Abstract
Alkene aminoarylation with a single, bifunctional reagent is a concise synthetic strategy. Despite the synthetic brevity of aminoarylation, combined with opportunities for stereoselective alkene functionalization, few methodologies have deviated from an intramolecular aminometallation-arylation reaction design. To contrast this paradigm, we report a protocol utilizing photoredox catalysis for the aminoarylation of electron-rich alkenes with arylsulfonylacetamides. This process is understood to operate through chemo- and regioselective alkene radical cation trapping with nucleophilic arylsulfonylacetamides, wherein a subsequent Smiles-Truce reaction transfers a variety of aryl groups in high diastereoselectivity. As this process is driven by visible light, employs readily-available starting materials, and demonstrates convergent synthesis, it is well-suited to impact a variety of synthetic endeavors.
One Sentence Summary:
Photoredox catalysis activates arylsulfonylacetamides to provide both the arene and amine for alkene difunctionalization.
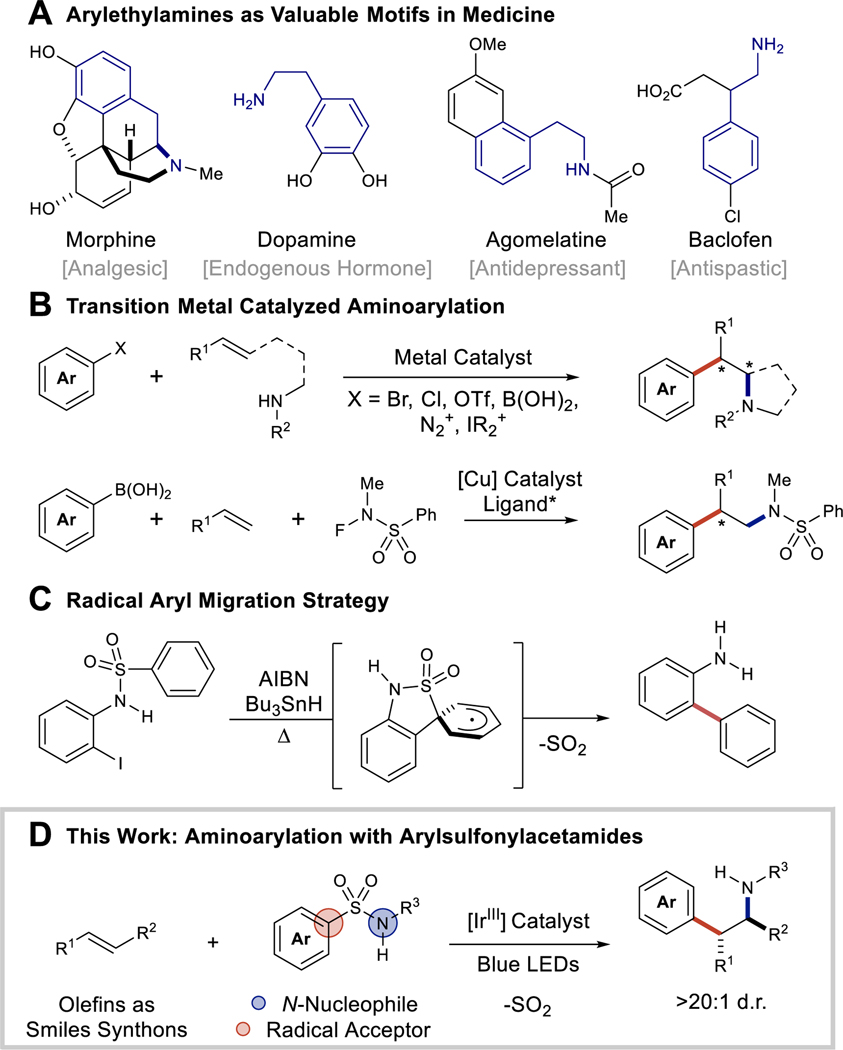
The arylethylamine motif is conserved in dopamine, serotonin, and many opioid receptor drugs responsible for modulating pain sensation and treating neurobehavioral disorders (Figure 1A) (1,2). In light of the opioid epidemic, the climate surrounding opioid pain medications is conflicted. It is noteworthy that frontline medications treating opioid addiction contain such arylethylamine substructures (naltrexone and buprenorphine) (3–5). With this rationale, continued drug development in the arylethylamine chemical space is necessary for general hit-to-lead exploration and the discovery of new and safer medicines. Conventional methods to synthesize arylethylamines employ multi-step homologation and reductive amination sequences. Alternatively, alkene aminoarylation, particularly of anethole and other biomass-derived alkenes, allows for direct access to this medicinally desirable functionality. Development of methodologies to rapidly construct two new bonds (C‒C and C–N) in a single operation from feedstock chemicals can improve and expedite the discovery of new arylethylamine-based small molecule therapeutics.
Figure 1. Strategies to access arylethylamines.
(A) Arylethylamines as valuable motifs. (B) Current approaches toward arylethylamines with transition-metal catalysis. (C) The Smiles-Truce rearrangement as an aryl migration strategy. (D) The method proposed herein. Me, methyl; Ph, phenyl; AIBN, azobisisobutyronitrile; HSnBu3, tributyltin hydride.
Alkene aminoarylation has been demonstrated with palladium (6,7), copper (8–10), nickel (11,12), and gold (13), in which alkenes are activated by the transition metal to facilitate a stereoselective amine cyclization, followed by a two-electron metal-mediated arylation event (Figure 1B). The metals used in these aminoarylation platforms control stereoselectivity and activate the alkene for reactivity, while suppressing proto-demetallation or β-hydride elimination pathways that hinder desired C–C bond formation. Amides and amines are more nucleophilic than the alkene coupling partner; thus, elevated temperatures are often necessary to facilitate ligand substitution to unite the reactants in the initial amination event (14). Despite robust investigation, these methods are generally limited by the need for directing groups and intramolecular reaction designs which restrict the products to pyrrolidine and piperidine structures. Recently transformations effecting intermolecular aminoarylation and carboamination have been accomplished in which the alkene is decoupled from the arylation and amination reagents. In one case, Lin and Liu demonstrated an enantioselective copper(I) catalyzed aminoarylation of vinyl arenes relying upon pre-oxidized sulfonamide reactants (N-fluoro-N-methylbenzenesulfonamide) (Figure 1B) (9). Separately, Rovis and Piou demonstrated an intermolecular carboamination using N-enoxyphthalimides and Rh(III) catalysis (15).
Photocatalysis and radical-based chemistry has proven similarly influential in alkene difunctionalization. The simplest strategy is Meerwein aminoarylation, a Markovnikov-selective reaction that begins with the reductive generation of a radical from a suitable precursor (arene diazonium salt or diaryliodonium salt) followed by radical-polar crossover and carbocation trapping with acetonitrile solvent (16). These reactions are regioselective, but devoid of stereoselectivity. Photocatalytic anti-Markovnikov selective alkene hydro- and carboamination reactions have been recently demonstrated by both Knowles (17–21) and Nicewicz (22–24). These approaches represent contrasting C–N bond formation strategies, while employing a common catalytic cycle. Knowles et. al. has demonstrated both aminium radical cation and amidyl radical generation for the addition to olefins. In both cases, nitrogen centered radicals couple with π-systems to generate β-amino radicals that are rapidly trapped with a H-atom transfer reagent. Successful H-atom transfer reagents are minimally nucleophilic to prevent thiol-ene reactivity. Nitrogen radical based chemistry is particularly challenging as both alkene addition and allylic H-atom abstraction are kinetically competitive processes (25), thus excesses of the alkene component or intramolecular amino-cyclization is often necessary for success. Additionally, amine and amide oxidation generates a more reactive, but no more nucleophilic nitrogen atom. In contrast, Nicewicz et. al. has targeted alkene single electron oxidation, a similarly rapid process to amide or amine oxidation. This approach benefits from converting the alkene to a more electrophilic species in solution, necessitating lower equivalents of the nitrogen nucleophile to conduct alkene difunctionalization.
To contrast the widely investigated field of transition metal-mediated aminoarylation and build upon the successes of photocatalytic alkene difunctionalization chemistry, we were inspired by the possibility of a radical Smiles-Truce rearrangement to provide alkene aminoarylation products in a diastereoselective fashion. Traditionally, the Truce variant of the Smiles rearrangement is a nucleophilic aromatic substitution effected by benzylic lithiation of ortho-tolyl-arylsulfones (26). The rearrangement is more broadly applicable to ipso-substitution reactions with aryl sulfides, sulfoxides, sulfones, and amides. Pennell and Motherwell furthered the utility of this transformation by demonstrating that aryl radicals are also capable of the same arene transposition (27) (Figure 1C). Although there are numerous intramolecular examples of radical Smiles-Truce reactions (28–33), many of these reactions employ net reductive conditions, generate a stoichiometric amount of waste, and rely upon a substrate design that tethers the radical precursor to the aryl-sulfonate derivative. Realizing this intramolecular tether can be formed via in situ oxidation of an alkene and subsequent nucleophilic trapping with an arylsulfonylacetamide (34), we sought to design a photocatalyzed radical Smiles-Truce reaction which showcases the utility of arylsulfonylacetamides as capable reagents for both C–N and C–C bond formation in aminoarylation (Figure 1D).
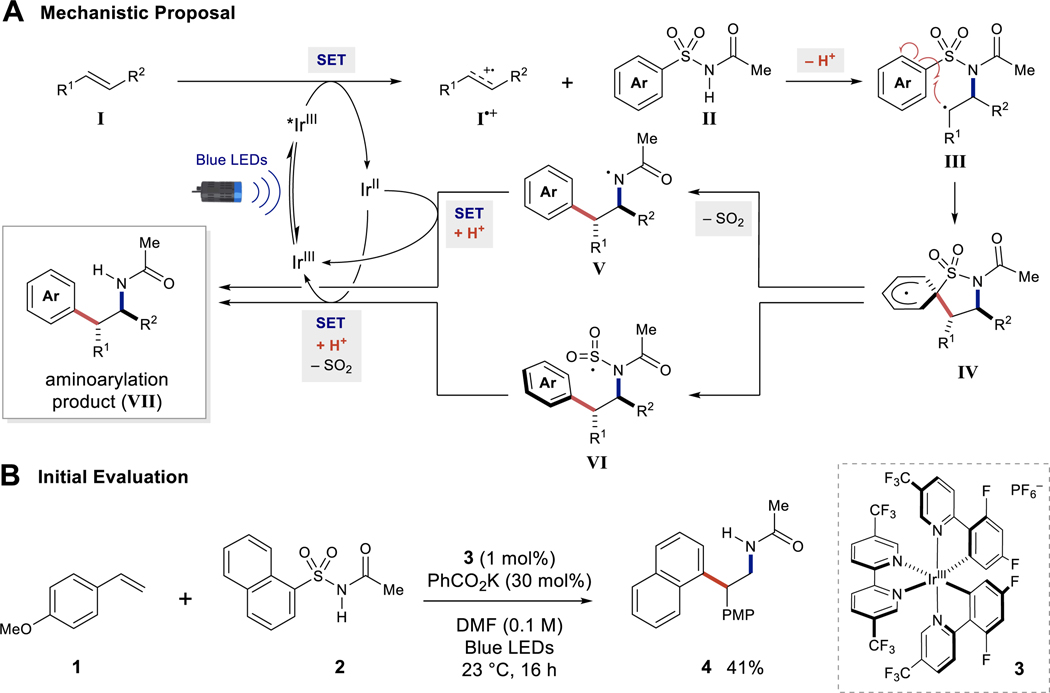
A general catalytic cycle was postulated to begin with an oxidation event between a photoexcited catalyst (*IrIII) and an alkene (I) (Figure 2A) (23,24). Single electron oxidation of the alkene would enable nucleophilic addition of an arylsulfonylacetamide (II) to afford the desired β–alkyl radical intermediate (III) (35–37). This radical is poised for regioselective cyclization onto the ipso-position of the appended arene to generate IV (38). Lastly, an entropically-favored desulfonylation can proceed via two plausible pathways to generate the aminoarylation product (VII): rapid radical desulfonylation from IV to generate nitrogen-centered radical V followed by catalyst turnover; or homolytic fragmentation of the CAr–S bond to furnish VI, which can turnover the catalyst and undergo desulfonylation to VII. Exploiting both the electronic activation of the sulfonylated arene unit and tunable nucleophilicity of the nitrogen motif allows for this photoredox catalysis platform to promote both the C–N and C–C bond forming events with arylsulfonylacetamides.
Figure 2. Proposed reaction design for aminoarylation with arylsulfonylacetamides.
(A) Proposed reaction mechanism. (B) Initial reaction evaluation. PMP, p-methoxyphenyl; Me, methyl; Ph, phenyl.
To realize the proposed aminoarylation reactivity, reaction optimization was first conducted with vinyl anisole (1) (Ep/2=1.6 V vs SCE) (39) and 1-naphthylsulfonylacetamide (2) (Table S1). A potent photooxidant, [Ir(dF(CF3ppy)2)(5,5’-CF3-bpy)]PF6 (3) (IrII/III*=1.68 V vs SCE in MeCN) (40) was initially selected for alkene radical cation formation (Figure 2B). Early optimization experiments lent evidence to the chemoselectivity of this reaction; excess loading of arylsulfonylacetamide and base were unnecessary (Table S1). Nearly equivalent stoichiometry between 1 and 2 afforded the highest yield for the optimization product 4. A base screen revealed potassium acetate, benzoate, and tribasic phosphate as superior bases to the less basic potassium trifluoroacetate and potassium phosphate (mono- or di-basic). The reaction was incompatible with pyridine, or stronger alkoxide bases, as photocatalyst decomposition was observed. Reaction dilution past 0.1 M slowed the rate of product formation, while reaction concentrations greater than 0.1 M inhibited product formation. Further optimization proved that less oxidizing photocatalysts such as [Ru(bpy)3]Cl2 (RuI/II* = 0.77 V vs SCE in MeCN), [Ir(dF(CF3ppy)2)(dtbbpy)]PF6 (IrII/III* = 0.89 V vs SCE in MeCN), [Ir(ppy)2(dtbbpy)]PF6 (IrII/III* = 0.31 V vs SCE in MeCN) (41,47), were unable to catalyze this transformation. Employment of Fukuzumi’s catalyst (PC•/PC* = 1.88 V vs SCE in MeCN) (42) did produce 4 in 13% yield. Finally, H-atom donor additives such as 1,4-cyclohexadiene and isopropanol did not improve upon the established conditions for the optimization product 4. Exclusion of either light or photocatalyst failed to promote aminoarylation (Table S1). With the proof-of-concept established, we identified the acyl group, among a range of amides and carbamates, as the optimal activating group for the sulfonamide reagent in this transformation (Figure 3, 4–7). We reasoned that the acidity and the steric encumbrance of the sulfonamide activating group control the nucleophilicity of the arylsulfonylacetamide.
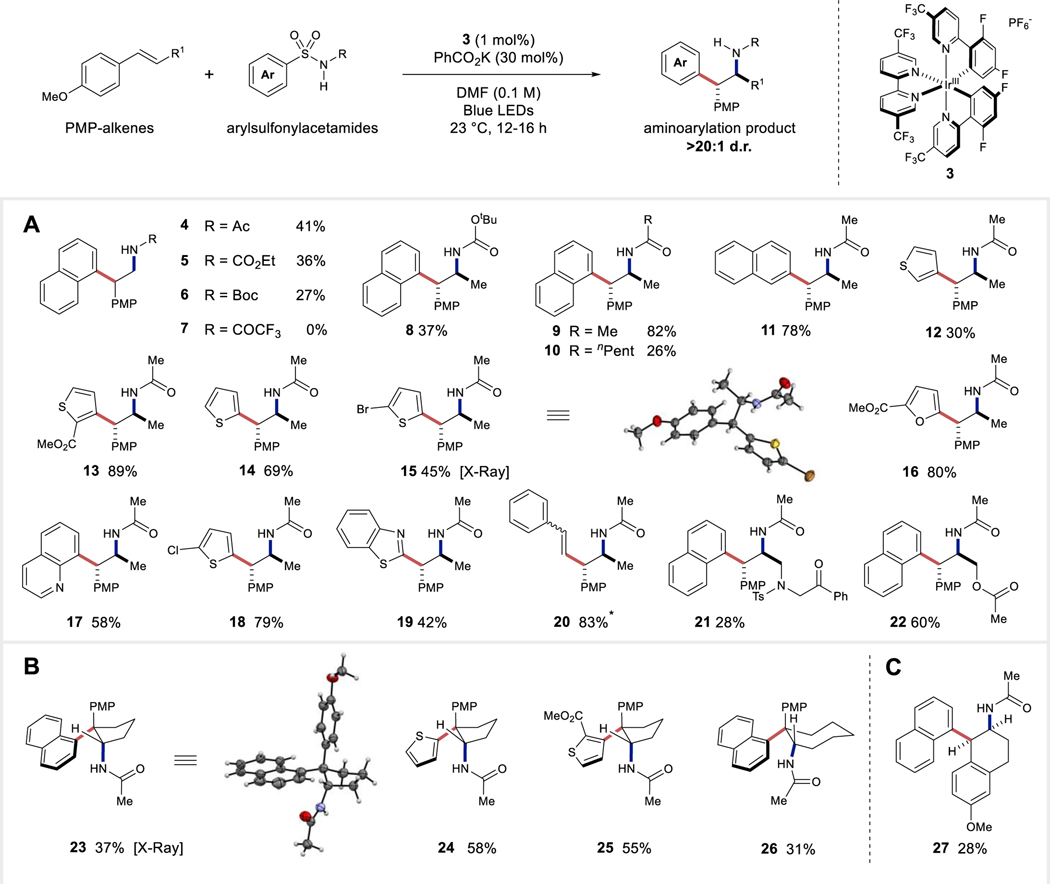
Figure 3. Exploration of substrate scope.
All yields are isolated yields. Relative configurations of products were assigned by analogy to 15 and 23. (A) Evaluation of scope of aryl group. (B) Scope of cyclic trans-PMP alkenes. (C) Scope of a compatible cyclic cis-PMP alkene. PMP, p-methoxyphenyl; Ac, acetyl; Boc, tert-butyloxycarbonyl; Me, methyl. nPen, pentyl; Ph, phenyl, Ts, tosyl. *2:1 mixture of E/Z alkene diastereomers.
A substantial increase in aminoarylation was observed when using 1,2-disubstituted p-methoxyphenyl alkenes in comparison to 1 (Figure 3A). This substitution allowed us to realize the aryl transfer of several groups including 1-naphthyl (4–6, 8–10, 21, 22), 2-napthyl (11), 3-thiophenyl (12, 13), 2-thiophenyl (14, 15, 18), 2- furanyl (16), 8-quinolino (17), 2-benzothiazole (19), and β-styrene (20) all in greater than 20:1 diastereoselectivity. X-Ray crystallographic analysis of 15 was found to show a syn-configuration between the 5-bromothiophene and the acetamide groups supporting the stereochemical assignment. Use of cyclic (E)-alkenes allowed for the synthesis of cyclic arylethylamines (23–26) containing two contiguous stereocenters, one of which is quaternary (Figure 3B). Furthermore, the cis-diastereomer 27 can be formed when a cyclic (Z)-alkene is used as the oxidizable alkene substrate partner (Figure 3C). Preparation of arylethylamine 21 containing an N-tosyl amide showcases the chemoselective nature of this aminoarylation, while the successful isolation of 22 suggests that nucleophiles tethered to the alkene are well tolerated under the reaction conditions. The current aminoarylation conditions are not amenable to benzenesulfonylacetamides, likely due to the increased enthalpic barrier for dearomatization during the initial radical cyclization (Figure S3).
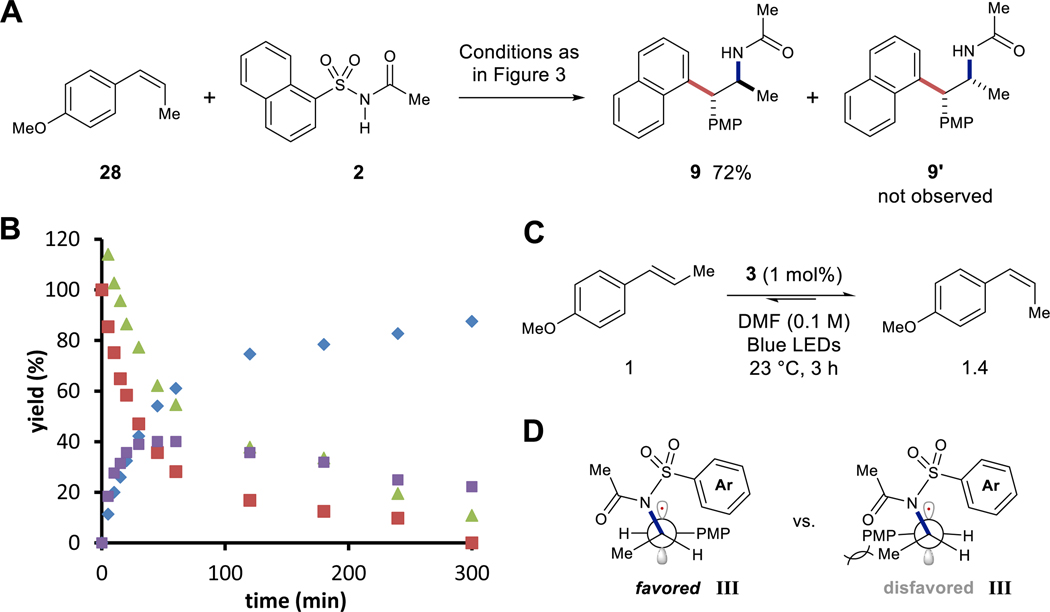
To provide mechanistic insight, several studies were carried out to understand the efficiency and high diastereoselectivity of this transformation. We hypothesized that both acyclic (Z)- and (E)-alkenes would convert to the same trans-aminoarylation diastereomer due to bond rotation outcompeting cyclization of intermediate III. Notably, performing the titleaminoarylation with (Z)-anethole afforded a nearly identical yield of 9 (72%), in comparison to (E)-anethole (82%), while diastereomer 9’ was not observed (Figure 4A). Reaction progress analysis by 1H-NMR spectroscopy of (Z)-anethole aminoarylation reveals that (E)-anethole is generated during the reaction (Figure 4B). Based on this observation, we examined the rates of isomerization for each anethole isomer to the photostationary state (Figure 4C). This revealed a photostationary state of 1.4:1 (Z:E), with the initial rate of (Z)-anethole isomerization being much faster than (E)-anethole isomerization (Figure S4, S8-S10) (43). Furthermore, initial rate analysis of aminoarylation shows alkene consumption to be slower (Figure S6) than (Z)-anethole isomerization (Figure S8, S9). These data suggest the diastereoselectivity arises from either, but not exclusively, a kinetically favored generation of (E)-anethole radical cation and subsequent aminoarylation; or a thermodynamic preference of radical intermediate III to adopt an anti-periplanar conformation between the para-methoxyphenyl (PMP) and methyl substituents prior to cyclization (Figure 4D). One other competing possibility is that (E)-anethole radical cation reacts with 2 faster than (Z)-anethole radical cation. Overall, these mechanistic details describe how the combination of a Smiles-Truce aryl transfer and radical cation chemistry can be combined into a highly diastereoconvergent alkene aminoarylation.
Figure 4. Experiments to probe reaction mechanism.
(A) Aminoarylation with (Z)-anethole (28). (B) Tracking reaction progress for aminoarylation with (Z)-anethole (28) (▲ = 2, ■ = (Z)-anethole, ♦ = 9, ■ = (E)-anethole). (C) Determination of the photostationary state for anethole isomers catalyzed by 3. (D) Favored and disfavored conformations for intermediate III.
In conclusion, given the current availability of sulfonamide building blocks along with the ubiquity of alkenes as feedstock substrates, we view the method to be a highly enabling platform for research efforts synthesizing the arylethylamine pharmacophore diastereoselectively in a single operation.
Supplementary Material
Acknowledgments:
The authors are grateful to Dr. Jeff W. Kampf for assistance with X-ray crystallographic analyses.
Funding:
The authors acknowledge the financial support for this research from the NIH NIGMS (R01-GM096129), the Camille Dreyfus Teacher-Scholar Award Program, and the University of Michigan. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship for RCM (Grant No DGE 1256260).
Footnotes
Competing interests: The authors declare no competing financial interests.
Data and materials availability: X-ray data for compounds 15 and 23 are available free of charge from the Cambridge Crystallographic Data Centre under CCDC 1572215 and 1572214 respectively.
References and Notes:
- 1.Dalley JW, Everitt BJ, Dopamine receptors in the learning, memory and drug reward circuitry. Semin. Cell Dev. Biol 20, 403–410 (1980). [DOI] [PubMed] [Google Scholar]
- 2.Zhang A, Neumeyer JL, Baldessarini RJ, Recent progress in development of dopamine receptor subtype-selective agents: potential therapeutics for neurological and psychiatric disorders. Chem. Rev 107, 274–302 (2007). [DOI] [PubMed] [Google Scholar]
- 3.National Institute on Drug Abuse. Effective Treatments for Opioid Addiction. https://www.drugabuse.gov/publications/effective-treatments-opioid-addiction/effective-treatments-opioid-addiction. (accessed June 13, 2018)
- 4.Spahn V, Del Vecchio G, Labuz D, Rodriguez-Gaztelumendi A, Massaly N, Temp J, Durmaz V, Sabri P, Reidelbach M, Machelska H, Weber M, Stein CA, A nontoxic pain killer designed by modeling of pathological receptor conformations. Science 355, 966–969 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Ding H, Czoty PW, Kiguchi N, Cami-Kobeci G, Sukhtankar DD, Nader MA, Husbands SM, Ko M-C, A novel orvinol analog, BU08028, as a safe opioid analgesic without abuse liability in primates. PNAS 113, E5511–E5518 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schultz DM, Wolfe JP, Recent developments in palladium-catalyzed alkene aminoarylation reactions for the synthesis of nitrogen heterocycles. Synthesis 44, 351–361 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Z, Wang Y, Wang Z, Zeng T, Liu P, Engle KM, Catalytic intermolecular carboamination of unactivated alkenes via directed aminopalladation. J. Am. Chem. Soc 139, 11261–11270 (2017). [DOI] [PubMed] [Google Scholar]
- 8.S. R. Chemler, The enantioselective intramolecular aminative functionalization of unactivated alkenes, dienes, allenes and alkynes for the synthesis of chiral nitrogen heterocycles. Org. Biomol. Chem 7, 3009–3019 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D, Wu L, Wang F, Wan X, Chen P, Lin Z, Liu G, Asymmetric copper-catalyzed intermolecular aminoarylation of styrenes: efficient access to optical 2,2-diarylethylamines. J. Am. Chem. Soc 139, 6811–6814 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Sleet CE, Tambar UK, Copper-catalyzed aminothiolation of 1,3-dienes via a dihydrothiazine intermediate. Angew. Chem. Int. Ed 56, 5536–5540 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang H-B, Pathipati SR, Selander N, Nickel-catalyzed 1,2-aminoarylation of oxime ester-tethered alkenes with boronic acids. ACS Catal. 12, 8441–8445 (2017). [Google Scholar]
- 12.Tasker SZ, Jamison TF, Highly regioselective indoline synthesis under nickel/photoredox dual catalysis. J. Am. Chem. Soc 137, 9531–9534 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tkatchouk E, Mankad NP, Benitez D, Goddard III WA, Toste FD, Two metals are better than one in the gold catalyzed oxidative heteroarylation of alkenes. J. Am. Chem. Soc 133, 14293–14300 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Müller TE, Beller M, Metal-Initiated Amination of Alkenes and Alkynes. Chem. Rev 98, 675–703 (1998). [DOI] [PubMed] [Google Scholar]
- 15.Piou T, Rovis T, Rhodium-catalysed syn-carboamination of alkenes via a transient directing group. Nature 527, 86–90 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kindt S, Heinrich MR, Recent advances in Meerwein arylation chemistry. Synthesis 48, 1597–1606 (2016). [Google Scholar]
- 17.Musacchio AJ, Lainhart BC, Zhang X, Naguib SG, Sherwood TC, Knowles RR, Catalytic intermolecular hydroaminations of unactivated olefins with secondary alkyl amines. Science 355, 727–730 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musacchio AJ, Nguyen QL, Beard GH, Knowles RR, Catalytic olefin hydroamination with aminium radical cations: a photoredox method for direct C–N bond formation. J. Am. Chem. Soc 136, 12217–12220 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Zhu Q, Graff DE, Knowles RR, Intermolecular anti-Markovnikov hydroamination of unactivated alkenes with sulfonamides enabled by proton-coupled electron transfer. J. Am. Chem. Soc 140, 741–747 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi GJ, Knowles RR, Catalytic alkene carboaminations enabled by oxidative proton-coupled electron transfer. J. Am. Chem. Soc 137, 9226–9229 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller DC, Choi GJ, Orbe SH, Knowles RR, Catalytic olefin hydroamidation enabled by proton-coupled electron transfer. J. Am. Chem. Soc 137, 13492–13495 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Margrey KA, Nicewicz DA, A general approach to catalytic alkene anti-Markovnikov hydrofunctionalization reactions via acridinium photoredox catalysis. Acc. Chem. Res 49, 1997–2006 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Nguyen TM, Manohar N, Nicewicz DA, Anti-Markovnikov hydroamination of alkenes catalyzed by a two-component organic photoredox system: direct access to phenethylamine derivatives. Angew. Chem. Int. Ed 53, 6198–6120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gesmundo NJ, Grandjean J-MM, Nicewicz DA, Amide and amine nucleophiles in polar radical crossover cycloadditions: synthesis of γ-lactams and pyrrolidines. Org. Lett 17, 1316–1319 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Zard SZ, Recent progress in the generation and use of nitrogen-centered radicals. Chem Soc. Rev 37, 1603–1618 (2008)]. [DOI] [PubMed] [Google Scholar]
- 26.Plesniak K, Zarecki A, Wicha J, The Smiles rearrangement and the Julia-Kocienski olefination reaction, Top. Curr. Chem 275, 163–250 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Motherwell WB, Pennell AMK, A novel route to biaryls via intramolecular free radical ipso-substitution reactions. J. Chem. Soc. Chem. Commun 1991, 877–879. [Google Scholar]
- 28.Holden CM, Greaney MF, Modern aspects of the Smiles rearrangement. Chem Eur J. 23, 8992–9008 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Henderson ARP, Kosowan JR, Wood TE, The Truce-Smiles rearrangement and related reactions: a review. Can. J. Chem 95, 483–504 (2017). [Google Scholar]
- 30.Snape TJ, A truce on the Smiles rearrangement: revisiting an old reaction – the Truce-Smiles rearrangement. Chem. Soc. Rev 37, 2452–2458 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Allart-Simon I, Gérard S, Sapi J, Radical Smiles rearrangement: An update. Molecules 21, 878–889 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Douglas JJ, Albright H, Sevrin MJ, Cole KP, Stephenson CRJ, A visible-light-mediated radical Smiles rearrangement and its application to the synthesis of a difluorosubstituted spirocyclic ORL-1 antagonist. Angew. Chem. Int. Ed 54 14898–14902 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Douglas JJ, Sevrin MJ, Cole KP, Stephenson CRJ, Preparative scale demonstration and mechanistic investigation of a visible light-mediated radical Smiles rearrangement. Org. Process Res. Dev 20, 1148–1155 (2016). [Google Scholar]
- 34.Rabet PTG, Boyd S, Greaney MF, Metal-free intermolecular aminoarylation of alkynes. Angew. Chem. Int. Ed 56, 4183–4186 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Johnston LJ, Schepp NP, Reactivities of radical cations: characterization of styrene radical cations and measurements of their reactivity toward nucleophiles. J. Am. Chem. Soc 115, 6564–6571 (1993). [Google Scholar]
- 36.Schepp NP, Johnston LJ, Reactivity of radical cations. Effect of radical cation and alkene structure on the absolute rate constants of radical cation mediated cycloaddition reactions. J. Am. Chem. Soc 118, 2872–2881 (1996). [Google Scholar]
- 37.Xu H-C, Moeller KD, Intramolecular anodic olefin coupling reactions: the use of a nitrogen trapping group. J. Am. Chem. Soc 130, 13542–13543 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Chen Z-M, Zhang X-M, Tu Y-Q, Radical aryl migration reactions and synthetic applications. Chem. Soc. Rev 44, 5520–5245 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Roth HG, Romero NA, Nicewicz DA, Experimental and calculated electrochemical potentials of common organic molecules for applications to single electron redox chemistry. Synlett 27, 714–723. (2016). [Google Scholar]
- 40.Choi GJ, Zhu Z, Miller DC, Gu CJ, Knowles RR, Catalytic alkylation of remote C–H bonds enabled by proton-coupled electron transfer. Nature 539, 268–271 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prier CK, Rankic DA, MacMillan DWC, Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev 113, 5322–5363 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukuzumi S, Ohkubo K, Suenobu T, Kato K, Fujitsuka M, Ito O, Photoalkylation of 10-alkylacridinium ion via a charge-shift type of photoinduced electron transfer controlled by solvent polarity. J. Am. Chem. Soc 123, 8459–8467 (2001). [DOI] [PubMed] [Google Scholar]
- 43.Lewis FD, Kojima M, Photodimerization of singlet trans- and cis-anethole. Concerted or stepwise? J. Am. Chem. Soc 110, 8660–8664 (1988). [Google Scholar]
- 44.Lowry MS, Goldsmith JI, Slinker JD, Rohl R, Pascal RA, Malliaras GG, Bernhard S, Single-layer electroluminescent devices and photoinduced hydrogen production from an ionic Iridium (III) complex. Chem. Mater 17, 5712–5719 (2005). [Google Scholar]
- 45.Romero NA, Nicewicz DA, Organic Photoredox Catalysis. Chem Rev. 116, 10075–10166 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Monos TM, Sun AC, McAtee RC, Devery JJ III, Stephenson CRJ, Microwave-assisted synthesis of heteroleptic Ir(III)+ polypyridyl complexes. J. Org. Chem 81, 6988–6994 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Blum TR, Zhu Y, Nordeen SA, Yoon TP, Photocatalytic synthesis of dihydrobenzofurans by oxidative [3+2] cycloaddition of phenols. Angew. Chem. Int 53, 11056–11059 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vassilikogiannakis G, Hatzimarinaki M, Orfanopoulos M, Mechanism of the [2+2] photocycloaddition of fullerene C60 with styrenes. J. Org. Chem 65, 8180–8187 (2000). [DOI] [PubMed] [Google Scholar]
- 49.Tallineau J, Bashiardes G, Coustard J-M, Lecournué F, A one-pot preparation of aryl- and heteroarylcycloalkenes: application to the total synthesis of (±)-Laurokamurene B. Synlett 17, 2761–2764 (2009). [Google Scholar]
- 50.Tarantino KT, Liu P, Knowles RR, Catalytic ketyl-olefin cyclizations enabled by proton-coupled electron transfer. J. Am. Chem. Soc 135 10022–10025 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Hopkins PB, Fuchs PL, Chlorosulfenylation-dehydrochlorination reactions. New and improved methodology for the synthesis of unsaturated aryl sulfides and aryl sulfones. J. Org. Chem 43, 1208–1217 (1978). [Google Scholar]
- 52.Sheldrick GM Crystal structure refinement with SHELXL. Acta Cryst. C71, 3–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.CrystalClear Expert 2.0 r16, Rigaku Americas and Rigaku Corporation (2014), Rigaku Americas, 9009, TX, USA: 77381–5209, Rigaku Tokyo, 196–8666, Japan. [Google Scholar]
- 54.CrysAlisPro 1.171.38.41 (Rigaku Oxford Diffraction, 2015).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.