Abstract
Staphylococcus aureus is a very common Gram-positive bacterium, and S. aureus infections play an extremely important role in a variety of diseases. This paper describes the types of virulence factors involved, the inflammatory cells activated, the process of host cell death, and the associated diseases caused by S. aureus. S. aureus can secrete a variety of enterotoxins and other toxins to trigger inflammatory responses and activate inflammatory cells, such as keratinocytes, helper T cells, innate lymphoid cells, macrophages, dendritic cells, mast cells, neutrophils, eosinophils, and basophils. Activated inflammatory cells can express various cytokines and induce an inflammatory response. S. aureus can also induce host cell death through pyroptosis, apoptosis, necroptosis, autophagy, etc. This article discusses S. aureus and MRSA (methicillin-resistant S. aureus) in atopic dermatitis, psoriasis, pulmonary cystic fibrosis, allergic asthma, food poisoning, sarcoidosis, multiple sclerosis, and osteomyelitis. Summarizing the pathogenic mechanism of Staphylococcus aureus provides a basis for the targeted treatment of Staphylococcus aureus infection.
Keywords: Staphylococcus aureus, toxin, inflammatory cells, pyroptosis, apoptosis, necroptosis, autophagy
1. Introduction
Staphylococcus aureus (S. aureus), a Gram-positive bacterium, is one of the most notorious human pathogens, causing illnesses ranging from mild skin and wound infections to fatal sepsis or multi-organ failure.
S. aureus secretes a variety of toxins and enzymes and some proteins and peptides that also have certain virulence effects [1]. Different virulence factors play different roles in different diseases. In addition to the above virulence components, S. aureus itself has virulence effects. The proliferation of S. aureus at the site of infection leads to a dysbiosis of the flora at the site of infection, which drives a range of diseases [2]. S. aureus has a virulence regulator, Agr (auxotrophic gene regulator), which is a population sensing system. Agr upregulates many toxins and virulence determinants when bacterial cell density reaches a certain threshold, leading to the exacerbation of disease [3,4].
Inflammatory cells play an important role in S. aureus infection. S. aureus infection and toxins can activate a variety of inflammatory cells, such as keratinocytes [5], helper T cells [6], innate lymphoid cells (ILCs) [7], macrophages [8], dendritic cells (DCs) [9], mast cells [10], neutrophils [11], eosinophils [12], and basophils [13], which release inflammatory factors that accumulate at the site of infection and cause an inflammatory response. During infection, S. aureus can also induce host cell death through programmed forms of cell death, such as pyroptosis [14], apoptosis [15], necroptosis [16], and autophagy [17].
S. aureus leads to a variety of different infections ranging in severity from mild to fatal. A distinctive feature of S. aureus or methicillin-resistant S. aureus (MRSA) is its considerable reservoir of virulence factors that can lead to atopic dermatitis (AD) [18], psoriasis [19], pulmonary cystic fibrosis (CF) [20], allergic asthma [21], pneumonia [22], food poisoning [23], chronic granulomatous disease (CGD) [24], osteomyelitis [25], diabetic foot infections (DFIs), and many other diseases.
In this article, we focus on the types of immune cells and cell death mechanisms activated by S. aureus in various human diseases. All the abbreviations are mentioned in Table 1. We wrote this article to explain the relationship between S. aureus and related diseases and to describe the mechanism of action of S. aureus in related diseases. We hope it will provide some help in the treatment of related diseases.
Table 1.
List of abbreviations and their full forms used in this review.
| Abbreviation | Full Name | Abbreviation | Full Name |
|---|---|---|---|
| 5-HT | 5-hydroxytryptamine | MMPs | Matrix metal proteinases |
| AAV | Associated vasculitis | MPO | Myeloperoxidase |
| Atopic dermatitis | MRSA | Methicillin-resistant S. aureus | |
| AdsA | Adenosine synthase A | MSCRAM | Mucin matrix molecules |
| Agr | Auxotrophic gene regulator | MSCRAMM | Microbial surface components recognizing adhesive matrix molecules |
| AMP | Antimicrobial peptide | NETs | Neutrophil extracellular traps |
| AMs | Alveolar macrophages | NF-κB | Nuclear factor kappa B |
| ANCA | Antineutrophil cytoplasmic antibody | NK | Natural killer cells |
| BCL-6 | B-cell lymphoma-6 | NO | Nitric oxide |
| BMECs | Bovine mammary epithelial cells | Nuc | Nuclease |
| CF | Cystic fibrosis | PA | Protein A |
| CFTR | Conductance regulator | PAMPs | Pathogen-associated molecular patterns |
| CGD | Chronic granulomatous disease | PBP2a | Penicillin-binding protein |
| Coa | Coagulase | PCD | Programmed cell death |
| CpG | Cytosine–phosphate–guanine | PD-1 | Programmed death 1 |
| CRSwNP | Chronic rhinosinusitis with nasal polyposis | PFTs | Pore-forming toxins |
| CWA | Cell wall-anchored | PGN | Peptidoglycan |
| CXCR5 | CXC chemokine receptor 5 | PI3K | Phosphatidylinositol 3-kinase |
| DAMPs | Damage-associated molecular patterns | PIP | Phosphatidylinositol phosphate |
| DCs | Dendritic cells | PMN | Polymorphonuclear leukocytes |
| DFI | Diabetic foot infection | PRRs | Pattern recognition receptors |
| Dsg1 | Desmoglein 1 | PSMs | Phenol-soluble modulins |
| Eap | Extracellular adhesion protein | PVL | Panton–Valentine leukocidin |
| ECP | Eosinophil cationic protein | RIPK1 | Receptor-interacting protein kinase 1 |
| EDIN | Epidermal differentiation inhibitor | ROS | Reactive oxygen specie |
| eDNA | Extracellular DNA | S. aureus | Staphylococcus aureus |
| EPO | Eosinophil peroxidase | SAgs | Superantigens |
| ESS | Early-secretion antigen-6 secretion system | Sbi | Second immunoglobulin-binding protein |
| ETs | Exfoliative toxins | SEs | Staphyloccocal enterotoxins |
| FPR2 | Formylpeptide receptor 2 | Socs3 | Suppressor of cytokine signaling 3 |
| GC | Germinal center | SpA | Staphylococcal protein A |
| G-CSF | Granulocyte colony-stimulating factor | Spls | Serine protease-like proteins |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor | SspB | Staphopain B |
| GPA | Granulomatous polyangiitis | SSSS | Staphylococcal scalded skin syndrome |
| Hla | α-hemolysin | TCA | Tricarboxylic acid |
| Hlb | β-hemolysin | Tem | Memory T |
| Hlg | γ-hemolysin | TER | Transmembrane electrical |
| I-CAM | Intercellular adhesion molecules | Tfh | Follicular helper T |
| IFN-γ | Interferon γ | TGF-β | Transforming growth factor β |
| lgE | Immunoglobulin-E | Th | T helper |
| IL | Interleukin | TLR | Toll-like receptor |
| ILCs | Innate lymphoid cells | TNF | Tumor necrosis factor |
| iPLA2 | Inhibition of calcium-dependent phospholipase A2 | TNFR1 | Tumor necrosis factor receptor 1 |
| JNK | Jun N-terminal kinase | Treg | Regulatory T |
| LCs | Langerhans cells | TSST-1 | Toxic shock syndrome toxin 1 |
| LTA | Lipoteichoic acid | V-CAM | Vascular cell adhesion molecules |
| MDP | Muramyl dipeptide | VEGF | Vascular endothelial growth factor |
| MLKL | Mixed-spectrum kinase-like protein |
2. Virulence Factors of S. aureus
The virulence factors of S. aureus can be divided into the following categories: (1) secreted virulence factors, including toxins and superantigens, the main function of which is to disrupt host cell membranes and induce target cell lysis and inflammation [26]; (2) extracellular enzymes, the main function of which is to break down host molecules for nutrition, promote bacterial survival and dissemination, etc. [26]; (3) surface proteins of S. aureus, whose main functions are adhesion, invasion, and immune escape [27], and (4) pathogen-associated molecular patterns (PAMPs), which promote inflammatory responses [28].
2.1. Secreted Virulence Factors
S. aureus can secrete a variety of enzymes and virulence factors that affect the immune system, leading to immune system dysregulation and the proliferation of auto-reactive T cells, as well as the development or progression of chronic autoimmune diseases. Virulence factors of S. aureus include pore-forming toxins (PFTs) [29], phenol-soluble modulins (PSMs) [30], exfoliative toxins (ETs) [31], and superantigens (SAgs) [32] that activate different types of immune cells and cause several different inflammatory and infectious diseases.
2.1.1. PFTs
PFTs are important virulence factors secreted by bacteria that lead to cell lysis by forming pore structures in eukaryotic cell membranes. PFTs exert their toxic effects mainly by altering the permeability of cell membranes, leading to cell death [29]. However, the disruption of cell permeability is often preceded by the release of cytokines and the activation of intracellular protein kinases. PFTs include α-hemolysin (Hla) [33], β-hemolysin (Hlb), γ-hemolysin (Hlg) [34,35], α-toxin [36], and Panton–Valentine leukocidin (PVL) [37].
Hla, Hlb, and Hlg
Haemolysin is a pore-forming toxin, also known as a membrane-disrupting toxin. Haemolysin is a substance that lyses red blood cells and releases hemoglobin, a sensitive, complementary fixed antibody that binds specifically to the antigen type of the red blood cell. This antibody can be produced by stimulation of the surface antigen and can cause red blood cells to lyse and release hemoglobin [38]. Haemolysin includes Hla, Hlb, and Hlg.
Hla can induce the formation of small pores in the cell membrane, leading to the rapid release of K+ ions as well as inducing the production of interleukin-1β (IL-1β), IL-6, and IL-8 [33,39,40,41]. Activated endothelial and epithelial cells induce the activation of caspase-1 and the production of NLRP3-inflammasome through the release of nitric oxide (NO), leading to extracellular Ca2+ influx, the production of pro-inflammatory cytokines, and the pyroptosis of monocytes. Ca2+ influx also activates caspase-12, activates caspase-3, and induces apoptosis. Hla also acts on Toll-like receptor (TLR) 3/4 and mediates necroptosis [41,42,43,44,45,46].
Hlb and Hlg can promote inflammasome formation containing procaspase-1, ASC, and NLRP3. Inflammasome activation promotes the release of IL-1β and IL-18. Activated caspase-1 can also cleave GSDMD to form the N-terminal cleavage product of GSDMD, and these together induce pyroptosis [34,35].
-
2.
α-Toxin
α-Toxin is a lecithin enzyme that breaks down lecithin, which is an important component of cell membranes. Therefore, α-toxin can damage the cell membrane of many cells, causing hemolysis, tissue necrosis, and vascular endothelial cell damage, increasing vascular permeability, and causing edema. α-Toxin activates macrophages and induces the activation of Th1 via CXCL-10. Th1 cells express interferon-γ (IFN-γ) in AD. α-Toxin induces early phagosomes (Rab5, Rab22b) to form autophagosomes (ILC3, Rab7), which induces cellular autophagy [36]. α-Toxin also activates RIPK3, caspase-8, and caspase-12, mediating necroptosis and apoptosis. α-Toxin acts in AD, granulomatous polyangiitis (GPA), pneumonia, chronic sinusitis, sepsis, and other diseases [47,48,49,50,51,52,53].
-
3.
PVL
PVL is a pore-forming toxin produced by S. aureus that causes leukocyte destruction [37]. PVL can induce the lysis of macrophages and neutrophils, leading to cell death [54,55]. PVL activates receptor-interacting serine threonine kinase 1 (RIPK1), RIPK3, and mixed-lineage kinase-like protein (MLKL) through tumor necrosis factor receptor 1 (TNFR1) and TLR3/4, forming a complex that leads to necroptosis. PVL can also lead to apoptosis. PVL also stimulates K+ efflux, NLRP3 inflammasome production, and caspase-1 activation, leading to pyroptosis [35,56,57,58,59,60,61]. PVL causes pneumonia through the above cell death mechanisms [58,62,63,64,65,66].
2.1.2. PSMs
PSMs are a family of amphipathic alpha-helical peptides found in Staphylococci [30]. PSMs contribute to biofilm structure and the propagation of biofilm-associated infections. PSMs include PSMα, PSMβ, and PSMγ.
PSMα
PSMα can induce necroptosis in atopic dermatitis and pneumonia [5,67,68,69,70]. PSMα induces the release of IL-1α and IL-36α from keratinocytes and induces the release of the pro-inflammatory cytokine IL-17, which mediates the skin inflammatory response to cause S. aureus infection [5]. PSMα induces the activation of keratinocytes and neutrophils, resulting in a series of pro-inflammatory responses (including cytokine production, leukocyte activation, and neutrophil chemotaxis) [71,72].
PSMα also activates and phosphorylates MLKL, as well as increases the expression and secretion of lactate dehydrogenase. Through MLKL and lactate dehydrogenase, PSMα can induce neutrophil necroptosis. The necroptosis of cells is the main pathological manifestation of S. aureus pneumonia [68].
-
2.
PSMβ
PSMβ has an important role in the formation of biofilms. PSMβ activates and induces neutrophil aggregation through formyl-peptide receptor 2 (FPR2), which induces an inflammatory response [73]. In addition to the role of surfactant, PSMβ can also lyse erythrocytes and destroy them. However, in addition to the common properties of PSM, the unique role of PSMβ in S. aureus itself has not yet been investigated [30].
-
3.
PSMγ (δ Toxin)
The S. aureus δ toxin is a member of the PSM family. The δ toxin is cytolytic to neutrophils and erythrocytes.
The δ toxin is cytolytic to neutrophils and erythrocytes. The staphylococcal δ-toxin promotes allergic skin disease in mice by inducing mast cell degranulation [74]. The δ toxin is an enterotoxin that is cytotoxic and increases cellular cAMP expression to inhibit water absorption in the ileum by altering the concentration of Na+ and Cl− in the mucosa, causing diarrhea [75].
2.1.3. Proteases
ETs
ETs are extremely specific serine proteases secreted by S. aureus. ETs can play a role in AD [31]. ETs are the primary toxins that play a role in staphylococcal scalded skin syndrome (SSSS), an abscessing skin disease [76].
ETs specifically recognize and hydrolyze the cell adhesion molecule desmoglein 1 (Dsg1), causing the dissociation of keratinocytes in human and animal skin and promoting skin infection by S. aureus. Three distinct ET subtypes (ETA, ETB, and ETD) were identified in S. aureus [77].
-
2.
Serine Protease-Like Proteins (Spls)
Spls play a role in T cells and result in asthma [78]. Spls recognize and hydrolyze desmoplastic proteins in the superficial skin layer, inducing skin peeling and blister formation [79,80,81,82,83]. Spls trigger IgE antibody responses in most asthmatics. Peripheral blood T cells produce Th 2 cytokines after Spls stimulation. Therefore, Spls are considered to be triggering allergens in the allergic airway response to S. aureus [78]. T cells of CF patients produced more TH2 cytokines after stimulation by Spls [84]. Increased IgE concentrations of Spls were detected in the serum of CF patients relative to healthy controls [84].
-
3.
Staphopain B (SspB)
SspB is a human strain of S. aureus that secretes papain-like proteases, and SspB has bacterial virulence. SspB can activate macrophages and lead to apoptosis [85]. SspB may contribute to the recruitment of host cells, including immunomodulatory pDCs and/or macrophages, which contribute to the initiation and maintenance of a chronic inflammatory state by S. aureus [86].
2.1.4. SAgs
SAgs are a class of antigenic substances composed of bacterial exotoxins and retroviral proteins. They bind to most T cells and provide signals for T cell activation. SAgs are highly efficient T-cell mitogens and manipulate the host immune system. They can directly activate T lymphocytes, triggering the release of a large number of pro-inflammatory cytokines, such as IFN-γ, IL-2, and tumor necrosis factor (TNF) [32,87,88]. SAgs include Staphyloccucal enterotoxins (SEs) and toxic shock syndrome toxin 1 (TSST-1).
SEs
SEs include SEA, SEB, SEC, SEG, SHE, SEI, SEM, SEO, and SEQ (Table 2). SEA and SEB can play a role in Th1, Th2, and Th22 cells and induce apoptosis. In nasal polyposis, SEB can induce IL-21 expression, and IL-21 also induces differentiation of Th17 [89,90]. SEA can lead to AD and asthma. SEB can cause AD, asthma, and chronic sinusitis. SEC can also lead to AD and cancer [91,92,93]. SEA serves as a potent stimulant of PBMCs and induces the release of large amounts of cytokines and chemokines through the Src, ERK, and STAT pathways [94]. SEB, SEG, SHE, SEI, SEM, SEO, and SEQ can also lead to food poisoning [95,96,97,98,99,100].
Table 2.
The mechanisms and characteristics of different types of SEs.
| SEs | Functions |
|---|---|
| SEA | Anti-protein hydrolase, supercarcinogenic and emetic toxin, causes food poisoning, but no diarrheal activity |
| SEB | Increase in the number of T lymphocytes, as well as the concentration of pro-inflammatory cytokines and inflammatory mediators in the intestinal mucosa; increase mucosal permeability and intestinal secretion |
| SEC | Supercarcinogenic and emetic toxin, but no diarrheal activity |
| SEG | Reversible destruction of intestinal cell ultrastructure |
| SEH | Causes food poisoning |
| SEI | Reversibly disrupts enterocyte ultrastructure and exhibits weak emetic activity |
| SEM | Strong emetic potential |
| SEO | Has emetic activity |
| SEQ | Significantly stable for heat treatment and digestive enzyme degradation, and exhibits significant supercarcinogenic and emetic activity |
-
2.
TSST-1
TSST-1 is a bacterial SAg produced and secreted by S. aureus. TSST-1 can activate CD4+ T cells to produce large amounts of cytokines and lead to a systemic toxic response. In AD, TSST-1 can lead to B lymphocytes and keratinocytes [13,50].
2.1.5. Secreted Enzymes (Exoenzymes) and Effectors
In addition to toxins, S. aureus secretes many virulence factors with proenzymatic effects. These proenzymatic virulence factors can be broadly classified into two types: cofactors, which are used to activate host enzymes, and enzymes that lyse and destroy host cells and tissues. Secreted enzymes (exoenzymes) and cofactors act on different substances with different specific mechanisms of action, but their main function is to break down host cells and tissues to obtain nutrients for their growth, reproduction, and propagation [26]. Secreted enzymes and effectors include EsxA, EsxB, coagulase (Coa), nuclease (Nuc), and adenosine synthase (AdsA), and staphopain (SspB).
EsxA and EsxB
EsxA and EsxB are small acidic proteins secreted by the early-secretion antigen-6 secretion system (ESS) as potential T-cell antigens of S. aureus. ESS is the basic virulence factor of S. aureus. EsxA and EsxB mediate the release of S. aureus from host cells, which can cause apoptosis [101,102].
-
2.
Coa
Coa is an enzyme produced by S. aureus. Coa has thrombospondin-like activity and coagulates plasma treated with citric or oxalic acid. Coa can induce coagulation with vascular hemophilia factor binding protein [103]. Coa can lead to apoptosis [104].
-
3.
Nuc and AdsA
Nuc can disrupt biofilms by breaking down extracellular DNA (eDNA) as well as mediating the escape of S. aureus from the NET. The NET is an innate immune defense mechanism by means of which invading pathogens are removed [105,106]. Moreover, Nuc can degrade DNA in abscesses or NETs.
The degradation product, nucleotide monophosphate, can be a substrate for the synthesis of another adenylate, synthase A (AsdA). AdsA degrades DNA into deoxyadenosine, which induces the apoptosis of macrophages around NETs by caspase-3 activation. Thus, AsdA can promote the survival of S. aureus [107]. Nuc and AdsA can induce bacteraemia and nephrapostasis [108].
-
4.
Extracellular Adhesion Protein (Eap)
Eap is the substance that mediates the adhesion of bacteria to host cells. In the early stages of infection, S. aureus adheres and colonizes through the expression of Eap, secondary to infection. Eap can act on T cells and lung epithelial cells. It can also work in psoriasis and CF [109].
2.2. Surface Proteins of S. aureus (Cell Wall-Anchored (CWA) Proteins)
Invasion of organs and tissues by S. aureus from the blood stream requires not only immune evasion but also adhesion. Biofilm formation is an important way for S. aureus to maintain infection. Adhesion, proliferation, and detachment are the main processes of biofilm formation.
Surface proteins of S. aureus constitute a range of virulence factors, known as CWA proteins. CWA proteins play a key role in the adhesion phase. The sorting signal of CWA proteins is responsible for covalently coupling proteins to peptidoglycan (PGN) [110]. There are 24 different CWA proteins on the surface of S. aureus, including microbial surface components recognizing adhesive matrix molecules (MSCRAMMs), the near iron transporter (NEAT) motif protein family, three-helical bundle motif protein A, G5-E repeat family, legume-lectin, and cadherin-like domain protein [111]. CWA proteins play an important role in extracellular matrix (ECM) adhesion, host cell invasion, and immune response evasion. Therefore, targeting CWA proteins with vaccines can counteract S. aureus infections [112].
2.2.1. MSCRAMM
MSCRAMMs are a series of proteins with similar amino acid sequences and very similar structures and functions. MSCRAMMs consist of two folded subdomains similar to IgG, and the two folded subdomains are adjacent to each other. MSCRAMM plays an important role in tissue invasion during S. aureus infection, including enabling S. aureus to adhere to and invade host cells and tissues, evade host immune attack, and induce biofilm formation [110]. Therefore, targeting the MSCRAMM protein is an alternative immunotherapeutic direction for the therapy of S. aureus infections [113].
2.2.2. Staphylococcal Protein A (SpA)
SPA is a protein that forms the cell wall of S. aureus. It was found that SpA can form a complex with human immunoglobulin, and the complex formed by the two can induce the necrosis of various immune cells in the body [114]. SpA can induce apoptosis and cause osteomyelitis [115,116,117,118].
The second immunoglobulin-binding protein (Sbi) belongs to SpA and consists of four triple-helix bundles arranged in tandem. Two of the triple-helix bundles bind similarly to those of SpA and IgG. Sbi non-covalently binds lipoteichoic acid (LTA), which then binds to the cell envelope and helps S. aureus to evade attack by the host immune system.
S. aureus Sbi can induce IL-33 production from keratinocytes in AD [119]. IL-33 can induce itch. IL-33 is a type 2 cytokine and a major regulator of chronic itch [120]. Sbi-inducible expression of IL-33 causes pruritus in AD [121]. It is therefore a virulence factor that promotes type 2 immune response.
2.3. PAMPs
PAMPs are bacterial-specific structures that typically activate TLRs, which act as neutrophil activators [28]. PAMPs of S. aureus include triacyl lipopetides, diacyl lipoproteins, and LTA.
2.3.1. Triacyl Lipopetides and Diacyl Lipoproteins
Triacyl lipopetides and diacyl lipoproteins are components of the Staphylococcal cell wall that provide structural integrity and protection against virulence organisms from the host immune system. Triacyl lipopetides and diacyl lipoproteins can be distinguished by subtle differences in TLR 1 and TLR 6 interactions with TLR2 [122]. Triacyl lipopetides and diacyl lipoproteins can induce activation of the TLR 2 and NLRP3 inflammasome. NLRP3 activation induces pyroptosis. Lipopetides and diacyl lipoproteins can induce the activation of TLR 2 and the NLRP3 inflammasome [35,69,123]. Triacyl lipopetides and diacyl lipoproteins can make use of sepsis [69,124].
2.3.2. LTA
LTA is an adhesion affinity agent associated with surface adhesion and a modulator of the bacteria’s own cell wall lysis enzymes (Muramidases). LTA is released from the bacteria after rupture and death. LTA can induce the phenomenon of passive immune killing. LTA promotes macrophage activation and the expression of IL-1β and IL-18 [125]. LTA activates neutrophils and macrophages and leads to pyroptosis. LTA can also work in food poisoning [23,125,126,127].
2.3.3. PGN
PGN is the main structural component of the cell wall [128], a glycopolymer that maintains the shape of the bacteria. PGN is also a key factor in bacterial recognition by the host immune system [129].
PGN induces the activation of keratinocytes, Langerhans cells (LCs), mast cells, macrophages, CD4+T cells, Th1 cells, and microglia, and induces the release of several cytokines, including TNF-α, IL-1, and IL-4 [17,130,131,132,133,134,135,136,137,138,139]. PGN causes autophagy, which drives AD, sepsis, pneumonia, and psoriasis [17,130,131,132,133,140,141]
S. aureus contains large amounts of SpA, which evades phagocytosis and uptake by phagocytes by binding to immunoglobulin (Ig) molecules and then covering the bacterial surface. Peptidoglycan can promote the B-cell superantigen activity of SpA [142].
A summary of the above factors activating immune cell types and the types of induced cell death and induced diseases is shown in Table 3.
Table 3.
Inflammatory cell types and cell death mechanisms involved in different Staphylococcus aureus virulence factors.
| Classification | Virulence Factors | Cell Types | Cell Death Mechanisms | Diseases | Refs. | |
|---|---|---|---|---|---|---|
| Secreted Virulence factors |
PFT | Hla | Keratinocytes Macrophages |
Pyroptosis Necroptosis Autophagy Apoptosis |
[43,143,144,145,146] | |
| Hlb | Pyroptosis | [34] | ||||
| Hlg | Pyroptosis | [35] | ||||
| α-Toxin | Th1, Th2 Neutrophils Macrophages |
Apoptosis Necroptosis Autophagy |
AD GPA Pneumonia Chronic sinusitis sepsis |
[47,48,49,50,51,52,53] | ||
| PVL | DCs Macrophages |
Apoptosis | Pneumonia | [58,62,63,64,65,66] | ||
| PSMs | PSMα | Keratinocytes Neutrophils |
Necroptosis | AD Pneumonia |
[5,67,68,69,70] | |
| PSMβ | Neutrophil | [73] | ||||
| PSMγ (δ toxin) |
Mast cells Keratinocytes |
AD Diarrhea |
[74,75,147] | |||
| SAgs | SEA | B cells CD4+ T cells Keratinocytes Eosinophils |
Apoptosis | AD Asthma Food poisoning |
[13,91,92,148,149] | |
| SEB | Th1, Th2 Eosinophils Proximal renal tubular epithelial cells |
Apoptosis | Chronic sinusitis AD Asthma |
[15,92,93,149] | ||
| SEC | B cells | AD Food poisoning |
[13,150] | |||
| SEG | Food poisoning | [95] | ||||
| SEH | Bovine mammary gland epithelial cells | Apoptosis | Food poisoning | [96] | ||
| SEI | Food poisoning | [95,97] | ||||
| SEM | Apoptosis | Food poisoning | [98,99] | |||
| SEO | Food poisoning | [97] | ||||
| SEQ | Food poisoning | [100] | ||||
| TSST-1 | B cells Keratinocytes |
AD | [13,50] | |||
| Proteases | ETs | AD | [31] | |||
| Spls | T cells | Asthma SSSS |
[78] [76] |
|||
| SspB | Macrophages | Apoptosis | [85] | |||
| Secreted Enzymes (proteins) |
EsxA, EsxB | Apoptosis | [101,102] | |||
| Coa | Apoptosis | [104] | ||||
| Eap | T cells Lung epithelial cells |
Psoriasis CF |
[109] | |||
| Nuc | Neutrophils Macrophages |
Apoptosis | Bacteraemia Nephrapostasis |
[108] | ||
| AdsA | Neutrophils Macrophages |
Apoptosis | Bacteraemia Nephrapostasis |
[107] | ||
| Cell wall components | Surface Proteins (CWA) |
Sbi | Keratinocytes | AD | [119] | |
| SpA | Tumor cells | Apoptosis | [115,116,117,118] | |||
| PAMPs | Triacyl lipopetides | Pyroptosis | Sepsis | [69,124] | ||
| Diacyl lipoproteins | Pyroptosis | Sepsis | [69,151] | |||
| LTA | Neutrophils Macrophages B cell |
Pyroptosis | Food poisoning | [23,125,126,127] | ||
| PGN | LCs Mast cells Macrophages Macrophages |
Autophagy | AD Sepsis Pneumonia Psoriasis |
[17,130,131,132,133,140,141] |
Note: The virulence factors of S. aureus, i.e., PFTs, PSMs, ETs, SAgs, enzymes, effectors, PAMPs, proteins, and peptides, induce different kinds of cellular activation and different forms of cell death in different diseases.
3. Different Inflammatory Cell Types Involved in S. aureus Pathogenesis
The cells involved in S. aureus infection and inflammation include keratinocytes, T cells (helper T cells, ILCs), macrophages, DCs, mast cells, neutrophils, eosinophils, and basophils.
3.1. Keratinocytes/Epithelial Cells
Keratinocytes are the main cellular components that make up the epidermis. S. aureus expresses PSMα, which is a group of secreted virulence peptides.
In AD, S. aureus accumulates in the lysosomes of keratinocytes and induces IL-1α secretion via TLR9 [152]. S. aureus expresses PSMα acting on keratinocytes, induces IL-1α and IL-36α release from keratinocytes via myd88 signaling, induces γδt cell and ILC3 production of IL-17, and promotes neutrophil infiltration [5]. In addition, S. aureus promotes IL-36α secretion from keratinocytes and promotes IgE production and allergic inflammatory response [70]. S. aureus promotes IL-1β expression by keratinocytes and induces skin regeneration through keratinocyte-dependent IL-1R-MyD88 signaling [153].
S. aureus Sbi promotes IL33 secretion by keratinocytes, and IL-33 promotes type 2 immune responses [119,154]. IL-33 also has an important role in pruritus [155]. S. aureus diacylated lipoprotein is a TLR2 and TLR6 ligand, which activates keratinocytes via TLR2-TLR6 heterodimers to produce TSLP, which promotes T(H)2-type inflammation and thus AD [156]. The keratinocyte-expressed TSLP acts directly on TRPA1-positive sensory neurons, triggering intense pruritus-induced scratching [157].
In nasal polyp tissue, S. aureus can directly induce the release of the epithelial cell-derived cytokines TSLP and IL-33 by binding to TLR 2, thereby potentially propagating the expression of type 2 cytokines IL-5 and IL-13 in nasal polyp tissue [158,159,160,161].
3.2. Helper T Cells (Th Cells)
3.2.1. T Helper 1 (Th1) Cells
Th1 cells are CD4+ cells that mainly secrete IL-2, IFN-γ, and TNF-β (tumor necrosis factor β), which are involved in regulating cellular immunity, assisting in the differentiation of cytotoxic T cells, mediating cellular immune responses, and participating in delayed hypersensitivity reactions. IFN-γ, IL-2, and TNF-β can drive inflammatory responses [162].
S. aureus infection can induce Th1-type inflammatory responses in different diseases. Enterotoxin B (SEB) induces Th1 activation in chronic sinusitis; α-toxin promotes Th1 activation via CXCL10 in AD [48,49,93,163]. Activated Th1 cells release the cytokine IFN-γ. IFN-γ is a driver of chronic inflammation in the chronic phase of AD, and the overexpression of IFN-γ can lead to recurrent inflammation and pruritus, causing lichenoid degeneration of the skin [13,164]. In contrast, psoriasis is also a chronic disease mediated by Th1 cytokines, and IL-12 mediates the differentiation of Th1 cells [165].
3.2.2. Th2 Cells
Th2 cells (which are CD4+ T cells) are important cells in the type 2 inflammatory pathway. th2 secretes IL-4, IL-5, and IL-13 and stimulates type 2 immunity, as evidenced by the production of high levels of IgE and eosinophils. Type 2 immunity is a specific immune response that includes both innate and adaptive immunity and contributes to the formation of an immune barrier on the mucosal surface to a clear response to pathogens [166]. Th2 cells are formed by the induced differentiation of Th0 cells by IL-4 produced by basophils, eosinophils, mast cells, natural killer cells (NK), or already differentiated Th2 cells.
In AD infection with S. aureus, keratinocytes can express TSLP, IL33 [119,154], and IL-19 [167], which can induce the Th2 expression of IL-4, IL-10, IL-13, and IL-31, causing an inflammatory response. IL-4 and IL-31 play important roles in itchiness [158,159,167]. S. aureus was found to induce the expression of IL-8, IL-19, and IL-22, which induced increased expression of Th2 cytokines [131,167,168]. S. aureus PGN action on LCs induces Th2 cells in the skin via CCL17, and it induces IL-18 stimulation of CD4+ T cells to produce IL-4 to induce Th2 cytokine expression [130,131]. Staphylococcal α-toxin mediates the expression of Th2 cytokines IL-4 and IL-13 through STAT6, leading to increased keratin-forming cell death [169].
It was found that antigen-specific regulatory T (Treg) cells can also induce Th2 cell proliferation [170]. S. aureus PGN action on LCs induces Th2 cells in the skin via CCL17, and it induces the IL-18 stimulation of CD4+ T cells to produce IL-4, which induces Th2 cytokine expression [130,131]. In addition, staphylococcal α-toxin mediates the expression of Th2 cytokines IL-4 and IL-13 through STAT6, leading to increased keratin-forming cell death [169]. SEB can also act on eosinophils, monocytes, and Treg cells to induce Th2 cytokine expression and induce the Th2 proliferation and secretion of IL-10 to aggravate skin inflammation [170,171]. In asthma, eosinophils induce the activation of Th2 and express IL-5 in conjunction with basophils [172]. In chronic sinusitis, Th2 cytokines can activate B cells, release IgE, and indirectly mediate eosinophil inflammation [7]. Th2 cytokines can reduce filamentous protein expression in keratinocytes, further exacerbating the disruption of skin barrier function [173]. Th2 expresses IL-4, IL-10, IL-13, and IL-31, inducing epidermal thickening, sensitization, inflammation, and pruritus [174,175]. Th2 also reduces the expression of AMP, HBD-2, HBD-3, filamentous polymerase, and epidermal proteins, further promoting the proliferation of S. aureus and exacerbating flora imbalance. The activation of keratinocytes increased the expression of endogenous serine proteases, induced inflammatory responses and tissue damage, and released various cytokines. Serine protease V8 and the serine protease strip toxin cleave corneal adhesion proteins, including DSG-1, leading to increased desquamation [158,159].
3.2.3. Th17 Cells
Th17 is a newly discovered subpopulation of T cells that secrete IL-17, which is important in autoimmune diseases and the body’s defense response. Transforming growth factor β (TGF-β), IL-6, IL-23, and IL-21 play active roles in the differentiation and formation of Th17 cells, while IFN-γ, IL-4, cytokine signaling (IFN-γ), IL-4, suppressor of cytokine signaling 3 (Socs3), and IL-2 inhibit its differentiation [176].
Th17 cells have a crucial role in host defense. Dysregulated Th17 responses mediate various autoimmune and inflammatory diseases. IL-6, TGF-β, and IL-23, secreted by macrophages and DCs, induce the activation and differentiation of Th17. Th17 cells are recruited into the skin and interact with keratinocytes and fibroblasts to promote epidermal tissue repair [164]. Th17 cells also express IL-17, IL-22, and IL-26. IL-17 can coordinate local tissue by upregulating pro-inflammatory cytokines and chemokines (including IL-1β, IL-6, TNF-α, granulocyte-macrophage colony-stimulating factor (GM-CSF), KC/CXCL1, MCP-1/CCL2, MIP-2/CXCL2, MCP-3/CCL7, and MIP-3α/CCL20, and matrix metal proteinases (MMPs)), enabling the migration of activated T cells through the ECM [177,178,179].
S. aureus triggers a strong Th17-type response in skin effector T cells, inducing Th17 to produce IL-17A, IL-17F, and IL-22 [180]. S. aureus promotes keratinocyte expression of IL1β, promotes differentiation of Th17, and promotes skin inflammation [181]. SEB induces IL-21 secretion by Follicular helper T (Tfh), induces differentiation of Th17, and acts in nasal polyp disease [89,90].
In septic arthritis caused by S. aureus infection, S. aureus activates immune cell-specific macrophages and DCs to release pro-inflammatory mediators, such as TNF-α, IL-1β, IL-6, and IL-21, which induce RORγt, RORγt, and CD4 T cells to differentiate into Th17 cells [182,183,184]. Th17 cells produce the pro-inflammatory cytokine IL-17, which directly stimulates arthritic inflammation by binding to receptors on immune cells, stimulating the production of more pro-inflammatory cytokines, chemokines, and other inflammatory mediators, including NO and MMPs, while TNF-α, IL-1β, and IL-6 upregulate MMPs, which can promote cartilage degradation and enhance joint destruction [185,186].
IL-17 activates macrophages to express TNF-α and IL-1β as well as fibroblasts to produce IL-6, IL-8, and MMPs; it also stimulates blood endothelial cells to produce chelators and the p38 MAPK-dependent expression of VCAM-1 and ICAM-1, which contribute to immune cell evasion from blood to tissue [180]. In mouse studies, ICAM-1 was upregulated on endothelial cells in the diseased skin of CD18 (β2-integrin) (CD18hypo) mice, whereas in vivo S. aureus extracellular adhesion protein prevented T cell vascular migration to the inflamed skin of CD18 (β2-integrin) (CD18hypo) mice but did not inhibit their proliferation and activation [109]. IL-17 also promoted epithelial cell stimulation by IL-8 and granulocyte colony-stimulating factor (G-CSF), induced neutrophil migration and activation, and synergistically enhanced the induction of TNF-α. IL-22 not only triggers a pro-inflammatory response, but also inhibits terminal differentiation of keratinocytes and induces Th2-type inflammation [168].
In psoriasis, IL-17 can activate macrophages to express TNF-α and IL-1β, thereby inducing fibroblast activation [180]. In inflammatory areas of the skin, Th17 can increase the inflammatory response of the skin, and IL-17A can strongly induce IL-19 expression in keratinocytes. IL-19 also induces the expression of Th2 cytokines [167,187].
3.2.4. Tregs
Tregs, characterized by an expression of the forkhead transcription factor FOXP3 and IL-2R α chain CD25, play a central role in maintaining tolerance to self and preventing an overexcited inflammatory response to infection [188]. S. aureus induces the proliferation of effector memory T (Tem) cells and the activation of Treg cells and Th17 responses through cutaneous LCs [189,190]. Th17 and Treg cells antagonize each other, and Th17/Treg cell imbalance can trigger or exacerbate the disease process in AD [191]. After superantigenic stimulation of SEs, Treg cells lose their immunosuppressive activity, and a Treg induces Th2 proliferation and the secretion of IL-10 to aggravate the skin inflammatory response [170].
3.2.5. Tfh Cells
Tfh cells are a specialized subset of CD4+ T cells located in B cell follicles that induce B and T cell interactions and release cytokines to promote germinal center (GC) formation, promote the differentiation of GC B cells into memory B cells or plasma cells, and drive the maturation of high-affinity antibodies [192]. The Tfh cells express CXC chemokine receptor 5 (CXCR5) [193], ICOS [194], programmed death-1 (PD-1) [195], cytotoxic T lymphocyte antigen 4 (CTLA-4) [196], B-cell lymphoma-6 (BCL-6) [197], and IL-21 [90].
In S. aureus-infected chronic rhinosinusitis with nasal polyposis (CRSwNP), Tfh can migrate into lymphoid aggregates (lymphoid aggregates resemble germinal centers) and interact with B cells to induce the proliferation and differentiation of naive B cells to plasma cells [89]. Tfh is also involved in the B cell-mediated immune response induced by the S. aureus vaccine [198]. In nasal polyposis, IL-21 expression was increased after SEB stimulation. IL-21 can also induce the differentiation of Th17 [89,90].
However, there has been minimal research on this aspect of the mechanism of Tfh in S. aureus infection, and future research in this direction may allow the mechanism of S. aureus infection to be further deciphered and allow for more comprehensive counseling for clinical treatment.
3.2.6. Th9 Cells
Recently, researchers have identified another novel and unique population of immunomodulatory cells. Th9 cells were initially thought to be a subpopulation of Th2 cells that could produce IL-9 [199]. Th9 cells have been shown to act on many cell types associated with asthma, including T cells, B cells, mast cells, eosinophils, neutrophils, and epithelial cells, and therefore may be important in the pathophysiology of allergic asthma [200]. Moreover, Th9 has been found to be a major factor in regulating immunity to autoimmune diseases [201] and tumor immunity [202].
There are few studies on this aspect of the mechanism of S. aureus infection promoting Th9-type inflammatory response, which may be a direction for further research.
3.3. ILCs
ILCs are lymphocytes that do not express the diverse antigen receptor types expressed on T and B cells [203]. ILCs comprise a heterogeneous population of immune cells that maintain barrier function and can initiate a protective or pathological immune response in response to infection [204].
ILCs can be divided into ILC1, ILC2, and ILC3 types. In cell-mediated effector immunity, ILC1, ILC2, and ILC3 are involved in type 1, type 2, and type 17 immune responses, respectively [205]. S. aureus and S. aureus muramyl dipeptide (MDP) can activate ILC2 and promote type 2 immune response [161]. In AD, PSMα induces the production of ILC3, which is involved in the mediation of skin inflammation [5]. In patients with AD infection with S. aureus, the expansion of ILC3 is involved in mediating skin inflammation [13].
However, little is known about the relationship between S. aureus infection and ILCs. This could be another direction in the study of the mechanism of S. aureus infection in the future.
3.4. Macrophages
Macrophages are cellular components of the innate immune system and are present in almost all tissues, contributing to immunity, repair, and homeostasis [206]. When S. aureus infects humans, epithelial cells recognize the invading S. aureus through pathogen recognition receptors (PRRs), which induce the production of pro-inflammatory cytokines and chemokines, leading to the recruitment and activation of phagocytes, including GM-CSF, G-CSF, IL-1β, IL-6, and IL-8 [8].
Phagocytosis of S. aureus triggers the TLR2-dependent signaling and activation of the NLRP3 inflammasome, leading to the recruitment of ASC and the activation of cystathione-1, causing cells to release cytokines IL-1β and IL-18 and inducing apoptosis [207,208]. S. aureus was found to survive and replicate in macrophages, which deliver nutrients to lysosomal-engulfing S. aureus to promote bacterial growth, and promote bacterial persistence during infection by limiting reactive oxygen species (ROS) and RNS production by macrophages through lipoic acid synthesis [209,210].
3.5. DCs
DCs are the most powerful specialized antigen-presenting cells in the body and are highly efficient in the uptake, processing, and presentation of antigens. Immature DCs have a strong migratory capacity and mature DCs can effectively activate initial T cells and are central to the initiation, regulation, and maintenance of the immune response. They are usually found in small numbers in contact with external skin and their immature forms can be found in the blood. When activated, they move to the lymphoid tissue to interact with T and B cells to stimulate and control the appropriate immune response.
S. aureus activates macrophages and DCs to release pro-inflammatory mediators, such as TNF-α, IL-1β, IL-6, and IL-21, which induce RORγt to produce IL-17A, IL-17F, and IL-21 and induce CD4 T cells to differentiate into Th17 cells [182,183,184].
TSLP released by keratinocytes in AD is a potent activator of DCs, triggering the production of Th2-attracting chemokines, such as CCL17/TARC and CCL22/MDC, and inducing Th2 differentiation through upregulation of these cells by OX40L, which activates Th2 cells [9]. In experiments with psoriatic mice, S. aureus Eap was found to disrupt cell–cell contacts between T cells and DCs in vitro, blocking T cell extravasation into inflamed skin to inhibit psoriasis [109].
LCs are the major DCs in the normal epidermis, and DCs are the major antigen-presenting cells [211]. Epidermal exposure to S. aureus induces the proliferation of effector memory T (Tem) cells and limited Treg cell activation and Th17 responses, and LCs directly interact with S. aureus via the pattern recognition receptor langerin (CD207), which interacts directly with S. aureus [189,190]. LCs express TLRs that recognize bacterial and viral products, and the TLR2-mediated transduction of S. aureus-derived signals is severely impaired in LCs with AD skin [212]. The DC-mediated blockade of human T cell activation and proliferation, PVL, targets DCs to blunt CD4+ T lymphocyte activation and kills DCs, leading to impaired T cell responses and increased infection [213].
3.6. Mast Cells
Mast cells are granulocytes that disintegrate to release granules and the substances in the granules. Such granules in the blood contain heparin, histamine, and 5-hydroxytryptamine, which can drive tachyphylactic allergic reactions (inflammation) in tissues, especially in asthma. Mast cells are the main effector cells of inflammation, and mast cells act as antigen-presenting cells and induce Th1 and Th2 cell development.
In patients with AD, S. aureus infection leads to an increase in the number of mast cells, which proliferate within mast cells and mediate Th1 cell development as well as the development of chronic inflammation, leading to the release of Th1 cytokines and the upregulation of IFN, manifested as edema within the lamina propria [214,215]. In mast cells, PGN from S. aureus stimulates mast cells in a TLR2-dependent manner, producing TNF-α, IL-4, IL-5, IL-6, and IL-13, and S. aureus also triggers TNFα and IL-8 release by binding to CD48 [132,133].
The δ toxin induces mast cell degranulation, which is dependent on intracellular PI3K activation and free calcium ion influx. δ toxin-induced mast cell degranulation differs from conventional IgE cross-linking in that this action does not require the presence of an antigen. IgE enhances δ toxin-induced mast cell degranulation, promotes IgE and IL-4 production, and leads to skin inflammation. However, AD is a chronic inflammatory skin disease caused by mast cells, leading to immunoglobulin-E (lgE)-mediated hypersensitivity [74,147].
S. aureus-expressed SAgs can also trigger mast cell degranulation via IgE and FcεR, and SEs may also trigger direct histamine release from mast cell degranulation via unknown receptors [10]. In asthma, the airways are hyperreactive, and mast cells release histamine and various cytokines that attract the accumulation of eosinophils, causing epithelial cell damage and respiratory distress [216,217].
Although mast cells may help clear the infection, S. aureus may use mast cells to evade detection and immune clearance.
3.7. Neutrophils
Neutrophils are a type of myeloid leukocyte and some of the major responders in acute inflammation. Activated neutrophils mediate inflammation by synthesizing and secreting cytokines, chemokines, leukotrienes, and prostaglandins. Neutrophils synthesize and secrete the chemokine CXCL8 to recruit more neutrophils and express IL-1, IL-6, IL-12, TGF-β, and TNF-α, reactivating neutrophils and other cells of the immune system [218].
S. aureus can recruit and activate neutrophils at the site of infection [219]. In AD and pneumonia, PSMα can induce the expression of IL-1α and IL-36 and induce neutrophil death, leading to disease exacerbation [67,68]. γδ T cells mediate IL-17 responses and induce neutrophil recruitment, pro-inflammatory cytokines IL-1α, IL-1β, and TNF, and host defense peptides, while rapid neutrophil recruitment enhances S. aureus colonization in the skin [11]. IL-17 also promotes IL-8 and G-CSF stimulation of epithelial cells, induces neutrophil migration and activation, synergistically enhances TNF-α induction, and increases antimicrobial peptide (AMP) production [180].
Neutrophils induce IL-20 expression, which contributes to psoriasis, wound healing, and anti-inflammatory effects [165]. S. aureus LTA promotes the expression of neutrophil factors TNF-α and IL-8. IL-8 also attracts neutrophils to accumulate in the intestine, leading to the activation and attraction of polymorphonuclear leukocytes (PMN), causing an inflammatory response in the intestine during food poisoning [23]. The α toxin produces IL-1β via TLR2, NOD2, FPR1, and ASC/NLRP3 inflammasome induced by neutrophils, and IL-1β can induce thymic stromal lymphopoietin and contribute to abscess formation [178,220]. S. aureus α toxin produces IL-1β via TLR2, NOD2, FPR1, and ASC/NLRP3 inflammasomes induced by neutrophils expressing IL-1β [220]. In GPA, the activation of neutrophils, by expressing neutrophil extracellular trap products (NET-derived products), plays a role in the disease [221].
3.8. Eosinophils (Eos)
Eosinophils are considered to be the effector cells associated with infection and the cause of tissue damage. Eosinophils can express a range of ligand receptors that play a role in cell growth, adhesion, chemotaxis, degranulation, and intercellular interactions. Eosinophils synthesize, store, and secrete cytokines, chemokines, and growth factors. Eosinophils can function as antigen-presenting cells and can regulate the immune system [222].
In AD, S. aureus produces exotoxins that can amplify the allergic response by directly activating other immune cells, such as eosinophils. Eosinophil inflammation can be induced by SEB. Eosinophils lead to epidermal damage, tissue swelling, and inflammatory cell recruitment through the release of toxic mediators, including eosinophil cationic protein (ECP), major basic proteins, eosinophil-derived neurotoxins, and eosinophil peroxidase (EPO) [223,224]. In addition, activated eosinophils can promote antimicrobial defense by releasing mitochondrial DNA associated with granule proteins [12].
In asthma, eosinophils are not only involved in the release of granulins, lipid mediators, ROS, cytokines, and growth factors that trigger the Th2 response [172]. The IL-5 cytokines of Th2, GM-CSF, and IL-3 induce the eosinophil response and eosinophil maturation. IL-3 and GM-CSF also induce eosinophil recruitment [225,226].
In CRSwNP, S. aureus can also cause eosinophil inflammation by inducing IgE [7].
3.9. Basophils
Basophils are a type of leukocyte that originate from bone marrow hematopoietic pluripotent stem cells that differentiate and mature in the bone marrow and enter the bloodstream. Basophils are important cells for S. aureus respecting the virulence of AD and asthma [3,216].
S. aureus SAgs, including SEA, SEB, SEC, and TSST-1, also directly activate B lymphocytes and induce specific IgE-dependent mast cells and basophil degranulation to release histamine, further exacerbating AD and promoting adaptive cellular and humoral type 2 immunity [13].
NOD2 and TLR2 ligands trigger basophil activation by interacting with dermal fibroblasts in AD-like skin inflammation [227]. S. aureus was found to induce skin basophil aggregation and increase IL-4 expression. Basophil-derived IL-4 inhibited skin IL-17A production by TCRγδ+ cells and promoted S. aureus infection of the skin. Basophils secrete IL-6 to promote Th17 responses and inhibit the IL-17A production of IL-4 via STAT6 inhibition of the IL-17A promoter to promote S. aureus infection [228], while Toll-like expressing receptor-expressing epidermal keratinocytes recognize invasion and respond to LTA by inducing the expression of cytokines such as TSLP, which leads to basophil recruitment and IL-4 production [126].
3.10. B Cells
In inflammatory and neoplastic diseases, B cells play a regulatory role by secreting regulatory cytokines, such as IL-10, or by relying on the secretion of antibodies. B cells can act in concert with other immunomodulatory cells, such as Treg cells. TSST-1 can induce B cell apoptosis [229]. LTA inhibits LPS-induced B cell proliferation by reducing ERK phosphorylation through the TLR2 signaling pathway [230]. The S. aureus-induced activation of Th2 triggers B cells to produce IgE in response to allergens and autoantigens, indirectly mediating eosinophil inflammation [7]. Tfh and ILCs can also induce B cell differentiation [89,216].
In the disease response to S. aureus infection, there are many other cells involved besides those mentioned above. As a major human pathogen, S. aureus induces cell activation in various cell types, releases various cytokines, and causes apoptosis, which is important in S. aureus infections. However, the cellular mechanisms are not yet clear, including the role of Th9, Tfh, and ILCs in the pathogenesis of S. aureus. This may be another direction for studies on S. aureus infection in the future.
A summary of the inflammatory cells activated by S. aureus in several diseases is shown in Figure 1.
Figure 1.
Inflammatory cell types in the pathogenesis of Staphylococcus aureus. Different virulence factors of S. aureus can induce activation of Tfh, Th1, Th2, Th9, and Th17 cells, which play a role in chronic sinusitis, AD, asthma, itch, psoriasis, septic arthritis, and CGD. Eos: eosinophils, Bas: basophils, MC: mast cells, Mø: macrophage.
4. Types of Host Cell Death Caused by S. aureus
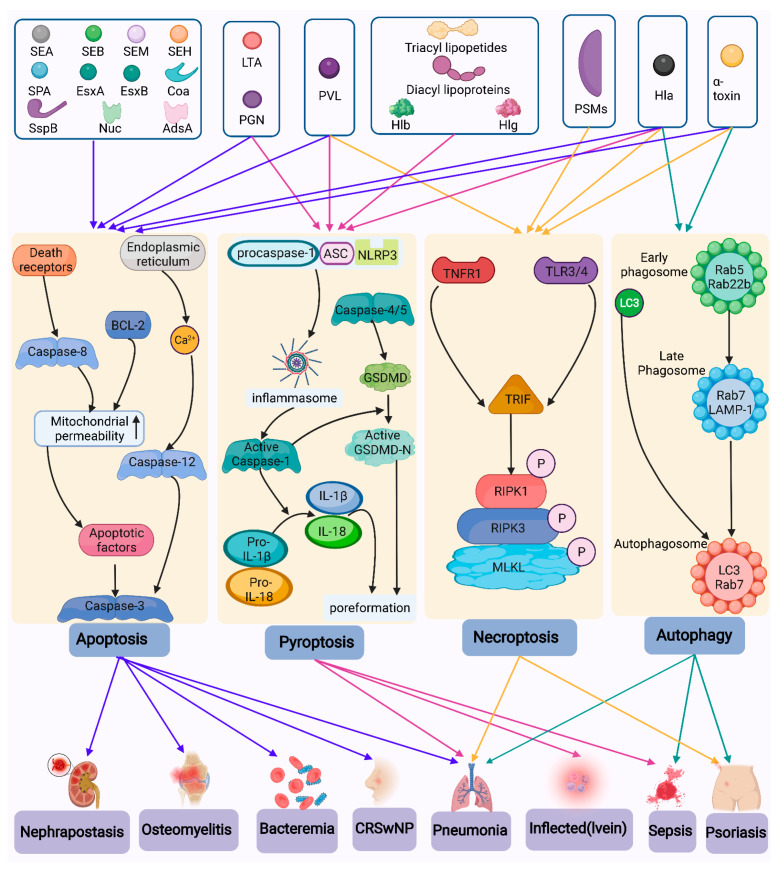
S. aureus can induce cell death modalities, such as pyroptosis, apoptosis, necroptosis, and autophagy, which in many ways have substantial impacts on the pathogenesis and clinical manifestations of staphylococcal disease. An increasing number of studies have shown that the various cell death modalities are interrelated [231]. This suggests that the various modes of cell death do not exist in isolation.
4.1. Pyroptosis
4.1.1. Activation of Inflammasomes Caused by S. aureus
The inflammasome is a caspase-1-activated multiprotein platform that responds to many exogenous and endogenous danger signals [232]. Inflammasomes may be important in the development of diseases and may be an important trigger for disease development. Inflammasomes activate the caspase protease caspase-1, which subsequently activates the proinflammatory cytokines IL-1β and IL-18 and induces pyroptosis [233,234].
Inflammasomes can recognize the uncontrolled release of damage-associated molecular patterns (DAMPs) or PAMPs through pattern recognition receptors (PRRs) [235]. Their main components include NLRP1 [236], NLRP2 [237], NLRP3 [238], NLRP4 [239], NLRP6 [240], NLRP7 [241], NLRP12 [242], NLRC4 [243], AIM2 [244], IFI16 [245], and Pyrin [246,247]. Among these, NLRP3 [35] and NLRP6 [125] play an important role in S. aureus infection.
S. aureus acts through two signaling pathways to activate inflammasomes to promote AD: (1) Signal 1: S. aureus acts on TLRs and TNFR1, interacts with NOD2, activates NF-kB, induces gene transcription, produces Pro-IL-1β, and synthesizes raw materials for the inflammasomes. (2) Signal 2: PFTs, purine receptors, and lysosome rupture act together to cause potassium ion efflux, which, together with the inflammasome raw materials produced by signal 1, forms the inflammasome. Inflammasomes activate caspase-1, which subsequently activates the proinflammatory cytokines IL-1β and IL-18 and induces pyroptosis [233,234]. The NLRP3 inflammasome was found to promote IL-1β activation at the site of S. aureus skin infection in mice by mediating neutrophil recruitment to the site of skin infection [220,248].
Inflammasomes can suppress the expression of TSLP, which regulates Th1 and Th2 cells. The gene expression of NLRP3 and ASC is significantly reduced during Th2 cytokine (IL-4, IL-5, and IL-13) stimulation in human keratinocytes. Therefore, NLRP3 inflammasome activity can be inhibited by suppressing the expression of NLRP3, ASC, inflammatory cystein-1, and IL-1β in keratinocytes [249,250].
Munoz-Planillo et al. [35] found that SEs activated the NLRP3 inflammasome in macrophages. When bound to bacterial lipoproteins, enterotoxins SEA and SEB activated the inflammasome. This activation was not dependent on the P2X7 receptor or the TLR junction MyD88, suggesting that SEs may bypass the P2X7 receptor activation pathway for the NLRP3 inflammasome.
4.1.2. Pyroptosis
Pyroptosis is a new form of programmed cell death caused by the inflammasome, which is characterized by the dependence on inflammatory cysteases (mainly caspase-1, 4, 5, and 11) and accompanied by the release of large amounts of pro-inflammatory factors. The morphological features, occurrence, and regulatory mechanisms of pyroptosis death are different from other cell death modalities, such as apoptosis and necrosis. The activation of caspases by the inflammasome leads to the cleavage of gasdermin proteins, the activation of gasdermin proteins, the translocation of activated gasdermin proteins to the membrane, the formation of pores, cell swelling, cytoplasmic outflow, and finally cell membrane rupture and cell death. There are also classical and non-classical pathways for cell death. The classical pathway, the caspase-1 pathway, recruits and activates caspase-1 through inflammasomes that sense danger. Caspase-1 cleaves and activates inflammatory factors, such as IL-18 and IL-1β, which cleave the N-terminal sequence of GSDMD and bind to the membrane to create membrane pores, leading to pyroptosis. In the non-classical pathway, human-derived caspase-4,5 and murine-derived caspase-11, on the other hand, can be activated by direct contact with bacterial LPS, etc., which then cleaves the GSDMD and indirectly activates caspase-1, triggering pyroptosis. The GSDMD of the Gasdermin protein family is cleaved by inflammatory cystathionases and exhibits pore-forming activity to drive pyroptosis [251]. S. aureus can activate NLRP3 and lead to MAC-T cell pyroptosis via the K+ efflux pathway, and the homeostatic phenotype of resident macrophages can also orchestrate the early inflammatory response via the inflammasome pathway and subsequent pyroptosis [252,253]. It was found that the S. aureus associated-PAMPs, i.e., LTA [125], triacyl lipopetides [124], diacyl lipoproteins [151], and PGN [140], and S. aureus toxins, enzymes, and effectors, i.e., PVL [62],α-Hla [143], Hlb [34], and Hlg [35], can mediate pyroptosis [16].
S. aureus, which is phagocytosed by macrophages, increase the expression of NLRP3 and caspase-1 (Casp-1 p20), the accumulation of GSDMD-NT, and the release of IL-1β and IL-18 in macrophages and induce macrophage pyroptosis [254]. The LTA-induced activation of the NLRP6 inflammasome promotes the expression of caspase-1, IL-1β, and IL-18 in macrophages, inducing host cell pyroptosis [125]. In sepsis and venous infections, the shedding of lipopeptides from bacterial surfactants induces the activation of TLR 2 [69]. Lipoproteins from S. aureus are essential for growth and immunity. The lysozyme promotes activation of the NLRP3 inflammasome in the presence of lipoproteins and the induction of caspase-1 [35,123]. Furthermore, PGN strongly induces NLRP3 inflammasome expression in macrophages in S. aureus skin infections [140]. PVL exposure activates NLRP3, and binding to monocytes and macrophages leads to the release of the caspase-1-dependent pro-inflammatory cytokines IL-1β and IL-18, which induce macrophage death and exacerbate pneumonia [58,62]. It was found that S. aureus also induced keratinocyte pyroptosis via a caspase-1-dependent form of inflammatory cell death [143]. Activation of the NLRP3 inflammasome and caspase-1 by S. aureus toxin Hla induces the production of IL-1β and IL-18 and pyroptosis [14].
4.2. Necroptosis
Necroptosis, a form of programmed inflammatory cell death, was originally identified as an alternative to apoptosis following the involvement of death domain receptors. The classical necrosis pathway consists of RIPK1–RIPK3–MLKL. The necrosis pathway is triggered downstream of the death structural domain receptors (e.g., TNF receptors and Fas) and TLR3/4. Active RIPK1 is recruited in oligomeric complexes containing FADD, caspase-8, and caspase-10. In the absence of caspase-8, RIPK1 recruits and phosphorylates RIPK3 to form a RIPoptosome, which then phosphorylates MLKL to form a necrosome. MLKL oligomers translocate to phosphatidylinositol phosphate (PIP)-rich patches at the plasma membrane, forming macropores that lead to necrotic cell death by allowing ion inward flow, cell swelling, and membrane lysis, followed by the uncontrolled release of intracellular material [255].
PVL [63], Hla [43], and PSMs [69] induce the necroptosis of host cells after S. aureus infection [16]. In pneumonia and psoriasis, S. aureus infection induces necroptosis through RIP1/RIP3/MLKL signaling. Phagocytosis by S. aureus triggers the programmed necrosis of neutrophils. Bacterial pathogens increase their chances of survival by disrupting host programmed cell death (PCD). S. aureus is recognized by homologous host receptors, and the bacteria adhere and enter non-phagocytic cells, triggering programmed cellular necrosis through either intrinsic pathway mitochondrial damage or the extrinsic pathway in response to cell surface receptor engagement [16,63,255,256]. TNF and TLR3/TLR4 activation triggered necroptosis through a downstream signaling complex containing RIPK1, RIPK3, and MLKL. Whereas c-Jun N-terminal kinase (JNK1 and JNK2) activity significantly enhanced programmed cell death induced by TNF and TLRs, RIPK1 and RIPK3 promoted cell death-independent JNK activation in macrophages [257]. However, it has also been suggested that programmed cellular necrosis is a host cell death mechanism independent of the caspase and that glycolysis and mitochondrial ROS production are sufficient to induce programmed cellular necrosis [258]. PSMs can also induce cell death through formylpeptide receptor 2 (FPR2) by inducing the secretion of TNF-α [68].
4.3. Apoptosis
Apoptosis is a programmed cell death process that relies on a cascade of caspase endopeptidase activity called cystathionase. As a major human pathogen, S. aureus can induce apoptosis during infection through various pathways, which are also important in S. aureus infections [259]. It has been shown that apoptosis is essential in certain diseases caused by S. aureus, such as AD and sepsis [260]. Apoptosis of the host immune system may promote S. aureus infection, and the apoptosis of tissue cells may also reduce the immune response by affecting cytokine production and T-cell differentiation [253]. Thus, apoptosis can significantly influence the pathogenesis of S. aureus. With the exception of certain cellular components of S. aureus that may promote apoptosis (e.g., SpA [115]), most apoptotic processes caused by S. aureus are derived from different species of secreted toxins. Among the known S. aureus bacteria that have many virulence components that activate FADD, MyD88, RIPK3, caspase-8, and caspase-3 showed pro-apoptotic activity. The components include α-toxin [47], PVL [64], SEs (SEA [148], SEB [15], SEM [98], and SEH [96]), SpA [115], EsxA [101], Coa [104], Nuc, AdsA [108], SspB [85], Hla [144], and PGN [261].
S. aureus plays a role in osteomyelitis nephrapostasis, cancer, and bacteremia through apoptosis [16]. In pneumonia, S. aureus α-toxin inhibits the ability of alveolar macrophages (AMs) to clear neutrophils at the site of infection, induces the activation of caspase-3 in lymphocytes and monocytes, mediates cell death, activates caspase-8 and -9, and induces the release of cytochrome C [144,262].
PVL is an important virulence factor for the induction of apoptosis, acting on NLRP3 to induce macrophage death and IL-1β secretion during the course of pneumonia disease; PVL induces the release of the apoptotic proteins cytochrome c and Smac/DIABLO in mitochondria [62,65].
SEA triggers TCR-mediated cell death in CD4+ T cells [148]. SEB induces the differential expression of Rnd3 and RhoA in proximal renal tubular epithelial cells and induces actin stress fiber assembly and apoptosis [15]. In bovine mammary epithelial cells infected with S. aureus, SEM induces apoptosis by decreasing the mitochondrial membrane potential (ΔΨm) as well as the intracellular ATP concentration and activating caspase-3 [98]. SEH was also found to induce apoptosis in bovine mammary epithelial cells in vitro [96].
The SpA of S. aureus induces the release of apoptotic TNF-α and NO, induces apoptosis in EAC cells by upregulating pro-apoptotic factors (p53 and Bax) and downregulating anti-apoptotic factors (Bcl-2), induces the activation of caspase-3, and induces apoptosis in tumor cells, thus playing a role in cancer [115]. Bcl-2 inhibits neutral sphingomyelinase and ceramide-induced cytochrome c release, resulting in a delay in apoptosis; however, Bax accelerates cytochrome c release and caspase activation to induce apoptosis [116]. SpA is also closely associated with the disease process of osteomyelitis due to S. aureus infection [117].
In the immune response to S. aureus infection, EsxA regulates the production of cytokines and apoptotic processes, induces apoptosis, and, together with EsxB, mediates the release of S. aureus from host cells [101,102].
In bacteraemia and renal abscesses, S. aureus can induce caspase-3 production by secreting Nuc and AdsA. Neutrophil extracellular traps produce cytotoxic deoxyadenylation (dAdo) to induce caspase-3 production. Caspase-3 can trigger the apoptosis of macrophages [107,108].
SspB selectively cleaves CD11b on phagocytes, induces the phagocyte expression of phosphatidylserine and membrane-linked protein I, and induces apoptosis [85].
PGN activates the TLR2-mediated PP2A-ASK1-JNK-AP-1-C/EBPβ cascade, the macrophage expression of COX-2, and IL-6 expression [134,135]. PGN can also induce IL-1β, IL-8, and TNF-α expression through TLR2 signaling and induce the apoptosis of THP-1 cells [136].
4.4. Autophagy
Autophagy is the process of isolating cellular components into lysosomes for degradation and is an evolutionarily conserved mechanism for adapting to unfavorable microenvironmental conditions [263]. In S. aureus infection, host autophagy occurs for S. aureus defense. The autophagy receptor induces selective autophagic degradation of S. aureus by specifically recognizing the entering cells. S. aureus is recruited to the autophagosome membrane by early phagosomes bearing Rab5 and Rab22b to LC3 labeled by Rab7 and LAMP-1 in the presence of Hla. S. aureus can inhibit the fusion of autophagosomes with lysosomes to form autolysosomes and thus replicate within the autophagosome without being killed [264,265]. However, S. aureus can inhibit autophagosome maturation and fusion with lysosomes, escape from autophagosomes into the cytoplasm, and lead to host cell death, which does not rely on caspase [266]. In contrast, the pore-forming toxin Hla can promote autophagy by decreasing intracellular cAMP levels, leading to decreased activation of the protein Epac [145,146]. S. aureus induces inflammatory response and autophagy in macrophages by TLR2 through multiple signaling pathways, including the JNK and PI3K signaling pathways [267,268]. It was found that unmethylated cytosine-phosphate-guanine (CpG) in the JNK/P38 signaling pathway promotes phagocytosis and autophagy in S. aureus-stimulated macrophages [139]. Endothelial cells with defective autophagy have been found to be more susceptible to the S. aureus α-toxin [36]. In bovine mammary epithelial cells (BMECs), S. aureus evades autophagic degradation by inhibiting autophagic flux and disrupting lysosomal function [269]. In PMN, intracellular survival of S. aureus was associated with the accumulation of autophagic flux markers LC3-II and p62 [36].
In sepsis and pneumonia, the PGN of S. aureus and TLR2 ligands also acts on the central nervous system, activating microglia and inducing autophagy and autophagy-dependent cell death [17]. In contrast, S. aureus strains with high levels of Agr activity are able to block the autophagic flux, leading to the accumulation of autophagosomes and promoting the spread of S. aureus infection [270]. MRSA can promote the degradation of inflammasome components through autophagy and promote their persistence in keratinocytes [271].
4.5. Ferroptosis and Cuprotosis
Ferroptosis is a novel form of programmed cell death that is iron ion-dependent and distinct from apoptosis, necroptosis, and autophagy. Ferroptosis is an iron-dependent form of regulated cell death caused by unrestricted lipid peroxidation and subsequent membrane damage. Ferroptosis is induced through an extrinsic pathway regulating transporter protein initiation and an intrinsic pathway blocking the expression or activity of intracellular antioxidant enzymes [272]. Altering glutathione levels by disrupting caspase countertransport protein (GPX4) or using iron inhibitor-1 specific inhibition can increase intracellular ROS levels in response to free intracellular iron, leading to the oxidative degradation of lipids in cell membranes and oxidative cell death [16].
S. aureus can be deregulated by the expression of ROS-dependent, and loss of function mutations within the phagocytic NADPH oxidase NOX2 can lead to impaired production of ROS [273,274,275]. However, there are few studies on the mechanism of S. aureus in iron necrosis, so the virulence mechanism of S. aureus could be a direction for future studies.
New research has identified a new mechanism of cell death called cuprotosis, in which excess copper causes mitochondrial proteins to accumulate and drives cell death [276]. Cuprotosis occurs through the direct binding of copper to the lipid acylated component of the tricarboxylic acid (TCA) cycle, leading to lipid acylated protein aggregation and the subsequent loss of iron-sulfur cluster proteins, resulting in proteotoxic stress and ultimately cell death [277]. In contrast, in S. aureus, ClpC ATPase regulates the TCA cycle and then regenerates and enters the bacterial death phase after fixation through functional TCA cycle metabolism [278,279]. Whether there is a corresponding link between S. aureus and cuprotosis remains to be determined.
A summary of the cell death mechanisms activated by S. aureus in several diseases is shown in Figure 2.
Figure 2.
Types of cell death induced by S. aureus. The multiple toxins of S. aureus can induce multiple modes of cell death, including apoptosis, necroptosis, pyroptosis, and cell autophagy, and play a role in diseases as diverse as renal abscesses, septic arthritis, cancer, bacteremia, chronic sinusitis, pneumonia, venous infections, sepsis, psoriasis, and diseases caused by MRSA. Blue arrows represent apoptotic pathways; purple arrows represent the pyroptosis cell death pathway; yellow arrows represent the necroptotic pathway; green arrows represent the autophagic pathway.
5. Basic Types of Diseases Caused by S. aureus and Virulence Mechanisms
5.1. Skin Disease
5.1.1. AD
AD is the most common chronic inflammatory skin disease with a lifetime prevalence of up to 20% and a significant impact on quality of life. AD is characterized by intense pruritus, impaired epidermal barrier function, recurrent eczema, and fluctuations in the course of the disease. The pathogenesis of AD is mainly associated with dysbiosis of the skin microbiota (especially overgrowth of S. aureus), systemic immune response, and neuroinflammation associated with itchiness [280,281].
The role of S. aureus in AD is unquestionable, and studies have shown that nearly 100% of patients with AD present colonization of the skin with S. aureus [282].
In AD, the colonization of the skin by S. aureus and its secretion of SAgs/toxins have different virulence mechanisms. In AD, S. aureus activates TLR 2 on genes controlled by nuclear factor kappa B (NF-κB), which induces cytokine release from keratinocytes [18]. S. aureus has been isolated from AD patients and found to adhere to keratinocytes in a clumping factor B-dependent manner. IL-1β induces the activation of Th17 and the release of IL-17 and induces neutrophil recruitment, S100A8/A9, and chemokine activation [10,70,153]. Moreover, S. aureus colonization of the skin mucosa was found to directly induce epithelial cell-derived cytokine release by binding to TLR 2.
Skin S. aureus colonization resulting in pH changes may serve as a predictor of the increased severity of AD. Skin pH is tightly controlled by intrinsic factors that limit the abundance of S. aureus due to high S. aureus load, especially during the growth of S. aureus outbreaks [283].
The S. aureus α toxin, TSST-1, PSMs, SpA, second immunoglobulin, SEs, and ETs play a role in AD [13]. The S. aureus α-toxin induces Th1 responses in AD and promotes Th1 polarization by inducing chemokine (C-X-C motif) ligand 10 (CXCL10) in macrophages [48,49]. It was found that the α-toxin and PVL can also induce apoptosis [229]. TSST-1 binds to CD40 on keratinocytes and upregulates chemokine gene expression, releasing IL-9 and MIP-3α, which activates T lymphocytes and macrophages, leading to immune dysregulation [50]. Staphylococcal epidermal ETs may also contribute to the deterioration of AD [31].
S. aureus expresses PSMα, which enhances the survival and dissemination of S. aureus in invasive infections and is required to trigger skin inflammation [5,67]. PSMα had a cytolytic effect on neutrophils and enhanced the expression of inflammatory molecules in a mouse model of skin necrosis [67]. PSMα induces the release of IL-36α, and released IL-36α and IL-4 act together to trigger IgE class conversion in B cells and to promote plasma cell differentiation and elevated serum IgE levels [5,70].
S. aureus Sbi is the major staphylococcal-derived virulence factor that directly drives IL-33 release from human keratinocytes and promotes AD [119].
S. aureus superantigen SEs can directly stimulate skin keratinocytes, LCs, skin vascular endothelial cells, and macrophages, including the expression of pro-inflammatory factors TNF-α, IL-10, intercellular adhesion molecules (I-CAM), and vascular cell adhesion molecules (V-CAM), which promote the entry of inflammatory cells into skin tissue, leading to the development of AD. SEs can also lead to the upregulation of the T lymphocytes of associated antigens, prompting memory T lymphocytes to travel to AD lesions and become activated. The upregulation and activation of T lymphocytes induces and exacerbates the inflammation in the lesions [284]. SEA induced an inflammatory response in HaCaT keratinocytes and HUVECs, inducing a significant upregulation of E-selectin, ICAM-1, MCP-1, IL-6, and IL-8 expression. SEB induced a strong inflammatory response in the skin, inducing the upregulation of ICAM-1 and VCAM-1 in dermal vessels [149]. SEB-mediated skin inflammation is T cell-dependent and promotes IL-17 secretion [180,285].
S. aureus expresses the δ-toxin, which stimulates cytokine release from keratinocytes and triggers an inflammatory response in keratinocytes as well as B-cell proliferation and cytokine release [10]. In contrast, Th22 (CD4+IL-22+IL-17AIFN-γ) cells from AD patients inhibit staphylococcal SEA and SEB responses [92]. Th22 expressed increased IL-22, as well as IL-4, IL-5, IL-13, and IL-31.
5.1.2. Psoriasis
Psoriasis is an immune-mediated skin disease stimulated by environmental factors and controlled by multiple genes, usually presenting as scaly erythematous plaques or patches confined to one location or widely distributed throughout the body. Most patients have exacerbations or relapses in the winter and remission in the summer. Studies have found that psoriasis is often accompanied by the colonization of the skin with S. aureus, leading to exacerbation of the lesions [286].
IL-17 is the major effector cytokine in the pathogenesis of psoriatic disease [19]. The microbial community on psoriatic skin is very different from that on healthy skin. The loss of community stability and the reduction in immunomodulatory bacteria (e.g., Staphylococcus epidermidis) may lead to a higher colonization of pathogens such as S. aureus, which may exacerbate skin inflammation along the Th17 axis [168].
Psoriasis is also a Th1-induced chronic disease in which PGN from S. aureus induces the expression of human cathepsin LL37 and vascular endothelial growth factor (VEGF) in keratinocytes, activates Th1 cells, and expresses IL-13 [137,138]. S. aureus also acts on keratinocytes to induce IFN-γ expression by Th1 and IL-12-mediated differentiation of Th1 [165,287]. S. aureus can also induce monocytes to express IL-12, an inducer of the differentiation of Th cells to Th1 [165,288]. In psoriasis, S. aureus infection can also be mediated by the RIPK1/RIPK3/MLKL-mediated necrotizing apoptosis of keratinocytes, and RIPK1 mediates keratinocyte death via TNF [256,289].
5.2. Respiratory Diseases
5.2.1. Asthma
It is well known that allergic asthma is usually mediated by Th2 cells, leading to the production of cytokines IL-4, IL-5, IL-9, and IL-13. The activation of B cells triggers the release of allergen-specific IgE antibodies and induces the degranulation of eosinophils, mast cells, and basophils, leading to airway smooth muscle contraction, epithelial cell dysfunction, and excessive mucus secretion [216].
SEs can penetrate the mucosal barrier under the right conditions and induce continuous eosinophilic inflammation and IgE formation. In polyp samples containing SEA-specific IgE antibodies, severe eosinophilic inflammation, manifested by the upregulation of IL-5, eosinophil chemokines, eosinophil cationic protein (ECP), and caspase-leukotriene synthesis, was observed, thus contributing to the severity of asthma [91]. The study finds that rapid αβ T-cell responses can coordinate pulmonary innate immunity to SEA [21]. Nasal SEB increases the serum concentrations of IL-4, IL-5, and IFN-γ, while bronchial SEB increases the serum titers of specific IgE and total IgE. Exacerbation of asthma is associated with the elevated expression of IL-5, IL-4, IFN-γ, IL-12 p40, eosinophil chemokine-1, and TGF-β mRNA in the bronchial tubes [93].
S. aureus also manipulates airway mucosal immunology at different levels through its proteins, inducing the activation of airway epithelium and the release of TSLP, IL-25, and IL-33, leading to sustained immune responses in DCs and ILC2s and the activation of type 2 immune responses [290,291]. In other studies, it was found that airway epithelial cells can release IL-33 and activate ILCs by their receptor ST2, followed by releasing type 2 cytokines, mast cell degranulation, and the massive activation of local B cells and IgE formation, attracting an accumulation of eosinophils.
Extracellular traps are then released, increasing epithelial cell damage and leading to disease-persistence Charcot–Leyden crystals [216]. Staphylococcal Spls cause an IgE antibody response and stimulate Th2 cytokine production by T cells, whereas S. aureus Hla causes low or absent concentrations of reactive Th1/Th17 cytokines [78].
However, the mechanism of cell death in asthma by S. aureus infection is still minimally investigated today, so the mechanism of asthma with S. aureus infection is worth investigating.
5.2.2. Chronic Rhinosinusitis
Chronic sinusitis is a chronic inflammatory disease of the sinus mucosa, and S. aureus is found to colonize the mucosa in most patients with chronic sinusitis with nasal polyps. S. aureus binding to TLR 2 directly induces the release of epithelial cell-derived cytokines and promotes the expression of TSLP, IL-5, IL-13, and IL-33 in nasal polyp tissue [160]. TSLP may also act directly on ion channel TRPA1-positive sensory neurons to trigger powerful pruritic behavior [157]. SEB induces Th1 and Th2 pro-inflammatory responses in nasal polyps and increases the serum concentrations of IL-4, IL-5, and IFN-γ [93,163]. The α-toxin induces nasal polyp cells to produce large amounts of IL-5, IL-13, IFN-γ, IL-17A, and IL-10, which are involved in the inflammatory response [51].
In patients with chronic CRSwNP, superantigenic SEs activate T cells to release the Th2 cytokines IL-4, IL-5, and IL-13, which activate antigen-specific B cells and produce polyclonal IgE. IgE acts on mast cells to release chemokines (eosinophil chemokines), leading to eosinophil inflammation [7]. In contrast, T lymphocytes located in nasal polyp tissue have higher T cell receptor V-β amplification, enhancing polyclonal IgE production and contributing to sustained Th2 inflammation [292].
In chronic sinusitis, insufficient IFN-γ-induced autophagy can lead to the p62-dependent apoptosis of epithelial cells, while TNF-α and IFN-γ-induced necroptosis may promote the production and release of numerous pro-inflammatory cytokines and lead to neutrophil infiltration to exacerbate inflammation in CRSwNP [293,294]. SEB can also stimulate IL-21 expression, and IL-21 can also induce Th17 differentiation [89,90].
5.2.3. Pneumonia
In pneumonia with S. aureus infection, staphylococcal Hla is an important virulence factor in severe S. aureus pneumonia [22]. S. aureus Hla evades ATP and P2X7 receptors in the presence of lipoproteins to induce cystathione-1 activation via NLRP3 [35]. The activation of inflammasomes induces the secretion of pro-inflammatory cytokines IL-1β and IL-18, which induce cell death and lead to the release of endogenous pro-inflammatory molecules, such as chromatin-associated protein and high mobility group box 1 (HMGB1) [43]. Some kinds of S. aureus have also been found to produce an extracellular, two-component, heterodimeric perforating cytolytic toxin (a leukocidal toxin, PVL), which is extremely virulent, promotes necroptosis and apoptosis, kills human polymorphonuclear cells, and causes severe infections. In necrotizing pneumonia, PVL upregulates the expression of pro-inflammatory cytokines and contributes to the activation of the JAK/STAT pathway, thereby increasing the severity of pneumonia [66]. PVL can also lead to the rapid disruption of mitochondrial homeostasis and apoptosis induced by the activation of cystatase-9 and cystatase-3 [65]. PSMs induce the necroptosis of neutrophils through the activation of mixed-spectrum kinase-like protein (MLKL) phosphorylation and increased release of lactate dehydrogenase, which mediates TNF-α secretion via formyl peptide receptor 2 (FPR2) and leads to the exacerbation of pneumonia [68].
5.2.4. CF
Pulmonary infections with S. aureus are highly prevalent in patients with CF, leading to a much higher mortality rate in pulmonary CF. S. aureus widely expresses adhesion molecules that recognize, adhere to, and internalize in lung epithelial cells, thereby protect the bacteria from the host immune system and promote chronic infection [20]. Mast cells are activated in infected lung epithelia and release multiple inflammatory cytokines during S. aureus infection via the C-KIT (CD117, a tyrosine kinase type III)-activated phosphatidylinositol 3-kinase (PI3K)/AKT/P65 NF-κB signaling pathway [217]. IL-8 expression is increased, and staphylococci entering the lungs are surrounded by a large number of immune cells, macrophages, and a small amount of fibronectin/collagen, causing a strong immune response [295,296]. Intracellular S. aureus was found to induce host cell death in epithelial cells via caspase protease [297]. In contrast, macrophages are the main reservoir of S. aureus in the body, which not only fail to effectively kill engulfed S. aureus, but also induce S. aureus to become resistant to a variety of antibiotics [20]. S. aureus can form chronic infections through biofilm formation, transformation to small colony variants, the emergence of hypervariable strains, the downregulation of virulence genes, and the formation of heterogeneous bacterial populations [298].
CF is an immunodeficiency disease of autophagy with defective transmembrane conductance regulator (CFTR) genes. The defective gene allows autophagy mediated by ROS. Autophagy inhibits aggregate formation and creates inflammation in the lungs [299,300]. S. aureus regulates the expression of ROS, so S. aureus infection may have an effect on autophagy in CF [273,274].
5.3. Food Poisoning
S. aureus is a major cause of food poisoning, which usually occurs after the ingestion of different foods, especially processed meats and dairy products, which are contaminated with S. aureus due to improper handling or subsequent improper storage. S. aureus is an opportunistic Gram-positive bacterium that actively interacts with mucosal epithelial cells in the intestine, leading to an inflammatory response.
Upon exposure of gastrointestinal epithelial cells to S. aureus, epithelial cells express and secrete pro-inflammatory mediators (including IL-8), leading to the activation and attraction of PMN, which recruit multiple immune cells to inflamed intestinal tissues, resulting in intestinal inflammation [23].
LTA provides resistance to host bactericidal factors and promotes adhesion in epithelial cells, contributing to the colonization of S. aureus in the gastrointestinal tract [127]. Moreover, LTA can also enhance the inflammatory response of macrophages together with PGN, promote phagosome maturation through activation of the JAK-STAT pathway, and lead to an inflammatory response, leading to multi-organ dysfunction [141]. However, LTA can also lead to the continued accumulation of PMN at the site of inflammation, resulting in chronic inflammation [23].
Staphylococci can also induce small intestinal colitis by Rho-inactivated epidermal differentiation inhibitor (EDIN) toxin-mediated enterocytosis, which disrupts the epithelial barrier. Production of β-defensins by the intestinal epithelium contributes to host defense against staphylococcal invasion [301].
In contrast, the δ toxin was found to increase vascular permeability in guinea pig skin and cyclo-AMP levels in the ileum in guinea pig experiments. It also increases the bidirectional movement of Na+ and Cl− in the mucosa and inhibits water absorption, thus increasing the severity of diarrhea [75].
The Staphylococcal α-toxin also induces a decrease in transmembrane electrical impedance (TER) and a decrease in cellular connexin levels, resulting in a disruption of barrier integrity and a significant decrease in calcium inward flow and TER of intracellular calcium chelation regulation in human intestinal epithelial Caco-2 cells [52]. Elevated intracytoplasmic Ca2+ leads to increased mitochondrial Ca2+ concentrations and activation of calpain and caspase, resulting in cell lysis [302]. The α-toxin may lead to intestinal epithelial barrier dysfunction and promote the release of intestinal bacteria into the underlying tissues and blood, thus further worsening sepsis [53].
S. aureus infections in the intestinal tract expressing superantigenic SEs can cause acute gastroenteritis with a rapid onset of symptoms, causing nausea and severe vomiting, with diarrhea [303]. SEs reach the lamina propria through the cupula and epithelium of the intestinal epithelium and induce the release of 5-hydroxytryptamine (5-HT/5-hydroxytryptamine precursor) from mast cells; 5-hydroxytryptamine can interact with the vagus nerve to induce an emetic response [304]. Enterotoxin SAgs can also penetrate the intestinal lining, act on T cells, macrophages, and monocytes of the immune system, and activate local and systemic immune responses, resulting in significant T cell proliferation, the inhibition of IgM synthesis, and the release of inflammatory mediators (including histamine, leukotrienes, and neurointestinal peptide substance P), which can lead to vomiting, with the patient’s condition becoming more severe as more toxins are added [305,306,307,308]. SEs can also inhibit the reabsorption of water and electrolytes in the small intestine, leading to diarrhea, and the activation of the local immune system can also lead to gastrointestinal damage associated with SE intake [309]. The SEs SEA [94], SEC [150], SEM [99], SEO [97], and SEQ [100] have emetic activities. SEA, SEC, and SEQ also have supercarcinogenic activity. SEB increases numbers of T lymphocytes, concentrations of pro-inflammatory cytokines, and inflammatory mediators in the intestinal mucosa, and increases mucosal permeability and intestinal secretion [97,150,310,311,312]. SEI reversibly disrupts the enterocyte ultrastructure and exhibits weak emetic activity [95,97]. Together with SEH, both can lead to food poisoning.
It was found that microbial colonization of the intestinal epithelium could obtain nutrients by inducing apoptosis in intestinal epithelial cells and that mammalian nutrients released through cystathione-3/7-dependent apoptosis could promote the growth of a variety of Enterobacteriaceae [313]. Whether S. aureus infection in the intestinal tract has a similar effect, however, has not been studied.
5.4. Autoimmune Diseases
In GPA, the nasal carriage of S. aureus is a common trigger for the onset of GPA. In S. aureus infection, increased B lymphocyte stimulating factor leads to an increase in B lymphocytes, which secrete ANCA (ANCA is the virulence antibody associated with GPA). ANCA binds to protease 3 on the surface of neutrophils and monocytes, releasing ROS, proteases, cytokines, and neutrophil extracellular trap products (NET-derived products) [221]. Neutrophil extracellular traps (NETs) are extracellular structures composed of chromatin and granule proteins that bind and kill microorganisms which are distinct from necroptosis and apoptosis [314]. Autoreactivity to myeloperoxidase (MPO) can also lead to antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) [315].
In hereditary phagocytic dysfunction, which is a CGD, there is an increased susceptibility to bacterial and fungal infections and an increased susceptibility to granuloma formation due to an excessive inflammatory response [316]. Affecting recurrent skin, airways, lymph nodes, liver, brain, and bone, S. aureus infections are often life-threatening. Host immune cells undergo ROS-dependent regulation. Phagocytic ROS production is fundamental to the pathogen elimination and regulation of the inflammatory response to infection. The loss of function within the phagocytic NADPH oxidase NOX2 by mutations leads to the impaired production of ROS [273,274,275]. Respiratory bursts attack ROS produced by iron–sulfur cluster-containing proteins, including TCA cycle enzymes, resulting in reduced respiration, reduced ATP, and increased antibiotic tolerance [20]. In neutrophils, the calmodulin-mediated inhibition of calcium-dependent phospholipase A2 enzyme (iPLA2) controls granule and vesicle cytostasis in the phagosome and extracellular microenvironment while correcting defective intracellular and extracellular microbicidal activity. In patients with CGD presenting with defective production of ROS, the increase in non-oxidative killing pathways partially corrected their bactericidal defects [317]. OLFM4 (a neutrophil protein) negatively regulated host defense against bacterial infection and reduced immune defense against S. aureus in mice with CGD [24].
5.5. Osteomyelitis
In osteomyelitis, SpA binds directly to TNFR-1 expressed on osteoblasts, leading to the activation of NF-κB and translocation to the nucleus of osteoblasts, leading to the release of IL-6 (an important osteoprogenitor), the induction of apoptosis, and the inhibition of the mineralization of osteoblasts. Infected osteoblasts also increase the expression of RANKL (a key protein involved in the initiation of bone resorption), recruiting and activating bone-resorbing cells and osteoclasts, leading to bone loss [117,118]. S. aureus also inhibits the expression of alkaline phosphatase, collagen type I, osteocalcin, osteopontin, and osteocalcin, thereby inhibiting bone formation [25].
S. aureus also plays a role in nephrapostasis, cancer, bacteremia, pulmonary infections, venous infections, and subcutaneous infections by inducing apoptosis and in sepsis by inducing autophagy [16].
5.6. MRSA
MRSA and its antimicrobial resistance exacerbate global health threats, and drug abuse promotes the emergence of bacterial drug resistance. MRSA is the result of a large number of virulence factors produced by S. aureus with β-lactam resistance and the resistance of most clones to other antibiotic classes. MRSA has been shown to develop multiple mechanisms of resistance, including cell wall thickening, increased efflux pumps on the cell membrane, mutations in cytoplasmic drug targets, and the modification of drugs and changes in bacterial colonization status [318]. MRSA resistance usually arises through the acquisition of a non-native gene encoding a penicillin-binding protein (PBP2a) with a significantly reduced affinity for β-lactams. This resistance allows cell wall biosynthesis (the target of β-lactams) to continue even in the presence of certain inhibitory concentrations of antibiotics [319]. PBP2a, a PGN transpeptidase, is encoded by the mecA gene, which is carried on a unique mobile genetic element (SCCmec). MecA’s expression is controlled through a protein hydrolysis signaling pathway containing a sensor protein (MecR1) and an inhibitory factor (MecI) that allows S. aureus to become resistant to most members of the β-lactam class of antibiotics [320]. FMMs are formed by the interaction of membrane carotenoids with scaffolding proteins, and interference with FMM assembly using existing drugs interferes with PBP2a oligomerization and disables MRSA penicillin resistance in vitro and in vivo, leading to the sensitivity of MRSA infection to penicillin therapy [321]. MRSA infections remain a major global healthcare problem. Of concern is S. aureus bacteremia, which has high morbidity and mortality and can cause metastatic or complicated infections, such as infective endocarditis or sepsis. MRSA infections often lead to poorer clinical outcomes [322]. MRSA can promote the degradation of inflammasome components through autophagy and promote their persistence in keratinocytes [271]. Host cells can also prevent MRSA-induced sepsis by attenuating Th1 and Th17 responses through autophagy and by promoting mitochondrial autophagy [323,324]. IL-19, -20, and -24 signals through type I and type II IL-20 receptors inhibit cutaneous IL-1β and IL-17A production to promote MRSA infection in mice [325].
5.7. DFI
DFI is a very common and serious complication of diabetes mellitus, with a prevalence of up to 25% in diabetic patients [326]. DFI is caused by diabetes mellitus-induced lower limb vasculopathy, which triggers neuropathy and causes infection of the soft tissue and bone below the ankle joint. DFI can even lead to amputation in severe cases [327]. S. aureus is the most common infecting organism in DFI patients, with an infection rate of 42.1%, of which 70% are MRSA [328]. Endogenous flora is the main source of S. aureus in DFI patients. The nasal carriage of S. aureus is a risk factor for DFI. S. aureus infection with nasal carriage was detected in 30.87% of patients with a diabetic foot [329]. Among those patients infected with S. aureus, 40.85% were considered MRSA [329].
S. aureus is the most virulent pathogen in DFI and induces DFI by expressing a variety of virulence factors [330]. The detection of virulence factors Cap8, Sea, Sei, LukDE (leukocidin), and hlgv (haemolysin) allows for the grading of grade 1 and grade 2–4 ulcers. Toxicity factor testing can also predict wound outcomes. Therefore, the detection of virulence factors provides a useful basis for the treatment of diabetic foot ulcers [331]. Among these genes, SEA has been shown to have a strong pro-inflammatory effect [94]. The virulence factor epidermal cell differentiation inhibitor (EDIN), which can affect epithelial and endothelial cohesion by inhibiting RhoA, facilitates the spread of S. aureus to infect deeper tissues [332]. It was found that 71.4% of EDIN-positive DFI patients had moderate to severe infections and that 28.6% had mild infections [333]. Different strains of S. aureus pathogenic bacteria can produce three subtypes of EDIN, such as EDIN type A (EDIN-A), EDIN type B (EDIN-B), or EDIN type C (EDIN-C). The majority of EDIN-positive strains were found to be EDIN-B positive (86.7%), suggesting that EDIN-B is a co-predictive risk marker for DFI [333].
Treatment of DFI can begin with debridement of the wound, followed by irrigation and disinfection of the wound with an antiseptic and the use of antibiotics. The choice of antibiotics varies depending on the severity of the infected wound of DFI patients. Antibiotic treatment can be either oral or intravenous. Clindamycin is preferred for mild cases [334]. Gentamicin can be used to treat moderate and severe cases [335]. Vancomycin can be used for more severe DFI patients and can be used to combat MRSA infection [336].
MRSA is a drug-resistant organism of S. aureus and is highly resistant to most β-lactam antibiotics, as well as to a wide range of other anti-microbials. This makes MRSA infections difficult and costly to treat. MRSA can form a biofilm in vitro that is resistant to antibiotics, and biofilm formation is one of the main determinants of the prevalence of pathogens associated with medical device infections. Therefore, in the treatment of DFI, the inhibition of in vitro MRSA biofilm formation often requires high doses of broad-spectrum antibiotics in combination with multiple drugs. Ceflorin and gentamicin have also been found to have an inhibitory effect on biofilms isolated from diabetic feet [337].
Antimicrobial peptides (AMPs) are an adjunct to conventional therapies [338]. Nisin, a type of AMP, is produced by lactic acid bacteria. Nisin can inhibit S. aureus and other Gram-positive bacteria effectively. To prolong the half-life of Nisin in vivo, guar gum biogel has been used as a delivery agent. Nisin biogel was able to inhibit the formation of the biofilm of S. aureus isolated from DFI [339].
Chlorhexidine is widely used as a skin antiseptic and is one of the most commonly used disinfectants in the treatment of DFI. Furthermore, chlorhexidine can be used to inhibit S. aureus and MRSA infection [340]. In addition, chlorhexidine inhibits the formation of biofilms [341]. It was found that the combination of chlorhexidine and AMP niosomal-biogel was more efficient in inhibiting biofilm formation than either of them alone [342].
Another treatment option for alternative antibacterial drugs is the use of natural compounds from plants that have antibacterial properties. Urtica dioica and Lavandula angustifolia extracts were found to have an inhibitory effect on MRSA isolated from diabetic feet, suggesting that topical applications of lavender and nettle leaves could be used to treat DFI [343].
As there is no effective treatment for DFI, it is important to study the mechanisms of immune cells, cell death in S. aureus, and virulence factors, especially MRSA, for the treatment of DFI.
A summary of the diseases caused by S. aureus is shown in Figure 3.
Figure 3.
Types of diseases caused by S. aureus. S. aureus can cause a variety of systemic diseases, including skin diseases, respiratory diseases, food poisoning, autoimmune diseases, osteomyelitis, DFI, and the formation of MRSA.
6. Conclusions and Future Perspectives
In this review, we have summarized the virulence factors produced by S. aureus, the types of immune cells, the cell death mechanisms activated by S. aureus, and the pathogenesis of S. aureus in several diseases. A better understanding of these may contribute to targeted therapies for S. aureus-induced diseases in the future.
Two areas of investigation remain open for major advancement. First, the mechanisms of Th9, Tfh, and ILC cells activated by S. aureus and virulence factors remain largely unknown. Second, the roles of ferroptosis and copper-induced cell death in diseases caused by S. aureus remain poorly defined. These may become new directions for future research.
Acknowledgments
This work was supported by the National Natural Science Foundation of China under grants 82171764, 81871266, and 81301948; the Natural Science Foundation from the Guangdong Science and Technology Department (2021A1515012551); and a Guangzhou Science and Technology Project (202102010104).
Author Contributions
Conceptualization: X.L., H.C. and A.T.; writing—original draft preparation: X.L. and H.C.; writing—review and editing: X.L., H.C., J.Z., Y.H., Z.L. (Zhuoyi Lv), Z.L. (Zhengtong Liang), J.C., P.L., J.L., H.Y. and A.T.; supervision: X.L. and A.T.; funding acquisition: X.L. and A.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
A better understanding of the virulence factors of S. aureus, the types of immune cells, and the cell death mechanisms activated by S. aureus in various diseases may contribute to future targeted therapies for S. aureus-induced diseases.
Funding Statement
National Natural Science Foundation of China: 82171764, 81871266, 81301948; Guangzhou science and technology project: 202102010104; the Natural Science Foundation from Guangdong Science and Technology Department: 2021A1515012551.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lowy F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Shopsin B., Kreiswirth B.N. Molecular epidemiology of methicillin-resistant Staphylococcus aureus. Emerg. Infect. Dis. 2001;7:323–326. doi: 10.3201/eid0702.010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le K.Y., Otto M. Quorum-sensing regulation in staphylococci-an overview. Front. Microbiol. 2015;6:1174. doi: 10.3389/fmicb.2015.01174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Recsei P., Kreiswirth B., O’Reilly M., Schlievert P., Gruss A., Novick R.P. Regulation of exoprotein gene expression in Staphylococcus aureus by agar. Mol. Gen. Genet. 1986;202:58–61. doi: 10.1007/BF00330517. [DOI] [PubMed] [Google Scholar]
- 5.Nakagawa S., Matsumoto M., Katayama Y., Oguma R., Wakabayashi S., Nygaard T., Saijo S., Inohara N., Otto M., Matsue H., et al. Staphylococcus aureus Virulent PSMα Peptides Induce Keratinocyte Alarmin Release to Orchestrate IL-17-Dependent Skin Inflammation. Cell Host Microbe. 2017;22:667–677.e5. doi: 10.1016/j.chom.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peters N., Peters A.T. Atopic dermatitis. Allergy Asthma Proc. 2019;40:433–436. doi: 10.2500/aap.2019.40.4265. [DOI] [PubMed] [Google Scholar]
- 7.Fujieda S., Imoto Y., Kato Y., Ninomiya T., Tokunaga T., Tsutsumiuchi T., Yoshida K., Kidoguchi M., Takabayashi T. Eosinophilic chronic rhinosinusitis. Allergol. Int. 2019;68:403–412. doi: 10.1016/j.alit.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Pidwill G.R., Gibson J.F., Cole J., Renshaw S.A., Foster S.J. The Role of Macrophages in Staphylococcus aureus Infection. Front. Immunol. 2020;11:620339. doi: 10.3389/fimmu.2020.620339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tatsuno K., Fujiyama T., Yamaguchi H., Waki M., Tokura Y. TSLP Directly Interacts with Skin-Homing Th2 Cells Highly Expressing its Receptor to Enhance IL-4 Production in Atopic Dermatitis. J. Investig. Dermatol. 2015;135:3017–3024. doi: 10.1038/jid.2015.318. [DOI] [PubMed] [Google Scholar]
- 10.Geoghegan J.A., Irvine A.D., Foster T.J. Staphylococcus aureus and Atopic Dermatitis: A Complex and Evolving Relationship. Trends Microbiol. 2018;26:484–497. doi: 10.1016/j.tim.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Marchitto M.C., Dillen C.A., Liu H., Miller R.J., Archer N.K., Ortines R.V., Alphonse M.P., Marusina A.I., Merleev A.A., Wang Y., et al. Clonal Vγ6(+)Vδ4(+) T cells promote IL-17-mediated immunity against Staphylococcus aureus skin infection. Proc. Natl. Acad. Sci. USA. 2019;116:10917–10926. doi: 10.1073/pnas.1818256116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gevaert E., Zhang N., Krysko O., Lan F., Holtappels G., De Ruyck N., Nauwynck H., Yousefi S., Simon H.U., Bachert C. Extracellular eosinophilic traps in association with Staphylococcus aureus at the site of epithelial barrier defects in patients with severe airway inflammation. J. Allergy Clin. Immunol. 2017;139:1849–1860.e6. doi: 10.1016/j.jaci.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 13.Tian X., Huang Q., Liang J., Wang J., Zhang J., Yang Y., Ye Q., He S., Li J., Wu Z., et al. A review of the mechanisms of keratinocytes damage caused by Staphylococcus aureus infection in patients with atopic dermatitis. J. Leukoc. Biol. 2021;110:1163–1169. doi: 10.1002/JLB.3MR0921-030RRR. [DOI] [PubMed] [Google Scholar]
- 14.Kitur K., Wachtel S., Brown A., Wickersham M., Paulino F., Peñaloza H.F., Soong G., Bueno S., Parker D., Prince A. Necroptosis Promotes Staphylococcus aureus Clearance by Inhibiting Excessive Inflammatory Signaling. Cell Rep. 2016;16:2219–2230. doi: 10.1016/j.celrep.2016.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ionin B., Hammamieh R., Shupp J.W., Das R., Pontzer C.H., Jett M. Staphylococcal enterotoxin B causes differential expression of Rnd3 and RhoA in renal proximal tubule epithelial cells while inducing actin stress fiber assembly and apoptosis. Microb. Pathog. 2008;45:303–309. doi: 10.1016/j.micpath.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Soe Y.M., Bedoui S., Stinear T.P., Hachani A. Intracellular Staphylococcus aureus and host cell death pathways. Cell Microbiol. 2021;23:e13317. doi: 10.1111/cmi.13317. [DOI] [PubMed] [Google Scholar]
- 17.Arroyo D.S., Soria J.A., Gaviglio E.A., Garcia-Keller C., Cancela L.M., Rodriguez-Galan M.C., Wang J.M., Iribarren P. Toll-like receptor 2 ligands promote microglial cell death by inducing autophagy. FASEB J. 2013;27:299–312. doi: 10.1096/fj.12-214312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mempel M., Voelcker V., Köllisch G., Plank C., Rad R., Gerhard M., Schnopp C., Fraunberger P., Walli A.K., Ring J., et al. Toll-like receptor expression in human keratinocytes: Nuclear factor kappaB controlled gene activation by Staphylococcus aureus is toll-like receptor 2 but not toll-like receptor 4 or platelet activating factor receptor dependent. J. Investig. Dermatol. 2003;121:1389–1396. doi: 10.1111/j.1523-1747.2003.12630.x. [DOI] [PubMed] [Google Scholar]
- 19.Blauvelt A., Chiricozzi A. The Immunologic Role of IL-17 in Psoriasis and Psoriatic Arthritis Pathogenesis. Clin. Rev. Allergy Immunol. 2018;55:379–390. doi: 10.1007/s12016-018-8702-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rowe S.E., Wagner N.J., Li L., Beam J.E., Wilkinson A.D., Radlinski L.C., Zhang Q., Miao E.A., Conlon B.P. Reactive oxygen species induce antibiotic tolerance during systemic Staphylococcus aureus infection. Nat. Microbiol. 2020;5:282–290. doi: 10.1038/s41564-019-0627-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar S., Colpitts S.L., Ménoret A., Budelsky A.L., Lefrancois L., Vella A.T. Rapid αβ T-cell responses orchestrate innate immunity in response to Staphylococcal enterotoxin A. Mucosal Immunol. 2013;6:1006–1015. doi: 10.1038/mi.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kebaier C., Chamberland R.R., Allen I.C., Gao X., Broglie P.M., Hall J.D., Jania C., Doerschuk C.M., Tilley S.L., Duncan J.A. Staphylococcus aureus α-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J. Infect. Dis. 2012;205:807–817. doi: 10.1093/infdis/jir846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hattar K., Grandel U., Moeller A., Fink L., Iglhaut J., Hartung T., Morath S., Seeger W., Grimminger F., Sibelius U. Lipoteichoic acid (LTA) from Staphylococcus aureus stimulates human neutrophil cytokine release by a CD14-dependent, Toll-like-receptor-independent mechanism: Autocrine role of tumor necrosis factor-[alpha] in mediating LTA-induced interleukin-8 generation. Crit. Care Med. 2006;34:835–841. doi: 10.1097/01.CCM.0000202204.01230.44. [DOI] [PubMed] [Google Scholar]
- 24.Liu W., Yan M., Sugui J.A., Li H., Xu C., Joo J., Kwon-Chung K.J., Coleman W.G., Rodgers G.P. Olfm4 deletion enhances defense against Staphylococcus aureus in chronic granulomatous disease. J. Clin. Investig. 2013;123:3751–3755. doi: 10.1172/JCI68453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Widaa A., Claro T., Foster T.J., O’Brien F.J., Kerrigan S.W. Staphylococcus aureus protein A plays a critical role in mediating bone destruction and bone loss in osteomyelitis. PLoS ONE. 2012;7:e40586. doi: 10.1371/journal.pone.0040586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tam K., Torres V.J. Staphylococcus aureus Secreted Toxins and Extracellular Enzymes. Microbiol. Spectr. 2019;7:2. doi: 10.1128/microbiolspec.GPP3-0039-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foster T.J., Geoghegan J.A., Ganesh V.K., Höök M. Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 2014;12:49–62. doi: 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Askarian F., Wagner T., Johannessen M., Nizet V. Staphylococcus aureus modulation of innate immune responses through Toll-like (TLR), (NOD)-like (NLR) and C-type lectin (CLR) receptors. FEMS Microbiol. Rev. 2018;42:656–671. doi: 10.1093/femsre/fuy025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verma P., Gandhi S., Lata K., Chattopadhyay K. Pore-forming toxins in infection and immunity. Biochem. Soc. Trans. 2021;49:455–465. doi: 10.1042/BST20200836. [DOI] [PubMed] [Google Scholar]
- 30.Otto M. Phenol-soluble modulins. Int. J. Med. Microbiol. 2014;304:164–169. doi: 10.1016/j.ijmm.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yagi S., Wakaki N., Ikeda N., Takagi Y., Uchida H., Kato Y., Minamino M. Presence of staphylococcal exfoliative toxin A in sera of patients with atopic dermatitis. Clin. Exp. Allergy. 2004;34:984–993. doi: 10.1111/j.1365-2222.2004.1687.x. [DOI] [PubMed] [Google Scholar]
- 32.Spaulding A.R., Salgado-Pabón W., Kohler P.L., Horswill A.R., Leung D.Y., Schlievert P.M. Staphylococcal and streptococcal superantigen exotoxins. Clin. Microbiol. Rev. 2013;26:422–447. doi: 10.1128/CMR.00104-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cassidy P., Harshman S. Studies on the binding of staphylococcal 125I-labeled alpha-toxin to rabbit erythrocytes. Biochemistry. 1976;15:2348–2355. doi: 10.1021/bi00656a016. [DOI] [PubMed] [Google Scholar]
- 34.Becker K.A., Fahsel B., Kemper H., Mayeres J., Li C., Wilker B., Keitsch S., Soddemann M., Sehl C., Kohnen M., et al. Staphylococcus aureus Alpha-Toxin Disrupts Endothelial-Cell Tight Junctions via Acid Sphingomyelinase and Ceramide. Infect. Immun. 2018;86:e00606-17. doi: 10.1128/IAI.00606-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muñoz-Planillo R., Franchi L., Miller L.S., Núñez G. A critical role for hemolysins and bacterial lipoproteins in Staphylococcus aureus-induced activation of the Nlrp3 inflammasome. J. Immunol. 2009;183:3942–3948. doi: 10.4049/jimmunol.0900729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulcahy M.E., O’Brien E.C., O’Keeffe K.M., Vozza E.G., Leddy N., McLoughlin R.M. Manipulation of Autophagy and Apoptosis Facilitates Intracellular Survival of Staphylococcus aureus in Human Neutrophils. Front. Immunol. 2020;11:565545. doi: 10.3389/fimmu.2020.565545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adler A., Temper V., Block C.S., Abramson N., Moses A.E. Panton-Valentine leukocidin-producing Staphylococcus aureus. Emerg. Infect. Dis. 2006;12:1789–1790. doi: 10.3201/eid1211.060726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiseman G.M. The hemolysins of Staphylococcus aureus. Bacteriol. Rev. 1975;39:317–344. doi: 10.1128/br.39.4.317-344.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bubeck Wardenburg J., Patel R.J., Schneewind O. Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect. Immun. 2007;75:1040–1044. doi: 10.1128/IAI.01313-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lizak M., Yarovinsky T.O. Phospholipid scramblase 1 mediates type i interferon-induced protection against staphylococcal α-toxin. Cell Host Microbe. 2012;11:70–80. doi: 10.1016/j.chom.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhakdi S., Muhly M., Korom S., Hugo F. Release of interleukin-1 beta associated with potent cytocidal action of staphylococcal alpha-toxin on human monocytes. Infect. Immun. 1989;57:3512–3519. doi: 10.1128/iai.57.11.3512-3519.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suttorp N., Fuhrmann M., Tannert-Otto S., Grimminger F., Bhadki S. Pore-forming bacterial toxins potently induce release of nitric oxide in porcine endothelial cells. J. Exp. Med. 1993;178:337–341. doi: 10.1084/jem.178.1.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Craven R.R., Gao X., Allen I.C., Gris D., Bubeck Wardenburg J., McElvania-Tekippe E., Ting J.P., Duncan J.A. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS ONE. 2009;4:e7446. doi: 10.1371/journal.pone.0007446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suttorp N., Seeger W., Dewein E., Bhakdi S., Roka L. Staphylococcal alpha-toxin-induced PGI2 production in endothelial cells: Role of calcium. Am. J. Physiol. 1985;248:C127–C134. doi: 10.1152/ajpcell.1985.248.1.C127. [DOI] [PubMed] [Google Scholar]
- 45.Berube B.J., Bubeck Wardenburg J. Staphylococcus aureus α-toxin: Nearly a century of intrigue. Toxins. 2013;5:1140–1166. doi: 10.3390/toxins5061140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grimminger F., Rose F., Sibelius U., Meinhardt M., Pötzsch B., Spriestersbach R., Bhakdi S., Suttorp N., Seeger W. Human endothelial cell activation and mediator release in response to the bacterial exotoxins Escherichia coli hemolysin and staphylococcal alpha-toxin. J. Immunol. 1997;159:1909–1916. [PubMed] [Google Scholar]
- 47.Iacovache I., Bischofberger M., van der Goot F.G. Structure and assembly of pore-forming proteins. Curr. Opin. Struct. Biol. 2010;20:241–246. doi: 10.1016/j.sbi.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 48.Breuer K., Wittmann M., Kempe K., Kapp A., Mai U., Dittrich-Breiholz O., Kracht M., Mrabet-Dahbi S., Werfel T. Alpha-toxin is produced by skin colonizing Staphylococcus aureus and induces a T helper type 1 response in atopic dermatitis. Clin. Exp. Allergy. 2005;35:1088–1095. doi: 10.1111/j.1365-2222.2005.02295.x. [DOI] [PubMed] [Google Scholar]
- 49.Kasraie S., Niebuhr M., Kopfnagel V., Dittrich-Breiholz O., Kracht M., Werfel T. Macrophages from patients with atopic dermatitis show a reduced CXCL10 expression in response to staphylococcal α-toxin. Allergy. 2012;67:41–49. doi: 10.1111/j.1398-9995.2011.02710.x. [DOI] [PubMed] [Google Scholar]
- 50.Schlievert P.M., Roller R.J., Kilgore S.H., Villarreal M., Klingelhutz A.J., Leung D.Y.M. Staphylococcal TSST-1 Association with Eczema Herpeticum in Humans. mSphere. 2021;6:e00608-21. doi: 10.1128/mSphere.00608-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okano M., Fujiwara T., Kariya S., Higaki T., Haruna T., Matsushita O., Noda Y., Makihara S., Kanai K., Noyama Y., et al. Cellular responses to Staphylococcus aureus alpha-toxin in chronic rhinosinusitis with nasal polyps. Allergol. Int. 2014;63:563–573. doi: 10.2332/allergolint.14-OA-0703. [DOI] [PubMed] [Google Scholar]
- 52.Kang S.S., Noh S.Y., Park O.J., Yun C.H., Han S.H. Staphylococcus aureus induces IL-8 expression through its lipoproteins in the human intestinal epithelial cell, Caco-2. Cytokine. 2015;75:174–180. doi: 10.1016/j.cyto.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 53.Kwak Y.K., Vikström E., Magnusson K.E., Vécsey-Semjén B., Colque-Navarro P., Möllby R. The Staphylococcus aureus alpha-toxin perturbs the barrier function in Caco-2 epithelial cell monolayers by altering junctional integrity. Infect. Immun. 2012;80:1670–1680. doi: 10.1128/IAI.00001-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gillet Y., Issartel B., Vanhems P., Fournet J.C., Lina G., Bes M., Vandenesch F., Piémont Y., Brousse N., Floret D., et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002;359:753–759. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 55.Finck-Barbançon V., Duportail G., Meunier O., Colin D.A. Pore formation by a two-component leukocidin from Staphylococcus aureus within the membrane of human polymorphonuclear leukocytes. Biochim. Biophys. Acta. 1993;1182:275–282. doi: 10.1016/0925-4439(93)90069-D. [DOI] [PubMed] [Google Scholar]
- 56.Yanai M., Rocha M.A., Matolek A.Z., Chintalacharuvu A., Taira Y., Chintalacharuvu K., Beenhouwer D.O. Separately or combined, LukG/LukH is functionally unique compared to other staphylococcal bicomponent leukotoxins. PLoS ONE. 2014;9:e89308. doi: 10.1371/journal.pone.0089308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Melehani J.H., James D.B., DuMont A.L., Torres V.J., Duncan J.A. Staphylococcus aureus Leukocidin A/B (LukAB) Kills Human Monocytes via Host NLRP3 and ASC when Extracellular, but Not Intracellular. PLoS Pathog. 2015;11:e1004970. doi: 10.1371/journal.ppat.1004970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holzinger D., Gieldon L., Mysore V., Nippe N., Taxman D.J., Duncan J.A., Broglie P.M., Marketon K., Austermann J., Vogl T., et al. Staphylococcus aureus Panton-Valentine leukocidin induces an inflammatory response in human phagocytes via the NLRP3 inflammasome. J. Leukoc. Biol. 2012;92:1069–1081. doi: 10.1189/jlb.0112014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spaan A.N., Vrieling M., Wallet P., Badiou C., Reyes-Robles T., Ohneck E.A., Benito Y., de Haas C.J., Day C.J., Jennings M.P., et al. The staphylococcal toxins γ-haemolysin AB and CB differentially target phagocytes by employing specific chemokine receptors. Nat. Commun. 2014;5:5438. doi: 10.1038/ncomms6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Staali L., Monteil H., Colin D.A. The staphylococcal pore-forming leukotoxins open Ca2+ channels in the membrane of human polymorphonuclear neutrophils. J. Membr. Biol. 1998;162:209–216. doi: 10.1007/s002329900358. [DOI] [PubMed] [Google Scholar]
- 61.Noda M., Kato I., Hirayama T., Matsuda F. Mode of action of staphylococcal leukocidin: Effects of the S and F components on the activities of membrane-associated enzymes of rabbit polymorphonuclear leukocytes. Infect. Immun. 1982;35:38–45. doi: 10.1128/iai.35.1.38-45.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chow S.H., Deo P., Yeung A.T.Y., Kostoulias X.P., Jeon Y., Gao M.L., Seidi A., Olivier F.A.B., Sridhar S., Nethercott C., et al. Targeting NLRP3 and Staphylococcal pore-forming toxin receptors in human-induced pluripotent stem cell-derived macrophages. J. Leukoc. Biol. 2020;108:967–981. doi: 10.1002/JLB.4MA0420-497R. [DOI] [PubMed] [Google Scholar]
- 63.Kitur K., Parker D., Nieto P., Ahn D.S., Cohen T.S., Chung S., Wachtel S., Bueno S., Prince A. Toxin-induced necroptosis is a major mechanism of Staphylococcus aureus lung damage. PLoS Pathog. 2015;11:e1004820. doi: 10.1371/journal.ppat.1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shallcross L.J., Fragaszy E., Johnson A.M., Hayward A.C. The role of the Panton-Valentine leucocidin toxin in staphylococcal disease: A systematic review and meta-analysis. Lancet Infect. Dis. 2013;13:43–54. doi: 10.1016/S1473-3099(12)70238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Genestier A.L., Michallet M.C., Prévost G., Bellot G., Chalabreysse L., Peyrol S., Thivolet F., Etienne J., Lina G., Vallette F.M., et al. Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondria and induces Bax-independent apoptosis of human neutrophils. J. Clin. Investig. 2005;115:3117–3127. doi: 10.1172/JCI22684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang J., Zhang T., Zou X., Wu S., Zhu J. Panton-valentine leucocidin carrying Staphylococcus aureus causing necrotizing pneumonia inactivates the JAK/STAT signaling pathway and increases the expression of inflammatory cytokines. Infect. Genet. Evol. 2020;86:104582. doi: 10.1016/j.meegid.2020.104582. [DOI] [PubMed] [Google Scholar]
- 67.Gray B., Hall P., Gresham H. Targeting agr- and agr-Like quorum sensing systems for development of common therapeutics to treat multiple gram-positive bacterial infections. Sensors. 2013;13:5130–5166. doi: 10.3390/s130405130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou Y., Niu C., Ma B., Xue X., Li Z., Chen Z., Li F., Zhou S., Luo X., Hou Z. Inhibiting PSMα-induced neutrophil necroptosis protects mice with MRSA pneumonia by blocking the agr system. Cell Death Dis. 2018;9:362. doi: 10.1038/s41419-018-0398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hanzelmann D., Joo H.S., Franz-Wachtel M., Hertlein T., Stevanovic S., Macek B., Wolz C., Götz F., Otto M., Kretschmer D., et al. Toll-like receptor 2 activation depends on lipopeptide shedding by bacterial surfactants. Nat. Commun. 2016;7:12304. doi: 10.1038/ncomms12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patrick G.J., Liu H., Alphonse M.P., Dikeman D.A., Youn C., Otterson J.C., Wang Y., Ravipati A., Mazhar M., Denny G., et al. Epicutaneous Staphylococcus aureus induces IL-36 to enhance IgE production and ensuing allergic disease. J. Clin. Investig. 2021;131:e143334. doi: 10.1172/JCI143334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McKevitt A.I., Bjornson G.L., Mauracher C.A., Scheifele D.W. Amino acid sequence of a deltalike toxin from Staphylococcus epidermidis. Infect. Immun. 1990;58:1473–1475. doi: 10.1128/iai.58.5.1473-1475.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kretschmer D., Gleske A.K., Rautenberg M., Wang R., Köberle M., Bohn E., Schöneberg T., Rabiet M.J., Boulay F., Klebanoff S.J., et al. Human formyl peptide receptor 2 senses highly pathogenic Staphylococcus aureus. Cell Host Microbe. 2010;7:463–473. doi: 10.1016/j.chom.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rautenberg M., Joo H.S., Otto M., Peschel A. Neutrophil responses to staphylococcal pathogens and commensals via the formyl peptide receptor 2 relates to phenol-soluble modulin release and virulence. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2011;25:1254–1263. doi: 10.1096/fj.10-175208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nakamura Y., Oscherwitz J., Cease K.B., Chan S.M., Muñoz-Planillo R., Hasegawa M., Villaruz A.E., Cheung G.Y., McGavin M.J., Travers J.B., et al. Staphylococcus δ-toxin induces allergic skin disease by activating mast cells. Nature. 2013;503:397–401. doi: 10.1038/nature12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kapral F.A. Staphylococcus aureus delta toxin as an enterotoxin. Ciba. Found. Symp. 1985;112:215–229. doi: 10.1002/9780470720936.ch12. [DOI] [PubMed] [Google Scholar]
- 76.Ullah A., Khan A., Al-Harrasi A., Ullah K., Shabbir A. Three-Dimensional Structure Characterization and Inhibition Study of Exfoliative Toxin D from Staphylococcus aureus. Front. Pharmacol. 2022;13:800970. doi: 10.3389/fphar.2022.800970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Imanishi I., Nicolas A., Caetano A.B., Castro T.L.P., Tartaglia N.R., Mariutti R., Guédon E., Even S., Berkova N., Arni R.K., et al. Exfoliative toxin E, a new Staphylococcus aureus virulence factor with host-specific activity. Sci. Rep. 2019;9:16336. doi: 10.1038/s41598-019-52777-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stentzel S., Teufelberger A., Nordengrün M., Kolata J., Schmidt F., van Crombruggen K., Michalik S., Kumpfmüller J., Tischer S., Schweder T., et al. Staphylococcal serine protease-like proteins are pacemakers of allergic airway reactions to Staphylococcus aureus. J. Allergy Clin. Immunol. 2017;139:492–500.e8. doi: 10.1016/j.jaci.2016.03.045. [DOI] [PubMed] [Google Scholar]
- 79.Melish M.E., Glasgow L.A. Staphylococcal scalded skin syndrome: The expanded clinical syndrome. J. Pediatr. 1971;78:958–967. doi: 10.1016/S0022-3476(71)80425-0. [DOI] [PubMed] [Google Scholar]
- 80.Bukowski M., Wladyka B., Dubin G. Exfoliative toxins of Staphylococcus aureus. Toxins. 2010;2:1148–1165. doi: 10.3390/toxins2051148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nishifuji K., Sugai M., Amagai M. Staphylococcal exfoliative toxins: “molecular scissors” of bacteria that attack the cutaneous defense barrier in mammals. J. Dermatol. Sci. 2008;49:21–31. doi: 10.1016/j.jdermsci.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 82.Amagai M., Yamaguchi T., Hanakawa Y., Nishifuji K., Sugai M., Stanley J.R. Staphylococcal exfoliative toxin B specifically cleaves desmoglein 1. J. Investig. Dermatol. 2002;118:845–850. doi: 10.1046/j.1523-1747.2002.01751.x. [DOI] [PubMed] [Google Scholar]
- 83.Oliveira D., Borges A., Simões M. Staphylococcus aureus Toxins and Their Molecular Activity in Infectious Diseases. Toxins. 2018;10:252. doi: 10.3390/toxins10060252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nordengrün M., Abdurrahman G., Treffon J., Wächter H., Kahl B.C., Bröker B.M. Allergic Reactions to Serine Protease-Like Proteins of Staphylococcus aureus. Front. Immunol. 2021;12:651060. doi: 10.3389/fimmu.2021.651060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smagur J., Guzik K., Magiera L., Bzowska M., Gruca M., Thøgersen I.B., Enghild J.J., Potempa J. A new pathway of staphylococcal pathogenesis: Apoptosis-like death induced by Staphopain B in human neutrophils and monocytes. J. Innate Immun. 2009;1:98–108. doi: 10.1159/000181014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kulig P., Zabel B.A., Dubin G., Allen S.J., Ohyama T., Potempa J., Handel T.M., Butcher E.C., Cichy J. Staphylococcus aureus-derived staphopain B, a potent cysteine protease activator of plasma chemerin. J. Immunol. 2007;178:3713–3720. doi: 10.4049/jimmunol.178.6.3713. [DOI] [PubMed] [Google Scholar]
- 87.Bergdoll M.S., Crass B.A., Reiser R.F., Robbins R.N., Davis J.P. A new staphylococcal enterotoxin, enterotoxin F, associated with toxic-shock-syndrome Staphylococcus aureus isolates. Lancet. 1981;1:1017–1021. doi: 10.1016/S0140-6736(81)92186-3. [DOI] [PubMed] [Google Scholar]
- 88.McCormick J.K., Yarwood J.M., Schlievert P.M. Toxic shock syndrome and bacterial superantigens: An update. Annu Rev. Microbiol. 2001;55:77–104. doi: 10.1146/annurev.micro.55.1.77. [DOI] [PubMed] [Google Scholar]
- 89.Calus L., Derycke L., Dullaers M., Van Zele T., De Ruyck N., Pérez-Novo C., Holtappels G., De Vos G., Lambrecht B.N., Bachert C., et al. IL-21 Is Increased in Nasal Polyposis and after Stimulation with Staphylococcus aureus Enterotoxin B. Int. Arch. Allergy Immunol. 2017;174:161–169. doi: 10.1159/000481435. [DOI] [PubMed] [Google Scholar]
- 90.Bauquet A.T., Jin H., Paterson A.M., Mitsdoerffer M., Ho I.C., Sharpe A.H., Kuchroo V.K. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat. Immunol. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Van Zele T., Gevaert P., Watelet J.B., Claeys G., Holtappels G., Claeys C., van Cauwenberge P., Bachert C. Staphylococcus aureus colonization and IgE antibody formation to enterotoxins is increased in nasal polyposis. J. Allergy Clin. Immunol. 2004;114:981–983. doi: 10.1016/j.jaci.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 92.Orfali R.L., da Silva Oliveira L.M., de Lima J.F., de Carvalho G.C., Ramos Y.A.L., Pereira N.Z., Pereira N.V., Zaniboni M.C., Sotto M.N., da Silva Duarte A.J., et al. Staphylococcus aureus enterotoxins modulate IL-22-secreting cells in adults with atopic dermatitis. Sci. Rep. 2018;8:6665. doi: 10.1038/s41598-018-25125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hellings P.W., Hens G., Meyts I., Bullens D., Vanoirbeek J., Gevaert P., Jorissen M., Ceuppens J.L., Bachert C. Aggravation of bronchial eosinophilia in mice by nasal and bronchial exposure to Staphylococcus aureus enterotoxin B. Clin. Exp. Allergy. 2006;36:1063–1071. doi: 10.1111/j.1365-2222.2006.02527.x. [DOI] [PubMed] [Google Scholar]
- 94.Liu X., Wen Y., Wang D., Zhao Z., Jeffry J., Zeng L., Zou Z., Chen H., Tao A. Synergistic activation of Src, ERK and STAT pathways in PBMCs for Staphylococcal enterotoxin A induced production of cytokines and chemokines. Asian Pac. J. Allergy Immunol. 2020;38:52–63. doi: 10.12932/ap-220818-0396. [DOI] [PubMed] [Google Scholar]
- 95.Naik S., Smith F., Ho J., Croft N.M., Domizio P., Price E., Sanderson I.R., Meadows N.J. Staphylococcal enterotoxins G and I, a cause of severe but reversible neonatal enteropathy. Clin. Gastroenterol. Hepatol. 2008;6:251–254. doi: 10.1016/j.cgh.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 96.Liu Y., Chen W., Ali T., Alkasir R., Yin J., Liu G., Han B. Staphylococcal enterotoxin H induced apoptosis of bovine mammary epithelial cells in vitro. Toxins. 2014;6:3552–3567. doi: 10.3390/toxins6123552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hou F., Peng L., Jiang J., Chen T., Xu D., Huang Q., Ye C., Peng Y., Hu D.L., Fang R. ATP Facilitates Staphylococcal Enterotoxin O Induced Neutrophil IL-1β Secretion via NLRP3 Inflammasome Dependent Pathways. Front. Immunol. 2021;12:649235. doi: 10.3389/fimmu.2021.649235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao Y., Tang J., Yang D., Tang C., Chen J. Staphylococcal enterotoxin M induced inflammation and impairment of bovine mammary epithelial cells. J. Dairy Sci. 2020;103:8350–8359. doi: 10.3168/jds.2019-17444. [DOI] [PubMed] [Google Scholar]
- 99.Zhao Y., Zhu A., Tang J., Tang C., Chen J. Identification and measurement of staphylococcal enterotoxin M from Staphylococcus aureus isolate associated with staphylococcal food poisoning. Lett. Appl. Microbiol. 2017;65:27–34. doi: 10.1111/lam.12751. [DOI] [PubMed] [Google Scholar]
- 100.Hu D.L., Ono H.K., Isayama S., Okada R., Okamura M., Lei L.C., Liu Z.S., Zhang X.C., Liu M.Y., Cui J.C., et al. Biological characteristics of staphylococcal enterotoxin Q and its potential risk for food poisoning. J. Appl. Microbiol. 2017;122:1672–1679. doi: 10.1111/jam.13462. [DOI] [PubMed] [Google Scholar]
- 101.Cruciani M., Etna M.P., Camilli R., Giacomini E., Percario Z.A., Severa M., Sandini S., Rizzo F., Brandi V., Balsamo G., et al. Staphylococcus aureus Esx Factors Control Human Dendritic Cell Functions Conditioning Th1/Th17 Response. Front. Cell. Infect. Microbiol. 2017;7:330. doi: 10.3389/fcimb.2017.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Korea C.G., Balsamo G., Pezzicoli A., Merakou C., Tavarini S., Bagnoli F., Serruto D., Unnikrishnan M. Staphylococcal Esx proteins modulate apoptosis and release of intracellular Staphylococcus aureus during infection in epithelial cells. Infect. Immun. 2014;82:4144–4153. doi: 10.1128/IAI.01576-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Becker K., Heilmann C., Peters G. Coagulase-negative staphylococci. Clin. Microbiol. Rev. 2014;27:870–926. doi: 10.1128/CMR.00109-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jin T., Zhu Y.L., Li J., Shi J., He X.Q., Ding J., Xu Y.Q. Staphylococcal protein A, Panton-Valentine leukocidin and coagulase aggravate the bone loss and bone destruction in osteomyelitis. Cell Physiol. Biochem. 2013;32:322–333. doi: 10.1159/000354440. [DOI] [PubMed] [Google Scholar]
- 105.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 106.Berends E.T., Horswill A.R., Haste N.M., Monestier M., Nizet V., von Köckritz-Blickwede M. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. J. Innate Immun. 2010;2:576–586. doi: 10.1159/000319909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thammavongsa V., Missiakas D.M., Schneewind O. Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science. 2013;342:863–866. doi: 10.1126/science.1242255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Winstel V., Schneewind O., Missiakas D. Staphylococcus aureus Exploits the Host Apoptotic Pathway To Persist during Infection. mBio. 2019;10:e02270-19. doi: 10.1128/mBio.02270-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang H., von Rohrscheidt J., Roehrbein J., Peters T., Sindrilaru A., Kess D., Preissner K.T., Scharffetter-Kochanek K. Extracellular adherence protein of Staphylococcus aureus suppresses disease by inhibiting T-cell recruitment in a mouse model of psoriasis. J. Investig. Dermatol. 2010;130:743–754. doi: 10.1038/jid.2009.310. [DOI] [PubMed] [Google Scholar]
- 110.Foster T.J. The MSCRAMM Family of Cell-Wall-Anchored Surface Proteins of Gram-Positive Cocci. Trends Microbiol. 2019;27:927–941. doi: 10.1016/j.tim.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 111.Pazos M., Peters K. Peptidoglycan. Subcell. Biochem. 2019;92:127–168. doi: 10.1007/978-3-030-18768-2_5. [DOI] [PubMed] [Google Scholar]
- 112.Foster T.J. Surface Proteins of Staphylococcus aureus. Microbiol. Spectr. 2019;7:4. doi: 10.1128/microbiolspec.GPP3-0046-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rivas J.M., Speziale P., Patti J.M., Höök M. MSCRAMM--targeted vaccines and immunotherapy for staphylococcal infection. Curr. Opin. Drug Discov. Dev. 2004;7:223–227. [PubMed] [Google Scholar]
- 114.Fox P.G., Schiavetti F., Rappuoli R., McLoughlin R.M., Bagnoli F. Staphylococcal Protein A Induces Leukocyte Necrosis by Complexing with Human Immunoglobulins. mBio. 2021;12:e00899-21. doi: 10.1128/mBio.00899-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Das T., Sa G., Chattopadhyay S., Ray P.K. Protein A-induced apoptosis of cancer cells is effected by soluble immune mediators. Cancer Immunol. Immunother. 2002;51:376–380. doi: 10.1007/s00262-002-0288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sawada M., Nakashima S., Banno Y., Yamakawa H., Takenaka K., Shinoda J., Nishimura Y., Sakai N., Nozawa Y. Influence of Bax or Bcl-2 overexpression on the ceramide-dependent apoptotic pathway in glioma cells. Oncogene. 2000;19:3508–3520. doi: 10.1038/sj.onc.1203699. [DOI] [PubMed] [Google Scholar]
- 117.Claro T., Widaa A., McDonnell C., Foster T.J., O’Brien F.J., Kerrigan S.W. Staphylococcus aureus protein A binding to osteoblast tumour necrosis factor receptor 1 results in activation of nuclear factor kappa B and release of interleukin-6 in bone infection. Microbiology. 2013;159:147–154. doi: 10.1099/mic.0.063016-0. [DOI] [PubMed] [Google Scholar]
- 118.Claro T., Widaa A., O’Seaghdha M., Miajlovic H., Foster T.J., O’Brien F.J., Kerrigan S.W. Staphylococcus aureus protein A binds to osteoblasts and triggers signals that weaken bone in osteomyelitis. PLoS ONE. 2011;6:e18748. doi: 10.1371/journal.pone.0018748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Al Kindi A., Williams H., Matsuda K., Alkahtani A.M., Saville C., Bennett H., Alshammari Y., Tan S.Y., O’Neill C., Tanaka A., et al. Staphylococcus aureus second immunoglobulin-binding protein drives atopic dermatitis via IL-33. J. Allergy Clin. Immunol. 2021;147:1354–1368.e3. doi: 10.1016/j.jaci.2020.09.023. [DOI] [PubMed] [Google Scholar]
- 120.Garcovich S., Maurelli M., Gisondi P., Peris K., Yosipovitch G., Girolomoni G. Pruritus as a Distinctive Feature of Type 2 Inflammation. Vaccines. 2021;9:303. doi: 10.3390/vaccines9030303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Imai Y. Interleukin-33 in atopic dermatitis. J. Dermatol. Sci. 2019;96:2–7. doi: 10.1016/j.jdermsci.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 122.Takeda K., Takeuchi O., Akira S. Recognition of lipopeptides by Toll-like receptors. J. Endotoxin. Res. 2002;8:459–463. doi: 10.1177/09680519020080060101. [DOI] [PubMed] [Google Scholar]
- 123.Stoll H., Dengjel J., Nerz C., Götz F. Staphylococcus aureus deficient in lipidation of prelipoproteins is attenuated in growth and immune activation. Infect. Immun. 2005;73:2411–2423. doi: 10.1128/IAI.73.4.2411-2423.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Takeuchi O., Kawai T., Mühlradt P.F., Morr M., Radolf J.D., Zychlinsky A., Takeda K., Akira S. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 2001;13:933–940. doi: 10.1093/intimm/13.7.933. [DOI] [PubMed] [Google Scholar]
- 125.Hara H., Seregin S.S., Yang D., Fukase K., Chamaillard M., Alnemri E.S., Inohara N., Chen G.Y., Núñez G. The NLRP6 Inflammasome Recognizes Lipoteichoic Acid and Regulates Gram-Positive Pathogen Infection. Cell. 2018;175:1651–1664.e14. doi: 10.1016/j.cell.2018.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brauweiler A.M., Goleva E., Leung D.Y.M. Staphylococcus aureus Lipoteichoic Acid Initiates a TSLP-Basophil-IL4 Axis in the Skin. J. Investig. Dermatol. 2020;140:915–917.e2. doi: 10.1016/j.jid.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Misawa Y., Kelley K.A., Wang X., Wang L., Park W.B., Birtel J., Saslowsky D., Lee J.C. Staphylococcus aureus Colonization of the Mouse Gastrointestinal Tract Is Modulated by Wall Teichoic Acid, Capsule, and Surface Proteins. PLoS Pathog. 2015;11:e1005061. doi: 10.1371/journal.ppat.1005061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pasquina-Lemonche L., Burns J., Turner R.D., Kumar S., Tank R., Mullin N., Wilson J.S., Chakrabarti B., Bullough P.A., Foster S.J., et al. The architecture of the Gram-positive bacterial cell wall. Nature. 2020;582:294–297. doi: 10.1038/s41586-020-2236-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Covas G., Vaz F., Henriques G., Pinho M.G., Filipe S.R. Analysis of Cell Wall Teichoic Acids in Staphylococcus aureus. Methods Mol. Biol. 2016;1440:201–213. doi: 10.1007/978-1-4939-3676-2_15. [DOI] [PubMed] [Google Scholar]
- 130.Matsui K., Tofukuji S., Ikeda R. CCL17 production by mouse langerhans cells stimulated with Staphylococcus aureus cell wall components. Biol. Pharm. Bull. 2015;38:317–320. doi: 10.1248/bpb.b14-00614. [DOI] [PubMed] [Google Scholar]
- 131.Matsui K., Wirotesangthong M., Nishikawa A. Peptidoglycan from Staphylococcus aureus induces IL-4 production from murine spleen cells via an IL-18-dependent mechanism. Int. Arch. Allergy Immunol. 2008;146:262–266. doi: 10.1159/000116363. [DOI] [PubMed] [Google Scholar]
- 132.Supajatura V., Ushio H., Nakao A., Akira S., Okumura K., Ra C., Ogawa H. Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J. Clin. Investig. 2002;109:1351–1359. doi: 10.1172/JCI0214704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Biggs T.C., Abadalkareem R.S., Hayes S.M., Holding R.E., Lau L.C., Harries P.G., Allan R.N., Pender S.L.F., Walls A.F., Salib R.J. Staphylococcus aureus internalisation enhances bacterial survival through modulation of host immune responses and mast cell activation. Allergy. 2021;76:1893–1896. doi: 10.1111/all.14701. [DOI] [PubMed] [Google Scholar]
- 134.Lin H.Y., Tang C.H., Chen J.H., Chuang J.Y., Huang S.M., Tan T.W., Lai C.H., Lu D.Y. Peptidoglycan induces interleukin-6 expression through the TLR2 receptor, JNK, c-Jun, and AP-1 pathways in microglia. J. Cell. Physiol. 2011;226:1573–1582. doi: 10.1002/jcp.22489. [DOI] [PubMed] [Google Scholar]
- 135.Hsu M.J., Chang C.K., Chen M.C., Chen B.C., Ma H.P., Hong C.Y., Lin C.H. Apoptosis signal-regulating kinase 1 in peptidoglycan-induced COX-2 expression in macrophages. J. Leukoc. Biol. 2010;87:1069–1082. doi: 10.1189/jlb.1009668. [DOI] [PubMed] [Google Scholar]
- 136.Wang D., Xiao P.L., Duan H.X., Zhou M., Liu J., Li W., Luo K.L., Chen J.J., Hu J.Y. Peptidoglycans promotes human leukemic THP-1 cell apoptosis and differentiation. Asian Pac. J. Cancer Prev. 2012;13:6409–6413. doi: 10.7314/APJCP.2012.13.12.6409. [DOI] [PubMed] [Google Scholar]
- 137.Namazi M.R. Paradoxical exacerbation of psoriasis in AIDS: Proposed explanations including the potential roles of substance P and gram-negative bacteria. Autoimmunity. 2004;37:67–71. doi: 10.1080/08916930310001637986. [DOI] [PubMed] [Google Scholar]
- 138.Ruíz-González V., Cancino-Diaz J.C., Rodríguez-Martínez S., Cancino-Diaz M.E. Keratinocytes treated with peptidoglycan from Staphylococcus aureus produce vascular endothelial growth factor, and its expression is amplified by the subsequent production of interleukin-13. Int. J. Dermatol. 2009;48:846–854. doi: 10.1111/j.1365-4632.2008.03924.x. [DOI] [PubMed] [Google Scholar]
- 139.Wu H.M., Wang J., Zhang B., Fang L., Xu K., Liu R.Y. CpG-ODN promotes phagocytosis and autophagy through JNK/P38 signal pathway in Staphylococcus aureus-stimulated macrophage. Life Sci. 2016;161:51–59. doi: 10.1016/j.lfs.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 140.Müller S., Wolf A.J., Iliev I.D., Berg B.L., Underhill D.M., Liu G.Y. Poorly Cross-Linked Peptidoglycan in MRSA Due to mecA Induction Activates the Inflammasome and Exacerbates Immunopathology. Cell Host Microbe. 2015;18:604–612. doi: 10.1016/j.chom.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhu F., Zhou Y., Jiang C., Zhang X. Role of JAK-STAT signaling in maturation of phagosomes containing Staphylococcus aureus. Sci. Rep. 2015;5:14854. doi: 10.1038/srep14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shi M., Willing S.E., Kim H.K., Schneewind O., Missiakas D. Peptidoglycan Contribution to the B Cell Superantigen Activity of Staphylococcal Protein A. mBio. 2021;12:e00039-21. doi: 10.1128/mBio.00039-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Soong G., Chun J., Parker D., Prince A. Staphylococcus aureus activation of caspase 1/calpain signaling mediates invasion through human keratinocytes. J. Infect. Dis. 2012;205:1571–1579. doi: 10.1093/infdis/jis244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bantel H., Sinha B., Domschke W., Peters G., Schulze-Osthoff K., Jänicke R.U. α-Toxin is a mediator of Staphylococcus aureus-induced cell death and activates caspases via the i.intrinsic death pathway independently of death receptor signaling. J. Cell Biol. 2001;155:637–648. doi: 10.1083/jcb.200105081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mestre M.B., Colombo M.I. Staphylococcus aureus promotes autophagy by decreasing intracellular cAMP levels. Autophagy. 2012;8:1865–1867. doi: 10.4161/auto.22161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mestre M.B., Colombo M.I. cAMP and EPAC are key players in the regulation of the signal transduction pathway involved in the α-hemolysin autophagic response. PLoS Pathog. 2012;8:e1002664. doi: 10.1371/journal.ppat.1002664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.McFadden J.P., Noble W.C., Camp R.D. Superantigenic exotoxin-secreting potential of staphylococci isolated from atopic eczematous skin. Br. J. Dermatol. 1993;128:631–632. doi: 10.1111/j.1365-2133.1993.tb00257.x. [DOI] [PubMed] [Google Scholar]
- 148.Porichis F., Morou A., Baritaki S., Spandidos D.A., Krambovitis E. Activation-induced cell death signalling in CD4+ T cells by staphylococcal enterotoxin A. Toxicol. Lett. 2008;176:77–84. doi: 10.1016/j.toxlet.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 149.Lee H.W., Kim S.M., Kim J.M., Oh B.M., Kim J.Y., Jung H.J., Lim H.J., Kim B.S., Lee W.J., Lee S.J., et al. Potential Immunoinflammatory Role of Staphylococcal Enterotoxin A in Atopic Dermatitis: Immunohistopathological Analysis and in vitro Assay. Ann. Dermatol. 2013;25:173–180. doi: 10.5021/ad.2013.25.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Maina E.K., Hu D.L., Tsuji T., Omoe K., Nakane A. Staphylococcal enterotoxin A has potent superantigenic and emetic activities but not diarrheagenic activity. Int. J. Med. Microbiol. 2012;302:88–95. doi: 10.1016/j.ijmm.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 151.Bardoel B.W., Vos R., Bouman T., Aerts P.C., Bestebroer J., Huizinga E.G., Brondijk T.H., van Strijp J.A., de Haas C.J. Evasion of Toll-like receptor 2 activation by staphylococcal superantigen-like protein 3. J. Mol. Med. 2012;90:1109–1120. doi: 10.1007/s00109-012-0926-8. [DOI] [PubMed] [Google Scholar]
- 152.Moriwaki M., Iwamoto K., Niitsu Y., Matsushima A., Yanase Y., Hisatsune J., Sugai M., Hide M. Staphylococcus aureus from atopic dermatitis skin accumulates in the lysosomes of keratinocytes with induction of IL-1α secretion via TLR9. Allergy. 2019;74:560–571. doi: 10.1111/all.13622. [DOI] [PubMed] [Google Scholar]
- 153.Wang G., Sweren E., Liu H., Wier E., Alphonse M.P., Chen R., Islam N., Li A., Xue Y., Chen J., et al. Bacteria induce skin regeneration via IL-1β signaling. Cell Host Microbe. 2021;29:777–791.e6. doi: 10.1016/j.chom.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cayrol C., Girard J.P. Interleukin-33 (IL-33): A nuclear cytokine from the IL-1 family. Immunol. Rev. 2018;281:154–168. doi: 10.1111/imr.12619. [DOI] [PubMed] [Google Scholar]
- 155.Liu B., Tai Y., Achanta S., Kaelberer M.M., Caceres A.I., Shao X., Fang J., Jordt S.E. IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy. Proc. Natl. Acad. Sci. USA. 2016;113:E7572–E7579. doi: 10.1073/pnas.1606608113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vu A.T., Baba T., Chen X., Le T.A., Kinoshita H., Xie Y., Kamijo S., Hiramatsu K., Ikeda S., Ogawa H., et al. Staphylococcus aureus membrane and diacylated lipopeptide induce thymic stromal lymphopoietin in keratinocytes through the Toll-like receptor 2-Toll-like receptor 6 pathway. J. Allergy Clin. Immunol. 2010;126:985–993.e3. doi: 10.1016/j.jaci.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 157.Wilson S.R., Thé L., Batia L.M., Beattie K., Katibah G.E., McClain S.P., Pellegrino M., Estandian D.M., Bautista D.M. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155:285–295. doi: 10.1016/j.cell.2013.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Son E.D., Kim H.J., Park T., Shin K., Bae I.H., Lim K.M., Cho E.G., Lee T.R. Staphylococcus aureus inhibits terminal differentiation of normal human keratinocytes by stimulating interleukin-6 secretion. J. Dermatol. Sci. 2014;74:64–71. doi: 10.1016/j.jdermsci.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 159.Williams M.R., Nakatsuji T., Sanford J.A., Vrbanac A.F., Gallo R.L. Staphylococcus aureus Induces Increased Serine Protease Activity in Keratinocytes. J. Investig. Dermatol. 2017;137:377–384. doi: 10.1016/j.jid.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lan F., Zhang N., Holtappels G., De Ruyck N., Krysko O., Van Crombruggen K., Braun H., Johnston S.L., Papadopoulos N.G., Zhang L., et al. Staphylococcus aureus Induces a Mucosal Type 2 Immune Response via Epithelial Cell-derived Cytokines. Am. J. Respir. Crit Care Med. 2018;198:452–463. doi: 10.1164/rccm.201710-2112OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hardman C.S., Chen Y.L., Salimi M., Nahler J., Corridoni D., Jagielowicz M., Fonseka C.L., Johnson D., Repapi E., Cousins D.J., et al. IL-6 effector function of group 2 innate lymphoid cells (ILC2) is NOD2 dependent. Sci. Immunol. 2021;6:eabe5084. doi: 10.1126/sciimmunol.abe5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Romagnani S. T-cell subsets (Th1 versus Th2) Ann. Allergy Asthma Immunol. 2000;85:9–18; quiz 18, 21. doi: 10.1016/S1081-1206(10)62426-X. [DOI] [PubMed] [Google Scholar]
- 163.Pérez Novo C.A., Jedrzejczak-Czechowicz M., Lewandowska-Polak A., Claeys C., Holtappels G., Van Cauwenberge P., Kowalski M.L., Bachert C. T cell inflammatory response, Foxp3 and TNFRS18-L regulation of peripheral blood mononuclear cells from patients with nasal polyps-asthma after staphylococcal superantigen stimulation. Clin. Exp. Allergy. 2010;40:1323–1332. doi: 10.1111/j.1365-2222.2010.03577.x. [DOI] [PubMed] [Google Scholar]
- 164.Orciani M., Campanati A., Caffarini M., Ganzetti G., Consales V., Lucarini G., Offidani A., Di Primio R. T helper (Th)1, Th17 and Th2 imbalance in mesenchymal stem cells of adult patients with atopic dermatitis: At the origin of the problem. Br. J. Dermatol. 2017;176:1569–1576. doi: 10.1111/bjd.15078. [DOI] [PubMed] [Google Scholar]
- 165.Tada Y., Asahina A., Takekoshi T., Kishimoto E., Mitsui H., Saeki H., Komine M., Tamaki K. Interleukin 12 production by monocytes from patients with psoriasis and its inhibition by ciclosporin A. Br. J. Dermatol. 2006;154:1180–1183. doi: 10.1111/j.1365-2133.2006.07180.x. [DOI] [PubMed] [Google Scholar]
- 166.Stetson D.B., Voehringer D., Grogan J.L., Xu M., Reinhardt R.L., Scheu S., Kelly B.L., Locksley R.M. Th2 cells: Orchestrating barrier immunity. Adv. Immunol. 2004;83:163–189. doi: 10.1016/s0065-2776(04)83005-0. [DOI] [PubMed] [Google Scholar]
- 167.Kamijo H., Miyagaki T., Hayashi Y., Akatsuka T., Watanabe-Otobe S., Oka T., Shishido-Takahashi N., Suga H., Sugaya M., Sato S. Increased IL-26 Expression Promotes T Helper Type 17- and T Helper Type 2-Associated Cytokine Production by Keratinocytes in Atopic Dermatitis. J. Investig. Dermatol. 2020;140:636–644.e2. doi: 10.1016/j.jid.2019.07.713. [DOI] [PubMed] [Google Scholar]
- 168.Chang H.W., Yan D., Singh R., Liu J., Lu X., Ucmak D., Lee K., Afifi L., Fadrosh D., Leech J., et al. Alteration of the cutaneous microbiome in psoriasis and potential role in Th17 polarization. Microbiome. 2018;6:154. doi: 10.1186/s40168-018-0533-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Brauweiler A.M., Goleva E., Leung D.Y.M. Th2 cytokines increase Staphylococcus aureus alpha toxin-induced keratinocyte death through the signal transducer and activator of transcription 6 (STAT6) J. Investig. Dermatol. 2014;134:2114–2121. doi: 10.1038/jid.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ou L.S., Goleva E., Hall C., Leung D.Y. T regulatory cells in atopic dermatitis and subversion of their activity by superantigens. J. Allergy Clin. Immunol. 2004;113:756–763. doi: 10.1016/j.jaci.2004.01.772. [DOI] [PubMed] [Google Scholar]
- 171.Laouini D., Kawamoto S., Yalcindag A., Bryce P., Mizoguchi E., Oettgen H., Geha R.S. Epicutaneous sensitization with superantigen induces allergic skin inflammation. J. Allergy Clin. Immunol. 2003;112:981–987. doi: 10.1016/j.jaci.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 172.Jacobsen E.A., Ochkur S.I., Lee N.A., Lee J.J. Eosinophils and asthma. Curr. Allergy Asthma Rep. 2007;7:18–26. doi: 10.1007/s11882-007-0026-y. [DOI] [PubMed] [Google Scholar]
- 173.Warner J.A., McGirt L.Y., Beck L.A. Biomarkers of Th2 polarity are predictive of staphylococcal colonization in subjects with atopic dermatitis. Br. J. Dermatol. 2009;160:183–185. doi: 10.1111/j.1365-2133.2008.08905.x. [DOI] [PubMed] [Google Scholar]
- 174.Dubin C., Del Duca E., Guttman-Yassky E. The IL-4, IL-13 and IL-31 pathways in atopic dermatitis. Expert Rev. Clin. Immunol. 2021;17:835–852. doi: 10.1080/1744666X.2021.1940962. [DOI] [PubMed] [Google Scholar]
- 175.Iwaszko M., Biały S., Bogunia-Kubik K. Significance of Interleukin (IL)-4 and IL-13 in Inflammatory Arthritis. Cells. 2021;10:3000. doi: 10.3390/cells10113000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kimura A., Kishimoto T. Th17 cells in inflammation. Int. Immunopharmacol. 2011;11:319–322. doi: 10.1016/j.intimp.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 177.Tokura Y. Th17 cells and skin diseases. Nihon Rinsho Meneki Gakkai Kaishi. 2012;35:388–392. doi: 10.2177/jsci.35.388. [DOI] [PubMed] [Google Scholar]
- 178.Koga C., Kabashima K., Shiraishi N., Kobayashi M., Tokura Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J. Investig. Dermatol. 2008;128:2625–2630. doi: 10.1038/jid.2008.111. [DOI] [PubMed] [Google Scholar]
- 179.Orfali R.L., Yoshikawa F.S.Y., Oliveira L., Pereira N.Z., de Lima J.F., Ramos YÁ L., Duarte A., Sato M.N., Aoki V. Staphylococcal enterotoxins modulate the effector CD4(+) T cell response by reshaping the gene expression profile in adults with atopic dermatitis. Sci. Rep. 2019;9:13082. doi: 10.1038/s41598-019-49421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sugaya M. The Role of Th17-Related Cytokines in Atopic Dermatitis. Int. J. Mol. Sci. 2020;21:1314. doi: 10.3390/ijms21041314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Liu H., Archer N.K., Dillen C.A., Wang Y., Ashbaugh A.G., Ortines R.V., Kao T., Lee S.K., Cai S.S., Miller R.J., et al. Staphylococcus aureus Epicutaneous Exposure Drives Skin Inflammation via IL-36-Mediated T Cell Responses. Cell Host Microbe. 2017;22:653–666.e5. doi: 10.1016/j.chom.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Dey I., Bishayi B. Role of Th17 and Treg cells in septic arthritis and the impact of the Th17/Treg-derived cytokines in the pathogenesis of S. aureus induced septic arthritis in mice. Microb. Pathog. 2017;113:248–264. doi: 10.1016/j.micpath.2017.10.033. [DOI] [PubMed] [Google Scholar]
- 183.Dey I., Bishayi B. Role of different Th17 and Treg downstream signalling pathways in the pathogenesis of Staphylococcus aureus infection induced septic arthritis in mice. Exp. Mol. Pathol. 2020;116:104485. doi: 10.1016/j.yexmp.2020.104485. [DOI] [PubMed] [Google Scholar]
- 184.Lee G.R. The Balance of Th17 versus Treg Cells in Autoimmunity. Int. J. Mol. Sci. 2018;19:730. doi: 10.3390/ijms19030730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sultana S., Dey R., Bishayi B. Dual neutralization of TNFR-2 and MMP-2 regulates the severity of induced septic arthritis correlating alteration in the level of interferon gamma and interleukin-10 in terms of TNFR2 blocking. Immunol. Res. 2018;66:97–119. doi: 10.1007/s12026-017-8979-y. [DOI] [PubMed] [Google Scholar]
- 186.Ghosh R., Dey R., Sawoo R., Bishayi B. Neutralization of IL-17 and treatment with IL-2 protects septic arthritis by regulating free radical production and antioxidant enzymes in Th17 and Tregs: An immunomodulatory TLR2 versus TNFR response. Cell Immunol. 2021;370:104441. doi: 10.1016/j.cellimm.2021.104441. [DOI] [PubMed] [Google Scholar]
- 187.Saito S., Quadery A.F. Staphylococcus aureus Lipoprotein Induces Skin Inflammation, Accompanied with IFN-γ-Producing T Cell Accumulation through Dermal Dendritic Cells. Pathogens. 2018;7:64. doi: 10.3390/pathogens7030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Taylor A.L., Llewelyn M.J. Superantigen-induced proliferation of human CD4+CD25-T cells is followed by a switch to a functional regulatory phenotype. J. Immunol. 2010;185:6591–6598. doi: 10.4049/jimmunol.1002416. [DOI] [PubMed] [Google Scholar]
- 189.Seneschal J., Clark R.A., Gehad A., Baecher-Allan C.M., Kupper T.S. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity. 2012;36:873–884. doi: 10.1016/j.immuni.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.van Dalen R., De La Cruz Diaz J.S., Rumpret M., Fuchsberger F.F., van Teijlingen N.H., Hanske J., Rademacher C., Geijtenbeek T.B.H., van Strijp J.A.G., Weidenmaier C., et al. Langerhans Cells Sense Staphylococcus aureus Wall Teichoic Acid through Langerin To Induce Inflammatory Responses. mBio. 2019;10:e00330-19. doi: 10.1128/mBio.00330-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ma L., Xue H.B., Guan X.H., Shu C.M., Wang F., Zhang J.H., An R.Z. The Imbalance of Th17 cells and CD4(+) CD25(high) Foxp3(+) Treg cells in patients with atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2014;28:1079–1086. doi: 10.1111/jdv.12288. [DOI] [PubMed] [Google Scholar]
- 192.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Moser B. CXCR5, the Defining Marker for Follicular B Helper T (TFH) Cells. Front. Immunol. 2015;6:296. doi: 10.3389/fimmu.2015.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bossaller L., Burger J., Draeger R., Grimbacher B., Knoth R., Plebani A., Durandy A., Baumann U., Schlesier M., Welcher A.A., et al. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J. Immunol. 2006;177:4927–4932. doi: 10.4049/jimmunol.177.7.4927. [DOI] [PubMed] [Google Scholar]
- 195.Bennett F., Luxenberg D., Ling V., Wang I.M., Marquette K., Lowe D., Khan N., Veldman G., Jacobs K.A., Valge-Archer V.E., et al. Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: Attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J. Immunol. 2003;170:711–718. doi: 10.4049/jimmunol.170.2.711. [DOI] [PubMed] [Google Scholar]
- 196.Sage P.T., Paterson A.M., Lovitch S.B., Sharpe A.H. The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity. 2014;41:1026–1039. doi: 10.1016/j.immuni.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Johnston R.J., Poholek A.C., DiToro D., Yusuf I., Eto D., Barnett B., Dent A.L., Craft J., Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Dupont C.D., Scully I.L., Zimnisky R.M., Monian B., Rossitto C.P., O’Connell E.B., Jansen K.U., Anderson A.S., Love J.C. Two Vaccines for Staphylococcus aureus Induce a B-Cell-Mediated Immune Response. mSphere. 2018;3:e00217-18. doi: 10.1128/mSphere.00217-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chang H.C., Sehra S., Goswami R., Yao W., Yu Q., Stritesky G.L., Jabeen R., McKinley C., Ahyi A.N., Han L., et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat. Immunol. 2010;11:527–534. doi: 10.1038/ni.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Soussi-Gounni A., Kontolemos M., Hamid Q. Role of IL-9 in the pathophysiology of allergic diseases. J. Allergy Clin. Immunol. 2001;107:575–582. doi: 10.1067/mai.2001.114238. [DOI] [PubMed] [Google Scholar]
- 201.Jäger A., Dardalhon V., Sobel R.A., Bettelli E., Kuchroo V.K. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J. Immunol. 2009;183:7169–7177. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Purwar R., Schlapbach C., Xiao S., Kang H.S., Elyaman W., Jiang X., Jetten A.M., Khoury S.J., Fuhlbrigge R.C., Kuchroo V.K., et al. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat. Med. 2012;18:1248–1253. doi: 10.1038/nm.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Vivier E., Artis D., Colonna M., Diefenbach A., Di Santo J.P., Eberl G., Koyasu S., Locksley R.M., McKenzie A.N.J., Mebius R.E., et al. Innate Lymphoid Cells: 10 Years On. Cell. 2018;174:1054–1066. doi: 10.1016/j.cell.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 204.Ghaedi M., Takei F. Innate lymphoid cell development. J. Allergy Clin. Immunol. 2021;147:1549–1560. doi: 10.1016/j.jaci.2021.03.009. [DOI] [PubMed] [Google Scholar]
- 205.Annunziato F., Romagnani C., Romagnani S. The 3 major types of innate and adaptive cell-mediated effector immunity. J. Allergy Clin. Immunol. 2015;135:626–635. doi: 10.1016/j.jaci.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 206.Gentek R., Molawi K., Sieweke M.H. Tissue macrophage identity and self-renewal. Immunol. Rev. 2014;262:56–73. doi: 10.1111/imr.12224. [DOI] [PubMed] [Google Scholar]
- 207.Thurlow L.R., Hanke M.L., Fritz T., Angle A., Aldrich A., Williams S.H., Engebretsen I.L., Bayles K.W., Horswill A.R., Kielian T. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J. Immunol. 2011;186:6585–6596. doi: 10.4049/jimmunol.1002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wang X., Eagen W.J., Lee J.C. Orchestration of human macrophage NLRP3 inflammasome activation by Staphylococcus aureus extracellular vesicles. Proc. Natl. Acad. Sci. USA. 2020;117:3174–3184. doi: 10.1073/pnas.1915829117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Flannagan R.S., Heinrichs D.E. Macrophage-driven nutrient delivery to phagosomal Staphylococcus aureus supports bacterial growth. EMBO Rep. 2020;21:e50348. doi: 10.15252/embr.202050348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Grayczyk J.P., Alonzo F., 3rd Staphylococcus aureus Lipoic Acid Synthesis Limits Macrophage Reactive Oxygen and Nitrogen Species Production To Promote Survival during Infection. Infect. Immun. 2019;87:e00344-19. doi: 10.1128/iai.00344-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Iwamoto K., Nümm T.J., Koch S., Herrmann N., Leib N., Bieber T. Langerhans and inflammatory dendritic epidermal cells in atopic dermatitis are tolerized toward TLR2 activation. Allergy. 2018;73:2205–2213. doi: 10.1111/all.13460. [DOI] [PubMed] [Google Scholar]
- 212.Asahina A., Tamaki K. Role of Langerhans cells in cutaneous protective immunity: Is the reappraisal necessary? J. Dermatol. Sci. 2006;44:1–9. doi: 10.1016/j.jdermsci.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 213.Berends E.T.M., Zheng X., Zwack E.E., Ménager M.M., Cammer M., Shopsin B., Torres V.J. Staphylococcus aureus Impairs the Function of and Kills Human Dendritic Cells via the LukAB Toxin. mBio. 2019;10:e01918-18. doi: 10.1128/mBio.01918-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Matsui K., Kanai S., Ikuta M., Horikawa S. Mast Cells Stimulated with Peptidoglycan from Staphylococcus aureus Augment the Development of Th1 Cells. J. Pharm. Pharm. Sci. 2018;21:296–304. doi: 10.18433/jpps29951. [DOI] [PubMed] [Google Scholar]
- 215.Hayes S.M., Biggs T.C., Goldie S.P., Harries P.G., Walls A.F., Allan R.N., Pender S.L.F., Salib R.J. Staphylococcus aureus internalization in mast cells in nasal polyps: Characterization of interactions and potential mechanisms. J. Allergy Clin. Immunol. 2020;145:147–159. doi: 10.1016/j.jaci.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 216.Bachert C., Humbert M., Hanania N.A., Zhang N., Holgate S., Buhl R., Bröker B.M. Staphylococcus aureus and its IgE-inducing enterotoxins in asthma: Current knowledge. Eur. Respir. J. 2020;55:1901592. doi: 10.1183/13993003.01592-2019. [DOI] [PubMed] [Google Scholar]
- 217.Liu C., Yang L., Han Y., Ouyang W., Yin W., Xu F. Mast cells participate in regulation of lung-gut axis during Staphylococcus aureus pneumonia. Cell Prolif. 2019;52:e12565. doi: 10.1111/cpr.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lehman H.K., Segal B.H. The role of neutrophils in host defense and disease. J. Allergy Clin. Immunol. 2020;145:1535–1544. doi: 10.1016/j.jaci.2020.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Gough P., Ganesan S., Datta S.K. IL-20 Signaling in Activated Human Neutrophils Inhibits Neutrophil Migration and Function. J. Immunol. 2017;198:4373–4382. doi: 10.4049/jimmunol.1700253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Cho J.S., Guo Y., Ramos R.I., Hebroni F., Plaisier S.B., Xuan C., Granick J.L., Matsushima H., Takashima A., Iwakura Y., et al. Neutrophil-derived IL-1β is sufficient for abscess formation in immunity against Staphylococcus aureus in mice. PLoS Pathog. 2012;8:e1003047. doi: 10.1371/journal.ppat.1003047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lutalo P.M., D’Cruz D.P. Diagnosis and classification of granulomatosis with polyangiitis (aka Wegener’s granulomatosis) J. Autoimmun. 2014;48–49:94–98. doi: 10.1016/j.jaut.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 222.Ravin K.A., Loy M. The Eosinophil in Infection. Clin. Rev. Allergy Immunol. 2016;50:214–227. doi: 10.1007/s12016-015-8525-4. [DOI] [PubMed] [Google Scholar]
- 223.Gangwar R.S., Levi-Schaffer F. sCD48 is anti-inflammatory in Staphylococcus aureus Enterotoxin B-induced eosinophilic inflammation. Allergy. 2016;71:829–839. doi: 10.1111/all.12851. [DOI] [PubMed] [Google Scholar]
- 224.Prince L.R., Graham K.J., Connolly J., Anwar S., Ridley R., Sabroe I., Foster S.J., Whyte M.K. Staphylococcus aureus induces eosinophil cell death mediated by α-hemolysin. PLoS ONE. 2012;7:e31506. doi: 10.1371/journal.pone.0031506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kay A.B. TH2-type cytokines in asthma. Ann. N. Y. Acad. Sci. 1996;796:1–8. doi: 10.1111/j.1749-6632.1996.tb32561.x. [DOI] [PubMed] [Google Scholar]
- 226.Holgate S.T. Innate and adaptive immune responses in asthma. Nat. Med. 2012;18:673–683. doi: 10.1038/nm.2731. [DOI] [PubMed] [Google Scholar]
- 227.Jiao D., Wong C.K., Qiu H.N., Dong J., Cai Z., Chu M., Hon K.L., Tsang M.S., Lam C.W. NOD2 and TLR2 ligands trigger the activation of basophils and eosinophils by interacting with dermal fibroblasts in atopic dermatitis-like skin inflammation. Cell Mol. Immunol. 2016;13:535–550. doi: 10.1038/cmi.2015.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Leyva-Castillo J.M., Das M., Kane J., Strakosha M., Singh S., Wong D.S.H., Horswill A.R., Karasuyama H., Brombacher F., Miller L.S., et al. Basophil-derived IL-4 promotes cutaneous Staphylococcus aureus infection. JCI Insight. 2021;6:e149953. doi: 10.1172/jci.insight.149953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zhang X., Hu X., Rao X. Apoptosis induced by Staphylococcus aureus toxins. Microbiol. Res. 2017;205:19–24. doi: 10.1016/j.micres.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 230.Kang S.S., Kim S.K., Baik J.E., Ko E.B., Ahn K.B., Yun C.H., Han S.H. Staphylococcal LTA antagonizes the B cell-mitogenic potential of LPS. Sci. Rep. 2018;8:1496. doi: 10.1038/s41598-018-19653-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Place D.E., Lee S., Kanneganti T.D. PANoptosis in microbial infection. Curr. Opin. Microbiol. 2021;59:42–49. doi: 10.1016/j.mib.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Tsuchiya K., Hara H. The inflammasome and its regulation. Crit. Rev. Immunol. 2014;34:41–80. doi: 10.1615/CritRevImmunol.2013008686. [DOI] [PubMed] [Google Scholar]
- 233.Douglas T., Champagne C., Morizot A., Lapointe J.M., Saleh M. The Inflammatory Caspases-1 and -11 Mediate the Pathogenesis of Dermatitis in Sharpin-Deficient Mice. J. Immunol. 2015;195:2365–2373. doi: 10.4049/jimmunol.1500542. [DOI] [PubMed] [Google Scholar]
- 234.Vanaja S.K., Rathinam V.A., Fitzgerald K.A. Mechanisms of inflammasome activation: Recent advances and novel insights. Trends Cell Biol. 2015;25:308–315. doi: 10.1016/j.tcb.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Tang L., Zhou F. Inflammasomes in Common Immune-Related Skin Diseases. Front. Immunol. 2020;11:882. doi: 10.3389/fimmu.2020.00882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zhong F.L., Mamaï O., Sborgi L., Boussofara L., Hopkins R., Robinson K., Szeverényi I., Takeichi T., Balaji R., Lau A., et al. Germline NLRP1 Mutations Cause Skin Inflammatory and Cancer Susceptibility Syndromes via Inflammasome Activation. Cell. 2016;167:187–202.e17. doi: 10.1016/j.cell.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 237.Tilburgs T., Meissner T.B., Ferreira L.M.R., Mulder A., Musunuru K., Ye J., Strominger J.L. NLRP2 is a suppressor of NF-κB signaling and HLA-C expression in human trophoblasts. Biol. Reprod. 2017;96:831–842. doi: 10.1093/biolre/iox009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Sharma B.R., Kanneganti T.D. NLRP3 inflammasome in cancer and metabolic diseases. Nat. Immunol. 2021;22:550–559. doi: 10.1038/s41590-021-00886-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Jounai N., Kobiyama K., Shiina M., Ogata K., Ishii K.J., Takeshita F. NLRP4 negatively regulates autophagic processes through an association with beclin1. J. Immunol. 2011;186:1646–1655. doi: 10.4049/jimmunol.1001654. [DOI] [PubMed] [Google Scholar]
- 240.Li R., Zhu S. NLRP6 inflammasome. Mol. Asp. Med. 2020;76:100859. doi: 10.1016/j.mam.2020.100859. [DOI] [PubMed] [Google Scholar]
- 241.Radian A.D., de Almeida L., Dorfleutner A., Stehlik C. NLRP7 and related inflammasome activating pattern recognition receptors and their function in host defense and disease. Microbes Infect. 2013;15:630–639. doi: 10.1016/j.micinf.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Tuladhar S., Kanneganti T.D. NLRP12 in innate immunity and inflammation. Mol. Asp. Med. 2020;76:100887. doi: 10.1016/j.mam.2020.100887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Duncan J.A., Canna S.W. The NLRC4 Inflammasome. Immunol. Rev. 2018;281:115–123. doi: 10.1111/imr.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wang B., Tian Y., Yin Q. AIM2 Inflammasome Assembly and Signaling. Adv. Exp. Med. Biol. 2019;1172:143–155. doi: 10.1007/978-981-13-9367-9_7. [DOI] [PubMed] [Google Scholar]
- 245.Song Y., Wu X., Xu Y., Zhu J., Li J., Zou Z., Chen L., Zhang B., Hua C., Rui H., et al. HPV E7 inhibits cell pyroptosis by promoting TRIM21-mediated degradation and ubiquitination of the IFI16 inflammasome. Int. J. Biol. Sci. 2020;16:2924–2937. doi: 10.7150/ijbs.50074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Loeven N.A., Medici N.P., Bliska J.B. The pyrin inflammasome in host-microbe interactions. Curr. Opin. Microbiol. 2020;54:77–86. doi: 10.1016/j.mib.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Man S.M., Kanneganti T.D. Regulation of inflammasome activation. Immunol. Rev. 2015;265:6–21. doi: 10.1111/imr.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Stappers M.H., Thys Y., Oosting M., Plantinga T.S., Ioana M., Reimnitz P., Mouton J.W., Netea M.G., Joosten L.A., Gyssens I.C. TLR1, TLR2, and TLR6 gene polymorphisms are associated with increased susceptibility to complicated skin and skin structure infections. J. Infect. Dis. 2014;210:311–318. doi: 10.1093/infdis/jiu080. [DOI] [PubMed] [Google Scholar]
- 249.Niebuhr M., Baumert K., Heratizadeh A., Satzger I., Werfel T. Impaired NLRP3 inflammasome expression and function in atopic dermatitis due to Th2 milieu. Allergy. 2014;69:1058–1067. doi: 10.1111/all.12428. [DOI] [PubMed] [Google Scholar]
- 250.Schuepbach-Mallepell S., Philippe V., Brüggen M.C., Watanabe H., Roques S., Baldeschi C., Gaide O. Antagonistic effect of the inflammasome on thymic stromal lymphopoietin expression in the skin. J. Allergy Clin. Immunol. 2013;132:1348–1357. doi: 10.1016/j.jaci.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 251.Shi J., Zhao Y., Wang K., Shi X., Wang Y., Huang H., Zhuang Y., Cai T., Wang F., Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 252.Wang X., Liu M., Geng N., Du Y., Li Z., Gao X., Han B., Liu J., Liu Y. Staphylococcus aureus mediates pyroptosis in bovine mammary epithelial cell via activation of NLRP3 inflammasome. Vet. Res. 2022;53:10. doi: 10.1186/s13567-022-01027-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Accarias S., Lugo-Villarino G., Foucras G., Neyrolles O., Boullier S., Tabouret G. Pyroptosis of resident macrophages differentially orchestrates inflammatory responses to Staphylococcus aureus in resistant and susceptible mice. Eur. J. Immunol. 2015;45:794–806. doi: 10.1002/eji.201445098. [DOI] [PubMed] [Google Scholar]
- 254.Yao R., Chen Y., Hao H., Guo Z., Cheng X., Ma Y., Ji Q., Yang X., Wang Y., Li X., et al. Pathogenic effects of inhibition of mTORC1/STAT3 axis facilitates Staphylococcus aureus-induced pyroptosis in human macrophages. Cell Commun. Signal. 2020;18:187. doi: 10.1186/s12964-020-00677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Wang X., Yousefi S., Simon H.U. Necroptosis and neutrophil-associated disorders. Cell Death Dis. 2018;9:111. doi: 10.1038/s41419-017-0058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Duan X., Liu X., Liu N., Huang Y., Jin Z., Zhang S., Ming Z., Chen H. Inhibition of keratinocyte necroptosis mediated by RIPK1/RIPK3/MLKL provides a protective effect against psoriatic inflammation. Cell Death Dis. 2020;11:134. doi: 10.1038/s41419-020-2328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Cao M., Chen F., Xie N., Cao M.Y., Chen P., Lou Q., Zhao Y., He C., Zhang S., Song X., et al. c-Jun N-terminal kinases differentially regulate TNF- and TLRs-mediated necroptosis through their kinase-dependent and -independent activities. Cell Death Dis. 2018;9:1140. doi: 10.1038/s41419-018-1189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Wong Fok Lung T., Monk I.R., Acker K.P., Mu A., Wang N., Riquelme S.A., Pires S., Noguera L.P., Dach F., Gabryszewski S.J., et al. Staphylococcus aureus small colony variants impair host immunity by activating host cell glycolysis and inducing necroptosis. Nat. Microbiol. 2020;5:141–153. doi: 10.1038/s41564-019-0597-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Xu S.X., McCormick J.K. Staphylococcal superantigens in colonization and disease. Front. Cell Infect. Microbiol. 2012;2:52. doi: 10.3389/fcimb.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Ayala A., Wesche-Soldato D.E., Perl M., Lomas-Neira J.L., Swan R., Chung C.S. Blockade of apoptosis as a rational therapeutic strategy for the treatment of sepsis. Novartis Found. Symp. 2007;280:37–49; discussion 49–52, 160–164. doi: 10.1002/9780470059593.ch4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Dziarski R., Gupta D. Staphylococcus aureus peptidoglycan is a toll-like receptor 2 activator: A reevaluation. Infect. Immun. 2005;73:5212–5216. doi: 10.1128/IAI.73.8.5212-5216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Cohen T.S., Jones-Nelson O., Hotz M., Cheng L., Miller L.S., Suzich J., Stover C.K., Sellman B.R. S. aureus blocks efferocytosis of neutrophils by macrophages through the activity of its virulence factor alpha toxin. Sci. Rep. 2016;6:35466. doi: 10.1038/srep35466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Galluzzi L., Pietrocola F., Levine B., Kroemer G. Metabolic control of autophagy. Cell. 2014;159:1263–1276. doi: 10.1016/j.cell.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.López de Armentia M.M., Amaya C., Colombo M.I. Rab GTPases and the Autophagy Pathway: Bacterial Targets for a Suitable Biogenesis and Trafficking of Their Own Vacuoles. Cells. 2016;5:11. doi: 10.3390/cells5010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wang M., Fan Z., Han H. Autophagy in Staphylococcus aureus Infection. Front. Cell. Infect. Microbiol. 2021;11:750222. doi: 10.3389/fcimb.2021.750222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Schnaith A., Kashkar H., Leggio S.A., Addicks K., Krönke M., Krut O. Staphylococcus aureus subvert autophagy for induction of caspase-independent host cell death. J. Biol. Chem. 2007;282:2695–2706. doi: 10.1074/jbc.M609784200. [DOI] [PubMed] [Google Scholar]
- 267.Fang L., Wu H.M., Ding P.S., Liu R.Y. TLR2 mediates phagocytosis and autophagy through JNK signaling pathway in Staphylococcus aureus-stimulated RAW264.7 cells. Cell. Signal. 2014;26:806–814. doi: 10.1016/j.cellsig.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 268.Li S., Fang L., Wang J., Liu R. TLR2 modulates Staphylococcus aureus-induced inflammatory response and autophagy in macrophages through PI3K signaling pathway. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2017;33:1160–1164. [PubMed] [Google Scholar]
- 269.Geng N., Wang X., Yu X., Wang R., Zhu Y., Zhang M., Liu J., Liu Y. Staphylococcus aureus Avoids Autophagy Clearance of Bovine Mammary Epithelial Cells by Impairing Lysosomal Function. Front. Immunol. 2020;11:746. doi: 10.3389/fimmu.2020.00746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.O’Keeffe K.M., Wilk M.M., Leech J.M., Murphy A.G., Laabei M., Monk I.R., Massey R.C., Lindsay J.A., Foster T.J., Geoghegan J.A., et al. Manipulation of Autophagy in Phagocytes Facilitates Staphylococcus aureus Bloodstream Infection. Infect. Immun. 2015;83:3445–3457. doi: 10.1128/IAI.00358-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Soong G., Paulino F., Wachtel S., Parker D., Wickersham M., Zhang D., Brown A., Lauren C., Dowd M., West E., et al. Methicillin-resistant Staphylococcus aureus adaptation to human keratinocytes. mBio. 2015;6:e00289-15. doi: 10.1128/mBio.00289-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Tang D., Kroemer G. Ferroptosis. Curr. Biol. 2020;30:R1292–R1297. doi: 10.1016/j.cub.2020.09.068. [DOI] [PubMed] [Google Scholar]
- 273.Roos D. Chronic granulomatous disease. Br. Med. Bull. 2016;118:50–63. doi: 10.1093/bmb/ldw009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Buvelot H., Posfay-Barbe K.M., Linder P., Schrenzel J., Krause K.H. Staphylococcus aureus, phagocyte NADPH oxidase and chronic granulomatous disease. FEMS Microbiol. Rev. 2017;41:139–157. doi: 10.1093/femsre/fuw042. [DOI] [PubMed] [Google Scholar]
- 275.Talpur R., Bassett R., Duvic M. Prevalence and treatment of Staphylococcus aureus colonization in patients with mycosis fungoides and Sézary syndrome. Br. J. Dermatol. 2008;159:105–112. doi: 10.1111/j.1365-2133.2008.08612.x. [DOI] [PubMed] [Google Scholar]
- 276.Kahlson M.A., Dixon S.J. Copper-induced cell death. Science. 2022;375:1231–1232. doi: 10.1126/science.abo3959. [DOI] [PubMed] [Google Scholar]
- 277.Tsvetkov P., Coy S., Petrova B., Dreishpoon M., Verma A., Abdusamad M., Rossen J., Joesch-Cohen L., Humeidi R., Spangler R.D., et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. 2022;375:1254–1261. doi: 10.1126/science.abf0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Chatterjee I., Neumayer D., Herrmann M. Senescence of staphylococci: Using functional genomics to unravel the roles of ClpC ATPase during late stationary phase. Int. J. Med. Microbiol. 2010;300:130–136. doi: 10.1016/j.ijmm.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 279.Chatterjee I., Becker P., Grundmeier M., Bischoff M., Somerville G.A., Peters G., Sinha B., Harraghy N., Proctor R.A., Herrmann M. Staphylococcus aureus ClpC is required for stress resistance, aconitase activity, growth recovery, and death. J. Bacteriol. 2005;187:4488–4496. doi: 10.1128/JB.187.13.4488-4496.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Weidinger S., Beck L.A., Bieber T., Kabashima K., Irvine A.D. Atopic dermatitis. Nat. Rev. Dis. Primers. 2018;4:18003. doi: 10.1038/s41572-018-0001-z. [DOI] [PubMed] [Google Scholar]
- 281.Kim H.S., Yosipovitch G. The Skin Microbiota and Itch: Is There a Link? J. Clin. Med. 2020;9:1190. doi: 10.3390/jcm9041190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Ceccarelli F., Perricone C., Olivieri G., Cipriano E., Spinelli F.R., Valesini G., Conti F. Staphylococcus aureus Nasal Carriage and Autoimmune Diseases: From Pathogenic Mechanisms to Disease Susceptibility and Phenotype. Int. J. Mol. Sci. 2019;20:5624. doi: 10.3390/ijms20225624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Hülpüsch C., Tremmel K., Hammel G., Bhattacharyya M., de Tomassi A., Nussbaumer T., Neumann A.U., Reiger M., Traidl-Hoffmann C. Skin pH-dependent Staphylococcus aureus abundance as predictor for increasing atopic dermatitis severity. Allergy. 2020;75:2888–2898. doi: 10.1111/all.14461. [DOI] [PubMed] [Google Scholar]
- 284.Strickland I., Hauk P.J., Trumble A.E., Picker L.J., Leung D.Y. Evidence for superantigen involvement in skin homing of T cells in atopic dermatitis. J. Investig. Dermatol. 1999;112:249–253. doi: 10.1046/j.1523-1747.1999.00502.x. [DOI] [PubMed] [Google Scholar]
- 285.Seiti Yamada Yoshikawa F., Feitosa de Lima J., Notomi Sato M., Álefe Leuzzi Ramos Y., Aoki V., Leao Orfali R. Exploring the Role of Staphylococcus Aureus Toxins in Atopic Dermatitis. Toxins. 2019;11:321. doi: 10.3390/toxins11060321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Ng C.Y., Huang Y.H., Chu C.F., Wu T.C., Liu S.H. Risks for Staphylococcus aureus colonization in patients with psoriasis: A systematic review and meta-analysis. Br. J. Dermatol. 2017;177:967–977. doi: 10.1111/bjd.15366. [DOI] [PubMed] [Google Scholar]
- 287.Baker B.S., Laman J.D., Powles A., van der Fits L., Voerman J.S., Melief M.J., Fry L. Peptidoglycan and peptidoglycan-specific Th1 cells in psoriatic skin lesions. J. Pathol. 2006;209:174–181. doi: 10.1002/path.1954. [DOI] [PubMed] [Google Scholar]
- 288.Yilmaz V., Yentür S.P., Saruhan-Direskeneli G. IL-12 and IL-10 polymorphisms and their effects on cytokine production. Cytokine. 2005;30:188–194. doi: 10.1016/j.cyto.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 289.Schünke H., Göbel U., Dikic I., Pasparakis M. OTULIN inhibits RIPK1-mediated keratinocyte necroptosis to prevent skin inflammation in mice. Nat. Commun. 2021;12:5912. doi: 10.1038/s41467-021-25945-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Teufelberger A.R., Bröker B.M., Krysko D.V., Bachert C., Krysko O. Staphylococcus aureus Orchestrates Type 2 Airway Diseases. Trends Mol. Med. 2019;25:696–707. doi: 10.1016/j.molmed.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 291.Krysko O., Teufelberger A., Van Nevel S., Krysko D.V., Bachert C. Protease/antiprotease network in allergy: The role of Staphylococcus aureus protease-like proteins. Allergy. 2019;74:2077–2086. doi: 10.1111/all.13783. [DOI] [PubMed] [Google Scholar]
- 292.Poddighe D., Vangelista L. Staphylococcus aureus Infection and Persistence in Chronic Rhinosinusitis: Focus on Leukocidin ED. Toxins. 2020;12:678. doi: 10.3390/toxins12110678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Xie Y., Li M., Chen K., Zhu H., Tang M., Zhou C., Zheng Y., Wen J., Han M., Zhang J., et al. Necroptosis Underlies Neutrophilic Inflammation Associated with the Chronic Rhinosinusitis with Nasal Polyps (CRSwNP) J. Inflamm. Res. 2021;14:3969–3983. doi: 10.2147/JIR.S322875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Wang B.F., Cao P.P., Wang Z.C., Li Z.Y., Wang Z.Z., Ma J., Liao B., Deng Y.K., Long X.B., Xu K., et al. Interferon-γ-induced insufficient autophagy contributes to p62-dependent apoptosis of epithelial cells in chronic rhinosinusitis with nasal polyps. Allergy. 2017;72:1384–1397. doi: 10.1111/all.13153. [DOI] [PubMed] [Google Scholar]
- 295.Morgene M.F., Botelho-Nevers E., Grattard F., Pillet S., Berthelot P., Pozzetto B., Verhoeven P.O. Staphylococcus aureus colonization and non-influenza respiratory viruses: Interactions and synergism mechanisms. Virulence. 2018;9:1354–1363. doi: 10.1080/21505594.2018.1504561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Cigana C., Bianconi I., Baldan R., De Simone M., Riva C., Sipione B., Rossi G., Cirillo D.M., Bragonzi A. Staphylococcus aureus Impacts Pseudomonas aeruginosa Chronic Respiratory Disease in Murine Models. J. Infect. Dis. 2018;217:933–942. doi: 10.1093/infdis/jix621. [DOI] [PubMed] [Google Scholar]
- 297.Stelzner K., Boyny A., Hertlein T., Sroka A., Moldovan A., Paprotka K., Kessie D., Mehling H., Potempa J., Ohlsen K., et al. Intracellular Staphylococcus aureus employs the cysteine protease staphopain A to induce host cell death in epithelial cells. PLoS Pathog. 2021;17:e1009874. doi: 10.1371/journal.ppat.1009874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Goerke C., Wolz C. Adaptation of Staphylococcus aureus to the cystic fibrosis lung. Int. J. Med. Microbiol. 2010;300:520–525. doi: 10.1016/j.ijmm.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 299.Luciani A., Villella V.R., Esposito S., Brunetti-Pierri N., Medina D., Settembre C., Gavina M., Pulze L., Giardino I., Pettoello-Mantovani M., et al. Defective CFTR induces aggresome formation and lung inflammation in cystic fibrosis through ROS-mediated autophagy inhibition. Nat. Cell Biol. 2010;12:863–875. doi: 10.1038/ncb2090. [DOI] [PubMed] [Google Scholar]
- 300.Luciani A., Villella V.R., Esposito S., Brunetti-Pierri N., Medina D.L., Settembre C., Gavina M., Raia V., Ballabio A., Maiuri L. Cystic fibrosis: A disorder with defective autophagy. Autophagy. 2011;7:104–106. doi: 10.4161/auto.7.1.13987. [DOI] [PubMed] [Google Scholar]
- 301.Edwards L.A., O’Neill C., Furman M.A., Hicks S., Torrente F., Pérez-Machado M., Wellington E.M., Phillips A.D., Murch S.H. Enterotoxin-producing staphylococci cause intestinal inflammation by a combination of direct epithelial cytopathy and superantigen-mediated T-cell activation. Inflamm. Bowel Dis. 2012;18:624–640. doi: 10.1002/ibd.21852. [DOI] [PubMed] [Google Scholar]
- 302.Stelzner K., Winkler A.C., Liang C., Boyny A., Ade C.P., Dandekar T., Fraunholz M.J., Rudel T. Intracellular Staphylococcus aureus Perturbs the Host Cell Ca(2+) Homeostasis To Promote Cell Death. mBio. 2020;11:e02250-20. doi: 10.1128/mBio.02250-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Argudín M., Mendoza M.C., Rodicio M.R. Food poisoning and Staphylococcus aureus enterotoxins. Toxins. 2010;2:1751–1773. doi: 10.3390/toxins2071751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Fisher E.L., Otto M., Cheung G.Y.C. Basis of Virulence in Enterotoxin-Mediated Staphylococcal Food Poisoning. Front. Microbiol. 2018;9:436. doi: 10.3389/fmicb.2018.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Palmer E.D. The morphologic consequences of acute exogenous (Staphylococcic) gastroenteritis on the gastric mucosa. Gastroenterology. 1951;19:462–475. doi: 10.1016/S0016-5085(19)36408-X. [DOI] [PubMed] [Google Scholar]
- 306.Alber G., Scheuber P.H., Reck B., Sailer-Kramer B., Hartmann A., Hammer D.K. Role of substance P in immediate-type skin reactions induced by staphylococcal enterotoxin B in unsensitized monkeys. J. Allergy Clin. Immunol. 1989;84:880–885. doi: 10.1016/0091-6749(89)90383-7. [DOI] [PubMed] [Google Scholar]
- 307.Jett M., Brinkley W., Neill R., Gemski P., Hunt R. Staphylococcus aureus enterotoxin B challenge of monkeys: Correlation of plasma levels of arachidonic acid cascade products with occurrence of illness. Infect. Immun. 1990;58:3494–3499. doi: 10.1128/iai.58.11.3494-3499.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Scheuber P.H., Golecki J.R., Kickhöfen B., Scheel D., Beck G., Hammer D.K. Skin reactivity of unsensitized monkeys upon challenge with staphylococcal enterotoxin B: A new approach for investigating the site of toxin action. Infect. Immun. 1985;50:869–876. doi: 10.1128/iai.50.3.869-876.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Holmberg S.D., Blake P.A. Staphylococcal food poisoning in the United States. New facts and old misconceptions. JAMA. 1984;251:487–489. doi: 10.1001/jama.1984.03340280037024. [DOI] [PubMed] [Google Scholar]
- 310.Pérez-Bosque A., Moretó M. A rat model of mild intestinal inflammation induced by Staphylococcus aureus enterotoxin B. Proc. Nutr. Soc. 2010;69:447–453. doi: 10.1017/S0029665110001849. [DOI] [PubMed] [Google Scholar]
- 311.Kadariya J., Smith T.C., Thapaliya D. Staphylococcus aureus and staphylococcal food-borne disease: An ongoing challenge in public health. BioMed Res. Int. 2014;2014:827965. doi: 10.1155/2014/827965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Elahi S., Fujikawa H. Effects of Lactic Acid and Salt on Enterotoxin A Production and Growth of Staphylococcus aureus. J. Food Sci. 2019;84:3233–3240. doi: 10.1111/1750-3841.14829. [DOI] [PubMed] [Google Scholar]
- 313.Anderson C.J., Medina C.B., Barron B.J., Karvelyte L., Aaes T.L., Lambertz I., Perry J.S.A., Mehrotra P., Gonçalves A., Lemeire K., et al. Microbes exploit death-induced nutrient release by gut epithelial cells. Nature. 2021;596:262–267. doi: 10.1038/s41586-021-03785-9. [DOI] [PubMed] [Google Scholar]
- 314.Fuchs T.A., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V., Weinrauch Y., Brinkmann V., Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Ooi J.D., Jiang J.H., Eggenhuizen P.J., Chua L.L., van Timmeren M., Loh K.L., O’Sullivan K.M., Gan P.Y., Zhong Y., Tsyganov K., et al. A plasmid-encoded peptide from Staphylococcus aureus induces anti-myeloperoxidase nephritogenic autoimmunity. Nat. Commun. 2019;10:3392. doi: 10.1038/s41467-019-11255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Farinelli G., Jofra Hernandez R., Rossi A., Ranucci S., Sanvito F., Migliavacca M., Brombin C., Pramov A., Di Serio C., Bovolenta C., et al. Lentiviral Vector Gene Therapy Protects XCGD Mice from Acute Staphylococcus aureus Pneumonia and Inflammatory Response. Mol. Ther. 2016;24:1873–1880. doi: 10.1038/mt.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Harfi I., D’Hondt S., Sariban E. iPLA(2) Activation Mediates Granular Exocytosis and Corrects Microbicidal Defects in ROS-Deficient and CGD Human Neutrophils. J. Clin. Immunol. 2019;39:486–493. doi: 10.1007/s10875-019-00630-7. [DOI] [PubMed] [Google Scholar]
- 318.Gao Y., Chen Y., Cao Y., Mo A., Peng Q. Potentials of nanotechnology in treatment of methicillin-resistant Staphylococcus aureus. Eur. J. Med. Chem. 2021;213:113056. doi: 10.1016/j.ejmech.2020.113056. [DOI] [PubMed] [Google Scholar]
- 319.Peacock S.J., Paterson G.K. Mechanisms of Methicillin Resistance in Staphylococcus aureus. Annu. Rev. Biochem. 2015;84:577–601. doi: 10.1146/annurev-biochem-060614-034516. [DOI] [PubMed] [Google Scholar]
- 320.Lee A.S., de Lencastre H., Garau J., Kluytmans J., Malhotra-Kumar S., Peschel A., Harbarth S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers. 2018;4:18033. doi: 10.1038/nrdp.2018.33. [DOI] [PubMed] [Google Scholar]
- 321.García-Fernández E., Koch G., Wagner R.M., Fekete A., Stengel S.T., Schneider J., Mielich-Süss B., Geibel S., Markert S.M., Stigloher C., et al. Membrane Microdomain Disassembly Inhibits MRSA Antibiotic Resistance. Cell. 2017;171:1354–1367.e20. doi: 10.1016/j.cell.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Hassoun A., Linden P.K., Friedman B. Incidence, prevalence, and management of MRSA bacteremia across patient populations-a review of recent developments in MRSA management and treatment. Crit. Care. 2017;21:211. doi: 10.1186/s13054-017-1801-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Zhang S., Huang X., Xiu H., Zhang Z., Zhang K., Cai J., Cai Z., Chen Z., Zhang Z., Cui W., et al. The attenuation of Th1 and Th17 responses via autophagy protects against methicillin-resistant Staphylococcus aureus-induced sepsis. Microbes Infect. 2021;23:104833. doi: 10.1016/j.micinf.2021.104833. [DOI] [PubMed] [Google Scholar]
- 324.Chang A.L., Ulrich A., Suliman H.B., Piantadosi C.A. Redox regulation of mitophagy in the lung during murine Staphylococcus aureus sepsis. Free Radic. Biol. Med. 2015;78:179–189. doi: 10.1016/j.freeradbiomed.2014.10.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Myles I.A., Fontecilla N.M., Valdez P.A., Vithayathil P.J., Naik S., Belkaid Y., Ouyang W., Datta S.K. Signaling via the IL-20 receptor inhibits cutaneous production of IL-1β and IL-17A to promote infection with methicillin-resistant Staphylococcus aureus. Nat. Immunol. 2013;14:804–811. doi: 10.1038/ni.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Singh N., Armstrong D.G., Lipsky B.A. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 327.Gemechu F.W., Seemant F., Curley C.A. Diabetic foot infections. Am. Fam. Physician. 2013;88:177–184. [PubMed] [Google Scholar]
- 328.Lavery L.A., Fontaine J.L., Bhavan K., Kim P.J., Williams J.R., Hunt N.A. Risk factors for methicillin-resistant Staphylococcus aureus in diabetic foot infections. Diabet. Foot Ankle. 2014;5:23575. doi: 10.3402/dfa.v5.23575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Taha A.B. Relationship and susceptibility profile of Staphylococcus aureus infection diabetic foot ulcers with Staphylococcus aureus nasal carriage. Foot. 2013;23:11–16. doi: 10.1016/j.foot.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 330.Lipsky B.A., Berendt A.R., Deery H.G., Embil J.M., Joseph W.S., Karchmer A.W., LeFrock J.L., Lew D.P., Mader J.T., Norden C., et al. Diagnosis and treatment of diabetic foot infections. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2004;39:885–910. doi: 10.1086/424846. [DOI] [PubMed] [Google Scholar]
- 331.Sotto A., Lina G., Richard J.L., Combescure C., Bourg G., Vidal L., Jourdan N., Etienne J., Lavigne J.P. Virulence potential of Staphylococcus aureus strains isolated from diabetic foot ulcers: A new paradigm. Diabetes Care. 2008;31:2318–2324. doi: 10.2337/dc08-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Munro P., Benchetrit M., Nahori M.A., Stefani C., Clément R., Michiels J.F., Landraud L., Dussurget O., Lemichez E. The Staphylococcus aureus epidermal cell differentiation inhibitor toxin promotes formation of infection foci in a mouse model of bacteremia. Infect. Immun. 2010;78:3404–3411. doi: 10.1128/IAI.00319-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Messad N., Landraud L., Canivet B., Lina G., Richard J.L., Sotto A., Lavigne J.P., Lemichez E. Distribution of edin in Staphylococcus aureus isolated from diabetic foot ulcers. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2013;19:875–880. doi: 10.1111/1469-0691.12084. [DOI] [PubMed] [Google Scholar]
- 334.Bader M.S. Diabetic foot infection. Am. Fam. Physician. 2008;78:71–79. [PubMed] [Google Scholar]
- 335.Uçkay I., Kressmann B., Malacarne S., Toumanova A., Jaafar J., Lew D., Lipsky B.A. A randomized, controlled study to investigate the efficacy and safety of a topical gentamicin-collagen sponge in combination with systemic antibiotic therapy in diabetic patients with a moderate or severe foot ulcer infection. BMC Infect. Dis. 2018;18:361. doi: 10.1186/s12879-018-3253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Binda E., Marinelli F., Marcone G.L. Old and New Glycopeptide Antibiotics: Action and Resistance. Antibiotics. 2014;3:572–594. doi: 10.3390/antibiotics3040572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Mottola C., Matias C.S., Mendes J.J., Melo-Cristino J., Tavares L., Cavaco-Silva P., Oliveira M. Susceptibility patterns of Staphylococcus aureus biofilms in diabetic foot infections. BMC Microbiol. 2016;16:119. doi: 10.1186/s12866-016-0737-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Batoni G., Maisetta G., Esin S. Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochim. Biophys. Acta. 2016;1858:1044–1060. doi: 10.1016/j.bbamem.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 339.Santos R., Gomes D., Macedo H., Barros D., Tibério C., Veiga A.S., Tavares L., Castanho M., Oliveira M. Guar gum as a new antimicrobial peptide delivery system against diabetic foot ulcers Staphylococcus aureus isolates. J. Med. Microbiol. 2016;65:1092–1099. doi: 10.1099/jmm.0.000329. [DOI] [PubMed] [Google Scholar]
- 340.Touzel R.E., Sutton J.M., Wand M.E. Establishment of a multi-species biofilm model to evaluate chlorhexidine efficacy. J. Hosp. Infect. 2016;92:154–160. doi: 10.1016/j.jhin.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 341.Bonez P.C., Dos Santos Alves C.F., Dalmolin T.V., Agertt V.A., Mizdal C.R., Flores Vda C., Marques J.B., Santos R.C., Anraku de Campos M.M. Chlorhexidine activity against bacterial biofilms. Am. J. Infect. Control. 2013;41:e119–e122. doi: 10.1016/j.ajic.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 342.Santos R., Ruza D., Cunha E., Tavares L., Oliveira M. Diabetic foot infections: Application of a nisin-biogel to complement the activity of conventional antibiotics and antiseptics against Staphylococcus aureus biofilms. PLoS ONE. 2019;14:e0220000. doi: 10.1371/journal.pone.0220000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Zenão S., Aires A., Dias C., Saavedra M.J., Fernandes C. Antibacterial potential of Urtica dioica and Lavandula angustifolia extracts against methicillin resistant Staphylococcus aureus isolated from diabetic foot ulcers. J. Herb. Med. 2017;10:53–58. doi: 10.1016/j.hermed.2017.05.003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.