Abstract
BACKGROUND/OBJECTIVES:
Low-level, in-utero exposure to toxic metals such as lead (Pb) and mercury (Hg) is widespread in the US and worldwide; and, individually, was found to be obesogenic in children. To address the literature gaps on the health effects of co-exposure to low-level toxic metals and the lack of intervention strategy, we aimed to investigate the association between in-utero co-exposure to Hg, Pb, cadmium (Cd) and childhood overweight or obesity (OWO) and whether adequate maternal micronutrients (selenium (Se) and folate) can be protective.
SUBJECTS/METHODS:
This study included 1442 mother-child pairs from the Boston Birth Cohort, a predominantly urban, low-income, Black, and Hispanic population, who were enrolled at birth and followed prospectively up to age 15 years. Bayesian kernel machine regression (BKMR) was applied to estimate individual and joint effects of exposures to metals and micronutrients on childhood OWO while adjusting for pertinent covariables. Stratified analyses by maternal OWO and micronutrient status were performed to identify sensitive subgroups.
RESULTS:
In this sample of understudied US children, low-level in-utero co-exposure to Hg, Pb, and Cd was widespread. Besides individual positive associations of maternal Hg and Pb exposure with offspring OWO, BKMR clearly indicated a positive dose-response association between in-utero co-exposure to the three toxic metals and childhood OWO. Notably, the metal mixture-OWO association was more pronounced in children born to mothers with OWO; and in such a setting, the association was greatly attenuated if mothers had higher Se and folate levels.
CONCLUSIONS:
In this prospective cohort of US children at high-risk of toxic metal exposure and OWO, we demonstrated that among children born to mothers with OWO, low-level in-utero co-exposure to Hg, Pb, and Cd increased the risk of childhood OWO; and that adequate maternal Se and folate levels mitigated the risk of childhood OWO.
INTRODUCTION
Mercury (Hg), lead (Pb), and cadmium (Cd) have been identified by the World Health Organization (WHO) and by the US Agency for Toxic Substances and Disease Registry (ATSDR)(1, 2) as among the most toxic heavy metals that are of public health significance. The National Health and Nutrition Examination Survey (NHANES), a representative sample of US children and adults, showed that exposure to these toxic metals is ubiquitous, where 85.2%, 99.5%, and 69.8% of the population had detectable levels of Hg, Pb, and Cd in blood respectively; similar exposure is observed in the Boston Birth Cohort (BBC) (3, 4). In particular, Black women, including women of reproductive age, have been shown to have higher Hg, Pb, and Cd levels than non-Hispanic Whites women (5–8). This disparity is of clinical and public health concern because maternal exposure to these toxic metals can affect both pregnancy outcomes and a child’s long-term health due to a high degree of transplacental passage of these toxic metals. Further, this worry is compounded by the act of fetal bioaccumulation (3, 9, 10). Hg and Pb are known neurotoxins (11, 12). In recent years, studies conducted by us (13, 14) and other researchers (15) have also revealed that Hg and Pb could act as obesogens even at relatively low-levels. We found that prenatal exposure to low-levels of Hg and Pb, individually, was associated with an increased risk of overweight or obesity (OWO) in children in a dose-response fashion; there was no indication of safe exposure levels to these metals.
This study extends previous research by addressing critical knowledge gaps and analytical challenges regarding prenatal exposure to multiple environmental toxic metals and the risk of OWO in childhood. Most published studies have been limited to the investigations of single metal exposures. The importance of co-exposures is underscored by the National Institute of Environmental Health Sciences (NIEHS) (16) and supported by the NHANES finding that 66.8% of the US population had at least two metals detectable among Hg, Pb, Cd, and 32.7% had all three metals detected in blood and/or urine specimens (4).
Another research gap is related to the fact that there has been a lack of clear and effective strategies to reduce the adverse health effects of low-level toxic metals exposure to Hg, Pb, and Cd in both clinical and public health settings. Nutrition has been proposed as a safe, simple, and inexpensive means to alleviate the detrimental effects of environmental toxicants (17, 18). However, there is a critical need for evidence to support the role of micronutrients in counteracting the health effects of toxic metal exposures. Our study focuses on two micronutrients with such potential. Selenium (Se) is an essential trace element used increasingly in enriched foods, supplements, and fertilizers in the US (19–21). Studies have demonstrated that Se deficiency increases the risk of childhood OWO (22–24). To our knowledge, this is the first study to examine the role of Se in the setting of toxic metal co-exposure and childhood OWO. Folate is another relevant micronutrient that may reduce the adverse impacts of Hg and Pb on the developing child (25). We previously published that adequate maternal folate mitigated the risk of inter-generational obesity in the presence of prenatal Hg or Pb exposure (13, 14). While these findings are promising, more information is needed to determine if such a protective effect can also be seen when toxic metal exposures are analyzed as a mixture since most environmental exposures do not occur independently. As such, only by analyzing these toxic metals and micronutrients simultaneously as a mixture will we be able to delineate their individual and joint effects on child OWO.
This study addresses these knowledge gaps by investigating the associations between maternal co-exposures to Hg, Pb, Cd, and child risk of OWO status. Moreover, we examined the potential protective role of adequate maternal micronutrient (Se and folate) status. Accomplishing this requires more advanced analytical methods since conventional regression methods are limited in their ability to reveal individual and joint effects of co-exposures or account for nonlinear associations and interactions among the exposures. Hence, model misspecification is common when using conventional methods to study mixture exposures. To address this, we applied a novel statistical approach, Bayesian kernel machine regression (BKMR) (26, 27), to elucidate both individual and joint effects within a mixture of toxic and nutritional exposures. This study examined a) the individual association of maternal Hg, Pb, Cd, Se, folate exposure with offspring OWO risks; b) their pairwise effects on childhood OWO; c) the dose-response relationships between maternal co-exposure to Hg, Pb, Cd with childhood OWO, given that most people were co-exposed to metal mixtures; and d) estimate whether maternal OWO and micronutrients status (Se, folate) alter the relationships.
To the best of our knowledge, this is the first study to investigate in-utero co-exposures to toxic metals (Hg, Pb, Cd) on childhood OWO and whether maternal micronutrients (Se, folate) could modify the association in a large sample of understudied, underrepresented US minority children who are at high-risk of toxic metal exposure and OWO.
MATERIALS/SUBJECTS AND METHODS
Study population
The study population included 1442 mother-child pairs from the BBC, a predominantly urban, low-income, Black, and Hispanic population enrolled at birth and followed prospectively. Participants were recruited from 2002 to 2018. More detailed information about participant enrollment in the BBC has been described elsewhere (28). In short, after obtaining written informed consent, the research staff approached and recruited mother-infant pairs 24 to 72 h after delivery. Mothers completed a standardized questionnaire interview conducted by trained research staff, which included social-demographic variables, dietary and supplemental vitamin intake, smoking status, and alcohol drinking. Maternal blood samples were also collected. Maternal and child electronic medical records (EMR) were reviewed and abstracted using a standard form. There were 3349 children enrolled in the BBC who received pediatric care and consented to follow up at the Boston Medical Center (BMC) from birth up to age 21 years. Among these children, 1551 have available data for metal levels (i.e., Hg, Pb, Cd, Se) in red blood cells (RBCs). Of those, we excluded 109 children without any body mass index (BMI) data between the ages of 2 and 15 years. The final sample for this report included 1442 mother-child pairs who completed at least one postnatal well-child care visit beyond the age of 2 years at the BMC and had data on maternal BMI and metals biomarkers. A flow chart of the study population, registered in clinicaltrials.gov (#NCT03228875), including the inclusion and exclusion criteria, is shown in Fig. S1 in the Supplement. Institutional Review Boards of the Boston Medical Center and the Johns Hopkins Bloomberg School of Public Health approved the study.
Measurement of maternal RBC metal levels
Maternal Hg, Pb, Cd, and Se levels were measured using inductively coupled plasma mass spectrometry in RBC samples obtained within 24 to 72 hours postpartum period. RBC metals have the advantage of being long-term biomarkers of metal exposure (given RBC lifespan is 120 days (29)) and free from hemodilution during pregnancy (as compared to whole blood), and there were high correlations between maternal and cord RBCs for Hg, Pb, and Se (Spearman correlation r = 0.96, 0.89, and 0.72, respectively; p<0.0001) (3). More detailed information about measurements of metal levels in RBCs has been described previously (3, 13, 14). Briefly, plasma and RBCs were separated by centrifugation and kept frozen at − 80 °C in vials certified to be free of these metal elements. The samples were then transported on dry ice to the Public Health and Environmental Laboratories in the New Jersey Department of Health (NJ, USA), where they were measured following the standard CLIA-certified protocols of the NJ Biomonitoring Program and strict quality control and assurance procedures. We measured all four elements in the same run. Intraassay coefficients of variation (CV) were ≤ 5%. The limits of detection were 0.100 ug/L for Cd; 0.07 ug/dL for Pb; 0.280 ug/L for Hg, and 24.5 ug/L for Se. There are 123 and 166 samples below LOD for Cd and Hg, respectively. For those below LOD, we assigned a numeric value of LOD divided by the square root of 2 per conventional practice in environmental epidemiological studies. All the study samples are detectable for Pb, Se, and folate.
Ascertainment of maternal plasma folate concentrations
Maternal plasma folate levels were measured in archived plasma samples obtained within 24 to 72 hours postpartum period using a MAGLUMI 2000 Analyzer (Snibe Co., Ltd., Shenzhen, China), with an inter-assay CV that was smaller than 4%, as detailed in a previous publication (30).
Definition and assessment of covariates
We included important maternal characteristics and child’s birth outcomes as our covariates. Maternal variables, including maternal age, BMI, race/ethnicity, marital status, education level, pre-pregnancy height and weight, smoking status, parity, and fish intake level, were defined based on answers to the maternal questionnaire interview within a few days after delivery. Maternal race/ethnicity was categorized as Black (including African American or Haitian), White, Hispanic, or other. Maternal pre-pregnancy BMI was calculated based on the mother’s height and weight before pregnancy, which was grouped into non-OWO (BMI < 25 kg/m2) and OWO (BMI ≥ 25 kg/m2). Breastfeeding was assessed within the first two years of follow-up visits, and we categorized it into breastfeeding only, both breastfeeding and formula feeding, and formula feeding only. The child’s sex, birthweight, and preterm birth status were retrieved from the EMR. Low birthweight was defined as birthweight less than 2,500 grams. Preterm birth was defined as a birth occurring at < 37 weeks of gestation.
Definitions of overweight or obesity in childhood
Trained medical staff performed the child weight and height measurements using standard clinical protocols and equipment during well-child care visits and documented them in the EMR. BMI z-scores and percentiles were calculated using US national reference data for age and sex (31). The varying length of postnatal follow-up was because the BBC used rolling enrollment. Since OWO in older childhood is more likely to persist into adulthood, child OWO status was defined as a BMI ≥ 85th percentile for age and sex based on the last follow-up visit record beyond age 2 years (32), with age ranging from 2 years to 15 years.
Statistical analysis
Population characteristics, including demographic and clinical data, are presented as either n (%) or mean ± standard deviation (SD) for the total sample and subgroups defined by OWO in childhood. For non-normal continuous variables (Hg, Pb, Cd, Se, folate), geometric mean and geometric standard deviations are provided. The p-values were calculated using the two-sided t-test for normal continuous variables and log-transformed non-normal continuous variables, and χ2 test for categorical variables.
We first estimated the individual effects of metals (Hg, Pb, Cd) and micronutrient biomarkers (Se, folate) on child OWO status using multivariable generalized linear models (GLMs) in the total study population and in subgroups defined by maternal pre-pregnancy OWO status (no, yes). Since this is a prospective cohort study, we adopted Poisson regression with robust error variance (33) to estimate relative risk (RR) and 95% confidence intervals (CI). We categorized maternal toxic metal levels and micronutrient concentrations in tertiles and grouped them into low (T1) versus high (T2–T3) in the subsequent analyses; their detailed concentration ranges were reported in Table S1. The analysis was adjusted for relevant covariates, including maternal age, BMI, race/ethnicity, marital status, education level, parity, breastfeeding type, smoking status, fish intake level, child’s sex, low birthweight, and preterm birth status. The associations of covariates with maternal heavy metals, micronutrients, and child OWO status can be found in Table S2. In addition, to examine if the association differs by maternal pre-pregnancy OWO status (yes/no), we performed stratified analyses by maternal OWO. We also assessed the bivariate combined effects of maternal pre-pregnancy OWO, toxic metals, and micronutrients on child OWO status using the modified Poisson model.
To investigate the potential non-linearity and non-additive effects of the toxic metal mixture and micronutrient exposure on child OWO status, we adopted the novel BKMR model (26, 27, 34). This model was chosen because it can flexibly estimate the joint health effects of multiple exposures using a non-parametric kernel function. We used the bkmr package in R, generating four Markov Chain Monte Carlo (MCMC) chains in parallel, each with a different random seed. We included 100,000 iterations in each MCMC chain and adopted the default prior specification in the package (27). We also verified BKMR model convergence by inspecting the trace, autocorrelation, density, and Gelman-Rubin plots (Fig. S3-S8 in the Supplement). Since the metal and micronutrient biomarker data were skewed, we used z-scores of the log-transformed data in the model.
Given that prior studies by us (14) and others (35) have indicated the dominant role of maternal pre-pregnancy OWO in child OWO, we first stratified the study population based on maternal pre-pregnancy OWO status. Next, within each stratum, to estimate the independent effects of Hg, Pb, Cd, Se, and folate on child OWO, we estimated the univariate dose-response relationships of each biomarker while holding the others constant at the median. To assess pairwise interactions among biomarkers, we estimated the bivariate dose-response relationships of each biomarker while fixing a second biomarker at various quantiles (10th to 90th) and holding the remaining biomarkers at their median values. To investigate the overall effect of the toxic metal mixture, we plotted the overall effect curves of toxic metals on child OWO, obtained by the difference in the response when all the exposures are fixed at a specific quantile (10th to 90th), as compared to when all the exposures are fixed at their median value. In addition, to explore the potential protective effect of maternal Se and folate levels against the toxicity of the metal mixture, we studied the overall effects of toxic metals on different population groups stratified by maternal Se and folate levels. In all analyses using BKMR, we included maternal age, BMI, race/ethnicity, marital status, education level, parity, breastfeeding type, smoking status, fish intake, child’s sex, low birthweight, and preterm status as the covariates. We also conducted univariate dose-response analyses among children born to Black mothers and non-Black mothers and among boys and girls.
RESULTS
Characteristics of the study population
The study population included 1442 mother-child pairs, of which 722 (50.1%) children were boys. Table 1 presents the population characteristics, stratified by child OWO status. The prevalence of OWO among the 1442 children was 42.4%. Over half (52.4%) of the mothers were OWO pre-pregnancy; 967 mothers (67.1%) were Black. More than half (58.3%) of mothers were multiparous. About 17% of mothers ever smoked during pregnancy. Approximately half (48.8%) of mothers had fish intake more than one day per week. Compared with mothers of children without OWO, mothers of children with OWO were more likely to be OWO during pregnancy, older at delivery, and a smoker. Mothers of children with OWO also had relatively higher Hg, Pb, and Cd blood levels but lower folate values than mothers of children without OWO. Table S3 showed comparable population characteristics between the total sample (N=3349) and the study sample (N=1442).
Table 1.
Population characteristics of the 1,442 mother-child pairs, stratified by child overweight or obesity (OWO) status.
| Characteristic | Total Sample (n=1442) | Children Without OWO (n=830) | Children With OWO (n=612) | p-Value* | Adjusted p-Value** |
|---|---|---|---|---|---|
| Maternal | |||||
| Pregnancy BMI category, n (%) | <0.0001 | <0.0001 | |||
| Mothers without OWO | 687 (47.6) | 478 (57.6) | 209 (34.2) | ||
| Mothers with OWO | 755 (52.4) | 352 (42.4) | 403 (65.8) | ||
| BMI, mean ± SD, kg/m2 | 26.74 ± 6.55 | 25.41 ± 5.89 | 28.55 ± 6.97 | <0.0001 | <0.0001 |
| Maternal age, n (%) | 0.015 | 0.052 | |||
| <20 | 123 (8.5) | 85 (10.2) | 38 (6.2) | ||
| >=35 | 263 (18.2) | 141 (17.0) | 122 (19.9) | ||
| 20–35 | 1056 (73.2) | 604 (72.8) | 452 (73.9) | ||
| Race/ethnicity, n (%) | 0.33 | 0.49 | |||
| Black | 967 (67.1) | 555 (66.9) | 412 (67.3) | ||
| White | 74 (5.1) | 50 (6.0) | 24 (3.9) | ||
| Hispanic | 291 (20.2) | 163 (19.6) | 128 (20.9) | ||
| Others | 110 (7.6) | 62 (7.5) | 48 (7.8) | ||
| Marital status, n (%) | 0.83 | 0.93 | |||
| Not Married | 970 (67.3) | 556 (67.0) | 414 (67.6) | ||
| Married | 472 (32.7) | 274 (33.0) | 198 (32.4) | ||
| Educational level, n (%) | 0.31 | 0.49 | |||
| Below high school | 381 (26.4) | 216 (26.0) | 165 (27.0) | ||
| High school | 551 (38.2) | 307 (37.0) | 244 (39.9) | ||
| Above high school | 510 (35.4) | 307 (37.0) | 203 (33.2) | ||
| Parity, n (%) | 0.39 | 0.53 | |||
| Nulliparous | 602 (41.7) | 355 (42.8) | 247 (40.4) | ||
| Multiparous | 840 (58.3) | 475 (57.2) | 365 (59.6) | ||
| Breastfeeding type, n (%) | 0.016 | 0.052 | |||
| Breastfeeding only | 112 (7.8) | 74 (8.9) | 38 (6.2) | ||
| Both breastfeeding and formula feeding | 987 (68.4) | 578 (69.6) | 409 (66.8) | ||
| Formula feeding only | 343 (23.8) | 178 (21.4) | 165 (27.0) | ||
| Smoking, n (%) | 0.043 | 0.12 | |||
| Never | 1192 (82.7) | 701 (84.5) | 491 (80.2) | ||
| Continuous & Quitter | 250 (17.3) | 129 (15.5) | 121 (19.8) | ||
| Fish intake, n (%) | 0.98 | 0.99 | |||
| None | 322 (22.3) | 187 (22.5) | 135 (22.1) | ||
| <= 1 day/week | 416 (28.8) | 239 (28.8) | 177 (28.9) | ||
| > 1 day/week | 704 (48.8) | 404 (48.7) | 300 (49.0) | ||
| Mercury, mean (SD)***, μg/L | 2.02 (2.57) | 1.94 (2.53) | 2.14 (2.61) | 0.053 | 0.13 |
| Lead, mean (SD), μg/dL | 2.68 (1.90) | 2.58 (1.90) | 2.81 (1.90) | 0.013 | 0.052 |
| Cadmium, mean (SD), μg/L | 0.70 (1.97) | 0.68 (1.94) | 0.72 (2.00) | 0.17 | 0.29 |
| Selenium, mean (SD), μg/L | 284.07 (1.22) | 284.04 (1.22) | 284.10 (1.22) | 0.98 | 0.99 |
| Folate, mean (SD), nmol/L | 32.21 (1.69) | 32.86 (1.69) | 31.34 (1.67) | 0.089 | 0.17 |
| Child | |||||
| Sex, n (%) | 0.74 | 0.93 | |||
| Female | 720 (49.9) | 418 (50.4) | 302 (49.3) | ||
| Male | 722 (50.1) | 412 (49.6) | 310 (50.7) | ||
| Preterm birth, n (%) | 348 (24.1) | 203 (24.5) | 145 (23.7) | 0.78 | 0.93 |
| Low birth weight, n (%) | 328 (22.7) | 203 (24.5) | 125 (20.4) | 0.081 | 0.17 |
| BMI Z score, mean ± SD | 0.73 ± 1.22 | −0.11 ± 0.86 | 1.86 ± 0.53 | <0.0001 | <0.0001 |
The p-values were calculated using the two-sided t-test for normal continuous variables and log-transformed non-normal continuous variables and χ^2 test for categorical variables.
The adjusted p-values were obtained using Benjamini-Hochberg false discovery rate (FDR) correction (70).
For non-normal continuous variables (Hg, Pb, Cd, Se, folate), geometric mean and SDs are provided.
Conventional regression analysis to assess the association between toxic metals or micronutrients and child OWO, with and without covariate adjustment
As shown in Fig. 1, in the stratified analyses by maternal OWO, Pb showed a positive association with child OWO risk, as indicated by the relative risk (RR) for Pb (1.45; 95% CI: 1.11–1.89; p=0.0067) among mothers without OWO. Conversely, folate showed a weak protective association among children born to mothers with OWO. In a combined analysis of the effect of maternal OWO status and toxic metal exposures or micronutrient biomarkers on child OWO, we observed an additive association between maternal pre-pregnancy OWO and Hg or Pb levels and a counteractive association of maternal Se and folate against the effect of maternal OWO on child OWO. Children of mothers with OWO and with high Hg levels (1.36–27.8 μg/L) had an adjusted RR of 1.63 (95% CI: 1.26–2.10; p=0.0002) compared to those with mothers without OWO and low Hg levels (0.392–1.36 μg/L). Children of mothers with OWO and with high Pb (1.93–24.8 μg/dL) had an increased risk of OWO (RR=1.94; 95% CI: 1.49–2.53; p<0.0001) compared to those with mothers without OWO and with low Pb levels (0.582–1.93 μg/dL). The potential protective effect of higher maternal levels of Se and folate was suggested among children of mothers with OWO.
Fig. 1. Associations of toxic metal and micronutrient exposures with child OWO, stratified or combined by maternal pre-pregnancy OWO (N = 1442), using conventional regression analysis.
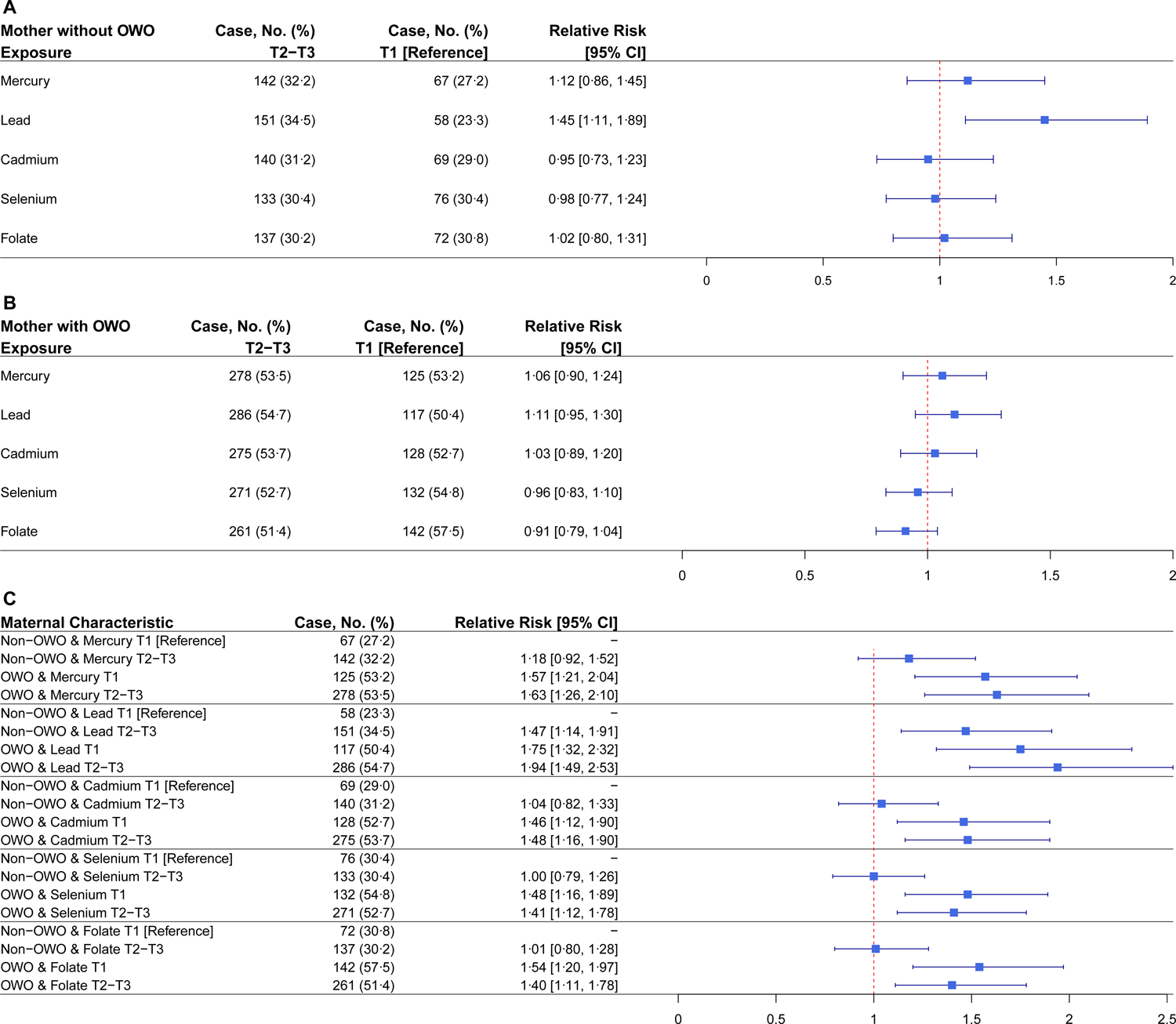
Adjusted for maternal age, BMI, race/ethnicity, marital status, education level, parity, breastfeeding type, smoking status, fish intake, child’s sex, low birthweight, and preterm status. (A) Children born to mothers without OWO (N=687). Children born to mothers with T1 biomarker were the reference groups. (B) Children born to mothers with OWO (N=755). Children born to mothers with T1 biomarker were the reference groups. (C) Children born to mothers without OWO with T1 biomarker were the reference groups. Refer to Table S4 and S5 for p-values.
Bayesian kernel machine regression analysis
The correlation heatmap (Fig. S9) showed moderate correlations among maternal Hg, Pb, Cd, Se, and folate. Therefore, we applied the BKMR method to further investigate individual and joint effects between these biomarkers, accounting for potential interactions and nonlinear effects, adjusting for covariates, and stratifying by mothers with and without OWO.
We first examined the univariate dose-response functions (Fig. 2) for each biomarker (Hg, Pb, Cd, Se, folate) while holding all others at their median value (36). Comparing Fig. 2A and Fig. 2B, we found that the estimated effects (both positive and negative) of exposures to toxic metals and micronutrients on the risk of child OWO were much stronger among children born to mothers with OWO compared to those of mothers without OWO. Among children born to mothers with OWO, Hg and Pb exposure levels were associated with an increased risk of childhood OWO. In contrast, Se and folate exposure exhibited protective associations with OWO in childhood. Among children of mothers without OWO, there was no association between maternal Hg, Pb, Cd, Se, and folate levels and child OWO.
Fig. 2. Univariate dose-response function (95% Credible Intervals, CrIs) of toxic metal (Hg, Pb, Cd) and micronutrient (Se, folate) exposures with child overweight or obesity (OWO).
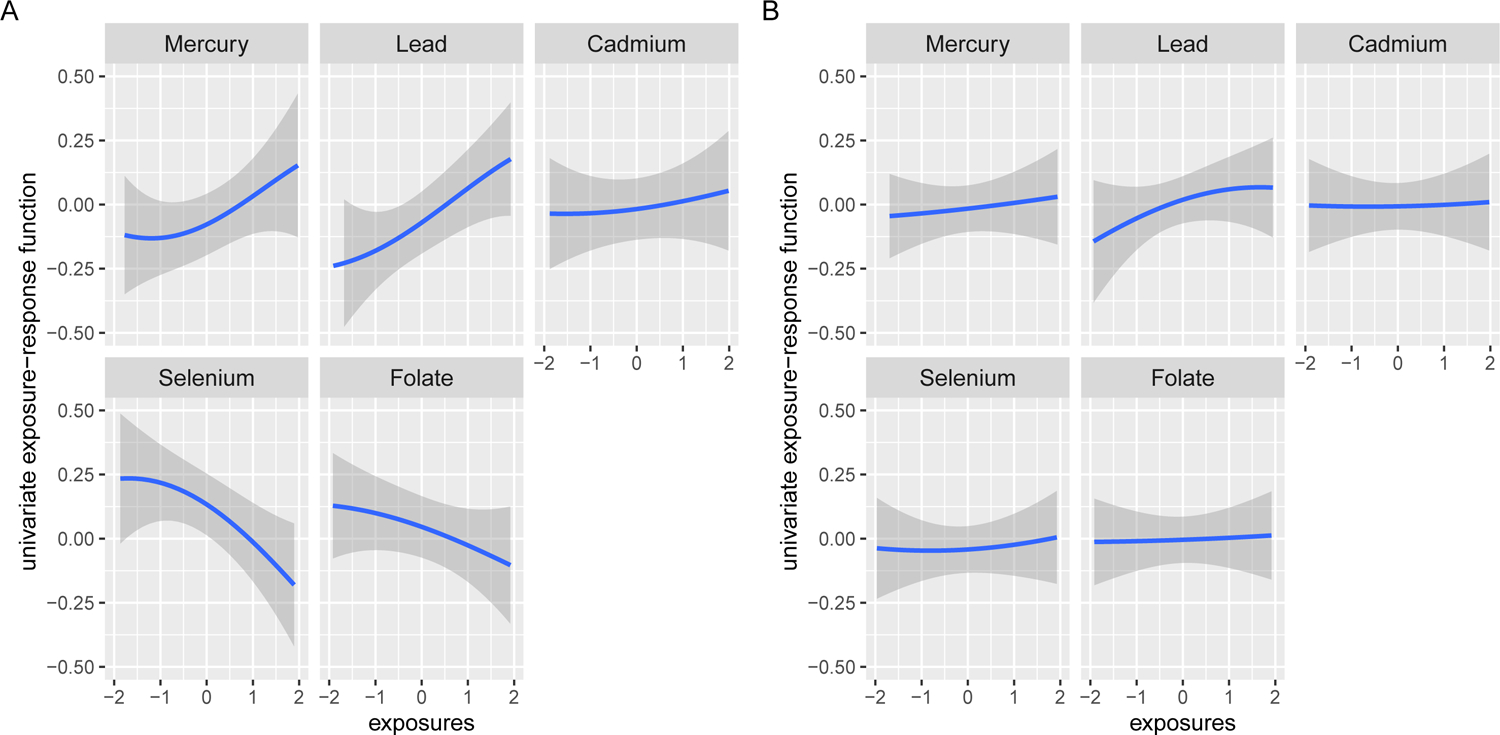
(A) Children born to mothers with OWO (N=755). (B) Children born to mothers without OWO (N=687). The univariate dose-response functions were assessed by fixing the remaining exposures at their median values and including maternal age, BMI, race/ethnicity, marital status, education level, parity, breastfeeding type, smoking status, fish intake, child’s sex, low birthweight, and preterm status as the covariates. The x-axis was limited to a range from −2 to 2, which reflects the z-score. Refer to the Supplement for the full range plot (Fig. S10). 95% CrIs are indicated by the shaded areas.
Next, we performed BKMR analysis using bivariate dose-response functions to further explore the interactions among pairs of exposures. Here, we investigated the dose-response function of a single exposure while fixing the second exposure at various quantiles and holding the remaining exposures at their median values. As shown in Fig. 3, we revealed several interactions among exposures that could not be observed in the univariate analyses. For example, among children of mothers with OWO, the slope of Cd became positive and steeper at higher quantiles of Pb, indicating a potential interaction between Pb and Cd in their association with child OWO. We also revealed that the positive association between Pb and child OWO was amplified in the presence of higher levels of Cd. At the same time, higher levels of maternal Se counteracted the association of Pb with child OWO. Similarly, higher levels of folate also mitigated the association of Pb and Hg with child OWO and augmented the protective effect of Se on child OWO, as shown in the last row of the Fig. 3 plot.
Fig. 3. Bivariate dose-response function of toxic metal (Hg, Pb, Cd) and micronutrient (Se, folate) exposures on child overweight or obesity (OWO) among children born to mothers with OWO.
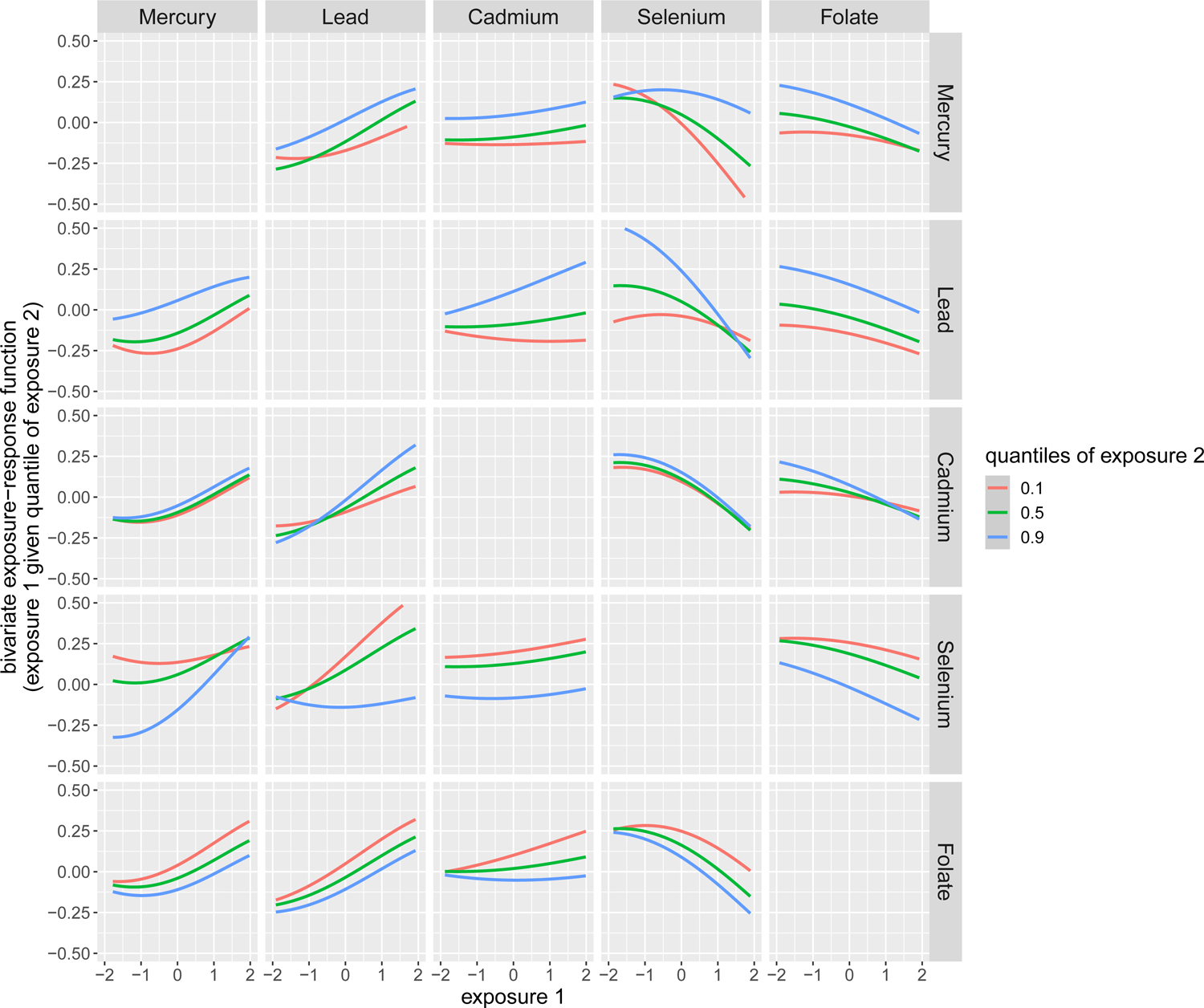
The bivariate dose-response function was obtained by plotting the dose-response function of a single predictor (exposure 1) when the second predictor (exposure 2) was fixed at various quantiles as labeled, while the remaining predictors were fixed to their median values. Maternal age, BMI, race/ethnicity, marital status, education level, parity, breastfeeding type, smoking status, fish intake, child’s sex, low birthweight, and preterm status were included as the covariates. The x-axis was limited to a range from −2 to 2, which reflects the z-score. Refer to the Supplement for the full range plot (Fig. S11).
Lastly, we investigated the protective effect of maternal Se and folate levels against the toxic metal mixture (instead of individual metals), respectively, among children born to mothers with and without OWO. As shown in Fig. 4A, we obtained an overall exposure-OWO association curve of the toxic metal mixture (Hg, Pb, Cd) by comparing the association when all the toxic metals were fixed at a specific quantile (ranging from 10th to 90th) with the association when all the toxic metals were fixed at their median value. Next, we generated exposure-OWO association curves corresponding to two groups of children stratified by maternal Se tertiles (T1 versus T2–T3) and fixed folate levels at their median value. We found that child OWO increased when all toxic metal exposures increased from the 10th to the 90th percentile in the low vs. high Se group. Moreover, the strength of association (as reflected by the slope of the curve) was greater when mothers had lower Se levels (129–260 μg/L) compared to when mothers had higher Se levels (260–624 μg/L). Similarly, when the study children were stratified into low vs. high maternal folate groups, and maternal Se level was fixed at its median, an even greater difference in the strength of the association was observed between the high (T2–T3: 28.6–186 nmol/L) vs. low (T1: 6.64–28.6 nmol/L) folate group, as shown in Fig. 4B.
Fig. 4. Overall effect (95% Credible Intervals, CrIs) of toxic metals (Hg, Pb, Cd) on child overweight or obesity (OWO) among children born to mothers with OWO, stratified by maternal Se (Panel A) and folate levels (Panel B).
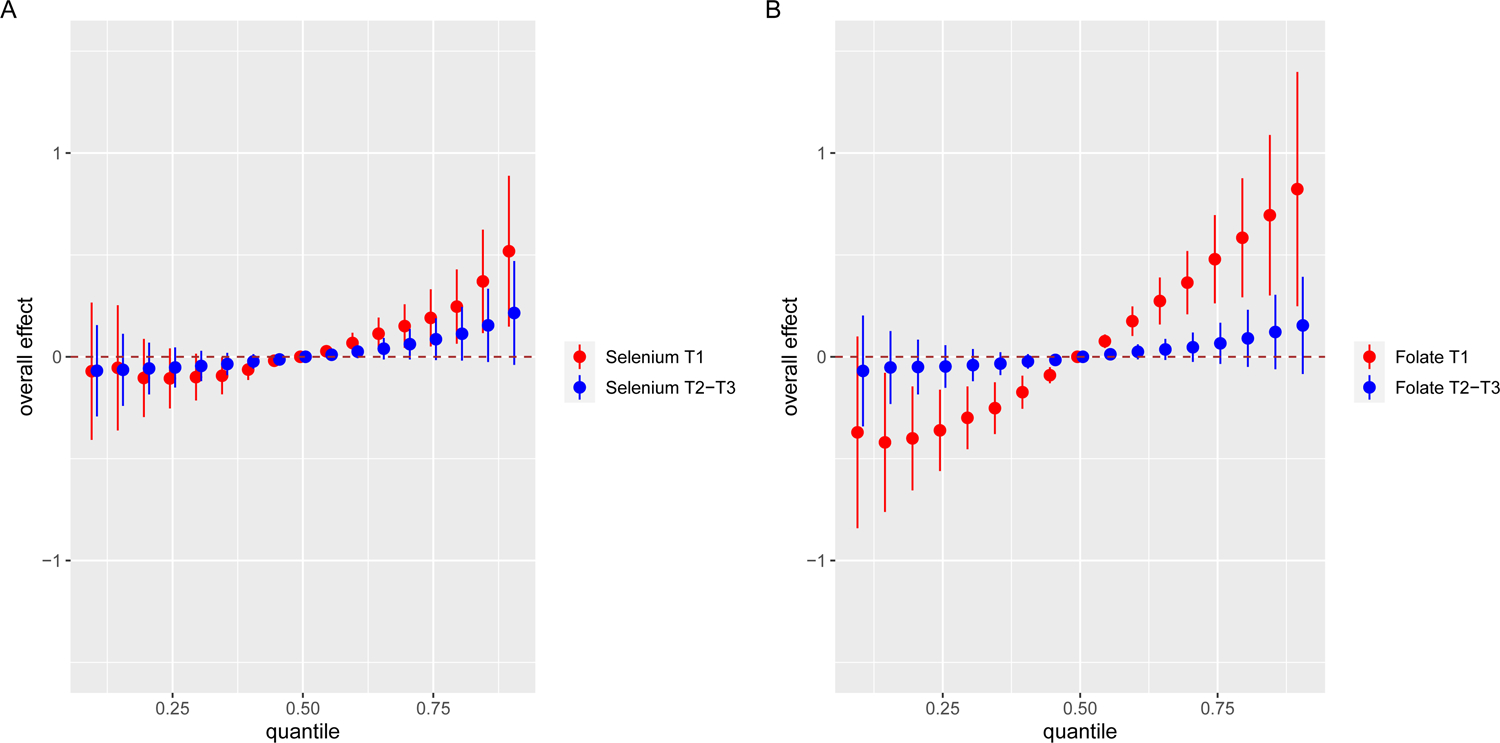
The overall effect (95% CrIs) of toxic metals (Hg, Pb, Cd) on child OWO is defined as the difference in the response when all the exposures are fixed at a specific quantile (ranging from 0.10 to 0.90), as compared to when all the exposures are fixed at their median value. Maternal age, BMI, race/ethnicity, marital status, education level, parity, breastfeeding type, smoking status, fish intake, child’s sex, low birthweight, and preterm status were included as the covariates. (A) Red group shows the overall effect on OWO risk among children whose mothers have OWO and have Se levels in T1, while the blue group shows the overall effect among children of mothers with OWO and with Se levels in T2–T3. (B) Red group shows the overall effect on OWO risk among children whose mothers have OWO and have folate levels in T1, while the blue group shows the overall effect among children of mothers with OWO and with folate levels in T2–T3. Note: Although the blue and red groups in (A) and (B) have the same quantiles (from 0.10 to 0.90), the blue group was slightly right-shifted to make the two sets of CrIs more clearly visible.
Sensitivity analysis
Fig. 2B, Fig. S12, Fig. S13 showed the univariate, bivariate, and overall dose-response functions among children born to mothers without OWO. The results suggested that among children born to mothers without OWO, the obesogenic effect of heavy metals (Hg, Pb, Cd) is weaker, and the protective effects of maternal Se and folate were not observed. Fig. S14 shows a higher estimated positive effect of Hg and lower negative effect of Se on the risk of child OWO among children born to non-Black mothers compared to Black mothers. Only small differences were revealed for Pb and Cd among boys and girls (Fig. S15). We removed post-exposure covariates (i.e., preterm birth, birthweight) from the covariates list in the BKMR model; the results (Fig. S16-S18) suggested consistent trends. Since diet is one source that contributes to metal exposure, and dietary habits influence the toxic metals (Hg, Pb, and Cd) exposures (37, 38), we further adjusted our BKMR models by including maternal Mediterranean dietary scores (39) as an additional covariate. The associations between maternal exposures and offspring OWO remained essentially unchanged after adding Mediterranean dietary scores (Fig. S19-S21). We also conducted our BKMR models to study childhood obesity (defined as BMI percentile greater than 95th) compared to children less than 85th in relation to maternal exposure biomarkers. Fig. S22-S24 showed consistent results as observed for childhood OWO. Since we used tertiles of maternal Se and folate levels to demonstrate their protective effects, we re-ran the BKMR model by using quartiles (Q1 vs. Q2–Q4) of Se and folate to assess the robustness of the association. Consistent protective effects of maternal Se and folate against the toxic metal mixture could be observed in Fig. S25. We also explored the overall effects of heavy metal mixtures (Hg, Pb, Cd) on offspring OWO risk under different levels of micronutrients through weighted quantile sum (WQS) regression (40), which is another novel method to study the overall effect of co-exposures, and the results (Fig. S26) were consistent with BKMR.
DISCUSSION
This study underscores the value of considering co-exposures to environmental toxic metals and micronutrients to evaluate health effects and identify novel, safe, and effective intervention strategies. In BBC, a US urban, low-income, underrepresented population, this study found a positive dose-response association between maternal exposure to low-level toxic metal mixture (Hg, Pb, Cd) and child risk of OWO, especially among children born to mothers with OWO. This work also demonstrated that adequate maternal Se or folate levels could mitigate the obesogenic effects of these toxic metals on children born to mothers with OWO. A particular strength of this study is our ability to apply BKMR, a novel method, to examine the relationships between exposure to toxic metal mixtures, micronutrients, and child OWO in US Black and Hispanic mother-child dyads. This population is disproportionally affected by exposures to these toxic metals (4) and has a high burden of cardiometabolic morbidities (41) as well as inadequate folate nutrition as reflected by maternal lower plasma folate levels compared to non-Hispanic Whites (42). To the best of our knowledge, this is the first study to investigate the joint effects of maternal exposures to toxic metals (Hg, Pb, Cd) and micronutrients (Se, folate) on child OWO in these under-studied, under-reported, and under-represented, yet high-risk populations. This study has made the following new contributions to the field.
First, we showed that in this high-risk US minority birth cohort, exposures to toxic metals (Hg, Pb, Cd) are widespread and do not occur in isolation, underscoring the importance of addressing the health effects of co-exposures. Second, we demonstrated the importance of considering maternal OWO status as an effect modifier in studying prenatal toxic metal and micronutrient exposures and child OWO. Third, our findings raise the possibility that maternal Se and folate nutrition may counteract the effect of toxic metal exposures on childhood OWO among children born to mothers with OWO, which, if further confirmed, will have important clinical and public health implications. Finally, this study employed both conventional regression models and novel BKMR. While we obtained consistent findings, we found that BKMR enhanced our ability to identify interactions among multiple co-exposures and allowed us to estimate their overall combined effects, which is essential to reflect real-world scenarios.
Most previous related studies were focused on a single toxic metal exposure. Although there are some prior investigations of the effects of maternal exposure to multiple metals on offspring birthweight (43–45), neurodevelopment (46–49), and childhood blood pressure (10), few studies directly explored the association between maternal multiple metals exposures and childhood OWO. This study, using BKMR, revealed that collectively, maternal Hg, Pb, and Cd exposures were associated with an increased risk of child OWO. However, it should be noted that the exposure levels of these toxic metals are low on average. Nevertheless, we demonstrated a clear dose-response relationship, underscoring that there are no safe exposure levels for these toxic metals. These findings are biologically plausible and consistent with previous studies (13, 14). For example, human studies by us and others showed a positive association between Hg (15, 50), Pb (51, 52), and obesity, and animal studies of perinatal Pb exposure showed a similar association with bodyweight (53, 54).
To date, in both clinical and public health settings, there has been a lack of clear and effective strategies to reduce the adverse health effects of low-level toxic metal exposure such as Hg, Pb, and Cd. Nutrition has been proposed as a safe, simple, and inexpensive means to alleviate the detrimental effects of environmental toxicants (17, 18). As underscored by a recent review (37), childhood obesity is due to multifactorial and multidimensional factors, including environmental pollution and diet. Consistently, findings of our study emphasize the need to consider micronutrient status in assessing the obesogenic effects of toxic metals. Our findings also raise the prospective that optimizing micronutrient status could be an intervention strategy in the setting of environmental obesogenic exposure. One study has indicated that folate may reduce the adverse impacts of Hg and Pb on the developing child (25). Folate is an essential B vitamin involved in DNA methylation, nucleic acid synthesis, cellular growth, differentiation, and repair (55). It also has antioxidant and anti-inflammatory properties and improves endothelial functions (56–59). The demand for folate increases during pregnancy due to fetal and placental growth and uterus enlargement (60). We have published findings showing that high folic acid intake (prenatal vitamins and over-the-counter multivitamins) in two or more periods (preconception, 1st, 2nd, and 3rd trimesters) was associated with higher folate concentration (42) and adequate maternal folate mitigated the risk of intergenerational obesity in the presence of prenatal Hg or Pb exposure among children of mothers with OWO (13, 14). Many studies have also demonstrated that Se deficiency increases the risk of childhood OWO (22–24). To our knowledge, this is the first study that demonstrates the protective effects of Se and folate in the setting of toxic metal co-exposure.
Methodologically, most studies of maternal exposure to multiple metals have adopted the approach in which all potential interaction terms are added to a multivariable generalized linear model (46, 47) or a WQS regression model (61, 62). However, as shown in our analyses using conventional linear regression models, it is difficult to account for the complex and nonlinear interactions of multiple exposures (48). WQS is able to quantify the summed mixture effect, but it assumes that any effects of the exposures on the outcome are all in the same direction (62, 63), which may result in biased estimates (64). Using BKMR, we revealed new findings that cannot be readily observed using conventional methods, such as the interactions of Cd with Pb and Hg. We also demonstrated that maternal OWO enhanced the effects of toxic metals on the risk of child OWO. Among children born to mothers with OWO, we showed that Se and folate could mitigate the obesogenic effect of toxic metal co-exposure on child OWO.
Our study also has limitations. First, we measured the levels of metals in RBCs as long-term biomarkers of metal exposure; given that RBCs are the primary storage site for these metals, the RBCs’ life span is about 120 days (29). However, it would be ideal if we could also measure metals in serum; using Pb as an example, it is the exchangeable fraction. In this way, we could compare which exposure measure is more closely linked to health effects, in our case, childhood OWO. Second, we measured maternal RBC metal levels at only one time point, within 24 to 72 hours after delivery, as a proxy for in-utero 2nd and 3rd trimester exposure. However, the 3rd trimester is a critical period for fetal weight gain and adiposity development (65) and there were high correlations between maternal and cord RBCs for Hg, Pb, and Se (3). Third, we did not measure metal exposures during childhood, which may also contribute to child OWO. Fourth, we did not measure all metals, like zinc and arsenic. We were unable to determine the sources of maternal toxic exposures. While diet is one of the main sources that contributes to metal exposure, and dietary habits influence the exposure to toxic metals (Hg, Pb, and Cd) and micronutrients (Se and folate) (37, 38), we were unable to conduct a systematic dietary analysis. Based on the literature, Hg exposure can occur through ingestion of certain fish, shellfish, or marine mammals (66) or other contaminated foods. While Pb is not as common in fish as Hg, it can still be found in spices, fruit (canned and fresh), and other foods. Imported cookware, plates, and pottery were other potential exposure resources as well (67). In the US, diet is the main source of Cd exposure among nonsmokers (68). Leafy vegetables, staples contain relatively high levels of Cd, and shellfish and organ meats (liver and kidney) intake also could increase Cd exposures. Smokers are likely to have higher Cd exposures as tobacco leaves naturally accumulate high amounts of Cd. Future studies are needed to systematically explore the potential maternal diet and toxicants interactions in offspring OWO outcomes. We measured total Hg, not methylmercury (MeHg), though Hg concentrations in RBCs are the best biomarker of MeHg exposure insofar as ~ 80% of MeHg is stored in red blood cells (14). Future studies are also needed to further consider co-occurring maternal exposure to other endocrine disruptors such as PFAS (69).This was a prospective observational study. Even though we adjusted for important confounders, such as maternal age, BMI, race, education level, and fish intake, we could not consider all the potential confounders. Moreover, our study population consisted of predominantly urban, low-income Black and Hispanic mothers and their children. While the investigation of this understudied population is critical for understanding and eliminating health disparities in this population, caution is needed when generalizing our findings to populations with different characteristics. Additional studies are warranted to confirm these associations in other populations. Furthermore, the reasons why these associations are more pronounced in children born to mothers with OWO are not clear. We speculate that it could be due in part to shared genetic susceptibility, or other environmental confounders. We raise this as a potential hypothesis for future studies to consider.
In summary, this study demonstrated an apparent dose-response association between maternal exposure to low-level toxic metal mixture (Hg, Pb, Cd) and increased risk of child OWO. Importantly, this association was mainly seen among children born to mothers with OWO, underscoring that children born to mothers with OWO may be particularly susceptible to toxic metal exposures, even at low-levels. In this setting, we further revealed that adequate maternal Se or folate levels could mitigate the obesogenic effects of these toxic metals. Our findings warrant additional investigation, if confirmed, would provide the critical evidence needed to inform environmental regulation of toxic metals and clinical and public health guidelines to improve early screening, prevention, and mitigation of prenatal toxic metal exposures in US urban, high-risk minority populations.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank all study participants in the Boston Birth Cohort. The authors also acknowledge the nursing staff at Labor and Delivery of the Boston Medical Center and the field team for their contributions to the Boston Birth Cohort. The Boston Birth Cohort (the parent study) was supported in part by the National Institutes of Health (NIH) grants (R21ES011666, 2R01HD041702, R21HD066471, R01HD086013, R01HD098232, R01ES031272, and R01ES031521); and the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) (UJ2MC31074). Dr. Guoying Wang is also supported by grant R03ES029594 from the NIH/National Institute of Environmental Health Science. This study was possible in part due to funding for programmatic analytical capacity and capability through Cooperative Agreement #CDC-RFA-EH14-140203 between the New Jersey Department of Health (NJDOH) Public Health and Environmental Laboratories (PHEL) and the Centers for Disease Control and Prevention (CDC) States Biomonitoring Grant Program. This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by any funding agencies.
Footnotes
Clinical Trial Registry number and website where it was obtained
Competing Financial Interests
The authors declare they have no actual or potential competing financial interests.
CODE AVAILABILITY
The R code supporting the findings of this study are available upon request from the corresponding author.
DATA AVAILABILITY STATEMENT
The data, data dictionary, and analytical programs for this manuscript are not currently available to the public. However, they can be made available upon reasonable request and after the review and approval of the institutional review board.
REFERENCES
- 1.WHO (World Health Organization). Preventing disease through healthy environment: Action is needed on chemicals of major public health concern 2010. [Available from: http://www.who.int/ipcs/features/chemicals_concern/en/.
- 2.ATSDR (Agency for Toxic Substances and Disease Registry). Substance Priority List 2020. [Available from: https://www.atsdr.cdc.gov/spl/index.html.
- 3.Chen Z, Myers R, Wei T, Bind E, Kassim P, Wang G, et al. Placental transfer and concentrations of cadmium, mercury, lead, and selenium in mothers, newborns, and young children. J Expo Sci Environ Epidemiol. 2014;24(5):537–44. 10.1038/jes.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shim YK, Lewin MD, Ruiz P, Eichner JE, Mumtaz MM. Prevalence and associated demographic characteristics of exposure to multiple metals and their species in human populations: The United States NHANES, 2007–2012. J Toxicol Environ Health A. 2017;80(9):502–12. 10.1080/15287394.2017.1330581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bulka CM, Persky VW, Daviglus ML, Durazo-Arvizu RA, Argos M. Multiple metal exposures and metabolic syndrome: A cross-sectional analysis of the National Health and Nutrition Examination Survey 2011–2014. Environ Res. 2019;168:397–405. 10.1016/j.envres.2018.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lanphear BP, Rauch S, Auinger P, Allen RW, Hornung RW. Low-level lead exposure and mortality in US adults: a population-based cohort study. Lancet Public Health. 2018;3(4):e177–e84. 10.1016/s2468-2667(18)30025-2. [DOI] [PubMed] [Google Scholar]
- 7.Mahaffey KR, Clickner RP, Bodurow CC. Blood organic mercury and dietary mercury intake: National Health and Nutrition Examination Survey, 1999 and 2000. Environ Health Perspect. 2004;112(5):562–70. 10.1289/ehp.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oulhote Y, Mergler D, Bouchard MF. Sex- and age-differences in blood manganese levels in the U.S. general population: national health and nutrition examination survey 2011–2012. Environ Health. 2014;13:87. 10.1186/1476-069x-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breton CV, Farzan SF. Invited Perspective: Metal Mixtures and Child Health: The Complex Interplay of Essential and Toxic Elements. Environmental Health Perspectives. 2021;129(6):061301. 10.1289/EHP9629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang M, Liu T, Wang G, Buckley JP, Guallar E, Hong X, et al. In Utero Exposure to Heavy Metals and Trace Elements and Childhood Blood Pressure in a U.S. Urban, Low-Income, Minority Birth Cohort. Environmental Health Perspectives. 2021;129(6):067005. 10.1289/EHP8325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanders T, Liu Y, Buchner V, Tchounwou PB. Neurotoxic effects and biomarkers of lead exposure: a review. Rev Environ Health. 2009;24(1):15–45. 10.1515/reveh.2009.24.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang LW. Neurotoxic effects of mercury-a review. Environmental research. 1977;14(3):329–73. 10.1016/0013-9351(77)90044-5. [DOI] [PubMed] [Google Scholar]
- 13.Wang G, DiBari J, Bind E, Steffens AM, Mukherjee J, Azuine RE, et al. Association Between Maternal Exposure to Lead, Maternal Folate Status, and Intergenerational Risk of Childhood Overweight and Obesity. JAMA Netw Open. 2019;2(10):e1912343. 10.1001/jamanetworkopen.2019.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang G, DiBari J, Bind E, Steffens AM, Mukherjee J, Bartell TR, et al. In utero exposure to mercury and childhood overweight or obesity: counteracting effect of maternal folate status. BMC Med. 2019;17(1):216. 10.1186/s12916-019-1442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S, Yoon JH, Won JU, Lee W, Lee JH, Seok H, et al. The Association Between Blood Mercury Levels and Risk for Overweight in a General Adult Population: Results from the Korean National Health and Nutrition Examination Survey. Biol Trace Elem Res. 2016;171(2):251–61. 10.1007/s12011-015-0530-1. [DOI] [PubMed] [Google Scholar]
- 16.NIH (National Institutes of Health). Strategic Plan 2018–2023: Advancing Environmental Health Sciences Improving Health 2018. [Available from: https://www.niehs.nih.gov/about/strategicplan/strategicplan20182023_508.pdf.
- 17.Furst A. Can nutrition affect chemical toxicity? Int J Toxicol. 2002;21(5):419–24. 10.1080/10915810290096649. [DOI] [PubMed] [Google Scholar]
- 18.Hennig B, Ettinger AS, Jandacek RJ, Koo S, McClain C, Seifried H, et al. Using nutrition for intervention and prevention against environmental chemical toxicity and associated diseases. Environ Health Perspect. 2007;115(4):493–5. 10.1289/ehp.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aguirre JD, Culotta VC. Battles with iron: manganese in oxidative stress protection. J Biol Chem. 2012;287(17):13541–8. 10.1074/jbc.R111.312181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark LC, Combs GF Jr., Turnbull BW, Slate EH, Chalker DK, Chow J, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. Jama. 1996;276(24):1957–63. 10.1001/jama.1996.03540240035027. [DOI] [PubMed] [Google Scholar]
- 21.Rayman MP. The importance of selenium to human health. Lancet. 2000;356(9225):233–41. 10.1016/s0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- 22.Blazewicz A, Klatka M, Astel A, Korona-Glowniak I, Dolliver W, Szwerc W, et al. Serum and urinary selenium levels in obese children: a cross-sectional study. J Trace Elem Med Biol. 2015;29:116–22. 10.1016/j.jtemb.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 23.Ortega RM, Rodriguez-Rodriguez E, Aparicio A, Jimenez-Ortega AI, Palmeros C, Perea JM, et al. Young children with excess of weight show an impaired selenium status. Int J Vitam Nutr Res. 2012;82(2):121–9. 10.1024/0300-9831/a000101. [DOI] [PubMed] [Google Scholar]
- 24.Azab SF, Saleh SH, Elsaeed WF, Elshafie MA, Sherief LM, Esh AM. Serum trace elements in obese Egyptian children: a case-control study. Ital J Pediatr. 2014;40:20. 10.1186/1824-7288-40-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ouyang F, Longnecker MP, Venners SA, Johnson S, Korrick S, Zhang J, et al. Preconception serum 1,1,1-trichloro-2,2,bis(p-chlorophenyl)ethane and B-vitamin status: independent and joint effects on women’s reproductive outcomes. Am J Clin Nutr. 2014;100(6):1470–8. 10.3945/ajcn.114.088377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. 2015;16(3):493–508. 10.1093/biostatistics/kxu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bobb JF, Claus Henn B, Valeri L, Coull BA. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ Health. 2018;17(1):67. 10.1186/s12940-018-0413-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang G, Divall S, Radovick S, Paige D, Ning Y, Chen Z, et al. Preterm birth and random plasma insulin levels at birth and in early childhood. JAMA. 2014;311(6):587–96. 10.1001/jama.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shemin D, Rittenberg D. The life span of the human red blood cell. J Biol Chem. 1946;166(2):627–36. [PubMed] [Google Scholar]
- 30.Huo Y, Li J, Qin X, Huang Y, Wang X, Gottesman RF, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. Jama. 2015;313(13):1325–35. 10.1001/jama.2015.2274. [DOI] [PubMed] [Google Scholar]
- 31.CDC (Centers for Disease Control and Prevention). CDC growth chart 2000 [updated November 26, 2013. 2000:[Available from: https://www.cdc.gov/growthcharts/.
- 32.CDC. Overweight & obesity: defining childhood obesity. Centers for Disease Control and Prevention; 2018. [Available from: https://www.cdc.gov/obesity/childhood/defining.html. [Google Scholar]
- 33.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 34.Kupsco A, Kioumourtzoglou M-A, Just AC, Amarasiriwardena C, Estrada-Gutierrez G, Cantoral A, et al. Prenatal Metal Concentrations and Childhood Cardiometabolic Risk Using Bayesian Kernel Machine Regression to Assess Mixture and Interaction Effects. Epidemiology. 2019;30(2). 10.1097/EDE.0000000000000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre-Pregnancy Body Mass Index in Relation to Infant Birth Weight and Offspring Overweight/Obesity: A Systematic Review and Meta-Analysis. PLOS ONE. 2013;8(4):e61627. 10.1371/journal.pone.0061627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott JG, Berger JO. Bayes and empirical-Bayes multiplicity adjustment in the variable-selection problem. The Annals of Statistics. 2010;38(5):2587–619, 33. 10.1214/10-AOS792. [DOI] [Google Scholar]
- 37.Martinez-Esquivel A, Trujillo-Silva DJ, Cilia-Lopez VG. Impact of environmental pollution on the obesogenic environment. Nutr Rev. 2022. 10.1093/nutrit/nuac003. [DOI] [PubMed] [Google Scholar]
- 38.Bjermo H, Sand S, Nalsen C, Lundh T, Enghardt Barbieri H, Pearson M, et al. Lead, mercury, and cadmium in blood and their relation to diet among Swedish adults. Food Chem Toxicol. 2013;57:161–9. 10.1016/j.fct.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 39.Rhee DK, Ji Y, Hong X, Pearson C, Wang X, Caulfield LE. Mediterranean-Style Diet and Birth Outcomes in an Urban, Multiethnic, and Low-Income US Population. Nutrients. 2021;13(4). 10.3390/nu13041188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carrico C, Gennings C, Wheeler DC, Factor-Litvak P. Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. J Agric Biol Environ Stat. 2015;20(1):100–20. 10.1007/s13253-014-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.CDC. Pregnancy Complications 2020. [updated August 13, 2020. Available from: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pregnancy-complications.html.
- 42.Cheng TL, Mistry KB, Wang G, Zuckerman B, Wang X. Folate Nutrition Status in Mothers of the Boston Birth Cohort, Sample of a US Urban Low-Income Population. Am J Public Health. 2018;108(6):799–807. 10.2105/AJPH.2018.304355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shirai S, Suzuki Y, Yoshinaga J, Mizumoto Y. Maternal exposure to low-level heavy metals during pregnancy and birth size. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2010;45(11):1468–74. 10.1080/10934529.2010.500942. [DOI] [PubMed] [Google Scholar]
- 44.Hu X, Zheng T, Cheng Y, Holford T, Lin S, Leaderer B, et al. Distributions of heavy metals in maternal and cord blood and the association with infant birth weight in China. J Reprod Med. 2015;60(1–2):21–9. https://www.ncbi.nlm.nih.gov/pubmed/25745747. [PMC free article] [PubMed] [Google Scholar]
- 45.Luo Y, McCullough LE, Tzeng JY, Darrah T, Vengosh A, Maguire RL, et al. Maternal blood cadmium, lead and arsenic levels, nutrient combinations, and offspring birthweight. BMC Public Health. 2017;17(1):354. 10.1186/s12889-017-4225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freire C, Amaya E, Gil F, Murcia M, S LL, Casas M, et al. Placental metal concentrations and birth outcomes: The Environment and Childhood (INMA) project. Int J Hyg Environ Health. 2019;222(3):468–78. 10.1016/j.ijheh.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 47.Freire C, Amaya E, Gil F, Fernandez MF, Murcia M, Llop S, et al. Prenatal co-exposure to neurotoxic metals and neurodevelopment in preschool children: The Environment and Childhood (INMA) Project. Sci Total Environ. 2018;621:340–51. 10.1016/j.scitotenv.2017.11.273. [DOI] [PubMed] [Google Scholar]
- 48.Valeri L, Mazumdar MM, Bobb JF, Claus Henn B, Rodrigues E, Sharif OIA, et al. The Joint Effect of Prenatal Exposure to Metal Mixtures on Neurodevelopmental Outcomes at 20–40 Months of Age: Evidence from Rural Bangladesh. Environ Health Perspect. 2017;125(6):067015. 10.1289/EHP614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vecchi Brumatti L, Rosolen V, Mariuz M, Piscianz E, Valencic E, Bin M, et al. Impact of Methylmercury and Other Heavy Metals Exposure on Neurocognitive Function in Children Aged 7 Years: Study Protocol of the Follow-up. J Epidemiol. 2021;31(2):157–63. 10.2188/jea.JE20190284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cho S, Jacobs DR Jr., Park K. Population correlates of circulating mercury levels in Korean adults: the Korea National Health and Nutrition Examination Survey IV. BMC Public Health. 2014;14:527. 10.1186/1471-2458-14-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim R, Hu H, Rotnitzky A, Bellinger D, Needleman H. A longitudinal study of chronic lead exposure and physical growth in Boston children. Environ Health Perspect. 1995;103(10):952–7. 10.1289/ehp.95103952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang N, Chen C, Nie X, Han B, Li Q, Chen Y, et al. Blood lead level and its association with body mass index and obesity in China - Results from SPECT-China study. Sci Rep. 2015;5:18299. 10.1038/srep18299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Faulk C, Barks A, Sanchez BN, Zhang Z, Anderson OS, Peterson KE, et al. Perinatal lead (Pb) exposure results in sex-specific effects on food intake, fat, weight, and insulin response across the murine life-course. PLoS One. 2014;9(8):e104273. 10.1371/journal.pone.0104273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu J, Wen XW, Faulk C, Boehnke K, Zhang H, Dolinoy DC, et al. Perinatal Lead Exposure Alters Gut Microbiota Composition and Results in Sex-specific Bodyweight Increases in Adult Mice. Toxicol Sci. 2016;151(2):324–33. 10.1093/toxsci/kfw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crider KS, Yang TP, Berry RJ, Bailey LB. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate’s role. Adv Nutr. 2012;3(1):21–38. 10.3945/an.111.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Antoniades C, Shirodaria C, Warrick N, Cai S, Bono Jd, Lee J, et al. 5-Methyltetrahydrofolate Rapidly Improves Endothelial Function and Decreases Superoxide Production in Human Vessels. Circulation. 2006;114(11):1193–201. 10.1161/CIRCULATIONAHA.106.612325. [DOI] [PubMed] [Google Scholar]
- 57.Homocysteine Lowering Trialists’ Collaboration. Dose-dependent effects of folic acid on blood concentrations of homocysteine: a meta-analysis of the randomized trials. Am J Clin Nutr. 2005;82(4):806–12. 10.1093/ajcn/82.4.806. [DOI] [PubMed] [Google Scholar]
- 58.Joshi R, Adhikari S, Patro BS, Chattopadhyay S, Mukherjee T. Free radical scavenging behavior of folic acid: evidence for possible antioxidant activity. Free Radic Biol Med. 2001;30(12):1390–9. 10.1016/s0891-5849(01)00543-3. [DOI] [PubMed] [Google Scholar]
- 59.Zhao M, Chen YH, Dong XT, Zhou J, Chen X, Wang H, et al. Folic acid protects against lipopolysaccharide-induced preterm delivery and intrauterine growth restriction through its anti-inflammatory effect in mice. PLoS One. 2013;8(12):e82713. 10.1371/journal.pone.0082713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Greenberg JA, Bell SJ, Guan Y, Yu YH. Folic Acid supplementation and pregnancy: more than just neural tube defect prevention. Rev Obstet Gynecol. 2011;4(2):52–9. [PMC free article] [PubMed] [Google Scholar]
- 61.Horton MK, Hsu L, Claus Henn B, Margolis A, Austin C, Svensson K, et al. Dentine biomarkers of prenatal and early childhood exposure to manganese, zinc and lead and childhood behavior. Environ Int. 2018;121(Pt 1):148–58. 10.1016/j.envint.2018.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y, Dong T, Hu W, Wang X, Xu B, Lin Z, et al. Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: Comparison of three statistical models. Environment International. 2019;123:325–36. 10.1016/j.envint.2018.11.076. [DOI] [PubMed] [Google Scholar]
- 63.Czarnota J, Gennings C, Wheeler DC. Assessment of weighted quantile sum regression for modeling chemical mixtures and cancer risk. Cancer Inform. 2015;14(Suppl 2):159–71. 10.4137/CIN.S17295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ. A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environ Health Perspect. 2020;128(4):47004. 10.1289/ehp5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gillman MW, Ludwig DS. How early should obesity prevention start? N Engl J Med. 2013;369(23):2173–5. 10.1056/NEJMp1310577. [DOI] [PubMed] [Google Scholar]
- 66.Risher JF, De Rosa CT, Jones DE, Murray HE. Updated toxicological profile for mercury. Toxicol Ind Health. 1999;15(5):480–2. 10.1177/074823379901500503. [DOI] [PubMed] [Google Scholar]
- 67.Abadin H, Ashizawa A, Stevens YW, Llados F, Diamond G, Sage G, et al. Toxicological Profile for Lead. Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles. Atlanta (GA) 2007. [PubMed] [Google Scholar]
- 68.Faroon O, Ashizawa A, Wright S, Tucker P, Jenkins K, Ingerman L, et al. Toxicological Profile for Cadmium. Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles. Atlanta (GA) 2012. [PubMed] [Google Scholar]
- 69.Zhang M, Chang H, Wang G, et al. Longitudinal trajectories and determinants of plasma per- and polyfluoroalkyl substance (PFAS) levels from birth to early childhood and metabolomic associations: A pilot study in the Boston Birth Cohort. Precis Nutr 2022;1(1): e00003. 10.1097/PN9.0000000000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological). 1995;57(1):289–300. 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data, data dictionary, and analytical programs for this manuscript are not currently available to the public. However, they can be made available upon reasonable request and after the review and approval of the institutional review board.


