Abstract
Gum hypertrophy is a very frequent condition linked to orthodontic treatment, especially in teenagers, and the same time, about 80% of young adults are affected by acne vulgaris, a chronic inflammatory skin disease, typically treated with antibacterial therapy. The use of probiotics has gained popularity in the medical field, and many studies have demonstrated its effectiveness, such as the positive effects of some bacterial strains belonging to Lactobacillus species. The aim of this study is to document the effect of Lactobacillus reuteri (L. reuteri) on facial skin that was randomly observed in two orthodontic patients. We present two case reports of a 14-year-old female patient and a 15-year-old male patient suffering from acne vulgaris who, during fixed orthodontic treatment, showed clinical signs of gingivitis with high values of Full Mouth Plaque Score (FMPS) and Bleeding on Probing (BOP). The patients were treated first with professional oral hygiene sessions and Scaling and Root Planing (SRP) procedures, and then with the administration of a formulate containing L. reuteri as a probiotic. The follow-up was made at four weeks. During the follow-up analysis, both patients showed a significant clinical remission for gum hypertrophy and skin acne vulgaris.
Keywords: gingival hypertrophy, acne vulgaris, antibiotic resistance, probiotics, microbioma, microbiota, Lactobacillus reuteri, host-microbial symbiosis
1. Introduction
During fixed orthodontic treatment the amount of plaque is considered an important risk factor that can lead to the development of chronic hyperplastic gingivitis increasing the prevalence of periodontopathogens [1,2]. Several authors have demonstrated that the accumulation of microbial plaque on the teeth surface is strictly connected to gingivitis and periodontitis [3,4,5]. Many teenagers present with gingival infections linked to poor oral hygiene, hormonal changes related to the puberty period and sometimes due to orthodontic treatments, that can cause moderate to advanced periodontal destruction. Orthodontic treatment improves the occlusal and maxillary relationship, the chewing function and also leads to an improvement in the aesthetics of the face; however, harmful effects on the periodontium have been documented.
The presence of fixed appliances and the quantity and quality of the oral microbiota are linked to the control of oral hygiene. Dental braces or retainers placement promotes plaque retention, and consequently, strongly microbial changes in the oral and subgingival microbiota; furthermore, certain specific bacterial species, such as Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans), Porphyromonas gingivalis (P. gingivalis), Tannerella forsythia (T. forsythia), Prevotella intermedia (P. intermedia), and Treponema denticola (T. denticola), which are associated with severe forms of this illness, mainly contribute to developing periodontal diseases [6,7]. Among these main periodontal pathogens which constitute the oral biofilm, the gram-negative anaerobic bacterium Porphyromonas gingivalis play a key role in the pathogenesis of periodontal disease and is mostly associated with the chronic form of periodontitis. This non-motile bacterium is an opportunistic oral pathogen that is capable of producing a large number of virulence factors (lipopolysaccharide, capsule, gingipains and fimbriae) and causing gingival inflammation. It is believed that it induces the destruction of periodontal tissues by influencing the host inflammatory response. In fact, the immunological host response is overactivated or blocked by P. gingivalis and the other periodontopathogenic species, resulting in temporary or persistent changes and damage to the periodontal tissues that can be seen in histopathological analysis [8]. In this context, SRP treatment performed with the use of manual and ultrasonic instrumentation can be considered the gold-standard therapy for eliminating plaque biofilm. In fact, scaling of teeth surfaces and debridement of root surfaces demonstrated both clinical and microbiological benefits. [9] It is also important to educate the patient with instructions about domiciliary oral hygiene management in order to reduce the inflammatory indices. It is also advisable to act only on plaque deposits trying to avoid damaging soft tissues. In recent years, probiotics showed promising results for the reduction of bacterial load and balancing oral dysbiosis. [10]
Over 80% of teenagers and young adults suffer from acne [11,12]. Acne vulgaris is the eighth most common disease in the world. Acne can be classified into two different types based on the presence of inflammation or not. In the non-inflammatory type, the hair follicle is obstructed but the basement membrane is not damaged; the inflammatory type is instead characterized by papules and/or pustules and we can have damage on the hair follicles and dermal tissues can be observed. Propionibacterium acnes (P. acnes) can survive and reproduce in deep hypoxic tissue for ≥6 months [13]. Acne vulgaris is clinically characterized by blackheads, inflammatory nodules, pustules and papules on the face, chest and back. In western countries, there is a wider prevalence of this condition; it may be related to diets rich in carbohydrates that increase the insulin-like growth factor signal (IGF-1) [12,14,15].
Some hormones such as dihydrotestosterone and testosterone stimulate the sebum production from sebocytes [16,17]. Interleukin-1 produced by sebaceous glands causes inflammation, hyperkeratinization and formation of comedones. P. acnes, also called Cutibacterium acnes, is a gram-positive anaerobic bacterium that belongs to the skin’s normal flora, but in sebaceous glands and hair follicles, it is found in greater quantity [18]. It is also present in the oral cavity, in the conjunctiva, in the bowel and in the external ear canal [19]. It is considered an opportunistic pathogen that can cause a range of infections, including those in the bones, joints and mouth [20]. In the study of Testa M et al., P. acnes was found in the oral microbiota composition of a group of forty-five children aged between 6 and 14 (13 boys and 32 girls) [21].
There are many medications proposed for acne treatment, due to the need to find a solution to a chronic disease that often causes scars on the skin [22,23].
Due to the growing problem of resistant bacteria, the restricted use of antibiotics is recommended for both oral and topical routes [24,25].
Tetracycline antibiotics, such as minocycline and doxycycline, are commonly used as the gold standard for the therapy of moderate to severe acne [26]. Recent studies showed that the antibiotic treatment duration often exceeds the recommendations [27,28,29,30]. Many new therapies proposed for acne pathogenesis include sebum-suppressing and anti-inflammatory phytochemicals, laser and light therapy. The research interest in probiotics has increased in the medical field in the last 20 years, especially in their effects on several organs and systems. In the near future, it is possible that therapies for P. acnes and acne vulgaris could include probiotics as a gold standard [31].
2. Case Report
A 14-year-old female patient and a 15-year-old male patient in systemic good health undergoing treatment with fixed orthodontics, on clinical examination, had numerous sites with bacterial plaque stagnation around the orthodontic brackets, gingival volume enlargement and bleeding on probing (Figure 1 and Figure 2). To improve the clinical picture of gingivitis, a session of professional oral hygiene with SRP was performed with instruction in the correct domiciliary oral hygiene maneuvers (DOH) to be practiced at least twice a day. Specifically, we advised and instructed patients in the use of the rotating-oscillating electric toothbrush with a soft head and in the correct use of spongy dental floss (super floss) equipped with a rigid end to be inserted above or below the orthodontic wire, sliding the spongy part under the gumline of hypertrophic gums. At the end of the DOH, patients were instructed to slowly dissolve a topical probiotic in the mouth. The prescribed topical probiotic was a non-medical commercial formula containing L. reuteri ATCC PTA 5289 (American Type Culture Collection, Manassas, VA, USA) in tablets to be taken twice a day, for four weeks (GUM® PerioBalance® Sunstar Italia, Saronno, Italy). Patients were advised not to drink, eat, or rinse their mouths for 60 min after the application. It was also recommended not to rinse the oral cavity with antibacterial mouthwashes in combination with probiotics for the 4 weeks of treatment. Patients were subsequently monitored at 1 week (T1), two (T2) and four weeks (T3). The periodontal clinical parameters were measured and recorded at T0 and re-evaluated at T3 with the online periodontal file “Periodontalchart-online.com” (https://periodontalchart-online.com/uk/, accessed on 30 May 2022). At the four-week follow-up, in both cases there was a marked improvement in hypertrophic gingivitis with a reduction in periodontal epidemiological parameters (Figure 3 and Figure 4). Both patients showed regression of gingival hypertrophy at one month follow-up (100%). The mean plaque index (PI) value before therapy was 69.5%, and at one month it was 1.5%. The mean BoP at T0 was 29% and at T3, one month after treatment, it was 1%. Furthermore, the reduction in inflammation of acne-affected skin was observed surprisingly as both patients also suffered from acne vulgaris. The female patient showed a less dry and brighter skin already after the first week of probiotic therapy; at 2 weeks the improvement was so marked we decided to photograph the skin of the face for an objective evaluation (Figure 5); at T3 a further reduction of acne was observed (Figure 6). Similarly, based on previous results, the male patient received the same probiotic treatment as the female patient (Figure 7). In this second case also, an improvement in skin inflammation was evident; the skin was velvety, hydrated, luminous and almost free of pimples (Figure 8). The patients expressed a high degree of satisfaction with the probiotic treatment, both for gingivitis and especially for acne vulgaris.
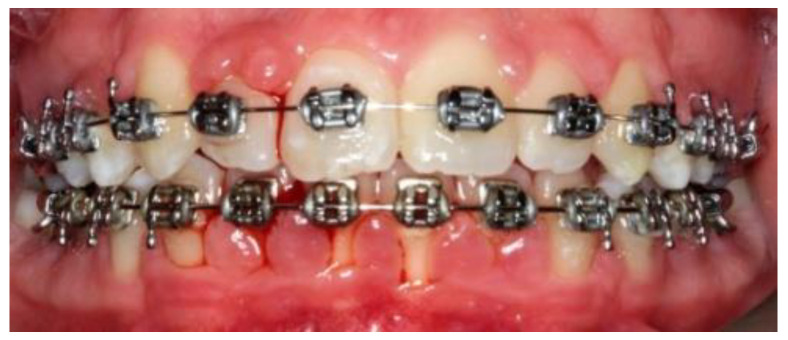
Figure 1.
Patient n.1, T0: particular gingival swelling, accompanied by bleeding and abundant accumulation of bacterial plaque.
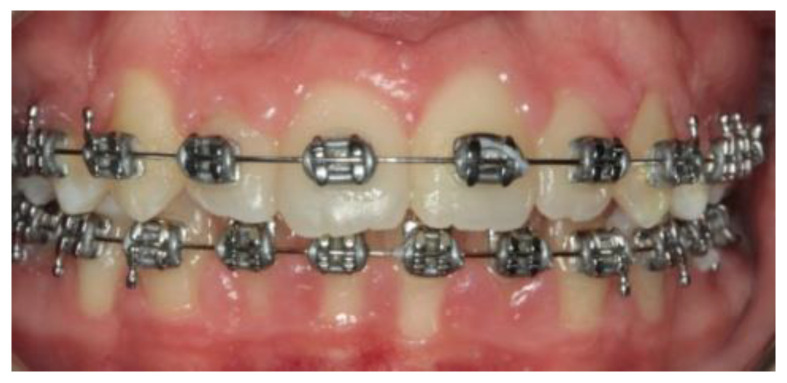
Figure 2.
Patient n.2, T0: gingival hypertrophy localized to the lower incisors.
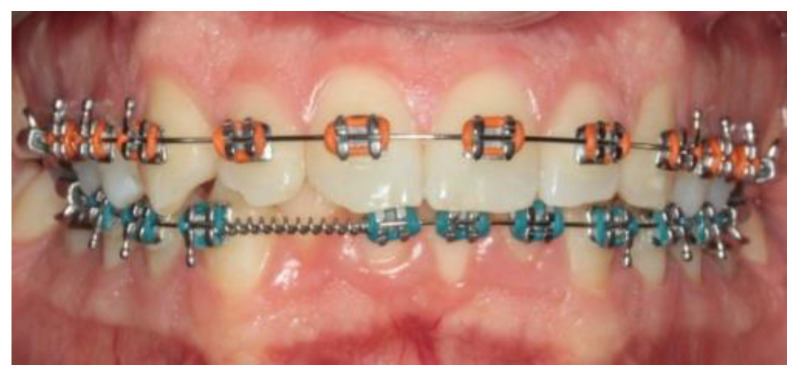
Figure 3.
Patient n.1 control at 4 weeks (T3): zero bleeding, reduction of periodontal indices.
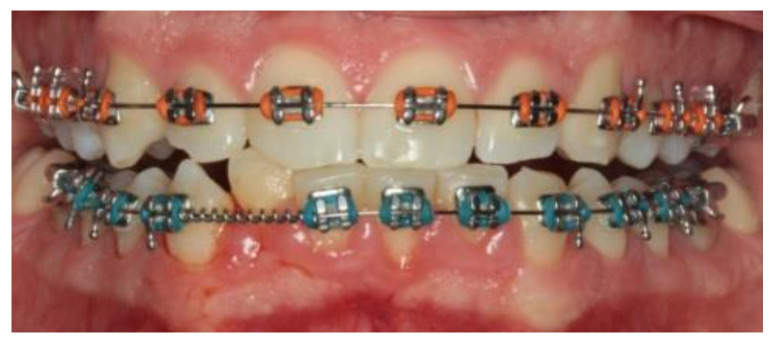
Figure 4.
Patient n.2, T3: at the 4-week check-up, the gums of the fifth sextant were healed.
Figure 5.
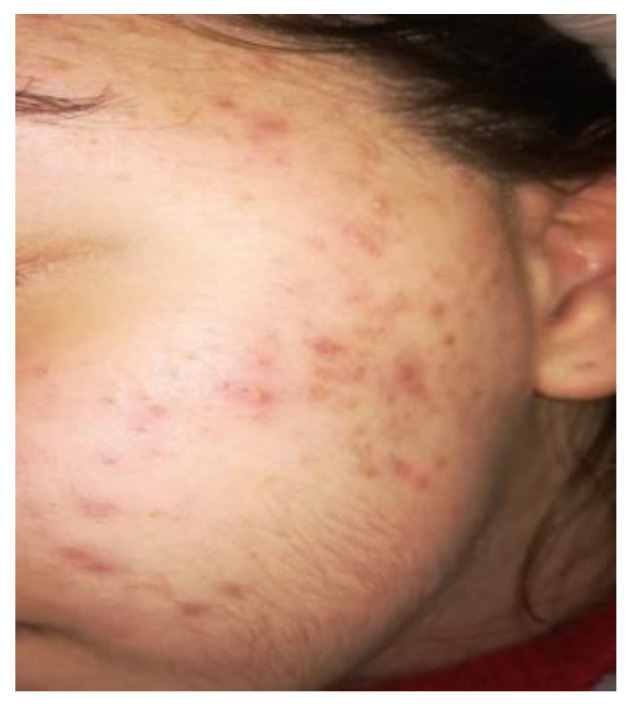
Two weeks follow up, patient n.1, T2: patient 2 weeks after the start of therapy with L. reuteri.
Figure 6.
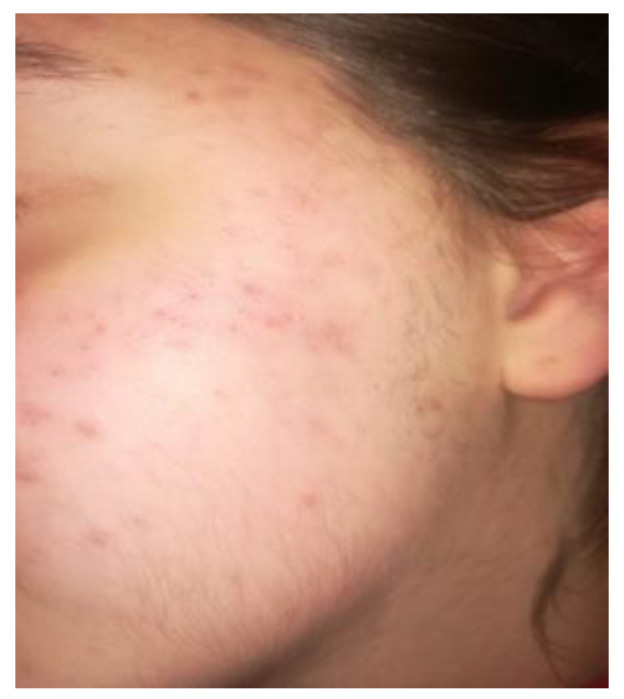
T3 patient n.1: picture of the patient’s skin with acne reduction four weeks after therapy with L. reuteri.
Figure 7.
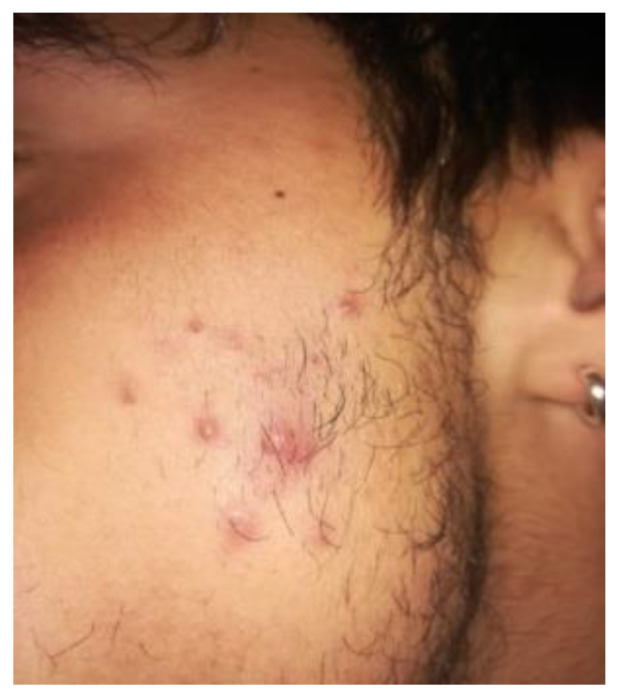
Patient n. 2 T0: acne with pustules before treatment with L. reuteri.
Figure 8.
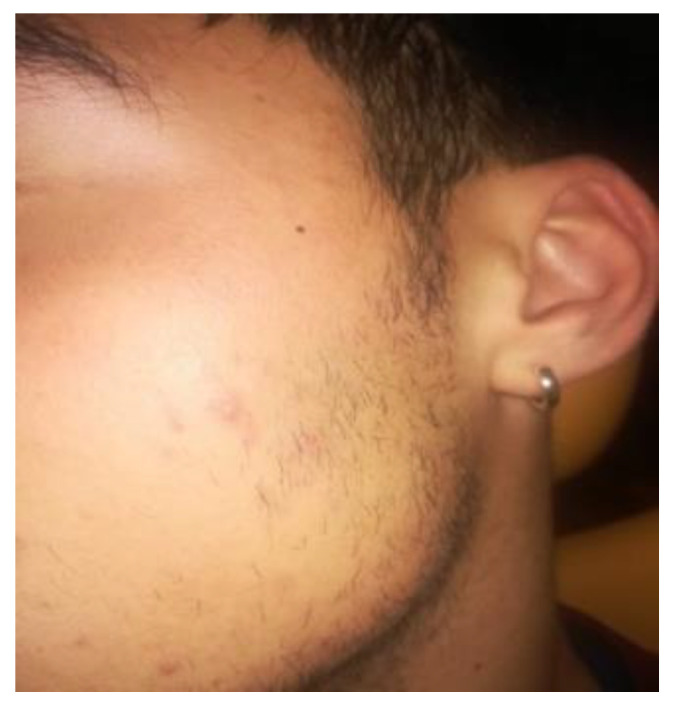
T3 patient n.2: almost complete cure of acne after 4 weeks of treatment with L. reuteri.
3. Discussion
Probiotics are defined as “live microorganisms that, when administered in sufficient concentration, confer a health benefit on the host”. Over the last decade, there has been an increased interest in the use of probiotics especially for periodontal health [32,33]. A recent systematic review showed a positive effect of a combination probiotic of two L. reuteri strains as an additive treatment to scaling and root planning [34]. The randomized clinical trials unambiguously showed that in periodontitis patients, this probiotic leads to an improvement of clinical parameters such as a sensible reduction of pocket probing depth after non-surgical mechanical therapy and at the microbiological and immunological level [34,35,36,37]. Vivekananda et al., showed that L. reuteri leads to a significant decrease in the CFU (Colony-forming units) of several periodontal pathgens such as A. actinomycetemcomitans, P. gingivalis and P. intermedia [36]. In addition, this probiotic reduced specific parameters associated with inflammation, such as MMP-8 levels in the gingival crevicular fluid [38]. In the pilot study of Sinesi A. et al., 14 adolescent patients with gingival hypertrophy in fixed orthodontic treatment were treated with SRP and topical L. reuteri. All the patients showed regression of gingival hypertrophy at the one month follow-up (100%). The initial FMPS value (T0) was 69.71%, and at one month it had fallen to 18.57%. The BoP at T0 was 37.85% and at one month after treatment it was 2.35% [39].
Future research should focus on the underlying immunomodulatory mechanisms of this positive clinical effect of probiotics [40,41].
Human skin microbiota could play an important role in maintaining skin health and preventing premature aging. Although we do not know the actual mechanism of probiotics, some authors have hypothesized that the anti-inflammatory effects are linked to the stimulation of regulatory T cells and the release of IL-10 cytokines. Probiotic strains containing Lactobacillus, Bifidobacterium or Streptococcus and other commensal skin bacteria, have demonstrated cutaneous immunoregulatory effects [42].
Several probiotics can have positive effects on the epidermal tissue. These effects could be connected to the presence of lipoteic acid, which is common in Lactobacillus species [43]. One study highlighted that Lactobacillus plantarum (L. plantarum) HY7714 is able to prevent UV-induced photoaging by inhibiting MMP-1 expression in dermal fibroblasts [43]. In another study, the authors conclude that oral supplementation of the same type of L. plantarum administered for 12 weeks in 110 subjects resulted in greater skin elasticity and hydration [44]. Another study on Lactobacillus sakei LTA showed that this microorganism is able to reverse UV-induced skin aging through its immunomodulating effect on monocytes [45]. Topical and oral antibiotics are the first choice in traditional acne treatment protocols. While effective, this approach risks antibiotic resistance and destruction of the microbiome with different systemic collateral events. Given the role of intestinal dysbiosis in inflammatory skin conditions, supplementing probiotics represents a promising alternative or adjuvant approach to treating acne. In a study of 300 patients investigating the administration of Lactobacillus acidophilus and Lactobacillus bulgaricus for acne, an improvement in acne was observed in 80% of subjects. Streptococcus salivarius and Lactococcus HY449 both produce an inhibitory substance similar to bacteriocins, which inhibits the growth of P. acnes [46,47]. In another clinical study, patients treated with Lactobacillus and Bifidobacterium species probiotics together with oral antibiotics showed a significantly greater reduction in acne lesion number than in an antibiotic-only control group [48].
We know that probiotics can interfere with the pathogenesis of acne also through immunomodulatory and anti-inflammatory actions. For example, in vitro studies on Streptococcus salivarius activity, a commensal microbe, found an important inhibition of IL-8 secretion, suppression of the NF-κB pathway and downregulation of some genes associated with the adhesion of other bacteria to epidermal surfaces.
Probiotics such as Lactobacillus rhamnosus SP1 can also reduce the glycemic load, reduce the IGF-1 signaling and finally reduce the proliferation of keratinocytes and hyperplasia of the sebaceous glands [49]. Khmaladze I et al. performed a comparative study on live and lysated products of the probiotic strain L. reuteri DSM 17938 in topical skin applications, in which a decrease of IL-6 and IL-8 was observed but live L. reuteri DSM 17938 had an antimicrobial action against pathogenic skin bacteria (Staphylococcus aureus, Streptococcus pyogenes M1 etc.), whereas the lysate had no antimicrobial effect. Therefore, it is hypothesized that L. reuteri DSM 17938 may be useful for general skin health and for improving the skin barrier [50]. Kang MS et al. successfully examined the inhibitory effects of L. reuteri on the proliferation of P. acnes and S. epidermidis, which are closely linked to the production of organic acids. Overall, these results suggest that L. reuteri may be a useful probiotic agent for controlling the growth of bacteria involved in skin and gum inflammation [38,51].
All in vitro studies have shown that probiotics successfully inhibited skin and wound pathogens and promoted healing. The exogenous and oral application of probiotics has shown a reduction in infections, especially if used as an adjuvant to antibiotic therapy, and therefore, the use of probiotics in this field remains worthy of further studies [52].
4. Conclusions
These two cases suggest that L. reuteri had a positive effect on the growth control of bacteria involved in acne vulgaris in teenagers and gingival plaque-dependent hypertrophic gingivitis. The results also suggest that professional oral hygiene treatment combined with the topical probiotic L. reuteri, could benefit the microbiota eubiosis status, which manifests itself, in particular, on the cutaneous and oral microbiota with a reduction of inflammation and swelling of the gums and pimples; it also prevents the worsening of these two conditions. These cases highlight a possible correlation in the etiopathogenesis of these two conditions.
This preliminary work could be a useful base protocol for the management of patients simultaneously affected by gum hypertrophy and refractory skin acne vulgaris. In this context, a multidisciplinary approach based on various medical fields remains important to promote the microbial symbiosis of the host. No firm conclusion can be drawn from a such small sample but our study offers interesting outcomes for further research.
Author Contributions
G.M.: Conceptualization, writing—original draft preparation, data curation; G.A.: writing—original draft preparation; M.P.: writing—review and editing; G.D.: supervision and writing; G.O.: writing—review and editing and supervision; C.C.: Conceptualization, writing—review and editing, methodology. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical review and approval were waived for this study because it is a case report in which we have reported the results obtained using a commercial product, that does not require a medical prescription.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhao L., Wang X.Y., Xu Y., Meng S. Relationship of Orthodontic Treatment and Periodontal Soft Tissue Health. West China J. Stomatol. 2018;36:595–601. doi: 10.7518/hxkq.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mártha K., Mezei T., Jánosi K. A histological analysis of gingival condition associated with orthodontic treatment. Rom. J. Morphol. Embryol. 2013;54((Suppl. 3)):823–827. [PubMed] [Google Scholar]
- 3.Bogren A., Teles R.P., Torresyap G., Haffajee A.D., Socransky S.S., Wennström J.L. Clinical and Microbiologic Changes Associated With the Combined Use of a Powered Toothbrush and a Triclosan/Copolymer Dentifrice: A 3-Year Prospective Study. J. Periodontol. 2007;78:1708–1717. doi: 10.1902/jop.2007.070028. [DOI] [PubMed] [Google Scholar]
- 4.Jansson H. Studies on periodontitis and analyses of individuals at risk for periodontal diseases. Swed. Dent. J. Suppl. 2006;180:5–49. [PubMed] [Google Scholar]
- 5.Butera A., Gallo S., Pascadopoli M., Luraghi G., Scribante A. Ozonized Water Administration in Peri-Implant Mucositis Sites: A Randomized Clinical Trial. Appl. Sci. 2021;11:7812. doi: 10.3390/app11177812. [DOI] [Google Scholar]
- 6.Freitas A.O., Marquezan M., Mda C.N., Alviano D.S., Maia L.C. The influence of orthodontic fixed appliances on the oral microbiota: A systematic review. Dent. Press J. Orthod. 2014;19:46–55. doi: 10.1590/2176-9451.19.2.046-055.oar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cerroni S., Pasquantonio G., Condo’ R., Cerroni L. Orthodontic Fixed Appliance and Periodontal Status: An Updated Systematic Review. Open Dent. J. 2018;12:614–622. doi: 10.2174/1745017901814010614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.How K.Y., Song K.P., Chan K.G. Porphyromonas gingivalis: An Overview of Periodontopathic Pathogen below the Gum Line. Front. Microbiol. 2016;7:53. doi: 10.3389/fmicb.2016.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butera A., Maiorani C., Natoli V., Bruni A., Coscione C., Magliano G., Giacobbo G., Morelli A., Moressa S., Scribante A. Bio-Inspired Systems in Nonsurgical Periodontal Therapy to Reduce Contaminated Aerosol during COVID-19: A Comprehensive and Bibliometric Review. J. Clin. Med. 2020;9:3914. doi: 10.3390/jcm9123914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scribante A., Butera A., Alovisi M. Customized Minimally Invasive Protocols for the Clinical and Microbiological Management of the Oral Microbiota. Microorganisms. 2022;10:675. doi: 10.3390/microorganisms10040675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan J.K., Bhate K. A global perspective on the epidemiology of acne. Br. J. Dermatol. 2015;172((Suppl. 1)):3–12. doi: 10.1111/bjd.13462. [DOI] [PubMed] [Google Scholar]
- 12.Zaenglein A.L., Pathy A.L., Schlosser B.J., Alikhan A., Baldwin H.E., Berson D.S., Bowe W.P., Graber E.M., Harper J.C., Kang S., et al. Guidelines of care for the management of acne vulgaris. J. Am. Acad. Dermatol. 2016;74:945–973.e33. doi: 10.1016/j.jaad.2015.12.037. [DOI] [PubMed] [Google Scholar]
- 13.Hsiao K.-H., Huang C.-M., Lee Y.-H. Development of Rifampicin-Indocyanine Green-Loaded Perfluorocarbon Nanodroplets for Photo-Chemo-Probiotic Antimicrobial Therapy. Front. Pharmacol. 2018;9:1254. doi: 10.3389/fphar.2018.01254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melnik B.C. Linking diet to acne metabolomics, inflammation, and comedogenesis: An update. Clin. Cosmet. Investig. Dermatol. 2015;8:371–388. doi: 10.2147/CCID.S69135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agamia N., Abdallah D., Sorour O.S., Mourad B., Younan D.Y. Skin expression of mammalian target of rapamycin and forkhead box transcription factor O1, and serum insulin-like growth factor-1 in patients with acne vulgaris and their relationship with diet. Br. J. Dermatol. 2016;174:1299–1307. doi: 10.1111/bjd.14409. [DOI] [PubMed] [Google Scholar]
- 16.Thiboutot D., Gollnick H., Bettoli V., Dréno B., Kang S., Leyden J.J., Shalita A.R., Lozada V.T., Berson D., Finlay A., et al. New insights into the management of acne: An update from the Global Alliance to Improve Outcomes in Acne group. J. Am. Acad. Dermatol. 2009;60((Suppl. 5)):S1–S50. doi: 10.1016/j.jaad.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 17.Gollnick H.P., Bettoli V., Lambert J., Araviiskaia E., Binic I., Dessinioti C., Galadari I., Ganceviciene R., Ilter N., Kaegi M., et al. A consensus-based practical and daily guide for the treatment of acne patients. J. Eur. Acad. Dermatol. Venereol. 2016;30:1480–1490. doi: 10.1111/jdv.13675. [DOI] [PubMed] [Google Scholar]
- 18.Dreno B., Gollnick H., Kang S., Thiboutot D., Bettoli V., Torres V., Leyden J. Understanding innate immunity and inflammation in acne: Implications for management. J. Eur. Acad. Dermatol. Venereol. 2015;29((Suppl 4)):3–11. doi: 10.1111/jdv.13190. [DOI] [PubMed] [Google Scholar]
- 19.Achermann Y., Goldstein E.J.C., Coenye T., Shirtliff M.E. Propionibacterium acnes: From Commensal to Opportunistic Biofilm-Associated Implant Pathogen. Clin. Microbiol. Rev. 2014;27:419–440. doi: 10.1128/CMR.00092-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perry A., Lambert P. Propionibacterium acnes: Infection beyond the skin. Expert Rev. Anti-Infect. Ther. 2011;9:1149–1156. doi: 10.1586/eri.11.137. [DOI] [PubMed] [Google Scholar]
- 21.Testa M., Erbiti S., Delgado A., Cardenas I. Evaluation of oral microbiota in undernourished and eutrophic children using checkerboard DNA-DNA hybridization. Anaerobe. 2016;42:55–59. doi: 10.1016/j.anaerobe.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Thiboutot D.M., Dréno B., Abanmi A., Alexis A.F., Araviiskaia E., Cabal M.I.B., Bettoli V., Casintahan F., Chow S., da Costa A., et al. Practical management of acne for clinicians: An international consensus from the Global Alliance to Improve Outcomes in Acne. J. Am. Acad. Dermatol. 2018;78((Suppl. 2)):S1–S23.e1. doi: 10.1016/j.jaad.2017.09.078. [DOI] [PubMed] [Google Scholar]
- 23.Sevimli Dikicier B. Topical treatment of acne vulgaris: Efficiency, side effects, and adherence rate. J. Int. Med. Res. 2019;47:2987–2992. doi: 10.1177/0300060519847367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellis S.R., Nguyen M., Vaughn A.R., Notay M., Burney W.A., Sandhu S., Sivamani R.K. The Skin and Gut Microbiome and Its Role in Common Dermatologic Conditions. Microorganisms. 2019;7:550. doi: 10.3390/microorganisms7110550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stangeland K.Z., Huldt-Nystrøm T., Li X., Danielsen K. Behandling av akne [Treatment of acne] Tidsskrift for Den Norske Legeforening. Sep 9, 2019. [DOI] [PubMed]
- 26.Barbieri J.S., Hoffstad O., Margolis D.J. Duration of oral tetracycline-class antibiotic therapy and use of topical retinoids for the treatment of acne among general practitioners (GP): A retrospective cohort study. J. Am. Acad. Dermatol. 2016;75:1142–1150.e1. doi: 10.1016/j.jaad.2016.06.057. [DOI] [PubMed] [Google Scholar]
- 27.Lee Y.H., Liu G., Thiboutot D.M., Leslie D., Kirby J.S. A retrospective analysis of the duration of oral antibiotic therapy for the treatment of acne among adolescents: Investigating practice gaps and potential cost-savings. J. Am. Acad. Dermatol. 2014;71:70–76. doi: 10.1016/j.jaad.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 28.Straight C.E., Lee Y.H., Liu G., Kirby J.S. Duration of oral antibiotic therapy for the treatment of adult acne: A retrospective analysis investigating adherence to guideline recommendations and opportunities for cost-savings. J. Am. Acad. Dermatol. 2015;72:822–827. doi: 10.1016/j.jaad.2015.01.048. [DOI] [PubMed] [Google Scholar]
- 29.Barbieri J.S., James W.D., Margolis D.J. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: A retrospective analysis, 2004–2013. J. Am. Acad. Dermatol. 2017;77:456–463.e4. doi: 10.1016/j.jaad.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 30.Chien A.L., Tsai J., Leung S., Mongodin E.F., Nelson A., Kang S., Garza L. Association of Systemic Antibiotic Treatment of Acne With Skin Microbiota Characteristics. JAMA Dermatol. 2019;155:425–434. doi: 10.1001/jamadermatol.2018.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trivedi M.K., Bosanac S.S., Sivamani R.K., Larsen L.N. Emerging Therapies for Acne Vulgaris. Am. J. Clin. Dermatol. 2018;19:505–516. doi: 10.1007/s40257-018-0345-x. [DOI] [PubMed] [Google Scholar]
- 32.Friedrich A.D., Paz M.L., Leoni J., Maglio D.H.G. Message in a Bottle: Dialog between Intestine and Skin Modulated by Probiotics. Int. J. Mol. Sci. 2017;18:1067. doi: 10.3390/ijms18061067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 34.Martin-Cabezas R., Davideau J.-L., Tenenbaum H., Huck O. Clinical efficacy of probiotics as an adjunctive therapy to non-surgical periodontal treatment of chronic periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2016;43:520–530. doi: 10.1111/jcpe.12545. [DOI] [PubMed] [Google Scholar]
- 35.Tekce M., Ince G., Gürsoy H., Ipci S.D., Cakar G., Kadir T., Yilmaz S. Clinical and microbiological effects of probiotic lozenges in the treatment of chronic periodontitis: A 1-year follow-up study. J. Clin. Periodontol. 2015;42:363–372. doi: 10.1111/jcpe.12387. [DOI] [PubMed] [Google Scholar]
- 36.Vivekananda M.R., Vandana K.L., Bhat K. Effect of the probiotic Lactobacilli reuteri (Prodentis) in the management of periodontal disease: A preliminary randomized clinical trial. J. Oral Microbiol. 2010;2:5344. doi: 10.3402/jom.v2i0.5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ince G., Gürsoy H., Ipçi D., Cakar G., Emekli-Alturfan E., Yılmaz S. Clinical and Biochemical Evaluation of Lozenges Containing Lactobacillus reuteri as an Adjunct to Non-Surgical Periodontal Therapy in Chronic Periodontitis. J. Periodontol. 2015;86:746–754. doi: 10.1902/jop.2015.140612. [DOI] [PubMed] [Google Scholar]
- 38.Laleman I., Pauwels M., Quirynen M., Teughels W. A dual-strain Lactobacilli reuteri probiotic improves the treatment of residual pockets: A randomized controlled clinical trial. J. Clin. Periodontol. 2020;47:43–53. doi: 10.1111/jcpe.13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sinesi A., Mosaico G., Cont M., Cefola S., Mautarelli G., Casu C. Gum Hypertrophy in Patients in Fixed Orthodontic Therapy Treated with Topical Probiotic Lactobacillus Reuteri: A Pilot Study. Proceedings. 2019;35:40. doi: 10.3390/proceedings2019035040. [DOI] [Google Scholar]
- 40.Huang M.-C.J., Tang J. Probiotics in personal care products. Microbiol. Discov. 2015;3:5. doi: 10.7243/2052-6180-3-5. [DOI] [Google Scholar]
- 41.Lee G.R., Maarouf M., Hendricks A.J., Lee D.E., Shi V.Y. Topical probiotics: The unknowns behind their rising popularity. Dermatol. Online J. 2019;25 doi: 10.5070/D3255044062. [DOI] [PubMed] [Google Scholar]
- 42.Jeong J.H., Lee C.Y., Chung D.K. Probiotic Lactic Acid Bacteria and Skin Health. Crit. Rev. Food Sci. Nutr. 2016;56:2331–2337. doi: 10.1080/10408398.2013.834874. [DOI] [PubMed] [Google Scholar]
- 43.Kim H.M., Lee D.E., Park S.D., Kim Y.-T., Kim Y.J., Jeong J.W., Jang S.S., Ahn Y.-T., Sim J.-H., Huh C.-S., et al. Oral Administration of Lactobacillus plantarum HY7714 Protects Hairless Mouse Against Ultraviolet B-Induced Photoaging. J. Microbiol. Biotechnol. 2014;24:1583–1591. doi: 10.4014/jmb.1406.06038. [DOI] [PubMed] [Google Scholar]
- 44.Lee D.E., Huh C.-S., Ra J., Choi I.-D., Jeong J.-W., Kim S.-H., Ryu J.H., Seo Y.K., Koh J.S., Lee J.-H., et al. Clinical Evidence of Effects of Lactobacillus plantarum HY7714 on Skin Aging: A Randomized, Double Blind, Placebo-Controlled Study. J. Microbiol. Biotechnol. 2015;25:2160–2168. doi: 10.4014/jmb.1509.09021. [DOI] [PubMed] [Google Scholar]
- 45.You G.-E., Jung B.-J., Kim H.-R., Kim H.-G., Chung D.K. Lactobacillus sakei Lipoteichoic Acid Inhibits MMP-1 Induced by UVA in Normal Dermal Fibroblasts of Human. J. Microbiol. Biotechnol. 2013;23:1357–1364. doi: 10.4014/jmb.1306.06026. [DOI] [PubMed] [Google Scholar]
- 46.Bowe W., Patel N.B., Logan A. Acne vulgaris, probiotics and the gut-brain-skin axis: From anecdote to translational medicine. Benef. Microbes. 2014;5:185–199. doi: 10.3920/BM2012.0060. [DOI] [PubMed] [Google Scholar]
- 47.Kober M.-M., Bowe W.P. The effect of probiotics on immune regulation, acne, and photoaging. Int. J. Womens Dermatol. 2015;1:85–89. doi: 10.1016/j.ijwd.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jung G.W., Tse J.E., Guiha I., Rao J. Prospective, Randomized, Open-Label Trial Comparing the Safety, Efficacy, and Tolerability of an Acne Treatment Regimen with and without a Probiotic Supplement and Minocycline in Subjects with Mild to Moderate Acne. J. Cutan. Med. Surg. 2013;17:114–122. doi: 10.2310/7750.2012.12026. [DOI] [PubMed] [Google Scholar]
- 49.Salem I., Ramser A., Isham N., Ghannoum M.A. The Gut Microbiome as a Major Regulator of the Gut-Skin Axis. Front. Microbiol. 2018;9:1459. doi: 10.3389/fmicb.2018.01459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khmaladze I., Butler É., Fabre S., Gillbro J.M. Lactobacillus reuteri DSM 17938—A comparative study on the effect of probiotics and lysates on human skin. Exp. Dermatol. 2019;28:822–828. doi: 10.1111/exd.13950. [DOI] [PubMed] [Google Scholar]
- 51.Kang M.-S., Oh J.-S., Lee S.-W., Lim H.-S., Choi N.-K., Kim S.-M. Effect of Lactobacillus reuteri on the proliferation of Propionibacterium acnes and Staphylococcus epidermidis. J. Microbiol. 2012;50:137–142. doi: 10.1007/s12275-012-1286-3. [DOI] [PubMed] [Google Scholar]
- 52.Fijan S., Frauwallner A., Langerholc T., Krebs B., ter Haar J.A., Heschl A., Mičetić Turk D., Rogelj I. Efficacy of Using Probiotics with Antagonistic Activity against Pathogens of Wound Infections: An Integrative Review of Literature. BioMed Res. Int. 2019;2019:7585486. doi: 10.1155/2019/7585486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.