Abstract
Unprecedented bacterial targets are urgently needed to overcome the resistance crisis. Herein we systematically mine pyridoxal phosphate‐dependent enzymes (PLP‐DEs) in bacteria to focus on a target class which is involved in crucial metabolic processes. For this, we tailored eight pyridoxal (PL) probes bearing modifications at various positions. Overall, the probes exceeded the performance of a previous generation and provided a detailed map of PLP‐DEs in clinically relevant pathogens including challenging Gram‐negative strains. Putative PLP‐DEs with unknown function were exemplarily characterized via in‐depth enzymatic assays. Finally, we screened a panel of PLP binders for antibiotic activity and unravelled the targets of hit molecules. Here, an uncharacterized enzyme, essential for bacterial growth, was assigned as PLP‐dependent cysteine desulfurase and confirmed to be inhibited by the marketed drug phenelzine. Our approach provides a basis for deciphering novel PLP‐DEs as essential antibiotic targets along with corresponding ways to decipher small molecule inhibitors.
Keywords: Antibiotic Compound Screening, Cofactors, Enzyme Characterisation, Proteomics, Pyridoxal Phosphate-Dependent Enzymes
Unprecedented targets are needed to fight the bacterial pandemic. A suite of pyridoxal probes that infiltrate the cellular metabolism of critical pathogens and get incorporated in pyridoxal‐phosphate dependent enzymes is applied. Chemoproteomics facilitated the functional characterization of previously unknown members and the identification of essential targets with antibiotic hit molecules.
Introduction
With the rise of antimicrobial resistance (AMR), new strategies to fight bacterial pathogens are desperately needed.[ 2 , 3 , 4 , 5 ] Most of the currently prescribed antibiotics address a limited number of cellular targets such as cell wall, DNA and protein biosynthesis.[ 6 , 7 ] Although initially effective against a broad range of bacteria, their constant use and abuse has led to the development of multiresistant strains, which bear versatile strategies to render these antibiotics ineffective. This problem is especially severe when it comes to the treatment of infections caused by Gram‐negative bacteria, where two cell membranes effectively block the uptake of small molecules.[ 3 , 8 , 9 ] The development of new treatment options against Gram‐negative strains such as Pseudomonas aeruginosa, for which no antibiotics have been introduced for decades, is an extremely urgent task and therefore ranked as a critical priority by the WHO. [10] Thus, unprecedented modes‐of‐action beyond the limited scope of current drug targets are desperately needed in order to overcome this daunting crisis. [4] However, drug discovery efforts in this direction are challenged by several obstacles. For example, a large number of bacterial proteins lack a firm functional assignment, which complicates their validation as drug targets. Additionally, the vast number of proteins with important roles for cellular physiology complicates prioritization for those being druggable and most promising for development. [5] Moreover, chemical inhibitors, which have been identified in vitro often exhibit a limited uptake especially in case of Gram‐negative bacteria. [9]
A novel concept focuses on the systematic mining of cofactor dependent enzymes in bacteria to focus on a target class, which is known to bind ligands (druggability), acquires these ligands from the media (uptake) and is involved in crucial metabolic processes (relevance).[ 11 , 12 ] This concept was previously introduced for pyridoxal phosphate (PLP), a cofactor which catalyses about 238 enzymatic reactions accounting for 4 % of all classified activities.[ 13 , 14 ] PLP‐dependent enzymes (PLP‐DEs) bind their cofactor via an active site lysine residue to form a Schiff‐base (internal aldimine) which upon substrate binding is displaced to form an external aldimine. [15] Here, the PLP cofactor acts as an electron sink facilitating a variety of chemical reactions (Figure 1A). [16] Due to this versatility, PLP is crucial for central metabolic processes in prokaryotic and eukaryotic cells including cell wall and neurotransmitter biosynthesis, respectively.[ 7 , 17 ] Accordingly, PLP‐DEs represent promising therapeutic targets with several drugs on the market. [18] In order to obtain an inventory of PLP‐DEs, assign novel functions and mine for promising targets, we recently introduced modified pyridoxal (PL) probes, which we functionalized with alkyne or azide moieties at the C2′‐position for application in activity‐based protein profiling (ABPP) (Figure 1B).[ 11 , 13 , 19 ]
Figure 1.
PL probe library and activation by pyridoxal kinase. A) Activation of PL occurs by phosphorylation with pyridoxal kinase (PLK) to form PLP, which can bind PLP‐DEs via an internal aldimine to an active site lysine residue. Reaction with substrate amines leads to the formation of external aldimines, which can undergo various reactions via quinonoid structures. B) Structures of first [13] and second generation PL probes applied in this study. Type A probes are derivatised at the C2′, type B probes at the C6 and one type C probe is modified at the C3′. C) Activation of PL probes by pyridoxal kinases (E. coli pdxK or S. aureus SaPLK) were monitored by measuring UV/Vis absorbance over time (every 40 min, 20 cycles, n=3, mean±SEM). Phosphorylated species absorb at around 395 nm, unphosphorylated around 320 nm. Phosphorylation efficiency is depicted by comparing the absorbance [a.u.] from phosphorylated species (PLX, X=1–13) at the beginning (PLXPinit ) and at the end (PLXPend ). For PL6 SaPLK phosphorylation, the difference of initial and final absorbance at wavelengths of the unphosphorylated compound is depicted. For PL13, phosphorylated compound absorbed at 320 nm. Raw data of all compounds are shown in Figure SI1, 2.
The probes were taken up by Staphylococcus aureus cells bearing a mutation in their de novo PLP biosynthesis (pdxS transposon, TnpdxS), phosphorylated to the corresponding PLP probes by pyridoxal kinase (PLK) and subsequently incorporated into PLP‐DEs to form the internal aldimine. Reductive amination upon cell lysis and copper‐assisted azide‐alkyne click chemistry (CuAAC) or Staudinger ligation (SL) with bio‐orthogonal biotin tags facilitated the detection of about 73 % of all known PLP‐DEs in S. aureus with high confidence. In addition, several enzymes of previously unknown function could be assigned as putative PLP‐DE. While this initial study showed the fidelity of the new methodology, major challenges such as the labelling in Gram‐negative bacteria, labelling in wild type (wt) strains devoid of the de novo mutation, functional assignment of unknown PLP‐DEs and discovery of new antibiotic compounds against essential targets remained.
We here designed and synthesized 8 new PL probes, which enhanced the overall coverage of PLP‐DEs based on their structural diversity. Interestingly, some enzymes could be enriched selectively with certain probes. Together with a refined methodology, these probes not only facilitated labelling in Gram‐negative pathogens but also in wt cells as exemplified for the critical pathogen P. aeruginosa. We deciphered the function of several previously unknown PLP‐DEs and identified antibiotically active inhibitors along with their cellular targets via competitive profiling with our probes.
Results and Discussion
Synthesis of a Chemically Diverse PL Probe Library
Based on a previously published crystal structure of S. aureus alanine racemase (alr) in complex with PL1 and PL2, [13] we hypothesized that other PLP‐DEs could also exhibit extra space in the cofactor binding pockets to accommodate larger substituents and thereby achieve specificity for certain enzyme subclasses. Previous pilot experiments with two probes PL4 and PL5 bearing large alkyl or benzyl substituents at the C2′ position of the pyridine ring, revealed that they are able to undergo phosphorylation by S. aureus PLK (SaPLK) (Figure SI1). [13]
In order to maximize the capture of known and unknown PLP‐DEs and achieve sub‐class specificity, we designed and synthesized probes with diverse substitutions at the C2′, C3′ and C6 position including differently spaced alkyl and triazole linkers equipped with a terminal alkyne for CuAAC (Figure 1B). The synthesis of type A probes followed the procedures described by Hoegl et al. with slight modifications.[ 13 , 21 , 23 ] In addition, we exploited the modification of the pyridine C6 site by the synthesis of several alkyl and triazole probes. These type B derivatives were obtained by a nucleophilic substitution. Prior to that step, pivaloyl protected PL was activated by iso‐butyl chloroformate (IBCF) as described previously. [21] The set was complemented by one additional probe exhibiting an alkylated C3‐hydroxy group. The synthesis of this type C probe, PL13, was accomplished by adding propargyl bromide and potassium carbonate to PL (Scheme 1). [22]
Scheme 1.
Synthesis scheme for derivatisation of PL to type A, B and C ABPP probes. (Type A) PL hydrochloride is protected with a methoxymethyl (MOM) group and as a methyl acetal, which is then alkylated at the C2′ position as described previously.[ 13 , 20 ] The compound is further modified with an azide linker. Final deprotection leads to type A probes. (Type B) PL hydrochloride is protected to the pivaloyl (Piv) ester, oxidized to the N‐oxide with meta‐chloroperbenzoic acid (m‐CPBA), activated by iso‐butyl chloroformate (IBCF) and then reacted with an alkyne‐Grignard reagent to form C6 modified PL scaffolds. [21] Final, basic deprotection leads to type B derivatives. (Type C) Deprotonation of PL hydrochloride at the C3 alcohol and substitution with propargyl bromide yields type C probe PL13. [22]
In total, we obtained 13 probes for comprehensive PLP‐DE detection (Figure 1B). Prior to in situ labelling studies, we tested if probes bearing structural modifications at all three sites were still recognized by SaPLK/E. coli PLK (pdxK) and phosphorylated into the corresponding PLP derivatives (Figure 1C). Satisfyingly, we observed phosphorylation of all probes except for PL11 and PL12 emphasizing that larger modifications at C6 position are not tolerated. Interestingly, differences in substrate specificity of SaPLK vs. pdxK were observed, e.g. in case of PL9 and PL13 (Figure 1C).
Screen of Library against Gram‐Positive S. aureus Unravels an Enhanced Scope and Individual Target Preferences
We anticipated that lowering the concentration of endogenous PL in growth media would in turn maximize the incorporation of probes into PLP‐DEs under physiological conditions. In order to optimize our previously established labelling procedure towards this direction, we tested the effect of different media and PL2 concentrations in a panel of bacterial strains. Chemically defined media (CDM) with a minimal PL supplementation of 250 nM provided reliable growth conditions for all tested strains, no matter if the wt or mutant cells devoid of the PLP de novo synthesis were used, i.e. E. coli (wt and ΔpdxJ), P. aeruginosa (wt) and S. aureus (wt and TnpdxS) (Figure SI3A–C).
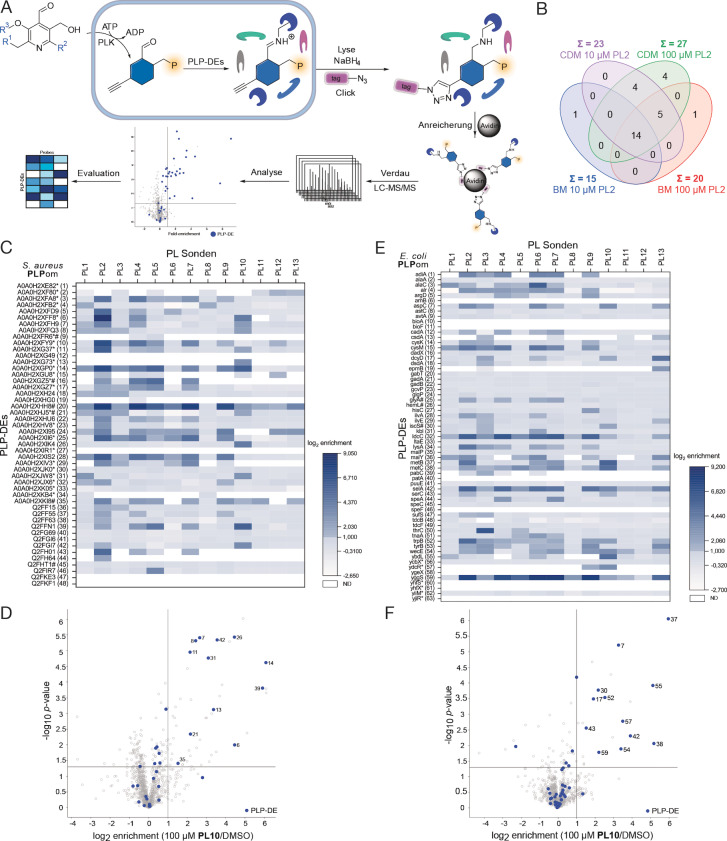
We next performed quantitative labelling with the set conditions to unravel and assign the cellular inventory of PLP‐DEs. S. aureus TnpdxS cells were incubated with 100 μM probe, grown for 2 h, lysed using lysostaphin and clicked to biotin azide or ligated to biotin phosphine (in case of PL3) [24] for subsequent enrichment of labelled proteins via avidin beads (Figure 2A). Tryptic digest, analysis via LC‐MS/MS and label‐free quantification (LFQ) revealed a characteristic pattern of significantly enriched proteins [cut‐off criteria: log2=1 (2‐fold enrichment) and p‐value<0.05] which are visualized in corresponding volcano plots (Figure SI4). First, we benchmarked our refined conditions with the previously established workflow. In total, we could increase the number of significantly enriched enzymes from 20 (B‐medium, 100 μM PL2) to 27 (CDM, [25] 100 μM PL2) and demonstrate that 100 μM is an ideal concentration to achieve a high coverage (Figure 2B). We next tested all of the probes under the optimized conditions in S. aureus USA300 (TnpdxS), which is devoid of de novo PLP biosynthesis. [26] Importantly, MS data analysis of the probe library not only revealed an increased overall coverage but also enhanced specificity of individual probes for certain PLP‐DEs. For example, cysteine synthase (13), an enzyme crucial for cysteine biosynthesis, [27] was detected for the first time by PL10 (Figure 2C, D, SI4, Table SI1).
Figure 2.
S. aureus and E. coli PLPome profiling. A) PL probes are taken up by the cells, get phosphorylated by PLK and subsequently function as PLP surrogates. After cell lysis, internal aldimines are reduced by sodium borohydride for downstream processing. Copper‐assisted azide‐alkyne click chemistry or Staudinger ligation to enrichment tags allows proteomic analysis and PLPome profiling. B) Comparison of enriched PLP‐DEs in S. aureus using different media (B medium, BM or chemically defined medium, CDM) and different PL2 concentrations (10 and 100 μM). C) Heatmap representing log2 enrichment values of all known or putative PLP‐DEs in S. aureus for all 13 PL probes (n=3). Enriched proteins have log2 enrichment values higher or equal 1. Proteins not detected (ND) at all are coloured white. PLP‐DEs with a putative function or which are poorly characterised are marked with an asterisk. Essential enzymes are marked with a #. [26] D) Volcano plot of PL10 enrichment at 100 μM in S. aureus USA300 TnpdxS compared to DMSO representing the t‐test results (criteria: log2 enrichment>1 and p‐value<0.05, n=3). Blue colours depict PLP‐DEs. Assigned numbers refer to Table SI1. Remaining plots are shown in Figure SI4. E) Heatmap representing log2 enrichment values of all known or putative PLP‐DEs in E. coli for all 13 PL probes (n=3). Enriched proteins have log2 enrichment values higher or equal 1. Proteins not detected (ND) at all are coloured white. PLP‐DEs with a putative function or which are poorly characterised are marked with an asterisk. Essential enzymes are marked with a #. [28] F) Volcano plot of PL10 enrichment at 100 μM in E. coli K12 ΔpdxJ compared to DMSO representing the t‐test results (criteria: log2 enrichment>1 and p‐value<0.05, n=3). Blue colours depict PLP‐DEs. Assigned numbers refer to Table SI2. Remaining plots are shown in Figure SI6.
Similarly, other important enzymes such as A0A0H2XFH9 (7), a Cys/Met metabolism PLP‐dependent enzyme, and A0A0H2XGZ5 (16), an essential gluconate operon transcriptional repressor, were detected solely with our refined labelling protocol. [26]
Moreover, A0A0H2XF80 (2), an uncharacterized helix‐turn‐helix (HTH)‐type transcriptional regulator [29] was only found by novel probes PL12 and PL13, respectively, emphasizing the need for structural diversity to occupy different PLP‐binding sites.
Overall, we were able to enhance the coverage of known PLP‐DEs by 7 additional proteins which represents a significant improvement gained by the new probe designs as well as the refined methodology. [30] With these promising tools at hand, we commenced to analyze the PLPome in challenging Gram‐negative bacteria.
Probes Label Intracellular PLP‐DEs in Gram‐Negative Escherichia coli and Wild Type Pseudomonas aeruginosa
In case of Gram‐negative bacteria, we selected an E. coli K12 mutant strain devoid of de novo PLP biosynthesis (E. coli K12 ΔpdxJ), [31] similar to our S. aureus approach. First, we established growth conditions in CDM [32] and found 250 nM of PL supplement as minimal concentration to maintain growth (Figure SI3A). Second, we applied the set conditions to monitor the growth of selected PL probes (Figure SI5A). Third, we exemplarily performed gel‐based labelling of probe PL2, PL3 and PL13 in E. coli ΔpdxJ as well as in wt cells. Upon probe treatment, CuAAC to rhodamine azide (PL2, PL13) or SL with rhodamine phosphine (PL3) and fluorescent SDS‐PAGE analysis, several labelled protein bands were observed (Figure SI5B). Interestingly, also in case of wt bacteria, specific bands could be detected at the highest probe concentration indicating that probe uptake could be sufficient to compete with endogenous PLP derived from de novo synthesis. While the intriguing perspective on wt cell labelling is followed up later in this study, we here focused on E. coli K12 ΔpdxJ for quantitative LC‐MS/MS analysis to compare the performance directly with S. aureus. For this, we utilized the refined workflow as described above and incubated all 13 probes individually with live cells at 100 μM for 2 h. A closer inspection of the corresponding volcano plots revealed a significant enrichment of 38 enzymes comprising 61 % of functionally assigned or predicted PLP‐DEs in E. coli (Figure 2E, F, Figure SI6, Table SI2).[ 30 , 33 ]
Importantly, this coverage reflects the individual labelling preferences of all probes highlighting that their diversity is key to access the various binding sites. As already observed for S. aureus, the extent of labelling greatly varied.
Although PL2 and PL3 labelled the majority of PLP‐DEs, 29 in total (77 % of all enriched), our structurally tailored probes accessed relevant subclasses, which would otherwise have escaped our attention. Many of these enzymes serve important functions, for example, alaC, an aminotransferase involved in the biosynthesis of alanine, [34] was solely enriched by PL6, metB, a cystathionine gamma‐synthase crucial for methionine biosynthesis [35] as well as iscS, a cysteine desulfurase essential for iron‐sulfur cluster formation, [36] were exclusively enriched by PL10. While PL6 exhibits an extended linker at C2′‐position, PL10 belongs to a group of probes bearing the tag at the C6‐position of the pyridine ring, highlighting the versatility of different structural analogues to satisfy different binding site requirements. Of note, we also identified several proteins of unknown function such as the uncharacterized HTH‐type transcriptional regulator ydcR, which was found with probe PL10. Based on the overall coverage, we rationalized that the majority of the E. coli and S. aureus labelling performance was contributed by a set of 6 probes (PL1, PL2, PL3, PL6, PL10, PL13). We therefore focused our following studies on this optimal set of compounds and commenced with the mining of the P. aeruginosa PAO1 PLPome. Here, we deliberately used the wt strain in order to decipher if sufficient competition with endogenous PLP can be achieved for efficient protein labelling.
Satisfyingly, labelling with 100 μM of each probe resulted in an overall coverage of 42 significantly enriched proteins, which reflect 51 % of the known and predicted P. aeruginosa PLP‐DEs (Figure 3A, B, Figure SI7, Table SI3). [30] Foremost, three probes PL2, PL6 and PL10 contributed to the majority of hits. These included e.g. aruH, a transaminase involved in arginine degradation [41] (PL10), ilvA2, a threonine dehydratase inferred from homology [42] (PL13), and PA0394, a PLP homeostasis protein annotated by UniRule [43] (PL2, PL6), which was also highly enriched in E. coli (yggS) and S. aureus (A0A0H2XHH8). Interestingly, PL10 again was able to enrich putative transcriptional regulators (e.g. PA2032, PA2100, PA2897 and PA5283, annotation by InterPro [29] ). It seems that the modification of the C6 position is a preferred motif for the recognition of this enzyme class. Further, PA5313, a probable PLP dependent aminotransferase (InterPro [29] and GO [42] ) was solely enriched by PL10.
Figure 3.

P. aeruginosa PLPome profiling. A) Heatmap representing log2 enrichment values of all known or putative PLP‐DEs in P. aeruginosa PAO1 for selected 6 PL probes (n=3). Enriched proteins have log2 enrichment values higher or equal 1. Proteins not detected (ND) at all are coloured white. PLP‐DEs with a putative function or which are poorly characterised are marked with an asterisk. Essential enzymes are marked with a #. [37] Remaining plots are shown in Figure SI7. Assigned numbers refer to Table SI3. B) Volcano plot of PL10 enrichment at 100 μM in P. aeruginosa compared to DMSO representing the t‐test results (criteria: log2 enrichment>1 and p‐value<0.05, n=3).
Overall, these studies demonstrate that PL probes can be applied under our refined conditions to directly label PLP‐DEs in wt cells preventing the use of de novo biosynthesis mutants. This eases the application of this method to diverse organisms without their prior genetic manipulation.
Functional Analysis of Uncharacterized Proteins
With a large panel of uncharacterized PLP‐binding proteins from three organisms, we selected candidates for a closer inspection on their functional role. First, PLP binding of all overexpressed enzymes was confirmed via intact protein MS (IP‐MS, Figure 4A). We started our studies with E. coli ydcR which was identified by PL10 and is annotated as an uncharacterized HTH‐type transcriptional regulator (TR) (by InterPro [29] and GO [42] ). We not only confirmed the binding to PLP but also determined a high‐affinity interaction with a specific promoter region upstream of a putrescin transporter via electrophoretic mobility shift assays (EMSA) (Figure 4B, Figure SI8). These results are in line with the known roles of related TRs in the PLP‐dependent transcription of genes involved in e.g. gamma‐aminobutyric acid metabolism. [44]
Figure 4.
Characterization of enzymes with unknown function. A) PLP loading states of overexpressed proteins were determined by IP‐MS as described previously. [13] B) EMSA gel under UV‐illumination. Concentrations are given in nM. The interaction of ydcR with the ydcR promoter region fragment PRydcR is clearly visible starting at 50 nM ydcR. The two lines (*) represent shifted DNA‐protein complexes. [38] As negative controls, we included samples w/o ydcR (lane 1), w/o PRydcR (lane 2), w/ PRydcR‐ydcS instead of PRydcR (lane 5) and one sample with yjiR instead of ydcR (lane 6). To exclude unspecific DNA binding, we added 2 μg μL−1 salmon sperm DNA (SSDNA) to each sample (except lane 7). Samples containing PLP (lane 3, 10 eq) and PLP and putrescine (lane 4, 10 eq PLP, 400 eq) were included (see also Figure SI8). C) LC‐MS/MS analysis of products after incubation of PA2683 with l‐Ser, d‐Ser, l‐Thr or d‐Thr and additional AMP and PLP at 37 °C. The MS2 signals of products (pyruvate or oxobutanoate) were integrated and plotted as individual values (n=3). Heat control (HC) samples were treated equally except for incubating the protein for 5 min at 95 °C prior to addition of substrates. D) Michaelis–Menten kinetics of PA2683 (n=3, mean±SEM) were calculated in Graphpad Prism (see also Figure SI9). E) Spectroscopic properties of PLP species during transamination.[ 39 , 40 ] F) UV/Vis screening from 300 to 600 nm of aminotransferases PA3659 and PA3798 with 20 l‐amino acids (AA) and a mixture of 11 d‐amino acids (each 10 equivalents). Changes in absorbance at 390 or 430 nm (external aldimine) were calculated and normalised. G) LC‐MS/MS analysis of transaminated products after incubation of PA3659 with l‐Trp and PA3798 with l‐Gln (n=3). Heat control (HC) samples were treated equally except for incubating the protein for 5 min at 95 °C prior to addition of substrates.
Next, we evaluated a putative enzyme from P. aeruginosa, PA2683 identified by probes PL1 and PL2, which showed homology to the family of serine deaminases (by GO [42] ) (Figure SI9A). [42] The enzyme was overexpressed and indeed turnover of l‐serine was observed together with NADH consumption within a coupled assay. Moreover, the formation of the expected products pyruvate and oxobutanoate was confirmed by LC‐MS/MS (Figure 4C, Figure SI9B).
PA2683 exhibited both serine and threonine dehydratase activity, with a preference for l‐enantiomers. Moreover, we could show, that the catalysis was accelerated by adding adenosine monophosphate (AMP) and magnesium [45] which enabled the measurement of Michaelis–Menten kinetics (Figure 4D).
Furthermore, two putative aminotransferases (ATs) (by InterPro [29] and GO [42] ), PA3798 and PA3659, identified in P. aeruginosa, were cloned and overexpressed for a closer inspection of substrate scope. PLP has different absorbance properties during its catalytic cycle in transamination due to different electronic states.[ 39 , 40 ] This fact can be used in order to study substrate specificities of aminotransferases, because pyridoxamine phosphate (PMP) is formed after the catalytic turnover, which absorbs at around 330 nm. Simultaneously, absorbance of the external aldimine at around 390–430 nm decreases (Figure 4E). Incubation of the holo‐enzymes with 20 natural l‐amino acids and a mixture of 11 selected d‐amino acids revealed substrate specificities for predominantly l‐Gln, l‐Met, l‐Trp in case of PA3798 and l‐Asp, l‐Cys, l‐Glu, l‐Trp in case of PA3659 via UV/Vis based detection of the external aldimine (Figure 4F). No conversion of d‐amino acids was observed. Enzymes treated with hydroxylamine to displace the cofactor were included as inactive controls. Furthermore, substrates of both aminotransferases were selected and their products quantified via LC‐MS/MS (Figure 4G, Figure SI10).
Although PLP‐DE mining narrowed down the number of possible reactions of uncharacterized proteins to only those catalyzed by this cofactor, follow up studies are still required for full functional assignment. Here, we could confirm previously uncharacterized enzymes or enzymes with a putative PLP dependency as PLP‐DEs and provide insights into their catalysis.
Screening of a Pyridoxal‐Targeting Library Unravels Novel Antibiotic Hits
A major goal of our PLP‐DE profiling approach is the identification of novel antibacterial drug targets together with tailored antibiotic hit molecules. Given the wealth of inhibitors designed to address PLP‐DEs in diverse organisms including drugs such as carbidopa, [18] we rationalized that repurposing of these molecules for the inhibition of bacterial PLP‐DEs would be an intriguing starting point for the identification of new antibacterial hit compounds. We thus collected a library of 53 molecules consisting of existing drugs as well as other previously described PLP‐DE inhibitors, direct binders of the PLP cofactor or analogues thereof (Table SI4).
These compounds were pre‐screened against bacteria highlighted in this study at 500 μM with hits occurring in S. aureus USA300, E. coli K12 and P. aeruginosa PAO1 (Table SI5). Hit compounds were then fine‐screened down to 25 μM resulting in hits in all 3 strains (Figure 5A).
Figure 5.
MIC screen and target validation. A) Compound MIC‐screen against S. aureus, E. coli and P. aeruginosa. Structures of hit‐compounds and their corresponding MIC values are shown. B) LFQ intensities of selected PLP‐DEs in DMSO, PL2 treated and phenelzine treated S. aureus samples (n=3). For competitive labelling samples, cells were incubated with phenelzine for 30 min prior to 2 h PL2 labelling. Essential proteins are marked with a #. [26] Raw data are given in Table SI7–9 and Figure SI12A. C) Activity of A0A0H2XII6 after incubation with different concentrations of phenelzine (n=3). Quantification of cadaverine was conducted by LC‐MS/MS (MS2 signal, parallel reaction monitoring PRM). IC50 values were calculated in Graphpad Prism 5.03 using the log(inhibitor) vs. response‐Variable slope function (four parameters). Heat control (HC) samples were treated equally except for incubating the protein for 5 min at 95 °C prior to addition of substrates. D) LC‐MS analysis of alanine after incubation of A0A0H2XHJ5 with l‐Cys (n=3). Heat control (HC) samples were treated equally except for incubating the protein for 5 min at 95 °C prior to addition of substrates. E) Activity of A0A0H2XHJ5 (sufS) after incubation with different concentrations of phenelzine (n=3). Quantification of alanine was conducted by LC/MS (MS1 signal, single ion monitoring SIM). IC50 values were calculated in Graphpad Prism 5.03 using the log(inhibitor) vs. response‐variable slope function (four parameters).
The approved drugs phenelzine, a nonselective monoamine oxidase inhibitor, [18] benserazide, a structurally related PLP‐dependent DOPA decarboxylase inhibitor [18] and CCG‐50014, [46] a mycobacterial alanine racemase inhibitor, were shown to exhibit moderate antibiotic activity against Gram‐positive bacterium S. aureus (Figure 5A). Benserazide is a prodrug which forms a hydrazone with the electrophilic PLP aldehyde in DOPA decarboxylase.[ 18 , 47 ] Similarly, phenelzine exhibits a hydrazine group, which was reported to bind PLP in the cell. [48] Interestingly, the synthesis of 11 phenelzine derivatives bearing structural modifications at the aromatic ring position or different alkyl chain lengths were less active suggesting that structural features and not mere hydrazine reactivity are responsible for the biological effect (Figure 5A, Figure SI11, Figure SI12A Table SI6). [49]
In order to decipher their targets in living bacteria, we applied phenelzine, benserazide and CCG‐50014 (all three in S. aureus) in competitive profiling. We selected PL2 for these studies due to its high coverage and applied the respective inhibitors in three different molar ratios. Interestingly, all competitive experiments revealed a significant, concentration‐dependent displacement of several PLP‐DEs, highlighting the interaction with this enzyme class (Figure 5B, Figure SI12B,C, Table SI7–SI15). For example, competition studies with phenelzine in S. aureus revealed strong displacement of several PLP‐DEs such as Orn/Lys/Arg decarboxylase (A0A0H2XII6). The enzyme was overexpressed and inhibition could be validated in an in vitro targeted metabolomics assay with an IC50 of 53.05±1.05 μM (Figure 5C).
Most importantly, among the hits was A0A0H2XHJ5, an uncharacterized enzyme, which is essential for cell growth. [26] A closer search revealed that A0A0H2XHJ5 exhibits homology to an essential cysteine desulfurase (sufS) (Figure SI13).[ 42 , 50 ] In order to assign its function and test if phenelzine targets this enzyme, we cloned and overexpressed A0A0H2XHJ5 and investigated its activity in a corresponding assay. Alanine formation was measured after incubation of A0A0H2XHJ5 with a FeS assembly protein (sufU), l‐Cys, dithiothreitol (DTT) and PLP at 37 °C. [51] Alanine formation could indeed be reliably quantified by LC‐MS confirming desulfurase activity (Figure 5D). Finally, with an assigned function and established activity assay at hand we confirmed the inhibition of A0A0H2XHJ5 enzymatic activity by phenelzine with an IC50 of 14.70±1.22 μM (Figure 5E).
Given the essential role of cysteine desulfurase for bacterial viability, its inhibition by phenelzine likely contributes to the overall antibiotic effects of this compound.
The mass spectrometry proteomics data have been deposited to the ProteomeXchange consortium via the PRIDE partner repository [1] with the dataset identifier PXD029832.
Conclusion
We here demonstrate the utility of cofactor mining for discovering unprecedented antibiotic targets on the example of PLP‐DEs. Our probe design showcases the importance of introducing structural diversity at various positions of the PL scaffold to access different binding sites. With this tailored approach, we managed a coverage of up to 71 % of all PLP‐DEs and mapped their binding to certain structural motifs. In fact, these maps may already guide the design of improved PL analogues specific for a single enzyme class. Uptake was not only achieved in pathogenic S. aureus but also in Gram‐negative E. coli and P. aeruginosa strains. Our improved labelling conditions furthermore facilitated the detection of PLP‐DEs in wild type cells superseding the use of PLP biosynthesis mutants. This now opens new possibilities of utilizing the methodology in the profiling of diverse bacterial communities to not only decipher their PLP‐DE inventory but also monitor their inhibition by small molecules in situ. Our pilot screen for new PLP‐DE inhibitors already revealed several hits which we could trace down to the corresponding cellular target. In case of the marketed drug phenelzine, we demonstrated the inhibition of a previously uncharacterized cysteine desulfurase, an enzyme which lacks homology in human cells,[ 50 , 52 ] and thus represents a prime antibiotic target along with an initial inhibitory scaffold.
Tools which allow the number of possible enzymatic transformations to be narrowed down are ideal starting points for functional annotations. Thus, we were able to exemplarily assign the function of previously uncharacterized enzymes including aminotransferases, a deaminase and a transcriptional regulator. In summary, our approach provides access to the unrestricted, comprehensive and rapid screening of new antibacterial targets in diverse pathogenic bacteria.
Conflict of interest
The authors declare no conflict of interest.
1.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
This project received funding from the European Research Council (ERC) and the European Union's Horizon 2020 research and innovation program (grant agreement no. 725085, CHEMMINE, ERC consolidator grant). M.P. acknowledges a PhD fellowship from the Studienstiftung des deutschen Volkes. The authors thank Prof. Dr. Kirsten Jung (LMU) for providing the E. coli knockout strain. We thank Mona Wolff and Katja Bäuml for technical assistance as well as intern Patrick Raunft for helping with synthesis. The authors thank Dr. Stephan Hacker and Dr. Stuart Ruddell for critical proofreading of the manuscript. Open Access funding enabled and organized by Projekt DEAL.
M. Pfanzelt, T. E. Maher, R. M. Absmeier, M. Schwarz, S. A. Sieber, Angew. Chem. Int. Ed. 2022, 61, e202117724; Angew. Chem. 2022, 134, e202117724.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Perez-Riverol Y., Csordas A., Bai J., Bernal-Llinares M., Hewapathirana S., Kundu D. J., Inuganti A., Griss J., Mayer G., Eisenacher M. et al., Nucleic Acids Res. 2019, 47, D442–D450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.
- 2a. Lakemeyer M., Zhao W., Mandl F. A., Hammann P., Sieber S. A., Angew. Chem. Int. Ed. 2018, 57, 14440–14475; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 14642–14682; [Google Scholar]
- 2b. Lewis K., Cell 2020, 181, 29–45; [DOI] [PubMed] [Google Scholar]
- 2c. Barra A. L. C., Dantas L. D. O. C., Morão L. G., Gutierrez R. F., Polikarpov I., Wrenger C., Nascimento A. S., Front. Public Health 2020, 8, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tacconelli E., Carrara E., Savoldi A., Harbarth S., Mendelson M., Monnet D. L., Pulcini C., Kahlmeter G., Kluytmans J., Carmeli Y. et al., Lancet Infect. Dis. 2018, 18, 318–327. [DOI] [PubMed] [Google Scholar]
- 4. Luepke K. H., Suda K. J., Boucher H., Russo R. L., Bonney M. W., Hunt T. D., Mohr J. F., Pharmacotherapy 2017, 37, 71–84. [DOI] [PubMed] [Google Scholar]
- 5. Payne D. J., Gwynn M. N., Holmes D. J., Pompliano D. L., Nat. Rev. Drug Discovery 2007, 6, 29–40. [DOI] [PubMed] [Google Scholar]
- 6. Le P., Kunold E., Macsics R., Rox K., Jennings M. C., Ugur I., Reinecke M., Chaves-Moreno D., Hackl M. W., Fetzer C. et al., Nat. Chem. 2020, 12, 145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kotnik M., Anderluh P. S., Prezelj A., Curr. Pharm. Des. 2007, 13, 2283–2309. [DOI] [PubMed] [Google Scholar]
- 8. Slama T. G., Crit. Care Clin. 2008, 12 Suppl 4, S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao S., Adamiak J. W., Bonifay V., Mehla J., Zgurskaya H. I., Tan D. S., Nat. Chem. Biol. 2020, 16, 1293–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.
- 10a. Mulani M. S., Kamble E. E., Kumkar S. N., Tawre M. S., Pardesi K. R., Front. Microbiol. 2019, 10, 539; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10b. Pang Z., Raudonis R., Glick B. R., Lin T.-J., Cheng Z., Biotechnol. Adv. 2019, 37, 177–192. [DOI] [PubMed] [Google Scholar]
- 11. Benns H. J., Wincott C. J., Tate E. W., Child M. A., Curr. Opin. Chem. Biol. 2021, 60, 20–29. [DOI] [PubMed] [Google Scholar]
- 12. Depaix A., Kowalska J., Molecules 2019, 24, 4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoegl A., Nodwell M. B., Kirsch V. C., Bach N. C., Pfanzelt M., Stahl M., Schneider S., Sieber S. A., Nat. Chem. 2018, 10, 1234–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Percudani R., Peracchi A., EMBO Rep. 2003, 4, 850–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oliveira E. F., Cerqueira Nuno M. F. S. A., Fernandes P. A., Ramos M. J., J. Am. Chem. Soc. 2011, 133, 15496–15505. [DOI] [PubMed] [Google Scholar]
- 16.
- 16a. Toney M. D., Arch. Biochem. Biophys. 2005, 433, 279–287; [DOI] [PubMed] [Google Scholar]
- 16b. Percudani R., Peracchi A., BMC Bioinf. 2009, 10, 273; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16c. Du Y.-L., Ryan K. S., Nat. Prod. Rep. 2019, 36, 430–457. [DOI] [PubMed] [Google Scholar]
- 17.
- 17a. Di Salvo M. L., Budisa N., Beilstein Bozen Symposium 2012, 27–66; [Google Scholar]
- 17b. Walsh C. T., J. Biol. Chem. 1989, 264, 2393–2396. [PubMed] [Google Scholar]
- 18. Amadasi A., Bertoldi M., Contestabile R., Bettati S., Cellini B., Di Salvo M. L., Borri-Voltattorni C., Bossa F., Mozzarelli A., Curr. Med. Chem. 2007, 14, 1291–1324. [DOI] [PubMed] [Google Scholar]
- 19.
- 19a. Cravatt B. F., Wright A. T., Kozarich J. W., Annu. Rev. Biochem. 2008, 77, 383–414; [DOI] [PubMed] [Google Scholar]
- 19b. Fonović M., Bogyo M., Expert Rev. Proteomics 2008, 5, 721–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heyl D., Luz E., Harris S. A., Folkers K., J. Am. Chem. Soc. 1951, 73, 3430–3433. [Google Scholar]
- 21. Kim Y.-C., Jacobson K. A., Synthesis 2000, 2000, 119–122. [Google Scholar]
- 22. Saha M., Hossain M. S., Bandyopadhyay S., Angew. Chem. Int. Ed. 2021, 60, 5220–5224; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2021, 133, 5280–5284. [Google Scholar]
- 23. Himo F., Lovell T., Hilgraf R., Rostovtsev V. V., Noodleman L., Sharpless K. B., Fokin V. V., J. Am. Chem. Soc. 2005, 127, 210–216. [DOI] [PubMed] [Google Scholar]
- 24. Saxon E., Science 2000, 287, 2007–2010. [DOI] [PubMed] [Google Scholar]
- 25. Liebeke M., Dörries K., Zühlke D., Bernhardt J., Fuchs S., Pané-Farré J., Engelmann S., Völker U., Bode R., Dandekar T. et al., Mol. BioSyst. 2011, 7, 1241–1253. [DOI] [PubMed] [Google Scholar]
- 26. Fey P. D., Endres J. L., Yajjala V. K., Widhelm T. J., Boissy R. J., Bose J. L., Bayles K. W., mBio 2013, 4, e00537-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lithgow J. K., Hayhurst E. J., Cohen G., Aharonowitz Y., Foster S. J., J. Bacteriol. 2004, 186, 1579–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.
- 28a. Gerdes S. Y., Scholle M. D., Campbell J. W., Balázsi G., Ravasz E., Daugherty M. D., Somera A. L., Kyrpides N. C., Anderson I., Gelfand M. S. et al., J. Bacteriol. 2003, 185, 5673–5684; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28b. Hashimoto M., Ichimura T., Mizoguchi H., Tanaka K., Fujimitsu K., Keyamura K., Ote T., Yamakawa T., Yamazaki Y., Mori H. et al., Mol. Microbiol. 2005, 55, 137–149; [DOI] [PubMed] [Google Scholar]
- 28c. Kang Y., Durfee T., Glasner J. D., Qiu Y., Frisch D., Winterberg K. M., Blattner F. R., J. Bacteriol. 2004, 186, 4921–4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blum M., Chang H.-Y., Chuguransky S., Grego T., Kandasaamy S., Mitchell A., Nuka G., Paysan-Lafosse T., Qureshi M., Raj S. et al., Nucleic Acids Res. 2021, 49, D344–D354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bateman A., Martin M.-J., Orchard S., Magrane M., Agivetova R., Ahmad S., Alpi E., Bowler-Barnett E. H., Britto R., Bursteinas B. et al., Nucleic Acids Res. 2021, 49, D480–D489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., Baba M., Datsenko K. A., Tomita M., Wanner B. L., Mori H., Mol. Syst. Biol. 2006, 2, 2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arie Geerlof, “M9 mineral medium”, can be found under https://www.helmholtz-muenchen.de/fileadmin/PEPF/Protocols/M9-medium 150510.pdf, 2010.
- 33. Tramonti A., Nardella C., Di Salvo M. L., Barile A., D'Alessio F., de Crécy-Lagard V., Contestabile R., EcoSal Plus 2021, 9, eESP-0004-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim S. H., Schneider B. L., Reitzer L., J. Bacteriol. 2010, 192, 5304–5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Holbrook E. L., Greene R. C., Krueger J. H., Biochemistry 1990, 29, 435–442. [DOI] [PubMed] [Google Scholar]
- 36. Takahashi Y., Nakamura M., J. Biochem. 1999, 126, 917–926. [DOI] [PubMed] [Google Scholar]
- 37. Held K., Ramage E., Jacobs M., Gallagher L., Manoil C., J. Bacteriol. 2012, 194, 6387–6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tramonti A., Nardella C., Di Salvo M. L., Pascarella S., Contestabile R., FEBS J. 2018, 285, 3925–3944. [DOI] [PubMed] [Google Scholar]
- 39. Griswold W. R., Toney M. D., J. Am. Chem. Soc. 2011, 133, 14823–14830. [DOI] [PubMed] [Google Scholar]
- 40. Mozzarelli A., Bettati S., Chem. Rec. 2006, 6, 275–287. [DOI] [PubMed] [Google Scholar]
- 41. Yang Z., Lu C.-D., J. Bacteriol. 2007, 189, 3954–3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gaudet P., Livstone M. S., Lewis S. E., Thomas P. D., Briefings Bioinf. 2011, 12, 449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. MacDougall A., Volynkin V., Saidi R., Poggioli D., Zellner H., Hatton-Ellis E., Joshi V., O'Donovan C., Orchard S., Auchincloss A. H. et al., Bioinformatics 2021, 36, 5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Suvorova I. A., Rodionov D. A., Microb. Genomics 2016, 2, e000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Goto M., Yamauchi T., Kamiya N., Miyahara I., Yoshimura T., Mihara H., Kurihara T., Hirotsu K., Esaki N., J. Biol. Chem. 2009, 284, 25944–25952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.
- 46a. Ciustea M., Mootien S., Rosato A. E., Perez O., Cirillo P., Yeung K. R., Ledizet M., Cynamon M. H., Aristoff P. A., Koski R. A. et al., Biochem. Pharmacol. 2012, 83, 368–377; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46b. Lee Y., Mootien S., Shoen C., Destefano M., Cirillo P., Asojo O. A., Yeung K. R., Ledizet M., Cynamon M. H., Aristoff P. A. et al., Biochem. Pharmacol. 2013, 86, 222–230; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46c. Azam M. A., Jayaram U., J. Enzyme Inhib. Med. Chem. 2016, 31, 517–526. [DOI] [PubMed] [Google Scholar]
- 47.
- 47a. Bender D. A., Smith W. R., Biochem. Soc. Trans. 1978, 6, 120–122; [DOI] [PubMed] [Google Scholar]
- 47b. Vilter R. W., Vitam. Horm. 1964, 22, 797–805; [DOI] [PubMed] [Google Scholar]
- 47c. Schwartz D. E., Brandt R., Arzneim.-Forsch. 1978, 28, 302–307. [PubMed] [Google Scholar]
- 48. Malcolm D. E., Yu P. H., Bowen R. C., O'Donovan C., Hawkes J., Hussein M., J. Psychiatry Neurosci. 1994, 19, 332–334. [PMC free article] [PubMed] [Google Scholar]
- 49. Prusevich P., Kalin J. H., Ming S. A., Basso M., Givens J., Li X., Hu J., Taylor M. S., Cieniewicz A. M., Hsiao P.-Y. et al., ACS Chem. Biol. 2014, 9, 1284–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Roberts C. A., Al-Tameemi H. M., Mashruwala A. A., Rosario-Cruz Z., Chauhan U., Sause W. E., Torres V. J., Belden W. J., Boyd J. M., Infect. Immun. 2017, 85, e00100-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.
- 51a. Selbach B., Earles E., Dos Santos P. C., Biochemistry 2010, 49, 8794–8802; [DOI] [PubMed] [Google Scholar]
- 51b. Albrecht A. G., Netz D. J. A., Miethke M., Pierik A. J., Burghaus O., Peuckert F., Lill R., Marahiel M. A., J. Bacteriol. 2010, 192, 1643–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.
- 52a.NCBI Resource Coordinators, Nucleic Acids Res. 2018, 46, D8–D13; [DOI] [PMC free article] [PubMed]
- 52b. Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J., J. Mol. Biol. 1990, 215, 403–410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.