Summary
Background
Previous studies have demonstrated a substantial economic impact of irritable bowel syndrome (IBS).
Aims
To provide contemporaneous estimates of direct healthcare costs of IBS in the United Kingdom.
Methods
We collected demographic, gastrointestinal and psychological symptoms, quality of life and healthcare usage data from adults with Rome IV or Rome III IBS in the United Kingdom. We calculated the mean annual direct healthcare costs of IBS per person and used contemporaneous IBS prevalence data, together with census data, to estimate annual direct costs of IBS. We also examined predictors of higher costs.
Results
The mean annual direct cost of IBS per person among 752 individuals with Rome IV IBS was £556.65 (SD £1023.92) and £474.16 (SD £897.86) for 995 individuals with Rome III IBS. We estimate the annual direct healthcare cost of IBS in the United Kingdom is £1.27 billion if the Rome IV criteria are used to define IBS, and £2.07 billion using Rome III. Among individuals with Rome IV IBS, mean annual costs were higher in those with opiate use (£907.90 vs £470.58, p < 0.001), more severe symptoms (p < 0.001 for trend), a shorter duration of IBS (1 year, £1227.14 vs >5 years £501.60, p = 0.002), lower quality of life (p < 0.001 for trend), and higher depression, somatisation and gastrointestinal symptom‐specific anxiety scores (P < 0.001 for trend for all).
Conclusion
We estimate annual direct healthcare costs of IBS of between £1.3 and £2 billion in the United Kingdom.
Abstract
Using data from over 750 individuals we provide a contemporaneous estimate of the mean annual direct costs of managing IBS to the United Kingdom health service of more than £1.2 billion, if the Rome IV criteria are used to define IBS, and more than £2 billion with Rome III. Mean annual direct healthcare costs were higher in those who used opiates, and those with more severe IBS symptoms, lower quality of life, a shorter duration of disease, and higher levels of depression, somatisation, and gastrointestinal symptom‐specific anxiety. Effective multidisciplinary management of IBS is important to reduce the economic burden that the condition represents to the healthcare system.

1. INTRODUCTION
Irritable bowel syndrome (IBS) is a disorder of gut–brain interaction, affecting between 5% and 10% of the world’s population, 1 and characterised by recurrent abdominal pain associated with a change in either stool form or frequency. 2 The pathophysiology is complex and incompletely understood. 3 A diagnosis of IBS is made using symptom‐based criteria, proposed by the Rome Foundation. 4 As a result of the likely different underlying pathophysiological abnormalities, but similar phenotypes, patients with IBS form a heterogenous group of individuals and current treatment approaches focus on addressing the predominant gastrointestinal symptom(s) reported by the individual. 5 , 6
Most treatments for IBS are of limited efficacy when tested in randomised controlled trials. 7 , 8 , 9 , 10 , 11 Partly as a result, IBS is chronic, with a relapsing and remitting course for most patients, leading to high consultation rates. 12 Quality of life is affected to the same degree as organic gastrointestinal conditions, such as Crohn’s disease, 13 and the impact of IBS on work productivity is considerable. 14 Because the symptoms of IBS can be confused with certain organic gastrointestinal diseases, it is often perceived as a diagnosis of exclusion among some clinicians, leading to unnecessary investigations and therefore costs, associated with making a diagnosis. 15 In addition, novel drugs are often expensive. For these reasons, IBS represents a substantial burden to both healthcare systems and society.
Previous studies have estimated the costs to the health service of IBS in various countries. 16 , 17 , 18 , 19 However, most have used a top‐down approach, relying on coding of a diagnosis of IBS in existing databases, but it has been shown this approach is prone to error due to inaccurate coding. 20 This also means that gold standard criteria for the diagnosis of IBS, such as the Rome criteria, have not been applied in many of these studies. In addition, most studies recruited patients from secondary care settings and are, therefore, not representative of all patients with IBS, many of whom either never consult a doctor or are managed solely in primary care. 21 Although previous studies have attempted to estimate direct healthcare costs of IBS in the United Kingdom, 22 , 23 , 24 , 25 only two have been conducted in the last 20 years. One of these reported total annual healthcare costs, 25 rather than costs related to IBS specifically. The second included patients with symptoms suggestive of, but not diagnostic for, IBS, such as constipation, change in bowel habit or abdominal pain in isolation. 24 Finally, few studies have examined patients factors that predict higher direct costs. 26 , 27
Assessing the burden of IBS on the healthcare system is important, not only to plan healthcare resource allocation but also to provide a rationale for adequate funding for IBS research from grant‐giving bodies. We, therefore, conducted a cross‐sectional survey to estimate the mean annual direct costs of IBS to the health service per person, extrapolating these across the entire United Kingdom adult population, to provide a contemporaneous approximation of the burden of IBS on the United Kingdom healthcare system, as well as examining predictors of higher costs.
2. METHODS
2.1. Participants and setting
We recruited individuals registered with ContactME‐IBS, a national United Kingdom registry of 4280 members with IBS who have expressed an interest in participating in research. We have reported data from this cohort previously. 28 Briefly, individuals find out about the registry via numerous sources, including primary care practices, specialist hospital clinics, posters in pharmacies or social media. Individuals enrol online by completing a short questionnaire about their bowel symptoms and providing contact details. Among all registrants, 2268 (53%) have seen their general practitioner (GP), and 1455 (34%) a gastroenterologist with IBS. The registry is run by County Durham and Darlington NHS Foundation Trust. The only exclusion criterion for this study was an inability to understand written English. We contacted all registered individuals via electronic mailshot, in July 2021, directing them to a website where they could access study information. Those willing to participate completed an online questionnaire, with responses stored in an online database. We sent reminder emails to all non‐responders in August 2021 and completed recruitment in September 2021. Participants were given a chance to win one of three gift cards (worth £200, £100 or £50) in return for completing the questionnaire. The University of Leeds research ethics committee approved the study in March 2021 (MREC 20‐051).
2.2. Data collection and synthesis
2.2.1. Demographic and symptom data
We collected basic demographic data, including age, sex, lifestyle (tobacco and alcohol consumption), ethnicity, marital status, educational level and annual income. We defined the presence of IBS utilising both the Rome III and Rome IV questionnaires, 29 , 30 via the scoring algorithms proposed for their use. 4 , 31 We categorised the IBS subtype according to the proportion of time stools were abnormal on the Bristol stool form scale, as recommended. 29 We asked all participants to report the number of years since their diagnosis of IBS, and whether they used opiates, as well as their most troublesome symptom from a list of five possibilities, including abdominal pain, constipation, diarrhoea, bloating or urgency.
2.2.2. IBS symptom severity
We assessed symptom severity using the IBS severity scoring system (IBS‐SSS), 32 which measures presence, severity and frequency of abdominal pain, presence and severity of abdominal distension, satisfaction with bowel habit and how much IBS symptoms are affecting, or interfering with, the individual’s life. The IBS‐SSS gives a maximum score of 500 points, with <75 points indicating remission of symptoms, 75–174 mild symptoms, 175–299 moderate symptoms and 300–500 severe symptoms.
2.2.3. Mood, somatic symptoms and gastrointestinal symptom‐specific anxiety
We collected anxiety and depression data using the Hospital Anxiety And Depression Scale (HADS). 33 The total HADS score ranges from 0 to 21 for either anxiety or depression. We categorised severity for each into normal (total HADS depression or anxiety score 0–7), borderline normal (8–10) or abnormal (≥11), as recommended. 33 We collected somatic symptom data using the Patient Health Questionnaire‐12 (PHQ‐12), 34 which is derived from the validated Patient Health Questionnaire‐15. 35 The total PHQ‐12 score ranges from 0 to 24 and we categorised severity into high (total PHQ‐12 ≥13), medium (8–12), low (4–7) or minimal (≤3). We measured gastrointestinal symptom‐specific anxiety with the Visceral Sensitivity Index (VSI). 36 There are 15 items, with replies to each provided on a 6‐point scale from “strongly disagree” (score 0) to “strongly agree” (score 5). As there are no validated cutoffs to define low, medium or high levels of gastrointestinal symptom‐specific anxiety, we divided these data into equally sized tertiles.
2.2.4. IBS‐specific quality of life
We used the irritable bowel syndrome quality of life (IBS‐QOL). This is a validated IBS‐specific questionnaire measuring health‐related quality of life, 37 , 38 consisting of 34 items, each ranked on a 5‐point Likert scale ranging from 0 to 4. The total possible score ranges from 0 to 136, with lower scores indicating better quality of life. The 34 items are based on the following eight variables: dysphoria, interference with activity, body image, health worry, food avoidance, social reactions, sexual activity and relationships. Scores were transformed to a 0 to 100‐point scale with 0 indicating worst, and 100 best, quality of life. Again, as there are no validated cutoffs to define low, medium or high levels of quality of life, we divided data into equally sized tertiles.
2.2.5. Annual direct costs
We collected data on healthcare usage related to IBS only over the 12 months prior to recruitment to the study (See the questionnaire in the Material S1). We carefully instructed participants to report the number of appointments with healthcare professionals (GPs, gastroenterologists, specialist nurses, dietitians or psychologists), number of investigations (blood tests, stool tests, endoscopies, abdominal ultrasounds, computed tomography scans, magnetic resonance imaging scans, hydrogen breath tests or 23‐seleno‐25‐homo‐tauro‐cholic acid scans), number of unplanned emergency department attendances or inpatient admissions (including length of stay), and over the counter and prescribed medication usage (months) only in relation to their IBS. We applied costs for GP appointments from Unit Costs of Health and Social Care 2020, 39 and all other appointments, investigations, emergency department attendances and unplanned inpatient days in secondary care using NHS 2019/20 National Cost Collection Data. 40 We assumed that all the appointments for IBS were follow‐up appointments, which cost less than a new patient appointment. Unit costs for appointments, investigations and hospital attendances are provided in Table 1. We applied the lowest price for a 1‐month supply of each IBS‐related medication using the online version of the British National Formulary (BNF). 41 These are provided in Table 2.
TABLE 1.
Unit costs (in UK pounds) for IBS‐related appointments, investigations and unplanned hospital attendances or admissions 39 , 40
| Cost (£) | |
|---|---|
| Follow‐up appointment with a GP | 33.00 |
| Follow‐up appointment with a gastroenterologist | 148.12 |
| Follow‐up appointment with a specialist nurse | 127.91 |
| Follow‐up appointment with a dietician | 83.03 |
| Follow‐up appointment with a psychologist | 179.84 |
| Blood test | 1.81 |
| Stool test | 8.09 |
| Gastroscopy | 482.23 |
| Colonoscopy | 559.35 |
| Hydrogen breath test | 57.96 |
| Abdominal ultrasound | 62.39 |
| Abdominal computed tomography | 114.36 |
| Abdominal magnetic resonance imaging | 144.29 |
| 23‐seleno‐25‐homo‐tauro‐cholic acid scan | 367.73 |
| Emergency department attendance | 220.53 |
| Inpatient admission under gastroenterology | 1551.77 |
TABLE 2.
Unit costs (in UK pounds) for a 1‐month supply of IBS‐related medications 41
| Cost (£) | |
|---|---|
| Loperamide | 1.68 |
| Sodium picosulfate | 4.62 |
| Bisacodyl | 1.67 |
| Polyethylene glycol | 2.99 |
| Hyoscine | 9.63 |
| Alverine | 7.64 |
| Mebeverine | 4.39 |
| Dicycloverine | 30.00 |
| Ispaghula | 3.24 |
| Peppermint oil | 4.95 |
| Amitriptyline | 1.08 |
| Nortriptyline | 1.00 |
| Imipramine | 2.15 |
| Fluoxetine | 0.50 |
| Paroxetine | 1.26 |
| Sertraline | 0.80 |
| Citalopram | 1.02 |
| Escitalopram | 1.55 |
| Lubiprostone | 53.48 |
| Linaclotide | 37.56 |
| Prucalopride | 47.62 |
| Eluxadoline | 88.20 |
2.3. Statistical analysis
We used contemporaneous prevalence data for Rome IV and Rome III IBS in the United Kingdom, derived from the Rome Foundation three‐nation prevalence study, 42 to extrapolate total annual direct costs per person from this study across the entire United Kingdom adult population, using published census data. 43 , 44 , 45 In the current study, the majority of participants had consulted with a doctor, which may skew the costs. We, therefore, contacted the authors of the three‐nation Rome Foundation study to obtain consultation rates with a doctor for IBS among those meeting either the Rome IV or Rome III criteria only for the United Kingdom population recruited into that study (data on file, personal communication: Dr Olafur Palsson, University of North Carolina, Chapel Hill, NC, USA) to perform a more conservative sensitivity analysis of annual direct costs. 42 Finally, among those with Rome IV IBS we examined the mean annual direct costs per individual according to demographic characteristics, gastrointestinal symptoms, psychological comorbidity and quality of life. We compared to mean annual direct costs using an independent samples t‐test or one‐way ANOVA, depending on the number of groups being compared. We used a logistic regression model, controlling for all baseline data, to examine predictors of the above mean annual direct costs in those with Rome IV IBS, and reported the results with odds ratios (ORs) with 95% confidence intervals (CIs). We used a p < 0.01 to define statistical significance and performed all analyses using SPSS for Windows (version 27.0, SPSS).
RESULTS
In total, 1278 (29.9%) of 4280 registrants (mean age 47.2 years (range 18–89 years), 1086 (85.0%) female) who had IBS by self‐report responded and completed the questionnaire. Of these, 995 (77.9%) and 752 (58.8%) met Rome III and IV criteria for IBS, respectively, and only their data were considered in all further analyses. Among those meeting Rome IV criteria for IBS, the mean age was 45.3 years (range 18–81 years) and 655 (87.1%) were female. In total, 136 (18.1%) had IBS with constipation (IBS‐C), 306 (40.7%) IBS with diarrhoea (IBS‐D), and 301 (40.0%) IBS with mixed bowel habits (IBS‐M). The mean IBS‐SSS score was 293.1 (SD 95.1). Among those meeting Rome III criteria, the mean age was 46.5 years (range 18–85 years) and 852 (85.6%) were female. There were 185 (18.6%) with IBS‐C, 414 (41.6%) with IBS‐D and 382 (38.4%) with IBS‐M. The mean IBS‐SSS score was 266.1 (SD 102.8).
2.4. Mean annual direct costs from IBS
The mean annual direct costs of IBS among individuals with Rome IV IBS was £556.65 per person (SD £1023.92) with appointments with healthcare professionals accounting for £224.48 (40.3%) of total costs, investigations £157.69 (28.3%), unplanned hospital attendances £101.85 (18.3%), and IBS‐related medications £72.60 (13.1%), (Figure 1). The prevalence of Rome IV‐defined IBS in the United Kingdom is 4.6%, 42 and there are 49,711,000 adults aged 18 years and over, meaning there are likely to be 2,286,706 individuals with Rome IV IBS in the United Kingdom. Applying these cost data resulted in an estimate of total annual direct costs of IBS to the health service of £1,272,894,895. In our sensitivity analysis, assuming 2.8% of the United Kingdom adult population have Rome IV IBS and will consult a physician, as per the United Kingdom population recruited into the three‐nation Rome Foundation study (data on file, personal communication: Dr Olafur Palsson, University of North Carolina, Chapel Hill, NC, USA), 42 there are likely to be 1,391,908 individuals with Rome IV IBS who have consulted a physician in the United Kingdom. Applying cost data to these figures yielded estimated total annual direct costs of IBS to the health service of £774,805,588.
FIGURE 1.
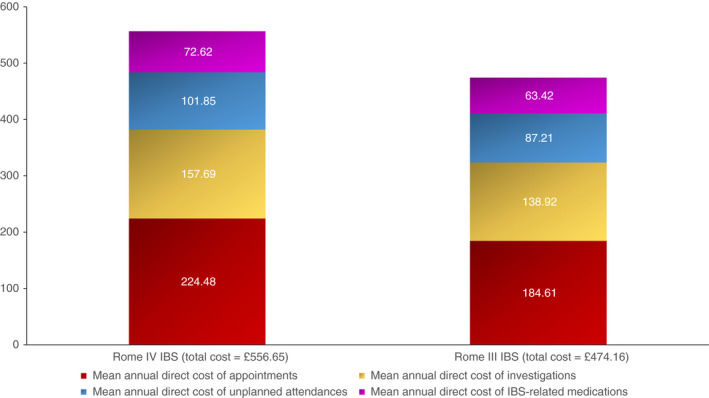
Mean annual direct costs of IBS among 752 individuals with Rome IV IBS and 995 individuals with Rome III IBS
The annual mean direct costs of IBS among individuals with Rome III IBS were lower at £474.16 per person (SD £897.86), with appointments with healthcare professionals costing £184.61 (38.9% of costs), investigations £138.92 (29.3%), unplanned hospital attendances £87.21 (18.4%) and IBS‐related medications £63.42 (13.4%) (Figure 1). Prevalence rates of Rome III‐defined IBS in the United Kingdom are 8.8%, 42 meaning there are 4,374,568 adults with Rome III IBS. Applying our costs data to these figures yielded estimated total annual direct costs to the health service for IBS of £2,074,245,163. Even when we performed a sensitivity analysis assuming 4.7% of the United Kingdom adult population have Rome III IBS and will consult a physician (data on file, personal communication: Dr Olafur Palsson, University of North Carolina, Chapel Hill, NC, USA), 42 meaning there are likely to be 2,336,417 individuals with Rome III IBS who have consulted a physician in the United Kingdom, total annual direct costs were estimated at £1,107,835,485.
2.5. Mean annual direct costs for individuals with Rome IV‐defined IBS according to demographics, gastrointestinal symptoms and psychological comorbidities
Annual mean direct healthcare costs for individuals with Rome IV IBS were not associated with sex, or level of education but were significantly higher in smokers (£845.13 vs £521.34, p = 0.007), those who did not drink alcohol (£747.79 vs. £420.36, p < 0.001), and those who were not married (£702.19 vs. £477.45, p = 0.004) (Table 3). There was no association between costs and IBS subtype, most troublesome symptom or whether IBS had been triggered after an acute enteric infection. However, mean costs were higher in those who used opiates (£907.90 vs. £470.58, p < 0.001), and those with more severe symptoms (severe, £724.03 vs moderate, £448.76 vs mild, £277.96 vs remission, £19.38, p < 0.001 for trend). Costs of IBS reduced significantly as the duration of a diagnosis of IBS increased, although even among those who were diagnosed >5 years ago mean annual direct costs were estimated at over £500 per year. We observed higher mean costs in those with abnormal HADS depression scores (abnormal, £953.69 vs borderline, £609.77 vs normal, £355.10, p < 0.001 for trend), higher somatisation scores (severe, £799.47 vs moderate, £508.80 vs mild, £365.91 vs low, £325.52, p < 0.001 for trend), and higher VSI scores (high, £765.86 vs medium, £459.86 vs low, £434.89, p < 0.001 for trend) but not abnormal HADS anxiety scores. Finally, costs increased significantly with reductions in IBS‐related quality of life (low, £858.61 vs medium, £585.97 vs high, £251.82, p < 0.001 for trend). Following logistic regression, older participants (OR per year = 1.02; 95% CI 1.01–1.04) were more likely to have above mean costs and those with higher IBS‐related quality of life (OR = 0.29; 95% CI 0.14–0.61) less likely.
TABLE 3.
Direct healthcare costs of IBS (in UK pounds), as defined by Rome IV criteria, according to demographics, symptom characteristics, psychological comorbidity and quality of life
| Annual mean cost per person, SD (£UK) | p valuea | |
|---|---|---|
| Sex | ||
| Male (n = 97) | 517.79 (879.01) | 0.69 |
| Female (n = 655) | 562.40 (1044.14) | |
| Smoker | ||
| Yes (n = 82) | 845.13 (1330.12) | 0.007 |
| No (n = 670) | 521.34 (975.33) | |
| Alcohol use | ||
| Yes (n = 439) | 420.36 (797.18) | <0.001 |
| No (n = 313) | 747.79 (1252.46) | |
| Married | ||
| Yes (n = 487) | 477.45 (859.26) | 0.004 |
| No (n = 265) | 702.19 (1261.11) | |
| University or postgraduate level of education | ||
| Yes (n = 314) | 489.17 (973.18) | 0.13 |
| No (n = 438) | 605.02 (1057.25) | |
| Annual income of £30,000 or more | ||
| Yes (n = 197) | 404.14 (823.55) | 0.014 |
| No (n = 483) | 609.49 (1046.89) | |
| IBS subtype | ||
| IBS‐C (n = 136) | 558.86 (1159.35) | 0.75 |
| IBS‐D (n = 306) | 522.75 (941.01) | |
| IBS‐M (n = 301) | 586.22 (1043.37) | |
| IBS after acute enteric infection | ||
| Yes (n = 91) | 665.27 (942.49) | 0.33 |
| No (n = 465) | 547.87 (1065.70) | |
| Most troublesome symptom | ||
| Abdominal pain (n = 169) | 686.02 (1191.23) | 0.31 |
| Constipation (n = 53) | 518.47 (810.23) | |
| Diarrhoea (n = 117) | 566.34 (1055.48) | |
| Bloating/distension (n = 218) | 457.65 (885.32) | |
| Urgency (n = 195) | 559.74 (1041.55) | |
| Opiate use | ||
| Yes (n = 148) | 907.90 (1391.88) | <0.001 |
| No (n = 604) | 470.58 (892.05) | |
| Duration of IBS diagnosis, year(s) | ||
| 1 (n = 25) | 1227.14 (1954.19) | 0.002 |
| 2 (n = 41) | 919.39 (1508.42) | |
| 3 (n = 54) | 449.03 (740.93) | |
| 4 (n = 33) | 701.18 (1420.32) | |
| 5 (n = 38) | 564.24 (854.99) | |
| >5 (n = 561) | 501.60 (910.60) | |
| Severity on IBS‐SSS | ||
| Remission (n = 7) | 19.38 (21.88) | <0.001 |
| Mild (n = 86) | 277.96 (639.10) | |
| Moderate (n = 300) | 448.76 (859.30) | |
| Severe (n = 359) | 724.03 (1193.10) | |
| HADS anxiety categories | ||
| Normal (n = 200) | 438.63 (1072.39) | 0.08 |
| Borderline abnormal (n = 174) | 521.50 (839.28) | |
| Abnormal (n = 378) | 635.26 (1069.61) | |
| HADS depression categories | ||
| Normal (n = 404) | 355.10 (836.24) | <0.001 |
| Borderline abnormal (n = 165) | 609.77 (877.43) | |
| Abnormal (n = 183) | 953.69 (1353.78) | |
| PHQ‐12 severity | ||
| Low (n = 36) | 325.52 (671.59) | <0.001 |
| Mild (n = 176) | 365.91 (785.65) | |
| Moderate (n = 307) | 508.80 (898.70) | |
| Severe (n = 233) | 799.47 (1302.59) | |
| VSI scores | ||
| Low (n = 247) | 434.89 (983.63) | <0.001 |
| Medium (n = 247) | 459.86 (924.15) | |
| High (n = 258) | 765.86 (1119.30) | |
| IBS‐QOL scores | ||
| Low (n = 239) | 858.61 (1210.79) | <0.001 |
| Medium (n = 252) | 585.97 (1152.04) | |
| High (n = 261) | 251.82 (476.60) | |
p value for independent samples t‐test or one‐way ANOVA.
3. DISCUSSION
We recruited individuals with IBS, defined according to validated criteria, 46 , 47 to estimate the mean annual direct costs of IBS to the health service per person and extrapolated these results across the entire United Kingdom adult population using contemporaneous IBS prevalence data, as well as published United Kingdom census data. The mean annual direct cost among individuals meeting Rome IV criteria for IBS was over £500 per person and almost £475 per person for those with Rome III IBS. For Rome IV IBS, 40% of direct costs were made up of appointments with healthcare professionals, 28% investigations, 18% unplanned hospital attendances and 13% medications. Using these data, we estimate the total annual direct healthcare cost of IBS in the United Kingdom to be more than £1.2 billion if the Rome IV criteria are used to define IBS, and more than £2 billion using the Rome III criteria, due to the higher prevalence of IBS when these are applied. Even when we performed a sensitivity analysis, using prevalence data for only those who are likely to consult a doctor with IBS, total annual direct costs were estimated at £0.75 billion with Rome IV and £1.1 billion with Rome III. Mean direct costs were significantly higher in smokers, those who did not drink alcohol, those who were unmarried, those who used opiates and those with a shorter duration of IBS. In terms of psychological comorbidity, higher depression, somatisation and gastrointestinal symptom‐specific anxiety scores were associated with higher mean direct costs. Finally, those with more severe IBS symptoms and lower quality of life had significantly higher direct costs.
We recruited almost 1000 individuals who self‐identified as having IBS and who also met either Rome IV or III criteria for IBS. We used validated questionnaires and obtained near‐complete data for the variables of interest because of the use of mandatory fields in our online questionnaire. Some of the recruited participants had never seen a doctor for their IBS, some had seen their GP, and some had seen a gastroenterologist. This, together with the fact that the duration of diagnosis of IBS was variable, implies this sample is likely to be representative of individuals with IBS in the United Kingdom. We used a bottom‐up approach, where we collected data concerning each individual appointment, investigation or medication used and applied the relevant unit cost to these items to estimate the direct healthcare cost of IBS across the United Kingdom, rather than a top‐down approach, in which one identifies participants with IBS via diagnostic coding and applies the average cost assigned to such a diagnosis. This bottom‐up approach to estimate costs meant that we did not have to rely on national databases, which are prone to coding errors, 20 and we were able to capture all IBS‐related healthcare resource use, as well as over the counter medications, which are used commonly by individuals with IBS. 48 This approach has been used previously in a study examining the economic impact of functional dyspepsia. 49
To capture a representative sample of individuals with IBS, rather than selecting only those from secondary care, we used a national United Kingdom registry. This methodology has some weaknesses. We were unable to check participants’ medical records to rule out other organic diseases that present with similar symptoms, such as coeliac disease or inflammatory bowel disease. Three‐quarters of participants met either the Rome IV or Rome III criteria. Although IBS is more prevalent than coeliac disease or inflammatory bowel disease, current United Kingdom national guidance recommends ruling out these diseases in individuals, before a diagnosis of IBS is made. 5 , 6 In addition, almost 90% of ContactME‐IBS registrants have seen a GP or gastroenterologist for their IBS and nearly 80% of our participants had a diagnosis of IBS for 5 years or longer. We believe, therefore, that participants in this study genuinely had IBS. Using an online questionnaire meant that we were unable to assess how many individuals visited our webpage but chose not to complete the questionnaire. As all participants were United Kingdom residents, the results are not applicable to individuals with IBS outside the United Kingdom. Minority ethnic groups were underrepresented in our study population, and this could have affected our results because of racial disparity in healthcare utilisation among patients with IBS. 50
Individuals joining ContactME‐IBS, compared with the wider population of IBS patients, and those who responded to our survey, compared to non‐respondents, may be more proactive in managing their IBS and may have higher healthcare use. In addition, although we used validated questionnaires to examine the presence and severity of IBS and psychological comorbidities, and to assess IBS‐related quality of life, we relied on self‐report to collect IBS‐related resource use retrospectively. Although it is impossible to eliminate errors in recall, we sought to minimise them by limiting the recall period to 12 months immediately prior to questionnaire completion, as accuracy generally decreases the longer the recall period. 51 Moreover, to limit variations in understanding that might affect recall, we ensured that questions designed to capture healthcare resource use were clear and precise. Importantly, self‐reported healthcare utilisation has been shown to be accurate and reliable for hospital and specialist visits, although GP visits may be at risk of underreporting. 52 The cost data were positively skewed, meaning that the mean cost per individual will have been affected by a small proportion of participants with higher costs. Despite this, we chose to use the mean cost, as using a median would involve applying the cost data from a single individual from our sample to estimate the cost of IBS across the United Kingdom. We felt a mean cost, although affected by outliers with higher costs, better reflected a real‐life situation with a large proportion of patients with low levels of healthcare usage and a small number of individuals with higher costs. As a result of some of this, the total annual direct costs we report may be an overestimate. However, we took steps to reduce this. First, we assumed all appointments were follow‐ups, which are cheaper than new patient appointments, used the cheapest drug price available from the BNF, including for over‐the‐counter medications, and did not consider out of pocket expenses, complementary or alternative medicines or other indirect costs. Second, we used United Kingdom census data from 2011 because the latest 2021 census results are, as yet, unpublished. The population of individuals aged 18 years or over is likely to have increased further in the last decade. Third, we performed a more conservative sensitivity analysis using prevalence data for Rome IV or Rome III IBS only for those who will consult a doctor in the United Kingdom, 42 because our costs may have been skewed by the fact that most responders in our study had consulted their GP or a gastroenterologist. Even in this analysis, costs were estimated at between £0.75 and £1.1 billion per year. Finally, 75% of participants were diagnosed more than 5 years prior to study recruitment, reflecting the chronicity of IBS, and costs were significantly lower in this group.
Several studies have attempted to estimate direct healthcare costs of IBS in the United Kingdom. 22 , 23 , 24 , 25 Importantly, only two of these have been carried out in the last 20 years, and one reported a total annual healthcare cost 3 years before and 3 years after a patient’s first appointment with a gastroenterologist with IBS, 25 rather than costs related to IBS per se. The second study reported a mean annual cost of IBS per patient of £383.20, but the authors also included patients with symptoms that were only suggestive of IBS, including constipation, change in bowel habit or abdominal pain in isolation. 24 Both studies used a top‐down approach, meaning that the Rome criteria for IBS were not applied, as the authors relied on accurate coding in existing databases. In addition, both used data from patients who had seen a gastroenterologist in secondary care for their IBS, which is not representative of all patients with IBS, because many either do not see a doctor at all or are managed solely in primary care. 21
Although it is difficult to compare costs across countries using different currencies and healthcare systems over different periods of time, all prior studies conducted elsewhere have shown, consistently, substantial costs associated with the care of individuals with IBS. 16 , 17 , 18 Annual direct healthcare costs estimates per patient in the most contemporaneous review, including studies conducted elsewhere, were between $742 and $7547 in the USA, £90 to £316 in the United Kingdom, €567to €862 in France, $259 in Canada, €791 in Germany and $92 in Iran. 17 To the best of our knowledge, no previous study has used the Rome IV criteria to estimate the annual direct cost of IBS. We identified one study using the Rome III criteria, which reported annual direct costs for IBS of ¥12,761.14 (approximately £1450) in 105 patients with IBS recruited from a university hospital in China. 19 The mean annual direct healthcare costs of IBS per patient observed in our study are of a similar magnitude to that estimated for patients with functional dyspepsia in the USA, which was $699 (approximately £525) per patient. 49 Finally, to put our findings in context with other chronic diseases, our estimate for the annual direct cost of IBS per patient, irrespective of the criteria used to define its presence, is lower than that for asthma and COPD (£808), 53 type 1 diabetes mellitus (£1323), and type 2 diabetes mellitus (£1080) in the United Kingdom. 54
Few studies have examined associations between patient demographics, symptom characteristics and costs. 26 , 27 These were published some time ago, and therefore used the Manning, Rome I or Rome II criteria to diagnose IBS, but their results are similar. Johansson et al. reported a positive correlation between both severity of IBS and the presence of somatic symptoms and costs, 26 whilst Lepen et al. demonstrated a negative correlation between quality of life, based on the IBS‐QOL, and costs, 27 and no association between sex or IBS subtype and costs. Our results demonstrated that those with opiate use, more severe symptoms and lower quality of life had higher annual mean costs. This probably reflects higher levels of consultations, investigations and medication usage in this group of patients. Mean annual cost correlated negatively with the duration of IBS, likely because of more frequent consultations and investigations at the onset of symptoms. The fact that those with higher depression, somatisation and gastrointestinal symptom‐specific anxiety scores had higher mean annual direct healthcare costs related to IBS may relate to confounding factors like the severity of IBS symptoms, which is known to be associated with both costs and psychological comorbidities. 34 , 55 , 56
Mean annual direct costs among individuals with Rome IV IBS were higher than for those with Rome III IBS, probably because the stricter Rome IV criteria select patients with more severe symptoms. 57 We have demonstrated that those with Rome IV IBS are more likely to consult their GP or a gastroenterologist for their IBS symptoms and are more likely to cycle through multiple treatments, which may partially explain the higher mean annual costs in those with Rome IV IBS. 58 Despite this, the annual direct cost to the United Kingdom health service we estimated was higher using the Rome III criteria because of the higher prevalence of Rome III IBS. 42 Overall costs of IBS are substantial, likely as a result of the chronicity of symptoms, 12 the non‐fatal nature of IBS, 59 , 60 the relatively high prevalence of the condition, the inappropriate use of exhaustive investigation to reach a diagnosis, 15 , 61 and the lack of a cure. Money spent on medications was lowest compared with other costs. This may reflect the continued use of older medications, due to their familiarity, as well as the lack of availability of newer effective drugs, due to a perceived lack of demand for these in the United Kingdom by pharmaceutical companies, meaning that they are no longer marketed.
Our study has important implications. The costs of IBS we estimated would represent 0.7% or 1.2%, for those with Rome IV IBS or Rome III IBS, respectively, of the total budget for Health and Social Care spending across all four nations in the United Kingdom for 2019 to 2020. 62 , 63 , 64 , 65 Clinicians should, therefore, be encouraged to make a positive diagnosis of IBS in the absence of alarm symptoms or signs, rather than regarding IBS as a diagnosis of exclusion that requires numerous investigations, driving management costs. 66 Careful explanation of symptoms, active listening, being empathetic, educating patients, offering reassurance and managing expectations are key to reducing multiple healthcare episodes. 67 , 68 The high levels of spending on IBS highlight the need for the optimised management of the condition, including a multidisciplinary approach and improved access to evidence‐based treatments, such as eluxadoline, ramosetron, plecanatide and tenapanor, which are yet to be licensed in the United Kingdom. Compared with seeing a gastroenterologist alone, multidisciplinary care may not only reduce treatment costs for IBS, 69 , 70 but also unplanned hospital attendances. Finally, the high cost of IBS should be an impetus for funding bodies to commission more research into both the causes and management of IBS, especially considering that research monies for IBS in both Europe and the USA are considerably lower than those for less prevalent gastrointestinal conditions, such as coeliac disease. 71
In summary, our study provides a contemporaneous estimate of the mean annual direct costs of managing IBS per person to the United Kingdom health service with data extrapolated across the entire United Kingdom adult population. We estimate direct healthcare costs of IBS of more than £1.2 billion, if the Rome IV criteria were used to define IBS, and more than £2 billion with Rome III. Mean annual direct healthcare costs were higher in those who used opiates, and those with more severe IBS symptoms, lower quality of life, a shorter duration of disease, and higher levels of depression, somatisation and gastrointestinal symptom‐specific anxiety. Effective multidisciplinary management of IBS is important to reduce the economic burden that the condition represents on the healthcare system.
AUTHOR CONTRIBUTIONS
Vivek Goodoory: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); project administration (equal); writing – original draft (equal); writing – review and editing (equal). Cho Ee Ng: Conceptualization (equal); data curation (equal); writing – review and editing (equal). Christopher Black: Conceptualization (equal); formal analysis (equal); methodology (equal); supervision (equal); writing – review and editing (equal). Alexander Ford: Conceptualization (equal); data curation (equal); formal analysis (equal); funding acquisition (equal); investigation (equal); methodology (equal); project administration (equal); supervision (equal); validation (lead); writing – original draft (equal); writing – review and editing (equal).
AUTHORSHIP
Guarantor of the article: Alexander C. Ford.
Supporting information
Data S1
ACKNOWLEDGEMENTS
We are grateful to the patients who gave their time freely to answer our questionnaire. We thank Dr Olafur Palsson for answering our queries about his study.
Declaration of personal interests: Vivek C. Goodoory, Cho Ee Ng, Christopher J. Black and Alexander C. Ford: none.
Goodoory VC, Ng CE, Black CJ, Ford AC. Direct healthcare costs of Rome IV or Rome III‐defined irritable bowel syndrome in the United Kingdom. Aliment Pharmacol Ther. 2022;56:110–120. 10.1111/apt.16939
Christopher J. Black and Alexander C. Ford denote joint last author.
The Handling Editor for this article was Dr Colin Howden, and it was accepted for publication after full peer‐review.
Funding informationUnrestricted research monies were provided by Tillotts Pharma UK Ltd. The funder had no input into the concept, design, analysis or reporting of the study.
REFERENCES
- 1. Oka P, Parr H, Barberio B, Black CJ, Savarino EV, Ford AC. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: a systematic review and meta‐analysis. Lancet Gastroenterol Hepatol. 2020;5(10):908–17. [DOI] [PubMed] [Google Scholar]
- 2. Ford AC, Sperber AD, Corsetti M, Camilleri M. Irritable bowel syndrome. Lancet. 2020;396(10263):1675–88. [DOI] [PubMed] [Google Scholar]
- 3. Holtmann GJ, Ford AC, Talley NJ. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol Hepatol. 2016;1(2):133–46. [DOI] [PubMed] [Google Scholar]
- 4. Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, et al. Bowel disorders. Gastroenterology. 2016;150(6):1393–407. [DOI] [PubMed] [Google Scholar]
- 5. Vasant DH, Paine PA, Black CJ, Houghton LA, Everitt HA, Corsetti M, et al. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut. 2021;70(7):1214–40. [DOI] [PubMed] [Google Scholar]
- 6. Hookway C, Buckner S, Crosland P, Longson D. Irritable bowel syndrome in adults in primary care: summary of updated NICE guidance. BMJ. 2015;350:h701. [DOI] [PubMed] [Google Scholar]
- 7. Black CJ, Burr NE, Quigley EMM, Moayyedi P, Houghton LA, Ford AC. Efficacy of secretagogues in patients with irritable bowel syndrome with constipation: systematic review and network meta‐analysis. Gastroenterology. 2018;155(6):1753–63. [DOI] [PubMed] [Google Scholar]
- 8. Black CJ, Burr NE, Camilleri M, Earnest DL, Quigley EM, Moayyedi P, et al. Efficacy of pharmacological therapies in patients with IBS with diarrhoea or mixed stool pattern: systematic review and network meta‐analysis. Gut. 2020;69(1):74–82. [DOI] [PubMed] [Google Scholar]
- 9. Black CJ, Staudacher HM, Ford AC. Efficacy of a low FODMAP diet in irritable bowel syndrome: systematic review and network meta‐analysis. Gut. 2021. 10.1136/gutjnl-2021-325,214 [DOI] [PubMed] [Google Scholar]
- 10. Black CJ, Thakur ER, Houghton LA, Quigley EMM, Moayyedi P, Ford AC. Efficacy of psychological therapies for irritable bowel syndrome: systematic review and network meta‐analysis. Gut. 2020;69(8):1441–51. [DOI] [PubMed] [Google Scholar]
- 11. Black CJ, Yuan Y, Selinger CP, Camilleri M, Quigley EMM, Moayyedi P, et al. Efficacy of soluble fibre, antispasmodic drugs, and gut‐brain neuromodulators in irritable bowel syndrome: a systematic review and network meta‐analysis. Lancet Gastroenterol Hepatol. 2020;5(2):117–31. [DOI] [PubMed] [Google Scholar]
- 12. Ford AC, Forman D, Bailey AG, Axon AT, Moayyedi P. Irritable bowel syndrome: a 10‐yr natural history of symptoms and factors that influence consultation behavior. Am J Gastroenterol. 2008;103(5):1229–39. quiz 40. [DOI] [PubMed] [Google Scholar]
- 13. Pace F, Molteni P, Bollani S, Sarzi‐Puttini P, Stockbrugger R, Bianchi Porro G, et al. Inflammatory bowel disease versus irritable bowel syndrome: a hospital‐based, case–control study of disease impact on quality of life. Scand J Gastroenterol. 2003;38(10):1031–8. [DOI] [PubMed] [Google Scholar]
- 14. Frandemark A, Tornblom H, Jakobsson S, Simren M. Work productivity and activity impairment in irritable bowel syndrome (IBS): a multifaceted problem. Am J Gastroenterol. 2018;113(10):1540–9. [DOI] [PubMed] [Google Scholar]
- 15. Spiegel BM, Farid M, Esrailian E, Talley J, Chang L. Is irritable bowel syndrome a diagnosis of exclusion? A survey of primary care providers, gastroenterologists, and IBS experts. Am J Gastroenterol. 2010;105(4):848–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Flacco ME, Manzoli L, De Giorgio R, Gasbarrini A, Cicchetti A, Bravi F, et al. Costs of irritable bowel syndrome in European countries with universal healthcare coverage: a meta‐analysis. Eur Rev Med Pharmacol Sci. 2019;23(7):2986–3000. [DOI] [PubMed] [Google Scholar]
- 17. Canavan C, West J, Card T. Review article: the economic impact of the irritable bowel syndrome. Aliment Pharmacol Ther. 2014;40(9):1023–34. [DOI] [PubMed] [Google Scholar]
- 18. Inadomi JM, Fennerty MB, Bjorkman D. Systematic review: the economic impact of irritable bowel syndrome. Aliment Pharmacol Ther. 2003;18(7):671–82. [DOI] [PubMed] [Google Scholar]
- 19. Zhang F, Xiang W, Li CY, Li SC. Economic burden of irritable bowel syndrome in China. World J Gastroenterol. 2016;22(47):10450–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Caldwell I, Collins J, Rance M, Dew RM. The Management of Irritable Bowel Syndrome (IBS) in England: a real world study in primary care clinical practice. Value Health. 2014;17(7):A582. [DOI] [PubMed] [Google Scholar]
- 21. Thompson WG, Heaton KW, Smyth GT, Smyth C. Irritable bowel syndrome: the view from general practice. Eur J Gastroenterol Hepatol. 1997;9(7):689–92. [DOI] [PubMed] [Google Scholar]
- 22. Wells NE, Hahn BA, Whorwell PJ. Clinical economics review: irritable bowel syndrome. Aliment Pharmacol Ther. 1997;11(6):1019–30. [DOI] [PubMed] [Google Scholar]
- 23. Akehurst RL, Brazier JE, Mathers N, O’Keefe C, Kaltenthaler E, Morgan A, et al. Health‐related quality of life and cost impact of irritable bowel syndrome in a UK primary care setting. Pharmacoeconomics. 2002;20(7):455–62. [DOI] [PubMed] [Google Scholar]
- 24. Soubieres A, Wilson P, Poullis A, Wilkins J, Rance M. Burden of irritable bowel syndrome in an increasingly cost‐aware National Health Service. Frontline Gastroenterol. 2015;6(4):246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Canavan C, West J, Card T. Calculating Total health service utilisation and costs from routinely collected electronic health records using the example of patients with irritable bowel syndrome before and after their first gastroenterology appointment. Pharmacoeconomics. 2016;34(2):181–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johansson PA, Farup PG, Bracco A, Vandvik PO. How does comorbidity affect cost of health care in patients with irritable bowel syndrome? A cohort study in general practice. BMC Gastroenterol. 2010;10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Le Pen C, Ruszniewski P, Gaudin AF, Amouretti M, Bommelaer G, Frexinos J, et al. The burden cost of French patients suffering from irritable bowel syndrome. Scand J Gastroenterol. 2004;39(4):336–43. [DOI] [PubMed] [Google Scholar]
- 28. Goodoory VC, Ng CE, Black CJ, Ford AC. Willingness to accept risk with medication in return for cure of symptoms among patients with Rome IV irritable bowel syndrome. Aliment Pharmacol Ther. 2022;55:1311–9. 10.1111/apt.16816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Palsson OS, Whitehead WE, van Tilburg MA, Chang L, Chey W, Crowell MD, et al. Rome IV diagnostic questionnaires and tables for investigators and clinicians. Gastroenterology. 2016;150:1481–91. [DOI] [PubMed] [Google Scholar]
- 30. Whitehead WE, and the Validation Working Team Committee in association with the Rome Questionnaire C . Development and validation of the Rome III diagnostic questionnaire. In: Drossman DA, editor. Rome III: The functional gastrointestinal disorders. Virginia: Degnon Associates Inc; 2006. pp. 835–53. [Google Scholar]
- 31. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–91. [DOI] [PubMed] [Google Scholar]
- 32. Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11(2):395–402. [DOI] [PubMed] [Google Scholar]
- 33. Zigmond AS, Snaith RP. The Hospital Anxiety And Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–70. [DOI] [PubMed] [Google Scholar]
- 34. Spiller RC, Humes DJ, Campbell E, Hastings M, Neal KR, Dukes GE, et al. The patient health questionnaire 12 somatic symptom scale as a predictor of symptom severity and consulting behaviour in patients with irritable bowel syndrome and symptomatic diverticular disease. Aliment Pharmacol Ther. 2010;32(6):811–20. [DOI] [PubMed] [Google Scholar]
- 35. Kroenke K, Spitzer RL, Williams JB. The PHQ‐15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64(2):258–66. [DOI] [PubMed] [Google Scholar]
- 36. Labus JS, Bolus R, Chang L, Wiklund I, Naesdal J, Mayer EA, et al. The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom‐specific anxiety scale. Aliment Pharmacol Ther. 2004;20:89–97. [DOI] [PubMed] [Google Scholar]
- 37. Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43(2):400–11. [DOI] [PubMed] [Google Scholar]
- 38. Drossman DA, Patrick DL, Whitehead WE, Toner BB, Diamant NE, Hu Y, et al. Further validation of the IBS‐QOL: a disease‐specific quality‐of‐life questionnaire. Am J Gastroenterol. 2000;95(4):999–1007. [DOI] [PubMed] [Google Scholar]
- 39. Curtis L, Burns A. Unit Costs of Health & Social Care 2020. Unit Costs of Health and Social Care Canterbury: Personal Social Services Research Unit (PSSRU), University of Kent; 2020. [updated January 31, 2021] [cited September 14, 2021]. https://www.pssru.ac.uk/project‐pages/unit‐costs/unit‐costs‐2020/.
- 40. National Health Service (NHS) National cost collection for the NHS [Internet]. England: NHS; 2020 [cited October 15 2021]. https://www.england.nhs.uk/national‐cost‐collection/#ncc1819. [Google Scholar]
- 41. National Institue for Health and Care Excellance (NICE) . British National Formulary (BNF) [Internet]. London: NICE; 2021. [cited October 15, 2021]. 2021. Available from: https://bnf.nice.org.uk. [Google Scholar]
- 42. Palsson OS, Whitehead W, Tornblom H, Sperber AD, Simren M. Prevalence of Rome IV functional bowel disorders among adults in the United States, Canada, and the United Kingdom. Gastroenterology. 2020;158(5):1262–73. e3. [DOI] [PubMed] [Google Scholar]
- 43. United Kingdom Government Age groups [Internet]. London: United Kingdom Government; 2020 [cited January 18 2022]. https://www.ethnicity‐facts‐figures.service.gov.uk/uk‐population‐by‐ethnicity/demographics/age‐groups/latest [Google Scholar]
- 44. Scotland’s Census . Census table data [Internet]. Edinburgh: Scotland’s Census; 2011 [cited January 21, 2022]. 2014; https://www.scotlandscensus.gov.uk/census‐results/download‐data/census‐table‐data/#section2. [Google Scholar]
- 45. Northern Ireland Statistics and Research Agency (NISRA) 2011 census – population tables [Internet]. Belfast: NISRA; 2011 [cited January 21, 2022], 2014. https://www.nisra.gov.uk/publications/2011‐census‐population‐tables. [Google Scholar]
- 46. Black CJ, Craig O, Gracie DJ, Ford AC. Comparison of the Rome IV criteria with the Rome III criteria for the diagnosis of irritable bowel syndrome in secondary care. Gut. 2021;70(6):1110–6. [DOI] [PubMed] [Google Scholar]
- 47. Ford AC, Bercik P, Morgan DG, Bolino C, Pintos‐Sanchez MI, Moayyedi P. Validation of the Rome III criteria for the diagnosis of irritable bowel syndrome in secondary care. Gastroenterology 2013;145(6):1262–70 e1. [DOI] [PubMed] [Google Scholar]
- 48. Andrews EB, Eaton SC, Hollis KA, Hopkins JS, Ameen V, Hamm LR, et al. Prevalence and demographics of irritable bowel syndrome: results from a large web‐based survey. Aliment Pharmacol Ther. 2005;22(10):935–42. [DOI] [PubMed] [Google Scholar]
- 49. Lacy BE, Weiser KT, Kennedy AT, Crowell MD, Talley NJ. Functional dyspepsia: the economic impact to patients. Aliment Pharmacol Ther. 2013;38(2):170–7. [DOI] [PubMed] [Google Scholar]
- 50. Silvernale C, Kuo B, Staller K. Racial disparity in healthcare utilization among patients with irritable bowel syndrome: results from a multicenter cohort. Neurogastroenterol Motil. 2021;33(5):e14039. [DOI] [PubMed] [Google Scholar]
- 51. Clarke PM, Fiebig DG, Gerdtham UG. Optimal recall length in survey design. J Health Econ. 2008;27(5):1275–84. [DOI] [PubMed] [Google Scholar]
- 52. Roberts RO, Bergstralh EJ, Schmidt L, Jacobsen SJ. Comparison of self‐reported and medical record health care utilization measures. J Clin Epidemiol. 1996;49(9):989–95. [DOI] [PubMed] [Google Scholar]
- 53. Lewis A, Torvinen S, Dekhuijzen PN, Chrystyn H, Watson AT, Blackney M, et al. The economic burden of asthma and chronic obstructive pulmonary disease and the impact of poor inhalation technique with commonly prescribed dry powder inhalers in three European countries. BMC Health Serv Res. 2016;16:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Currie CJ, Gale EA, Poole CD. Estimation of primary care treatment costs and treatment efficacy for people with type 1 and type 2 diabetes in the United Kingdom from 1997 to 2007*. Diabet Med. 2010;27(8):938–48. [DOI] [PubMed] [Google Scholar]
- 55. Goodoory VC, Mikocka‐Walus A, Yiannakou Y, Houghton LA, Black CJ, Ford AC. Impact of psychological comorbidity on the prognosis of irritable bowel syndrome. Am J Gastroenterol. 2021;116(7):1485–94. [DOI] [PubMed] [Google Scholar]
- 56. Jerndal P, Ringstrom G, Agerforz P, Karpefors M, Akkermans LM, Bayati A, et al. Gastrointestinal‐specific anxiety: an important factor for severity of GI symptoms and quality of life in IBS. Neurogastroenterol Motil. 2010;22(6):646–e179. [DOI] [PubMed] [Google Scholar]
- 57. Black CJ, Yiannakou Y, Houghton LA, Ford AC. Epidemiological, clinical, and psychological characteristics of individuals with self‐reported irritable bowel syndrome based on the Rome IV vs Rome III criteria. Clin Gastroenterol Hepatol 2020;18(2):392–8 e2. [DOI] [PubMed] [Google Scholar]
- 58. Goodoory VC, Yiannakou Y, Houghton LA, Black CJ, Ford AC. Natural history and disease impact of Rome IV versus Rome III irritable bowel syndrome: a longitudinal follow‐up study. Clin Gastroenterol Hepatol. 2021;20:569–77.e3. 10.1016/j.cgh.2021.04.043 [DOI] [PubMed] [Google Scholar]
- 59. Ford AC, Forman D, Bailey AG, Axon AT, Moayyedi P. Effect of dyspepsia on survival: a longitudinal 10‐year follow‐up study. Am J Gastroenterol. 2012;107(6):912–21. [DOI] [PubMed] [Google Scholar]
- 60. Staller K, Olen O, Soderling J, Roelstraete B, Tornblom H, Khalili H, et al. Mortality risk in irritable bowel syndrome: results from a Nationwide prospective cohort study. Am J Gastroenterol. 2020;115(5):746–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Black CJ, Ford AC. Rational investigations in irritable bowel syndrome. Frontline Gastroenterol. 2020;11(2):140–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. The King’s Fund The NHS budget and how it has changed [Internet]. London: The King’s Fund; 2021 [cited January 27 2022]. 2021. https://www.kingsfund.org.uk/projects/nhs‐in‐a‐nutshell/nhs‐budget. [Google Scholar]
- 63. Public Health Scotland Scottish health service costs [Internet]. Edinburgh: Scottish Health Service Costs; 2021 [cited January 27 2022]. 2021. https://publichealthscotland.scot/publications/scottish‐health‐service‐costs/scottish‐health‐service‐costs‐costsbook‐2020‐april‐2019‐to‐march‐2020/. [Google Scholar]
- 64. Llywodraeth Cymru (Welsh Government) , NHS expenditure programme budgets: April 2019 to March 2020 [Cardiff]: Llywodraeth Cymru (Welsh Government); 2021. [cited January 27 2022]. 2021. https://gov.wales/nhs‐expenditure‐programme‐budgets‐april‐2019‐march‐2020.
- 65. Health and Social Care Board . 2019/20 Annual report & Accounts. [Internet]. Belfast: Health and Social Care Board; 2020 [cited January 27, 2022]. http://www.hscboard.hscni.net/download/PUBLICATIONS/CORPORATEANDFINANCIAL/Annual‐Report‐and‐Accounts‐2019‐2020.pdf. [Google Scholar]
- 66. Begtrup LM, Engsbro AL, Kjeldsen J, Larsen PV. Schaffalitzky de Muckadell O, Bytzer P, et al. a positive diagnostic strategy is noninferior to a strategy of exclusion for patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2013;11(8):956–62. e1. [DOI] [PubMed] [Google Scholar]
- 67. Drossman DA. 2012 David sun lecture: helping your patient by helping yourself—how to improve the patient‐physician relationship by optimizing communication skills. Am J Gastroenterol. 2013;108(4):521–8. [DOI] [PubMed] [Google Scholar]
- 68. Drossman DA, Chang L, Deutsch JK, Ford AC, Halpert A, Kroenke K, et al. A review of the evidence and recommendations on communication skills and the patient‐provider relationship: a Rome foundation working team report. Gastroenterology. 2021;161(5):1670–88. e7. [DOI] [PubMed] [Google Scholar]
- 69. Basnayake C, Kamm MA, Stanley A, Wilson‐O’Brien A, Burrell K, Lees‐Trinca I, et al. Standard gastroenterologist versus multidisciplinary treatment for functional gastrointestinal disorders (MANTRA): an open‐label, single‐Centre, randomised controlled trial. Lancet Gastroenterol Hepatol. 2020;5(10):890–9. [DOI] [PubMed] [Google Scholar]
- 70. Basnayake C, Kamm MA, Stanley A, Wilson‐O’Brien A, Burrell K, Lees‐Trinca I, et al. Long‐term outcome of multidisciplinary versus standard gastroenterologist care for functional gastrointestinal disorders: a randomized trial. Clin Gastroenterol Hepatol. 2021. 10.1016/j.cgh.2021.12.005 [DOI] [PubMed] [Google Scholar]
- 71. The Lancet Gastroenterology H . Unmet needs of patients with irritable bowel syndrome. Lancet Gastroenterol Hepatol. 2018;3(9):587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1


