Abstract
Understanding why some wounds are hard to heal is important for improving care and developing more effective treatments. The method of sample collection used is an integral step in the research process and thus may affect the results obtained. The primary objective of this study was to summarise and map the methods currently used to sample wound fluid for protein profiling and analysis. Eligible studies were those that used a sampling method to collect wound fluid from any human wound for analysis of proteins. A search for eligible studies was performed using MEDLINE, Embase and CINAHL Plus in May 2020. All references were screened for eligibility by one reviewer, followed by discussion and consensus with a second reviewer. Quantitative data were mapped and visualised using appropriate software and summarised via a narrative summary. After screening, 280 studies were included in this review. The most commonly used group of wound fluid collection methods were vacuum, drainage or use of other external devices, with surgical wounds being the most common sample source. Other frequently used collection methods were extraction from absorbent materials, collection beneath an occlusive dressing and direct collection of wound fluid. This scoping review highlights the variety of methods used for wound fluid collection. Many studies had small sample sizes and short sample collection periods; these weaknesses have hampered the discovery and validation of novel biomarkers. Future research should aim to assess the reproducibility and feasibility of sampling and analytical methods for use in larger longitudinal studies.
Keywords: complex wound, surgical wound, wound biomarker, wound fluid collection, wound proteomics
Abbreviations
- BRC
Biomedical Research Centre
- TIDieR
Template for Intervention Description and Replication
1. INTRODUCTION
1.1. Rationale
Wounds are disruptions to the integrity of the skin and may compromise its structure and function, depending on wound severity. 1 Wounds can be classed as open or closed, with closed wounds having their edges bought together and held (e.g. with stiches) and open, or complex, wounds left to heal by secondary intention. Complex wounds, which include leg, foot, and pressure ulcers, can take months to heal and in some cases will not heal. The treatment of complex wounds is typically time‐ and resource‐intensive, and their chronicity can be distressing for those directly affected. 2 , 3 , 4 Biomarkers can give an indication of a person's biological state and may be useful for understanding, or predicting, the healing trajectory of a wound. 5 Proteomic profiling of the wound micro‐environment is one avenue for possible identification and validation of biomarkers 6 as well as being useful for uncovering the potential mechanism(s) of delayed healing. 7 Therefore, investigating the proteomic profile of the wound environment has become an important focus in wound research. 8 , 9 , 10
There are a number of sample types, which are collected for investigations of the wound environment. Wound fluid is commonly used for protein profiling and analysis 11 and has many characteristics that make it an ideal sample type for biomarker identification. 12 , 13 Although there are multiple methods of collecting wound fluid for research purposes, there has been no comprehensive overview, which clearly presents their details and pattern of use. Furthermore, a large dataset, which numerically summarises the collection methods used for wound healing research, may be useful to ascertain whether more stringent guidelines are required for standardisation of the current methods. 14 Therefore, a scoping review was performed to systematically map the methods used for wound fluid collection and analyses and to identify whether any gaps in the research base existed.
1.2. Objectives
The primary objective of the scoping review was to answer the question:
1. Which sample collection methods have been used to collect wound fluid for the analysis of protein expression or activity?Three related sub‐questions were further addressed as follows:
a. What study designs and sampling regimens have been used (number of samples and frequency of sampling)?
b. How were wound fluid samples processed and stored before proteomic analysis?
c. Which proteomic analysis methods have been used to analyse wound fluid, and what is the relationship between collection and analysis methods?
2. MATERIALS AND METHODS
A protocol was first developed outlining how the scoping review would be carried out. This protocol was registered with the open science framework on 27 May 2021 (https://osf.io/5qywx). The scoping review was reported with reference to the PRISMA extension for scoping reviews (PRISMA‐ScR) checklist 15 (Appendix S1).
2.1. Study inclusion criteria
Studies were eligible for inclusion in this review if they used a sampling method to collect wound fluid from any human wound for analysis of proteins or for proteomics (including endogenous protein expression/activity and expression/activity of their inhibitors). No restrictions were applied to study design, sample size, publication date or participant characteristics. Studies of other tissue types, e.g., from biopsy, were excluded. Only primary publications were accepted; however, reviews identified by the initial search were screened for potentially relevant studies. Finally, only full‐text articles written in, or available in, the English language were included in this review.
2.2. Search strategy
Three separate databases: Ovid (MEDLINE), Ovid (Embase) and EBSCO (CINAHL plus) were searched on 21 May 2020. The search strategy was developed by an information specialist and the search terms selected based on the above inclusion criteria. This strategy allowed for inclusion of a broad range of studies that could then be screened for relevance. The search was initially split into two separate categories: open wounds and surgical wounds. The initial search strategy used for each database, including all search terms, is outlined in the supplementary material along with the relevant search results (Appendix S2).
2.3. Screening
All articles were de‐duplicated in Endnote X9 (Clarivate Analytics, Philadelphia, PA, USA) and the resulting citations were uploaded to review software (Covidence; Covidence, Melbourne, VIC, Australia). Titles and abstracts were then screened for eligibility against the inclusion criteria by one reviewer (J.H.). Potentially eligible studies were obtained as full‐text articles for further screening against the inclusion criteria. A second researcher (KTM) screened 10% of the articles at the title and abstract stage and again at the full‐text stage to ensure consistency. Any study selection disagreements were resolved by discussion and consensus of both reviewers.
2.4. Data extraction and presentation
Data were extracted by one reviewer and charted using a piloted data charting form. This form was jointly developed by all reviewers (J.H., J.C.D., N.C. and R.E.B.W.), using Microsoft Access (Microsoft, Redmond, WA, USA), and created with reference to The Template for Intervention Description and Replication (TIDieR) checklist and guide. 16 The variables extracted include article information (paper title, authors, and year), study information (number of participants, number of wounds, type of wounds, dressings used), collection method data (collection setting, method(s) used, volume (ml) and total protein concentration (mg/ml) of wound fluid collected, duration and frequency of collection, etc.), sample processing characteristics (processing requirements and storage conditions) and analysis methods (number of unique proteins identified and assay methods used). The study period was also calculated from the charted data, as the time between the first and last sample collection.
The data from these variables were then organised into groups based on their key characteristics. We subsequently produced 10 wound type, six collection method and 12 assay method umbrella groupings to summarise the data (Tables S1–S3). All subsequent data analysis was carried out using these groupings.
The findings were then mapped and visualised using RStudio (RStudio, Boston, MA, USA) and a descriptive numerical summary of the data given as recommended by Arksey and O'Malley. 17 Furthermore, a narrative summary outlines the significance of the findings as well as the impact that they may have on future studies in this area. 18
3. RESULTS
Duplicates (1075) were removed from the 2160 potentially eligible articles, leaving 1085 records for initial screening by titles and abstracts. After excluding a further 520 records, we screened the full text of 565 articles, with 280 included and subjected to data extraction (Figure 1). 8 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 , 190 , 191 , 192 , 193 , 194 , 195 , 196 , 197 , 198 , 199 , 200 , 201 , 202 , 203 , 204 , 205 , 206 , 207 , 208 , 209 , 210 , 211 , 212 , 213 , 214 , 215 , 216 , 217 , 218 , 219 , 220 , 221 , 222 , 223 , 224 , 225 , 226 , 227 , 228 , 229 , 230 , 231 , 232 , 233 , 234 , 235 , 236 , 237 , 238 , 239 , 240 , 241 , 242 , 243 , 244 , 245 , 246 , 247 , 248 , 249 , 250 , 251 , 252 , 253 , 254 , 255 , 256 , 257 , 258 , 259 , 260 , 261 , 262 , 263 , 264 , 265 , 266 , 267 , 268 , 269 , 270 , 271 , 272 , 273 , 274 , 275 , 276 , 277 , 278 , 279 , 280 , 281 , 282 , 283 , 284 , 285 , 286 , 287 , 288 , 289 , 290 , 291 , 292 , 293 , 294 , 295 , 296 , 297 All data relevant for answering the questions set in the scoping review protocol were extracted and are presented in the supplementary material (Table S4).
FIGURE 1.
PRISMA flow diagram outlining how sources of evidence were selected
3.1. Overall study characteristics
The key characteristics of the included studies are summarised in Table 1. Of the 280 included studies, 203 (73%) were conducted in the last 20 years and only eight were published in 1990 or before. Hospitals were the most commonly reported setting for sample collection, although 91 studies did not report where collection took place. Of the 270 studies that reported sample size, over half had 20 participants or fewer and only five studies reported recruitment of 100 participants or more. There was large variety in the wound types studied, with a total of 409 wound groups reported across all 280 studies. There were eight instances where the type of wound was not reported, leaving 401 wound groups which could be identified. The most commonly studied groups were surgical wounds and venous leg ulcers, making up 32% (130/401) and 24% (96/401) of the identified wound groups, respectively.
TABLE 1.
Summary of the key characteristics of all included studies
| Study characteristics (N = 280) | n |
|---|---|
| Publication year | |
| 1990 and earlier | 8 |
| 1991–1995 | 24 |
| 1996–2000 | 45 |
| 2001–2005 | 34 |
| 2006–2010 | 50 |
| 2011–2015 | 67 |
| 2016–2020 | 52 |
| Total | 280 |
| Setting | |
| Burn centre | 10 |
| Community clinic | 14 |
| Hospital | 115 |
| Medical centre | 32 |
| Nursing facility | 6 |
| Other settings a | 3 |
| Unknown setting | 91 |
| Wound healing centre | 16 |
| Total | 287 b |
| Number of participants | |
| 1–20 | 139 |
| 21–40 | 92 |
| 41–60 | 24 |
| 61–80 | 6 |
| 81–100 | 4 |
| Over 100 | 5 |
| Unknown | 10 |
| Total | 280 |
| Wound group(s) studied | |
| Amputation or traumatic wound | 15 |
| Arterial ulcer | 5 |
| Artificial wound | 13 |
| Burn wound | 34 |
| Dental wound | 11 |
| Foot ulcer | 37 |
| Mixed vessel disease ulcer | 9 |
| Other wounds c | 17 |
| Pressure ulcer | 34 |
| Surgical wound | 130 |
| Unknown wound | 8 |
| Venous leg ulcer | 96 |
| Total | 409 d |
‘Other settings’ were defined as those that could not be classed in any of the other setting groups.
Some studies utilised multiple settings for collection. Therefore, the total number of settings (n = 287) exceeds the total number of studies (n = 280).
‘Other wounds’ were defined as those that could not be classed in any of the other wound groups.
Some studies investigated multiple wound groups. Therefore, the total number of wound groups studied (n = 409) exceeds the total number of studies (n = 280).
3.2. Review questions
3.2.1. Which sample collection methods have been used to collect wound fluid for the analysis of protein expression or activity?
Six distinct types of collection were identified (Figure 2A). Four studies used methods that could not be categorised into any of the six groups 19 , 20 , 21 , 22 and a further six studies did not report the collection method used for a specific wound. 23 , 24 , 25 , 26 , 27 , 28
FIGURE 2.
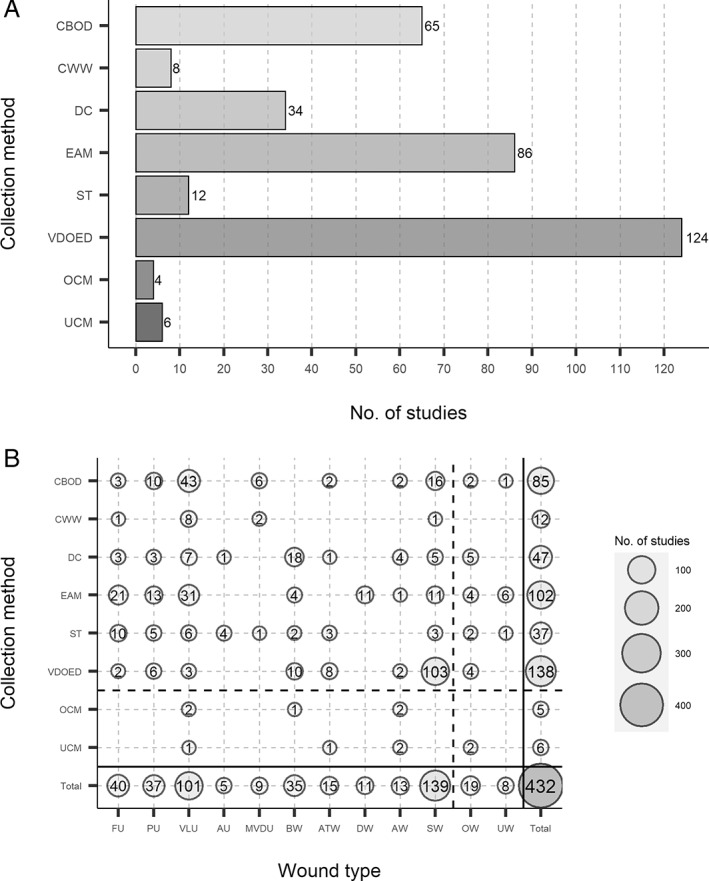
The number of studies that utilised each collection method for all included studies and all investigated wound groups. Counts were carried out for (A) the number of studies, which utilised each collection method and (B) the number and type of investigated wound groups, which utilised each collection method. Collection methods and wound types were grouped into classes as mentioned previously (Tables S1 and S2) based on description similarities. The total number of times a collection method was used in combination with all different wound groups was calculated, as well as the total number of times a wound group was used in combination with all different collection methods. As some of these studies used multiple collection methods and multiple wound groups, the totals for (A) (n = 339) and (B) (n = 432) may exceed the total number of studies (n = 280). AW, artificial wound; AU, arterial ulcer; ATW, amputation or traumatic wound; BW, burn wound; CBOD, collection beneath an occlusive dressing; CWW, collection of wound washout; DW, dental wound; DC, direct collection; EAM, extraction from absorbent materials; FU, foot ulcer; MVDU, mixed vessel disease ulcer; OW, other wounds; OCM, other collection methods; PU, pressure ulcer; ST, swab technique; SW, surgical wound; UW, unknown wound; UCM, unknown collection method; VDOED, vacuum, drainage or other external device; VLU, venous leg ulcer
Vacuum, drainage, or other external devices were used in 124 of the 280 (44%) studies and thus were the most frequently used methods of sample collection 22 , 23 , 24 , 25 , 26 , 27 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 ; the vast majority of sample collections using this method (75%, 103/138), were from surgical wounds (Figure 2B). Three other frequently used methods were extraction from absorbent material (31%, 86/280), 8 , 20 , 37 , 81 , 89 , 136 , 138 , 144 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 , 190 , 191 , 192 , 193 , 194 , 195 , 196 , 197 , 198 , 199 , 200 , 201 , 202 , 203 , 204 , 205 , 206 , 207 , 208 , 209 , 210 , 211 , 212 , 213 , 214 , 215 , 216 , 217 , 218 , 219 , 220 , 221 , 222 , 223 collection beneath occlusive dressing (23%, 65/280) 22 , 24 , 27 , 29 , 33 , 36 , 41 , 43 , 53 , 62 , 64 , 66 , 67 , 68 , 72 , 77 , 80 , 86 , 88 , 95 , 107 , 111 , 114 , 116 , 129 , 145 , 146 , 169 , 174 , 224 , 225 , 226 , 227 , 228 , 229 , 230 , 231 , 232 , 233 , 234 , 235 , 236 , 237 , 238 , 239 , 240 , 241 , 242 , 243 , 244 , 245 , 246 , 247 , 248 , 249 , 250 , 251 , 252 , 253 , 254 , 255 , 256 , 257 , 258 , 259 and direct collection (12%, 34/280). 23 , 26 , 42 , 53 , 99 , 113 , 198 , 225 , 242 , 245 , 248 , 251 , 260 , 261 , 262 , 263 , 264 , 265 , 266 , 267 , 268 , 269 , 270 , 271 , 272 , 273 , 274 , 275 , 276 , 277 , 278 , 279 , 280 , 281 The wound groups most frequently sampled using extraction from absorbent material or collection beneath occlusive dressing were ulcers: either pressure, foot, or most commonly, venous leg ulcers, whereas direct collection was more often used to sample burns. The two other defined methods, swab technique (4%, 12/280) 129 , 214 , 227 , 282 , 283 , 284 , 285 , 286 , 287 , 288 , 289 , 290 and collection of wound washout (3%, 8/280) 273 , 291 , 292 , 293 , 294 , 295 , 296 , 297 were used in only a small proportion of all included studies.
What study designs and sampling regiments have been used (number of samples and frequency of wound sampling)?
Tables 2 and 3 summarise the sampling regimens of all investigated wound groups (n = 409) from the included studies. Of these wound groups, 17 did not record the number of samples collected per wound and 26 did not clearly report the time between each sample collection, leaving a total of 392 and 383 investigated wound groups with reported sampling details for the data presented in Tables 2 and 3, respectively. Most of the studies were cross‐sectional and took only one sample per wound. Where studies took multiple samples from each wound over time, the number of samples collected was usually small, typically 2–3. Collecting over 10 samples per wound was uncommon and only occurred in nine of the investigated wound groups. The time between each collection, for longitudinal designs, was typically 1–3 days accounting for 21% (82/383) of the wound groups with reported sampling details. Consequently, most longitudinal studies had short study durations and small sample sizes.
TABLE 2.
Number of samples collected per wound, for each wound type in all included studies, grouped by collection method
| No. of samples collected (per wound) | Collection beneath occlusive dressing | Collection of wound washout | Direct collection | Extraction from absorbent materials | Swab technique | Vacuum, drainage or other external device | Other collection methods | Unknown collection method | All studies a |
|---|---|---|---|---|---|---|---|---|---|
| % (n b ) | % (n) | % (n) | % (n) | % (n) | % (n) | % (n) | % (n) | % (n) | |
| 1 | 54 (46) | 58 (7) | 83 (39) | 49 (50) | 78 (29) | 41 (57) | 60 (3) | 83 (5) | 53 (215) |
| 2–3 | 18 (15) | 33 (4) | 2 (1) | 10 (10) | 19 (7) | 16 (22) | 20 (1) | 0 (0) | 14 (59) |
| 4–5 | 6 (5) | 0 (0) | 11 (5) | 15 (15) | 0 (0) | 9 (13) | 0 (0) | 0 (0) | 9 (38) |
| 6–10 | 6 (5) | 0 (0) | 0 (0) | 11 (11) | 0 (0) | 8 (11) | 0 (0) | 0 (0) | 7 (27) |
| >10 | 0 (0) | 0 (0) | 0 (0) | 5 (5) | 3 (1) | 1 (2) | 20 (1) | 0 (0) | 2 (9) |
| Varied c | 5 (4) | 8 (1) | 2 (1) | 8 (8) | 0 (0) | 17 (24) | 0 (0) | 17 (1) | 11 (44) |
| Unknown | 12 (10) | 0 (0) | 2 (1) | 3 (3) | 0 (0) | 7 (9) | 0 (0) | 0 (0) | 4 (17) |
| Total | 100 (85) | 100 (12) | 100 (47) | 100 (102) | 100 (37) | 100 (138) | 100 (5) | 100 (6) | 100 (409 d ) |
‘All studies’ refers to the number of wound groups studied regardless of collection method used.
n = number of wound groups studied.
‘Varied’ refers to the collection of different numbers of samples for different wounds in a group.
Some studies used multiple collection methods. Therefore, the sum total of wound groups studied for all collection methods (n = 432) is higher than the total number of wound groups for all studies (n = 409), as some wound groups have been counted multiple times.
TABLE 3.
Time taken between each sample collection, for each wound type in all included studies, grouped by collection method
| Time between sample collections | Collection beneath occlusive dressing | Collection of wound washout | Direct collection | Extraction from absorbent materials | Swab technique | Vacuum, drainage or other external device | Other collection methods | Unknown collection method | All studies a |
|---|---|---|---|---|---|---|---|---|---|
| % (n b ) | % (n) | % (n) | % (n) | % (n) | % (n) | % (n) | % (n) | % (n) | |
| <1 day | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 9 (12) | 0 (0) | 0 (0) | 3 (12) |
| 1–3 days | 14 (12) | 0 (0) | 4 (2) | 11 (11) | 0 (0) | 41 (57) | 0 (0) | 17 (1) | 20 (82) |
| 4–6 days | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (2) |
| 1–2 weeks | 6 (5) | 33 (4) | 9 (4) | 14 (14) | 5 (2) | 1 (2) | 0 (0) | 0 (0) | 8 (31) |
| 2–4 weeks | 13 (11) | 0 (0) | 2 (1) | 2 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (14) |
| >1 month | 5 (4) | 0 (0) | 0 (0) | 3 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (7) |
| Varied c | 1 (1) | 0 (0) | 0 (0) | 9 (9) | 0 (0) | 2 (3) | 20 (1) | 0 (0) | 3 (13) |
| All at once d | 0 (0) | 0 (0) | 0 (0) | 6 (6) | 0 (0) | 0 (0) | 20 (1) | 0 (0) | 2 (7) |
| N/A e | 54 (46) | 58 (7) | 83 (39) | 49 (50) | 78 (29) | 41 (57) | 60 (3) | 83 (5) | 53 (215) |
| Unknown | 7 (6) | 8 (1) | 2 (1) | 6 (6) | 16 (6) | 4 (6) | 0 (0) | 0 (0) | 6 (26) |
| Total | 100 (85) | 100 (12) | 100 (47) | 100 (102) | 100 (37) | 100 (138) | 100 (5) | 100 (6) | 100 (409 f ) |
‘All studies’ refers to the number of wound groups studied regardless of collection method used.
n = number of wound groups studied.
If there was not a specific time between each collection which made up the majority, then the time was charted as ‘Varied’.
‘All at once’ refers to all instances where multiple samples were collected at the same time.
‘N/A' refers to all instances where only one sample was collected.
Some studies used multiple collection methods. Therefore, the sum total of studied wound groups for all collection methods (n = 432) is higher than the total number of wound groups for all studies (n = 409), as some wound groups have been counted multiple times.
How are wound fluid samples processed and stored before proteomic analysis?
Sixteen studies did not report how samples were processed and four did not report storage conditions (Tables 4 and 5) leaving a total of 264 and 276 studies with reported sample conditions, respectively. The majority of studies which reported these conditions processed samples immediately (71%, 188/264) and used ultra‐low freezer temperatures of −70°C or −80°C for sample storage (68%, 189/276). A further 16% (43/276) stored samples at −20°C and only four studies kept samples at over 0°C. Finally, only 14% (38/276) of studies processed and analysed samples immediately with no storage of samples before the initial analysis.
TABLE 4.
The sample processing characteristics of all studies, grouped by collection method
| Immediate processing? | Collection beneath occlusive dressing | Collection of wound washout | Direct collection | Extraction from absorbent materials | Swab technique | Vacuum, drainage or other external device | Other collection methods | Unknown collection method | All studies a |
|---|---|---|---|---|---|---|---|---|---|
| % (n b ) | % (n) | % (n) | % (n) | % (n) | % (n) | % (n) | % (n) | % (n) | |
| No | 14 (9) | 12 (1) | 26 (9) | 37 (32) | 42 (5) | 17 (21) | 25 (1) | 0 (0) | 26 (73) |
| Yes | 83 (54) | 88 (7) | 56 (19) | 58 (50) | 42 (5) | 76 (94) | 50 (2) | 100 (6) | 67 (188) |
| If possible c | 2 (1) | 0 (0) | 6 (2) | 1 (1) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 1 (3) |
| Unknown | 2 (1) | 0 (0) | 12 (4) | 3 (3) | 17 (2) | 6 (8) | 25 (1) | 0 (0) | 6 (16) |
| Total | 100 (65) | 100 (8) | 100 (34) | 100 (86) | 100 (12) | 100 (124) | 100 (4) | 100 (6) | 100 (280 d ) |
‘All studies’ refers to the number of wound groups studied regardless of collection method used.
n = Number of studies.
‘If possible’ refers to studies which attempted immediate processing but where it may not have been possible for all samples.
Some studies used multiple collection methods. Therefore, the sum total of studies for all collection methods (n = 339) is higher than the total number of studies (n = 280), as some studies have been counted multiple times.
TABLE 5.
The sample storage conditions of all studies, grouped by collection method
| Sample storage conditions | Collection beneath occlusive dressing | Collection of wound washout | Direct collection | Extraction from absorbent materials | Swab technique | Vacuum, drainage or other external device | Other collection methods | Unknown collection method | All studies a |
|---|---|---|---|---|---|---|---|---|---|
| % (n b ) | % (n) | % (n) | % (n) | % (n) | % (n) | % (n) | % (n) | % (n) | |
| −80°C | 43 (28) | 75 (6) | 24 (8) | 59 (51) | 42 (5) | 44 (54) | 25 (1) | 33 (2) | 48 (134) |
| −70°C | 26 (17) | 0 (0) | 32 (11) | 13 (11) | 8 (1) | 23 (28) | 25 (1) | 33 (2) | 20 (55) |
| −20°C | 20 (13) | 0 (0) | 21 (7) | 15 (13) | 17 (2) | 16 (20) | 25 (1) | 17 (1) | 15 (43) |
| 4°C | 2 (1) | 0 (0) | 0 (0) | 1 (1) | 8 (1) | 1 (1) | 0 (0) | 0 (0) | 1 (3) |
| 16°C | 0 (0) | 0 (0) | 3 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (1) |
| Other c | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 8 (1) | 1 (1) | 0 (0) | 0 (0) | 1 (2) |
| N/A d | 8 (5) | 12 (1) | 18 (6) | 12 (10) | 17 (2) | 15 (19) | 25 (1) | 17 (1) | 14 (38) |
| Unknown | 2 (1) | 12 (1) | 3 (1) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 1 (4) |
| Total | 100 (65) | 100 (8) | 100 (34) | 100 (86) | 100 (12) | 100 (124) | 100 (4) | 100 (6) | 100 (280 e ) |
‘All studies’ refers to the number of wound groups studied regardless of collection method used.
n = Number of studies.
‘Other’ sample storage conditions were those that could not be defined by any of the other charted categories.
‘N/A’ refers to studies which did not store their samples before processing or initial analysis.
Some studies used multiple collection methods. Therefore, the sum total of studies for all collection methods (n = 339) is higher than the total number of studies (n = 280), as some studies have been counted multiple times.
Which proteomic analysis methods are used on the collected samples and does this influence the method of collection used?
Immunoassays were the most frequently used method of analysis for all collection methods (Figure 3A). Other commonly used assay methods included absorbance or enzyme activity assays, immunoblot and zymography. Some of the assay methods were only utilised by studies using specific collection methods. For example, immunoelectrophoresis was only utilised for sample analysis when either direct collection or vacuum, drainage or other external devices were used to obtain samples. The majority of studies used collection – assay combinations which, on average, identified relatively few unique proteins (Figure 3B). Whilst techniques such as mass spectrometry, which when used in an untargeted workflow aim for complete proteome coverage, were utilised by relatively few studies. 298 When it was used, mass spectrometry was most frequently combined with collection via extraction from absorbent materials and the average number of unique protein identifications was highest when using this collection – assay approach. The number of unique proteins identified in each study did not correlate with either volume (ml) or total protein concentration (mg/ml) of wound fluid collected (Figures S1 and S2). Although these details of collection were only recorded for 100 and 41 of the 280 included studies, respectively.
FIGURE 3.
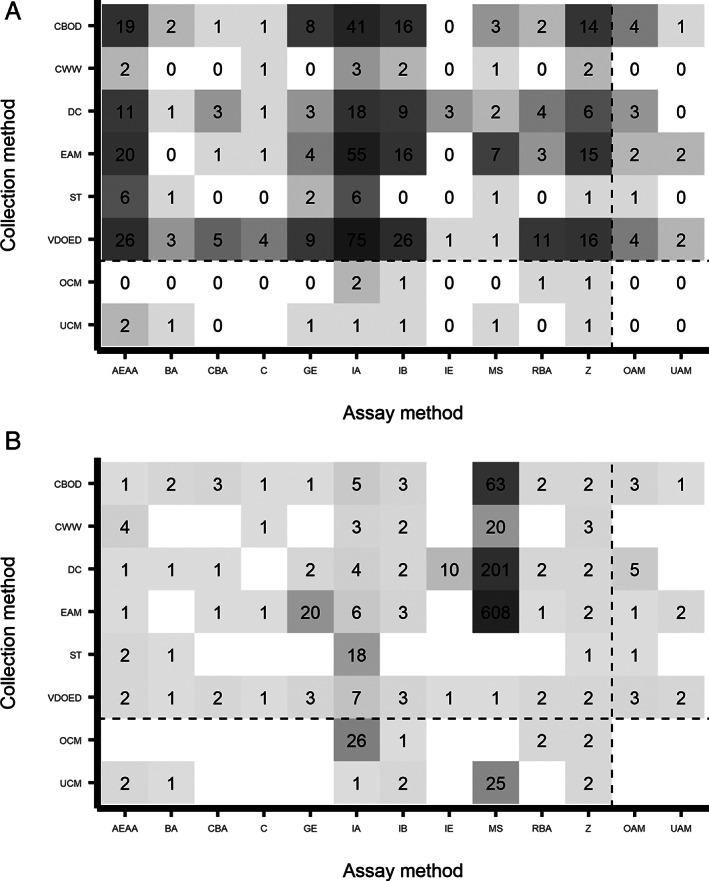
The number of studies that utilised each combination of assay and collection methods and the average number of unique proteins identified by each of these combinations. All studies from which data was extracted were included here (n = 280). (A) Counts were carried out for the number of studies which utilised each collection—assay combination. Some studies may have used multiple collection and/or assay methods. Therefore, the total for all combinations used (n = 561) may exceed the total number of studies (n = 280). (B) The average number of unique proteins identified in studies which used each collection—assay combination were calculated. Mean averages for each combination were rounded to the nearest integer value. Combinations which were not utilised by any studies, or where no unique protein identifications were reported, are denoted by a blank space. AEAA, absorbance or enzyme activity assay; BA, bioassay, CBA, cell‐based assay; C, chromatography; CBOD, collection beneath an occlusive dressing; CWW, collection of wound washout; DC, direct collection; EAM, extraction from absorbent materials; GE, gel electrophoresis; IA, immunoassay; IB, immunoblot; IE, immunoelectrophoresis; MS, mass spectrometry; OAM, other assay methods; OCM, other collection methods; RBA, radiation‐based assay; ST, swab technique; UAM, unknown assay method; UCM, unknown collection method; VDOED, vacuum, drainage or other external device; Z, zymography
4. DISCUSSION
4.1. Summary and implication of findings
We included 280 eligible studies, which collected wound fluid samples from human participants. Mapping of the data has highlighted the considerable heterogeneity present in wound fluid sampling, with at least six methodologically distinct collection methods being used. There was further variation within each of these groups with the details of collection differing between studies. This heterogeneity of activity and reporting impacts on confident comparisons between studies: various sampling methods are likely to influence the recovery of individual analytes, especially if processing and storage methods were also disparate. 129 , 299 Comparisons may only be possible between studies using similar sampling, processing and storage methods and could limit the value of the body of evidence. 14
Some sample collection methods are more commonly used than others. Rationales to support choice of collection method are not clear but may be primarily driven by wound type. The most commonly used collection methods – vacuum, drainage or other external devices – are primarily used for collection of wound fluid from surgical wounds (the most commonly chosen wound type). Drainage of fluid from surgical wounds is already common clinical practise, 300 where large volumes of exudate can be produced thus providing readily available samples for non‐invasive collection. 14 Exudate volume may also influence the collection method used for other wound types. For example, direct collection of wound fluid will typically require a larger volume than other methods, which may explain its use in sampling from burns or venous leg ulcers, which generally produce higher exudate volumes than some other non‐surgical wound types. 301
Many of the included studies utilised a cross‐sectional design, with samples taken at a single time point. 302 Studies designed in this manner are usually easy and inexpensive to set up but are not an appropriate design for assessing associations between potentially prognostic factors (such as biomarkers) and clinical outcomes, 303 nor wound healing research more broadly. Timing of sample collection is crucial, particularly as wounds will be in one of several possible healing phases, 304 with protein concentrations fluctuating accordingly. 222 Taking samples during only one of these phases will thus miss the complexity inherent in the healing process and may provide misleading results. Longitudinal follow‐up is also crucial to enable meaningful clinical endpoints such as complete wound healing to be assessed.
Although some of the included studies 160 , 164 , 231 , 237 , 290 did embed sample collection in a longitudinal design, the number of samples collected was typically low and the study periods short. This means although the evidence base we have identified is large, it does not identify an obvious wound fluid sampling approach for longer studies. Therefore, further work could focus on exploring optimal wound fluid sampling methods to be used in longitudinal studies in terms of being valid and operationally feasible in clinical settings.
For untargeted proteomics and biomarker discovery studies, techniques that allow for identification and measurement of large protein numbers simultaneously, such as mass spectrometry or multiplex immunoassays, are often favoured. 305 The relatively low number of studies which utilised such techniques therefore suggests that these study types are in the minority for wound fluid investigations. As measured wound fluid characteristics (wound fluid volume and total protein concentration) did not affect the number of proteins identified by a study, the choice of collection method may be due to other factors, such as the type of wound sampled.
Future work in this area should focus on identification of valid sampling approaches for wound proteomic studies ensuring that research waste is minimised by learning from studies that have already been conducted and reported. Whilst the 280 included studies in this scoping review represent large investment of time and other resources, the heterogeneity and size and scope of the evidence limits the value of the existing evidence for guiding sampling approaches for future work, thus perpetuating these issues. To support the conduct of rigorous biomarker identification and validation studies, sampling and analytical processes need to be proven accurate, reproducible and feasible for use in larger cohorts. 306 There is also a lack of recognised guidelines for the collection of wound fluid, using the included methods. Therefore, the development of a set of sampling standards may benefit further work in this area. Future studies that aim to link wound fluid sampling analysis to clinical outcomes should also draw on epidemiological as well as biological methodologies to ensure application of rigorous prognostic research methodology. 307
4.2. Strengths and limitations
This review is the first of its kind to summarise the methods used to sample, process, store and analyse fluid from human wounds. We followed a systematic, pre‐determined approach to the review, documented in a protocol, and ensured consistency by, for example, developing and piloting a data extraction form to best capture all data relevant for addressing the review questions.
Some deviations were made from the original protocol on commencement of data collection, extraction, and charting but none of these were considered to introduce bias into the process. Firstly, it was decided that related evidence that has supported development or use of the method would not be included, as this was outside the scope of the review; however, the data may be useful for research in this area. Furthermore, animal studies that used wound fluid for proteomics research were omitted, as data from these were deemed not relevant in answering the questions posed. As stated in the protocol, all articles not written in English and those not accessible as full texts were removed during the screening stage. These articles may include information relevant to this scoping review but were removed due to time and resource constraints.
Additionally, screening was carried out by one reviewer which increases the risk of missing relevant studies 308 , 309 and the possibility of selection bias. 310 To minimise the likelihood of this, a second reviewer screened 10% of the articles at the abstract and full‐text stages. Disagreements were uncommon, but where these occurred consensus between both reviewers was required before moving on and these decisions influenced the rest of the screening process. Furthermore, a single screening system was time intensive. To streamline the process in future scoping reviews, it may be preferable to use screening software, which employs a text‐mining tool to prioritise potentially eligible studies. 311 , 312
As the amount of data extracted was relatively large, groupings were created for some of the charted variables. Groups were created to clarify and summarise the key elements and allow clear visualisation of the extracted data, although grouping of some of these variables may be viewed as subjective. To minimise the risk of bias, all groupings were created by one researcher before consultation and acceptance by two other researchers. However, grouping the data in this way masks any variations within a group that may have been of interest.
5. CONCLUSIONS
This scoping review successfully mapped and numerically summarised the methods used for collection of wound fluid samples for protein analysis. This demonstrated that a large variety of collection methods have been employed, with some methods being used far more frequently than others. The use of specific methods may be dictated by the type of wound under study and the clinical characteristics of that wound. The majority of studies used small sample sizes and had short study periods, with many opting for a cross‐sectional design. Many proteomics studies, such as those for the identification and validation of biomarkers, require larger longitudinal designs to yield useful results. This review therefore highlights the requirement for progression in wound healing research to focus on larger cohort sizes over extended study periods, to acquire robust data for wound proteomics investigations. The data presented herein should reduce research waste by allowing researchers to learn from past studies and to address knowledge gaps in our existing understanding of complex wounds. Future work should aim to rigorously assess sampling methods and associated analytical techniques for use in large prognosis studies, with the possibility to create a set of sampling standards to ensure consistency between studies.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Appendix S1. Supporting Information
ACKNOWLEDGMENTS
The authors would like to thank Sophie Bishop, our information specialist, for developing and implementing the search strategy. This work was funded by the NIHR Manchester Biomedical Research Centre (BRC) (IS‐BRC‐1215‐20007). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health.
Harvey J, Mellody KT, Cullum N, Watson REB, Dumville J. Wound fluid sampling methods for proteomic studies: A scoping review. Wound Rep Reg. 2022;30(3):317‐333. doi: 10.1111/wrr.13009
Funding information NIHR Manchester Biomedical Research Centre, Grant/Award Number: IS‐BRC‐1215‐20007
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article
REFERENCES
- 1. Lazarus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol. 1994;130(4):489‐493. [PubMed] [Google Scholar]
- 2. Guest JF, Fuller GW, Vowden P. Venous leg ulcer management in clinical practice in the UK: costs and outcomes. Int Wound J. 2018;15(1):29‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kerr M, Barron E, Chadwick P, et al. The cost of diabetic foot ulcers and amputations to the National Health Service in England. Diabet Med. 2019;36(8):995‐1002. [DOI] [PubMed] [Google Scholar]
- 4. Dealey C, Posnett J, Walker A. The cost of pressure ulcers in the United Kingdom. J Wound Care. 2012;21(6):261‐266. [DOI] [PubMed] [Google Scholar]
- 5. Patel S, Maheshwari A, Chandra A. Biomarkers for wound healing and their evaluation. J Wound Care. 2016;25(1):46‐55. [DOI] [PubMed] [Google Scholar]
- 6. Magni F, Van Der Burgt YE, Chinello C, et al. Biomarkers discovery by peptide and protein profiling in biological fluids based on functionalized magnetic beads purification and mass spectrometry. Blood Transfus. 2010;8(Suppl 3):s92‐s97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parker TJ, Sampson DL, Broszczak D, et al. A fragment of the LG3 peptide of Endorepellin is present in the urine of physically active mining workers: a potential marker of physical activity. PLoS One. 2012;7(3):e33714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Edsberg LE, Wyffels JT, Brogan MS, Fries KM. Analysis of the proteomic profile of chronic pressure ulcers. Wound Repair Regen. 2012;20(3):378‐401. [DOI] [PubMed] [Google Scholar]
- 9. Berberich B, Thriene K, Gretzmeier C, et al. Proteomic profiling of fibroblasts isolated from chronic wounds identifies disease‐relevant signaling pathways. J Invest Dermatol. 2020;140(11):2280‐90.e4. [DOI] [PubMed] [Google Scholar]
- 10. Pleat JM, Hebestreit HF, Dwek RA. Identifying protein production in wound healing: current techniques. J Wound Care. 2002;11(2):63‐66. [DOI] [PubMed] [Google Scholar]
- 11. Staiano‐Coico L, Higgins PJ, Schwartz SB, Zimm AJ, Goncalves J. Wound fluids: a reflection of the state of healing. Ostomy Wound Manage. 2000;46((1A Suppl)):85S‐93S. quiz 4S‐5S. [PubMed] [Google Scholar]
- 12. Verma M, Patel P, Verma M. Biomarkers in prostate cancer epidemiology. Cancer. 2011;3:3773‐3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lindley LE, Stojadinovic O, Pastar I, Tomic‐Canic M. Biology and biomarkers for wound healing. Plast Reconstr Surg. 2016;138(3 Suppl):18S‐28S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ramsay S, Cowan L, Davidson JM, Nanney L, Schultz G. Wound samples: moving towards a standardised method of collection and analysis. Int Wound J. 2016;13(5):880‐891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA‐ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467‐473. [DOI] [PubMed] [Google Scholar]
- 16. Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
- 17. Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19‐32. [Google Scholar]
- 18. Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rasmussen LH, Jensen LT, Avnstorp C, Karlsmark T, Peters K, Hørslev‐Petersen K. Collagen types I and III propeptides as markers of healing in chronic leg ulcers. A noninvasive method for the determination of procollagen propeptides in wound fluid–influence of growth hormone. Ann Surg. 1992;216(6):684‐691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van den Broek LJ, Kroeze KL, Waaijman T, et al. Differential response of human adipose tissue‐derived mesenchymal stem cells, dermal fibroblasts, and keratinocytes to burn wound exudates: potential role of skin‐specific chemokine CCL27. Tissue Eng Part A. 2014;20(1–2):197‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carvalho B, Aleshi P, Horstman DJ, Angst MS. Effect of a preemptive femoral nerve block on cytokine release and hyperalgesia in experimentally inflamed skin of human volunteers. Reg Anesth Pain Med. 2010;35(6):514‐519. [DOI] [PubMed] [Google Scholar]
- 22. Wysocki AB. Wound fluids and the pathogenesis of chronic wounds. J Wound Ostomy Continence Nurs. 1996;23(6):283‐290. [DOI] [PubMed] [Google Scholar]
- 23. Hasmann A, Wehrschuetz‐Sigl E, Marold A, et al. Analysis of myeloperoxidase activity in wound fluids as a marker of infection. Ann Clin Biochem. 2013;50(3):245‐254. [DOI] [PubMed] [Google Scholar]
- 24. Grinnell F, Ho CH, Wysocki A. Degradation of fibronectin and vitronectin in chronic wound fluid: analysis by cell blotting, immunoblotting, and cell adhesion assays. J Invest Dermatol. 1992;98(4):410‐416. [DOI] [PubMed] [Google Scholar]
- 25. Cowin AJ, Hatzirodos N, Rigden J, Fitridge R, Belford DA. Etanercept decreases tumor necrosis factor‐alpha activity in chronic wound fluid. Wound Repair Regen. 2006;14(4):421‐426. [DOI] [PubMed] [Google Scholar]
- 26. Hasmann A, Gewessler U, Hulla E, et al. Sensor materials for the detection of human neutrophil elastase and cathepsin G activity in wound fluid. Exp Dermatol. 2011;20(6):508‐513. [DOI] [PubMed] [Google Scholar]
- 27. Wysocki AB, Staiano‐Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP‐2 and MMP‐9. J Invest Dermatol. 1993;101(1):64‐68. [DOI] [PubMed] [Google Scholar]
- 28. Chromy BA, Eldridge A, Forsberg JA, et al. Wound outcome in combat injuries is associated with a unique set of protein biomarkers. J Transl Med. 2013;11(1):281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yager DR, Chen SM, Ward SI, Olutoye OO, Diegelmann RF, Kelman CI. Ability of chronic wound fluids to degrade peptide growth factors is associated with increased levels of elastase activity and diminished levels of proteinase inhibitors. Wound Repair Regen. 1997;5(1):23‐32. [DOI] [PubMed] [Google Scholar]
- 30. Wicke C, Wagner S, Trabold O, et al. Age‐dependency of insulin‐like growth factors, insulin‐like growth factor–binding proteins, and acid labile subunit in plasma and wounds of surgical patients. Wound Repair Regen. 2002;10(6):360‐365. [DOI] [PubMed] [Google Scholar]
- 31. Petrlova J, Hansen FC, van der Plas MJA, et al. Aggregation of thrombin‐derived C‐terminal fragments as a previously undisclosed host defense mechanism. Proc Natl Acad Sci. 2017;114(21):E4213‐E4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haukipuro K, Risteli L, Kairaluoma MI, Risteli J. Aminoterminal propeptide of type III procollagen in healing wound in humans. Ann Surg. 1987;206(6):752‐756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Trengove NJ, Stacey MC, Macauley S, et al. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen. 1999;7(6):442‐452. [DOI] [PubMed] [Google Scholar]
- 34. Lassig AAD, Joseph AM, Lindgren BR, Yueh B. Association of oral cavity and oropharyngeal cancer biomarkers in surgical drain fluid with patient outcomes. JAMA Otolaryngol Head Neck Surg. 2017;143(7):670‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lisboa FA, Forsberg JA, Brown TS, Gage FA, Potter BK, Elster EA. Bilateral lower‐extremity amputation wounds are associated with distinct local and systemic cytokine response. Surgery. 2013;154(2):282‐290. [DOI] [PubMed] [Google Scholar]
- 36. Tarnuzzer RW, Schultz GS. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen. 1996;4(3):321‐325. [DOI] [PubMed] [Google Scholar]
- 37. Hardwicke J, Moseley R, Stephens P, Harding K, Duncan R, Thomas DW. Bioresponsive dextrin‐rhEGF conjugates: in vitro evaluation in models relevant to its proposed use as a treatment for chronic wounds. Mol Pharm. 2010;7(3):699‐707. [DOI] [PubMed] [Google Scholar]
- 38. Powerski MJ, Henrich D, Sander A, Wastl D, Ludwig K, Marzi I. CD133+CD34+ stem cells are mobilized after musculoskeletal surgery and target endothelium activated by surgical wound fluid. Langenbecks Arch Surg. 2011;396(3):379‐387. [DOI] [PubMed] [Google Scholar]
- 39. Grimstad Ø, Sandanger Ø, Ryan L, et al. Cellular sources and inducers of cytokines present in acute wound fluid. Wound Repair Regen. 2011;19(3):337‐347. [DOI] [PubMed] [Google Scholar]
- 40. Aiba‐Kojima E, Tsuno NH, Inoue K, et al. Characterization of wound drainage fluids as a source of soluble factors associated with wound healing: comparison with platelet‐rich plasma and potential use in cell culture. Wound Repair Regen. 2007;15(4):511‐520. [DOI] [PubMed] [Google Scholar]
- 41. Serra R, Buffone G, Falcone D, et al. Chronic venous leg ulcers are associated with high levels of metalloproteinases‐9 and neutrophil gelatinase‐associated lipocalin. Wound Repair Regen. 2013;21(3):395‐401. [DOI] [PubMed] [Google Scholar]
- 42. Ihlberg L, Haukipuro K, Risteli L, Oikarinen A, Kairaluoma MI, Risteli J. Collagen synthesis in intact skin is suppressed during wound healing. Ann Surg. 1993;217(4):397‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grinnell F, Zhu M, Parks WC. Collagenase‐1 complexes with alpha2‐macroglobulin in the acute and chronic wound environments. J Invest Dermatol. 1998;110(5):771‐776. [DOI] [PubMed] [Google Scholar]
- 44. Kulcenty K, Piotrowski I, Wróblewska JP, Wasiewicz J, Suchorska AWM. The composition of surgical wound fluids from breast cancer patients is affected by intraoperative radiotherapy treatment and depends on the molecular subtype of breast cancer. Cancers (Basel). 2019;12(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carvalho B, Clark DJ, Yeomans DC, Angst MS. Continuous subcutaneous instillation of bupivacaine compared to saline reduces interleukin 10 and increases substance P in surgical wounds after cesarean delivery. Anesth Analg. 2010;111(6):1452‐1459. [DOI] [PubMed] [Google Scholar]
- 46. Pajulo OT, Pulkki KJ, Alanen MS, et al. Correlation between interleukin‐6 and matrix metalloproteinase‐9 in early wound healing in children. Wound Repair Regen. 1999;7(6):453‐457. [DOI] [PubMed] [Google Scholar]
- 47. Sexton KW, Spear M, Pollins AC, et al. The correlation of matrix metalloproteinase 9‐to‐albumin ratio in wound fluid with postsurgical complications after body contouring. Plast Reconstr Surg. 2014;134(4):530e‐538e. [DOI] [PubMed] [Google Scholar]
- 48. Di Vita G, Patti R, D'Agostino P, et al. Cytokines and growth factors in wound drainage fluid from patients undergoing incisional hernia repair. Wound Repair Regen. 2006;14(3):259‐264. [DOI] [PubMed] [Google Scholar]
- 49. Picardo M, Grey AM, McGurk M, Ellis I, Schor SL. Detection of migration stimulating activity in wound fluid. Exp Mol Pathol. 1992;57(1):8‐21. [DOI] [PubMed] [Google Scholar]
- 50. Grinnell F, Baxter CR, Zhu M, Yin HL. Detection of the Actin scavenger system in burn wound fluid. Wound Repair Regen. 1993;1(4):236‐243. [DOI] [PubMed] [Google Scholar]
- 51. Vogt PM, Lehnhardt M, Wagner D, Jansen V, Krieg M, Steinau HU. Determination of endogenous growth factors in human wound fluid: temporal presence and profiles of secretion. Plast Reconstr Surg. 1998;102(1):117‐123. [DOI] [PubMed] [Google Scholar]
- 52. Bodendorf S, Born G, Hannappel E. Determination of thymosin beta4 and protein in human wound fluid after abdominal surgery. Ann N Y Acad Sci. 2007;1112:418‐424. [DOI] [PubMed] [Google Scholar]
- 53. Nissen NN, Gamelli RL, Polverini PJ, DiPietro LA. Differential angiogenic and proliferative activity of surgical and burn wound fluids. J Trauma Acute Care Surg. 2003;54(6):1205‐1210. [DOI] [PubMed] [Google Scholar]
- 54. Prager MD, Sabeh F, Baxter CR, Atiles L, Hartline B. Dipeptidyl peptidase IV and aminopeptidase in burn wound exudates: implications for wound healing. J Trauma. 1994;36(5):629‐633. [DOI] [PubMed] [Google Scholar]
- 55. Meesters A, den Bosch‐Meevissen Y, Weijzen CAH, et al. The effect of mindfulness‐based stress reduction on wound healing: a preliminary study. J Behav Med. 2018;41(3):385‐397. [DOI] [PubMed] [Google Scholar]
- 56. Scherzed A, Hackenberg S, Froelich K, et al. The effect of wound fluid on adipose‐derived stem cells in vitro: a study in human cell materials. Tissue Eng Part C Methods. 2011;17(8):809‐817. [DOI] [PubMed] [Google Scholar]
- 57. Rohde C, Chiang A, Adipoju O, Casper D, Pilla AA. Effects of pulsed electromagnetic fields on interleukin‐1 beta and postoperative pain: a double‐blind, placebo‐controlled, pilot study in breast reduction patients. Plast Reconstr Surg. 2010;125(6):1620‐1629. [DOI] [PubMed] [Google Scholar]
- 58. Bridges M Jr, Morris D, Hall JR, Deitch EA. Effects of wound exudates on in vitro immune parameters. J Surg Res. 1987;43(2):133‐138. [DOI] [PubMed] [Google Scholar]
- 59. Prager MD, Herring M, Germany B, Baxter CR. Elastase and alpha 1‐protease inhibitor in burn wound exudates. J Burn Care Rehabil. 1991;12(4):300‐305. [DOI] [PubMed] [Google Scholar]
- 60. Awad H, Abas M, Elgharably H, et al. Endogenous opioids in wound‐site neutrophils of sternotomy patients. PLoS One. 2012;7(10):e47569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gallo RL, Dorschner RA, Takashima S, Klagsbrun M, Eriksson E, Bernfield M. Endothelial cell surface alkaline phosphatase activity is induced by IL‐6 released during wound repair. J Invest Dermatol. 1997;109(4):597‐603. [DOI] [PubMed] [Google Scholar]
- 62. James TJ, Hughes MA, Cherry GW, Taylor RP. Evidence of oxidative stress in chronic venous ulcers. Wound Repair Regen. 2003;11(3):172‐176. [DOI] [PubMed] [Google Scholar]
- 63. Lassig AAD, Lindgren BR, Itabiyi R, Joseph AM, Gupta K. Excessive inflammation portends complications: wound cytokines and head and neck surgery outcomes. Laryngoscope. 2019;129(7):E238‐e46. [DOI] [PubMed] [Google Scholar]
- 64. Lauer G, Sollberg S, Cole M, et al. Expression and proteolysis of vascular endothelial growth factor is increased in chronic wounds. J Invest Dermatol. 2000;115(1):12‐18. [DOI] [PubMed] [Google Scholar]
- 65. Pallua N, Ulrich D. Expression of basic fibroblast growth factor and transforming growth factor‐Beta 1 in patients with fasciocutaneous and muscle flaps. Plast Reconstr Surg. 2003;111(1):79‐82. discussion 3–4. [DOI] [PubMed] [Google Scholar]
- 66. Grinnell F, Zhu M. Fibronectin degradation in chronic wounds depends on the relative levels of elastase, alpha1‐proteinase inhibitor, and alpha2‐macroglobulin. J Invest Dermatol. 1996;106(2):335‐341. [DOI] [PubMed] [Google Scholar]
- 67. Jafari P, Muller C, Grognuz A, et al. First insights into human fingertip regeneration by Echo‐Doppler imaging and wound microenvironment assessment. Int J Mol Sci. 2017;18(5):1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nuutila K, Siltanen A, Peura M, et al. Gene expression profiling of negative‐pressure‐treated skin graft donor site wounds. Burns. 2013;39(4):687‐693. [DOI] [PubMed] [Google Scholar]
- 69. Ure I, Partsch B, Wolff K, Petzelbauer P. Granulocyte/macrophage colony‐stimulating factor increases wound‐fluid interleukin 8 in normal subjects but does not accelerate wound healing. Br J Dermatol. 1998;138(2):277‐282. [DOI] [PubMed] [Google Scholar]
- 70. Dalhoff A, Frank G, Luckhaus G. The granuloma pouch: an in vivo model for pharmacokinetic and chemotherapeutic investigations I biochemical and histological characterization. Infection. 1982;10(6):354‐360. [DOI] [PubMed] [Google Scholar]
- 71. Baker EA, El Gaddal S, Aitken DG, Leaper DJ. Growth factor profiles in intraperitoneal drainage fluid following colorectal surgery: relationship to wound healing and surgery. Wound Repair Regen. 2003;11(4):261‐267. [DOI] [PubMed] [Google Scholar]
- 72. Lundqvist K, Herwald H, Sonesson A, Schmidtchen A. Heparin binding protein is increased in chronic leg ulcer fluid and released from granulocytes by secreted products of Pseudomonas aeruginosa. Thromb Haemost. 2004;92(2):281‐287. [DOI] [PubMed] [Google Scholar]
- 73. Wang D, Hu K, Gao N, et al. High throughput screening of cytokines, chemokines and matrix metalloproteinases in wound fluid induced by mammary surgery. Oncotarget. 2015;6(30):29296‐29310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Akinci B, Terzi C, Sevindik G, et al. Hyperglycemia is associated with lower levels of urokinase‐type plasminogen activator and urokinase‐type plasminogen activator receptor in wound fluid. J Diabetes Complications. 2014;28(6):844‐849. [DOI] [PubMed] [Google Scholar]
- 75. Grinnell F, Zhu M. Identification of neutrophil elastase as the proteinase in burn wound fluid responsible for degradation of fibronectin. J Invest Dermatol. 1994;103(2):155‐161. [DOI] [PubMed] [Google Scholar]
- 76. He Y, Young PK, Grinnell F. Identification of proteinase 3 as the major caseinolytic activity in acute human wound fluid. J Invest Dermatol. 1998;110(1):67‐71. [DOI] [PubMed] [Google Scholar]
- 77. Drinkwater SL, Burnand KG, Ding R, Smith A. Increased but ineffectual angiogenic drive in nonhealing venous leg ulcers. J Vasc Surg. 2003;38(5):1106‐1112. [DOI] [PubMed] [Google Scholar]
- 78. Leiblein M, Ponelies N, Johnson T, et al. Increased extracellular ubiquitin in surgical wound fluid provides a chemotactic signal for myeloid dendritic cells. Eur J Trauma Emerg Surg. 2020;46(1):153‐163. [DOI] [PubMed] [Google Scholar]
- 79. Wiik HT, Kaarteenaho‐Wiik RL, Mertaniemi PE, Risteli JP, Syrjälä HP, Haukipuro KA. Increased postoperative concentrations of tenascin‐C in wound fluid. J Trauma. 2004;56(4):901‐905. [DOI] [PubMed] [Google Scholar]
- 80. Subramaniam K, Pech CM, Stacey MC, Wallace HJ. Induction of MMP‐1, MMP‐3 and TIMP‐1 in normal dermal fibroblasts by chronic venous leg ulcer wound fluid*. Int Wound J. 2008;5(1):79‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Debats IB, Booi D, Deutz NE, Buurman WA, Boeckx WD, van der Hulst RR. Infected chronic wounds show different local and systemic arginine conversion compared with acute wounds. J Surg Res. 2006;134(2):205‐214. [DOI] [PubMed] [Google Scholar]
- 82. Hawksworth JS, Stojadinovic A, Gage FA, et al. Inflammatory biomarkers in combat wound healing. Ann Surg. 2009;250(6):1002‐1007. [DOI] [PubMed] [Google Scholar]
- 83. Evans KN, Forsberg JA, Potter BK, et al. Inflammatory cytokine and chemokine expression is associated with heterotopic ossification in high‐energy penetrating war injuries. J Orthop Trauma. 2012;26(11):e204‐e213. [DOI] [PubMed] [Google Scholar]
- 84. Brown TS, Hawksworth JS, Sheppard FR, Tadaki DK, Elster E. Inflammatory response is associated with critical colonization in combat wounds. Surg Infect (Larchmt). 2011;12(5):351‐357. [DOI] [PubMed] [Google Scholar]
- 85. Schönfelder U, Abel M, Wiegand C, Klemm D, Elsner P, Hipler UC. Influence of selected wound dressings on PMN elastase in chronic wound fluid and their antioxidative potential in vitro. Biomaterials. 2005;26(33):6664‐6673. [DOI] [PubMed] [Google Scholar]
- 86. Barone EJ, Yager DR, Pozez AL, et al. Interleukin‐1alpha and collagenase activity are elevated in chronic wounds. Plast Reconstr Surg. 1998;102(4):1023‐1027. discussion 8‐9. [PubMed] [Google Scholar]
- 87. Mateo RB, Reichner JS, Albina JE. Interleukin‐6 activity in wounds. Am J Physiol. 1994;266(6 Pt 2):R1840‐R1844. [DOI] [PubMed] [Google Scholar]
- 88. Yeoh‐Ellerton S, Stacey MC. Iron and 8‐isoprostane levels in acute and chronic wounds. J Invest Dermatol. 2003;121(4):918‐925. [DOI] [PubMed] [Google Scholar]
- 89. Grieb G, Simons D, Eckert L, et al. Levels of macrophage migration inhibitory factor and glucocorticoids in chronic wound patients and their potential interactions with impaired wound endothelial progenitor cell migration. Wound Repair Regen. 2012;20(5):707‐714. [DOI] [PubMed] [Google Scholar]
- 90. Holzheimer RG, Steinmetz W. Local and systemic concentrations of pro‐ and anti‐inflammatory cytokines in human wounds. Eur J Med Res. 2000;5(8):347‐355. [PubMed] [Google Scholar]
- 91. Hourigan LA, Hourigan L, Linfoot JA, et al. Loss of protein, immunoglobulins, and electrolytes in exudates from negative pressure wound therapy. Nutr Clin Pract. 2010;25(5):510‐516. [DOI] [PubMed] [Google Scholar]
- 92. Agren MS, Jorgensen LN, Andersen M, Viljanto J, Gottrup F. Matrix metalloproteinase 9 level predicts optimal collagen deposition during early wound repair in humans. Br J Surg. 1998;85(1):68‐71. [DOI] [PubMed] [Google Scholar]
- 93. Young PK, Grinnell F. Metalloproteinase activation cascade after burn injury: a longitudinal analysis of the human wound environment. J Invest Dermatol. 1994;103(5):660‐664. [DOI] [PubMed] [Google Scholar]
- 94. Utz ER, Elster EA, Tadaki DK, et al. Metalloproteinase expression is associated with traumatic wound failure. J Surg Res. 2010;159(2):633‐639. [DOI] [PubMed] [Google Scholar]
- 95. Nwomeh BC, Liang HX, Cohen IK, Yager DR. MMP‐8 is the predominant collagenase in healing wounds and nonhealing ulcers. J Surg Res. 1999;81(2):189‐195. [DOI] [PubMed] [Google Scholar]
- 96. Di Vita G, Patti R, D'Agostino P, et al. Modifications in the production of cytokines and growth factors in drainage fluids following mesh implantation after incisional hernia repair. Am J Surg. 2006;191(6):785‐790. [DOI] [PubMed] [Google Scholar]
- 97. Edwards JV, Yager DR, Cohen IK, et al. Modified cotton gauze dressings that selectively absorb neutrophil elastase activity in solution. Wound Repair Regen. 2001;9(1):50‐58. [DOI] [PubMed] [Google Scholar]
- 98. Starlinger P, Alidzanovic L, Schauer D, et al. Neoadjuvant bevacizumab persistently inactivates VEGF at the time of surgery despite preoperative cessation. Br J Cancer. 2012;107(6):961‐966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hasmann A, Wehrschuetz‐Sigl E, Kanzler G, et al. Novel peptidoglycan‐based diagnostic devices for detection of wound infection. Diagn Microbiol Infect Dis. 2011;71(1):12‐23. [DOI] [PubMed] [Google Scholar]
- 100. Flohé SB, Bangen JM, Flohé S, Agrawal H, Bergmann K, Schade FU. Origin of immunomodulation after soft tissue trauma: potential involvement of extracellular heat‐shock proteins. Shock. 2007;27(5):494‐502. [DOI] [PubMed] [Google Scholar]
- 101. Evans KN, Potter BK, Brown TS, Davis TA, Elster EA, Forsberg JA. Osteogenic gene expression correlates with development of heterotopic ossification in war wounds. Clin Orthop Relat Res. 2014;472(2):396‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Segatto I, Berton S, Sonego M, et al. p70S6 kinase mediates breast cancer cell survival in response to surgical wound fluid stimulation. Mol Oncol. 2014;8(3):766‐780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lucas VS, McCain N, Elswick RK, Pozez AL. Perceived stress and surgical wound cytokine patterns. Plast Surg Nurs. 2018;38(2):55‐72. [DOI] [PubMed] [Google Scholar]
- 104. Zhang H, Lu J, Wu Q, et al. A perioperative small dose of dexamethasone enhances postoperative recovery by reducing volume and inflammatory contents in wound drainage after thyroid surgery: a double‐blinded, randomized prospective study. World J Surg. 2019;43(7):1721‐1727. [DOI] [PubMed] [Google Scholar]
- 105. Andres BM, Taub DD, Gurkan I, Wenz JF. Postoperative fever after total knee arthroplasty: the role of cytokines. Clin Orthop Relat Res. 2003;415:221‐231. [DOI] [PubMed] [Google Scholar]
- 106. Carvalho B, Lemmens HJ, Ting V, Angst MS. Postoperative subcutaneous instillation of low‐dose ketorolac but not hydromorphone reduces wound exudate concentrations of interleukin‐6 and interleukin‐10 and improves analgesia following cesarean delivery. J Pain. 2013;14(1):48‐56. [DOI] [PubMed] [Google Scholar]
- 107. Tarlton JF, Vickery CJ, Leaper DJ, Bailey AJ. Postsurgical wound progression monitored by temporal changes in the expression of matrix metalloproteinase‐9. Br J Dermatol. 1997;137(4):506‐516. [DOI] [PubMed] [Google Scholar]
- 108. Baker EA, El‐Gaddal S, Williams L, Leaper DJ. Profiles of inflammatory cytokines following colorectal surgery: relationship with wound healing and outcome. Wound Repair Regen. 2006;14(5):566‐572. [DOI] [PubMed] [Google Scholar]
- 109. Baker EA, Leaper DJ. Profiles of matrix metalloproteinases and their tissue inhibitors in intraperitoneal drainage fluid: relationship to wound healing. Wound Repair Regen. 2003;11(4):268‐274. [DOI] [PubMed] [Google Scholar]
- 110. Liu T, Yang F, Li Z, Yi C, Bai X. A prospective pilot study to evaluate wound outcomes and levels of serum C‐reactive protein and interleukin‐6 in the wound fluid of patients with trauma‐related chronic wounds. Ostomy Wound Manage. 2014;60(6):30‐37. [PubMed] [Google Scholar]
- 111. Schmidtchen A, Frick IM, Andersson E, Tapper H, Björck L. Proteinases of common pathogenic bacteria degrade and inactivate the antibacterial peptide LL‐37. Mol Microbiol. 2002;46(1):157‐168. [DOI] [PubMed] [Google Scholar]
- 112. Baker EA, Leaper DJ. Proteinases, their inhibitors, and cytokine profiles in acute wound fluid. Wound Repair Regen. 2000;8(5):392‐398. [DOI] [PubMed] [Google Scholar]
- 113. Prager MD, Baxter CR, Hartline B. Proteolytic activity in burn wound exudates and comparison of fibrin degradation products and protease inhibitors in exudates and sera. J Burn Care Rehabil. 1994;15(2):130‐136. [DOI] [PubMed] [Google Scholar]
- 114. Saravanan R, Adav SS, Choong YK, et al. Proteolytic signatures define unique thrombin‐derived peptides present in human wound fluid in vivo. Sci Rep. 2017;7(1):13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Chromy BA, Eldridge A, Forsberg JA, et al. Proteomic sample preparation for blast wound characterization. Proteome Sci. 2014;12(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Werthén M, Davoudi M, Sonesson A, et al. Pseudomonas aeruginosa‐induced infection and degradation of human wound fluid and skin proteins ex vivo are eradicated by a synthetic cationic polymer. J Antimicrob Chemother. 2004;54(4):772‐779. [DOI] [PubMed] [Google Scholar]
- 117. Broadbent E, Petrie KJ, Alley PG, Booth RJ. Psychological stress impairs early wound repair following surgery. Psychosom Med. 2003;65(5):865‐869. [DOI] [PubMed] [Google Scholar]
- 118. Rohde CH, Taylor EM, Alonso A, Ascherman JA, Hardy KL, Pilla AA. Pulsed electromagnetic fields reduce postoperative interleukin‐1β, pain, and inflammation: a double‐blind, placebo‐controlled study in TRAM flap breast reconstruction patients. Plast Reconstr Surg. 2015;135(5):808e‐817e. [DOI] [PubMed] [Google Scholar]
- 119. Lehnhardt M, Jafari HJ, Druecke D, et al. A qualitative and quantitative analysis of protein loss in human burn wounds. Burns. 2005;31(2):159‐167. [DOI] [PubMed] [Google Scholar]
- 120. Cerveró‐Ferragut S, López‐Riquelme N, Martín‐Tomás E, et al. Quantitative analysis of blood cells and inflammatory factors in wounds. J Wound Care. 2017;26(3):121‐125. [DOI] [PubMed] [Google Scholar]
- 121. Koschwanez H, Robinson H, Beban G, et al. Randomized clinical trial of expressive writing on wound healing following bariatric surgery. Health Psychol. 2017;36(7):630‐640. [DOI] [PubMed] [Google Scholar]
- 122. Zhu ZY, Xue JX, Yu LX, et al. Reducing postsurgical exudate in breast cancer patients by using san Huang decoction to ameliorate inflammatory status: a prospective clinical trial. Curr Oncol. 2018;25(6):e507‐e515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Subramanian SV, Fitzgerald ML, Bernfield M. Regulated shedding of Syndecan‐1 and ‐4 Ectodomains by thrombin and growth factor receptor activation *. J Biol Chem. 1997;272(23):14713‐14720. [DOI] [PubMed] [Google Scholar]
- 124. Hormbrey E, Han C, Roberts A, McGrouther DA, Harris AL. The relationship of human wound vascular endothelial growth factor (VEGF) after breast cancer surgery to circulating VEGF and angiogenesis. Clin Cancer Res. 2003;9(12):4332‐4339. [PubMed] [Google Scholar]
- 125. Arai M, Ogita‐Nakanishi H, Lee K, et al. Role of cytokines in lavage or drainage fluid after hemithyroidectomy in wound healing: involvement of histamine in the acceleration and delay of wound healing. Wound Repair Regen. 2012;20(2):158‐165. [DOI] [PubMed] [Google Scholar]
- 126. Tokunaga A, Onda M, Fujita I, et al. Sequential changes in the cell mediators of peritoneal and wound fluids after surgery. Surg Today. 1993;23(9):841‐844. [DOI] [PubMed] [Google Scholar]
- 127. Karayiannakis AJ, Zbar A, Polychronidis A, Simopoulos C. Serum and drainage fluid vascular endothelial growth factor levels in early surgical wounds. Eur Surg Res. 2003;35(6):492‐496. [DOI] [PubMed] [Google Scholar]
- 128. Lisowska B, Siewruk K, Lisowski A. Substance P and acute pain in patients undergoing orthopedic surgery. Plos One. 2016;11(1):e0146400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Schmohl M, Beckert S, Joos TO, Königsrainer A, Schneiderhan‐Marra N, Löffler MW. Superficial wound swabbing: a novel method of sampling and processing wound fluid for subsequent immunoassay analysis in diabetic foot ulcerations. Diabetes Care. 2012;35(11):2113‐2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Segatto I, Berton S, Sonego M, et al. Surgery‐induced wound response promotes stem‐like and tumor‐initiating features of breast cancer cells, via STAT3 signaling. Oncotarget. 2014;5(15):6267‐6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kainulainen V, Wang H, Schick C, Bernfield M. Syndecans, heparan sulfate proteoglycans, maintain the proteolytic balance of acute wound fluids. J Biol Chem. 1998;273(19):11563‐11569. [DOI] [PubMed] [Google Scholar]
- 132. Haukipuro K. Synthesis of collagen types I and III in reincised wounds in humans. Br J Surg. 1991;78(6):708‐712. [DOI] [PubMed] [Google Scholar]
- 133. Haukipuro K, Melkko J, Risteli L, Kairaluoma M, Risteli J. Synthesis of type I collagen in healing wounds in humans. Ann Surg. 1991;213(1):75‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Wu FP, Hoekman K, Sietses C, et al. Systemic and peritoneal angiogenic response after laparoscopic or conventional colon resection in cancer patients: a prospective, randomized trial. Dis Colon Rectum. 2004;47(10):1670‐1674. [DOI] [PubMed] [Google Scholar]
- 135. Baker EA, Kumar S, Melling AC, Whetter D, Leaper DJ. Temporal and quantitative profiles of growth factors and metalloproteinases in acute wound fluid after mastectomy. Wound Repair Regen. 2008;16(1):95‐101. [DOI] [PubMed] [Google Scholar]
- 136. Wysocki AB, Kusakabe AO, Chang S, Tuan TL. Temporal expression of urokinase plasminogen activator, plasminogen activator inhibitor and gelatinase‐B in chronic wound fluid switches from a chronic to acute wound profile with progression to healing. Wound Repair Regen. 1999;7(3):154‐165. [DOI] [PubMed] [Google Scholar]
- 137. Scherer SD, Bauer J, Schmaus A, et al. TGF‐β1 is present at high levels in wound fluid from breast cancer patients immediately post‐surgery, and is not increased by intraoperative radiation therapy (IORT). PLoS One. 2016;11(9):e0162221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Bullen EC, Longaker MT, Updike DL, et al. Tissue inhibitor of metalloproteinases‐1 is decreased and activated gelatinases are increased in chronic wounds. J Invest Dermatol. 1995;104(2):236‐240. [DOI] [PubMed] [Google Scholar]
- 139. Lassig AAD, Bechtold JE, Lindgren BR, et al. Tobacco exposure and wound healing in head and neck surgical wounds. Laryngoscope. 2018;128(3):618‐625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Matsuoka J, Grotendorst GR. Two peptides related to platelet‐derived growth factor are present in human wound fluid. Proc Natl Acad Sci U S A. 1989;86(12):4416‐4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol. 1998;152(6):1445‐1452. [PMC free article] [PubMed] [Google Scholar]
- 142. Wu FP, Hoekman K, Meijer S, Cuesta MA. VEGF and endostatin levels in wound fluid and plasma after breast surgery. Angiogenesis. 2003;6(4):255‐257. [DOI] [PubMed] [Google Scholar]
- 143. Scherzad A, Gehrke T, Meyer T, et al. Wound fluid enhances cancer cell proliferation via activation of STAT3 signal pathway in vitro. Oncol Rep. 2019;41(5):2919‐2926. [DOI] [PubMed] [Google Scholar]
- 144. Hoffman R, Starkey S, Coad J. Wound fluid from venous leg ulcers degrades plasminogen and reduces plasmin generation by keratinocytes. J Invest Dermatol. 1998;111(6):1140‐1144. [DOI] [PubMed] [Google Scholar]
- 145. Yager DR, Zhang LY, Liang HX, Diegelmann RF, Cohen IK. Wound fluids from human pressure ulcers contain elevated matrix metalloproteinase levels and activity compared to surgical wound fluids. J Invest Dermatol. 1996;107(5):743‐748. [DOI] [PubMed] [Google Scholar]
- 146. Rao CN, Ladin DA, Chilukuri K, Woodley DT, Liu YY, Hou ZZ. α1‐antitrypsin is degraded and non‐functional in chronic wounds but intact and functional in acute wounds: the inhibitor protects fibronectin from degradation by chronic wound fluid enzymes. J Invest Dermatol. 1995;105(4):572‐578. [DOI] [PubMed] [Google Scholar]
- 147. Mikhal'chik EV, Piterskaya JA, Lipatova VA, Pen'kov LY, Ibragimova GA, Korkina LG. Activity of antioxidant enzymes in the wound in patients with deep burns. Bull Exp Biol Med. 2009;147(6):753‐756. [DOI] [PubMed] [Google Scholar]
- 148. Wyffels JT, Fries KM, Randall JS, et al. Analysis of pressure ulcer wound fluid using two‐dimensional electrophoresis. Int Wound J. 2010;7(4):236‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Morelli T, Neiva R, Nevins ML, et al. Angiogenic biomarkers and healing of living cellular constructs. J Dent Res. 2011;90(4):456‐462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Asadi MR, Torkaman G, Hedayati M, Mohajeri‐Tehrani MR, Ahmadi M, Gohardani RF. Angiogenic effects of low‐intensity cathodal direct current on ischemic diabetic foot ulcers: a randomized controlled trial. Diabetes Res Clin Pract. 2017;127:147‐155. [DOI] [PubMed] [Google Scholar]
- 151. Kitamura A, Minematsu T, Nakagami G, et al. Assessing subclinical inflammation by peroxidase detection in patients with pressure ulcers. J Wound Care. 2019;28(9):586‐591. [DOI] [PubMed] [Google Scholar]
- 152. Tamai N, Akase T, Minematsu T, et al. Association between components of exudates and periwound moisture‐associated dermatitis in breast cancer patients with malignant Fungating wounds. Biol Res Nurs. 2016;18(2):199‐206. [DOI] [PubMed] [Google Scholar]
- 153. Fazekas R, Molnár B, Kőhidai L, et al. Blood flow kinetics of a xenogeneic collagen matrix following a vestibuloplasty procedure in the human gingiva—an explorative study. Oral Dis. 2019;25(7):1780‐1788. [DOI] [PubMed] [Google Scholar]
- 154. Krisp C, Kubutat C, Kyas A, Steinsträßer L, Jacobsen F, Wolters D. Boric acid gel enrichment of glycosylated proteins in human wound fluids. J Proteomics. 2011;74(4):502‐509. [DOI] [PubMed] [Google Scholar]
- 155. Goto T, Tamai N, Nakagami G, et al. Can wound exudate from venous leg ulcers measure wound pain status?: a pilot study. PLoS One. 2016;11(12):e0167478‐e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Dellavia C, Canciani E, Rasperini G, Pagni G, Malvezzi M, Pellegrini G. CEMP‐1 levels in periodontal wound fluid during the early phase of healing: prospective clinical trial. Mediators Inflamm. 2019;2019:1737306‐1737308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Hodde JP, Hiles MC, Metzger DW. Characterization of the local wound environment following treatment of chronic leg ulcers with SIS wound matrix. J Tissue Viability. 2020;29(1):42‐47. [DOI] [PubMed] [Google Scholar]
- 158. Fivenson DP, Faria DT, Nickoloff BJ, et al. Chemokine and inflammatory cytokine changes during chronic wound healing. Wound Repair Regen. 1997;5(4):310‐322. [DOI] [PubMed] [Google Scholar]
- 159. Schmidtchen A. Chronic ulcers: a method for sampling and analysis of wound fluid. Acta Derm Venereol. 1999;79(4):291‐295. [DOI] [PubMed] [Google Scholar]
- 160. Ligi D, Mosti G, Croce L, Raffetto JD, Mannello F. Chronic venous disease ‐ part II: proteolytic biomarkers in wound healing. Biochim Biophys Acta. 2016;1862(10):1900‐1908. [DOI] [PubMed] [Google Scholar]
- 161. Ligi D, Croce L, Mosti G, Raffetto JD, Mannello F. Chronic venous insufficiency: transforming growth factor‐β isoforms and soluble Endoglin concentration in different states of wound healing. Int J Mol Sci. 2017;18(10):2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Seah CC, Phillips TJ, Howard CE, et al. Chronic wound fluid suppresses proliferation of dermal fibroblasts through a Ras‐mediated signaling pathway. J Invest Dermatol. 2005;124(2):466‐474. [DOI] [PubMed] [Google Scholar]
- 163. Saglam M, Kantarci A, Dundar N, Hakki SS. Clinical and biochemical effects of diode laser as an adjunct to nonsurgical treatment of chronic periodontitis: a randomized, controlled clinical trial. Lasers Med Sci. 2014;29(1):37‐46. [DOI] [PubMed] [Google Scholar]
- 164. Sabino F, Egli FE, Savickas S, et al. Comparative degradomics of porcine and human wound exudates unravels biomarker candidates for assessment of wound healing progression in trauma patients. J Invest Dermatol. 2018;138(2):413‐422. [DOI] [PubMed] [Google Scholar]
- 165. Mikhal'chik EV, Piterskaya JA, Budkevich LY, et al. Comparative study of cytokine content in the plasma and wound exudate from children with severe burns. Bull Exp Biol Med. 2009;148(5):771‐775. [DOI] [PubMed] [Google Scholar]
- 166. Moseley R, Hilton JR, Waddington RJ, Harding KG, Stephens P, Thomas DW. Comparison of oxidative stress biomarker profiles between acute and chronic wound environments. Wound Repair Regen. 2004;12(4):419‐429. [DOI] [PubMed] [Google Scholar]
- 167. Tao Q, Ren J, Ji Z, Wang B, Zheng Y, Li J. Continuous topical irrigation for severely infected wound healing. J Surg Res. 2015;198(2):535‐540. [DOI] [PubMed] [Google Scholar]
- 168. McInnes RL, Cullen BM, Hill KE, et al. Contrasting host immuno‐inflammatory responses to bacterial challenge within venous and diabetic ulcers. Wound Repair Regen. 2014;22(1):58‐69. [DOI] [PubMed] [Google Scholar]
- 169. Schmidtchen A. Degradation of antiproteinases, complement and fibronectin in chronic leg ulcers. Acta Derm Venereol. 2000;80(3):179‐184. [DOI] [PubMed] [Google Scholar]
- 170. Cooper DM, Yu EZ, Hennessey P, Ko F, Robson MC. Determination of endogenous cytokines in chronic wounds. Ann Surg. 1994;219(6):688‐692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Rakmanee T, Olsen I, Griffiths GS, Donos N. Development and validation of a multiplex bead assay for measuring growth mediators in wound fluid. Analyst. 2010;135(1):182‐188. [DOI] [PubMed] [Google Scholar]
- 172. Wall SJ, Bevan D, Thomas DW, Harding KG, Edwards DR, Murphy G. Differential expression of matrix metalloproteinases during impaired wound healing of the diabetes mouse. J Invest Dermatol. 2002;119(1):91‐98. [DOI] [PubMed] [Google Scholar]
- 173. Wiegand C, Bittenger K, Galiano RD, Driver VR, Gibbons GW. Does noncontact low‐frequency ultrasound therapy contribute to wound healing at the molecular level? Wound Repair Regen. 2017;25(5):871‐882. [DOI] [PubMed] [Google Scholar]
- 174. Zillmer R, Trøstrup H, Karlsmark T, Ifversen P, Agren MS. Duration of wound fluid secretion from chronic venous leg ulcers is critical for interleukin‐1α, interleukin‐1β, interleukin‐8 levels and fibroblast activation. Arch Dermatol Res. 2011;303(8):601‐606. [DOI] [PubMed] [Google Scholar]
- 175. Kaner D, Soudan M, Zhao H, Gaßmann G, Schönhauser A, Friedmann A. Early healing events after periodontal surgery: observations on soft tissue healing, microcirculation, and wound fluid cytokine levels. Int J Mol Sci. 2017;18(2):283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Ambrosch A, Halevy D, Fwity B, Brin T, Lobmann R. Effect of daptomycin on local interleukin‐6, matrix metalloproteinase‐9, and metallopeptidase inhibitor 1 in patients with MRSA‐infected diabetic foot. Int J Low Extrem Wounds. 2014;13(1):12‐16. [DOI] [PubMed] [Google Scholar]
- 177. Smeets R, Ulrich D, Unglaub F, Wöltje M, Pallua N. Effect of oxidised regenerated cellulose/collagen matrix on proteases in wound exudate of patients with chronic venous ulceration. Int Wound J. 2008;5(2):195‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Ulrich D, Smeets R, Unglaub F, Wöltje M, Pallua N. Effect of oxidized regenerated cellulose/collagen matrix on proteases in wound exudate of patients with diabetic foot ulcers. J Wound Ostomy Continence Nurs. 2011;38(5):522‐528. [DOI] [PubMed] [Google Scholar]
- 179. Keskiner I, Lutfioğlu M, Aydogdu A, Saygun NI, Serdar MA. Effect of Photobiomodulation on transforming growth factor‐β1, platelet‐derived growth factor‐BB, and Interleukin‐8 release in palatal wounds after free gingival graft harvesting: a randomized clinical study. Photomed Laser Surg. 2016;34(6):263‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Cooke JW, Sarment DP, Whitesman LA, et al. Effect of rhPDGF‐BB delivery on mediators of periodontal wound repair. Tissue Eng. 2006;12(6):1441‐1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Sarment DP, Cooke JW, Miller SE, et al. Effect of rhPDGF‐BB on bone turnover during periodontal repair. J Clin Periodontol. 2006;33(2):135‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Rauten AM, Silosi I, Stratul SI, et al. Expression of Pentraxin 3 and thrombospondin 1 in gingival Crevicular fluid during wound healing after Gingivectomy in Postorthodontic patients. J Immunol Res. 2016;2016:4072543‐4072547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Ruf MT, Andreoli A, Vujic G, Itin P, Pluschke G, Schmid P. Exudate collection using wound sponges‐an easy, non‐invasive and reliable method to explore protease activities in ulcers. Wound Repair Regen. 2017;25(2):320‐326. [DOI] [PubMed] [Google Scholar]
- 184. Stanley CM, Wang Y, Pal S, et al. Fibronectin fragmentation is a feature of periodontal disease sites and diabetic foot and leg wounds and modifies cell behavior. J Periodontol. 2008;79(5):861‐875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Steinsträßer L, Jacobsen F, Hirsch T, et al. Immunodepletion of high‐abundant proteins from acute and chronic wound fluids to elucidate low‐abundant regulators in wound healing. BMC Res Notes. 2010;3:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Mwaura B, Mahendran B, Hynes N, et al. The impact of differential expression of extracellular matrix metalloproteinase inducer, matrix metalloproteinase‐2, tissue inhibitor of matrix metalloproteinase‐2 and PDGF‐AA on the chronicity of venous leg ulcers. Eur J Vasc Endovasc Surg. 2006;31(3):306‐310. [DOI] [PubMed] [Google Scholar]
- 187. Pellegrini G, Rasperini G, Pagni G, et al. Local wound healing biomarkers for real‐time assessment of periodontal regeneration: pilot study. J Periodontal Res. 2017;52(3):388‐396. [DOI] [PubMed] [Google Scholar]
- 188. Muller M, Trocme C, Lardy B, Morel F, Halimi S, Benhamou PY. Matrix metalloproteinases and diabetic foot ulcers: the ratio of MMP‐1 to TIMP‐1 is a predictor of wound healing. Diabet Med. 2008;25(4):419‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Cullen B, Smith R, McCulloch E, Silcock D, Morrison L. Mechanism of action of PROMOGRAN, a protease modulating matrix, for the treatment of diabetic foot ulcers. Wound Repair Regen. 2002;10(1):16‐25. [DOI] [PubMed] [Google Scholar]
- 190. Raffetto JD, Vasquez R, Goodwin DG, Menzoian JO. Mitogen‐activated protein kinase pathway regulates cell proliferation in venous ulcer fibroblasts. Vasc Endovascular Surg. 2006;40(1):59‐66. [DOI] [PubMed] [Google Scholar]
- 191. Krisp C, McKay MJ, Wolters DA, Molloy MP. Multidimensional protein identification technology‐selected reaction monitoring improving detection and quantification for protein biomarker studies. Anal Chem. 2012;84(3):1592‐1600. [DOI] [PubMed] [Google Scholar]
- 192. Smith E, Hoffman R. Multiple fragments related to angiostatin and endostatin in fluid from venous leg ulcers. Wound Repair Regen. 2005;13(2):148‐157. [DOI] [PubMed] [Google Scholar]
- 193. Pukstad BS, Ryan L, Flo TH, et al. Non‐healing is associated with persistent stimulation of the innate immune response in chronic venous leg ulcers. J Dermatol Sci. 2010;59(2):115‐122. [DOI] [PubMed] [Google Scholar]
- 194. Yao M, Hasturk H, Kantarci A, et al. A pilot study evaluating non‐contact low‐frequency ultrasound and underlying molecular mechanism on diabetic foot ulcers. Int Wound J. 2014;11(6):586‐593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Edsberg LE, Wyffels JT, Ogrin R, Craven BC, Houghton P. A pilot study evaluating protein abundance in pressure ulcer fluid from people with and without spinal cord injury. J Spinal Cord Med. 2015;38(4):456‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Twetman S, Pedersen AML, Yucel‐Lindberg T. Probiotic supplements containing lactobacillus reuteri does not affect the levels of matrix metalloproteinases and interferons in oral wound healing. BMC Res Notes. 2018;11(1):759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Tarlton JF, Bailey AJ, Crawford E, Jones D, Moore K, Harding KD. Prognostic value of markers of collagen remodeling in venous ulcers. Wound Repair Regen. 1999;7(5):347‐355. [DOI] [PubMed] [Google Scholar]
- 198. Oono T, Fujiwara Y, Yoshioka T, Arata J. Prolidase activity in chronic wound and blister fluids. J Dermatol. 1997;24(10):626‐629. [DOI] [PubMed] [Google Scholar]
- 199. Mendez MV, Raffetto JD, Phillips T, Menzoian JO, Park HY. The proliferative capacity of neonatal skin fibroblasts is reduced after exposure to venous ulcer wound fluid: a potential mechanism for senescence in venous ulcers. J Vasc Surg. 1999;30(4):734‐743. [DOI] [PubMed] [Google Scholar]
- 200. Kloeters O, Unglaub F, de Laat E, van Abeelen M, Ulrich D. Prospective and randomised evaluation of the protease‐modulating effect of oxidised regenerated cellulose/collagen matrix treatment in pressure sore ulcers. Int Wound J. 2016;13(6):1231‐1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Driver VR, Yao M, Kantarci A, Gu G, Park N, Hasturk H. A prospective, randomized clinical study evaluating the effect of transdermal continuous oxygen therapy on biological processes and foot ulcer healing in persons with diabetes mellitus. Ostomy Wound Manage. 2013;59(11):19‐26. [PubMed] [Google Scholar]
- 202. Ganesh K, Das A, Dickerson R, et al. Prostaglandin E₂ induces oncostatin M expression in human chronic wound macrophages through Axl receptor tyrosine kinase pathway. J Immunol. 2012;189(5):2563‐2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Boeringer T, Gould LJ, Koria P. Protease‐resistant growth factor formulations for the healing of chronic wounds. Adv Wound Care (New Rochelle). 2020;9(11):612‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Moor AN, Vachon DJ, Gould LJ. Proteolytic activity in wound fluids and tissues derived from chronic venous leg ulcers. Wound Repair Regen. 2009;17(6):832‐839. [DOI] [PubMed] [Google Scholar]
- 205. Krisp C, Jacobsen F, McKay MJ, Molloy MP, Steinstraesser L, Wolters DA. Proteome analysis reveals antiangiogenic environments in chronic wounds of diabetes mellitus type 2 patients. Proteomics. 2013;13(17):2670‐2681. [DOI] [PubMed] [Google Scholar]
- 206. Piatkowski A, Ulrich D, Seidel D, Abel M, Pallua N, Andriessen A. Randomised, controlled pilot to compare collagen and foam in stagnating pressure ulcers. J Wound Care. 2012;21(10):505‐511. [DOI] [PubMed] [Google Scholar]
- 207. Gottrup F, Cullen BM, Karlsmark T, Bischoff‐Mikkelsen M, Nisbet L, Gibson MC. Randomized controlled trial on collagen/oxidized regenerated cellulose/silver treatment. Wound Repair Regen. 2013;21(2):216‐225. [DOI] [PubMed] [Google Scholar]
- 208. Ladwig GP, Robson MC, Liu R, Kuhn MA, Muir DF, Schultz GS. Ratios of activated matrix metalloproteinase‐9 to tissue inhibitor of matrix metalloproteinase‐1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair Regen. 2002;10(1):26‐37. [DOI] [PubMed] [Google Scholar]
- 209. Caulfield RH, Tyler MP, Austyn JM, Dziewulski P, McGrouther DA. The relationship between protease/anti‐protease profile, angiogenesis and re‐epithelialisation in acute burn wounds. Burns. 2008;34(4):474‐486. [DOI] [PubMed] [Google Scholar]
- 210. Debats IB, Wolfs TG, Gotoh T, Cleutjens JP, Peutz‐Kootstra CJ, van der Hulst RR. Role of arginine in superficial wound healing in man. Nitric Oxide. 2009;21(3–4):175‐183. [DOI] [PubMed] [Google Scholar]
- 211. Cullen B, Watt PW, Lundqvist C, et al. The role of oxidised regenerated cellulose/collagen in chronic wound repair and its potential mechanism of action. Int J Biochem Cell Biol. 2002;34(12):1544‐1556. [DOI] [PubMed] [Google Scholar]
- 212. Mouës CM, van Toorenenbergen AW, Heule F, Hop WC, Hovius SE. The role of topical negative pressure in wound repair: expression of biochemical markers in wound fluid during wound healing. Wound Repair Regen. 2008;16(4):488‐494. [DOI] [PubMed] [Google Scholar]
- 213. Trøstrup H, Holstein P, Christophersen L, et al. S100A8/A9 is an important host defence mediator in neuropathic foot ulcers in patients with type 2 diabetes mellitus. Arch Dermatol Res. 2016;308(5):347‐355. [DOI] [PubMed] [Google Scholar]
- 214. Wildeboer D, Hill KE, Jeganathan F, et al. Specific protease activity indicates the degree of Pseudomonas aeruginosa infection in chronic infected wounds. Eur J Clin Microbiol Infect Dis. 2012;31(9):2183‐2189. [DOI] [PubMed] [Google Scholar]
- 215. De Mattei M, Ongaro A, Magaldi S, et al. Time‐ and dose‐dependent effects of chronic wound fluid on human adult dermal fibroblasts. Dermatol Surg. 2008;34(3):347‐356. [DOI] [PubMed] [Google Scholar]
- 216. Henshaw FR, Bolton T, Nube V, et al. Topical application of the bee hive protectant propolis is well tolerated and improves human diabetic foot ulcer healing in a prospective feasibility study. J Diabetes Complications. 2014;28(6):850‐857. [DOI] [PubMed] [Google Scholar]
- 217. Henshaw FR, Boughton P, Lo L, McLennan SV, Twigg SM. Topically applied connective tissue growth factor/CCN2 improves diabetic preclinical cutaneous wound healing: potential role for CTGF in human diabetic foot ulcer healing. J Diabetes Res. 2015;2015:236238‐236210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Trøstrup H, Holstein P, Karlsmark T, Moser C, Ågren MS. Uncontrolled gelatin degradation in non‐healing chronic wounds. J Wound Care. 2018;27(11):724‐734. [DOI] [PubMed] [Google Scholar]
- 219. Hoffman R, Noble J, Eagle M. The use of proteases as prognostic markers for the healing of venous leg ulcers. J Wound Care. 1999;8(6):273‐276. [DOI] [PubMed] [Google Scholar]
- 220. Labler L, Rancan M, Mica L, Härter L, Mihic‐Probst D, Keel M. Vacuum‐assisted closure therapy increases local interleukin‐8 and vascular endothelial growth factor levels in traumatic wounds. J Trauma. 2009;66(3):749‐757. [DOI] [PubMed] [Google Scholar]
- 221. Minematsu T, Nakagami G, Yamamoto Y, et al. Wound blotting: a convenient biochemical assessment tool for protein components in exudate of chronic wounds. Wound Repair Regen. 2013;21(2):329‐334. [DOI] [PubMed] [Google Scholar]
- 222. Jindatanmanusan P, Luanraksa S, Boonsiri T, Nimmanon T, Arnutti P. Wound fluid matrix metalloproteinase‐9 as a potential predictive marker for the poor healing outcome in diabetic foot ulcers. Patholog Res Int. 2018;2018:1631325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Tan Q, Wang W, Yang C, et al. α‐Ketoglutarate is associated with delayed wound healing in diabetes. Clin Endocrinol (Oxf). 2016;85(1):54‐61. [DOI] [PubMed] [Google Scholar]
- 224. Buchstein N, Hoffmann D, Smola H, et al. Alternative proteolytic processing of hepatocyte growth factor during wound repair. Am J Pathol. 2009;174(6):2116‐2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Rennekampff HO, Hansbrough JF, Kiessig V, Doré C, Sticherling M, Schröder JM. Bioactive interleukin‐8 is expressed in wounds and enhances wound healing. J Surg Res. 2000;93(1):41‐54. [DOI] [PubMed] [Google Scholar]
- 226. Trengove NJ, Langton SR, Stacey MC. Biochemical analysis of wound fluid from nonhealing and healing chronic leg ulcers. Wound Repair Regen. 1996;4(2):234‐239. [DOI] [PubMed] [Google Scholar]
- 227. Broszczak D, Stupar D, Compay ALL, et al. Biochemical profiling of proteins and metabolites in wound exudates from chronic wound environments. Wound Pract Res. 2012;20:62‐72. [Google Scholar]
- 228. Kilpadi DV, Stechmiller J, Childress B, et al. Composition of wound fluid from pressure ulcers treated with negative pressure wound therapy using VAC (R) therapy in home health or extended care patients: a pilot study. Wounds. 2006;18:119‐128. [Google Scholar]
- 229. Harris IR, Yee KC, Walters CE, et al. Cytokine and protease levels in healing and non‐healing chronic venous leg ulcers. Exp Dermatol. 1995;4(6):342‐349. [DOI] [PubMed] [Google Scholar]
- 230. Weckroth M, Vaheri A, Myöhänen H, Tukiainen E, Sirén V. Differential effects of acute and chronic wound fluids on urokinase‐type plasminogen activator, urokinase‐type plasminogen activator receptor, and tissue‐type plasminogen activator in cultured human keratinocytes and fibroblasts. Wound Repair Regen. 2001;9(4):314‐322. [DOI] [PubMed] [Google Scholar]
- 231. Eming SA, Koch M, Krieger A, et al. Differential proteomic analysis distinguishes tissue repair biomarker signatures in wound exudates obtained from Normal healing and chronic wounds. J Proteome Res. 2010;9(9):4758‐4766. [DOI] [PubMed] [Google Scholar]
- 232. Grzela T, Niderla‐Bielinska J, Litwiniuk M, White R. The direct inhibition of MMP‐2 and MMP‐9 by an enzyme alginogel: a possible mechanism of healing support for venous leg ulcers. J Wound Care. 2014;23(5):280‐2‐284‐5. [DOI] [PubMed] [Google Scholar]
- 233. Iizaka S, Sanada H, Minematsu T, et al. Do nutritional markers in wound fluid reflect pressure ulcer status? Wound Repair Regen. 2010;18(1):31‐37. [DOI] [PubMed] [Google Scholar]
- 234. Nwomeh BC, Liang HX, Diegelmann RF, Cohen IK, Yager DR. Dynamics of the matrix metalloproteinases MMP‐1 and MMP‐8 in acute open human dermal wounds. Wound Repair Regen. 1998;6(2):127‐134. [DOI] [PubMed] [Google Scholar]
- 235. Agarwal P, Schulz JN, Blumbach K, et al. Enhanced deposition of cartilage oligomeric matrix protein is a common feature in fibrotic skin pathologies. Matrix Biol. 2013;32(6):325‐331. [DOI] [PubMed] [Google Scholar]
- 236. McDaniel JC, Szalacha L, Sales M, Roy S, Chafee S, Parinandi N. EPA + DHA supplementation reduces PMN activation in microenvironment of chronic venous leg ulcers: a randomized, double‐blind, controlled study. Wound Repair Regen. 2017;25(4):680‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Stacey MC, Phillips SA, Farrokhyar F, Swaine JM. Evaluation of wound fluid biomarkers to determine healing in adults with venous leg ulcers: a prospective study. Wound Repair Regen. 2019;27(5):509‐518. [DOI] [PubMed] [Google Scholar]
- 238. Lohmann N, Schirmer L, Atallah P, et al. Glycosaminoglycan‐based hydrogels capture inflammatory chemokines and rescue defective wound healing in mice. Sci Transl Med. 2017;9(386):eaai9044. [DOI] [PubMed] [Google Scholar]
- 239. Edwards JV, Bopp AF, Batiste S, et al. Inhibition of elastase by a synthetic cotton‐bound serine protease inhibitor: in vitro kinetics and inhibitor release. Wound Repair Regen. 1999;7(2):106‐118. [DOI] [PubMed] [Google Scholar]
- 240. Eming S, Smola H, Hartmann B, et al. The inhibition of matrix metalloproteinase activity in chronic wounds by a polyacrylate superabsorber. Biomaterials. 2008;29(19):2932‐2940. [DOI] [PubMed] [Google Scholar]
- 241. Grande R, Brachini G, Sterpetti AV, et al. Local release of metalloproteinases and their inhibitors after a successful revascularisation procedure. Int Wound J. 2020;17(1):149‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Weckroth M, Vaheri A, Lauharanta J, Sorsa T, Konttinen YT. Matrix metalloproteinases, gelatinase and collagenase, in chronic leg ulcers. J Invest Dermatol. 1996;106(5):1119‐1124. [DOI] [PubMed] [Google Scholar]
- 243. Trengove NJ, Bielefeldt‐Ohmann H, Stacey MC. Mitogenic activity and cytokine levels in non‐healing and healing chronic leg ulcers. Wound Repair Regen. 2000;8(1):13‐25. [DOI] [PubMed] [Google Scholar]
- 244. Sadler GM, Wallace HJ, Stacey MC. Oral doxycycline for the treatment of chronic leg ulceration. Arch Dermatol Res. 2012;304(6):487‐493. [DOI] [PubMed] [Google Scholar]
- 245. Palolahti M, Lauharanta J, Stephens RW, Kuusela P, Vaheri A. Proteolytic activity in leg ulcer exudate. Exp Dermatol. 1993;2(1):29‐37. [DOI] [PubMed] [Google Scholar]
- 246. Grayson LS, Hansbrough JF, Zapata‐Sirvent RL, Dore CA, Morgan JL, Nicolson MA. Quantitation of cytokine levels in skin graft donor site wound fluid. Burns. 1993;19(5):401‐405. [DOI] [PubMed] [Google Scholar]
- 247. Senet P, Bon FX, Benbunan M, et al. Randomized trial and local biological effect of autologous platelets used as adjuvant therapy for chronic venous leg ulcers. J Vasc Surg. 2003;38(6):1342‐1348. [DOI] [PubMed] [Google Scholar]
- 248. Bodnár E, Bakondi E, Kovács K, et al. Redox profiling reveals clear differences between molecular patterns of wound fluids from acute and chronic wounds. Oxid Med Cell Longev. 2018;2018:5286785‐5286712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Gohel MS, Windhaber RA, Tarlton JF, Whyman MR, Poskitt KR. The relationship between cytokine concentrations and wound healing in chronic venous ulceration. J Vasc Surg. 2008;48(5):1272‐1277. [DOI] [PubMed] [Google Scholar]
- 250. Amato B, Coretti G, Compagna R, et al. Role of matrix metalloproteinases in non‐healing venous ulcers. Int Wound J. 2015;12(6):641‐645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Rennekampff HO, Hansbrough JF, Woods V Jr, Doré C, Kiessig V, Schröder JM. Role of melanoma growth stimulatory activity (MGSA/gro) on keratinocyte function in wound healing. Arch Dermatol Res. 1997;289(4):204‐212. [DOI] [PubMed] [Google Scholar]
- 252. Trøstrup H, Lundquist R, Christensen LH, et al. S100A8/A9 deficiency in nonhealing venous leg ulcers uncovered by multiplexed antibody microarray profiling. Br J Dermatol. 2011;165(2):292‐301. [DOI] [PubMed] [Google Scholar]
- 253. James TJ, Hughes MA, Cherry GW, Taylor RP. Simple biochemical markers to assess chronic wounds. Wound Repair Regen. 2000;8(4):264‐269. [DOI] [PubMed] [Google Scholar]
- 254. Ahmad A, Saha P, Evans C, et al. The soluble urokinase plasminogen activator receptor and its fragments in venous ulcers. J Vasc Surg Venous Lymphat Disord. 2015;3(2):190‐197. [DOI] [PubMed] [Google Scholar]
- 255. Grönberg A, Zettergren L, Agren MS. Stability of the cathelicidin peptide LL‐37 in a non‐healing wound environment. Acta Derm Venereol. 2011;91(5):511‐515. [DOI] [PubMed] [Google Scholar]
- 256. Ono I, Gunji H, Zhang JZ, Maruyama K, Kaneko F. Studies on cytokines related to wound healing in donor site wound fluid. J Dermatol Sci. 1995;10(3):241‐245. [DOI] [PubMed] [Google Scholar]
- 257. Latijnhouwers MA, Bergers M, Veenhuis RT, Beekman B, Ankersmit‐Ter Horst MF, Schalkwijk J. Tenascin‐C degradation in chronic wounds is dependent on serine proteinase activity. Arch Dermatol Res. 1998;290(9):490‐496. [DOI] [PubMed] [Google Scholar]
- 258. Krishnaswami S, Ly QP, Rothman VL, Tuszynski GP. Thrombospondin‐1 promotes proliferative healing through stabilization of PDGF. J Surg Res. 2002;107(1):124‐130. [DOI] [PubMed] [Google Scholar]
- 259. Chin GA, Thigpin T, Perrin K, Moldawer L, Schultz G. Treatment of chronic ulcers in diabetic patients with a topical metalloproteinase inhibitor, Doxycycline. Wounds. 2003;15:315‐323. [Google Scholar]
- 260. Pan S‐C, Wu L‐W, Chen C‐L, Shieh S‐J, Chiu H‐Y. Angiogenin expression in burn blister fluid: implications for its role in burn wound neovascularization. Wound Repair Regen. 2012;20(5):731‐739. [DOI] [PubMed] [Google Scholar]
- 261. Frohm M, Gunne H, Bergman AC, et al. Biochemical and antibacterial analysis of human wound and blister fluid. Eur J Biochem. 1996;237(1):86‐92. [DOI] [PubMed] [Google Scholar]
- 262. Zang T, Broszczak DA, Cuttle L, Broadbent JA, Tanzer C, Parker TJ. The blister fluid proteome of paediatric burns. J Proteomics. 2016;146:122‐132. [DOI] [PubMed] [Google Scholar]
- 263. Forsberg JA, Elster EA, Andersen RC, et al. Correlation of procalcitonin and cytokine expression with dehiscence of wartime extremity wounds. J Bone Joint Surg Am. 2008;90(3):580‐588. [DOI] [PubMed] [Google Scholar]
- 264. Deitch EA, Emmett M. Early protein alteration in blister fluid and serum associated with burn injury. J Trauma. 1986;26(1):34‐39. [DOI] [PubMed] [Google Scholar]
- 265. Koivukangas V, Oikarinen A, Risteli J, Haukipuro K. Effect of jaundice and its resolution on wound re‐epithelization, skin collagen synthesis, and serum collagen propeptide levels in patients with neoplastic pancreaticobiliary obstruction. J Surg Res. 2005;124(2):237‐243. [DOI] [PubMed] [Google Scholar]
- 266. Deitch EA, Lu Q, Xu DZ, Specian RD. Effect of local and systemic burn microenvironment on neutrophil activation as assessed by complement receptor expression and morphology. J Trauma. 1990;30(3):259‐268. [DOI] [PubMed] [Google Scholar]
- 267. Zaleska K, Suchorska WM, Przybyła A, Murawa D. Effect of surgical wound fluids after intraoperative electron radiotherapy on the cancer stem cell phenotype in a panel of human breast cancer cell lines. Oncol Lett. 2016;12(5):3707‐3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Inoue M, Zhou LJ, Gunji H, Ono I, Kaneko F. Effects of cytokines in burn blister fluids on fibroblast proliferation and their inhibition with the use of neutralizing antibodies. Wound Repair Regen. 1996;4(4):426‐432. [DOI] [PubMed] [Google Scholar]
- 269. Tanzer C, Sampson DL, Broadbent JA, et al. Evaluation of haemoglobin in blister fluid as an indicator of paediatric burn wound depth. Burns. 2015;41(5):1114‐1121. [DOI] [PubMed] [Google Scholar]
- 270. Robson MC, Heggers JP. Evaluation of hand frostbite blister fluid as a clue to pathogenesis. J Hand Surg Am. 1981;6(1):43‐47. [DOI] [PubMed] [Google Scholar]
- 271. McDaniel JC, Massey K, Nicolaou A. Fish oil supplementation alters levels of lipid mediators of inflammation in microenvironment of acute human wounds. Wound Repair Regen. 2011;19(2):189‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Ortega MR, Ganz T, Milner SM. Human beta defensin is absent in burn blister fluid. Burns. 2000;26(8):724‐726. [DOI] [PubMed] [Google Scholar]
- 273. Rayment EA, Upton Z, Shooter GK. Increased matrix metalloproteinase‐9 (MMP‐9) activity observed in chronic wound fluid is related to the clinical severity of the ulcer. Br J Dermatol. 2008;158(5):951‐961. [DOI] [PubMed] [Google Scholar]
- 274. Wilson Y, Goberdhan N, Dawson RA, Smith J, Freedlander E, Mac NS. Investigation of the presence and role of calmodulin and other mitogens in human burn blister fluid. J Burn Care Rehabil. 1994;15(4):303‐314. [DOI] [PubMed] [Google Scholar]
- 275. Wallace HJ, Stacey MC. Levels of tumor necrosis factor‐alpha (TNF‐alpha) and soluble TNF receptors in chronic venous leg ulcers–correlations to healing status. J Invest Dermatol. 1998;110(3):292‐296. [DOI] [PubMed] [Google Scholar]
- 276. Varelias A, Cowin AJ, Adams D, et al. Mitogenic bovine whey extract modulates matrix metalloproteinase‐2, −9, and tissue inhibitor of matrix metalloproteinase‐2 levels in chronic leg ulcers. Wound Repair Regen. 2006;14(1):28‐37. [DOI] [PubMed] [Google Scholar]
- 277. McCarthy DW, Downing MT, Brigstock DR, et al. Production of heparin‐binding epidermal growth factor‐like growth factor (HB‐EGF) at sites of thermal injury in pediatric patients. J Invest Dermatol. 1996;106(1):49‐56. [DOI] [PubMed] [Google Scholar]
- 278. Shakespeare PG, Levick PL, Vaitheespara RB. Proteins in blister fluid. Burns. 1978;4(4):254‐260. [Google Scholar]
- 279. Ono I, Gunji H, Zhang JZ, Maruyama K, Kaneko F. A study of cytokines in burn blister fluid related to wound healing. Burns. 1995;21(5):352‐355. [DOI] [PubMed] [Google Scholar]
- 280. Smith TJ, Wilson MA, Young AJ, Montain SJ. A suction blister model reliably assesses skin barrier restoration and immune response. J Immunol Methods. 2015;417:124‐130. [DOI] [PubMed] [Google Scholar]
- 281. Reiss MJ, Han Y‐P, Garner WL. α1‐Antichymotrypsin activity correlates with and may modulate matrix metalloproteinase‐9 in human acute wounds. Wound Repair Regen. 2009;17(3):418‐426. [DOI] [PubMed] [Google Scholar]
- 282. Serena TE, Cullen BM, Bayliff SW, et al. Defining a new diagnostic assessment parameter for wound care: elevated protease activity, an indicator of nonhealing, for targeted protease‐modulating treatment. Wound Repair Regen. 2016;24(3):589‐595. [DOI] [PubMed] [Google Scholar]
- 283. Metcalf DG, Haalboom M, Bowler PG, et al. Elevated wound fluid pH correlates with increased risk of wound infection. Wound Med. 2019;26(1):100166. [Google Scholar]
- 284. Li J‐y, Wang Z‐j, Deng A‐p, Li Y‐m. ENA‐78 is a novel predictor of wound healing in patients with diabetic foot ulcers. J Diabetes Res. 2019;2019:2695436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Banerjee A, McNish S, Shanmugam VK. Interferon‐gamma (IFN‐γ) is elevated in wound exudate from hidradenitis Suppurativa. Immunol Invest. 2017;46(2):149‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Ingram JR, Cawley S, Coulman E, et al. Levels of wound calprotectin and other inflammatory biomarkers aid in deciding which patients with a diabetic foot ulcer need antibiotic therapy (INDUCE study). Diabet Med. 2018;35(2):255‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Schiffer D, Verient V, Luschnig D, et al. Lysozyme‐responsive polymer systems for detection of infection. Eng Life Sci. 2015;15(4):368‐375. [Google Scholar]
- 288. Bernatchez SF, Menon V, Stoffel J, et al. Nitric oxide levels in wound fluid may reflect the healing trajectory. Wound Repair Regen. 2013;21(3):410‐417. [DOI] [PubMed] [Google Scholar]
- 289. Heinzle A, Papen‐Botterhuis NE, Schiffer D, et al. Novel protease‐based diagnostic devices for detection of wound infection. Wound Repair Regen. 2013;21(3):482‐489. [DOI] [PubMed] [Google Scholar]
- 290. Wang X, Li J, Wang Z, Deng A. Wound exudate CXCL6: a potential biomarker for wound healing of diabetic foot ulcers. Biomark Med. 2019;13(3):167‐174. [DOI] [PubMed] [Google Scholar]
- 291. Rayment EA, Dargaville TR, Shooter GK, George GA, Upton Z. Attenuation of protease activity in chronic wound fluid with bisphosphonate‐functionalised hydrogels. Biomaterials. 2008;29(12):1785‐1795. [DOI] [PubMed] [Google Scholar]
- 292. Fernandez ML, Broadbent JA, Shooter GK, Malda J, Upton Z. Development of an enhanced proteomic method to detect prognostic and diagnostic markers of healing in chronic wound fluid. Br J Dermatol. 2008;158(2):281‐290. [DOI] [PubMed] [Google Scholar]
- 293. Fernandez ML, Upton Z, Edwards H, Finlayson K, Shooter GK. Elevated uric acid correlates with wound severity. Int Wound J. 2012;9(2):139‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Ambrosch A, Lobmann R, Pott A, Preissler J. Interleukin‐6 concentrations in wound fluids rather than serological markers are useful in assessing bacterial triggers of ulcer inflammation. Int Wound J. 2008;5(1):99‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Huttunen M, Harvima IT. Mast cell tryptase and chymase in chronic leg ulcers: chymase is potentially destructive to epithelium and is controlled by proteinase inhibitors. Br J Dermatol. 2005;152(6):1149‐1160. [DOI] [PubMed] [Google Scholar]
- 296. Cao Y, Croll TI, Rizzi SC, et al. A peptidomimetic inhibitor of matrix metalloproteinases containing a tetherable linker group. J Biomed Mater Res A. 2011;96A(4):663‐672. [DOI] [PubMed] [Google Scholar]
- 297. Wiegand C, Schönfelder U, Abel M, Ruth P, Kaatz M, Hipler UC. Protease and pro‐inflammatory cytokine concentrations are elevated in chronic compared to acute wounds and can be modulated by collagen type I in vitro. Arch Dermatol Res. 2010;302(6):419‐428. [DOI] [PubMed] [Google Scholar]
- 298. Sobsey CA, Ibrahim S, Richard VR, et al. Targeted and untargeted proteomics approaches in biomarker development. Proteomics. 2020;20(9):1900029. [DOI] [PubMed] [Google Scholar]
- 299. Kisand K, Kerna I, Kumm J, Jonsson H, Tamm A. Impact of cryopreservation on serum concentration of matrix metalloproteinases (MMP)‐7, TIMP‐1, vascular growth factors (VEGF) and VEGF‐R2 in biobank samples. Clin Chem Lab Med. 2011;49(2):229‐235. [DOI] [PubMed] [Google Scholar]
- 300. Durai R, Mownah A, Ng PC. Use of drains in surgery: a review. J Perioper Pract. 2009;19(6):180‐186. [DOI] [PubMed] [Google Scholar]
- 301. Nichols E. Wound assessment part 2: exudate. General Wound Care. 2016;11(1):36‐41. [Google Scholar]
- 302. Setia MS. Methodology series module 3: cross‐sectional studies. Indian J Dermatol. 2016;61(3):261‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Levin KA. Study design III: cross‐sectional studies. Evid Based Dent. 2006;7(1):24‐25. [DOI] [PubMed] [Google Scholar]
- 304. Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Fu Q, Schoenhoff FS, Savage WJ, Zhang P, Van Eyk JE. Multiplex assays for biomarker research and clinical application: translational science coming of age. PROTEOMICS – Clinical Applications. 2010;4(3):271‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Jones SR, Carley S, Harrison M. An introduction to power and sample size estimation. Emerg Med J. 2003;20(5):453‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Westby MJ, Norman G, Dumville JC, Stubbs N, Cullum N. Protease‐modulating matrix treatments for healing venous leg ulcers. Cochrane Database Syst Rev. 2016;12(12):CD011918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Waffenschmidt S, Knelangen M, Sieben W, Bühn S, Pieper D. Single screening versus conventional double screening for study selection in systematic reviews: a methodological systematic review. BMC Med Res Methodol. 2019;19(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Stoll CRT, Izadi S, Fowler S, Green P, Suls J, Colditz GA. The value of a second reviewer for study selection in systematic reviews. Res Synth Methods. 2019;10(4):539‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Buscemi N, Hartling L, Vandermeer B, Tjosvold L, Klassen TP. Single data extraction generated more errors than double data extraction in systematic reviews. J Clin Epidemiol. 2006;59(7):697‐703. [DOI] [PubMed] [Google Scholar]
- 311. Shemilt I, Khan N, Park S, Thomas J. Use of cost‐effectiveness analysis to compare the efficiency of study identification methods in systematic reviews. Syst Rev. 2016;5(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Waffenschmidt S, Hausner E, Sieben W, Jaschinski T, Knelangen M, Overesch I. Effective study selection using text mining or a single‐screening approach: a study protocol. Syst Rev. 2018;7(1):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article


