Summary
Background and Aims
Vedolizumab is a gut‐selective treatment approved for Crohn’s disease (CD) and ulcerative colitis (UC). Recently, a subcutaneous formulation of vedolizumab was approved. The aims of this study were to evaluate efficacy, safety, pharmacokinetics, patient experience and costs following a switch from intravenous to subcutaneous vedolizumab treatment.
Methods
Patients were switched from intravenous to subcutaneous vedolizumab maintenance treatment and followed prospectively for 6 months and a subgroup for 12 months. The primary endpoint was change in faecal calprotectin levels. Furthermore, we evaluated clinical disease activity, remission rates, plasma CRP, drug persistence, adverse events, local injection reactions, serum drug concentrations, patient satisfaction, quality‐of‐life and treatment costs.
Results
Eighty‐nine patients were included (48 CD; 41 UC). Faecal calprotectin decreased significantly in CD but not in UC. Clinical indices, remission rates, plasma CRP levels and quality‐of‐life scores remained unchanged. Patients that had been on standard compared to optimised IV vedolizumab dosing displayed similar outcomes on standard SC dosing. Drug persistence at 6 and 12 months was 95.5% and 88.5%, respectively. Frequencies of adverse events were similar before and after the switch. No serious adverse events occurred. Transient severe local injection reactions were experienced by 1.2% of patients. Median vedolizumab trough levels were 2.3 times higher on subcutaneous compared to intravenous treatment. Patient satisfaction was generally high. Annualised treatment costs were reduced by 15% following the switch.
Conclusions
The switch from intravenous to subcutaneous vedolizumab could be done with preserved therapeutic effectiveness, safety, high patient satisfaction and low discontinuation rate, at a reduced cost.
This prospective real‐world study examined switching patients with IBD from maintenance IV vedolizumab treatment to SC vedolizumab. The following was studied: efficacy, safety, pharmacokinetics, patient experience, and costs. All patients, including those that had been on optimized IV dosing, were switched to the standard SC dose. The switch could be done with preserved therapeutic effectiveness, preserved safety, high patient satisfaction, and low discontinuation rate, at a reduced cost.

1. INTRODUCTION
The inflammatory process of Crohn’s disease (CD) or ulcerative colitis (UC), which are the two main forms of inflammatory bowel disease (IBD), is thought to be driven by the infiltration of dysregulated proinflammatory immune cells into the inflamed intestinal tissue. 1 This infiltration is facilitated by the interaction between the integrin α4β7, which is expressed on several circulating immune cell subsets including previously activated T‐cells, and its counterreceptor Mucosal Addressin Cell Adhesion Molecule‐1 (MAdCAM‐1), which is selectively expressed on the endothelial cells of intestinal venules. 1
Vedolizumab is a humanised monoclonal IgG1 antibody that binds to α4β7 and inhibits the interaction with MAdCAM‐1. This prevents α4β7‐expressing immune cells from extravasating which leads to a decrease in local inflammatory activity. 2 More recently, vedolizumab has been shown to modulate innate immunity, including macrophage and dendritic cell populations, in addition to adaptive immunity. 3 The limited expression pattern of MAdCAM‐1 is thought to account for vedolizumab’s gut‐specific immunosuppressive effect which in turn translates into a beneficial safety profile. 2
Vedolizumab is approved for the treatment of patients with moderate‐to‐severe CD or UC, where treatment with conventional therapy or an anti‐TNF agent has failed. Vedolizumab was originally developed for administration via intravenous (IV) infusions. Recently, a formulation for subcutaneous (SC) administration was approved for maintenance treatment following at least two IV infusions. This approval was based on the phase III trials VISIBLE 1 and VISIBLE 2 which evaluated SC vedolizumab treatment after two initial IV doses in CD and UC patients, respectively. 4 , 5 The proportion of subjects in clinical remission 52 weeks after the start of treatment, which was the primary endpoint, was significantly higher in the SC vedolizumab‐treated group compared to the placebo group, in both trials. 4 , 5 Median trough concentrations at steady state during SC vedolizumab were 30.2 and 34.6 μg/ml for CD and UC patients, respectively, which was substantially higher than the median trough level during IV treatment presented in VISIBLE 1 (11.1 μg/ml). 4 , 5 In contrast, the average serum concentrations over time were rather similar (39.8 and 32.2 μg/ml, during SC and IV treatment, respectively). 4 , 5 Finally, there were no new safety issues observed, other than the incidence of injection‐site reaction frequencies. 4 , 5 However, data on patient experience or satisfaction were not presented in the VISIBLE publications, and studies to investigate the efficacy and safety of switching patients from maintenance IV to maintenance SC vedolizumab treatment in a real‐world setting are scarce. To our knowledge, there is only one report on the topic, and in that study, the follow‐up time after the switch was only 12 weeks. 6 Given that the half‐life of the drug is approximately 26 days and that the wash‐out period is several months, such a short follow‐up time may not be adequate to examine the effectiveness of the SC formulation. Nevertheless, the authors described a 52% increase (p < 0.01) in the patients' faecal calprotectin levels at the end of their study which was rather unexpected. 6 Thus, further studies are warranted.
There are several potential benefits with SC as compared to IV administration of vedolizumab including a reduced burden of health care resources and increased patient convenience. Potential caveats with SC treatment are increased difficulties to ensure therapy compliance, fewer built‐in disease follow‐up visits, possible IV treatment preference and local skin reactions to SC injections.
The aims of this study were to assess efficacy, safety, pharmacokinetics, patient experience, patient satisfaction, and potential cost savings following a switch from IV to SC vedolizumab treatment in patients with inflammatory bowel disease in a real‐world setting.
2. METHODS
2.1. Study design and study population
This was a prospective observational cohort study of a switch from maintenance IV to SC vedolizumab treatment in a population of adult IBD patients. The study was conducted at the Skane University Hospital, Sweden, with a 6‐month follow‐up period. Consecutive patients were approached regarding participation. Inclusion criteria comprised signed informed consent; diagnosis of CD, UC or IBD‐unclassified; and ongoing maintenance treatment with IV vedolizumab (previously received ≥3 doses of IV vedolizumab). Exclusion criteria comprised noticeable difficulties handling an SC injector pen, inability to give informed consent, or inability to comply with study procedures. The study was performed in accordance with the principles of the Declaration of Helsinki. Ethical permission was granted by the regional research ethics committee in Lund, Sweden (DNR 2018/761). All patients provided written informed consent to participation before study entry.
Patients were enrolled between December 2020 and June 2021. The study included a baseline visit, scheduled at the time‐point when the patient should have received the next dose of IV vedolizumab, and a follow‐up visit 6 months after the switch. A subset of patients, that was the first to be enrolled, could be evaluated also after 12 months. Additional visits were scheduled on demand in case of a suspected disease flare or potential side effects. All patients were switched to a dose of 108 mg vedolizumab SC every 2 weeks regardless of previous dose or dosing interval on IV vedolizumab. All conventional IBD treatments were permitted during the study and changes in the treatment regimen were allowed if clinically indicated. Dose optimization of SC vedolizumab to 108 mg weekly was done at the discretion of the treating physician based on a combination of clinical symptoms and biomarker levels. Any changes in treatment regimen throughout the study period were recorded.
Baseline data recorded included diagnosis (CD, UC or IBD‐unclassified), gender, age at diagnosis, age at inclusion, weight, height, smoking status, time on IV vedolizumab before the switch, IV vedolizumab dose and dosing interval at inclusion, previous and current IBD treatment, disease characteristics according to the Montreal classification, and disease activity at baseline.
2.2. Study endpoints and definitions
The primary endpoint was change in disease activity defined by faecal calprotectin levels at 6 months after the switch to SC vedolizumab treatment. Faecal calprotectin is a sensitive and non‐subjective measure of disease activity that is not affected by placebo or nocebo effects. It reacts to an early increase in subclinical inflammatory activity and is expected to change earlier than clinical symptom levels in the event of a diminished therapeutic effect.
Secondary endpoints were [all refer to 6 months of follow‐up unless otherwise indicated] (a) change in remission rates defined by a faecal calprotectin <150 μg/g and clinical disease activity indices: for CD patient‐based Harvey Bradshaw Index (HBI) 7 , 8 ≤4 or Patient‐Reported Outcomes (PRO)2‐CD ≤11 9 and for UC Simple Clinical Colitis Activity Index (SCCAI) 10 ≤2 or PRO2‐UC = 0. 9 PRO2 scores were applied in accordance with the STRIDE documents. 9 Briefly, the PRO2‐CD 11 is the sum of the daily soft or liquid stool frequency and abdominal pain (multiplied by the weighting factors 2 and 5, respectively) items from the Crohn’s Disease Activity Index, whereas the PRO2‐UC 12 is the simple sum of the stool frequency and rectal bleeding items from the Mayo score; (b) change in the laboratory biomarker plasma C‐reactive protein (CRP); (c) change in clinical disease activity defined by the patient‐based HBI for CD and the SCCAI for UC, as well as symptom levels according to the Patient‐Reported Outcomes (PRO)‐2 criteria as described in the STRIDE documents 9 ; (d) subgroup analyses of patients that were dose optimised on IV vedolizumab, and patients with perianal CD, respectively; (e) subgroup analysis of patients that completed 12 months of follow‐up, including drug persistence (11 patients that had not completed 12 months of follow‐up but remained on the drug were censored), and evaluation of faecal calprotectin and plasma CRP levels. When all patients had completed the first 6 months of follow‐up, the study was closed and thus not all patients completed 12 months of follow‐up; (f) adverse events and local injection reactions; (g) serum vedolizumab trough levels and relation to faecal calprotectin levels (patients categorised into quartiles based on serum vedolizumab trough levels and median faecal calprotectin levels calculated per quartile), and SC vedolizumab dose optimization rates; (h) patient experience of switching from IV to SC treatment, overall injection experience and patient satisfaction with various aspects of the injector pen [see below for details]; (i) health‐related quality‐of‐life using the Short Health Scale (SHS) 13 , 14 which is a validated four‐item questionnaire (symptom burden, social function, disease‐related worry and general well‐being; each item scored 0–5 with an SHS composite score ranging from 0 to 20) and (j) annualised cost savings per patient with SC as compared to IV treatment.
Faecal calprotectin levels were analysed using an enzyme‐linked immunosorbent assay (ELISA; PhiCal, Calpro AS). Serum vedolizumab trough levels were analysed by a Clinical and Laboratory Standards Institute (CLSI) validated in‐house developed chemiluminescence ELISA at the Karolinska Institute (Stockholm, Sweden). Both methods are used in clinical routine care and the analyses were performed in clinical laboratories.
Adverse events that occurred after study entry considered related or of possible relation to SC vedolizumab treatment or switch were documented. Local injection reactions including discomfort, pain, burning sensation and erythema; and patient satisfaction with the injector pen, overall injection experience, the experience of switching from IV to SC treatment; were evaluated using structured questionnaires (see Table 1 and Table S1 for details on items, questions asked and response options). The questions and response options were adapted from previously published questionnaires used in similar studies. 15 , 16 , 17
TABLE 1.
Incidence of adverse events and local injection reactions during study
| All patients (IBD) | Crohn’s disease | Ulcerative colitis | |
|---|---|---|---|
| Local injection reactions (adapted from Dehoratius et al.),16 n (%) | |||
| Discomfort | |||
| None | 60 (72.3) | 35 (74.5) | 25 (69.4) |
| Mild | 18 (21.7) | 10 (21.3) | 8 (22.2) |
| Moderate | 4 (4.8) | 2 (4.3) | 2 (5.6) |
| Severe | 1 (1.2) | 0 (0.0) | 1 (2.8) |
| Pain | |||
| None | 51 (61.4) | 29 (61.7) | 22 (61.1) |
| Mild | 22 (26.5) | 14 (29.8) | 8 (22.2) |
| Moderate | 9 (10.8) | 3 (6.4) | 6 (16.7) |
| Severe | 1 (1.2) | 1 (2.1) | 0 (0.0) |
| Burning sensation | |||
| None | 40 (48.2) | 22 (46.8) | 18 (50.0) |
| Mild | 35 (42.2) | 20 (42.6) | 15 (41.7) |
| Moderate | 7 (8.4) | 4 (8.5) | 3 (8.3) |
| Severe | 1 (1.2) | 1 (2.1) | 0 (0.0) |
| Erythema | |||
| None | 57 (68.7) | 36 (76.6) | 21 (58.3) |
| Mild | 16 (19.3) | 5 (10.6) | 11 (30.6) |
| Moderate | 9 (10.8) | 6 (12.8) | 3 (8.3) |
| Severe | 1 (1.2) | 0 (0.0) | 1 (2.8) |
| Other adverse events, n (%) | |||
| Fatigue | 8 (9.0) | 6 (12.5) | 2 (4.9) |
| Headache | 3 (3.4) | 1 (2.1) | 1 (2.4) |
| Nausea | 3 (3.4) | 3 (6.3) | 0 (0.0) |
| Rash | 3 (3.4) | 2 (4.2) | 1 (2.4) |
| Arthralgia | 2 (2.2) | 2 (4.2) | 0 (0.0) |
| Clostridium difficile enteritis | 1 (1.1) | 1 (2.1) | 0 (0.0) |
| Early satiety | 1 (1.1) | 1 (2.1) | 0 (0.0) |
| Herpes labialis | 1 (1.1) | 1 (2.1) | 0 (0.0) |
| Hyperhidrosis | 1 (1.1) | 1 (2.1) | 0 (0.0) |
| Muscle weakness/faintness | 1 (1.1) | 0 (0.0) | 1 (2.4) |
| Nasal congestion | 1 (1.1) | 1 (2.1) | 0 (0.0) |
| Nasal ulceration | 1 (1.1) | 0 (0.0) | 1 (2.4) |
| Non‐productive cough | 1 (1.1) | 1 (2.1) | 0 (0.0) |
| Paresthesia | 1 (1.1) | 0 (0.0) | 1 (2.4) |
| Photosensitivity | 1 (1.1) | 0 (0.0) | 1 (2.4) |
| Pruritus | 1 (1.1) | 1 (2.1) | 0 (0.0) |
| Sleep disturbance | 1 (1.1) | 0 (0.0) | 1 (2.4) |
| Weight loss | 1 (1.1) | 1 (2.1) | 0 (0.0) |
| Xerostomia | 1 (1.1) | 0 (0.0) | 1 (2.4) |
Abbreviations: IBD, inflammatory bowel disease; n, number of patients.
Annualised treatment costs for IV treatment included the cost of an appointment with a nurse for drug administration and the cost of the drug. Treatment costs of SC treatment included annual drug costs only. The rates of patients that were dose escalated on IV and SC treatment, respectively, were accounted for in the calculation.
2.3. Statistical analyses
Data are presented as mean values with standard deviation (SD), or median values with interquartile range (IQR) as appropriate. Prism 9 for Mac OS X version 9.3.1 (GraphPad Software, Inc.) was used for statistical analyses and to graph data. The paired‐samples Student’s t‐test alternatively the Wilcoxon matched‐pair signed‐rank test was used to compare baseline and follow‐up data for changes in laboratory biomarkers, disease activity indices and quality‐of‐life scores as appropriate depending on data scale type and data distribution. Missing data are shown by presenting numbers of data points in the figures. The complete case analysis method was applied, which together with an account of discontinued patients and data point numbers presented, was deemed to give the most adequate description of the cohort. 18 , 19 The approach was verified by performing sensitivity analyses comprising best‐ and worst‐case scenario calculations (i.e. missing data equals relapse and remission, respectively), neither of which altered the statistical significance as compared with the complete case analysis. 19 Regarding faecal calprotectin‐missing data, the missing‐at‐random assumption is plausible and the complete case analysis is thus adequate to apply. 20 The Kruskal–Wallis test was used to assess differences in faecal calprotectin levels between patient groups stratified by serum vedolizumab trough level quartiles during IV and SC vedolizumab treatment, respectively. The chi‐square test was used to compare remission rates between groups. Kaplan–Meier survival analysis was used to calculate drug persistence. A statistically significant test result was defined by p < 0.05.
3. RESULTS
Eighty‐nine patients (48 patients with CD, 41 patients with UC and no patients with IBD‐unclassified) were included in the study. In total, 102 patients were approached regarding participation. Twelve patients declined participation, and one patient did not meet the inclusion criteria (Figure 1). Baseline patient characteristics, clinical disease activity scores, laboratory biomarkers, concomitant IBD treatment and time on IV vedolizumab are presented in Table 2. Details on previous IBD treatments and changes during follow‐up are presented in Table S2. The median time on IV vedolizumab before the switch was 26.1 months (IQR 9.5–52.9). The wide IQR illustrates heterogeneity in terms of previous exposure time to vedolizumab. At baseline, four patients received low dose oral prednisolone (1.25–10 mg daily), and three patients received oral budesonide. Prednisolone could be discontinued in one of these patients. Results refer to 6 months follow‐up, unless otherwise specified.
FIGURE 1.
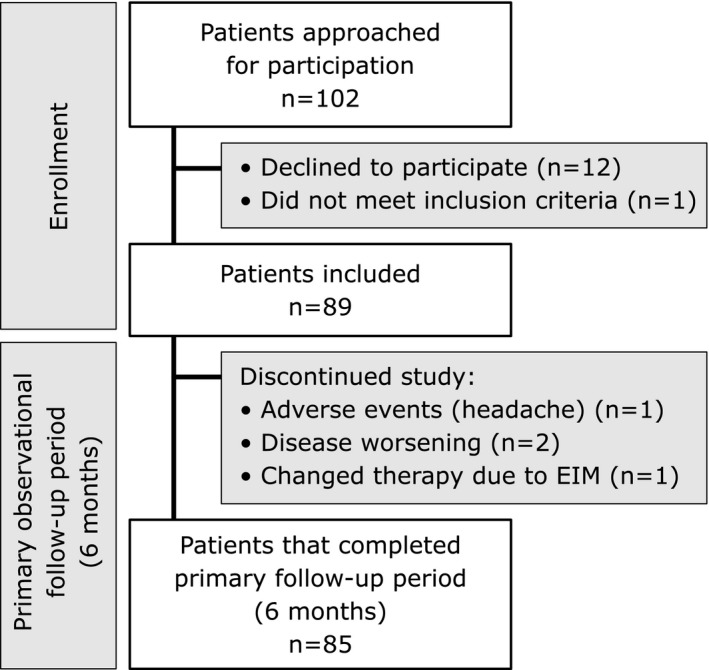
Flow chart of study design, enrollment of consecutive patients, patients discontinued and reasons for discontinuation during the primary observational follow‐up period of 6 months. Abbreviations: EIM, extraintestinal manifestations; n, number of patients
TABLE 2.
Demographics and disease characteristics at baseline (study inclusion)
| Baseline demographics and disease characteristics | All patients (IBD) | Crohn’s disease | Ulcerative colitis |
|---|---|---|---|
| IBD diagnosis, n (%) | 89 (100) | 48 (53.9) | 41 (46.1) |
| Age at baseline (years), Md (range) | 44 (20–82) | 46.5 (23–82) | 37 (20–82) |
| Age at diagnosis (years), Md (range) | 24 (9–76) | 23.5 (9–74) | 26 (14–76) |
| Height at baseline (cm), Mn (SD) | 174.2 (±11.6) | 173.2 (±11.5) | 175.5 (±11–8) |
| Weight at baseline (kg), Mn (SD) | 79.4 (±16.2) | 77.7 (±17.5) | 81.5 (±14.4) |
| Female:Male ratio, n (%) | 43:46 (48.3:51.7) | 25:23 (52.1:47.9) | 18:23 (43.9:56.1) |
| Montreal classification, n (%) | |||
| Age at diagnosis | |||
| A1 (<17 years) | 10 (11.2) | 7 (14.6) | 3 (7.3) |
| A2 (17–40 years) | 62 (69.7) | 36 (75) | 26 (63.4) |
| A3 (>40 years) | 17 (19.1) | 5 (10.4) | 12 (29.3) |
| Disease location (Crohn’s disease) | |||
| L1 (ileal ± cecal disease) | 10 (20.8) | ||
| L2 (colonic) | 12 (25) | ||
| L3 (ileocolonic) | 19 (39.6) | ||
| L3L4 (ileocolonic and upper gastrointestinal tract) | 7 (14.6) | ||
| Disease behaviour (Crohn’s disease) | |||
| B1 (uncomplicated) | 28 (58.3) | ||
| B2 (structuring) | 14 (29.2) | ||
| B3 (penetrating) | 1 (2.1) | ||
| B2B3 (stricturing, penetrating) | 5 (10.4) | ||
| p (perianal) a | 10 (20.9) | ||
| Disease extent (ulcerative colitis) | |||
| E1 (proctitis) | 5 (12.2) | ||
| E2 (left‐sided) | 15 (36.6) | ||
| E3 (extensive/pancolitis) | 21 (51.2) | ||
| Smoking status, n (%) | |||
| Never | 53 (59.6) | 29 (60.4) | 24 (58.5) |
| Previous | 29 (32.6) | 13 (27.1) | 16 (39.0) |
| Active | 7 (7.9) | 6 (12.5) | 1 (2.4) |
| Baseline data | |||
| Faecal calprotectin (μg/g), Median (IQR) | 39.0 (12.5–135) | 64.0 (12.5–238.5) | 12.5 (12.5–96.5) |
| Plasma CRP (mg/L), Median (IQR) | 2.0 (2.0–2.0) | 2.0 (2.0–2.0) | 2.0 (2.0–2.0) |
| Serum vedolizumab trough levels (μg/ml), Median (IQR) | 8.1 (5.2–14.0) | 8.7 (4.9–14.0) | 7.9 (5.3–12.5) |
| Patient‐based Harvey Bradshaw Index, Median (IQR) | 3.0 (1.0–5.8) | ||
| PRO2‐CD, Median (IQR) | 5.0 (0.5–12.5) | ||
| Simple Clinical Colitis Activity Index, Median (IQR) | 2.0 (1.0–3.0) | ||
| PRO2‐UC, Median (IQR) | 0.0 (0.0–0.0) | ||
| Short Health Scale composite score, Mean (SD) | 4.7 (±3.1) | 5.1 (±3.0) | 4.2 (±3.2) |
| IBD treatment at baseline | |||
| Conventional oral treatment, n (%) | |||
| 5‐aminosalisylic acid | 31 (34.8) | 9 (18.8) | 22 (53.7) |
| Azathioprine | 11 (12.4) | 3 (6.3) | 8 (19.5) |
| 6‐mercaptopurine | 2 (2.2) | 0 (0.0) | 2 (4.9) |
| Methotrexate | 2 (2.2) | 0 (0.0) | 2 (4.9) |
| Prednisolone | 4 (4.5) | 2 (4.2) | 2 (4.9) |
| Budesonide | 3 (3.4) | 2 (4.2) | 1 (2.4) |
| Topical treatment, n (%) | |||
| 5‐aminosalisylic acid | 3 (3.4) | 0 (0.0) | 3 (7.3) |
| Corticosteroids b | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Concomitant biologic treatment, n (%) | |||
| Adalimumab | 2 (2.2) | 1 (2.1) | 1 (2.4) |
| Time on IV vedolizumab (months), Median (IQR) | 26.1 (9.5–52.9) | 26.1 (8.7–53.1) | 27.4 (10.8–51.5) |
| IV vedolizumab dose | |||
| 300 mg, n (%) | 87 (97.8) | 46 (95.8) | 41 (100) |
| 600 mg, n (%) | 2 (2.2) | 2 (4.2) | 0 (0) |
| IV vedolizumab infusion interval, n (%) | |||
| 8 weeks | 71 (79.8) | 38 (79.2) | 33 (80.5) |
| 4 weeks | 9 (10.1) | 2 (4.2) | 7 (17.1) |
| Other (5–7 weeks) | 9 (10.1) | 8 (16.7) | 1 (2.4) |
Abbreviations: CRP, C‐reactive protein; IBD, inflammatory bowel disease; IQR, interquartile range; IV, intravenous; n, number of patients; PRO2‐CD, Crohn’s Disease Activity Index‐based patient‐reported outcome for Crohn’s disease; PRO2‐UC, Mayo‐based patient‐reported outcome for ulcerative colitis; SD, standard deviation.
May coexist with B1‐B3.
Prednisolone or budesonide.
3.1. Changes in disease activity and remission rates 6 months after the switch
For the cohort as a whole and the subgroup of CD patients, significant decreases in faecal calprotectin median levels were observed following the switch, whereas no change was seen in UC patients (Figure 2A). The remission rates as defined by faecal calprotectin remained stable for all three groups (Figure 2B). A subgroup analysis of faecal calprotectin levels in patients with limited ileal CD showed a median of 145 μg/g (IQR 48–281) at baseline and 94 μg/g (IQR 50.0–175.5) at follow‐up. The corresponding values for the entire CD group were 64.0 (IQR 12.5–238.5) μg/g and 49.0 μg/g (IQR 12.5–161.8), respectively. Analyses of plasma CRP levels did not show any significant changes before compared with after switch for the cohort as a whole, nor in the CD and UC subgroups (data not shown).
FIGURE 2.
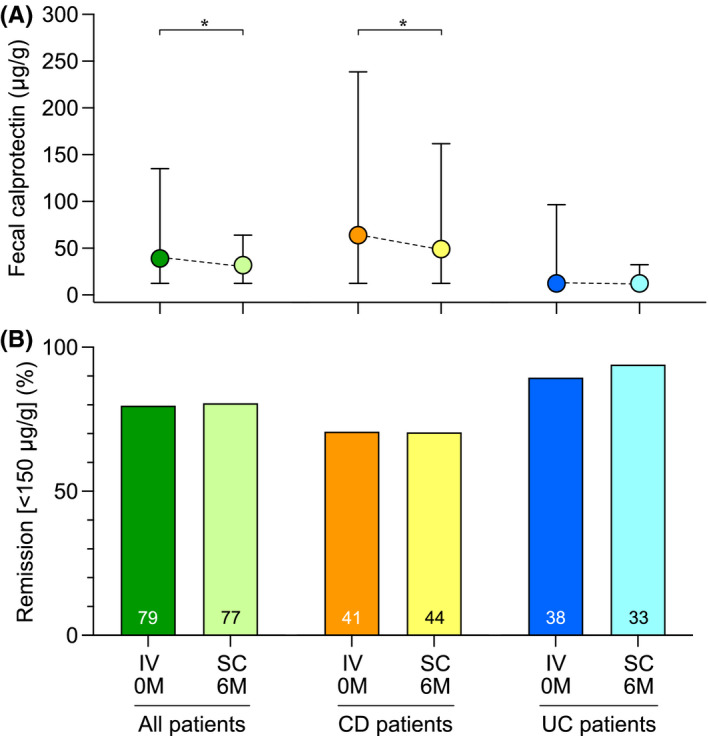
Analysis of faecal calprotectin levels and remission rates defined by faecal calprotectin at baseline and 6 months after the switch from IV to SC vedolizumab treatment. (A) Faecal calprotectin levels presented as median values with IQR. (B) Proportions of patients in remission as defined by a faecal calprotectin level of <150 μg/g. Abbreviations: CD, Crohn’s disease; IQR, interquartile range; IV, intravenous; M, months; SC, subcutaneous; UC, ulcerative colitis. *p < 0.05
Clinical disease activity in patients with CD, as measured by average patient‐based HBI and PRO2‐CD scores, remained unchanged (Figure 3A). Patients with UC displayed a statistically significant improvement in clinical symptoms according to the SCCAI although the median score remained unchanged, while the PRO2‐UC score did not show a significant change (Figure 3C). There were no statistically significant changes in the proportions of patients displaying clinical remission, as defined by patient‐based HBI ≤4 or PRO2‐CD ≤11 for patients with CD, and by SCCAI ≤2 or PRO2‐UC = 0 for patients with UC (Figure 3B,D).
FIGURE 3.
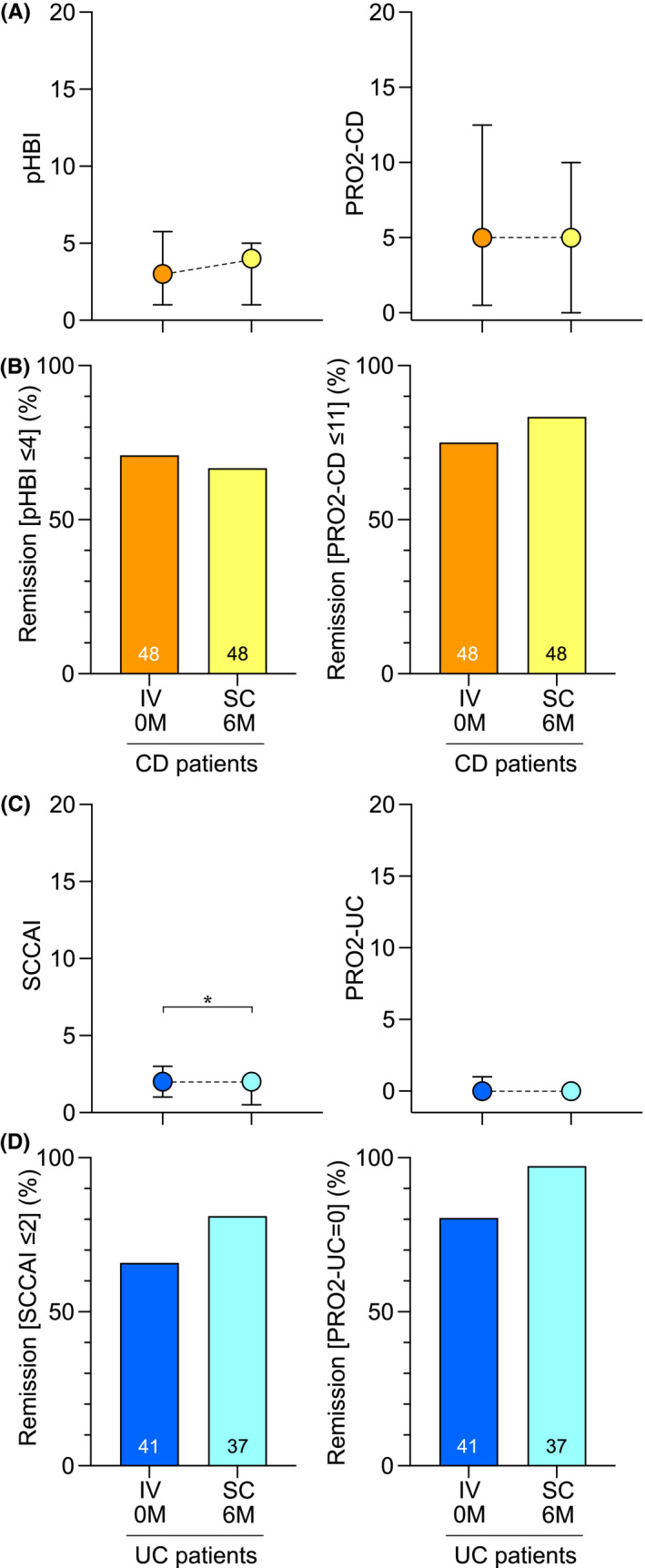
Analysis of clinical disease activity index score levels and remission rates at baseline and 6 months after the switch from IV to SC vedolizumab treatment. (A) Patient‐based HBI and PRO2‐CD scores for CD patients presented as median values with IQR. (B) Proportions of CD patients in remission as defined by a patient‐based HBI ≤4 or PRO2‐CD ≤11. (C) SCCAI and PRO2‐UC scores for UC patients presented as median values with IQR. (D) Proportions of UC patients in remission as defined by an SCCAI ≤2 or PRO2‐UC = 0. Abbreviations: CD, Crohn’s disease; IQR, interquartile range; IV, intravenous; M, months; pHBI, patient‐based Harvey Bradshaw index; PRO2‐CD, Crohn’s Disease Activity Index‐based patient‐reported outcomes‐2; PRO2‐UC, Mayo‐based patient‐reported outcomes‐2; SC, subcutaneous; SCCAI, simple clinical colitis activity index; UC, ulcerative colitis. *p < 0.05
At baseline, 10 patients had a diagnosis of perianal CD. Three of these had active perianal disease at baseline, and at follow‐up, this number was two.
3.2. Subgroup analyses of patients on optimised and standard dosing of IV vedolizumab
Twenty patients were on optimised IV vedolizumab dosing at baseline. At follow‐up, there was no significant change in their faecal calprotectin levels, whereas the 69 patients that had been on standard IV vedolizumab dosing showed a small but significant decrease (Figure 4A). Faecal calprotectin remission rates (Figure 4A) and plasma CRP median values (data not shown) remained unchanged in both groups. Clinical remission rates remained stable at follow‐up (Figure 4B,C) except for the PRO2‐UC remission rate for UC patients that had been on standard IV vedolizumab dosing, where a statistically significant improvement was seen (Figure 4C).
FIGURE 4.
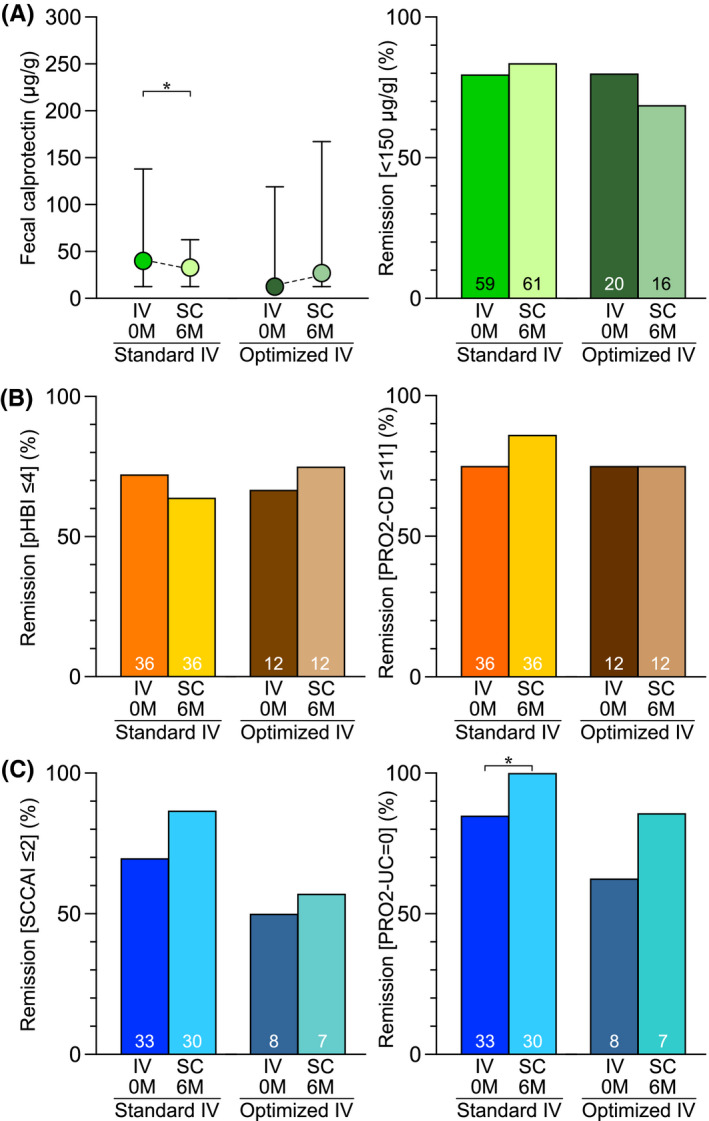
Subgroup analysis of faecal calprotectin and clinical disease activity levels at baseline and 6 months after the switch from IV to SC vedolizumab treatment for patients on standard versus optimised dosing of IV vedolizumab. (A) Faecal calprotectin levels presented as median values with IQR, and proportions of patients in remission as defined by a faecal calprotectin level of <150 μg/g, respectively. (B) Proportions of CD patients in remission as defined by a patient‐based HBI ≤4 or PRO2‐CD ≤11. (C) Proportions of UC patients in remission as defined by an SCCAI ≤2 or PRO2‐UC = 0. Abbreviations: CD, Crohn’s disease; IQR, interquartile range; IV, intravenous; M, months; pHBI, patient‐based Harvey Bradshaw index; PRO2‐CD, Crohn’s disease activity index‐based patient‐reported outcomes‐2; PRO2‐UC, Mayo‐based patient‐reported outcomes‐2; SC, subcutaneous; SCCAI, simple clinical colitis activity index; UC, ulcerative colitis. *p < 0.05
3.3. SC vedolizumab dose optimization
Dose optimization of SC vedolizumab to 108 mg weekly was deemed indicated in 10.1% of patients (6 CD, 3 UC). Three of these (1 CD, 2 UC) belonged to the subgroup of 20 patients (22.5%) that had been on intensified IV vedolizumab dosing before the switch, whereas the other 17 (85%) remained on standard SC dosing. All patients with perianal CD (n = 10) remained on treatment, but 3/10 were dose optimised to SC vedolizumab 108 mg weekly. No patient required hospitalisation or IV corticosteroid treatment due to disease worsening during the study.
3.4. Drug persistence and biomarker levels at 12 months after the switch
A subset of patients was evaluated at 12 months after the switch, in addition to the more comprehensive evaluation after the primary follow‐up period of 6 months. Drug persistence at 6 and 12 months were 95.5% (85/89) and 88.5% (69/78), respectively, for the whole cohort (Figure 5). Reasons for drug discontinuation (n = 9) were disease worsening in five patients, change in treatment regimen due to extraintestinal manifestations (present before the switch) in one patient, local injection reaction in one patient and adverse events in two patients (headache and repeated infections). There were no statistically significant changes in plasma CRP levels for the whole cohort (n = 50), CD (n = 27) or UC (n = 23); or in faecal calprotectin levels for the whole cohort (n = 20), CD (n = 10) or UC (n = 10), at 12 months (data not shown).
FIGURE 5.
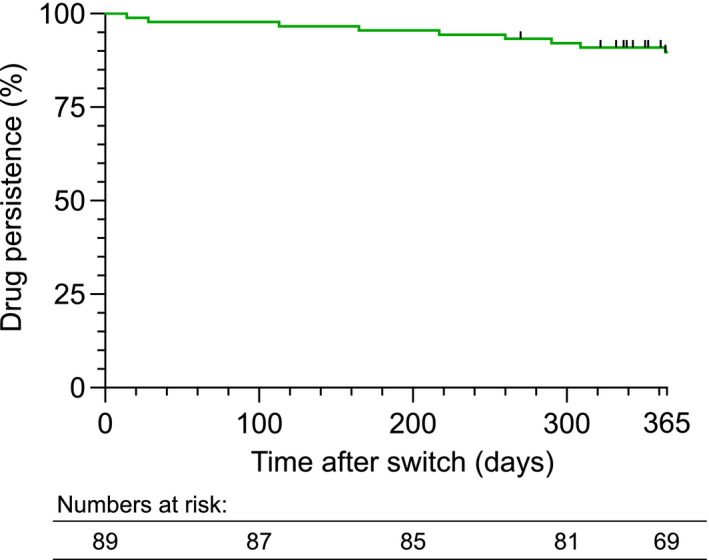
Kaplan–Meier survival curve for drug persistence after the switch from IV to SC vedolizumab treatment. Events refer to treatment discontinuation. Censored points refer to patients that have not yet completed 12 months of follow‐up
3.5. Serum vedolizumab concentrations before and after the switch
Median serum vedolizumab trough levels at steady state on IV treatment (i.e. at baseline) were 8.1 μg/ml (IQR 5.2–14 μg/ml) for the whole cohort, 8.7 μg/ml (IQR 4.9–14.0 μg/ml) in CD patients, and 7.9 μg/ml (IQR 5.3–12.5 μg/ml) in UC patients. Median serum vedolizumab trough levels at steady state on SC treatment (i.e. at 6 months follow‐up) were 19.0 μg/ml (IQR 13.0–23.0 μg/ml) for the whole cohort, 19.0 μg/ml (IQR 12.0–22.8 μg/ml) in CD patients, and 18.5 μg/ml (IQR 15.0–23.8 μg/ml) in UC patients. When patients were on IV treatment, we found significantly higher faecal calprotectin levels, primarily among CD patients, in the quartiles with the lowest serum vedolizumab trough levels compared to those with higher serum vedolizumab trough levels (Figure 6A). This relationship was not observed after patients had been on SC treatment for 6 months (Figure 6B).
FIGURE 6.
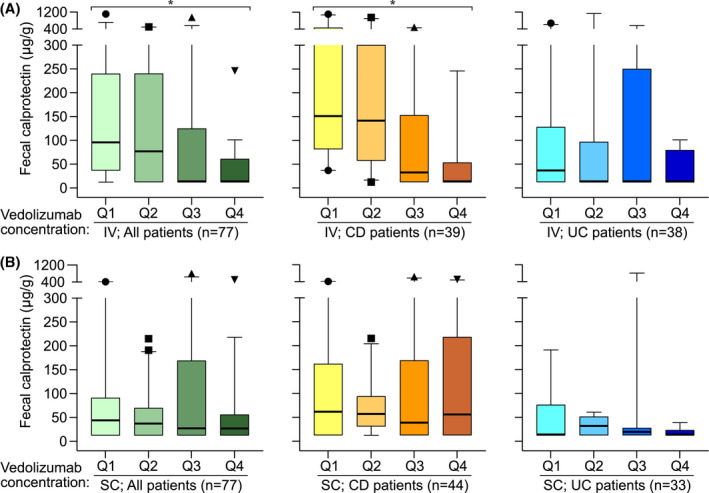
Associations between serum vedolizumab trough concentration quartiles (Q1 lowest; Q4 highest) and faecal calprotectin levels during IV and SC treatment for the entire cohort (all patients), and for the subgroups of CD and UC patients. (A) IV vedolizumab treatment. (B) SC vedolizumab treatment. Data are presented as median values with IQR (box), 1.5 × IQR (whiskers) and outliers. Abbreviations: CD, Crohn’s disease; IQR, interquartile range; IV, intravenous; Q, quartile; SC, subcutaneous; UC, ulcerative colitis. *p < 0.05
3.6. Quality‐of‐life and patient satisfaction regarding the switch and injector pen
We observed no differences in the SHS composite score or separate SHS items (Figure S1). Patient satisfaction with the injector pen (all eight categories; see Table S1 for details) was high with 94.1%–100% of patients responding “strongly agree”, “agree” or “agree to some extent” (Figure 7A). Overall satisfaction with the injection experience was generally high with 96.4% of the patients reporting being satisfied or very satisfied (Figure 7B). Generally, patients favoured SC administration over IV (Figure 7C). Only 2.4–9.4% of patients reported a slight preference for IV treatment over SC. Conversely, 55.3% of patients experienced SC treatment as slightly or clearly more effective, and 85.9% slightly or clearly more convenient (Figure 7C). Regarding overall feeling of security, no preference was the most frequent response (58.8%) and a slight preference for IV treatment was reported by 9.4% of patients (Figure 7C). Taking all aspects that follow with the given route of administration into account, 83.3% of patients reported a preference for SC over IV treatment, whereas the opposite was true for 2.4% of patients (Figure 7C).
FIGURE 7.
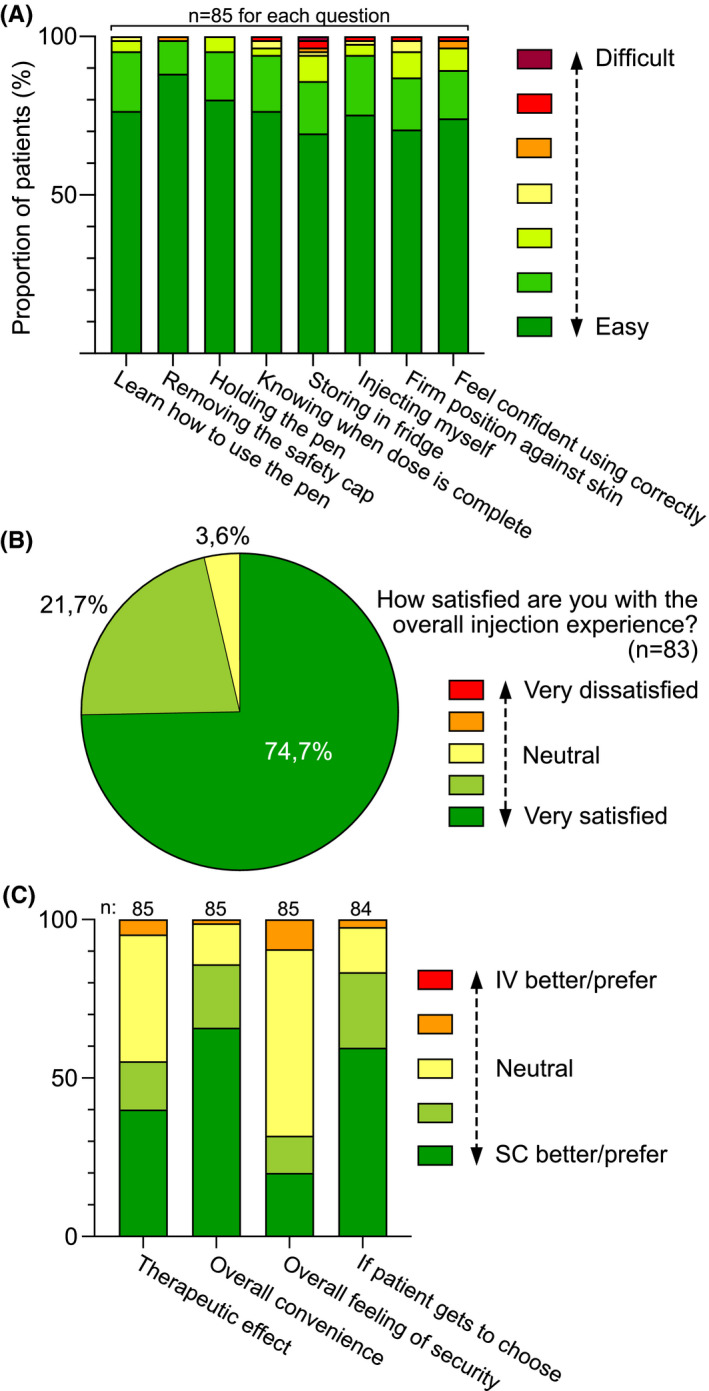
Patient satisfaction regarding various aspects of handling the vedolizumab injector pen, overall satisfaction with the injection experience, and patient preference comparing various aspects of IV versus SC vedolizumab treatment, based on questionnaires (Table S1). Data are presented for all patients (entire IBD cohort) collectively. (A) Aspects of handling the vedolizumab injector pen are perceived as easy or difficult to varying degrees. Data are presented as proportions of patients reporting a specific response option ranging from easy to difficult. (B) Satisfaction with the injection experience presented as proportions of patients reporting a specific response option ranging from very satisfied to very dissatisfied. (C) Patient preference comparing IV versus SC treatment in terms of patient‐perceived therapeutic effect, overall convenience, overall feeling of security and patient preference overall. Data are presented as proportions of patients reporting a specific response option ranging from clear preference for SC treatment to clear preference for IV treatment, with slight preference or no preference as response options in‐between. Abbreviations: IBD, inflammatory bowel disease; IV, intravenous, SC, subcutaneous
3.7. Adverse events experienced on IV and SC vedolizumab treatment
All reported adverse events are presented in Table 1. Adverse events, excluding local injection reactions, occurred in 15 (31.3%) of CD patients and 10 (24.4%) of UC patients. The corresponding rates for IV treatment were 27.1% and 22.0%, respectively. Some patients reported several side effects. The most common complaint was fatigue, followed by headache, nausea and rash. No serious adverse events were reported. Data on local skin reactions including discomfort, pain, burning sensation and erythema are presented in Table 1. The vast majority experienced none or only mild local injection reactions (88.0%–94.0% of patients, depending on the type of local injection reaction). Similar numbers were seen among CD and UC patients. Severe local symptoms were reported in only 1.2% of patients for the various subcategories. There was one case of drug discontinuation due to local injection reactions at 12 months of follow‐up, but none during the first 6 months.
3.8. Annualised costs related to SC as compared with IV vedolizumab treatment
In Sweden, the current fee for a visit to a registered nurse for administration of an IV infusion is approximately €290; the annual drug cost for IV vedolizumab (standard dosing) is approximately €13,700; and the annual drug cost for SC vedolizumab (standard dosing) is approximately €13,800. During IV vedolizumab treatment, 22.5% of patients required dose optimization (one infusion of 300 mg every 4–7 weeks or 600 mg every 8 weeks), and during SC vedolizumab treatment 10.1% were dose optimised to 108 mg of SC vedolizumab once weekly. Taking these factors into account, the annualised cost of maintenance treatment with SC vedolizumab was 15.0% lower than for maintenance treatment with IV vedolizumab.
4. DISCUSSION
The phase III VISIBLE studies investigated de novo treatment with SC vedolizumab. However, studies on switching patients that are on established maintenance treatment with IV vedolizumab to SC treatment are largely lacking. We report on a switch from IV to SC vedolizumab maintenance treatment in 89 adult IBD patients in a real‐world setting with a follow‐up time of 6 months and for a subgroup 12 months. Our results show that the levels of therapeutic efficacy, quality‐of‐life and adverse events were highly similar before as compared to after the switch with a high degree of drug persistence, and that the patients were in general very satisfied with being transferred to self‐administered SC treatment.
Faecal calprotectin levels showed a statistically significant decrease for the cohort as a whole and for CD patients, while levels remained unchanged for UC patients. However, absolute levels were low and the observed decreases may not be clinically relevant. On the other hand, subclinical changes in faecal calprotectin levels are considered to precede changes in clinical disease activity 21 , 22 , 23 , 24 and decreases of this type may further stabilise a state of clinical remission. Drug persistence was high with 95.5% of patients remaining on treatment after 6 months, and 88.5% after 12 months. These numbers are in line with those reported for IV vedolizumab treatment, ranging from 60–95% after 12 months. 25 , 26 , 27
Our evaluation of the outcome of the switch included thorough investigations of clinical disease activity, applying two activity indices for CD (patient‐based HBI and PRO2‐CD) and two for UC (SCCAI and PRO2‐UC), analysed by both median values and by proportions of patients in remission. All of these analyses corroborated the faecal calprotectin data, showing either unchanged disease activity levels after the switch in both CD and UC patients, or as in the case of SCCAI levels, a statistically significant improvement. For the 10 patients with perianal CD, the patient‐based HBI questionnaire was of particular interest since it included separate questions regarding active perianal fistula, perianal abscesses and anal fissures. At baseline, 3/10 had an active perianal disease but after the switch the number was 2/10 at follow‐up. From the literature, it seems that vedolizumab may have some therapeutic effect on perianal CD in a subset of patients, but that the effectiveness overall is moderate. 28 , 29 , 30 , 31 Our results do not contradict this view, but one should be cautious regarding conclusions given the low number of patients with perianal CD.
We also performed a subgroup analysis of patients with limited ileal CD, which showed measurable levels of faecal calprotectin with a numerical decrease after the switch. This confirms that faecal calprotectin was an adequate readout parameter also for this subset of CD patients, which has been debated but several recent studies have shown that faecal calprotectin is a sensitive measure of disease activity also in cases with limited ileal disease. 32 , 33 , 34 , 35
The SC dose of 108 mg every 2 weeks was chosen by the manufacturer with the intent to provide patients with similar average serum concentrations at steady state, as the IV dose of 300 mg every 8 weeks. 4 However, it is unclear whether average serum concentrations directly translate into levels of therapeutic efficacy. In addition, it is possible that the altered pharmacokinetics that comes with SC administration affect the various mechanisms of action of vedolizumab (i.e. those suggested to take place in the circulation compared to within tissues) in different ways. 1 , 2 , 3 Although the VISIBLE studies gave us an indication as to what serum levels are common with SC vedolizumab at standard dose, the studies did not address which levels are therapeutically optimal. In our study, serum vedolizumab trough concentrations at steady state were approximately twice as high during SC as compared with IV treatment. These findings are in line with the VISIBLE studies 4 , 5 and the study by Ventress et al. 6 Our results suggest an inverse relationship between serum vedolizumab trough levels and faecal calprotectin levels with vedolizumab given IV. However, after 6 months with SC vedolizumab at standard dose, this correlation was not seen. This change was primarily observed in CD patients, which also displayed a statistically significant decrease in faecal calprotectin levels after having switched to SC treatment. Our subgroup analyses of patients that had been on optimised IV dosing did not show any signs of disease worsening in terms of remission rates, clinical index scores, biomarker levels or drug persistence, after the switch to standard SC dosing. Interestingly, patients on standard IV dosing showed a statistically significant improvement in faecal calprotectin levels on SC treatment, while other outcome measures remained unchanged. Taken together, these results suggest that some patients were underdosed when being on standard IV dosing and that they were more adequately dosed (or potentially overdosed at the group level) on SC standard dosing.
Ventress et al. suggested that the first dose of SC vedolizumab should be given 28 days after the last IV dose. This was based on the assumption that the serum drug concentrations observed in the VISIBLE studies are therapeutically the most appropriate (although this has not been studied) and that the drug levels should be kept at or above this limit. 6 With IV vedolizumab, patients' drug levels are below this limit during the entire second half of the 8‐week dosing interval. There is no evidence that the limited time that serum concentrations are below the SC serum steady‐state levels in the VISIBLE studies increases the risk of relapse, nor for dose‐dependent toxicity, if the first SC dose is given in close proximity to the last IV dose. 36 , 37 Thus, with current knowledge and with support from our data we would argue that the first SC dose may be administered when the next IV dose would have been given, or earlier due to potential practical aspects.
In our cohort, the rates of adverse events were similar before and after the switch, and no serious adverse events were reported. Some of the reported symptoms, including fatigue, headache, nausea and arthralgia, were transient and lasted 1–2 days after injection. The time to the maximum serum concentration after administration of vedolizumab subcutaneously is on average 7 days with a variation between 3–14 days. 36 Such transient reactions are thus not likely to be an effect of the drug per se but more likely representing nonpharmacological potentially immune‐mediated adverse effects, 38 , 39 alternatively representing a nocebo effect. 40 Investigating local injection reactions, moderate discomfort, pain or burning sensations were reported by 5%–11% of patients, and corresponding severe reactions by 1% of the patients. Conversely, 88%–94% reported none or only mild local injection reactions. These results are in line with the VISIBLE studies as well as with data for other SC biologics. 41 SC vedolizumab contains citrate which has been pointed out to be causing pain, but studies that have addressed this issue underscore that citrate is merely one of several factors that may affect potential pain sensation at SC injection and that some of the studies attributing pain at the injection site to citrate are difficult to interpret since citrate was one of several factors modified. 42 , 43 , 44 Other factors that may be equally important in this context are other buffers commonly used such as phosphate and histidine, the buffer concentration, injected volume, solution temperature, pH, osmolality, needle gauge, injector device, injection speed, injection technique and low body weight. 42 , 43 , 44
We also investigated various aspects of the patients' experience with the injector pen and the switch from IV to SC treatment. Overall patients found the injector pen to be user‐friendly and they were very satisfied with switching to SC treatment, which was reflected in all aspects explored. However, this dataset can also be used to illuminate the group of patients, albeit small (2%–9%), that preferred IV infusions. Thus, SC administration may not be the best option for all patients. 45 One caveat with SC treatment is that it may be more difficult to ensure patient compliance. 46 , 47 Hence, for patients where compliance historically has been a problem or if risk factors for non‐adherence are present, IV therapy may be advisable. 48
Evaluations of health‐related quality‐of‐life using the SHS instrument 13 , 14 showed no statistically significant differences after the switch, but there was a slight numerical trend towards improved overall quality‐of‐life as well as better symptom‐related and social function‐related quality‐of‐life in patients with CD. These findings underscore the high level of satisfaction regarding the switch.
The annualised cost of SC vedolizumab maintenance treatment was calculated to be 15.0% lower than for IV maintenance treatment. Another structural benefit was that nurse resources were liberated for other work tasks. This was especially valuable in times of a pandemic when the number of nurses at the outpatient clinic had to be diminished to enable staffing of Covid‐19 wards. In addition, avoiding hospital visits in this context was desirable to prevent transmission of the virus.
This study had some important limitations. Firstly, there was no control group that was continued on IV treatment. Secondly, patients were not evaluated endoscopically. Lastly, anti‐drug antibodies were not measured.
In conclusion, this study shows that a switch from IV to SC vedolizumab maintenance treatment can be done with maintained efficacy, safety and tolerability, including in patients on optimised IV vedolizumab dosing. In addition, patient satisfaction regarding the switch was overall high, although for a small proportion of patients IV treatment may be advisable. The appropriate window for serum vedolizumab concentration to target for combined optimal efficacy, patient convenience and cost‐effectiveness during SC maintenance treatment, is still unclear and should be addressed in future studies.
AUTHOR CONTRIBUTIONS
VB contributed with acquisition, analysis and interpretation of data, as well as study design and writing the manuscript. JH was involved in study design, data analysis and data interpretation. DK was involved in study design and data interpretation. JM contributed with the detailed planning of the study including conceptualization and design, analysis and interpretation of data. All authors contributed intellectually to the work carried out within the framework of this study, critically revised the manuscript and approved the final version.
AUTHORSHIP
Guarantor of the article: Jan Marsal.
Supporting information
FigureS 1
TableS 1
TableS 2
ACKNOWLEDGEMENTS
We would especially like to thank research nurses Ida Kapusta and Ann Tornberg at the Gastroenterology Outpatient Clinic at Skane University Hospital for their excellent work in managing patient contact and communication throughout the study as well as for being instrumental in the data capturing process. We would also like to thank the staff at the same outpatient clinic for facilitating this project and for all the hard work during the pandemic.
Declaration of personal interests: DK has received speaker honoraria from Norgine. JH has received speaker honoraria from Takeda and has served as a consultant for Janssen‐Cilag. JM has served as a speaker, consultant or advisory board member for AbbVie, Bayer, Bristol‐Myers Squibb, Hospira, Janssen‐Cilag, Merck Sharp & Dohme, Pfizer, Sandoz, Takeda and Union Chimique Belge, and has received grant support from AbbVie, Calpro AS, Fresenius Kabi, Pfizer, SVAR Life Science and Takeda (not for this study).
Declaration of funding interests: This work was made possible through the financial support from the Healthcare Region of Southern Sweden, and by grants to researchers in the public health care from the Swedish government (ALFSKANE‐539811) to Jan Marsal.
Bergqvist V, Holmgren J, Klintman D, Marsal J. Real‐world data on switching from intravenous to subcutaneous vedolizumab treatment in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2022;55:1389–1401. 10.1111/apt.16927
Funding information
This work was made possible through financial support from the Healthcare Region of Southern Sweden, and by grants to researchers in public health care from the Swedish government (ALFSKANE‐539811) to Jan Marsal.
The Handling Editor for this article was Dr Rohit Loomba, and it was accepted for publication after full peer‐review.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
REFERENCES
- 1. Luzentales‐Simpson M, Pang YCF, Zhang A, Sousa JA, Sly LM. Vedolizumab: potential mechanisms of action for reducing pathological inflammation in inflammatory bowel diseases. Front Cell Dev Biol. 2021;9:612830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wyant T, Fedyk E, Abhyankar B. An overview of the mechanism of action of the monoclonal antibody vedolizumab. J Crohns Colitis. 2016;10(12):1437–44. [DOI] [PubMed] [Google Scholar]
- 3. Zeissig S, Rosati E, Dowds CM, Aden K, Bethge J, Schulte B, et al. Vedolizumab is associated with changes in innate rather than adaptive immunity in patients with inflammatory bowel disease. Gut. 2019;68(1):25–39. [DOI] [PubMed] [Google Scholar]
- 4. Sandborn WJ, Baert F, Danese S, Krznarić Ž, Kobayashi T, Yao X, et al. Efficacy and safety of vedolizumab subcutaneous formulation in a randomized trial of patients with ulcerative colitis. Gastroenterology. 2020;158(3):562–572 e512. [DOI] [PubMed] [Google Scholar]
- 5. Vermeire S, D’Haens G, Baert F, Danese S, Kobayashi T, Loftus EV Jr, et al. Efficacy and safety of subcutaneous vedolizumab in patients with moderately to severely active Crohn’s disease: results from the VISIBLE 2 randomised trial. J Crohns Colitis. 2022;16(1):27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ventress E, Young D, Rahmany S, Harris C, Bettey M, Smith T, et al. Transitioning from intRavenous to subcutAneous VEdolizumab in patients with infLammatory bowEl diSeaSe (TRAVELESS). J Crohns Colitis. 2021; jjab224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bjorkesten CG, Nieminen U, Turunen U, Arkkila P, Sipponen T, Farkkila M. Surrogate markers and clinical indices, alone or combined, as indicators for endoscopic remission in anti‐TNF‐treated luminal Crohn’s disease. Scand J Gastroenterol. 2012;47(5):528–37. [DOI] [PubMed] [Google Scholar]
- 8. Harvey RF, Bradshaw JM. A simple index of Crohn’s‐disease activity. Lancet. 1980;1(8167):514. [DOI] [PubMed] [Google Scholar]
- 9. Turner D, Ricciuto A, Lewis A, D'Amico F, Dhaliwal J, Griffiths AM, et al. STRIDE‐II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat‐to‐target strategies in IBD. Gastroenterology. 2021;160(5):1570–83. [DOI] [PubMed] [Google Scholar]
- 10. Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut. 1998;43(1):29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khanna R, Zou G, D’Haens G, et al. A retrospective analysis: the development of patient reported outcome measures for the assessment of Crohn’s disease activity. Aliment Pharmacol Ther. 2015;41(1):77–86. [DOI] [PubMed] [Google Scholar]
- 12. Jairath V, Khanna R, Zou GY, Stitt L, Mosli M, Vandervoort MK, et al. Development of interim patient‐reported outcome measures for the assessment of ulcerative colitis disease activity in clinical trials. Aliment Pharmacol Ther. 2015;42(10):1200–10. [DOI] [PubMed] [Google Scholar]
- 13. Hjortswang H, Jarnerot G, Curman B, et al. The short health scale: a valid measure of subjective health in ulcerative colitis. Scand J Gastroenterol. 2006;41(10):1196–203. [DOI] [PubMed] [Google Scholar]
- 14. Stjernman H, Granno C, Jarnerot G, et al. Short health scale: a valid, reliable, and responsive instrument for subjective health assessment in Crohn’s disease. Inflamm Bowel Dis. 2008;14(1):47–52. [DOI] [PubMed] [Google Scholar]
- 15. Callis Duffin K, Bukhalo M, Bobonich MA, Shrom D, Zhao F, Kershner J, et al. Usability of a novel disposable autoinjector device for ixekizumab: results from a qualitative study and an open‐label clinical trial, including patient‐reported experience. Med Devices (Auckl). 2016;9:361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dehoratius RJ, Brent LH, Curtis JR, Ellis LA, Tang KL. Satisfaction with subcutaneous golimumab and its auto‐injector among rheumatoid arthritis patients with inadequate response to adalimumab or etanercept. Patient. 2018;11(3):361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tischer B, Mehl A. Patients' and nurses' preferences for autoinjectors for rheumatoid arthritis: results of a European survey. Patient Prefer Adherence. 2018;12:1413–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Papp KA, Fonjallaz P, Casset‐Semanaz F, Krueger JG, Wittkowski KM. Analytical approaches to reporting long‐term clinical trial data. Curr Med Res Opin. 2008;24(7):2001–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thabane L, Mbuagbaw L, Zhang S, Samaan Z, Marcucci M, Ye C, et al. A tutorial on sensitivity analyses in clinical trials: the what, why, when and how. BMC Med Res Methodol. 2013;13:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fu EL, van Diepen M, Xu Y, Trevisan M, Dekker FW, Zoccali C, et al. Pharmacoepidemiology for nephrologists (part 2): potential biases and how to overcome them. Clin Kidney J. 2021;14(5):1317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Vos M, Louis EJ, Jahnsen J, et al. Consecutive fecal calprotectin measurements to predict relapse in patients with ulcerative colitis receiving infliximab maintenance therapy. Inflamm Bowel Dis. 2013;19(10):2111–7. [DOI] [PubMed] [Google Scholar]
- 22. Mao R, Xiao YL, Gao X, Chen BL, He Y, Yang L, et al. Fecal calprotectin in predicting relapse of inflammatory bowel diseases: a meta‐analysis of prospective studies. Inflamm Bowel Dis. 2012;18(10):1894–9. [DOI] [PubMed] [Google Scholar]
- 23. Molander P, Farkkila M, Ristimaki A, et al. Does fecal calprotectin predict short‐term relapse after stopping TNFalpha‐blocking agents in inflammatory bowel disease patients in deep remission? J Crohns Colitis. 2015;9(1):33–40. [DOI] [PubMed] [Google Scholar]
- 24. Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000;119(1):15–22. [DOI] [PubMed] [Google Scholar]
- 25. Eriksson C, Marsal J, Bergemalm D, Vigren L, Björk J, Eberhardson M, et al. Long‐term effectiveness of vedolizumab in inflammatory bowel disease: a national study based on the Swedish National Quality Registry for inflammatory bowel disease (SWIBREG). Scand J Gastroenterol. 2017;52(6–7):722–9. [DOI] [PubMed] [Google Scholar]
- 26. Eriksson C, Rundquist S, Lykiardopoulos V, Udumyan R, Karlén P, Grip O, Söderman C, Almer S, Hertervig E, Marsal J, Gunnarsson J, Malmgren C, Delin J, Strid H, Sjöberg M, Öberg D, Bergemalm D, Hjortswang H, Halfvarson J, The SWIBREG SVEAH Study Group Real‐world effectiveness of vedolizumab in inflammatory bowel disease: week 52 results from the Swedish prospective multicentre SVEAH study. Therap Adv Gastroenterol 2021;14:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reinglas J, Gonczi L, Verdon C, Bessissow T, Afif W, Wild G, et al. Low rate of drug discontinuation, frequent need for dose adjustment, and no association with development of new arthralgia in patients treated with vedolizumab: results from a tertiary referral IBD center. Dig Dis Sci. 2020;65(7):2046–53. [DOI] [PubMed] [Google Scholar]
- 28. Ayoub F, Odenwald M, Micic D, Dalal SR, Pekow J, Cohen RD, et al. Vedolizumab for perianal fistulizing Crohn’s disease: systematic review and meta‐analysis. Intest Res. 2022;1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chapuis‐Biron C, Bourrier A, Nachury M, Nancey S, Bouhnik Y, Serrero M, et al. Vedolizumab for perianal Crohn’s disease: a multicentre cohort study in 151 patients. Aliment Pharmacol Ther. 2020;51(7):719–27. [DOI] [PubMed] [Google Scholar]
- 30. Feagan BG, Schwartz D, Danese S, Rubin DT, Lissoos TW, Xu J, et al. Efficacy of vedolizumab in Fistulising Crohn’s disease: exploratory analyses of data from GEMINI 2. J Crohns Colitis. 2018;12(5):621–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schwartz DA, Peyrin‐Biroulet L, Lasch K, Adsul S, Danese S. Efficacy and safety of 2 vedolizumab intravenous regimens for perianal fistulizing Crohn’s disease: ENTERPRISE study. Clin Gastroenterol Hepatol. 2021;S1542‐3565(21)01042‐9. [DOI] [PubMed] [Google Scholar]
- 32. Buisson A, Mak WY, Andersen MJ, et al. Fecal calprotectin is highly effective to detect endoscopic ulcerations in Crohn’s disease regardless of disease location. Inflamm Bowel Dis. 2021;27(7):1008–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Iwamoto F, Matsuoka K, Motobayashi M, Takenaka K, Kuno T, Tanaka K, et al. Prediction of disease activity of Crohn’s disease through fecal calprotectin evaluated by balloon‐assisted endoscopy. J Gastroenterol Hepatol. 2018;33(12):1984–9. [DOI] [PubMed] [Google Scholar]
- 34. Jung ES, Lee SP, Kae SH, Kim JH, Kim HS, Jang HJ. Diagnostic accuracy of fecal calprotectin for the detection of small bowel Crohn’s disease through capsule endoscopy: an updated meta‐analysis and systematic review. Gut Liver. 2021;15(5):732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kopylov U, Yung DE, Engel T, Avni T, Battat R, Ben‐Horin S, et al. Fecal calprotectin for the prediction of small‐bowel Crohn’s disease by capsule endoscopy: a systematic review and meta‐analysis. Eur J Gastroenterol Hepatol. 2016;28(10):1137–44. [DOI] [PubMed] [Google Scholar]
- 36. Entyvio (vedolizumab) ‐ Summary of product characteristics (SPC). European Medicines Agency (EMA). https://www.ema.europa.eu/en/documents/product‐information/entyvio‐epar‐product‐information_en.pdf. Accessed February 1, 2022.
- 37. Colombel JF, Sands BE, Rutgeerts P, Sandborn W, Danese S, D'Haens G, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017;66(5):839–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Johnson DE. Biotherapeutics: Challenges and opportunities for predictive toxicology of monoclonal antibodies. Int J Mol Sci 2018;19(11):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Patel SV, Khan DA. Adverse reactions to biologic therapy. Immunol Allergy Clin North Am. 2017;37(2):397–412. [DOI] [PubMed] [Google Scholar]
- 40. Fleischmann R, Jairath V, Mysler E, Nicholls D, Declerck P. Nonmedical switching from originators to biosimilars: does the nocebo effect explain treatment failures and adverse events in rheumatology and gastroenterology? Rheumatol Ther. 2020;7(1):35–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thomaidou E, Ramot Y. Injection site reactions with the use of biological agents. Dermatol Ther. 2019;32(2):e12817. [DOI] [PubMed] [Google Scholar]
- 42. Shi GH, Pisupati K, Parker JG, Corvari VJ, Payne CD, Xu W, et al. Subcutaneous injection site pain of formulation matrices. Pharm Res. 2021;38(5):779–93. [DOI] [PubMed] [Google Scholar]
- 43. St Clair‐Jones A, Prignano F, Goncalves J, Paul M, Sewerin P. Understanding and Minimising injection‐site pain following subcutaneous Administration of Biologics: a narrative review. Rheumatol Ther. 2020;7(4):741–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Usach I, Martinez R, Festini T, Peris JE. Subcutaneous injection of drugs: literature review of factors influencing pain sensation at the injection site. Adv Ther. 2019;36(11):2986–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Allen PB, Lindsay H, Tham TC. How do patients with inflammatory bowel disease want their biological therapy administered? BMC Gastroenterol. 2010;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van der Have M, Oldenburg B, Kaptein AA, Jansen JM, Scheffer RCH, van Tuyl BA, et al. Non‐adherence to anti‐TNF therapy is associated with illness perceptions and clinical outcomes in outpatients with inflammatory bowel disease: results from a prospective multicentre study. J Crohns Colitis. 2016;10(5):549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wentworth BJ, Buerlein RCD, Tuskey AG, Overby MA, Smolkin ME, Behm BW. Nonadherence to biologic therapies in inflammatory bowel disease. Inflamm Bowel Dis. 2018;24(9):2053–61. [DOI] [PubMed] [Google Scholar]
- 48. Shah NB, Haydek J, Slaughter J, Ashton JR Jr, Zuckerman AD, Wong R, et al. Risk factors for medication nonadherence to self‐injectable biologic therapy in adult patients with inflammatory bowel disease. Inflamm Bowel Dis. 2020;26(2):314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FigureS 1
TableS 1
TableS 2
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.


