Abstract
The microorganisms inhabiting the gastrointestinal tract (GIT) of ruminants have a mutualistic relationship with the host that influences the efficiency and health of the ruminants. The GIT microbiota interacts with the host immune system to influence not only the GIT, but other organs in the body as well. The objective of this review is to highlight the importance of the role the gastrointestinal microbiota plays in modulating the health of a host through communication with different organs in the body through the microbiome-gut-organ axes. Among other things, the GIT microbiota produces metabolites for the host and prevents the colonization of pathogens. In order to prevent dysbiosis of the GIT microbiota, gut microbial therapies can be utilized to re-introduce beneficial bacteria and regain homeostasis within the rumen environment and promote gastrointestinal health. Additionally, controlling GIT dysbiosis can aid the immune system in preventing disfunction in other organ systems in the body through the microbiome-gut-brain axis, the microbiome-gut-lung axis, the microbiome-gut-mammary axis, and the microbiome-gut-reproductive axis.
Keywords: ruminant, microbiome-gut-organ axes, microbiome-gut-lung axis, microbiome-gut-brain axis, microbiome-gut-mammary axis, microbiome-gut-reproductive axis
1. Introduction
The ruminant animal has a unique set of evolutionary advantages that allow them to digest and utilize plant biomass that is undigestible by monogastric animals [1]. These advantages come from the rumen, which acts as a fermentation chamber to degrade feedstuffs and absorb organic acids before they reach the glandular stomach [2,3]. In order to degrade feedstuffs, the rumen is inhabited by a variety of microorganisms (i.e., bacteria, protozoa, fungi, and archaea) that degrade the plant materials and produce metabolites the host animal can utilize for both maintenance and growth [2,3,4]. The rumen accounts for an estimated 80% of the total volume of the gastrointestinal tract (GIT) of the ruminant and is the site in which more than 85% of the short chain fatty acid (SCFA) production in the GIT occurs [5]. In addition, approximately 50% of a ruminant’s protein requirement is produced from GIT microbial fermentation in the form of microbial protein [6,7].
The relationship between microbes colonizing the GIT can be commensal, mutualistic, competitive, and/or predatory [8,9]. Many bacteria produce bacteriocins (i.e., an antimicrobial peptide) that target other bacteria inhabiting a similar niche, providing a competitive advantage to the bacteriocin-producing bacteria [3]. However, these microbes can coexist and benefit from each other by utilizing the end products produced by microbes occupying a different niche in a process known as cross-feeding [10,11]. The microbe–microbe interactions enhance their collective ability to maximally degrade feedstuffs and provide energy and protein to the host animal.
The symbiotic relationship existing between microorganisms colonizing the GIT and the host is mainly mutualistic in nature [12]. The metabolites produced by this microbiota play an important role in host physiology by maintaining homeostasis with the host’s immune system [13]. Due to its influence on the immune system, the GIT is the site of the majority of pathogenic entrance into the host. In order to promote animal health, the GIT microbiota can be modulated to improve the functioning of the immune system and maintain homeostasis within the host’s body [14].
The microbes’ influence is not limited to the scope of the GIT but expands to all areas and organ systems of the body [13]. Currently, research is beginning to highlight the importance of microbiome-gut-organ axes in animals. The objective of this review is to highlight the importance of the role the gastrointestinal microbial population plays in modulating the health of a host through communication with different organs in the body through the microbiome-gut-organ axes. Although the microbiome-gut-organ axes are important in terms of all animals and humans, the main focus of this review is how these axes can be utilized in ruminant animals.
2. The Gut Microbiota
2.1. Development of the Gut Microbiota
Ruminants are born with an undeveloped, nonfunctional rumen; however, the microbial populations begin to establish shortly after birth [3,15]. Despite misconception, ruminants are not born with a sterile gastrointestinal tract. During parturition, a calf is exposed to microbes from the vaginal canal and perineum, which include microorganisms such as Truperella pyogenes and species from the genera Staphylococcus, Clostridium, Bacteroides, Ureaplasma, and Mannheimia [16,17,18,19]. The calf’s microbiota continues to be colonized by microbes derived from the skin during suckling and the oral cavity during licking [17]. This introduces bacteria commonly found on the skin of the udder, Citrobacter spp. and Leuconostoc spp., which have been detected in the GIT of calves as early as 6 h after birth [20]. In addition to bacterial species being introduced from a calf’s dam, eukaryotic species, protozoa (e.g., Entodinium), and fungi (e.g., Neocallimastix) found in the GIT are introduced through saliva as the mother licks her calf [21,22].
Another major source of bacterial colonization is colostrum which typically contains species from Lactobacillus, Bifidobacterium, Staphylococcus, Escherichia coli, and Streptococcus uberis [23]. Of note, some of these bacterial groups are generally classified as opportunistic pathogens that can take advantage of the uninhabited GIT of newborn ruminants. For example, E. coli begins to colonize the rumen as early as 24 h after birth [24]. These pathogens can cause gastrointestinal distress early in calves [25]. Previous research detected many opportunistic pathogenic species, including Escherichia and Salmonella, present in the hindgut of 1-week-old calves, contributing to digestive issues [25].
As a calf’s GIT microbiota matures, there is a change in the composition of the GIT microbiota. The initial pathogenic bacterial species that are generally facultative anaerobes able to utilize oxygen are soon replaced by the more beneficial species that are strict anaerobes [15,26]. With the change in bacteria, the rumen environment becomes anaerobic, with few species, including fungi being able to utilize oxygen. These more beneficial bacterial species (i.e., amylolytic bacteria, lactate utilizers, sulfate-reducing bacteria, and xylan and pectin fermenting bacteria) start out at much lower concentrations but soon dominate the ruminal environment as early as three days of age [3]. The fecal microbiota is dominated by the phyla Bacteroidetes, Firmicutes, and Proteobacteria, with Bifidobacterium, Bacteroides, and Lactobacillus being the most prevalent genera during a calf’s first four weeks of age [27,28,29,30].
2.2. Dietary Effects on the Gut Microbiota
As a ruminant’s GIT microbial population continues to establish, diet becomes a key factor contributing to its composition [31,32,33]. When the diet of a ruminant is altered, the GIT microbiota is altered as a result. One of the most dramatic dietary shifts that occurs during the beef production system is the change from a forage-based diet to a concentrate-based feedlot-finishing ration. During this transition, the GIT microbiota becomes unstable resulting in dysbiosis, or an imbalance, allowing GIT distress to occur [34,35]. Many studies have found that the microbial composition of the GIT is altered by age and diet [36,37,38,39]. Previous research revealed that the fecal microbial composition of steers at weaning is different from the microbial composition at yearling and slaughter, which can be attributed to dietary differences [37].
The shift in the microbial composition from a dietary change can affect specific bacterial species with the GIT microbiota. Many bacterial species can be described as either generalists, bacteria able to degrade a wide variety of substrates and thrive in a wide variety of environments, or specialists, bacteria that are only able to degrade a very specific set of substrates that occupy a narrow niche [40]. The abundance of these bacterial species is determined by the nutrients in the diet, which provides a competitive advantage to certain bacteria in the rumen. Earlier in life, when ruminants are fed a predominately forage-based diet, cellulolytic bacteria, including Ruminococcus albus, R. flavefaciens, and Fibrobacter succinogenes, tend to dominate since they are better able to degrade and utilize forages [41,42,43]. The fecal microbial population is made up of mainly the family Ruminococcaceae [37], which has many genes present that can bind cellulose, hemicellulose, and xylan, making this family particularly adapted to degrading plant materials [44,45,46]. Once cattle arrive at the feedlot, the diet shifts to a predominately concentrate-based diet and ruminal fermentation must rapidly change as well. Instead of degrading mainly cellulose and hemicellulose, fermentation must shift to mainly degrading starch and soluble sugars [31,47,48]. After this switch to a high concentrate diet, amylolytic bacteria dominate with Ruminobacter amylophilus, Streptococcus bovis, Succinomonas amylolytica, Butyrivibrio fibrosolvens, Selenomonas ruminantium, and many species from the genus Prevotella being to dominate the ruminal niche [49].
2.3. Effects on the Immune System
Calves are born with an immature but functional immune system [50]. While in utero, the central organs (e.g., bone marrow and thymus) are fully developed. However, the peripheral organs (e.g., lymph nodes, spleen, and mucosa-associated lymphoid tissues, including the gut-associated mucosal tissue [GALT]), do not fully develop until they are exposed to antigens after birth [51]. The developing GIT microbial population plays a vital role in regulating and activating a calf’s immune system during the early stages of life [17]. Previous research has discovered that the hindgut is crucial for the development of the immune system in monogastric animals [52]. Research is now revealing that the microbial consortium in the hindgut of cattle is equally as important in the development of the calf’s immune system [53], which plays a vital role in the GIT health, feed digestion, and energy production [54].
The epithelial cells in the GIT are joined by an intercellular junction called tight junctions, which are comprised of proteins (e.g., occludin, claudins, zonula occludens) and adhesion molecules. The tight junctions allow the passage of nutrients, ions, and water through the bloodstream while preventing the passage of microbes and their peptides [55,56]. Right after birth, a calf’s tight junctions are not fully formed, and passage into the bloodstream is increased, which improves a calf’s ability to absorb nutrients, immunoglobulins, and leukocytes from the colostrum [57,58]. This permeability begins to decrease around 24 to 36 h after birth and continues to decrease during the calf’s first month [59]. Research has shown that the presence of some bacterial species (e.g., Lactobacillus spp. and Bifodobacterium spp.) and their metabolites can promote the activation of these tight junction proteins [60,61,62]. This can be very important during the first few weeks of life when the intestinal permeability is just starting to decrease. The colostrum calves receive during the early stages of life plays an important role in host immunity by increasing the hindgut abundance of probiotic species such as Bifidobacterium while decreasing the hindgut abundance of opportunistic pathogenic bacteria E. coli and Escherichia-Shigella [63]. Robseburia and Oscillospira have been found to have genes involved in the regulation of host immunity and metabolism, while SCFA receptor genes decrease inflammation and increase intestinal barrier function [14], which is vital during the early stages of development.
Proinflammatory (e.g., interleukin [IL]-8) and anti-inflammatory (e.g., IL-10) cytokines are upregulated during the first week of a calf’s life [64]. These are important because IL-8 works to activate and attract neutrophils into the interstitial space in the body [65], and IL-10 prohibits proinflammatory molecules (e.g., INF-g, TNF-α, IL-6) from activating and prevents the immune system’s ability to recognize antigens [66]. Bacterial species aid the immune system in these functions because Lactobacillus and Bifidobacterium activate IL-10, which helps prevent the immune system from activating a proinflammatory response to beneficial bacteria within the GIT microbiota [67]. Both Lactobacillus and Bifidobacterium are introduced to the GIT through colostrum [23], and both play an important role in the early stages of the establishment of the GIT microbiota, which can contribute to controlling the ruminant’s immune response and preventing any unnecessary inflammatory responses to “self” antigens.
Not only does the GIT microbial population aid in establishing the calf’s immune system in the first few weeks of its life, but it also continues to play a vital role in maintaining and regulating the immune system as cattle mature and grow. As calves are transitioned from a milk-based diet to either a forage- or concentrate-based diet, both the calf’s GIT microbiota and the expression of genes related to intestinal immunity are impacted [68]. The expression of immune genes has been linked to the abundance of luminal bacteria and bacteria correlated with GIT in health in dairy calves [69]. IgA, a secretory immunoglobulin that plays a large role in intestinal immunity, aids the immune system in regulating the relationship between both beneficial and pathogenic bacteria [70]. If this immunoglobulin is removed from the GIT, bacteria can increase uncontrollably in abundance while the immune system upregulates the expression of proinflammatory cytokines [71]. Recovery of IgA will restore intestinal homeostasis by returning commensal bacterial species populations and eliminating inflammation [72].
Another key feature of the gastrointestinal system that helps maintain health and proper functioning is mucus, which provides a barrier to tight junctions and aids in maintaining gut integrity [69,73]. Although beneficial for host health, if the production of mucus becomes abnormal, then the GIT can experience distress and disease [73,74]. The GIT microbial population plays a vital role in the production of intestinal mucus. When comparing germ-free and conventionally raised mice, the mice without functional microbiota had less mucus lining their intestinal epithelial cells [75,76]. Meanwhile, when germ-free mice are exposed to bacterial molecules (e.g., lipopolysaccharides and peptidoglycans), their intestinal mucus production is restabilized [75,77]. These results highlight the importance of the GIT microbiota and its metabolites in promoting and maintaining intestinal mucus production.
Due to the communication between the GIT microbiota and the host’s immune system, any disturbance in the equilibrium of the microbiota or the immune system will affect both. Suppose calves are suddenly switched to a different diet and their GIT microbial population is not given time to acclimate. In that case, the expression of claudin and occludin is downregulated in the mucosal barrier of the intestines resulting in an increase in gut permeability [78]. The decreased GIT integrity can be attributed to an increase in the host’s inflammatory response due to an interaction with pathogenic bacteria and their metabolites, or with pro-inflammatory cytokines [68]. Although the immune’s pro-inflammatory response is a defense mechanism designed to target foreign invaders (e.g., pathogens) and works in conjunction with the GIT microbiota to prevent pathogenic invasion and gastrointestinal dysbiosis, the inflammatory response can become too upregulated and negatively impact host health [79]. Another detrimental interaction between the GIT microbiota and the host’s immune system can occur during the onset of rumen acidosis. This digestive issue is caused when the rumen pH drops and becomes acidic as a result of an abundance of rapidly digestible carbohydrates in the diet producing an abundance of lactic acid (acute acidosis) or volatile fatty acids (subacute acidosis) [54]. As a result, the number of cellulolytic bacteria can decrease, and the number of amylolytic bacteria can increase, resulting in the microbiota experiencing dysbiosis [80]. Additionally, as the pH of the rumen decreases, the rumen epithelial cells become damaged, preventing the absorption of nutrients that can ultimately have negative impacts on the host’s performance and health [54]. This highlights the importance of maintaining balance in the microbial populations in the gut and in the host’s immune system to increase growth and health in the host animal.
2.4. Therapies to Modulate GIT Health
Since so much interplay exists between the GIT microbial population and the host’s immune system, GIT microbial therapies can be utilized to improve the overall health of a host. Three main types of therapies are utilized to modulate the GIT microbiota—probiotics, prebiotics, and gut microbial transplants. They range from the introduction of a few key species or fermentable products [81,82,83] to a complete functional microbial population [84,85,86,87,88]. Recently, research has focused on how these therapies can be utilized to stabilize the GIT microbiota to prevent diseases [89]. These therapies mainly work by preventing dysbiosis in the GIT microbial community by preventing an increase in harmful bacteria and a decrease in beneficial bacteria, which is especially valuable when the host is undergoing stress.
2.4.1. Probiotics
One of the most commercially available and widely used GIT microbiota therapies available to promote human and animal health is probiotics, or direct-fed microbials (DFM). Probiotics are living microorganisms found naturally in the GIT that have a direct or indirect impact on host health. They can be mono- or mixed cultures usually comprised of bacteria or fungi [83,90]. Probiotics work by producing metabolites that stimulate the growth of commensal bacteria, inhibiting proliferation and colonization of pathogenic bacteria, regulating gastrointestinal pH, promoting mucus production, and improving the function of intestinal epithelial cells [91]. In livestock production, probiotics are valuable tools utilized to improve GIT health, feed efficiency, and milk quality [92,93]. Additionally, they are crucial to preventing dysbiosis from occurring in the GIT microbiota as a result of stressful events such as transportation [94].
In ruminant animals, probiotics can act as alternatives to antimicrobial feed additives by limiting the colonization of pathogenic species. S. cerevisiae has been found to improve ruminant production by increasing ruminal pH [95], which is beneficial in both beef and dairy production by preventing acidosis when introducing more starch into the diet. S. cerevisiae can aid in nutrient utilization by increasing ruminal fermentation [96]. The introduction of Lactobacillus probiotics to the diet of calves has been shown to increase growth and prevent immunocompetence [97]. Megasphaera elsdenii and Butyrivibiro fibrosolvens have been found to redirect SCFA production from lactate to butyrate, which increases ruminal pH preventing subacute rumen acidosis [98]. Although studies have controversial results on the effects of probiotics in the diet of ruminants, adding probiotics to the diet prior to a stressful event such as weaning or a diet change stabilizes the ruminal ecosystem preventing dysbiosis.
2.4.2. Prebiotics
The practice of introducing substrates that bacteria utilize into the GIT instead of the bacterial species themselves is increasing in popularity. These substrates are called prebiotics because they cause the “prebiotic effect,” which is “the selective stimulation of growth and/or activity of one or a limited number of species in the gut microbiota that confer(s) health benefits to the host” [99]. Prebiotics are comprised of non-starch polysaccharides (NSP) or oligosaccharides. Prebiotics are introduced into the diet to be fermented and utilized by beneficial bacteria to improve the health of the GIT [91]. Therefore, to truly be considered a prebiotic, the product must be indigestible by the host, fermentable by commensal GIT microbiota, and increase the growth of beneficial bacteria in the gut [100,101].
Like probiotics, prebiotics can positively impact cattle production and health. They are usually introduced into ruminant diets alongside probiotics to increase substrates that can be utilized by the microorganisms contained in the probiotic to improve its efficacy [81]. Prebiotics can be fed to increase weight gain and feed efficiency and reduce scours and respiratory diseases [102,103,104]. Fructose oligosaccharides (FOS) have previously been shown to lessen enteric issues in calves [105] and decrease colonization of many pathogenic bacteria, including Salmonella and E. coli [106]. Adding Galactosyl-lactose (GL) to milk replacers has previously been shown to increase growth while improving overall health status of dairy calves [102]. Prebiotics, also, work best as a preemptive therapeutic to prevent GIT dysbiosis during a stressful time for the host.
2.4.3. Gut Microbial Transplants
The utilization of sequencing technology has provided many recent advances to our knowledge of the role of individual microorganisms within the GIT microbiota. However, all the bacteria comprising the GIT microbiota have yet to be elucidated [107]. Currently, we are only able to culture an estimated 23–40% of the microbes within the rumen [107]; therefore, we are limited in our ability to develop gut therapies like probiotics and prebiotics since we still have not identified every microorganism or their function in the GIT. To overcome our lack of knowledge, gut microbial transplants can be utilized. Within human and small animal medicine, gut microbial transplantation has become a very promising avenue for GIT therapies [84,85,86,87,88]. In terms of cattle production, there can be two types of gut microbial transplants—fecal matter transplants (FMT) and ruminal fluid transplants (RFT).
In ruminant animals, RFT is the most popular form of gut microbial transplants. This introduces rumen fluid from a healthy donor into a recipient experiencing dysbiosis [108]. In sheep experiencing acidosis, an RFT accelerated rumen fermentation, decreased dysbiosis, and repaired damage to the ruminal epithelial cells [109]. An RFT is also a valuable tool prior to weaning in lambs by increasing starch degrading bacteria, thus improving the digestibility of starch-containing diets and increasing propionate production by ruminal microbes [110]. This study also found that an RFT led to faster organ development, especially the hindgut and liver. Although not as commonly used in ruminants, FMT treatment can also be used in cattle. Research has shown an FMT can be an effective treatment for diarrhea [111]. Additionally, this study found that an FMT treatment in a calf’s early life can potentially improve growth performance. Although the idea of inoculation with GIT contents dates back as early as the 1700s [112], research is still needed to fully understand the validity of using an RFT or an FMT as a therapeutic for modulating host health.
3. Microbiome-Gut-Organ Axes
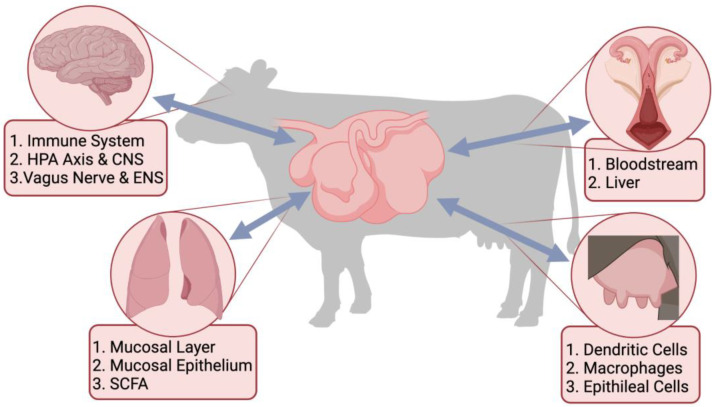
Recently, there has been a major drive towards understanding the complex synergistic relationship that exists between the GIT microbial population and the host. The GIT microbiota interacts with all aspects of the body through the different microbiome-gut-organ axes (MGOA). The metabolites produced by the GIT microbiota send signals throughout the body to different organs, which affect the immune system and host physiology [113,114]. This interaction between the microbes colonizing the GIT and the immune system impacts organs throughout the host and forms an “axis” that can send signals [115]. Some examples of these axes in cattle include the established gut-brain axis and gut-lung axis [116] and the proposed gut-mammary axis and gut-reproductive axis (Figure 1).
Figure 1.
Proposed links between the gastrointestinal tract microbiota and different organ systems through the microbiome-gut-organ axes, including the microbiome-gut-brain axis (MGOA), the microbiome-gut-lung axis, the microbiome-gut-reproductive axis, and the microbiome-gut-mammary axis. Included are pathways, cells, and metabolites important for the bi-directional communication of the MGOA.
The different MGOA enable bidirectional communication between the GIT and different organs that occur through signaling pathways [117,118]. The metabolites produced in the GIT by the microbes can have a direct impact on the host’s risk of infection [119]. Dietary or environmental stressors can alter the species diversity within the GIT, leaving the microbiota susceptible to pathogenic colonization [119,120]. Ultimately, any alterations within the gut microbial population can have cascading detrimental effects on the health of the host through these different axes. Therefore, it is imperative we understand the mechanisms of the microbiota within the GIT and its communication with other organs to ensure the health and well-being of animals.
3.1. Microbiome-Gut-Brain Axis
One of the most extensively studied gut-organ-axes within all mammalian systems is the microbiome-gut-brain axis (MGBA). This axis serves as a bi-directional link where signals and metabolites can be sent between the brain and the gut. Previous research has revealed the microbes within the GIT play an important role in many processes within the brain of the host, including the development of the brain, neural processes, pain processes, the hypothalamic-pituitary-adrenal (HPA) axis, and behavior [121]. Due to its impact on the brain, the MGBA has been increasing in popularity as a tool to modulate brain function and, ultimately, host health.
There are three different pathways that serve as routes of communication for the MGBA [122]. The first is the immunoregulatory pathway, where the immune system interacts with the microbiota and affects the production of cytokines, cytokinetic reaction factors, and prostaglandin E2 [123], which subsequently alters brain function [13]. The second is the neuroendocrine pathway which involves both the HPA axis and the central nervous system (CNS). The intestines of mammals serve as one of the largest endocrine organs in the body by possessing over 20 different types of enteroendocrine cells [124]. Additionally, the microbes within the gut regulate the production of many neurotransmitters (e.g., cortisol) through the HPA axis and CNS [125]. The last pathway is connected through the vagus nerve and enteric nervous system (ENS). The ENS forms synapses with the vagus nerve, which allows communication between the microbes and the brain [125]. Ruminal fermentation produces metabolites that are toxic to the brain (e.g., ammonia and D-lactic acid), which can travel through this pathway to negatively impact brain function and the host’s stress response and quality of sleep [126]. Additionally, sensory neurons can send signals through the CNS to control gut motility and hormone secretion [13].
The role the MGBA plays in modulating host health has been studied thoroughly in humans; however, research investigating the MGBA has expanded to animals. This communication can occur when there is an infection in the GIT microbiota that negatively impacts the brain and increases sickness behavior or when the GIT microbiota is healthy and promotes brain function. Research utilizing germ-free animals has shown that the inclusion of probiotics and prebiotics results in behavioral changes [127]. Throughout livestock production, there are many stressful events (e.g., weaning and transportation) that are unavoidable. Research has shown that after transportation, there is an increase in cortisol, adrenocorticotropic hormone (ACTH), and pro-inflammatory cytokines (i.e., IL-6, TNF-α, IL-1β) in multiple breeds of beef cattle [128]. This study also found an abundance of ruminal Lactobacillus that were positively correlated with IL-6 and IL-4. In addition, probiotics or prebiotics can be added to the diet of ruminants to prevent the signaling of the HPA axis to increase anxiety. A study utilizing dairy calves found supplementing the calf’s diet with a multispecies probiotic prior to weaning improved growth, decreased the incidence of diarrhea, affected the fecal microbiota (e.g., increased abundances of Bifidobacterium, Lactobacillus, Collinsella, and Saccharomyces), and reduced serum concentration (i.e., IgA, IgG, and IgM) [129]. Ultimately the impact of the GIT microbiota on behavior in animals directly impacts the overall health of the host since the microbes directly impact the immune system of the host [130].
In addition to general behavior being affected by the GIT microbiota, feed behavior is directly impacted by the microbial population within the GIT, which directly impacts the feed efficiency of livestock. An increase in pathogenic bacteria within the GIT microbiota results in an increase in sickness behavior followed by a reduction in feed intake [127]. When ruminants are fed a high-starch diet, they can experience ruminal acidosis, which can negatively influence feed behavior by decreasing both feed intake and the time spent ruminating [131]. This can be reversed by an RFT by introducing healthy microbes back into the GIT microbiota. This increases feed intake while decreasing inflammation, ultimately promoting host health [132]. In addition to an RFT, the utilization of a probiotic, S. cerevisiae, can inhibit the negative effects of ruminal acidosis (e.g., reduced pH) by increasing ruminal pH [92,133]. This influences the MGBA by increasing feed behavior by increasing the time spent ruminating and decreasing the time between feedings [134].
3.2. Microbiome-Gut-Lung Axis
Another MGOA discovered more recently is the microbiome-gut-lung-axis (MGLA). Due to the inability to culture microbes from the lungs of healthy hosts, the lungs were originally thought to be sterile unless an infection was present [135]. With the utilization of sequencing technologies (e.g., whole genome sequencing and 16S rRNA gene sequencing), researchers were able to discover that the lungs were inhabited by a community of commensal microbes existing in healthy individuals [136]. These microbial communities play a protective role in the respiratory tract, preventing the colonization of bacteria or viruses, which can cause disease [137]. Much like the GIT microbiota, the respiratory microbiota plays a role in regulating the activation of both the innate and adaptive immune responses [138,139].
The respiratory tract is divided into two parts based on location and function: the upper respiratory tract (URT) and the lower respiratory tract (LRT) [140]. The URT is comprised of the nasal cavity, paranasal sinuses, nasal passages, nasopharynx, oropharynx, tonsils, and upper portion of the larynx. In contrast, the LRT contains the larynx, trachea, bronchi, bronchioles, and alveoli [141]. Much like their function, the URT and LRT are colonized by different microbes [142,143,144] shortly after birth [145]. The most abundant phyla of the LRT are Bacteroidetes and Firmicutes, which is similar to the oral cavity microbiota suggesting the oral cavity plays a role in the development of the lung microbiota [136,146,147]. On the other hand, the microbial population of the nasal cavity is mainly comprised of the phyla Firmicutes and Actinobacteria, which more closely resembles the microbial population of the skin [148,149,150], suggesting the development of the nasal cavity microbiota is influenced by the skin microbiota. Due to differences in the suspected sources of colonization of the URT and LRT, researchers need to be cautious when drawing conclusions about the microbiota of one location based on the other. However, research has revealed correlations between microbes within the URT and LRT that can influence both microbial communities [144]. Other factors are known to influence the development of the respiratory microbiota, including diet [151], genetics, age [145], vaccination administration, management, and environment [152,153].
Although signaling through the MGLA travels bi-directionally, the majority of the communication between the microbial populations of the GIT and respiratory tract travels from the gut to the lungs [135]. The specific mechanisms and pathways involved in the MGLA in cattle remain undiscovered. Still, micro-aspiration, inhalation of bacteria, and transfusion of bacteria through mucosal cells play an important role in the communication [154]. Additionally, the lymphatic system and bloodstream play an important role in communication between the GIT and respiratory tract by carrying bacteria and bacterial metabolites from the GIT to the lungs [155].
Within the respiratory tract, the nasopharyngeal mucosal layer serves as the first line of defense against pathogenic colonization by capturing particles that are inhaled through the respiratory tract and moving them back up into nasal and oral cavities [141]. The mucus contains immune cells, including antimicrobial peptides, glycoproteins, and IgA, that help maintain homeostasis in the respiratory microbiota [156,157]. The second line of defense is the mucosal epithelium which produces molecules that trigger innate and adaptive immune responses to improve barrier function [158,159,160,161]. Not only do the respiratory tract epithelial cells, but luminal and mucosal surface macrophages and dendritic cells express innate patter-recognition receptors that work to identify and clear pathogenic microorganisms [161,162]. The commensal microbes comprising the microbiota of the respiratory tract, mucosal epithelium, and the immune system communicate to promote respiratory health, reduce inflammation, and maintain a functioning microbiota [141].
One of the main ways the GIT microbiota influences the immune system and, thus, the respiratory tract through the MGLA is with SCFA [135]. SCFA are important for maintaining intestinal integrity and preventing inflammation in both the gut and the respiratory tract [163,164,165]. SCFA can enhance the intestinal epithelial barrier function within the gut by increasing mucus production by goblet cells [166,167] and strengthening tight junctions [168]. Additionally, SCFA increase IgA production by enhancing plasma B cells metabolism to ensure the intestines are protected from inflammation [169,170]. An individual SCFA, butyrate, aids the intestinal epithelium in suppressing inflammation to maintain homeostasis [171]. SCFA also promote intestinal homeostasis through a positive feedback loop directing the metabolism of intestinal cells toward increased fatty acid ß-oxidation [172]. Butyrate supplementation has the ability to improve epithelial integrity while also increasing the host’s defense mechanisms [173].
Due to the numerous stressors calves are exposed to during weaning, it serves as one of the most influential times for respiratory microbiota development [151,152,174,175]. The composition of the URT is majorly affected by the first 40 days after arrival at the feedlot [176]. This is due to the stress, exposure to diseases, and dietary changes that occur when calves arrive at the feedlot that can result in dysbiosis in the URT, which weakens a calf’s immune response and allow pathogens in the URT to migrate into the LRT [146,154,177,178]. In dairy calves experiencing illness, there was an increase in Mannheimia, Moraella, and Mycoplasma in their URT compared to that of healthy calves [179]. Additionally, the URT of calves that later developed pneumonia was inhabited by a greater number of bacteria at three days of age than calves that remained healthy.
Bovine raspatory disease (BRD) is one of the most significant health concerns that can occur in weaned calves or feedlot cattle shortly after transportation [180]. After years of research to eradicate the disease, BRD remains one of the leading causes of morbidity, mortality, welfare issues, and economic losses within beef production [181]. It is influenced by a combination of factors, including the host, environment, and management [175,180,182]. The bacteria causing BRD are common commensals (e.g., Mycoplasma, Mannheimia, Histophilus, and Pasteurella) within the URT microbiota of healthy and sick cattle that can translocate from the URT to the LRT through inhalation after the host undergoes stress resulting in the development of pneumonia [177,179,183,184,185]. One example of how these commensals cause disease is in the case of Mannheimia haemolytica. After the stressors occurring after arrival at a feedlot occur and cause dysbiosis, M. haemolytica rapidly proliferates within the URT [186]. It then travels to the bronchial epithelial cells, where it damages tight junction proteins, causes lesions in the lungs, releases leukotoxins and lipopolysaccharides, which cause further damage to the respiratory tract, and triggers the host’s immune response causing inflammation. Due to this disease occurring as a result of a stressful event to the host, the MGLA may play a role in the development of this disease; therefore, the GIT microbiota may aid producers in helping eradicate the disease.
Several studies have shown that employing management strategies can positively impact the nasopharyngeal microbial diversity in calves post-weaning [141]. Management strategies can thus help the immune system maintain respiratory health during stressful times throughout the production cycle that leaves the host susceptible to diseases. Research has shown that dietary changes that influence the GIT microbiota shortly after weaning can also have an impact on the respiratory microbiota [151]. One example is preconditioning weaned calves for nine weeks prior to entry into a feedlot with selenium-fortified alfalfa hay can positively impact the microbial population in the nasal cavity [151]. This suggests fortifying the GIT microbiota prior to stress can help prevent respiratory disease through the MGLA.
3.3. Microbiome-Gut-Mammary Axis
As research on the GIT microbiota continues to progress, it becomes evident that the microbial population plays a major role in the functioning and disease prevention in other organs besides the brain and the lungs. Within cattle production, the mammary gland is an organ with major importance not only for cattle in general but also for producers in terms of milk production. Much like the GIT and the respiratory tract, the mammary system is also inhabited by a community of microbes [187]. Research into the milk microbiota has begun to suggest communication between the GIT microbiota and the mammary microbiota through a microbiome-gut-mammary axis (MGMA).
The microbiota of the milk is influenced by direct and indirect contact. Direct contact comes from contact with the surface of the teat from milking machines or other dairy equipment [188]. Indirect contact stems from environmental factors, including bedding, feces, forage, drinking water, washing water, air, and the milker itself [189,190]. A study of healthy Holstein Friesian and Rendena cows showed the milk microbiota was influenced by breed [188]. Although there were differences present, Firmicutes was the most abundant phylum, with Streptococcus being the most abundant genus. Milk from cows infected with mastitis was dominated by the order Enterobacteriales, followed by Pseudomonadales, Bacillales, and Lactobacillales [191]. In the purebred cattle, E. coli was the most abundant genus, followed by Pseudomonas aeruginosa, P. mendocina, Shigella flexneri, and Bacillus cereus; whereas, in the crossbred cattle, the most dominant genus was Staphylococcus aureus followed by Klebsiella pneumoniae, S. epidermidis, and E. coli. Although the majority of the bacteria detected are pathogenic, there are still other commensal strains present. Previous studies have found some of the commensals can come from direct contact with skin or can travel from the intestinal lumen to the mammary gland through the communication of the MGMA [187,192].
The mechanisms by which the mammary gland and the GIT microbiota communicate with each other has yet to be fully elucidated. The current knowledge of the route of communication between these two organs is from research done in humans and mice. A previous study reported that bacteria travel from the GIT to the mammary system via dendritic cells and macrophages [193]. These cell types, which are in the GALT, can internalize bacteria from the GIT microbiota and translocate it to a different location, such as the mammary gland [194,195]. This suggests an infection within the mammary system may affect the integrity of the epithelial cells allowing translocation of pathogenic bacteria through the MGMA.
Within dairy production, mastitis is the most impactful disease for dairy producers [196]. It causes a major economic loss worldwide by decreasing both the quality and quantity of milk [197]. A study of healthy and infected dairy cows revealed the microbiota of milk from infected quarters has more variation compared to the milk microbiota of healthy quarters, suggesting the milk microbiota experiences dysbiosis when infection occurs [198]. When a quarter is infected, the microbial community can become dominant by a specific microbial taxon. When comparing milk and skin microbiotas from cows with mastitis, Staphylococcus spp. and Debaryomyces spp. were shared between them [198], indicating a link between the skin and milk microbiota.
There is debate on the validity of mammary microbiota, with some theories speculating bacteria sequenced from the mammary gland being contamination introduced during sample collection, sample processing, or DNA extraction [199,200]. When determining if bacteria isolated from the mammary gland is part of a community of microorganisms or a pathogen infecting a sterile environment, researchers need to objectively evaluate the validity of their results to determine if the differences are biological in nature, if the relationship is causative, what the mechanism of action is, how effective the experimental design was at answering the hypothesis, and if any potential factors could be contributing to the experimental differences [201]. Research has shown sequencing samples using different methods can result in differences [202], so it is imperative to maintain proper sterile techniques to ensure accuracy when performing microbiome studies [107]. However, despite this debate and the many theories surrounding it, the mammary gland can still be impacted by the GIT microbiota through the MGMA. This impact may be direct by influencing the colonization of the mammary gland or indirect by the GIT causing systemic inflammation after gut dysbiosis [68], which can lead to inflammation in the mammary system and potential for disease.
3.4. Microbiome-Gut-Reproductive Axis
Like the MGMA, the relationship that exists between the GIT microbiota and the reproductive system remains relatively unexplored. Much like the GIT microbial population, the reproductive microbiota can experience dysbiosis where a disease state occurs. Research has begun to explore the interactions that exist between the GIT microbiota and the reproductive microbiota, which can be referred to as the microbiome-gut-reproductive axis (MGRA), and its role in preventing pathogenic colonization and promoting host health. With the importance of reproductive health for both beef and dairy production, the MGRA could majorly impact the reproductive efficiency of cattle.
For a long period of time, much like the lungs, the reproductive tract of healthy females was considered sterile [203,204,205]. With recent advances in sequencing technologies, there has been increasing evidence that the reproductive tract of humans and animals is inhabited by a resident microbiota [206]. This microbial population begins to colonize the reproductive tract shortly after birth. At birth, the barrier of the cervix is compromised, which can allow microbes to travel from the vagina, the environment, feces, or skin into the reproductive tract [207,208].
The different sections of the reproductive tract have distinct microbial populations; however, they can still influence each other. The vaginal niche is mainly comprised of the phyla Firmicutes, Bacteroidetes, and Proteobacteria [18,209]. A study of Bos Indicus breeds found the most abundant bacterial genera in the vagina were Aeribacillus, Bacillus, Clostridium, Bacteroides, and Ruminococcus [210]. Very similarly, the most dominant phyla in the cervix have been found to be Proteobacteria, Bacteroidetes, and Firmicutes [211]. Meanwhile, the uterus has been found to be colonized by the phyla Proteobacteria, Tenericutes, Firmicutes, Bacteroidetes, Fusobacteria, and Actinobacteria [212].
Previous research has highlighted the bloodstream as a major route of communication for the MGRA [213]. This study found the bloodstream contained a microbiota that was colonized with the main pathogens within the uterine microbiota, such as Bacteroides, Porphyromonas, and Fusobacterium, which are found in the blood shortly after calving, suggesting the bloodstream has a role in bacteria translocation. Additionally, some bacteria which cause liver abscesses in cattle, T. pyogenes and F. necrophorum, have been isolated in the uterus, suggesting these bacteria travel from the liver to the uterus through the bloodstream [214]. The hematogenous pathway can be utilized to carry bacteria from the GIT microbiota to the reproductive tract microbiota; thus, the GIT microbial population influences the composition of the vaginal and uterine microbial populations [18]. For example, Bacteroides heparinolyticus, identified in both the feces and blood of dairy cows, is assumed to be part of the uterine microbiota.
Through the MGRA, the GIT microbiota plays a major role in the functioning of the reproductive system. Research in dairy cows found a genetic similarity in strains of E. coli found in the GIT and uterus, suggesting the GIT aids in the colonization of the uterine microbiota through ascending colonization of bacteria by the lower genital tract [215]. Butyrate supplementation in postpartum cows positively influenced breeding capacity by re-establishing estrous and restoring ovarian function earlier than in supplemented cows [216]. The ratio of circulating follicle-stimulating hormone (FSH)/luteinizing hormone (LH) has been positively correlated with systemic lipopolysaccharide (LPS) and the bacterial genera Actinobacteria, Bacteroides, and Streptococcus [217]. A study in male sheep found that supplementing the diet with SCFA, including acetate, propionate, and butyrate, increased the secretion of LH and FSH [218]. Previous research in dairy cows found the uterine and vaginal microbiotas were most similar in composition seven days postpartum [219]. Additionally, cows that later developed endometritis at 21 days postpartum had a delay in uterine and vaginal microbiota differentiation and a decrease in bacterial diversity at seven days postpartum. The presence of Bacteroides, Poryphomonas, and Fusobacteria was associated with metritis occurring after a decrease in bacterial richness, causing uterine dysbiosis [220]. This indicates these two environments may indicate future reproductive diseases. Specific bacterial families (Lachnospiraceae and Rikenellaceae) and genera (Acinetobacter, Bacillus, Oscillospira, CF231, and 5–7NS) have been identified as an indication of a healthy vaginal microbiota and have the potential to be a therapeutic target [221]. Research has demonstrated that the MGRA may be a valuable tool to improve reproductive efficiency in cattle herds by preventing reproductive diseases and increasing hormone secretion.
4. Conclusions
The many microbiome-gut-organ axes throughout a host highlight the importance of the GIT microbial populations in regulating homeostasis by preventing colonization of pathogenic species, thus decreasing disease/infection prevalence. By stabilizing the GIT microbiota, all organ systems in the host can function at optimal levels. These axes can therefore be a novel target for therapeutics to prevent certain diseases or infections. Although this research has come a long way in the past couple of years, there is still much that remains unknown about the microbiome-gut-organ axes, specifically in ruminants. As improvements are made in sequencing techniques, we can gain a more comprehensive understanding of all the microbes with the GIT and other organs. Additionally, with advances, we can further elucidate the roles the microbes play in regulating the host immune system as well as the numerous organ systems throughout the body. With the addition of future research, producers will be able to target the many MGOA to increase overall health and efficiency in their herds.
Author Contributions
Writing—original draft preparation, C.B.W.; writing—review and editing, V.E.R., T.D.P. and J.M.L.; visualization, C.B.W.; supervision, J.M.L.; All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
C.B.W. assistantship was supported by the National Science Foundation: Grant No. DGE-1545433.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hungate R.E. The Rumen and Its Microbes. Elsevier; Amsterdam, The Netherlands: 1966. [Google Scholar]
- 2.Flint H.J. The rumen microbial ecosystem—some recent developments. Trends Microbiol. 1997;5:483–488. doi: 10.1016/S0966-842X(97)01159-1. [DOI] [PubMed] [Google Scholar]
- 3.Mizrahi I. The Prokaryotes: Prokaryotic Biology and Symbiotic Associations. Springer; Berlin/Heidelberg, Germany: 2013. Rumen Symbioses; pp. 533–544. [Google Scholar]
- 4.Mackie R.I. Mutualistic fermentative digestion in the gastrointestinal tract: Diversity and evolution. Integr. Comp. Biol. 2002;42:319–326. doi: 10.1093/icb/42.2.319. [DOI] [PubMed] [Google Scholar]
- 5.Oh J., Hume I., Torell D. Development of microbial activity in the alimentary tract of lambs. J. Anim. Sci. 1972;35:450–459. doi: 10.2527/jas1972.352450x. [DOI] [PubMed] [Google Scholar]
- 6.Russell J.B., Hespell R.B. Microbial rumen fermentation. J. Dairy Sci. 1981;64:1153–1169. doi: 10.3168/jds.S0022-0302(81)82694-X. [DOI] [PubMed] [Google Scholar]
- 7.Yeoman C.J., White B.A. Gastrointestinal tract microbiota and probiotics in production animals. Annu. Rev. Anim. Biosci. 2014;2:469–486. doi: 10.1146/annurev-animal-022513-114149. [DOI] [PubMed] [Google Scholar]
- 8.Hungate R. Handbook of Physiology No. 5. Oxford University Press; New York, NY, USA: 1968. Ruminal fermentation; pp. 2725–2745. [Google Scholar]
- 9.Russell J. Aspects of Digestive Physiology in Ruminants. Cornell University Press; Ithaca, NY, USA: 1988. Ecology of rumen microorganisms: Energy use; pp. 74–98. [Google Scholar]
- 10.Bryant M.P., Wolin E.A., Wolin M.J., Wolfe R.S. Methanobacillus omelianskii, a symbiotic association of two species of bacteria. Arch. Microbiol. 1967;59:20–31. doi: 10.1007/BF00406313. [DOI] [PubMed] [Google Scholar]
- 11.Iannotti E.L., Kafkewitz D., Wolin M.J., Bryant M.P. Glucose fermentation products of Ruminococcus albus grown in continuous culture with Vibrio succinogenes: Changes caused by interspecies transfer of H2. J. Bacteriol. 1973;114:1231–1240. doi: 10.1128/jb.114.3.1231-1240.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sender R., Fuchs S., Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14:e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das P., Ranjan R. Role of Gut Microbiome in Improving Animal Health and Productivity. Indian J. Anim. Health. 2020;59:146–155. doi: 10.36062/ijah.59.2SPL.2020.146-155. [DOI] [Google Scholar]
- 14.Fan P., Nelson C.D., Driver J.D., Elzo A.E., Penagaricano F., Jeong K.C. Host genetics exerts lifelong effects upon hindgut microbiota and its association with bovine growth and immunity. ISME J. 2021;15:2306–2321. doi: 10.1038/s41396-021-00925-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fonty G., Gouet P., Jouany J.-P., Senaud J. Establishment of the microflora and anaerobic fungi in the rumen of lambs. Microbiology. 1987;133:1835–1843. doi: 10.1099/00221287-133-7-1835. [DOI] [Google Scholar]
- 16.Bicalho M.L.S., Santin T., Rodrigues M.X., Marques C.E., Lima S.F., Bicalho R.C. Dynamics of the microbiota found in the vaginas of dairy cows during the transition period: Associations with uterine diseases and reproductive outcome. J. Dairy Sci. 2017;100:3043–3058. doi: 10.3168/jds.2016-11623. [DOI] [PubMed] [Google Scholar]
- 17.Gomez D.E., Galvão K.N., Rodriguez-Lecompte J.C., Costa M.C. The cattle microbiota and the immune system: An evolving field. Vet. Clin. Food Anim. Pract. 2019;35:485–505. doi: 10.1016/j.cvfa.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Laguardia-Nascimento M., Branco K.M.G.R., Gasparini M.R., Giannattasio-Ferraz S., Leite L.R., Araujo F.M.G., Salim A.C.D.M., Nicoli J.R., De Oliveira G.C., Barbosa-Stancioli E.F. Vaginal microbiome characterization of nellore cattle using metagenomic analysis. PLoS ONE. 2015;10:e0143294. doi: 10.1371/journal.pone.0143294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zambrano-Nava S., Bosco’n-Ocando J., Nava J. Normal bacterial flora from vaginas of Criollo Limonero cows. Trop. Anim. Health Prod. 2011;43:291–294. doi: 10.1007/s11250-010-9701-4. [DOI] [PubMed] [Google Scholar]
- 20.Mayer M., Abenthum A., Matthes J.M., Kleeberger D., Ege M.J., Hölzel C., Bauer J., Schwaiger K. Development and genetic influence of the rectal bacterial flora of newborn calves. Vet. Microbiol. 2012;161:179–185. doi: 10.1016/j.vetmic.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 21.Dehority B.A. Rumen Microbiology. Nottingham University Press; Nottingham, UK: 2003. [Google Scholar]
- 22.Trinci A.P., Lowe S.E., Milne A., Theodorou M.K. Growth and survival of rumen fungi. Biosystems. 1988;21:357–363. doi: 10.1016/0303-2647(88)90033-0. [DOI] [PubMed] [Google Scholar]
- 23.Fecteau G., Baillargeon P., Higgins R., Paré J., Fortin M. Bacterial contamination of colostrum fed to newborn calves in Que´bec dairy herds. Can. Vet. J. 2002;43:523–527. [PMC free article] [PubMed] [Google Scholar]
- 24.Minato H., Otsuka M., Shirasaka S., Itabashi H., Mitsumori M. Colonization of microorganisms in the rumen of young calves. J. Gen. Appl. Microbiol. 1992;38:447–456. doi: 10.2323/jgam.38.447. [DOI] [Google Scholar]
- 25.Song Y., Malmuthuge N., Steele M.A., Guan L.L. Shift of hindgut microbiota and microbial short chain fatty acids profiles in dairy calves from birth to pre-weaning. FEMS Microbiol. Ecol. 2018;94:fix179. doi: 10.1093/femsec/fix179. [DOI] [PubMed] [Google Scholar]
- 26.Bryant M., Small N., Bouma C., Robinson I. Studies on the composition of the ruminal flora and fauna of young calves. J. Dairy Sci. 1958;41:1747–1767. doi: 10.3168/jds.S0022-0302(58)91160-3. [DOI] [Google Scholar]
- 27.Alipour M.J., Jalanka J., Pessa-Morikawa T., Kokkonen T., Satokari R., Hynönen U., Iivanainen A., Niku M. The composition of the perinatal intestinal microbiota in cattle. Sci. Rep. 2018;8:10437. doi: 10.1038/s41598-018-28733-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dias J., Marcondes M.I., Motta de Souza S., Cardoso da Mata e Silva B., Fontes Noronha M., Tassinari Resende R., Machado F.S., Cuquetto Mantovani H., Dill-McFarland K.A., Suen G. Bacterial community dynamics across the gastrointestinal tracts of dairy calves during preweaning development. Appl. Environ. Microbiol. 2018;84:e02675-17. doi: 10.1128/AEM.02675-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klein-Jöbstl D., Schornsteiner E., Mann E., Wagner M., Drillich M., Schmitz-Esser S. Pyrosequencing reveals diverse fecal microbiota in Simmental calves during early development. Front. Microbiol. 2014;5:622. doi: 10.3389/fmicb.2014.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oikonomou G., Bicalho M.L., Meira E., Rossi R., Foditsch C., Machado V.S., Teixeira A.G.V., Santisteban C., Schukken Y., Bicalho R.C. Microbiota of cow’s milk; distinguishing healthy, sub-clinically and clinically diseased quarters. PLoS ONE. 2014;9:e85904. doi: 10.1371/journal.pone.0085904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carberry C.A., Kenny D.A., Han S., McCabe M.S., Waters S.M. Effect of phenotypic residual feed intake and dietary forage content on the rumen microbial community of beef cattle. Appl. Environ. Microbiol. 2012;78:4949–4958. doi: 10.1128/AEM.07759-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Menezes A.B., Lewis E., O’Donovan M., O’Neill B.F., Clipson N., Doyle E.M. Microbiome analysis of dairy cows fed pasture or total mixed ration diets. FEMS Microbiol. Ecol. 2011;78:256–265. doi: 10.1111/j.1574-6941.2011.01151.x. [DOI] [PubMed] [Google Scholar]
- 33.Tajima K., Aminov R.I., Nagamine T., Matsui H., Nakamura M., Benno Y. Diet-dependent shifts in the bacterial population of the rumen revealed with real-time PCR. Appl. Environ. Microbiol. 2001;67:2766–2774. doi: 10.1128/AEM.67.6.2766-2774.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagaraja T.G., Chengappa M.M. Liver abscesses in feedlot cattle: A review. J. Anim. Sci. 1998;76:287–298. doi: 10.2527/1998.761287x. [DOI] [PubMed] [Google Scholar]
- 35.USDA/APHIS . Feedlot Health Management Report: Part II: Cattle on Feed Evaluation. USDA/APHIS/Veterinary Services, Centers for Epidemiology and Animal Health; Fort Collins, CO, USA: 1995. N172.0195. [Google Scholar]
- 36.Durso L.M., Miller D.N., Schmidt T.B., Callaway T. Tracking bacteria through the entire gastrointestinal tract of a beef steer. Agric. Environ. Lett. 2017;2:170016. doi: 10.2134/ael2017.05.0016. [DOI] [Google Scholar]
- 37.Welch C.B., Lourenco J.M., Krause T.R., Seidel D.S., Fluharty F.L., Pringle T.D., Callaway T.R. Evaluation of the Fecal Bacterial Communities of Angus Steers with Divergent Feed Efficiencies Across the Lifespan from Weaning to Slaughter. Front. Vet. Sci. 2021;8:597405. doi: 10.3389/fvets.2021.597405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou M., Hernandez-Sanabria E., Guan L.L. Assessment of the microbial ecology of ruminal methanogens in cattle with different feed efficiencies. Appl. Environ. Microbiol. 2009;75:6524–6533. doi: 10.1128/AEM.02815-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lourenco J.M., Krause T.R., Welch C.B., Callaway T.R., Pringle T.D. Longitudinal Changes of the Ruminal Microbiota in Angus Beef Steers. Animals. 2022;12:1066. doi: 10.3390/ani12091066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weimer P.J. Redundancy, resilience, and host specificity of the ruminal microbiota: Implications for engineering improved ruminal fermentations. Front. Microbiol. 2015;6:296. doi: 10.3389/fmicb.2015.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flint H.J., Bayer E.A. Plant cell wall breakdown by anaerobic microorganisms from the mammalian digestive tract. Ann. N. Y. Acad. Sci. 2008;1125:280–288. doi: 10.1196/annals.1419.022. [DOI] [PubMed] [Google Scholar]
- 42.Flint H.J., Bayer E.A., Rincon M.T., Lamed R., White B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Microbiol. 2008;6:121–131. doi: 10.1038/nrmicro1817. [DOI] [PubMed] [Google Scholar]
- 43.Hobson P.N., Stewart C.S. The Rumen Microbial Ecosystem. Springer Science & Business Media; New York, NY, USA: 2012. [Google Scholar]
- 44.Biddle A., Stewart L., Blanchard J.L., Leschine S. Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity. 2013;5:627–640. doi: 10.3390/d5030627. [DOI] [Google Scholar]
- 45.Brulc J.M., Antonopoulos D.A., Miller M.E.B., Wilson M.K., Yannarell A.C., Dinsdale E.A., Edwards R.E., Frank E.D., Emerson J.B., Wacklin P., et al. Gene-centric metagenomics of the fiber-adherent bovine rumen microbiome reveals forage specific glycoside hydrolases. Proc. Nat. Acad. Sci. USA. 2009;106:1948–1953. doi: 10.1073/pnas.0806191105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ding S.-Y., Rincon M.T., Lamed R., Martin J.C., McCrae S.I., Aurilia V., Shoham Y., Bayer E.A., Flint H.J. Cellulosomal scaffolding-like proteins from Ruminococcus flavefaciens. J. Bacteriol. 2001;183:1945–1953. doi: 10.1128/JB.183.6.1945-1953.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernando S.C., Purvis H.T., Najar F.Z., Sukharnikov L.O., Krehbiel C.R., Nagaraja T.G., Roe B.A., DeSilva U. Rumen microbial population dynamics during adaptation to a high-grain diet. Appl. Environ. Microbiol. 2010;76:7482–7490. doi: 10.1128/AEM.00388-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McAllister T. Learning more about rumen bugs: Genetic and environmental factors affecting rumen bugs. South. Alta. Beef Rev. 2000;2 [Google Scholar]
- 49.Krause D.O., Denman S., Mackie R.I., Morrison M., Rae A.L., Attwood G., McSweeney C. Opportunities to improve fiber degradation in the rumen: Microbiology, ecology, and genomics. FEMS Microbiol. Rev. 2003;27:663–693. doi: 10.1016/S0168-6445(03)00072-X. [DOI] [PubMed] [Google Scholar]
- 50.Barrington G.M., Parish S.M. Bovine neonatal immunology. Vet. Clin. N. Am. Food Anim. Pract. 2001;17:463–476. doi: 10.1016/S0749-0720(15)30001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Felippe M.J. The immune system. In: Felippe M.J., editor. Equine Clinical Immunology. Wiley Blackwell; Ames, IA, USA: 2015. pp. 1–10. [Google Scholar]
- 52.Mulder I.E., Schmidt B., Lewis M., Delday M., Stokes C.R., Bailey M., Aminov R., Gill B.P., Pluske J., Mayer C.-D., et al. Restricting microbial exposure in early life negates the immune benefits associated with gut colonization in environments of high microbial diversity. PLoS ONE. 2011;6:e28279. doi: 10.1371/journal.pone.0028279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malmuthuge N., Guan L.L. Understanding host-microbial interactions in rumen: Searching the best opportunity for microbiota manipulation. J. Anim. Sci. Biotechnol. 2017;8:8. doi: 10.1186/s40104-016-0135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Hara E., Neves A.L., Song Y., Guan L.L. The role of the gut microbiome in cattle production and health: Driver or passenger? Annu. Rev. Anim. Biosci. 2020;8:199–220. doi: 10.1146/annurev-animal-021419-083952. [DOI] [PubMed] [Google Scholar]
- 55.Rajasekaran S.A., Beyenbach K.W., Rajasekaran A.K. Interactions of tight junctions with membrane channels and transporters. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2008;1778:757–769. doi: 10.1016/j.bbamem.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 56.Sawada N., Murata M., Kikuchi K., Osanai M., Tobioka H., Kojima T., Chiba H. Tight junctions and human diseases. Med. Electron Microsc. 2003;36:147–156. doi: 10.1007/s00795-003-0219-y. [DOI] [PubMed] [Google Scholar]
- 57.Bush L.J., Staley T.E. Absorption of colostral immunoglobulins in newborn calves. J. Dairy Sci. 1980;63:672–680. doi: 10.3168/jds.S0022-0302(80)82989-4. [DOI] [PubMed] [Google Scholar]
- 58.Besser T.E., Gay C.C. The importance of colostrum to the health of the neonatal calf. Vet. Clin. N. Am. Food Anim. Pract. 1994;10:107–117. doi: 10.1016/S0749-0720(15)30591-0. [DOI] [PubMed] [Google Scholar]
- 59.Araujo G., Yunta C., Terré M., Mereu A., Ipharraguerre I., Bach A. Intestinal permeability and incidence of diarrhea in newborn calves. J. Dairy Sci. 2015;98:7309–7317. doi: 10.3168/jds.2015-9666. [DOI] [PubMed] [Google Scholar]
- 60.Ewaschuk J.B., Diaz H., Meddings L., Diederichs B., Dmytrash A., Backer J., Langen M.L.-V., Madsen K.L. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;295:1025–1034. doi: 10.1152/ajpgi.90227.2008. [DOI] [PubMed] [Google Scholar]
- 61.Miyauchi E., O’Callaghan J., Buttó L.F., Hurley G., Melgar S., Tanabe S., Shanahan F., Nally K., O’Toole P.W. Mechanism of protection of transepithelial barrier function by Lactobacillus salivarius: Strain dependence and attenuation by bacteriocin production. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;303:1029–1041. doi: 10.1152/ajpgi.00003.2012. [DOI] [PubMed] [Google Scholar]
- 62.Sultana R., McBain A.J., O’Neill C.A. Strain-dependent augmentation of tight junction barrier function in human primary epidermal keratinocytes by Lactobacillus and Bifidobacterium lysates. Appl. Environ. Microbiol. 2013;79:4887–4894. doi: 10.1128/AEM.00982-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Song Y., Malmuthuge N., Li F., Guan L.L. Colostrum feeding shapes the hindgut microbiota of dairy calves during the first 12 h of life. FEMS Microbiol. Ecol. 2019;95:fiy203. doi: 10.1093/femsec/fiy203. [DOI] [PubMed] [Google Scholar]
- 64.Liang G., Malmuthuge N., Bao H., Stothard P., Griebel P.J., Guan L.L. Transcriptome analysis reveals regional and temporal differences in mucosal immune system development in the small intestine of neonatal calves. BMC Genom. 2016;17:602. doi: 10.1186/s12864-016-2957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stillie R., Farooq S.M., Gordon J.R., Stadnyk A.W. The functional significance behind expressing two IL-8 receptor types on PMN. J. Leukoc. Biol. 2009;86:529–543. doi: 10.1189/jlb.0208125. [DOI] [PubMed] [Google Scholar]
- 66.Iyer S.S., Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012;32:23–63. doi: 10.1615/CritRevImmunol.v32.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smits H.H., Engering A., Van Der Kleij D., De Jong E.C., Schipper K., Van Capel T.M.M., Zaat B.A.J., Yazdanbakhsh M., Wierenga E.A., Van Kooyk Y., et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J. Allergy Clin. Immunol. 2005;115:1260–1267. doi: 10.1016/j.jaci.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 68.Marchiando A.M., Graham W.V., Turner J.R. Epithelial barriers in homeostasis and disease. Annu. Rev. Pathol. 2010;5:119–144. doi: 10.1146/annurev.pathol.4.110807.092135. [DOI] [PubMed] [Google Scholar]
- 69.Okumura R., Takeda K. Maintenance of intestinal homeostasis by mucosal barriers. Inflamm. Regen. 2018;38:5. doi: 10.1186/s41232-018-0063-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakajima A., Vogelzang A., Maruya M., Miyajima M., Murata M., Son A., Kuwahara T., Tsuruyama T., Yamada S., Matsuura M., et al. IgA regulates the composition and metabolic function of gut microbiota by promoting symbiosis between bacteria. J. Exp. Med. 2018;215:2019–2034. doi: 10.1084/jem.20180427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cunningham-Rundles C. Physiology of IgA and IgA deficiency. J. Clin. Immunol. 2001;21:303–309. doi: 10.1023/A:1012241117984. [DOI] [PubMed] [Google Scholar]
- 72.Shroff K.E., Meslin K., Cebra J.J. Commensal enteric bacteria engender a self-limiting humoral mucosal immune response while permanently colonizing the gut. Infect. Immun. 1995;63:3904–3913. doi: 10.1128/iai.63.10.3904-3913.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bergstrom K.S.B., Kissoon-Singh V., Gibson D.L., Ma C., Montero M., Sham H.P., Ryz N., Huang T., Velcich A., Finlay B.B., et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010;6:e1000902. doi: 10.1371/journal.ppat.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zarepour M., Bhullar K., Montero M., Ma C., Huang T., Velcich A., Xia L., Vallance B.A. The mucin Muc2 limits pathogen burdens and epithelial barrier dysfunction during Salmonella enterica serovar Typhimurium colitis. Infect. Immun. 2013;81:3672–3683. doi: 10.1128/IAI.00854-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Enss M.L., Grosse-Siestrup H., Schmidt-Wittig U., Gärtner K. Changes in colonic mucins of germfree rats in response to the introduction of a “normal” rat microbial flora. Rat colonic mucin. J. Exp. Anim. Sci. 1992;35:110–119. [PubMed] [Google Scholar]
- 76.Szentkuti L., Riedesel H., Enss M.L., Gaertner K., Von Engelhardt W. Pre-epithelial mucus layer in the colon of conventional and germ-free rats. Histochem. J. 1990;22:491–497. doi: 10.1007/BF01007234. [DOI] [PubMed] [Google Scholar]
- 77.Enss M.L., Schmidt-Wittig U., Müller H., Mai U.E., Coenen M., Hedrich H.J. Response of germfree rat colonic mucous cells to peroral endotoxin application. Eur. J. Cell Biol. 1996;71:99–104. [PubMed] [Google Scholar]
- 78.Malmuthuge N., Li M., Goonewardene L.A., Oba M., Guan L.L. Effect of calf starter feeding on gut microbial diversity and expression of genes involved in host immune responses and tight junctions in dairy calves during weaning transition. J. Dairy Sci. 2013;96:3189–3200. doi: 10.3168/jds.2012-6200. [DOI] [PubMed] [Google Scholar]
- 79.Chen L., Deng H., Cui H., Fang J., Zuo Z., Deng J., Li Y., Wang X., Zhao L. Inflammatory responses and inflammation associated diseases in organs. Oncotarget. 2017;9:7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ogunade I., Pech-Cervantes A., Schweickart H. Metatranscriptomic Analysis of Sub-Acute Ruminal Acidosis in Beef Cattle. Animals. 2019;9:232. doi: 10.3390/ani9050232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gupta R.C., Srivastava A., Lall R., editors. Nutraceuticals in Veterinary Medicine. Volume 2109 Springer; Cham, Switzerland: 2019. [Google Scholar]
- 82.Anadón A., Martínez-Larrañaga M.R., Aresi M.M.A. Chapter 54. Prebiotics: Safety and toxicity considerations. In: Gupta R.C., editor. Nutraceuticals: Efficacy, Safety and Toxicity. Academic; Amsterdam, The Netherlands: 2016. pp. 757–775. [Google Scholar]
- 83.Fuller R. The Lactic Acid Bacteria. Volume 1. Springer; Boston, MA, USA: 1992. The effect of probiotics on the gut micro-ecology of farm animals; pp. 171–192. [Google Scholar]
- 84.Hamilton M.J., Weingarden A.R., Unno T., Khoruts A., Sadowsky M.J. High-throughput DNA sequence analysis reveals stable engraftment of gut microbiota following transplantation of previously frozen fecal bacteria. Gut Microbes. 2013;4:125–135. doi: 10.4161/gmic.23571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liou A.P., Paziuk M., Luevano J.M.J., Machineni S., Turnbaugh P.J., Kaplan L.M. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci. Transl. Med. 2013;5:178ra41. doi: 10.1126/scitranslmed.3005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Manichanh C., Reeder J., Gibert P., Varela E., Llopis M., Antolin M., Guigo R., Knight R., Guarner F. Reshaping the gut microbiome with bacterial transplantation and antibiotic intake. Genome Res. 2010;20:1411–1419. doi: 10.1101/gr.107987.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weingarden A., González A., Vázquez-Baeza Y., Weiss S., Humphry G., Berg-Lyons D., Knights D., Unno T., Bobr A., Kang J., et al. Dynamic changes in short- and long-term bacterial composition following fecal microbiota transplantation for recurrent Clostridium difficile infection. Microbiome. 2015;3:10. doi: 10.1186/s40168-015-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Willing B.P., Vacharaksa A., Croxen M., Thanachayanont T., Finlay B.B. Altering host resistance to infections through microbial transplantation. PLoS ONE. 2011;6:e26988. doi: 10.1371/journal.pone.0026988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fong W., Li Q., Yu J. Gut microbiota modulation: A novel strategy for prevention and treatment of colorectal cancer. Oncogene. 2020;39:4925–4943. doi: 10.1038/s41388-020-1341-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Anadón A., Martínez-Larrañaga M.R., Arés I., Martínez M.A. Chapter 1. Prebiotics and probiotics: An assessment of their safety and health benefits. In: Ross Watson R., Preedy V.R., editors. Probiotics, Prebiotics, and Synbiotics: Bioactive Foods in Health Promotion. Academic; San Diego, CA, USA: 2016. pp. 3–23. [Google Scholar]
- 91.Kraimi N., Calandreau L., Zemb O., Germain K., Dupont C., Velge P., Guitton E., Lavillatte S., Parias C., Leterrier C. Leterrier. Effects of a gut microbiota transfer on emotional reactivity in Japanese quails (Coturnix japonica) J. Exp. Biol. 2019;222:jeb.202879. doi: 10.1242/jeb.202879. [DOI] [PubMed] [Google Scholar]
- 92.De Angelis M., Piccolo M., Vannini L., Siragusa S., De Giacomo A., Serrazzanetti D.I., Cristofori F., Guerzoni M.E., Gobbetti M., Francavilla R. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS ONE. 2013;8:e76993. doi: 10.1371/journal.pone.0076993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Signorini M., Soto L., Zbrun M., Sequeira G., Rosmini M., Frizzo L. Impact of probiotic administration on the health and fecal microbiota of young calves: A meta-analysis of randomized controlled trials of lactic acid bacteria. Res. Vet. Sci. 2012;93:250–258. doi: 10.1016/j.rvsc.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 94.Faubladier C., Chaucheyras-Durand F., da Veiga L., Julliand V. Julliand. Effect of transportation on fecal bacterial communities and fermentative activities in horses: Impact of Saccharomyces cerevisiae CNCM I-1077 supplementation. J. Anim. Sci. 2013;91:1736–1744. doi: 10.2527/jas.2012-5720. [DOI] [PubMed] [Google Scholar]
- 95.Julien C., Marden J.P., Auclair E., Moncoulon R., Cauquil L., Peyraud J.L., Bayourthe C. Interaction between live yeast and dietary rumen degradable protein level: Effects on diet utilization in early-lactating dairy cows. Agric. Sci. 2015;6:1–13. doi: 10.4236/as.2015.61001. [DOI] [Google Scholar]
- 96.Adeyemi J.A., Peters S.O., De Donato M., Cervantes A.P., Ogunade I.M. Effects of a blend of Saccharomyces cerevisiae-based direct-fed microbial and fermentation products on plasma carbonyl-metabolome and fecal bacterial community of beef steers. J. Anim. Sci. Biotechnol. 2020;11:14. doi: 10.1186/s40104-019-0419-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Al-Saiady M.Y. Effect of probiotic bacteria on immunoglobulin G concentration and other blood components of Newborn calves. J. Anim. Vet. Adv. 2010;9:604–609. doi: 10.3923/javaa.2010.604.609. [DOI] [Google Scholar]
- 98.Chen L., Shen Y., Wang C., Ding L., Zhao F., Wang M., Fu J., Wang H. Megasphaera elsdenii Lactate Degradation Pattern Shifts in Rumen Acidosis Models. Front. Microbiol. 2019;10:162. doi: 10.3389/fmicb.2019.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roberfroid M. Prebiotics: The concept revisited. J. Nutr. 2007;137:830–837. doi: 10.1093/jn/137.3.830S. [DOI] [PubMed] [Google Scholar]
- 100.Macfarlane S.M.G.T., Macfarlane G.T., Cummings J.T. Prebiotics in the gastrointestinal tract. Aliment Pharmacol. Ther. 2006;24:701–714. doi: 10.1111/j.1365-2036.2006.03042.x. [DOI] [PubMed] [Google Scholar]
- 101.De Vrese M., Schrezenmeir J. Probiotics, prebiotics, and synbiotics. Adv. Biochem. Eng. Biotechnol. 2008;111:1–66. doi: 10.1007/10_2008_097. [DOI] [PubMed] [Google Scholar]
- 102.Quigley J.D., III, Drewry J.J., Murray L.M., Ivey S.J. Body weight gain, feed efficiency, and fecal scores of dairy calves in response to galactosyllactose or antibiotics in milk replacers. J. Dairy Sci. 1997;80:1751–1754. doi: 10.3168/jds.S0022-0302(97)76108-3. [DOI] [PubMed] [Google Scholar]
- 103.Ghosh S., Mehla R.K. Influence of dietary supplementation of prebiotics (mannooligosaccharide) on the performance of crossbred calves. Trop. Anim. Health Prod. 2012;44:617–622. doi: 10.1007/s11250-011-9944-8. [DOI] [PubMed] [Google Scholar]
- 104.Roodposhti P.M., Dabiri N. Effects of probiotic and prebiotic on average daily gain, fecal shedding of Escherichia coli, and immune system status in newborn female calves. Asian-Australas. J Anim. Sci. 2012;25:1255–1261. doi: 10.5713/ajas.2011.11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Quigley J.D., III, Kost C.J., Wolfe T.A. Effects of spray-dried animal plasma in milk replacers or additives containing serum and oligosaccharides on growth and health of calves. J. Dairy Sci. 2002;85:413–421. doi: 10.3168/jds.S0022-0302(02)74089-7. [DOI] [PubMed] [Google Scholar]
- 106.Hartemink R., Van Laere K.M.J., Rombouts F.M. Growth of enterobacteria on fructo-oligosaccharides. J. Appl. Microbiol. 1997;83:367–374. doi: 10.1046/j.1365-2672.1997.00239.x. [DOI] [PubMed] [Google Scholar]
- 107.Lourenco J.M., Welch C.B. Using microbiome information to understand and improve animal performance. Ital. J. Anim. Sci. 2022;21:899–913. doi: 10.1080/1828051X.2022.2077147. [DOI] [Google Scholar]
- 108.Falony G., Joossens M., Vieira-Silva S., Wang J., Darzi Y., Faust K., Kurilshikov A., Bonder M.J., Valles-Colomer M., Vandeputte D.J.S. Population-level analysis of gut microbiome variation. Science. 2016;352:560–564. doi: 10.1126/science.aad3503. [DOI] [PubMed] [Google Scholar]
- 109.Liu J., Li H., Zhu W., Mao S. Dynamic changes in rumen fermentation and bacterial community following rumen fluid transplantation in a sheep model of rumen acidosis: Implications for rumen health in ruminants. FASEB J. 2019;33:8453–8467. doi: 10.1096/fj.201802456R. [DOI] [PubMed] [Google Scholar]
- 110.Yu S., Shi W., Yang B., Gao G., Chen H., Cao L., Yu Z., Wang J. Effects of repeated oral inoculation of artificially fed lambs with lyophilized rumen fluid on growth performance, rumen fermentation, microbial population and organ development. Anim. Feed Sci. Technol. 2020;264:114465. doi: 10.1016/j.anifeedsci.2020.114465. [DOI] [Google Scholar]
- 111.Kim H.S., Whon T.W., Sung H., Jeong Y.-S., Jung E.S., Shin N.-R., Hyun D.-W., Kim P.S., Lee J.-Y., Lee C.H., et al. Longitudinal evaluation of fecal microbiota transplantation for ameliorating calf diarrhea and improving growth performance. Nat. Commun. 2021;12:161. doi: 10.1038/s41467-020-20389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brag S., Hansen H. Treatment of ruminal indigestion according to popular belief in Sweden. Rev. Sci. Tech. 1994;13:529–535. doi: 10.20506/rst.13.2.782. [DOI] [PubMed] [Google Scholar]
- 113.Forkosh E., Ilan Y. The heart-gut axis: New target for atherosclerosis and congestive heart failure therapy. Open Heart. 2019;6:e000993. doi: 10.1136/openhrt-2018-000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schroeder B.O., Bäckhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016;22:1079. doi: 10.1038/nm.4185. [DOI] [PubMed] [Google Scholar]
- 115.Nicholson J.K., Holmes E., Kinross J., Burcelin R., Gibson G., Jia W., Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 116.Ahlawat S., Sharma K.K. Gut–organ axis: A microbial outreach and networking. Lett. Appl. Microbiol. 2021;72:636–668. doi: 10.1111/lam.13333. [DOI] [PubMed] [Google Scholar]
- 117.El Aidy S., Dinan T.G., Cryan J.F. Gut microbiota: The conductor in the orchestra of immune-neuroendocrine communication. Clin. Ther. 2015;37:954–967. doi: 10.1016/j.clinthera.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 118.Wells J.M., Rossi O., Meijerink M., Van Baarlen P. Epithelial crosstalk at the microbiota-mucosal interface. Proc. Natl. Acad. Sci. USA. 2011;108:4607–4614. doi: 10.1073/pnas.1000092107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Clemente J.C., Ursell L.K., Parfrey L.W., Knight R. The impact of the gut microbiota on human health: An integrative view. Cell. 2012;148:1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Conlon M., Bird A. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2015;7:17–44. doi: 10.3390/nu7010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bienenstock J., Kunze W., Forsythe P. Microbiota and the gut-brain axis. Nutr. Rev. 2015;73:28–31. doi: 10.1093/nutrit/nuv019. [DOI] [PubMed] [Google Scholar]
- 122.Bercik P. The microbiota-gut-brain axis: Learning from intestinal bacteria? Gut. 2011;60:288–290. doi: 10.1136/gut.2010.226779. [DOI] [PubMed] [Google Scholar]
- 123.Feng Q., Chen W.D., Wang Y.D. Gut microbiota: An integral moderator in health and disease. Front. Microbiol. 2018;9:151. doi: 10.3389/fmicb.2018.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Raybould H.E. Gut chemosensing: Interactions between gut endocrine cells and visceral afferents. Auton. Neurosci. 2010;153:41–46. doi: 10.1016/j.autneu.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Powley T.L., Wang X.Y., Fox E.A., Phillips R.J., Liu L.W.C., Huizinga J.D. Ultrastructural evidence for communication between intramuscular vagal mechanoreceptors and interstitial cells of Cajal in the rat fundus. Neurogastroenterol. Motil. 2008;20:69–79. doi: 10.1111/j.1365-2982.2007.00990.x. [DOI] [PubMed] [Google Scholar]
- 126.Wang Y., Kasper L.H. The role of microbiome in central nervous system disorders. Brain Behav. Immun. 2014;38:1–12. doi: 10.1016/j.bbi.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kraimi N., Dawkins M., Gebhardt-Henrich S.G., Velge P., Rychlik I., Volf J., Creach P., Smith A., Colles F., Leterrier C. Influence of the microbiota-gut-brain axis on behavior and welfare in farm animals: A review. Physiol. Behav. 2019;210:112658. doi: 10.1016/j.physbeh.2019.112658. [DOI] [PubMed] [Google Scholar]
- 128.Li F., Shah A.M., Wang Z., Peng Q., Hu R., Zou H., Tan C., Zhang X., Liao Y., Zeng L., et al. Effects of Land Transport Stress on Variations in Ruminal Microbe Diversity and Immune Functions in Different Breeds of Cattle. Animals. 2019;9:599. doi: 10.3390/ani9090599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wu Y., Wang L., Luo R., Chen H., Nie C., Niu J., Chen C., Xu Y., Li X., Zhang W. Effect of a Multispecies Probiotic Mixture on the Growth and Incidence of Diarrhea, Immune Function, and Fecal Microbiota of Pre-weaning Dairy Calves. Front. Microbiol. 2021;12:681014. doi: 10.3389/fmicb.2021.681014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Han Z., Willer T., Li L., Pielsticker C., Rychlik I., Velge P., Kaspers B., Rautenschlein S. Rautenschlein. Influence of the gut microbiota composition on Campylobacter jejuni colonization in chickens. Infect. Immun. 2017;85:e00380-17. doi: 10.1128/IAI.00380-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nagata R., Kim Y.-H., Ohkubo A., Kushibiki S., Ichijo T., Sato S. Effects of repeated subacute ruminal acidosis challenges on the adaptation of the rumen bacterial community in Holstein bulls. J. Dairy Sci. 2018;101:4424–4436. doi: 10.3168/jds.2017-13859. [DOI] [PubMed] [Google Scholar]
- 132.Thomson R.G. Rumenitis in cattle. Can. Vet. J. 1967;8:189–192. [PMC free article] [PubMed] [Google Scholar]
- 133.Desnoyers M., Giger-Reverdin S., Sauvant D., Bertin G., Duvaux-Ponter C. The influence of acidosis and live yeast (Saccharomyces cerevisiae) supplementation on time-budget and feeding behaviour of dairy goats receiving two diets of differing concentrate proportion. Appl. Anim. Behav. Sci. 2009;121:108–119. doi: 10.1016/j.applanim.2009.09.001. [DOI] [Google Scholar]
- 134.Devries T., Chevaux E. Modification of the feeding behavior of dairy cows through live yeast supplementation. J. Dairy Sci. 2014;97:6499–6510. doi: 10.3168/jds.2014-8226. [DOI] [PubMed] [Google Scholar]
- 135.Dang A.T., Marsland B.J. Microbes, metabolites, and the gut–lung axis. Mucosal Immunol. 2019;12:843–850. doi: 10.1038/s41385-019-0160-6. [DOI] [PubMed] [Google Scholar]
- 136.Morris A., Beck J.M., Schloss P.D., Campbell T.B., Crothers K., Curtis J.L., Flores S.C., Fontenot A.P., Ghedin E., Huang L., et al. Comparison of the respiratory microbiome in healthy non-smokers and smokers. Am. J. Respir. Crit. Care Med. 2013;187:1067–1075. doi: 10.1164/rccm.201210-1913OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schuijt T.J., Lankelma J.M., Scicluna B.P., e Melo F.D.S., Roelofs J.J., de Boer J.D., Hoogendijk A.J., de Beer R., de Vos A., Belzer C., et al. The gut microbiota plays a protective role in the host defense against pneumococcal pneumonia. Gut. 2016;65:575–583. doi: 10.1136/gutjnl-2015-309728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen L.W., Chen P.H., Hsu C.M. Commensal microflora contribute to host defense against Escherichia coli pneumonia through Toll-like receptors. Shock. 2011;36:67–75. doi: 10.1097/SHK.0b013e3182184ee7. [DOI] [PubMed] [Google Scholar]
- 139.Ichinohe T., Pang I.K., Kumamoto Y., Peaper D.R., Ho J.H., Murray T.S., Iwasaki A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. USA. 2011;108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Beers M.H. The Merck Manual of Diagnosis and Therapy. Merck Research Laboratories; Whitehouse Station, NJ, USA: 2001. [Google Scholar]
- 141.Zeineldin M., Lowe J., Aldridge B. Contribution of the mucosal microbiota to bovine respiratory health. Trends Microbiol. 2019;27:753–770. doi: 10.1016/j.tim.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 142.Goddard A.F., Staudinger B.J., Dowd S.E., Joshi-Datar A., Wolcott R.D., Aitken M.L., Fligner C.L., Singh P.K. Direct sampling of cystic fibrosis lungs indicates that DNA- based analyses of upper-airway specimens can misrepresent lung microbiota. Proc. Natl. Acad. Sci. USA. 2012;109:13769–13774. doi: 10.1073/pnas.1107435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nicola I., Cerutti F., Grego E., Bertone I., Gianella P., D’Angelo A., Peletto S., Bellino C. Characterization of the upper and lower respiratory tract microbiota in Piedmontese calves. Microbiome. 2017;5:152. doi: 10.1186/s40168-017-0372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zeineldin M.M., Lowe J.F., Grimmer E.D., De Godoy M.R.C., Ghanem M.M., El-Raof Y.M.A., Aldridge B.M. Relationship between nasopharyngeal and bronchoalveolar microbial communities in clinically healthy feedlot cattle. BMC Microbiol. 2017;17:138. doi: 10.1186/s12866-017-1042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Woldehiwet Z., Mamache B., Rowan T.G. Effects of age, environmental temperature and relative humidity on the colonization of the nose and trachea of calves by Mycoplasma spp. Br. Vet. J. 1990;146:419–424. doi: 10.1016/0007-1935(90)90030-7. [DOI] [PubMed] [Google Scholar]
- 146.Dickson R.P., Erb-Downward J.R., Martinez F.J., Huffnagle G.B. The microbiome and the respiratory tract. Annu. Rev. Physiol. 2016;78:481–504. doi: 10.1146/annurev-physiol-021115-105238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dewhirst F.E., Chen T., Izard J., Paster B.J., Tanner A.C., Yu W.H., Lakshmanan A., Wade W.G. The human oral microbiome. J. Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lemon K.P., Klepac-Ceraj V., Schiffer H.K., Brodie E.L., Lynch S.V., Kolter R. Comparative analyses of the bacterial microbiota of the human nostril and oropharynx. mBio. 2010;1:e00129-10. doi: 10.1128/mBio.00129-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rasmussen T.T., Kirkeby L.P., Poulsen K., Reinholdt J., Kilian M. Resident aerobic microbiota of the adult human nasal cavity. APMIS. 2000;108:663–675. doi: 10.1034/j.1600-0463.2000.d01-13.x. [DOI] [PubMed] [Google Scholar]
- 150.Grice E.A., Segre J.A. The skin microbiome. Nat. Rev. Microbiol. 2011;9:244–253. doi: 10.1038/nrmicro2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hall J.A., Isaiah A., Estill C.T., Pirelli G.J., Suchodolski J.S. Weaned beef calves fed selenium-biofortified alfalfa hay have an enriched nasal microbiota compared with healthy controls. PLoS ONE. 2017;12:e0179215. doi: 10.1371/journal.pone.0179215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Timsit E., Holman D.B., Hallewell J., Alexander T.W. The nasopharyngeal microbiota in feedlot cattle and its role in respiratory health. Anim. Front. 2016;6:44–50. doi: 10.2527/af.2016-0022. [DOI] [Google Scholar]
- 153.McMullen C., Orsel K., Alexander T.W., van der Meer F., Plastow G., Timsit E. Evolution of the nasopharyngeal bacterial microbiota of beef calves from spring processing to 40 days after feedlot arrival. Vet. Microbiol. 2018;225:139–148. doi: 10.1016/j.vetmic.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 154.Bassis C.M., Erb-Downward J.R., Dickson R.P., Freeman C.M., Schmidt T.M., Young V.B., Beck J.M., Curtis J.L., Huffnagle G.B. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. mBio. 2015;6:e00037-15. doi: 10.1128/mBio.00037-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.He Y., Wen Q., Yao F., Xu D., Huang Y., Wang J. Gut- lung axis: The microbial contributions and clinical implications. Crit. Rev. Microbiol. 2017;43:81–95. doi: 10.1080/1040841X.2016.1176988. [DOI] [PubMed] [Google Scholar]
- 156.Krishnamoorthy N., Khare A., Oriss T.B., Raundhal M., Morse C., Yarlagadda M., Wenzel S.E., Moore M.L., Peebles R.S., Ray A., et al. Early infection with respiratory syncytial virus impairs regulatory T cell function and increases susceptibility to allergic asthma. Nat. Med. 2012;18:1525–1530. doi: 10.1038/nm.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Roy M.G., Livraghi-Butrico A., Fletcher A.A., McElwee M.M., Evans S.E., Boerner R.M., Alexander S.N., Bellinghausen L.K., Song A.S., Petrova Y.M., et al. Muc5b is required for airway defense. Nature. 2014;505:412–416. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Eisele N.A., Anderson D.M. Host defense and the airway epithelium: Frontline responses that protect against bacterial invasion and pneumonia. J. Pathog. 2011;2011:249802. doi: 10.4061/2011/249802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Vareille M., Kieninger E., Edwards M.R., Regamey N. The airway epithelium: Soldier in the fight against respiratory viruses. Clin. Microbiol. Rev. 2011;24:210–229. doi: 10.1128/CMR.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Osman R., Malmuthuge N., Gonzalez-Cano P., Griebel P. Development and function of the mucosal immune system in the upper respiratory tract of neonatal calves. Annu. Rev. Anim. Biosci. 2018;6:141–155. doi: 10.1146/annurev-animal-030117-014611. [DOI] [PubMed] [Google Scholar]
- 161.Uehara A., Fujimoto Y., Fukase K., Takada H. Various human epithelial cells express functional Toll-like receptors, NOD1 and NOD2 to produce anti-microbial peptides, but not proinflammatory cytokines. Mol. Immunol. 2007;44:3100–3111. doi: 10.1016/j.molimm.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 162.Ackermann M.R., Derscheid R., Roth J.A. Innate immunology of bovine respiratory disease. Vet. Clin. N. Am. Food Anim. Pract. 2010;26:215–228. doi: 10.1016/j.cvfa.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Maslowski K.M., Vieira A.T., Ng A., Kranich J., Sierro F., Yu D., Schilter H.C., Rolph M.S., Mackay F., Artis D., et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fukuda S., Toh H., Hase K., Oshima K., Nakanishi Y., Yoshimura K., Tobe T., Clarke J.M., Topping D.L., Suzuki T., et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 165.Trompette A., Gollwitzer E.S., Yadava K., Sichelstiel A.K., Sprenger N., Ngom-Bru C., Blanchard C., Junt T., Nicod L.P., Harris N.L., et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 166.Gaudier E., Jarry A., Blottière H.M., de Coppet P., Buisine M.P., Aubert J.P., Laboisse C., Cherbut C., Hoebler C. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G1168–G1174. doi: 10.1152/ajpgi.00219.2004. [DOI] [PubMed] [Google Scholar]
- 167.Willemsen L.E.M., Koetsier M.A., Van Deventer S.J.H., Van Tol E.A.F. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut. 2003;52:1442–1447. doi: 10.1136/gut.52.10.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ohata A., Usami M., Miyoshi M. Short-chain fatty acids alter tight junction permeability in intestinal monolayer cells via lipoxygenase activation. Nutrition. 2005;21:838–847. doi: 10.1016/j.nut.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 169.Kim M., Qie Y., Park J., Kim C.H. Gut Microbial metabolites fuel host antibody responses. Cell Host Microbe. 2016;20:202–214. doi: 10.1016/j.chom.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wu W., Sun M., Chen F., Cao A.T., Liu H., Zhao Y., Huang X., Xiao Y., Yao S., Zhao Q., et al. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol. 2017;10:946–956. doi: 10.1038/mi.2016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Donohoe D.R., Collins L.B., Wali A., Bigler R., Sun W., Bultman S.J. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol. Cell. 2012;48:612–626. doi: 10.1016/j.molcel.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Byndloss M.X., Olsan E.E., Rivera-Chávez F., Tiffany C.R., Cevallos S.A., Lokken K.L., Torres T.P., Byndloss A.J., Faber F., Gao Y., et al. Microbiota-activated PPAR-gamma signaling inhibits dysbiotic Enterobacteriaceae expansion. Science. 2017;357:570–575. doi: 10.1126/science.aam9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Guilloteau P., Martin L., Eeckhaut V., Ducatelle R., Zabielski R., Van Immerseel F. From the gut to the peripheral tissues: The multiple effects of butyrate. Nutr. Res. Rev. 2010;23:366–384. doi: 10.1017/S0954422410000247. [DOI] [PubMed] [Google Scholar]
- 174.Meale S.J., Li S., Azevedo P., Derakhshani H., Plaizier J.C., Steele M., Khafipour E. Does weaning age affect the development of ruminal and fecal microbiomes in dairy calves? J. Anim. Sci. 2016;94:785. doi: 10.2527/jam2016-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zeineldin M., Lowe J., de Godoy M., Maradiaga N., Ramirez C., Ghanem M., Abd El-Raof Y., Al-dridge B. Disparity in the nasopharyngeal microbiota between healthy cattle on feed, at entry processing and with respiratory disease. Vet. Microbiol. 2017;208:30–37. doi: 10.1016/j.vetmic.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 176.Timsit E., Workentine M., Schryvers A.B., Holman D.B., van der Meer F., Alexander T.W. Evolution of the nasopharyngeal microbiota of beef cattle from weaning to 40 days after arrival at a feedlot. Vet. Microbiol. 2016;187:75–81. doi: 10.1016/j.vetmic.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 177.Caswell J.L. Failure of respiratory defenses in the pathogenesis of bacterial pneumonia of cattle. Vet. Pathol. 2014;51:393–409. doi: 10.1177/0300985813502821. [DOI] [PubMed] [Google Scholar]
- 178.Lyte M. The effect of stress on microbial growth. Anim. Health Res. Rev. 2014;15:172–174. doi: 10.1017/S146625231400019X. [DOI] [PubMed] [Google Scholar]
- 179.Lima S.F., Teixeira A.G.V., Higgins C.H., Lima F.S., Bicalho R.C. The upper respiratory tract microbiome and its potential role in bovine respiratory disease and otitis media. Sci. Rep. 2016;6:29050. doi: 10.1038/srep29050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Buckham Sporer K.R., Weber P.S.D., Burton J.L., Earley B., Crowe M.A. Transportation of young beef bulls alters circulating physiological parameters that may be effective biomarkers of stress. J. Anim. Sci. 2008;86:1325–1334. doi: 10.2527/jas.2007-0762. [DOI] [PubMed] [Google Scholar]
- 181.Apley M. The clinical syndrome of BRD: What it is and what it is not. Anim. Health Res. Rev. 2014;15:135–137. doi: 10.1017/S1466252314000152. [DOI] [PubMed] [Google Scholar]
- 182.Zeineldin M.M., El-Raof Y.M.A., El-attar H.A., Ghanem M.M. Lung ultrasonography and computer-aided scoring system as a diagnostic aid for bovine respiratory disease in feedlot cattle. Glob. Vet. 2016;17:588–594. doi: 10.5829/idosi.gv.2016.588.594. [DOI] [Google Scholar]
- 183.Alexander T.W., Plaizier J.C. The importance of microbiota in ruminant production. Anim. Front. 2016;6:4. doi: 10.2527/af.2016-0016. [DOI] [Google Scholar]
- 184.Rice J.A., Carrasco-Medina L., Hodgins D.C., Shewen P.E. Mannheimia haemolytica and bovine respiratory disease. Anim. Health Res. Rev. 2007;8:117–128. doi: 10.1017/S1466252307001375. [DOI] [PubMed] [Google Scholar]
- 185.Timsit E., Workentine M., van der Meer F., Alexander T. Distinct bacterial metacommunities inhabit the upper and lower respiratory tracts of healthy feedlot cattle and those diagnosed with bronchopneumonia. Vet. Microbiol. 2018;221:105–113. doi: 10.1016/j.vetmic.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 186.Chai J., Capik S.F., Kegley B., Richeson J., Powell J., Zhao J. Bovine respiratory microbiota of feedlot cattle and its association with disease. Vet. Res. 2022;53:4. doi: 10.1186/s13567-021-01020-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Addis M.F., Tanca A., Uzzau S., Oikonomou G., Bicalho R.C., Moroni P. The bovine milk microbiota: Insights and perspectives from -omics studies. Mol. BioSyst. 2016;12:2359–2372. doi: 10.1039/C6MB00217J. [DOI] [PubMed] [Google Scholar]
- 188.Cremonesi P., Ceccarani C., Curone G., Severgnini M., Pollera C., Bronzo V., Riva F., Addis M.F., Filipe J., Amadori M., et al. Milk microbiome diversity and bacterial group prevalence in a comparison between healthy Holstein Friesian and Rendena cows. PLoS ONE. 2018;13:e0205054. doi: 10.1371/journal.pone.0205054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Montel M.C., Buchin S., Mallet A., Delbes-Paus C., Vuitton D.A., Desmasures N., Berthier F. Traditional cheeses: Rich and diverse microbiota with associated benefits. Int. J. Food Microbiol. 2014;177:136–154. doi: 10.1016/j.ijfoodmicro.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 190.Quigley L., O’Sullivan O., Beresford T.P., Ross R.P., Fitzgerald G.F., Cotter P.D. Molecular approaches to analysing the microbial composition of raw milk and raw milk cheese. Int. J. Food Microbiol. 2011;150:81–94. doi: 10.1016/j.ijfoodmicro.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 191.Bhatt V., Ahir V., Koringa P., Jakhesara S., Rank D., Nauriyal D., Kunjadia A., Joshi C. Milk microbiome signatures of subclinical mastitis-affected cattle analysed by shotgun sequencing. J. Appl. Microbiol. 2012;112:639–650. doi: 10.1111/j.1365-2672.2012.05244.x. [DOI] [PubMed] [Google Scholar]
- 192.Young W., Hine B.C., Wallace O.A., Callaghan M., Bibiloni R. Transfer of intestinal bacterial components to mammary secretions in the cow. PeerJ. 2015;3:e888. doi: 10.7717/peerj.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rodriguez J.M. The origin of human milk bacteria: Is there a bacterial entero-mammary pathway during late pregnancy and lactation? Adv. Nutr. 2014;5:779–784. doi: 10.3945/an.114.007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Roux M.E., Mcwilliams M., Phillips-Quaglita J.M., Weisz-Carrington P., Lamm M.E. Origin of IgA-secreting plasma cells in the mammary gland. J. Exp. Med. 1977;146:1311–1322. doi: 10.1084/jem.146.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Macpherson A.J., Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 196.Hu X., Guo J., Zhao C., Jiang P., Maimai T., Yanyi L., Cao Y., Fu Y., Zhang N. The gut microbiota contributes to the development of Staphylococcus aureus-induced mastitis in mice. ISME J. 2020;14:1897–1910. doi: 10.1038/s41396-020-0651-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Melchior M., Vaarkamp H., Fink-Gremmels J. Biofilms: A role in recurrent mastitis infections? Vet. J. 2006;171:398–407. doi: 10.1016/j.tvjl.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 198.Andrews T., Neher D.A., Weicht T.R., Barlow J.W. Mammary microbiome of lactating organic dairy cows varies by time, tissue site, and infection status. PLoS ONE. 2019;14:e0225001. doi: 10.1371/journal.pone.0225001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hillerton J.E. Whatever happened to mastitis pathogenesis? J. Dairy Res. 2020;87:273–276. doi: 10.1017/S0022029920000746. [DOI] [Google Scholar]
- 200.Rainard P. Mammary microbiota of dairy ruminants: Fact or fiction? Vet. Res. 2017;48:25. doi: 10.1186/s13567-017-0429-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hanage W.P. Microbiology: Microbiome science needs a healthy dose of scepticism. Nature. 2014;512:247–248. doi: 10.1038/512247a. [DOI] [PubMed] [Google Scholar]
- 202.Taponen S., McGuinness D., Hiitiö H., Simojoki H., Zadoks R., Pyorala S. Bovine milk microbiome: A more complex issue than expected. Vet. Res. 2019;50:44. doi: 10.1186/s13567-019-0662-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Schoenmakers S., Steegers-Theunissen R., Faas M. The matter of the reproductive microbiome. Obstet. Med. 2019;12:107–115. doi: 10.1177/1753495X18775899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chen C., Song X., Wei W., Zhong H., Dai J., Lan Z., Li F., Yu X., Feng Q., Wang Z., et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017;8:875. doi: 10.1038/s41467-017-00901-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Scolari F., Attardo G.M., Aksoy E., Weiss B., Savini G., Takac P., Abd-Alla A., Parker A.G., Aksoy S., Malacrida A.R. Symbiotic microbes affect the expression of male reproductive genes in Glossina m. morsitans. BMC Microbiol. 2018;18:169. doi: 10.1186/s12866-018-1289-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Appiah M., Wang J., Lu W. Microflora in the reproductive tract of cattle: A review. Agriculture. 2020;10:232. doi: 10.3390/agriculture10060232. [DOI] [Google Scholar]
- 207.Piersanti R.L., Bromfield J.J. The Consequence of Postpartum Uterine Disease on Dairy Cow Fertility. EDIS. 2019;2019:107174. doi: 10.32473/edis-an354-2019. [DOI] [Google Scholar]
- 208.Sheldon I.M., Dobson H. Postpartum uterine health in cattle. Anim. Reprod. Sci. 2004;82–83:295–306. doi: 10.1016/j.anireprosci.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 209.Nesengani L.T., Wang J., Yang Y., Yang L., Lu W. Unravelling vaginal microbial genetic diversity and abundance between Holstein and Fleckvieh cattle. RSC Adv. 2017;7:56137–56143. doi: 10.1039/C7RA10553C. [DOI] [Google Scholar]
- 210.Giannattasio-Ferraz S., Laguardia-Nascimento M., Gasparini M.R., Leite L.R., Araujo F.M.G., de Matos Salim A.C., de Oliveira A.P., Nicoli J.R., de Oliveira G.C., da Fonseca F.G., et al. A common vaginal microbiota composition among breeds of Bos taurus indicus (Gyr and Nellore) Braz. J. Microbiol. 2019;50:1115–1124. doi: 10.1007/s42770-019-00120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wang Y., Wang J., Li H., Fu K., Pang B., Yang Y., Liu Y., Tian W., Cao R. Characterization of the cervical bacterial community in dairy cows with metritis and during different physiological phases. Theriogenology. 2018;108:306–313. doi: 10.1016/j.theriogenology.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 212.Santos T.M., Bicalho R.C. Diversity and succession of bacterial communities in the uterine fluid of postpartum metritic, endometritic and healthy dairy cows. PLoS ONE. 2012;7:e53048. doi: 10.1371/journal.pone.0053048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Jeon S.J., Cunha F., Vieira-Neto A., Bicalho R.C., Lima S., Bicalho M.L., Galvão K.N. Blood as a route of transmission of uterine pathogens from the gut to the uterus in cows. Microbiome. 2017;5:109. doi: 10.1186/s40168-017-0328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Nagaraja T., Lechtenberg K.F. Liver abscesses in feedlot cattle. Vet. Clin. N. Am. Food Anim. Pract. 2007;23:351–369. doi: 10.1016/j.cvfa.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 215.Jones K., Cunha F., Jeon S.J., Pérez-Báez J., Casaro S., Fan P., Liu T., Lee S., Jeong K.C., Yang Y., et al. Tracing the source and route of uterine colonization by exploring the genetic relationship of Escherichia coli isolated from the reproductive and gastrointestinal tract of dairy cows. Vet. Microbiol. 2020;266:109355. doi: 10.1016/j.vetmic.2022.109355. [DOI] [PubMed] [Google Scholar]
- 216.Ulfina G.G., Kimothi S.P., Oberoi P.S., Baithalu R.K., Kumaresan A., Mohanty T.K., Imtiwati P., Dang A.K. Modulation of post-partum reproductive performance in dairy cows through supplementation of long- or short-chain fatty acids during transition period. J. Anim. Physiol. Anim. Nutr. 2015;99:1056–1064. doi: 10.1111/jpn.12304. [DOI] [PubMed] [Google Scholar]
- 217.Wang T., Sha L., Li Y., Zhu L., Wang Z., Li K., Lu H., Bao T., Guo L., Zhang X., et al. Dietary alinolenic acid-rich flaxseed oil exerts beneficial effects on polycystic ovary syndrome through sex steroid hormones—microbiota—inflammation Axis in rats. Front. Endocrinol. 2020;11:284. doi: 10.3389/fendo.2020.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Boukhliq R., Martin G.B. Administration of fatty acids and gonadotrophin secretion in the mature ram. Anim. Reprod. Sci. 1997;49:143–159. doi: 10.1016/S0378-4320(97)00065-1. [DOI] [PubMed] [Google Scholar]
- 219.Miranda-CasoLuengo R., Lu J., Williams E.J., Miranda-CasoLuengo A.A., Carrington S.D., Evans A.C., Meijer W.G. Delayed differentiation of vaginal and uterine microbiomes in dairy cows developing postpartum endometritis. PLoS ONE. 2019;14:e0200974. doi: 10.1371/journal.pone.0200974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Galvão K.N., Bicalho R.C., Jeon S.J. Symposium review: The uterine microbiome associated with the development of uterine disease in dairy cows. J. Dairy Sci. 2019;102:11786–11797. doi: 10.3168/jds.2019-17106. [DOI] [PubMed] [Google Scholar]
- 221.Moreno C.G., Luque A.T., Galvão K.N., Otero M.C. Bacterial communities from vagina of dairy healthy heifers and cows with impaired reproductive performance. Res. Vet. Sci. 2022;142:15–23. doi: 10.1016/j.rvsc.2021.11.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.