Abstract
The root-associated biological control bacterium Pseudomonas aureofaciens 30-84 produces a range of exoproducts, including protease and phenazines. Phenazine antibiotic biosynthesis by phzXYFABCD is regulated in part by the PhzR-PhzI quorum-sensing system. Mutants defective in phzR or phzI produce very low levels of phenazines but wild-type levels of exoprotease. In the present study, a second genomic region of strain 30-84 was identified that, when present in trans, increased β-galactosidase activity in a genomic phzB::lacZ reporter and partially restored phenazine production to a phzR mutant. Sequence analysis identified two adjacent genes, csaR and csaI, that encode members of the LuxR-LuxI family of regulatory proteins. No putative promoter region is present upstream of the csaI start codon and no lux box-like element was found in either the csaR promoter or the 30-bp intergenic region between csaR and csaI. Both the PhzR-PhzI and CsaR-CsaI systems are regulated by the GacS-GacA two-component regulatory system. In contrast to the multicopy effects of csaR and csaI in trans, a genomic csaR mutant (30-84R2) and a csaI mutant (30-84I2) did not exhibit altered phenazine production in vitro or in situ, indicating that the CsaR-CsaI system is not involved in phenazine regulation in strain 30-84. Both mutants also produced wild-type levels of protease. However, disruption of both csaI and phzI or both csaR and phzR eliminated both phenazine and protease production completely. Thus, the two quorum-sensing systems do not interact for phenazine regulation but do interact for protease regulation. Additionally, the CsaI N-acylhomoserine lactone (AHL) signal was not recognized by the phenazine AHL reporter 30-84I/Z but was recognized by the AHL reporters Chromobacterium violaceum CV026 and Agrobacterium tumefaciens A136(pCF240). Inactivation of csaR resulted in a smooth mucoid colony phenotype and formation of cell aggregates in broth, suggesting that CsaR is involved in regulating biosynthesis of cell surface components. Strain 30-84I/I2 exhibited mucoid colony and clumping phenotypes similar to those of 30-84R2. Both phenotypes were reversed by complementation with csaR-csaI or by the addition of the CsaI AHL signal. Both quorum-sensing systems play a role in colonization by strain 30-84. Whereas loss of PhzR resulted in a 6.6-fold decrease in colonization by strain 30-84 on wheat roots in natural soil, a phzR csaR double mutant resulted in a 47-fold decrease. These data suggest that gene(s) regulated by the CsaR-CsaI system also plays a role in the rhizosphere competence of P. aureofaciens 30-84.
Numerous plant- and animal-associated bacteria regulate the expression of specific sets of genes in response to their own population densities, a phenomenon termed quorum sensing (10, 33). Most quorum-sensing systems thus far identified in gram-negative bacteria employ N-acylhomoserine lactones (AHL) as signaling molecules. AHL signals, which differ in the length and substitution of their acyl side chains, are generated by a single enzyme (a member of the LuxI protein family) (11, 25, 30). These signals accumulate with increasing cell density and upon reaching a threshold concentration bind a transcriptional regulator that in turn activates or represses target gene expression. Over 30 bacterial species have been shown to use quorum-sensing circuits to regulate diverse functions, including bioluminescence, virulence factor production, plasmid conjugal transfer, biofilm formation, motility, symbiosis, and antibiotic production (7).
Pseudomonas aureofaciens strain 30-84, isolated from the wheat rhizosphere, is a biological control agent effective in inhibiting Gaeumannomyces graminis var. tritici, the causal agent of take-all disease of wheat (34). The production of three phenazine antibiotics by strain 30-84 is responsible for its suppressive capacity (34) and its ability to persist on wheat roots (21). In addition to phenazines, this bacterium has been found to produce exoprotease, siderophores, and hydrogen cyanide (6). However, the specific roles of these compounds (all of which were reported to be responsible for disease suppression by other bacterial biocontrol agents [40]) in the antagonism of strain 30-84 against plant pathogens are unknown. Phenazine antibiotic biosynthesis in strain 30-84 is regulated at multiple levels. The PhzR-PhzI quorum-sensing system regulates phenazine production in a cell density-dependent manner (35, 42). The phzR gene encodes a transcriptional regulator of the phenazine operon, and phzI encodes an AHL synthase that directs the synthesis of the signal hexanoylhomoserine lactone (HHL). Upon binding HHL, PhzR becomes activated, thereby inducing transcription of the phenazine genes. The GacS-GacA two-component signal transduction system is also involved in controlling phenazine production, partly via regulating transcription of phzI and partly via other regulatory elements (6). Mutation of gacS or gacA has pleiotropic effects, eliminating production of HHL, phenazines, exoprotease, and HCN and increasing fluorescence (6). However, phzI and phzR null mutants produced wild-type levels of protease, HCN, and siderophores (unpublished data). Production of these compounds is regulated in a cell density-dependent manner in a number of other bacterial species (5, 17, 22). Interestingly, phzI and phzR null mutants produced phenazines at low levels on a certain medium. Recently, several bacteria were shown to harbor two or more quorum-sensing systems that regulate expression of the same or different factors (12, 15, 37). Taken together, the above results suggested that strain 30-84 might contain an additional regulatory system acting independently or cooperatively with the PhzR-PhzI system to mediate secondary metabolite production.
In this study, we report the identification of a second quorum-sensing system, CsaR-CsaI, in P. aureofaciens strain 30-84. The nature of the interaction between CsaR-CsaI and PhzR-PhzI in regulating phenazine and exoprotease production and rhizosphere colonization was examined. In addition, several phenotypes regulated specifically by the CsaR-CsaI system were identified.
MATERIALS AND METHODS
Strains and plasmids.
The bacterial strains and plasmids used are listed in Table 1. P. aureofaciens strain 30-84, a spontaneous rifampin-resistant mutant of the wild-type strain (35), and its derivatives were grown at 28°C in Luria-Bertani (LB) medium (19), King's B medium (KMB) (14), M9 minimal medium (19), AB minimal medium (38), skim milk-water agar (6), or pigment production medium (PPM-D) (42). Chromobacterium violaceum CV026 (16) and Agrobacterium tumefaciens A136(pCF240) (9) were grown at 28°C in LB or AB medium. Escherichia coli strains were cultured in LB medium at 37°C. Where applicable, antibiotics were used at the following concentrations (in micrograms per milliliter): for E. coli, ampicillin at 100, gentamicin (GM) at 25, kanamycin (KM) at 50, and tetracycline (TC) at 25; for P. aureofaciens, KM at 50, rifampin at 100, TC at 50, and GM at 50; for A. tumefaciens, KM at 150, spectinomycin at 50, and TC at 10.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| P. aureofaciens | W. Bockus | |
| 30-84 | Wild type, plant disease biocontrol agent, Rifr | W. W. Bockus |
| 30-84Z | phzB::lacZ genomic fusion | 35 |
| 30-84Ice | phzB::inaZ genomic fusion | 43 |
| 30-84I | phzI::npt genomic fusion, Kmr | 42 |
| 30-84Z/I | phzB::lacZ and phzI::npt genomic fusion, Kmr | 42 |
| 30-84Ice/I | phzB::inaZ and phzI::npt genomic fusion, Kmr | 43 |
| 30-84R | phzR::Tn5lacZ genomic fusion, Kmr | 35 |
| 30-84I2 | csaI::uidA-Gm genomic fusion, Gmr | This study |
| 30-84Ice/I2 | phzB::inaZ and csaI::uidA-Gm genomic fusion, Gmr | This study |
| 30-84R2 | csaR::uidA-Gm genomic fusion, Gmr | This study |
| 30-84Ice/R2 | phzB::inaZ and csaR::uidA-Gm genomic fusion, Gmr | This study |
| 30-84I/I2 | phzI::npt and csaI::uidA-Gm genomic fusion, Kmr Gmr | This study |
| 30-84I/R2 | phzI::npt and csaR::uidA-Gm genomic fusion, Kmr Gmr | This study |
| 30-84I2/R | csaI::uidA-Gm and phzI::npt genomic fusion, Gmr Kmr | This study |
| 30-84R/R2 | phzR::Tn5lacZ and csaR::uidA-Gm genomic fusion, Kmr Gmr | This study |
| 30-84 gacA | gacA::npt genomic fusion, Kmr | 6 |
| 30-84Z/sgacA | phzB::lacZ genomic fusion, spontaneous gacA mutant of 30-84Z | 6 |
| C. violaceum CV026 | Double mini-Tn5 mutant from C. violaceum ATCC 31532, AHL biosensor | 16 |
| A. tumefaciens A136 | Ti plasmid-less, pCF240traA::lacZ113, pCF251 | 9 |
| E. coli | ||
| DH5α | F−recA1 endA1 hsdR17 supE44 thi-1 gyrA96 relA1 Δ(argE-lacZYA)169 φ80lacZΔM15 | Gibco-BRL |
| MC1061 | F−araD139 Δ(araABC-leu)7679 galU galK Δ(lac) X74 rpsL thi | Gibco-BRL |
| Plasmids | ||
| pLAFR3 | IncP1, Tcr | 39 |
| pMGm | ColE1, Gmr | 26 |
| pWM3 | ColE1, Apr, uidA transcriptional fusion cassette | 23 |
| pUC18 | ColE1, Apr | 44 |
| pLSP5-5 | pLAFR3 carrying a 29-kb fragment of 30–84 chromosomal DNA that contains csaI and csaR | This study |
| pZZG5-5-2 | pLAFR3 carrying the 7.15-kb EcoR1 fragment from pLSP5-5 that contains csaI and csaR | This study |
| PZZG3 | pLAFR3 carrying the 5-kb PstI-HindIII fragment from pIC20H5-5-2 that contains csaI and csaR | This study |
| pZZG11 | pLAFR3 carrying the 1-kb SphI-EcoRV fragment that contains csaR | This study |
| pZZGP18-1 | pUC18 carrying the 5-kb PstI-HindIII fragment that contains csaI and csaR | This study |
| pZZGP18-2 | pZZGP18-1 containing the introduced SacI site in csaI | This study |
| pZZGP18-3 | pZZGP18-1 containing the introduced SacI site in csaR | This study |
| pWM3-Gm | pWM3 carrying the 2-kb SalI fragment from pMGm, Gmr | This study |
| pZZGI-Sac | pLAFR3 carrying the 5-kb PstI-HindIII fragment from pZZGP18-2 | This study |
| pZZGR-Sac | pLAFR3 carrying the 5-kb PstI-HindIII fragment from pZZGP18-3 | This study |
| pZZGI-uidAGm | pZZGI-Sac carrying the 4-kb SacI fragment from pWM3-Gm that contains uidA-Gm | This study |
| pZZGR-uidAGm | pZZGR-Sac carrying the 4-kb SacI fragment from pWM3-Gm that contains uidA-Gm | This study |
| pUC18-csaI | pUC18 containing the 2.5-kb SacI-SphI fragment from pZZGP18–4 | This study |
| pLSPphzB-inaZ | pLAFR3 containing the phzB::inaZ fusion | 43 |
Screening for the presence of a new luxR-luxI homologue.
A cosmid library of strain 30-84 was mobilized into the indicator phzB::lacZ reporter 30-84Z or the phzR mutant 30-84R through triparental mating as described previously (35). In the case of 30-84Z, the Rifr and Tcr transconjugants were inoculated on M9 agar containing TC and 4% (wt/vol) 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). After incubation (24 h), β-galactosidase activity of the transconjugants was determined by examining the blue intensity and presence or absence of a blue halo around the colonies. When strain 30-84R was used, transconjugants were assessed for phenazine production on LB agar supplemented with TC by the orange intensity on and around the colonies.
DNA manipulations.
DNA isolations, restriction enzyme digestions, agarose gel electrophoresis, ligations, transformations, and Southern hybridizations were carried out as described previously (6, 34).
Oligonucleotides for PCR and DNA sequencing were synthesized by Gibco-BRL (Gaithersburg, Md.). DNA sequencing was performed at the University of Arizona Biotechnology Center using an Applied Biosystems automatic DNA sequencer (model 373A, version 1.2.1.). Sequence analysis was performed with the University of Wisconsin Genetics Computer Group Software packages (version 9.1).
Construction of 30-84I2, 30-84R2, and double mutants.
Since Kmr and lacZ were used previously to construct strains 30-84I (phzI::npt) and 30-84R (phzR::Tn5lacZ), mutants with mutations in csaR and csaI were constructed using a β-glucuronidase-and-gentamicin resistance (uidA-Gm) cartridge constructed in our laboratory. A 2-kb SalI fragment from pMGm (26) was inserted into the SalI site of pWM3 (23), resulting in pWM3-Gm. The 5-kb PstI-HindIII fragment from pZZG5-5-2 (Fig. 1A) was ligated into pUC18, resulting in pZZGP18-1. PCR primers were designed to create a SacI restriction site in either the csaI or csaR coding region in pZZGP18-1, as described in the QuickChange site-directed mutagenesis kit (Stratagene), yielding pZZGP18-2 and pZZGP18-3, respectively. The primers for introduction of the SacI site (underlined) in csaI are 5′-CACGCCGGCGCTGGAGCTCGTTATTTCCTGC-3′ (forward) and 5′-GCAGGAAATAACGAGCTCCAGCGCCGGCGTG-3′ (reverse), and those for csaR are 5′-GCCTTGTTCGGCAAGAGCTCGGTGTTGTG-3′ (forward) and 5′-CACAACACCGAGCTCTTGCCGAACAAGGC-3′ (reverse). The 5-kb PstI-HindIII fragment with the engineered SacI site in csaI or in csaR was ligated into pLAFR3 to create pZZGI-Sac or pZZGR-Sac. The csaI or csaR genes were disrupted by insertion of the 4-kb SacI uidA-Gm cartridge from pMW3-Gm into the engineered SacI site in csaI contained in pZZGI-Sac and in csaR contained in pZZGR-Sac. The resultant plasmids, pZZGI-uidAGm and pZZGR-uidAGm (Fig. 1B), were mobilized into P. aureofaciens via triparental mating and introduced into the genome via homologous recombination. Mutants resistant to GM but sensitive to TC were selected and verified by Southern hybridization (data not shown). Two mutants, named 30-84I2 (csaI) and 30-84R2 (csaR), were selected. Double mutants 30-84I/I2 (phzI csaI), 30-84R/R2 (phzR csaR), 30-84I/R2 (phzI csaR), and 30-84I2/R (csaI phzR) were constructed analogously as described above and confirmed by Southern hybridization (data not shown).
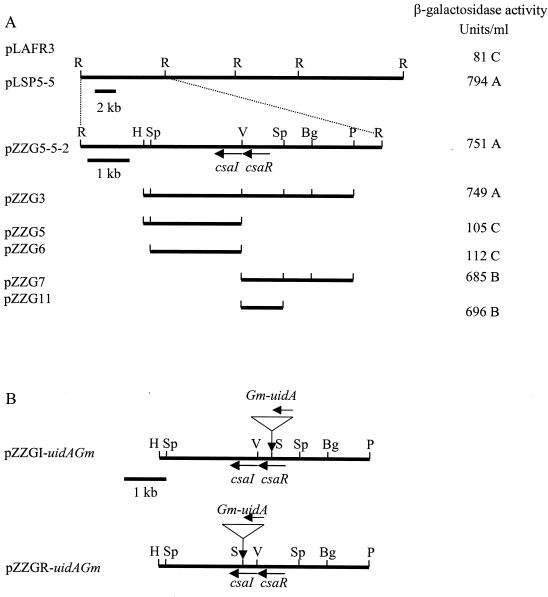
FIG. 1.
Localization and physical map of the regions containing csaI or csaR and construction of disrupted versions of csaI and csaR. Arrows represent the location and orientation of the genes. (A) The location of the regions leading to elevated β-galactosidase activity was determined by measuring the effects of the indicated subclones of pLSP5-5 on β-galactosidase expression in the phzB::lacZ reporter strain 30-84Z. Letters indicate significant differences between values. (B) csaR and csaI loci from pZZG3 and insertional mutation of csaR and csaI. Restriction enzyme abbreviations: R, EcoRI; H, HindIII; Sp, SphI; V, EcoRV, Bg, BglII, P, PstI; S, SacI.
Assays for exoproducts.
Exoprotease activity was assessed qualitatively on skim milk agar (6) and quantitatively by a modified Lowry method (41) using the substrate 1% (wt/vol) casein in 50 mM Tris-acetate (pH 7.5), prepared as described by Belew and Porath (4). One enzyme unit of exoprotease activity was defined as the amount of enzyme that liberated 1 μg of tyrosine per min at 30°C.
Phenazine production was qualitatively determined by examining orange color on PPM-D medium and quantified from cell extracts as described previously (34). HCN production and cell fluorescence indicative of siderophores were determined as described previously (6).
Colony morphology of strain 30-84 and its mutant derivatives was determined on KMB, AB, LB, or PPM-D plates. After 2 days, cultures were examined for various characteristics, such as mucoidy, shininess, and roughness. To determine whether bacterial cells clumped, strains were cultured in KMB for 24 h. Three microliters of culture was spotted onto a slide, air dried, and stained with Congo red. The cells were examined for distribution, the presence of capsules, and aggregation under light microscopy. For each culture, three slides (five fields per slide) were examined.
AHL detection and quantification.
An AHL donor strain, 30-84Ice/I2 (phzI+ csaI:uidA-Gm phzB::inaZ), was constructed by introduction of pLSPphzB-inaZ (42) into 30-84I2 via triparental mating and was verified via Southern hybridization (data not shown). To determine whether csaI can direct synthesis of a diffusible signal, the 3.2-kb SphI fragment containing csaI was excised from pZZGP18-3 and ligated into pUC18 (44) in the same orientation as in pZZGP18-3. The resultant plasmid was digested with SacI and religated, generating pUC18-csaI, which carries csaI under the control of the vector lacZ promoter. E. coli DH5α(pUC18-csaI) was used as a CsaI AHL donor strain.
The AHL-dependent reporter strains 30-84I/I2 (phzI::npt csaI::uidA-Gm), 30-84I/Z (phzI::npt phzB::lacZ), and C. violaceum CV026 (cviI mutant) were tested for their ability to respond to exogenously added AHL compounds in a cross-feeding assay. Strains 30-84Ice/I (phzB::inaZ phzI::npt csaI+) and 30-84Ice/I2 (phzB::inaZ csaI::uidA-Gm phzI+) were used as the AHL donor strains. AHL donor strains were streaked onto one side of a plate, while the reporter strain was streaked onto the other side of the plate. When 30-84I/I2 was used as the AHL recipient, the assay was performed on PPM-D agar to examine phenazine production. Skim milk plates and KMB plates were used for examination of exoprotease activity and colony surface roughness of the double I mutant, respectively. When strain 30-84I/Z was used, the recipient and donor were streaked onto PPM-D plus X-Gal. When CV026 was the recipient, the assay was performed on LB broth. The plates were incubated 1 to 2 days before being evaluated for cross-communication.
To quantify AHL production, culture supernatants of P. aureofaciens or E. coli strains were extracted with acidified ethyl acetate as described previously (32). AHL extracts added to LB broth were tested for activation of β-galactosidase activity in strains 30-84I/Z and A. tumefaciens A136(pCF240). β-Galactosidase activity was measured as described by Miller (24). Strain 30-84Z/sgacA (6), a spontaneous gacA mutant of 30-84Z, was used as a negative reporter, and the extract from E. coli DH5α(pUC18) was used as a negative control.
Growth chamber assays for rhizosphere colonization and phenazine production.
Wheat seeds (cv. Penewawa) were surface sterilized and treated with bacterial cultures as described previously (32). Briefly, treated seeds were sown in 30- by 30-cm plastic cones (two seeds per cone) containing 25 cm of steam-pasteurized or natural field soil mixed with vermiculite and sand in equal volumes and moistened with 10 ml of sterile 1/3 Hoagland's solution (13) prior to planting. Each treatment was repeated in at least four cones, which were arranged in a randomized complete block design. The seeds were covered with a 1-cm layer of the potting medium and incubated in a Conviron growth chamber (20°C during the light period and 15°C during the dark period, ∼75% relative humidity, 12-h photoperiod). Four weeks after emergence, the roots were aseptically excised, and bacterial populations were determined on KMB plus rifampin as described previously (32).
The effect of the CsaR-CsaI system on phenazine operon expression in situ was determined by comparing ice nucleation activities of the isogenic reporter strains 30-84Ice/R2 (csaR::uidA-Gm phzB::inaZ) and 30-84Ice/I2 with that of 30-84Ice (phzB::inaZ) in the wheat rhizosphere. Wheat seeds were prepared, treated, and grown as described above. Twenty days after emergence, bacterial populations and ice nucleation activities were determined. Ice nucleation activity was expressed as ice nucleation frequency calculated on a per-cell level, as described by Lindgren et al. (18).
Statistical analysis.
All experiments described above were repeated at least once. Data from the two experiments were pooled, and analysis of variance was used to analyze data. Fisher's least significant difference and Duncan's multiple range tests were used to compare means.
Nucleotide sequence accession number.
The nucleotide sequences of the csaI and csaR genes have been deposited in GenBank (accession no. AY040629).
RESULTS
Identification of CsaR and CsaI.
A cosmid library of strain 30-84 was mobilized into the reporter strains 30-84Z and 30-84R to search for genomic regions able to enhance phenazine production. Strain 30-84Z and strain 30-84R transconjugants were tested for β-galactosidase activity and for phenazine production, respectively. A single cosmid (pLSP5-5) when present in trans in strain 30-84Z resulted in a dark blue colony surrounded by a blue halo (data not shown). When cultured in M9 broth, 30-84Z(pLSP5-5) expressed ninefold-higher β-galactosidase activity than 30-84Z(pLAFR3) (Fig. 1A). The same cosmid restored phenazine production to strain 30-84R, as indicated by an orange colony compared to the white 30-84R(pLARF3) colony (data not shown). Analysis of pLSP5-5 indicated the presence of a 29-kb insert comprised of EcoRI fragments of 5, 7.2, 7.5, and 10 kb, respectively. Only the 7.2-kb fragment in pZZG5-5-2 was effective in enhancing β-galactosidase activity in strain 30-84Z (Fig. 1A). Further deletion analysis revealed that the 2.2-kb SphI-EcoRV fragment and the 1-kb EcoRV-SphI fragment resulted in elevated β-galactosidase activity.
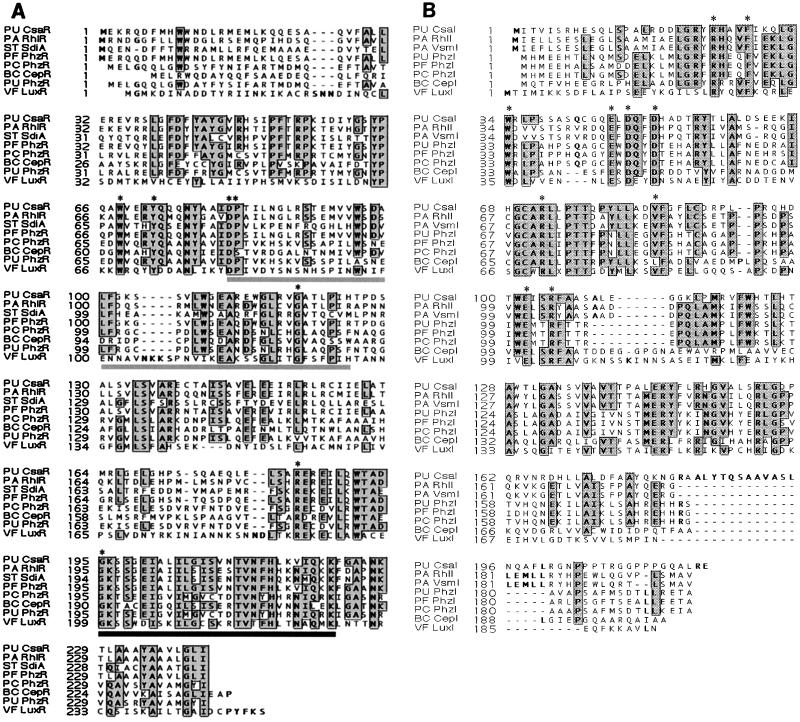
DNA sequence analysis of the 1,034-bp EcoRV-SphI fragment revealed the presence of one 723-nucleotide open reading frame (ORF), designated CsaR. A putative ribosome binding site (RBS), AGGA, is located eight nucleotides upstream from the CsaR start codon. Potential promoter sequences for csaR include a −10 region (TAGATT) and −35 region (TTGACA). This 723-nucleotide ORF can encode a 241-amino-acid protein with a predicted molecular mass of 27.2 kDa. BLAST searches revealed similarity between the deduced amino acid sequence of CsaR and diverse members of the LuxR family (Fig. 2A). It has 67, 37, 36, 35, 35, and 33% identity (80, 52, 53, 52, and 51% similarity) with Pseudomonas aeruginosa RhlR (27), Salmonella enterica serovar Typhimurium SdiA (GenBank accession number U88651), P. aureofaciens 30-84 PhzR (35), Pseudomonas fluorescens PhzR (20), Pseudomonas chlororaphis PhzR (GenBank accession number AF195615), and Burkholderia cepacia CepR (17), respectively.
FIG. 2.
Multiple alignment of CsaR and CsaI with other LuxR-LuxI family members. Regions in which amino acids are identical in at least five proteins are boxed and shaded. (A) Alignment of CsaR with seven other LuxR family members. The gray bar below LuxR residues 81 to 129 represents the conserved autoinducer-binding domain (10). The black bar below LuxR residues 199 to 226 represents the putative helix-turn-helix region of the DNA-binding domain. The seven invariant amino acids of LuxR homologs are indicated with asterisks (8). Abbreviations: PA RhlR, P. aeruginosa PAOI RhlR (L08962); PA VsmR, P. aeruginosa VsmR (U15644); ST SdiA, S. enterica serovar Typhimurium SdiA (U88651); PF PhzR, P. fluorescens 2-79 PhzR (L48616); BC CepR, B. cepacia CepR (AF01954); PU PhzR, P. aureofaciens PU PhzR (L32729); VF LuxR, Vibrio fischeri LuxR (Y00509). (B) Alignment of CsaI with seven representative LuxI family members. The 10 invariant amino acids characteristic of LuxI homologs are labeled with asterisks (29). Abbreviations: PA RhlI, P. aeruginosa PAOI RhlI (AE004768); PA VsmI, P. aeruginosa VsmI (U15644); PU PhzI, P. aureofaciens 30-84 PhzI (L33724); PF PhzI, P. fluorescens 2-79 PhzI (L48616); PC PhzI, P. chlororaphis PhzI (AF195615); BC CepI, B. cepacia CepI (AF019654); VF LuxI, V. fischeri LuxI (Y00509).
A second ORF was found in the 2,204-bp SphI-EcoRV fragment adjacent to csaR and was designated csaI. The CsaI ORF contains 657 nucleotides and potentially encodes a 219-amino-acid protein of 24.4 kDa. Since the translational start codon is located only five nucleotides downstream from the EcoRV site, the RBS and promoter region are probably outside the fragment. When the sequences from both fragments were assembled, the CsaI ORF was found to be located only 30 bp downstream from the CsaR ORF and contained a putative RBS, TAAGGA, 9 bp upstream from the start codon. No promoter region was identified in the 30-bp region, indicating that csaI may be cotranscribed with csaR. No consensus lux box sequence (12) was found in either the 30-bp intergenic region or the csaR promoter region. CsaI shared significant homology with numerous AHL synthases (Fig. 2B). It has 53, 43, 39, 39, and 39% identity (69%, 56, 58, 55, 57, and 56% similarity) to P. aeruginosa RhlI (28), B. cepacia CepI (17), P. aureofaciens PhzI (42), P. fluorescens PhzI (20), and P. chlororaphis PhzI (AF195615), respectively.
CsaR-CsaI is not required for phenazine gene expression.
To determine whether the CsaR-CsaI quorum-sensing system is involved in regulating production of phenazines or other factors, we individually disrupted the genomic csaI locus and csaR locus by inserting a uidA-Gm cartridge in the genes, resulting in strains 30-84I2 (csaI::uidA-Gm) and 30-84R2 (csaR::uidA-Gm). Similarly, four double mutants, 30-84I/I2 (phzI::km csaI::uidA-Gm), 30-84I/R2 (phzI::npt csaR::uidA-Gm), 30-84I2/R (csaI::uidA-Gm phzR::Tn5lacZ), and 30-84R/R2 (phzR::Tn5lacZ csaR::uidA-Gm), were constructed. All mutants were verified by Southern hybridization using a 2-kb DNA fragment containing csaI and csaR as a probe, with all csaR mutants displaying fragments of 4.9 and 6.2 kb and all csaI mutants displaying fragments of 5.3 and 5.8 kb.
When cultured on agar medium, loss of either csaR or csaI had no effect on phenazine production compared to that of strain 30-84 (data not shown). Both mutants initially produced slightly lower levels of the antibiotics than 30-84 in PPM-D broth after 24 h, but no significant differences in the amount of phenazines was detected among the three strains after 72 h (Table 2). As found previously, loss of phzR or phzI resulted in ca. 10% of the wild-type levels of phenazines after 72 h on PPM-D medium. In contrast to the single mutants, all double mutants (phzI csaI, phzI csaR, csaI phzR, and phzR csaR strains) failed to produce detectable phenazines on PPM-D at any time, as judged by colony color (data not shown) and the absence of detectable absorbance (optical density at 467 nm) of culture extracts (Table 2).
TABLE 2.
Phenotypes of P. aureofaciens 30-84 and its derivatives
| Strain | Genotype | Absorbance at 367 nmac
|
Protease activitybc (U/ml) | Colony morphologyd | Clumping phenotypee | |
|---|---|---|---|---|---|---|
| After 24 h | After 72 h | |||||
| 30-84 | Wild type | 6.46 ± 0.09 A | 3.05 ± 0.14 A | 19.80 ± 1.83 A | Semidry and rough | − |
| 30-84I | phzI::npt | 0.04 ± 0.00 C | 0.30 ± 0.02 B | 21.13 ± 2.91 A | Semidry and rough | − |
| 30-84R | phzR::Tn5lacZ | 0.04 ± 0.00 C | 0.24 ± 0.01 B | 18.59 ± 2.68 A | Semidry and rough | − |
| 30-84I2 | csaI::uidA-Gm | 5.96 ± 0.18 B | 2.80 ± 0.28 A | 19.06 ± 1.56 A | Semidry and rough | − |
| 30-84R2 | csaR::uidA-Gm | 5.99 ± 0.33 B | 2.89 ± 0.22 A | 19.72 ± 1.98 A | Shiny and mucoid | + |
| 30-84I/I2 | phzI::npt csaI::uidA-Gm | 0.02 ± 0.00 C | 0.03 ± 0.00 C | 0.71 ± 0.00 B | Shiny and mucoid | + |
| 30-84I/R2 | phzI::npt csaR::uidA-Gm | 0.03 ± 0.01 C | 0.05 ± 0.01 C | 18.64 ± 1.78 A | Shiny and mucoid | + |
| 30-84I2/R | csaI::uidA-Gm phzR::Tn5lacZ | 0.03 ± 0.01 C | 0.05 ± 0.01 C | 21.04 ± 2.75 A | Semidry and rough | − |
| 30-84R/R2 | phzR::Tn5lacZ csaR::uidA-Gm | 0.02 ± 0.01 C | 0.02 ± 0.01 C | 0.35 ± 0.00 B | Shiny and mucoid | + |
| 30-84.gacA | gacA::npt | 0.02 ± 0.01 C | 0.03 ± 0.01 C | 0.00 ± 0.00 B | Shiny and mucoid | + |
Phenazines were extracted from supernatants of 24- and 72-h-old bacterial cultures grown in PPM-D and quantified by measuring absorbance at 367 nm.
Exoprotease activity in supernatants of 24-h-old broth cultures was determined by a modified Lowry method using casein as the substrate.
All values are means ± standard deviations of two experiments, with three replicates per experiment. Values followed by the same letter in a column are not significantly different (P = 0.05).
Bacterial strains were cultured on KMB agar, and 2 days later the colonies were examined for roughness and mucoidy.
Bacterial strains were cultured on KMB broth, and 1 day later the cultures were stained with Congo red and observed for the aggregation of bacterial cells.
To determine if csaR in trans restored phenazine production, pZZG11 (Fig. 1A) was introduced into strains 30-84I/R2 (phzI csaR), 30-84R/R2 (phzR csaR), and 30-84I/I2 (phzI csaI). When cultured in PPM-D, 30-84I/R2(pZZG11) and 30-84R/R2(pZZG11) produced phenazines at ca. 40% of the wild-type level. Strain 30-84I/I2(pZZG11) did not produce detectable phenazine after 24 h (Table 3) but did produce low levels after 48 h (data not shown). Strains 30-84I/I2(pZZG3) and 30-84I2/R(pZZG3) were assayed for phenazine production. The presence of csaR csaI in trans also restored production to ca. 40% of wild-type levels (Table 3). This partial complementation also occurred in LB medium and KMB (data not shown).
TABLE 3.
Complementation of P. aureofaciens 30-84 derivatives with csaR or csaR-csaI in trans
| Strainf | Genotype (host/plasmid) | Absorbance at 367 nmac | Protease activitybc | Colony morphologyd | Clumping phenotypee |
|---|---|---|---|---|---|
| 30-84(pLAFR3) | Wild type | 5.91 ± 0.29 A | + | Semidry and rough | − |
| 30-84R(pLAFR3) | phzR::Tn5lacZ | 0.03 ± 0.01 C | + | Semidry and rough | − |
| 30-84R2(pZZG11) | csaR::uidA-Gm/csaR+ | 5.98 ± 0.39 A | + | Semidry and rough | − |
| 30-84I/I2(pZZG11) | phzI::npt csaI::uidA-Gm/csaR+ | 0.04 ± 0.01 C | − | Semidry and rough | − |
| 30-84I/I2(pZZG3) | phzI::npt csaI::uidA-Gm/csaR+ csaI+ | 2.67 ± 0.11 B | − | Semidry and rough | − |
| 30-84I/R2(pZZG11) | phzI::npt csaR::uidA-Gm/csaR+ | 2.36 ± 0.20 B | + | Semidry and rough | − |
| 30-84I2/R(pZZG3) | csaI::uidA-Gm phzR:Tn5lacZ/csaR+csaI+ | 2.57 ± 0.20 B | + | Semidry and rough | − |
| 30-84R/R2(pZZG11) | phzR::Tn5lacZ, csaR::uidA-Gm/csaR+ | 2.42 ± 0.19 B | − | Semidry and rough | − |
| 30-84.gacA(pZZG3) | gacA::npt/csaR+csaI+ | 0.03 ± 0.00 C | − | Shiny and mucoid | + |
Phenazines were extracted from supernatants of 24-h-old bacterial cultures grown in PPM-D and quantified by measuring absorbance at 367 nm.
Exoprotease activity in supernatants of 24-h-old broth cultures was determined by a modified Lowry method using casein as the substrate.
All values are means ± standard deviations of two experiments, with three replicates per experiment. Values followed by the same letter in a column are not significantly different (P = 0.05).
Bacterial strains were cultured on KMB agar, and 2 days later the colonies were examined for roughness and mucoidy.
Bacterial strains were cultured on KMB broth for 24 h, and the cultures were stained with Congo red and observed for the presence of the clumping phenotype.
The presence of the vector pLAFR3 alone had no effect on any of the strains listed (data not shown).
The ice nucleation reporter strains 30-84Ice/I2 (phzB::inaZ csaI::uidA-Gm) and 30-84Ice/R2 (phzB::inaZ csaR::uidA-Gm) were used to determine whether csaI or csaR is required for phenazine gene expression in the wheat rhizosphere. These strains, together with strain 30-84Ice, contain a genomic phzB::inaZ fusion and synthesize ice nucleation protein under the control of the phenazine operon promoter. The amount of ice nucleation activity (InaZ) is proportional to the amount of phenazines produced by the csaI or csaR mutation. Twenty days after emergence, high InaZ activity (−1.6 to −1.8 log nuclei/cell) was detected from each bacterial strain isolated from roots in comparison to the negative control strain 30-84.gacA (6), which showed no activity. However, there was no significant difference in ice nucleation activity (−1.61, −1.78, and −1.59 log nuclei per cell for 30-84Ice, 30-84Ice/I2, and 30-84Ice/R2, respectively) among the three strains. These results are consistent with the effect of mutations in csaI or csaR in strain 30-84 on phenazine production in vitro, further indicating that the CsaR-CsaI system is not required for phenazine biosynthesis by strain 30-84.
CsaR and CsaI are involved in exoprotease activity.
Exoprotease activity was compared between strain 30-84 and mutant derivatives. Strain 30-84 and derivatives with single null mutations in csaI, csaR, phzI, or phzR or with phzI csaR or csaI phzR double mutations produced extracellular protease, as shown by the presence of indistinguishable clear zones on skim milk agar (data not shown). However, phzI csaI and phzR csaR double mutants failed to produce any clear zone. Quantitative assays of culture supernatants showed that all single mutants and the csaI phzR and phzI csaR double mutants exhibited levels of proteolysis similar to those of strain 30-84 (Table 2). The double mutants in which both AHL synthases (PhzI and CsaI) or both transcriptional activators (PhzR and CsaR) were inactivated had significantly diminished protease activity comparable to that of a GacA null mutant (30-84.gacA). Surprisingly, introduction of csaI-csaR into the phzI csaI double mutant or introduction of csaR in trans into the phzR csaR double mutant failed to restore protease activity (Table 3).
No differences in HCN production or fluorescence between strain 30-84 and any of the single or double mutants were detected (data not shown).
CsaR and the AHL signal are required for expression of two surface traits.
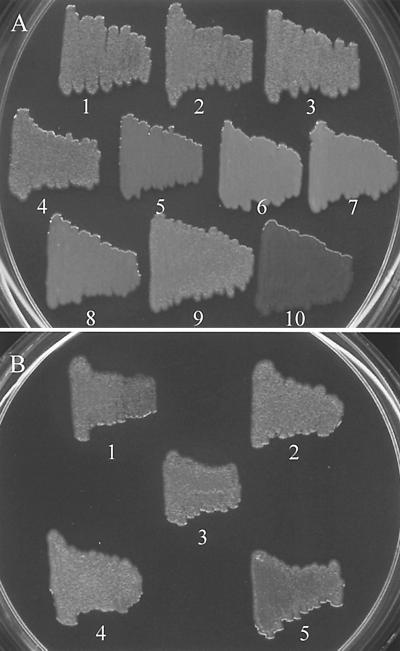
Mutations in csaR, regardless of other alterations, exhibited a shiny, mucoid phenotype on KMB agar in contrast to the rough, semidry phenotype of strain 30-84 (Fig. 3A). Like the csaR mutants, strain 30-84I/I2 also showed a mucoid phenotype (Fig. 3A). Strains that contained a functional csaR and at least one AHL synthase gene, such as 30-84I, 30-84R, 30-84I2, and 30-84I2/R, produced bacterial colonies with a rough, semidry surface (Fig. 3A). Each of the above strains also showed the same phenotype on AB agar (data not shown). In contrast, all the mutant strains and strain 30-84 displayed the same mucoid phenotype when grown on PPM-D or LB agar (data not shown). Strain 30-84(pZZG11) containing multiple copies of csaR showed a rough phenotype not only on KMB and AB agar but also on PPM-D and LB agar (data not shown). Complementation of the csaR mutation in 30-84R2, 30-84R/R2, and 30-84I/R2 by pZZG11 resulted in colonies with surface morphologies indistinguishable from that of strain 30-84 on KMB agar (Fig. 3B). These strains maintained the rough morphology on PPM-D agar (data not shown). In addition, the presence of multiple copies of csaI csaR or csaR alone in the phzI csaI double mutant also resulted in a rough phenotype (Fig. 3B).
FIG. 3.
Colony morphology of 30-84 and derivatives on KMB agar. Photographs were taken after 48 h. (A) 30-84 and various mutants. Bacterial strains: 1, 30-84; 2, 30-84I (phzI); 3, 30-84R (phzR); 4, 30-84I2 (csaI); 5, 30-84R2 (csaR); 6, 30-84R/R2 (phzR csaR); 7, 30-84I/I2 (phzI csaI); 8, 30-84I/R2 (phzI csaR); 9, 30-84I2/R (csaI phzR); and 10, 30-84.gacA. (B) Complementation of 30-84I/I2 and csaR mutants. Bacterial strains: 1, 30-84R2(pZZG11); 2, 30-84R/R2(pZZG11); 3, 30-84I/I2(pZZG3); 4, 30-84I/I2(pZZG11); and 5, 30-84I/R2(pZZG11). Plasmid pZZG11 contains a functional csaR, while pZZG3 contains both csaR and csaI.
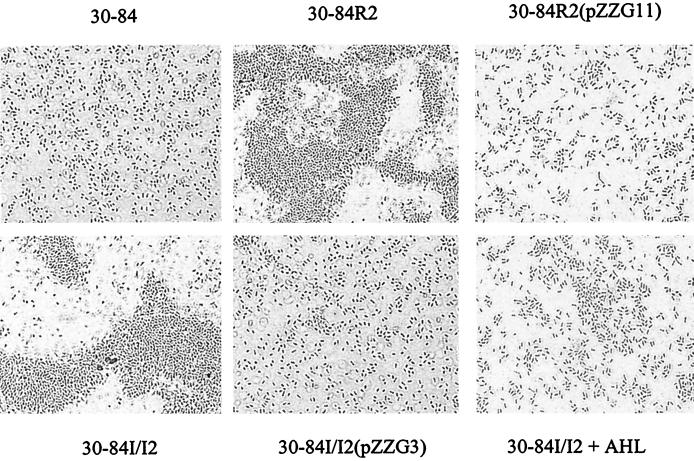
All strains produced capsules when grown in KMB, as indicated by the presence of a white envelope surrounding the cell (data not shown). Both the csaR and csaI phzI mutants displayed a clumping phenotype with cell aggregates, in contrast to cells of strain 30-84, which were uniformly distributed (Fig. 4). Introduction of functional copies of csaR or csaI csaR into these mutants restored the wild-type phenotype. Interestingly, the csaI mutant did not clump, suggesting that phzI may complement this defect. The addition of AHL-containing ethyl acetate extracts from strain E. coli DH5α(pUC18-csaI) reversed the clumping phenotype in the csaI phzI mutant, unlike an equivalent amount of strain DH5α(pUC18) extract (data not shown).
FIG. 4.
Micrographs of P. aureofaciens 30-84 and derivatives. Bacteria were grown in KMB broth for 24 h and stained with Congo red prior to microscopic observation.
CsaI directs the synthesis of a diffusible signal.
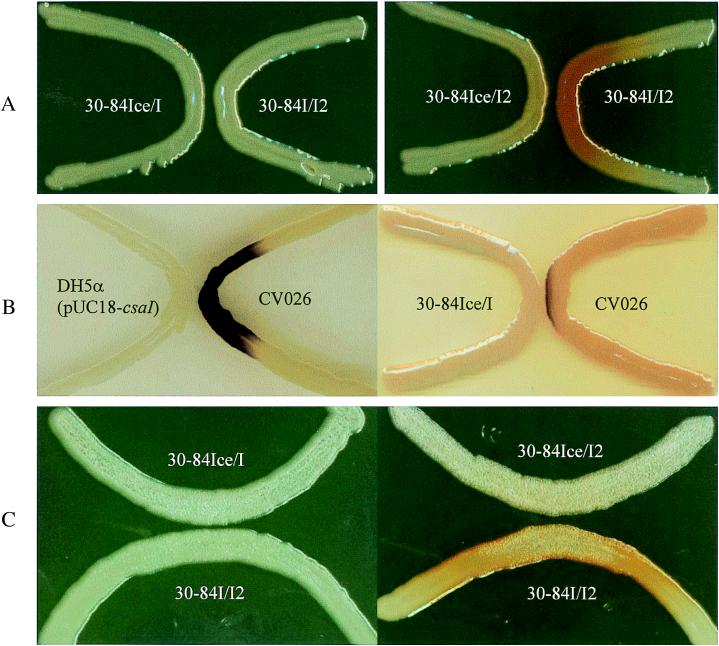
Several methods were employed to determine whether CsaI is responsible for the production of a diffusible AHL signal. The AHL-specific reporter strains 30-84I/I2, 30-84I/Z, A136(pCF240), and CV026 and the differential AHL donor strains 30-84Ice/I and 30-84Ice/I2 were used in a cross-feeding assay, in which an AHL donor strain and an AHL reporter strain were “V” streaked onto the same agar plate. Diffusion of AHL signals from 30-84Ice/I2 (phzI+ csaI) but not from strain 30-84Ice/I (phzI csaI+) or DH5α(pUC18-csaI) stimulated phenazine production in 30-84I/I2 (Fig. 5A) or β-galactosidase activity in 30-84I/Z (data not shown). These data indicate that the PhzI-generated signal, not the CsaI-generated signal, specifically activates PhzR to induce phenazine operon expression. When C. violaceum CV026 was used as the AHL sensor, production of violacein was restored by the presence of either signal. To further verify the presence and specificity of the csaI signal, E. coli DH5α(pUC18-csaI) was used as an AHL donor. This strain did not effectively induce phenazine production by 30-84I/I2 but did rapidly induce violacein production by CV026 (Fig. 5B) in comparison to strain DH5α(pUC18), which did not affect either strain (data not shown).
FIG. 5.
Assay for the presence and specificity of AHL signals generated by CsaI and PhzI. (A) Activation of phenazine biosynthesis in 30-84I/I2 (phzI csaI) on PPM-D agar by AHL signal diffused from 30-84Ice/I (phzI) or 30-84Ice/I2 (csaI) cultures. (B) Activation of violacein biosynthesis in C. violaceum CV026 on LB agar by AHL signal from E. coli DH5α(pUC18-csaI) or 30-84Ice/I. (C) Restoration of 30-84I/I2 to a rough phenotype on KMB agar by exogenous AHL from 30-84Ice/I or 30-84Ice/I2 cultures.
In a separate cross-feeding assay, the double phzI csaI mutant was restored to the wild-type semidry, rough colony morphology on KMB or AB agar when cross-streaked with 30-84Ice/I (phzI), 30-84Ice/I2 (csaI), or DH5α(pUC18-csaI) (Fig. 5C).
AHL signal production by 30-84Ice/I (csaI+) and 30-84Ice/I2 (phzI+) was quantified by determining their effects on β-galactosidase activity in 30-84I/Z and A. tumefaciens A136(pCF240). The amount of β-galactosidase activity in the reporters is correlated to the specificity and amount of the AHL signal present. AHL extracts from 30-84Ice/I2 (phzI+) significantly stimulated phzB::lacZ expression in 30-84I/Z compared to that of 30-84I/Z without extract (796 ± 32 versus 45 ± 8 U/ml). In contrast, the 30-84Ice/I (csaI+) AHL extract only slightly elevated β-galactosidase activity in 30-84I/Z (108 ± 12 U/ml). When A136(pCF240) was used as the reporter, AHL extracts from both strains markedly improved traA::lacZ expression, with their effects on β-galactosidase activity in A136(pCF240) being virtually equal (3,743 ± 316 versus 3,693 ± 260 U/ml). As expected, addition of extracts of either signal had no effect on β-galactosidase activity in 30-84Z/sgacA (11 ± 3 U/ml for each).
Consistent with the above observations, the presence of AHL extracts from E. coli DH5α(pUC18-csaI) caused a 10-fold increase in traA::lacZ expression in A136(pCF240) but had little effect on phzB::lacZ expression in 30-84I/Z, although the above data suggest that DH5α(pUC18-csaI) synthesizes high levels of AHL (data not shown).
Colonization of the wheat rhizosphere.
The various mutants were compared to strain 30-84 for survival and colonization of the wheat rhizosphere. When the seeds were sown in potting mix containing pasteurized soil, the phzI, csaI, phzR, and csaR single null mutants colonized the roots at levels comparable to that of strain 30-84. However, fivefold-lower population levels were detected on the roots colonized by the double phzI csaI and phzR csaR mutants (Table 4).
TABLE 4.
Bacterial populations of 30-84 and its derivatives recovered from the wheat rhizospherea
| Strain | Genotype | Log CFU/g of rootsb
|
|
|---|---|---|---|
| Pasteurized soil | Natural soil | ||
| 30-84 | Wild type | 7.68 ± 0.27 A | 7.14 ± 0.26 A |
| 30-84I | phzI::npt | 7.60 ± 0.25 A | 6.42 ± 0.23 B |
| 30-84R | phzR::Tn5lacZ | 7.54 ± 0.26 A | 6.32 ± 0.23 B |
| 30-84I2 | csaI::uidA-Gm | 7.54 ± 0.24 A | 6.20 ± 0.29 B |
| 30-84R2 | csaR::uidA-Gm | 7.63 ± 0.21 A | 6.89 ± 0.28 A |
| 30-84I/I2 | phzI::npt csaI::uidA-Gm | 6.98 ± 0.29 B | 5.44 ± 0.28 C |
| 30-84R/R2 | phzR::Tn5lacZ csaR::uidA-Gm | 6.94 ± 0.30 B | 5.46 ± 0.23 C |
Pregerminated wheat seeds were treated with a bacterial suspension and sown in potting mix containing pasteurized or natural soil contained in plastic cones. The cones were placed in a growth chamber. Twenty days after emergence, root samples were collected and bacteria were isolated.
All values are means ± standard deviations of two experiments, with four replicates per experiment. Values followed by the same letter in each column are not significantly different (P = 0.05).
When plants were grown in potting soil containing natural soil, overall bacterial rhizosphere populations were lower than those from roots grown in pasteurized soil (Table 4). Except for the csaR mutant, which established population numbers similar to those of the wild type, all the other mutants showed significantly lower root colonization abilities than 30-84. Similar to observations seen in the pasteurized soil, the double I and double R mutants were the least effective in colonizing and surviving in the wheat rhizosphere, with their population densities being at least 47-fold lower than that of 30-84 isolated from roots in the same soil (Table 4).
DISCUSSION
Our previous research identified the PhzR-PhzI quorum-sensing system responsible for controlling phenazine antibiotic production in P. aureofaciens strain 30-84 (35, 42). In the present study, we identified a second quorum-sensing regulatory system, termed CsaR-CsaI (for “cell surface alterations”), which is only marginally involved in phenazine regulation. The primary function of the CsaR-CsaI system appears to be the regulation of exoprotease production in conjunction with the PhzR-PhzI system and also the regulation of cell surface properties.
CsaI and CsaR were most similar to RhlI and RhlR, respectively, the second quorum-sensing system discovered in P. aeruginosa (16, 31). However, these two quorum-sensing systems differ from each other. While rhlI and rhlR are separated by 181 bp and rhlI has its own promoter (28), csaR and csaI are separated by only 30 bp and csaI has an RBS but no promoter, suggesting that csaI expression is dependent on csaR. The RhlR-RhlI system is primarily responsible for regulating rhamnolipid production in P. aeruginosa, but P. aureofaciens strain 30-84 does not synthesize rhamnolipids. Finally, in P. aeruginosa the LasR-LasI and RhlR-RhlI systems exist in a hierarchical relationship, while PhzR-PhzI and CsaR-CsaI appear to function independently.
The CsaR-CsaI system is responsible for the low but detectable phenazine production observed in phzI and phzR null mutants in PPM-D medium. However, csaI or csaR null mutants produced phenazines in only slightly smaller amounts in PPM-D than the wild-type strain, and disruption of phzI and csaI or phzR and csaR completely eliminated antibiotic production in this medium. The presence of multiple copies of csaR-csaI in trans in 30-84I/I2 (Table 3) and 30-84R/R2 (data not shown) only partially restored phenazine production, indicating that the CsaR-CsaI system cannot substitute for the PhzR-PhzI system for phenazine production. Evidence that CsaI is an AHL synthase includes activation of the AHL-specific reporters C. violaceum CV026 and A. tumefaciens A136(pCF240) by AHL extracts of the phzI mutant 30-84I and E. coli DH5α(pUC18-csaI) (Fig. 5). The AHL signals generated by CsaI and PhzI cannot activate PhzR and CsaR, respectively, to induce phenazine biosynthesis, as evidenced by the fact that phenazine production in double mutants containing one functional AHL synthase and the noncognate regulator was abolished. These data suggest that unlike for PhzR-PhzI, phenazine regulation is not the primary role of CsaR-CsaI.
Analogous to the PhzR-PhzI system, while multiple copies of csaR-csaI in trans did enhance β-galactosidase activity and phenazine production in strains 30-84Z and 30-84R, respectively, neither restored detectable phenazine production in strain 30-84.gacA (Table 3). These data indicate that both quorum sensing systems require GacS-GacA in order to function.
Mutation of either phzI or csaI or of either phzR or csaR had no effect on exoprotease production by strain 30-84, indicating the two quorum-sensing systems may interact to regulate exoprotease production (Table 2). However, disruption of phzI and csaI or phzR and csaR abolished exoprotease activity, indicating that exoprotease in strain 30-84 is under AHL-mediated regulation. It is interesting that the two quorum-sensing systems appear to be able to interact for exoprotease production while they are unable to interact for phenazine production. This suggests that both CsaI and PhzI signals are capable of activating their noncognate R proteins to induce protease activity. This is different from P. aeruginosa, in which both las and rhl systems are involved in regulating exoprotease activity (5, 31). Although the las system regulated lasB and lasA and the rhl system regulated lasB, the R proteins were not significantly activated by their noncognate AHL to induce transcription of the respective protease genes (31).
A further complication in exoprotease regulation was the observation that AHL produced by CsaI or PhzI (or both) failed to restore exoprotease activity in 30-84I/I2. Furthermore, multiple copies of csaR in trans failed to restore proteolysis in 30-84R/R2, and so did the introduction of csaR-csaI in trans in 30-84I/I2. This is in contrast to the phenazine phenotype discussed above and the colony surface phenotype discussed below, and it differs from the situation in other bacteria, in which exoprotease activity in an I or R mutant can be restored by the respective I or R gene (5, 17). This may reflect additional as-yet-unknown levels of exoprotease regulation in strain 30-84. A similar observation was reported in that lipase activity was not restored in a B. cepacia cepI or cepR mutant by addition of AHL signal or introduction of the appropriate gene (17).
Unlike strain 30-84, mutants defective in csaR, regardless of csaI, phzR, or phzI, exhibited a mucoid colony morphology and a clumping phenotype in KMB. This suggested that csaR mutants are altered in some cell surface property. Additionally, a csaI phzI double mutant demonstrated a similar smooth and clumping phenotype, whereas variants with mutations in csaI or phzI alone did not. This suggest that colony smoothness and the clumping phenotype are controlled by CsaR and that either the CsaI or PhzI signal can interact with CsaR to regulate these traits. It is currently unclear whether the smooth phenotype is due to the csaR mutation allowing a trait to be expressed or, more likely, the loss of a trait. In Pantoea stewartii, EsaR represses transcription of extracellular polysaccharide (2, 3), in B. cepacia the CepR-CepI system represses siderophore production (17), and in Rhodobacter sphaeroides a cepI mutant overproduces exopolysaccharide (3, 36), while a ypsR mutant of Yersinia pseudotuberculosis exhibits increased cell aggregation, motility, and flagellin production (1). Microscopic observation did not reveal any obvious differences in cell capsules between the csaR mutant and wild-type strain 30-84 (data not shown). Fatty acid analysis (Microbial ID, Inc.) indicated that the csaR mutant had alterations in fatty acid composition or relative percentages of some fatty acids compared to 30-84, although both strains were still clearly P. aureofaciens (data not shown). Comparison of the csaR mutant with 30-84 did not reveal motility differences (data not shown).
Disruption of phzI, phzR, or csaI resulted in reduced colonization of the wheat rhizosphere in natural soil, with the most notable reduction being seen in the phzI csaI and phzR csaR double mutants. These results indicate that quorum sensing plays an important role in the survival of strain 30-84 and competition with other microorganisms in situ. Interestingly, the csaR mutant still colonized roots to levels similar to those of strain 30-84. The reason for this is unclear, but one possible hypothesis is that aggregation of the csaR mutants may have enabled them to persist over the course of the experiment. In potting soil containing pasteurized soil, all single mutants colonized wheat roots to the same level as the wild type. This may reflect the lack of competition with other rhizosphere microflora. However, mutations in phzI csaI or phzR csaR resulted in lower bacterial survival in this pasteurized soil, demonstrating that both quorum-sensing systems play a role in rhizosphere survival.
This work provides a new example of a microorganism that employs two unrelated AHL-mediated quorum-sensing circuits to regulate multiple functions. The existence of two nonhierarchical regulatory systems that interact to control some behaviors but not others has important implications for the spatial and temporal control of gene expression in the bacterium. Future studies will focus on determining how the CsaR-CsaI and PhzR-PhzI systems interact with each other and their effect on rhizosphere colonization and biocontrol activity.
ACKNOWLEDGMENTS
We thank Francoise Blachere, Scott Chancey, Patricia Figuli, and Cheryl Whistler for technical assistance. We also thank Christina Kennedy and Elizabeth Pierson for critical reviews of the manuscript.
This work was supported by USDA NRI-CGP grant 98-02129.
REFERENCES
- 1.Atkinson S, Throup J P, Stewart G S A B, Williams P. A hierarchical quorum-sensing system in Yersinia pseudotuberculosis is involved in the regulation of motility and clumping. Mol Microbiol. 1999;33:1267–1277. doi: 10.1046/j.1365-2958.1999.01578.x. [DOI] [PubMed] [Google Scholar]
- 2.Beck von Bodman S, Farrand S K. Capsular polysaccharide biosynthesis and pathogenicity in Erwinia stewartii require induction by an N-acylhomoserine lactone autoinducer. J Bacteriol. 1995;177:5000–5008. doi: 10.1128/jb.177.17.5000-5008.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck von Bodman S, Majerczak D R, Coplin D L. A negative regulator mediates quorum-sensing control of exopolysaccharide production in Pantoea stewartii subsp. stewartii. Proc Natl Acad Sci USA. 1998;95:7687–7692. doi: 10.1073/pnas.95.13.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belew M, Porath J. Extracellular proteinase from Penicillium notatum. Methods Enzymol. 1970;19:576–581. [Google Scholar]
- 5.Brint J M, Ohman D E. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under control of RhlR-RhlI, another set of regulators in strain PAOI with homology to the autoinducer-response LuxR-LuxI family. J Bacteriol. 1995;177:7155–7163. doi: 10.1128/jb.177.24.7155-7163.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chancey S T, Wood D W, Pierson L S., III Two-component transcriptional regulation of N-acyl-homoserine lactone production in Pseudomonas aureofaciens. Appl Environ Microbiol. 1999;65:2294–2299. doi: 10.1128/aem.65.6.2294-2299.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eberl L. N-Acyl homoserine lactone-mediated gene regulation in Gram-negative bacteria. Syst Appl Microbiol. 1999;22:493–506. doi: 10.1016/S0723-2020(99)80001-0. [DOI] [PubMed] [Google Scholar]
- 8.Flavier A B, Ganova-Raeva L M, Schell M A, Denny T P. Hierarchical autoinduction in Ralstonia solanacearum: control of N-acyl-homoserine lactone production by a novel autoregulatory system responsive to 3-hydroxypalmitic acid methyl ester. J Bacteriol. 1997;179:7089–7097. doi: 10.1128/jb.179.22.7089-7097.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuqua W C, Winans S C. A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J Bacteriol. 1994;176:2796–2806. doi: 10.1128/jb.176.10.2796-2806.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuqua W C, Winans S C, Greenberg E P. Census and consensus in bacterial ecosystems: the Lux-LuxI family of quorum sensing transcriptional regulators. Annu Rev Microbiol. 1996;50:727–751. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 11.Gilson L, Kuo A, Dunlop P V. AinS and a new family of autoinducer synthesis proteins. J Bacteriol. 1995;177:6946–6951. doi: 10.1128/jb.177.23.6946-6951.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray K M, Passador L, Iglewski B H, Greenberg E P. Interchangeability and specificity of components from the quorum-sensing regulatory systems of Vibrio fischeri and Pseudomonas aeruginosa. J Bacteriol. 1994;176:3076–3080. doi: 10.1128/jb.176.10.3076-3080.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoagland D R, Arnon K I. The water-culture method for growing plants without soil. UC Agricultural Experiment Station circular 347, revised ed. Davis, Calif: UC Agricultural Experiment Station; 1950. [Google Scholar]
- 14.King E O, Ward M K, Raney D E. Two simple media for the demonstration of pyocyanin and fluorescein. J Lab Clin Med. 1954;44:301–307. [PubMed] [Google Scholar]
- 15.Kuo A, Blough N V, Dunlap P V. Multiple N-acyl-l-homoserine lactone autoinducer of luminescence in the marine symbiotic bacterium Vibrio fischeri. J Bacteriol. 1994;176:7558–7565. doi: 10.1128/jb.176.24.7558-7565.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Latifi A, Winson M K, Foglino M, Bycroft B W, Stewart G S A B, Lazdunski A, Williams P. Multiple homologues of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1. Mol Microbiol. 1995;17:333–343. doi: 10.1111/j.1365-2958.1995.mmi_17020333.x. [DOI] [PubMed] [Google Scholar]
- 17.Lewenza S, Conway B, Greenberg E P, Sokol P A. Quorum sensing in Burkholderia cepacia: identification of the LuxRI homologs CepRI. J Bacteriol. 1999;181:748–756. doi: 10.1128/jb.181.3.748-756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindgren P B, Frederick R, Govindaragan A G, Panopoulos N J, Staskawicz B J, Lindow S E. An ice nucleation reporter gene system: identification of inducible pathogenicity genes in Pseudomonas syringae pv. phaseolicola. EMBO J. 1989;8:1291–1301. doi: 10.1002/j.1460-2075.1989.tb03508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 20.Mavrodi D V, Ksenzenko V N, Bonsall R I, Cook R J, Boronin A M, Thomashow L S. A seven-gene locus for synthesis of phenazine-1-carboxylic acid by Pseudomonas fluorescens 2-79. J Bacteriol. 1998;180:2541–2548. doi: 10.1128/jb.180.9.2541-2548.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazzola M, Cook R J, Thomashow L S, Weller D M, Pierson L S. Contribution of phenazine antibiotic biosynthesis to the ecological competence of fluorescent pseudomonads in soil habitats. Appl Environ Microbiol. 1992;58:2616–2624. doi: 10.1128/aem.58.8.2616-2624.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLean K H, Winson M K, Fish L, Taylor A, Chhabra S R, Camara M, Daykin M, Lamb J H, Swift S, Bycroft B W, Stewart G S A B, Williams P. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology. 1997;143:3703–3711. doi: 10.1099/00221287-143-12-3703. [DOI] [PubMed] [Google Scholar]
- 23.Metcalf W M, Wanner B L. Construction of new β-glucuronidase cassettes for making transcriptional fusions and their use with methods for allele replacement. Gene. 1993;129:17–25. doi: 10.1016/0378-1119(93)90691-u. [DOI] [PubMed] [Google Scholar]
- 24.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 352–355. [Google Scholar]
- 25.Morè M I, Finger L D, Stryker J L, Fuqua C, Eberhard A, Winans S C. Enzymatic synthesis of a quorum-sensing autoinducer through use of defined substrates. Science. 1996;272:1655–1658. doi: 10.1126/science.272.5268.1655. [DOI] [PubMed] [Google Scholar]
- 26.Murillo J, Shen H, Gerhold D, Sharma A, Cooksey D, Keen N T. Characterization of pPT23B, the plasmid involved in syringolide production by Pseudomonas syringae pv. tomato PT23. Gene. 1994;31:275–287. doi: 10.1006/plas.1994.1029. [DOI] [PubMed] [Google Scholar]
- 27.Ochsner U A, Koch A K, Fiechter A, Reiser J. Isolation and characterization of a regulatory gene affecting rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. J Bacteriol. 1994;176:2044–2054. doi: 10.1128/jb.176.7.2044-2054.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ochsner U A, Reiser J. Autoinducer-mediated regulation of rhamnolipid biosynthesis in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1995;92:6424–6428. doi: 10.1073/pnas.92.14.6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parsek M R, Schaefer A L, Greenberg E P. Analysis of random and site-directed mutations in rhlI, a Pseudomonas aeruginosa gene encoding an acylhomoserine lactone synthase. Mol Microbiol. 1997;26:301–310. doi: 10.1046/j.1365-2958.1997.5741935.x. [DOI] [PubMed] [Google Scholar]
- 30.Parsek M R, Val D L, Hanzelka B L, Cronan J E, Jr, Greenberg E P. Acyl homoserine-lactone quorum-sensing signal generation. Proc Natl Acad Sci USA. 1999;96:4360–4365. doi: 10.1073/pnas.96.8.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pearson J P, Pesci E C, Iglewski B H. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J Bacteriol. 1997;179:5756–5767. doi: 10.1128/jb.179.18.5756-5767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pierson E A, Wood D W, Cannon J A, Blachere F M, Pierson L S., III Interpopulation signaling via N-acyl-homoserine lactone among bacteria in the wheat rhizosphere. Mol Plant-Microbe Interact. 1998;11:1078–1084. [Google Scholar]
- 33.Pierson L S, III, Wood D W, Pierson E A. Homoserine lactone-mediated gene regulation in plant-associated bacteria. Annu Rev Phytopathol. 1998;36:207–225. doi: 10.1146/annurev.phyto.36.1.207. [DOI] [PubMed] [Google Scholar]
- 34.Pierson L S, III, Thomashow L S. Cloning and heterologous expression of phenazine biosynthetic locus from Pseudomonas aureofaciens 30-84. Mol Plant-Microbe Interact. 1992;5:330–339. doi: 10.1094/mpmi-5-330. [DOI] [PubMed] [Google Scholar]
- 35.Pierson L S, III, Keppenne V D, Wood D W. Phenazine antibiotic biosynthesis in Pseudomonas aureofaciens 30-84 is regulated by PhzR in response to cell density. J Bacteriol. 1994;176:3966–3974. doi: 10.1128/jb.176.13.3966-3974.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puskas A, Greenberg E P, Kaplan S, Schaeffer A L. A quorum-sensing system in the free-living photosynthetic bacterium Rhodobacter sphaeroides. J Bacteriol. 1997;179:7530–7537. doi: 10.1128/jb.179.23.7530-7537.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosemeyer V, Michiels J, Verreth C, Vanderleyden J. luxI- and luxR-homologous genes of Rhizobium etli CNPAF-52 contribute to synthesis of autoinducer molecules and nodulation of Phaseolus vulgaris. J Bacteriol. 1998;180:815–821. doi: 10.1128/jb.180.4.815-821.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schleif R F, Wensink P C. Practical methods in molecular biology. New York, N.Y: Springer-Verlag; 1981. [Google Scholar]
- 39.Staskawicz B, Dahlbreck D, Keen N, Napoli C. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J Bacteriol. 1987;169:5789–5794. doi: 10.1128/jb.169.12.5789-5794.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weller D M. Biological control of soilborne pathogens in the rhizosphere with bacteria. Annu Rev Phytopathol. 1988;26:379–407. [Google Scholar]
- 41.Whooley M A, O'Callaghan J A, McLoughlin A J. Effect of substrate on the regulation of exoprotease production by Pseudomonas aeruginosa ATCC 10145. J Gen Microbiol. 1983;129:981–988. doi: 10.1099/00221287-129-4-981. [DOI] [PubMed] [Google Scholar]
- 42.Wood D W, Pierson L S., III The phzI gene of Pseudomonas aureofaciens 30-84 is responsible for the production of a diffusible signal required for phenazine antibiotic production. Gene. 1996;168:49–53. doi: 10.1016/0378-1119(95)00754-7. [DOI] [PubMed] [Google Scholar]
- 43.Wood D W, Gong F, Daykin M M, Williams P, Pierson L S., III N-Acyl-homoserine lactone-mediated regulation of phenazine gene expression by Pseudomonas aureofaciens 30-84 in the wheat rhizosphere. J Bacteriol. 1997;179:7603–7670. doi: 10.1128/jb.179.24.7663-7670.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]