Key Points
Question
Is nonoperative management of uncomplicated acute appendicitis comparable with surgical appendectomy in adult patients?
Findings
In this systematic review and meta-analysis including 8 randomized clinical trials, there were no significant differences in treatment success or major complications between operative and nonoperative management of acute uncomplicated appendicitis. However, length of stay and recurrence were significantly higher in the nonoperative management group.
Meaning
Nonoperative management with antibiotics may be an alternative option to appendectomy in adult patients presenting with uncomplicated acute appendicitis, noting the possibility of longer hospital stays and potentially higher rates of recurrent appendicitis.
This systematic review and meta-analysis evaluates the findings of randomized clinical trials on the efficacy and safety of nonoperative management vs appendectomy for patients with acute uncomplicated appendicitis.
Abstract
Importance
Appendectomy remains the standard of care for uncomplicated acute appendicitis despite several randomized clinical trials pointing to the safety and efficacy of nonoperative management of this disease. A meta-analysis of randomized clinical trials may contribute to the body of evidence and help surgeons select which patients may benefit from surgical and nonsurgical treatment.
Objective
To assess the efficacy and safety of nonoperative management vs appendectomy for acute uncomplicated appendicitis.
Data Sources
A systematic review was conducted using indexed sources (Embase and PubMed) to search for published randomized clinical trials in English comparing nonoperative management with appendectomy in adult patients presenting with uncomplicated acute appendicitis. To increase sensitivity, no limits were set for outcomes reported, sex, or year of publication. All nonrandomized or quasi-randomized trials were excluded, and validated primers were used.
Study Selection
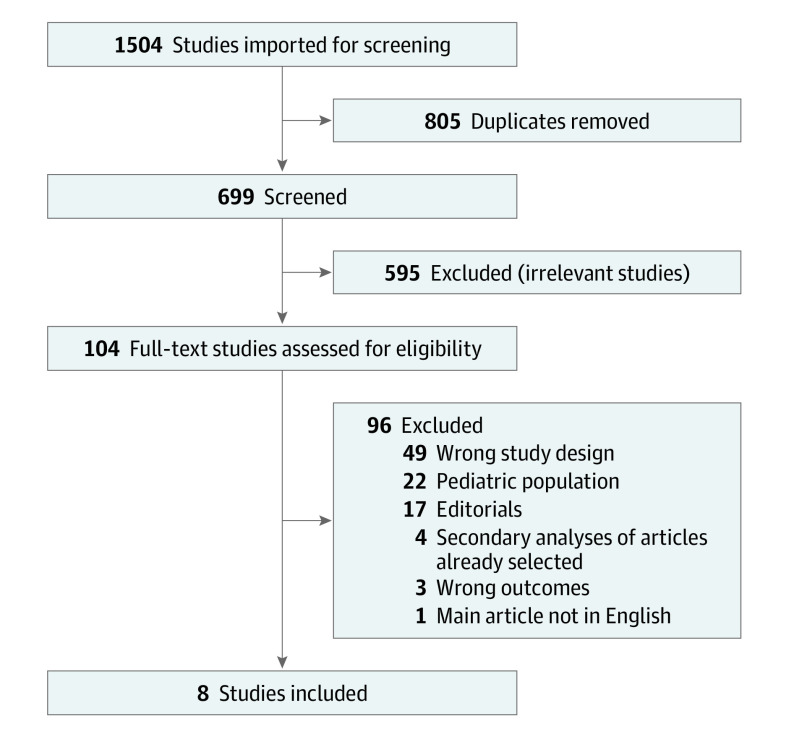
Among 1504 studies imported for screening, 805 were duplicates, and 595 were excluded for irrelevancy. A further 96 were excluded after full-text review, mainly owing to wrong study design or inclusion of pediatric populations. Eight studies met the inclusion criteria and were selected for the meta-analysis.
Data Extraction and Synthesis
Meta-extraction was conducted with independent extraction by multiple reviewers using the Covidence platform for systematic reviews and in accordance with PRISMA guidelines. Data were pooled by a random-effects model.
Main Outcomes and Measures
Treatment success and major adverse effects at 30 days’ follow-up.
Results
The main outcome (treatment success proportion at 30 days of follow-up) was not significantly different in the operative and nonoperative management cohorts (risk ratio [RR], 0.85; 95% CI, 0.66-1.11). Likewise, the percentage of major adverse effects was similar in both cohorts (RR, 0.72; 95% CI, 0.29-1.79). However, in the nonoperative management group, length of stay was significantly longer (RR, 1.48; 95% CI, 1.26-1.70), and a median cumulative incidence of 18% of recurrent appendicitis was observed.
Conclusions and Relevance
These results point to the general safety and efficacy of nonoperative management of uncomplicated acute appendicitis. However, this strategy may be associated with an increase in duration of hospital stay and a higher rate of recurrent appendicitis. This meta-analysis may help inform decision-making in nonoperative management of uncomplicated acute appendicitis.
Introduction
Appendicitis may present in many forms and with variable complications, rendering optimal treatment challenging. Although surgery has historically been the standard treatment regardless of presentation, multiple studies have suggested that nonoperative options may be used in both complicated and uncomplicated appendicitis.1,2 Appendectomy is generally well tolerated, yet because it is a surgical intervention, there are substantial intraoperative and postoperative risks, such as infection, scars, and delayed recovery.3,4
New scoring models and enhanced imaging in computed tomography permit accurate diagnosis of appendicitis and its manifestations in most patients.5 For uncomplicated appendicitis, there has been an effort to consider nonoperative management.6,7 Antibiotic-only treatment may be an attractive option to patients, as it may be associated with avoidance of surgical scars, reduction of postoperative pain, and faster recovery.6 It may also provide potential benefits to health care systems by reducing the burden on operating rooms, sparing the use of personal protective gear, and reducing overall costs. At this time, a nonoperative approach for uncomplicated appendicitis with antibiotic treatment is regarded as a safe alternative in select patients.8,9,10,11,12
Several randomized clinical trials have been conducted to address the respective risk-benefit ratio of antibiotic treatment compared with surgical approaches. However, the wide range of treatment failure rates (7% to 39%) have led to confusion about overall effectiveness of nonoperative treatment and rendered these analyses difficult to interpret.8,9,10,11,12 In addition, these trials had limitations related to the heterogeneity of patient characteristics, variable interventions (antibiotics), and nonstandard follow-up, affecting the consistency of the results. We performed a meta-analysis to address the question as to whether antibiotic therapy in adult patients with uncomplicated appendicitis is comparable with surgical treatment. We performed a systematic review with strict inclusion and exclusion criteria to evaluate the outcomes of surgical and nonsurgical approaches.
Methods
Study Design
We conducted a systematic review of randomized clinical trials evaluating the efficacy of antibiotic treatment in the management of acute uncomplicated appendicitis. We published the statistical analysis plan and a priori hypotheses for the current study before unblinding results through the Covidence platform for systematic reviews at Harvard Countway Library.13 Our research protocol was built using validated primers for randomized clinical trials according to the Cochrane database.14 Specifically, we included search terms for nonoperative vs operative management of acute uncomplicated appendicitis. Our inclusion criteria for this protocol were randomized clinical trials with adult patients aged 18 years and older presenting with acute uncomplicated appendicitis. The types of interventions compared were conservative (oral, intramuscular, intravenous, or combined antibiotic treatment) vs surgical treatment (laparoscopic, robotic, or open appendectomy). There were no exclusions based on sex. We excluded nonrandomized or quasi-randomized clinical trials, patients presenting with complicated appendicitis (perforation, abscess, or peritonitis), hemodynamically unstable patients (ie, those with shock or septic shock), pediatric patients, and patients with appendicoliths. We did include a small set of adolescent patients aged 16 to 17 years from the COMMA trial,6 because they comprised a small subset of the overall population (less than 10%) and did not demonstrate significant clinical and biological differences from the adult population. We also used overall results from the CODA collaborative,8 which included a subset of patients with appendicoliths. We made this choice because of the low prevalence of appendicoliths (27%) and similar clinical outcomes compared with patients without appendicoliths. The full protocol is available in the eAppendix in the Supplement. Each trial supplied results regarding each treatment in different reporting forms. Before pooling data, we compared trial protocols, case-report forms, and data dictionaries to identify any recoding needed. We then provided a detailed data-set specification to each trial team to prepare the data file for pooling. We converted the results to similar risk ratio (RR) calculation to obtain comparable results. We conducted a bias analysis of the 8 randomized clinical trials using the Cochrane risk of bias 2 tool.
Primary Outcome
Our prespecified primary outcome measure was all-cause mortality at 30 days. However, owing to extremely low mortality rates, we did not have adequate power to report this outcome. For this reason, we used the outcome of treatment success as defined by each individual trial protocol, which included resolution of abdominal pain, no complications, and improvement of inflammatory markers, among other variables. We analyzed treatment success proportion at 30 days (or at the longest time of follow-up when different times were reported) as our primary outcome. All studies had similar primary outcomes and definitions of treatment success. In 6 studies, treatment success was defined as the resolution of appendicitis symptoms without recurrence of pain, no complications, and improvement of inflammatory markers. The CODA collaborative8 used a more comprehensive definition of treatment success, through a broad questionnaire of health status. Conversely, the clinical trial by Vons et al15 used a narrow definition of treatment success: the absence of peritonitis during 30 days’ follow-up. Detailed definitions for each study are listed in the Table. The choice of the primary outcome and the choice for the longest period of follow-up were standardized as per the Cochrane Library protocol for meta-analyses.20
Table. Primary Outcome Definitions.
| Source | Definition of treatment success |
|---|---|
| CODA Collaborative,8 2020 | 30-d Health status, as assessed with the use of the European Quality-of-Life 5-Dimensions questionnaire. |
| Livingston et al,9 2018 | Resolution of acute appendicitis resulting in discharge from the hospital without the need for surgical intervention and no recurrent appendicitis during a minimum follow-up of 1 y. |
| Styrud et al,16 2006 | Resolution of appendicitis symptoms in 24 h and absence of recurrence, pain, and complications at up to 1 y of follow-up. |
| Vons et al,15 2011 | The rate of peritonitis that occurred within 30 d of treatment initiation. |
| Ceresoli et al,17 2019 | Success rate of the treatment, defined as the resolution of symptoms (no abdominal pain or fever) and resolution of inflammatory markers (white blood cell count <10 000/μLa and C-reactive protein <1 mg/dLb) within 2 wk after appendectomy in the surgical arm or from the third dose of ertapenem without other treatments in the antibiotic arm. |
| O’Leary et al,6 2021 | The primary end point for the trial evaluated the success rate of antibiotic treatment only for acute uncomplicated appendicitis at 1-y follow-up. In the operative treatment arm, the primary end point was defined as successful appendectomy, which was expected to be 100%. |
| Ericksson et al,18 1995 | Resolution of pain and decrease in white blood cell count and C-reactive protein. |
| Hansson et al,192009 | Efficacy for antibiotic treatment was defined as definite improvement without the need for surgery within a median follow-up of 1 y. Efficacy for surgical treatment was confirmed appendicitis at operation or another appropriate surgical indication for operation. |
To convert to ×109/L, multiply by 0.001.
To convert to mg/L, multiply by 10.
Secondary Outcomes
For secondary outcomes, we analyzed the RRs for major complications in both groups and compared in-hospital length of stay for both groups. Because in-hospital length of stay was reported with substantial differences between trials, we contacted the authors of the trials to report the RRs of length of stay by Poisson regression models with robust standard errors. Our study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.21
Statistical Analysis
For our primary outcome, we report RRs obtained through χ2 tests. We conducted all analyses using the intention-to-treat population and determined heterogeneity among trials by fitting a fixed interaction between treatment and trial, and 95% CIs were adopted. When the studies did not report the outcome of interest, we included them as missing values and removed them for the analysis. We conducted logarithmic transformation of RRs and the confidence level boundaries for inclusion in the analysis. We built forest plots for the primary and secondary outcomes using the random effect meta-analysis command. We built a funnel plot and conducted the Egger test for the primary outcome to estimate publication bias. We also conducted a meta-regression to estimate study heterogeneity by year of publication, study size, and time of follow-up. We include detailed statistical analyses plans and results in the eAppendix in the Supplement. All analysis were performed with Stata version 17 (StataCorp).
Results
Systematic Review Results
Of the 1504 studies imported for our systematic review, 8 met the inclusion criteria and were included in the meta-analysis.6,8,9,15,16,17,18,19 The main reasons for exclusion were wrong study design (observational or nonrandomized) and the inclusion of pediatric patients. In our search protocol, we did screen for specific outcomes to increase sensitivity. Figure 1 summarizes inclusion and exclusion of trials. The trials were conducted between 1995 and 2021 in Europe and North America. The date of last database search was December 2021.
Figure 1. Systematic Review Flowchart.
Primary Outcome
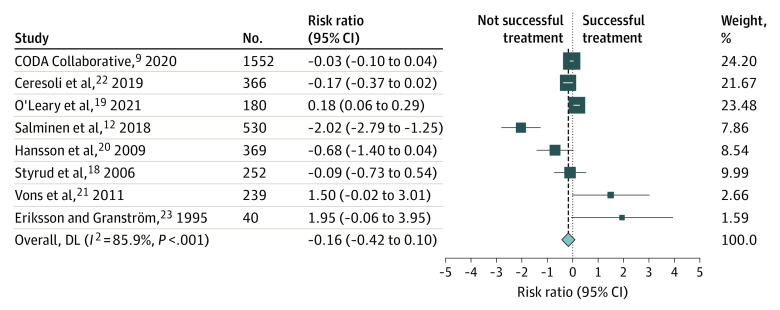
The primary outcome is summarized in Figure 2. The overall RR for the percentage of successful treatment at 30 days (or the longest period of follow-up when different time points were reported) did not differ between the antibiotic group and the surgery group (RR, 0.85; 95% CI, 0.66-1.11). Of the 8 trials in the meta-analysis, 6 did not find statistically significant differences for the primary outcome between the intervention and control groups. One study presented a statistically significant benefit with surgical treatment, and 1 pointed to superiority of antibiotic treatment. Our meta-analysis presented a high degree of outcome variation between studies. The estimated I2 was 85.9%, indicating that the outcome variation may be because of heterogeneity between studies.
Figure 2. Risk Ratios for Treatment Success.
Risk ratios with 95% CIs for treatment success proportions across all included studies. Weights were obtained through random-effects model. DL indicates DerSimonian-Laird estimator.
Because the studies reported different follow-up periods (4 reported the main outcome at 12 months, 3 at 30 days, and 1 at 15 days), we constructed a subgroup forest plot for the primary outcome. All RRs for treatment success were similar to our primary outcome. However, we noticed a nonsignificant trend toward better outcomes for the antibiotic group at the longest period of follow-up (12 months). Although the largest-sample trial in this long follow-up subset (the APPAC trial11) found that most patients treated conservatively did not require surgery during the first year of follow-up, antibiotic treatment did not meet the prespecified criterion for noninferiority compared with appendectomy. The subgroup analysis can be found in eFigures 1 and 2 in the Supplement.
Secondary Outcomes
Major Adverse Effects
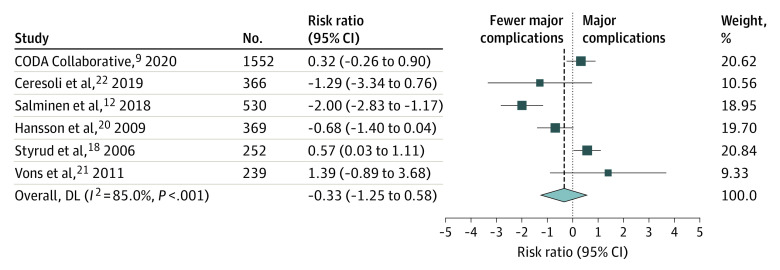
Of the 8 selected clinical trials, 6 reported incidence of major adverse effects between the intervention and comparison groups at 30 days (or at the longest period of follow-up). In the meta-analysis of these 6 trials, antibiotic treatment was associated with a nonsignificant trend toward lower percentages of major adverse effects compared with surgical treatment (RR, 0.72; 95% CI, 0.29-1.79). Of the 6 trials included in the analysis, 1 pointed to significant superiority of the antibiotic treatment, and 1 to the superiority of surgical treatment. The 4 remaining trials demonstrated no statistically significant difference between the intervention and comparison groups. This secondary outcome is represented in Figure 3.
Figure 3. Risk Ratios for Major Complications.
Risk ratios with 95% CIs for major complication proportions across all studies. Weights were obtained through random-effects model. DL indicates DerSimonian-Laird estimator.
Length of Hospital Stay and Cost Analysis
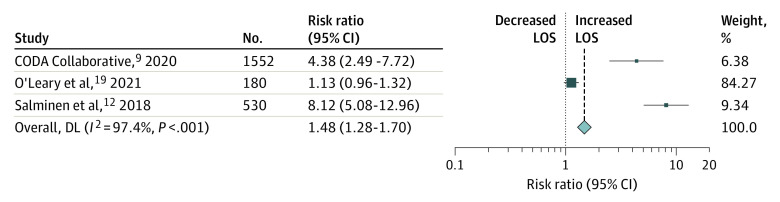
Among the 8 included trials, 3 reported total hospital length of stay. To compare similar variables and obtain a standard result, we conducted a meta-analysis of RRs for the length of hospital stay obtained by Poisson regression models as reported by the CODA collaborative.8
After direct contact with the authors of the COMMA trial6 and the APPAC trial,11 we obtained comparable RRs. In the meta-analysis of the CODA, COMMA, and APPAC trials, surgical treatment was associated with a significantly shorter length of hospital stay (RR, 1.48; 95% CI, 1.26-1.70). The results for length of hospital stay are presented in Figure 4. Regrettably, we were not able to conduct a formal meta-analysis of cost of care owing to different currencies and cost analysis in each trial. We may only report the subjective finding that each of those 3 trials pointed to a significant increase in cost of care with nonoperative management, in addition to speculating that longer lengths of stay may be associated with increased cost of care.
Figure 4. Risk Ratios for Increased Length of Stay (LOS).
Risk ratios with 95% CIs of increased length of stay across studies. Weights were obtained through random-effects model. DL indicates DerSimonian-Laird estimator; LOS, length of stay.
Heterogeneity and Publication Bias Analysis
The Funnel plot and the Egger test did not show any evidence of publication bias or small studies effect. There was no significant evidence for small studies effect. We conducted a meta-regression to analyze if study heterogeneity was because of the study population size, year of publication, or time of follow-up. Our analysis did not show significant evidence for heterogeneity owing to any of these variables.
Recurrent Appendicitis in Antibiotic-Treated Cohort
Among the 8 included trials, 4 reported considerable rates of recurrent appendicitis with the need for appendectomy in the nonoperative treated cohort. We conducted a descriptive analysis of cumulative incidence of recurrent appendicitis followed by appendectomy at the longest period of follow-up in each trial, ranging to up to 1 year of follow-up. The median (range) rate for recurrent appendicitis was 18% (7%-29%) in the CODA collaborative.8 The detailed results for recurrence rates are summarized in eFigure 3 in the Supplement.
Risk of Bias Analysis
Overall, there was a low risk of bias in the included studies. The most significant source of bias was detection bias owing to the open-label design of 1 study and missing information from 2 others. We also detected risk of attrition bias owing to incomplete reporting of data in 2 studies. Risk of bias is described the eTable in the Supplement.
Discussion
In this systematic review and meta-analysis of 8 randomized clinical trials conducted between 1995 and 2021, nonoperative treatment was not statistically different compared with operative treatment in the management of acute uncomplicated appendicitis based on our prespecified definition of treatment success and major adverse events. The RRs for treatment success and major complications did not differ between treatment groups. Our meta-analysis revealed significant heterogeneity, which could not be traced to study population size, year of publication, or follow-up period. We believe that internal differences in diagnostic methods and interventions between the studies may be responsible for some of the observed heterogeneity. We did not find evidence for publication bias.
A nonsignificant trend toward better outcomes was observed in the nonoperative treatment group compared with the surgical group, specifically a higher incidence of treatment success with longer periods of follow-up. This may be because of the higher incidence of long-term complications in the surgical group, such as incisional hernias or adhesions. However, the risk of rehospitalization for appendicitis must also be considered, which was estimated to be 18%. Given the short period of follow-up for many of the included studies, the true incidence of recurrent appendicitis remains unknown. Further research is needed to review the long-term risks associated with both approaches, particularly nonoperative treatment.
Considering the incidence of major adverse effects, our meta-analysis pointed to relative safety with both operative and nonoperative approaches for acute uncomplicated appendicitis. Mortality was very low in both approaches and across trials such that it could not be estimated or compared. For this reason, we used the outcome of treatment success as defined by each individual trial protocol, which included resolution of abdominal pain, no complications, and improvement of inflammatory markers, among other variables. Unfortunately, a more useful index of quality of life or other patient-reported outcome might be more valuable in each individual decision-making approach. A patient-centered discussion considering the substantial rate of recurrent appendicitis with antibiotics only must be prioritized. Patients should be informed that despite promising outcomes with nonoperative management of acute appendicitis, nearly 1 of 5 patients treated nonoperatively in this review eventually experienced recurrent appendicitis symptoms. It is also crucial to mention the importance of the local infrastructure conditions in selecting a nonoperative approach, and to closely monitor patients with serial physical examinations and a 24/7 availability of imaging, interventional radiology, laboratory testing, and the flexibility to change treatment approach as needed. In addition, patients should also have easy access to rehospitalization if necessary. All of the trials included in this study were conducted in Europe and North America, which likely include facilities that have all the structure and personnel to perform the nonoperative approach. In locations lacking these conditions, the surgical approach should be first recommended.
Cost and resource use must be considered before expanding our findings to a societal perspective. Our meta-analysis revealed an increase in length of stay with the nonoperative approach. Data are scarce regarding the cost-effectiveness of nonoperative approaches to uncomplicated appendicitis. In an observational study from the United Kingdom,18 a decrease in cost was noted with nonoperative appendicitis management. However, that study also revealed similar length of stay and a higher incidence of unplanned readmissions during 90 days of follow-up with nonoperative treatment. Given the ongoing COVID-19 pandemic, nonoperative management of appendicitis during times when surgical theaters and intensive care units are limited may be of considerable benefit to our health systems.22 In fact, a reduced incidence of acute appendicitis has been reported during the COVID-19 pandemic, as noted in a retrospective analysis showing a decreased incidence of acute appendicitis by 40% following the outbreak.23 Similarly, an observational study in Italy showed a significant drop in hospital admission for acute appendicitis (46%) and a reduction in the incidence of noncomplicated appendicitis (56%) and appendectomy rate (from 17.3% to 6.1%). The nonoperative treatment rate remained similar (12.1% vs 11.6%).24 A retrospective study in Germany evaluating 9797 patients also showed a decrease of 50% in the incidence of noncomplicated appendicitis.25 None of these studies reported an increase in the number of complicated cases during this time period. Outpatient treatment with antibiotics or even spontaneous resolution in patients with mild symptoms could explain these documented declines during the pandemic, in which many patients refrained from seeking hospital care.
Although our prespecified protocol excluded patients with appendicoliths owing to an expected higher failure incidence with conservative treatment in these patients, some trials did not come to this same conclusion. Two randomized clinical trials included in our meta-analysis conducted a subgroup analysis of patients with appendicoliths. In the CODA collaborative,8 the investigators noted an equivalence in outcomes for patients with appendicoliths, while the clinical trial by Vons et al15 demonstrated a slight increase in conservative treatment failure for patients with appendicoliths. As this meta-analysis did not specifically seek to answer the question of equivalence in the setting of appendicoliths, further research is needed to better guide management in this clinical situation.
Limitations
Our study has limitations related to the included trials and other considerations germane to meta-analyses. We only included English-language articles in our systematic review, which limits generalizability, and the inclusion of only Embase and PubMed as databases may be considered a less robust systematic search. In addition, the high degree of heterogeneity between the trials, especially regarding the definition of treatment success, limits conclusions. Owing to lack of detailed operative reports in the trials, we could not estimate the prevalence of more advanced surgical techniques, such as robotic appendectomy, in operative management. These differing interventions might present different outcomes. Additionally, we were not able to specifically analyze the interventions and outcomes within the recurrent appendicitis groups, particularly for the rate of missed neoplasms and surgical morbidity. However, our study overcomes the limitations of previous studies by generating a summary estimate of the efficacy of both treatments for acute uncomplicated appendicitis in adult patients. Compared with previously published meta-analyses26,27 our systematic review protocol was designed to be more sensitive in retrieving information. We chose to not set outcome limits for the literature review, as this process may lead to bias in reporting results. In addition, our study included the recently published CODA collaborative,8 which is to our knowledge the largest published randomized clinical trial on the topic to date. We also present a unique analysis of length of stay, and reports outcomes at a short (up to 30 days) and longer-term (up to 12 months) follow-up.
Conclusions
In summary, nonoperative management with antibiotics may be an alternative strategy for the treatment of acute uncomplicated appendicitis. Although we did not find significantly different proportions of treatment success and major complications for either treatment approach, we noticed a trend toward greater success of treatment for nonoperative management when considering longer follow-up periods. This advantage must be considered within the context of recurrent risk of appendicitis with nonoperative management found in this study. In addition, operative management was associated with significantly shorter length of stay compared with antibiotic-only treatment. Practicing surgeons can use these summary data to guide patients presenting with acute uncomplicated appendicitis.
eAppendix. Protocol and Statistical Analysis Plan
eFigure 1. RR for Treatment Success (30 days of Follow-up)
eFigure 2. RR for Treatment Success (12 months)
eFigure 3. Recurrent Appendicitis Rate in Antibiotics Group
eTable 1. Bias Table
References
- 1.Wagner M, Tubre DJ, Asensio JA. Evolution and current trends in the management of acute appendicitis. Surg Clin North Am. 2018;98(5):1005-1023. doi: 10.1016/j.suc.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 2.Livingston EH, Fomby TB, Woodward WA, Haley RW. Epidemiological similarities between appendicitis and diverticulitis suggesting a common underlying pathogenesis. Arch Surg. 2011;146(3):308-314. doi: 10.1001/archsurg.2011.2 [DOI] [PubMed] [Google Scholar]
- 3.Margenthaler JA, Longo WE, Virgo KS, et al. Risk factors for adverse outcomes after the surgical treatment of appendicitis in adults. Ann Surg. 2003;238(1):59-66. doi: 10.1097/01.SLA.0000074961.50020.f8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sammalkorpi HE, Mentula P, Leppäniemi A. A new adult appendicitis score improves diagnostic accuracy of acute appendicitis—a prospective study. BMC Gastroenterol. 2014;14:114. doi: 10.1186/1471-230X-14-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rud B, Vejborg TS, Rappeport ED, Reitsma JB, Wille-Jørgensen P. Computed tomography for diagnosis of acute appendicitis in adults. Cochrane Database Syst Rev. 2019(11):CD009977. doi: 10.1002/14651858.CD009977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Leary DP, Walsh SM, Bolger J, et al. A randomized clinical trial evaluating the efficacy and quality of life of antibiotic-only treatment of acute uncomplicated appendicitis: results of the COMMA trial. Ann Surg. 2021;274(2):240-247. doi: 10.1097/SLA.0000000000004785 [DOI] [PubMed] [Google Scholar]
- 7.Apisarnthanarak P, Suvannarerg V, Pattaranutaporn P, Charoensak A, Raman SS, Apisarnthanarak A. Alvarado score: can it reduce unnecessary CT scans for evaluation of acute appendicitis? Am J Emerg Med. 2015;33(2):266-270. doi: 10.1016/j.ajem.2014.11.056 [DOI] [PubMed] [Google Scholar]
- 8.CODA Collaborative . A randomized trial comparing antibiotics with appendectomy for appendicitis. N Engl J Med. 2020;383(20):1907-1919. doi: 10.1056/NEJMoa2014320 [DOI] [PubMed] [Google Scholar]
- 9.Livingston EH. Antibiotic treatment for uncomplicated appendicitis really works: results from 5 years of observation in the APPAC trial. JAMA. 2018;320(12):1245-1246. doi: 10.1001/jama.2018.13368 [DOI] [PubMed] [Google Scholar]
- 10.Di Saverio S, Sibilio A, Giorgini E, et al. The NOTA study (Non Operative Treatment for Acute Appendicitis): prospective study on the efficacy and safety of antibiotics (amoxicillin and clavulanic acid) for treating patients with right lower quadrant abdominal pain and long-term follow-up of conservatively treated suspected appendicitis. Ann Surg. 2014;260(1):109-117. doi: 10.1097/SLA.0000000000000560 [DOI] [PubMed] [Google Scholar]
- 11.Salminen P, Tuominen R, Paajanen H, et al. Five-year follow-up of antibiotic therapy for uncomplicated acute appendicitis in the APPAC randomized clinical trial. JAMA. 2018;320(12):1259-1265. doi: 10.1001/jama.2018.13201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prechal D, Damirov F, Grilli M, Ronellenfitsch U. Antibiotic therapy for acute uncomplicated appendicitis: a systematic review and meta-analysis. Int J Colorectal Dis. 2019;34(6):963-971. doi: 10.1007/s00384-019-03296-0 [DOI] [PubMed] [Google Scholar]
- 13.Harvard Countway Library . Non-Operative versus Operative Management of Acute Appendicitis—Systematic Review and Meta-Analysis. Accessed November 20, 2021. https://app.covidence.org/reviews/180453
- 14.Harvard Countway Library . Systematic Reviews and Meta Analysis. Accessed November 20, 2021. https://guides.library.harvard.edu/meta-analysis/protocol
- 15.Vons C, Barry C, Maitre S, et al. Amoxicillin plus clavulanic acid versus appendicectomy for treatment of acute uncomplicated appendicitis: an open-label, non-inferiority, randomised controlled trial. Lancet. 2011;377(9777):1573-1579. doi: 10.1016/S0140-6736(11)60410-8 [DOI] [PubMed] [Google Scholar]
- 16.Styrud J, Eriksson S, Nilsson I, et al. Appendectomy versus antibiotic treatment in acute appendicitis. a prospective multicenter randomized controlled trial. World J Surg. 2006;30(6):1033-1037. doi: 10.1007/s00268-005-0304-6 [DOI] [PubMed] [Google Scholar]
- 17.Ceresoli M, Pisano M, Allievi N, et al. Never put equipoise in appendix! final results of ASAA (antibiotics vs. surgery for uncomplicated acute appendicitis in adults) randomized controlled trial. Updates Surg. 2019;71(2):381-387. doi: 10.1007/s13304-018-00614-z [DOI] [PubMed] [Google Scholar]
- 18.Eriksson S, Granström L. Randomized controlled trial of appendicectomy versus antibiotic therapy for acute appendicitis. Br J Surg. 1995;82(2):166-169. doi: 10.1002/bjs.1800820207 [DOI] [PubMed] [Google Scholar]
- 19.Hansson J, Körner U, Khorram-Manesh A, Solberg A, Lundholm K. Randomized clinical trial of antibiotic therapy versus appendicectomy as primary treatment of acute appendicitis in unselected patients. Br J Surg. 2009;96(5):473-481. doi: 10.1002/bjs.6482 [DOI] [PubMed] [Google Scholar]
- 20.Doleman B, Fonnes S, Lund J, et al. Appendectomy versus antibiotic treatment for acute appendicitis. Cochrane Database Syst Rev. 2021;9. doi: 10.1002/14651858.CD015038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Javanmard-Emamghissi H, Hollyman M, Boyd-Carson H, et al. Antibiotics as first-line alternative to appendicectomy in adult appendicitis: 90-day follow-up from a prospective, multicentre cohort study. Br J Surg. 2021;108(11):1351–1359. doi: 10.1093/bjs/znab287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tankel J, Keinan A, Blich O, et al. The decreasing incidence of acute appendicitis during COVID-19: a retrospective multi-centre study. World J Surg. 2020;44(8):2458-2463. doi: 10.1007/s00268-020-05599-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ceresoli M, Coccolini F, Magnone S, et al. ; Appendicitis-COVID study group . The decrease of non-complicated acute appendicitis and the negative appendectomy rate during pandemic. Eur J Trauma Emerg Surg. 2021;47(5):1359-1365. doi: 10.1007/s00068-021-01663-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maneck M, Günster C, Meyer H-J, Heidecke C-D, Rolle U. Influence of COVID-19 confinement measures on appendectomies in Germany—a claims data analysis of 9797 patients. Langenbecks Arch Surg. 2021;406(2):385-391. doi: 10.1007/s00423-020-02041-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Z, Sun F, Ai S, Wang J, Guan W, Liu S. Meta-analysis of studies comparing conservative treatment with antibiotics and appendectomy for acute appendicitis in the adult. BMC Surg. 2019;19(1):110. doi: 10.1186/s12893-019-0578-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Podda M, Cillara N, Di Saverio S, et al. ; ACOI (Italian Society of Hospital Surgeons) Study Group on Acute Appendicitis . Antibiotics-first strategy for uncomplicated acute appendicitis in adults is associated with increased rates of peritonitis at surgery. a systematic review with meta-analysis of randomized controlled trials comparing appendectomy and non-operative management with antibiotics. Surgeon. 2017;15(5):303-314. doi: 10.1016/j.surge.2017.02.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Protocol and Statistical Analysis Plan
eFigure 1. RR for Treatment Success (30 days of Follow-up)
eFigure 2. RR for Treatment Success (12 months)
eFigure 3. Recurrent Appendicitis Rate in Antibiotics Group
eTable 1. Bias Table