Introduction
This best practice report aims to review the current literature and provide healthcare providers with background information and a practical, evidence-based algorithmic approach to evaluation and management of patients with nocturia. Given the multifactorial etiology of nocturia, these patients benefit from a multimodal and multidisciplinary approach to treat underlying diseases or conditions that may be contributing to symptoms, and to target management to improve symptoms.
Methods
A panel of content experts was convened to determine the scope and focus of this narrative review. Data was obtained from published original trials and meta-analyses identified through a literature search using PubMed, Medline, and the Cochrane Library database. Bibliographies of relevant articles were also reviewed to identify any other relevant important studies. Quality of evidence was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework. While overall, there was a lack of high-quality evidence to inform aspects of this review, the strength of recommendations was supported by clinical principles, fundamental pathophysiology, and consensus expert opinion.
Background
The International Continence Society (ICS) defines nocturia as waking one or more times to void during the hours of sleep, with each void preceded and followed by sleep.1 Although voiding even once at night is considered nocturia, some literature has shown that fewer than two voids per night does not result in significant patient bother.2 However, the degree of bother experienced by individual patients will vary according to factors beyond just the number of voids per night. Nocturia is associated with impaired quality of life3 and even mortality.4 This association of nocturia with mortality has been reported to be independent of bother, and should be noted even if patients are not bothered.5 Patients, therefore, may benefit from assessment for nocturia even if they don’t independently report nocturia, as bother is often what motivates patients to raise nocturia as an issue with their care providers.
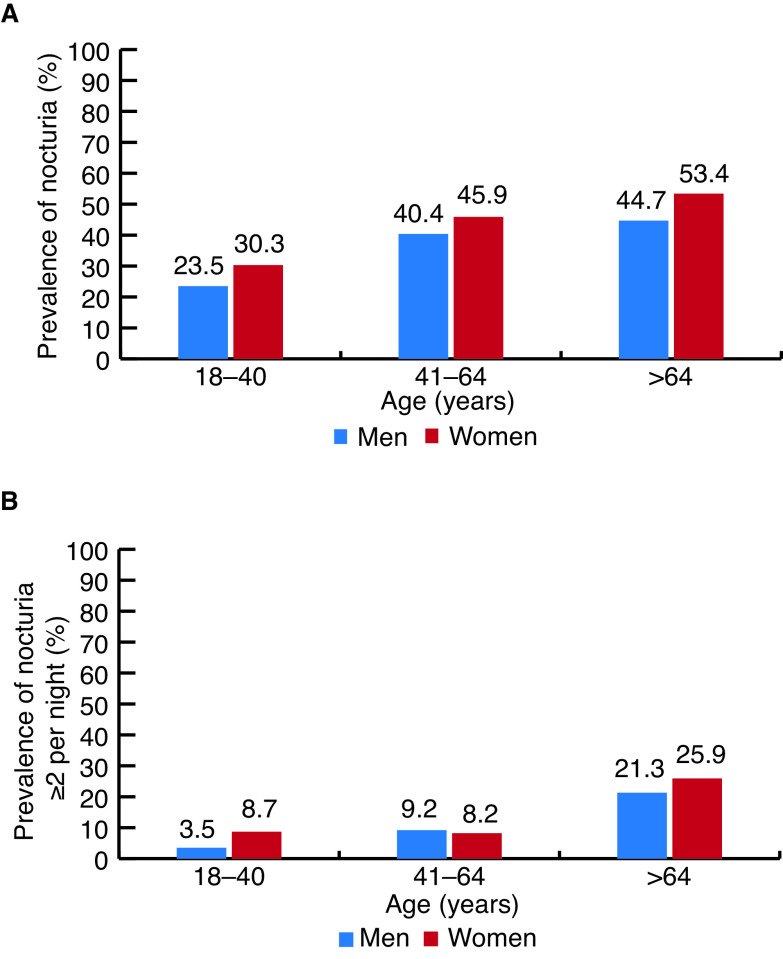
Depending on the patient population and definition of nocturia used, prevalence varies significantly.6–8 From a Canadian survey of 1000 adults, prevalence, using the ICS definition of one or more episodes per night, was estimated to be 36.4% in all adults and 49.5% in adults over age 65. When defined as two or more episodes per night, prevalence was 9.1% in all adults and 23.8% in adults over the age of 65.9 Nocturia by any definition increases with age, particularly in men (Figure 1).6,7,9
Figure 1.
Prevalence of nocturia (A) one or more times per night; and (B) two or more times per night in Canadian adults, by age and sex.9
Normal renal function allows for a circadian production of urine, with concentration of urine at night. This function is age-dependent, typically established by age 3–5, and leads to decreased nocturnal urine volume. Arginine vasopressin (AVP, or antidiuretic hormone [ADH]) is released from the posterior pituitary and serves as the primary hormone regulating renal water excretion. Factors such as high serum osmolality, hypovolemia, and angiotensin II stimulate AVP, leading to water reabsorption, whereas factors such as atrial natriuretic peptide (ANP/ANH), prostaglandin E2 (PGE2), and hypercalcemia inhibit AVP, leading to diuresis.10 With aging, however, the circadian rhythm of release of these hormones is blunted, which can increase nocturnal urine production.
Normal bladder function requires adequate bladder storage and emptying abilities. This requires coordination of multiple components, including the central and peripheral nervous systems, detrusor smooth muscle function, and urethral and pelvic floor function. In addition, the bladder urothelium seems to have a role in regulating urinary function and possibly contributing to AVP-mediated water homeostasis.10 Important considerations for the general patient population are that bladder capacity may diminish with age, and nocturnal detrusor overactivity may occur in patients with underlying overactive bladder (OAB).
Recommendation: Patients presenting with nocturia should be counselled on the multifactorial mechanisms that may contribute to nocturia, and clinicians should consider the symptom in the context of the patient’s comorbidities, as well as dietary, lifestyle, and voiding behaviors. Consultation should elicit the patient’s degree of bother/impact on quality of life (strong recommendation, moderate level of evidence).
Initial assessment and investigations
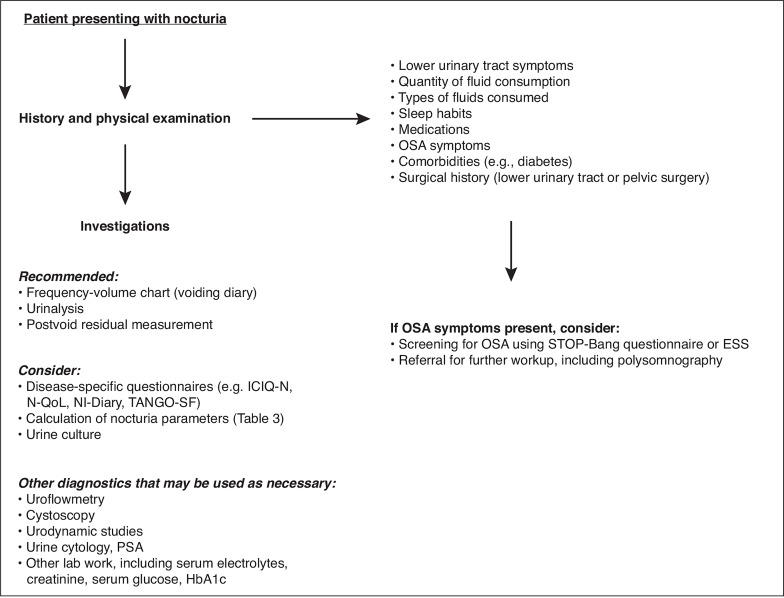
The multifactorial nature of nocturia complicates its diagnosis and treatment. An initial assessment with a thorough history and physical examination can clarify its etiology, whether it be urological or non-urological (Figure 2, Table 1). A detailed history is particularly important given the multiple possible contributing etiologies of bothersome nocturia, and patients should be evaluated for: 1) quantity, type, and time of fluid consumption (e.g., water, alcohol, caffeine, energy drinks); 2) lower urinary tract symptoms (LUTS) (storage, voiding, post-micturition); 3) sleep habits (including quantity, quality, and use of pharmacological sleep aids); 4) medications, including dosage schedule; 5) symptoms of obstructive sleep apnea (OSA), such as loud snoring; 6) cardiovascular conditions; 7) diabetes; 8) any prior lower urinary tract or pelvic surgeries; and 9) pain.11
Figure 2.

Initial workup of patients presenting with nocturia. ESS: Epworth Sleepiness Scale; ICIQ-N: International Consultation on Incontinence Questionnaire-Nocturia; N-QoL: Nocturia Quality of Life Questionnaire; NI-Diary: Nocturia Impact Diary; OSA: obstructive sleep apnea; PSA: prostate-specific antigen; TANGO SF: Targeting the Individual’s Aetiology of Nocturia to Guide Outcomes Short Form.
Table 1.
| Urological causes (resulting in diminished global or nocturnal bladder capacity) | Non-urological causes (resulting in global or nocturnal polyuria, or sleep disruption) |
|---|---|
| Overactive bladder Benign prostatic hyperplasia Ureteral or bladder calculi Learned voiding dysfunction Neurogenic voiding dysfunction Nocturnal detrusor overactivity |
Heart failure Peripheral edema Diabetes mellitus (uncontrolled/ poorly controlled) Diabetes insipidus Primary polydipsia Obstructive sleep apnea Medication effects Chronic pain Neurologic disorders Nocturnal polyuria |
Patients should be specifically asked to quantify the number of times they wake to void at night and if they drink large amounts of liquid throughout the day or in the evening before going to sleep.12 They should be asked about their occupation, at what time they go to bed, and at what time they wake up in the morning. Patients should also be asked about their emotional state, as depression/anxiety and nocturia appear to be frequently associated.13
Insomnia and daytime tiredness may be indirect symptoms of nocturia or OSA.14 Undiagnosed OSA may be screened using the STOP-Bang questionnaire14 and confirmed with in-laboratory polysomnography, which is considered the gold-standard diagnostic tool.15 Patients suspected of having OSA can be referred to a sleep medicine expert for further evaluation. This is further discussed in a later section.
Current medications should be reviewed (types and timing) to identify iatrogenic pharmacological causes that may contribute to nocturia through increasing diuresis (e.g., diuretics, angiotensin-converting enzyme [ACE] inhibitors, lithium), changing vesico-sphincteric function (e.g., acetylcholinesterase inhibitors), increasing water retention and peripheral edema (e.g., calcium channel blockers, steroids), or interfering with sleep (e.g., central nervous system [CNS] stimulants, such as methylphenidate, psychotropics, antiepileptics, decongestants). A non-exhaustive list of medications that may affect nocturia and their associated mechanisms is provided in Table 2.16
Table 2.
| Mechanism | Drugs |
|---|---|
| Diuresis (either via increased free water or osmotic clearance) | Diuretics, progesterone, melatonin, ACE inhibitors, lithium, SGLT-2 inhibitors |
| Antidiuresis (either via decreased free water or osmotic clearance) | ddAVP, testosterone, estrogens, antipsychotics, chemotherapeutics, antidepressants, antiepileptics, opiates, calcium channel blockers, beta adrenoreceptor antagonists, NSAIDs, lithium, melatonin, corticosteroids, thiazolidinediones |
| Edema | Caffeine, alcohol, anticholinesterase inhibitors, cyclophosphamide, ketamine |
| Central nervous system effects (e.g., insomnia) | Antiepileptics, psychotropic agents, stimulants, antihypertensives (alpha- and beta-blockers), decongestants, hormones (corticosteroids, thyroid hormones), caffeine |
| Precipitating lower urinary tract symptoms | Caffeine, alcohol, anticholinesterase inhibitors, cyclophosphamide, ketamine |
ACE: angiotensin converting enzyme; ddAVP: desmopressin acetate; NSAIDs: non-steroidal anti-inflammatory drugs; SGLT-2: sodium–glucose co-transporter 2.
Recommendation: Consultation should include a thorough history, including assessment of urinary symptoms, fluid intake (quantity, type, and timing), medications, and sleep habits in relation to nocturia (strong recommendation, low level of evidence).
On physical examination, attention should be given to blood pressure measurement (hypertension or orthostatic hypotension), obesity, large neck circumference, simple assessment of cognitive and motor capacity, abdominal exam for bladder distension, digital rectal exam (DRE) and external genital examination in men (assessing for benign prostatic hyperplasia [BPH], phimosis, meatal stenosis, etc.), pelvic examination in women (assessing for vaginal atrophy, pelvic organ prolapse, etc.), and lower leg edema, to help clarify the underlying etiology.14
As per the ICS consensus, use of disease-specific questionnaires is recommended to help assess nocturia bother and severity, and to follow treatment progress. These include the International Consultation on Incontinence Questionnaire Nocturia Module, the Nocturia Quality-of-Life questionnaire, and the Nocturia Impact Diary.17 Recently, the Targeting the Individual’s Aetiology of Nocturia to Guide Outcomes (TANGO) questionnaire has been developed to assist in diagnosing non-lower urinary tract contributing components of nocturia. The TANGO Short-Form (TANGO SF) is a patient-administered screening tool consisting of 22 statements across four domains (cardio/metabolic, sleep, urinary tract, and wellbeing domains) that has been validated in English and Spanish.18
A frequency-volume chart, or voiding diary, is the most important objective diagnostic tool when investigating a patient for nocturia. The frequency-volume chart will help clarify the underlying pathophysiology by differentiating global or nocturnal polyuria from reduced bladder capacity.19 Over a 72-hour period, patients must record: 1) time and volume of each voided urine; 2) volume and type of fluid intake; 3) any episodes of incontinence; and 4) sleep and wake-up times.20 Clinicians should give clear explanations on how to complete a voiding diary in order to obtain an accurate and useful portrait of the typical patient daily voiding experience. See Appendix for a voiding diary template (available at cuaj.ca).
For the purposes of calculations, the first morning void is included in the nocturnal urine volume produced but should not be considered a nocturia event.21 The total urine volume (TUV) refers to the total volume of urine produced during a 24-hour period and the nocturnal urine volume (NUV) is the total volume of urine produced at night. The maximum voided volume (MVV) is the largest single voided volume in a 24-hour period and serves as a representation of bladder capacity. The nocturia index (Ni) is calculated by dividing the NUV by MVV; if this is value is >1, nocturia or enuresis will occur because the MVV has been exceeded by NUV (Table 3). For example:
Table 3.
Parameters obtainable from 72-hour voiding diary and calculations required to classify nocturia
| Term | Definition | Clinical application |
|---|---|---|
| 24-h urine volume | Total volume voided over 24 h | 24-h urine volume >40 ml/ kg is diagnostic of global polyuria |
| NUV* | Total volume of urine voided during the night, including the first morning void | Alternative definitions of NP:
|
| MVV | Largest single voided volume over 24 h, day or night (thus representative of bladder capacity) | Low MVV indicates reduced global bladder capacity |
| Ni* | Ni=NUV/MVV | Ni >1 suggests nocturia due to mismatch between production and capacity during sleep |
| ANV | Number of nocturnal voids, excluding first morning void | Accurate measure of nocturnal frequency (superior to questionnaires) |
| PNV | PNV=Ni-1 | Used to determine nocturnal bladder capacity |
| NBCi | NBCi=ANV-PNV | NBCi >0 indicates reduced nocturnal bladder capacity |
| NPi | NPi=NUV/(24 h urine volume) | NPi >20–33% diagnostic of NP (age dependent) |
An NUV exceeding bladder capacity or a Ni >1 leads to nocturia.
ANV: actual number of nightly voids; MVV: maximum voided volume; NBCi: nocturnal bladder capacity index; Ni: nocturia index; NP: nocturnal polyuria; NPi: nocturnal polyuria index; NUV: nocturnal urine volume; PNV: predicted number of nightly voids. Adapted with permission from Dani et al.110
– If a patient’s MVV is 400 mL and he produces 1000 mL of urine at night (NUV=1000 mL), his nocturia index is 2.5 (1000/400=2.5).
– As the Ni is >1, he will have nocturia because the volume of urine he produces at night is more than his bladder capacity.
In line with the multidisciplinary nature of nocturia, several investigations and therapeutic options may be considered depending on the most likely etiology, as illustrated in Figure 3.17 All patients should have a urinalysis and postvoid residual (PVR) urine measurement, and patients with microhematuria should be referred to a urologist for assessment as per the Canadian Urology Association (CUA) hematuria guidelines.22 Urine culture should be done if infection is suspected based on urinalysis. Urine microscopy and subsequently cytology should be considered if blood is seen on urinalysis. Prostate-specific antigen (PSA) may be ordered as part of BPH assessment if suspected based on history or physical exam. Other laboratory investigations to consider include serum electrolytes and creatinine, serum glucose/HbA1c, or urinary osmolality, as nocturia can be the result of nephrogenic diabetes insipidus, hypercalciuria, or polyuria due to renal disease.23
Figure 3.
Multimodal approach to management of nocturia, organized by disease domains with subsequent pathways for treatment and consideration of specialist referral. Note that nocturia is often multifactorial and requires workup and management across multiple domains to achieve patient treatment goals. Clinical specialties listed are not exhaustive and may vary by region. 5ARI: 5-alpha-reductase inhibitors; BOO: bladder outlet obstruction; CPAP: continuous positive airway pressure; OAB: overactive bladder; OSA: obstructive sleep apnea; PCP: primary care practitioner.
Sex hormones (serum luteinizing hormone [LH], follicle-stimulating hormone [FSH], testosterone, estrogen) are involved in regulation of diuresis through sodium and water excretion/reabsorption, and levels can be measured in patients with signs or symptoms of menopause or andropause, as hormone replacement or other treatment may help address menopause-related or andropause-related nocturia.17,24 Measuring plasma B-type natriuretic peptide (BNP) levels has been suggested since they are considered a marker of cardiac insufficiency and intravascular volume overload, but data are inconsistent and this merits further study prior to advocating its clinical utility.25 Additional tests may be necessary in individual patients to evaluate the exact origin of nocturia in the setting of significant LUTS, such as non-invasive uroflowmetry, cystoscopy, or urodynamics.
Recommendation: All patients presenting with nocturia should undergo physical examination to assess for contributory etiologies. Initial assessment should include a frequency-volume chart, urinalysis, and PVR. Disease-specific questionnaires should be used to ascertain bother and severity to guide initial treatment and subsequent response. Cystoscopy and urodynamics are not usually necessary for patients with nocturia (strong recommendation, low level of evidence).
Classification of nocturia
It is clinically useful to classify nocturia based on etiology, as this allows for a better understanding of the multifactorial nature of this condition and helps direct individualized treatment. Information obtained from an accurately completed 72-hour voiding diary guides classification into one of the following groups: global polyuria, nocturnal polyuria, reduced bladder capacity, sleep disorders, or mixed disorders. Table 3 outlines the parameters and calculations required to categorize nocturia.
Global polyuria
Global polyuria (GP) is defined as a 24-hour urine output >40 ml/kg. The causes of GP include diabetes mellitus (DM), diabetes insipidus (DI), and primary polydipsia (PPD). Management of GP depends on a precise determination of its underlying cause, and in many cases can be managed by primary care or referred to an internal medicine specialist. The investigation of GP is first approached by performing an overnight water deprivation test, with restriction of all fluid intake for eight hours or until 5% of body mass has been lost, followed by administration of 2 mcg of intramuscular desmopressin and measurement of urine and plasma osmolality.11 If a normal urine osmolality (>600–800 mOsm/kg) is recorded, then the diagnosis is PPD and the patient can be instructed to fluid restrict. Primary polydipsia may be psychogenic or dipsogenic: the latter is often secondary to brain insults (e.g., trauma, surgery, neoplasia, radiation) that impact the osmoregulation of thirst.
If the first morning urine osmolality is low (<600 mOsm/kg), then DM should be ruled out by measuring serum glucose and hemoglobin A1C. Abnormally low urine osmolality with normal glucose testing indicates DI. DI is secondary to insufficient synthesis or secretion of ADH (central DI), or abnormal response of the kidneys to circulating ADH (nephrogenic DI). The type of DI is determined by measuring the urine osmolality in response to desmopressin 40 mcg intranasally or 0.4 mg orally (renal concentrating capacity test), which will normalize the urine osmolality in central but not nephrogenic DI. Central DI may be idiopathic or secondary to trauma, pituitary neoplasms, or vascular, infiltrative, or infectious processes. Nephrogenic DI may be caused by chronic kidney disease, excess prostaglandin or ANP, hypercalcemia, hypokalemia, lithium toxicity, or tetracycline use.
Nocturnal polyuria
When urine overproduction occurs only at night, it is termed nocturnal polyuria (NP). A number of bladder diary-based definitions exist, and their diagnostic accuracy continues to be explored and debated. The widely accepted ICS definition of NP is a NP index (NPi) >33% in those >65 years old and NPi >20% in those 25–65 years old (Table 3).26 In other words, NP occurs when adults aged 25–65 years old produce more than 20% of their total urine volume overnight, or when adults over 65 years old produce more than 33% of their total urine volume overnight. Other definitions include nocturnal urine production >90 ml/hr, NUV >6.4 ml/kg, and NUV >0.9 ml/min.27 NP is present in the majority of patients with nocturia.6 The differential diagnosis for NP includes edema-forming states (e.g., congestive heart failure, nephrotic syndrome), OSA, neurodegenerative disease (e.g., Parkinson’s, Alzheimer’s), chronic kidney disease, autonomic neuropathy, venous stasis, and idiopathic NP thought to be caused by deficient nocturnal ADH secretion.
Reduced bladder capacity
Reduced bladder capacity is caused by a wide variety of conditions. Bladder outlet obstruction and detrusor under-activity can affect the time between voids via incomplete bladder emptying, whereas other conditions, such as neurogenic bladder, OAB syndrome, and cystitis, may diminish functional bladder capacity via involuntary detrusor contractions. Bladder sensory disorders, such as bladder pain syndrome, may also reduce functional capacity. Reduced bladder capacity may be constant or nocturnal only; the reasons for this are not well-understood. A low MVV is indicative of overall reduced bladder capacity. Nocturnal bladder capacity is reduced relative to MVV when nocturnal bladder capacity index (NBCi) is >0. Consensus does not yet exist, but NBCi >1.3 has been proposed as the threshold at which reduced nocturnal bladder capacity contributes to nocturia.28 NBCi is calculated by subtracting predicted number of nightly voids (PNV) from actual number of nightly voids (ANV) (Table 3). For example:
– If a patient has a MVV of 400 mL and her NUV is 1000 mL, her Ni is 2.5 (Ni= NUV/MVV=1000/400=2.5).
– Her PNV would be 1.5 (PNV=Ni-1=2.5–1=1.5).
– If she actually wakes up four times per night (ANV=4), the NBCi is 2.5 (NBCi=ANV-PNV=4–1.5=2.5).
– As her NBCi is >1.3, reduced nocturnal bladder capacity likely contributes to her nocturia.
Sleep disorders
Sleep disorders have the potential to drive nocturnal micturition. However, nocturnal voiding in the context of sleep disturbance is typically habitual and done out of convenience vs. need. Patients with suspected diagnoses of sleep disorders can be further worked up by primary care and/or sleep medicine specialists.29,30
Mixed disorders
The etiology of nocturia is multifactorial in many patients with this condition.21,23 Patients may require subspecialist referral for workup of contributing etiologies.
Recommendation: Clinicians investigating nocturia should aim to classify nocturia based on etiology with the guidance of a detailed patient history, physical examination, and frequency-volume chart. Further workup and management of specific causes of nocturia may involve the patient’s primary care practitioner, urologist, other specialists, and allied health professionals as appropriate (strong recommendation, moderate level of evidence).
Behavioral and lifestyle contributors to nocturia
Numerous behavioral and lifestyle factors may play a role in nocturia, mostly by increasing NP, but also by contributing to an underlying sleep disturbance or diminished bladder capacity. Recommendations to make behavioral or lifestyle changes must be tailored to the individual and the underlying contributors to nocturia. Management recommendations to consider are listed in Table 4.
Table 4.
Summary of behavioral and lifestyle management considerations for patients with nocturia
| Problem | Recommendation |
|---|---|
| Nocturnal polyuria | Evening fluid restriction (none for 2 hr prior to bedtime) Total daily fluid restriction to 2 L/day, or reduce 25% from baseline Reducing dietary salt intake Reducing evening protein intake Restriction/avoidance of caffeine and alcohol Mobilizing peripheral edema fluid (evening leg elevation, support stockings) |
| Diminished bladder capacity | Pre-emptive voiding or clean intermittent catheterization at bedtime Pelvic floor muscle training Bladder training |
| Sleep disturbance | Improving sleep hygiene (e.g., establishing a relaxing bedtime routine, minimizing electronics before bed) Treatment of hot flashes in post-menopausal women |
| Multifactorial | Management of chronic lifestyle-related diseases (e.g., diabetes, hypertension, cardiac disease) Weight loss Increasing exercise and fitness |
Restricting evening fluids is recommended to slow urine production during sleep. Furthermore, there is good evidence to support the provision of guidance on fluid intake and reduction of total daily fluid consumption (2 L/day or 25% reduction from baseline), and this is considered a central recommendation for initial management.14,17,31–34
Small studies confirm that sodium intake correlates with diurnal leg edema and NUV.35 Patients with high salt intake should reduce their daily sodium consumption in order to see a beneficial reduction of nocturnal voids. Along with salt and water, protein consumption may also play a role. Patients with NP demonstrate higher nighttime excretion of urea, indicating that reduction of protein intake in the evening may be beneficial.36 Other dietary interventions, including reduction of alcohol, caffeine, and carbonated beverages, may improve symptoms in some patients; however, the data is limited.37 Limiting caffeine intake, in particular, should be recommended, as caffeine has specifically been shown to be associated with nocturia.38 Caffeine acts not only as a diuretic increasing urine volume, but also has multiple cellular effects that increase bladder contractility.39
Peripheral edema should be evaluated and treated according to the underlying cause (e.g., dietary, venous insufficiency, heart failure).14 General recommendations include late afternoon/evening lower limb elevation (legs above the heart level for 1–3 hours prior to bedtime), use of compression stockings, and diuretics (taken mid-afternoon).14,40–42 These treatments will lead to reduction in nighttime voids in most cases.
Obesity is an independent risk factor for nocturia, with level 1 evidence.43–47 Obesity contributes to OSA, which can lead to NP, as well as to other chronic disorders, along with their required treatments, which may also contribute to nocturia. There is no consensus, however, as to the role of weight loss or the most appropriate way to implement it in the setting of nocturia. The ICS summarizes that an appropriate theoretical diet for an obese patient with high body mass index (BMI) would be a well-balanced, calorie-restricted diet to avoid high output of urea and salt. Other diets need to be critically appraised in the patient with nocturia; for example, many advocate for high water intake, which could worsen nocturia, or low fat, which will not directly affect diuresis. Further research is needed in this area to produce evidence-informed recommendations, especially in the context of a multicultural population with significant variation in individual diets. Exercise and fitness are important elements of weight control and improved sleep habits and can contribute to the management of related chronic diseases, such as DM.17
Common sense would dictate that going to bed with a completely empty bladder reduces one’s likelihood of having to void in the middle of the night. Bladder emptying should be assessed in all patients with nocturia; those who consistently carry significantly elevated residual volumes may benefit from treatment of bladder outlet obstruction (e.g., BPH treatment with alpha-blockers) or performing intermittent catheterization at bedtime. The evidence for bladder training in patients with nocturia comes indirectly from its use in treating OAB, and it is commonly recommended.48,49 Bladder training may be most effective as an adjunct to OAB drugs or other modalities, such as biofeedback and/or electrical stimulation.50,51 Pelvic floor muscle training with biofeedback as part of a behavioral therapy program has proven effective in treating all types of urinary incontinence, and the literature suggests that it can also reduce OAB symptoms, including nocturia, independently or by augmenting the effect of oral OAB agents.52–56
Lifestyle-related diseases, especially uncontrolled hypertension, are associated with an increased risk of NP.57,58 In a Chinese study of 9637 adults, nocturia was associated with cardiovascular disease, hypertension, and DM. Sporting activities were protective in this cohort.59 Efforts should be undertaken to ensure that these chronic comorbid conditions are optimally managed, including any related lifestyle modifications. Management of these conditions, and guidance on appropriate lifestyle/behavioral modifications, is optimally carried out by primary care providers or respective subspecialists.
Recommendations:
– Management of nocturia should be guided by severity and bother. Initial treatment should focus on conservative measures directed at behavioral, lifestyle, and dietary modifications. Patients should be counselled on: total daily and evening fluid restriction (2 L/day or 25% reduction from baseline, and restricting fluids 2+ hours prior to bedtime); bladder training; weight loss and physical activity; management of peripheral edema; and dietary changes, including normal salt intake and decreasing evening protein intake(strong recommendation, moderate level of evidence).
– Counselling on voiding habits and management of coexisting lower urinary tract disorders (e.g., OAB) are optimally performed by urologists; involvement of a patient’s primary care practitioner ± referral to other specialists is appropriate for further lifestyle counselling and management of comorbidities(weak recommendation, very low level of evidence).
Heart failure, fluid status, and other contributing medical conditions
Many authors have drawn an association between cardiovascular morbidity (DM, increased BMI, hypertension, cardiovascular disease, and stroke) and nocturia, but the causality of one vs. the other has not been clearly established.60,61 There are several cardiorenal conditions that lead to NP due to alterations of fluid handling by the kidneys, renal function, and associated diuresis. The underlying pathophysiological changes responsible for this association are numerous. Hypertension leads to changes in glomerular filtration and tubular transport, resetting the renal pressure-natriuresis relationship, and thus leading to NP.62 Repeated arousal from sleep to urinate further raises blood pressure, creating a vicious cycle.63 Addressing the underlying etiology of nocturia increases the likelihood of producing a meaningful improvement in patient symptoms and bother. Indeed, Victor et al demonstrated that the rates of nocturia in men with untreated hypertension or medically treated uncontrolled hypertension were higher than in men with normotension or medically controlled hypertension.64
Heart failure results in atrial stretch and release of ANP, a hormone produced by the heart that stimulates vasodilation and diuresis through the renin-angiotensin axis. Nocturia is common in patients with heart failure, with more than 80% of individuals with stable heart failure reporting one or more nightly episodes of nocturia.65 Lifestyle modifications, such as physical activity, weight loss, and salt restriction, along with postural drainage stockings to decrease peripheral edema, may decrease nocturia episodes.17
Metabolic syndrome and diabetes, which may contribute to nocturia, are associated with oxidative stress, which results in mitochondrial dysfunction in the kidney and bladder.10 This mitochondrial dysfunction may affect AVP-mediated water homeostasis leading to nocturia.10 Patients with DM may develop GP, but diabetes can also contribute to renal tubular dysfunction associated with NP.66
Chronic kidney disease in patients prevents the expected decline in blood pressure during sleep (i.e., non-dipping).67,68 The blunting of nocturnal blood pressure dipping can predispose patients to nocturia.64 This results in enhanced natriuresis and related osmotic diuresis during the night, causing NP.69 Dietary salt, protein, and calorie restrictions are generally advised in chronic renal disease.
Several drugs can alter renal handling of fluid and are, therefore, associated with increased risk of NP, including calcium channel blockers, lithium, and diuretics. Beta-blockers can affect bladder function by decreasing its capacity.7 Additionally, several medications, including antidepressants (monoamine oxidase inhibitors, trazodone), certain antihypertensives (minoxidil, hydralazine, dihydropyridine calcium antagonists, alpha-blockers, antiadrenergic drugs, and non-dihydropyridine calcium antagonists), antivirals (acyclovir), hormonal therapies (sex hormones), non-steroidal anti-inflammatory drugs (NSAIDs) (celecoxib, ibuprofen), and some chemotherapeutics and immunologic agents, can cause peripheral edema, which can further result in postural diuresis.17
The polyuria present in these conditions may also lead to morphological and functional changes of the bladder that can contribute to further voiding dysfunction.70 Interestingly, nocturia has been found to be a marker for increased risk of chronic heart diseases in younger men in later life, as well as death in older men.71
Management of reduced bladder capacity
Management of common conditions that cause reduced functional bladder capacity are covered in detail in other dedicated publications on adult OAB and male LUTS.48,72 The causes for reduced global and nocturnal bladder capacity are numerous but worthy of thoughtful consideration, as they are often modifiable. Treatment of bladder outlet obstruction, involuntary detrusor contractions (OAB and neurogenic lower urinary tract dysfunction [NLUTD]), dysfunctional voiding, cystitis, urolithiasis, and lower urinary tract malignancies all have the potential to increase bladder capacity.
Bladder outlet obstruction
Oral pharmacotherapies for men with bladder outlet obstruction in the setting of prostate enlargement and bothersome nocturia can be considered for management. Options, including monotherapy with an alpha-1-blocker or 5-alpha-reductase inhibitor, or combination therapy with both, have demonstrated only modest clinical improvement in nocturia in this setting, and patients should be counselled on this.73–75
While the exact mechanism of action remains unclear, NSAIDs appear to be effective in men with prostate enlargement and nocturia that is not related to NP. Falahatkar et al conducted a randomized controlled trial that randomized men with pharmacologically BPH and refractory nocturia to celecoxib 100 mg nightly or placebo for one month. The authors found that men in the celecoxib arm had a statistically significant reduction in nocturnal voids (5.17 to 2.5) when compared to placebo (5.30 to 5.12). Men randomized to celecoxib also demonstrated a statistically significant reduction in International Prostate Symptoms Scores compared to the placebo arm (i.e., 18.2 to 15.5 vs. 18.4 to 18).76 However, a daily NSAID is not recommended long-term due to potential serious adverse effects.77
Surgical procedures, such as transurethral resection of prostate (TURP), which alleviate bladder outlet obstruction may improve nocturia by decreasing PVR, reducing detrusor overactivity, and modulating sensory afferents in the bladder neck and prostatic urethra. Improvements in nocturia appear modest,78 and as such, surgery should be only be offered to those with other indications for intervention. Studies demonstrate after treatment of BPH, nocturia usually improves at a lower frequency and degree than other LUTS, with reported decreased in nocturia episodes ranging from −0.8 to −1.1.78–82 The caveat is that these studies included patients with multiple bothersome symptoms due to BPH and not patients with isolated or predominant nocturia. Predictors of persistent nocturia after TURP include pre-existing sleep disorders, increased prostate size, metabolic syndrome, and smoking.79,81
Overactive bladder
A meta-analysis evaluating the use of various antimuscarinic agents in patients with nocturia revealed that most antimuscarinics demonstrate no benefit over placebo. Trospium chloride was the most efficacious pharmacological therapy but only reduced nocturnal voids by 0.24 when compared to placebo.83 More recently, fesoterodine was shown to reduce nocturnal voids and improve sleep quality, but only in those without NP.84
Mirabegron, a beta-3-adrenergic agonist, has been shown to significantly reduce mean nocturnal voids (−0.39 episodes) when compared with placebo.85 Mirabegron has also been shown to be effective in reducing nocturia (−0.6 episodes) when used as an add-on to antimuscarinic monotherapy.86
Intradetrusor onabotulinumtoxinA appears to have only a modest effect on nocturia in patients with OAB. A systematic review and meta-analysis found only a 0.25 reduction in mean nocturnal voids compared with placebo.87
Although data on existing pharmacological agents used in the treatment of conditions associated with a reduced bladder capacity do not suggest a significant benefit in their use for patients with NP, urologists should consider treatment of bladder outlet obstruction and OAB where appropriate. Given that these patients often present with a constellation of bothersome LUTS, treatment of these disorders may result in improvement of several LUTS, including nocturia.
Recommendation: Patients presenting with nocturia as part of a constellation of multiple LUTS should be evaluated and treated appropriately for lower urinary tract disorders (e.g., OAB, bladder outlet obstruction, NLUTD) (strong recommendation, low level of evidence).
OSA and other sleep disorders
OSA is a condition where there is repetitive upper airway closure in sleep, either completely (apnea) or partially (hypopnea). This commonly leads to poor sleep quality, nocturnal hypoxemia, and daytime symptoms, such as fatigue. Patients with OSA frequently complain of nocturia, among other, more commonly described symptoms, such as snoring and frequent awakening.
One retrospective review analyzing over 1000 consecutive patients presenting to a sleep clinic found that the sensitivity of nocturia as a predictor of OSA is comparable to that of snoring (84.4 vs. 82.6%). Among this population, the combination of nocturia and snoring as a predictive factor for OSA demonstrated a sensitivity of 97.4%.88 Similarly, the converse appears to be true as well. An apnea/hypopnea index (AHI) >15 (indicating at least moderate OSA) in one multicenter, retrospective study had an odds ratio of 3.15 (1.90–4.81, p=0.025) for nocturia in multivariate analysis.89
One well-known postulate for the physiological basis of this association involves the cardiac response during upper airway obstruction. During an apneic event, the respiratory muscles produce a Müller’s maneuver, whereby the negative intrathoracic pressure can reach as high as −80c mH2O, causing stretch of the atria and ventricles.90 This leads to increased release of atrial and brain natriuretic peptides from the atria and ventricles, respectively. These hormones are well-known to inhibit the secretion of ADH. Sleep disruption itself may also reduce ADH production; there is evidence to suggest that patients with OSA, when compared to controls, demonstrate a reduced serum level of copeptin, which is a C-terminal fragment of ADH.91
Another mechanism may be that hypoxia is caused by increased airway pressure, which in turn, causes pulmonary vasoconstriction. This leads to increased right atrial transmural pressure with a resulting increase in ANP production and ultimately increased renal sodium and water excretion, thus leading to NP.92
A less well-known possible mechanism is the effect of intermittent hypoxia on the bladder itself. A study in mouse models showed that intermittent hypoxia led to detrusor instability, bladder non-compliance, and increased spontaneous contractions.93 This is thought to be related to oxidative stress caused by tissue hypoxia.
Screening for OSA is commonly done with the STOP-Bang questionnaire, consisting of eight simple questions that can be easily performed in the clinic setting.15 Confirmatory testing for OSA involves polysomnography. This is typically performed as a level 1 sleep study, conducted in a specialized testing center. In regions where level 1 polysomnography is not available, unattended level 3 studies are often performed at home instead, however, electroencephalogram monitoring for sleep staging is not usually included in a level 3 study. Monitoring channels include flow signal to detect apnea and hypopnea, respiratory effort to differentiate between obstructive and central apnea, and oximetry monitoring. The most effective treatment for OSA is continuous positive airway pressure (CPAP) therapy.94
To further support that OSA is a risk factor for nocturia, there is evidence that treatment of OSA leads to improvement of nocturia. A 2015 systemic review found that CPAP had a significant decrease in the frequency of nocturia (−2.82, confidence interval [CI] −2.42 to −2.15, p<0.00001) and urine volume (−183.12 mL, CI −248.27 to −117.98, p<0.00001).95 A 2020 prospective, non-controlled, pre- and post-treatment comparison study also found that after one year of CPAP use, the percentage of patients who experience two or more voids per night decreased from 73% to 51% (p<0.001).94 A recent, prospective, non-randomized, control study also showed that post-ischemic stroke, CPAP treatment improved patients’ NPi and urine output.96
In addition to OSA, insomnia and other sleep disorders also increase in prevalence with age.30,97 A careful evaluation of sleep disorders should be undertaken in the evaluation of patients with nocturia, and treatment should be directed accordingly, most commonly under the direction of the primary care provider or a sleep specialist. As well, sleep disturbance can be a cause or consequence of nocturia, and thus on history, it is important to differentiate if the urge to void wakes the patient or if they simply void after waking for another reason. Fundamentals of good sleep hygiene that can be broadly recommended are summarized in a review by Wolkove et al and are available in Table 5.29 In peri-/post-menopausal women, hot flashes may contribute to sleep disturbances, and these can be managed by cooling techniques such as cold packs and changes to sleepwear and the environment (e.g., bedroom fan, air conditioning).98 Avoidance of alcohol and spicy or hot foods is also beneficial in these patients.99
Table 5.
Fundamentals of good sleep hygiene
| What to do | What to avoid |
|---|---|
|
|
Adapted with permission from Wolkove et al. CMAJ 2007;176:1449–54.29
Recommendation: Patients with nocturia should be screened for OSA, and the need for further evaluation, management, or specialist referral can be determined by the primary care provider (strong recommendation, very low level of evidence).
Pharmacological treatment of NP
Several conditions (such as OSA, increased fluid intake prior to bed, and peripheral edema) can increase nocturnal urine output and should be actively managed as part of a treatment plan for NP. Desmopressin (DDAVP™) is a synthetic form of AVP, with a duration of action of approximately 6–14 hours. It works by binding the vasopressin type 2 receptor in the distal tubule of the nephron, which increases the activation of aquaporin channels; this then leads to the reabsorption of water and a reduction in urine volume.100 Recent sex-specific systematic reviews have evaluated the evidence on the clinical efficacy of desmopressin. In women, there was a significant reduction in nocturnal voids (on average 0.5 fewer voids/night compared to placebo) and a significant increase in the duration of the first period of sleep by about one hour.101 The magnitude of effect was quite similar in men,102 and likely has similar efficacy as alpha-blockers in terms of reducing episodes of nocturia in men. In male patients, randomized trials have demonstrated that desmopressin may be synergistic with alpha-blockers,103 however, this has been questioned by systematic reviews.102 It is important to note that many of these clinical trials were carried out in people with nocturia and not specifically NP. Other formulations of desmopressin are available specifically for nocturia, such as a nasal spray104 and a rapidly dissolving tablet,105 with similar efficacy.
Desmopressin formulations are generally well-tolerated. Recommended starting dose is 50–100 mcg taken orally one hour before bedtime, which can be titrated to 200 mcg as desired. Some sublingual formulations (DDAVP melt) have recently been discontinued in Canada and are variably available at this time; dosing is slightly different, with a 60 mcg starting dose and possible titration to a maximum dose of 240 mcg. Intranasal desmopressin has not been adequately studied in NP to be included in these recommendations.
Potential side effects include nausea, diarrhea, dizziness, hypertension, and hyponatremia. Care should be taken in patients with severe liver disease, heart failure, and renal failure. Hyponatremia is the primary side effect of concern, as it can be fatal when severe. Risk factors for clinically significant hyponatremia include increased age (significantly higher after age 65), renal dysfunction, small body mass, female sex, and baseline low-normal sodium levels.106 Desmopressin for management of nocturia should not be used in patients with an estimated glomerular filtration rate <50 mL/min/1.73m2, as the risk of hyponatremia is increased.17 In a large population-based study in the U.S. (which may better represent the real-world use of this medication than clinical trials), hyponatremia occurred in 2.5% of desmopressin users within 30 days, and new cases of hyponatremia continued to occur with long-term use.107 A conservative approach to hyponatremia monitoring is to measure serum sodium levels at baseline and then at followup after one week, one month, and then every six months; this can be tailored to the patient’s individual risk.108,109 If desmopressin dose is changed, hyponatremia monitoring should be restarted again with serum sodium levels at baseline, one week, one month, and then every six months.
Two other, less studied treatment options are also available.109 First, diuretics (such as furosemide) can be given six hours prior to bedtime to increase fluid excretion. Patients must be monitored for potential electrolyte abnormalities and blood pressure and renal function changes. Second, imipramine before bed increases sodium and water reabsorption, and may help with sleep. Physicians should consider whether a baseline electrocardiogram is necessary to assess for the QTc interval and be aware that impramine can potentiate the hyponatremia risk of desmopressin.
Recommendation: Patients with NP should be worked up and optimally managed for contributing etiologies. Those with refractory NP impacting quality of life can be offered pharmacological therapy, including desmopressin, diuretics, and imipramine. Clinicians should counsel patients on potential adverse effects and monitor signs, symptoms, and lab work as needed for each medication. Urologists can consider involvement of primary care physicians or subspecialists for pharmacological management (strong recommendation, low level of evidence).
Summary
Nocturia remains a difficult-to-treat symptom with significant impact on not just quality of life but also morbidity and mortality. It has classically been considered a symptom of the lower urinary tract, and thus has been most commonly managed by urologists. However, the majority of patients have nocturia due at least in part to NP, and many have multiple contributing factors resulting in their symptoms. These multifactorial etiologies are best managed by a multidisciplinary team led by primary care physicians and urologists, and involving geriatricians, sleep specialists, pulmonologists, cardiologists, and endocrinologists as needed. Regardless of etiology, a large proportion of patients respond well to conservative strategies, including behavioral and lifestyle modifications.
Supplementary Information
Footnotes
Appendix available at cuaj.ca
Prior to publication, this best practice report was reviewed by the CUA Guidelines Committee, external experts, and the CUA Board of Directors.
Competing interests: Dr. Nguyen has been a speaker for Astellas and Pfizer. Dr. Nadeau has been an advisory board member for Allergan, Astellas, Boston Scientific, Ferring, and Pfizer; has been a speaker for Allergan, Astellas, Boston Scientific, Ferring, Laborie, Pfizer, Red Leaf Medical, and Searchlight Pharma; has received payment from Allergan, Boston Scientific, Red Leaf Medical, and Searchlight Pharma (preceptorships); and has received a research grant from Pfizer. Dr. Cox has been an advisory board member for Astellas, Pfizer, and TEVA; a speaker for Astellas and Pfizer; and is the site lead of a clinical trial supported by Aquinox. Dr. Campeau has been an advisory board member for Allergan, Astellas, and Coloplast; and has been a speaker for Astellas and Coloplast. Dr. Carlson has been an advisory board member for and received honoraria from Abbvie. The remaining authors do not report any competing personal or financial interests related to this work.
References
- 1.Van Kerrebroeck P, Abrams P, Chaikin D, et al. The standardization of terminology in nocturia: report from the standardization subcommittee of the International Continence Society. BJU Int. 2002;90(Suppl3):11–5. doi: 10.1046/j.1464-410X.90.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 2.Tikkinen KA, Johnson TM, 2nd, Tammela TL, et al. Nocturia frequency, bother, and quality of life: How often is too often? A population-based study in Finland. Eur Urol. 2010;57:488–96. doi: 10.1016/j.eururo.2009.03.080. [DOI] [PubMed] [Google Scholar]
- 3.Kupelian V, Rosen RC, Link CL, et al. Association of urological symptoms and chronic illness in men and women: Contributions of symptom severity and duration — results from the BACH Survey. J Urol. 2009;181:694–700. doi: 10.1016/j.juro.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kupelian V, Fitzgerald MP, Kaplan SA, et al. Association of nocturia and mortality: Results from the Third National Health and Nutrition Examination Survey. J Urol. 2011;185:571–7. doi: 10.1016/j.juro.2010.09.108. [DOI] [PubMed] [Google Scholar]
- 5.Akerla J, Pesonen JS, Poyhonen A, et al. Lower urinary tract symptoms and mortality among Finnish men: The roles of symptom severity and bother. J Urol. 2022;207:1285–94. doi: 10.1097/JU.0000000000002450. [DOI] [PubMed] [Google Scholar]
- 6.Zumrutbas AE, Bozkurt AI, Alkis O, et al. The prevalence of nocturia and nocturnal polyuria: Can new cutoff values be suggested according to age and sex? Int Neurourol J. 2016;20:304–10. doi: 10.5213/inj.1632558.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornu JN, Abrams P, Chapple CR, et al. A contemporary assessment of nocturia: Definition, epidemiology, pathophysiology, and management — a systematic review and meta-analysis. Eur Urol. 2012;62:877–90. doi: 10.1016/j.eururo.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Coyne KS, Zhou Z, Bhattacharyya SK, et al. The prevalence of nocturia and its effect on health-related quality of life and sleep in a community sample in the USA. BJU Int. 2003;92:948–54. doi: 10.1111/j.1464-410X.2003.04527.x. [DOI] [PubMed] [Google Scholar]
- 9.Herschorn S, Gajewski J, Schulz J, et al. A population-based study of urinary symptoms and incontinence: The Canadian Urinary Bladder Survey. BJU Int. 2008;101:52–8. doi: 10.1111/j.1464-410X.2007.07198.x. [DOI] [PubMed] [Google Scholar]
- 10.Birder LA, Van Kerrebroeck PEV. Pathophysiological mechanisms of nocturia and nocturnal polyuria: The contribution of cellular function, the urinary bladder urothelium, and circadian rhythm. Urology. 2019;133S:14–23. doi: 10.1016/j.urology.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 11.Aizen JLC, Olugbade K, Weiss JP. Evaluation and Management of Nocturia. AUA Update Series. 2015;34:353–60. [Google Scholar]
- 12.Bergman AM, Sih AM, Weiss JP. Nocturia: An overview of evaluation and treatment. Bladder. 2015;2:e13. doi: 10.14440/bladder.2015.42. [DOI] [Google Scholar]
- 13.Breyer BN, Shindel AW, Erickson BA, et al. The association of depression, anxiety, and nocturia: A systematic review. J Urol. 2013;190:953–7. doi: 10.1016/j.juro.2013.03.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oelke M, De Wachter S, Drake MJ, et al. Int J Clin Pract. 2017;71:e13027. doi: 10.1111/ijcp.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagappa M, Liao P, Wong J, et al. Validation of the STOP-Bang questionnaire as a screening tool for obstructive sleep apnea among different populations: A systematic review and meta-analysis. PLoS One. 2015;10:e0143697. doi: 10.1371/journal.pone.0143697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bower WF, Everaert K, Ong TJ, et al. Questions to ask a patient with nocturia. Aust J Gen Pract. 2018;47:465–9. doi: 10.31128/AJGP-01-18-4448. [DOI] [PubMed] [Google Scholar]
- 17.Everaert K, Hervé F, Bosch R, et al. International Continence Society consensus on the diagnosis and treatment of nocturia. Neurourol Urodyn. 2019;38:478–98. doi: 10.1002/nau.23939. [DOI] [PubMed] [Google Scholar]
- 18.Bower WF, Rose GE, Ervin CF, et al. TANGO — a screening tool to identify comorbidities on the causal pathway of nocturia. BJU Int. 2017;119:933–41. doi: 10.1111/bju.13774. [DOI] [PubMed] [Google Scholar]
- 19.Oelke M, Adler E, Marschall-Kehrel D, et al. Nocturia: State-of-the-art and critical analysis of current assessment and treatment strategies. World J Urol. 2014;32:1109–17. doi: 10.1007/s00345-014-1396-0. [DOI] [PubMed] [Google Scholar]
- 20.Everaert K, Goessaert AS, Denys MA. Nocturia. Pract Funct Urol. 2016:377–92. doi: 10.1007/978-3-319-25430-2_15. [DOI] [Google Scholar]
- 21.Weiss JP, Lee CL, Blaivas JG. Nocturia in adults. AUA Update Series. 2008;27:85–92. [Google Scholar]
- 22.Wollin T, Laroche B, Psooy K. Canadian guidelines for the management of asymptomatic microscopic hematuria in adults. Can Urol Assoc J. 2009;3:77–80. doi: 10.5489/cuaj.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiss JP, Everaert K. Management of nocturia and nocturnal polyuria. Urology. 2019;133s:24–33. doi: 10.1016/j.urology.2019.09.022. [DOI] [PubMed] [Google Scholar]
- 24.Everaert K, Herve F, Bower W, et al. How can we develop a more clinically useful and robust algorithm for diagnosing and treating nocturia? ICI-RS 2017. Neurourol Urodyn. 2018;37:S46–59. doi: 10.1002/nau.23569. [DOI] [PubMed] [Google Scholar]
- 25.Weiss JP, Monaghan TF, Epstein MR, et al. Future considerations in nocturia and nocturnal polyuria. Urology. 2019;133S:34–42. doi: 10.1016/j.urology.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Hashim H, Blanker MH, Drake MJ, et al. International Continence Society (ICS) report on the terminology for nocturia and nocturnal lower urinary tract function. Neurourol Urodyn. 2019;38:499–508. doi: 10.1002/nau.23917. [DOI] [PubMed] [Google Scholar]
- 27.Hofmeester I, Kollen BJ, Steffens MG, et al. Impact of the International Continence Society (ICS) report on the standardization of terminology in nocturia on the quality of reports on nocturia and nocturnal polyuria: A systematic review. BJU Int. 2015;115:520–36. doi: 10.1111/bju.12753. [DOI] [PubMed] [Google Scholar]
- 28.Burton C, Weiss JP, Parsons M, et al. Reference values for the nocturnal bladder capacity index. Neurourol Urodyn. 2011 Jan;30:52–7. doi: 10.1002/nau.20924. [DOI] [PubMed] [Google Scholar]
- 29.Wolkove N, Elkholy O, Baltzan M, et al. Sleep and aging: 2. Management of sleep disorders in older people. CMAJ. 2007;176:1449–54. doi: 10.1503/cmaj.070335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolkove N, Elkholy O, Baltzan M, et al. Sleep and aging: 1. Sleep disorders commonly found in older people. CMAJ. 2007;176:1299–304. doi: 10.1503/cmaj.060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho SY, Lee SL, Kim IS, et al. Short-term effects of systematized behavioral modification program for nocturia: A prospective study. Neurourol Urodyn. 2012;31:64–8. doi: 10.1002/nau.21186. [DOI] [PubMed] [Google Scholar]
- 32.Hashim H, Abrams P. How should patients with an overactive bladder manipulate their fluid intake? BJU Int. 2008;102:62–6. doi: 10.1111/j.1464-410X.2008.07463.x. [DOI] [PubMed] [Google Scholar]
- 33.Katz EG, MacLachlan LS. Nocturnal enuresis in the adult. Curr Urol Rep. 2020;21:31. doi: 10.1007/s11934-020-00983-2. [DOI] [PubMed] [Google Scholar]
- 34.Tani M, Hirayama A, Torimoto K, et al. Guidance on water intake effectively improves urinary frequency in patients with nocturia. Int J Urol. 2014;21:595–600. doi: 10.1111/iju.12387. [DOI] [PubMed] [Google Scholar]
- 35.Yoshikawa M, Torimoto K, Hirayama A, et al. Daily salt intake is associated with leg edema and nocturnal urinary volume in elderly men. Neurourol Urodyn. 2020;39:1550–6. doi: 10.1002/nau.24401. [DOI] [PubMed] [Google Scholar]
- 36.Alwis US, Delanghe J, Dossche L, et al. Could evening dietary protein intake play a role in nocturnal polyuria? J Clin Med. 2020 Aug 5;9:2532. doi: 10.3390/jcm9082532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson D, Hanna-Mitchell A, Rantell A, et al. Are we justified in suggesting change to caffeine, alcohol, and carbonated drink intake in lower urinary tract disease? Report from the ICI-RS 2015. Neurourol Urodyn. 2017;36:876–81. doi: 10.1002/nau.23149. [DOI] [PubMed] [Google Scholar]
- 38.Liao YM, Dougherty MC, Biemer PP, et al. Factors related to lower urinary tract symptoms among a sample of employed women in Taipei. Neurourol Urodyn. 2008;27:52–9. doi: 10.1002/nau.20457. [DOI] [PubMed] [Google Scholar]
- 39.Lohsiriwat S, Hirunsai M, Chaiyaprasithi B. Effect of caffeine on bladder function in patients with overactive bladder symptoms. Urol Ann. 2011;3:14–8. doi: 10.4103/0974-7796.75862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reynard JM, Cannon A, Yang Q, et al. A novel therapy for nocturnal polyuria: A double-blind, randomized trial of frusemide against placebo. Br J Urol. 1998;81:215–8. doi: 10.1046/j.1464-410X.1998.00511.x. [DOI] [PubMed] [Google Scholar]
- 41.Torimoto K, Hirayama A, Samma S, et al. The relationship between nocturnal polyuria and the distribution of body fluid: Assessment by bioelectric impedance analysis. J Urol. 2009;181:219–24. doi: 10.1016/j.juro.2008.09.031. discussion 224. [DOI] [PubMed] [Google Scholar]
- 42.Viaene A, Roggeman S, Goessaert AS, et al. Conservative treatment for leg oedema and the effect on nocturnal polyuria in patients with spinal cord injury. BJU Int. 2019;123:E43–50. doi: 10.1111/bju.14672. [DOI] [PubMed] [Google Scholar]
- 43.Burgio KL, Johnson TM, 2nd, Goode PS, et al. Prevalence and correlates of nocturia in community-dwelling older adults. J Am Geriatr Soc. 2010;58:861–6. doi: 10.1111/j.1532-5415.2010.02822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cruz R, Garcia-Rosa M, Faria C. Nocturia: Prevalence and associated factors in community-dwelling subjects — a population-based study. Rev Assoc Med Bras. 2020;66:830–7. doi: 10.1590/1806-9282.66.6.830. [DOI] [PubMed] [Google Scholar]
- 45.Madhu C, Coyne K, Hashim H, et al. Nocturia: Risk factors and associated comorbidities; findings from the EpiLUTS study. Int J Clin Pract. 2015;69:1508–16. doi: 10.1111/ijcp.12727. [DOI] [PubMed] [Google Scholar]
- 46.Tikkinen KA, Auvinen A, Johnson TM, 2nd, et al. A systematic evaluation of factors associated with nocturia — the population-based FINNO study. Am J Epidemiol. 2009;170:361–8. doi: 10.1093/aje/kwp133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaughan CP, Auvinen A, Cartwright R, et al. Impact of obesity on urinary storage symptoms: Results from the FINNO study. J Urol. 2013 Apr;189:1377–82. doi: 10.1016/j.juro.2012.10.058. [DOI] [PubMed] [Google Scholar]
- 48.Corcos J, Przydacz M, Campeau L, et al. CUA guideline on adult overactive bladder. Can Urol Assoc J. 2017;11:E142–73. doi: 10.5489/cuaj.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gormley EA, Lightner DJ, Burgio KL, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. J Urol. 2012;188:2455–63. doi: 10.1016/j.juro.2012.09.079. [DOI] [PubMed] [Google Scholar]
- 50.Firinci S, Yildiz N, Alkan H, et al. Which combination is most effective in women with idiopathic overactive bladder, including bladder training, biofeedback, and electrical stimulation? A prospective randomized controlled trial. Neurourol Urodyn. 2020;39:2498–2508. doi: 10.1002/nau.24522. [DOI] [PubMed] [Google Scholar]
- 51.Rai BP, Cody JD, Alhasso A, et al. Anticholinergic drugs versus non-drug active therapies for non-neurogenic overactive bladder syndrome in adults. Cochrane Database Syst Rev. 2012;12:CD003193. doi: 10.1002/14651858.CD003193.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burgio KL, Goode PS, Locher JL, et al. Behavioral training with and without biofeedback in the treatment of urge incontinence in older women: A randomized controlled trial. JAMA. 2002;288:2293–9. doi: 10.1001/jama.288.18.2293. [DOI] [PubMed] [Google Scholar]
- 53.Burgio KL, Kraus SR, Johnson TM, 2nd, et al. Effectiveness of combined behavioral and drug therapy for overactive bladder symptoms in men: A randomized clinical trial. JAMA Intern Med. 2020;180:411–9. doi: 10.1001/jamainternmed.2019.6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burgio KL, Locher JL, Goode PS, et al. Behavioral vs. drug treatment for urge urinary incontinence in older women: A randomized controlled trial. JAMA. 1998;280:1995–2000. doi: 10.1001/jama.280.23.1995. [DOI] [PubMed] [Google Scholar]
- 55.Dumoulin C, Cacciari LP, Hay-Smith EJC. Pelvic floor muscle training vs. no treatment, or inactive control treatments for urinary incontinence in women. Cochrane Database Syst Rev. 2018;10:CD005654. doi: 10.1002/14651858.CD005654.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson TM, 2nd, Burgio KL, Redden DT, et al. Effects of behavioral and drug therapy on nocturia in older incontinent women. J Am Geriatr Soc. 2005;53:846–50. doi: 10.1111/j.1532-5415.2005.53260.x. [DOI] [PubMed] [Google Scholar]
- 57.Natsume O. A clinical investigation of nocturnal polyuria in patients with nocturia: A diurnal variation in arginine vasopressin secretion and its relevance to mean blood pressure. J Urol. 2006;176:660–4. doi: 10.1016/j.juro.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 58.Yokoyama O, Nishizawa O, Homma Y, et al. Nocturnal polyuria and hypertension in patients with lifestyle related diseases and overactive bladder. J Urol. 2017;197:423–31. doi: 10.1016/j.juro.2016.08.087. [DOI] [PubMed] [Google Scholar]
- 59.Wen L, Wen YB, Wang ZM, et al. Risk factors of nocturia (two or more voids per night) in Chinese people older than 40 years. Neurourol Urodyn. 2015;34:566–70. doi: 10.1002/nau.22623. [DOI] [PubMed] [Google Scholar]
- 60.Parthasarathy S, Fitzgerald M, Goodwin JL, et al. Nocturia, sleep-disordered breathing, and cardiovascular morbidity in a community-based cohort. PLoS One. 2012;7:e30969. doi: 10.1371/journal.pone.0030969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fitzgerald MP, Litman HJ, Link CL, et al. The association of nocturia with cardiac disease, diabetes, body mass index, age, and diuretic use: Results from the BACH survey. J Urol. 2007 Apr;177:1385–9. doi: 10.1016/j.juro.2006.11.057. [DOI] [PubMed] [Google Scholar]
- 62.Feldstein CA. Nocturia in arterial hypertension: a prevalent, underreported, and sometimes underestimated association. J Am Soc Hypertens. 2013;7:75–84. doi: 10.1016/j.jash.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 63.Kamperis K, Hagstroem S, Radvanska E, et al. Excess diuresis and natriuresis during acute sleep deprivation in healthy adults. Am J Physiol Renal Physiol. 2010;299:F404–11. doi: 10.1152/ajprenal.00126.2010. [DOI] [PubMed] [Google Scholar]
- 64.Victor RG, Li N, Blyler CA, et al. Nocturia as an unrecognized symptom of uncontrolled hypertension in black men aged 35–49 years. J Am Heart Assoc. 2019;8:e010794. doi: 10.1161/JAHA.118.010794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Redeker NS, Adams L, Berkowitz R, et al. Nocturia, sleep and daytime function in stable heart failure. J Cardiac Fail. 2012;18:569–75. doi: 10.1016/j.cardfail.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aoki Y, Yokoyama O. Metabolic syndrome and nocturia. Low Urin Tract Sympt. 2012;4(Suppl1):11–5. doi: 10.1111/j.1757-5672.2011.00118.x. [DOI] [PubMed] [Google Scholar]
- 67.Agarwal R, Light RP, Bills JE, et al. Nocturia, nocturnal activity, and non-dipping. Hypertension. 2009;54:646–51. doi: 10.1161/HYPERTENSIONAHA.109.135822. [DOI] [PubMed] [Google Scholar]
- 68.Takayama M, Omori S, Iwasaki K, et al. Relationship between nocturnal polyuria and non-dipping blood pressure in male patients with lower urinary tract symptoms. Low Urin Tract Sympt. 2019;11:O98–102. doi: 10.1111/luts.12225. [DOI] [PubMed] [Google Scholar]
- 69.Fukuda M, Motokawa M, Miyagi S, et al. Polynocturia in chronic kidney disease is related to natriuresis rather than to water diuresis. Nephrol Dial Transplant. 2006;21:2172–7. doi: 10.1093/ndt/gfl165. [DOI] [PubMed] [Google Scholar]
- 70.Velasquez Flores M, Mossa AH, Cammisotto P, et al. Bladder overdistension with polyuria in a hypertensive rat model. Neurourol Urodyn. 2018;37:1904–12. doi: 10.1002/nau.23550. [DOI] [PubMed] [Google Scholar]
- 71.Lightner DJ, Krambeck AE, Jacobson DJ, et al. Nocturia is associated with an increased risk of coronary heart disease and death. BJU Int. 2012;110:848–53. doi: 10.1111/j.1464-410X.2011.10806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nickel JC, Aaron L, Barkin J, et al. Canadian Urological Association guideline on male lower urinary tract symptoms/benign prostatic hyperplasia (MLUTS/BPH): 2018 update. Can Urol Assoc J. 2018;12:303–12. doi: 10.5489/cuaj.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnson TM, 2nd, Jones K, Williford WO, et al. Changes in nocturia from medical treatment of benign prostatic hyperplasia: Secondary analysis of the Department of Veterans Affairs Cooperative Study Trial. J Urol. 2003;170:145–8. doi: 10.1097/01.ju.0000069827.09120.79. [DOI] [PubMed] [Google Scholar]
- 74.Johnson TM, 2nd, Burrows PK, Kusek JW, et al. The effect of doxazosin, finasteride, and combination therapy on nocturia in men with benign prostatic hyperplasia. J Urol. 2007;178:2045–50. doi: 10.1016/j.juro.2007.07.013. discussion 2050–1. [DOI] [PubMed] [Google Scholar]
- 75.Eisenhardt A, Schneider T, Cruz F, et al. Consistent and significant improvement of nighttime voiding frequency (nocturia) with silodosin in men with LUTS suggestive of BPH: Pooled analysis of three randomized, placebo-controlled, double-blind, phase 3 studies. World J Urol. 2014;32:1119–25. doi: 10.1007/s00345-013-1228-7. [DOI] [PubMed] [Google Scholar]
- 76.Falahatkar S, Mokhtari G, Pourreza F, et al. Celecoxib for treatment of nocturia caused by benign prostatic hyperplasia: A prospective, randomized, double-blind, placebo-controlled study. Urology. 2008;72:813–6. doi: 10.1016/j.urology.2008.04.069. [DOI] [PubMed] [Google Scholar]
- 77.Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem Pharmacol. 2020;180:114147. doi: 10.1016/j.bcp.2020.114147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Simaioforidis V, Papatsoris AG, Chrisofos M, et al. Tamsulosin vs. transurethral resection of the prostate: Effect on nocturia as a result of benign prostatic hyperplasia. Int J Urol. 2011;18:243–8. doi: 10.1111/j.1442-2042.2010.02704.x. [DOI] [PubMed] [Google Scholar]
- 79.De Nunzio C, Tema G, Lombardo R, et al. Metabolic syndrome and smoking are associated with persistence of nocturia after transurethral resection of the prostate. Neurourol Urodyn. 2019;38:1692–9. doi: 10.1002/nau.24041. [DOI] [PubMed] [Google Scholar]
- 80.Margel D, Lifshitz D, Brown N, et al. Predictors of nocturia quality of life before and shortly after prostatectomy. Urology. 2007;70:493–7. doi: 10.1016/j.urology.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 81.Wada N, Numata A, Hou K, et al. Nocturia and sleep quality after transurethral resection of the prostate. Int J Urol. 2014;21:81–5. doi: 10.1111/iju.12185. [DOI] [PubMed] [Google Scholar]
- 82.Yoshida M, Inadome A, Masunaga K, et al. Effectiveness of tamsulosin hydrochloride and its mechanism in improving nocturia associated with lower urinary tract symptoms/benign prostatic hyperplasia. Neurourol Urodyn. 2010;29:1276–81. doi: 10.1002/nau.20872. [DOI] [PubMed] [Google Scholar]
- 83.Buser N, Ivic S, Kessler TM, et al. Efficacy and adverse events of antimuscarinics for treating overactive bladder: Network meta-analyses. Eur Urol. 2012;62:1040–60. doi: 10.1016/j.eururo.2012.08.060. [DOI] [PubMed] [Google Scholar]
- 84.Yokoyama O, Hiro S, Hotta S, et al. Efficacy of fesoterodine on nocturia and quality of sleep in Asian patients with overactive bladder. Urology. 2014;83:750–5. doi: 10.1016/j.urology.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 85.Chapple CR, Amarenco G, Lopez Aramburu MA, et al. A proof-of-concept study: Mirabegron, a new therapy for overactive bladder. Neurourol Urodyn. 2013;32:1116–22. doi: 10.1002/nau.22373. [DOI] [PubMed] [Google Scholar]
- 86.Yamaguchi O, Kakizaki H, Homma Y, et al. Safety and efficacy of mirabegron as ‘add-on’ therapy in patients with overactive bladder treated with solifenacin: A post-marketing, open-label study in Japan (MILAI study) BJU Int. 2015;116:612–22. doi: 10.1111/bju.13068. [DOI] [PubMed] [Google Scholar]
- 87.Sun Y, Luo D, Tang C, et al. The safety and efficiency of onabotulinumtoxinA for the treatment of overactive bladder: A systematic review and meta-analysis. Int Urol Nephrol. 2015;47:1779–88. doi: 10.1007/s11255-015-1125-7. [DOI] [PubMed] [Google Scholar]
- 88.Romero E, Krakow B, Haynes P, et al. Nocturia and snoring: Predictive symptoms for obstructive sleep apnea. Sleep Breath. 2010;14:337–43. doi: 10.1007/s11325-009-0310-2. [DOI] [PubMed] [Google Scholar]
- 89.Arslan B, Gezmis CT, Cetin B, et al. Is obstructive sleep apnea syndrome related to nocturia? Low Urin Tract Sympt. 2019;11:139–42. doi: 10.1111/luts.12250. [DOI] [PubMed] [Google Scholar]
- 90.Shiomi T, Guilleminault C, Stoohs R, et al. Leftward shift of the interventricular septum and pulsus paradoxus in obstructive sleep apnea syndrome. Chest. 1991;100:894–902. doi: 10.1378/chest.100.4.894. [DOI] [PubMed] [Google Scholar]
- 91.Ozben S, Guvenc TS, Huseyinoglu N, et al. Low serum copeptin levels in patients with obstructive sleep apnea. Sleep Breath. 2013;17:1187–92. doi: 10.1007/s11325-013-0822-7. [DOI] [PubMed] [Google Scholar]
- 92.Marshall SD, Weiss JP. Nocturia. In: Partin AW, Dmochowski RR, Kavoussi MD, Peters CA, editors. Urology. 12 ed. chap 119 Elsevier; 2020. pp. 2664–2678. Campbell-Walsh-Wein. [Google Scholar]
- 93.Witthaus MW, Nipa F, Yang JH, et al. Bladder oxidative stress in sleep apnea contributes to detrusor instability and nocturia. J Urol. 2015;193:1692–9. doi: 10.1016/j.juro.2014.11.055. [DOI] [PubMed] [Google Scholar]
- 94.Vrooman OPJ, van Balken MR, van Koeveringe GA, et al. The effect of continuous positive airway pressure on nocturia in patients with obstructive sleep apnea syndrome. Neurourol Urodyn. 2020;39:1124–8. doi: 10.1002/nau.24329. [DOI] [PubMed] [Google Scholar]
- 95.Wang T, Huang W, Zong H, et al. The efficacy of continuous positive airway pressure therapy on nocturia in patients with obstructive sleep apnea: A systematic review and meta-analysis. Int Neurourol J. 2015;19:178–84. doi: 10.5213/inj.2015.19.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu CC, Huang CY, Kuo WK, et al. Continuous positive airway pressure improves nocturnal polyuria in ischemic stroke patients with obstructive sleep apnea. Clin Interv Aging. 2019;14:241–7. doi: 10.2147/CIA.S193448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miner B, Kryger MH. Sleep in the aging population. Sleep Med Clin. 2017;12:31–8. doi: 10.1016/j.jsmc.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pauwaert K, Goessaert AS, Ghijselings L, et al. Nocturia through the menopausal transition and beyond: A narrative review. Int Urogynecol J. 2021;32:1097–1106. doi: 10.1007/s00192-020-04640-7. [DOI] [PubMed] [Google Scholar]
- 99.Carpenter JGM, Maki PM, Newton KM, et al. Nonhormonal management of menopause-associated vasomotor symptoms. Menopause. 2015;22:1155–74. doi: 10.1097/GME.0000000000000546. [DOI] [PubMed] [Google Scholar]
- 100.Chung E. Desmopressin and nocturnal voiding dysfunction: Clinical evidence and safety profile in the treatment of nocturia. Expert Opin Pharmacother. 2018;19:291–8. doi: 10.1080/14656566.2018.1429406. [DOI] [PubMed] [Google Scholar]
- 101.Cai X, Tian Y, Nie M, et al. Efficacy and safety of desmopressin in women with nocturia: A systematic review and meta-analysis of randomized controlled trials. Int Urol Nephrol. 2019;51:1913–23. doi: 10.1007/s11255-019-02242-x. [DOI] [PubMed] [Google Scholar]
- 102.Han J, Jung JH, Bakker CJ, et al. Desmopressin for treating nocturia in men. BJU Int. 2018;122:549–59. doi: 10.1111/bju.14183. [DOI] [PubMed] [Google Scholar]
- 103.Kim JC, Cho KJ, Lee JG, et al. Efficacy and safety of desmopressin add-on therapy for men with persistent nocturia on alpha-blocker monotherapy for lower urinary tract symptoms: A randomized, double-blind, placebo-controlled study. J Urol. 2017;197:459–64. doi: 10.1016/j.juro.2016.08.116. [DOI] [PubMed] [Google Scholar]
- 104.Kaminetsky J, Fein S, Dmochowski R, et al. Efficacy and safety of SER120 nasal spray in patients with nocturia: Pooled analysis of 2 randomized, double-blind, placebo controlled, phase 3 trials. J Urol. 2018;200:604–11. doi: 10.1016/j.juro.2018.04.050. [DOI] [PubMed] [Google Scholar]
- 105.Weiss JP, van der Meulen EA, Juul KV. Low-dose desmopressin orally disintegrating tablet: Suggested clinically meaningful benefit in patients with nocturia due to nocturnal polyuria. Eur Urol Focus. 2020;6:1006–12. doi: 10.1016/j.euf.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 106.Rembratt A, Riis A, Norgaard JP. Desmopressin treatment in nocturia; an analysis of risk factors for hyponatremia. Neurourol Urodyn. 2006;25:105–9. doi: 10.1002/nau.20168. [DOI] [PubMed] [Google Scholar]
- 107.Fralick M, Schneeweiss S, Wallis CJD, et al. Desmopressin and the risk of hyponatremia: A population-based cohort study. PLoS Med. 2019;16:e1002930. doi: 10.1371/journal.pmed.1002930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Juul KV, Malmberg A, van der Meulen E, et al. Low-dose desmopressin combined with serum sodium monitoring can prevent clinically significant hyponatraemia in patients treated for nocturia. BJU Int. 2017;119:776–84. doi: 10.1111/bju.13718. [DOI] [PubMed] [Google Scholar]
- 109.Li ESW, Flores VX, Weiss JP. Current guidelines and treatment paradigms for nocturnal polyuria: A “NEW” disease state for US physicians, patients and payers. Int J Clin Pract. 2019;73:e13337. doi: 10.1111/ijcp.13337. [DOI] [PubMed] [Google Scholar]
- 110.Dani H, Esdaille A, Weiss JP. Nocturia: Etiology and treatment in adults. Nat Rev Urol. 2016;13:573–83. doi: 10.1038/nrurol.2016.134. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.