Abstract
Compromised stability of pharmaceutical formulations loaded with volatiles is a serious problem associated with devices designed to deliver volatile compounds. The present study has been focused to evaluate the stability potential of matrix-type polymeric patches composed of volatile ethyl anthranilate for prophylaxis against vector-borne diseases. Ethyl anthranilate-loaded matrix-type polymeric patches were fabricated by solvent evaporation method on an impermeable backing membrane and attached to temporary release liners. Stability testing of the polymeric patches was performed as per the International Conference on Harmonization (ICH) guidelines for 6 months under accelerated conditions. In addition, the quantification of residual solvents was also performed as per the ICH guidelines. After conducting the stability studies for 6 months, the optimized patches showed the best possible results with respect to uniformity of drug content, physical appearance, and other analytical parameters. Furthermore, the amount of residual solvent was found well below the accepted limit. Thus, the present report outlined the analytical parameters to be evaluated to ensure the stability of a certain devices consisting of volatile compounds.
Keywords: Controlled release, Ethyl 2-aminobenzoate, Insect repellent, ICH guidelines, Polymeric patch
1. Introduction
Pharmaceutical formulations designed to deliver volatile compounds, such as insect repellents, herbicides, pesticides, essential oils, flavours, perfumes, and pheromones, are subjected to degradation because the volatiles are lost easily due to their inherent properties as well as due to the influence of different environmental factors, including heat and humidity. Thus, the stability potential of a very important pharmaceutical formulation is compromised, which is highly undesirable. With the advent of modern technology, both natural (e.g., ethyl cellulose, gelatin, dextran, etc.) and synthetic polymers (e.g., polyvinyl alcohol, polyvinyl chloride, etc.) have been widely used in different fields as biomaterials, controlled drug delivery, in protection of functional ingredients, etc. [1–3]. However, the end product designed with polymeric materials should remain relatively stable to storage conditions over a long period. Polymeric materials used for designing different devices not only enhance the physical–chemical stability but also the safety by entrapping the volatile compounds in their matrix, thereby releasing the volatiles at a desired controlled rate [4].
Stability testing is a significant prerequisite criterion that warrants the efficiency and safety of a particular pharmaceutical product to sustain its properties throughout its declared shelf life under predefined conditions [5–8]. Stability testing of pharmaceutical products is a complex set of procedures involving scientific expertise, considerable cost, and time in order to build in quality, efficacy, and safety in a drug formulation [6,9]. The chemical, physical, and microbiological aspects of stability are generally assessed by adhering to the International Conference on Harmonization (ICH) guidelines with two primary goals: (1) selection of an effective formulation and packaging material; and (2) estimation of shelf life and suitable storage conditions for the formulation [10]. A comprehensive development plan includes pharmaceutical analysis and stability studies that not only ensure the identity, potency, and purity of ingredients as well as those of the formulated products but also determine scientific and commercial success of a pharmaceutical product [11,12].
Ethyl anthranilate (EA, CAS. 87-25-2), chemically known as ethyl 2-aminobenzoate, is a new member in the realm of entomology and has drawn significant attention in repellent research in the recent years; it is considered as an improved alternative to DEET, chemically known as diethyltoluamide [13–16]. Insect repellent formulation containing EA has not been attempted till date in any form; therefore, there is a vast opportunity in the formulation development. However, because of the volatile nature of EA, fabrication of a controlled-release insect repellent formulation is difficult. Membrane-moderated matrix-type monolithic polymeric device or otherwise known as “polymeric patch” is a newer invention and has been a subject of scientific investigations recently. The polymeric patch is a versatile, solid-state, asymmetric (relatively small molecules of one or more active principles mixed with large polymer molecules), controlled-release diffusing system in which the active material is dispersed in a rate-controlling polymer matrix [17]. A polymeric patch is a promising and an attractive option to deliver volatile compounds in a controlled-release manner [17–20]. Its advantage over the traditional control measures is that it may be affixed to hat, collar, cuffs, shirt, belt, pant, boots, socks, and certainly may also be affixed to furniture, walls, or appliances too [21].
In the present study, EA-loaded polymeric patch has been developed in our laboratory for prophylaxis against different vector-borne diseases, and their stability potential was evaluated as per the ICH guidelines [10]. Further, in the present investigation, the residual solvents were quantified to assure the quality attributes of developed patches as per the ICH guidelines [22]. The study would be useful for the routine analysis of formulation containing EA not only in topical prophylactic polymeric patch formulation but also in other dosage forms.
Thus, the overall study put forward a one-step novel method to confirm the stability aspects of an emerging insect repellent prophylactic patch.
2. Methods and materials
2.1. Materials
The following chemicals were purchased from different chemical suppliers and used as received. EA, phthalate esters, acetonitrile, and water were purchased from Sigma-Aldrich (Sigma-Aldrich Chemical Co., St. Louis, MO, USA). Chloroform, dimethyl sulfoxide, ethyl cellulose, and polyvinylpyrrolidone K-30 were purchased from HiMedia (HiMedia Laboratories Pvt. Ltd, Maharashtra, India). A 3M double-coated polyethylene tape 9766 was received from 3M (3M, St. Paul, USA) as a gift sample. All reagents and solvents used were of analytical grade.
2.2. Methods
2.2.1. Preparation of matrix-type polymeric patch
Matrix-type polymeric patch was prepared by dry casting solvent evaporation method with slight modifications, as per the method described by Chattopadhyay et al [23,24]. Briefly, varied ratio of ethyl cellulose and polyvinylpyrrolidone K-30 was dissolved in a solvent of chloroform and water (96:4). After stirring for a certain period of time, EA and phthalate ester, which was used as a plasticizer, were added to the resultant mixture with continuous stirring at room temperature until a homogeneous mixture was obtained. This mixture was degassed and subsequently moulded into a ring in a customized mould with defined surface area and thickness over a horizontal surface of suitable backing membrane, followed by solvent evaporation at ambient temperature for next the 48 hours. The rate of evaporation was controlled by inverting a funnel. The formed polymeric patches were separated and attached to a temporary release liner and finally stored in desiccators under vacuum until analysis.
2.2.2. Accelerated stability-testing studies
The best optimized matrix-type polymeric patches were subjected to accelerated stability testing for 6 months as per the ICH guidelines at 40 ± 2°C and relative humidity of 75 ± 5% [10]. The samples were placed inside a stability chamber (Thermolab Scientific Equipments Pvt. Ltd, Maharashtra, India) in a sealed aluminum pouch and collected at different time intervals (0, 15, 30, 60, 90, 180 days) for physical appearance, surface morphology, EA content uniformity, etc. At Day 0 and after Day 180, fourier transform infrared spectroscopy (FTIR), scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM-EDX), differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), and X-ray powder diffraction (XRD) studies were performed to confirm the stability of the matrix-type polymeric patches (Table 1).
Table 1.
Points of analysis.
| Points of analysis | Analysis |
|---|---|
| 1 | Ethyl anthranilate content |
| 2 | Visual inspection |
| 3 | SEM-EDX |
| 4 | FTIR |
| 5 | DSC-TGA |
| 6 | XRD |
DSC-TGA = differential scanning calorimetry with thermogravimetric analysis; FTIR = fourier transform infrared spectroscopy; SEM-EDX = scanning electron microscopy with energy dispersive X-ray spectroscopy; XRD = X-ray powder diffraction.
2.2.3. Content uniformity of EA
EA-loaded optimized patches were stored as per the study guidelines, followed by the periodical (0, 15, 30, 60, 90, 180 days) collection of samples for the assessment of content uniformity of EA. The content uniformity of EA was assured by reversed phase high-performance liquid chromatography (Agilent Technology, 1260 Infinity, Germany) with the method developed in our laboratory, as per the ICH guidelines [10], using an isocratic mode of elution with the following chromatographic conditions: (1) mobile phase composition: mixture of acetonitrile and water at a ratio of 75:25 (v/v); (2) flow rate: 1 mL/min; (3) injection volume: 20 μL; (4) detection wavelength: 220 nm using diode array detector; (5) column: ZORBAX eclipse plus C18 column (250 mm × 4.6 mm) with mean particle size of 5μm; (6) guard column: ZORBAX eclipse plus C18 guard column (12.5 mm × 4.6 mm) with mean particle size of 5μm; and (7) chromatographic software: Open LAB CDS chemstation chromatographic software (version C.01.04).
For high-performance liquid chromatography (HPLC) analysis, the polymeric patches were transferred to 100 mL volumetric flasks and extracted with 20 mL of 1% sulfuric acid in methanol. The flasks were shaken mechanically for about 2 hours, followed by centrifugation at 6000g for 15 minutes, and the supernatants were collected. The supernatants were transferred to 10 mL volumetric flask and further diluted with mobile phase. The aliquots were filtered through 13 mm × 0.45 μm nylon syringe filter (HiMedia Laboratories Pvt. Ltd, Maharashtra, India) and stored under refrigeration at 4°C (±0.5°C) until use.
2.2.4. Physical appearance
Polymeric patches were visually inspected at regular intervals to observe for the alteration of colour, shape, clarity, smoothness, uniformity, flexibility, and other physical changes that may occur during the period of storage.
2.2.5. SEM-EDX analysis
The surface morphology, structure, and chemical transformation and chemical mapping in terms of inorganic chemistry of polymeric patches were examined using JEOL SEM-EDX (JSM-6390 LV, SEM-EDX, MA, USA). From the developed patch, required size was cut and mounted onto the specimen stub using double-sided adhesive tape. The surface of the polymeric patches was coated with platinum using sputter coater to prevent charging followed by scanning electron microscopy. EDX identifies the elemental composition of polymeric patch and is used as an alternative tool to substantiate the integrity of the desired patch.
2.2.6. FTIR analysis
Physical and chemical interactions between the different components of polymeric patches, such as polymers, plasticizers and EA, were assessed using FTIR (Thermo Scientific Instruments, Maharashtra, India). The sample was placed in the sample holder and scanned in the wave number region 4000–500 cm−1 at the scan speed of 2 mm/s and resolution of 4 cm−1.
2.2.7. Thermal analysis
Initially, the samples were heated to remove moisture, and each sample (4–10 mg) was placed on an aluminum cassette and sealed hermetically. DSC and TGA of polymeric patches were performed using a DSC-50 (Shimadzu DSC-50) and TGA-50 (Shimadzu TGA-50) analyzer, respectively, at a constant nitrogen flow (30 mL/min) and heating at a rate of 10°C/min up to 600°C. Midpoint criterion was used to reproduce the glass transition temperature (Tg) for each sample.
2.2.8. XRD analysis
Polymeric patches were analyzed using an X-ray diffractometer (Miniflex, Rigaku Corporation, Japan) at room temperature (25°C). The samples were characterised in powdered form, and the polymeric patches were analyzed for their diffraction patterns. The samples were placed in sample holder and XRD patterns were recorded at 5°C/min over the range of 2θ = 5–80°C.
2.2.9. Analysis of residual solvents
Residual solvents play an important role in the synthesis of drugs and in product formulations; however, they do not possess any therapeutic benefits. In addition, they pose a serious problem that is toxicity as many of them exhibit toxic or environmentally hazardous properties [25]. Therefore, all residual solvents should be removed to the extent possible to meet product specifications, good manufacturing practices, or other quality-based requirements. Thus, the determination of residual solvents in pharmaceuticals has become one of the most important tasks in quality assurance/quality control (QA/QC) in the pharmaceutical industry [22]. Analytical procedures based on gas chromatography (GC) are the most popular and chemically specific for determination of residual solvents. In routine QC, GC with flame ionization detection (FID) is preferred, whereas mass spectrometry (MS) can be used for screening and identification [26,27].
EA-loaded matrix-type polymeric patches evaluated in the present investigation were fabricated using chloroform (Class II residual solvents) as a solvent. Therefore, it is very important to quantify the levels of chloroform in polymeric patches. The quantification experiments were performed on a model GC-Trace GC Ultra (Thermo Scientific, USA), as per the method described elsewhere with slight modifications [28]. Listed below are the GC conditions used for the quantification of chloroform: (1) detector: FID system and detector temperature was kept at 240°C; (2) column: TR-5MS capillary column (30 m × 0.25 mm ID) with 0.25 μm film thickness (Thermo Scientific, USA); (3) oven temperature program: 70°C held for 1 minute, followed by increasing the temperature at 10°C/min to 220°C, at 5°C/min to 270°C, and kept at this temperature for 5 minutes; (4) injection port: the injection port temperature was set at 230°C, and its inlet was operated under the split mode (split ratio: 10:1); (5) carrier gas: nitrogen and its on-column flow rate was set at 38.3 cm/s; (6) total run time: 30 minutes; and (7) chromatographic software: Chrom Card chromatographic software (version 2.9).
For the analysis of residual solvents in pharmaceutical products, the drug substance or drug product is typically dissolved in a high-boiling point (low-volatility) solvent, such as dimethyl acetamide, dimethyl sulfoxide (DMSO), 1,3-dimethyl-2-imidazolidinone, benzyl alcohol, hexane, ethylene glycol, etc. Using high-boiling point solvents has the advantage that the solvent peak will elute later, thus not interfering with the earlier eluting analyte peaks. Therefore, the standard solution of chloroform was prepared in DMSO at the concentration of 600 μg/mL (600 μg/mL is the prescribed concentration for the preparation of standard solution of Class II solvents). Aliquots of chloroform (100 μg/mL and 200 μg/mL) were further prepared by properly diluting the standard solution.
The polymeric patches were also extracted with 20 mL DMSO in 100 mL volumetric flasks. The flasks were shaken mechanically for about 2 hours, followed by centrifugation at 6000g for 15 minutes, and the supernatants were collected. Next, the supernatants were filtered through 13 mm × 0.45 μm nylon syringe filter (HiMedia Laboratories Pvt. Ltd, Maharashtra, India) and subsequently injected for GC analysis. Each sample was analyzed three times.
3. Results and discussion
The best optimized matrix-type polymeric patch loaded with EA was subjected to accelerated stability testing as per the ICH guidelines [10]. The stability testing was performed for 6 months by storing the patches at 40 ± 2°C and relative humidity of 75 ± 5%. After 6 months, the optimized formulation was found stable in terms of drug content uniformity and cumulative percentage release of EA. In addition, the stability of the optimized patch was further ascertained by defining integrity and compatibility of the final formulation by means of FTIR, DSC, TGA, and XRD analysis. The surface morphology and the chemical mapping further vouched the stability of the formulation (SEM-EDX studies).
3.1. Content uniformity of EA
EA content uniformity during 0–180 days storage was determined using the HPLC method developed in our laboratory. The content of EA in the best optimized matrix-type polymeric patches at day 0, 15, 30, 60, 90 and 180 were 79.70 ± 1.6%, 77.15 ± 3.8%, 76.61 ± 2.38%, 73.35 ± 3.3%, 78.92 ± 1.04%, and 72.35 ± 2.3%, resepectively (Table 2). During the predefined storage conditions, no significant changes in EA content were observed in the patches. The observed variations were due to the manual drug loading processes. EA was uniformly distributed throughout the polymeric matrix, and the uniformity parameters were relatively identical to each other with minimal standard deviation values. Temperature and humidity did not show any adverse effects on the polymeric patches. No degradation products were obtained in HPLC analysis after 6 months of stress conditions. Minute variations were observed but within accepted limits.
Table 2.
Points of analysis performed for accelerated stability testing for 6 months.
| Points of analysis | Duration (mo) | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| 0 | 0.5 | 1.0 | 2.0 | 3.0 | 6.0 | |
| 1 | 79.70 ± 1.68 | 77.15 ± 3.8 | 76.61 ± 2.3 | 73.35 ± 3.3 | 78.92 ± 1.04 | 72.35 ± 2.33 |
| 2 | Complies | Complies | Complies | Complies | Complies | Complies |
| 3 | Complies | — | — | — | — | Complies |
| 4 | Complies | — | — | — | — | Complies |
| 5 | Complies | — | — | — | — | Complies |
| 6 | Complies | — | — | — | — | Complies |
3.2. Physical appearances
The physical appearances of the best optimized patches were visually observed, and no alteration could be witnessed in terms of colour, shape, clarity, smoothness, uniformity, flexibility, etc. during the period of analysis. No brown or red tint coloration, which appears due to heat and light sensitivity of EA, over the surface of polymeric patches was observed under accelerated stability testing for 6 months. This might be because of the rigidity of polymeric matrix where EA was compactly entrapped, preventing the presence of free EA on the surface of the polymeric patches. This was further established by the DSC-TGA and XRD analysis. These studies revealed that EA was homogenously dispersed over the polymeric matrix system. No corresponding peaks of the pristine EA were observed in DSC-TGA and XRD analysis, indicating the absence of free EA.
3.3. SEM-EDX analysis
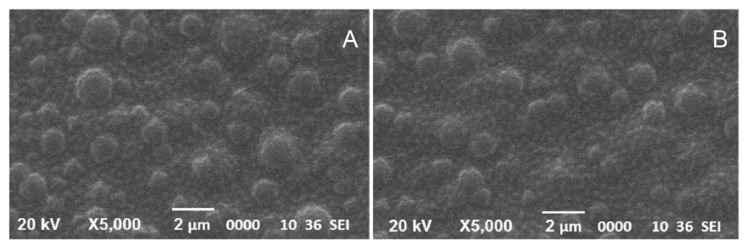
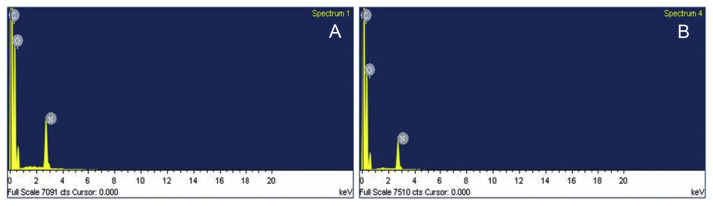
SEM analysis was performed at 0 month and after 6 months to visualize the distribution of EA throughout the polymeric matrix. Throughout the phase of stability testing, the polymeric patches maintained its stability, including smoothness, homogeneity, and uniformity (Figure 1). The integrity of EA-loaded polymeric patches was further confirmed by EDX analysis at 0 month and after 6 months. In EDX analysis, the atomic percentage of C, O and N were found to be 63.78, 28.17 and 8.05, respectively. We did not observe any variation in corresponding peaks of C and O, however, we observed a little variation in the characteristic peak of nitrogen (Figure 2). The observed variation is due to the loading of patches in hermetically-sealed aluminum bags.
Figure 1.
Scanning electron microscopy (A) at Day 0 and (B) at Day 180.
Figure 2.
Energy dispersive X-ray spectroscopy (A) at Day 0 and (b) at Day 180.
3.4. FTIR analysis
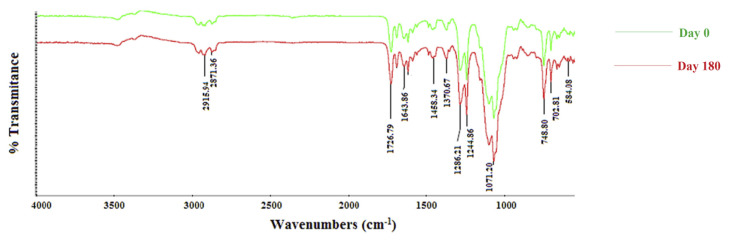
FTIR spectra of optimized polymeric patches were collected at 0 month and after 6 months (Figure 3). The optimized patches remained stable during the stability testing for 6 months. Small shift in the corresponding peaks (2915.94 cm−1, 1071.20 cm−1) were observed, which might be due to the drug loading process or the complete loss of organic solvent system.
Figure 3.
Fourier transform infrared spectroscopy at Day 0 and Day 180.
3.5. DSC and TGA analysis
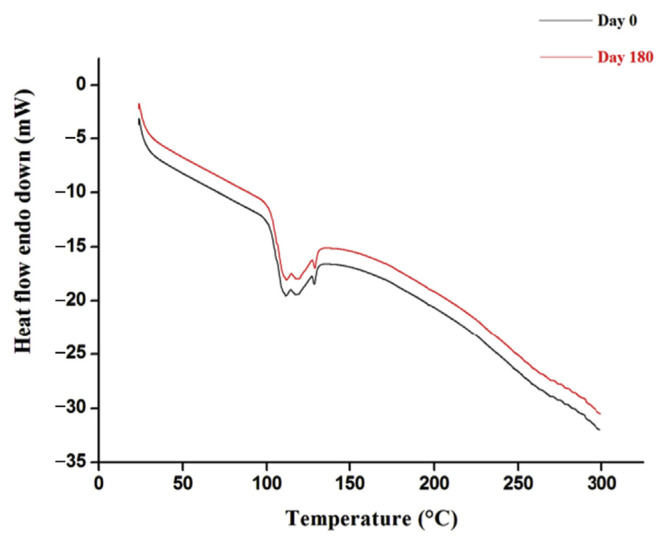
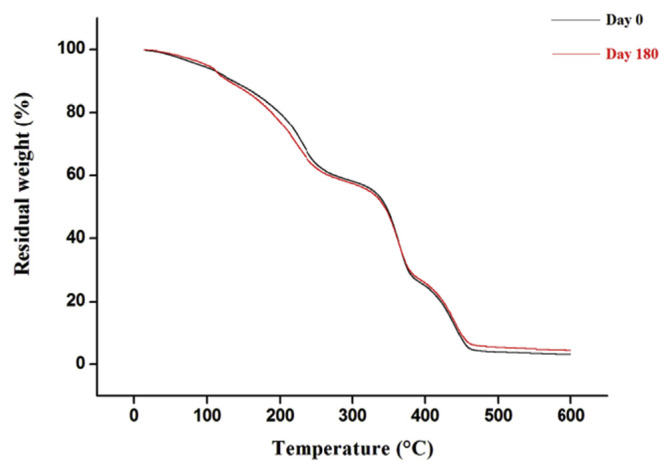
No significant changes were observed in DSC analysis, especially in the glass transition temperature (Tg) (Figure 4). Moreover, the TGA profiles of the optimized polymeric patches, before and after stability testing, showed the same structure, demonstrating the stability of the polymeric patches during analysis (Figure 5).
Figure 4.
Differential scanning calorimetry at Day 0 and Day 180.
Figure 5.
Thermo gravimetric analysis at Day 0 and Day 180.
3.6. XRD analysis
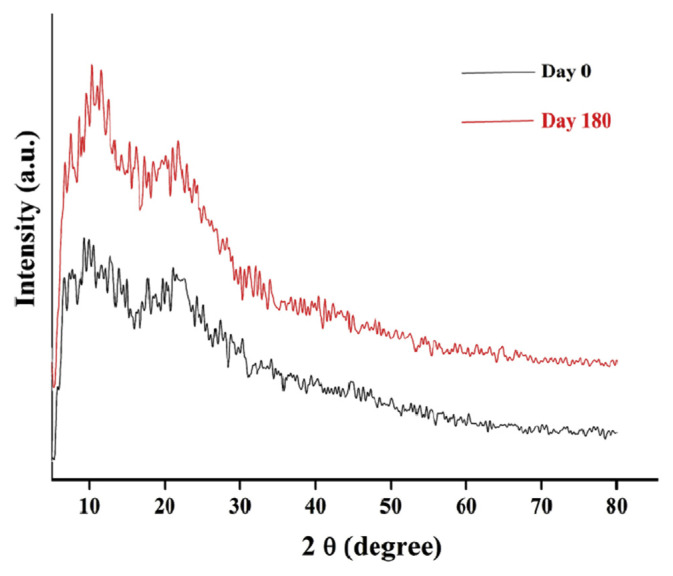
The X-ray diffractograms of optimized polymeric patches were collected before and after stability testing and compared with each other. No distinct changes were observed, signifying the stability and non-crystallinity of the polymeric patches during storage (Figure 6).
Figure 6.
X-ray diffraction at Day 0 and Day 180.
3.7. Analysis of residual solvents
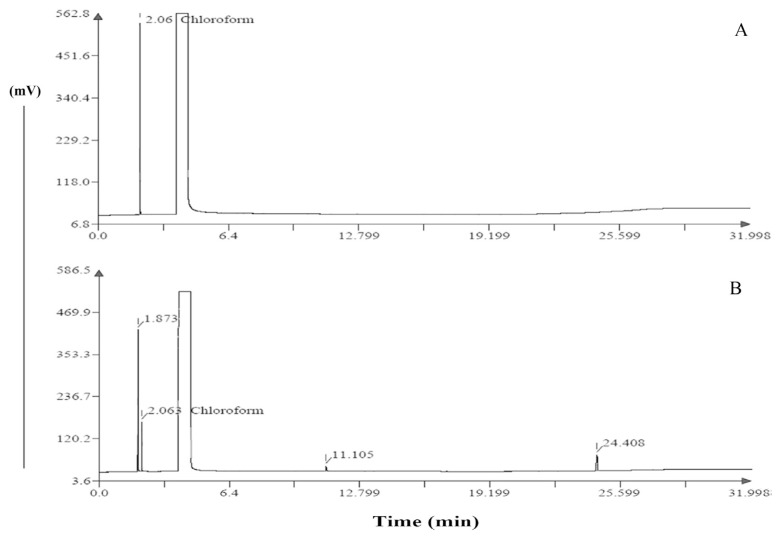
Residual solvents routinely used in the manufacture of pharmaceuticals pose a problem and must be removed. However, complete removal is quite difficult or impossible. Therefore, the ICH guidelines recommend the use of less toxic solvents and describes levels considered to be toxicologically acceptable for some residual solvents [22]. However, in certain cases, such as topical and short-term (30 days or less) applications, higher levels of residual solvents may be acceptable. As per the ICH guidelines, the acceptable limit of chloroform is 60 μg/mL [22]. The standard solution of chloroform showed a distinguished peak at the retention time of 2.06 minutes (Figure 7A). The corresponding chromatogram of the EA-loaded matrix-type polymeric patch also showed a peak at the same retention time of 2.06 minutes (Figure 7B), thus indicating the presence of chloroform. The estimated amount of chloroform was found to be 38.23 ± 2.6 μg/mL.
Figure 7.
Chromatogram of (A) standard solution of chloroform (100 μg/mL) and (B) patch.
4. Conclusion
The work presented here, reports for the first time, the stability testing profiles of matrix-type polymeric patches loaded with EA as per the ICH guidelines (40 ± 2°C/75 ± 5% for ≥ 6 months) [10]. The study showed successful results and the optimized polymeric patches remained stable under stress conditions for 6 months with no significant changes. In addition, the presence of lower limit of residual solvent i.e., the chloroform, confirms the suitability and applicability of EA-loaded matrix-type polymeric patches. Thus, the present report can be of practical utility in confirming the QC attributes of newly developed drug substances or drug products, conforming to the regulatory guidelines.
Acknowledgments
The author highly acknowledges the Defence Research Laboratory, Assam, India for providing all the necessary facilities for this research work and the administration of Dibrugarh University, Assam, India for providing the necessary administrative support for carrying out his PhD work. The author is also grateful to the University Grant Commission (UGC), New Delhi, India for providing Maulana Azad National Fellowship for conducting the PhD work (Award no: MANF-2012-13-MUS-ASS-15143). Dr Prasanta Raul, Scientist-C, Defence Research Laboratory, Assam, India is gratefully acknowledged for the GC analysis of chloroform.
Funding Statement
The author is also grateful to the University Grant Commission (UGC), New Delhi, India for providing Maulana Azad National Fellowship for conducting the PhD work (Award no: MANF-2012-13-MUS-ASS-15143).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
REFERENCES
- 1. Siepmann J, Siepmann F. Mathematical modelling of drug dissolution. Int J Pharm. 2013;65:94–7. doi: 10.1016/j.ijpharm.2013.04.044. [DOI] [PubMed] [Google Scholar]
- 2. Siepmann F, Siepmann J, Walther M, MacRae RJ, Bodmeier R. Polymer blends for controlled release coatings. J Controlled Release. 2008;125:1–15. doi: 10.1016/j.jconrel.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 3. Siepmann F, Hoffmann A, Leclercq B, Carlin B, Siepmann J. How to adjust desired drug release patterns from ethyl cellulose-coated dosage forms. J Controlled Release. 2007;119:182–9. doi: 10.1016/j.jconrel.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 4. Perez MF, Sanchez MV, Cespedes FF, Fernandez ID. Ethyl cellulose and lignin as bearer polymers in controlled release formulations of chloridazon. Carbohydr Polym. 2011;83:1672–9. [Google Scholar]
- 5. Kommanaboyina B, Rhodes CT. Trends in stability testing, with emphasis on stability during distribution and storage. Drug Dev Ind Pharm. 1999;25:857–68. doi: 10.1081/ddc-100102246. [DOI] [PubMed] [Google Scholar]
- 6. Matthews BR. Regulatory aspects of stability testing in Europe. Drug Dev Ind Pharm. 1999;25:831–56. doi: 10.1081/ddc-100102245. [DOI] [PubMed] [Google Scholar]
- 7. Banerjee S, Chattopadhyay P, Ghosh A, Bhattacharya SS, Kundu A, Veer V. Accelerated stability testing of a transdermal patch composed of eserine and paralidoxime chloride for prophylaxis against (±) anatoxin A poisoning. J Food Drug Anal. 2014;22:264–70. [Google Scholar]
- 8. Koyu H, Haznedaroglu MZ. Investigation of impact of storage conditions on Hypericum perforatum L. dried total extract. J Food Drug Anal. 2015;23:545–51. doi: 10.1016/j.jfda.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singh S, Bakshi M. Guidance on conduct of stress tests to determine inherent stability of drugs. Pharm Tech. 2000;24:1–14. [Google Scholar]
- 10.ICH Guideline Q1A(R2) Stability testing of new drug substances and products. Geneva: 2000. [Accessed 7 Sep 2015]. Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q1A_R2/Step4/Q1A_R2__Guideline.pdf. [Google Scholar]
- 11. Bott RF, Oliveira WP. Storage conditions for stability testing of pharmaceuticals in hot and humid regions. Drug Dev Ind Pharm. 2007;33:393–401. doi: 10.1080/03639040600975022. [DOI] [PubMed] [Google Scholar]
- 12. Lee G. Drug stability, principles and practices. Eur J Pharm. 2002;53:256–7. [Google Scholar]
- 13. Api AM, Belsito D, Bhatia S, Bruze M, Calow P, Dagli ML, Dekant W, Fryer AD, Kromidas L, Cava SL, Lalko JF, Lapczynski A, Liebler DC, Miyachi Y, Politano VT, Ritacco G, Salvito D, Shen J, Schultz TW, Sipes IG, Wall B, Wilcox DK. RIFM fragrance ingredient safety assessment, ethyl anthranilate, CAS registry number 87-25-2. Food Chem Toxicol. 2015;82:S97–104. doi: 10.1016/j.fct.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 14. Afify A, Horlacher B, Roller J, Galizia CG. Different repellents for Aedes aegypti against blood-feeding and oviposition. PLoS ONE. 2014;9:103765–71. doi: 10.1371/journal.pone.0103765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guda T, Kain P, Sharma KR, Pham CK, Ray A.Repellent compound with larger protective zone than DEET identified through activity screening of Ir40a neurons, does not require Or function. bio Rxiv. 2015. p. 017145. [DOI]
- 16. Kain P, Boyle SM, Tharadra SK, Guda T, Pham C, Dahanukar A, Ray A. Odour receptors and neurons for DEET and new insect repellents. Nature. 2013;502:507–12. doi: 10.1038/nature12594. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17. Tramon C. Modeling the controlled release of essential oils from a polymer matrix-A special case. Ind Crops Prod. 2014;61:23–30. [Google Scholar]
- 18. Ma Y, Zhao F, Zeng B. Electro deposition of poly(3,4-ethylenedioxythiophene) on a stainless steel wire for solid phase microextraction and GC determination of some esters with high boiling points. Talanta. 2013;104:27–31. doi: 10.1016/j.talanta.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 19. Zhao S, Wu M, Zeng B. Electrochemical preparation of polyaniline-polypyrrole solid-phase microextraction coating and its application in the GC determination of several esters. Talanta. 2013;117:146–51. doi: 10.1016/j.talanta.2013.08.060. [DOI] [PubMed] [Google Scholar]
- 20. Siepmann J, Lecomte F, Bodmeier R. Diffusion-controlled drug delivery systems: Calculation of the required composition to achieve desired release profiles. J Controlled Release. 1999;60:379–89. doi: 10.1016/s0168-3659(99)00093-0. [DOI] [PubMed] [Google Scholar]
- 21. Chen CM, Hsien T. Mosquito repellent patch. 20070036833Al. US Patent US. 2007
- 22.ICH Guideline Q3C (R5) Impurities: Guideline for residual solvents. Geneva: 2011. [Accessed 22 Oct 2016]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/03/WC500104258.pdf. [Google Scholar]
- 23. Chattopadhyay P, Dhiman S, Borah S, Rabha B, Chaurasia A, Veer V. Essential oil based polymeric patch development and evaluating its repellent activity against mosquitoes. Acta Trop. 2015;147:45–53. doi: 10.1016/j.actatropica.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 24. Chattopadhyay P, Dhiman S, Devi KA, Banerjee S, Rabha B, Chaurasia A, Veer V. Ultra low concentration deltamethrin loaded patch development and evaluation of its repellency against dengue vector Aedes (S) albopictus. Parasit Vectors. 2013;6:284–93. doi: 10.1186/1756-3305-6-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tankiewicz M, Namieśnik J, Sawicki W. Analytical procedures for the quality control of pharmaceuticals in terms of residual solvents content-Challenges and recent developments. Trends Anal Chem. 2016;80:328–44. [Google Scholar]
- 26.Akyar I. Wide spectra of quality control. Croatia: European Union; 2011. [Google Scholar]
- 27. Grodowska K, Parczewski A. Organic solvents in the pharmaceutical industry. Acta Pol Pharm. 2010;67:3–12. [PubMed] [Google Scholar]
- 28. Grodowska K, Parczewski A. Analytical methods for residual solvents determination in pharmaceutical products. Acta Pol Pharm. 2010;67:13–26. [PubMed] [Google Scholar]