Abstract
Background
Patients with Polycystic ovary syndrome (PCOS) are predisposed to the development of several mental comorbidities such as depression. According to several studies, PCOS can be managed by improving insulin sensitivity. The insulin-sensitizing effect of vitamin K has been reported in recent studies. Therefore, in the current trial, we assessed the effect of administrating vitamin K2 (Menaquinone-7) on depression status in women afflicted with PCOS.
Methods
Eighty-four PCOS women were allocated into the intervention and comparison groups; the intervention group (n = 42) administered 90 µg/day Menaquinone-7, and the comparison group (n = 42) consumed placebo capsules (containing avesil) for 8 weeks. In this randomized, double blind, placebo-controlled clinical trial, depression status was measured by BECK depression inventory-II (BDI-II) before and after 8 weeks of intervention.
Results
Consumption of Menaquinone-7 in comparison with the placebo capsules significantly improved depression status (P = 0.012).
Conclusion
This clinical study reported the advantageous effect of Menaquinone-7 administration on depression status in PCOS patients.
Trial registration The present study was registered at http://www.IRCT.ir on 06/06/2018 (registration number: IRCT20170916036204N5).
Keywords: Vitamin K2, Polycystic ovary syndrome, Depression
Background
Polycystic ovary syndrome (PCOS) is a frequent endocrinopathy with the prevalence of 9–18% among women of reproductive age [1–3].
This syndrome, according to the Rotterdam criteria, is characterized by the presence of at least 2 features of the following characteristics: 1. polycystic ovaries 2. oligo or anovulation 3. biochemical and/or clinical symptoms of hyperandrogenism [4, 5]. In addition to these features, an array of feminine problems including hirsutism, acne and scalp hair thinning and infertility can prone PCOS patients to several mental disorders, namely anxiety, bipolar disorder, depression, and eating disorders [6]. Also, PCOS can increase the risk of pregnancy complications [7].
Although the root cause of PCOS is not completely comprehended, insulin resistance (IR) has been reported as the leading cause of PCOS according to a number of studies [8]. IR in these patients can create hyperinsulinemia which increases serum free androgen levels through increasing the hepatic generation of sex hormone binding globulin (SHBG), and androgen production by ovarian theca cells [9].
Changing life-style is the first-line strategy for managing PCOS. Even small changes in diet and physical activity can improve insulin resistance which leads to ameliorate the complications of PCOS [6, 10]. Also, diet restriction and fasting might improve the metabolic imbalance typical of PCOS [11, 12].
The use of insulin-sensitizers has gained growing attention because of their significant efficiency on PCOS [13, 14]. Recently, a number of studies reported the insulin-sensitizing effect of vitamin K [15]. Based on these studies, vitamin K can enhance insulin sensitivity through inducing adiponectin expression [16].
Phylloquinone (vitamin K1) and menaquinone (vitamin K2) are two natural forms of vitamin K.
The current recommendations for vitamin K dietary intake are according to the daily dose which is vital for preventing bleeding [17]; in normal situations such as normal pregnancy, there is no need for vitamin K supplementation [18].
It has been demonstrated that vitamin K can be mainly found in the form of menaquinone in brain [19]. Gancheva et al. investigated the effect of vitamin K on depression for the first time. In this animal study, menaquinone-7 administration prevented the anxiety and depression progression in rats suffering from metabolic syndrome [20].
It should be noted that no paper has reported the effect of menaquinone-7 on depression status in PCOS patients. Therefore, the effect of menaquinone-7 administration on depression status in PCOS patients has been investigated in this clinical study.
Materials and methods
Participants
Between July and September 2016, the present study was performed on 84 women who were referred to the infertility clinic of Ghadir Mother & Child Hospital in Shiraz, Iran.
PCOS women aged 18–40 years who were diagnosed based on the Rotterdam criteria were eligible to participate in this study [21]. Exclusion criteria consisted of following specific physical activity program or diet for three months prior to the beginning of the study, administrating medications or supplements that were likely to affect glucose metabolism, bone metabolism, lipid profile, and ovarian function, and using any antibiotics and anticoagulant drugs such as warfarin for 3 months prior to the study. Pregnant/lactating individuals, and patients with Cushing syndrome, thyroid disorders, congenital adrenal hyperplasia, hypertension, and diabetes mellitus were also excluded from participating in this study.
This trial was accomplished in accordance with the Declaration of Helsinki and good clinical practice guidelines. The local Ethics Committee of Shiraz University of Medical Sciences reviewed and approved the protocol of this study (IR.SUMS.REC.1397.102). This trial was recorded in the Iranian registry of clinical trials (first registration date: 04-06-2018, registration number: IRCT20170916036204N5).
The protocol of this study was described for all the participants; therefore, they signed the inform consent consciously.
Thirty-seven women were calculated to be in each group based on the decreased level of serum glucose in a previous study [22] with 0.05 significance level and power of 80%. Eventually, 42 women were determined in each study arm considering a 15% dropout rate.
Study design
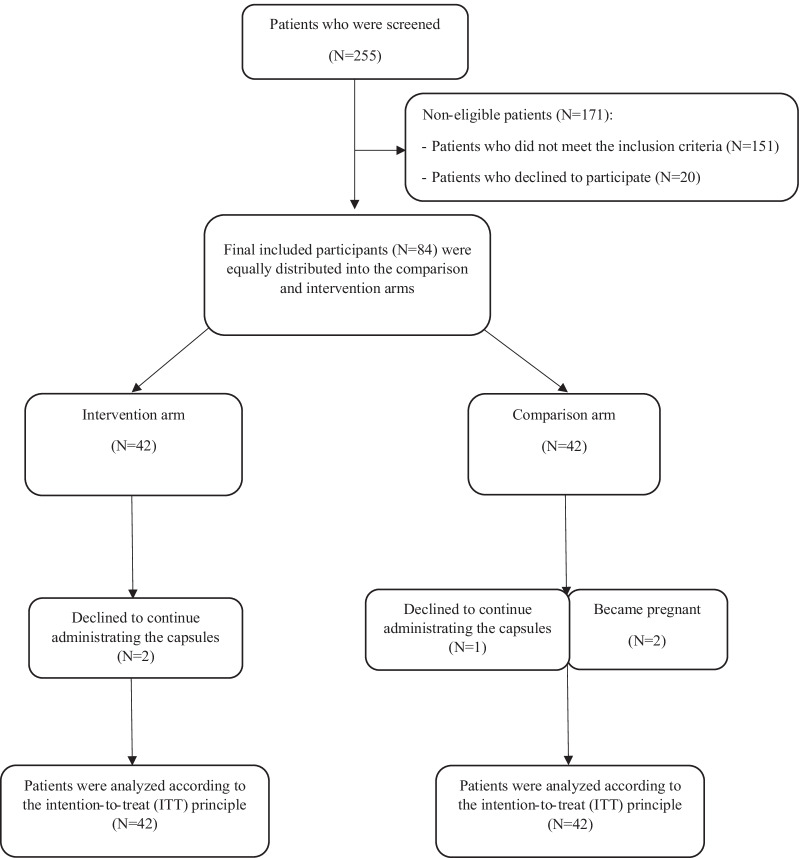
Totally, 255 patients with PCOS were screened and eighty-four subjects participated in this randomized, double-blind, placebo-controlled clinical trial, as shown in the consort flow diagram (Fig. 1).
Fig. 1.
Flowchart of the participants
All the eligible patients were evenly randomized into two arms of comparison and intervention by block randomization. This process was performed with a fixed block size of 4 using random allocation software [23]. Subjects in the intervention group administered one capsule (90 µg of Menaquinone-7), and participants in the comparison arm consumed a placebo capsule (avesil) on a daily basis for 8 weeks.
All the capsules were equal regarding size (size 0), color (white), aroma and weight. Arian Salamat Sina Company (Tehran, Iran) provided all these capsules specifically for the present trial in order to blind the patients, investigators and infertility clinic staff for group assignment. A leaflet including some PCOS dietary recommendations was given to each participant. We asked the participants not to alter their usual diet and physical activity level, and report any side-effect within the 8-week study period.
A daily text message was forwarded to each patient’s contact number to evaluate participants’ adherence. Moreover, the patients were asked to return their capsule packets after the intervention period, and if they had consumed 90 percent of the capsules, we assumed them adherent.
Three-day dietary records were completed by participants before and after this trial. We computed the patients’ daily energy and intakes of macronutrients (carbohydrate, fat and protein) and micronutrients (vitamin K and vitamin D) by means of Nutritionist 4 software (first data bank, San Bruno, CA, USA) that was adjusted for Persian foods [24]. In addition, the level of physical activity was evaluated through completing the validated version of International Physical Activity Questionnaire (IPAQ) at baseline of the trial [25, 26].
Measurement of parameters
Anthropometry and depression assessments
We measured weight (to 0.1 kg) and height (to 0.1 cm) using Seca scales (Hamburg, Germany) for each patient after an overnight fast before and after this clinical study. Moreover, Body mass index (BMI) was calculated through dividing weight (kg) by height (m2). For assessing the intensity of depression, all participants completed BECK depression inventory-II (BDI-II) at baseline and at the end of the study [27].
Blood sampling and vitamin K assessment
After 12 h of fasting, 7 ml of venous blood were collected before the trial. Blood samples were centrifuged at 2,000 g/min for 10 min, and the separated serum samples were frozen at − 80 °C.
Serum level of vitamin K was measured by ELISA kit (ZellBio, Catalog No. RK00737).
Statistical analysis
All of the statistical analyses were done by means of statistical package for the social sciences, version 21 (SPSS, Inc. Chicago, USA). The parametric information was reported as mean ± SD and the non-parametric information was shown as median (IQR).
We performed Levene’s and Kolmogorov–Smirnov tests for checking homoscedasticity of data and normal/abnormal distribution. For comparing the variables between study groups, we used an independent sample t-test to compare the normally distributed data, and the Mann–Whitney U-test for skewed data. A pair t-test was performed for comparing the parametric information of depression status in each study arm before and after the intervention.
Results
Eighty-four PCOS women were eligible to participate in this study. During this study, five patients dropped out; two participants in the MK-7 arm were reluctant to administer the capsules, and two women became pregnant and a participant was lost to follow-up in the comparison arm (Fig. 1).
Almost 90% of the participants in each study arm completely followed the protocol of this study. The participants did not report any adverse effect during this 8-week trial.
Baseline data related to the participants can be observed in Table 1; there were not any significant differences between the intervention and comparison arms regarding anthropometric measurements, physical activity level, age, fasting blood sugar, serum vitamin K2 and depression status.
Table 1.
Baseline data in the intervention and comparison arms
| Variables | Intervention arm (n = 42) | Comparison arm (n = 42) | Comparison between groups (P-value)* |
|---|---|---|---|
| Age (y) | 28 (26–30) | 27 (24–28) | 0.71 |
| Weight (kg) | 68.02 ± 11.28 | 70.47 ± 14.08 | 0.39 |
| Body Mass Index (kg/m2) | 25.93 ± 4.18 | 27.31 ± 4.78 | 0.16 |
| Physical activity level (MET-min/week) | 594 (198–1282.5) | 462 (231–997.5) | 0.5 |
| Fasting blood sugar (mg/dl) | 90.32 ± 7.52 | 89.19 ± 8.5 | 0.52 |
| Serum level of vitamin K (ng/ml) | 436.95 (400–495.7) | 434.3 (401.2–492.02) | 0.85 |
| Depression status | 16.9 ± 7.918 | 13.78 ± 9.05 | 0.1 |
*Parametric data were analyzed by independent samples t test, and non-parametric data were Mann–Whitney U test
P-values less than 0.05 were considered as the significant level of differences
Information is shown as mean ± SD or median (IQR)
Table 2 shows depression status before and after this 8-week trial in all participants.
Table 2.
Comparison of depression status in the participated at baseline and at the end of the intervention
| Variable | Vitamin-administered arm (n = 42) | Placebo-administered arm (n = 42) | P-value** | ||||
|---|---|---|---|---|---|---|---|
| Before the intervention | At the end of the intervention | P-value* | Before the intervention | At the end of the intervention | P-value* | ||
| Depression status | 16.9 ± 7.918 | 14.975 ± 8.3 | 0.013 | 13.78 ± 9.05 | 14.02 ± 9.54 | 0.84 | 0.012 |
*Data were analyzed by paired samples t test
**Data were analyzed by independent samples t test
P-values less than 0.05 were presumed as the significant level of differences
Information is shown as mean ± SD
Consumption of MK-7 in comparison with the placebo capsules significantly improved depression status (P = 0.012).
Regarding reported macro-nutrient and micro-nutrient intakes, there were no significant differences between the intervention and comparison groups during the study.
Discussion
To our knowledge, this clinical study is the first trial that has investigated the effect of vitamin K on depression status in PCOS patients. The findings of this trial reported that an eight-week administration of MK-7 significantly improved depression status in PCOS women.
A number of previous studies have shown that PCOS patients may experience depression [28]. Factors such as inflammation and neurotransmitter dysfunction were discussed to be involved in depression pathogenesis [29].
The NF-KB pathway can be regulated by vitamin K, as an anti-inflammatory agent, which can suppress the expression of inflammatory cytokines [30]. Moreover, lack of vitamin K in brain can increase ceramides; as the concentration of brain ceramides increases, inflammatory cytokines namely IL-2 and IL-6, and reactive oxygen species (ROS) production can be increased [30–32].
In addition, several studies have shown that vitamin K can enhance nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) through activating protein kinase A; it has been hypothesized that a decline in BDNF and NGF in hippocampus can be associated with depressive behaviors [31, 32]. In line with the mentioned results, Silvia M Gancheva et al. [20] reported that the 10-week administration of vitamin K2 by oral gavage prevented the development of depression in rats afflicted with metabolic syndrome. Also, Turker et al., found out that individuals with atrial fibrillation administrating warfarin, a vitamin K antagonist, were more likely to suffer from depression compared to patients with atrial fibrillation consuming dabigatran, a non-vitamin K anticoagulant [33].
Along with these findings, the current study has shown the anti-depressive effect of vitamin K2 in PCOS women. On the contrary, Rubio-López et al. reported a positive association between depressive symptoms and the dietary intake of vitamin K in Spanish children [34]. The contradiction between these results might be because of the difference in depression questionnaires (BECK vs CES-DS) and target subjects (PCOS women vs children).
A few studies hypothesized that insulin resistance (IR) might be related to depression in women with PCOS [35]. Regarding the impact of vitamin K on the metabolism of glucose, this fat-soluble vitamin can improve insulin sensitivity by increasing adiponectin expression [36]. Plus, osteocalcin, a vitamin K-dependent protein, induces insulin secretion by affecting pancreatic β-cells [36–38]. Having said all that, in our previous study [24], fasting blood sugar was not significantly affected by 8 weeks of MK-7 consumption compared to administrating placebo capsules in PCOS patients. In line with the mentioned study, Shahdadian et al. [39] in a systematic review and meta-analysis reported that vitamin K administration could not significantly affect glycemic control within healthy individuals. In another study [40], after 4 weeks of vitamin k2 administration by healthy young men, insulin sensitivity increased but fasting blood glucose did not significantly change. Also, Knapen et al. did not observe any significant alteration in fasting blood sugar after three years of MK-7 supplementation in postmenopausal women [41]. In our previous study [24], all the participants had normal serum vitamin K level at baseline; it can be hypothesized that vitamin K administration may have greater effects on fasting blood sugar in vitamin K deficient individuals.
Based on our knowledge, this paper has investigated the effect of vitamin K on depression status for the first time; this can be considered as a study strength. Lack of funding was the limitation of our study as we could not measure serum levels of osteocalcin, adiponectin, and inflammatory markers to better interpret the potential mechanisms led to improve our patients’ depression status. Further studies of the issue are still required; clinical studies with longer intervention periods and larger sample sizes are needed for confirming the anti-depressant effect of vitamin K in PCOS women.
Conclusion
This clinical study reported the possible anti-depressant effect of vitamin K2 in PCOS women for the first time. However, additional clinical studies with longer intervention periods and larger sample sizes should be performed to confirm the anti-depressant effect of vitamin K in PCOS women.
Acknowledgements
The authors would like to thank the patients who participated in this study.
Abbreviations
- PCOS
Polycystic ovary syndrome
- IR
Insulin resistance
- SHBG
Sex hormone binding globulin
- IPAQ
International Physical Activity Questionnaire
- BMI
Body mass index
- BDI-II
BECK depression inventory-II
- ROS
Reactive oxygen species
- NGF
Nerve growth factor
- BDNF
Brain-derived neurotrophic factor
Author contributions
F T: Investigation, Visualization, Software, Writing the Original Draft, Data Curation. B N. J: Resources, Methodology, Writing—Review & Editing. N H: Conceptualization, Methodology, Project Administration, Writing—Review & Editing, Supervision. G H: Resources, Software, Writing the Original Draft. All authors real and approved the manuscript.
Funding
This study was funded by the Vice Chancellor of Research and Technology Department at Shiraz University of Medical Sciences, Shiraz, Iran (Grant No #1396-01-87-15343).
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The present study was approved by the local Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran (IR.SUMS.REC.1397.102). The written informed consent, which was provided for this trial, was received from all the participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Asuncion M, Calvo RM, San Millan JL, Sancho J, Avila S, Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85(7):2434–2438. doi: 10.1210/jcem.85.7.6682. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89(6):2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 3.March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):544–551. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 4.Esmaeilinezhad Z, Barati-Boldaji R, Brett N, de Zepetnek J, Bellissimo N, Babajafari S, et al. The effect of synbiotics pomegranate juice on cardiovascular risk factors in PCOS patients: a randomized, triple-blinded, controlled trial. J Endocrinol Investig. 2020;43(4):539–548. doi: 10.1007/s40618-019-01139-x. [DOI] [PubMed] [Google Scholar]
- 5.Banaszewska B, Wrotyńska-Barczyńska J, Spaczynski RZ, Pawelczyk L, Duleba AJ. Effects of resveratrol on polycystic ovary syndrome: a double-blind, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2016;101(11):4322–4328. doi: 10.1210/jc.2016-1858. [DOI] [PubMed] [Google Scholar]
- 6.Deeks AA, Gibson-Helm ME, Teede HJ. Anxiety and depression in polycystic ovary syndrome: a comprehensive investigation. Fertil Steril. 2010;93(7):2421–2423. doi: 10.1016/j.fertnstert.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 7.D'Alterio MN, Sigilli M, Succu AG, Ghisu V, Laganà AS, Sorrentino F, et al. Pregnancy outcomes in women with polycystic ovarian syndrome (PCOS). Minerva Obstet Gynecol. 2021. [DOI] [PubMed]
- 8.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33(6):981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2014;6:1. doi: 10.2147/CLEP.S37559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taddei C, Zhou B, Bixby H, Carrillo-Larco RM, Jackson RT, Farzadfar F, et al. Repositioning of the global epicentre of non-optimal cholesterol. Nature. 2020;582:73–77. doi: 10.1038/s41586-020-2338-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiofalo B, Laganà AS, Palmara V, Granese R, Corrado G, Mancini E, et al. Fasting as possible complementary approach for polycystic ovary syndrome: Hope or hype? Med Hypotheses. 2017;105:1–3. doi: 10.1016/j.mehy.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Muscogiuri G, Palomba S, Laganà AS, Orio F. Current insights into inositol isoforms, Mediterranean and ketogenic diets for polycystic ovary syndrome: from bench to bedside. Curr Pharm Des. 2016;22(36):5554–5557. doi: 10.2174/1381612822666160720160634. [DOI] [PubMed] [Google Scholar]
- 13.Laganà AS, Rossetti P, Buscema M, La Vignera S, Condorelli RA, Gullo G, et al. Metabolism and ovarian function in PCOS women: a therapeutic approach with inositols. Int J Endocrinol. 2016;2016. [DOI] [PMC free article] [PubMed]
- 14.Paul C, Lagana AS, Maniglio P, Triolo O, Brady DM. Inositol’s and other nutraceuticals’ synergistic actions counteract insulin resistance in polycystic ovarian syndrome and metabolic syndrome: state-of-the-art and future perspectives. Gynecol Endocrinol. 2016;32(6):431–438. doi: 10.3109/09513590.2016.1144741. [DOI] [PubMed] [Google Scholar]
- 15.Schwalfenberg GK. Vitamins K1 and K2: The emerging group of vitamins required for human health. J Nutr Metab. 2017;2017. [DOI] [PMC free article] [PubMed]
- 16.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130(3):456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vermeer CV. Vitamin K: the effect on health beyond coagulation—an overview. Food Nutr Res. 2012;56(1):5329. doi: 10.3402/fnr.v56i0.5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shahrook S, Ota E, Hanada N, Sawada K, Mori R. Vitamin K supplementation during pregnancy for improving outcomes: a systematic review and meta-analysis. Sci Rep. 2018;8(1):1–11. doi: 10.1038/s41598-018-29616-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferland G. Vitamin K and the nervous system: an overview of its actions. Adv Nutr. 2012;3(2):204–212. doi: 10.3945/an.111.001784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gancheva SM, Zhelyazkova-Savova MD. Vitamin K2 improves anxiety and depression but not cognition in rats with metabolic syndrome: A role of blood glucose? Folia Med. 2016;58(4):264–272. doi: 10.1515/folmed-2016-0032. [DOI] [PubMed] [Google Scholar]
- 21.Fr DD, Tarlatzis R. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1). [DOI] [PubMed]
- 22.Rasekhi H, Karandish M, Jalali M, Mohammad-Shahi M, Zarei M, Saki A, et al. The effect of vitamin K1 supplementation on sensitivity and insulin resistance via osteocalcin in prediabetic women: a double-blind randomized controlled clinical trial. Eur J Clin Nutr. 2015;69(8):891. doi: 10.1038/ejcn.2015.17. [DOI] [PubMed] [Google Scholar]
- 23.Saghaei M. Random allocation software for parallel group randomized trials. BMC Med Res Methodol. 2004;4(1):26. doi: 10.1186/1471-2288-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarkesh F, Namavar Jahromi B, Hejazi N, Tabatabaee H. Beneficial health effects of Menaquinone-7 on body composition, glycemic indices, lipid profile, and endocrine markers in polycystic ovary syndrome patients. Food Sci Nutr. 2020;8(10):5612–5621. doi: 10.1002/fsn3.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karyani AK, Matin BK, Soltani S, Rezaei S, Soofi M, Salimi Y, et al. Socioeconomic gradient in physical activity: findings from the PERSIAN cohort study. BMC Public Health. 2019;19(1):1312. doi: 10.1186/s12889-019-7715-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagströmer M, Oja P, Sjöström M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. 2006;9(6):755–762. doi: 10.1079/PHN2005898. [DOI] [PubMed] [Google Scholar]
- 27.Mirghafourvand M, Charandabi SM-A, Aliasghari F. Predictors of depression in Iranian women with Polycystic ovarian syndrome. Commun Mental Health J. 2018;54(8):1274–83. [DOI] [PubMed]
- 28.Dokras A, Clifton S, Futterweit W, Wild R. Increased risk for abnormal depression scores in women with polycystic ovary syndrome: a systematic review and meta-analysis. Obstet Gynecol. 2011;117(1):145–152. doi: 10.1097/AOG.0b013e318202b0a4. [DOI] [PubMed] [Google Scholar]
- 29.Peng G-j, Tian J-s, Gao X-x, Zhou Y-z, Qin X-m. Research on the pathological mechanism and drug treatment mechanism of depression. Curr Neuropharmacol. 2015;13(4):514–523. doi: 10.2174/1570159X1304150831120428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsang CK, Kamei Y. Novel effect of vitamin K1 (phylloquinone) and vitamin K2 (menaquinone) on promoting nerve growth factor-mediated neurite outgrowth from PC12D cells. Neurosci Lett. 2002;323(1):9–12. doi: 10.1016/S0304-3940(01)02550-2. [DOI] [PubMed] [Google Scholar]
- 31.Shirayama Y, Chen AC-H, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22(8):3251–61. [DOI] [PMC free article] [PubMed]
- 32.Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF) Pharmacol Biochem Behav. 1997;56(1):131–137. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- 33.Turker Y, Ekinozu I, Aytekin S, Turker Y, Basar C, Baltaci D, et al. Comparison of changes in anxiety and depression level between dabigatran and warfarin use in patients with atrial fibrillation. Clin Appl Thromb Hemost. 2017;23(2):164–167. doi: 10.1177/1076029615600792. [DOI] [PubMed] [Google Scholar]
- 34.Rubio-López N, Morales-Suárez-Varela M, Pico Y, Livianos-Aldana L, Llopis-González A. Nutrient intake and depression symptoms in Spanish children: the ANIVA study. Int J Environ Res Public Health. 2016;13(3):352. doi: 10.3390/ijerph13030352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenwood EA, Pasch LA, Cedars MI, Legro RS, Eisenberg E, Huddleston HG, et al. Insulin resistance is associated with depression risk in polycystic ovary syndrome. Fertil Steril. 2018;110(1):27–34. doi: 10.1016/j.fertnstert.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manna P, Kalita J. Beneficial role of vitamin K supplementation on insulin sensitivity, glucose metabolism, and the reduced risk of type 2 diabetes: a review. Nutrition. 2016;32(7–8):732–739. doi: 10.1016/j.nut.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 37.Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates β cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci. 2008;105(13):5266–5270. doi: 10.1073/pnas.0711119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Booth SL, Centi A, Smith SR, Gundberg C. The role of osteocalcin in human glucose metabolism: Marker or mediator? Nat Rev Endocrinol. 2013;9(1):43–55. doi: 10.1038/nrendo.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shahdadian F, Mohammadi H, Rouhani MH. Effect of Vitamin K Supplementation on glycemic control: a systematic review and meta-analysis of clinical trials. Horm Metab Res. 2018;50(03):227–235. doi: 10.1055/s-0044-100616. [DOI] [PubMed] [Google Scholar]
- 40.Choi HJ, Yu J, Choi H, An JH, Kim SW, Park KS, et al. Vitamin K2 supplementation improves insulin sensitivity via osteocalcin metabolism: a placebo-controlled trial. Diabetes Care. 2011;34(9):e147-e. [DOI] [PMC free article] [PubMed]
- 41.Knapen M, Jardon K, Vermeer C. Vitamin K-induced effects on body fat and weight: results from a 3-year vitamin K2 intervention study. Eur J Clin Nutr. 2018;72(1):136–141. doi: 10.1038/ejcn.2017.146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.