Abstract
Resting cells of the sulfate-reducing bacterium Desulfovibrio fructosovorans grown in the absence of sulfate had a very high Tc(VII)-reducing activity, which led to the formation of an insoluble black precipitate. The involvement of a periplasmic hydrogenase in Tc(VII) reduction was indicated (i) by the requirement for hydrogen as an electron donor, (ii) by the tolerance of this activity to oxygen, and (iii) by the inhibition of this activity by Cu(II). Moreover, a mutant carrying a deletion in the nickel-iron hydrogenase operon showed a dramatic decrease in the rate of Tc(VII) reduction. The restoration of Tc(VII) reduction by complementation of this mutation with nickel-iron hydrogenase genes demonstrated the specific involvement of the periplasmic nickel-iron hydrogenase in the mechanism in vivo. The Tc(VII)-reducing activity was also observed with cell extracts in the presence of hydrogen. Under these conditions, Tc(VII) was reduced enzymatically to soluble Tc(V) or precipitated to an insoluble black precipitate, depending on the chemical nature of the buffer used. The purified nickel-iron hydrogenase performed Tc(VII) reduction and precipitation at high rates. These series of genetic and biochemical approaches demonstrated that the periplasmic nickel-iron hydrogenase of sulfate-reducing bacteria functions as a Tc(VII) reductase. The role of cytochrome c3 in the mechanism is also discussed.
Technetium (99Tc) is a fission product of 235U formed during the generation of nuclear power. The solubility and mobility of Tc are highly dependent upon its redox state. Under oxic conditions, Tc is present in its most stable form, the pertechnetate anion [Tc(VII)O4−]. This form, which is highly soluble and mobile in the environment, can enter the food chain as a sulfate analogue (2, 20, 34). These properties, coupled with its long half-life (2.13 × 105 years), make contamination by Tc one of the major factors in the long-term impact of the nuclear fuel cycle. One approach to remove 99Tc from aqueous solution is to reduce the pertechnetate form Tc(VII) into the insoluble, low-valence form Tc(IV). This can be achieved by abiotic (5) or biotic (19, 20) processes.
Abiotic reduction involves electron transfer between Fe(II)-containing minerals and Tc(VII) (3). The most efficient mineral appears to be magnetite, particularly when it is anodically polarized (5). Biotic precipitation of pertechnetate, probably its reduction into a low-valence, insoluble Tc oxide, has been reported for several species of bacteria during the past few years. These include species such as Geobacter metallireducens (14), Geobacter sulfurreducens (17), Escherichia coli (13), Desulfovibrio desulfuricans (16), Shewanella putrefaciens (33), and Deinococcus radiodurans (9). Both indirect (chemical) and direct (enzymatic) reduction processes have been observed, depending on the bacterial growth conditions. Chemical processes have been clearly demonstrated in the case of the sulfate-reducing bacterium D. desulfuricans and in the case of the metal-reducing bacterium G. sulfurreducens (15, 17). Cultures of D. desulfuricans supplied with sulfate and lactate as electron acceptor and donor, respectively, precipitated Tc extracellularly as an insoluble sulfide. In this case, the Tc sulfide results from the chemical reaction between H2S, formed during reduction of sulfate, and TcO4− (19). A chemical reduction of Tc(VII) by the Fe(II) is also observed during reduction of Fe(III) by G. sulfurreducens (17). In addition, enzymatic reduction of Tc(VII) has been reported for different bacterial species. There are several lines of evidence indicating that this process involves hydrogenase, an enzyme which reversibly catalyzes the splitting of molecular hydrogen into protons and electrons. Indeed, hydrogen is an effective electron donor for Tc(VII) reduction for E. coli, D. desulfuricans, and S. putrefaciens (13, 15, 16, 33). Similarly, in the case of G. sulfurreducens, Tc(VII) reduction has an exclusive requirement for hydrogen as the electron donor, unlike the reduction of Fe(III), which can be coupled to the oxidation of different organic electron donors (17). The most convincing evidence has come from the work of Lloyd et al. (13), who showed that mutants of E. coli defective in the synthesis of transcription factor FNR, of molybdenum cofactor, or of formate dehydrogenase H were unable to reduce Tc(VII), indicating a role for the formate-hydrogenlyase complex in the reduction.
Desulfovibrio fructosovorans (24) is a sulfate-reducing bacterium amenable to molecular biological study. Three different hydrogenases have been identified in this bacterium: [NiFe] and [Fe] hydrogenases (1, 10, 27), localized in the periplasm, and a heterotetrameric NADP-reducing [Fe] hydrogenase, localized in the cytoplasm (4, 21, 22). In the present work, we have combined physiological, genetic, and biochemical approaches to determine, at the molecular level, the exact roles of these different hydrogenases in Tc(VII) reduction and precipitation.
MATERIALS AND METHODS
Growth of organisms and preparation of extracts.
The wild-type strain D. fructosovorans DSM 3604 (24), D. fructosovorans strain MR400 carrying a deletion in the nickel-iron hydrogenase operon (26), or strain MR400 complemented with the nickel-iron hydrogenase genes (28) was grown for 72 h at 37°C in stoppered 100-ml bottles supplemented, when required, with kanamycin and gentamicin (50 μg/ml) in a minimal medium defined by Widdel and Pfennig (32). For Tc(VII) reduction assay, the strains were subcultured three times in a medium containing 20 mM fructose as an electron donor and 20 mM fumarate as an electron acceptor.
D. fructosovorans cells grown to an optical density at 600 nm of 1.0 (20 g [wet weight]) were collected by centrifugation at 2,000 × g, washed twice with Tris-HCl (10 mM, pH 7.6), and stored at −80°C before use. Unless otherwise noted, all operations were performed under air at 4°C. Freshly thawed cells were passed twice in a French pressure cell at 1,000 lb/in2 pressure in the presence of a few crystals of DNase. Cell debris were removed by centrifugation at 4,000 × g for 30 min, and the supernatant (crude extract) was then centrifuged at 120,000 × g for 1 h. The resulting soluble fraction was used for purification and for Tc(VII) reduction.
Tc(VII) reduction by resting cell suspensions or purified proteins.
Bacteria grown for 72 h at 37°C were transferred anaerobically in a centrifuge tube stoppered with a rubber septum (Suba seal no. 37; Aldrich) and washed four times in 50 mM Tris-HCl buffer (pH 8.0). The bacterial pellet was resuspended anaerobically in either Tris-HCl (20 mM, pH 8.0 or 8.5), MES (morpholineethanesulfonic acid) (20 mM, pH 5.5), MOPS (morpholinepropanesulfonic acid) (20 mM, pH 6.5 or 7.5), or citrate-sodium phosphate buffer (20 mM, pH 4.5) to a concentration of about 0.5 mg of cells (dry weight) per ml. Aliquots (1.9 ml) of the washed cell suspension were transferred under nitrogen to 10-ml serum bottles sealed with butyl rubber stoppers. Electron donors (fructose, fumarate, lactate, pyruvate, or formate) were added from concentrated stock solutions to a final concentration of 10 mM. For these experiments, all of the bottles were depleted of oxygen by three cycles of vacuum-nitrogen and then flushed under nitrogen for 10 min. When hydrogen was supplied as an electron donor for metal reduction, the gas was flushed into the headspace of the bottles for 20 min with resting cell suspensions or for 180 min with soluble extracts or purified proteins in order to activate the nickel-iron hydrogenase, as described by Fernandez et al. (6) and Hatchikian et al. (10). A solution (100 μl) of ammonium pertechnetate (NH4TcO4) (Amersham Life Science Products, Orsay, France, and NEN Life Science Products, Paris, France), deaerated by flushing with argon 10 min before use, was added to a final concentration of 1 mM for cell suspensions. Concentrations of 250 μM to 6 mM were used for Km and Vmax determination, and a concentration of 0.5 mM was used for reduction by soluble extracts or by purified proteins.
Measurements of Tc.
Total Tc in solution was assayed by autoradiography with a STORM 840 PhosphorImager (Molecular Dynamics) as described by Lloyd and Macaskie (14). Tc uptake was expressed as the percentage or the concentration of Tc remaining in solution after centrifugation in an Eppendorf 5415C centrifuge (14,000 rpm, 20 min) in comparison with total Tc. Tc(VII) (Rf = 0.7) was also separated from reduced, nonmobile [Tc(V); Rf = 0.0] and mobile [mainly Tc(IV); Rf = 0.9] soluble Tc species using paper chromatography (29) prior to autoradiography and quantification using a PhosphorImager. In most experiments, concentrations of Tc were also quantified using a Packard 1900 TR analyzer. Each sample (10 μl) was added to a glass scintillation vial with 10 ml of Ultima Gold or Insta-Gel-Plus scintillation fluid (Packard Instrument S. A., Rungis, France). Disintegration counts per minute were recorded at between 20 and 250 keV for 5 min.
Purification of cytochrome c3 and [NiFe] hydrogenase.
Pure cytochrome c3 and [NiFe] hydrogenase were obtained in four steps, and all operations were performed at pH 7.6. In the first step, the soluble fraction was loaded on a DEAE-Sepharose (Pharmacia) column. This column retains all of the hydrogenase but not the cytochrome c3. The fraction containing cytochrome c3 was subsequently loaded on an SP-Sepharose (Pharmacia) column equilibrated with Tris-HCl (10 mM, pH 7.6) and eluted at 200 mM NaCl. The eluted fraction was then concentrated in a 15-ml Centriprep YM-10 (Centrifugal Filter Device; Amicon) and filtered through a Sephacryl S-200 high-resolution column (Pharmacia). Finally, cytochrome was loaded on a hydroxylapatite (Bio-Rad) column and eluted at 200 mM potassium phosphate (pH 7.6). Purified cytochrome exhibited a broad single band of 16.5 kDa in sodium dodecyl sulfate–15% polyacrylamide gel electrophoresis and a purity index (A553reduced − A570reduced/A280oxidized) of 3.02.
The hydrogenase fraction was eluted from the first DEAE-Sepharose column with 100 mM NaCl. This fraction was subsequently loaded on a Q-Sepharose (Pharmacia) column equilibrated with Tris-HCl (10 mM, pH 7.6) and eluted at 320 mM NaCl. The eluted fraction was then concentrated in a 50-ml ultrafiltration cell with a PM 30 membrane (Amicon) and filtered through a Sephacryl S-200 high-resolution column (Pharmacia). Finally, hydrogenase was loaded on a hydroxylapatite (Bio-Rad) column and eluted at 180 mM potassium phosphate (pH 7.6). The hydrogenase was judged to be homogeneous by the following criteria: (i) native polyacrylamide gel electrophoresis giving a single band of protein which catalyzed the hydrogen-dependent reduction of methyl viologen, (ii) the presence of two single bands at 29 and 60 kDa after sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and (iii) an absorbance ratio (A400/A280) equal to 0.28.
Analytical procedures.
Protein concentrations were measured with a bicinchoninic acid assay kit (Pierce) by the method of Smith et al. (30), using bovine serum albumin as a standard. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was carried out by the method of Laemmli (11). The hydrogen uptake activity was visualized in the gel after native 7.5% polyacrylamide gel electrophoresis as previously described (4). UV-visible spectra of the pure nickel-iron hydrogenase and the cytochrome c3 were recorded on a Varian spectrophotometer. The hydrogen uptake activity was measured spectrophotometrically by monitoring the reduction of methyl viologen (6) at 30°C in a tightly closed quartz cuvette filled with 1 ml of reaction buffer (50 mM Tris-HCl [pH 8], 1 mM methyl viologen) bubbled for 20 min with H2. The proton-deuterium (H-D) exchange reaction was measured in whole-cell suspensions by a mass spectrometric method as described previously (31). Production of H2 and HD was used to calculate the exchange activity.
RESULTS
Microbial reduction of technetium: physiology and kinetic parameters.
In order to study exclusively the enzymatic contribution to Tc(VII) reduction by sulfate-reducing bacteria, chemical Tc(VII) reduction was prevented by growing D. fructosovorans in a mineral medium in the absence of sulfate. Cells grown under these conditions had a greyish color with no black precipitate of iron sulfide. These conditions were preferred to those used by Lloyd et al. (15) for D. desulfuricans, which did not completely abolish the formation of iron sulfide in the case of D. fructosovorans (data not shown).
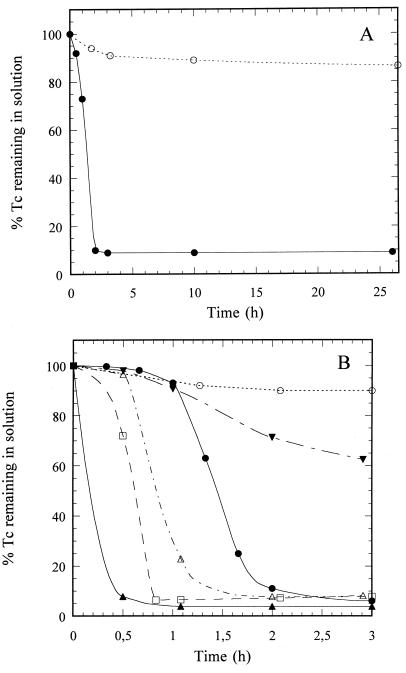
After 2 h of incubation under hydrogen, approximately 92% of the Tc(VII) (1 mM) present was reduced by a resting cell suspension of D. fructosovorans to an insoluble black precipitate (Fig. 1A), as observed in the case of S. putrefaciens (33). On the other hand, only a minor reduction (13%) occurred after 24 h when hydrogen was replaced by nitrogen (Fig. 1A and Table 1). Tc(VII) reduction occurred between pH 5.5 and 8.0 (Fig. 1B) and between 10 and 40°C (data not shown). The highest rate was observed at pH 5.5 and between 30 and 40°C, where an apparent Km of 2 mM and a maximal velocity of 7 mmol of Tc(VII) reduced per g (dry weight) of bacteria per h were determined using a Lineweaver-Burk plot.
FIG. 1.
(A) Tc(VII) reduction and precipitation by resting cells of D. fructosovorans supplied with hydrogen (closed circles) as an electron donor. Control cultures with nitrogen (open circles) contained no added electron donor. The cells were incubated for 24 h at 23°C and pH 8.0. (B) Effect of pH on Tc(VII) reduction by D. fructosovorans. Hydrogen was supplied as the electron donor, and the cells were incubated at room temperature (23°C) and pH 4.5 (open circles), 5.5 (closed triangles), 6.5 (open squares), 7.5 (open triangles), 8.0 (closed circles), or 8.5 (inverted closed triangles).
TABLE 1.
Effect of electron donor on Tc(VII) reduction and precipitation by resting cells of D. fructosovoransa
| Electron donor | Tc remaining in solution (mM) |
|---|---|
| Hydrogen | 0.08 |
| Pyruvate | 0.74 |
| Lactate | 0.81 |
| Fructose | 0.87 |
| Formate | 0.91 |
| Fumarate | 0.97 |
| None (control with nitrogen) | 0.87 |
Cells were incubated for 24 h at pH 5.5 and 30°C with 1 mM Tc(VII).
Several compounds, such as fructose, lactate, pyruvate, fumarate, and formate, which are efficient electron donors for sulfate reduction in D. fructosovorans (24) were tested for Tc(VII) reduction (Table 1). Only hydrogen appeared to be an efficient electron donor for Tc(VII) reduction (Table 1), suggesting the involvement of a hydrogenase in this process.
Inhibitors and genetic determinants.
The Tc(VII) reduction activity of D. fructosovorans was not inhibited by exposure of the cells to air, which indicated oxygen tolerance of the enzyme (Fig. 2). Moreover, this activity was irreversibly inhibited by a 10-min preincubation of the cells with 0.5 mM CuCl2, a specific inhibitor of the periplasmic hydrogen uptake activity in vivo (8) (Fig. 2). These results excluded a possible role of NADP-reducing hydrogenase in the reduction mechanism, as this enzyme is cytoplasmic and oxygen sensitive (4, 22). To investigate the role of the [NiFe] hydrogenase, which is periplasmic and oxygen tolerant and is the major hydrogenase produced by D. fructosovorans (10), Tc(VII) reduction by strain MR400, which carries a specific deletion of the structural genes of this hydrogenase (26), was studied. This deletion strain showed a dramatic decrease in the rate of reduction (Fig. 2): only 20% of the Tc(VII) was reduced in 3 h, although iron-only hydrogenase activity still represented about 16% of the wild-type level in both the hydrogen uptake and deuterium-hydrogen exchange activities (data not shown). Moreover, the complementation of strain MR400 by the nickel-iron hydrogenase genes carried on a multicopy plasmid (28) restored hydrogenase activity (hydrogen uptake and deuterium exchange) (data not shown) and Tc(VII) reductase activity (Fig. 2) to wild-type levels. These results demonstrated the essential role of the nickel-iron hydrogenase in the in vivo reduction of Tc(VII) by the sulfate-reducing bacterium D. fructosovorans.
FIG. 2.
Effect of hydrogenase inhibitors or hydrogenase contents on Tc(VII) reduction in D. fructosovorans. Cells were incubated at 23°C and pH 5.5 with hydrogen supplied as an electron donor. Open circles, wild-type strain (control); closed circles, wild-type strain after preincubation with air; closed squares, wild-type strain after preincubation with 0.5 mM CuCl2; closed triangles, mutant MR400 with the nickel-iron hydrogenase genes deleted; open triangles, complemented mutant MR400.
Reduction and precipitation of Tc(VII) by soluble extracts and purified proteins.
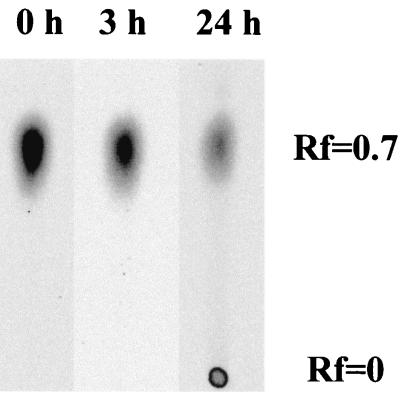
In order to provide biochemical evidence of the involvement of the [NiFe] hydrogenase and possibly other electron carriers in the biological reduction of Tc(VII), this activity was tested in different fractions: (i) crude extract (soluble and membranes proteins) and (ii) soluble fraction (supernatant from centrifugation at 120,000 × g) suspended in MOPS (20 mM, pH 6.5). In both cases, 85% of the Tc(VII) was reduced and precipitated as shown by the appearance of brownish particles after 18 h of incubation in the presence of hydrogen. No reduction or precipitation occurred when hydrogen was omitted. This experiment demonstrates that reduction of Tc(VII) is performed in vitro by soluble proteins. This further shows that the precipitation of reduced forms of Tc does not necessitate the presence of membranes as nucleation sites. On the other hand, the precipitation process is highly dependent on the buffer used. Indeed, when Tris-HCl (50 mM, pH 8.0) was used, 80% of the Tc(VII) (Rf = 0.7) was reduced to Tc(V) (Rf = 0) in the first 24 h as shown by paper chromatography (Fig. 3) (29), but no precipitation occurred. This result indicates that Tc(VII) reduction mediated by the soluble fraction is not obligately followed by precipitation. The precipitation process seems to be dependent mainly on the chemical nature of the buffer used and the pH. This behavior is different from that observed in vivo, where the precipitation of Tc is independent of the buffer used (Fig. 1B).
FIG. 3.
Chromatograms of Tc(VII) reduction by soluble fractions of D. fructosovorans at pH 8.0. Soluble proteins were incubated at 23°C and pH 8.0 (20 mM Tris-HCl) with hydrogen as an electron donor and 0.5 mM Tc(VII). Tc(VII), Rf = 0.7; Tc(V), Rf = 0.0.
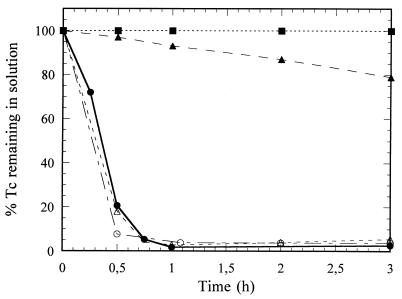
To identify the proteins and enzymes involved in Tc(VII) reduction in vitro, we have tested the involvement of the purified [NiFe] hydrogenase and its physiological electron acceptor (12, 25), cytochrome c3, in this process. Pure hydrogenase at a high concentration (3.9 μM) exhibited a high Tc(VII)-reducing activity [95% of Tc(VII) reduced in 2 h] (Table 2). Diluted hydrogenase (0.4 μM) reduced Tc(VII) more slowly, and 95% of the Tc(VII) was reduced only after 18 h. Preincubation with Cu(II) inhibited 85% of this activity. This value corresponded to the level of inhibition of methyl viologen reduction observed with Desulfovibrio gigas [NiFe] hydrogenase (7). On the other hand, purified cytochrome c3 alone (0.4 μM) did not precipitate or reduce Tc(VII) with hydrogen. In the presence of both [NiFe] hydrogenase and cytochrome c3 at low concentrations, reduction of Tc(VII) occurred in less than 1 h (Table 2).
TABLE 2.
Tc(VII) reduction and precipitation by soluble proteins of D. fructosovoransa
| Protein(s) tested | Concn
|
% Tc remaining in solution after (h):
|
||||
|---|---|---|---|---|---|---|
| mg/ml | μM | 0 | 1 | 2 | 18 | |
| Soluble fraction | 0.35 | 100 | 70 | 60 | 15 | |
| [NiFe] hydrogenase | 0.35 | 3.9 | 100 | 20 | 5 | 5 |
| [NiFe] hydrogenase | 0.037 | 0.4 | 100 | 100 | 100 | 5 |
| [NiFe] hydrogenase + 0.5 mM CuCl2 | 0.037 | 0.4 | 100 | 100 | 100 | 85 |
| Cytochrome c3 | 0.006 | 0.4 | 100 | 100 | 100 | 100 |
| Cytocrome c3 + [NiFe] hydrogenase | 0.006 + 0.037 | 0.4 + 0.4 | 100 | 5 | 5 | 5 |
Proteins were incubated at 30°C and pH 6.5 (20 mM MOPS) with hydrogen as an electron donor and 0.5 mM Tc(VII) as an electron acceptor.
The high reducing activity observed under such conditions may be related to the reactivation of the hydrogenase in the presence of cytochrome c3 (6). Indeed, the addition of the oxidative agent Tc(VII) (TcO4−/TcO2, E′o = +0.748 V) may have induced some inactivation of the hydrogenase, which is more readily reactivated in the presence of its physiological electron acceptor, cytochrome c3 (6).
DISCUSSION
In the present work, we report that D. fructosovorans reduces Tc(VII) and removes it efficiently from solution. The reduction process occurs at wide ranges of temperature (10 to 40°C) and pH (5.5 to 8.0). The optimum pH (around pH 5.5) probably reflects the best affinity of hydrogenase for TcO4−. Reduction of Tc(VII) with increasing Tc concentrations gave an apparent Km of 2 mM, which is slightly higher than but consistent with the Km of 0.5 mM determined by Lloyd et al. (18) with E. coli and D. desulfuricans supplied with formate as an electron donor. At a high concentration of TcO4− (6 mM), D. fructosovorans exhibits the highest rate of reduction described so far, i.e., 7 mmol of Tc reduced per g (dry weight) of bacteria per h. The corresponding values for E. coli and D. desulfuricans were 12.5 and 800 μmol of Tc reduced per g (dry weight) of bacteria per h, respectively (18). The high efficiency of the Tc(VII)-reducing activity of D. fructosovorans within wide ranges of pH and temperature makes this bacterium a good candidate for the removal of this radionuclide from solution in a bioremediation process.
The Tc(VII)-reducing activity of D. fructosovorans requires the presence of hydrogen as an electron donor, whereas organic electron donors such as lactate, pyruvate, fumarate, fructose, and formate are inefficient (Table 1). The essential role of the nickel-iron hydrogenase in the process of reduction of Tc(VII) was further proved by genetic and biochemical studies. This role is supported by (i) in vivo and in vitro inhibition of the activity by Cu(II), (ii) oxygen tolerance of the activity, (iii) the dramatic decrease of Tc(VII) reduction in a mutant lacking [NiFe] hydrogenase structural genes, and (iv) demonstration of direct reduction by purified [NiFe] hydrogenase. This is the first report which demonstrates the reduction and removal of Tc(VII) from solution with purified nickel-iron hydrogenase. Reduction of Tc(VII) at the expense of molecular hydrogen is the most efficient and most widespread mechanism in the bacteria tested so far. Even though alternative electron donors can be used by several species, such as E. coli (13), S. putrefaciens (14, 33), and D. desulfuricans (16), the oxidation of these organic substrates often leads to the production of hydrogen or formate. Therefore, hydrogenase (alone or in a formate-hydrogenlyase complex) appears to be the major component involved in enzymatic Tc(VII) reduction.
In addition to the ability to reduce Tc(VII), selenite- and chromate-reducing activities have been reported for the [Fe] hydrogenase of Clostridium pasteurianum (35) and the [Fe] hydrogenase of sulfate-reducing bacteria (23), respectively. Metal reductase activity of hydrogenases therefore appears to be a widespread property.
ACKNOWLEDGMENTS
We gratefully acknowledge Bernard Dimon and Patrick Carrier for the mass spectrometric measurements and Jean-Pierre Bélaïch for his support and helpful discussions.
Marc Rousset is Laboratoire de Recherche Conventionné avec le CEA no. 25V.
REFERENCES
- 1.Casalot L, Hatchikian C E, Forget N, de Philip P, Dermoun Z, Bélaïch J P, Rousset M. Molecular study and partial characterization of iron-only hydrogenase in Desulfovibrio fructosovorans. Anaerobe. 1998;4:45–55. doi: 10.1006/anae.1997.0137. [DOI] [PubMed] [Google Scholar]
- 2.Cataldo D A, Garland T R, Wildung R E, Wildung R E, Fellows R J. Comparative metabolic behaviour and interrelationships of Tc and S in soybean plants. Heath Physics. 1989;57:281–288. doi: 10.1097/00004032-198908000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Cui D, Ericksen T E. Reduction of pertechnetate in solution by heterogeneous electron transfer from Fe(II)-containing geological material. Environ Sci Technol. 1996;30:2263–2269. [Google Scholar]
- 4.De Luca G, de Philip P, Rousset M, Bélaïch J P, Dermoun Z. The NADP-reducing hydrogenase of Desulfovibrio fructosovorans: evidence for a native complex with hydrogen-dependent methyl viologen activity. Biochem Biophys Res Commun. 1998;248:591–596. doi: 10.1006/bbrc.1998.9022. [DOI] [PubMed] [Google Scholar]
- 5.Farell J, Bostick W D, Jarabek R J, Fiedor J N. Electrosorption and reduction by anodically polarized magnetite. Environ Sci Technol. 1999;33:1244–1249. [Google Scholar]
- 6.Fernandez V M, Hatchikian C E, Cammack R. Properties and reactivation of two different deactivated forms of Desulfovibrio gigas hydrogenase. Biochim Biophys Acta. 1985;832:69–79. [Google Scholar]
- 7.Fernandez V M, Rua M L, Reyes P, Cammack R, Hatchikian C E. Inhibition of Desulfovibrio gigas hydrogenase with copper salts and other metal ions. Eur J Biochem. 1989;185:449–454. doi: 10.1111/j.1432-1033.1989.tb15135.x. [DOI] [PubMed] [Google Scholar]
- 8.Fitz R M, Cypionka H. Generation of a proton gradient in Desulfovibrio vulgaris. Arch Microbiol. 1991;155:444–448. [Google Scholar]
- 9.Fredrickson J K, Kostandarithes H M, Li S W, Plymale A E, Maly M J. Reduction of Fe(III), Cr(VI), U(VI), and Tc(VII) by Deinococcus radiodurans R1. Appl Environ Microbiol. 2000;66:2006–2011. doi: 10.1128/aem.66.5.2006-2011.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatchikian C E, Traore A S, Fernandez V M, Cammack R. Characterization of the nickel-iron periplasmic hydrogenase from Desulfovibrio fructosovorans. Eur J Biochem. 1990;187:635–643. doi: 10.1111/j.1432-1033.1990.tb15347.x. [DOI] [PubMed] [Google Scholar]
- 11.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 12.LeGall J, Payne W J, Chen L, Liu M Y, Xavier A V. Localization and specificity of cytochromes and other electron transfer proteins from sulfate-reducing bacteria. Biochimie. 1994;76:655–665. doi: 10.1016/0300-9084(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 13.Lloyd J R, Cole J A, Macaskie L E. Reduction and removal of heptavalent technetium from solution by Escherichia coli. J Bacteriol. 1997;179:2014–2021. doi: 10.1128/jb.179.6.2014-2021.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lloyd J R, Macaskie L E. A novel phosphorimager-based technique for monitoring the microbial reduction of technetium. Appl Environ Microbiol. 1996;62:578–583. doi: 10.1128/aem.62.2.578-582.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lloyd J R, Nolting H-F, Solé V A, Bosecker K, Macaskie L E. Technetium reduction and precipitation by sulfate-reducing bacteria. Geomicrobiol J. 1998;15:43–56. [Google Scholar]
- 16.Lloyd J R, Ridley J, Khizniak T, Lyalikova N N, Macaskie L E. Reduction of technetium by Desulfovibrio desulfuricans: biocatalyst characterization and use in a flowthrough bioreactor. Appl Environ Microbiol. 1999;65:2691–2696. doi: 10.1128/aem.65.6.2691-2696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lloyd J R, Sole V A, Van Praagh C V, Lovley D R. Direct and Fe(II)-mediated reduction of technetium by Fe(III)-reducing bacteria. Appl Environ Microbiol. 2000;66:3743–3749. doi: 10.1128/aem.66.9.3743-3749.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lloyd J R, Thomas G H, Finlay J A, Cole J A, Macaskie L E. Microbial reduction of technetium by Escherichia coli and Desulfovibrio desulfuricans: enhancement via the use of high-activity strains and effect of process parameters. Biotechnol Bioeng. 1999;66:122–130. [PubMed] [Google Scholar]
- 19.Lovley D R. Dissimilatory metal reduction. Annu Rev Microbiol. 1993;47:263–290. doi: 10.1146/annurev.mi.47.100193.001403. [DOI] [PubMed] [Google Scholar]
- 20.Macaskie L E. The application of biotechnology to the treatment of wastes produced from the nuclear fuel cycle: biodegradation and bioaccumulation as a means of treating radionuclide-containing streams. Crit Rev Biotechnol. 1991;11:41–112. doi: 10.3109/07388559109069183. [DOI] [PubMed] [Google Scholar]
- 21.Malki S, De Luca G, Fardeau M L, Rousset M, Bélaïch J P, Dermoun Z. Physiological characteristics and growth behaviour of single and double hydrogenase mutants of Desulfovibrio fructosovorans. Arch Microbiol. 1997;167:38–45. doi: 10.1007/s002030050414. [DOI] [PubMed] [Google Scholar]
- 22.Malki S, Saimmaime I, De Luca G, Rousset M, Dermoun Z, Bélaïch J P. Characterization of an operon encoding a NADP-reducing hydrogenase in Desulfovibrio fructosovorans. J Bacteriol. 1995;177:2628–2636. doi: 10.1128/jb.177.10.2628-2636.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michel C, Brugna M, Aubert C, Bernadac A, Bruschi M. Enzymatic reduction of chromate: comparative studies using sulfate-reducing bacteria. Key role of polyheme cytochromes c and hydrogenases. Appl Microbiol Biotechnol. 2001;55:95–100. doi: 10.1007/s002530000467. [DOI] [PubMed] [Google Scholar]
- 24.Ollivier B, Cord-Ruwisch R, Hatchikian E C, Garcia J-L. Characterization of Desulfovibrio fructosovorans sp. nov. Arch Microbiol. 1988;150:26–31. [Google Scholar]
- 25.Peck H D., Jr . Bioenergetic strategies of the sulfate-reducing bacteria. In: Odom J M, Singleton R, editors. Sulfate-reducing bacteria: contemporary perspectives. New York, N.Y: Springer-Verlag; 1993. pp. 41–76. [Google Scholar]
- 26.Rousset M, Dermoun Z, Chippaux M, Bélaïch J P. Marker exchange mutagenesis of the hydN genes in Desulfovibrio fructosovorans. Mol Microbiol. 1991;5:1735–1740. doi: 10.1111/j.1365-2958.1991.tb01922.x. [DOI] [PubMed] [Google Scholar]
- 27.Rousset M, Dermoun Z, Hatchikian C E, Bélaïch J P. Cloning and sequencing of the locus encoding the large and small subunit genes of the periplasmic [NiFe] hydrogenase from Desulfovibrio fructosovorans. Gene. 1990;94:95–101. doi: 10.1016/0378-1119(90)90473-5. [DOI] [PubMed] [Google Scholar]
- 28.Rousset M, Montet Y, Guigliarelli B, Forget N, Asso M, Bertrand P, Fontecilla-Camps J C, Hatchikian C E. [3Fe-4S] to [4Fe-4S] cluster conversion in Desulfovibrio fructosovorans [NiFe] hydrogenase by site-directed mutagenesis. Proc Natl Acad Sci USA. 1998;95:11625–11630. doi: 10.1073/pnas.95.20.11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shukla S K. Ion exchange paper chromatography of Tc(IV), Tc(V) and Tc(VII) in hydrochloric acid. J Chromatogr. 1966;21:92–97. doi: 10.1016/s0021-9673(01)91264-6. [DOI] [PubMed] [Google Scholar]
- 30.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 31.Vignais P M, Dimon B, Zorin N A, Colbeau A, Elsen S. HupUV proteins of Rhodobacter capsulatus can bind H2: evidence from the H-D exchange reaction. J Bacteriol. 1997;179:290–292. doi: 10.1128/jb.179.1.290-292.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Widdel F, Pfennig N. Dissimilatory sulfate- or sulfur-reducing bacteria. In: Krieg N R, Holt J G, editors. Bergey's manual of systematic bacteriology. 9th ed. Baltimore, Md: Williams & Wilkins; 1984. pp. 663–679. [Google Scholar]
- 33.Wildung R E, Gorby Y A, Krupka K M, Hess N J, Li S W, Plymale A E, McKinley J P, Fredrikson J K. Effect of electron donor and solution chemistry on products of dissimilatory reduction of technetium by Shewanella putrefaciens. Appl Environ Microbiol. 2000;66:2451–2460. doi: 10.1128/aem.66.6.2451-2460.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wildung R E, McFadden K M, Garland T R. Technetium sources and behaviour in the environment. J Environ Qual. 1979;8:156–161. [Google Scholar]
- 35.Yanke L J, Bryant R D, Laishley E J. Hydrogenase I of Clostridium pasteurianum functions as a novel selenite reductase. Anaerobe. 1995;1:61–67. doi: 10.1016/s1075-9964(95)80457-9. [DOI] [PubMed] [Google Scholar]