Abstract
There are an estimated 5.4 million snakebite cases every year. People with snakebite envenoming suffer from severe complications, or even death. Although some review articles cover several topics of snakebite envenoming, a review of the cases regarding cerebral complications, especially rare syndromes, is lacking. Here, we overview 35 cases of snakebite by front-fanged snakes, including Bothrops, Daboia, Cerastes, Deinagkistrodon, Trimeresurus, and Crotalus in the Viperidae family; Bungarus and Naja in the Elapidae family, and Homoroselaps (rare cases) in the Lamprophiidae family. We also review three rare cases of snakebite by rear-fanged snakes, including Oxybelis and Leptodeira in the Colubridae family. In the cases of viper bites, most patients (17/24) were diagnosed with ischemic stroke and intracranial hemorrhage, leading to six deaths. We then discuss the potential underlying molecular mechanisms that cause these complications. In cases of elapid bites, neural, cardiac, and ophthalmic disorders are the main complications. Due to the small amount of venom injection and the inability to deep bite, all the rear-fanged snakebites did not develop any severe complications. To date, antivenom (AV) is the most effective therapy for snakebite envenoming. In the six cases of viper and elapid bites that did not receive AV, three cases (two by viper and one by elapid) resulted in death. This indicates that AV treatment is the key to survival after a venomous snakebite. Lastly, we also discuss several studies of therapeutic agents against snakebite-envenoming-induced complications, which could be potential adjuvants along with AV treatment. This article organizes the diagnosis of hemotoxic and neurotoxic envenoming, which may help ER doctors determine the treatment for unidentified snakebite.
Keywords: snakebite envenoming, hemotoxin, neurotoxin, ischemic stroke, intracranial hemorrhage, anti-venom serum
1. Introduction
Snakes are limbless reptiles that can be found on all continents and oceans except Antarctica. Among the 3700 species of snakes, 15% of them are estimated to be venomous [1]. On the continents, Viperidae and Elapidae are the major venomous snake families in the world [2]. Several hundred thousand people suffer from snakebite envenoming every year, and 5% of victims die due to the accompanying complications [3,4]. Among the five continents, southern Asia unfortunately reports the most deaths from snakebites [3]. However, with the invention of antivenom (AV), survival rates following a venomous snakebite have significantly increased [5].
Snake venoms are complex mixtures of enzymes, lipids, nucleotides, and carbohydrates. There are three main toxins in snake venom, including hemotoxins, neurotoxins, and cytotoxins [6], which lead to systematic damage, including cerebral complications. Among the complications, cerebral hemorrhage, ischemic stroke, cerebral infarction, and secondary inflammation frequently occur after viper envenomings due to hemotoxic enzymes such as snake-venom metalloproteinases (SVMPs) [7], coagulant enzymes [8], and proteolytic enzyme toxicity [9]. Without appropriate treatment, these compounds cause severe irreversible brain edema and fatality [10,11,12,13,14].
Some venomous snake bites treated with AV still result in fatality. Therefore, there remains an unmet need to develop an adjuvant therapy for snakebite envenoming. Most viper-bite cases studied, in both clinical reports and animal studies, were reported and diagnosed with hemorrhage and tissue necrosis in skin and muscle [15,16,17]. Little is known about viper-bite-induced complications in the central nervous system (CNS). In this article, we review a series of clinical snakebite cases that were reported after 2000, focusing on the cerebral complications caused by hemotoxic and neurotoxic envenoming, and describe a few cases of CNS and PNS complications of snakebite envenoming. Finally, we discuss other potential therapeutic strategies for snakebite treatment.
2. Envenoming by Snakes That Mainly Have Hemotoxic Venoms
Here, we review snakebites by three genera of the Viperidae family (Table 1).
Table 1.
Brain and nervous-system complications following Viperidae envenoming.
| Complications | Snake Species | Sex/Age | Bite Mark | Other Symptoms | Presuming Venom Components |
Outcome | Antivenom/Amount | Ref. |
|---|---|---|---|---|---|---|---|---|
| Cerebral infarction |
Bothrops lanceolatus (Fer-de-Lance) |
M/74 | Elbow | Atrial fibrillation | SVMPs, Hemotoxin | Dead | IV Antivenom/80 mL | [11] |
| Cerebral infarction |
Trimeresurus stejnegeri (Asian palm pit viper) |
F/49 | Foot | Speech disturbances | SVMPs, Hemotoxin, | Recovered | IV Antivenom/3 vials | [18] |
| Intracranial hemorrhage |
Bothrops jararacussu (Jararacussu) |
M/52 | Foot | Sudden loss of consciousness | SVMPs, Hemotoxin | Recovered | IV Antivenom/Unclear | [10] |
| Subarachnoid hemorrhage |
Bothrops atrox (Fer-de-Lance) |
F/59 | Foot | Hypothermia, Bradycardia and Hypotension | SVMPs, Hemotoxin | Dead | IV Antivenom/80 mL | [19] |
| Cerebral edema and hemorrhage |
Crotalus adamanteus (Eastern diamondback rattlesnake) |
M/54 | Hand | Facial fasciculations | SVMPs, Hemotoxin, Neurotoxin | Dead | IV Antivenom/16 vials | [14] |
| Intracranial hemorrhage | Unidentified species | F/48 | Hand | Exotropia | SVMPs, Hemotoxin, | Recover with minor visual complication | IV Antivenom/Unclear | [20] |
| ADEM | Deinagkistrodon acutus | M/50 | Foot | Severe pain | SVMPs, Hemotoxin | Recovered | IV Antivenom/1 vial | [21] |
ADEM: Acute demyelinating encephalomyelitis; AHL: Acute hemorrhagic leukoencephalitis; SVMPs: Snake-venom metalloproteinases; PLA2: Phospholipase A2.
2.1. Bothrops
Bothrops, commonly known as pit viper, fer-de-lance “spearhead” in French, is often found in central and south America. In Brazil, around 26,000 snakebites by Bothrops jararaca are recorded each year [22]. Due to the snake-venom metalloproteinases (SVMPs) and hemotoxins in venom, intracranial hemorrhage and infarction are the main brain complications after snakebite. The fatality rate of Bothrops bites is estimated to be about 0.51% [23]. Here we reviewed three cases of Bothrops bites that happened in Brazil and Martinique.
2.2. Deinagkistrodon
Deinagkistrodon acutus is a Viperidae family snake endemic to Southeast Asia. It is also called the “hundred pacer” because locals believe that the victims who are bitten will only be able to walk 100 steps before dying. Although D. acutus presents hemotoxins in the venom [24], fatality is unusual after D. acutus bite. Here, we review one case of D. acutus bite that happened in China.
2.3. Trimeresurus
Trimeresurus stejnegeri, also called bamboo viper, is endemic to southern China and Taiwan. Due to the similar color of T. stejnegeri to bamboo, victims are typically bitten while walking in a bamboo forest. SVMPs and hemotoxins are the main toxins in the T. stejnegeri venom. A bite wound usually becomes swollen and gradually undergoes necrosis [25]. The severity of the wound depends on the amount of venom injected and the depth of the bite. Here, we review one case of T. stejnegeri bite that happened in China.
3. Envenoming by Snakes That Mainly Have Neurotoxic Venoms
Here, we review snakebites by two different genera of the Elapidae family (Table 2).
Table 2.
Brain and nervous-system complications following Elapidae envenoming.
| Complications | Snake Species | Sex/Age | Bite Mark | Other Symptoms | Presuming Venom Components | Outcome | Antivenom/Amount | Ref. |
|---|---|---|---|---|---|---|---|---|
| Respiratory paralysis, ptosis |
Bungarus candidus (Malaya Krait) |
M/35 | Hand | Tachycardia, prolonged micturition | Neurotoxin, PLA2 | Permanent mydriasis | IV Antivenom/6 vials | [26] |
| Respiratory paralysis, ptosis | M/41 | Hand | Tachycardia | Permanent mydriasis | IV Antivenom/3 vials | |||
| Prolonged cerebral anoxia | F/12 | Hand | Periodic convulsions | Permanent brain damage | Unclear | |||
| Hyponatremia and cerebral edema |
Bungarus multicinctus (Many-banded Krait) |
F/17 | Foot | Seizure | Neurotoxin | Dead | N/A | [27] |
| Hypoxic ischemic encephalopathy |
Bungarus caeruleus (Indian Krait) |
M/15 | Foot | Paraplegia | Neurotoxin | Loss of vision | IV Antivenom/1 vial | [28] |
| PRES |
Bungarus caeruleus (Indian Krait) |
M/10 | Ear | Hypertension | Neurotoxin | Recovered with vision blurring | IV Antivenom/10 vials | [29] |
| EMNS |
Bungarus caeruleus (Indian Krait, prediction) |
M/38 | N/K | Difficulty in swallowing and double vision | Neurotoxin | Recovered | IV Antivenom/9 vials | [30] |
| EMNS | F/27 | N/K | Mydriasis | Recovered | IV Antivenom/Unclear | |||
| Temporary brain death |
Naja haje arabica (Arabian cobra) |
F/57 | Hand | Breathing ceased | Neurotoxin | Recovered | IV Antivenom/50 vials | [31] |
EMNS: Early-morning neuroparalytic syndrome; N/A: Not Applied; N/K: Not known; PLA2: Phospholipase A2; PRES: Posterior reversible encephalopathy syndrome.
3.1. Bungarus
Three cases were reported in Thailand that involved Bungarus Candidus [26], which is also called Malaya Krait and is often found in Southeast Asia. Two patients were retreated with AV but still suffered from respiratory paralysis and ptosis and were discharged with permanent mydriasis. Another patient did not receive AV and was diagnosed with prolonged cerebral anoxia. Unfortunately, she was discharged with permanent brain damage. Other symptoms such as tachycardia and periodic convulsions were also noted in the report. Neurotoxins are enriched in Elapid snakes’ venom [32]. Phospholipase A2 (PLA2) is one of the neurotoxins found in the venom of B. Candidus, which causes neural disorders [33]. A previous review article also pointed out that PLA2 may cause ophthalmic disorders [34], which explains why the patients were discharged with permanent mydriasis.
In India, four patients were bitten by B. caeruleus (Indian Krait), one of the most common snakes in Bangladesh and India. A 15-year-old boy was bitten by B. caeruleus and immediately sent to the hospital to receive AV treatment [28]. After one vial of AV treatment, he had respiratory difficulty, perhaps due to a hypersensitivity reaction. He was diagnosed with hypoxia ischemic encephalopathy along with paraplegia. He was then discharged with vision loss in both eyes. Another 10-year-old boy was bitten by B. caeruleus on his ear while sleeping and sent to the hospital to receive a total of 10 vials of AV [29]. He was then diagnosed with PRES, a rare syndrome associated with Elapid snakebites. He recovered and was discharged 10 days later with minor vision blurring. Another two adult cases of B. caeruleus bite were treated with AV and diagnosed with early-morning neuroparalytic syndrome (EMNS) [30]. Although having difficulty in swallowing, double vision, and mydriasis, they were discharged with a full recovery.
In Vietnam, a 17-year-old girl was bitten by B. multicinctus (Many-banded Krait) and sent to the hospital a few hours later [27]. She did not receive AV treatment due to an unavailability at that time in Vietnam. She was stable for the first 2 days and suddenly deteriorated with seizures and a coma. She was then diagnosed with hyponatremia and cerebral edema. Unfortunately, she still did not make it 18 days later due to lack of AV treatment.
3.2. Naja
Naja, also known as cobra, is one of the most common elapid snakes on the planet. To date, there are about 38 species of Naja [35]. Here, we review a case of a 57-year-old woman that was bitten by Naja arabica (Arabian cobra) [31]. She was sent to the hospital one hour after N. arabica bite and immediately received multiple vials of AV treatment. After 6 h of intensive care (30 vials of AV), she was still diagnosed with brain death along with respiratory failure. She then received extensive AV treatment; up to 50 vials in 24 h. Fortunately, her neurologic status gradually recovered by the second day and her respiratory muscles started to function by the third day. She was then discharged from the ICU and eventually returned home with a full recovery.
4. Envenoming by Snakes That Have Both Hemotoxic and Neurotoxic Venoms
Here we review snakebites by 3 genera of the Viperidae family and 1 genus of the Lamprophiidae family (Table 3).
Table 3.
Brain and nervous-system complications following Viperidae and Lamprophiidae bite: both hemotoxic and neurotoxic venoms.
| Complications | Snake Species | Sex/Age | Bite Mark | Other Symptoms | Presuming Venom Components |
Outcome | Antivenom/Amount | Ref. |
|---|---|---|---|---|---|---|---|---|
| Ischemic stroke |
Daboia russelii (Russell’s Viper) |
M/18 | Foot | Sever pain | Hemotoxin | Recovered with minor complications | IV Antivenom/6 vials | [36] |
| Ischemic stroke |
Daboia russelii (Russell’s Viper) |
F/40 | Foot | Hypotonia of the limbs | SVMPs, Hemotoxin | Significant ataxia of gait | IV Antivenom/24 vials | [37] |
| Ischemic Stroke |
Daboia russelii (Russell’s Viper) |
M/70 | Foot | Ptosis, Seizure | Hemotoxin, Neurotoxin | Recovered | IV Antivenom/20 vials | [38] |
| Ischemic Stroke | M/55 | Foot | Ptosis, Speech disturbances | Hemotoxin, Neurotoxin | Recovered | IV Antivenom/35 vials | ||
| Ischemic stroke |
Cerastes cerastes (Desert horned viper) |
F/5 | Foot | Thrombocytopenia, Acute anemia | SVMPs, Hemotoxin, PLA2 | Dead | N/A | [13] |
| Thalamic and cerebral infarctions |
Daboia russelii (Russell’s Viper) |
M/55 | Foot | N/A | SVMPs, Hemotoxin | Recovered | IV Antivenom/26 vials | [39] |
| Cerebral infarction |
Daboia russelii (Russell’s Viper) |
F/27 | Foot | Gerstmann’s syndrome | Hemotoxin, Neurotoxin | Recovered | IV Antivenom/Unclear | [40] |
| Cerebral infarction |
Daboia russelii (Russell’s Viper, prediction) |
M/50 | Foot | Status epilepticus | SVMPs, Hemotoxin | Motor deficit | N/K | [41] |
| Ischemic brain infarction |
Daboia russelii (Russell’s Viper) Mis-identified at beginning |
M/43 | Foot | Ptosis and ophthalmoplegia |
SVMPs, Hemotoxin, Neurotoxin |
Dead | IV Antivenom/35 vials | [12] |
| Cerebral infarction |
Cerastes cerastes (Desert horned viper) |
F/32 | Hand | Tonic-clonic seizures | SVMPs, Hemotoxin, PLA2 | Dead | N/A | [13] |
| Cerebral infarction | M/51 | Foot | Hemodynamic shock | Recovered | N/A | |||
| ADEM |
Daboia russelii (Russell’s Viper) |
M/36 | Foot | Hematemesis and gum bleeding | SVMPs, Hemotoxin | Recovered | IV Antivenom/30 vials | [42] |
| AHL |
Daboia russelii (Russell’s Viper, prediction) |
M/44 | Foot | Hemorrhagic necrosis | SVMPs, Hemotoxin | Recovered | N/K | [43] |
| PRES |
Cerastes cerastes (Desert horned viper) |
F/23 | Foot | Reduction in visual acuity | SVMPs, Hemotoxin,PLA2 | Recovered with visual impairment | IV Antivenom/Unclear | [44] |
| Myokymia |
Crotalus oreganus abyssus
(Grand Canyon rattlesnake) |
M/46 | Hand | Pain, swelling, and erythema of his left hand | Hemotoxin, Neurotoxin | Recovered | IV Antivenom/20 vials | [45] |
| fasciotomy-requiring compartment syndrome | Rattlesnake | W/32 | Hand | Somnolent, febrile, suffering of headache, tachypnoea | Hemotoxin, Neurotoxin | Recovered with complications | N/A | [46] |
| Bilateral ptosis | Crotalus cerastes (sidewinder rattlesnake) | M/56 | Foot | Burning and tingling pain | Hemotoxin, Neurotoxin | Recovered | N/A | [47] |
| Numbness |
Lamprophiidae Homoroselaps lacteus (Spotted harlequin snake) |
M/Adult | Palm | Slight burning sensation, swelling | SVMPs, PLA2 | Recovered | N/A | [48] |
| Subcutaneous ecchymoses | M/Adult | Thumb | Swelling | Recovered | N/A |
ADEM: Acute demyelinating encephalomyelitis; AHL: Acute hemorrhagic leukoencephalitis; SVMPs: Snake-venom metalloproteinases; N/A: Not Applied; N/K: Not known; PLA2: Phospholipase A2; PRES: Posterior reversible encephalopathy syndrome.
4.1. Daboia
Daboia russelii, also called Russell’s viper, is one of the most common vipers found in India [49] and is the greatest contributor to accidental snakebites [50]. To shorten the time needed to treat an unidentified snakebite, a group in Taiwan utilized avian and mammal antibodies to develop a rapid and sensitive diagnostic tool for a Russell’s viper’s snakebite [51]. Although D. russelii belongs to the family of Viperidae, several neurotoxins are identified in the viper’s venom [52] which also cause neural disorders such as ptosis and ophthalmoplegia [12,38]. Here, we review nine cases of D. russelii snakebites in India and one case in Sri Lanka. Since D. russelii is usually found on farms, 8 of the 10 patients were male farmers, and all were bitten on their feet. Because AV against D. russelii is well-established, most of the patients received AV during hospitalization and recovered with no or minor complications.
4.2. Cerastes
Cerastes cerastes, also called the desert horned viper due to its appearance, is endemic in the deserts of northern Africa and parts of the Arabian Peninsula. Cerastes is considered a venomous snake due to its hemotoxin venoms. In addition to SVMPs and hemotoxin, phospholipase A2 (PLA2) was found to be the main constituent of the venom [53]. Here we reviewed four patients who suffered from Cerastes bites. Receiving no AV treatment, two patients died after alternative medical treatments, and one recovered without any neurological complications. The patient who received AV and other medications was discharged with visual impairment, which might have been caused by PLA2 toxicity [34].
4.3. Crotalus
Crotalus adamanteus, also called Eastern diamondback rattlesnake, is endemic to the southeastern United States. Although AV against C. adamanteus is available, there are still fatalities due to snakebite-envenoming-induced intracranial hemorrhage [54]. Most venomous snakebite victims in the United States are bitten by a member of the rattlesnake family. However, with the development of AV, death from a C. adamanteus bite is rare [55]. Here we review one case of C. adamanteus bite that happened in the state of Florida. In addition to hemotoxins, neurotoxins are also the main components in the venom of Crotalus. Without severe cerebral complications, rattlesnake bites cause myokymia [45], fasciotomy-requiring compartment syndrome [46] and bilateral ptosis [47]. Even without AV treatment, they were all recovered.
4.4. Homoroselaps
Homoroselaps, belonging to the Lamprophiidae family, is mainly endemic to the Republic of South Africa. Despite having front fangs [56], Homoroselaps bite is unlikely to cause life-threatening effects due to the tiny amount of venom injected. Two adult males were bitten by Homoroselaps lacteus on their hands in South Africa [48]. One had numbness on his finger but recovered soon after. However, another patient had severe subcutaneous ecchymoses for 4 days. The ecchymoses were visible for two weeks on his hand before he recovered. Although the composition of the venom in H. lacteus has yet to be studied, SVMP and PLA2 were predicted in the venom due to the symptoms caused by the snakebite [48]. Other old studies of H. lacteus bite also reported bruises and/or ecchymoses on patients’ hands, which confirm the predictive components of the venom.
5. Envenoming by Rear-Fanged Snakes
Here we review three cases of rear-fanged snakebites by two genera of the Colubridae family (Table 4).
Table 4.
Complications following rear-fanged Colubridae envenoming.
| Complications | Snake Species | Sex/Age | Bite Mark | Other Symptoms | Presuming Venom Components |
Outcome | Antivenom | Ref. |
|---|---|---|---|---|---|---|---|---|
| Dizziness |
Oxybelis fulgidus (Colubridae) |
M/67 | Arm | Moderate pain, tachycardiac, local bleeding | Hemotoxin, 3FTx (fulgimotoxin) |
Recovered | N/A | [57] |
| Hand edema |
Leptodeira annulata (Colubridae) Banded cat-eye snake |
F/29 | Hand | Burning sensation and itching, pain | SVMPs, Hemotoxin | Recovered | N/A | [58] |
| CRPS |
Leptodeira annulata (Colubridae) Banded cat-eye snake |
M/44 | Arm | Fever, chills, nausea, and light-headedness. | SVMPs, Hemotoxin, PLA2 | Recovered | N/A | [59] |
CRPS: Complex regional pain syndrome; PLA2: Phospholipase A2; SVMPs: Snake-venom metalloproteinases; 3FTx: Three-finger toxins, N/A: Not Applied.
5.1. Oxybelis
Oxybelis, commonly known as the vine snake, is a genus of colubrid snakes mainly endemic to the Americas. Here, we review a case of 67-year-old male that was bitten on his arm by Oxybelis fulgidus in Brazil [57]. He came to the local hospital with bite-site bleeding and dizziness. Only taking painkiller pills, he recovered and was discharged in 2 days. No neurologic complication was observed during his hospitalization. Both hemotoxic and neurotoxic proteins such as L-amino acid oxidase (LAAO), PIII-SVMP, cysteine-Rich Secretory Proteins (CRiSP), and three-finger toxins (3FTx, fulgimotoxin) were identified in the venom of O. fulgidus [60,61].
5.2. Leptodeira
Leptodeira, commonly called the cat-eye snake, is a genus of colubrid snakes mainly endemic to Mexico and Central America. Here, we report two cases of Leptodeira bite, one by Leptodeira annulate and one by unknown species. A 29-year-old female was bitten on her finger by L. annulate while trying to catch the snake during fieldwork in Colombia [58]. She had edema on her whole hand 18 min after the bite and was treated with a single intravenous dose of hydrocortisone. She recovered and was discharged after 12 h and no systemic symptoms were referred. Another case was a 44-year-old male that was bitten by unidentified Leptodeira on his arm [59]. He had fever, chills, nausea, and light-headedness. He was then discharged soon later. However, a few months later, he complained about hyperalgesia, swelling, alternating cool and warm sensation, and dermal changes, and was then diagnosed with complex regional pain syndrome (CRPS), which is a rare case reported by snakebites in the world [59]. In addition, two cases of pediatric CRPS (<18-year-old) were reported to be bitten by a viper and southern Pacific rattlesnake in Turkey [62] and the US [63], respectively.
5.3. Other Studies of Colubridae Family
Compared with the venoms of many front-fanged snakes, the toxic nature of the venom in rear-fanged colubrid snakes is less clearly understood. It may be because of the difficulty of collecting the venom from rear-fanged snakes and of the tiny amount of venom for each injection. Like Viperiade snakes, SVMP is one of the main toxins in the venom, which was discovered in several colubrid species including Dispholidus typus [64], Thamnodynastes strigatus [65] and Philodryas species [66]. In addition to SVMP, other toxins such as serine proteases, LAAOs, PLA2, C-type lectin-like proteins and 3FTx were identified in colubrid snakes [60,61,67]. Although no clinical case of cerebral complications was reported by colubrid bite due to a tiny amount of envenoming, an animal model of venom (Philodryas patagoniensis) injection demonstrates the hemotoxic features of the venom, leading to multiple hemorrhages in the cerebellum and cerebrum [68].
6. Brain Complications
We review 35 cases of brain complications caused by toxic snake proteins, including hemotoxins (Table 1), neurotoxins (Table 2), and both toxins (Table 3 and Table 4).
6.1. Cerebral Infarction and Ischemic Stroke
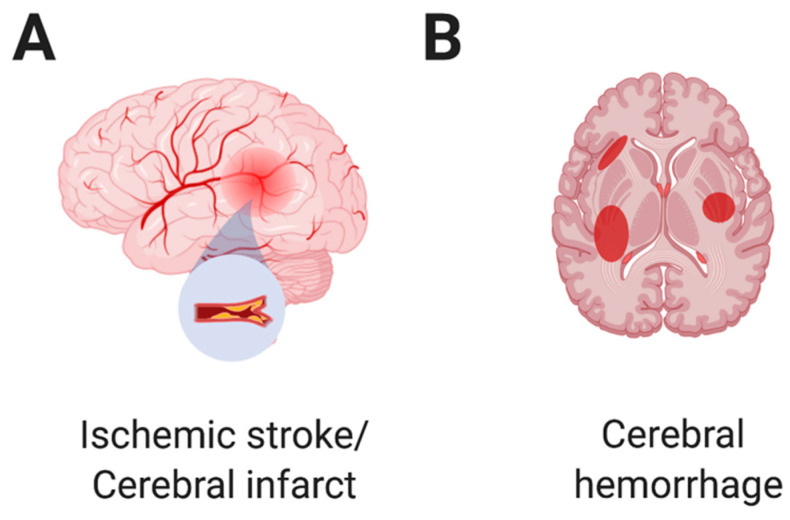
There are two main types of strokes: ischemic stroke and hemorrhagic stroke. Ischemic stroke, also called cerebral infarction (Figure 1A), occurs because of disrupted blood flow to the brain due to a clot or clots in the vessel. Ischemic stroke causes necrosis in the affected area of brain tissues, leading to irreversible neural damage. Unfortunately, cerebral infarction or ischemic stroke is the most frequent CNS complication following viper envenoming [69] due to abnormal activation of thrombocytes, such as platelet aggregation [70]. Among the proteins in the viper venom, Snake C-type lectin-like proteins (snaclecs) are considered to be the cause of ischemic stroke by activating thrombocytes or platelets [71,72]. Snaclecs are mainly expressed in the venoms of vipers and colubrids [73,74]. Regarding the vipers reported in this review, snaclecs were identified in Bothrops [75,76], Daboia [77], Crotalus [78], and Trimeresurus [71]. In addition, procoagulant proteases, one type of snake-venom serine proteases (SVSPs), were reported to be involved in affecting blood coagulation [79,80]. Rather than ischemic stroke, hemorrhagins are the toxic components of Viperidae snake venom, which were reported to cause endothelial damage and an increase in vascular permeability, leading to hemorrhagic stroke [81,82]. Here, we review 13 cases of ischemic stroke or cerebral infarction, depending on the authors’ preference, followed by viper envenoming.
Figure 1.
Main cerebral complications of snakebite envenoming, ischemic stroke, and cerebral hemorrhage.
Among five patients diagnosed with ischemic stroke after snakebite, four of them were bitten by D. russelii. They received AV treatment in the hospital and recovered with no or minor complications [36,37,38]. Although SVMPs and hemotoxins are the main toxins in the venom of D. russelii, two patients were also diagnosed with neural disorders such as ptosis, seizure, and speech disturbances [38]. These may have been caused by the neurotoxins in the venom. PLA2 is the most abundant component in the venom of D. russelii. The proteolytic activity of PLA2 promotes degradation and depletion of fibrin (ogen) resulting in a coagulopathy [83].
Another 5-year-old girl in Morocco was bitten by Cerastes and sent to the hospital 4 days later. She was diagnosed with ischemic stroke and developed thrombocytopenia and acute anemia [13]. She did not receive any AV treatment and died on day 7. Unavailability of the AV and delayed hospitalization may have been the causes of her fatality.
In India, four patients were bitten by D. russelii and diagnosed with cerebral infarction during hospitalization [12,39,40,41]. Three patients received AV treatment; two of them recovered with no complications. Unfortunately, one patient died even after 35 vials of AV administration due to misidentifying the snake species at the beginning of treatment [12]. One patient did not receive any AV and recovered with motor deficit [41]. The neurotoxins of D. russelii also caused neural complications such as Gerstmann’s syndrome [40] and ptosis [12] in patients.
In Martinique, in the French Caribbean, a case of Bothorps Ianceolatus snakebite occurred in a 74-year-old man who was sent to the hospital where he lost consciousness for 2 days. Specific AV treatment was given without reaction. A magnetic-resonance imaging (MRI) diffusion-weighted scan showed multiple cerebral infarcts, and he died 10 days later [11]. The likely cause of death was cardiogenic shock resulting from massive myocardial infarction. The fatal outcome, in this case, may have been avoided by early antivenom therapy [84].
A case report in Morocco showed that two patients were diagnosed with cerebral infarction after C. Cerastes bites [13]. The 32-year-old woman was sent to a local hospital one day after snakebite. She developed generalized tonic-clonic seizures on her way to the hospital. She did not receive any AV treatment and died eventually. Another 51-year-old man was sent to hospital with hemodynamic shock. He did not receive any AV but was treated with fresh frozen plasma and units of platelet administration. Fortunately, he was discharged with full recovery.
A 49-year-old woman in China was bitten by T. stejnegeri and sent to the hospital, where she received AV treatment. She had speech disturbances during hospitalization and was diagnosed with cerebral infarction [18]. After 2 weeks in the hospital, she was discharged and followed up 3 months later with a full recovery.
6.2. Brain Hemorrhage
SVMPs are zinc-dependent enzymes belonging to the metzincin family [85] that are mainly expressed in Viperinae and Crotalinae [6]. Based on the size and domain structure, three known SVMPs are identified in P-I (20–30 kDa), P-II (30–60 kDa) and P-III (60–100 kDa) classes [7,75]. In some vipers, SVMPs and anticoagulant toxins exist in their venoms [75]. Once bitten by those vipers, patients are likely to suffer from a severe cerebral hemorrhage (Figure 1B) due to vascular endothelium damage and coagulation disorder.
In Brazil, a 52-year-old man was sent to the hospital 2 days after a juvenile B. jararaca bite. He was treated with AV and diagnosed with intracranial hemorrhage because of the proteolytic, hemorrhagic, and coagulant activity in the venom [10]. An earlier case reported that the venom of juvenile Bothrops shows higher hemorrhagic but lower proteolytic activity than adult Bothrops [86], which explains why this patient had intracranial bleeding without overt local manifestations. Luckily, he was discharged after having recovered. Observing the cases above, only one patient survived following a snakebite from a juvenile Bothorps. Another 59-year-old woman who became the victim of a Bothorps atrox bite was sent to the hospital one and half hours after a snakebite and was immediately treated with AV. However, she died on the second day due to severe subarachnoid hemorrhage [19].
A 54-year-old man in Florida was bitten by C. adamanteus and sent to the hospital within an hour, with immediate AV treatment. He was diagnosed with cerebral edema and hemorrhage [14]. Although he received 16 vials of AV, he still died on day 7. The patient in this case responded very well to AV and was prepared to return home on day 5. However, multiple small cerebral hemorrhages occurred on the morning of day 6; on day 7 he passed away. A 48-year-old woman in India was bitten by an unidentified snake and was diagnosed with intracranial hemorrhage and exotropia in the hospital [20]. She received AV treatment and transfusion of fresh frozen plasma to correct the suspected venom-induced consumptive coagulopathy. Ten days later, she was discharged and recovered with minor visual complications. Based on the hemotoxic and neurotoxic responses, she was likely bitten by D. russelii.
6.3. Acute Demyelinating Encephalomyelitis (ADEM)
Acute demyelinating encephalomyelitis (ADEM) is an autoimmune-mediated nervous-system disease often occurring after animal or insect bites, which is usually seen in children [87]. However, a 36-year-old man was bitten by D. russelii and diagnosed with ADEM on day 8 [42]. He stayed in the hospital for 20 days, receiving 30 vials of AV, and was discharged with full recovery. Adverse reactions to AV such as anaphylaxis (which happens within 10–180 min) and immune complex diseases (happens within 5–25 days) were reported [88]. Due to the delayed observation (day 8) of ADEM in the patient, the authors of this case report postulated that the inflammatory responses due to the AV were a more likely cause of ADEM than direct neurotoxicity of D. russelii venom.
In China, a 50-year-old man was bitten by D. acutus and sent to the hospital one hour after the snakebite. He experienced severe pain and was treated with AV. Eventually, he was diagnosed with ADEM [21]. After appropriate medical care, he was discharged and followed up one month later with full recovery. Although SVMPs and hemotoxin are present in snake venom, his treatment with AV and other medicines in a very short period minimized venomous toxicity.
6.4. Acute Hemorrhagic Leukoencephalitis (AHL)
Similar to ADEM, acute hemorrhagic leukoencephalitis (AHL) is a fulminant inflammatory disease of cerebral white matter. It is more commonly seen in adults and has a high rate of fatality [89]. A 44-year-old man was bitten by D. russelii and later diagnosed with AHL [43]. Despite multiple hemorrhagic foci in his brain, he recovered rapidly with AV treatment. He was discharged after 28 days with great improvement.
6.5. Posterior Reversible Encephalopathy Syndrome (PRES)
Posterior reversible encephalopathy syndrome (PRES) is a rare syndrome characterized by multiple symptoms such as headaches, altered consciousness, and focal neurological deficits [69]. Many reported cases of PRES are caused by animal bites [90], scorpion stings [91], and wasp attacks [92]. A case in Egypt showed that a 23-year-old pregnant woman was bitten by C. Cerastes and sent to the hospital immediately, where she received AV treatment. Among her complications, she complained of a reduction in visual acuity. Based on CT imaging, a very faint hypodense area in the posterior part of the parietal and occipital lobes was found and she was diagnosed with PRES [44]. After one week, she was discharged with significant improvement. Although the author claimed that the underlying mechanism of PRES was unclear in this case, PLA2 in the C. Cerastes’ venom seems likely to have been the cause of PRES.
6.6. Early Morning Neuroparalytic Syndrome (EMNS)
Two rare cases of EMNS were diagnosed in Indian Krait bites. The patients who suffer from EMNS usually do not have bite marks on their bodies, which makes the diagnosis complicated [93]. We also review a case of Naja bite, which caused a case of temporary brain death that was rescued by 50 vials of AV [31]. In fact, Bungarus and Naja bites commonly cause death in India due to neurotoxin-induced respiratory muscle paralysis [94]. On the contrary to viper bites, more than 50% of the elapid bites in this review were on the hand, suggesting that the patients may have tried to capture them.
6.7. Other Complications by Snakebite Envenoming
Hemotoxins and SVMPs are the main toxins in the venom of viper snakes [95]. Most vipers generate both SVMPs and hemotoxins. For example, both coagulant enzyme [8,96] and anticoagulant enzyme [97,98] were identified in Bothrops’ venom. Due to both hemorrhagic and coagulant features of the venoms, viper bites examined in the 21 cases of this review caused cerebral infarction, ischemic stroke, and cerebral hemorrhage, leading to six deaths and five recoveries with complications. Another interesting observation is that most viper bites happened on the foot (17/21), suggesting that most victims were bitten while standing or walking.
In addition to cerebral complications, complications in the spinal cord (a part of CNS) also occurred after snakebite envenoming. Acute-onset flaccid quadriparesis [29] and acute flaccid paraplegia [99], which would cause weakness of the respiratory and pharyngeal muscles, were reported by snakebites of elapid and viper, respectively. The snake PLA2 also induces inflammatory responses, leading to spinal pain [100,101]. Another article further confirmed the effects of PLA2 on CNS complications [102]. However, a case of spinal-cord complication (myelopathy) after snakebite in India was the result of AV treatment [103]. Thus, the necessity of using AV will be a critical determination for snakebites due to the tiny amount of venom injection.
In addition, other inflammatory responses such as ADEM and AHL are other complications that are noted in viper bites; however, these might be due to the side effects of AV treatment. Among the cases in this review, a rare case of viper bite was diagnosed with PRES, which may have been the result of a minor or undetectable cerebral infarct or hemorrhage.
Ocular complications also occur after hemotoxic envenoming. A review article indicated that subconjunctival hemorrhage, vitreous hemorrhage, central retinal artery occlusion (CRAO), and macular infarction were diagnosed in patients who suffered from viper bites [34]. Rarely, few patients are diagnosed with acute-angle-closure glaucoma (AACG) after viper bites [104,105], which might be due to ciliary-body edema-induced intraocular pressure elevation.
Neurotoxins are the main component of venom in Elapidae [32]. In this review, most patients who showed neurotoxic symptoms were bitten by elapids (Table 2). However, neurotoxic influences were also observed in some viper bites such as D. russelii [12,38] and C. cerastes [13,44] (Table 3). Among the neurotoxins, PLA2 and its isomers are identified in the venom of D. russelii and C. cerastes [106,107]. Fortunately, most neurotoxic influences are recoverable after appropriate treatment. In this review, there is only a rare death caused by B. multicinctus bite due to lack of AV at that time [27]. Most of the patients had respiratory paralysis, ptosis, cerebral anoxia, and hypoxic encephalopathy. Not only in viper bites, PRES was also observed in the elapid bite [29], suggesting that PRES is a common syndrome of snakebite, no matter whether it is neurotoxic or hemotoxic. However, not every neurotoxic envenoming causes severe complications. In some cases of snakebites, it merely causes local PNS numbness on the bitten finger [48].
7. Treatment
To date, intravenous injection of AV is the most effective treatment for snakebites [108]. In this review, of the six patients who did not receive AV treatment, four of them died. However, some patients were not able to complete the whole AV treatment process due to hypersensitive reactions to AV [28]. Although treated with AV, the misidentification of the snake species at the beginning causes irreversible damage, leading to a fatality [12]. Such iatrogenic causes of snakebite, either by a mistake in diagnosis [12] or by mistreatments [109,110], make the cases worse. Neostigmine, a cholinesterase inhibitor for overcoming paralysis of skeletal muscle, is commonly used to cotreat with AV when patients have neural disorders such as ptosis and muscle weakness [26,29,30,31,38,40]. Hemotoxin-induced ischemic stroke and cerebral infarction seem to be the main CNS complications (15/21) following a viper bite. Aspirin is a known anticoagulant agent and is used against ischemic stroke [111] and cerebral infarction [112]. Thus, aspirin could be an adjuvant for viper bite. SVMPs are the main components in the venom that lead to hemorrhage by proteolytic destruction of the basal membrane and extracellular matrix around the capillary and vessels [113]. Several studies have reported that using chelators to neutralize the cations such as Ca2+, Mg2+, and Zn2+, the cofactors in the active sites of the hemotoxic enzymes, could be a potential therapeutic strategy [114,115,116]. Although EDTA and DTPA can neutralize the hemotoxic activities in vitro, no significant improvement was observed in tissue necrosis or increases of survival in vivo [117]. Moreover, excessive administration of EDTA may cause electrolyte imbalance in patients. A previous study showed that a premix of sodium silicate complex (SSC) with PM could reduce subdermal hemorrhage and elongate the survival in a rodent model of PM envenoming [17], suggesting that SSC may not only inhibit hemotoxic effects but also alleviate neurotoxic influences. Thus, SSC could be an adjuvant as first aid for viper snakebites.
Recently, a promising study showed that 2,3-dimercapto-1-propanesulfonic acid (DMPS), a strong metal chelator, can block the enzymatic activity of SVMPs in vitro and prolong the survival rate of animals that suffered from snake-venom envenoming [118]. Varespladib (LY315920) was demonstrated to show PLA2 inhibition against 55 to 60 snake venoms and significantly increase the survival rate of venom-injected rodents [119,120,121]. Later, an orally bioavailable prodrug of Varespladib (methyl-Varespladib; LY333013) was developed and it shows similar efficacy in abrogating or delaying neurotoxic manifestations induced by snake venoms [122]. Both Varespladib [123] and DMPS [124] are in clinical trials for snakebite. In addition, an animal experiment reported that hyperbaric oxygen (HBO) is a potential adjuvant along with AV, showing more prominent protective effects on brain damage caused by D. acutus envenoming [125]. α-neurotoxin causes a reversible blockage of acetylcholine receptors, leading to central-nervous-system disorders [126]. A synthetic recombinant peptide against short-chain α-neurotoxin (ScNtx) was developed to neutralize the venoms of diverse genera such as Micrurus, Dendroaspis, Naja, and Walterinnesia [127]. Another proteomics study further pointed out the protein landscapes of the venom, which provides a platform for synaptic inhibitor development [128].
8. Discussion and Conclusions
Cerebral infarctions and hemorrhages are the main causes leading to fatal cases of viper bite. Both hemotoxins and neurotoxins contribute to those severe lethal brain complications (Figure 2) [75]. Although most patients were bitten on the foot or toe by vipers (17 of 21), the toxins still circulate to the brain and cause cerebral complications. To reduce accidental bites, always wearing shoes and pants while walking could be a strategy for preventing viper snakebites. Another rare case of viper (Protobothrops muscrosquamatus) snakebite to the head did not show any cerebral complications [129], suggesting that the site of the bite close to the brain may not be associated with snakebite-envenoming-induced cerebral complications. In addition, delayed treatment due to initial misidentification of the snake can cause irreversible tragedy [12]. Identification of features between venomous and nonvenomous [2] snakes might also be helpful to avoid snakebite envenoming and provide the best information for treatment. Finally, in a total of 33 cases of viper and elapid bite, 6 patients did not receive AV, leading to 3 deaths; this indicates that immediate hospitalization and treatment with AV remains crucial for survival after snakebite.
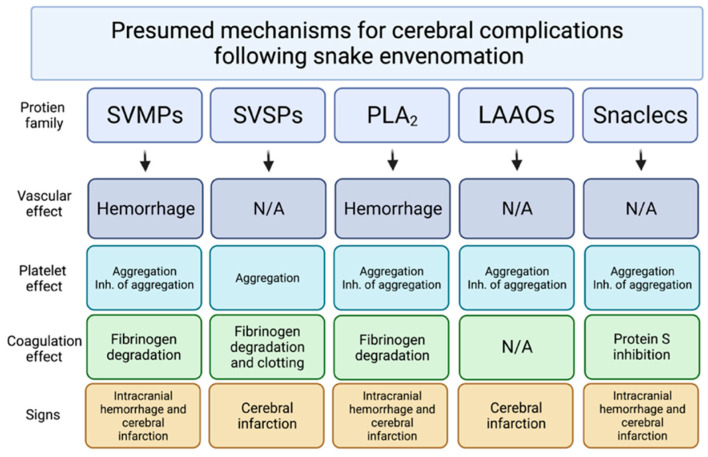
Figure 2.
Presumed mechanisms for cerebral complications following snakebite envenoming. SVMPs: Snake-venom metalloproteinases; SVSPs: Snake-venom serine proteases; PLA2: Phospholipase A2; LAAOs: L-amino-acid oxidase; Snaclecs: Snake C-type lectin-like proteins; N/A: Not applied. The figure is adapted from the previously described [75].
Author Contributions
Literature collection: Y.-K.H., H.-C.C. and C.-C.L.; drafting and revising of the manuscript: Y.-K.H., Y.-C.C., A.T.T. and K.-C.C.; All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
All the authors declare no competing interests.
Key Contribution
In this review article, we overview the case studies of cerebral complications after snakebite envenomings, including hemotoxic or neurotoxic, or both. This article also discusses the therapeutic strategies for snakebites.
Funding Statement
The work was supported by the NIH Core Grant P30-EY008098, the Eye and Ear Foundation of Pittsburgh, an unrestricted grant from Research to Prevent Blindness, New York, NY, USA and from Kaohsiung Municipal Ta-Tung Hospital (kmtth-107-020 and kmtth-108-022).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Russell F.E. When a snake strikes. Emerg. Med. 1990;22:21–43. [Google Scholar]
- 2.Gold B.S., Barish R.A., Dart R.C. North American snake envenomation: Diagnosis, treatment, and management. Emerg. Med. Clin. N. Am. 2004;22:423–443. doi: 10.1016/j.emc.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Kasturiratne A., Wickremasinghe A.R., de Silva N., Gunawardena N.K., Pathmeswaran A., Premaratna R., Savioli L., Lalloo D.G., de Silva H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5:e218. doi: 10.1371/journal.pmed.0050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tu A.T. Venoms: Chemistry and Molecular Biology. John. Wiley & Sons, Inc.; New York, NY, USA: 1977. p. 560. [Google Scholar]
- 5.Park K.H., Shin H., Kang H., Kim C., Choi H.J., Yoo K., Oh J., Lim T.H. Effectiveness of repeated antivenom therapy for snakebite-related systemic complications. J. Int. Med. Res. 2019;47:4808–4814. doi: 10.1177/0300060519870012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tasoulis T., Isbister G.K. A Review and Database of Snake Venom Proteomes. Toxins. 2017;9:290. doi: 10.3390/toxins9090290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Markland F.S., Jr., Swenson S. Snake venom metalloproteinases. Toxicon. 2013;62:3–18. doi: 10.1016/j.toxicon.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Stocker K., Barlow G.H. The coagulant enzyme from Bothrops atrox venom (batroxobin) Methods Enzym. 1976;45:214–223. doi: 10.1016/s0076-6879(76)45021-8. [DOI] [PubMed] [Google Scholar]
- 9.Serrano S.M. The long road of research on snake venom serine proteinases. Toxicon. 2013;62:19–26. doi: 10.1016/j.toxicon.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Silveira G.G., Machado C.R., Tuyama M., Lima M.A. Intracranial Bleeding Following Bothrops sp. Snakebite. Neurologist. 2016;21:11–12. doi: 10.1097/NRL.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 11.Malbranque S., Piercecchi-Marti M.D., Thomas L., Barbey C., Courcier D., Bucher B., Ridarch A., Smadja D., Warrell D.A. Fatal diffuse thrombotic microangiopathy after a bite by the "Fer-de-Lance" pit viper (Bothrops lanceolatus) of Martinique. Am. J. Trop. Med. Hyg. 2008;78:856–861. doi: 10.4269/ajtmh.2008.78.856. [DOI] [PubMed] [Google Scholar]
- 12.Namal Rathnayaka R.M., Kularatne S.A., Kumarasinghe K.D., Ranaweera J., Nishanthi Ranathunga P.E. Ischemic brain infarcts and intracranial haemorrhages following Russell’s viper (Daboia russelii) bite in Sri Lanka. Toxicon. 2017;125:70–73. doi: 10.1016/j.toxicon.2016.11.253. [DOI] [PubMed] [Google Scholar]
- 13.Rebahi H., Nejmi H., Abouelhassan T., Hasni K., Samkaoui M.A. Severe envenomation by Cerastes cerastes viper: An unusual mechanism of acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2014;23:169–172. doi: 10.1016/j.jstrokecerebrovasdis.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Kitchens C., Eskin T. Fatality in a case of envenomation by Crotalus adamanteus initially successfully treated with polyvalent ovine antivenom followed by recurrence of defibrinogenation syndrome. J. Med. Toxicol. 2008;4:180–183. doi: 10.1007/BF03161198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waiddyanatha S., Silva A., Siribaddana S., Isbister G.K. Long-term Effects of Snake Envenoming. Toxins. 2019;11:193. doi: 10.3390/toxins11040193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutierrez J.M., Rucavado A., Escalante T., Herrera C., Fernandez J., Lomonte B., Fox J.W. Unresolved issues in the understanding of the pathogenesis of local tissue damage induced by snake venoms. Toxicon. 2018;148:123–131. doi: 10.1016/j.toxicon.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y.C., Wang T.Y., Huang Y.K., Chang K.C., Chen M.H., Liu C.C., Liu K.L., Yang Y.H., Yen D.H., Fan J.S. Effects of Sodium Silicate Complex against Hemorrhagic Activities Induced by Protobothrops mucrosquamatus Venom. Toxins. 2021;13:59. doi: 10.3390/toxins13010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng X., Hu J., Liang X., Wu Y., Yan M., Zhu M., Fu Y. Acute cerebral infarction following a Trimeresurus stejnegeri snakebite: A case report. Medicine. 2019;98:e15684. doi: 10.1097/MD.0000000000015684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva de Oliveira S., Freitas-de-Sousa L.A., Alves E.C., de Lima Ferreira L.C., da Silva I.M., de Lacerda M.V.G., Fan H.W., Moura-da-Silva A.M., Monteiro W.M. Fatal stroke after Bothrops snakebite in the Amazonas state, Brazil: A case report. Toxicon. 2017;138:102–106. doi: 10.1016/j.toxicon.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 20.Sinha S., Naik B.B., Ghanekar J. Wall eyed bilateral internuclear ophthalmoplegia (WEBINO) syndrome as a false localising sign in intracranial haemorrhage due to snake bite. BMJ Case Rep. 2021;14:e244830. doi: 10.1136/bcr-2021-244830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu A., Shan R., Huang D., Zhou J., Keenoo A., Qin J. Case report: Acute demyelinating encephalomyelitis following viper bite. Medicine. 2016;95:e5310. doi: 10.1097/MD.0000000000005310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gewin V. Handling snakes for science. Nature. 2021;600:352. doi: 10.1038/d41586-021-03629-6. [DOI] [Google Scholar]
- 23.Magalhaes S.F.V., Peixoto H.M., Moura N., Monteiro W.M., de Oliveira M.R.F. Snakebite envenomation in the Brazilian Amazon: A descriptive study. Trans. R. Soc. Trop. Med. Hyg. 2019;113:143–151. doi: 10.1093/trstmh/try121. [DOI] [PubMed] [Google Scholar]
- 24.Huang J., Song W., Hua H., Yin X., Huang F., Alolga R.N. Antithrombotic and anticoagulant effects of a novel protein isolated from the venom of the Deinagkistrodon acutus snake. Biomed. Pharmacother. 2021;138:111527. doi: 10.1016/j.biopha.2021.111527. [DOI] [PubMed] [Google Scholar]
- 25.Chiang L.C., Tsai W.J., Liu P.Y., Ho C.H., Su H.Y., Lai C.S., Lai K.L., Lin W.L., Lee C.H., Yang Y.Y., et al. Envenomation by Trimeresurus stejnegeri stejnegeri: Clinical manifestations, treatment and associated factors for wound necrosis. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020;26:e20200043. doi: 10.1590/1678-9199-jvatitd-2020-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laothong C., Sitprija V. Decreased parasympathetic activities in Malayan krait (Bungarus candidus) envenoming. Toxicon. 2001;39:1353–1357. doi: 10.1016/S0041-0101(01)00087-3. [DOI] [PubMed] [Google Scholar]
- 27.Hojer J., Tran Hung H., Warrell D. Life-threatening hyponatremia after krait bite envenoming—A new syndrome. Clin. Toxicol. 2010;48:956–957. doi: 10.3109/15563650.2010.533677. [DOI] [PubMed] [Google Scholar]
- 28.Samanta S.K., Mahapatra N.C., Fariduddin K., Mazumdar D.B., Mandal K. Cortical blindness and paraplegia following hypoxic ischemic encephalopathy as a complication of common krait bite. Nepal. J. Ophthalmol. 2011;3:206–209. doi: 10.3126/nepjoph.v3i2.5280. [DOI] [PubMed] [Google Scholar]
- 29.Kaushik J.S., Chakrabarty B., Gulati S., Patel H., Lodha R., Pai G., Kumar A. Unusual late neurological complication in a child after an Indian krait bite. Pediatr. Neurol. 2014;51:130–132. doi: 10.1016/j.pediatrneurol.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 30.Anadure R.K., Narayanan C.S., Hande V., Singhal A., Varadaraj G. Two Cases of Early Morning Neuroparalytic Syndrome (EMNS) in the Tropics—Masquerading as Brain Death. J. Assoc. Physicians India. 2018;66:92–95. [PubMed] [Google Scholar]
- 31.ALfaifi M.S., ALOtaibi A.E., AlQahtani S.A., ALShahrani O.A., ALSharani K.M., ALbshabshi A.O., ALZahrani H.M., ALAli H.E. Cobra snakebite mimicking brain death treated with a novel combination of polyvalent snake antivenom and anticholinesterase. Am. J. Emerg. Med. 2020;38:2490.e5–2490.e7. doi: 10.1016/j.ajem.2020.05.111. [DOI] [PubMed] [Google Scholar]
- 32.Ratanabanangkoon K. A Quest for a Universal Plasma-Derived Antivenom Against All Elapid Neurotoxic Snake Venoms. Front. Immunol. 2021;12:668328. doi: 10.3389/fimmu.2021.668328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hermann P.M., Watson S.N., Wildering W.C. Phospholipase A2—Nexus of aging, oxidative stress, neuronal excitability, and functional decline of the aging nervous system? Insights from a snail model system of neuronal aging and age-associated memory impairment. Front. Genet. 2014;5:419. doi: 10.3389/fgene.2014.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang K.C., Huang Y.K., Chen Y.W., Chen M.H., Tu A.T., Chen Y.C. Venom Ophthalmia and Ocular Complications Caused by Snake Venom. Toxins. 2020;12:576. doi: 10.3390/toxins12090576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wüster W., Chirio L., Trape J.F., Ineich I., Jackson K., Greenbaum E., Barron C., Kusamba C., Nagy Z.T., Storey R., et al. Integration of nuclear and mitochondrial gene sequences and morphology reveals unexpected diversity in the forest cobra (Naja melanoleuca) species complex in Central and West Africa (Serpentes: Elapidae) Zootaxa. 2018;4455:68–98. doi: 10.11646/zootaxa.4455.1.3. [DOI] [PubMed] [Google Scholar]
- 36.Narang S.K., Paleti S., Azeez Asad M.A., Samina T. Acute ischemic infarct in the middle cerebral artery territory following a Russell’s viper bite. Neurol. India. 2009;57:479–480. doi: 10.4103/0028-3886.55594. [DOI] [PubMed] [Google Scholar]
- 37.Gouda S., Pandit V., Seshadri S., Valsalan R., Vikas M. Posterior circulation ischemic stroke following Russell’s viper envenomation. Ann. Indian Acad. Neurol. 2011;14:301–303. doi: 10.4103/0972-2327.91957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pothukuchi V.K., Kumar A., Teja C., Verma A. A Rare Case Series of Ischemic Stroke Following Russell’s Viper Snake Bite in India. Acta. Med. Indones. 2017;49:343–346. [PubMed] [Google Scholar]
- 39.Ittyachen A.M., Jose M.B. Thalamic infarction following a Russell’s viper bite. Southeast. Asian J. Trop. Med. Public Health. 2012;43:1201–1204. [PubMed] [Google Scholar]
- 40.Das S.K., Khaskil S., Mukhopadhyay S., Chakrabarti S. A patient of Russell’s viper envenomation presenting with cortical venous thrombosis: An extremely uncommon presentation. J. Postgrad. Med. 2013;59:235–236. doi: 10.4103/0022-3859.118051. [DOI] [PubMed] [Google Scholar]
- 41.Lahiri D., Sawale V.M., Dubey S., Roy B.K., Das S.K. Status epilepticus and bilateral middle cerebral artery infarction: A rare presentation after viper bite. Ann. Afr. Med. 2019;18:111–114. doi: 10.4103/aam.aam_21_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tripathy S., Routray P.K., Mohapatra A.K., Mohapatra M., Dash S.C. Acute demyelinating encephalomyelitis after anti-venom therapy in Russell’s viper bite. J. Med. Toxicol. 2010;6:318–321. doi: 10.1007/s13181-010-0015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prabhakar A.T., Kamanahalli R., Sivadasan A., Joseph E., Viggeswarpu S. Non-fatal acute haemorrhagic leukoencephalitis following snake bite: A case report. Trop. Doct. 2016;46:57–59. doi: 10.1177/0049475515577987. [DOI] [PubMed] [Google Scholar]
- 44.Ibrahim A.M., ElSefi T.T., Ghanem M., Fayed A.M., Shaban N.A. A Horned Viper Bite Victim with PRES. Case Rep. Neurol. Med. 2017;2017:1835796. doi: 10.1155/2017/1835796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heise C.W., Cunningham C., Ruha A.M., O’Connor A.D. One Bite, Two Patients: Disparate Clinical Courses Following Simultaneous Crotalus oreganus abyssus Envenomation. Wilderness Environ. Med. 2020;31:354–357. doi: 10.1016/j.wem.2020.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Tincu R.C., Ghiorghiu Z., Tomescu D., Macovei R.A. The Compartment Syndrome Associated with Deep Vein Thrombosis due to Rattlesnake Bite: A Case Report. Balk. Med. J. 2017;34:367–370. doi: 10.4274/balkanmedj.2016.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bosak A.R., Ruha A.M., Graeme K.A. A case of neurotoxicity following envenomation by the Sidewinder rattlesnake, Crotalus cerastes. J. Med. Toxicol. 2014;10:229–231. doi: 10.1007/s13181-013-0373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tilbury C.R., Peacock F., Harvey J. Envenomation by the spotted harlequin snake, Homoroselaps lacteus (Linnaeus) 1754 (Serpentes: Atractaspidinae) Toxicon. 2021;198:151–155. doi: 10.1016/j.toxicon.2021.04.020. [DOI] [PubMed] [Google Scholar]
- 49.Chauhan V., Thakur S. The North-South divide in snake bite envenomation in India. J. Emerg. Trauma Shock. 2016;9:151–154. doi: 10.4103/0974-2700.193350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suraweera W., Warrell D., Whitaker R., Menon G., Rodrigues R., Fu S.H., Begum R., Sati P., Piyasena K., Bhatia M., et al. Trends in snakebite deaths in India from 2000 to 2019 in a nationally representative mortality study. Elife. 2020;9:e54076. doi: 10.7554/eLife.54076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin J.H., Lo C.M., Chuang S.H., Chiang C.H., Wang S.D., Lin T.Y., Liao J.W., Hung D.Z. Collocation of avian and mammal antibodies to develop a rapid and sensitive diagnostic tool for Russell’s Vipers Snakebite. PLoS Negl. Trop. Dis. 2020;14:e0008701. doi: 10.1371/journal.pntd.0008701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silva A., Kuruppu S., Othman I., Goode R.J., Hodgson W.C., Isbister G.K. Neurotoxicity in Sri Lankan Russell’s Viper (Daboia russelii) Envenoming is Primarily due to U1-viperitoxin-Dr1a, a Pre-Synaptic Neurotoxin. Neurotox. Res. 2017;31:11–19. doi: 10.1007/s12640-016-9650-4. [DOI] [PubMed] [Google Scholar]
- 53.Fatah C., Samah S., Fatima L.D. Antiplatelet and anticoagulant activities of two phospholipase A2s purified from Cerastes cerastes venom: Structure-function relationship. J. Biochem. Mol. Toxicol. 2018;32:e22219. doi: 10.1002/jbt.22219. [DOI] [PubMed] [Google Scholar]
- 54.Keating G.M. Crotalidae polyvalent immune Fab: In patients with North American crotaline envenomation. BioDrugs. 2011;25:69–76. doi: 10.2165/11207250-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 55.Boyer L.V., Seifert S.A., Clark R.F., McNally J.T., Williams S.R., Nordt S.P., Walter F.G., Dart R.C. Recurrent and persistent coagulopathy following pit viper envenomation. Arch. Intern. Med. 1999;159:706–710. doi: 10.1001/archinte.159.7.706. [DOI] [PubMed] [Google Scholar]
- 56.Portillo F., Stanley E.L., Branch W.R., Conradie W., Rodel M.O., Penner J., Barej M.F., Kusamba C., Muninga W.M., Aristote M.M., et al. Evolutionary history of burrowing asps (Lamprophiidae: Atractaspidinae) with emphasis on fang evolution and prey selection. PLoS ONE. 2019;14:e0214889. doi: 10.1371/journal.pone.0214889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silva K.V., Said R.D.C., Assy J., Duarte M.R., Torrez P.P.Q., Franca F.O.S. A case of envenomation caused by Oxybelis fulgidus (Serpentes, Colubridae) in Brazilian Amazon. Rev. Soc. Bras. Med. Trop. 2019;52:e20180423. doi: 10.1590/0037-8682-0426-2018. [DOI] [PubMed] [Google Scholar]
- 58.Angarita-Sierra T., Montanez-Mendez A., Toro-Sanchez T., Rodriguez-Vargas A. A case of envenomation by the false fer-de-lance snake Leptodeira annulata (Linnaeus, 1758) in the department of La Guajira, Colombia. Biomedica. 2020;40:20–26. doi: 10.7705/biomedica.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lazaro R.P. Complex Regional Pain Syndrome Following Snakebite: A Putatively Rare Complication of Envenomation and Review of the Literature. Int. Med. Case Rep. J. 2020;13:603–607. doi: 10.2147/IMCRJ.S275591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heyborne W.H., Mackessy S.P. Venoms of New World Vinesnakes (Oxybelis aeneus and O. fulgidus) Toxicon. 2021;190:22–30. doi: 10.1016/j.toxicon.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 61.Heyborne W.H., Mackessy S.P. Identification and characterization of a taxon-specific three-finger toxin from the venom of the Green Vinesnake (Oxybelis fulgidus; family Colubridae) Biochimie. 2013;95:1923–1932. doi: 10.1016/j.biochi.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 62.Ipek S., Gungor S., Gullu U.U., Dalkiran T., Mercan M., Demiray S., Gurbuz Y. Snakebites in Pediatric Patients in Kahramanmaras: Is Pro-brain Natriuretic Peptide a Prognostic Biomarker for Snakebites? Cureus. 2022;14:e21570. doi: 10.7759/cureus.21570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.House L.M., Lewin M.R., Naidu R.K., Beqaj H. Complex regional pain syndrome following southern pacific rattlesnake (C. oreganus helleri) envenoming. Clin. Case Rep. 2021;9:e05019. doi: 10.1002/ccr3.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kamiguti A.S., Theakston R.D., Sherman N., Fox J.W. Mass spectrophotometric evidence for P-III/P-IV metalloproteinases in the venom of the Boomslang (Dispholidus typus) Toxicon. 2000;38:1613–1620. doi: 10.1016/S0041-0101(00)00089-1. [DOI] [PubMed] [Google Scholar]
- 65.Ching A.T., Paes Leme A.F., Zelanis A., Rocha M.M., Furtado Mde F., Silva D.A., Trugilho M.R., da Rocha S.L., Perales J., Ho P.L., et al. Venomics profiling of Thamnodynastes strigatus unveils matrix metalloproteinases and other novel proteins recruited to the toxin arsenal of rear-fanged snakes. J. Proteome Res. 2012;11:1152–1162. doi: 10.1021/pr200876c. [DOI] [PubMed] [Google Scholar]
- 66.Urra F.A., Miranda-Calle A.B., Araya-Maturana R. Philodryas (Serpentes: Dipsadidae) Envenomation, a Neglected Issue in Chile. Toxins. 2019;11:697. doi: 10.3390/toxins11120697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bastida J., Crampet A., Meneghel M., Morais V. Preliminary Biochemical and Venomic Characterization of the Venom of Phalotris lemniscatus (Serpentes, Colubridae) Curr. Top. Med. Chem. 2019;19:1981–1989. doi: 10.2174/1568026619666190802143252. [DOI] [PubMed] [Google Scholar]
- 68.Peichoto M.E., Teibler P., Ruiz R., Leiva L., Acosta O. Systemic pathological alterations caused by Philodryas patagoniensis colubrid snake venom in rats. Toxicon. 2006;48:520–528. doi: 10.1016/j.toxicon.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 69.Al-Sadawi M., Mohamadpour M., Zhyvotovska A., Ahmad T., Schechter J., Soliman Y., McFarlane S.I. Cerebrovascular Accident and Snake Envenomation: A Scoping Study. Int. J. Clin. Res. Trials. 2019;4:133. doi: 10.15344/2456-8007/2019/133. [DOI] [Google Scholar]
- 70.Abumiya T., Fitridge R., Mazur C., Copeland B.R., Koziol J.A., Tschopp J.F., Pierschbacher M.D., del Zoppo G.J. Integrin alpha(IIb)beta(3) inhibitor preserves microvascular patency in experimental acute focal cerebral ischemia. Stroke. 2000;31:1402–1409. doi: 10.1161/01.STR.31.6.1402. discussion 1409–1410. [DOI] [PubMed] [Google Scholar]
- 71.Tian H., Liu M., Li J., Xu R., Long C., Li H., Mwangi J., Lu Q., Lai R., Shen C. Snake C-Type Lectins Potentially Contribute to the Prey Immobilization in Protobothrops mucrosquamatus and Trimeresurus stejnegeri Venoms. Toxins. 2020;12:105. doi: 10.3390/toxins12020105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clemetson K.J. Snaclecs (snake C-type lectins) that inhibit or activate platelets by binding to receptors. Toxicon. 2010;56:1236–1246. doi: 10.1016/j.toxicon.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 73.Del Brutto O.H., Del Brutto V.J. Neurological complications of venomous snake bites: A review. Acta Neurol. Scand. 2012;125:363–372. doi: 10.1111/j.1600-0404.2011.01593.x. [DOI] [PubMed] [Google Scholar]
- 74.Fry B.G., Wuster W. Assembling an arsenal: Origin and evolution of the snake venom proteome inferred from phylogenetic analysis of toxin sequences. Mol. Biol. Evol. 2004;21:870–883. doi: 10.1093/molbev/msh091. [DOI] [PubMed] [Google Scholar]
- 75.Larreche S., Chippaux J.P., Chevillard L., Mathe S., Resiere D., Siguret V., Megarbane B. Bleeding and Thrombosis: Insights into Pathophysiology of Bothrops Venom-Related Hemostasis Disorders. Int. J. Mol. Sci. 2021;22:9643. doi: 10.3390/ijms22179643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rodrigues C.R., Teixeira-Ferreira A., Vargas F.F.R., Guerra-Duarte C., Costal-Oliveira F., Stransky S., Lopes-de-Souza L., Dutra A.A.A., Yarleque A., Bonilla C., et al. Proteomic profile, biological activities and antigenic analysis of the venom from Bothriopsis bilineata smaragdina (“loro machaco”), a pitviper snake from Peru. J. Proteomics. 2018;187:171–181. doi: 10.1016/j.jprot.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 77.Kalita B., Singh S., Patra A., Mukherjee A.K. Quantitative proteomic analysis and antivenom study revealing that neurotoxic phospholipase A2 enzymes, the major toxin class of Russell’s viper venom from southern India, shows the least immuno-recognition and neutralization by commercial polyvalent antivenom. Int. J. Biol. Macromol. 2018;118:375–385. doi: 10.1016/j.ijbiomac.2018.06.083. [DOI] [PubMed] [Google Scholar]
- 78.Rodrigues C.R., Molina Molina D.A., de Souza D.L.N., Cardenas J., Costal-Oliveira F., Guerra-Duarte C., Chavez-Olortegui C. Biological and proteomic characterization of the venom from Peruvian Andes rattlesnake Crotalus durissus. Toxicon. 2022;207:31–42. doi: 10.1016/j.toxicon.2021.12.008. [DOI] [PubMed] [Google Scholar]
- 79.Kini R.M. Serine proteases affecting blood coagulation and fibrinolysis from snake venoms. Pathophysiol. Haemost. Thromb. 2005;34:200–204. doi: 10.1159/000092424. [DOI] [PubMed] [Google Scholar]
- 80.Sajevic T., Leonardi A., Krizaj I. Haemostatically active proteins in snake venoms. Toxicon. 2011;57:627–645. doi: 10.1016/j.toxicon.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 81.Warrell D.A. Snake bite. Lancet. 2010;375:77–88. doi: 10.1016/S0140-6736(09)61754-2. [DOI] [PubMed] [Google Scholar]
- 82.Paul G., Paul B.S., Puri S. Snake bite and stroke: Our experience of two cases. Indian J. Crit. Care Med. 2014;18:257–258. doi: 10.4103/0972-5229.130585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alangode A., Reick M., Reick M. Sodium oleate, arachidonate, and linoleate enhance fibrinogenolysis by Russell’s viper venom proteinases and inhibit FXIIIa; a role for phospholipase A2 in venom induced consumption coagulopathy. Toxicon. 2020;186:83–93. doi: 10.1016/j.toxicon.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 84.Thomas L., Tyburn B., Bucher B., Pecout F., Ketterle J., Rieux D., Smadja D., Garnier D., Plumelle Y. Prevention of thromboses in human patients with Bothrops lanceolatus envenoming in Martinique: Failure of anticoagulants and efficacy of a monospecific antivenom. Research Group on Snake Bites in Martinique. Am. J. Trop. Med. Hyg. 1995;52:419–426. doi: 10.4269/ajtmh.1995.52.419. [DOI] [PubMed] [Google Scholar]
- 85.Fox J.W., Serrano S.M. Structural considerations of the snake venom metalloproteinases, key members of the M12 reprolysin family of metalloproteinases. Toxicon. 2005;45:969–985. doi: 10.1016/j.toxicon.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 86.Kouyoumdjian J.A., Polizelli C. [Snake bites by Bothrops moojeni: Correlation of the clinical picture with the snake size] Rev. Inst. Med. Trop Sao Paulo. 1989;31:84–90. doi: 10.1590/S0036-46651989000200004. [DOI] [PubMed] [Google Scholar]
- 87.Cole J., Evans E., Mwangi M., Mar S. Acute Disseminated Encephalomyelitis in Children: An Updated Review Based on Current Diagnostic Criteria. Pediatr. Neurol. 2019;100:26–34. doi: 10.1016/j.pediatrneurol.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 88.Weatherall D.J., Ledingham J., Warrell D.A. Injuries, Envenoming, Poisoning, and Allergic Reactions Caused by Animals. Oxford University Press; Oxford, UK: 1996. [Google Scholar]
- 89.Grzonka P., Scholz M.C., De Marchis G.M., Tisljar K., Ruegg S., Marsch S., Fladt J., Sutter R. Acute Hemorrhagic Leukoencephalitis: A Case and Systematic Review of the Literature. Front. Neurol. 2020;11:899. doi: 10.3389/fneur.2020.00899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Del Brutto O.H. Reversible posterior leukoencephalopathy after venomous bites and stings. Neurotoxicology. 2013;39:10. doi: 10.1016/j.neuro.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 91.Porcello Marrone L.C., Marrone B.F., Neto F.K., Costa F.C., Thome G.G., Aramburu M.B., Schilling L.P., Pascoal T.A., Gadonski G., Huf Marrone A.C., et al. Posterior reversible encephalopathy syndrome following a scorpion sting. J. Neuroimaging. 2013;23:535–536. doi: 10.1111/jon.12017. [DOI] [PubMed] [Google Scholar]
- 92.Loh H.H., Tan C.H. Acute renal failure and posterior reversible encephalopathy syndrome following multiple wasp stings: A case report. Med. J. Malaysia. 2012;67:133–135. [PubMed] [Google Scholar]
- 93.Haneef M., George D.E., Babu A.S. Early morning neuroparalytic syndrome. Indian J. Pediatr. 2009;76:1072. doi: 10.1007/s12098-009-0210-2. [DOI] [PubMed] [Google Scholar]
- 94.Bawaskar H.S., Bawaskar P.H. Envenoming by the common krait (Bungarus caeruleus) and Asian cobra (Naja naja): Clinical manifestations and their management in a rural setting. Wilderness Environ. Med. 2004;15:257–266. doi: 10.1580/1080-6032(2004)015[0257:EBTCKB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 95.Damm M., Hempel B.F., Sussmuth R.D. Old World Vipers-A Review about Snake Venom Proteomics of Viperinae and Their Variations. Toxins. 2021;13:427. doi: 10.3390/toxins13060427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.You K.E., Koo M.A., Lee D.H., Kwon B.J., Lee M.H., Hyon S.H., Seomun Y., Kim J.T., Park J.C. The effective control of a bleeding injury using a medical adhesive containing batroxobin. Biomed. Mater. 2014;9:025002. doi: 10.1088/1748-6041/9/2/025002. [DOI] [PubMed] [Google Scholar]
- 97.Guan J., Song S., Wang W., Ji X., Meng R. Cerebral venous sinus thrombosis due to external compression of internal jugular vein. J. Int. Med. Res. 2021;49:3000605211006609. doi: 10.1177/03000605211006609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Song S.Y., Dornbos D., 3rd, Lan D., Jiao B.L., Wan S.L., Guo Y.B., Ding Y.C., Yang Q., Ji X.M., Meng R. High-Resolution Magnetic Resonance Black Blood Thrombus Imaging and Serum D-Dimer in the Confirmation of Acute Cortical Vein Thrombosis. Front. Neurol. 2021;12:680040. doi: 10.3389/fneur.2021.680040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Singh S., Chattopadhya A., Sud A., Wanchu A., Bambery P. Acute paraplegia following viper bite. J. Assoc. Physicians India. 2002;50:1427–1429. [PubMed] [Google Scholar]
- 100.Chacur M., Gutierrez J.M., Milligan E.D., Wieseler-Frank J., Britto L.R., Maier S.F., Watkins L.R., Cury Y. Snake venom components enhance pain upon subcutaneous injection: An initial examination of spinal cord mediators. Pain. 2004;111:65–76. doi: 10.1016/j.pain.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 101.Chacur M., Milligan E.D., Sloan E.M., Wieseler-Frank J., Barrientos R.M., Martin D., Poole S., Lomonte B., Gutierrez J.M., Maier S.F., et al. Snake venom phospholipase A2s (Asp49 and Lys49) induce mechanical allodynia upon peri-sciatic administration: Involvement of spinal cord glia, proinflammatory cytokines and nitric oxide. Pain. 2004;108:180–191. doi: 10.1016/j.pain.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 102.Harris J.B., Scott-Davey T. Secreted phospholipases A2 of snake venoms: Effects on the peripheral neuromuscular system with comments on the role of phospholipases A2 in disorders of the CNS and their uses in industry. Toxins. 2013;5:2533–2571. doi: 10.3390/toxins5122533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Biswas R., Irodi A., Paul A., Ghimere G., Joshi K.R., Alurkar V.M., Shetty K.J. Anti-venom-induced myelopathy in a semipoisonous snakebite. Int. J. Clin. Pract. 2004;58:645–646. doi: 10.1111/j.1368-5031.2004.00201.x. [DOI] [PubMed] [Google Scholar]
- 104.Aye M.T.H., Naing T., Myint K.T. Unusual ocular manifestations following viper bite. BMJ Case Rep. 2018;2018:bcr2018225040. doi: 10.1136/bcr-2018-225040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Olcaysu O.O., Cadirci K., Altun A., Durur Karakaya A., Bayramlar H. Unilateral Optic Neuropathy and Acute Angle-Closure Glaucoma following Snake Envenomation. Case Rep. Ophthalmol. Med. 2015;2015:687829. doi: 10.1155/2015/687829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kumar J.R., Basavarajappa B.S., Vishwanath B.S., Gowda T.V. Biochemical and pharmacological characterization of three toxic phospholipase A2s from Daboia russelii snake venom. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2015;168:28–38. doi: 10.1016/j.cbpc.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 107.Cherifi F., Namane A., Laraba-Djebari F. Isolation, functional characterization and proteomic identification of CC2-PLA(2) from Cerastes cerastes venom: A basic platelet-aggregation-inhibiting factor. Protein J. 2014;33:61–74. doi: 10.1007/s10930-013-9534-x. [DOI] [PubMed] [Google Scholar]
- 108.Fry B.G. Snakebite: When the Human Touch Becomes a Bad Touch. Toxins. 2018;10:170. doi: 10.3390/toxins10040170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bhattacharya S., Krishnamurthy A., Gopalakrishnan M., Kalra S., Kantroo V., Aggarwal S., Surana V. Endocrine and Metabolic Manifestations of Snakebite Envenoming. Am. J. Trop. Med. Hyg. 2020;103:1388–1396. doi: 10.4269/ajtmh.20-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Frank H.A. Snakebite or frostbite: What are we doing? An evaluation of cryotherapy for envenomation. Calif. Med. 1971;114:25–27. [PMC free article] [PubMed] [Google Scholar]
- 111.Yang Y., Huang Z., Zhang X. Efficacy and safety of clopidogrel and/or aspirin for ischemic stroke/transient ischemic attack: An overview of systematic reviews and meta-analysis. Medicine. 2021;100:e27804. doi: 10.1097/MD.0000000000027804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ryu W.S., Schellingerhout D., Hong K.S., Jeong S.W., Kim B.J., Kim J.T., Lee K.B., Park T.H., Park S.S., Park J.M., et al. Relation of Pre-Stroke Aspirin Use With Cerebral Infarct Volume and Functional Outcomes. Ann. Neurol. 2021;90:763–776. doi: 10.1002/ana.26219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gutierrez J.M., Escalante T., Rucavado A., Herrera C. Hemorrhage Caused by Snake Venom Metalloproteinases: A Journey of Discovery and Understanding. Toxins. 2016;8:93. doi: 10.3390/toxins8040093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bjarnason J.B., Tu A.T. Hemorrhagic toxins from Western diamondback rattlesnake (Crotalus atrox) venom: Isolation and characterization of five toxins and the role of zinc in hemorrhagic toxin e. Biochemistry. 1978;17:3395–3404. doi: 10.1021/bi00609a033. [DOI] [PubMed] [Google Scholar]
- 115.Friederich C., Tu A.T. Role of metals in snake venoms for hemorrhagic, esterase and proteolytic activities. Biochem. Pharmacol. 1971;20:1549–1556. doi: 10.1016/0006-2952(71)90283-8. [DOI] [PubMed] [Google Scholar]
- 116.Ownby C.L., Tu A.T., Kainer R.A. Effect of diethylenetriaminepentaacetic acid and procaine on hemorrhage induced by rattlesnake venom. J. Clin. Pharmacol. 1975;15:419–426. doi: 10.1002/j.1552-4604.1975.tb02363.x. [DOI] [PubMed] [Google Scholar]
- 117.Ownby C.L. In: The Merck Manual of Diagnosis and Therapy. 13th ed. Talbott R.B.J.H., editor. Merck & Co., Incorp; Rahway, NJ, USA: 1972. [Google Scholar]
- 118.Albulescu L.O., Hale M.S., Ainsworth S., Alsolaiss J., Crittenden E., Calvete J.J., Evans C., Wilkinson M.C., Harrison R.A., Kool J., et al. Preclinical validation of a repurposed metal chelator as an early-intervention therapeutic for hemotoxic snakebite. Sci. Transl. Med. 2020;12:eaay8314. doi: 10.1126/scitranslmed.aay8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lewin M., Samuel S., Merkel J., Bickler P. Varespladib (LY315920) Appears to Be a Potent, Broad-Spectrum, Inhibitor of Snake Venom Phospholipase A2 and a Possible Pre-Referral Treatment for Envenomation. Toxins. 2016;8:248. doi: 10.3390/toxins8090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Salvador G.H.M., Gomes A.A.S., Bryan-Quiros W., Fernandez J., Lewin M.R., Gutierrez J.M., Lomonte B., Fontes M.R.M. Structural basis for phospholipase A2-like toxin inhibition by the synthetic compound Varespladib (LY315920) Sci. Rep. 2019;9:17203. doi: 10.1038/s41598-019-53755-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zinenko O., Tovstukha I., Korniyenko Y. PLA2 Inhibitor Varespladib as an Alternative to the Antivenom Treatment for Bites from Nikolsky’s Viper Vipera berus nikolskii. Toxins. 2020;12:356. doi: 10.3390/toxins12060356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gutierrez J.M., Lewin M.R., Williams D.J., Lomonte B. Varespladib (LY315920) and Methyl Varespladib (LY333013) Abrogate or Delay Lethality Induced by Presynaptically Acting Neurotoxic Snake Venoms. Toxins. 2020;12:131. doi: 10.3390/toxins12020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gutierrez J.M., Albulescu L.O., Clare R.H., Casewell N.R., Abd El-Aziz T.M., Escalante T., Rucavado A. The Search for Natural and Synthetic Inhibitors That Would Complement Antivenoms as Therapeutics for Snakebite Envenoming. Toxins. 2021;13:451. doi: 10.3390/toxins13070451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Abouyannis M., FitzGerald R., Ngama M., Mwangudzah H., Nyambura Y.K., Ngome S., Riako D., Babu L., Lewa F., Else L., et al. TRUE-1: Trial of Repurposed Unithiol for snakebite Envenoming phase 1 (safety, tolerability, pharmacokinetics and pharmacodynamics in healthy Kenyan adults) Wellcome Open Res. 2022;7:90. doi: 10.12688/wellcomeopenres.17682.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li M., Xie Z.H., Yu A.Y., He D.P. Increased Efficacy of Antivenom Combined with Hyperbaric Oxygen on Deinagkistrodon acutus Envenomation in Adult Rats. Chin. Med. J. (Engl.) 2018;131:323–329. doi: 10.4103/0366-6999.223840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bekbossynova A., Zharylgap A., Filchakova O. Venom-Derived Neurotoxins Targeting Nicotinic Acetylcholine Receptors. Molecules. 2021;26:3373. doi: 10.3390/molecules26113373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.de la Rosa G., Olvera F., Archundia I.G., Lomonte B., Alagon A., Corzo G. Horse immunization with short-chain consensus alpha-neurotoxin generates antibodies against broad spectrum of elapid venomous species. Nat. Commun. 2019;10:3642. doi: 10.1038/s41467-019-11639-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Montoni F., Andreotti D.Z., Eichler R., Santos W.D.S., Kisaki C.Y., Arcos S.S.S., Lima I.F., Soares M.A.M., Nishiyama-Jr M.Y., Nava-Rodrigues D., et al. The impact of rattlesnake venom on mice cerebellum proteomics points to synaptic inhibition and tissue damage. J. Proteomics. 2020;221:103779. doi: 10.1016/j.jprot.2020.103779. [DOI] [PubMed] [Google Scholar]
- 129.Yeh Y.T., Chen M.H., Chang J.C., Fan J.S., Yen D.H., Chen Y.C. Protobothrops mucrosquamatus Bites to the Head: Clinical Spectrum from Case Series. Am. J. Trop. Med. Hyg. 2018;99:753–755. doi: 10.4269/ajtmh.18-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in this article.