Western blotting illustrated that decitabine and aspirin inhibited the β-catenin/STAT3 pathway (Figure 3). In addition, decitabine and aspirin increased the phosphorylation of β-catenin and decreased the expression of β-catenin and STAT3 (Figure 3a). We therefore demonstrated that decitabine and aspirin could inhibit tumor growth and metastasis via regulation of Wnt/β-catenin signaling and inactivation of STAT3. These results indicated that β-catenin/STAT3 signaling could be a potential therapeutic target for cancer treatment.
Figure 3.
Decitabine and aspirin decreases STAT3/β-catenin signaling activation in lung cancer cells. A549 and H1299 cells were treated with or without decitabine (10 mM) and/or aspirin (2 mM), and the whole-cell lysates from each group were prepared and analyzed by western blotting to determine the expression of STAT3, β-catenin, and p-β-catenin. The data are representative of at least three independent experiments.
The anti-tumor activity of decitabine and aspirin combination therapy against human A549 xenografts
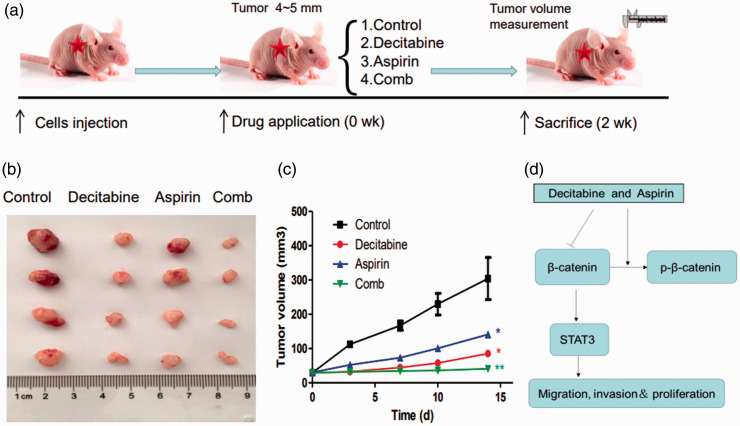
To further characterize the anti-cancer efficacy of decitabine and aspirin combination treatment, the in vivo activity of the drugs was evaluated in an A549 lung cancer xenograft model. Mice bearing tumors were treated with decitabine and/or aspirin (Figure 4a). As presented in Figure 4b, the growth of tumor xenografts was slower in the decitabine and aspirin groups than in the control group. This suggested that decitabine and aspirin could suppress the growth of lung cancer. The growth of tumor xenografts in the combination treatment group was slowest among the four groups (Figure 4c). Thus, the anti-cancer efficacy of decitabine and aspirin was further validated in vivo in A549 xenografts.
Figure 4.
The anti-tumor activity of decitabine and aspirin combination therapy against human A549 xenografts. A549 cells (1 × 106 in 100 μL of PBS) were injected subcutaneously into the flanks of mice. The xenografts were harvested after 2 weeks. (a) Schematic figure of the xenograft model used in this study. (b) Representative images of the xenografts. (c) Tumor volume was measured once every 2 days and calculated as V = 1/2 (width2 × length) and (d) The schematic illustration of the mechanism by which decitabine and aspirin regulate NSCLC cell growth and metastasis.
Discussion
Accumulating evidence has revealed the inhibitory effects of decitabine or aspirin alone on lung cancer.3,18 However, the effects of combined decitabine and aspirin therapy on lung cancer remain unclear. In this study, we investigated the effects of combined treatment with decitabine and aspirin in NSCLC in vitro and in vivo. The results of our study revealed that the regimen decreased NSCLC cell viability and inhibited the migration and invasion of lung cancer cells. Moreover, the combination of decitabine and aspirin had a significant inhibitory effect on in vivo tumor growth. Therefore, this drug combination may have a synergistic and powerful anti-cancer effect on lung cancer. However, the signaling mechanisms underlying the combined effects of these drugs on NSCLC cells are not well understood.
Previous research demonstrated that STAT3 was regulated by the β‐catenin pathway at the transcriptional level, suggesting that STAT3 was a target of β-catenin, and β-catenin/STAT3 signaling could be a potential therapeutic target for cancer treatment.22,23 These results indicated that decitabine and aspirin could inhibit the β-catenin/STAT3 pathway in NSCLC cells. Decitabine and aspirin increased the phosphorylation of β-catenin and decreased the expression of β-catenin and STAT3. These results demonstrated that the inhibition of β-catenin/STAT3 signaling could be a potential therapeutic strategy for NSCLC (Figure 4d).
In summary, our study revealed a novel mechanism of lung cancer treatment using decitabine and aspirin. The results indicated that this combination therapy inhibited tumor growth and metastasis through the β-catenin/STAT3 signaling pathway. The findings of our study emphasize the potential usefulness of the combination of decitabine and aspirin for NSCLC therapy.
Footnotes
Declaration of conflicting interest: The authors report no conflicts of interest in this work.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Project of Jiaxing Leading Medica Talents (2019-lj-05), the 2019 Jiaxing Key Discipiline of Medicine-Oncology (Supporting Subject) 2019-zc-11, and the Jiaxing Key Laboratory of tumor radiotherapy.
ORCID iD
References
- 1.Siegel R, Miller K, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017; 67: 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022; 72: 7–33. [DOI] [PubMed] [Google Scholar]
- 3.Sun Q, Zhang W, Wang L, et al. Hypermethylated CD36 gene affected the progression of lung cancer. Gene 2018; 678: 395–406. [DOI] [PubMed] [Google Scholar]
- 4.Nie J, Liu L, Li X, et al. Decitabine, a new star in epigenetic therapy: the clinical application and biological mechanism in solid tumors. Cancer Lett 2014; 354: 12–20. [DOI] [PubMed] [Google Scholar]
- 5.Yu J, Qin B, Moyer AM, et al. DNA methyltransferase expression in triple-negative breast cancer predicts sensitivity to decitabine . J Clin Invest 2018; 128: 2376–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lillo Osuna MA, Garcia-Lopez J, El Ayachi I, et al. Activation of Estrogen Receptor Alpha by Decitabine Inhibits Osteosarcoma Growth and Metastasis. Cancer Res 2019; 79: 1054–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin DY, Sung Kang H, Kim GY, et al. Decitabine, a DNA methyltransferases inhibitor, induces cell cycle arrest at G2/M phase through p53-independent pathway in human cancer cells. Biomed Pharmacother 2013; 67: 305–311. [DOI] [PubMed] [Google Scholar]
- 8.Zhang N, Liu Y, Wang Y, et al. Decitabine reverses TGF-β1-induced epithelial-mesenchymal transition in non-small-cell lung cancer by regulating miR-200/ZEB axis. Drug Des Devel Ther 2017; 11: 969–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hua H, Zhang H, Kong Q, et al. Complex roles of the old drug aspirin in cancer chemoprevention and therapy. Med Res Rev 2019; 39: 114–145. [DOI] [PubMed] [Google Scholar]
- 10.Maddison P. Effects of aspirin on small-cell lung cancer mortality and metastatic presentation. Lung Cancer 2017; 106: 67–69. [DOI] [PubMed] [Google Scholar]
- 11.Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev 2004; 56: 387–437. [DOI] [PubMed] [Google Scholar]
- 12.Wennogle LP, Liang H, Quintavalla JC, et al. Comparison of recombinant cyclooxygenase-2 to native isoforms: aspirin labeling of the active site. FEBS Lett 1995; 371: 315–320. [DOI] [PubMed] [Google Scholar]
- 13.Jiang W, Yan Y, Chen M, et al. Aspirin enhances the sensitivity of colon cancer cells to cisplatin by abrogating the binding of NF-κB to the COX-2 promoter. Aging (Albany NY) 2020; 12: 611–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rapp J, Jaromi L, Kvell K, et al. WNT signaling – lung cancer is no exception. Respir Res 2017; 18: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Song X, Yue W, et al. Fibulin-5 inhibits Wnt/β-catenin signaling in lung cancer. Oncotarget 2015; 6: 15022–15034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SJ, Kang HG, Kim K, et al. Crosstalk between WNT and STAT3 is mediated by galectin-3 in tumor progression. Gastric Cancer 2021; 5: 1050–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gan H, Lin L, Hu N, et al. Aspirin ameliorates lung cancer by targeting the miR-98/WNT1 axis. Thorac Cancer 2019; 10: 744–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Lv C, Dong Y, et al. Aspirin-targeted PD-L1 in lung cancer growth inhibition. Thorac Cancer 2020; 11: 1587–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang R, Wang S, Wang N, et al. CCL5 derived from tumor-associated macrophages promotes prostate cancer stem cells and metastasis via activating β-catenin/STAT3 signaling. Cell Death Dis 2020; 11: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi J, Su Q, Han F, et al. MiR-337 suppresses pancreatic cancer development via STAT3/Wnt/β-catenin axis. Anticancer Drugs 2021; 32: 681–692. [DOI] [PubMed] [Google Scholar]
- 21.Habibie, Yokoyama S, Abdelhamed S, et al. Survivin suppression through STAT3/β-catenin is essential for resveratrol-induced melanoma apoptosis. Int J Oncol 2014; 45: 895–901. [DOI] [PubMed] [Google Scholar]
- 22.Han L, Yue X, Zhou X, et al. MicroRNA-21 expression is regulated by β-catenin/STAT3 pathway and promotes glioma cell invasion by direct targeting RECK. CNS Neurosci Ther 2012; 18: 573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan S, Zhou C, Zhang W, et al. beta-Catenin/TCF pathway upregulates STAT3 expression in human esophageal squamous cell carcinoma. Cancer Lett 2008; 271: 85–97. [DOI] [PubMed] [Google Scholar]