Abstract
Plasma phosphorylated tau 181 (P-tau181) and 217 (P-tau217) are indicators of both amyloid and tau pathology in clinical settings, but their performance in heterogeneous community-based populations is unclear. We examined P-tau181 and P-tau217 (N=1,329, aged 30 to 98 years), in the population-based Mayo Clinic Study of Aging. Continuous, unadjusted, plasma P-tau181 and P-tau217 predicted abnormal amyloid PET (AUROC=0.81–0.86) and tau PET entorhinal cortex (AUROC>0.80), but was less predictive of a tau PET temporal region of interest (AUROC<0.70). Multiple comorbidities were associated with higher plasma P-tau181 and P-tau217 levels; the difference between participants with versus without chronic kidney disease (CKD) was similar to participants with versus without elevated brain amyloid. The exclusion of participants with CKD and other comorbidities effected the establishment of a normal reference range and cut-points. Understanding the impact of comorbidities on P-tau181 and P-tau217 levels is important for their future interpretation in the context of clinical screening, diagnosis, or prognosis at the population level.
Introduction
Several studies have reported that plasma phosphorylated tau 181 (P-tau181) and 217 (P-tau217) are indicators of both amyloid and tau pathology across the clinical Alzheimer’s disease (AD) spectrum and can differentiate AD dementia from other neurodegenerative diseases including frontotemporal lobe dementia1–11. Some studies suggest that P-tau217 has better discriminative accuracy for AD dementia than P-tau1815,8. However, these studies have primarily utilized clinical cohorts of patients with subjective memory complaints, mild cognitive impairment (MCI) or AD dementia. A major benefit of the use of blood-based biomarkers in screening for AD pathology, or diagnosis, is that collection of blood is less invasive and costly than cerebrospinal fluid or neuroimaging markers, and more feasible at the primary care levels where most individuals will present with cognitive symptoms12,13. Thus, there is a need to better understand the predictive value of plasma P-tau181 and P-tau217 in community-based populations.
Community-based studies are also more heterogenous, with participants often exhibiting more comorbidities than those enrolled in more clinically-based studies. As blood-based biomarkers of AD pathology enter clinical use, it is essential to understand what factors influence the levels of these blood biomarkers for interpretation of the results14,15. This is particularly important for the establishment of reference ranges. For example, it is important to understand whether levels differ with age and by sex, or whether conditions such as chronic kidney disease (CKD), which can affect the clearance of proteins, should be considered. Moreover, continuous plasma P-tau variables have been utilized for prediction of AD pathology in studies to date. The development of cutpoints for screening or diagnostic purposes is needed. Ultimately P-tau cutpoints will vary based on the specific assay and platform utilized. However, understanding who the reference population should be and whether certain medical conditions need to be considered in the interpretation of the plasma P-tau level will be relevant to all.
In the present analysis of 1,329 participants, aged 30 to 98 years, enrolled in the population-based Mayo Clinic Study of Aging (MCSA), we had several goals. First, we sought to identify what factors (e.g., age, APOE, comorbidities) might affect the interpretation of plasma P-tau181 and P-tau217 in a large community-based sample. Second, we examined the predictive accuracy of continuous plasma P-tau181 and P-tau217 for amyloid and tau PET in a large community-based sample. Last, we determined whether the factors found to be associated with plasma P-tau181 and P-tau217 will need to be considered in the development of cutpoints for predicting amyloid and tau PET. To further examine the temporality of the P-tau biomarkers to AD pathology, we examined tau PET in the entorhinal cortex (ERC) to assess the earliest neurofibrillary tangle pathology, as well as tau PET in a meta region of interest (ROI) to assess an area slightly later affected.
Results
Participant characteristics.
The characteristics of the 1,329 MCSA participants, by clinical diagnosis, are shown in Table 1. The median age (IQR; range) of the cohort was 73.2 (53.5, 81.3; 30.7, 97.9) years, 730 (54.9%) were male, and 26.6% had an APOE ε4 allele. There were 153 (11.5%) participants with a clinical diagnosis of MCI and 15 (1.1%) with dementia (10 AD dementia, 2 Lewy body dementia, 1 other dementia, and 2 indeterminate). Of the 1,329 participants, 1,051 (79.1%) had a concurrent amyloid PET scan with the plasma P-tau measures. There were 495 (37.2%) participants with a concurrent tau PET scan, all of whom also had amyloid PET. We dichotomized participants as A+ based on a cutoff of >1.48 standard uptake value ratio (SUVR) using the reliable worsening method16. Participants were dichotomized as T+ based on the tau PET temporal meta ROI defined as >1.29 SUVR and entorhinal cortex (ERC) defined as >1.27 SUVR based on autopsy diagnosis and Braak NFT stage17.
Table 1 |.
Participant characteristics, median (IQR) or N (%), by clinical diagnosis
| CU (N=1161) | MCI (N=153) | Dementia (N=15) | All (N=1329) | ||
|---|---|---|---|---|---|
| Characteristics | Median (IQR)/N(%) | Median (IQR)/N(%) | Median (IQR)/N(%) | Median (IQR)/N(%) | P-value |
| Age | 70.9 (48.4, 79.8) | 80.8 (75.8, 86.1) | 83.5 (81.5, 85.0) | 73.2 (53.5, 81.3) | <0.001 |
| Male | 629 (54.2%) | 88 (57.5%) | 13 (86.7%) | 730 (54.9%) | 0.030 |
| Education | 16 (13, 17) | 13 (12, 16) | 13 (12, 16) | 15 (13, 17) | <0.001 |
| Presence of APOE ε4 allele | 283/1138 (24.9%) | 58/153 (37.9%) | 7/15 (46.7%) | 348/1306 (26.6%) | <0.001 |
| Race | 0.823 | ||||
| White | 1124 (96.8%) | 149 (97.4%) | 14 (93.3%) | 1287 (96.8%) | |
| Black | 9 (0.8%) | 1 (0.7%) | 0 (0.0%) | 10 (0.8%) | |
| Other | 28 (2.4%) | 3 (2.0%) | 1 (6.7%) | 32 (2.4%) | |
| Ethnicity: Non-Hispanic | 1144 (98.5%) | 152 (99.3%) | 15 (100.0%) | 1311 (98.6%) | 0.782 |
| Plasma P-tau181 (pg/ml) | 1.00 (0.80, 1.32) | 1.61 (1.05, 2.47) | 2.01 (1.45, 3.40) | 1.03 (0.81, 1.44) | <0.001 |
| Plasma P-tau217 (pg/ml) | 0.14 (0.11, 0.19) | 0.24 (0.14, 0.39) | 0.40 (0.15, 0.72) | 0.14 (0.11, 0.21) | <0.001 |
| Body mass index, kg/m2 | 27.6 (24.8, 31.3) | 26.5 (23.9, 29.8) | 26.5 (23.6, 29.1) | 27.5 (24.7, 31.1) | 0.010 |
| Charlson comorbidity index | 2 (0, 4) | 4 (2, 6) | 5 (3, 10) | 2 (0, 4) | <0.001 |
| Beck Anxiety Inventory, total | 1 (0, 3) | 2 (0, 5) | 2 (0, 5) | 1 (0, 4) | <0.001 |
| Depression | 64 (5.5%) | 17 (11.1%) | 3 (21.4%) | 84 (6.3%) | 0.002 |
| Current/former smoker | 469 (40.4%) | 61 (39.9%) | 7 (46.7%) | 537 (40.4%) | 0.877 |
| Hypertension | 600 (51.7%) | 116 (75.8%) | 11 (73.3%) | 727 (54.7%) | <0.001 |
| Chronic kidney disease | 79 (6.8%) | 17 (11.1%) | 2 (13.3%) | 98 (7.4%) | 0.110 |
| Dyslipidemia | 777 (67.0%) | 120 (78.4%) | 14 (93.3%) | 911 (68.6%) | 0.002 |
| Diabetes | 156 (13.4%) | 33 (21.6%) | 5 (33.3%) | 194 (14.6%) | 0.003 |
| Stroke | 62 (5.3%) | 21 (13.7%) | 2 (13.3%) | 85 (6.4%) | <0.001 |
| Myocardial infarction | 103 (9.6%) | 30 (19.9%) | 2 (13.3%) | 135 (10.9%) | <0.001 |
| Atrial Fibrillation | 103 (9.6%) | 29 (19.2%) | 4 (26.7%) | 136 (11.0%) | <0.001 |
| Head trauma | 253 (23.4%) | 43 (31.4%) | 4 (28.6%) | 300 (24.4%) | 0.120 |
| Cancer | 251 (21.6%) | 46 (30.1%) | 10 (66.7%) | 307 (23.1%) | <0.001 |
| Amyloid PET ≥ 1.48 | 303/892 (34.0%) | 99/144 (68.8%) | 12/15 (80.0%) | 414/1051 (39.4%) | <0.001 |
| Tau PET ≥ 1.29 | 55/462 (11.9%) | 9/32 (28.1%) | 1/1 (100.0%) | 65/495 (13.1%) | 0.003 |
| ERC tau PET ≥ 1.27 | 40/462 (8.7%) | 8/32 (25.0%) | 1/1 (100.0%) | 49/495 (9.9%) | 0.001 |
CU, cognitively unimpaired; IQR, interquartile range; MCI, mild cognitive impairment; ERC, entorhinal cortex.
Plasma ptau with clinical diagnosis and amyloid or tau PET.
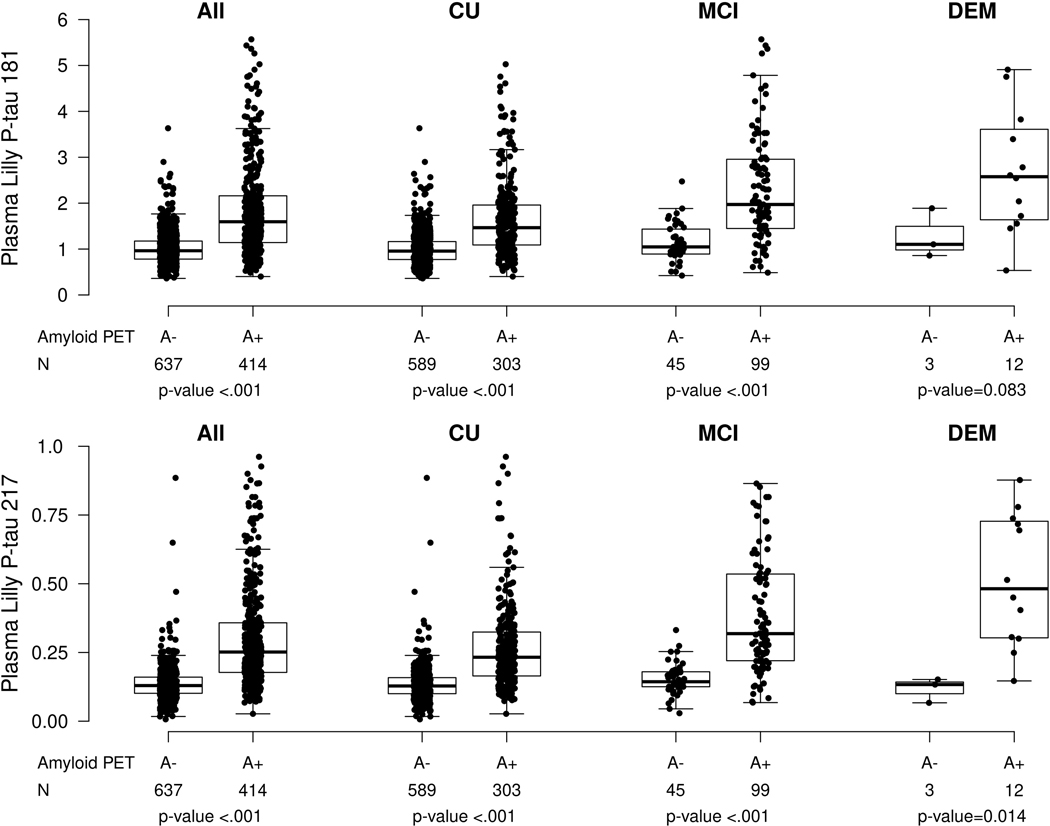
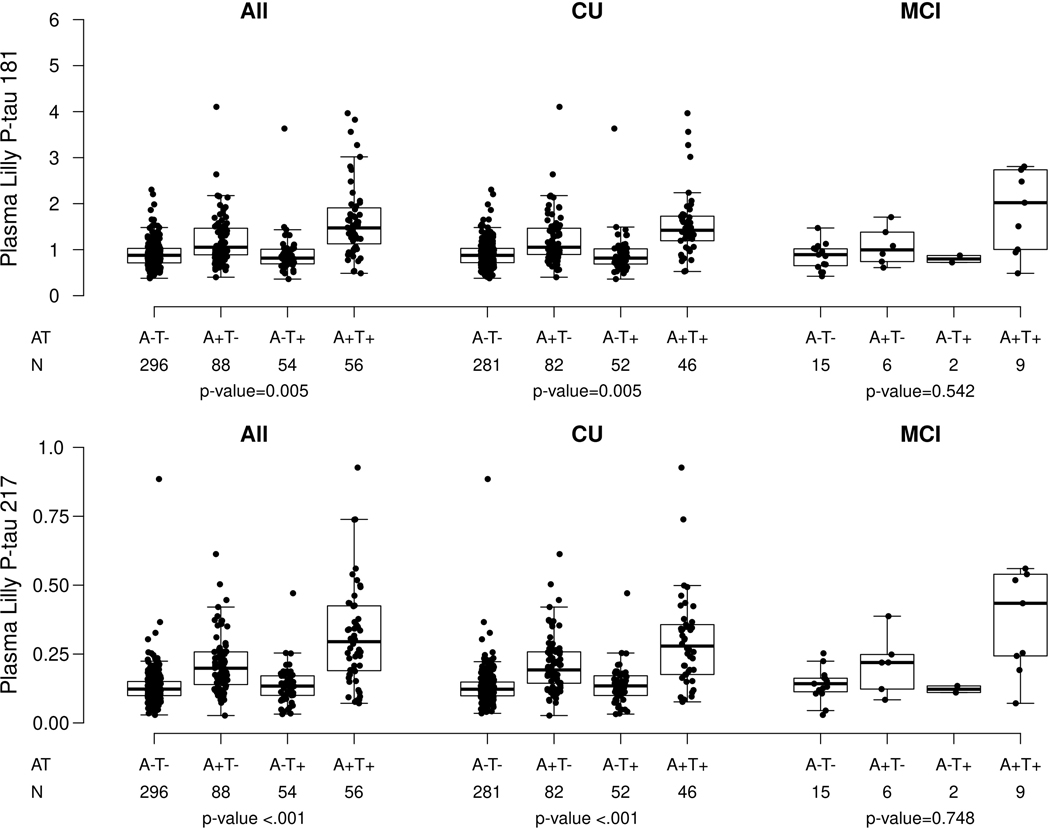
Plasma P-tau181 and P-tau217 were highly correlated (Spearman rho = 0.80, P < 0.001). Similar to previous reports, median levels of plasma P-tau181 and P-tau217 were higher among those with MCI versus CU (P-tau181: 1.61 versus 1.00, P < 0.001; P-tau217: 0.24 versus 0.14, P < 0.001) or dementia versus CU (P-tau181: 2.04 versus 1.00, P < 0.001; P-tau217: 0.40 versus 0.14, P < 0.001). For dementia versus MCI, median levels of P-tau181 (2.04 versus 1.61, P = 0.130) and P-tau217 (0.40 versus 0.24, P = 0.080) did not differ (Table 1). Further, within each clinical diagnosis (except for P-tau181 and dementia), both plasma P-tau181 and P-tau217 were significantly higher among those with elevated brain amyloid (A+) compared to those without (A−) (Table 2 and Extended Data Fig. 1). Among the CU and MCI participants with both amyloid and tau PET, P-tau181 and P-tau217 levels stratified by amyloid (A) and tau (T) (based on the tau PET temporal meta ROI) are shown in Extended Data Fig. 2 and Supplementary Table 1. Among CU and MCI combined and CU only, the A+T+ group had higher levels of both P-tau181 and P-tau217 compared to each of the three other groups (A−T−, A+T−, A−T+). Participants who were A+T− had significantly higher levels than A−T− and A−T+. Median levels of P-tau181 and P- tau217 did not differ by AT status among MCI only, but the sample size was small (N = 32).
Table 2 |.
Mean (SD) P-tau 181 and 217 levels by group and percent with abnormal values by group using different methods for selecting cutpoints
| Characteristic | CU A− N=589 |
CU A+ N=303 |
MCI or DEM A− N=48 |
MCI or DEM A+ N=111 |
Total N=1051 |
P-value |
|---|---|---|---|---|---|---|
| Plasma biomarker levels | ||||||
| Plasma Lilly P-tau181 | 1.02 (0.37) | 1.65 (0.83) | 1.16 (0.41) | 2.64 (3.49) | 1.38 (1.35) | <0.001a |
| Plasma Lilly P-tau217 | 0.13 (0.06) | 0.27 (0.16) | 0.15 (0.06) | 0.46 (0.61) | 0.21 (0.24) | <0.001a |
| Without stroke/MI/CKD | ||||||
| P-tau 181 >=1.57 | 42 (7.1%) | 135 (44.6%) | 9 (18.8%) | 78 (70.3%) | 264 (25.1%) | <0.001b |
| P-tau 217 >=0.25 | 19 (3.2%) | 134 (44.2%) | 4 (8.3%) | 77 (69.4%) | 234 (22.3%) | <0.001c |
| With stroke/MI/CKD | ||||||
| P-tau 181 >=1.75 | 22 (3.7%) | 103 (34.0%) | 4 (8.3%) | 69 (62.2%) | 198 (18.8%) | <0.001c |
| P-tau 217 >=0.26 | 10 (1.7%) | 120 (39.6%) | 2 (4.2%) | 73 (65.8%) | 205 (19.5%) | <0.001c |
Numbers indicate N (%) unless otherwise noted.
Anova F-test.
Chi-square.
Fisher exact. All p-values are two-sided and are not adjusted for multiple comparisons.
CKD, chronic kidney disease; CU, cognitively unimpaired; DEM, dementia; MI, myocardial infarction; SD, standard deviation.
Accuracy of P-tau181 and P-tau217 for amyloid and tau PET.
Supplementary Tables 2–4 provide the accuracy of continuous measures of P-tau181 and P-tau217 for abnormal amyloid PET, tau PET temporal meta ROI, and ERC tau PET. Plasma P-tau217 more accurately predicted abnormal amyloid PET (A+) than P-tau181 (area under the receiver operating characteristic [AUROC]: 0.855 versus 0.805, P < 0.001). Although P-tau217 was also more predictive than P-tau181 for tau PET temporal meta ROI (AUROC 0.694 versus 0.660, P = 0.133) and tau PET ERC (AUROC 0.857 versus 0.827, P = 0.155), the results were not statistically significant. Adding age, sex, and APOE to the model with either plasma P-tau181 or P-tau217 significantly increased the accuracy of predicting abnormal amyloid PET and tau PET ERC, but not tau PET temporal meta ROI. In fact, for tau PET temporal meta ROI, the combination of age, sex, and APOE was a significantly better predictor than P-tau 181 (AUROC 0.788 versus 0.660, P < 0.001) or P-217 (AUROC 0.788 versus 0.694, P < 0.001) alone.
Associations of plasma ptau with age.
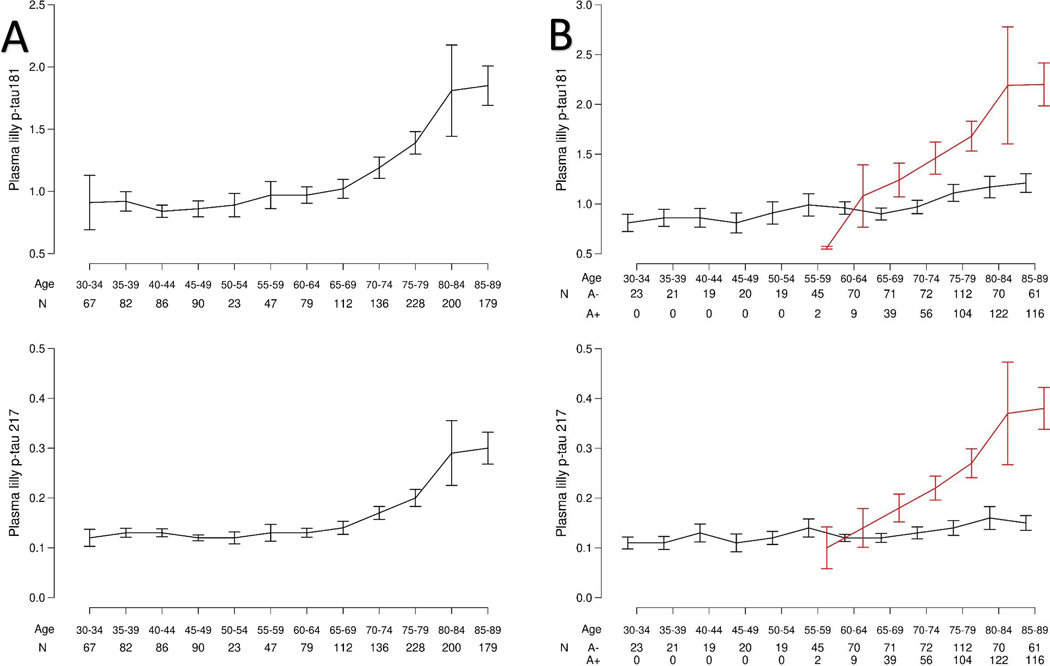
Examining plasma P-tau181 and P-tau217 levels from the age of 30 to 98 years, both biomarkers increased with age starting between the ages of 65 and 70 (Fig. 1A). However, in additional analyses stratified by amyloid PET status, the increase in both P-tau markers with age was markedly increased for A+ compared to A− participants (Fig. 1B). There was a significant difference for slope of P-tau181 with age for A+ versus A− participants (0.43 versus 0.06, P < 0.001). This corresponded to a 7.2-fold greater increase with age for A+ versus A− participants. For P-tau217, the slope was also higher for A+ versus A− (0.48 versus 0.04, P < 0.001) such that P-tau217 levels increased with age 12 times faster in A+ participants compared to A− participants.
Fig. 1. Plasma P-tau181 and P-tau217 levels by age.
Fig. 1A shows the plasma P-tau181 and −217 levels by age alone. Fig. 1B shows the plasma P-tau181 and −217 levels by age and elevated brain amyloid based on PiB-PET>1.48 standard uptake value ratio. Black indicates A− and red indicates A+. Data are presented as mean values +/− Standard error of the mean (SEM).
Associations of plasma ptau with other covariates.
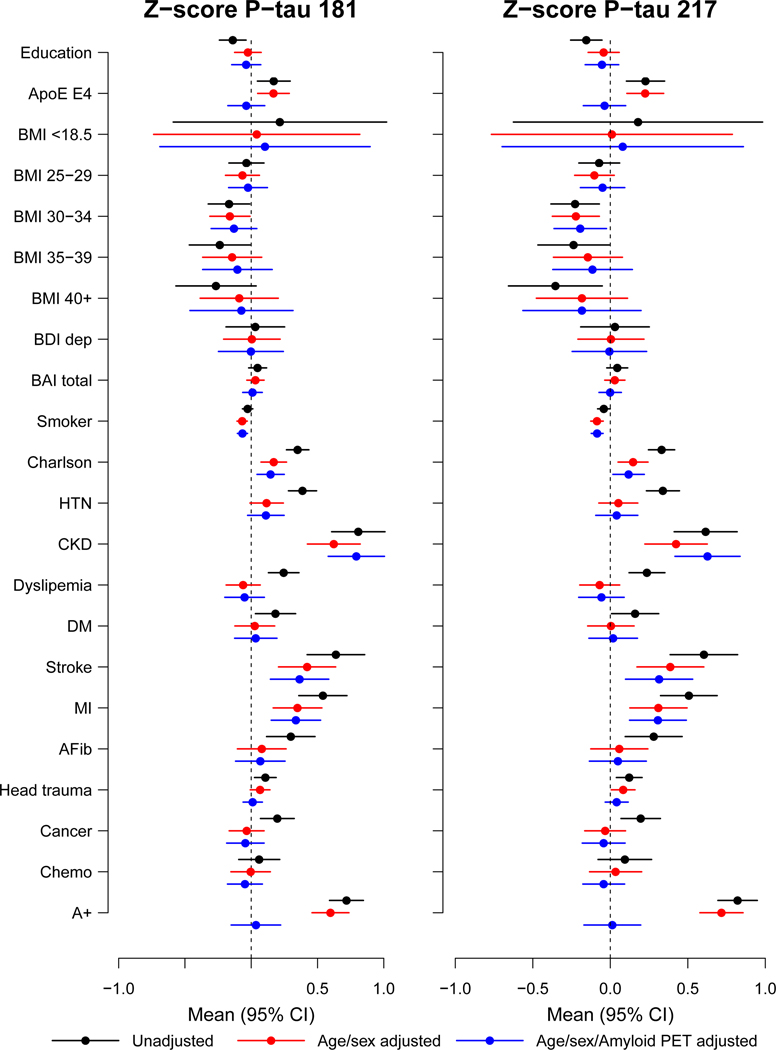
We next examined the associations of plasma P-tau181 or P-tau217 with several health-related factors, conditions, and APOE genotype. Plasma P-tau181 and P-tau217 were z-scored for comparison of the coefficients. The unadjusted, age- and sex-adjusted, and age-, sex- and amyloid PET-adjusted associations between z-scored P-tau181 or P-tau217 with several factors were similar when including the entire sample (n = 1,329) and the subset of 1,051 with concurrent amyloid PET. To better provide an indication of the influence of each variable on z-scored plasma Ptau181 and P-tau217, we include the subset of those with amyloid PET to demonstrate the difference in P-tau levels between those who were and were not A+ (see Fig. 2). The unadjusted and age-, sex-, and amyloid PET-adjusted associations between plasma P-tau181 and P-tau217 among all participants are shown in Supplementary Table 5 and Supplementary Table 6; associations stratified by clinical diagnosis (CU and MCI/dementia) are shown in Supplementary Table 7.
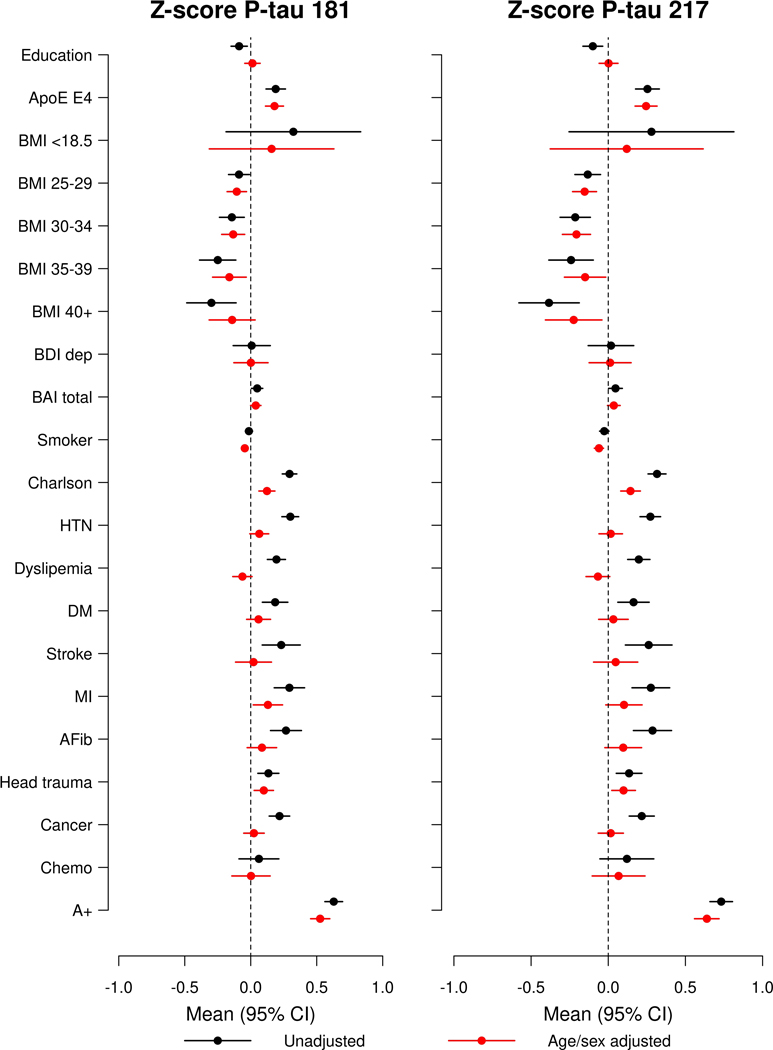
Fig. 2. Forest plots of associations between multiple factors and plasma P-tau181 and P- tau217 using linear regression among all participants (N=1329 unique biological participants).
Black lines indicate univariable associations, red lines indicate associations after adjustment for age and sex, and blue lines indicate associations after adjustment for age, sex, and amyloid PET. Means and 95% confidence intervals are provided. A+, Elevated amyloid PET; AFib, atrial fibrillation; BAI total, Beck Anxiety Inventory; BDI dep, Beck Depression Inventory; BMI, body mass index; Chemo, chemotherapy for those with a cancer diagnosis; CKD, chronic kidney disease; DM, diabetes mellitus; HTN, hypertension; MI, myocardial infarction.
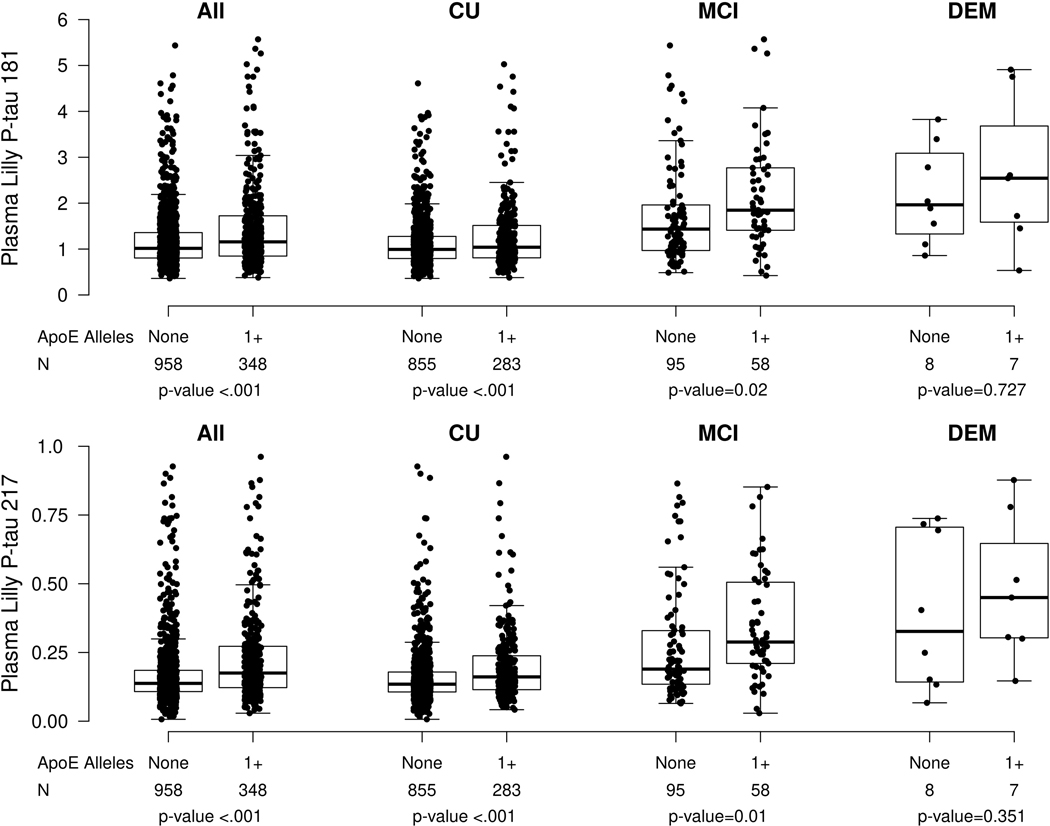
Among all participants, CU only, and MCI only, those who had at least one APOE ε4 allele had significantly higher P-tau181 and p-tau 217 measures compared to those without (Extended Data Fig. 3, Supplementary Table 5, and Supplementary Table 6). Chronic kidney disease (CKD), hypertension, stroke, or myocardial infarction (MI) were most strongly associated with higher plasma P-tau 181 and P-tau 217 levels. The mean P-tau181 level for those with versus without CKD (b = 0.81) was greater than the mean difference between those who were A+ versus A− (b = 0.72); the mean P-tau217 level was also elevated in those with CKD (b = 0.62 higher for CKD versus no CKD), but not as large as the difference between A+ and A− (b = 0.82 for A+ versus A−). Mean P-tau181 and P-tau217 levels were also elevated for those with versus without a history of clinical stroke (P-tau181: 0.64; P-tau217: 0.60) and those with versus without a history of MI (P-tau181: 0.54; P-tau217: 0.51). Notably, additional adjustment for elevated amyloid PET did not change the association between any of these factors and plasma P-tau181 or P-tau217 levels.
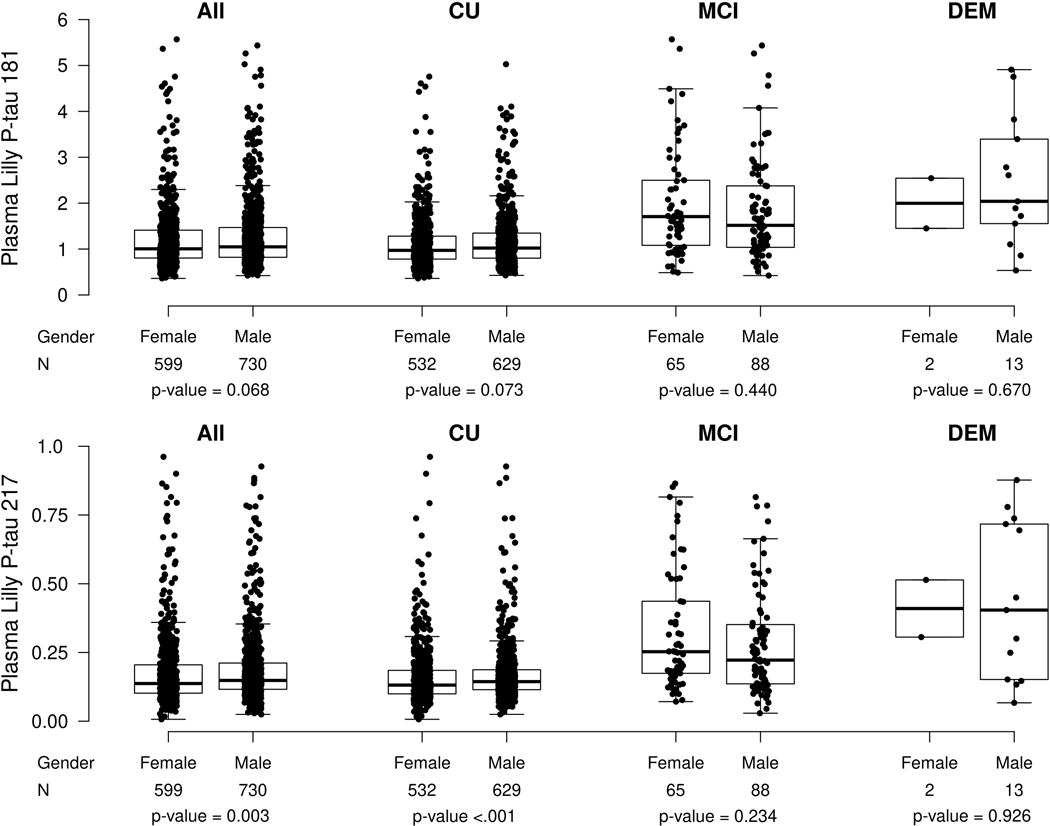
There were no significant differences in plasma P-tau181 levels by sex in the entire cohort, or when subset by clinical diagnosis (Extended Data Fig. 4). Plasma P-tau217 levels were higher among males than females for the entire cohort (median [Q1, Q3] among males: 0.148 [0.116, 0.212], females: 0.137 [0.102, 0.210], P = 0.003) and among CU only (median [Q1, Q3] among males: 0.144 [0.115, 0.187], females: 0.131 [0.099, 0.185], P = 0.003). There were no sex differences in the MCI or dementia groups. Notably, after excluding participants with a diagnosis of CKD or a history of stroke or MI, the sex differences were slightly attenuated but plasma P-tau217 levels were still higher for males in the entire cohort (median [Q1, Q3] among males: 0.142 [0.112, 0.193], females: 0.131 [0.099, 0.191], P = 0.01), and among CU participants (median [Q1, Q3] among males: 0.139 [0.110, 0.180], females: 0.127 [0.098, 0.179], P = 0.004).
When examining the effect of other variables on plasma P-tau181 and P-tau-217, a self- reported history of head trauma was associated with greater mean levels of P-tau217 (see Fig. 2, Supplementary Table 5, and Supplementary Table 6). Higher body mass index (BMI) and more years of education were associated with lower P-tau181 and P-tau217 in unadjusted models but were insignificant after age- and sex-adjustment. Similarly, diabetes, atrial fibrillation, and cancer were associated with higher P-tau181 and P-tau217 levels in unadjusted models but were no longer significant after adjustment for age and sex.
We further assessed the impact of CKD on the above associations. Because individuals with CKD often have hypertension and other comorbidities, we repeated the age- and sex- adjusted analyses after excluding the 98 participants with CKD (Extended Data Fig. 5, Supplementary Table 5, Supplementary Table 6). Although MI was still associated with elevated P-tau181 and Ptau217 levels, hypertension and stroke were not. Notably, having a high BMI was even more strongly associated with lower plasma P-tau181 and P-tau217 levels.
Evaluation of cutpoints for plasma P-tau181 and P-tau217.
We next used the large number of CU and A− participants in the study to define a normal range for plasma P-tau181 and P-tau217 between ages 30–90. Abnormal plasma P-tau levels were defined as >1.96 standard deviations above the mean in patients who were CU and A−, after including and then excluding participants with a diagnosis of CKD or a history of MI or stroke. We evaluated the diagnostic interpretation pertaining to amyloid and tau pathology as measured by PET in the context of these two approaches for establishing normal ranges. The cutpoint for P-tau181 when including all participants was ≥1.75 pg/ml; it decreased by 10% to ≥1.57 pg/ml after excluding participants with any of the three comorbidities (Table 2 and Table 3). In contrast, the cutpoint for P-tau217 changed little when either including participants with comorbidities (≥0.26 pg/ml) or excluding those participants (≥0.25 pg/ml), a difference of 4%. The Youden Index was improved for all three PET outcomes when the normal range was established without participants with the three comorbidities known to effect biomarker levels. As expected with lower cutpoint values, sensitivity, and negative predictive value (NPV) were slightly increased and specificity and positive predictive value (PPV) slightly decreased. Table 4 provides the NPV and PPV of the cutpoints (after excluding participants with a diagnosis of CKD, MI or stroke) by age groups to demonstrate the impact of different prevalences of elevated brain amyloid on PPV and NPV.
Table 3 |.
Prediction of amyloid and tau PET abnormalities with dichotomous plasma P-tau181 and P-tau217 results using different methods for selecting cutpoints.
| P-tau Measure | Negative N (%) | Positive N (%) | Sens | Spec | PPV | NPV | Youden | Unadjusted AUROC | Adjusted * AUROC | Adj onlya AUROC |
|---|---|---|---|---|---|---|---|---|---|---|
| Amyloid PET >=1.48 | ||||||||||
| Cutpoints defined as 1.96 standard deviations above the mean among participants who were CU, A−, and without history or stroke, myocardial infarction, or chronic kidney disease | ||||||||||
| P-tau 181 >=1.57 | 51 / 637 (8.0%) | 213 / 414 (51.4%) | 51.4 | 91.9 | 80.6 | 74.4 | 43.3 | 0.717 (0.691, 0.744) | 0.825 (0.801, 0.850) | 0.754 (0.726, 0.783) |
| P-tau 217 >=0.25 | 23 / 637 (3.6%) | 211 / 414 (51.0%) | 50.9 | 96.3 | 90.1 | 75.1 | 47.2 | 0.737 (0.712, 0.762) | 0.837 (0.813, 0.861) | 0.754 (0.726, 0.783) |
|
| ||||||||||
| Cutpoints defined as 1.96 standard deviations above the mean among all participants who were CU and A− | ||||||||||
| P-tau 181 >=1.75 | 26 / 637 (4.1%) | 172 / 414 (41.5%) | 41.5 | 95.9 | 86.8 | 71.6 | 37.4 | 0.687 (0.662, 0.712) | 0.819 (0.794, 0.844) | 0.754 (0.726, 0.783) |
| P-tau 217 >=0.26 | 12 / 637 (1.9%) | 193 / 414 (46.6%) | 46.6 | 98.1 | 94.1 | 73.8 | 44.7 | 0.724 (0.699, 0.748) | 0.833 (0.809, 0.858) | 0.754 (0.726, 0.783) |
|
| ||||||||||
| Temporal Meta ROI Tau PET >=1.29 | ||||||||||
| Cutpoints defined as 1.96 standard deviations above the mean among participants who were CU, A−, and without history or stroke, myocardial infarction, or chronic kidney disease | ||||||||||
| P-tau 181 >=1.57 | 30 / 430 (7.0%) | 18 / 65 (27.7%) | 27.6 | 93.0 | 37.5 | 89.4 | 20.6 | 0.604 (0.547, 0.660) | 0.798 (0.746, 0.850) | 0.788 (0.736, 0.839) |
| P-tau 217 >=0.25 | 43/430 (10.0%) | 26/65 (40.0%) | 40.0 | 90.0 | 37.6 | 90.8 | 30.0 | 0.650 (0.588, 0.712) | 0.804 (0.752, 0.855) | 0.788 (0.736, 0.839) |
|
| ||||||||||
| Cutpoints defined as 1.96 standard deviations above the mean among all participants who were CU and A− | ||||||||||
| P-tau 181 >=1.75 | 21 / 430 (4.9%) | 13 / 65 (20.0%) | 20.0 | 95.1 | 38.2 | 88.7 | 15.1 | 0.576 (0.526, 0.626) | 0.792 (0.739, 0.844) | 0.788 (0.736, 0.839) |
| P-tau 217 >=0.26 | 33 / 430 (7.7%) | 23 / 65 (35.4%) | 35.3 | 92.3 | 41.0 | 90.4 | 27.6 | 0.639 (0.579, 0.698) | 0.804 (0.753, 0.855) | 0.788 (0.736, 0.839) |
|
| ||||||||||
| ERC Tau PET >=1.27 | ||||||||||
| Cutpoints defined as 1.96 standard deviations above the mean among participants who were CU, A−, and without history or stroke, myocardial infarction, or chronic kidney disease | ||||||||||
| P-tau 181 >=1.57 | 24 / 446 (5.4%) | 24 / 49 (49.0%) | 48.9 | 94.6 | 50.0 | 94.4 | 43.5 | 0.718 (0.647, 0.789) | 0.872 (0.825, 0.919) | 0.800 (0.748, 0.852) |
| P-tau 217 >=0.25 | 38/446 (8.5%) | 31 / 49 (63.3%) | 63.2 | 91.4 | 44.9 | 95.7 | 54.6 | 0.774 (0.704, 0.843) | 0.869 (0.823, 0.916) | 0.800 (0.748, 0.852) |
|
| ||||||||||
| Cutpoints defined as 1.96 standard deviations above the mean among all participants who were CU and A− | ||||||||||
| P-tau 181 >=1.75 | 16 / 446 (3.6%) | 18 / 49 (36.7%) | 36.7 | 96.4 | 52.9 | 93.2 | 33.1 | 0.666 (0.597, 0.734) | 0.857 (0.808, 0.906) | 0.800 (0.748, 0.852) |
| P-tau 217 >=0.26 | 28 / 446 (6.3%) | 28 / 49 (57.1%) | 57.1 | 93.7 | 50.0 | 95.2 | 50.8 | 0.754 (0.683, 0.825) | 0.872 (0.826, 0.918) | 0.800 (0.748, 0.852) |
Adjusted variables included age and sex. The Adj only column is the AUROC for age and sex alone.
A−, not having elevated brain amyloid, CU, cognitively unimpaired; ERC, entorhinal cortex; NPV, negative predictive value; PPV, positive predictive value; ROI, Region of Interest; Sens, sensitivity; Spec, specificity.
Table 4 |.
Negative and positive predictive values of abnormal amyloid PET for plasma P-tau 181 and P-tau 217 by age group
| Subgroup | P-tau Cutpoint | A− | A+ | Prevalence | PPV | NPV |
|---|---|---|---|---|---|---|
| All (N=1051) | P-tau 181 <1.57 | 586 (92.0%) | 201 (48.6%) | 414/1051 (39.4%) | 213/264 (80.7%) | 586/787 (74.5%) |
| P-tau 181 >=1.57 | 51 (8.0%) | 213 (51.4%) | ||||
| P-tau 217 <0.25 | 614 (96.4%) | 203 (49.0%) | 414/1051 (39.4%) | 211/234 (90.2%) | 614/817 (75.2%) | |
| P-tau 217 >=0.25 | 23 (3.6%) | 211 (51.0%) | ||||
|
| ||||||
| <70 years (N=338) | P-tau 181 <1.57 | 286 (97.6%) | 37 (82.2%) | 45/338 (13.3%) | 8/15 (53.3%) | 286/323 (88.5%) |
| P-tau 181 >=1.57 | 7 (2.4%) | 8 (17.8%) | ||||
| P-tau 217 <0.25 | 291 (99.3%) | 37 (82.2%) | 45/338 (13.3%) | 8/10 (80.0%) | 291/328 (88.7%) | |
| P-tau 217 >=0.25 | 2 (0.7%) | 8 (17.8%) | ||||
|
| ||||||
| 70–77 years (N=261) | P-tau 181 <1.57 | 146 (90.7%) | 49 (49.0%) | 100/261 (38.3%) | 51/66 (77.3%) | 146/195 (74.9%) |
| P-tau 181 >=1.57 | 15 (9.3%) | 51 (51.0%) | ||||
| P-tau 217 <0.25 | 155 (96.3%) | 59 (59.0%) | 100/261 (38.3%) | 41/47 (87.2%) | 155/214 (72.4%) | |
| P-tau 217 >=0.25 | 6 (3.7%) | 41 (41.0%) | ||||
|
| ||||||
| 78–83 years (N=242) | P-tau 181 <1.57 | 97 (89.0%) | 64 (48.1%) | 133/242 (55.0%) | 69/81 (85.2%) | 97/161 (60.2%) |
| P-tau 181 >=1.57 | 12 (11.0%) | 69 (51.9%) | ||||
| P-tau 217 <0.25 | 101 (92.7%) | 60 (45.1%) | 133/242 (55.0%) | 73/81 (90.1%) | 101/161 (62.7%) | |
| P-tau 217 >=0.25 | 8 (7.3%) | 73 (54.9%) | ||||
|
| ||||||
| 84+ years (N=210) | P-tau 181 <1.57 | 57 (77.0%) | 51 (37.5%) | 136/210 (64.8%) | 85/102 (83.3%) | 57/108 (52.8%) |
| P-tau 181 >=1.57 | 17 (23.0%) | 85 (62.5%) | ||||
| P-tau 217 <0.25 | 67 (90.5%) | 47 (34.6%) | 136/210 (64.8%) | 89/96 (92.7%) | 67/114 (58.8%) | |
| P-tau 217 >=0.25 | 7 (9.5%) | 89 (65.4%) | ||||
Abnormal amyloid PET (A+) was defined as standard uptake value ratio (SUVR)>1.48 using PiB-PET. PPV, positive predictive value; NPV, negative predictive value.
Discussion
In this study, we assessed factors that might affect the interpretation of the plasma markers at the population level, and we established normal ranges of plasma P-tau181 and P-tau217. We then examined their diagnostic use as a predictor of elevated amyloid and tau PET in a large community-based cohort. We had four main findings. First, plasma P-tau181 and P-tau217 increased with age starting between the ages of 65 and 70 years, but the increase was most pronounced among those with elevated brain amyloid (A+). Second, multiple comorbidities were associated with higher plasma P-tau181 and P-tau217 levels; a diagnosis of CKD or a history of MI or clinical stroke had the largest effect on the plasma biomarkers. Third, the exclusion of participants with any of the three comorbidities (CKD, MI, stroke) had an effect on establishing a normal range for P-tau. Last, both P-tau measures were excellent predictors of elevated brain amyloid (AUROC>0.80) and ERC tau PET (AUROC>0.80) in a community-based cohort, but less so for a tau PET temporal meta ROI (AUROC<0.70).
Previous studies have shown correlations between increasing levels of plasma P-tau181 and P-tau217 with increasing age18,19. However, studies have not assessed whether the associations with age differ by elevated brain amyloid. In the present study, we showed that the primary increase with age was among individuals with elevated brain amyloid. The small increases with age among those without elevated brain amyloid likely reflects the effects of aging-related comorbidities, as discussed below, or possibly sub-threshold levels of brain amyloid. Regardless, these results do suggest the utility of using amyloid-negative individuals across the age-span as a reference group for developing reference intervals and clinical decision limits.
Multiple comorbidities were found to be associated with elevated plasma P-tau181 and P-tau217 level, with a history of CKD, MI, and stroke having the biggest effect. Indeed, the difference in P-tau levels between those with and without elevated brain amyloid was almost the same as participants with versus without CKD. Going forward, it will be important to consider that the effect of some of the variables examined on the blood biomarker levels may be because they are risk factors for AD pathology whereas the influence of others may be due to physiological processes. For example, the effect of APOE on plasma P-tau181 and P-tau217 is likely due to the increased risk of amyloidosis associated with AD for those with an APOE ε4 allele and the specificity of these plasma P-tau markers for the 3R/4R tauopathy found in AD as compared to the 3R or 4R found in FTD. By comparison, CKD most likely has an impact on blood P-tau levels via a reduced clearance of proteins in the blood. CKD has also been shown to be associated with higher levels of plasma Aβ40, Aβ42, neurofilament light chain, and total tau15. It is less clear why participants with a history of MI or clinical stroke have elevated P-tau levels. Understanding the etiology and impact of each factor on the blood biomarker levels, will be important for their future interpretation in the context of clinical screening, diagnosis, or prognosis at the population level. If these factors are not considered, a patient could have a false-positive result.
Few studies have examined cutpoints for the plasma P-tau biomarkers, but this is a critical step before incorporating these biomarkers at the population level for clinical use. Because cutpoints can differ based on the assay, P-tau isoform, and the specific diagnostic or prognostic use, the cutpoints identified in this study are not considered final by any means. Instead, our aim was to determine normal ranges and whether any of the comorbidities found to be associated with plasma P-tau would need to be considered. This single normal range can then be used universally for multiple diagnostic and prognostic interpretations across studies. Because we were focused on the population, we defined ‘abnormal’ P-tau as 1.96 standard deviations above the mean (>97.5th percentile) among participants who were CU and A−, a commonly used method to develop reference intervals and clinical decision limits20–22. Compared to including all CU A−, excluding those with a history of CKD, MI, or stroke had a significant effect on the cutpoint for P-tau181, but less so for P-tau217. A potential explanation is the greater specificity of P-tau217 for AD pathology compared with P-tau181, which is exhibited through fold change differences in this study and other studies comparing large populations of CU A− vs AD A+4,19. Excluding the participants with a history of CKD, MI, or stroke resulted in higher Youden index, and higher AUROC for amyloid PET, temporal meta ROI tau PET, and ERC tau PET. These results suggest that CKD, MI, and stroke need to be considered in the interpretation of the plasma P-tau levels. One way may be by somehow adjusting the plasma markers for these factors (or creatinine clearance in the case of CKD) or developing cutpoints for use in the presence of various conditions or diseases. In addition, physician education of factors and how they affect the levels of these markers is needed to obtain the best interpretation.
Several studies have shown that plasma P-tau181 and P-tau217 are good predictors of elevated brain amyloid and tau, measured by PET1–11. However, these studies have generally been comprised of clinical samples, which tend to be younger and not as generalizable to the population. Additionally, different cutpoints were established for each specific outcome. In the present study, we also found that abnormal levels of plasma P-tau181 and P-tau217 were predictive of amyloid PET (AUROC>0.80), with P-tau217 being significantly more accurate than P-tau181. This finding is similar to a recently published paper of 593 participants that reported P-tau217 was a greater predictor of amyloid PET than P-tau18123. With regards to tau PET, we found good prediction of both P-tau181 and P-tau217 for ERC tau PET (AUROC>0.80), but less so for a tau PET temporal meta ROI that included the ERC (AUROC<0.70). A potential explanation is that plasma P-tau changes occur earlier and are likely to precede Tau PET signal. Since the ERC is typically the earliest region affected by neurofibrillary tangles in AD and measurable by PET, it should show improved association with plasma P-tau compared with the regions that are affected later in the disease process and are included in the meta ROI. Indeed, this aligns with the best predictive value of plasma P-tau for amyloid PET because amyloid deposition is thought to prior to neurofibrillary tangles.
Key strengths of the study include the large sample size of community-based residents aged 30–98 years old, large number of participants with concurrent plasma P-tau measures and amyloid (n = 1,051) and tau (n = 495), and the abstraction of comorbidities from medical record review as compared to self-report which is less reliable. However, a limitation of the study is that the cohort is primarily of European decent (>95%) and results may not be generalizable to other populations. It is important to assay plasma P-tau measures across diverse populations (including race/ethnicity and socioeconomic status [SES]) to better determine what the reference intervals should be21. The prevalence and incidence of CKD, MI, and stroke differ by race/ethnicity, geography, and SES so the sampling of the population and consideration of these differences will be imperative.
Methods
Study participants.
The Mayo Clinic Study on Aging (MCSA) is a prospective population-based study examining the epidemiology of cognitive decline and risk of MCI among residents living in Olmsted County, Minnesota24. In 2004, Olmsted County residents between the ages of 70 and 89 were enumerated using the Rochester Epidemiology Project (REP) medical records-linkage system in an age- and sex-stratified random sampling design25. The study was extended to include those aged 50 and older in 2012, and to those 30 and older in 2015. The present analysis includes all participants with measures of both plasma P-tau181 and P-tau217. The study was approved by Mayo Clinic and Olmsted Medical Center Institutional Review Boards. Written informed consent was obtained from all participants and a small remuneration was provided for their time.
Participant assessment.
MCSA visits include an interview by a study coordinator, physician examination, and neuropsychological testing24. Participant demographics (age, sex, and years of education) and medical history were ascertained at the in-clinic examination. The cognitive battery included 9 tests covering 4 domains: memory, language, executive function, and visuospatial. Sample-specific z-scores for all cognitive tests were calculated; domain-specific z-scores were created by averaging the z-scores for the individual tests within each domain. A global cognitive z-score was created by averaging the z-scores of the four domains.
Participant demographics (age, sex, and years of education) were ascertained at the in-clinic examination. Participants’ height (cm) and weight (kg) were measured and used to calculate body mass index (BMI) (kg/m2). Nurse abstractors confirmed medical conditions (hypertension, dyslipidemia, diabetes, stroke, myocardial infarction, atrial fibrillation, cancer, and chemotherapy) based on medical record review using the REP medical records-linkage system25. CKD was determined using an electronic-based algorithm that incorporate all medical record information. The Charlson Comorbidity Index was also determined using the REP medical records-linkage system26. Depressive symptoms were assessed using the Beck Depression Inventory-II (BDI-II)27; participants with a score of ≥13 were considered to have depression. Anxiety symptoms were assessed using the Beck Anxiety Inventory (BAI)28. Apolipoprotein E (APOE) ε4 genotyping was performed from a blood sample.
Mild Cognitive Impairment (MCI) and dementia diagnoses.
Clinical diagnoses were determined by a consensus committee of those who evaluated each participant. Cognitive performance was compared with the age-adjusted scores of cognitively unimpaired (CU) individuals previously obtained using Mayo’s Older American Normative Studies29. Participants with scores around 1.0 SD below the age-specific mean in the general population were considered for possible cognitive impairment. The operational definition of MCI was based on clinical judgment including a history from the patient and informant. Published criteria were used for the diagnosis: cognitive complaint, cognitive function not normal for age, essentially normal functional activities, no dementia30. A final decision was made after considering education, occupation, visual or hearing deficits, and reviewing all other participant information. The diagnosis of dementia was based on published criteria31. Participants who performed in the normal range and did not meet criteria for MCI or dementia were deemed CU. The consensus committee was blinded to blood P-tau and neuroimaging results when determining the clinical diagnosis, and also to clinical information and diagnoses from previous study visits.
Plasma P-tau 181 and P-tau217 assay.
Blood was collected in-clinic after an overnight fast. The blood was centrifuged, resulting plasma aliquoted, and stored at −80°C. Both P-tau181 and P-tau217 levels were measured in duplicate on the MSD platform by electrochemiluminescence using proprietary assays developed by Lilly Research Laboratories 11. Samples were diluted 1:2 and 50 uL of diluted sample was used for each replicate. The assay was performed on a streptavidin small spot plate using the Meso Scale Discovery platform. P-tau181 used Biotinylated-AT270 (mIgG1, 1 ug/mL, Thermo Scientific cat. MN1050) as the capture and P-tau217 used Biotinylated-IBA493 (anti-phosphorylated Thr217 tau monoclonal antibody developed by Lilly Research Laboratories, 0.5 ug/mL) as the capture. In this study, both assays used SULFO-4G10-E2 (anti-tau monoclonal antibody developed by Lilly Research Laboratories, 0.02 ug/mL) as the detector. Each assay was calibrated using a unique synthetic P-tau peptide coupled with a polyethylene glycol linker to a second tau peptide matching amino acid 111–130 according to the Tau441 sequence numbering.
Amyloid and Tau PET imaging.
Aβ PiB-PET and Tau PET images were acquired using a PET/CT scanner (DRX, GE Healthcare) operating in 3-dimensional mode32. Pittsburgh compound B (PiB)–PET scan, consisting of 4 5-minute dynamic frames, was acquired from 40 to 60 minutes after injection33,34. Tau PET was performed using AV1451 and images were acquired from 80–100 minutes after injection.
Quantitative image analyses for PiB and AV1451 were done using our in-house fully automated image processing pipeline35. A global cortical PiB-PET retention ratio was computed by calculating the median uptake over voxels in the prefrontal, orbitofrontal, parietal, temporal, anterior cingulate, and posterior cingulate/precuneus regions of interest for each participant and dividing this by the median uptake over voxels in the cerebellar crus. A tau PET temporal meta region of interest (ROI) included the amygdala, entorhinal cortex, fusiform, parahippocampal, and inferior temporal and middle temporal gyri. In addition, we also examined the entorhinal cortex (ERC) as a single ROI. No partial volume correction was used. The atlas and image recognition steps were based on a 3D T1-weighted volume MRI sequence. We dichotomized participants as A+ based on a cutoff of >1.48 standard uptake value ratio (SUVR) using the reliable worsening method16. Participants were dichotomized as T+ based on the tau PET temporal meta ROI defined as >1.29 SUVR and ERC defined as >1.27 SUVR based on autopsy diagnosis and Braak NFT stage17.
Statistical analyses.
Data were descriptively summarized using frequencies and percentages for categorical data and medians and interquartile ranges for continuous data. Data distributions across clinical diagnoses were compared using chi-square or Fisher exact tests (where appropriate) for categorical variables and Kruskal-Wallis tests for continuous variables. We summarized relevant variables including patient demographics, comorbidities, plasma biomarkers, and brain imaging data. We descriptively summarized mean plasma P-tau measures by patient age in 5-year categories beginning at age 30–34 through age 85+. We visually presented the mean P-tau181 and P-tau217 measures by age in the overall sample, and additionally by amyloid PET status (A− and A+). Boxplots of P-tau measures by diagnosis, abnormal amyloid PET, presence of APOE allele, abnormal A:T status, and sex were also created.
We analyzed the predicative accuracy of each biomarker as a continuous variable for abnormal amyloid PET, temporal meta ROI tau PET, and ERC tau PET using logistic regression models. Each biomarker was included in unadjusted, age adjusted, age/sex adjusted, and age/sex/APOE adjusted model. Models were fit overall and subset to CU only and MCI only. Direct AUROC measures between biomarkers were compared using the concordance function36.
Associations between baseline patient characteristics and P-tau181 or P-tau217 measures were analyzed using linear regression models. Covariates analyzed included age, sex, education, APOE, BMI, BDI depression, BAI score, smoking status, Charlson score, and comorbidities. Each covariate was included in an unadjusted model, as well as a model that additionally adjusted for the effects of age and gender. We performed these comparisons once overall and stratified by clinical diagnosis (CU only and MCI/DEM). We repeated the analyses after exclusion of participants with a diagnosis of CKD.
To determine the predictive accuracy of dichotomized P-tau181 and p-tau217 for abnormal amyloid PET, temporal meta ROI tau PET, and ERC tau PET, we estimated the sensitivity, specificity, PPV, NPV, Youden’s index, unadjusted area under the receiver operating curve (AUROC) (e.g., P-tau181 included as the only covariate), and age/sex adjusted AUROC (e.g., P-tau181 + Age + Sex) for each biomarker and for each outcome. To determine the cutpoint for each biomarker, we defined abnormal as 1.96 standard deviations above the mean in patients who were CU, A−, and without history or stroke, MI, or chronic kidney disease. We replicated the analysis and included all CU A− participants to determine the effect of the comorbidities on the cutpoint. All analyses were completed using SAS version 9.4 (SAS Institute Inc., Cary, NC) and R version 3.6.2. A two-sided P-value < 0.05 was considered statistically significant.
Reporting Summary.
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Raw and analyzed de-identified data from the Mayo Clinic Study on Aging can be requested using the following link: https://ras-rdrs.mayo.edu/Request/IndexRequest. The request will be reviewed by the Mayo Clinic Study on Aging investigators and Mayo Clinic to verify whether the request is subject to any intellectual property or confidentiality obligations. A data sharing agreement must be obtained prior to release.
Code availability
No custom code or mathematical algorithm that was central to the conclusions was used in this study.
Extended Data
Extended Data Fig. 1. Plasma P-tau181 and P-tau217 levels by clinical diagnosis and amyloid PET status.
Abnormal amyloid PET (A+) was defined as standard uptake value ratio (SUVR)>1.48 using PiB-PET. P-values are from two-sided Wilcoxon rank-sum tests without adjustment for multiple comparisons. Box plots display the median, 25th percentile, 75th percentile (middle, bottom, and top bars of the box), and the whiskers go out to 1.5 times the interquartile range (75th percentile – 25th percentile) from the 25th and 75th percentile. CU, cognitively unimpaired; MCI, mild cognitive impairment; DEM, dementia.
Extended Data Fig. 2. Plasma P-tau181 and P-tau217 levels by clinical diagnosis and amyloid and tau PET status.
Abnormal amyloid PET (A+) was defined as standard uptake value ratio (SUVR)>1.48 using PiB-PET. Abnormal tau PET (T+) meta region of interest (ROI) was defined as standard uptake value ratio (SUVR)≥1.29 using AV1451, and included the amygdala, entorhinal cortex, fusiform, parahippocampal, and inferior temporal and middle temporal gyri. P-values are from two-sided Kruskal-Wallis tests. Box plots display the median, 25th percentile, 75th percentile (middle, bottom, and top bars of the box), and the whiskers go out to 1.5 times the interquartile range (75th percentile – 25th percentile) from the 25th and 75th percentile. CU, cognitively unimpaired; MCI, mild cognitive impairment.
Extended Data Fig. 3. Plasma P-tau181 and P-tau217 levels by presence of an APOE ε4 allele.
P-values are from two-sided Wilcoxon rank-sum tests without adjustment for multiple comparisons. Box plots display the median, 25th percentile, 75th percentile (middle, bottom, and top bars of the box), and the whiskers go out to 1.5 times the interquartile range (75th percentile – 25th percentile) from the 25th and 75th percentile. CU, cognitively unimpaired; MCI, mild cognitive impairment; DEM, dementia.
Extended Data Fig. 4. Plasma P-tau181 and P-tau217 levels by sex.
P-values are from two-sided Wilcoxon rank-sum tests without adjustment for multiple comparisons. Box plots display the median, 25th percentile, 75th percentile (middle, bottom, and top bars of the box), and the whiskers go out to 1.5 times the interquartile range (75th percentile – 25th percentile) from the 25th and 75th percentile. CU, cognitively unimpaired; MCI, mild cognitive impairment; DEM, dementia.
Extended Data Fig. 5. Forest plots of associations between multiple factors and plasma P-tau181 and P-tau217 levels using linear regression after excluding all participants with chronic kidney disease (N=1231).
Black lines indicate univariable associations and red lines indicate associations after adjustment for age and sex. Means and 95% confidence intervals are provided. A+, Elevated amyloid PET; AFib, atrial fibrillation; BAI total, Beck Anxiety Inventory; BDI dep, Beck Depression Inventory; BMI, body mass index; Chemo, chemotherapy for those with a cancer diagnosis; CKD, chronic kidney disease; DM, diabetes mellitus; HTN, hypertension; MI, myocardial infarction.
Supplementary Material
Acknowledgements
Funding for this study was provided by grants from the National Institutes of Health (U01 AG006786 [MMM, CRJ, RCP], R37 AG011378 [CRJ], R01 NS097495 [PV], P30 AG062677 [RCP], and R01 AG041851 [CRJ, DSK]) and the GHR Foundation [RCP]. This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01 AG034676. MSD P-tau181 and P-tau217 assays were performed at Eli Lilly and Company.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study, and all authors had final responsibility for the decision to submit for publication.
Competing Interests
Dr Mielke has served as a consultant for Biogen, Brain Protection Company, and LabCorp. Dr Dage was previously an employee and is still a minor shareholder of Eli Lilly and Company. Dr Dage is an inventor on patents or patent applications of Eli Lilly and Company relating to the assays, methods, reagents and / or compositions of matter used in this work. Dr Dage has served as a consultant for Karuna Therapeutics and received research support from ADx Neurosciences, Roche Diagnostics and Eli Lilly and Company. Mr Frank and Dr. Algeciras-Schimnich report no conflicts. Dr Knopman serves on a Data Safety Monitoring Board for the DIAN study. He serves on a Data Safety monitoring Board for Biogen but receives no personal compensation. He is an investigator in clinical trials sponsored by Biogen, Lilly Pharmaceuticals and the University of Southern California. He serves as a consultant for Roche, Samus Therapeutics, Third Rock and Alzeca Biosciences but receives no personal compensation. Dr Lowe received consulting fees from Bayer Schering Pharma, Piramal Life Sciences and Merck Research and grants from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals and the NIH. Guojun Bu serves as a consultant for AbbView, E-Scape, and SciNeuro. Prashanti Vemuri received speaking fees from Miller Medical Communications, LLC. Dr Graff-Radford receives NIH funding and serves as Assistant Editor for Neurology. Dr Jack Jr serves on an independent data monitoring board for Roche, has served as a speaker for Eisai, and consulted for Biogen, but he receives no personal compensation from any commercial entity. He receives research support from NIH and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic. Dr Petersen received consulting fees from Hoffman-La Roche, Inc, Merck, Inc, Genentech, Inc, Biogen, Inc, GE Healthcare and Eisai, Inc.
References
- 1.Mielke MM et al. Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement. 14, 989–997 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janelidze S. et al. Plasma P-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat. Med 26, 379–386 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Karikari TK et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol 19, 422–433 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Thijssen EH et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat. Med 26, 387–397 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmqvist S. et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA 324, 772–781 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattsson-Carlgren N. et al. Longitudinal plasma p-tau217 is increased in early stages of Alzheimer’s disease. Brain 143, 3234–3241 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moscoso A. et al. Time course of phosphorylated-tau181 in blood across the Alzheimer’s disease spectrum. Brain 144, 325–339 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barthelemy NR, Horie K, Sato C. & Bateman RJ Blood plasma phosphorylated-tau isoforms track CNS change in Alzheimer’s disease. J. Exp. Med 217, e20200861 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benussi A. et al. Diagnostic and prognostic value of serum NfL and p-Tau181 in frontotemporal lobar degeneration. J Neurol Neurosurg Psychiatry 91, 960–967 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Simren J. et al. The diagnostic and prognostic capabilities of plasma biomarkers in Alzheimer’s disease. Alzheimers Dement. 17, 1145–1156 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mielke MM et al. Comparison of plasma phosphorylated tau species with amyloid and tau positron emission tomography, neurodegeneration, vascular pathology, and cognitive outcomes. JAMA Neurol. 78, 1108–1117 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Bryant SE et al. Blood-based biomarkers in Alzheimer disease: current state of the science and a novel collaborative paradigm for advancing from discovery to clinic. Alzheimer’s & dementia 13, 45–58 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teunissen CE et al. Blood-based biomarkers for Alzheimer’s disease: towards clinical implementation. Lancet Neurol 21, 66–77 (2022). [DOI] [PubMed] [Google Scholar]
- 14.Mielke MM Consideration of sex differences in the measurement and interpretation of Alzheimer disease-related biofluid-based biomarkers. J Appl Lab Med 5, 158–169 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Syrjanen JA et al. Associations of amyloid and neurodegeneration plasma biomarkers with comorbidities. Alzheimers Dement. (2021). [DOI] [PMC free article] [PubMed]
- 16.Jack CR Jr. et al. Defining imaging biomarker cut points for brain aging and Alzheimer’s disease. Alzheimers Dement. 13, 205–216 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowe VJ et al. Tau-positron emission tomography correlates with neuropathology findings. Alzheimers Dement. 16, 561–571 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ossenkoppele R. et al. Tau PET correlates with different Alzheimer’s disease-related features compared to CSF and plasma p-tau biomarkers. EMBO Mol. Med 13, e14398 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brickman AM et al. Plasma p-tau181, p-tau217, and other blood-based Alzheimer’s disease biomarkers in a multi-ethnic, community study. Alzheimers Dement. 17, 1353–1364 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozarda Y. Reference intervals: current status, recent developments and future considerations. Biochem Med (Zagreb) 26, 5–16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manrai AK, Patel CJ & Ioannidis JPA In the era of precision medicine and big data, who is normal? JAMA 319, 1981–1982 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshihara T. et al. Cerebrospinal fluid protein concentration in healthy older Japanese volunteers. Int J Environ Res Public Health 18, 8683 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thijssen EH et al. Plasma phosphorylated tau 217 and phosphorylated tau 181 as biomarkers in Alzheimer’s disease and frontotemporal lobar degeneration: a retrospective diagnostic performance study. Lancet Neurol 20, 739–752 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts RO et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 30, 58–69 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.St. Sauver JL et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int. J. Epidemiol 41, 1614–1624 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charlson ME, Pompei P, Ales KL & MacKenzie CR A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis 40, 373–383 (1987). [DOI] [PubMed] [Google Scholar]
- 27.Beck AT, Steer RA & Brown GK Manual for the Beck Depression Inventory-II, (The Psychological Corporation Harcourt Brace & Company, San Antonio, TX, 1996). [Google Scholar]
- 28.Beck AT & Steer RA Beck Anxiety Inventory Manual, (The Psychological Corporation Harcourt Brace & Company, San Antonio, TX, 1993). [Google Scholar]
- 29.Ivnik RJ et al. Mayo’s Older Americans Normative Studies: updated AVLT norms for ages 56 to 97. Clinical Neuropsychologist 6, 83–104 (1992). [Google Scholar]
- 30.Petersen RC Mild cognitive impairment as a diagnostic entity. J. Intern. Med 256, 183–194 (2004). [DOI] [PubMed] [Google Scholar]
- 31.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), (American Psychiatric Association, Washington, D.C., 1994). [Google Scholar]
- 32.Lowe VJ et al. Comparison of 18F-FDG and PiB PET in cognitive impairment. J. Nucl.Med 50, 878–886 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Price JC et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J. Cereb. Blood Flow Metab. 25, 1528–1547 (2005). [DOI] [PubMed] [Google Scholar]
- 34.McNamee RL et al. Consideration of optimal time window for Pittsburgh compound B PET summed uptake measurements. J. Nucl. Med 50, 348–355 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jack CR Jr. et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain 131, 665–680 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Therneau TM A Package for Survival Analysis in R. R package version 3.2–7, https://CRAN.R-project.org/package=survival. (2020)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw and analyzed de-identified data from the Mayo Clinic Study on Aging can be requested using the following link: https://ras-rdrs.mayo.edu/Request/IndexRequest. The request will be reviewed by the Mayo Clinic Study on Aging investigators and Mayo Clinic to verify whether the request is subject to any intellectual property or confidentiality obligations. A data sharing agreement must be obtained prior to release.