Abstract
Two Janus‐associated kinase inhibitors (JAKi) (initially ruxolitinib and, more recently, fedratinib) have been approved as treatment options for patients who have intermediate‐risk and high‐risk myelofibrosis (MF), with pivotal trials demonstrating improvements in spleen volume, disease symptoms, and quality of life. At the same time, however, clinical trial experiences with JAKi agents in MF have demonstrated a high frequency of discontinuations because of adverse events or progressive disease. In addition, overall survival benefits and clinical and molecular predictors of response have not been established in this population, for which the disease burden is high and treatment options are limited. Consistently poor outcomes have been documented after JAKi discontinuation, with survival durations after ruxolitinib ranging from 11 to 16 months across several studies. To address such a high unmet therapeutic need, various non‐JAKi agents are being actively explored (in combination with ruxolitinib in first‐line or salvage settings and/or as monotherapy in JAKi‐pretreated patients) in phase 3 clinical trials, including pelabresib (a bromodomain and extraterminal domain inhibitor), navitoclax (a B‐cell lymphoma 2/B‐cell lymphoma 2‐xL inhibitor), parsaclisib (a phosphoinositide 3‐kinase inhibitor), navtemadlin (formerly KRT‐232; a murine double‐minute chromosome 2 inhibitor), and imetelstat (a telomerase inhibitor). The breadth of data expected from these trials will provide insight into the ability of non‐JAKi treatments to modify the natural history of MF.
Keywords: biomarkers, fedratinib, Janus kinase inhibitor, myelofibrosis, ruxolitinib, safety, survival
Short abstract
Janus‐associated kinase inhibitor (JAKi) agents improved spleen volume and symptoms in patients who had myelofibrosis but were associated with high discontinuation rates, a lack of predictors of response, and poor outcomes after discontinuation. Non‐JAKi agents, in combination with or subsequent to JAK agents, were being actively explored in phase 3 clinical trials.
Introduction
Myelofibrosis (MF) is a clonal stem cell disease characterized by bone marrow fibrosis and a heterogeneous disease phenotype, with a variable degree of splenomegaly, cytopenias, and constitutional symptoms that significantly affect quality of life and survival. Currently, allogeneic hematopoietic stem cell transplantation is the only treatment capable of inducing long‐term remission of MF. However, the majority of patients are ineligible for hematopoietic stem cell transplantation because of advanced age and/or the presence of comorbidities. Two Janus‐associated kinase (JAK) inhibitors (JAKi), ruxolitinib 1 , 2 and, more recently, fedratinib, 3 , 4 have been approved for the treatment of intermediate‐risk and high‐risk MF—reducing spleen volume and improving disease‐related symptoms. With responses to ruxolitinib typically observed within the first 3 to 6 months after therapy initiation, 1 , 2 it has been suggested that, for patients who have not had a reduction in spleen size or an improvement in symptoms after that period, alternative therapies should be considered. 5 For patients who progress to blast phase disease during ruxolitinib treatment, survival is typically measured in weeks to months. 6 Suboptimal adherence to ruxolitinib, translating into undertreatment and associated poor outcomes, is also a concern in clinical practice. 7
Defining progressive disease (PD) in MF poses clinical challenges. 8 The International Working Group for Myeloproliferative Neoplasms Research and Treatment criteria (2013 revision) are focused on new or worsening splenomegaly and leukemic transformation as signs of progression. However, PD may take other forms, including worsening anemia and/or thrombocytopenia, progressive myeloproliferative neoplasms symptoms or leukocytosis, or extramedullary hematopoiesis, compromising organ function or causing pain. In recent years, stringent criteria for ruxolitinib failure have been adopted in the design and analysis of some clinical trials 9 , 10 ; however, discordance among clinicians in defining ruxolitinib failure persists in real‐world practice. 11 Here, we explore the characteristics and outcomes of patients with MF who discontinue JAKi treatment because of resistance, progression, or intolerance.
Overview of Key Clinical Trial Experiences with Approved JAK Inhibitors
Ruxolitinib
Efficacy
Primary and follow‐up results of pivotal phase 3 clinical trials, referred to as COMFORT‐I (ClinicalTrials.gov identifier NCT00952289) and COMFORT‐II (ClinicalTrials.gov identifier NCT00934544), 1 , 2 , 12 , 13 , 14 , 15 , 16 collectively demonstrate reduced spleen volume, improved MF‐related symptoms and quality‐of‐life measures, and prolonged overall survival in patients with intermediate‐2–risk or high‐risk MF compared with controls (Table 1). 1 , 2 , 13 , 15 A combined analysis of COMFORT‐I and COMFORT‐II demonstrated a significant survival benefit for ruxolitinib as frontline treatment for patients with MF (5.3 vs 2.4 years for controls), irrespective of baseline anemia status or transfusion requirements at week 24. 16 The phase 3b expanded‐access JUMP study (ClinicalTrials.gov identifier NCT01493414) demonstrated that ruxolitinib confers meaningful improvements in spleen length and symptoms, which were also observed in a low‐platelet‐count cohort, and symptomatic benefits also apply to patients without splenomegaly. 17
TABLE 1.
Key Efficacy Findings from Pivotal Clinical Trials of Ruxolitinib in Myelofibrosis
| Study | Population | Treatment | Spleen Response Rates | Survival |
|---|---|---|---|---|
| COMFORT‐I |
|
Ruxolitinib (15 or 20 mg BID [per platelet count]) or placebo | — | — |
|
||||
|
||||
| Primary results (Verstovsek 2012 2 ) | — | — | SVR ≥35% at wk 24: | |
|
|
|||
|
|
|||
| 5‐y update (Verstovsek 2017 15 ) | — | — | SVR ≥35% at any time during study: | Median OS: |
|
|
|||
|
||||
|
||||
| COMFORT‐II |
|
Ruxolitinib (15 or 20 mg BID [per platelet count]) or BAT | — | — |
|
||||
|
||||
| Primary results (Harrison 2012 1 ) | — | — | SVR ≥35% at wk 48: | |
|
|
|||
|
|
|||
| 5‐y update (Harrison 2016 13 ) | — | — | SVR ≥35% at any time during study: | Median OS: |
|
|
|||
|
|
Abbreviations: ASCT, allogeneic stem cell transplantation; BAT, best available therapy; BID, twice daily; CI, confidence interval; HR, hazard ratio; JAKi, Janus‐associated kinase inhibitor; NR, not reached; OS, overall survival; PET‐MF, postessential thrombocythemia myelofibrosis; PMF, primary myelofibrosis; PPV‐MF, postpolycythemia vera myelofibrosis; SVR, spleen volume reduction.
Safety
At the 5‐year data cutoff in COMFORT‐I, ruxolitinib treatment was ongoing in 27.7% of patients who originally were randomized to receive ruxolitinib and 25.2% of patients who crossed over from ruxolitinib to placebo. 15 Death was the most common reason for early discontinuation, followed by PD, and adverse events (AEs). AEs led to ruxolitinib discontinuation in approximately one‐third of patients who were randomized or crossed over to ruxolitinib, a rate that was substantially higher than the 12.6% AE‐related discontinuation rate with placebo. Nearly 3% of ruxolitinib‐randomized patients discontinued treatment for acute myeloid leukemia or anemia (2.6% each), and nearly 4% discontinued treatment for acute myeloid leukemia or thrombocytopenia (3.6% each) in the ruxolitinib crossover group. In COMFORT‐II, early discontinuations before 5 years of ruxolitinib were because of AEs and PD in 24% and 22% of patients, respectively. 13 Overall, AE‐related study discontinuations occurred in 25% of patients who received ruxolitinib (in the randomized and extension phases or after crossover from best available therapy [BAT]), most commonly for thrombocytopenia (3.7%) or for anemia, splenomegaly, pneumonia, or prostate cancer (1% each). No new safety concerns were identified in the phase 3b expanded‐access JUMP study. 17 Long‐term safety has been described in the real‐world setting, supporting an AE‐related discontinuation rate <10% but more a frequent need for dose adjustment (approximately 25% of patients). 18
Fedratinib
Efficacy
Fedratinib was evaluated in a pivotal phase 3 clinical trial, JAKARTA (ClinicalTrials.gov identifier NCT01437787) and demonstrated spleen volume reductions along with symptomatic and quality‐of‐life benefits relative to placebo in patients with intermediate‐2–risk or high‐risk, primary or secondary MF (Table 2). 3 , 4 , 9 , 19 , 20 Of note, the JAKARTA trial had been terminated in 2013 in response to a clinical hold on development because of a suspected emergence of Wernicke encephalopathy; however, the hold was lifted in 2017 after consideration of additional safety data (supporting that these cases were in patients receiving 500 mg daily), thus resuming the regulatory submission process. 4 , 19 Reanalyzed efficacy results from JAKARTA, which formed the basis for the US Food and Drug Administration approval of fedratinib 400 mg daily, showed a 24‐week spleen response rate of 47% (vs 1% with placebo) or 37% when confirmed with 4‐week scans, along with a symptom response rate of 40%. 19 A subsequent phase 2 clinical trial specifically in ruxolitinib‐resistant or ruxolitinib‐intolerant patients, JAKARTA‐2 (ClinicalTrials.gov identifier NCT01523171), met its primary end point of spleen response in the primary analysis 3 and in an updated analysis using stringent criteria for ruxolitinib failure (Table 2), 9 with quality‐of‐life benefits also demonstrated. 21
TABLE 2.
Key Efficacy Findings from Pivotal Clinical Trials of Fedratinib in Myelofibrosis
| Study | Population | Treatment | Spleen Response Rates | Survival |
|---|---|---|---|---|
| JAKARTA |
|
Fedratinib 400 or 500 mg QD (per randomization) or placebo | — | — |
|
||||
| Primary results (Pardanani 2015 4 ) | SVR ≥35% at wk 24 (and confirmed after 4 wks): |
|
||
|
|
|||
|
|
|||
|
||||
| Updated analysis (Pardanani 2021 19 ) | SVR ≥35% at wk 24 (and confirmed after 4 wks): | Not reported | ||
|
||||
|
||||
| JAKARTA‐2 |
|
Fedratinib (400 mg QD) | — | — |
|
||||
|
||||
| Primary results (Harrison 2017 3 ) | SVR ≥35% at wk 24: |
|
||
|
||||
|
||||
|
||||
| Updated analysis (Harrison 2020 9 ) | SVR ≥35% at wk 24: | Not reported | ||
|
||||
|
||||
|
Abbreviations: ITT, intention‐to‐treat; OS, overall survival; PET‐MF, postessential thrombocythemia myelofibrosis; PMF, primary myelofibrosis; PPV‐MF, postpolycythemia vera myelofibrosis; QD, daily; SVR, spleen volume reduction.
This was a subset of patients from the ITT population who were ruxolitinib‐intolerant or were classified as having relapsed/refractory disease according to the more stringent criteria, revised from the original analysis.
This was a subset of patients from the stringent criteria cohort who reached cycle 6 or discontinued before cycle 6 for reasons other than study terminated by the sponsor.
Survival data have been presented for patients receiving fedratinib 400 mg daily in JAKARTA and JAKARTA‐2. Although the results were confounded by the clinical hold on fedratinib development and crossover to fedratinib in JAKARTA, first‐line fedratinib showed a significant progression‐free survival benefit and appeared to confer an overall survival benefit when used early (based on interpretation of the separation of the overall survival curves, even after the point of crossover from placebo to fedratinib). 22 Outcomes in ruxolitinib‐pretreated patients, including a median overall survival that had not been reached and 1‐year and 18‐month overall survival rates of 84% and 67%, respectively, were encouraging.
Of note, efficacy results are awaited from additional ongoing phase 3 evaluations of fedratinib in MF, including a single‐arm trial of the long‐term efficacy and safety of fedratinib in ruxolitinib‐pretreated patients (FREEDOM; ClinicalTrials.gov identifier NCT03755518) and an open‐label, randomized comparison of fedratinib versus other active MF therapies, including ruxolitinib, in ruxolitinib‐pretreated patients (FREEDOM2; ClinicalTrials.gov identifier NCT03952039).
Safety
In the initial analysis of JAKARTA, fedratinib treatment was ongoing in 67% of patients originally randomized to fedratinib 400 mg daily and in 90% (27 of 30) of patients who crossed over from placebo to fedratinib 400 mg. 4 In the cohort randomized to fedratinib 400 mg daily, most of the discontinuations by the end of week 24 were for AEs (13 of 21 patients). In the reanalyzed JAKARTA safety data, patients randomized to fedratinib had AE‐related treatment interruption and dose reduction rates 21% and 14%, respectively, mostly for gastrointestinal AEs (eg, diarrhea; responsible for 5% and 4% of patients interrupting or dose‐reducing treatment) or anemia (the most common cause of dose reductions; 6% of patients). 19 Thirteen patients, or 14%, had permanent discontinuations of fedratinib: 3 of these patients had cardiac failure, and 2 each discontinued for thrombocytopenia, myocardial ischemia, diarrhea, or increased blood creatinine. In JAKARTA‐2, most study discontinuations were related to the aforementioned fedratinib clinical hold (65%), followed by AEs (19%), and PD (6%). 9 AEs leading to treatment interruption, dose reduction, permanent discontinuation, or death were reported in 26%, 39%, 20%, and 7% of patients, respectively. Like in the JAKARTA study, the most common reasons for dose interruptions or reductions were gastrointestinal AEs or anemia. AEs resulting in permanent discontinuation in >1 patient were diarrhea (n = 2) and thrombocytopenia (n = 2), and the AE‐related deaths included PD (n = 4) and cardiopulmonary AEs that were considered to be unrelated to fedratinib. No cases of Wernicke encephalopathy were reported.
Safety and tolerability data for fedratinib 400 mg daily have been presented from the phase 3b FREEDOM trial, which, unlike early clinical trials of fedratinib, included AE mitigation strategies for gastrointestinal events (prophylactic or symptomatic use of antiemetic and antidiarrheal agents) as well as monitoring and management of thiamine level reductions and surveillance for Wernicke encephalopathy. 23 The most common gastrointestinal AEs were constipation (47%), diarrhea (35%), nausea (26%), abdominal pain (24%), and vomiting (18%), all of grade 1 or 2 severity, except for 1 case of abdominal pain, which was not considered to be related to fedratinib. Diarrhea, nausea, and vomiting were primarily seen during the first cycle, with rates falling below 5% after cycle 1 for nausea/vomiting and after cycle 2 for diarrhea and with no occurrences at cycle 6. Anemia was the most common all‐grade (32%) and grade 3 and 4 (21%) nongastrointestinal AE. Oral thiamine supplementation was used therapeutically in 5 patients who had thiamine level reductions (with prophylactic or empirical use in 5 additional patients), effectively preventing the need for fedratinib dose reduction or interruption, and there were no reports of Wernicke encephalopathy.
Determining Predictors of Response to JAK Inhibitor Treatment
Clinical Characteristics
Benefits for ruxolitinib were demonstrated across all clinical subgroups evaluated in both COMFORT‐I (Fig. 1) and COMFORT‐II. 1 , 24 In analyzing predictors of response in the phase 3b expanded‐access JUMP study, higher spleen response rates were observed with the use of ruxolitinib in patients with lower International Prognostic Scoring System risk (43.1% for low/intermediate‐1 risk vs 30.6% for intermediate‐2/high risk; adjusted odds ratio [OR], 0.65; 95% CI, 0.44‐0.95), earlier in treatment (40.2% for first‐line vs 31.5% for second‐line or later therapy; adjusted OR, 0.53; 95% CI, 0.38‐0.75), or at a higher total daily dose after 12 weeks (41.3% for >20 mg daily vs 30.4% for ≤20 mg daily; adjusted OR, 0.47; 95% CI, 0.33‐0.68). 25 These characteristics, which were predictive of spleen responses, however, were not predictive of symptomatic responses. In a large cohort of patients treated with ruxolitinib across 23 European Hematology Centers, whether treated within or outside of a clinical trial, patients with a lower disease burden state were more likely to achieve spleen or symptom responses, whereas ruxolitinib dosing appeared to affect spleen responses but not symptom responses. 26 By using this same European clinical database, a separate analysis was conducted that stratified patients into 3 groups based on type of spleen response (stable, unstable, or never achieved). 27 Characteristics of statistical significance for patients who attained a spleen response (stable or unstable) versus no response included lower frequencies of high‐risk disease (OR, 0.45; P = .01) or splenomegaly >10 cm (OR, 0.24; P = .001), higher frequencies of early initiation of ruxolitinib within 2 years of diagnosis (OR, 0.51; P = .04), and having received higher 12‐week ruxolitinib doses of ≥15 mg (OR, 1.98; P = .03). Additional findings derived from these European data are that responses to ruxolitinib do not appear to differ based on primary versus secondary MF (although the latter have been shown to have a lower propensity for developing ruxolitinib‐induced cytopenia) 28 and that comorbidities and body mass index are not predictive of spleen or symptom response. 29
Figure 1.
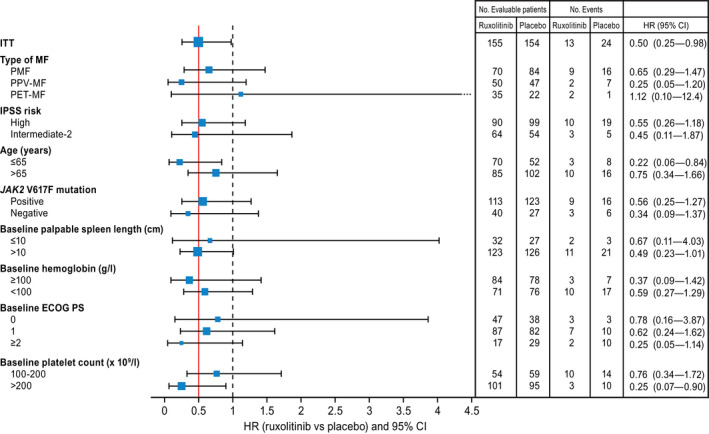
This is a forest plot of survival by patient subgroup in the COMFORT‐I trial. The red line represents the hazard ratio (HR) of the intent‐to‐treat (ITT) population, and the dashed line represents an HR of 1.0. The squares represent the HR and sample size for each subgroup, where the area of the square is proportional to the subgroup sample size. CI indicates confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; IPSS, International Prognostic Scoring System; ITT, intention‐to‐treat; JAK, Janus‐associated kinase; PET‐MF, postessential thrombocythemia myelofibrosis; PMF, primary myelofibrosis; PPV‐MF, postpolycythemia vera myelofibrosis. Reproduced from: Verstovsek S, Mesa RA, Gotlib J, et al. The clinical benefit of ruxolitinib across patient subgroups: analysis of a placebo‐controlled, phase III study in patients with myelofibrosis. Br J Haematol. 2013;161:508‐516. 24 © 2013 Wiley‐Blackwell.
Limited published data are available to draw any insights into clinical predictors of response to fedratinib. In JAKARTA‐2, an ad hoc analysis found that duration of prior ruxolitinib therapy and baseline spleen size did not substantially affect spleen responses with fedratinib. 3
Molecular Predictors
Accumulating data are providing insight into molecular predictors of response to ruxolitinib. In COMFORT‐I, JAK2 V617F (JAK2V617F ) mutation status seemed to influence response to ruxolitinib, with spleen response rates of 33% and 14% in positive and negative subgroups, respectively, with the investigators emphasizing the overall similarity of responses across subgroups and that longer follow‐up would be needed to determine the significance of baseline JAK2V617F allele burden. 1 Barosi et al evaluated predictive characteristics for spleen response in 69 consecutive patients receiving ruxolitinib for MF and associated splenomegaly as part of a clinical trial or off‐study. 30 In these patients, spleen response rates were 38%, 32%, and 41% at 3 months, 6 months, and 1 year, respectively. No significant associations were seen between spleen response and most baseline demographic and clinical characteristics. Although a hemoglobin concentration ≥100 g/L, higher ruxolitinib dose intensity, genotype other than CALR mutation, and JAK2V617F allele burden ≥50% were significantly associated with spleen response in univariable analyses, only dose intensity and JAK2V617F allele burden ≥50% were identified as independent predictors in multivariate analyses. For patients who had a JAK2V617F allele burden ≥50%, spleen response probability was 5.5‐fold higher compared with those wo had a burden <50% or any other mutation, and they represented 19 of the 22 patients who maintained response at the data cutoff.
A subset analysis of COMFORT‐II, focused on MF‐associated mutations, showed no impact of molecular profiles (including those of high molecular risk) on spleen or symptom responses, hematologic toxicity, or overall survival in ruxolitinib‐treated patients. 31 Conversely, Patel et al applied next‐generation sequencing to baseline bone marrow or peripheral blood samples derived from 95 patients who participated in a phase 1 and 2 study of ruxolitinib, finding an association between mutational burden and spleen response. 32 During that study, 72% of patients had achieved a spleen response. Spleen responses were less likely to occur among patients who had ≥1 mutation(s) in ASXL1, EZH2, or IDH1/IDH2 or ≥3 mutations of any type, with no associations involving JAK2, CALR, MPL, or triple‐negative mutation status. Further analysis of 20 long‐term responders who were continuing to receive ruxolitinib after a median of 6.4 years found that all had only 1 or 2 mutations, and 18 had no identifiable high‐molecular‐risk mutations, including ASXL1, DNMT3A, EZH2, or IDH1/IDH2.
Outcomes After JAKi Discontinuation
Recent observational data (capturing patients treated in the JUMP trial or off study) support that approximately one‐half of patients discontinue JAKi/ruxolitinib treatment within 3 years because of lack of response, loss of therapeutic effect or PD, or toxicities, including cytopenias. 33 Based on previously published phase 1 and 2 clinical trial data for ruxolitinib in MF, the 1‐year, 2‐year, and 3‐year discontinuation rates were 49%, 71% and 86%, respectively. 34 Of note, a phenomenon of ruxolitinib‐discontinuation syndrome has been described, in which some patients experience a symptomatic relapse and worsening splenomegaly along with potentially life‐threatening AEs (eg, respiratory distress, septic‐like shock, and disseminated intravascular coagulation‐like syndrome) from an acute postruxolitinib cytokine storm. 35 Real‐world data also indicate a rising burden of cytopenias after ruxolitinib discontinuation relative to the period of active treatment. 36 Ruxolitinib rechallenge may be a consideration for some patients who discontinue treatment, particularly those who initially discontinue for intolerance, although most will go on to permanently discontinue ruxolitinib. 37
Overall, outcomes after ruxolitinib discontinuation are poor, including poor overall survival in the range of 11 to 16 months. 36 , 38 , 39 , 40 In an early analysis of outcomes after ruxolitinib discontinuation in patients with MF enrolled in a phase 1 and 2 study, the median overall survival after discontinuation was 14 months. 39 Kuykendall et al, in evaluating salvage treatment options and clinical outcomes among patients with MF who received and discontinued ruxolitinib outside the context of a clinical trial, reported that the median overall survival after ruxolitinib discontinuation was 13 months. 38 Similarly, in a population‐based cohort study of Swedish and Norwegian patients with an MF diagnosis in the National Cancer Registries, the median relative survival (vs a matched general population) was 16 months among patients who discontinued ruxolitinib. 40 Most recently, a retrospective analysis of medical claims‐based data for the MF population provided insight into outcomes after ruxolitinib discontinuation as well as patient characteristics associated with an increased risk of PD or death, reporting a median overall survival of 11.1 months after ruxolitinib discontinuation. 36 In that retrospective analysis, significant predictors of poor overall survival included age older than 65 years at ruxolitinib discontinuation (hazard ratio, 3.8) and, to a lesser extent, a higher Charlson comorbidity index score (hazard ratio, 1.2). Both advanced age and higher comorbidities were also significant predictors of a composite outcome of treatment progression or death, along with female gender.
Overview of Ongoing Phase 3 Clinical Development Efforts of Non‐JAK Inhibitors in MF
Currently, most agents in phase 3 clinical development for MF are being evaluated as ruxolitinib‐based combination strategies in JAKi‐naive patients, as single agents to address spleen and/or symptom burden in the case of ruxolitinib discontinuation, or in combination with ruxolitinib to salvage suboptimal spleen and symptom response. Pelabresib, a bromodomain and extraterminal domain inhibitor, is being evaluated in combination with ruxolitinib in the placebo‐controlled phase 3 MANIFEST‐2 study in patients with ruxolitinib‐naive MF (ClinicalTrials.gov identifier NCT04603495). Phase 3 evaluation of navitoclax, a B‐cell lymphoma 2/B‐cell lymphoma 2‐xL inhibitor, includes a placebo‐controlled study of navitoclax plus ruxolitinib in JAKi‐naive patients (TRANSFORM‐1; ClinicalTrials.gov identifier NCT04472598) as well as a comparison of the combination with BAT as second‐line treatment in suboptimal responders to ruxolitinib monotherapy (TRANSFORM‐2; ClinicalTrials.gov identifier NCT04468984). The phosphoinositide 3‐kinase inhibitor parsaclisib is undergoing phase 3 evaluation in combination with ruxolitinib, both in the first‐line setting (LIMBER‐313; ClinicalTrials.gov identifier NCT04551066) and in a combination salvage setting (LIMBER‐304; ClinicalTrials.gov identifier NCT04551053). In addition, a randomized phase 3 study (Boreas; ClinicalTrials.gov identifier NCT03662126) is comparing the murine double‐minute chromosome 2 inhibitor navtemadlin (formerly KRT‐232) with BAT (hydroxyurea, chemotherapy, and supportive care, excluding JAKi) in patients with MF who are relapsed or refractory to JAKi treatment. All of these studies are evaluating spleen volume reduction as the primary outcome, building on the established benefits of ruxolitinib in terms of spleen and symptomatic benefit, with the added potential of demonstrating other aspects of disease modification using secondary end points, such as reduction in bone marrow fibrosis, modulation of the driver mutation allele fraction, and ultimately extending survival. Currently, reliable predictive biomarkers are not available to guide treatment decision making in MF; therefore, the potential to introduce multiple JAKi and non‐JAKi agents into the commercial space will add a welcomed layer of complexity to MF management.
Imetelstat, a telomerase inhibitor, is an important non‐JAKi in phase 3 testing of patients with MF who are refractory to JAKi treatment. The comparator arm of this randomized phase 3 trial (IMpactMF/MYF3001; ClinicalTrials.gov identifier NCT04576156) is BAT, excluding JAKi, and uniquely and importantly has a primary end point of overall survival—reflecting the reproducibly poor outcomes in those patients with MF who are refractory to JAKi and thus the urgent unmet need to extend survival. It is important to note that, in phase 2 testing of 2 doses of single‐agent imetelstat in JAKi relapsed/refractory MF, the median survival of 30 months achieved with imetelstat 9.4 mg/kg was not only favorable compared with the lower dose of 4.7 mg/kg (median survival, 20 months), 41 it was also prolonged relative to a propensity score‐matched, real‐world population of patients with MF (median survival, 12 months). 42 Importantly, this survival improvement was achieved with a median duration of 33 weeks of imetelstat therapy and was maintained with censoring for subsequent lines of therapy including transplantation, 41 , 42 suggesting that continued exposure to this infusional agent may not even be necessary to confer improvements in progression‐free and overall survival. Further analyses of the phase 2 imetelstat data showed reductions in bone marrow fibrosis and mutation variant allele frequency that correlated with prolongation of overall survival. 43 , 44 Whether a minimum duration of telomerase inhibition is required to alter the natural history of this PD and whether maintenance therapy with imetelstat can extend benefits will also need to be explored. Of note, preclinical data supporting synergy with a JAKi 45 has also inspired the ongoing phase 1b clinical trial evaluation of the addition of imetelstat to ruxolitinib after 12 weeks of JAKi therapy (IMproveMF).
Summary and Future Perspectives
JAKi agents have changed the treatment approach for MF, setting a standard of spleen and symptom burden alleviation, but they do not reliably alter the natural history of disease. The identification of reliable predictors of response have remained elusive. With high rates of ruxolitinib discontinuation by the third year of treatment, the prognostic outlook is poor for this patient population. After JAKi failure, the overarching treatment goal for many patients may be prolongation of life—representing an unmet need for which new therapies hold potential to confer a meaningful impact.
MF is broadly classified as primary MF or secondary MF, the latter of which includes both postessential thrombocythemia and postpolycythemia vera MF. It is appreciated that primary MF tends to behave more aggressively than secondary MF and is associated with shorter survival, 46 resulting in the development of a prognostic tool for secondary MF (known as MYSEC) that takes these nuances into account. 47 At the same time, however, current treatment algorithms for the management of MF do not distinguish between primary versus secondary MF. 48 A practical approach to MF therapy is presented in Figure 2. It is possible to envision a future with multiple approved JAKi options that can be personalized and sequenced according to the degree of cytopenias or driver mutation status, with the option to add therapies that can improve upon depth and duration of spleen or symptomatic benefit and may modify disease features, such as grade of bone marrow fibrosis and driver mutation burden, in subsets of patients. 49 However, current JAKi therapy options alone are unlikely to ensure disease course modification beyond an improvement in inflammatory cytokine‐driven systemic symptoms, reversal of cachexia, and recovery of performance status that largely underlies the survival benefit seen in the COMFORT trials. Treatments with non‐JAKi mechanisms of action that effectively deplete the malignant hematopoietic stem cell pool are required to salvage the poor outcomes uniformly reported across multiple independent studies after JAKi discontinuation. In the future, as meaningful outcome measures with salvage therapies are confirmed in prospective trials, it will then be natural to evaluate disease course‐modifying therapies earlier in the treatment paradigm, either in combination with a JAKi or perhaps even before JAKi therapy.
Figure 2.
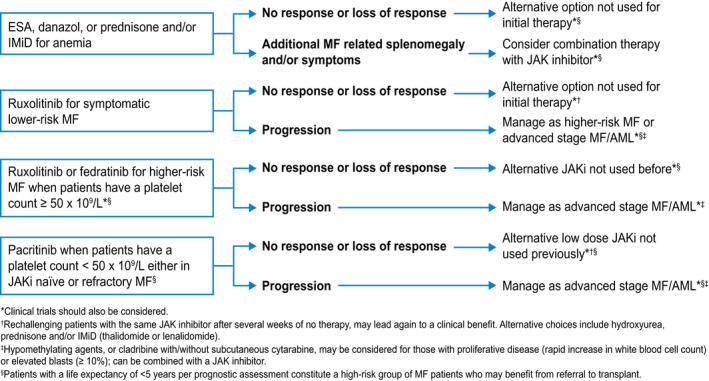
A practical approach to therapy for patients with myelofibrosis (MF) is illustrated. AML indicates acute myeloid leukemia; IMiD, immunomodulatory drug; JAKi, Janus‐associated kinase inhibitor.
Funding Support
Editorial support for this study was provided by funding from Geron Corporation.
Conflict of Interest Disclosures
John Mascarenhas reports research support from Incyte, CTI Bio, PharmaEssentia, Novartis, Roche, Kartos, AbbVie, Bristol‐Myers Squibb, Geron Corporation, Celgene, and Merck; and personal fees from Galecto, Incyte, Celgene, Bristol‐Myers Squibb, Novartis, Roche, Kartos, Geron Corporation, PharmaEssentia, Karyopharm, and CTI Bio outside the submitted work. Srdan Verstovsek reports research support from Incyte, CTI Bio, PharmaEssentia, Novartis, Roche, Kartos, Bristol‐Myers Squibb, Geron Corporation, NS Pharma, Sierra, Protagonist, Constellation, and Galecto and personal fees from Incyte, Celgene, Bristol‐Myers Squibb, Novartis, and Constellation outside the submitted work.
Mascarenhas JO, Verstovsek S. The clinical dilemma of JAK inhibitor failure in myelofibrosis: Predictive characteristics and outcomes. Cancer. 2022. 10.1002/cncr.34222
Editorial support for this publication was provided by Laurie Orloski, PharmD, of InSeption Group, funded by Geron Corporation.
The sponsor had no influence over the content of this article.
References
- 1. Harrison C, Kiladjian JJ, Al‐Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787‐798. doi: 10.1056/NEJMoa1110556 [DOI] [PubMed] [Google Scholar]
- 2. Verstovsek S, Mesa RA, Gotlib J, et al. A double‐blind, placebo‐controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799‐807. doi: 10.1056/NEJMoa1110557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harrison CN, Schaap N, Vannucchi AM, et al. Janus kinase‐2 inhibitor fedratinib in patients with myelofibrosis previously treated with ruxolitinib (JAKARTA‐2): a single‐arm, open‐label, non‐randomised, phase 2, multicentre study. Lancet Haematol. 2017;4:e317‐e324. doi: 10.1016/S2352-3026(17)30088-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pardanani A, Harrison C, Cortes JE, et al. Safety and efficacy of fedratinib in patients with primary or secondary myelofibrosis: a randomized clinical trial. JAMA Oncol. 2015;1:643‐651. doi: 10.1001/jamaoncol.2015.1590 [DOI] [PubMed] [Google Scholar]
- 5. Keohane C, Radia DH, Harrison CN. Treatment and management of myelofibrosis in the era of JAK inhibitors. Biologics. 2013;7:189‐198 [erratum in Biologics. 2013;7:231]. doi: 10.2147/BTT.S34942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Palandri F, Breccia M, Tiribelli M, et al. Risk factors for progression to blast phase and outcome in 589 patients with myelofibrosis treated with ruxolitinib: real‐world data. Hematol Oncol. 2020;38:372‐380. doi: 10.1002/hon.2737 [DOI] [PubMed] [Google Scholar]
- 7. Guglielmelli P, Palandri F, Selleri C, et al; ROMEI Study Group . Adherence to ruxolitinib, an oral JAK1/2 inhibitor, in patients with myelofibrosis: interim analysis from an Italian, prospective cohort study (ROMEI). Leuk Lymphoma. 2022;63:189‐198. doi: 10.1080/10428194.2021.1969388 [DOI] [PubMed] [Google Scholar]
- 8. Bose P, Verstovsek S. SOHO state of the art updates and next questions: identifying and treating “progression” in myelofibrosis. Clin Lymphoma Myeloma Leuk. 2021;21:641‐649. doi: 10.1016/j.clml.2021.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harrison CN, Schaap N, Vannucchi AM, et al. Fedratinib in patients with myelofibrosis previously treated with ruxolitinib: an updated analysis of the JAKARTA2 study using stringent criteria for ruxolitinib failure. Am J Hematol. 2020;95:594‐603. doi: 10.1002/ajh.25777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gerds AT, Savona MR, Scott BL, et al. Determining the recommended dose of pacritinib: results from the PAC203 dose‐finding trial in advanced myelofibrosis. Blood Adv. 2020;4:5825‐5835. doi: 10.1182/bloodadvances.2020003314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Breccia M, Barate C, Benevolo G, et al. Tracing the decision‐making process for myelofibrosis: diagnosis, stratification, and management of ruxolitinib therapy in real‐word practice. Ann Hematol. 2020;99:65‐72. doi: 10.1007/s00277-019-03847-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harrison CN, Mesa RA, Kiladjian JJ, et al. Health‐related quality of life and symptoms in patients with myelofibrosis treated with ruxolitinib versus best available therapy. Br J Haematol. 2013;162:229‐239. doi: 10.1111/bjh.12375 [DOI] [PubMed] [Google Scholar]
- 13. Harrison CN, Vannucchi AM, Kiladjian JJ, et al. Long‐term findings from COMFORT‐II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia. 2016;30:1701‐1707. doi: 10.1038/leu.2016.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mesa RA, Gotlib J, Gupta V, et al. Effect of ruxolitinib therapy on myelofibrosis‐related symptoms and other patient‐reported outcomes in COMFORT‐I: randomized, double‐blind, placebo‐controlled trial. J Clin Oncol. 2013;31:1285‐1292. doi: 10.1200/JCO.2012.44.4489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Verstovsek S, Mesa RA, Gotlib J, et al; COMFORT‐I Investigators . Long‐term treatment with ruxolitinib for patients with myelofibrosis: 5‐year update from the randomized, double‐blind, placebo‐controlled, phase 3 COMFORT‐I trial. J Hematol Oncol. 2017;10:55. doi: 10.1186/s13045-017-0417-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Verstovsek S, Gotlib J, Mesa RA, et al. Long‐term survival in patients treated with ruxolitinib for myelofibrosis: COMFORT‐I and ‐II pooled analyses. J Hematol Oncol. 2017;10:156. doi: 10.1186/s13045-017-0527-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Al‐Ali HK, Griesshammer M, Foltz L, et al. Primary analysis of JUMP, a phase 3b, expanded‐access study evaluating the safety and efficacy of ruxolitinib in patients with myelofibrosis, including those with low platelet counts. Br J Haematol. 2020;189:888‐903. doi: 10.1111/bjh.16462 [DOI] [PubMed] [Google Scholar]
- 18. Barraco F, Greil R, Herbrecht R, et al. Real‐world non‐interventional long‐term post‐authorisation safety study of ruxolitinib in myelofibrosis. Br J Haematol. 2020;191:764‐774. doi: 10.1111/bjh.16729 [DOI] [PubMed] [Google Scholar]
- 19. Pardanani A, Tefferi A, Masszi T, et al. Updated results of the placebo‐controlled, phase III JAKARTA trial of fedratinib in patients with intermediate‐2 or high‐risk myelofibrosis. Br J Haematol. 2021;195:244‐248. doi: 10.1111/bjh.17727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mesa RA, Schaap N, Vannucchi AM, et al. Patient‐reported effects of fedratinib, an oral, selective inhibitor of Janus kinase 2, on myelofibrosis‐related symptoms and health‐related quality of life in the randomized, placebo‐controlled, phase III JAKARTA trial. Hemasphere. 2021;5:e553. doi: 10.1097/HS9.0000000000000553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harrison CN, Schaap N, Vannucchi AM, et al. Fedratinib improves myelofibrosis‐related symptoms and health‐related quality of life in patients with myelofibrosis previously treated with ruxolitinib: patient‐reported outcomes from the phase II JAKARTA2 trial. Hemasphere. 2021;5:e562. doi: 10.1097/HS9.0000000000000562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harrison C, Kiladjian JJ, Verstovsek S, et al. Overall and progression‐free survival in patients treated with fedratinib as first‐line myelofibrosis (MF) therapy and after prior ruxolitinib (RUX): results from the JAKARTA and JAKARTA2 trials. HemaSphere. 2021;5(suppl 2):58. doi: 10.1097/HS9.0000000000000566 [DOI] [Google Scholar]
- 23. Gupta V, Yacoub A, Verstovsek S, et al. Safety and tolerability of fedratinib (FEDR), an oral inhibitor of Janus kinase 2 (JAK2), in patients with intermediate‐ or high‐risk myelofibrosis (MF) previously treated with ruxolitinib (RUX): results from the phase 3b FREEDOM trial. Blood. 2021;138(suppl 1):389. doi: 10.1182/blood-2021-147607 [DOI] [Google Scholar]
- 24. Verstovsek S, Mesa RA, Gotlib J, et al. The clinical benefit of ruxolitinib across patient subgroups: analysis of a placebo‐controlled, phase III study in patients with myelofibrosis. Br J Haematol. 2013;161:508‐516. doi: 10.1111/bjh.12274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gupta V, Griesshammer M, Martino B, et al. Analysis of predictors of response to ruxolitinib in patients with myelofibrosis in the phase 3b expanded‐access JUMP study. Leuk Lymphoma. 2021;62:918‐926. doi: 10.1080/10428194.2020.1845334 [DOI] [PubMed] [Google Scholar]
- 26. Palandri F, Palumbo GA, Bonifacio M, et al. Baseline factors associated with response to ruxolitinib: an independent study on 408 patients with myelofibrosis. Oncotarget. 2017;8:79073‐79086. doi: 10.18632/oncotarget.18674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Palandri F, Palumbo GA, Bonifacio M, et al. Durability of spleen response affects the outcome of ruxolitinib‐treated patients with myelofibrosis: results from a multicentre study on 284 patients. Leuk Res. 2018;74:86‐88. doi: 10.1016/j.leukres.2018.10.001 [DOI] [PubMed] [Google Scholar]
- 28. Palandri F, Palumbo GA, Iurlo A, et al. Differences in presenting features, outcome and prognostic models in patients with primary myelofibrosis and post‐polycythemia vera and/or post‐essential thrombocythemia myelofibrosis treated with ruxolitinib. new perspective of the MYSEC‐PM in a large multicenter study. Semin Hematol. 2018;55:248‐255. doi: 10.1053/j.seminhematol.2018.05.013 [DOI] [PubMed] [Google Scholar]
- 29. Breccia M, Bartoletti D, Bonifacio M, et al. Impact of comorbidities and body mass index in patients with myelofibrosis treated with ruxolitinib. Ann Hematol. 2019;98:889‐896. doi: 10.1007/s00277-018-3569-1 [DOI] [PubMed] [Google Scholar]
- 30. Barosi G, Klersy C, Villani L, et al. JAK2(V617F) allele burden ≥50% is associated with response to ruxolitinib in persons with MPN‐associated myelofibrosis and splenomegaly requiring therapy. Leukemia. 2016;30:1772‐1775. doi: 10.1038/leu.2016.45 [DOI] [PubMed] [Google Scholar]
- 31. Guglielmelli P, Biamonte F, Rotunno G, et al; COMFORT‐II Investigators; Associazione Italiana per la Ricerca sul Cancro Gruppo Italiano Malattie Mieloproliferative (AGIMM) Investigators . Impact of mutational status on outcomes in myelofibrosis patients treated with ruxolitinib in the COMFORT‐II study. Blood. 2014;123:2157‐2160. doi: 10.1182/blood-2013-11-536557 [DOI] [PubMed] [Google Scholar]
- 32. Patel KP, Newberry KJ, Luthra R, et al. Correlation of mutation profile and response in patients with myelofibrosis treated with ruxolitinib. Blood. 2015;126:790‐797. doi: 10.1182/blood-2015-03-633404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Palandri F, Breccia M, Bonifacio M, et al. Life after ruxolitinib: reasons for discontinuation, impact of disease phase, and outcomes in 218 patients with myelofibrosis. Cancer. 2020;126:1243‐1252. doi: 10.1002/cncr.32664 [DOI] [PubMed] [Google Scholar]
- 34. Abdelrahman RA, Begna KH, Al‐Kali A, Hogan WJ, Litzow MR, Tefferi A. Revised assessment of response and long‐term discontinuation rates among 111 patients with myelofibrosis treated with momelotinib or ruxolitinib. Leukemia. 2015;29:498‐500. doi: 10.1038/leu.2014.286 [DOI] [PubMed] [Google Scholar]
- 35. Palandri F, Palumbo GA, Elli EM, et al. Ruxolitinib discontinuation syndrome: incidence, risk factors, and management in 251 patients with myelofibrosis. Blood Cancer J. 2021;11:4. doi: 10.1038/s41408-020-00392-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mascarenhas J, Mehra M, He J, Potluri R, Loefgren C. Patient characteristics and outcomes after ruxolitinib discontinuation in patients with myelofibrosis. J Med Econ. 2020;23:721‐727. doi: 10.1080/13696998.2020.1741381 [DOI] [PubMed] [Google Scholar]
- 37. Palandri F, Tiribelli M, Breccia M, et al. Ruxolitinib rechallenge in resistant or intolerant patients with myelofibrosis: frequency, therapeutic effects, and impact on outcome. Cancer. 2021;127:2657‐2665. doi: 10.1002/cncr.33541 [DOI] [PubMed] [Google Scholar]
- 38. Kuykendall AT, Shah S, Talati C, et al. Between a rux and a hard place: evaluating salvage treatment and outcomes in myelofibrosis after ruxolitinib discontinuation. Ann Hematol. 2018;97:435‐441. doi: 10.1007/s00277-017-3194-4 [DOI] [PubMed] [Google Scholar]
- 39. Newberry KJ, Patel K, Masarova L, et al. Clonal evolution and outcomes in myelofibrosis after ruxolitinib discontinuation. Blood. 2017;130:1125‐1131. doi: 10.1182/blood-2017-05-783225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schain F, Vago E, Song C, et al. Survival outcomes in myelofibrosis patients treated with ruxolitinib: a population‐based cohort study in Sweden and Norway. Eur J Haematol. 2019;103:614‐619. doi: 10.1111/ejh.13330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mascarenhas J, Komrokji RS, Palandri F, et al. Randomized, single‐blind, multicenter phase II study of two doses of imetelstat in relapsed or refractory myelofibrosis. J Clin Oncol. 2021;39:2881‐2892. doi: 10.1200/JCO.20.02864 [DOI] [PubMed] [Google Scholar]
- 42. Kuykendall AT, Sun L, Mascarenhas J, et al. Favorable overall survival with imetelstat in relapsed/refractory myelofibrosis patients compared with real‐world data. Ann Hematol. 2022;101:139‐146. doi: 10.1007/s00277-021-04683-w [DOI] [PubMed] [Google Scholar]
- 43. Mascarenhas J, Komrokji RS, Cavo M, et al. Potential disease‐modifying activity of imetelstat demonstrated by reduction in cytogenetically abnormal clones and mutation burden leads to clinical benefits in relapsed/refractory myelofibrosis patients. Blood. 2020;136(suppl 1):39‐40. doi: 10.1182/blood-2020-138818 [DOI] [Google Scholar]
- 44. Mascarenhas J, Komrokji RS, Cavo M, et al. Favorable overall survival with imetelstat treatment correlates with other clinical benefits in intermediate 2 or high risk myelofibrosis relapsed/refractory to Janus kinase inhibitor [abstract]. Paper presented at: 62nd American Society of Hematology Annual Meeting (Virtual); December 5‐8, 2020. Acessed April 04, 2022. https://ash.confex.com/ash/2020/webprogram/Paper141013.html
- 45. Hu S, Huang F, Hoffman R, Wang X. Combination treatment with imetelstat, a telomerase inhibitor, and ruxolitinib depletes myelofibrosis hematopoietic stem cells and progenitor cells. Blood. 2019;134(suppl 1):2963. doi: 10.1182/blood-2019-126189 [DOI] [Google Scholar]
- 46. Price GL, Davis KL, Karve S, Pohl G, Walgren RA. Survival patterns in United States (US) Medicare enrollees with non‐CML myeloproliferative neoplasms (MPN). PLoS One. 2014;9:e90299. doi: 10.1371/journal.pone.0090299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Passamonti F, Giorgino T, Mora B, et al. A clinical‐molecular prognostic model to predict survival in patients with post polycythemia vera and post essential thrombocythemia myelofibrosis. Leukemia. 2017;31:2726‐2731. doi: 10.1038/leu.2017.169 [DOI] [PubMed] [Google Scholar]
- 48. National Comprehensive Cancer Network (NCCN) . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Myeloproliferative Neoplasms, Version 2.2021. NCCN; 2021. Acessed April 04, 2022.https://www.nccn.org [DOI] [PubMed] [Google Scholar]
- 49. Waksal JA, Harrison CN, Mascarenhas JO. Novel therapeutics and targets in myelofibrosis. Leuk Lymphoma. Published online December 2, 2021. doi: 10.1080/10428194.2021.2010068 [DOI] [PubMed] [Google Scholar]


