Abstract
Inorganic polyphosphate (polyP) is obtained by the polymerization of the terminal phosphate of ATP through the action of the enzyme polyphosphate kinase (PPK). Despite the presence of polyP in every living cell, a gene homologous to that of known PPKs is missing from the currently sequenced genomes of Eukarya, Archaea, and several bacteria. To further study the metabolism of polyP in Archaea, we followed the previously published purification procedure for a glycogen-bound protein of 57 kDa with PPK as well as glycosyl transferase (GT) activities from Sulfolobus acidocaldarius (R. Skórko, J. Osipiuk, and K. O. Stetter, J. Bacteriol. 171:5162–5164, 1989). In spite of using recently developed specific enzymatic methods to analyze polyP, we could not reproduce the reported PPK activity for the 57-kDa protein and the polyP presumed to be the product of the reaction most likely corresponded to glycogen-bound ATP under our experimental conditions. Furthermore, no PPK activity was found associated to any of the proteins bound to the glycogen-protein complex. We cloned the gene corresponding to the 57-kDa protein by using reverse genetics and functionally characterized it. The predicted product of the gene did not show similarity to any described PPK but to archaeal and bacterial glycogen synthases instead. In agreement with these results, the recombinant protein showed only GT activity. Interestingly, the GT from S. acidocaldarius was phosphorylated in vivo. In conclusion, our results convincingly demonstrate that the glycogen-protein complex of S. acidocaldarius does not contain a PPK activity and that what was previously reported as being glycogen-bound PPK is a bacterial enzyme-like thermostable glycogen synthase.
Polyphosphate (polyP) is a linear polymer of hundreds of orthophosphate residues, linked by high-energy phosphoanhydride bonds. Likely prevalent in prebiotic evolution, polyP is found in every living organism, including the domain Archaea (14). This ubiquity is explained by the variety of physiological functions it performs, among them providing a reservoir of phosphate (Pi), substituting for ATP in kinase reactions, and chelating metals. Also it has recently been established that polyP has a role in adjustments to growth in response to nutrient limitation and during stationary phase (6). The main enzymes involved in the metabolism of polyP in bacteria are the polyphosphate kinase (PPK) that catalyzes the reversible conversion of the terminal phosphate of ATP into polyP and the exopolyphosphatase (PPX) that processively hydrolyzes the terminal residues of polyP to liberate Pi. These enzymes from Escherichia coli have been purified, and their genes have been cloned (2, 3). Manipulation of the genes responsible for polyP metabolism has been proposed as a possible way to remove heavy metals or phosphate from contaminated environments (12).
At present almost nothing is known about the metabolism of polyP in the domain Archaea. A gene homologous to ppk has not been described so far in the finished or unfinished archaeal genomes. The reported purification of an enzyme identified as a glycogen-bound PPK from Sulfolobus acidocaldarius (28) is, to our knowledge, the only described PPK activity in Archaea (25, 27). This putative PPK of 57 kDa was shown to be active only in the presence of glycogen, a striking feature considering known PPKs. Also, the 57-kDa protein was described as a glycosyl transferase (GT) by the same laboratory (13). The possibility of finding a novel archaeal ppk gene prompted us to repurify this protein and characterize it. By using definitive accurate and specific enzymatic assays to analyze polyP (5), we found that the glycogen-protein complex from S. acidocaldarius does not contain a PPK activity. Instead, the protein previously thought to be a PPK is a glycogen synthase.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
S. acidocaldarius DSM 639 was heterotrophically grown in medium 88 (Deutsche Sammlung von Mikroorganismen und Zellkulturen) with 0.1% yeast extract and 0.2% sucrose, according to the method of Skórko et al. (28). For 32P-labeling purposes, growth was done in the same medium but the yeast extract was replaced by 2% amino acids and the concentration of Pi was diluted 1:100. E. coli strains JM109 and BL21(DE3)pLysS were cultivated in Luria-Bertani medium at 37°C.
Purification of the previously described glycogen-bound PPK activity from S. acidocaldarius.
The glycogen-protein complex containing the 57-kDa protein was extracted by two-step isopycnic CsCl gradient centrifugation, as described by Skórko et al. (28). A culture was grown to an optical density at 600 nm (OD600) of 0.8 and was harvested by centrifugation (7,000 × g for 30 min). The pellet was washed, resuspended in 1 volume of buffer D (50 mM Tris-acetate [pH 7], 1 mM phenylmethylsulfonyl fluoride, 1 mM EDTA), and sonicated five times for 30 s. The lysate was centrifuged (10,700 × g for 10 min) to eliminate cellular debris, and the supernatant (F1) was loaded into a step density gradient of CsCl (step densities were 1.79, 1.52, 1.30, and 1.11). After 2 h of centrifugation (100,000 × g) the glycogen was located as a sharp turbid band near the bottom of the tube. The glycogen contained in this band was dialyzed against buffer D, diluted, and sedimented by centrifugation (100,000 × g for 2 h at 4°C). The final pellet, composed of the glycogen-protein complex, was resuspended in 1 volume of 50 mM Tris-acetate (pH 7.0) and stored at −20°C as fraction F2.
For the next purification step, the glycogen band obtained after 2 h of centrifugation (100,000 × g) was collected and reloaded into an identical gradient of CsCl and centrifuged at 100,000 × g for 48 h at 4°C. The glycogen contained in the band resulting after this centrifugation was dialyzed against buffer D, diluted, and sedimented by centrifugation (100,000 × g for 2 h). The final pellet was resuspended in 1 volume of 50 mM Tris-acetate (pH 7.0) and stored at −20°C as fraction F3.
In vivo labeling of S. acidocaldarius with H332PO4.
A 200-ml culture of S. acidocaldarius was grown to an OD600 of 0.8, harvested by centrifugation, and resuspended in 40 ml of medium 88 with 0.02 mM H332PO4 (6.25 μCi/nmol). The cells were further incubated for 24 h and harvested. The radioactively labeled glycogen-protein complex was isolated by centrifugation for 2 h in CsCl as described above.
Assay for PPK activity.
PPK activity was determined by using the buffer, salts, and temperature conditions reported by Skórko et al. (28), except that the method described by Ahn and Kornberg was followed (1). A 250-μl reaction mixture containing 50 mM Tris-acetate (pH 7), 2 mM MnCl2, 10 mM KCl, and 1 mM [γ-32P]ATP (1.08 mCi/mmol; NEN) was incubated for 1 h at 70°C. After the mixture was cooled on ice for 5 min, the reaction was stopped with 250 μl of 7% HClO4 and 50 μl of 2-mg/ml bovine serum albumin. The acid-precipitated 32P-labeled material was collected on Whatman GF/C glass fiber filters and washed with 0.1 M pyrophosphate and 1 M HCl, followed by ethanol. Quantitation was done by liquid scintillation counting. One unit of enzyme was defined as the amount incorporating 1 pmol of phosphate from ATP into polyP per min at 70°C.
Assay for GT activity.
GT activity was assayed according to the method of König et al. (13). A 50-μl reaction mixture containing 50 mM Tris-acetate (pH 7), 1 mM EDTA, 22 mM NH4Cl, and 5 mM UDP-[U-14C]glucose (4 mCi/mol; Amersham) was incubated for 30 min at 70°C. The reaction was stopped with 117 μl of ethanol. Incorporation of [U-14C]glucose into the precipitated 14C material was quantified by liquid scintillation counting after collection on Whatman GF/C glass fiber filters and washing with 70% ethanol. One unit of enzyme was defined as the amount incorporating 1 pmol of glucose into glycogen per min at 70°C.
In vitro preparation of [32P]polyP750.
Radioactively labeled polyP was prepared as described by Ault-Riché et al. (6). According to the properties of E. coli PPK, the synthesized polyP has a uniform length of around 750 residues (16). A 0.35-ml reaction mixture contained 50 mM Tris-HCl (pH 7.4), 40 mM (NH4)2S04, 4 mM MgCl2, 40 mM creatine phosphate, 20 μg of creatine kinase per ml, 1 mM [γ-32P]ATP (14 μCi/nmol), and 35,000 U of purified recombinant PPK from E. coli (PPKEco) (16). After 30 min of incubation at 37°C the mixture was cooled on ice for 5 min, and the reaction was stopped by the addition of 35 μl of 0.5 M EDTA.
The polyP reaction mixture was loaded over a cushion of 1.9 ml of 2.5 M CsCl–50 mM Tris-HCl (pH 7.4)–10 mM EDTA. After centrifugation at 45,000 rpm for 4 h at 4°C in an AH-650 rotor (Sorvall), aliquots of 200 μl were taken and to each one was added 140 μl of isopropanol. After incubation at room temperature for 30 min and centrifugation at 13,000 rpm for 30 min in an Eppendorf centrifuge, the supernatants were removed and the pellets were washed twice with 600 μl of 70% ethanol, dried overnight in a vacuum-desiccator, and resuspended in 25 μl of distilled water.
Assay of polyP.
PolyP was assayed according to the method of Wurst et al. (32) in a 20-μl reaction mixture containing 20 mM Tris-HCl (pH 7.5), 5 mM Mg(CH3COO)2, 50 mM (NH4)2SO4, 200 μM [32P]polyP750, and 6,000 U of PPXSce (32). Conversion of polyP to Pi was analyzed by ascending thin layer chromatography (TLC) in polyethyleneimine-cellulose (Merck) using 0.75 M KH2P04, (pH 3.5) as the solvent. The products obtained were quantified after autoradiography.
Protein analysis.
Protein concentration was determined by the method of Bradford (CoomassiePlus Protein Assay Reagent; Pierce). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and staining with Coomassie blue were performed as described before (17). Two-dimensional nonequilibrium pH polyacrylamide gel electrophoresis (2-D NEPHGE) (pH 3 to 10 in the first dimension) was performed as described by O'Farrell et al. (20) and as used for S. acidocaldarius in our laboratory (21). The second dimension consisted of an SDS–11.5% PAGE, followed by staining with Coomassie blue and drying. When 32P-labeled proteins were analyzed, they were detected by autoradiography after 7 days of exposure.
Isolation of P60 from 2-D gels and amino-terminal amino acid sequencing.
The 57-kDa protein discussed in the work of Skórko et al. (28) migrated with a molecular mass of 60 kDa under our conditions and will be referred to as P60. The glycogen-protein complex was separated by 2-D PAGE, and the protein spots were cut out from the dried Coomassie blue-stained gels. After rehydration in 500 μl of 50 mM H3BO3–0.1% SDS for 2 h at room temperature, the spots were concentrated by SDS-PAGE. The proteins were electroblotted onto a polyvinylidene difluoride Inmobilon P (Millipore) membrane as described by Towbin et al. (30) by employing the Trans-Blot Cell system (Bio-Rad) in transfer buffer and application of a 0.8-A constant current for 48 min. For the generation of internal peptides from P60, the protein was subjected to partial proteolysis with endolysine C. The peptides were separated by high-pressure liquid chromatography. Amino-terminal end sequencing was performed in the Laboratoire de Microséquençage des Protéines of the Institut Pasteur.
DNA manipulations.
Restriction enzyme digestions and T4 DNA ligase reactions were performed according to the manufacturer's recommendations. Recombinant DNA techniques and Southern blotting were carried out according to standard laboratory procedures (23). Prehybridization and hybridization reactions were performed at 42°C with the DIG Easy Buffer (Roche). Digoxigenin-labeled probes were obtained by PCR as described by Roche with the nondegenerated primers P60ND1D (5′-GCTAGAGAAAGTAGCTAGTC-3′) and P60ND2R (5′-TATTTCAGCCCTATCCTCAGT-3′) deduced from the sequence of the S. acidocaldarius DNA fragment obtained by degenerate oligonucleotide primer (DOP)-PCR. Detection of digoxigenin-labeled DNA fragments was accomplished by using the DIG Luminescent Detection Kit as described by Roche.
The dideoxy chain termination method was employed to sequence DNA using [γ-33P]ATP and the dsDNA Cycle Sequencing System from GIBCO-BRL. The DNA sequences were compiled and analyzed with the University of Wisconsin GCG Package (version 9.1; Genetics Computer Group, Madison).
Primers and PCR conditions.
The oligonucleotide primers were purchased from Genset Corporation. Taq polymerase and Elongase were from Promega and GIBCO-BRL, respectively, and were used according to the manufacturer's recommendations. The DNA fragments were recovered from 1% agarose gels, purified with Wizard PCR Prep (Promega), and cloned into the pGEM-T vector (Promega). Twenty-mer DOPs were designed on the basis of P60 amino-terminal sequence determinations. Sixty picomoles of each nucleotide and 25 ng of S. acidocaldarius total DNA were used in 50-μl reaction mixtures.
The DOPs for DOP-PCR were P60NH2DD (5′-YTNAARCAYGTNTGGATGAT-3′), P6021DD (5′-ATHATHGAYWSNTGGAAYAT-3′), P6021DR (5′-ATRTTCCANSWRTCWATWAT-3′), P6027DD (5′-ACNGARGAYMGNGCNGARAT-3′), and P6027DR (5′-ARNACYTCNARYTCRTCRAA-3′).
DOP-PCR amplification conditions were 3 min at 95°C followed by 30 cycles at 95°C for 30 s, 40°C for 30 s, and 72°C for 30 s, and then 3 min at 72°C.
Amplification of flanking sequences was done by inverse PCR as was described before by Ochman et al. (19). Inverse PCRs with nondegenerate primers P60ND3R (5′-AGACTAGCTACTTTCTCTAC-3′) and P60ND5D (5′-CTTCTCTTCTGGTTCCATAG-3′) were performed on total S. acidocaldarius DNA digested by XhoI and religated as follows: 3 min at 95°C followed by 30 cycles at 95°C for 25 s, 67°C for 30 s, and 72°C for 1 min, and then 3 min at 72°C.
p60 gene cloning and expression.
We used the pET system from Novagen. The p60 gene was obtained by PCR using P60NNdeI (5′-TTAACATATGAAGAGATATGAAAGCCT-3′) and P60CAvaI2 (5′-AATACTCGAGAAATGATGCTAACAGTCTAT-3′) primers corresponding to the N-terminal and C-terminal end sequences of P60 and containing NdeI and AvaI restriction sites, respectively. We used Elongase (GIBCO-BRL) and a low number of amplification cycles to decrease sequence errors. After purification of the amplified DNA fragment and digestion by the corresponding restriction enzymes, the DNA fragment was ligated to the pET21b(+) vector previously digested with NdeI and AvaI. The ligation product [pET21b(+)P60 vector] was used to transform E. coli strain BL21(DE3)pLysS. The recombinant clones were selected on Luria-Bertani solid medium supplemented with ampicillin (50 μg/ml) and chloramphenicol (34 μg/ml). The induction and expression analysis was done in the presence or absence of 2 mM isopropyl-β-d-thiogalactopyranoside (IPTG) added when the cultures reached an OD600 of 0.6. Expression of the recombinant P60 (rP60) was determined in total cell fractions and membrane fractions.
Purification of rP60.
rP60 was purified under denaturing conditions as follows. A 400-ml culture of BL21(DE3)pLysS transformed with pET21b(+)P60 vector was grown to an OD600 of 0.5 and induced with 2 mM IPTG. Cells were harvested by centrifugation, and the pellet was resuspended in 40 ml of 1× binding buffer containing 5 mM imidazole, 0.5 M NaCl, and 20 mM Tris HCl (pH 7.9). Cell disruption was performed by sonication (three times for 30 s each). After centrifugation (20,000 × g for 15 min) the pellet (membrane fractions) was resuspended in 10 ml of 1× binding buffer containing 6 M urea and incubated for 1 h on an ice bath. The sample was centrifuged (40,000 × g for 20 min), and the supernatant, previously filtered through a 0.45-μm-pore-size Millipore filter, was applied onto a column containing 1.5 ml of His-Bind resin (Novagen). rP60 was eluted with 9 ml of elute buffer containing 300 mM imidazole, 0.25 mM NaCl, 10 mM Tris-HCl (pH 7.9), and 6 M urea. The collected fractions (0.5 ml) were analyzed by SDS-PAGE. Finally, rP60-containing fractions, which were essentially free from other proteins, were pooled and renatured by dialyzing the urea away in three sequential steps with 50 mM Tris-acetate (pH 7) buffer containing 4 M, 2 M, and no urea.
Sequence analysis.
Identity and similarity searching in databases was done using the BlastP program (4) from the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov) and from the Sulfolobus solfataricus genome site (http://niji.imb.nrc.ca/sulfolobus/). Multiple alignments were performed with ClustalW 1.8 (http://dot.imgen.bcm.tmc.edu:9331/multi-align/multi-align.html) and edited using BOXSHADE 3.21 (http://www.isrec.isb-sib.ch:8080/software/BOX_form.html). Searching of conserved domains was done with RPS-BLAST 2.1.2 (http://www.ncbi.nlm.nih.gov/Structure/cdd/). Identification of potential phosphorylation sites of P60 was done with Phosphobase (15) (http://www.cbs.dtu.dk/databases/PhosphoBase/index.html).
Nucleotide sequence accession number.
The nucleotide sequence of the p60 gene (glgA) from S. acidocaldarius is available in the EMBL database under accession no. AJ294724.
RESULTS
Purification of glycogen-bound P60 and assays of PPK and GT activities.
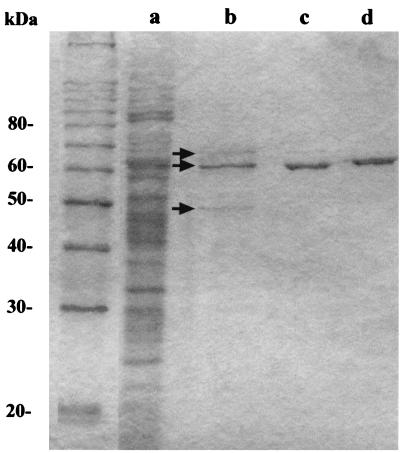
The two-step purification of the protein described as glycogen-bound PPK in S. acidocaldarius, was repeated essentially as described by Skórko et al. (28). After 2 h of CsCl centrifugation we obtained the previously reported sharp turbid band produced by the glycogen-protein complex. The presence of glycogen in this band was confirmed by acid hydrolysis during 2 h in 1 N HCl and enzymatic hydrolysis with amyloglucosidase (Sigma) rendering glucose as the product of the reaction (data not shown). After dialysis and sedimentation, the glycogen-protein fractions were analyzed by SDS-PAGE (Fig. 1). F2 was composed of three protein bands of 65, 60, and 50 kDa (Fig. 1), similar in molecular mass to those previously reported (61, 57, and 46.5 kDa, respectively) by Skórko et al. (28). As described before for the band of 57 kDa, reported to be the glycogen-bound PPK (28), after 48 h of CsCl centrifugation the resulting fraction (F3) was largely enriched in the band of 60 kDa (Fig. 1, lane c). Only a barely detectable band corresponding to the protein of 65 kDa was noticed. Thus, we assumed that this enriched protein of 60 kDa (P60) corresponded to the 57-kDa glycogen-bound protein described Skórko et al. (28).
FIG. 1.
Purification of the glycogen-bound P60. The different protein fractions obtained by the two-step isopycnic CsCl gradient centrifugation were analyzed by SDS-PAGE. Protein bands were visualized by staining with Coomassie blue. Lanes: a, total cell extract fraction (F1); b, 2-h CsCl centrifugation fraction (F2); c, 48-h CsCl centrifugation fraction (F3); d, purified recombinant P60 (rP60). Arrows, protein bands of 65, 60, and 50 kDa.
As the 57-kDa band was described to be composed by several polypeptides of different isoelectric points (28) and in view of the reported PPK (28) and GT activities (13) for the same protein, we assayed these fractions simultaneously for both activities (Table 1). Crude extracts from S. acidocaldarius showed GT activity and PPK activity (as determined by measuring the 32P-labeled acid-precipitable material). However, although these activities seemed to be present in the glycogen- bound complex (F2), the yields of the supposed PPK and GT activities were quite poor (0.2% for PPK and 5% for GT) indicating that only a minor fraction of these activities was bound to glycogen, specially in the case of PPK. The specific activity of PPK was 11 times higher in F2 and 5 times higher in F3 than in crude extracts. This indicates that during the centrifugation for 48 h in CsCl, a 50% loss of activity was observed for F3. In contrast, the GT activity was purified 644-fold. When the GT/PPK ratio was calculated it did not remain constant, clearly showing that the GT activity was being purified while PPK activity was not. This could be due to some kind of inactivation of the PPK activity or to the loss of the enzyme from the F3 fraction. In addition, our results cannot discard the possibility that P65, still present in very low amounts in F3 (Fig. 1), could be responsible for the PPK activity. Skórko et al. (28) attributed the PPK and GT activities to the band of 57 kDa. Their PPK activity was reported as 2.34 nmol of phosphate incorporated into polyP per h. However, the specific activity for this enzyme was not reported. We estimated this value, based on their assay conditions, to be 19,500 U/mg of protein, much higher than the value observed in our assays. This lack of congruence of our data with their previously reported results led us to investigate in more detail the method used by Skórko et al. (28) in measuring PPK activity and to compare it with the one we used. Briefly, to measure the synthesized polyP, they boiled in Laemmli's sample buffer a 21-μl reaction mixture containing the glycogen-protein complex and [γ-32P]ATP (0.9 μCi) for 3 min at 100°C and loaded it on a standard SDS–10% PAGE. The supposed polyP was detected as the radioactivity remaining at the origin of the running gel by autoradiography since, as they claimed, polyP failed to enter the SDS-polyacrylamide gel. Instead, to measure the supposed PPK activity, we used the same buffer and conditions as those described by Skórko et al. (28) except that a lower radioactivity (0.27 μCi of [γ-32P]ATP) was used, the acid-precipitated 32P-labeled compound present in the reaction mixture was filtered through glass fiber filters and washed, and the retained radioactivity was measured by liquid scintillation counting (see Materials and Methods). When we repeated the PPK assay exactly according to Skórko et al. (28), we noticed that no radioactivity was obtained in the stacking-running interface of the SDS-polyacrylamide gel, as they described for the polyP location, while the glycogen from S. acidocaldarius and a control glycogen from oyster (Sigma) were found at this position in the gel, as revealed by silver staining (data not shown). In addition, when 2 nmol of purified [32P]polyP750 (0.007 μCi/nmol) was loaded in the gel as a control, this compound did not remain in the stacking gel but ran out of the gel.
TABLE 1.
Enzymatic activities reported to be associated to the glycogen-protein complex from S. acidocaldarius
| Fraction | Total protein (mg) | PPKa
activity
|
GT activity
|
GT/PPK activity ratio | ||
|---|---|---|---|---|---|---|
| Total units | U/mg of protein | Total units | U/mg of protein | |||
| F1 | 116 | 44,660 | 385 | 208,916 | 1,801 | 4.7 |
| F2 | 0.02 | 84.46 | 4,223 | 10,054 | 502,700 | 119 |
| F3 | 0.01 | 18.43 | 1,843 | 11,600 | 1.16 × 106 | 629 |
The putative PPK activity was measured as the acid-precipitable 32P-labeled material, collected on Whatman GF/C glass fiber filters.
Analysis of the reaction products of the PPK assay.
Although the method for polyP synthesis measurement that we used has been tried with success in many previous works (2, 5, 16), it is still necessary to demonstrate that the acid-precipitated 32P corresponds to polyP. The purified overexpressed PPK (16) and PPX from E. coli (3) and the PPX from Saccharomyces cerevisiae (PPXSce) (32) have been successfully used as specific reagents in polyP analysis (5). Pure PPK allows the in vitro synthesis of [32P]polyP750 for use as a marker, while the nature of the putative synthesized polyP can be confirmed by treatment with PPX and analysis of the reaction product by TLC (5). Typically, the nature of polyP was previously analyzed by acid hydrolysis of the compound. However, the use of PPX as an enzymatic reagent to hydrolyze the polyP is much more specific.
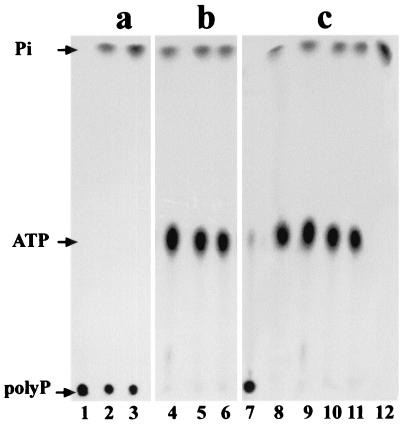
To accumulate the radioactively labeled material synthesized we performed the PPK assay with fraction F2, which had been shown to have the highest specific activity (Table 1), in the same conditions as described above but in a preparative manner as for the in vitro preparation of [32P]polyP750 (see Materials and Methods). We used 9 μg of the glycogen-bound proteins (F2) in a reaction mixture of 0.35 ml incubated for 30 min at 70°C. As a control, we ran in parallel the same reaction at 37°C using 35,000 U of purified PPKEco (16). To extract the polyP formed in each case, we loaded the reaction mixtures over a cushion of CsCl. After centrifugation, the gradient was divided in aliquots of 200 μl, precipitated with isopropanol, and washed. The pellets were resuspended in 20 μl of distilled water and quantified by liquid scintillation. The 32P-labeled compound was found to be present in the 200-μl fractions corresponding to the bottom of the CsCl gradients, as expected for polyP (6). These fractions were used as a substrate for the assay of polyP with PPXSce. Conversion of the putative polyP to Pi was followed by TLC (Fig. 2). When the polyP substrate obtained from the PPK assay using PPKEco was analyzed (Fig. 2a), the polyP spot, located at the origin of the TLC, clearly diminished with time by the action of PPX while Pi appeared concomitantly, confirming the identity of polyP (Fig. 2a, lanes 1, 2, and 3). However, no spot was observed in the location of polyP when the putative polyP substrate came from the reaction with fraction F2 (Fig. 2b). Instead, radioactive spots migrating with ATP and Pi were observed at all the times analyzed (Fig. 2b, lanes 4, 5, and 6). The observed Pi corresponded to partial hydrolysis of ATP as shown by a control tube without PPXSce (Fig. 2c, lanes 9, 10, and 11) and ATP run as a standard (Fig. 2c, lane 8). The putative polyP synthesized using fraction F2 therefore may correspond to the nonspecific binding of ATP to some isopropanol-precipitable compound, present at the bottom of the CsCl gradient, possibly the glycogen-protein complex.
FIG. 2.
TLC analysis of the reaction products obtained during PPK assay. [32P]polyP750 (panel a, lanes 1, 2, and 3), the 32P-labeled material obtained in the PPK assay using fraction F2 (panel b, lanes 4, 5, and 6 and panel c, lanes 9, 10, and 11) were incubated in the presence (a and b) or in the absence (c) of PPXSce at time zero (lanes 1, 4, and 9), at 5 min (lanes 2, 5, and 10), or at 15 min (lanes 3, 6, and 11). Standards used were [32P]polyP750 (lane 7), [γ-32P]ATP (lane 8), and H332PO4 (lane 12).
The evidence just presented strongly suggests that the glycogen-protein complex from S. acidocaldarius does not have any PPK activity. Therefore, our measurements of PPK activity present in fractions F2 and F3 (Table 1) may correspond to this nonspecific binding of ATP to the acid-precipitable glycogen-protein complex and not to a real PPK activity. Since it is also possible that some kind of enzymatic inhibition was present in our preparations of F2 and F3, we further investigated the identity of the proteins present in the glycogen-protein complex and genetically characterized P60.
Characterization of the polypeptides present in the glycogen-protein complex corresponding to fraction F2.
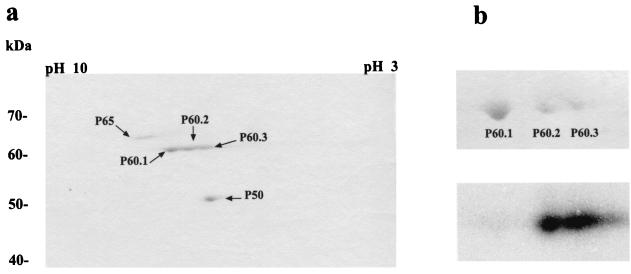
The glycogen-protein complex (F2) was analyzed by 2-D PAGE (Fig. 3). Three protein spots were resolved, P65, P60, and P50 (Fig. 3a), which migrated according to their previously observed molecular masses (Fig. 1). P60 was composed of at least three spots with the same molecular mass, P60.1, P60.2, and P60.3 (Fig. 3a and b, upper panel). This was in agreement with Skórko et al. (28), who reported that their 57-kDa protein (our P60) showed several bands by one-dimensional isoelectric focusing. The observed spots (P65, P60.1, P60.2, and P60.3) and P50 were excised from the gel and subjected to amino-terminal sequencing. The amino-terminal sequence of P60.1 was MKRYESLWFEDELKHVWMI. The amino-terminal sequences of P60.2 and P60.3 were both MKRYESLWF. These results indicate that P60 had different forms. These forms appeared to be more acidic, which is typical of phosphorylated proteins. To test this idea, we obtained the F2 fraction from an in vivo 32P-labeled culture and analyzed these proteins by 2-D NEPHGE (Fig 3b). Autoradiographic analysis (Fig. 3b, lower panel) clearly showed that the two more acidic spots (P60.2 and P60.3) were 32P labeled most likely due to phosphorylation. Taken together, these observations demonstrate that P60, the 57-kDa protein described by Skórko et al. (28) as a PPK and by König et al. (13) as a GT is composed of a single polypeptide chain that is probably phosphorylated.
FIG. 3.
Characterization of the polypeptides present in the glycogen-protein complex fraction F2. (a) Fraction F2 was analyzed by 2-D NEPHGE. Protein spots were visualized by staining with Coomassie blue. (b) Cells of S. acidocaldarius were labeled in vivo with H332PO4. Fraction F2 was obtained and analyzed by 2-D NEPHGE. Upper panel, portion of the 2-D gel containing P60.1, P60.2, and P60.3 stained with Coomassie blue; lower panel, autoradiography of the same gel showing 32P-labeled P60.2 and P60.3.
While no sequence was obtained for P65 due to insufficient material or to a blocked amino terminus, the amino-terminal sequence of P50 was RNVILGFEVH. With the sequences obtained for P60 and P50, no match was found against the available databases. A further search in the recently completed genome sequence of S. solfataricus genome database revealed that P50 and P60 match with the open reading frames (ORFs) bac04_023 and bac04_024, the former coding for a hypothetical alpha-amylase (amyA) of 447 amino acids (53.6 kDa; pI, 4.47) and the latter coding for a glycogen synthase (glgA) of 566 amino acids (65.6 kDa; pI, 6.73). These values are similar to the molecular masses and approximate pIs of the glycogen-bound proteins from S. acidocaldarius. These putative ORFs are consecutive in the genome and are probably clustered in the same operon. Other genes involved in the metabolism of glycogen, glgX (encoding for a glycogen-debranching enzyme) and glgC (ADP-glucose pyrophosphorylase), are adjacent to amyA but oriented in the opposite direction.
Isolation of the p60 gene and analysis of the deduced amino acid sequence of P60.
To clone the p60 gene we used the amino-terminal amino acid sequence of P60 and the amino-terminal sequences of two internal peptides to obtain degenerate oligonucleotide primers, which were then employed in DOP-PCR experiments using purified genomic DNA from S. acidocaldarius as a template. A 300-bp DNA fragment and an 800-bp DNA fragment were amplified with P60NH2DD and P60P21DR (for the former) and P60NH2DD and P60P27DR (for the latter). These two DNA fragments were cloned into the pGEM-T vector and sequenced. New primers were defined from the nucleotide sequence and employed to produce a digoxigenin probe, which was used in Southern blotting experiments against total DNA from S. acidocaldarius. After digestion with different restriction enzymes, only one DNA fragment hybridized with the probe, indicating that S. acidocaldarius strain 639 DSM carried a single copy of the p60 gene (data not shown). Two other primers, P60ND3R and P60ND5D, were defined and employed in reverse PCR experiments which allowed us to obtain the entire sequence of the p60 gene.
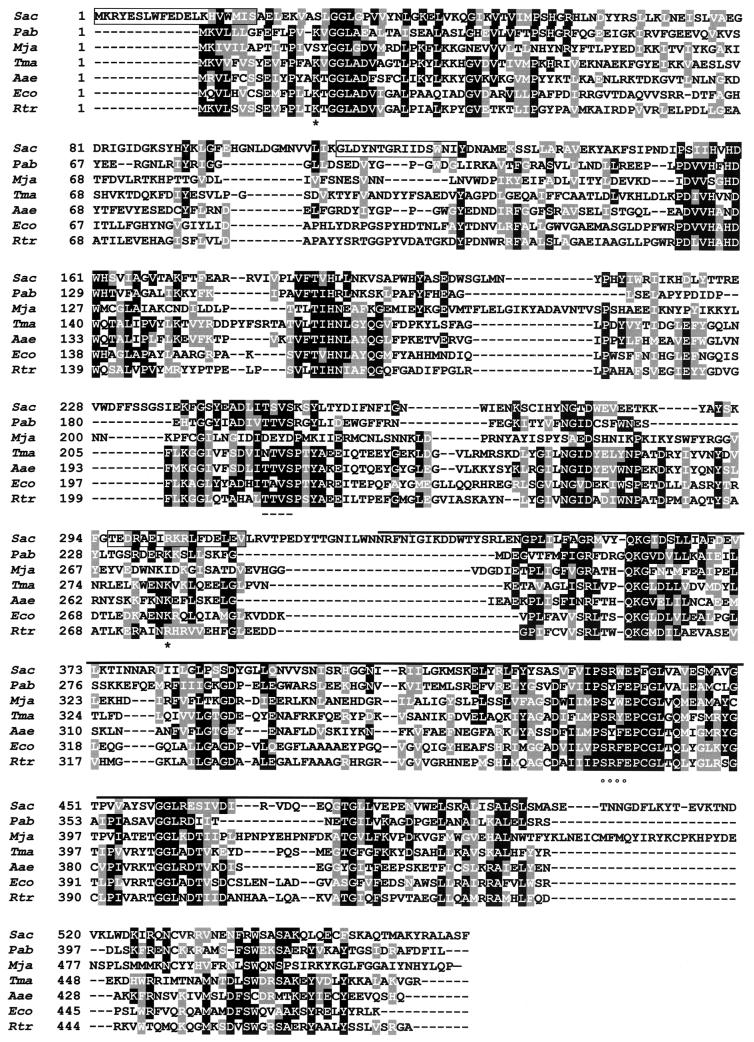
The 2,000-nucleotide sequence revealed the presence of one ORF of 1,698 bp that contained exactly the sequences of the initial peptides used to define the degenerate oligonucleotide primers (Fig. 4). This observation confirms that we isolated the gene coding for P60 protein. Searching in data banks with the BLASTP program indicated that P60 had a positive similarity to glycogen synthases from Archaea and Bacteria (Fig. 4) and a carboxyl-terminal portion of plant starch synthases. This was in agreement with the reported GT activity of the 57-kDa protein (13) and with our purification of GT activity (Table 1). Searching for conserved domains in P60 revealed the presence of the GT group 1 domain (expect value [E] = 2 × 10−23) from the Pfam database of protein domains (Fig. 4). This family comprises GTs from archaea, bacteria, fungi, and plants. The glycogen synthase from S. acidocaldarius showed 22% identity and 38% similarity (E = 9 × 10−9) to the glycogen synthase encoded by the glgA gene of E. coli. Two important sites have been described for E. coli GT: Lys 15, which forms part of the motif KXGG (where X represents any amino acid) and is involved in ADP-glucose binding (9), and Lys 277, which constitutes part of the proposed active site (10). Lys 277 is well conserved in all the analyzed bacterial and archaeal glycogen synthases (Fig. 4). Although the position of the equivalent Lys 277 is not clear in the sequence of the glycogen synthase of Methanococcus jannaschii, the alignment shows two Lys residues near this position, suggesting that this residue is also conserved in this protein. However, this is not the case for Lys 15, which appears to be lacking from the glycogen synthases of S. acidocaldarius and M. jannaschii.
FIG. 4.
Multiple alignment of bacterial and archaeal glycogen synthases. The P60 deduced amino acid sequence from S. acidocaldarius (Sac) was aligned (accession numbers in brackets) with other archaeal (Pab, Pyrococcus abyssi [CAB49000]; Mja, Methanococcus jannaschii [E64500]) and bacterial (Tma, Thermotoga maritima [AAD35976]; Aae, Aquifex aeolicus [AAC06894]; Eco, Escherichia coli [AAC76454]; Rtr, Rhizobium tropici [CAC17472]) glycogen synthases. Identical (shaded in black) and similar (shaded in grey) residues are indicated. The peptides which allowed us to isolate the p60 gene by reverse genetics are boxed. Conserved Lys 15 and 277 are indicated by an asterisk. The black bar over the P60 sequence represents the glycosyl transferase 1 domain. Putative phosphorylation sites for CKI (dashed lines) and CK2 (circles) are shown.
P60 was found to be labeled in vivo with H332PO4, suggesting a probable phosphorylation of the protein. This labeling was stable at pH 2.5 (data not shown), a characteristic not present in phospho-histidine proteins such as the PPK from E. coli phosphorylated in vitro (16). On the other side, mammalian and yeast glycogen synthases, which may be distant relatives of bacterial glycogen synthases, are regulated by phosphorylation in Ser and Thr residues (11, 29). Therefore, we searched for potential Ser/Thr phosphorylation sites of P60 in the PhosphoBase database (15). We found a potential casein kinase II (CKII) phosphorylation site that was remarkably conserved in all the glycogen synthases analyzed and a potential casein kinase I (CKI) phosphorylation site that was conserved in all sequences analyzed with the exception of the glycogen synthases of M. jannaschii and Thermotoga maritima (Fig. 4).
Cloning, expression, and functional analysis of the p60gene.
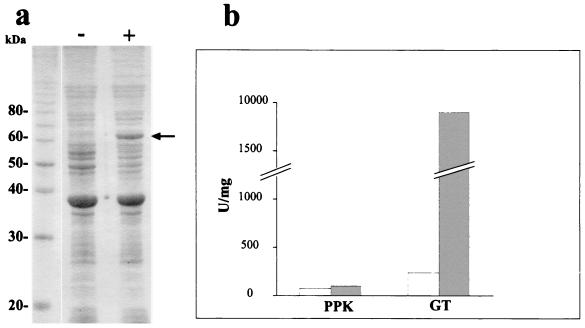
At present, no function for any glycogen synthase gene of the domain Archaea has been experimentally confirmed. Since gene disruption systems for S. acidocaldarius are not currently available, we decided to elucidate the functional properties of the product of the p60 gene by cloning and expressing it in a heterologous host. Therefore, we cloned the p60 gene in the expression vector pET21b(+) and expressed the recombinant protein (rP60) in E. coli host cells. The rP60, which was associated to the membrane fraction (Fig. 5a), was purified under denaturing conditions by nickel affinity chromatography (Fig. 1, lane d). The identity of the p60 gene was confirmed by sequencing both strands of the cloned insert in PeT21b(+), obtaining 100% identity with the previous 2,000-nucleotide sequence (data not shown).
FIG. 5.
Overexpression and functional analysis of rP60 in E. coli. (a) E. coli strain BL21(DE3)/pLysS transformed with pET21b(+) carrying a p60 insert was grown for 2 h in the presence (+) or in the absence (−) of 2 mM IPTG. Membrane fractions were analyzed by SDS-PAGE and stained with Coomassie blue. The arrow indicates the overexpressed rP60 protein. (b) PPK and GT activities, present in the membrane fractions of E. coli strain BL21(DE3)/pLysS transformed with pET21b(+) carrying a p60 insert in the presence (shaded bars) or in the absence (empty bars) of 2 mM IPTG, were measured at 70°C.
We measured PPK and GT activities of rP60 in membrane fractions of noninduced and induced cells. When the proposed activities were measured at 37°C neither PPK nor GT activity increased in the induced cells (not shown). Therefore, we assayed these enzymatic activities at 70°C. Under these conditions (Fig. 5b), there was a large increase in GT activity in the membrane fractions of induced cells. This was expected due to the similarity of P60 with glycogen synthases. However, the PPK activity was barely detectable, while the slight increase observed in induced cells was not significant. As Skórko et al. (28) reported that the enzyme was active only in the presence or glycogen, we also assayed these activities with the purified rP60 in the presence of 2% glycogen and/or 20 μM primer polyP750. However, the result obtained confirmed the absence of PPK activity. The GT specific activity of the purified rP60 was 750,000 U/mg of protein in the presence of 2% glycogen, a value in agreement with the native purified enzyme (fraction F3). Other reported specific activities of glycogen synthases range from 106 to 2 × 108 U/mg of protein depending on the sources and the experimental conditions used (8, 22).
DISCUSSION
Skórko et al. (28) reported that their glycogen-bound 57-kDa band was composed of several polypeptides possessing different isoelectric points. This observation allowed them to speculate on the possible existence of two different proteins with two different enzymatic activities (PPK and GT) or one protein with the ability to perform both reactions. In this paper, we demonstrate that the 57-kDa band contains 32P-labeled forms of a single polypeptide (P60). The gene coding for P60 turned out to be homologous to bacterial glycogen synthases and did not show similarity to any of the amino acid sequences of more than 15 known PPKs. Moreover, the native and the overexpressed P60 showed only GT activity. Taken together, these results demonstrate that the proposed glycogen-bound PPK from S. acidocaldarius is actually a bacterial-type glycogen synthase. The properties of the functional glycogen-bound glycogen synthase, which we describe for S. acidocaldarius, may lead to a revision of the current view of the prokaryotic glycogen synthases for two reasons. First, the conserved Lys 15 at the ADP-glucose binding site (9) is lacking in the glycogen synthase from S. acidocaldarius (P60) and from M. jannaschii. Second, P60 was labeled with H332PO4 in vivo, suggesting it to be phosphorylatable. The analysis of the bacterial-archaeal glycogen synthase sequences revealed the presence of highly conserved potential phosphorylation sites for CKI and CKII. Although experimental evidence is required in order to confirm this prediction, this observation could support a regulation of the bacterial-archaeal glycogen synthases by phosphorylation by a yet unidentified eukaryotic-like Ser/Thr protein kinase, a very probable phenomenon given the presence of several types of Ser/Thr protein kinases in bacterial and archaeal genomes (18, 26). In addition, the finding of a glycogen-bound alpha-amylase encoded by a gene clustered in the same operon suggests that this enzyme may fulfill the catabolic function of the glycogen phosphorylase (glgP) (24).
A possible explanation for a glycogen-bound PPK may reside in the nucleoside-diphosphate kinase activity of the PPK of E. coli (31). PPK, acting as a nucleoside-diphosphate kinase, might take part in glycogen metabolism, regenerating the ATP consumed in the synthesis of the ADP glucose, the precursor of glycogen synthesis. An ATP-regenerating role for PPK has been described for endogenous PPK from the E. coli RNA degradosome (7). Therefore, it seems reasonable that PPK might form part of other macromolecular complexes, such as those formed with glycogen. Although this speculative model of a glycogen-bound system for regenerating ATP seems possible, in this paper we demonstrate the absence of a glycogen-bound PPK activity in S. acidocaldarius.
Given the presence of polyP in S. acidocaldarius (14), the small amount of PPK-like activity detected in the crude extracts (this paper), and the absence of ppk genes in the S. solfataricus genome and other archaeal genomes, the existence of polyphosphate kinase in this microorganism and others of the domain Archaea remains controversial. The most reasonable route to achieve the identification of an enzyme involved in polyP synthesis in Archaea seems to be an alternative exhaustive purification. Also, the characterization of functional domains in the known PPKs from different organisms will be of great help in identifying a PPK-like gene(s) on the largely divergent genomic sequences of the domain Archaea.
ACKNOWLEDGMENTS
This research was supported by FONDECYT projects no. 2990035 and no. 1000679, project ICM P-99-031-F, and ICGEB (project CRP/CHI00-04, contract 01/001). S.C. was the recipient of a DAAD Ph.D. scholarship.
We are very grateful to Arthur Kornberg for spurring our involvement in polyP research and for kindly providing us with PPXSce and E. coli strain NR 100 in order to obtain [32P]polyP750.
REFERENCES
- 1.Ahn K, Kornberg A. Polyphosphate kinase from Escherichia coli. Purification and demonstration of a phosphoenzyme intermediate. J Biol Chem. 1990;265:11734–11739. [PubMed] [Google Scholar]
- 2.Akiyama M, Crooke E, Kornberg A. The polyphosphate kinase gene of Escherichia coli. Isolation and sequence of the ppkgene and membrane location of the protein. J Biol Chem. 1992;267:22556–22561. [PubMed] [Google Scholar]
- 3.Akiyama M, Crooke E, Kornberg A. An exopolyphosphatase of Escherichia coli. The enzyme and its ppxgene in a polyphosphate operon. J Biol Chem. 1993;268:633–639. [PubMed] [Google Scholar]
- 4.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D L. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ault-Riché D, Kornberg A. Definitive enzymatic assays in polyphosphate analysis. In: Schröder H C, Müller W E G, editors. Inorganic polyphosphates. Biochemistry, biology, bio/technology. Berlin, Germany: Springer-Verlag; 1999. pp. 241–251. [DOI] [PubMed] [Google Scholar]
- 6.Ault-Riché D, Fraley C D, Tzeng C-M, Kornberg A. A novel assay reveals multiple pathways regulating stress-induced accumulations of inorganic polyphosphate in Escherichia coli. J Bacteriol. 1998;180:1841–1847. doi: 10.1128/jb.180.7.1841-1847.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blum E, Py B, Carpousis A J, Higgins C F. Polyphosphate kinase is a component of the Escherichia coliRNA degradosome. Mol Microbiol. 1997;26:387–398. doi: 10.1046/j.1365-2958.1997.5901947.x. [DOI] [PubMed] [Google Scholar]
- 8.Fox J, Kawaguchi K, Greenberg E, Preiss J. Biosynthesis of bacterial glycogen. Purification and properties of the Escherichia coli B ADPglucose:1,4-alpha-d-glucan 4-alpha-glucosyltransferase. Biochemistry. 1976;15:849–856. doi: 10.1021/bi00649a019. [DOI] [PubMed] [Google Scholar]
- 9.Furukawa K, Tagaya M, Tanizawa K, Fukui T. Role of the conserved Lys-X-Gly-Gly sequence at the ADP-glucose-binding site in Escherichia coliglycogen synthase. J Biol Chem. 1993;268:23837–23842. [PubMed] [Google Scholar]
- 10.Furukawa K, Tagaya M, Tanizawa K, Fukui T. Identification of Lys277 at the active site of Escherichia coliglycogen synthase. Application of affinity labeling combined with site-directed mutagenesis. J Biol Chem. 1994;269:868–871. [PubMed] [Google Scholar]
- 11.Hardy T A, Roach P J. Control of yeast glycogen synthase-2 by COOH-terminal phosphorylation. J Biol Chem. 1993;268:23799–23805. [PubMed] [Google Scholar]
- 12.Keasling J D, Van Dien S J, Trelstad P, Renninger N, McMahon K. Application of polyphosphate metabolism to environmental and biotechnological problems. Biochemistry (Moscow) 2000;65:324–331. [PubMed] [Google Scholar]
- 13.König H, Skórko R, Zillig W, Reiter W. Glycogen in thermoacidophilic archaebacteria of the genera Sulfolobus, Thermoproteus, Desulfurococcus and Thermococcus. Arch Microbiol. 1982;132:297–303. [Google Scholar]
- 14.Kornberg A, Rao N N, Ault-Riché D. Inorganic polyphosphate: a molecule of many functions. Annu Rev Biochem. 1999;68:89–125. doi: 10.1146/annurev.biochem.68.1.89. [DOI] [PubMed] [Google Scholar]
- 15.Kreegipuu A, Blom N, Brunak S. PhosphoBase, a database of phosphorylation sites: release 2.0. Nucleic Acids Res. 1999;27:237–239. doi: 10.1093/nar/27.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumble K D, Ahn K, Kornberg A. Phosphohistidyl active sites in polyphosphate kinase of Escherichia coli. Proc Natl Acad Sci USA. 1996;93:14391–14395. doi: 10.1073/pnas.93.25.14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Leonard C J, Aravind L, Koonin E V. Novel families of putative protein kinases in bacteria and archaea: evolution of the “eukaryotic” protein kinase superfamily. Genome Res. 1998;8:1038–1047. doi: 10.1101/gr.8.10.1038. [DOI] [PubMed] [Google Scholar]
- 19.Ochman H, Medhora M M, Garza D, Hartl D L. Amplification of flanking sequences by inverse PCR. In: Innis M A, Gelfand D, Sninsky J J, White T, editors. PCR protocols, a guide to methods and applications. New York, N.Y: Academic Press, Inc.; 1990. pp. 219–227. [Google Scholar]
- 20.O'Farrell P Z, Goodman H M, O'Farrell P H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977;12:1133–1142. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- 21.Osorio G, Jerez C A. Adaptative response of the archaeon Sulfolobus acidocaldariusBC65 to phosphate starvation. Microbiology. 1996;142:1531–1536. doi: 10.1099/13500872-142-6-1531. [DOI] [PubMed] [Google Scholar]
- 22.Pollock C, Preiss J. The citrate-stimulated starch synthase of starchy maize kernels: purification and properties. Arch Biochem Biophys. 1980;204:578–588. doi: 10.1016/0003-9861(80)90070-3. [DOI] [PubMed] [Google Scholar]
- 23.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 24.Schinzel R, Nidetzky B. Bacterial α-glucan phosphorylases. FEMS Microbiol Lett. 1999;171:73–79. doi: 10.1111/j.1574-6968.1999.tb13414.x. [DOI] [PubMed] [Google Scholar]
- 25.Schröder H C, Lorenz B, Kurz L, Müller W E G. Inorganic polyphosphate in eukaryotes: enzymes, metabolism and function. In: Schröder H C, Müller W E G, editors. Inorganic polyphosphates. Biochemistry, biology, bio/technology. Berlin, Germany: Springer-Verlag; 1999. pp. 45–74. [DOI] [PubMed] [Google Scholar]
- 26.Shi L, Potts M, Kennelly P J. The serine, threonine, and/or tyrosine-specific protein kinases and protein phosphatases of prokaryotic organisms. A family portrait. FEMS Microbiol Rev. 1998;22:229–253. doi: 10.1111/j.1574-6976.1998.tb00369.x. [DOI] [PubMed] [Google Scholar]
- 27.Skórko R. Polyphosphate as a source of phosphoryl group in protein modification in archaebacterium Sulfolobus acidocaldarius. Biochimie. 1989;71:9–10. doi: 10.1016/0300-9084(89)90115-6. [DOI] [PubMed] [Google Scholar]
- 28.Skórko R, Osipiuk J, Stetter K O. Glycogen-bound polyphosphate kinase from the archaebacterium Sulfolobus acidocaldarius. J Bacteriol. 1989;171:5162–5164. doi: 10.1128/jb.171.9.5162-5164.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skurat A V, Wang Y, Roach P J. Rabbit skeletal muscle glycogen synthase expressed in COS cells. Identification of regulatory phosphorylation sites. J Biol Chem. 1994;269:2534–2542. [PubMed] [Google Scholar]
- 30.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocelllulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tzeng C-M, Kornberg A. The multiple activities of polyphosphate kinase of Escherichia coliand their subunit structure determined by radiation target analysis. J Biol Chem. 2000;275:3977–3983. doi: 10.1074/jbc.275.6.3977. [DOI] [PubMed] [Google Scholar]
- 32.Wurst H, Shiba T, Kornberg A. The gene for a major exopolyphosphatase of Saccharomyces cerevisiae. J Bacteriol. 1995;177:898–906. doi: 10.1128/jb.177.4.898-906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]