Summary
Red blood cells (RBCs) lose plasma membrane in the spleen as they age, but the cells and molecules involved are yet to be identified. Sickle cell disease and infection by Plasmodium falciparum cause oxidative stress that induces aggregates of cross‐linked proteins with N‐linked high‐mannose glycans (HMGs). These glycans can be recognised by mannose‐binding lectins, including the mannose receptor (CD206), expressed on macrophages and specialised phagocytic endothelial cells in the spleen to mediate the extravascular haemolysis characteristic of these diseases. We postulated this system might also mediate removal of molecules and membrane in healthy individuals. Surface expression of HMGs on RBCs from patients who had previously undergone splenectomy was therefore assessed: high levels were indeed observable as large membrane aggregates. Glycomic analysis by mass spectrometry identified a mixture of Man5‐9GlcNAc2 structures. HMG levels correlated well with manual pit counts (r = 0.75–0.85). To assess further whether HMGs might act as a splenic reticuloendothelial function test, we measured levels on RBCs from patients with potential functional hyposplenism, some of whom exhibited high levels that may indicate risk of complications.
Keywords: glycosylation, membrane editing, oxidative damage, red blood cells, splenectomy
INTRODUCTION
The red pulp of the spleen filters blood to remove pathogenic microorganisms and senescent or diseased red blood cells (RBCs). 1 , 2 Cells migrate from the cords into venous sinuses, then squeeze through interendothelial slits (IESs) on the sinus walls to return to systemic circulation. Several ligands have been implicated in uptake of whole cells, 3 , 4 , 5 , 6 , 7 including phosphatidylserine, autoantibodies and complement bound to oxidatively degraded molecules, adhesion molecules 8 and CD47, as well as physicochemical changes such as loss of membrane deformability. 9 The relative importance of these mechanisms remains uncertain. It is plausible that the importance of this process is such that extensive redundancy is necessary. 10 , 11
Splenic phagocytes also scavenge selected cellular constituents, failure of which results in retention of intracellular inclusions within RBCs such as Howell–Jolly, Heinz and Pappenheimer bodies. 7 A further mechanism removes RBC membrane, sometimes known as membrane editing, with surface areas, volumes and densities progressively decreasing with age. 12 Despite being described over 50 years ago, 1 the molecular and cellular bases for these processes are poorly understood. Some membrane loss results from vesiculation, 13 possibly mediated by weakened cohesion between the lipid bilayer and the cytoskeleton. 14 However, most membrane loss is thought to takes place in the spleen.
In this study, we investigate oligosaccharides, or glycans, carried on the surface of RBC from patients after splenectomy. Glycosylation of membrane proteins occurs in the endoplasmic reticulum (ER) and Golgi apparatus. In the ER, N‐glycosylation results in asparagine residues being linked to a chitobiose core capped by several mannose residues, which together are known as high mannoses. 15 In the Golgi apparatus of humans, mannose residues are trimmed back and are replaced by other sugars, mainly sialic acids, to form ‘complex’ glycans. Cells are thereby coated by a glycocalyx containing infrequent mannose residues, which contrasts with the often mannose‐rich cell walls of microbes. Phagocytic cells such as macrophages expressing mannose‐binding receptors are then able to use these lectins as pathogen recognition receptors. We recently reported that oxidative damage, in the contexts of both laboratory‐induced as well as sickle cell disease 16 and infection by malarial parasites, results in aggregates of cross‐linked RBC membrane proteins that are marked by high‐mannose glycans (HMGs). 17 HMGs induce uptake of cells by macrophages mediated, at least in part, by the mannose receptor (CD206). HMG levels correlated with extravascular, but not intravascular, haemolysis in sickle cell disease. 17 The mannose receptor is generally deemed to be more involved in recognition and uptake of smaller molecules and structures than whole cells. 18 These observations raise the possibility that the high‐mannose–mannose receptor ligand pair might also mediate removal of oxidised molecules and membrane editing in healthy individuals.
We therefore assessed the expression of glycans on the surface of RBCs from patients with poor splenic function as well as conditions where oxidative stress might also be important.
MATERIALS AND METHODS
Donors
Healthy controls, patients with sickle cell disease and the patient who underwent splenectomy during the study gave informed consent for the study (North of Scotland REC Number 11/NS/0026). Samples from patients with splenectomies carried out at least a year previous to the study, those used to assess functional hypoplenism and healthy controls (chosen on the basis of unrelated diagnoses with normal or near‐normal full blood count parameters) were obtained using the NHS Grampian Biorepository scheme (application number TR000224), which permits access to anonymised samples from routinely collected clinical samples, once all requested tests have been completed and tubes are ready for disposal. No extra blood samples were taken for research purposes in this scheme. A sample was also obtained from King's College Hospital, London, where patients were informed that discarded, anonymised samples might be used for research by displaying a written notice. All samples were collected into EDTA tubes (Becton‐Dickinson).
RBC isolation
For Percoll density fractionation and western blotting, blood was transferred into acid citrate dextrose solution (ACD, 455055; Grenier) and RBC purified by sodium metrizoate density gradient centrifugation (1.077 g/ml, Lymphoprep; 1114547 Axis‐Shield). Packed RBCs were diluted with an equal volume of Dulbecco's modified Eagle's medium (DMEM; 4.5 g/l glucose, l‐glutamine; 41965; Gibco), stored in ACD (9 ml RBC/DMEM per ACD tube) at 4°C and used within 3 days.
Lectins
Biotinylated lectins were purchased from Vector Laboratories. They include: Galanthus nivalis agglutinin (GNA, B‐1245, 4 μg/ml), Narcissus pseudonarcissus lectin (NPL, B‐1375, 4 μg/ml), Griffonia simplicifolia lectin II (G.Simp, B‐1215, 4 μg/ml), Solanum tuberosum lectin (STL, B‐1165, 20 ng/ml), Aleuria aurantia lectin (AAL, B‐1395, 33 ng/ml), Maackia amurensis lectin II (MAL II, B‐1265, 67 ng/ml), Sambucus nigra lectin (SNA, B‐1305‐2, 57 ng/ml), Sophora japonica lectin (S.Japan), Ricinus communis agglutinin I (dRCA, B‐1085‐5, no longer available, 50 ng/ml).
Flow cytometry
Whole‐blood flow cytometry assays were used for Figures 1A, 2A–C, 2E and 3A–C. Purified RBCs were used for Figures 2D and 4C,D. RBC gating was applied by forward and side scatter gating of both whole blood and purified RBC flow cytometry experiments. 17 Staining with anti‐glycophorin A (GPA) confirmed gates contained >99% RBCs (data not shown). For lectin flow cytometry, ~5 × 106 RBCs were washed three times in phosphate‐buffered saline and incubated for 10 min at room temperature in calcium buffer (10 mM HEPES, 150 mM NaCl2, 2.5 mM CaCl2, pH 7.4) containing 10% Carbo‐Free Blocking Solution (SP5040; Vector Laboratories), then stained in the dark for 30 min. Annexin V staining was carried out according to the manufacturer's instructions (640945; BioLegend). For biotinylated lectin staining, cells were then washed and incubated with streptavidin PE (0.67 μg/ml, 554 061; BD Pharmingen) for 30 min at room temperature in blocking buffer.
FIGURE 1.
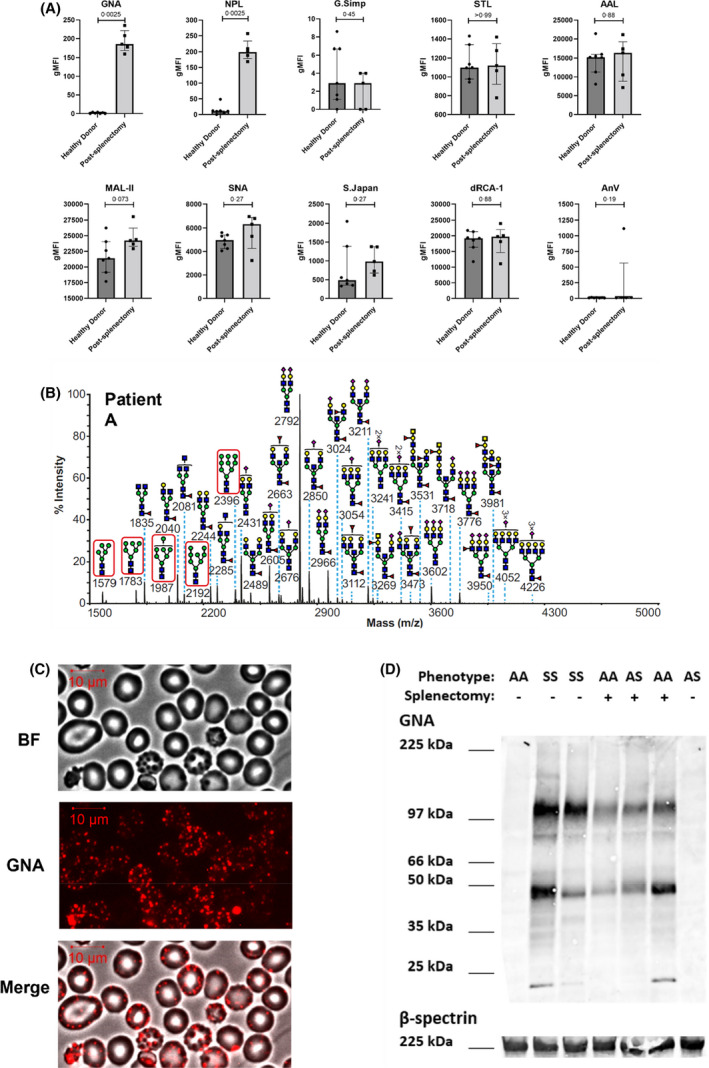
Red blood cells (RBCs) from patients after splenectomy express high levels of high‐mannose glycans. (A) Flow cytometric analysis of blood samples from seven healthy donors versus five patients after splenectomy. RBCs are stained with nine fluorescently labelled plant lectins that bind a range of glycans, detailed in the Materials and methods section. Lectins GNA and NPL bind terminal mannoses. Vertical axes show normalised geometric mean fluorescence (gMFI). Individual data points from individual samples measured on a single occasion are shown with medians ± interquartile ranges. p values above each graph test the differences between samples from donors with intact versus absent spleen, calculated using two tailed Mann–Whitney tests. (B) Matrix‐assisted laser desorption/ionisation time‐of‐flight (MALDI‐TOF) mass spectra (m/z versus relative intensity) for glycomic analysis of permethylated N‐glycans from membrane ghosts from red blood cells (RBCs) from a single patient after splenectomy (patient A). Red boxes indicate high‐mannose structures. All ions are [M + Na]+. Annotation uses conventional symbols for carbohydrates in accordance with the symbol nomenclature for glycans (SNFG) 23 : purple diamond, sialic acids; yellow circle, galactose; yellow square, N‐acetyl galactosamine; blue square, N‐acetyl glucosamine; green circle, mannose; red triangle, fucose. Only major structures are annotated for clarity. (C) Representative images of post‐splenectomy patient's RBCs stained with fluorescently labelled mannose‐binding lectin GNA. Bright field (BF), immunofluorescent (GNA) and merged (merge) views are shown. (D) Western blot from RBC ghosts probed with GNA (top) or β‐spectrin (bottom) from healthy controls (indicated HbAA and splenectomy −), patients after splenectomy (HbAA and splenectomy +, or HbAS indicating heterozygosity for the sickle cell allele and splenectomy +) and sickle cell disease patients (HbSS and splenectomy −). AAL, Aleuria aurantia lectin; dRCA, Ricinus communis agglutinin I; GNA, Galanthus nivalis agglutinin; G.Simp, Griffonia simplicifolia lectin II; MAL‐II, Maackia amurensis lectin II; NPL, Narcissus pseudonarcissus lectin; S.Japan, Sophora japonica lectin; SNA, Sambucus nigra lectin; STL, Solanum tuberosum lectin
FIGURE 2.
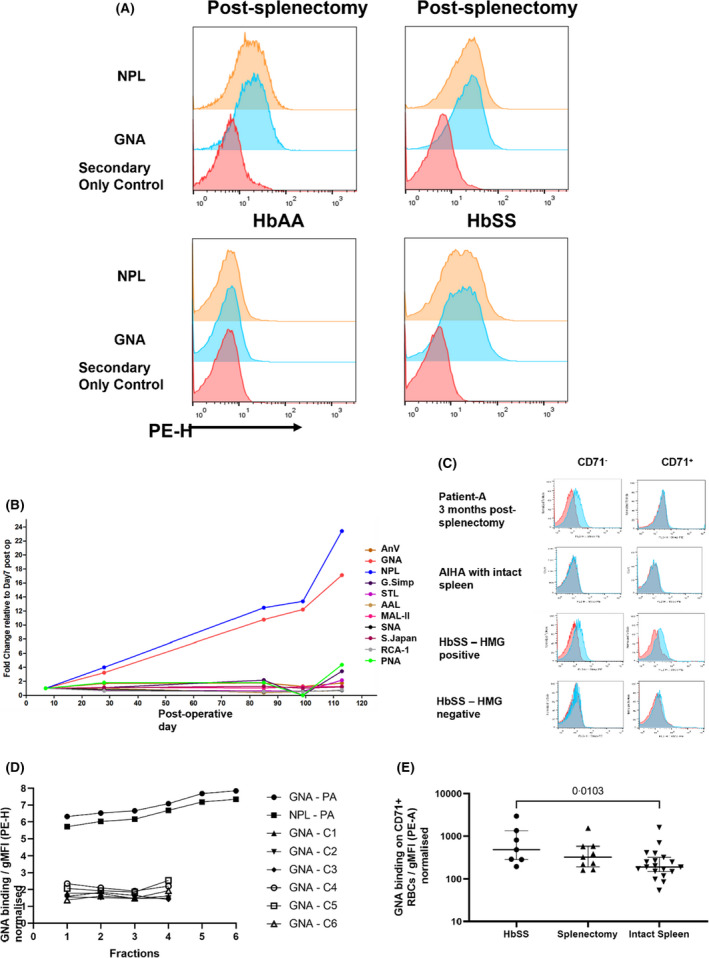
HMG expression after splenectomy. (A) Flow cytometric histograms of mannose‐binding lectin (NPL and GNA) binding to RBCs versus secondary stain‐only control for four patients: Two post‐splenectomy, a healthy donor and a patient with sickle disease (HbSS), both with intact spleens. PE‐H indicates intensity/height of phycoerythrin staining. (B) Time course of binding of lectins to RBCs obtained from patient A after undergoing splenectomy at day 0. RBCs stained with 1 of 10 fluorescently labelled plant lectins that bind a wide range of glycans, detailed in the Materials and methods section, or annexin‐V (AnV). Lectins GNA and NPL bind terminal mannoses. Lectin‐binding gMFI expressed as fold change compared to day 7 post splenectomy sample. (C) Examples of GNA staining of RBCs from three patients. From top to bottom: (i) patient A 4 months post splenectomy, (ii) control autoimmune haemolytic anaemia (AIHA) patient with intact spleen, (iii) patient with sickle cell disease. Staining of CD71+ cells, indicating reticulocytes, are compared with mature CD71− RBCs. GNA binding is indicated in blue and secondary antibody‐only control in red. (D) Surface HMG expression on RBCs obtained from patient A 3 months after splenectomy, marked PA, and six healthy controls with intact spleens, marked C1–6. Cells are fractionated by density, with fractions 1 to 6 indicating increasing density/age. HMG expression is measured by binding of lectins GNA or NPL, assessed by flow cytometry and expressed as normalised gMFI. PE‐H indicates intensity/height of phycoerythrin staining. (E) GNA expression on CD71+ RBCs from patients with sickle cell disease (HbSS, n = 7), post‐splenectomy (n = 9) and healthy controls with intact spleens (n = 19). Medians with interquartile ranges are shown; p value calculated using the Mann–Whitney test, non‐significant for splenectomy versus healthy controls. PE‐A indicates area under curve of phycoerythrin staining. AnV, Annexin‐V; g‐MFI, geometric mean fluorescence intensity; HMG, high‐mannose glycan; RBC, red blood cell. For abbreviations of lectins, see caption to Figure 1
FIGURE 3.
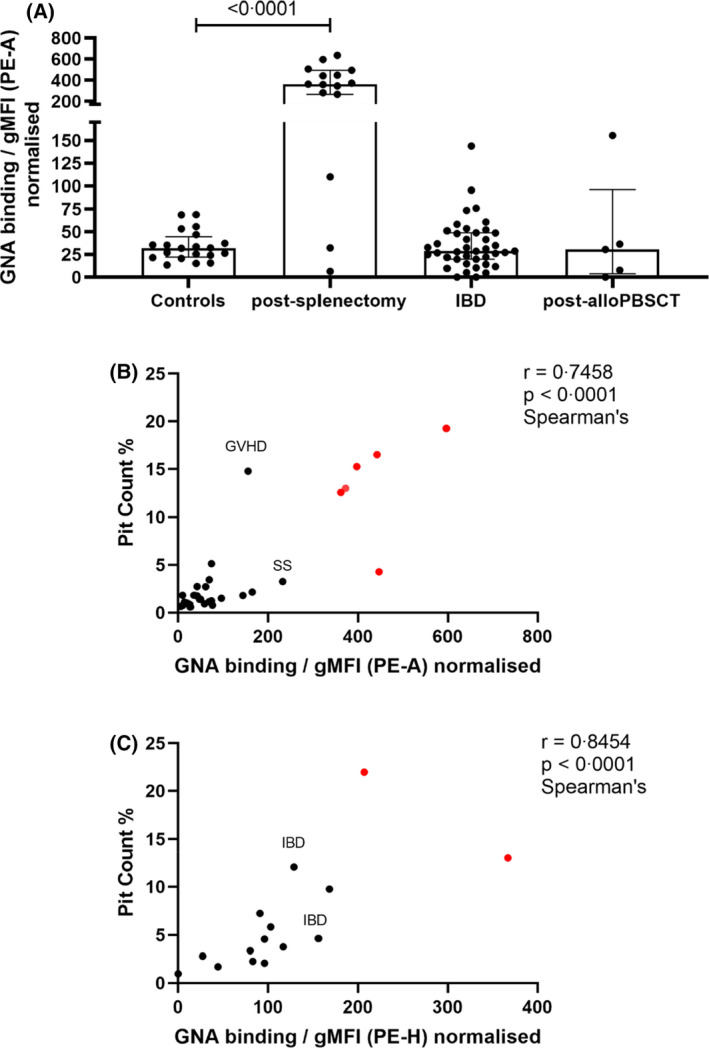
RBC HMG levels as a potential test of splenic function. (A) RBC HMG levels from healthy controls (n = 20), patients after splenectomy (n = 15) and patients with potential functional hyposplenism: inflammatory bowel disease (IBD) [Crohn's disease (n = 15), ulcerative colitis (n = 3), not further specified (n = 22)] and post‐allogeneic peripheral blood stem cell transplantation (post‐alloPBSCT; n = 5). Medians with interquartile ranges are shown; p value calculated using the Mann–Whitney test. (B) Correlation between pit counts and HMG levels measured by gMFI‐area under curve of GNA binding. Samples from post‐splenectomy donors shown in red, sample from donor with sickle cell disease marked SS and from patient with graft‐versus‐host disease (GVHD) (total n = 31). (C) Correlation between pit counts and HMG levels measured by gMFI‐peak height of GNA binding, measured using samples obtained independently of those used to measure area under curve in (B). Samples from post‐splenectomy donors shown in red, patients with inflammatory bowel disease marked IBD (total n = 15). gMFI, geometric mean fluorescence intensity; GNA, Galanthus nivalis agglutinin; HMG, high‐mannose glycan; PE‐A, area under curve of phycoerythrin staining; RBC, red blood cell
Red blood cell ghost preparation
Washed RBCs were subjected to ice‐cold hypotonic lysis in 20 mM Tris, pH 7.6, with protease inhibitor (05056489001; Roche). 19 Lysates were washed three times in lysis buffer (37 000 g, 4°C, 30 min) before resuspension. Protein concentrations were determined by protein BCA assay (23227; Pierce). No trypsinisation was performed before any glycan analysis.
Glycomic mass spectrometry
N‐linked glycan analysis from RBC ghosts was performed according to Jang‐Lee et al. 20 mass spectrometry (MS) and MS/MS data from the permethylated purified glycan fractions were acquired on a 4800 MALDI‐TOF/TOF mass spectrometer (Applied Biosystems). Data were processed using Data Explorer 4.9 Software (Applied Biosystems). The processed spectra were subjected to manual assignment and annotation with the aid of a glycobioinformatics tool, GlycoWorkBench. 21 Proposed assignments for the selected peaks were based on 12C isotopic composition together with knowledge of the biosynthetic pathways, and structures were confirmed by data obtained from MS/MS experiments.
Lectin/immuno‐blotting
Ghost preparations were mixed in equal volumes with sodium dodecyl sulphate (SDS) sample buffer containing 8 M urea 19 and heated at 100°C for 10 min. Western blotting used NuPage 4%–12% Bis‐Tris gel (Invitrogen; NP0312BOX) and transfer (30 V, 1 h) to polyvinylidene fluoride membrane (P 0.45 μm; 10600023 Amersham Hybond; GE Healthcare). Blots were probed with biotinylated GNA lectin (40 μg/ml, B1245; Vector Laboratories) and Streptavidin HRP (1:2500 dilution, 3999S; Cell Signalling) in calcium binding buffer (10 mM HEPES, 150 mM NaCl2, 2.5 mM CaCl2, pH 7.4) containing 1× Carbo‐Free Blocking Solution (Vector Laboratories; SP‐5040) and protease inhibitor cocktail (11836145001; Roche) before development in Amersham ECL Select substrate (RPN2235; GE Healthcare). 0.1% Tween‐20 was added in probing and washing steps. Loading of wells was normalised by protein concentration (~6 μg per sample).
Immunofluorescence microscopy
Cell surface GNA lectin binding for immunofluorescence was performed as for flow cytometry with minor alterations: 8 μg/ml GNA lectin and 1 μg/ml streptavidin PE were pre‐complexed overnight. Stained cells were washed and pulse‐centrifuged (<300 g) for 30 s (including acceleration), in 24‐well, flat‐bottom tissue culture plates (Greiner). Cells were imaged at 32× magnification using an immunofluorescence microscope (Zeiss AxioObserver Z1). Images were analysed by Zen (Black and Blue versions; Zeiss).
Statistical analysis
Statistical significance was assessed by either a two‐tailed Mann–Whitney test for unpaired group data or two paired Spearman's rank testsfor correlation analysis. Calculations were implemented in Prism version 5.04 (GraphPad Software).
RESULTS
High‐mannose glycan expression after splenectomy
A panel of nine fluorescently labelled lectins was used to investigate the surface glycans of RBCs from patients after splenectomies. Only two, GNA and NPL, both with high affinities for terminal mannose residues, 22 bound to a systematically greater extent than to RBCs from donors with intact spleens (Figure 1A). Another, Maackia amurensis lectin II (MAL‐II), which binds α2,3‐linked sialic acids, also bound RBCs from splenectomised patients at moderately greater levels, although this difference was not statistically significant and not investigated further. Phosphatidylserine, as judged by binding of annexin V, was also noted to be similar between the two groups. A glycomic analysis was made of the N‐linked glycans from the RBC ghosts of two patients post splenectomy. Matrix‐assisted laser desorption/ionisation time‐of‐flight/mass spectroscopy (MALDI‐TOF/MS) analysis of permethylated N‐glycans showed the presence of HMGs ranging from Man5GlcNAc2 to Man9GlcNAc2 structures (Figure 1B; Figure S1), which are the only glycans identified that would be predicted to bind lectins GNA and NPL. Binding of GNA was visualised using microscopy and found to comprise discrete patches (Figure 1C), similar to those previously observed in sickle cell disease. The structural basis for these HMGs was investigated further by lectin blotting, which showed that lectin‐binding activity comigrated with those for both full‐molecular‐weight spectrin, as well as several lower‐molecular‐weight bands (Figure 1D). The latter were similar in size to those previously observed in sickle cell disease, which arose from protease‐resistant, spectrin‐containing fragments derived from high‐molecular‐weight aggregates.
Kinetics of high‐mannose glycan accumulation
We noted that HMG expression on RBCs from post‐splenectomy patients comprised single peaks (Figure 2A), indicating that their accumulation affected all RBCs rather than a sub‐population with specific properties. The kinetics of this process was investigated using several approaches. A time course from 7 days to 4 months post splenectomy for autoimmune haemolytic anaemia (AIHA) for an individual (‘patient A’) yielded a linear increase of GNA and NPL binding, in contrast to that of other lectins and annexin V (Figure 2B). By 110 days post operatively, corresponding to the typical life span of RBCs, the level of HMGs was similar to those from patients whose splenectomies had been performed years previously. These data indicate HMG accumulation is a gradual process.
We also investigated the levels of HMG expression on RBCs with respect to the course of their life spans. RBCs newly released from the bone marrow can be identified by high surface levels of the transferrin receptor, CD71. Patient A's CD71+ cells 3 months post splenectomy did not express HMG, in contrast to their CD71− population (Figure 2C), indicating that the oxidative stress that resulted in surface HMG expression arose in RBCs older than reticulocytes. In addition, we noted that only a minority of RBCs expressed high CD71 levels, indicating there must be an alternative location for removing this molecule other than the spleen. 24 RBCs from a control patient with AIHA, but an intact spleen, did not express high levels of surface HMG on either CD71+ or CD71− fractions (Figure 2C). By contrast, RBCs from a patient with sickle cell disease exhibited high levels of HMGs on both CD71+ and CD71− RBCs (Figure 2C), showing that sufficient oxidative stress can accumulate at stages prior to and/or at the reticulocyte stage. We interpret this to indicate that, in contrast to patient A, pathologically high levels of oxidative stress are able exceed membrane‐editing capacity in the bone marrow. Finally, we used discontinuous density gradients to fractionate Patient A's RBCs from three months post splenectomy. All fractions expressed high levels of HMGs, although denser fractions expressed greater amounts (Figure 2D).
We extended these approaches to RBCs from other donors. All density fractions from six healthy donors exhibited low levels of HMGs (Figure 2D), suggesting that spleens are effective in removing oxidatively damaged membrane marked by HMGs at all stages in the life spans of RBCs. Average levels of HMG expression by CD71+ RBCs obtained from seven patients after splenectomies were similar to those from healthy donors and less than those observed from patients with sickle cell disease (Figure 2E). However, there was appreciable overlap, with only the difference between healthy and donors with sickle cell disease being statistically significant.
High‐mannose glycans as a splenic function test
The high levels of RBC HMGs observed post‐splenectomy and their ease of measurement suggested they might act as a useful test of splenic reticuloendothelial function. To explore this possibility, we measured RBC HMG expression from 13 more patients with previous splenectomies (Figure 3A). Ten of these yielded clearly high values. However, two of the 10 patients exhibited levels similar to those from healthy controls, which may reflect clinically important residual splenic function. As these samples were anonymised, we could not investigate this possibility further.
RBC HMG levels might also be useful to assess patients with anatomically intact spleens but possible functional hyposplenism. We therefore measured RBC HMGs in samples from patients with conditions previously reported to cause poor splenic function: inflammatory bowel disease (IBD) 25 and allogeneic haematopoietic stem cell transplantation. 26 Most patients with IBD had HMG levels within the range seen in healthy donors (Figure 3A). However, a few patients had higher levels (Figure 3A), which might justify prophylactic measures against infection. One of the five post‐transplant patients exhibited high levels of HMG expression (Figure 3A) and it is probably relevant that this sample was also labelled as having graft‐versus‐host disease. 26
Splenic function has been conventionally assessed by counting Howell–Jolly bodies using conventional microscopy or, less frequently, RBC pits using differential interference microscopy. 27 We compared HMG levels to values obtained using both of these techniques. Howell–Jolly bodies were infrequent (<0.1 per high‐power field) in all post‐splenectomy samples and not seen in any sample from patients with functional hyposplenism, in keeping with their known poor sensitivity. However, good correlations were observed between HMG expression and pit numbers (Figure 3B,C).
DISCUSSION
Here we report that RBCs from patients who have had splenectomies exhibit high levels of binding to lectins with high affinities for terminal mannose residues. The surface ligands appear to be N‐linked high mannoses covalently linked to oxidatively cross‐linked membrane protein aggregates.
Oxidatively damaged proteins can have pathological properties, are difficult to repair 28 and so must be degraded. Minor degrees of damage are generally recognised by chaperones that recruit ubiquitinases, which direct the damaged molecules to proteolysis by the proteasome. 29 , 30 More extensively damaged proteins form aggregates that cannot be digested by the proteasome and are delivered by chaperone‐mediated autophagy to lysosomes. 31 , 32 Removal of oxidatively damaged proteins in plasma membranes presents particular challenges. The proteasome has relatively limited access and delivery to lysosomes is more important 33 , 34 , 35 ; membrane is removed as part of this process. The extensive autophagy that takes place during terminal erythroid differentiation uses proteasomal degradation 35 and mature RBCs retain functional components of the system. However, uniquely among human cells, they lack lysosomes. Autophagic digestion is therefore unavailable, which raises the question as to how oxidatively damaged membrane aggregates resistant to proteasomal degradation are removed. As red cells age, membrane is removed in the spleen so becoming smaller and denser. 12 Our discovery that surface HMGs are removed by the spleen therefore implicates these glycans as candidate ligands involved in membrane editing as well as phagocytosis of whole cells. The only other implicated ligand, phosphatidylserine, is involved in the removal of autophagic vacuoles containing intracellular organelles. 36
The identities of the cognate prophagocytic ligands were not identified in this study. However, it is likely that the mannose receptor (CD206) is involved. CD206 is known to be important in the uptake of molecules and structures bearing high mannoses 37 and has been shown to mediate uptake of oxidised RBCs. 17 Its expression in human spleens is confined to LYVE‐1+ expressing cells lining the IESs that must be traversed for RBCs to exit the sinusoids. 38 Given the lack of CD206 expression elsewhere, the documented ability of endothelial cells to effect phagocytosis and their anatomical location, we believe these endothelial cells are the most plausible candidates to perform membrane editing. Other mannose‐binding lectins, such as dectin‐2, SIGNR1 39 and DC‐SIGN, 18 are also likely to have roles.
Red cells are especially prone to several forms of oxidative stress, at least in part because of the high concentrations of oxygen and iron in several valency states that cause high rates of reactive oxygen species production. 40 , 41 This situation pertains throughout the life span of red cells from the erythroblast stage onwards. Concerning the time course of appearance of HMGs with respect to the life span of RBCs, our data indicate most accumulate gradually in circulating erythrocytes from healthy individuals, with only low levels seen in immature CD71+ cells. In contrast, reticulocytes from patients with sickle cell disease exhibit high HMG levels, indicating higher levels of oxidative stress 16 at earlier stages of differentiation. In sickle cell disease, we presented evidence that HMGs mediated extravascular haemolysis. Whether there is a role for HMGs acting as an end‐of‐life signal remains to be determined. If so, the enhanced induction of HMGs by P. falciparum seen in RBCs from donors with sickle cell trait can be viewed as the sickle mutation exploiting the mechanisms of natural clearance of RBCs at the end of their lives.
It remains to be seen whether macrophage‐mediated removal of oxidised surface molecules is used in tissues other than blood. CD206 is a prototypical marker of M2 activation and so widely expressed by tissue macrophages, 42 including the bone marrow, whose macrophages are known to express CD206, at least in mice. 43 If so, the HMG expression we noted in some patients' reticulocytes presumably arises only if the capacity of this and other lysosomal systems is exceeded by differentiating erythroblasts.
Our work indicates that RBC HMG expression is a test of splenic reticuloendothelial function. The main complication of hyposplenism is overwhelming infection with encapsulated bacteria, notably Streptococcus pneumoniae. 25 , 44 Various mechanisms have been proposed, but none is generally accepted. 25 Together with the observation that the mannose receptor binds the cell wall of S. pneumoniae with high affinity, 39 our findings suggest that an important mechanism is loss of filtering function mediated by lectins on the surface of the cells lining the IESs. Measuring HMG levels may therefore be of direct clinical importance, in contrast to methods such as counting pits, 27 Howell–Jolly bodies or platelets. 45 Furthermore, and again in contrast to manually‐based methods, it should be automatable. The test may therefore be useful for checking complete removal of splenic function after splenectomies as well as stratifying treatments in situations where functional hyposplenism may be important, such as post‐haematopoietic stem cell transplantation 26 , 46 and inflammatory bowel disease. 25
In summary, we present data showing that physiological oxidative damage to RBCs results in membrane aggregates characterised by HMGs that are removed by passage through the spleen. Measurement of RBC HMGs therefore offers great potential as a test of splenic function.
AUTHOR CONTRIBUTIONS
Huan Cao performed the laboratory work, analysed the data and wrote the paper. Abhinav Mathur, Charlotte Robertson, Sadie Henderson, Louis‐Pierre Girard, Jin Hien Wong and Anne Dell performed laboratory work. Sonja Wright, John Brewin and David C. Rees provided samples. Aristotelis Antonopoulos, Adam Davie and Stuart M. Haslam performed and analysed the glycomic data. Mark A. Vickers supervised the project and wrote the paper.
Supporting information
Figure S1
Table S1
Table S2
ACKNOWLEDGEMENTS
This work was funded by Aberdeen University Development Trust and Friends of Anchor. The University of Aberdeen is applying for a patent based on this work.
Cao H, Mathur A, Robertson C, Antonopoulos A, Henderson S, Girard L‐P, et al. Measurement of erythrocyte membrane mannoses to assess splenic function. Br J Haematol. 2022;198:155–164. 10.1111/bjh.18164
REFERENCES
- 1. Crosby WH. Normal functions of the spleen relative to red blood cells: a review. Blood. 1959. April 01;14(4):399–408. [PubMed] [Google Scholar]
- 2. Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005. August 01;5(8):606–16. [DOI] [PubMed] [Google Scholar]
- 3. Bratosin D, Mazurier J, Tissier JP, Estaquier J, Huart JJ, Ameisen JC, et al. Cellular and molecular mechanisms of senescent erythrocyte phagocytosis by macrophages. A review. Biochimie. 1998. February 01;80(2):173–95. [DOI] [PubMed] [Google Scholar]
- 4. Lutz HU. Innate immune and non‐immune mediators of erythrocyte clearance. Cell Mol Biol (Noisy‐le‐Grand). 2004. March 01;50(2):107–16. [PubMed] [Google Scholar]
- 5. Lutz HU, Bogdanova A. Mechanisms tagging senescent red blood cells for clearance in healthy humans. Front Physiol. 2013. December;25(4):387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klei TR, Meinderts SM, van den Berg TK, van Bruggen R. From the cradle to the grave: the role of macrophages in erythropoiesis and erythrophagocytosis. Front Immunol. 2017. February 02;8(8):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Back DZ, Kostova EB, van Kraaij M, van den Berg TK, van Bruggen R. Of macrophages and red blood cells; a complex love story. Front Physiol. 2014. January;30(5):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klei TRL, de Back DZ, Asif PJ, Verkuijlen PJJH, Veldthuis M, Ligthart PC, et al. Glycophorin‐C sialylation regulates Lu/BCAM adhesive capacity during erythrocyte aging. Blood Adv. 2018. January 03;2(1):14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pivkin IV, Peng Z, Karniadakis GE, Buffet PA, Dao M, Suresh S. Biomechanics of red blood cells in human spleen and consequences for physiology and disease. Proc Natl Acad Sci U S A. 2016. July 12;113(28):7804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zimring JC. Turning over a new leaf on turning over RBCs. Blood. 2020. October 01;136(14):1569–70. [DOI] [PubMed] [Google Scholar]
- 11. Thiagarajan P, Parker CJ, Prchal JT. How do red blood cells die? Front Physiol. 2021. March;15(12):655393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Piomelli S, Seaman C. Mechanism of red blood cell aging: relationship of cell density and cell age. Am J Hematol. 1993. January 01;42(1):46–52. [DOI] [PubMed] [Google Scholar]
- 13. Asaro RJ, Zhu Q, Cabrales P. Erythrocyte aging, protection via vesiculation: an analysis methodology via oscillatory flow. Front Physiol. 2018. November;16(9):1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li H, Lu L, Li X, Buffet PA, Dao M, Karniadakis GE, et al. Mechanics of diseased red blood cells in human spleen and consequences for hereditary blood disorders. Proc Natl Acad Sci U S A. 2018. September 18;115(38):9574–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reily C, Stewart TJ, Renfrow MB, Novak J. Glycosylation in health and disease. Nat Rev Nephrol. 2019. June 01;15(6):346–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Strader MB, Jana S, Meng F, Heaven MR, Shet AS, Thein SL, et al. Post‐translational modification as a response to cellular stress induced by hemoglobin oxidation in sickle cell disease. Sci Rep. 2020. August 26;10(1):14218‐020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cao H, Antonopoulos A, Henderson S, Wassall H, Brewin J, Masson A, et al. Red blood cell mannoses as phagocytic ligands mediating both sickle cell anaemia and malaria resistance. Nat Commun. 2021. March 19;12(1):1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown GD, Crocker PR. Lectin receptors expressed on myeloid cells. Microbiol Spectr. 2016. October 01;4(5). 10.1128/microbiolspec.MCHD-0036 [DOI] [PubMed] [Google Scholar]
- 19. Barker RN, Casswell KM, Reid ME, Sokol RJ, Elson CJ. Identification of autoantigens in autoimmune haemolytic anaemia by a non‐radioisotope immunoprecipitation method. Br J Haematol 1992. September 01;82(1):126–132. [DOI] [PubMed] [Google Scholar]
- 20. Jang‐Lee J, North SJ, Sutton‐Smith M, Goldberg D, Panico M, Morris H, et al. Glycomic profiling of cells and tissues by mass spectrometry: fingerprinting and sequencing methodologies. Methods Enzymol. 2006;415:59–86. [DOI] [PubMed] [Google Scholar]
- 21. Ceroni A, Maass K, Geyer H, Geyer R, Dell A, Haslam SM. GlycoWorkbench: a tool for the computer‐assisted annotation of mass spectra of glycans. J Proteome Res. 2008. April 01;7(4):1650–9. [DOI] [PubMed] [Google Scholar]
- 22. Shibuya N, Goldstein IJ, Van Damme EJ, Peumans WJ. Binding properties of a mannose‐specific lectin from the snowdrop (Galanthus nivalis) bulb. J Biol Chem. 1988. January 15;263(2):728–34. [PubMed] [Google Scholar]
- 23. Neelamegham S, Aoki‐Kinoshita K, Bolton E, Frank M, Lisacek F, Lutteke T, et al. Updates to the symbol nomenclature for glycans guidelines. Glycobiology. 2019. August 20;29(9):620–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rhodes MM, Koury ST, Kopsombut P, Alford CE, Price JO, Koury MJ. Stress reticulocytes lose transferrin receptors by an extrinsic process involving spleen and macrophages. Am J Hematol. 2016. September 01;91(9):875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mathur A, McLean MH, Cao H, Vickers MA. Hyposplenism and gastrointestinal diseases: significance and mechanisms. Dig Dis 2021. May 25. [DOI] [PubMed] [Google Scholar]
- 26. Rozmus J, Mallhi K, Ke J, Schultz KR. Functional hyposplenism after hematopoietic stem cell transplantation. Bone Marrow Transplant 2015. October 01;50(10):1343–1347. [DOI] [PubMed] [Google Scholar]
- 27. Rogers DW, Serjeant BE, Serjeant GR. Early rise in the "pitted" red cell count as a guide to susceptibility to infection in childhood sickle cell anaemia. Arch Dis Child. 1982. May 01;57(5):338–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marques C, Guo W, Pereira P, Taylor A, Patterson C, Evans PC, et al. The triage of damaged proteins: degradation by the ubiquitin‐proteasome pathway or repair by molecular chaperones. FASEB J. 2006. April 01;20(6):741–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jung T, Hohn A, Grune T. The proteasome and the degradation of oxidized proteins: part II ‐ protein oxidation and proteasomal degradation. Redox Biol. 2014;2:99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pickering AM, Davies KJ. Degradation of damaged proteins: the main function of the 20S proteasome. Prog Mol Biol Transl Sci. 2012;109:227–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kaushik S, Cuervo AM. Chaperone‐mediated autophagy: a unique way to enter the lysosome world. Trends Cell Biol. 2012. August 01;22(8):407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jackson MP, Hewitt EW. Cellular proteostasis: degradation of misfolded proteins by lysosomes. Essays Biochem. 2016. October 15;60(2):173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Houck SA, Singh S, Cyr DM. Cellular responses to misfolded proteins and protein aggregates. Methods Mol Biol. 2012;832:455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Houck SA, Cyr DM. Mechanisms for quality control of misfolded transmembrane proteins. Biochim Biophys Acta. 2012. April 01;1818(4):1108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nguyen AT, Prado MA, Schmidt PJ, Sendamarai AK, Wilson‐Grady JT, Min M, et al. UBE2O remodels the proteome during terminal erythroid differentiation. Science. 2017. August 04;357(6350). 10.1126/science.aan0218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mankelow TJ, Griffiths RE, Trompeter S, Flatt JF, Cogan NM, Massey EJ, et al. Autophagic vesicles on mature human reticulocytes explain phosphatidylserine‐positive red cells in sickle cell disease. Blood. 2015. October 08;126(15):1831–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martinez‐Pomares L. The mannose receptor. J Leukoc Biol. 2012. December 01;92(6):1177–86. [DOI] [PubMed] [Google Scholar]
- 38. Martinez‐Pomares L, Hanitsch LG, Stillion R, Keshav S, Gordon S. Expression of mannose receptor and ligands for its cysteine‐rich domain in venous sinuses of human spleen. Lab Invest 2005. October 01;85(10):1238–1249. [DOI] [PubMed] [Google Scholar]
- 39. McGreal EP, Rosas M, Brown GD, Zamze S, Wong SY, Gordon S, et al. The carbohydrate‐recognition domain of Dectin‐2 is a C‐type lectin with specificity for high mannose. Glycobiology. 2006. May 01;16(5):422–30. [DOI] [PubMed] [Google Scholar]
- 40. Vona R, Sposi NM, Mattia L, Gambardella L, Straface E, Pietraforte D. Sickle cell disease: role of oxidative stress and antioxidant therapy. Antioxidants (Basel). 2021. February 16;10(2):296. 10.3390/antiox10020296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fujii J, Homma T, Kobayashi S, Warang P, Madkaikar M, Mukherjee MB. Erythrocytes as a preferential target of oxidative stress in blood. Free Radic Res. 2021. May 01;55(5):562–80. [DOI] [PubMed] [Google Scholar]
- 42. Gordon S, Pluddemann A. Tissue macrophages: heterogeneity and functions. BMC Biol. 2017. June 29;15(1):53‐017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bisgaard LS, Mogensen CK, Rosendahl A, Cucak H, Nielsen LB, Rasmussen SE, et al. Bone marrow‐derived and peritoneal macrophages have different inflammatory response to oxLDL and M1/M2 marker expression ‐ implications for atherosclerosis research. Sci Rep. 2016. October;13(6):35234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zamze S, Martinez‐Pomares L, Jones H, Taylor PR, Stillion RJ, Gordon S, et al. Recognition of bacterial capsular polysaccharides and lipopolysaccharides by the macrophage mannose receptor. J Biol Chem. 2002. November 01;277(44):41613–23. [DOI] [PubMed] [Google Scholar]
- 45. Mathur A, Samaranayake S, NPF S, Vickers MA. Investigating thrombocytosis. BMJ. 2019. July;04(366):l4183. [DOI] [PubMed] [Google Scholar]
- 46. Majhail NS, Rizzo JD, Lee SJ, Aljurf M, Atsuta Y, Bonfim C, et al. Recommended screening and preventive practices for long‐term survivors after hematopoietic cell transplantation. Rinsho Ketsueki. 2014. June 01;55(6):607–32. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Table S1
Table S2


