Abstract
Up to 30% of ischemic stroke cases are due to large vessel occlusion (LVO), causing significant morbidity. Studies have shown that the collateral circulation of patients with acute ischemic stroke (AIS) secondary to LVO can predict their clinical and radiological outcomes. The aim of this study is to identify baseline patient characteristics that can help predict the collateral status of these patients for improved triage. In this IRB approved retrospective study, consecutive patients presenting with AIS secondary to anterior circulation LVO were identified between September 2019 and August 2021. The baseline patient characteristics, laboratory values, imaging features and outcomes were collected using a manual chart review. From the 181 consecutive patients initially reviewed, 54 were confirmed with a clinical diagnosis of AIS and anterior circulation LVO. In patients with poor collateral status, the body mass index (BMI) was found to be significantly lower compared to those with good collateral status (26.4 ± 5.6 vs. 31.7 ± 12.3; p = 0.045). BMI of >35 kg/m2 was found to predict the presence of good collateral status. Age was found to be significantly higher (70.5 ± 9.6 vs. 58.9 ± 15.6; p = 0.034) in patients with poor collateral status and M1 strokes associated with older age and BMI.
Keywords: acute ischemic, hypoperfusion, collaterals status, hypoperfusion index
1. Introduction
Acute ischemic stroke (AIS) secondary to large vessel occlusion (LVO) comprises approximately 24–38% of all cases [1,2]. Mechanical thrombectomy (MT) has become the standard of care for the treatment of LVOs in anterior circulation strokes within 24 h of symptom onset, leading to improved clinical outcomes [3].
Collateral status (CS), for which digital subtraction angiography is considered the reference standard, has been shown to be an independent predictor for good outcome after MT. Good CS was found to be associated with functional independence, successful reperfusion, as well as both decreased symptomatic intracranial hemorrhage and mortality [4]. Poor CS is associated with increased mortality even after successful recanalization [5]. Collateral circulation is important as it provides the brain tissue with blood supply after the vessel supplying the area has been occluded. Good collateral flow can sustain the ischemic penumbra before reperfusion therapy, thereby minimizing the growth of the ischemic core and leading to less neurological deficit [6].
Recently, there has been an increase in the use of computed tomography perfusion (CTP) with the help of automatic post-processing software. CTP provides non-invasive quantitative and rapid measures to estimate the infarct core and potentially salvageable tissue. With the help of these software, a surrogate of CS has been identified which is called the hypoperfusion index (HI). HI is calculated as the ratio of time-to-maximum (Tmax) concentration of more than 10 s divided by the time-to-maximum concentration of more than 6 s [7]. The Tmax is an artificial perfusion parameter that reflects the time delay between the arrival of contrast bolus into the proximal large arterial circulation and the brain parenchyma, and is calculated through a deconvolution step using an arterial input function [8]. In the DEFUSE 2 cohort study, HI has shown to predict the rate of infarct growth and the functional outcome at 90 days in patients presenting with AIS secondary to LVO [8].
In previous studies, it has been shown that certain baseline patient characteristics and laboratory value changes are associated with increased risk of post-MT complications and worse functional outcomes [9,10]. It has been suggested that the patient characteristics and laboratory values can predict their CS. However, to the best of our knowledge, no study to date has systematically explored these relationships when utilizing HI as an indirect imaging surrogate for CS. The aim of our study was to explore these relationships in patients presenting with AIS secondary to anterior circulation LVO who underwent MT for their stroke management.
2. Materials and Methods
2.1. Study Population
The study population for this institutional review board (JHU-IRB00269637) approved respective study was consecutive patients with anterior circulation LVO who underwent baseline computed tomography angiography (CTA) and CTP followed by MT for their stroke management from September 2019 to August 2021. Anterior circulation LVO was defined as an occlusion of the intracranial internal carotid artery (ICA), M1 or proximal M2 segments of the middle cerebral artery (MCA).
2.2. Technical Parameters
Baseline comprehensive CT imaging was performed at the Johns Hopkins Hospital and Johns Hopkins Bayview Medical Centers using helical scanners on the Siemens Flash and/ or Drive (Siemens Healthineers, Erlangen, Germany). For non-contrast CT: helical mode at 5 mm slice thickness (ST), 120 kVp, 365 mAs, rotation time 1 s, acquisition time 6–8 s, collimation 128 × 0.6 mm, pitch value 0.55, scan direction craniocaudal. For CTP: injection of 50 mL non-ionic iodinated contrast with 30 mL saline flush at 5–6 mL/s with coverage of 70–100 mm at 5 mm ST. CTP parameters: 70 kVp, 200 effective mAs, rotation time 0.25 s, average acquisition time 60 s, collimation 48 × 1.2 mm, pitch value 0.7, 4D range 114 mm × 1.5 s. CTP images were then post-processed using RAPID commercial software (IschemaView, Menlo Park, CA, USA) for generating Tmax maps. For CTA head and neck: non-ionic iodinated contrast with 50–70 mL injected at 5–6 mL/s from the aortic arch through the vertex using a bolus triggered method at 3 mm ST. CTA parameters: 90/150 kVp with an Sn filter, quality reference mAs 180, rotation time 0.25 s, average acquisition time 3–5 s, collimation 128 × 0.6 mm, pitch value 0.7, scan direction craniocaudal.
2.3. Data Collection
The baseline and clinical data for each patient was collected with the help of a manual chart review performed by O.M.H. The variables collected for each patient included patient demographics, body mass index (BMI)), admission National Institutes of Health Stroke Scale (NIHSS), laboratory values, such as baseline hemoglobin level (Hb), hematocrit (Hct), white blood cell count (WBC), platelet count, platelet/WBC ratio, sodium concentration, potassium concentration, calcium concentration, random blood glucose level, blood urea nitrogen (BUN) level, creatinine level, blood pressure, heart rate, respiratory rate, blood oxygen level measure with SpO2 at admission, time from admission to CT, time from admission to IV tPA administration (if applicable), time from admission to groin puncture (if applicable), and groin puncture to recanalization time (if applicable). ASPECTS scores on noncontrast CT were calculated by a board certified neuroradiologist (V.S.Y.)
The CS was quantified using the HI, which was measured using the RAPID commercial software platform (IschemaView, Menlo Park, CA, USA) after post-processing the CTP images. The HI values were dichotomized into poor CS and good CS. Poor CS was defined as an HI of 0.4 or higher while good CS was an HI of less than 0.4.
2.4. Study Outcomes
The primary outcome measure was presence of good CS which was defined as HI of less than 0.4.
2.5. Statistical Analysis
The data was collected on a secure desktop using Microsoft Office Excel 2007 (Redmond, WA, USA) and analyzed using IBM SPSS statistics (Version 22.0, Chicago, IL, USA). Continuous variables were expressed using mean and SD or median and interquartile range (IQR) based on the distribution of the variable in question. Normality for all continuous variables was assessed using the Shapiro Wilk test. Quantitative data were compared using the independent t-test. Qualitative data were compared using Chi square or Fisher’s exact tests. Univariate analysis was initially applied to examine each of the baseline variables independently. Bonferroni corrections were applied for post-hoc tests as multiple comparisons were made on the same dependent variables to reduce the risk of type 1 errors Table 1. All p-values were two sided and the p value of <0.05 was considered to be statistically significant, unless multiple comparisons were made for which Bonferroni correction was applied, in such cases a p value of <0.017 was considered to be statistically significant.
Table 1.
Baseline demographics of the study population and their comparison based on the affected arterial territory.
| Variables | All Cases (N = 54) |
Arterial Territory | p-Value | |||
|---|---|---|---|---|---|---|
| ICA (N = 8) |
M1 (N = 26) |
Proximal M2 (N = 20) |
||||
| Age (years) | 67.9 ± 13.6 | 74.4 ± 15.7 | 64.7 ± 14.0 | 69.4 ± 11.5 | 0.175 | |
|
Male Sex
(n%) |
28 (51.9%) | 3 (37.5%) | 14 (53.8%) | 11 (55.0%) | 0.811 | |
|
Race
(n%) |
White/Caucasian | 23 (42.6%) | 3 (37.5%) | 9 (34.6%) | 11 (55.0%) | 0.577 |
| AfricanAmerican/Black | 30 (55.6%) | 5 (62.5%) | 16 (61.5%) | 9 (45.0%) | ||
| Asian | 1 (1.9%) | 0 (0.0%) | 1 (3.8%) | 0 (0.0%) | ||
| BMI (kg/m2) | 28.9 ± 9.7 | 33.2 ± 9.7 | 28.3 ± 11.2 | 27.9 ± 7.4 | 0.399 | |
| BMI grade | <30.0 | 36 (66.7%) | 5 (62.5%) | 19 (73.1%) | 12 (60.0%) | 0.624 |
| ≥30.0 | 18 (33.3%) | 3 (37.5%) | 7 (26.9%) | 8 (40.0%) | ||
| Hemoglobin level (gm/dL) | 12.4 ± 2.1 | 11.9 ± 3.0 | 11.9 ± 2.0 | 13.3 ± 1.4 | 0.061 | |
| Hematocrit (%) | 38.5 ± 5.7 | 36.4 ± 8.1 | 37.0 ± 5.2 | 41.3 ± 4.4 | 0.019 * p1: 0.799 p2: 0.047 p3: 0.005 * |
|
| WBC count (×103/mL) | 8.7 ± 3.0 | 8.9 ± 2.4 | 8.6 ± 3.0 | 8.7 ± 3.3 | 0.971 | |
| Platelet count (×103/mL) | 237.3 ± 79.3 | 223.9 ± 56.0 | 233.7 ± 70.5 | 247.4 ± 98.1 | 0.746 | |
| Platelet/WBC ratio | 29.2 ± 11.3 | 26.6 ± 8.7 | 29.1 ± 8.8 | 30.3 ± 15.0 | 0.740 | |
| Sodium level (mEq/L) | 139.2 ± 3.2 | 141.0 ± 4.2 | 138.3 ± 2.7 | 139.7 ± 3.1 | 0.085 | |
| Potassium level (mmol/L) | 4.1 ± 0.5 | 4.1 ± 0.4 | 4.1 ± 0.6 | 4.1 ± 0.4 | 0.964 | |
| Calcium level (mg/dL) | 8.8 ± 0.5 | 8.7 ± 0.5 | 8.9 ± 0.6 | 8.8 ± 0.5 | 0.725 | |
| Blood Glucose level (mg/dL) | 135.8 ± 65.1 | 118.3 ± 10.6 | 130.4 ± 73.6 | 149.8 ± 65.5 | 0.439 | |
| BUN/ creatinine ratio | 18.2 ± 7.8 | 17.5 ± 7.6 | 19.8 ± 8.5 | 16.5 ± 6.8 | 0.345 | |
| SBP (mmHg) | 148.2 ± 23.7 | 154.4 ± 21.2 | 144.2 ± 21.6 | 150.9 ± 27.3 | 0.468 | |
| DBP (mmHg) | 82.8 ± 19.9 | 88.0 ± 23.2 | 78.4 ± 18.6 | 86.4 ± 20.0 | 0.301 | |
| HR (beat/minute) | 80.6 ± 17.8 | 83.3 ± 20.8 | 80.9 ± 17.9 | 79.2 ± 17.2 | 0.857 | |
| RR (cycle/minute) | 17.6 ± 3.8 | 17.5 ± 4.3 | 17.6 ± 3.4 | 17.6 ± 4.3 | 0.997 | |
| SpO2 (%) | 97.9 ± 2.6 | 96.6 ± 4.1 | 98.1 ± 2.2 | 98.2 ± 2.4 | 0.329 | |
| NIHSS score | 15.0 ± 7.3 | 17.8 ± 5.7 | 15.5 ± 7.2 | 13.2 ± 7.9 | 0.307 | |
|
Left side improvement
(n%) |
32 (59.3%) | 4 (50.0%) | 16 (61.5%) | 12 (60.0%) | 0.866 | |
| HI | 0.3 ± 0.2 | 0.3 ± 0.2 | 0.3 ± 0.2 | 0.3 ± 0.2 | 0.990 | |
| Collaterals (n%) | Good | 26 (48.1%) | 4 (50.0%) | 13 (50.0%) | 9 (45.0%) | 0.933 |
| Poor | 28 (51.9%) | 4 (50.0%) | 13 (50.0%) | 11 (55.0%) | ||
| Hemorrhagic transformation (HT) within 48 H after MT, (n%) | 18 (33.3%) | 5 (62.5%) | 10 (38.5%) | 3 (15.0%) | 0.041 * p1: 0.231 p2: 0.012 * p3: 0.080 |
|
* Statistically significant (<0.05), p1 is ICA vs. M1, p2 is ICA vs. M2, p3 is M1 vs. M2.
3. Results
A total of 54 patients were included in the study cohort. Out of these, 8 (14.8%) patients had an ICA occlusion, 26 (48.1%) patients had a M1 occlusion, and 20 (37.0%) patients had a proximal M2 occlusion.
Table 1 shows the baseline characteristics of the patient cohort with comparison between patients with ICA, M1 and proximal M2 occlusions. The mean age of the patients was 67.9 ± 13.6 years. Fewer than half (48.1%, 26/54) of the patients had a good CS and collateral perfusion. There was no statistically significant difference in the baseline characteristics of patients with ICA, M1 and proximal M2 occlusions, except that the Hct was significantly higher in patients with proximal M2 strokes compared to those with M1 strokes (M1, 37.0 ± 5.2 vs. proximal M2, 41.3 ± 4.4; p = 0.005) and hemorrhagic transformation (HT) within 48 H after MT was significantly higher in patients with ICA occlusions compared to M2 occlusions (ICA, 62.5% (5/8) vs. proximal M2, 15% (3/20); p = 0.012).
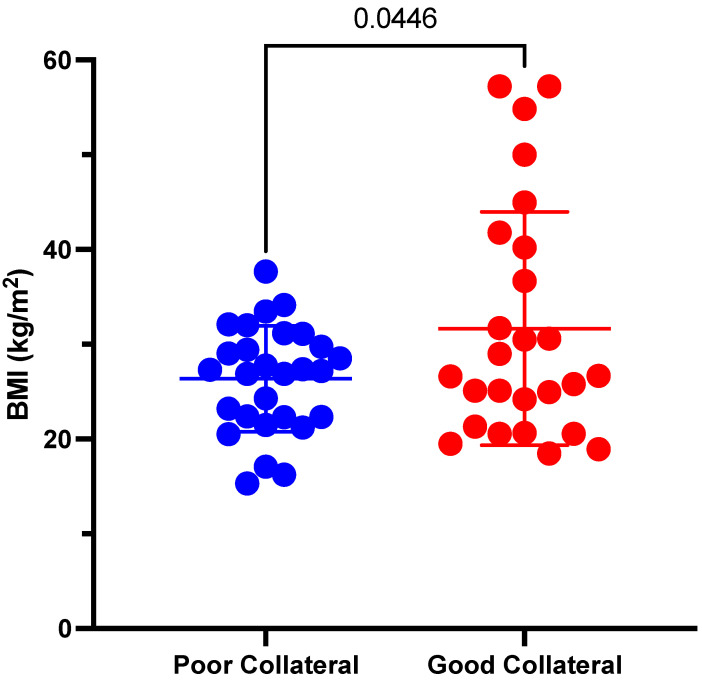
The difference in baseline characteristics of patients with poor and good collateral status is shown in Table 2. The BMI statistically was significantly lower (26.4 ± 5.6 vs. 31.7 ± 12.3; p = 0.045) in patients with poor CS compared to patients with good CS (Figure 1).
Table 2.
Comparison in the baseline characteristics according to the collateral status (HI).
| Variables | Perfusion | p-Value | ||
|---|---|---|---|---|
| Good (N = 26) |
Poor (N = 28) |
|||
| Age (years) | 70.7 ± 10.9 | 65.2 ± 15.3 | 0.135 | |
|
Male Sex
(n%) |
15 (57.7%) | 13 (46.4%) | 0.408 | |
|
Race
(n%) |
White/Caucasian | 14 (53.8%) | 9 (32.1%) | 0.099 |
| African American/Black | 11 (42.3%) | 19 (67.9%) | ||
| Asian | 1 (3.8%) | 0 (0.0%) | ||
| BMI (kg/m2) | 31.7 ± 12.3 | 26.4 ± 5.6 | 0.045 * | |
| BMI grade | <30.0 kg/m2 | 15 (57.7%) | 21 (75.0%) | 0.178 |
| ≥30.0 kg/m2 | 11 (42.3%) | 7 (25.0%) | ||
| Hemoglobin level (gm/dL) | 11.9 ± 2.4 | 12.9 ± 1.6 | 0.074 | |
| Hematocrit (%) | 37.4 ± 6.8 | 39.4 ± 4.4 | 0.205 | |
| WBC count (×103/mL) | 8.0 ± 2.4 | 9.3 ± 3.3 | 0.099 | |
| Platelet count (×103/mL) | 228.2 ± 79.1 | 245.8 ± 80.0 | 0.419 | |
| Platelet/WBC ratio | 30.4 ± 12.5 | 28.1 ± 10.2 | 0.468 | |
| Sodium level (mEq/L) | 139.9 ± 3.0 | 138.6 ± 3.3 | 0.131 | |
| Potassium level (mmol/L) | 4.1 ± 0.4 | 4.1 ± 0.6 | 0.817 | |
| Calcium level (mg/dL) | 8.7 ± 0.5 | 8.9 ± 0.6 | 0.403 | |
| Blood glucose level (mg/dL) | 127.4 ± 38.1 | 143.6 ± 82.8 | 0.368 | |
| BUN/creatinine ratio | 19.0 ± 6.7 | 17.5 ± 8.8 | 0.487 | |
| SBP (mmHg) | 150.0 ± 22.6 | 146.5 ± 25.1 | 0.597 | |
| DBP (mmHg) | 84.6 ± 20.3 | 81.1 ± 19.8 | 0.518 | |
| HR (beat/minute) | 81.0 ± 14.9 | 80.2 ± 20.4 | 0.867 | |
| RR (cycle/minute) | 17.0 ± 2.5 | 18.1 ± 4.7 | 0.280 | |
| SpO2 (%) | 97.8 ± 3.2 | 98.0 ± 2.0 | 0.790 | |
| NIHSS score | 13.3 ± 8.1 | 16.6 ± 6.2 | 0.102 | |
| ASPECTS score | 9.86 ± 0.14 | 9.2 ± 0.49 | 0.696 | |
| Time from door to CT (mins) | 18.28 ± 4.84 | 14 ± 5.34 | 0.499 | |
| Time from door to needle (IV TPA) (mins) | 74.28 ± 24.04 | 50.4 ± 10.67 | 0.908 | |
| Time from door to groin puncture (MT) (mins) | 167 ± 40.06 | 122 ± 17.16 | 0.317 | |
| Time from groin puncture to recanalization (mins) | 34.28 ± 7.86 | 38 ± 12.14 | 0.489 | |
| Mechanical Thrombectomy | 26/26 (100%) | 26/28 (92.3%) | 1 | |
| IV tPA | 8/26 (30.7%) | 10/28 (36.3%) | 0.758 | |
|
Site
(n%) |
Right | 8 (30.8%) | 14 (50.0%) | 0.151 |
| Left | 18 (69.2%) | 14 (50.0%) | ||
| Hemorrhagic transformation (HT) within 48 H after MT, (n%) | 9 (34.6%) | 9 (32.1%) | 0.847 | |
* Statistically significant (<0.05).
Figure 1.
Comparison of BMI in patients with poor vs. good collaterals.
The comparison in the CS of patients with occlusions in different arterial territories and their relationship to baseline characteristics is shown in Table 3. Age was significantly higher (70.5 ± 9.6 vs. 58.9 ± 15.6; p = 0.034) in patients with poor CS and M1 strokes, and BMI was significantly higher (39.7 ± 9.7 vs. 26.8 ± 3.9; p = 0.049) in patients with ICA occlusions and good CS. Right sided proximal M2 occlusions were significantly more common in patients with poor CS (63.6% (7/11) vs. 11.1% (1/9); p = 0.028) than those with good CS. Diagnostic performance based on vessel subgroup is shown in Table 4.
Table 3.
Comparison in the collateral status of patients with occlusions in different arterial territories and their relationship to baseline characteristics.
| Variables | ICA | M1 Artery | Proximal M2 Artery | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Good (N = 4) |
Poor (N = 4) |
p-Value | Good (N = 13) |
Poor (N = 13) |
p-Value | Good (N = 9) |
Poor (N = 11) |
p-Value | ||
| Age (years) | 72.3 ± 16.1 | 76.5 ± 17.5 | 0.733 | 70.5 ± 9.6 | 58.9 ± 15.6 | 0.034 * | 70.4 ± 11.8 | 68.5 ± 11.7 | 0.711 | |
|
Male Sex
(n%) |
1 (25.0%) | 2 (50.0%) | 0.999 | 8 (61.5%) | 6 (46.2%) | 0.431 | 6 (66.7%) | 5 (45.5%) | 0.406 | |
|
Race
(n%) |
White/
Caucasian |
2 (50.0%) | 1 (25.0%) | 0.999 | 6 (46.2%) | 3 (23.1%) | 0.226 | 6 (66.7%) | 5 (45.5%) | 0.406 |
| African American/Black | 2 (50.0%) | 3 (75.0%) | 6 (46.2%) | 10 (76.9%) | 3 (33.3%) | 6 (54.5%) | ||||
| Asian | 0 (0.0%) | 0 (0.0%) | 1 (7.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||||
| BMI (kg/m2) | 39.7 ± 9.7 | 26.8 ± 3.9 | 0.049 * | 31.7 ± 14.6 | 25.0 ± 4.9 | 0.138 | 28.0 ± 8.6 | 27.8 ± 6.8 | 0.944 | |
| BMI grade | <30.0 | 1 (25.0%) | 4 (100.0%) | 0.143 | 8 (61.5%) | 11 (84.6%) | 0.378 | 6 (66.7%) | 6 (54.5%) | 0.670 |
| ≥30.0 | 3 (75.0%) | 0 (0.0%) | 5 (38.5%) | 2 (15.4%) | 3 (33.3%) | 5 (45.5%) | ||||
| Hemoglobin level (gm/dL) | 10.6 ± 3.9 | 13.1 ± 1.1 | 0.296 | 11.4 ± 2.0 | 12.5 ± 1.9 | 0.188 | 13.2 ± 1.7 | 13.3 ± 1.2 | 0.796 | |
| Hematocrit (%) | 33.4 ± 10.8 | 39.3 ± 3.5 | 0.363 | 35.6 ± 4.7 | 38.4 ± 5.4 | 0.170 | 41.9 ± 5.8 | 40.7 ± 3.2 | 0.563 | |
| WBC count (×103/mL) | 8.7 ± 3.3 | 9.1 ± 1.7 | 0.826 | 8.2 ± 2.9 | 9.0 ± 3.2 | 0.505 | 7.3 ± 0.9 | 9.8 ± 4.1 | 0.098 | |
| Platelet count (×103/mL) | 214.0 ± 52.4 | 233.8 ± 65.7 | 0.655 | 228.6 ± 65.7 | 238.8 ± 77.4 | 0.722 | 233.8 ± 109.0 | 258.5 ± 92.1 | 0.590 | |
| Platelet/WBC ratio | 26.5 ± 7.3 | 26.8 ± 11.0 | 0.968 | 29.9 ± 8.3 | 28.3 ± 9.5 | 0.652 | 32.8 ± 18.7 | 28.4 ± 11.6 | 0.528 | |
| Sodium level (mEq/L) | 143.5 ± 4.0 | 138.5 ± 3.0 | 0.094 | 138.6 ± 2.2 | 138.1 ± 3.1 | 0.619 | 140.2 ± 2.4 | 139.3 ± 3.6 | 0.512 | |
| Potassium level (mmol/L) | 4.1 ± 0.4 | 4.0 ± 0.4 | 0.675 | 4.1 ± 0.4 | 4.1 ± 0.7 | 0.764 | 3.9 ± 0.4 | 4.2 ± 0.4 | 0.218 | |
| Calcium level (mg/dL) | 8.5 ± 0.5 | 9.0 ± 0.4 | 0.165 | 8.9 ± 0.5 | 8.8 ± 0.7 | 0.717 | 8.6 ± 0.5 | 8.9 ± 0.5 | 0.283 | |
| Blood glucose level (mg/dL) | 124.5 ± 5.2 | 112.0 ± 11.3 | 0.092 | 118.7 ± 20.9 | 142.2 ± 102.7 | 0.428 | 141.3 ± 59.4 | 156.7 ± 72.2 | 0.614 | |
| BUN/creatinine | 21.0 ± 6.0 | 14.0 ± 8.0 | 0.212 | 18.8 ± 5.8 | 20.8 ± 10.8 | 0.576 | 18.3 ± 8.6 | 14.9 ± 4.9 | 0.277 | |
| SBP (mmHg) | 145.0 ± 5.4 | 163.8 ± 28.0 | 0.236 | 150.1 ± 23.8 | 138.2 ± 18.2 | 0.167 | 152.0 ± 26.7 | 150.0 ± 29.1 | 0.876 | |
| DBP (mmHg) | 79.5 ± 27.7 | 96.5 ± 17.2 | 0.337 | 80.3 ± 15.7 | 76.5 ± 21.6 | 0.616 | 93.1 ± 22.5 | 80.8 ± 16.8 | 0.178 | |
| HR (beat/minute) | 81.0 ± 3.8 | 85.5 ± 31.3 | 0.785 | 83.2 ± 12.6 | 78.7 ± 22.2 | 0.535 | 78.0 ± 20.7 | 80.1 ± 14.8 | 0.795 | |
| RR (cycle/minute) | 17.8 ± 4.0 | 17.3 ± 5.2 | 0.884 | 17.0 ± 2.2 | 18.2 ± 4.3 | 0.366 | 16.7 ± 2.4 | 18.3 ± 5.3 | 0.387 | |
| SpO2 (%) | 96.3 ± 5.7 | 97.0 ± 2.4 | 0.816 | 97.9 ± 2.5 | 98.3 ± 2.0 | 0.667 | 98.3 ± 3.0 | 98.0 ± 1.9 | 0.766 | |
| NIHSS score | 16.8 ± 5.0 | 18.8 ± 7.0 | 0.658 | 13.2 ± 8.1 | 17.6 ± 5.8 | 0.123 | 11.9 ± 9.4 | 14.4 ± 6.4 | 0.503 | |
|
Site
(n%) |
Right | 1 (25.0%) | 3 (75.0%) | 0.486 | 6 (46.2%) | 4 (30.8%) | 0.420 | 1 (11.1%) | 7 (63.6%) | 0.028 * |
| Left | 3 (75.0%) | 1 (25.0%) | 7 (53.8%) | 9 (69.2%) | 8 (88.9%) | 4 (36.4%) | ||||
| Hemorrhagic transformation (HT) within 48 H after MT, (n%) | 2 (50.0%) | 3 (75.0%) | 0.999 | 6 (46.2%) | 4 (30.8%) | 0.420 | 1 (11.1%) | 2 (18.2%) | 0.362 | |
* Statistically significant (<0.05).
Table 4.
Diagnostic performance and characteristics of BMI in predicting poor collaterals.
| Characteristics | All Cases | ICA | M1 | Proximal M2 | ||||
|---|---|---|---|---|---|---|---|---|
| Value | 95% CI | Value | 95% CI | Value | 95% CI | Value | 95% CI | |
| Poor Collaterals from Good Collateral | ||||||||
| AUC | 0.560 | 0.401–0.720 | 0.813 | 0.465–1.000 | 0.550 | 0.320–0.781 | 0.465 | 0.199–0.730 |
| p-value | 0.446 | 0.149 | 0.663 | 0.790 | ||||
| Cut point | ≤35.0 | ≤35.0 | ≤35.0 | ≤35.0 | ||||
| Sensitivity | 96.4% | 81.7–99.9% | 100% | 39.8–100% | 100% | 75.3–100% | 90.9% | 58.7–99.8% |
| Specificity | 30.8% | 14.3–51.8% | 75.0% | 19.4–99.4% | 23.1% | 5.0–53.8% | 22.2% | 2.8–60.0% |
| DA | 64.8% | 50.6–77.3% | 87.5% | 47.3–99.7% | 61.5% | 40.6–79.8% | 60.0% | 36.1–80.9% |
| YI | 27.2% | 8.2–46.2% | 75.0% | 32.6–100% | 23.1% | 0.2–46.0% | 13.1% | 18.9–45.2% |
| PPV | 60.0% | 44.3–74.3% | 80.0% | 28.4–99.5% | 56.5% | 34.5–76.8% | 58.8% | 32.9–81.6% |
| NPV | 88.9% | 51.8–99.7% | 100% | 29.2–100% | 100% | 29.2–100% | 66.7% | 9.4–99.2% |
AUC: Area under the curve; CI: Confidence interval. DA: Diagnostic accuracy. YI: Youden’s Index. PPV: Positive Predictive value. NPV: Negative Predictive value.
4. Discussion
In this study, the relationships between baseline patient characteristics and CS were explored, and poor CS was found to be associated with lower BMI. Additionally, the baseline Hct of patients with proximal M2 occlusions was found to be higher than those with M1 occlusions, however, the reason for this association is not clear and needs to be explored in larger studies. The HT rate within 48 H after MT was significantly higher in patients with ICA occlusions compared to M2 occlusions, which differs from prior literature where the rate of HT has been shown to be similar between ICA and MCA occlusions [11]. The reason for this may be due to the much larger volume of territory at risk in ICA occlusions compared to M2 occlusions [12,13].
In patients suffering from AIS, collateral circulation plays an important role in maintaining the blood flow to the tissue at risk of becoming ischemic, and in reducing the risk of hemorrhagic transformation in patients undergoing MT [14]. HI has been shown to be a good surrogate for predicting the CS in patients with acute LVO [15]. Various previous studies have explored the association between patient baseline characteristics and the CS, however variable results have been reported, most likely due to the heterogenous nature of the patient population [16,17,18,19,20,21,22]. Analysis of one of the largest stroke registries, the MR CLEAN Trial and Registry, has shown that older age, male sex, high glucose levels and occlusion of the intracranial internal carotid artery terminus is associated with poor collateral grades as identified on CTA, however, association of various other baseline characteristics, such as body mass index and laboratory values, were not explored in that study [23].
The CS was characterized as poor in 54% of the patients included in this study. This may in part be explained by the older age of the patients included in this cohort. Older age has been associated with progressive loss of number of collaterals and their diameter, along with the increase in arterial tortuosity, all of which leads to an increased resistance in the collateral circulation [24].
Several studies in the literature have shown that higher BMI is associated with reduced cerebral flow and an increased risk of ischemic stroke [25,26,27,28]. However, the outcomes of strokes in patients with obesity have been shown to better than those without due to a multitude of reasons (termed the “obesity paradox”) [27,28,29,30,31]. The obesity paradox can, on a biological level, be explained by the protective effect of soluble tumor necrosis factor-alpha-receptors, which are secreted by the adipose tissue, and bind to the tumor necrosis factor-alpha circulating in the blood [32,33]. Obese patients also have elevated levels of serum lipoproteins and lipids that have been shown to play an important role in blocking the inflammatory cytokine cascade by binding to the liposaccharides in the blood, and this might be responsible for better outcomes in these patients [34,35,36]. Additionally, this study shows that the CS was better in patients with a higher BMI, which could be one of the reasons contributing to better mortality and morbidity outcomes after strokes in patients with obesity.
This study has several limitations to acknowledge. This study was a retrospective analysis at a single center, which can lead to sampling bias, however, we included consecutive patients in the study to minimize this. The data was collected with the help of a retrospective chart review, which can lead to some incorrect recording of data. The study included only patients with acute anterior circulation LVO comprising intracranial ICA, M1, and proximal M2 only. Therefore, these results are not applicable to patients with occlusions of other arterial territories. Patient outcome after MT was not assessed and therefore the results of this study cannot be used to predict the clinical outcomes of patients, although it is again important to note that several prior studies have shown that CS is an important predictive biomarker of patient outcomes, which is the purpose of utilizing CS for the current study. The CS of the patients was assessed utilizing an artificial index for CS estimation in HI. Although HI has been validated as a strong predictor of CS, it still requires further validation in larger prospective cohorts.
5. Conclusions
Patients with lower BMI and older age are associated with poor collateral status as predicted by the HI with the help of an automated software. Further investigations are necessary in larger cohorts to validate the results of this study.
Acknowledgments
This work has been accomplished with the support of the Johns Hopkins University School of Medicine Department of Radiology Physician Scientist Incubator Program.
Author Contributions
O.H.—Data Collection, Manuscript Writing, Editing; T.G.—Data Collection, Manuscript Writing, Editing; O.E.—Editing; R.W.—Editing, Formatting; A.A. (Alperen Aslan)—Editing; A.A. (Amara Ahmed)—Editing, Formatting; A.M.—Editing; V.Y.—Idea Generation, Data Collection, Manuscript Writing, Editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Johns Hopkins University School of Medicine (protocol code JHU-IRB00269637 and 3 September 2021.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Vivek Yedavalli serves as a consultant for MRIOnline (Cincinnati, OH, USA), RAPID (IschemaView, Menlo Park, CA, USA), and editorial board of Frontiers in Radiology. Tushar Garg serves on the editorial board of RadioGraphics and Pediatrics Oncall, receives unrelated conference travel support from Siemens Healthineers and unrelated salary support from the RSNA Research Seed Award.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Malhotra K., Gornbein J., Saver J.L. Ischemic Strokes Due to Large-Vessel Occlusions Contribute Disproportionately to Stroke-Related Dependence and Death: A Review. Front. Neurol. 2017;8:651. doi: 10.3389/fneur.2017.00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dozois A., Hampton L., Kingston C.W., Lambert G., Porcelli T.J., Sorenson D., Templin M., VonCannon S., Asimos A.W. PLUMBER Study (Prevalence of Large Vessel Occlusion Strokes in Mecklenburg County Emergency Response) Stroke. 2017;48:3397–3399. doi: 10.1161/STROKEAHA.117.018925. [DOI] [PubMed] [Google Scholar]
- 3.Berkhemer O.A., Fransen P.S.S., Beumer D., van den Berg L.A., Lingsma H.F., Yoo A.J., Schonewille W.J., Vos J.A., Nederkoorn P.J., Wermer M.J.H., et al. A Randomized Trial of Intraarterial Treatment for Acute Ischemic Stroke. N. Engl. J. Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 4.Qian J., Fan L., Zhang W., Wang J., Qiu J., Wang Y. A Meta-analysis of Collateral Status and Outcomes of Mechanical Thrombectomy. Acta Neurol. Scand. 2020;142:191–199. doi: 10.1111/ane.13255. [DOI] [PubMed] [Google Scholar]
- 5.Nordmeyer H., Webering N., Chapot R., Hadisurya J., Heddier M., Stracke P., Berger K., Isenmann S., Weber R. The Association between Collateral Status, Recanalization and Long Term Outcome in Stroke Patients Treated with Stent Retrievers-Are There Indications Not to Perform Thrombectomy Based on CT Angiography? J. Neuroradiol. 2017;44:217–222. doi: 10.1016/j.neurad.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Jung S., Gilgen M., Slotboom J., El-Koussy M., Zubler C., Kiefer C., Luedi R., Mono M.-L., Heldner M.R., Weck A., et al. Factors That Determine Penumbral Tissue Loss in Acute Ischaemic Stroke. Brain. 2013;136:3554–3560. doi: 10.1093/brain/awt246. [DOI] [PubMed] [Google Scholar]
- 7.Olivot J.M., Mlynash M., Inoue M., Marks M.P., Wheeler H.M., Kemp S., Straka M., Zaharchuk G., Bammer R., Lansberg M.G., et al. Hypoperfusion Intensity Ratio Predicts Infarct Progression and Functional Outcome in the DEFUSE 2 Cohort. Stroke. 2014;45:1018–1023. doi: 10.1161/STROKEAHA.113.003857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wouters A., Christensen S., Straka M., Mlynash M., Liggins J., Bammer R., Thijs V., Lemmens R., Albers G.W., Lansberg M.G. A Comparison of Relative Time to Peak and Tmax for Mismatch-Based Patient Selection. Front. Neurol. 2017;8:539. doi: 10.3389/fneur.2017.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zachrison K.S., Schwamm L.H., Xu H., Matsouaka R., Shah S., Smith E.E., Xian Y., Fonarow G.C., Saver J. Frequency, Characteristics, and Outcomes of Endovascular Thrombectomy in Patients with Stroke Beyond 6 Hours of Onset in US Clinical Practice. Stroke. 2021;52:3805–3814. doi: 10.1161/STROKEAHA.121.034069. [DOI] [PubMed] [Google Scholar]
- 10.Dutra B.G., Tolhuisen M.L., Alves H.C.B.R., Treurniet K.M., Kappelhof M., Yoo A.J., Jansen I.G.H., Dippel D.W.J., van Zwam W.H., van Oostenbrugge R.J., et al. Thrombus Imaging Characteristics and Outcomes in Acute Ischemic Stroke Patients Undergoing Endovascular Treatment. Stroke. 2019;50:2057–2064. doi: 10.1161/STROKEAHA.118.024247. [DOI] [PubMed] [Google Scholar]
- 11.Feng X., Ye G., Cao R., Qi P., Lu J., Chen J., Wang D. Identification of Predictors for Hemorrhagic Transformation in Patients with Acute Ischemic Stroke After Endovascular Therapy Using the Decision Tree Model. CIA. 2020;15:1611–1624. doi: 10.2147/CIA.S257931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nawabi J., Kniep H., Schön G., Flottmann F., Leischner H., Kabiri R., Sporns P., Kemmling A., Thomalla G., Fiehler J., et al. Hemorrhage After Endovascular Recanalization in Acute Stroke: Lesion Extent, Collaterals and Degree of Ischemic Water Uptake Mediate Tissue Vulnerability. Front. Neurol. 2019;10:569. doi: 10.3389/fneur.2019.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian B., Tian X., Shi Z., Peng W., Zhang X., Yang P., Li Z., Zhang X., Lou M., Yin C., et al. Clinical and Imaging Indicators of Hemorrhagic Transformation in Acute Ischemic Stroke After Endovascular Thrombectomy. Stroke. 2022;53:1674–1681. doi: 10.1161/STROKEAHA.121.035425. [DOI] [PubMed] [Google Scholar]
- 14.Iwasawa E., Ichijo M., Ishibashi S., Yokota T. Acute Development of Collateral Circulation and Therapeutic Prospects in Ischemic Stroke. Neural Regen Res. 2016;11:368–371. doi: 10.4103/1673-5374.179033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang C.-M., Chang Y.-M., Sung P.-S., Chen C.-H. Hypoperfusion Index Ratio as a Surrogate of Collateral Scoring on CT Angiogram in Large Vessel Stroke. JCM. 2021;10:1296. doi: 10.3390/jcm10061296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liebeskind D.S., Cotsonis G.A., Saver J.L., Lynn M.J., Cloft H.J., Chimowitz M.I. Investigators for the Warfarin—Aspirin Symptomatic Intracranial Disease (WASID) Collateral Circulation in Symptomatic Intracranial Atherosclerosis. J. Cereb. Blood Flow Metab. 2011;31:1293–1301. doi: 10.1038/jcbfm.2010.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H., Chai Q., Gutterman D.D., Liu Y. Elevated Glucose Impairs CAMP-Mediated Dilation by Reducing Kv Channel Activity in Rat Small Coronary Smooth Muscle Cells. Am. J. Physiol.-Heart Circ. Physiol. 2003;285:H1213–H1219. doi: 10.1152/ajpheart.00226.2003. [DOI] [PubMed] [Google Scholar]
- 18.Tsuruta R., Fujita M., Ono T., Koda Y., Koga Y., Yamamoto T., Nanba M., Shitara M., Kasaoka S., Maruyama I., et al. Hyperglycemia Enhances Excessive Superoxide Anion Radical Generation, Oxidative Stress, Early Inflammation, and Endothelial Injury in Forebrain Ischemia/Reperfusion Rats. Brain Res. 2010;1309:155–163. doi: 10.1016/j.brainres.2009.10.065. [DOI] [PubMed] [Google Scholar]
- 19.Brunner F., Tomandl B., Hanken K., Hildebrandt H., Kastrup A. Impact of Collateral Circulation on Early Outcome and Risk of Hemorrhagic Complications after Systemic Thrombolysis. Int. J. Stroke. 2014;9:992–998. doi: 10.1111/j.1747-4949.2012.00922.x. [DOI] [PubMed] [Google Scholar]
- 20.Romano J.G., Liebeskind D.S. Revascularization of Collaterals for Hemodynamic Stroke: Insight on Pathophysiology from the Carotid Occlusion Surgery Study. Stroke. 2012;43:1988–1991. doi: 10.1161/STROKEAHA.112.650119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ovbiagele B., Saver J.L., Starkman S., Kim D., Ali L.K., Jahan R., Duckwiler G.R., Vinuela F., Pineda S., Liebeskind D.S. Statin Enhancement of Collateralization in Acute Stroke. Neurology. 2007;68:2129–2131. doi: 10.1212/01.wnl.0000264931.34941.f0. [DOI] [PubMed] [Google Scholar]
- 22.Lima F.O., Furie K.L., Silva G.S., Lev M.H., Camargo É.C.S., Singhal A.B., Harris G.J., Halpern E.F., Koroshetz W.J., Smith W.S., et al. The Pattern of Leptomeningeal Collaterals on CT Angiography Is a Strong Predictor of Long-Term Functional Outcome in Stroke Patients with Large Vessel Intracranial Occlusion. Stroke. 2010;41:2316–2322. doi: 10.1161/STROKEAHA.110.592303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiegers E.J.A., Mulder M.J.H.L., Jansen I.G.H., Venema E., Compagne K.C.J., Berkhemer O.A., Emmer B.J., Marquering H.A., van Es A.C.G.M., Sprengers M.E., et al. Clinical and Imaging Determinants of Collateral Status in Patients with Acute Ischemic Stroke in MR CLEAN Trial and Registry. Stroke. 2020;51:1493–1502. doi: 10.1161/STROKEAHA.119.027483. [DOI] [PubMed] [Google Scholar]
- 24.Faber J.E., Zhang H., Lassance-Soares R.M., Prabhakar P., Najafi A.H., Burnett M.S., Epstein S.E. Aging Causes Collateral Rarefaction and Increased Severity of Ischemic Injury in Multiple Tissues. ATVB. 2011;31:1748–1756. doi: 10.1161/ATVBAHA.111.227314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selim M., Jones R., Novak P., Zhao P., Novak V. The Effects of Body Mass Index on Cerebral Blood Flow Velocity. Clin. Auton. Res. 2008;18:331–338. doi: 10.1007/s10286-008-0490-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alosco M.L., Spitznagel M.B., Raz N., Cohen R., Sweet L.H., Colbert L.H., Josephson R., van Dulmen M., Hughes J., Rosneck J., et al. Obesity Interacts with Cerebral Hypoperfusion to Exacerbate Cognitive Impairment in Older Adults with Heart Failure. Cerebrovasc. Dis. Extra. 2012;2:88–98. doi: 10.1159/000343222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurth T., Gaziano J.M., Berger K., Kase C.S., Rexrode K.M., Cook N.R., Buring J.E., Manson J.E. Body Mass Index and the Risk of Stroke in Men. Arch. Intern. Med. 2002;162:2557–2562. doi: 10.1001/archinte.162.22.2557. [DOI] [PubMed] [Google Scholar]
- 28.Strazzullo P., D’Elia L., Cairella G., Garbagnati F., Cappuccio F.P., Scalfi L. Excess Body Weight and Incidence of Stroke: Meta-Analysis of Prospective Studies with 2 Million Participants. Stroke. 2010;41:e418–e426. doi: 10.1161/STROKEAHA.109.576967. [DOI] [PubMed] [Google Scholar]
- 29.Towfighi A., Ovbiagele B. The Impact of Body Mass Index on Mortality after Stroke. Stroke. 2009;40:2704–2708. doi: 10.1161/STROKEAHA.109.550228. [DOI] [PubMed] [Google Scholar]
- 30.Ovbiagele B., Bath P.M., Cotton D., Vinisko R., Diener H.-C. Obesity and Recurrent Vascular Risk after a Recent Ischemic Stroke. Stroke. 2011;42:3397–3402. doi: 10.1161/STROKEAHA.111.624957. [DOI] [PubMed] [Google Scholar]
- 31.Vemmos K., Ntaios G., Spengos K., Savvari P., Vemmou A., Pappa T., Manios E., Georgiopoulos G., Alevizaki M. Association between Obesity and Mortality after Acute First-Ever Stroke: The Obesity-Stroke Paradox. Stroke. 2011;42:30–36. doi: 10.1161/STROKEAHA.110.593434. [DOI] [PubMed] [Google Scholar]
- 32.Carbone S., Lavie C.J., Arena R. Obesity and Heart Failure: Focus on the Obesity Paradox. Mayo Clin. Proc. 2017;92:266–279. doi: 10.1016/j.mayocp.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Oesch L., Tatlisumak T., Arnold M., Sarikaya H. Obesity Paradox in Stroke-Myth or Reality? A Systematic Review. PLoS ONE. 2017;12:e0171334. doi: 10.1371/journal.pone.0171334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parto P., Lavie C.J., Arena R., Bond S., Popovic D., Ventura H.O. Body Habitus in Heart Failure: Understanding the Mechanisms and Clinical Significance of the Obesity Paradox. Future Cardiol. 2016;12:639–653. doi: 10.2217/fca-2016-0029. [DOI] [PubMed] [Google Scholar]
- 35.Clark A.L., Fonarow G.C., Horwich T.B. Obesity and the Obesity Paradox in Heart Failure. Prog. Cardiovasc. Dis. 2014;56:409–414. doi: 10.1016/j.pcad.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Horwich T.B., Fonarow G.C., Hamilton M.A., MacLellan W.R., Woo M.A., Tillisch J.H. The Relationship between Obesity and Mortality in Patients with Heart Failure. J. Am. Coll. Cardiol. 2001;38:789–795. doi: 10.1016/S0735-1097(01)01448-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.