Abstract
Introduction: Rewarming from accidental hypothermia is often complicated by hypothermia-induced cardiovascular dysfunction, which could lead to shock. Current guidelines do not recommend any pharmacological treatment at core temperatures below 30°C, due to lack of knowledge. However, previous in vivo studies have shown promising results when using phosphodiesterase 3 (PDE3) inhibitors, which possess the combined effects of supporting cardiac function and alleviating the peripheral vascular resistance through changes in cyclic nucleotide levels. This study therefore aims to investigate whether PDE3 inhibitors milrinone, amrinone, and levosimendan are able to modulate cyclic nucleotide regulation in hypothermic settings.
Materials and methods: The effect of PDE3 inhibitors were studied by using recombinant phosphodiesterase enzymes and inverted erythrocyte membranes at six different temperatures—37°C, 34°C, 32°C, 28°C, 24°C, and 20°C- in order to evaluate the degree of enzymatic degradation, as well as measuring cellular efflux of both cAMP and cGMP. The resulting dose-response curves at every temperature were used to calculate IC50 and Ki values.
Results: Milrinone IC50 and Ki values for cGMP efflux were significantly lower at 24°C (IC50: 8.62 ± 2.69 µM) and 20°C (IC50: 7.35 ± 3.51 µM), compared to 37°C (IC50: 22.84 ± 1.52 µM). There were no significant changes in IC50 and Ki values for enzymatic breakdown of cAMP and cGMP.
Conclusion: Milrinone, amrinone and levosimendan, were all able to suppress enzymatic degradation and inhibit extrusion of cGMP and cAMP below 30°C. Our results show that these drugs have preserved effect on their target molecules during hypothermia, indicating that they could provide an important treatment option for hypothermia-induced cardiac dysfunction.
Keywords: hypothermia, phosphodiesterase 3 inhibitor, phosphodiesterase 3, ATP-binding cassette transporter, cyclic AMP, cyclic GMP, cardiovascular dysfunction
1 Introduction
Cold-related deaths have been recognized for several hundred years but it was not until late 19th century that hypothermia was defined, when clinical thermometry was made available (Guly, 2011). Today, accidental hypothermia is defined as an unintentional drop in core temperature below 35°C (Brown et al., 2012; Musi et al., 2021). Further, accidental hypothermia is classified in four different stages by The European Resuscitation Council -stage I—mild hypothermia (35°C–32°C), stage II—moderate hypothermia (32°C–28°C), stage III—severe hypothermia (core temperature below 28°C), and stage IV—severe hypothermia with no apparent vital signs (core temperature variable) (Lott et al., 2021). Mortality rates approach 40% in severe (<28°C) accidental hypothermia (Vassal et al., 2001; Van der Ploeg et al., 2010). One of the conditions that contribute to the high mortality is hypothermia-induced cardiac dysfunction (HCD) (Han et al., 2010), which is characterized by a decrease in cardiac output, in combination with profound increase in systemic vascular resistance (SVR) when rewarming patients. In worst case scenario, acute cardiovascular failure, so called rewarming shock, ensues (Blair, Montgomery and Swan, 1956; Tveita et al., 1996).
Various cardiovascular drugs used to treat heart failure and control blood pressure, target cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP). These are intracellular second messengers that are involved in numerous important processes, such as modulating cardiac contraction and vasoregulation. Intracellular levels of these cyclic nucleotides are regulated by the rate of synthesis and degradation by various phosphodiesterases and cellular efflux by transmembrane transporter proteins. Phosphodiesterase 3A (PDE3A), which is especially active in the cardiovascular system, is the main enzyme responsible for degrading cAMP. PDE5A, which resides abundantly in smooth muscle cells in the vascular system, metabolizes primarily cGMP (Omori and Kotera, 2007; Ya et al., 2016). cAMP and cGMP levels are also regulated through active transportation facilitated by transmembrane proteins. These ATP-binding cassette (ABC) transporters are one of the largest transport protein superfamilies (Vasiliou, Vasiliou and Nebert, 2009). ABCC4, which is present in many tissues, transports cAMP out of the cell and has an additional transport site with high Km for both cAMP and cGMP. ABCC5 transports cGMP with high affinity and cAMP with low affinity (Sager and Ravna, 2009).
Well-known β-receptor agonists, such as epinephrine and isoproterenol, increase cAMP levels through the adenylyl cyclase (AC) pathway. Studies in a rat model of severe hypothermia suggest that these drugs increase SVR without simultaneous positive inotropic effect (Dietrichs, Sager and Tveita, 2016) but species-dependent differences exist (Mohyuddin et al., 2021). More promisingly, in vivo studies performed in rat models, have demonstrated that PDE3 inhibitors, such as milrinone and levosimendan, have positive effects on stroke volume and cardiac output, almost restoring these parameters to baseline levels during rewarming (Detrichstrichs et al., 2014a; Dettrichstrichs et al., 2014b). Their mechanism of action is through inhibiting cyclic nucleotide breakdown, in contrast to the adrenergic agents, which stimulate their synthesis (Movsesian, 2016). The additional and positive effect of vasodilation by PDE3-inhibitors that was observed in these studies, have prompted research where sodium nitroprusside, a widely known nitric oxide (NO) donor, was studied in the same rat model. The results demonstrate clearly that reducing SVR, without directly supporting the heart, lead to a significant increase in cardiac output (Håheim et al., 2017). These findings indicate that, to treat HCD, it could be favourable to support the failing heart through an alternative route to that of the β-adrenoceptor agonists, and also promote attenuation of SVR. Thus, in the present study we wanted to investigate how PDE3-inhibitors affect cyclic nucleotide regulation in human cells during hypothermia, to improve treatment of severely hypothermic patients.
2 Materials and Methods
2.1 Temperature
The chosen temperatures of this study were selected to represent the full spectrum of severity, including normothermia; 37–34—32–28—24–20°C. Temperature was maintained at the chosen level by using a Grant Optima T100 heated circulating bath (Grant Instruments LTD., Shepreth, England). Separate experiments were carried out for all included temperatures and parallels. Exposure to the selected temperature lasted for 30 min.
2.2 Pharmaceuticals
Amrinone (Sigma-Aldrich, Steinheim, Germany), milrinone (United States Pharmacopeia (USP) Reference Standard, Rockville, United States), and levosimendan (Sigma-Aldrich, Steinheim, Germany) were used in seven different concentrations throughout the study, increasing by a factor 10 and ranging from 1.00E-09 to 1.00E-03 M (1 nM–1 mM), respectively.
2.3 Cells
Erythrocytes were chosen for the evaluation of intracellular access of the three drugs as well as cellular extrusion of cyclic nucleotides during hypothermia, since they contain both ABCC4 and ABCC5 transport molecules in the cell membrane (Köck et al., 2007). Blood was provided by Blodbanken (Department of Immunohematology and Transfusion Medicine, University Hospital of North Norway) where all participants were pre-screened and only admitted as donors if they were healthy. The blood was consistently collected from randomly assigned, pre-screened healthy blood donors. As described in our recent publication from the same project (Selli et al., 2021), the regional ethical committee found that ethical review and approval was not required for this study, as the study was performed according to local legislation and institutional requirements included in our agreement with Department of Immunohematology and Transfusion Medicine, University Hospital of North Norway. The participants provided their written informed consent to contribute before sampling at Blodbanken, and we only received anonymized blood samples for the experiments.
2.4 Experimental Protocols
Three independent experiments were conducted to determine the degree of intracellular access of the substances and cyclic nucleotide turnover. Each medication and control solution were tested in triplicates and at three independent experiments for each temperature. The study protocol has been described previously by our research group (Selli et al., 2021).
2.4.1 Intracellular Access
Fresh (<24 h) EDTA blood from healthy donors (n = 18) was obtained and washed with Krebs-Ringer-Phosphate-Buffer containing glucose (KRPB/G, pH 7.4) and centrifuged (10 min, 1,000 g) three consecutive times. Plasma and buffy coat were removed. KRPB/G was added to the cell solution to bring it to the ratio 1:2.5. Hematocrit (Hct) was measured and adjusted with additional buffer to reach 0.44, in order to give the final Hct of 0.40 in the incubation solution. 500 µl cell suspension (Hct 0.44) were added to test tubes, along with 50 µl of either milrinone (final concentration 10 µM), amrinone (final concentration 100 µM), levosimendan (final concentration 1 µM) or MQ-water (negative control) and incubated for 30 min in the chosen temperature. The reaction was stopped by placing the test tubes on ice and adding 4 ml of ice cold KRFB/G. The samples were subsequently centrifugated (5 min, 600 g), then washed with KRFB/G once more and centrifugation was repeated twice. 50 µl of the remaining red blood cell suspension was transferred to Eppendorf tubes. 50 µl 500 nM IS-Milrinone-d3 was added as an internal standard (TLC Pharmaceutical Standards Ltd.). 200 µl ZnSO4 was added to each test sample, to induce lysis of the cells, and mixed in a vortex mixer. 30 µl of the samples were used for measurement of protein concentration, the rest was mixed with 500 µl acetonitrile and centrifugated (2 min, 13400 g). Finally, 100 µl of the solution was collected for analysis using mass spectrometry (MS).
2.4.2 Cellular Efflux
The transport assay was performed using so called inside-out vesicles (IOVs), which were prepared from erythrocytes by using a modified version of the Steck IOV preparation (Steck, 1974). Freshly collected EDTA blood from healthy donors (n = 35) was prepared at 0°C–4°C, starting by separating the erythrocytes from plasma by centrifugation at 2,300 g for approximately 15 min. Plasma and buffy were disposed of and the remaining cells washed three times with 5 mM Tris- HCl and 113 mM KCl (pH 8.1), and subsequently centrifugated at 1,000 g. Lysis followed thereafter, with ten volumes of 5 mM Tris-HCl, 0.5 mM EGTA, 4 mM KCl (pH 8.1) and washed by repeated centrifugation at 20,000 g for 20 min and resuspension in the same buffer. Vesiculation was initiated by adding 39 volumes of a hypertonic buffer (0.5 µM Tris-HCl, pH 8.2) to one volume of cell suspension and completed by forcing the solution five times through a 27-gauge syringe needle to promote homogenization of the membranes. The resulting IOVs were separated from the right-side vesicles and ghosts by ultracentrifugation (100,000 g) overnight, using a density gradient, ranging from 1.048 g/ml to 1.146 g/ml (Nycodenz, Axis-Shield PoC, Oslo, Norway) in 5 mM Tris, 3 mM KCl, and 0.3 mM, EGTA. This procedure gathered the IOVs in the uppermost band, which was collected, washed and resuspended in 1.47 mM KH2PO4, 81 mM K2KPO4 and 140 mM KCl (pH 7.6). Sidedness was verified by using the acetylcholinesterase accessibility test (Ellman, 1961). The resulting IOVs, which still contained ABC-transporters in the membrane, enabled us to control the intracellular environment, in this case the surrounding medium, and collect the cyclic nucleotides within. Thus, IOVs were subsequently incubated for 60 min at the designated temperature, with or without 2.0 mM ATP, in the following mixture: 20 mM Tris-HCl, 10 mM MgCl2, 1 mM EGTA, 121 mM KCl. Radioactive labeled (3H)-cAMP or (3H)-cGMP (Perkin Elmer, Boston, MA, United States) were added to the incubation solutions in the concentration 20 and 2 μM, respectively, depending on the transporter examined, together with the appointed study drug in concentrations up to 1 mM. The assay was terminated by adding ice cold buffer (<4°C), containing 1.47 mM KH2PO4, 8.1 mM K2HPO4, and 140 mM KCl (pH 7.6). Next step was to collect the IOVs, which was done by filtration through a nitrocellulose membrane (Bio-Rad Laboratories, Feldkirchen, Germany), and then drying it. The resulting collection of radioactivity upon the filter, was later quantified by using a Packard TopCount NXT (Packard, Downers Grove, IL, United states) after adding scintillation fluid (MicroScint-O, PerkinElmer, Groningen, Netherlands).
2.4.3 Phosphodiesterase Assay
The study drugs were tested upon their ability to hinder cAMP and cGMP hydrolysis by inhibiting PDE3 and PDE5 respectively. Either 5 µM cAMP or cGMP (Sigma-Aldrich, St. Louis, MO, United States) were used as substrates for the enzymes. The reaction was initiated by adding PDE to the assay solution in designated Eppendorf tubes, containing fresh incubation buffer (10 mM Tris, 8.2 mM Propionic acid, 3 mM Magnesium acetate, 1.5 mM EGTA and 0.5 mg/ml BSA, 0.2 mM DTT) selected substrate (cAMP or cGMP) and inhibitor. The reaction was initiated by adding 0.016 units/µl incubate of PDE3 (Abcam, Cambridge, United Kingdom), or 0.022 units/µl incubate of PDE5 (Sigma-Aldrich, St. Louis, United States). Control samples were free of drug and were either with or without PDE. The incubation time was 30 min in the selected temperature. Reaction was stopped by adding 99.9% methanol to the solutions. Internal standards of cGMP-13C5, cAMP-13C5, AMP-13C5 (Toronto Research Chemicals Inc., Ontario, Canada) and GMP-15N5 (Sigma-Aldrich, St. Louis, MO, United States) were added to each sample, before MS analysis.
2.5 Mass Spectrometry Analysis
Liquid chromatography tandem mass spectrometry (LC-MS) was used to assess concentrations of the inhibitors for experiments performed to determine intracellular access of these drugs, as well as to quantify levels of cAMP/AMP and cGMP/GMP for the PDE3-and PDE5-inhibition plots. Internal standards were used, as described previously. There was a linearity from 0.2 nM to at least 2000 nM (r2 > 0.998) for cAMP, AMP, and cGMP. For GMP, linearity was present from 2 nM to at least 2000 nM (r2 > 0.998). Concerning PDE3-inhibitors, the linearity was found at 10 nM to at least 5,000 nM (r2 > 0.99). Lower limit of quantification (LLOQ) was 0.2 for cAMP, cGMP, and AMP, 2 nM for GMP and 10 nM for PDE3-inhibitors (2 µl injection volume).
2.6 Bioactivity
IC50 and Ki values were calculated for each of the drugs, both for their ability to inhibit cAMP- and cGMP- efflux, as well as PDE3 and PDE5 activity. IC50 values were derived according to Chou (1976), and Ki values according to Cheng and Prusoff (1973). Inhibition curves were created accordingly, both with and without adjustment for normothermic control.
2.7 Statistical Analysis
Intracellular concentrations of drugs were adjusted for protein concentrations in each sample. The total incubation concentrations were also adjusted for protein concentrations to evaluate the degree of access in percentage. Statistical analysis was performed using one-way ANOVA with Holm-Sidak multiple comparisons post hoc test at each temperature. ANOVA on ranks with Dunn post hoc test was used when the results were not normally distributed. The results are presented as means ± standard error of mean (SEM). p-values < 0.05 were considered significant results. Degrees of freedom (dF) is given as between group values. SigmaPlot 14.0 (Systat Software, San Jose, CA, United States) was used for all analysis, as well as creating the graphs.
3 Results
3.1 Intracellular Access
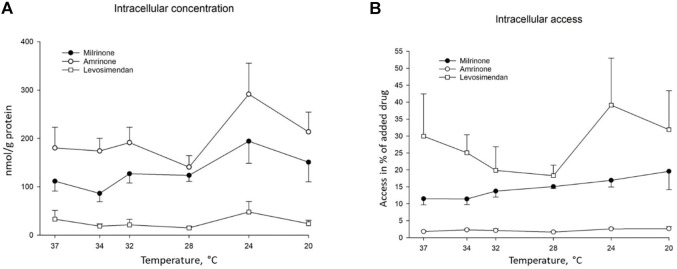
After 30 min of incubation, all study drugs were found in the IOVs at all temperatures. Decreasing the temperature from 37°C to 20°C did not significantly change their ability to reach their intracellular site of action (Figure 1). A higher percentage of levosimendan entered the cells compared to milrinone and amrinone at 34°C (25.06 ± 5.289 vs. 11.41 ± 1.675, ANOVA, dF = 2, F = 12.755, p = 0.047, and 2.303 ± 0.284, ANOVA, dF = 2, F = 12.755, p = 0.007), as well as at 28°C compared to amrinone (18.29 ± 3.122 vs. 1.66 ± 0.216, ANOVA, dF = 2, F = 23.133, p = 0.002). The degree of intracellular access was also significantly higher for milrinone, compared to amrinone, at 28°C (15.06 ± 0.544 vs. 1.66 ± 0.216, ANOVA, dF = 2, F = 23.133, p = 0.004).
FIGURE 1.
Temperature-dependent intracellular access of added milrinone, amrinone, and levosimendan, presented as means ± SEM (A) Intracellular concentration of milrinone, amrinone, and levosimendan in nmol/g protein at temperatures ranging from 37°C to 20°C, n = 3. (B) Intracellular access of milrinone, amrinone, and levosimendan in % of drug concentration per gram protein, compared to drug concentration per gram protein in the incubation solution at temperatures ranging from 37°C to 20°C, n = 3.
3.2 Intracellular Elimination by Phosphodiesterase
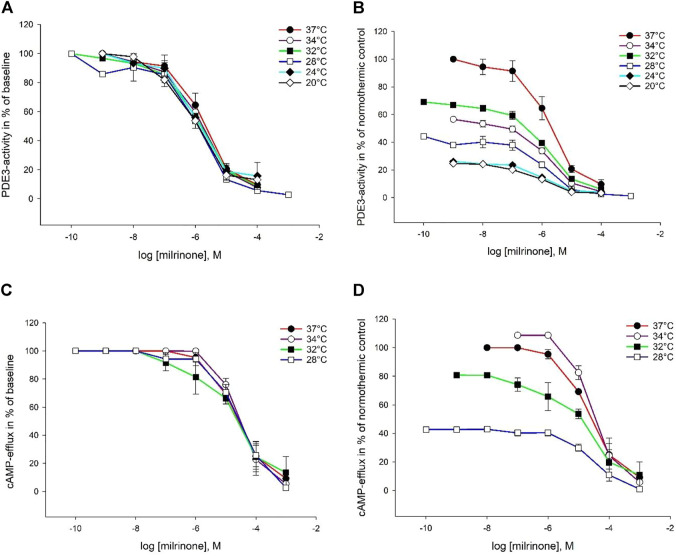
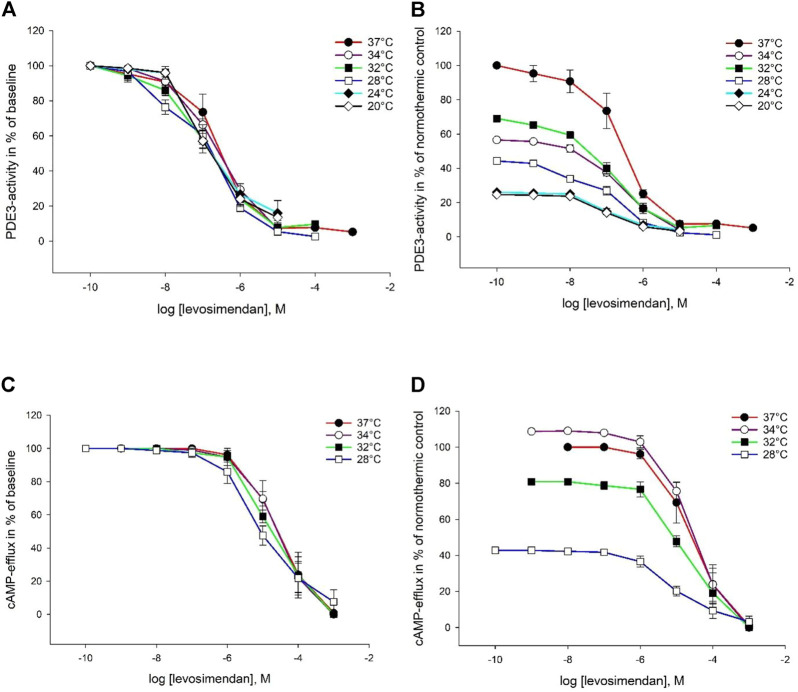
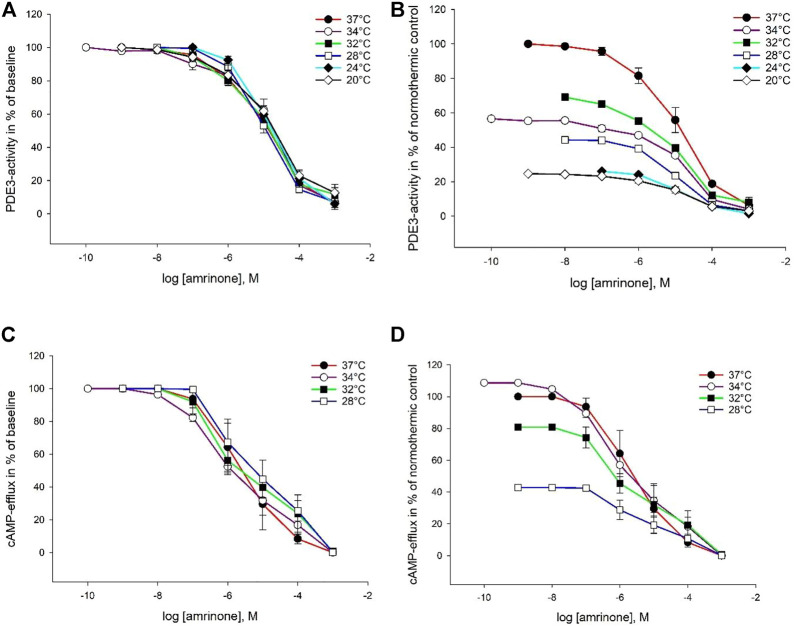
Milrinone, amrinone, and levosimendan were all able to inhibit both PDE enzymes at all six temperatures. Inhibition plots for PDE3 are depicted in Figures 2–4. IC50 and Ki values for PDE3 inhibition were not significantly different at hypothermia and the same was true for PDE5 inhibition. IC50 and Ki values for PDE3 inhibition by amrinone were significantly higher, regardless of temperature, compared to levosimendan (Table 2). At 34°C and 32°C, there were significant differences in values between amrinone and milrinone (IC50: 9.863 ± 1.709 µM vs. 1.771 ± 0.716 µM, ANOVA, dF = 2, F = 23.056, p = 0.004 and 15.07 ± 1.855 µM vs. 1.302 ± 0.357 µM, ANOVA, dF = 2, F = 56.783, p < 0.001). For PDE5 inhibition, milrinone had consistently higher IC50 and Ki values, compared to amrinone (Table 1), and they were also significantly higher at 24°C and 20°C, compared to levosimendan (IC50: 253 ± 32.92 µM vs. 148 ± 26.12 µM, ANOVA, dF = 2, F = 10.394, p = 0.045 and 330 ± 58.11 µM vs. 157 ± 27.45 µM, ANOVA, dF = 2, F = 9.906, p = 0.034).
FIGURE 2.
Temperature-dependent inhibition of PDE3 and cAMP-efflux by milrinone. The doses are in logarithm of the concentration in mol/L, presented as means ± SEM. (A) Inhibition curves for PDE3-activity at temperatures ranging from 37°C to 20°C. (B) Inhibition curves for PDE3-activity in % of normothermic inhibition curve at temperatures ranging from 37°C to 20°C. (C) Inhibition curves for ABCC4-activity at temperatures ranging from 37°C to 20°C. (D) Inhibition curves for ABCC4-activity in % of normothermic inhibition curve at temperatures ranging from 37°C to 20°C.
FIGURE 4.
Temperature-dependent inhibition of PDE3 and cAMP-efflux by levosimendan. The does are in logarithm of the concentration in mol/L, presented as means ± SEM. (A) Inhibition curves for PDE3-activity at temperatures ranging from 37°C to 20°C. (B) Inhibition curves for PDE3-activity in % of normothermic inhibition curve at temperatures ranging from 37°C to 20°C. (C) Inhibition curves for ABCC4-activity at temperatures ranging from 37°C to 20°C. (D) Inhibition curves for ABCC4-activity in % of normothermic inhibition curve at temperatures ranging from 37°C to 20°C.
TABLE 2.
IC50 and Ki values for inhibition of PDE3, PDE5, ABCC4 and ABCC5 at temperatures ranging from 37°C to 20°C.
| Amrinone | ||||||||
|---|---|---|---|---|---|---|---|---|
| PDE3 | PDE5 | ABCC4 | ABCC5 | |||||
| Temperature | IC50 | Ki | IC50 | Ki | IC50 | Ki | IC50 | Ki |
| 37°C | 10.55 ± 3.53● | 0.48 ± 0.16● | 110 ± 12.80 | 27.8 ± 3.25 | 4.65 ± 3.21* | 2.82 ± 1,946* | 2.20 ± 0.70 | 1.24 ± 0.39 |
| 34°C | 9.86 ± 1.71●* | 0.45 ± 0.078●* | 91.9 ± 3.65 | 23.3 ± 0.93 | 1.21 ± 0.10* | 0.73 ± 0.058* | 2.43 ± 0.75●* | 1.37 ± 0.43●* |
| 32°C | 15.07 ± 1.86●* | 0.69 ± 0.085●* | 84.5 ± 5.94 | 21.5 ± 1.51 | 5.49 ± 2.25 | 3.33 ± 1,362 | 1.75 ± 0.61* | 0.99 ± 0.35* |
| 28°C | 15.68 ± 2.25● | 0.72 ± 0.103● | 95.0 ± 7.29 | 24.1 ± 1.85 | 8.42 ± 4.81 | 5.11 ± 2.91 | 1.10 ± 0.33●* | 0.62 ± 0.18●* |
| 24°C | 24.87 ± 5.05● | 1.14 ± 0.23● | 99.1 ± 4.20 | 25.1 ± 1.07 | - | 1.10 ± 0.51 | 0.62 ± 0.29 | |
| 20°C | 26.44 ± 15.73● | 1.21 ± 0.72● | 105 ± 8.04 | 26.7 ± 2.04 | - | 5.44 ± 2.68 | 3.08 ± 1.52 | |
#Significant difference (p-value < 0.05) when compared to normothermic control.
*Significant difference (p < 0.05) when compared to milrinone.
•Significant difference (p < 0.05) when compared to levosimendan.
Values are presented as means ± SEM and given in µM.
TABLE 1.
IC50 and Ki values for inhibition of PDE3, PDE5, ABCC4, and ABCC5 at temperatures ranging from 37°C to 20°C.
| Milrinone | ||||||||
|---|---|---|---|---|---|---|---|---|
| PDE3 | PDE5 | ABCC4 | ABCC5 | |||||
| Temperature (°C) | IC50 | Ki | IC50 | Ki | IC50 | Ki | IC50 | Ki |
| 37 | 2.99 ± 1.28 | 0.14 ± 0.059 | 336 ± 58.60‡ | 85.3 ± 14.87‡ | 58.9 ± 13.09‡● | 35.69 ± 7.94 ‡● | 22.8 ± 1.52 | 12.91 ± 0.86 |
| 34 | 1.77 ± 0.72 ‡ | 0.081 ± 0.033‡ | 255 ± 29.84‡ | 64.6 ± 7.57‡ | 67.0 ± 22.65‡ | 40.65 ± 13.73‡ | 17,8E ± 1.73‡ | 10.04 ± 0.96‡ |
| 32 | 1.30 ± 0.36‡ | 0.060 ± 0.016‡ | 280 ± 48.33‡ | 70.9 ± 12.26‡ | 32.9 ± 14.61 | 19.95 ± 8.86 | 17.2 ± 3.02‡ | 9.70 ± 1.71‡ |
| 28 | 0.82 ± 0.31 | 0.038 ± 0.014 | 305 ± 72.47‡ | 77.4 ± 18.39‡ | 37.4 ± 24.52 | 22.67 ± 14.86 | 18.2 ± 2.03‡● | 10.26 ± 1.15‡● |
| 24 | 1.91 ± 0.73 | 0.087 ± 0.034 | 253 ± 32.92‡● | 64.2 ± 8.35‡● | - | 8.62 ± 2.69# | 4.87 ± 1.52# | |
| 20 | 2.15 ± 0.65 | 0.099 ± 0.030 | 330 ± 58.11‡● | 83.8 ± 14.75‡● | - | 7.35 ± 3.51# | 4.16 ± 1.98# | |
#Significant difference (p-value < 0.05) when compared to normothermic control.
‡Significant difference (p < 0.05) when compared to amrinone.
●Significant difference (p < 0.05) when compared to levosimendan.
Values are presented as means ± SEM and given in µM.
FIGURE 3.
Temperature-dependent inhibition of PDE3 and cAMP-efflux by amrinone. The doses are in logarithm of the concentration in mol/L, presented as means ± SEM. (A) Inhibition curves for PDE3-activity at temperatures ranging from 37°C to 20°C. (B) Inhibition curves for PDE3-activity in % of normothermic inhibition curve at temperatures ranging from 37°C to 20°C. (C) Inhibition curves for ABCC4-activity at temperatures ranging from 37°C to 20°C. (D) Inhibition curves for ABCC4-activity in % of normothermic inhibition curve at temperatures ranging from 37°C to 20°C.
3.3 Cellular Efflux
The IC50 and Ki values for inhibition of cyclic nucleotide extrusion showed no significant differences between 37°C and 28°C for all PDE3 inhibitors. At 24°C and 20°C, however, neither of the study drugs were able to inhibit cAMP-efflux. Milrinone had significantly higher capacity to inhibit cGMP-efflux at 24°C and 20°C, compared to normothermia (IC50: 22.8 ± 1.52 µM compared to 8.62 ± 2.69 µM, ANOVA, dF = 5, F = 5.762, p = 0.025 and 7.35 ± 3.51 µM, ANOVA, dF = 5, F = 5.762, p = 0.014) (Table 1). Amrinone had significantly lower IC50 and Ki values at 37°C–34°C for cAMP expulsion and at 34°C–28°C for cGMP-efflux, compared to milrinone (Table 2). Levosimendan had significantly higher values at 34°C and 28°C, compared to amrinone, for cGMP-extrusion (Table 3).
TABLE 3.
IC50 and Ki values for inhibition of PDE3, PDE5, ABCC4, and ABCC5 at temperatures ranging from 37°C to 20°C.
| Levosimendan | ||||||||
|---|---|---|---|---|---|---|---|---|
| PDE3 | PDE5 | ABCC5 | ABCC5 | |||||
| Temperature | IC50 | Ki | IC50 | Ki | IC50 | Ki | IC50 | Ki |
| 37°C | 0.33 ± 0.10‡ | 0.015 ± 0.00‡ | 155 ± 4.74 | 39.2 ± 1.20 | 9.41 ± 5.91* | 5.71 ± 3.58* | 28.5 ± 10.29 | 16.1 ± 5.82 |
| 34°C | 0.29 ± 0.067‡ | 0.013 ± 0.00‡ | 150 ± 13.45 | 38.0 ± 3.41 | 12.7 ± 6.60 | 7.73 ± 4.00 | 13.9 ± 1.72‡ | 7.86 ± 0.97‡ |
| 32°C | 0.19 ± 0.060‡ | 0 0088 ± 0.00‡ | 162 ± 22.51 | 41.2 ± 5.71 | 16.5 ± 5.56 | 10.0 ± 3.37 | 13.2 ± 3.99 | 7.45 ± 2.25 |
| 28°C | 0.101 ± 0.026‡ | 0.0046 ± 0.00‡ | 183 ± 23.04 | 46.4 ± 5.85 | 27.2 ± 21.34 | 16.5 ± 12.94 | 11.9 ± 1.35‡* | 6.74 ± 0.76‡* |
| 24°C | 0.35 ± 0.21‡ | 0.016 ± 0.01‡ | 148 ± 26.12* | 37.6 ± 6.63* | - | 18.4 ± 12.73 | 10.4 ± 7.20 | |
| 20°C | 0.35 ± 0.200‡ | 0.016 ± 0.01‡ | 157 ± 27.45* | 39.9 ± 6.97* | - | 7.15 ± 1.57 | 4.04 ± 0.89 | |
#Significant difference (p-value < 0.05) when compared to normothermic control.
*Significant difference (p < 0.05) when compared to milrinone.
‡Significant difference (p < 0.05) when compared to amrinone.
Values are presented as means ± SEM and given in µM.
3.4 Drug Selectivity
3.4.1 Phosphodiesterase Enzymes
Calculated IC50 ratios for the phosphodiesterase enzymes showed that notably higher concentrations of all three drugs were needed to inhibit PDE5, compared to PDE3, during normothermia. The same trend was seen at all studied temperatures and appeared to be accentuated for levosimendan from 32°C to 28°C where the IC50 ratio for PDE5/PDE3 inhibition doubled from 846 to 1820. Amrinone was the least selective drug with ratios spanning from 10.38 (37°C) to 3.98 (20°C) (Table 4).
TABLE 4.
Drug selectivity for milrinone (M), amrinone (A), and levosimendan (L) at temperatures ranging from 37°C to 20°C. Values are ratios between IC50-values for different elimination ways of cAMP and cGMP. Neither of the drugs inhibited ABCC4-activity below 28°C.
| Drug selectivity (IC50)/(IC50) | (PDE5)/(PDE3) inhibition | (cGMP-efflux)/(cAMP-efflux) inhibition | (cAMP-efflux)/(PDE3) inhibition | (cGMP-efflux)/(PDE5) inhibition | ||||||||
| Temperature | M | A | L | M | A | L | M | A | L | M | A | L |
| 37°C | 112 | 10.38 | 472 | 0.39 | 0.47 | 3.03 | 19.66 | 0.441 | 28.76 | 0.068 | 0.020 | 0.184 |
| 34°C | 144 | 9.32 | 510 | 0.27 | 2.01 | 1.09 | 37.86 | 0.123 | 43.45 | 0.070 | 0.026 | 0.093 |
| 32°C | 215 | 5.61 | 846 | 0.52 | 0.32 | 0.80 | 25.27 | 0.364 | 85.98 | 0.061 | 0.021 | 0.081 |
| 28°C | 370 | 6.06 | 1820 | 0.49 | 0.13 | 0.44 | 45.40 | 0.537 | 270.7 | 0.060 | 0.012 | 0.065 |
| 24°C | 133 | 3.98 | 427 | - | - | - | - | - | - | 0.034 | 0.011 | 0.124 |
| 20°C | 154 | 3.98 | 450 | - | - | - | - | - | - | 0.022 | 0.052 | 0.045 |
3.4.2 Cyclic Nucleotide Efflux
Concerning drug selectivity for cGMP/cAMP efflux, milrinone and amrinone appeared to inhibit cGMP extrusion at lower concentrations during normothermic conditions (ratio of 0.39 and 0.47, respectively). Inhibition ratio for levosimendan (3.03) indicated an opposite tendency, but when the temperature decreased, the ratio switched to 0.44 at 28°C.
3.4.3 Cyclic Adenosine Monophosphate Elimination
Higher concentrations (IC50) were needed to inhibit ABCC4 compared to PDE3, for milrinone (ratio of 19.66) and levosimendan (28.76) at 37°C. cAMP efflux/PDE3 ratio almost doubled at 34°C for milrinone (37.86) and increased steadily for levosimendan as the temperature fell (270.65 at 28°C). Higher IC50 values were consistently registered for inhibiting PDE3-activity by amrinone, compared to ABCC4-function, reflected in an IC50-ratio <1 at all temperatures.
3.4.4 Cyclic Guanosine Monophosphate Elimination
The calculated IC50 ratios indicated higher selectivity towards cGMP efflux inhibition than PDE5-mediated cGMP elimination for all medications at all temperatures.
4 Discussion
The present study shows that both levosimendan, milrinone and amrinone are able to reduce cAMP elimination during cooling to severe hypothermia (20°C). All drugs had intact, inhibitory effect on PDE3 and we observed no pharmacodynamic shift towards more potent effect on cGMP-metabolism relative to cAMP metabolism during hypothermic conditions. Our findings therefore suggest that PDE3-inhibition is a promising target to treat and prevent HCD, already from temperatures down to 20°C in severely hypothermic patients.
Current European guidelines for treating accidental hypothermia, do not recommend use of vasoactive drugs in patients with core temperatures below 30°C. As a result, hypothermic cardiac arrest patients should be treated according to the standard advanced life support (ALS) algorithm, but without any pharmacological aid. Once the core temperature reaches >30°C, it is recommended that adrenaline is administered every 6–10 min (instead of 3–5 min, as in normothermic conditions). When the patient is no longer hypothermic, ALS algorithm is followed as originally intended (Lott et al., 2021). In prehospital settings, the focus is to extricate and impede further cooling. Patients with signs of cardiovascular instability, cardiac arrest, or core temperatures <30°C, are transported directly to a hospital with stand-by extracorporeal life support (ECLS) for active rewarming. Remaining patients are triaged to the nearest, appropriate hospital for passive and/or minimally invasive active rewarming (Lott et al., 2021; Paal et al., 2022).
In accidental hypothermic patients, one pharmacological strategy to prevent or treat HCD is to elevate cardiac contractility (Dietrichs, Sager and Tveita, 2016). Amrinone, a PDE3-inhibitor, that is, also an important precursor for so-called novel cardiotonic agents, providing positive inotropic effect, has been used intravenously in short-term management of cardiac heart failure (Endoh, 2013). The same pharmacological mechanisms are displayed by milrinone, although it is 30 times more potent than amrinone and it is commonly used in intensive care units as a mean to treat cardiogenic shock (Young and Ward, 1988; Mathew et al., 2021). Levosimendan acts as a PDE3 inhibitor at higher concentrations (>0.3 µM). It is also being used for managing acutely decompensated congestive heart failure (Pathak et al., 2013). Being well-known and widely distributed, these three medications can provide means to treat hemodynamically unstable patients in prehospital settings, especially if the transfer time to nearest ECLS centre is several hours. Nations worldwide without ECLS-facilities are inherently at a disadvantage but can make use of treatment protocols with minimally invasive and cheap pharmacological therapy. Additionally, our findings could also improve more advanced ECLS-treatment of hypothermic patients. Human erythrocytes contain both β1-and β2-adrenergic receptors, with the latter being predominant (Sager, 1982; Bree et al., 1984). It has been shown that β2-receptor activation increases the level of cAMP intracellularly, and that there is a concentration-dependent increase in red blood cell filterability when stimulated with adrenaline (Tuvia et al., 1999; Horga et al., 2000). Thus, decreased elimination of cAMP through ABCC4, could provide a pharmacological strategy to increase erythrocyte-deformability and improve the microcirculation during ECLS-treatment of hypothermic patients.
The evidence for using pharmacological support in cold patients is scarce and the use of, e.g., adrenaline as inotropic support is mainly based on animal studies. The same goes for our study drugs. Various experiments have been conducted, both in vitro and in vivo. In rodent models, milrinone have a preserved effect on both systolic and diastolic heart function during cooling, down to 15°C and rewarming to 37°C (Tveita and Sieck, 2012; Dietrichs et al., 2014a). On the other hand, in isolated guinea pig hearts, milrinone was shown to have no inotropic effect at study temperatures 31°C and 34°C (Rieg et al., 2009). Interestingly, the latter study demonstrated a temperature-dependent elevation of cardiac inotropy, which could impede any further effect of pharmacological treatment. Studies exploring temperature-dependent effects of levosimendan, show that it has significant positive effects on the circulation in deeply hypothermic rats connected to cardiopulmonary bypass, when compared to adrenaline (Rungatscher et al., 2012). Levosimendan also increases organ blood flow during rewarming, besides contributing to higher CO (Håheim et al., 2020). Knowledge of the effects of amrinone during hypothermia are limited. One study examined whether amrinone could accelerate rewarming in patients after iatrogenic mild hypothermia during neurosurgical procedures (Inoue et al., 2002) but, as of today, no in vitro or in vivo experiments that investigates potential use of amrinone in accidental hypothermia, exists to our knowledge.
Our findings demonstrate that PDE3-inhibitors milrinone, amrinone, and levosimendan are all able to enter the cells and inhibit PDE3 down to 20°C. Furthermore, we show that their pharmacodynamic effects, in regulating cAMP- and cGMP-elimination, is intact during severe hypothermia and that the dose-response relationship is maintained. It is however likely that the pharmacokinetic properties of these drugs will differ from normothermic temperatures. In general, there is a higher risk of drug and metabolite accumulation in plasma because of diminished clearance at low core temperatures. The volume of distribution is often reduced. This mandates a lower dosage but at the same time, one has to take into account that receptor sensitivity could differ from normothermia (Mallet, 2002; van den Broek et al., 2010). The present study also shows reduced activity of both PDE-enzymes and ABCC-transporters with temperature reduction. Still, all PDE3-inhibitors had intact effect on these target molecules during severe hypothermia. This could provide a more physiological strategy to elevate cAMP levels in hypothermia and avoid an extensive and harmful increase of cAMP, seen with adrenaline-infusion (Dietrichs et al., 2015). Hence, use of PDE3-inhibitors is a promising treatment strategy as their pharmacodynamic effects appear unchanged during hypothermia. Before these drugs are considered for inclusion in guidelines for rewarming accidental hypothermia patients, it is imperative that their temperature-dependent pharmacokinetic properties are established and that their electrophysiological properties are tested in cardiomyocytes. Further, translational studies to assess optimal dosage regimes at different body temperatures and rewarming rates, are therefore necessary to avoid drug toxicity and therapy failure.
5 Conclusion
Milrinone, amrinone, and levosimendan are able to cross cell membranes and reach their cytosolic site of action, during severe hypothermia. At temperatures down to 20°C, they have maintained inhibitory effects on inhibiting cellular elimination of cAMP and cGMP, and provide a promising treatment strategy to treat and prevent hypothermia-induced cardiac dysfunction.
Acknowledgments
We thank the Department of Immunohematology and Transfusion Medicine, University Hospital of North Norway, for the contribution of blood used in the experiments.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AKK, ALS, NS, and TK conducted the experiments in the lab. RAL helped with technical issues and theoretical questions during experiments. O-MF analyzed the results using mass-spectrometry. The results and statistical analysis were interpreted by AKK and ESD. AWR, TT, GS, and ESD planned the research project. AKK, ALS, ESD, O-MF, NS, and GS contributed to the manuscript. All authors read and approved the manuscript.
Funding
The project was funded by the Norwegian Air Ambulance Foundation. Additional grant was awarded ALS from the medical research student program (Forskerlinjen) at UiT—The Arctic University of Norway. ESD received funds from the Northern Norwegian Health Authority (HNF1337–17).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Blair E., Montgomery A. V., Swan H. (1956). Posthypothermic Circulatory Failure. Circulation 13 (6), 909–915. 10.1161/01.cir.13.6.909 [DOI] [PubMed] [Google Scholar]
- Bree F., Gault I., d'Athis P., Tillement J. P. (1984). Beta Adrenoceptors of Human Red Blood Cells, Determination of Their Subtypes. Biochem. Pharmacol. 33 (24), 4045–4050. 10.1016/0006-2952(84)90019-4 [DOI] [PubMed] [Google Scholar]
- Brown D. J. A., Brugger H., Boyd J., Paal P. (2012). Accidental Hypothermia. N. Engl. J. Med. 367 (20), 1930–1938. 10.1136/bmj.1.5489.739-b10.1056/nejmra1114208 [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. (1973). Relationship between the Inhibition Constant (K1) and the Concentration of Inhibitor Which Causes 50 Per Cent Inhibition (I50) of an Enzymatic Reaction. Biochem. Pharmacol. 22 (23), 3099–3108. 10.1016/0006-2952(73)90196-2 [DOI] [PubMed] [Google Scholar]
- Chou T.-C. (1976). Derivation and Properties of Michaelis-Menten Type and Hill Type Equations for Reference Ligands. J. Theor. Biol. 59 (2), 253–276. 10.1016/0022-5193(76)90169-7 [DOI] [PubMed] [Google Scholar]
- Dietrichs E. S., Håheim B., Kondratiev T., Sieck G. C., Tveita T. (2014a). Cardiovascular Effects of Levosimendan during Rewarming from Hypothermia in Rat. Cryobiology 69 (3), 402–410. 10.1016/j.cryobiol.2014.09.007 [DOI] [PubMed] [Google Scholar]
- Dietrichs E. S., Kondratiev T., Tveita T. (2014b). Milrinone Ameliorates Cardiac Mechanical Dysfunction after Hypothermia in an Intact Rat Model. Cryobiology 69 (3), 361–366. 10.1016/j.cryobiol.2014.09.002 [DOI] [PubMed] [Google Scholar]
- Dietrichs E. S., Sager G., Tveita T. (2016). Altered Pharmacological Effects of Adrenergic Agonists during Hypothermia. Scand. J. Trauma Resusc. Emerg. Med. 24 (1), 143. 10.1186/s13049-016-0339-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrichs E. S., Schanche T., Kondratiev T., Gaustad S. E., Sager G., Tveita T. (2015). Negative Inotropic Effects of Epinephrine in the Presence of Increased β-adrenoceptor Sensitivity during Hypothermia in a Rat Model. Cryobiology 70 (1), 9–16. Epub 2014 Nov 13. 10.1016/j.cryobiol.2014.10.012 [DOI] [PubMed] [Google Scholar]
- Ellman G. L., Courtney K. D., Andres V., Featherstone R. M. (1961). A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 7, 88–95. 10.1016/0006-2952(61)90145-9 [DOI] [PubMed] [Google Scholar]
- Endoh M. (2013). Amrinone, Forerunner of Novel Cardiotonic Agents, Caused Paradigm Shift of Heart Failure Pharmacotherapy. Circ. Res. 113 (4), 358–361. 10.1161/CIRCRESAHA.113.301689 [DOI] [PubMed] [Google Scholar]
- Guly H. (2011). History of Accidental Hypothermia. Resuscitation 82 (1), 122–125. 10.1016/j.resuscitation.2010.09.465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håheim B., Kondratiev T., Dietrichs E. S., Tveita T. (2020). Comparison between Two Pharmacologic Strategies to Alleviate Rewarming Shock: Vasodilation vs. Inodilation. Front. Med. 7 (November), 566388. 10.3389/fmed.2020.566388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håheim B., Kondratiev T., Dietrichs E. S., Tveita T. (2017). The Beneficial Hemodynamic Effects of Afterload Reduction by Sodium Nitroprusside during Rewarming from Experimental Hypothermia. Cryobiol. Neth. 77, 75–81. 10.1016/j.cryobiol.2017.05.002 [DOI] [PubMed] [Google Scholar]
- Han Y.-S., Tveita T., Prakash Y. S., Sieck G. C. (2010). Mechanisms Underlying Hypothermia-Induced Cardiac Contractile Dysfunction. Am. J. Physiology-Heart Circulatory Physiology 298 (3), H890–H897. 10.1152/ajpheart.00805.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horga J. F., Gisbert J., De Agustín J. C., Hernández M., Zapater P. (2000). A Beta-2-Adrenergic Receptor Activates Adenylate Cyclase in Human Erythrocyte Membranes at Physiological Calcium Plasma Concentrations. Blood Cells, Mol. Dis. 26 (3), 223–228. 10.1006/bcmd.2000.0299 [DOI] [PubMed] [Google Scholar]
- Inoue S., Kawaguchi M., Sakamoto T., Kitaguchi K., Furuya H., Sakaki T. (2002). High-dose Amrinone Is Required to Accelerate Rewarming from Deliberate Mild Intraoperative Hypothermia for Neurosurgical Procedures. Anesthesiology 97 (1), 116–123. 10.1097/00000542-200207000-00017 [DOI] [PubMed] [Google Scholar]
- Köck K., Grube M., Jedlitschky G., Oevermann L., Siegmund W., Ritter C. A., et al. (2007). Expression of Adenosine Triphosphate-Binding Cassette (ABC) Drug Transporters in Peripheral Blood Cells: Relevance for Physiology and Pharmacotherapy. Clin. Pharmacokinet. 46 (6), 449–470. 10.2165/00003088-200746060-00001 [DOI] [PubMed] [Google Scholar]
- Lott C., Truhlář A., Alfonzo A., Barelli A., González-Salvado V., Hinkelbein J., et al. (2021). European Resuscitation Council Guidelines 2021: Cardiac Arrest in Special Circumstances. Resuscitation 161, 152–219. 10.1016/j.resuscitation.2021.02.011 [DOI] [PubMed] [Google Scholar]
- Mallet M. L. (2002). Pathophysiology of Accidental Hypothermia. QJM 95 (12), 775–785. 10.1093/qjmed/95.12.775 [DOI] [PubMed] [Google Scholar]
- Mathew R., Di Santo P., Jung R. G., Marbach J. A., Hutson J., Simard T., et al. (2021). Milrinone as Compared with Dobutamine in the Treatment of Cardiogenic Shock. N. Engl. J. Med. 385 (6), 516–525. 10.1056/NEJMoa2026845 [DOI] [PubMed] [Google Scholar]
- Mohyuddin R., Dietrichs E. S., Sundaram P., Kondratiev T., Figenschou M. F., Sieck G. C., et al. (2021). Cardiovascular Effects of Epinephrine During Experimental Hypothermia (32°C) with Spontaneous Circulation in an Intact Porcine Model. Front. Physiol. 12 (September). 10.3389/fphys.2021.718667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movsesian M. (2016). Novel Approaches to Targeting PDE3 in Cardiovascular Disease. Pharmacol. Ther. 163, 74–81. 10.1016/j.pharmthera.2016.03.014 [DOI] [PubMed] [Google Scholar]
- Musi M. E., Sheets A., Zafren K., Brugger H., Paal P., Hölzl N., et al. (2021). Clinical Staging of Accidental Hypothermia: The Revised Swiss System. Resuscitation 162 (May), 182–187. 10.1016/j.resuscitation.2021.02.038 [DOI] [PubMed] [Google Scholar]
- Omori K., Kotera J. (2007). Overview of PDEs and Their Regulation. Circulation Res. 100 (3), 309–327. 10.1161/01.RES.0000256354.95791.f1 [DOI] [PubMed] [Google Scholar]
- Paal P., Pasquier M., Darocha T., Lechner R., Kosinski S., Wallner B., et al. (2022). Accidental Hypothermia: 2021 Update. Int. J. Environ. Res. Public Health 19 (501), 501. 10.3390/ijerph19010501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak A., Lebrin M., Vaccaro A., Senard J. M., Despas F. (2013). Pharmacology of Levosimendan: Inotropic, Vasodilatory and Cardioprotective Effects. J. Clin. Pharm. Ther. 38 (5), 341–349. 10.1111/jcpt.12067 [DOI] [PubMed] [Google Scholar]
- Rieg A. D., Schroth S. C., Grottke O., Hein M., Ackermann D., Rossaint R., et al. (2009). Influence of Temperature on the Positive Inotropic Effect of Levosimendan, Dobutamine and Milrinone. Eur. J. Anaesthesiol. 26 (11), 946–953. 10.1097/EJA.0b013e328330e9a0 [DOI] [PubMed] [Google Scholar]
- Rungatscher A., Linardi D., Tessari M., Menon T., Luciani G. B., Mazzucco A., et al. (2012). Levosimendan Is Superior to Epinephrine in Improving Myocardial Function after Cardiopulmonary Bypass with Deep Hypothermic Circulatory Arrest in Rats. J. Thorac. Cardiovasc. Surg. 143 (1), 209–214. 10.1016/j.jtcvs.2011.09.020 [DOI] [PubMed] [Google Scholar]
- Sager G., Ravna A. (2009). Cellular Efflux of cAMP and cGMP - a Question about Selectivity. Mrmc 9 (8), 1009–1013. 10.2174/138955709788681654 [DOI] [PubMed] [Google Scholar]
- Sager G. (1982). Receptor Binding Sites for Beta-Adrenergic Ligands on Human Erythrocytes. Biochem. Pharmacol. 31 (1), 99–104. 10.1016/0006-2952(82)90243-X [DOI] [PubMed] [Google Scholar]
- Selli A. L., Kuzmiszyn A. K., Smaglyukova N., Kondratiev T. V., Fuskevåg O.-M., Lyså R. A., et al. (2021). Treatment of Cardiovascular Dysfunction with PDE5-Inhibitors - Temperature Dependent Effects on Transport and Metabolism of cAMP and cGMP. Front. Physiol. 12 (July), 695779. 10.3389/fphys.2021.695779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck T. L., Kant J. A. (1974). [16] Preparation of Impermeable Ghosts and Inside-Out Vesicles from Human Erythrocyte Membranes. Methods Enzym. 31, 172–180. 10.1016/0076-6879(74)31019-1 [DOI] [PubMed] [Google Scholar]
- Tuvia S., Moses A., Gulayev N., Levin S., Korenstein R. (1999). β‐Adrenergic Agonists Regulate Cell Membrane Fluctuations of Human Erythrocytes. J. Physiology 516 (3), 781–792. 10.1111/j.1469-7793.1999.0781u.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tveita T., Sieck G. C. (2012). Effects of Milrinone on Left Ventricular Cardiac Function during Cooling in an Intact Animal Model. Cryobiology 65 (1), 27–32. 10.1016/j.cryobiol.2012.03.007 [DOI] [PubMed] [Google Scholar]
- Tveita T., Skandfer M., Refsum H., Ytrehus K. (1996). Experimental Hypothermia and Rewarming: Changes in Mechanical Function and Metabolism of Rat Hearts. J. Appl. Physiology 80 (1), 91–297. 10.1152/jappl.1996.80.1.291 [DOI] [PubMed] [Google Scholar]
- van den Broek M. P. H., Groenendaal F., Egberts A. C. G., Rademaker C. M. A. (2010). Effects of Hypothermia on Pharmacokinetics and Pharmacodynamics: a Systematic Review of Preclinical and Clinical Studies. Clin. Pharmacokinet. 49 (5), 277–294. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg G.-J., Goslings J. C., Walpoth B. H., Bierens J. J. L. M. (2010). Accidental Hypothermia: Rewarming Treatments, Complications and Outcomes from One University Medical Centre. Resuscitation 81 (11), 1550–1555. 10.1016/j.resuscitation.2010.05.023 [DOI] [PubMed] [Google Scholar]
- Vasiliou V., Vasiliou K., Nebert D. W. (2008). Human ATP-Binding Cassette (ABC) Transporter Family. Hum. Genomics 3 (3), 281–290. 10.1186/1479-7364-3-3-281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassal T., Benoit-Gonin B., Carrat F., Guidet B., Maury E., Offenstadt G. (2001). Severe Accidental Hypothermia Treated in an ICU. Chest 120 (6), 1998–2003. 10.1378/chest.120.6.1998 [DOI] [PubMed] [Google Scholar]
- Yan K., Gao L.-N., Cui Y.-L., Zhang Y., Zhou X. (2016). The Cyclic AMP Signaling Pathway: Exploring Targets for Successful Drug Discovery (Review). Mol. Med. Rep. 13 (5), 3715–3723. 10.3892/mmr.2016.5005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Ward A. (1988). Milrinone. Drugs 36 (2), 158–192. 10.2165/00003495-198836020-00003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.