Summary
Background
Eczema (atopic dermatitis; AD) is a very common itchy skin condition affecting 1 in 5 children and up to 1 in 10 adults worldwide. The skin of eczema sufferers is prone to redness, irritation and dryness because it does not form an effective barrier, i.e. the ability of the skin to stop irritants, allergens and microorganisms getting into the body. Skin barrier dysfunction is a hallmark of AD. The regular and liberal (600 g/week for an adult) use of emollients is recommended for all patients with eczema), even between episodes of itching and redness, to soften and soothe the skin. In England alone, almost 9 million prescriptions for emollient creams were issued in 2018, at a cost of over £50 million. Despite this widespread use, relatively little is known about how commonly prescribed emollient creams affect the skin's barrier, and thus the role of moisturizers in AD development and progression remains unclear. We set out to compare three different types of emollient cream and a no‐treatment control.
Aim
To compare the barrier‐strengthening properties of a new moisturizer containing urea and glycerol (urea–glycerol cream; UGC), with those of a glycerol‐containing moisturizer (glycerol cream; GC), a simple paraffin cream (PC) with no humectant, and a no‐treatment control (NTC).
Methods
This was an observer‐blinded prospective Phase 2 within‐subject multilateral single‐centre randomized controlled trial in adults with AD (Clinical Trials #NCT03901144). The intervention involved 4 weeks of treatment, twice daily, with the three products applied to one of four areas on the forearms the (the fourth area was the untreated control, randomized allocation). Skin properties [dryness, transepidermal water loss (TEWL), hydration and natural moisturizing factor (NMF) levels] were assessed before, during and after treatment to see what happened to the skin's barrier. The primary outcome was skin sensitivity to the irritant sodium lauryl sulfate (SLS) after treatment. We performed tests on the skin before and after treatment to see what happened to the skin's barrier.
Results
In total, 49 patients were randomized, completed treatment and included in the analysis. UGC significantly reduced the response to SLS as indicated by a reduction in TEWL compared with NTC (−9.0 g/m2/h; 95% CI −12.56 to −5.49), with PC (−9.0 g/m2/h; 95% CI −12.60 to −5.44) and with GC −4.2 g/m2/h; 95% CI 7.76 to −0.63). Skin moisturization improved at sites treated with UGC compared with NTC and PC, and this was accompanied by concordant changes in dryness and NMF levels. Subgroup analysis suggested FLG‐dependent enhancement of treatment effects.
Conclusion
The study showed that not all emollient creams for eczema are equal. The simple paraffin‐based emollient, which represents the most widely prescribed type of emollient cream in England, had no effect on the skin's barrier and reduced the skin's NMF. UGC markedly improved the skin's barrier and protected against irritation. GC performed better than PC, but not as well as UGC. UGC strengthened the skin barrier through a mechanism involving increased NMF levels in the skin, and imparted protection from SLS‐induced irritation. By helping correct a major pathophysiological process, UGC has the potential to improve the long‐term control of AD. The results show that different emollient creams have different effects on our skin, and only certain types have the ability to improve the skin's barrier and protect against irritants that trigger eczema.
This study compares the barrier‐strengthening properties of a new moisturizer, containing urea and glycerol, to a glycerol‐containing moisturizer, a simple paraffin cream (no humectant), and no treatment. In contrast to the simple paraffin cream, the urea/glycerol cream strengthened the skin barrier through a mechanism involving increased natural moisturizing factor levels in the skin, and imparted protection from irritation. By helping correct a major pathophysiological process, the urea/glycerol cream has the potential to improve the long‐term control of atopic eczema.

Introduction
Atopic dermatitis (atopic eczema, AD) is a common inflammatory disease of the skin, characterized by persistent skin dryness and a reduced function of the skin as a barrier to the outside environment. The liberal use of moisturizers (emollients) is encouraged as a baseline therapy for AD, with anti‐inflammatory treatments added as required. 1 A growing, albeit still small, body of evidence has suggested that regular use of moisturizers can prolong the flare‐free period and reduce the amount of anti‐inflammatory treatment needed. This has stoked interest in moisturizers and supported the compelling hypothesis that, by correcting the skin barrier defect, the primary development of AD can be delayed or even prevented. 2 In recent years, a number of studies have investigated this hypothesis with varying results, and have raised important questions about the intervention itself. 2 Importantly, not all moisturizers have the same effect on the skin; some moisturizers impart superior hydration, 3 while some appear to exert no positive (or negative) biophysical effects, and others appear to increase skin permeability to irritants and increase skin infections. 4 , 5 , 6 Aqueous cream, for example, is associated with a high rate of adverse cutaneous reactions in children. 7 The variability of moisturizer effects could go a long way to explain the challenges patients experience in controlling the condition and the poor overall adherence with topical therapy. 8 In light of this, the aim of this work was to determine the barrier‐strengthening properties of a new moisturizer, containing the active ingredients urea and glycerol (urea–glycerol cream; UGC), compared with two reference creams, a glycerol‐containing moisturizer (glycerol cream; GC) and a simple paraffin cream [PC; containing no humectant, and with a no‐treatment control (NTC)].
Methods
The West Midlands–Edgbaston Research Ethics Committee approved the study (18/WM/0311), which was sponsored and funded by Perrigo Nordic. The study was pre‐registered on ClinicalTrials.gov (NCT03901144) and performed in accordance with the Helsinki Declaration of 1964, and its later amendments. No changes to the study design were made following commencement. All subjects provided informed consent to participate. Further details pertaining to the design and conduct of the study can be found in Supplementary Data S1. The study data are available from the authors on request.
Study design
This was an observer‐blinded prospective phase 2 within‐subject multilateral single‐centre randomized controlled trial to determine the superiority of the test treatment at strengthening the skin barrier compared with no treatment and two reference treatments in adults with a recent history of AD. All participants undertook all four treatment conditions. Each forearm (volar face) was divided into two areas, providing four possible treatment areas per subject (Supplementary Table S1). All treatment areas were clear of the signs of eczema at the start of treatment. Participants were required to treat each area for 28 days with the allocated study treatment (Table 1) or no treatment, according to a randomized scheme. Dosing was one fingertip unit (approximately 0.5 g) of each cream applied twice daily, once in the morning and once in the evening. The properties of the skin were assessed before (Day 1), during (Day 15, for tolerability only) and after (Day 29) treatment.
Table 1.
Treatments.
| Name | Manufacturer | Ingredients |
|---|---|---|
| UGC | Miniderm Duo, ACO Hud Nordic AB, Upplands Väsby, Sweden | Urea 20 mg/g and glycerol 200 mg/g (active ingredients) with butylene glycol, hydrogenated canola oil, dexpanthenol, medium‐chain triglycerides, cetostearyl alcohol, dimeticone, hard paraffin, glycerol monostearate, macrogol stearate, triacetin, carbomer, water |
| GC | Miniderm cream, ACO Hud Nordic AB, Upplands Väsby, Sweden | Glycerol 200 mg/g (active ingredient) with hydrogenated canola oil, cholesterol, glycerol monostearate, macrogol stearate, cetostearyl alcohol, dimeticone, light liquid paraffin, hard paraffin, white soft paraffin, ethyl parahydroxybenzoate, methyl parahydroxybenzoate, purified water |
| PC | Diprobase cream, Bayer plc, Berkshire UK | White soft paraffin with cetostearyl alcohol, liquid paraffin, macrogol cetostearyl ether, chlorocresol, sodium dihydrogen phosphate, sodium hydroxide, phosphoric acid and purified water |
GC, glycerol cream; PC, paraffin cream; UGC, urea–glycerol cream.
Outcomes
The primary outcome was skin sensitivity to the irritant SLS following 28 days of treatment. SLS 1% was applied to each pretreated (including NTC) skin area on Day 29 under patches that were left in place for 24 h, and the response to SLS was measured 24 h after patch removal on Day 31. Sensitivity to SLS was reported as the change in transepidermal water loss (TEWL) and redness from Day 29 to Day 31. The secondary outcomes were basal TEWL (change from baseline), skin moisturization (capacitance, change from baseline), skin surface dryness (change from baseline), natural moisturizing factor (NMF) levels (after treatment), participant‐reported tolerability (on Days 1, 15 and 29 of treatment), objective tolerability [Erythema Index (EI) from two‐dimensional images, change from baseline at Days 15 and 29 of treatment], amount of cream used and the number of adverse events after 28 days of treatment. Tertiary outcomes included the number of FLG loss‐of‐function mutations and descriptive tabulations of TEWL by mutation status if sufficient participants with mutations were detected. Further details on how these outcomes were measured can be found in Supplementary Data S1.
Statistical analysis
The trial was designed to detect a difference (in change) of 3.5 g/m2/h TEWL in the primary comparison between the test treatment and the NTC, at a 5% significance level (two‐sided) with > 90% power with an expected SD of the between‐group differences of approximately 6 g/m2/h (based on SD of within‐groups differences of approximately 8 g/m2/h and a correlation of approximately 0.7). A sample size of 40 participants was required and so the recruitment target was set at 50 to account for dropouts. For the secondary comparison between the test treatment and one of the two reference treatments, this sample also provided > 90% power to detect a difference of 2 g/m2/h TEWL, based upon an expected SD of 3 g/m2/h.
The primary analyses were performed using analysis of covariance (ANCOVA) with change (Day 31 minus Day 29) as the outcome, treatment as a factor, subject as a random effect and Day 29 included as a covariate. Key group comparisons (UGC vs. NTC, UGC vs. GC and UGC vs. PC) were calculated directly from the ANCOVA model. These analyses were carried out on both the full analysis set (FAS), presented here, and the per‐protocol set (PPS). The analysis on the PPS was considered supportive of the primary analysis carried out on the FAS (PPS data not shown).
The secondary analyses of resting skin barrier function (TEWL), skin moisturization (capacitance), skin surface dryness and EI (tolerability outcome) were performed using ANCOVA with change (Day 29 minus Day 1) as the outcome, Day 1 measurements as a covariate, treatment as a factor and subject as a random effect. The secondary analysis of NMF levels was performed using ANOVA with Day 29 NMF as the outcome, treatment as a factor and subject as a random effect. No correction for multiple tests was performed, and therefore the results from the secondary analyses should be interpreted cautiously referring to effect size and confidence intervals rather than nominal P values.
Results
Participants
Recruitment took place during the period February–May 2019, during which time 69 volunteers were formally assessed for eligibility; 49 of these were enrolled and completed the study (Fig. 1, Table 2). Treatment compliance was good overall, with all participants applying > 75% of the applications over 28 days. Total cream consumption is presented in Table 3, and protocol deviations are described in Supplementary Data S1.
Figure 1.
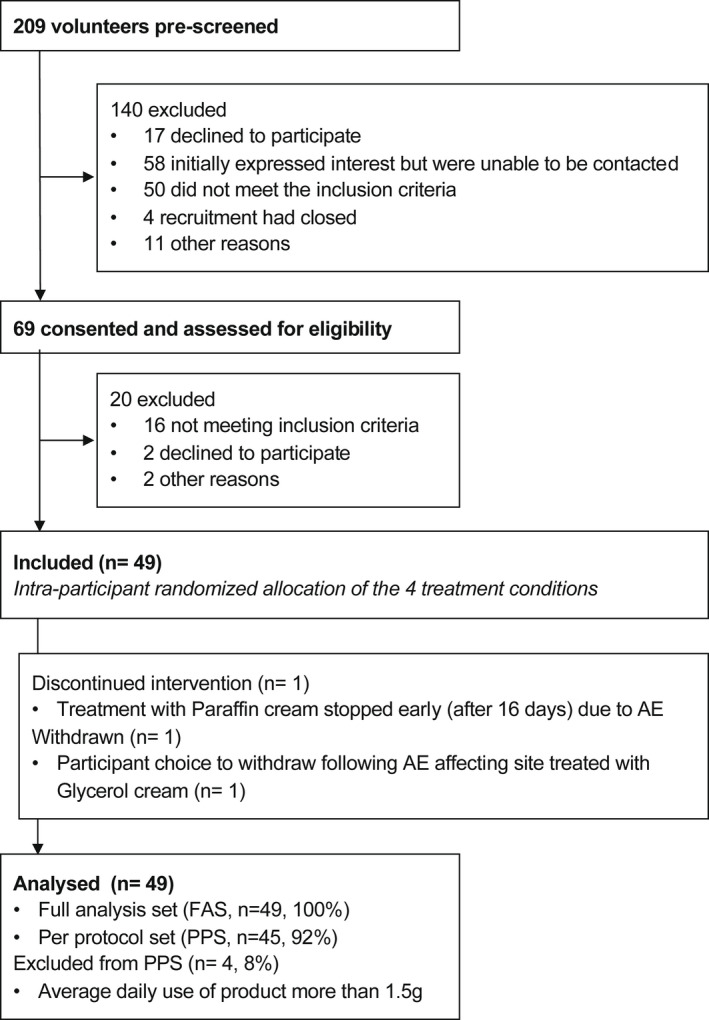
Trial flowchart. AE, adverse event; FAS, full analysis set; PPS, per‐protocol set.
Table 2.
Baseline characteristics of the study population.
| Characteristic | Full analysis set |
|---|---|
| Age, years a | 37.6 ± 16.26 (19–81) |
| Ethnicity, n (%) | |
| Asian | |
| Indian | 2 (4) |
| Any other | 2 (4) |
| Chinese | 1 (2) |
| Mixed | |
| White and Asian | 1 (2) |
| White and Black Caribbean | 1 (2) |
| Any other | 1 (2) |
| White | |
| British | 37 (76) |
| Any other | 4 (8) |
| Sex, n (%) | |
| Female | 29 (59) |
| Male | 20 (41) |
| IGA for severity, n (%) | |
| Clear (score 0) | 11 (22) |
| Almost clear (score 1) | 23 (47) |
| Mild (score 2) | 15 (31) |
| Time since last flare, months b | 1 (0–8) |
| No. of relapses/flares in past 12 months b | 4 (1 to > 20) |
| Nottingham Eczema Severity Score, n (%) | |
| Mild (score 3–8) | 41 (84) |
| Moderate, score 9–11 | 8 (16) |
| Self‐reported allergies, n (%) | 25 (51) |
| Filaggrin mutation status, n (%) | |
| wt/wt | 37 (77) |
| wt/flg− | 10 (21) |
| flg−/flg− | 1 (2) |
IGA, Investigator Global Assessment.
Mean ± SD (range).
Median (range).
Table 3.
Treatment compliance.
| UGC | GC | PC | |
|---|---|---|---|
| Total consumption, mean ± SD | 30.6 ± 7.54 | 26.1 ± 7.66 | 25.0 ± 5.90 |
| Daily use, mean ± SD | 1.15 ± 0.23 | 0.97 ± 0.25 | 0.94 ± 0.18 |
| Mean daily use < 0.5 g, n (%) | 0 | 0 | 0 |
| Mean daily use > 0.5 g, n (%) | 4 (8) | 2 (4) | 0 |
GC, glycerol cream; PC, paraffin cream; UGC, urea–glycerol cream.
Sodium lauryl sulfate challenge
After 28 days of treatment, skin barrier function was determined at each of the four treatment sites by measuring skin sensitivity to the irritant SLS when applied under a patch for 24 h.
At the untreated site, SLS challenge provoked a clear response, with mean ± SD increases in TEWL of 28.6 ± 13.67 g/m2/h, visible redness of 1.4 ± 0.68 points (measured on a four‐point visual scale from 0 to 3, and equating to slight to moderate uniform erythema), objective redness (measured with a Mexameter) of 99.0 ± 53.92 Mexameter units (MU) and redness determined from dermoscopic images of 16.75 ± 6.76 EI units (EIU) (Fig. 2, Supplementary Fig. S1 and Table S2).
Figure 2.
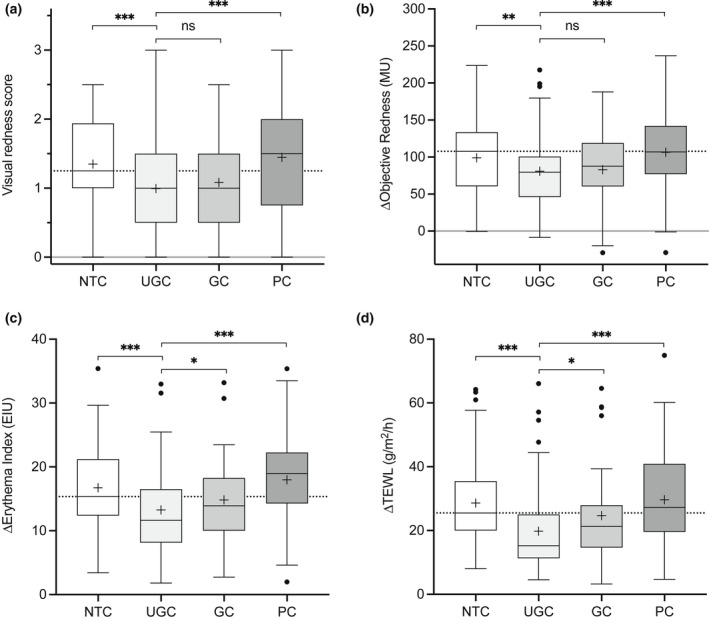
(a–d) Primary outcome of skin sensitivity after 28 days of treatment: (a) visual redness/erythema (Day 31); (b) change in objective redness measured with a Mexameter [in Mexameter units (MU), Day 31 minus Day 29]; (c) change in redness determined from dermoscopic images [Erythema Index units (EIU), Day 31 minus Day 29]; and (d) change in transepidermal water loss (TEWL) (Day 31 minus Day 29). Boxes indicate the median, 25th and 75th percentiles, with ‘+’ for the mean and whiskers showing 1.5 × interquartile range (IQR). Asterisks indicate the results of pairwise testing against UGC (test) (*P < 0.05, **P < 0.01, ***P < 0.001). GC, glycerol cream; NTC, no‐treatment control; PC, paraffin cream; UGC, urea–glycerol cream.
Pretreatment with UGC significantly reduced the response to SLS compared with NTC, displaying estimated effect sizes of TEWL −9.0 g/m2/h, and redness −19.08 MU, −3.54 EIU and −0.35 points (Table 4, Supplementary Figs S1 and S2, Supplementary Tables S2 and S3 for the PPS). The effect of pretreatment with UGC was also significantly different to that of PC (TEWL −9.02 g/m2/h; redness −27.04 MU, –4.74 EIU and −0.45 points for redness) and to a lesser extent GC (TEWL −4.194 g/m2/h; redness −1.748 EIU; the changes of −4.49 MU and −0.09 points for the other measures of redness were not significant). A post hoc analysis of change in TEWL with PC compared with NTC confirmed that PC had no significant effect on the response to SLS, despite a trend for elevated TEWL (29.6 vs. 28.65 g/m2/h) and redness (18.0 vs. 16.75 EIU). GC exhibited a significant effect compared with NTC, albeit with a smaller effect size than observed for UGC compared with NTC.
Table 4.
Summary of primary outcomes of skin sensitivity following treatment.
| Comparison | Estimated difference | 95% CI | P a |
|---|---|---|---|
| Visual redness score at Day 31 b | |||
| UGC vs. NTC | −0.35 | −0.56 to −0.15 | < 0.001 |
| UGC vs. GC | −0.09 | −0.29 to 0.115 | 0.39 |
| UGC vs. PC | −0.45 | −0.65 to −0.24 | < 0.001 |
| Objective redness, c MU; change from Day 29 to Day 31 d | |||
| UGC vs. NTC | −19.08 | −31.33 to −6.83 | < 0.01 |
| UGC vs. GC | −4.49 | −16.79 to 7.80 | 0.47 |
| UGC vs. PC | −27.035 | −39.37 to −14.70 | < 0.001 |
| Objective redness, e EIU; change from Day 29 to Day 31 d , f | |||
| UGC vs. NTC | −3.54 | −5.11 to −1.96 | < 0.001 |
| UGC vs. GC | −1.75 | −3.33 to −0.16 | 0.03 |
| UGC vs. PC | −4.74 | −6.33 to −3.16 | < 0.001 |
| TEWL, g/m2/h; change from Day 29 to Day 31 d | |||
| UGC vs. NTC | −9.03 | −12.56 to −5.49 | < 0.001 |
| UGC vs. GC | −4.19 | −7.76 to −0.63 | 0.02 |
| UGC vs. PC | −9.02 | −12.6 to −5.44 | < 0.001 |
| GC vs. NTC g | −4.83 | −8.41 to −1.25 | < 0.01 |
| PC vs. NTC g | −0.005 | −3.60 to 3.59 | 1.0 |
ANCOVA, analysis of covariance; AU, arbitrary unit; EI, Erythema Index; GC, glycerol cream; MU, Mexameter unit; NTC, no‐treatment control; PC, paraffin cream; UGC, urea–glycerol cream.
P < 0.01 values are displayed in bold.
No correction for multiple tests was performed, and therefore the results from the secondary analyses should be interpreted cautiously referring to effect size and CIs rather than nominal P values.
ANCOVA model includes fixed factor for treatment and random effect for patient.
Based on Mexameter readings.
ANCOVA model includes fixed factor for treatment, random effect for patient and covariate for Day 29 measure.
Based on EI.
Secondary outcome measure of skin sensitivity.
Post hoc analysis.
Transepidermal water loss and skin moisturization
The effect of treatment on basal TEWL and skin moisturization is presented in Fig. 3. Sites treated with UGC exhibited marginally but significantly smaller decreases in TEWL compared with NTC sites (estimated difference + 0.51 g/m2/h), but not compared with sites treated with GC or PC (Supplementary Table S4). A post hoc analysis confirmed similarly significant marginal differences between the sites treated with the two reference creams and NTC, which in all cases was attributed to a greater reduction in TEWL at the NTC site than the treated sites during the treatment period. Capacitance was significantly higher at sites treated with UGC compared with NTC [estimated + 5.74 arbitrary units (AU)] and PC (estimated + 5.30 AU), but not GC (estimated − 0.78 AU). This was mirrored by reductions in skin surface dryness and accompanied by increases in stratum corneum (SC) NMF levels that followed the same pattern. Unexpectedly, NMF levels were noticeably lower following treatment with PC compared with NTC, and were accompanied by marginally lower levels of capacitance and slightly higher levels of surface dryness on average.
Figure 3.
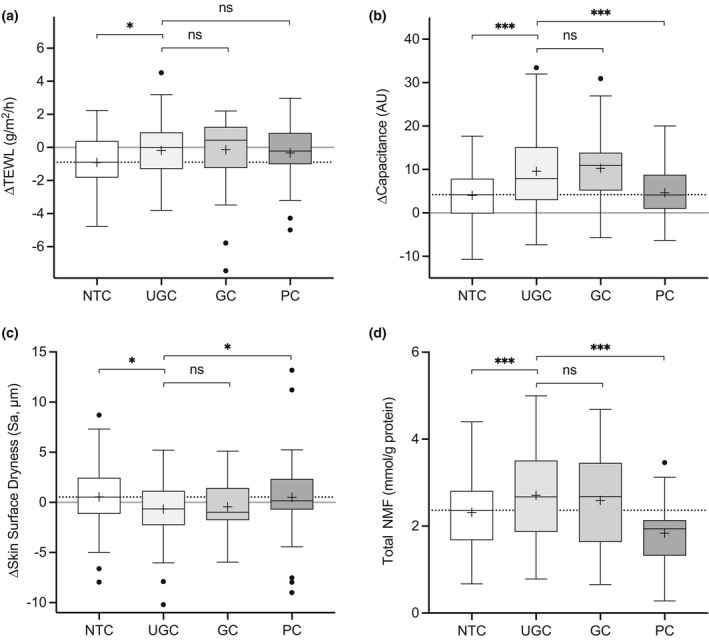
(a–d) Secondary outcomes: (a–c) change in (a) transepidermal water loss (TEWL), (b) skin hydration [capacitance in arbitrary units (AU)] and (c) skin surface dryness from Day 1 to Day 29, and (d) stratum corneum natural moisturizing factor (NMF) levels at Day 29. Boxes indicate the median, 25th and 75th percentiles, with ‘+’ for the mean and whiskers showing 1.5 × interquartile range. Asterisks indicate the results of pairwise testing against UGC (test) (*P < 0.05, **P < 0.01, ***P < 0.001). GC, glycerol cream; NTC, no‐treatment control; PC, paraffin cream; UGC, urea–glycerol cream.
Adverse effects
UGC was well tolerated with no evidence of stinging or redness (Supplementary Data S1, Supplementary Tables S5 and S6). Adverse events (AEs) were reported in 40 participants (82%), with 19 reporting AEs possibly or probably related to the treatments (Table S7). Where AEs could be related to a single treatment, the most common AEs were classed as ‘general disorders and administration site conditions’ (UGC 0%, GC 6% and PC 18%) and skin and subcutaneous tissue disorders (UGC 2%, GC 4% and PC 4%).
FLG mutation analysis
The analysis of treatment effects on skin sensitivity by FLG genotype was a tertiary outcome, and is presented in Table 4. There were 11 patients with FLG mutations affecting one or both alleles, and so these were grouped together (Table 5). This group exhibited slightly elevated basal TEWL, a slightly higher response to SLS (Supplementary Table S8) and a reduced level of NMF levels (Supplementary Table S9) as expected. The effect size for skin treatment with UGC vs. NTC was almost twice as large in the mutation group than in the wildtype group (−15.06 vs. −8.53 g/m2/h TEWL). This difference was even greater when UGC was compared with PC (−17.36 vs. −7.98 g/m2/h). In contrast to UGC, there was very little difference in effect size between GC and NTC.
Table 5.
Tertiary outcome of skin sensitivity (transepidermal water loss change from Day 29 to Day 31) by FLG genotype.
| Comparison | Estimated difference (95% CI) a | |
|---|---|---|
| Wildtype (n = 35) | FLG mutation (n = 11) | |
| UGC vs. NTC | −8.53 (−12.24 to −4.82)*** | −15.06 (−21.69 to −8.44)*** |
| UGC vs. GC | −2.94 (−6.66 to 0.78) | −8.39 (−15.05 to −1.74)* |
| UGC vs. PC | −7.99 (−11.72 to −4.24)*** | −17.36 (−23.99 to −10.73)*** |
| GC vs. NTC | −5.59 (−9.35 to −1.84) ** | −6.67 (−13.27 to −0.065)* |
| PC vs. NTC | −0.55 (−4.32 to 3.22) | 2.30 (−4.30 to 8.89) |
GC, glycerol cream; NTC, no‐treatment control; PC, paraffin cream; UGC, urea–glycerol cream.
No correction for multiple tests was performed, and therefore the results from the secondary analyses should be interpreted cautiously referring to effect size and CIs rather than nominal P values.
*P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
Treatment of the skin for 4 weeks with UGC imparted significant protection from SLS‐induced skin irritation compared with NTC and the two reference creams, as indicated by reduced levels of TEWL and redness. Challenging the skin with SLS is a validated approach to assessing skin barrier function. 9 , 10 The response to SLS is determined by the structure and function (permeability) of the skin. 11 Owing to a defective skin barrier, SLS more readily penetrates the skin of patients with AD to elicit irritation. 12 Irritants such as SLS are important triggers that exacerbate AD, and so there is a need for treatments that can enhance skin barrier function to reduce susceptibility to them. Unfortunately, some moisturizers increase the skin sensitivity to irritants and allergens. 13 , 14 However, there is good evidence that moisturizers with 5% urea can strengthen the skin barrier. 15 , 16 In addition, one of these moisturizers was found to significantly prolong the eczema‐free period compared with no treatment 10 and a reference moisturizer. 9 In the current study, we found that a moisturizer with just 2% urea and 20% glycerol exhibited comparable protective effects. The response to SLS was reduced by a clinically significant degree, with a 30% reduction in TEWL and 20% reduction in objective erythema. This also implies that by strengthening the skin barrier, pretreatment of the skin with UGC has the potential to delay relapses of AD. The lower urea concentration in our cream is important, as concentrations of ≥ 5% have been associated with a stinging sensation. We found virtually no evidence of adverse sensations upon application of UGC, and administration site conditions related to a single treatment were reported only at sites treated with the reference products.
Although the UGC was protective in all participants, the effect was almost twice as large in carriers of FLG mutations. GC was also protective, but to a significantly less extent, and was not affected by carriage of FLG mutations. This meant that the protective effect of UGC compared with GC was twice as large in patients with an FLG mutation compared with those without. Treatment with PC had no significant effect on the response to SLS challenge on average, and appeared to increase sensitivity in some patients, especially those with FLG gene mutations. Both UGC and GC, but not PC, delivered clinically significant improvements in skin moisturization, supported by decreased skin surface dryness and increased levels of NMF in the SC. The negative effects of simple emollients, like the PC tested here, have been raised previously and include inability to rehydrate the skin, elevation of TEWL and increased susceptibility to irritants. 5 , 13 , 14 , 17
The results of this study are consistent with the findings of a recent systematic review that found evidence to support the superiority of glycerol‐based moisturizers over moisturizers without a humectant for restoring skin hydration. 3 Although glycerol and urea are both effective humectants, urea has been found to have physiological effects, including the ability to enhance filaggrin expression by keratinocytes 18 and consequently increase NMF levels in the skin (NMF is a downstream product of filaggrin catabolism). 16 Both filaggrin expression and NMF levels are associated with skin barrier function and the severity of AD. 19 This study demonstrates that UGC significantly increases SC NMF levels, possibly through increased FLG expression, and suggests that this contributes to the positive effects on the skin. Participants in this study with FLG mutations exhibited both reduced NMF levels and raised TEWL, consistent with the literature. 19 The enhanced effect of UGC, but not GC, on the skin barrier in FLG mutation carriers suggests that urea can stimulate a more pronounced effect in these cases. FLG mutations are the most common risk factors for AD, and suppression of filaggrin expression is a downstream effect of proallergic inflammation in the context of AD, suggesting that treatment with UGC could contribute to the correction of pathophysiological events driving this condition. 20 Environmental modification of FLG‐associated risk in early life, such as by the hardness of water used for washing, has already been identified, and involves altered susceptibility to surfactants (such as SLS) used for washing. 21 Our study shows that emollient pretreatment can reduce that risk, and may therefore offer protection against the development of AD in early life.
In the products used, urea and glycerol are recognized as active ingredients; however, the formulations under investigation also contain excipients that could potentially influence the effects observed. For example, canola oil, present in both GC and UGC, helps restore normal skin barrier function after irritation, while dexpanthenol, present in UGC, has moisturizing and skin barrier‐enhancing properties. 22 , 23 This limits the generalizability of these findings to other urea–glycerol‐containing creams with different formulations. Another limitation of this study is its relatively small sample size performed at a single centre in a soft water area during the spring months with a predominantly white British middle‐aged adult population. There is no reason to believe that these effects would not be observed in other populations and settings; however, the scale may be different. For instance, hard water is associated with greater irritant responses to SLS, while the risk and severity of dermatitis increases in the winter, and advancing age (≥ 60 years old) and darker skin colour is associated with an increased risk of xerosis. 20 , 21 , 24 These factors are all likely to increase the scope for positive effects, and may increase the scale of responses to topical treatments. Further randomized studies will be required to draw firm conclusions and to confirm that FLG mutation status impacts the skin response to different moisturizers. We propose that this model of establishing the skin barrier properties of a moisturizer first, before progressing to large‐scale clinical trials, is a valuable approach for understanding moisturizer effects and selecting the best candidate treatment.
Conclusion
This study highlights that not all creams have positive effects on the skin barrier and the potential to improve the long‐term control of AD. PC and its generic equivalents are commonly used creams for AD, accounting for 22.3% of emollient cream prescriptions in England in 2018; 37% if including similar oil‐in‐water emollient preparations without humectants. 25 There is an absence of evidence supporting the use of these simple emollients for the treatment of AD, and this study finds that they may not improve the condition of the skin barrier and that they could in fact contribute to the increasing incidence of allergy and AD. UGC significantly strengthened the skin barrier, as indicated by a reduced susceptibility to the irritant SLS, through a mechanism involving, or resulting in, increased NMF levels in the skin. By helping to correct a major pathophysiological process in this condition, UGC has the potential to improve the long‐term control of AD, as already established for a moisturizer with 5% urea. By clearly defining the effects of three different types of moisturizer on the skin, the results of this study offer an insight into the variable findings of recent eczema prevention studies, 2 and suggest that moisturizer formulation is a critical factor in determining treatment success.
Conflict of interest
SGD has received research grants from, participated in advisory boards for, or has consulted with Almirall, Astellas Pharma, Bayer, Harvey Water Softeners, Hyphens Pharma, Leo Pharma, L'Oreal, Johnson & Johnson, MSD, Perrigo, Pfizer and Stiefel‐GSK. MJC is an investigator and consultant for Astellas, Almirall, Bayer, Boots, Galapagos, Hyphens, Johnson & Johnson, Leo Pharma, L'Oreal, Menlo, MSD, Novartis, Oxagen, Pfizer, Procter & Gamble, Perrigo, Regeneron Pharmaceuticals, Inc., Sanofi Genzyme and Stiefel‐GSK. AF, KC and TH are in the employment of Perrigo Nordic who funded this study and manufacture UGC and GC. PVA, RNT, LJK, KB, JC and IU declare no conflicts of interest.
What's already known about this topic?
Treatment with a skin barrier strengthening cream has been shown to prevent relapse of AD.
By contrast, some emollients, such as aqueous cream, disrupt the skin barrier and induce adverse skin reactions.
Early emollient interventions to prevent the development of AD have yielded conflicting findings.
A comparison of the effects of key classes of emollients, to each other and to untreated skin, on the skin barrier is needed.
What does this study add?
A simple paraffin‐based cream did not provide any skin benefits compared with no treatment and reduced the amount of NMFs.
A moisturizer containing urea and glycerol exhibited barrier‐strengthening effects, imparted greater skin moisturization and protected the skin from irritation.
The different effects of emollients on the skin barrier contribute to our understanding of why they are associated with different clinical outcomes, raising the importance of emollient choice.
Not all emollients exhibit skin barrier‐strengthening effects, highlighting their different therapeutic potential for treating and preventing conditions driven by skin barrier defects, such as AD.
Supporting information
Data S1. Supplementary Methods, Results, Discussion and References.
Figure S1. Treatment with test cream was well tolerated and reduced skin erythema in response to sodium lauryl sulfate exposure.
Figure S2. Relationship between Mexameter units and visual skin redness.
Figure S3. Relationship between erythema index and visual skin redness.
Table S1. Treatment allocation frequencies to the test sites.
Table S2. Summary statistics for the primary outcome measures.
Table S3. Summary of primary outcome measure of skin sensitivity for the per‐protocol set.
Table S4. Summary of secondary outcome measures.
Table S5. Summary of visual analogue scores for tolerability.
Table S6. Summary of Erythema Index change from Day 1 to Day 29.
Table S7. Adverse events (patient level) by system organ class and preferred term.
Table S8. Summary of transepidermal water loss (g/m2/h) by FLG genotype.
Table S9. Summary of stratum corneum natural moisturizing factor levels on Day 29 by FLG genotype.
Acknowledgement
We thank and are very grateful to all of our volunteers who have given up their time to take part in this study. This investigator‐led study was funded by Perrigo Nordic.
References
- 1. Wollenberg A, Christen‐Zäch S, Taieb A et al. ETFAD/EADV Eczema Task Force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol 2020; 34: 2717–44. [DOI] [PubMed] [Google Scholar]
- 2. Kelleher MM, Cro S, Van Vogt E et al. Skincare interventions in infants for preventing eczema and food allergy: a cochrane systematic review and individual participant data meta‐analysis. Clin Exp Allergy 2021; 51: 402–18. [DOI] [PubMed] [Google Scholar]
- 3. van Zuuren EJ, Fedorowicz Z, Christensen R et al. Emollients and moisturisers for eczema. Cochrane Database Syst Rev 2017; 2: CD012119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Loden M, Andersson AC, Andersson C et al. Instrumental and dermatologist evaluation of the effect of glycerine and urea on dry skin in atopic dermatitis. Skin Res Technol 2001; 7: 209–13. [PubMed] [Google Scholar]
- 5. Danby SG, Chalmers J, Brown K et al. A functional mechanistic study of the effect of emollients on the structure and function of the skin barrier. Br J Dermatol 2016; 175 : 1011–19. [DOI] [PubMed] [Google Scholar]
- 6. Larson AA, Dinulos JG. Cutaneous bacterial infections in the newborn. Curr Opin Pediatr 2005; 17: 481–5. [DOI] [PubMed] [Google Scholar]
- 7. Cork MJ, Timmins J, Holden C et al. An audit of adverse drug reactions to aqueous cream in children with atopic eczema. Pharm J 2003; 271: 746–7. [Google Scholar]
- 8. Snyder A, Farhangian M, Feldman SR. A review of patient adherence to topical therapies for treatment of atopic dermatitis. Cutis 2015; 96: 397–401. [PubMed] [Google Scholar]
- 9. Akerstrom U, Reitamo S, Langeland T et al. Comparison of moisturizing creams for the prevention of atopic dermatitis relapse: a randomized double‐blind controlled multicentre clinical trial. Acta Derm Venereol 2015; 95: 587–92. [DOI] [PubMed] [Google Scholar]
- 10. Wiren K, Nohlgard C, Nyberg F et al. Treatment with a barrier‐strengthening moisturizing cream delays relapse of atopic dermatitis: a prospective and randomized controlled clinical trial. J Eur Acad Dermatol Venereol 2009; 23: 1267–72. [DOI] [PubMed] [Google Scholar]
- 11. de Jongh CM, Jakasa I, Verberk MM, Kezic S. Variation in barrier impairment and inflammation of human skin as determined by sodium lauryl sulphate penetration rate. Br J Dermatol 2006; 154: 651–7. [DOI] [PubMed] [Google Scholar]
- 12. Jakasa I, de Jongh CM, Verberk MM et al. Percutaneous penetration of sodium lauryl sulphate is increased in uninvolved skin of patients with atopic dermatitis compared with control subjects. Br J Dermatol 2006; 155: 104–9. [DOI] [PubMed] [Google Scholar]
- 13. Held E, Agner T. Effect of moisturizers on skin susceptibility to irritants. Acta Derm Venereol 2001; 81: 104–7. [DOI] [PubMed] [Google Scholar]
- 14. Held E, Sveinsdottir S, Agner T. Effect of long‐term use of moisturizer on skin hydration, barrier function and susceptibility to irritants. Acta Derm Venereol 1999; 79: 49–51. [DOI] [PubMed] [Google Scholar]
- 15. Loden M. Barrier recovery and influence of irritant stimuli in skin treated with a moisturizing cream. Contact Dermatitis 1997; 36: 256–60. [DOI] [PubMed] [Google Scholar]
- 16. Danby SG, Brown K, Higgs‐Bayliss T et al. The effect of an emollient containing urea, ceramide NP, and lactate on skin barrier structure and function in older people with dry skin. Skin Pharmacol Physiol 2016; 29: 135–47. [DOI] [PubMed] [Google Scholar]
- 17. Gloor M, Hauth A, Gehring W. O/W emulsions compromise the stratum corneum barrier and improve drug penetration. Pharmazie 2003; 58: 709–15. [PubMed] [Google Scholar]
- 18. Grether‐Beck S, Felsner I, Brenden H et al. Urea uptake enhances barrier function and antimicrobial defense in humans by regulating epidermal gene expression. J Invest Dermatol 2012; 132: 1561–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kezic S, O'Regan GM, Yau N et al. Levels of filaggrin degradation products are influenced by both filaggrin genotype and atopic dermatitis severity. Allergy 2011; 66: 934–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Danby SG. Biological variation in skin barrier function: from A (atopic dermatitis) to X (xerosis). Curr Probl Dermatol 2016; 49: 47–60. [DOI] [PubMed] [Google Scholar]
- 21. Danby SG, Brown K, Wigley AM et al. The effect of water hardness on surfactant deposition following washing and subsequent skin irritation in atopic dermatitis patients and healthy controls. J Invest Dermatol 2018; 138: 68–77. [DOI] [PubMed] [Google Scholar]
- 22. Loden M, Andersson AC. Effect of topically applied lipids on surfactant‐irritated skin. Br J Dermatol 1996; 134: 215–20. [PubMed] [Google Scholar]
- 23. Proksch E, de Bony R, Trapp S, Boudon S. Topical use of dexpanthenol: a 70th anniversary article. J Dermatolog Treat 2017; 28: 766–73. [DOI] [PubMed] [Google Scholar]
- 24. Alexis AF, Woolery‐Lloyd H, Williams K et al. Racial/ethnic variations in skin barrier: implications for skin care recommendations in skin of color. J Drugs Dermatol 2021; 20: 932–8. [DOI] [PubMed] [Google Scholar]
- 25. NHS Digital . Prescription cost analysis England. 2018. Available at: https://digital.nhs.uk/data‐and‐information/publications/statistical/prescription‐cost‐analysis/2018 (accessed 7 March 2022). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplementary Methods, Results, Discussion and References.
Figure S1. Treatment with test cream was well tolerated and reduced skin erythema in response to sodium lauryl sulfate exposure.
Figure S2. Relationship between Mexameter units and visual skin redness.
Figure S3. Relationship between erythema index and visual skin redness.
Table S1. Treatment allocation frequencies to the test sites.
Table S2. Summary statistics for the primary outcome measures.
Table S3. Summary of primary outcome measure of skin sensitivity for the per‐protocol set.
Table S4. Summary of secondary outcome measures.
Table S5. Summary of visual analogue scores for tolerability.
Table S6. Summary of Erythema Index change from Day 1 to Day 29.
Table S7. Adverse events (patient level) by system organ class and preferred term.
Table S8. Summary of transepidermal water loss (g/m2/h) by FLG genotype.
Table S9. Summary of stratum corneum natural moisturizing factor levels on Day 29 by FLG genotype.


