Abstract
Diet-related metabolic diseases, and especially obesity, are metabolic disorders with multifactorial aetiologies. Diet has been a cornerstone in both the aetiology and management of this metabolic disorders. Rice, a staple food for over half of the world’s population, could be exploited as part of the solution to check this menace which has been skyrocketing in the last decade. The present study investigated nine forms of rice from three widely grown Malaysian rice cultivars for in vitro and in vivo (glycaemic index and load) properties that could translate clinically into a lower predisposition to diet-related diseases. The germinated brown forms of MRQ 74 and MR 84 rice cultivars had high amylose content percentages (25.7% and 25.0%), high relative percentage antioxidant scavenging abilities of 85.0% and 91.7%, relatively low glycaemic indices (67.6 and 64.3) and glycaemic load (32.3 and 30.1) values, and modest glucose uptake capabilities of 33.69% and 31.25%, respectively. The results show that all things being equal, rice cultivars that are germinated and high in amylose content when compared to their white and low amylose counterparts could translate into a lower predisposition to diet-related diseases from the dietary point of view in individuals who consume this cereal as a staple food.
Keywords: Brown rice, Diet-related metabolic diseases, Germinated brown rice, White rice
1. Introduction
Rice, which is the second most grown cereal in the world, is a staple food for over one-half of the world’s population and accounts for almost one-fifth of the total calorie intake in man [1]. In South-east Asia, it provides a staggering 76% of total calorie intake, making it the most important food in this part of the world [2]. Beyond Asia, the yearly consumption of rice has continued to increase steadily with the sub-Saharan Africa at the top of the list. In a similar pattern, rice consumption has steadily increased in both European Union countries and the United States, partly due to the influx of Asians to this part of the world and the shift in consumers’ choice from a protein-based diet to a more fibre-based one [3].
Obesity is a single important risk factor of type 2 diabetes and other diet-related metabolic diseases [4]. Over 90% of type 2 diabetics are either overweight or obese [5]. In 2014, the global prevalence of obesity (BMI × 30 kg/m2) in adults was well over 10%, with figures expected to rise [5]. Children have not been excluded from the scourge of obesity. In Africa, for example, where there has been a steady growth in the consumption of rice in the recent past, the number of obese and overweight children (<18 yrs) nearly doubled between 1990 and 2014 from about 5.4 million to about 10.6 million children [6]. Since rice is a staple food for over half of the world’s population [1], and the regions of the globe (Asia, Africa and the Eastern Mediterranean) who consume the cereal the most harbour a greater proportion of individuals with diet-related metabolic diseases like obesity and its sequelae [4], this could make the staple consumption of a peculiar rice cultivar a significant contributor to the development of obesity and other diet-related metabolic diseases.
Several studies have tried to link some beneficial or harmful effects to various cultivars of rice using a single parameter of the rice. These effects include the prevention of neuroleptic-induced extrapyramidal symptoms [7], a predisposition to diabetes [8], the development of chronic kidney diseases which are often not unconnected to presence of cadmium, arsenic and mercury in rice [9], possible linkage to cancer and a predisposition to obesity [10]. This study focused on the dual effect of amylose content value and germination status of a rice cultivar in relation to possible predisposition to diet-related diseases.
MRQ 76, MRQ 74 and MR 84 are among the most commonly consumed rice cultivars in Malaysia. They are relatively affordable and widely cultivated, as is true of rice cultivars consumed as a staple food in most parts of the world. The aim of this study is to investigate the composition of several white, brown and germinated brown rice cultivars in a bid to offer insight into the growing diet-related metabolic diseases in regions where rice is a staple food.
2. Materials and methodology
2.1. Material collection and germination
Brown and white rice cultivars of MRQ 74, MRQ 76, and MR 84 were obtained from the Malaysian Agricultural Research and Development Institute. The brown form of each cultivar was germinated to obtain its corresponding germinated brown form as described previously [11]. Briefly, the brown rice was washed and soaked in 0.05% of sodium hypochloride for 30 min, washed with tap water and then soaked in 0.5% hydrogen peroxide for 6 h and finally incubated for 18 h in an oven until it dried. The white rice, brown rice and germinated brown rice of each cultivar were size reduced using a laboratory blender (Wairing® model HGB2WTS3, Connecticut, USA).
2.2. Determination of amylose content
The amylose content of the rice cultivars was determined according to the International Standard Organisation [12] protocol. Briefly, 0.1 g of each sample, blank or standard was weighed in triplicate and placed in a 100 ml volumetric flask, before 1 ml of 95% ethanol and 9 ml of 1 M sodium hydroxide was added. The mixture was heated for 20 min in a boiling water bath and allowed to cool to room temperature before making it up to 100 ml with water. Then, 0.5 ml of the test, standard or blank solution was pipetted into a 10 ml test tube containing 5 ml of water. Next, 0.1 ml of 5% acetic acid and 0.2 ml of iodine (Lugol’s solution) was added to each test tube. An additional 4.2 ml of water was added to each test tube to make the volume up to 10 ml, mixed well with a vortex mixer and immediately measured at 720 nm against the blank. A calibration curve was obtained using standard graded amylose (Fluka chemicals, Germany) and the percentage amylose of the cultivars of the rice was extrapolated from the curve.
2.3. Proximate analysis
The moisture and ash contents were determined by drying 2 g of each sample in triplicate in an oven at 105 °C for 1 h to a constant weight; the loss of weight is used to calculate the moisture content of the sample. The ash content was determined by heating 2 g of each sample in same oven at 600 °C for 2 h. The percentage ash content was calculated based on dry weight.
The protein content was determined according to the Kjeldahl method using block digestion and steam distillation.
The crude fat content was determined using the Soxtex® extraction system. Briefly, 2 g of the samples in triplicate were weighed and placed into thimbles which were then placed into the extraction system for extraction and evaporation. The extraction cups were dried to a constant weight and the percentage of crude fat was calculated as follows.
The carbohydrate content was calculated by difference as shown below
2.4. Determination of heavy metals and mineral composition
About 0.5 g of the samples was transferred into each 55 ml self-regulating pressure control PFA vessel and digested with reagents consisting of 2.50 ml 65% HNO3, 0.5 ml 30% HCL, and 7.0 ml ultra-pure water. The mixture was cooled, filtered and stored at 4 °C. The heavy metal content was analysed using inductively-coupled plasma mass spectrometry (ICP-MS) with the Agilent 7500A series (Agilent Technologies, USA) using the following parameters: Plasma RF power 1550 W, Reflected power <15 W, Sampling depth 8.0 mm, Plasma gas flow 15 L/ min, Carrier gas flow 1.2 L/min, Collision gas type He, Collision gas flow 3.0–5.0 L/min, S/C temperature 2 °C.
2.5. Animal handling and feeding
Sixty male Sprague–Dawley rats weighing 160–200 g were purchased from the animal house of the Faculty of Veterinary Medicine, Universiti Putra Malaysia, Serdang, Malaysia. The animals were housed in pairs, in plastic cages, and maintained in a well-ventilated room with an approximate 12/12 h light/ dark cycle, at 25–30 °C. Approval for the use of animals was sought from the Institutional Animal Care and Use Committee (IACUC) of the Faculty of Medicine and Health Sciences, Universiti Putra Malaysia (Project approval number: UPM/IACUC/ AUP-R017/2016), and animals were handled as stipulated by the guidelines for the use of animals. Animals were allowed to acclimatize for 1 week on standard rat chow ad libitum and free access to water before the commencement of the experiment.
2.5.1. Glycaemic index (GI) determination
This was determined according to FAO/WHO [13]. The rats were randomly assigned to ten groups of six rats per group ensuring a minimum difference in mean weight among the groups and within each group. The rats were allowed to acclimatize to the new environment for one week. A night before the commencement of the experiment, the rats were fasted for 12 h and their initial plasma glucose concentrations were taken (t = 0) from the tail tip artery of the rats. The rats in all of the groups were administered 500 mg of d-glucose (Fluka chemicals, Germany) followed by plasma glucose concentration measurements from the tail tip artery at t = 30, 60, 90 and 120 min. The rats were thereafter given free access to standard rat chow and water ad libitum for 48 h. The above procedure was repeated by replacing 500 mg of glucose for each group with a rice cultivar type containing 500 mg of carbohydrate based on the proximate analysis. The GI was calculated by dividing the incremental area under 2 h glucose response curve of a particular rice cultivar in a particular rat by the incremental area under 2 h glucose response curve of the reference d-glucose in the same rat and multiplying the outcome by 100.
2.5.2. Glycaemic load (GL) derivation
The GL values of each rice cultivar was determined by dividing the mathematical product of its GI and the grams of available carbohydrate in 1000 kJ of the rice cultivar by 100.
2.6. Extraction of the cultivars of rice
Twenty five grams of each cultivar of the size reduced rice were extracted by either ultra-sonication-assisted water extraction (model: powersonic 505, Hwash technology, Seoul, Korea) at room temperature for 4 h, ultra-sonication assisted methanol (80% v/v) extraction at room temperature for 4 h or subcritical water extraction at 120 °C (Separex® Model 4377, Champigneulles, France). The sub-critical water extraction was accomplished at 150 °C, 100 bar pressure and 25 ml/min flow rate for 1 h. The resulting extracts were evaporated to dryness using a Buchi Syncore rotary evaporator (Switzerland).
2.7. Determination of 2, 2′-diphenyl-1-picrylhydrazyl (DPPH) radical-scavenging ability
The DPPH radical-scavenging ability of rice extracts were evaluated according to the method reported by Brand-Williams et al. [14], with slight modifications. The reaction mixture contained 1.5 ml DPPH (Sigma–Aldrich, St. Louis, MO, USA) working solution (4.73 mg of DPPH in 100 ml ethanol HPLC-grade) and 300 μL rice extract. The mixtures were shaken and incubated for 90 min in the dark at room temperature. Their absorbance was read at 515 nm relative to the control (as 100%) using a spectrophotometer (Pharmaspec UV-1700, Shimadzu, Japan). The percentage of radical-scavenging abilities was calculated by using the formula:
2.8. 2,2-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radical cation scavenging assay
The ABTS radical cation scavenging assay was analysed following a modified method of Pellegrini et al. [15]. A stable stock solution of ABTS radical cation was produced by reacting a 7 mM aqueous solution of ABTS (Sigma–Aldrich, St. Louis, MO, USA) with 2.45 Mm solution of potassium persulphate (Sigma–Aldrich, St. Louis, MO, USA) in the dark at room temperature for 12–16 h before use. Rice extract (120 μL) was mixed to react with 1.5 ml of a diluted ABTS radical cation solution (absorbance of 0.70 ± 0.02 AU at 734 nm). The absorbance at 734 nm of the mixture was measured after a 1 min reaction time. The gradient of the plot of percentage inhibition of absorbance vs. concentration plot for each sample was divided by the gradient of the plot for Trolox to obtain Trolox equivalent antioxidant capacity. Results were expressed as Trolox equivalents antioxidant capacity (TEAC) in mmol of Trolox (Sigma–Aldrich, St. Louis, MO, USA) per 100 g of flour.
2.9. Determination of total phenolic content (TPC)
The total phenolic content of the extracts was determined using Folin–Ciocalteu (Sigma–Aldrich, St. Louis, MO, USA) reagent as described by Sompong et al. [16] with little modification. Aliquot (120 μL) of the extract was added to 600 μL of freshly prepared 10% Folin–Ciocalteu reagent. Nine hundred and sixty microlitres of sodium carbonate solution (75 g/L) was added after waiting for 40 min. The absorbance of the resulting solution was read at 765 nm against methanol blank at room temperature. Gallic acid (Sigma–Aldrich, St. Louis, MO, USA) was used as the standard and results were expressed as milligrams of gallic acid equivalent per gram of dry weight of the sample.
2.10. Determination of the total flavonoid contents (TFC)
The total flavonoid content was determined using the Dowd method as adapted by Arvouet-Grand et al. [17]. Briefly, 500 μl of 2% Aluminium trichloride (AlCl3) (Labosi, Paris, France) in methanol was mixed with the same volume of the reconstituted plant extract (500 ppm), and incubated at room temperature for 10 min before reading the absorbance at 415 nm against a methanol blank. The total flavonoid content was determined using a standard curve with rutin (0–0.1 mg/ml) (Sigma–Aldrich, St. Louis, MO, USA) as the standard. The mean of three readings was expressed as mg of rutin equivalents (mg rutin/g DW).
2.11. Singular and interaction effects of the different factors of rice cultivars responsible for their antioxidant effect
The germination status of the rice cultivars, their extraction method and their cultivar types were subjected to a balanced analysis of variance (ANOVA) to investigate how each factor and its interaction with other factors contribute to the observed antioxidant indices.
2.12. Insulin secretion assay
Since oxidative stress has been demonstrated to play a major role in the pathophysiology of many diet-related metabolic diseases [18], only the methanolic extracts of the rice cultivars were chosen for the in vitro glucose handling experiments based on their superior antioxidant properties. After cytotoxicity assay, the insulin secretion assay was performed according to the method of Irwin et al. [19]. Briefly, clonal pancreatic BRIN-BD11 cells (American Type Culture Collection® CL-173™) (passage numbers 25–30) were used. The cells were cultured in RPMI-1640 culture medium (Sigma–Aldrich, St. Louis, MO, USA). Cells were washed with phosphate-buffered saline (PBS) (Gibco, Eggenstein-Leopoldshafen, Germany) prior to detachment from tissue culture flasks with the aid of 0.025% (wt/vol) trypsin containing 1 mM EDTA (Solar Biotech, Beijing, China) and seeded at 1.0 × 105 cells per well into 96-multiwell plates. Monolayers of cells were then cultured for 18 h at 37 °C in the absence or presence of glibenclamide and metformin (Sigma Aldrich, St. Louis, MO, USA) as indicated. Culture medium was replaced with 1 ml of a Krebs Ringer bicarbonate (KRB) buffer, consisting of (in mM) 115 NaCl, 4.7 KCl, 1.2 MgSO4, 1.28 CaCl2, 1.2 KH2PO4, 25 HEPES and 8.4% (wt/vol) NaHCO3 (pH 7.4) supplemented with 0.1% (wt/vol) bovine serum albumin (Sigma–Aldrich, St. Louis, MO, USA) and 1.1 mmol/l glucose. After 40 min pre-incubation at 37 °C, the buffer was replaced with 1 ml of KRB test buffer containing glucose and test agents. After 20 min incubation at 37 °C, aliquots of test buffer were removed and stored at −20 °C for insulin radioimmunoassay.
2.13. 3T3-L1 cell differentiation and glucose uptake
The differentiation and uptake assay was performed as described previously [20] with few modifications. Seeded cells were grown overnight and then treated with the methanolic rice cultivar extracts, rosiglitazone (Sigma–Aldrich, USA), apigenin (1:1000) (Sigma–Aldrich, St. Louis, MO, USA) or vehicle control in 100 μL of glucose free culture medium containing 150 μg/ml 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBDG) (Invitrogen Corporation, Carlsbad, CA, USA) and incubated for 90 min. The plates were centrifuged, the supernatant was aspirated and 200 μL of cell based assay buffer was added. The cellular uptake of 2-NBDG was measured using a multifunctional plate reader at excitation and emission wavelengths of 485 and 535 nm, respectively.
2.14. Statistical analysis
The study was carried out in triplicate. Results are expressed as the mean ± standard deviation. Statistical analysis of data was carried out using Minitab software application version 19 (Minitab Inc. Pennsylvania, USA). Analysis was performed using Pearson’s correlation, One-way analysis of variance (ANOVA) or balanced ANOVA, as appropriate for each analysis. Differences in mean were considered to be significant when p < 0.050. Values are represented as tables and figures.
3. Results
3.1. Amylose content
The percentage amylose composition of the of the rice cultivars is presented in Table 1. The amylose content of each cultivar of the rice was extrapolated from a calibration curve of rice amylose (standards) with known amylose concentration. MRQ 76 has the lowest amylose composition while MRQ 74 has the highest. As expected, germination and polishing did not significantly increase the amylose content of the rice as the brown and white form of each cultivar were not significantly different from their germinated counterpart. Based on the classification of the International Rice Research Institute (IRRI, 2009) on amylose, MRQ 74 and MR 84 could be classified as high amylose rice while MRQ 76 would fall into the low amylose group.
Table 1.
Proximate composition and percentage amylose composition of some Malaysian rice cultivars (g/100 g DM basis).
| Rice cultivar | Composition (mean ± SD) | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Ash | Moisture | Fat | Protein | Carbohydrates | Calorie (Kcal) | Amylose (%) | |
| MRQ 74 wr | 0.56 ± 0.009g | 12.89 ± 0.5a | 3.75 ± 0.2e | 8.08 ± 0.7d | 76.72 ± 0.1b | 356.93 ± 0.8h | 26.1 1.4a |
| MRQ 74 br | 1.83 ± 0.018a | 12.35 ± 0.4abc | 5.85 ± 0.3c | 9.58 ± 0.5bc | 69.39 ± 0.2f | 372.53 ± 0.5e | 25.7 ± 1.1a |
| MRQ 74 gbr | 1.55 ± 0.015d | 11.45 ± 0.5cd | 2.90 ± 0.1f | 10.69 ± 0.5abc | 72.43 ± 0.5d | 362.10 ± 0.6g | 25.7 ± 2.1a |
| MRQ 76 wr | 0.34 ± 0.009i | 12.39 ± 0.3abc | 3.60 ± 0.2e | 7.83 ± 0.3d | 75.85 ± 0.3c | 367.12 ± 0.7f | 12.5 ± 1.3c |
| MRQ 76 br | 1.67 ± 0.020c | 12.44 ± 0.5abc | 6.35 ± 0.2b | 9.83 ± 0.5abc | 69.71 ± 0.1f | 375.31 ± 0.6d | 12.5 ± 1.2c |
| MRQ 76 gbr | 1.47 ± 0.010e | 10.57 ± 0.6d | 5.85 ± 0.1c | 10.83 ± 0.7ab | 71.28 ± 0.2e | 381.09 ± 0.8c | 12.5 ± 0.9c |
| MR 84 wr | 0.39 ± 0.008h | 11.82 ± 0.8bc | 3.45 ± 0.1e | 9.83 ± 0.3abc | 77.51 ± 0.4a | 384.41 ± 0.8b | 25.7 ± 0.7a |
| MR 84 br | 1.80 ± 0.010b | 12.33 ± 0.7abc | 8.90 ± 0.3a | 9.50 ± 0.6c | 67.48 ± 0.2g | 388.02 ± 0.6a | 24.0 ± 1.1b |
| MR 84 gbr | 1.22 ± 0.007f | 12.49 ± 0.4ab | 4.15 ± 0.2d | 11.00 ± 0.5a | 71.14 ± 0.3e | 365.91 ± 0.5f | 25.0 ± 1.5ab |
Mean values within a column superscripted by the same letter are not significantly different at p < 0.050. wr = white rice, br = brown rice and gbr = germinated brown rice. SD = standard deviation.
3.2. Proximate analysis
The proximate composition of the rice cultivars is presented in Table 1. The moisture content ranged from 10.57% to 12.89%. All forms of MR 84 cultivar were not significantly different from one another. The white form of MRQ 74 and the germinated brown form of MRQ 76 were significantly different from their respective other forms. Except for the germinated brown form of MR 84 cultivar, all other forms of other cultivars were significantly different from one another in terms of their ash content. The fat content was significantly different among the cultivars and forms of rice, with the brown form of MR 84 cultivar being the highest. The protein content varied between 6.08% and 11.69% with the white form of each cultivar being significantly different from their brown and germinated brown form counterpart. The total carbohydrate content of all forms and cultivars of the rice was greater than 67%, which implies that all the cultivars are good sources of carbohydrates.
3.3. Mineral concentration
The mineral contents of the rice cultivars are presented in Table 2. The brown form of MRQ 74 cultivar had the highest concentration of magnesium while the white form of MRQ 76 cultivar had the lowest. There was a significant difference between the magnesium concentration of the white form of each cultivar and their unmilled counterparts. The calcium concentration had a similar trend to that of magnesium.
Table 2.
Concentration of heavy metals and minerals in various rice cultivars.
| Rice cultivar | Mineral concentration (g/100 g) | Heavy metal concentration (mg/kg) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Magnesium | Calcium | Cobalt | Lead | Arsenic | Cadmium | |
| MRQ 74 wr | 0.140 ± 0.0070cd | 0.070 ± 0.0050d | 1.313 ± 0.0160c | 0.039 ± 0.0003d | BDL | 0.016 ± 0.0009f |
| MRQ 74 br | 0.320 ± 0.0050a | 0.160 ± 0.0060a | 2.013 ± 0.0360ab | 0.050 ± 0.0008c | 0.048 ± 0.007a | 0.022 ± 0.0003e |
| MRQ 74 gbr | 0.300 ± 0.0080a | 0.150 ± 0.0070ab | 2.001 ± 0.1040ab | 0.051 ± 0.0008c | 0.041 ± 0.003a | 0.023 ± 0.0002e |
| MRQ 76 wr | 0.120 ± 0.0090d | 0.070 ± 0.0030d | 1.489 ± 0.0380e | 0.032 ± 0.0011e | BDL | 0.028 ± 0.0005d |
| MRQ 76 br | 0.260 ± 0.0060b | 0.090 ± 0.0030c | 2.127 ± 0.1370a | 0.054 ± 0.0035bc | 0.048 ± 0.002a | 0.038 ± 0.0003c |
| MRQ 76 gbr | 0.250 ± 0.0050b | 0.110 ± 0.0020c | 2.043 ± 0.0480ab | 0.058 ± 0.0022b | 0.051 ± 0.001a | 0.042 ± 0.0009c |
| MR 84 wr | 0.160 ± 0.0040c | 0.070 ± 0.0080d | 1.358 ± 0.0650c | 0.040 ± 0.0003d | BDL | 0.030 ± 0.0017d |
| MR 84 br | 0.290 ± 0.0070a | 0.140 ± 0.0040b | 1.866 ± 0.0320b | 0.070 ± 0.0052a | 0.043 ± 0.003a | 0.051 ± 0.0012b |
| MR 84 gbr | 0.290 ± 0.0060a | 0.140 ± 0.0020b | 2.130 ± 0.0420a | 0.072 ± 0.0006a | 0.044 ± 0.008a | 0.056 ± 0.0023a |
Mean values within a column superscripted by the same letter are not significantly different at p < 0.050. Values are mean ± SD. BDL = below detection limit, wr = white rice, br = brown rice and gbr = germinated brown rice.
3.4. Heavy metal concentration
The heavy metal concentrations of the rice cultivars are presented in Table 2. The heavy metal concentrations were well below the maximum permissible limit. Arsenic was below the detection limit for the white form of all cultivars of rice. On a general note, the white form of each cultivar of the rice was significantly lower in its heavy metal concentration when compared to its brown and germinated brown form counterpart. There was obviously no significant difference in individual heavy metal concentration between the brown and germinated brown form of the rice in each cultivar.
3.5. Glycaemic index and glycaemic load values
The values of the glycaemic index and glycaemic load are presented in Table 3 below. Irrespective of the germination status, the MRQ 76 rice had the highest GI and GL values. Except for the germinated brown form of MRQ 74, all other germinated brown forms of each cultivar had the lowest GI when compared to the white and brown form counterparts. Pearson correlation of the GI and GL values was 0.88 at a p-value of 0.000.
Table 3.
Glycaemic index and glycaemic load of some popular Malaysian rice cultivars.
| Rice cultivar | GI | SD | GI min | GI max | GL/1000 kJ |
|---|---|---|---|---|---|
| MRQ 74 wr | 65.6b | 3.51 | 62.0 | 69.0 | 33.4 |
| MRQ 74 br | 67.7b | 2.04 | 65.8 | 69.9 | 30.1 |
| MRQ 74 gbr | 67.6b | 2.27 | 66.0 | 70.2 | 32.3 |
| MRQ 76 wr | 84.7a | 2.81 | 81.5 | 86.8 | 41.8 |
| MRQ 76 br | 81.8a | 2.70 | 79.2 | 84.6 | 36.3 |
| MRQ 76 gbr | 80.1a | 4.60 | 75.7 | 84.9 | 38.6 |
| MR 84 wr | 67.5b | 1.30 | 66.1 | 68.7 | 32.5 |
| MR 84 br | 65.9b | 1.06 | 65.1 | 67.1 | 27.3 |
| MR 84 gbr | 64.3b | 1.87 | 62.9 | 66.4 | 30.1 |
Mean values within a column superscripted by the same letter are not significantly different at p < 0.050, wr: white rice; br: brown rice; gbr: germinated brown rice; SD: standard deviation; GI: glycaemic index; GL: glycaemic load; GI min: minimum GI value; GI max: maximum GI value; GL/1000 kJ: glycaemic load X gram glucose equivalents, derived based on a 1000 kJ portion size.
3.6. DPPH radical-scavenging ability
The percentage DPPH radical scavenging capacity of the rice extracts is presented in Table 4. Irrespective of the rice cultivar, the methanolic extracts had the highest DPPH scavenging ability percentage, while the sub-critical water extracts had the lowest. Within each method of extraction, the white form of each cultivar scavenged DPPH the least when compared to the brown and germinated brown forms. There is no significant difference between the scavenging ability of the rice cultivars.
Table 4.
Anti-oxidant indices of several Malaysian rice cultivar extracts.
| Rice cultivar | Extraction method | Mean ± SD | |||
|---|---|---|---|---|---|
|
| |||||
| TPC (mg GAE/g DW) | TFC (mg Rutin/g DW) | % DPPH scavenging ability | Mmol TEAC/g | ||
| MRQ 76 GBR | Me Ex | 44.60 ± 1.40b | 7.20 ± 0.30d | 90.10 ± 4.20ab | 0.27 ± 0.008a |
| MRQ 74 GBR | Me Ex | 54.10 ± 1.00a | 4.60 ± 0.20f | 85.00 ± 3.20bc | 0.27 ± 0.005a |
| MR 84 GBR | Me Ex | 38.50 ± 1.00cd | 8.10 ± 0.10c | 91.70 ± 3.60a | 0.28 ± 0.007a |
| MRQ 76 BR | Me Ex | 22.80 ± 1.30f | 6.10 ± 0.20e | 83.80 ± 2.30c | 0.25 ± 0.009b |
| MRQ 74 BR | Me Ex | 36.70 ± 0.80d | 9.30 ± 0.30b | 86.40 ± 3.20abc | 0.22 ± 0.004d |
| MR 84 BR | Me Ex | 27.20 ± 1.10e | 10.80 ± 0.30a | 87.00 ± 2.20abc | 0.23 ± 0.005c |
| MRQ 76 WR | Me Ex | 26.90 ± 1.40e | 2.00 ± 0.10i | 62.50 ± 2.70fg | 0.07 ± 0.003jk |
| MRQ 74 WR | Me Ex | 11.20 ± 0.70hi | 2.40 ± 0.10h | 35.80 ± 3.80i | 0.07 ± 0.002ijk |
| MR 84 WR | Me Ex | 16.40 ± 0.80g | 2.80 ± 0.10g | 57.60 ± 3.20g | 0.07 ± 0.003ij |
| MRQ 76 GBR | USE | 46.70 ± 0.80b | 2.90 ± 0.10g | 71.20 ± 2.20d | 0.17 ± 0.007f |
| MRQ 74 GBR | USE | 40.30 ± 0.60c | 2.90 ± 0.10g | 68.70 ± 3.80de | 0.17 ± 0.007ef |
| MR 84 GBR | USE | 23.70 ± 1.00f | 4.40 ± 0.10f | 69.00 ± 1.90de | 0.18 ± 0.006e |
| MRQ 76 BR | USE | 18.30 ± 0.70g | 1.30 ± 0.00j | 62.20 ± 2.80fg | 0.15 ± 0.008g |
| MRQ 74 BR | USE | 36.10 ± 0.80d | 2.30 ± 0.00hi | 63.10 ± 2.20efg | 0.17 ± 0.006ef |
| MR 84 BR | USE | 26.90 ± 0.50e | 2.40 ± 0.00h | 65.50 ± 3.00def | 0.17 ± 0.004ef |
| MRQ 76 WR | USE | 17.20 ± 0.60g | 0.80 ± 0.00k | 27.90 ± 2.10jklm | 0.04 ± 0.002l |
| MRQ 74 WR | USE | 13.10 ± 0.20h | 1.00 ± 0.00jk | 29.10 ± 1.00jkl | 0.04 ± 0.002l |
| MR 84 WR | USE | 9.20 ± 0.40ij | 0.20 ± 0.00l | 23.00 ± 2.60lmn | 0.04 ± 0.002l |
| MRQ 76 GBR | SWE | 8.10 ± 0.40j | 0.40 ± 0.00l | 30.90 ± 3.30ijk | 0.1 ± 0.004h |
| MRQ 74 GBR | SWE | 9.80 ± 0.40ij | 0.40 ± 0.00l | 24.00 ± 2.20klm | 0.10 ± 0.005h |
| MR 84 GBR | SWE | 7.60 ± 0.50j | 1.10 ± 0.00jk | 33.90 ± 3.20ij | 0.08 ± 0.003i |
| MRQ 76 BR | SWE | 3.50 ± 0.10k | 0.20 ± 0.00l | 22.30 ± 1.90mn | 0.09 ± 0.003h |
| MRQ 74 BR | SWE | 3.70 ± 0.30k | 0.20 ± 0.00l | 47.70 ± 2.60h | 0.09 ± 0.004h |
| MR 84 BR | SWE | 3.40 ± 0.30k | 0.30 ± 0.00l | 35.60 ± 2.40i | 0.06 ± 0.003k |
| MRQ 76 WR | SWE | 4.60 ± 0.40k | 0.10 ± 0.00l | 13.00 ± 2.20° | 0.02 ± 0.002mn |
| MRQ 74 WR | SWE | 3.00 ± 0.20k | 0.10 ± 0.00l | 18.60 ± 1.90no | 0.01 ± 0.007n |
| MR 84 WR | SWE | 2.70 ± 0.10k | 0.10 ± 0.00l | 14.20 ± 4.30° | 0.02 ± 0.002m |
Mean values within a column superscripted by the same letter are not significantly different at p < 0.050. wr = white rice, br = brown rice and gbr = germinated brown rice, SWE = sub-critical water extraction, USE = ultrasonicated assisted water extraction, Me Ex = 80% methanolic extraction, SD = standard deviation, TEAC = Trolox equivalent antioxidant capacity.
3.7. ABTS radical scavenging ability of the rice cultivar extracts in Trolox equivalence
The ABTS scavenging capacity of the rice extracts is presented in Table 4. The ABTS radical scavenging activity of the various rice cultivars followed a similar pattern when compared to the DPPH scavenging capacity. Irrespective of the rice cultivar, the methanolic extracts had the highest percentage of ABTS radical scavenging ability, while the sub-critical water extracts had the lowest percentage. The scavenging ability of the germinated brown form and the brown form of the rice cultivars significantly supersedes their white form counterpart. The cultivar type was not a determinant of the scavenging ability of the rice extracts.
3.8. Total phenolic and flavonoid content
Methanol was superior in phenolic extractions from the rice cultivars when compared to subcritical water extraction and ultra-sonication-assisted water extraction. The germinated brown form of the rice had significantly higher phenolics when compared to their brown and white form counterpart under the same extraction method, with sub-critical water extraction extracting the lowest level of phenolics. The flavonoid extraction, even though much lower than the phenolics, had about 65% positive correlation pattern when compared to the phenolics. The methanolic extraction method had the highest flavonoidal yield while the sub-critical water extraction method had the least. Irrespective of the cultivar form, there was barely a significant difference in the flavonoidal yield of the sub-critical water extraction.
3.9. Effect of the interaction of the rice cultivar properties on antioxidant indices
Table 5 shows the contribution of the singular and interaction effects of the properties of the rice cultivars. Overall, all measured properties of the rice contributed significantly to their respective antioxidant indices.
Table 5.
Interaction effect of germination status, cultivar type and extraction method on several antioxidant indices.
| Response | Factor/interaction | p-Value | R-square |
|---|---|---|---|
| TPC | Germination status | 0.001 | 95.63 |
| Extraction method | 0.000 | ||
| Cultivar | 0.000 | ||
| Germination status*extraction | 0.000 | ||
| Extraction*cultivar | 0.008 | ||
| Germination*cultivar | 0.000 | ||
| TFC | Germination status | 0.000 | 97.30 |
| Extraction method | 0.001 | ||
| Cultivar | 0.000 | ||
| Germination status*extraction | 0.000 | ||
| Extraction*cultivar | 0.001 | ||
| Germination status*cultivar | 0.000 | ||
| DPPH | Germination status | 0.000 | 97.93 |
| Extraction method | 0.000 | ||
| Cultivar | 0.244 | ||
| Germination status*extraction | 0.000 | ||
| Extraction*cultivar | 0.000 | ||
| Germination status*cultivar | 0.000 | ||
| TEAC | Germination status | 0.000 | 99.35 |
| Extraction method | 0.000 | ||
| Cultivar | 0.609 | ||
| Germination status*extraction | 0.000 | ||
| Extraction*cultivar | 0.000 | ||
| Germination status*cultivar | 0.007 |
TPC: total phenolic content, TFC: total flavonoid content, DPPH: 2, 2′-diphenyl-1-picrylhydrazyl radical-scavenging ability, TEAC: Trolox equivalent anti-oxidant capacity.
3.10. Insulin secretion
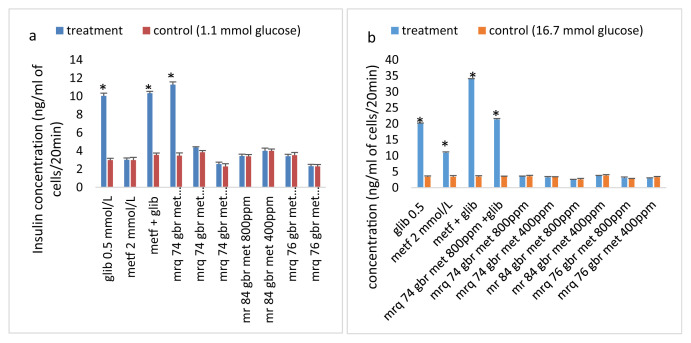
The effect of the rice cultivar extracts on the stimulation of insulin secretion is presented in Fig. 1. None of the rice cultivar extract was able to provoke significant insulin secretion when compared to their respective control after incubation for 20 min. Glibenclamide provoked significant insulin secretion by the cells at both low (1.1 mmol) and high (16.7 mmol) glucose concentration. Also, an additive provocation of insulin secretion was observed on cells co-treated with metformin and glibenclamide at a high glucose concentration but not at a low concentration. No such additive provocation of insulin release was observed when the extract of the germinated brown rice MRQ 76 was co-treated with glibenclamide at both low and high glucose concentrations.
Fig. 1.
Acute effects of rice cultivar extracts on stimulation of insulin release. (a) Following 40 min preincubation at 1.1 mM glucose, effects of the treatments stimulated insulin release that were tested during a 20-min incubation period (b). Values are mean ± SD. *p < 0.010 compared to respective control (1.1 or 16.7 mM glucose). metf: metformin; glib: glibenclamide; met: methanolic extract; gbr: germinated brown rice; ppm.
3.11. Glucose uptake study
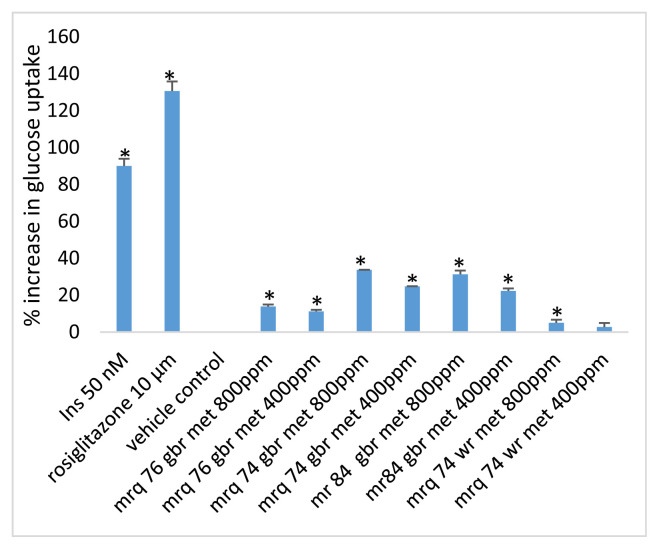
The result of the glucose uptake study is presented in Fig. 2. The results demonstrated varied glucose uptake ability by some of the extracts. Overall, the methanolic extracts evoked a graded response in glucose uptake capacity. At the dose of 800 ppm, the germinated brown rice form of MRQ 74 and MR 84 evoked a superior glucose uptake capacity when compared to the all other extracts.
Fig. 2.
Effect of insulin, rosiglitazone and rice cultivar extracts on glucose uptake. Glucose uptake activity was analysed by measuring the uptake of radiolabelled 2-deoxyglucose in differentiated 3T3-L1 cells. Cells incubated with vehicle (0.1% DMSO) alone were used to measure the basal rate of glucose uptake. Data shown are percentage increase in glucose uptake compared to the basal condition (vehicle control). Values are shown as the mean ± S.D.
*p < 0.010 compared to the vehicle control. gbr: germinated brown rice; ins: insulin; met: methanol; wr: white rice.
4. Discussion
Starch, a polysaccharide of utmost importance to mankind for calorie production, consists majorly of amylose and amylopectin. Rice has been classified based on their percentage amylose composition into high amylose rice (>25%), intermediate amylose rice (20–25%) and low amylose rice (<20%) [21]. The high amylose content of MR 84 and MRQ 74 would translate in vivo into a minimal gastro-intestinal absorption of its carbohydrate composition when compared to the low amylose MRQ 76, a key physiologic parameter that could determine how wide the net caloric value is among the rice cultivars and, by extension, a contributor to weight gain in people who consume these cereals as staple. The amylose content of rice has been demonstrated to significantly affect glycaemic metabolism [22] and consequently sustained post prandial glucose and insulin response to low amylose starch as against high amylose starch. Individuals who consume the likes of the white form of a low amylose rice (MRQ 76) daily, as is prevalent in regions where rice is a staple food, are likely to be predisposed to obesity. Byrnes et al. had previously demonstrated the development of insulin resistance in rats fed with high amylopectin starch [23] compared to those fed on low amylopectin. This gives further credence to the possible culpability of rice high in amylopectin in the development of diet-related diseases. Six of the nine forms of cultivars being investigated fall into the high amylose rice group, making them possible candidates for curbing diet-related diseases. High amylose rice cultivars would less predispose individuals for whom rice is their staple food to insulin resistance [23], obesity, and subsequently diabetes and cardiovascular disorders [24].
The ash content of the rice cultivars gives an insight into their mineral composition. The result shows that more than half its essential minerals are lost during milling and polishing which is the reason why the white rice form of each cultivar had the lowest ash value. These losses of minerals during the polishing could be a significant source for the less privileged in rural parts of the globe who depend on rice as their staple food.
Since most of rice’s protein is found within the endosperm, milling significantly decreased the protein content. Rice protein is nutritious partly because it contains essential amino acids like methionine and lysine. Although studies have linked elevated fasting branched chain amino acids like leucine, isoleucine and valine to obesity in children [25], they are quite insignificant in rice protein. Contrary to rice protein, rice fat is found mainly within the aleurone layer of the bran and consists mainly of unsaturated fatty acids. The milling process takes away almost half of the fat alongside the bran, as displayed in Table 2. This makes white rice poor in fat, necessary for intestinal fat soluble vitamin absorption and linoleic acid, an essential poly-unsaturated fatty acid required by the body. The concentrations of the heavy metals were well below the internationally accepted limits. The heavy metal composition in the non-white rice cultivars were significantly reduced due to polishing. This reduction in heavy metals due to polishing vouches for the safety of the long-term consumption of rice (white) grown in heavy metal polluted areas when compared to their unpolished counterparts.
Brownness and germination did not significantly affect the GI of the rice in each cultivar. This scenario was expected since both properties did not affect the carbohydrate content of the rice cultivars, a proximate parameter that majorly influences the GI. The GL paints a picture of the amount of glucose available for the provision of energy or for storage for later use from a specified quantity of food. There was a positive 0.88 Pearson correlation value between the GI and GL. Although a seemingly good predictor of post prandial glucose excursion, it is noteworthy to state that the dual factors of GI and GL of a particular food are influenced by a number of factors including cooking time, processing, the presence of micro- and macro-nutrients, eating habit, the time of harvest of the food, etc. Notwithstanding all of the constrains in ascertaining a food item’s true GI/GL position, it is worth mentioning that making a rice variety with high GI/GL a staple food will cause sustained hyperglycaemia over a long period of time, which could lead to diet-related diseases like obesity, insulin resistance and type 2 diabetes in the long-term.
The free radical scavenging ability, flavonoid content and total phenolic content of the rice cultivars followed a similar trend. Germinated brown rice cultivars extracted with methanol topped the group for all of the antioxidant parameters analysed. The richness of the germinated brown rice form of each cultivar in polyphenols could play an important role in protection against diet-related metabolic diseases, as emphasised by previous studies [26,27]. Diet-related metabolic diseases could be related to the consumption of white rice as staple, especially in low income countries and rural areas where other sources of these compounds are low. In fact, epidemiological studies and meta-analyses conducted towards the end of the last century shows that the consumption of diets rich in plant polyphenols offers some form of protection against the development of metabolic diseases [28]. Hyperleptinaemia, which is not uncommon in obesity and is a precursor to most diet-related metabolic diseases, triggering an increase in adhesion molecules. This signals monocytes to be recruited as macrophages in adipocytes. Macrophages then produce high amounts of peroxynitrite [29], which causes oxidative stress if not countered. Since rice is a staple food, the consumption of rice cultivars rich in polyphenols would go a long way towards curbing oxidative stress, one of the important contributors to diet-related metabolic diseases. Supporting this is a study which demonstrates that rice bran prevents high fat diet-induced inflammation and macrophage content in adipose tissue [30]. Overall, the singular and interaction effects among the germination status, cultivar and the method of extraction of the rice cultivars clearly show that all factors singularly, and in interactions significantly determine all of the measured antioxidant indices. The results showed that having a cultivar in the germinative brown form, extracted with methanol and from MR 84 and MRQ 76 was almost synonymous to having a good antioxidant index.
As presumed, none of the methanolic rice cultivar extracts provoked a significant insulin secretion in pancreatic beta cell lines. This is in line with previously reported results. Although Sugita et al. [31] demonstrated otherwise using a genetically modified rice which stimulated insulin secretion in a mouse pancreatic beta cell line.
Even though not potent when compared to rosiglitazone, the modest glucose uptake capacity of the germinated brown rice methanolic extract of MRQ 74 and MR 84 could play a long-term significant role in overweight and obese patients where rice is their staple food. This would likely retard progression to obesity and other diet-related metabolic disorders.
Diet-related metabolic diseases are often due to multifactorial causes [32]. Of these multifactorial causes, lack of energy balance has maintained a top position in the ranking [32]. There is no denying the fact that selecting a better quality rice such as one which is high in its amylose content, germinated and grown in areas where heavy metals are not prevalent, meaning that it could go a long way in retarding the progression of this epidemic in regions of the globe where rice is a staple food.
5. Conclusion
From the results and analysis presented above, the properties of germination and high amylose content, if present in a rice cultivar, as demonstrated in the germinated brown rice of cultivars of MRQ 74 and MR 84 cultivars, would less predispose persons who depend on them as a staple food to diet-related metabolic diseases.
Acknowledgement
The authors wish to sincerely acknowledge the contributions of Nor Asma Abdulrazak and Kim Wei Chan in the completion of this work. Also the effort of Dr Asfaliza Ramli is highly acknowledged for providing us with the samples. The authors wish to express their gratitude towards the Universiti Putra Malaysia (UPM) for sponsoring this research (vote no. 62166).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1. Bhattacharjee P, Singhal RS, Kulkarni PR. Basmati rice: a review. J Food Sci Technol. 2002;37(1):1–12. [Google Scholar]
- 2. Fitzgerald MA, McCouch SR, Hall RD. Not just a grain of rice: the quest for quality. Cell. 2009;14:133–9. doi: 10.1016/j.tplants.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 3.International Rice Research Institute. Trends in global rice consumption, 12. Rice Today. 2013. [Accessed 14 May 2017]. p. 1. Available at: http://irri.org/rice-today/trends-in-global-rice-consumption.
- 4. Wu Y, Ding Y, Tanaka Y, Zhang W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int J Med Sci. 2014;11(11):1185–200. doi: 10.7150/ijms.10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organisation. Obesity fact sheet 2014. Facts about overweight and obesity. Accessed from: http://www.who.int/mediacentre/factsheets/fs311/en/
- 6. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014;384(9945):766–81. doi: 10.1016/S0140-6736(14)60460-8.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Samad N. Rice bran oil prevents neuroleptic-induced extrapyramidal symptoms in rats: possible antioxidant mechanism. J Food Drug Anal. 2015;23:370–5. doi: 10.1016/j.jfda.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hu EA, Pan A, Malik V, Sun Q. White rice consumption and risk of type 2 diabetes: meta-analysis and systematic review. Brit Med J. 2012;344:e1454. doi: 10.1136/bmj.e1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li WC, Ouyang Y, Ye ZH. Accumulation of mercury and cadmium in rice from paddy soil near a mercury mine. Environ Toxicol Chem. 2014;33:2438–47. doi: 10.1002/etc.2706. [DOI] [PubMed] [Google Scholar]
- 10. Kolahdouzan M, Khosravi-Boroujeni H, Nikkar B, Zakizadeh E, Abedi B, Ghazavi N, et al. The association between dietary intake of white rice and central obesity in obese adults. ARYA Atheroscler. 2013;9(2):140–4. [PMC free article] [PubMed] [Google Scholar]
- 11. Imam MU, Musa SNA, Azmi NH, Ismail M. Effects of white rice, brown rice and germinated brown rice on antioxidant status of type 2 diabetic rats. Int J Mol Sci. 2012;13:12952–69. doi: 10.3390/ijms131012952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.International Standard Organisation. 2015. [Accessed 15 December 2016]. pp. 6647–51. www.iso.org/obp/ui/#iso:std:iso:6647:-1:ed-2:v1:en .
- 13. Food and Agriculture Organization/World Health Organization. Carbohydrates in human nutrition. Report of a joint FAO/WHO expert consultation. FAO Food and Nutrition Paper 66. 1998 [PubMed] [Google Scholar]
- 14. Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28(1):25–30. [Google Scholar]
- 15. Pellegrini N, Del-Rio D, Colombi B, Bianchi M, Brighenti F. Application of the 2,2-azino bis(3-ethylbenzothiazoline-6-sulfonic acid) radical cation assay to a flow injection system for the evaluation of antioxidant activity of some pure compounds and beverages. J Agric Food Chem. 2003;51(1):260–4. doi: 10.1021/jf020657z. [DOI] [PubMed] [Google Scholar]
- 16. Sompong R, Siebenhandl-Ehn S, Linsberger-Martin G, Berghofer E. Physicochemical and antioxidative properties of red and black rice varieties from Thailand, China and Sri Lanka. Food Chem. 2011;124(1):132–40. [Google Scholar]
- 17. Arvouet-Grand A, Vennat B, Pourrat A, Legret P. Standardization of propolis extract and identification of principal constituents. J Pharm Belg. 1994;49(6):462–8. [PubMed] [Google Scholar]
- 18. Bajaj S, Khan A. Antioxidants and diabetes. Indian J Endocrinol Metabol. 2012;16(2):S267–71. doi: 10.4103/2230-8210.104057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Irwin N, McKinney JM, Bailey CJ, Flatt PR, McClenaghan NH. Effects of metformin on BRIN-BD11 beta-cell insulin secretory desensitization induced by prolonged exposure to sulphonylureas. Diabetes Obes Metab. 2010;12:1066–71. doi: 10.1111/j.1463-1326.2010.01294.x. [DOI] [PubMed] [Google Scholar]
- 20. Hwang J, Do HJ, Kim OY, Chung JH, Lee JY, Park YS, et al. Fermented soy bean extract suppresses differentiation of 3T3-L1 preadipocytes and facilitates its glucose utilization. J Funct Foods. 2015;15:516–24. [Google Scholar]
- 21.International Rice Research Institute. Rice breeding course: grain quality. 2015. [Accessed 15 December 2016]. http://www.knowledgebank.irri.org/ricebreedingcourse/Grain_quality.htm .
- 22. Denardin CC, Boufleur N, Reckziegel P, da Silva LP, Walter M. Amylose content in rice (Oryza sativa) affects performance, glycemic and lipid metabolism in rats. Ciênc Rural. 2012;42:381–7. [Google Scholar]
- 23. Byrnes SE, Miller JC, Denyer GS. Amylopectin starch promotes the development of insulin resistance in rats. J Nutr. 1995;125(6):1430–7. doi: 10.1093/jn/125.6.1430. [DOI] [PubMed] [Google Scholar]
- 24. Wild SH, Byrne CD. Risk factors for diabetes and coronary heart disease. Brit Med J. 2006;333(7576):1009–11. doi: 10.1136/bmj.39024.568738.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McCormack SE, Shaham O, McCarthy MA, Deik AA, Wang TJ, Gerszten RE, et al. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Ped Obes. 2013;8(1):52–61. doi: 10.1111/j.2047-6310.2012.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2:270–8. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang YP, Lai HM. Bioactive compounds and antioxidative activity of colored rice bran. J Food Drug Anal. 2016;24:564–74. doi: 10.1016/j.jfda.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Graf BA, Milbury PE, Blumberg JB. Flavonols, flavonones, flavanones and human health: epidemological evidence. J Med Food. 2005;8(3):281–90. doi: 10.1089/jmf.2005.8.281. [DOI] [PubMed] [Google Scholar]
- 29. Ischiropoulos H, Zhu L, Beckman JS. Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys. 1992;298(2):446–51. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- 30. Justo ML, Claro C, Zeyda M, Stulnig TM, Herrera MD, Rodríguez-Rodríguez R. Rice bran prevents high-fat diet-induced inflammation and macrophage content in adipose tissue. Eur J Nutr. 2015 doi: 10.1007/s00394-015-1015-x. [DOI] [PubMed] [Google Scholar]
- 31. Sugita K, Endo-Kasaharaa S, Tadab Y, Lijunb Y, Yasudab H, Hayashic Y, et al. Genetically modified rice seeds accumulating GLP-1 analogue stimulate insulin secretion from a mouse pancreatic beta-cell line. FEBS Lett. 2005;579:1085–8. doi: 10.1016/j.febslet.2004.12.082. [DOI] [PubMed] [Google Scholar]
- 32. Grundy SM. Multifactorial causation of obesity: implications for prevention. Am J Clin Nutr. 1998;67:563S–72S. doi: 10.1093/ajcn/67.3.563S. [DOI] [PubMed] [Google Scholar]