Abstract
Alkaloids are a wide family of basic N-containing natural products, whose research has revealed bioactive compounds of pharmacological interest. Studies on these compounds have focused more attention on those produced by plants, although other types of organisms have also been proven to synthesize bioactive alkaloids, such as animals, marine organisms, bacteria, and fungi. This review covers the findings of the last 20 years (2002–2022) related to the isolation, structures, and biological activities of the alkaloids produced by mushrooms, a fungal subgroup, and their potential to develop drugs and agrochemicals. In some cases, the synthesis of the reviewed compounds and structure−activity relationship studies have been described.
Keywords: fungi, mushrooms, alkaloids, structure, biological activity, structure-activity relationship, potential practical application
1. Introduction
Natural sources have a great diversity of N-containing compounds. Numerous studies have been performed on the isolation and chemical and biological characterization, and these studies are still increasing. These investigations have also confirmed that families such as alkaloids [1], peptides [2], phenoxazines [3], amines [4], or nitrogenous sesquiterpenoids [5] could show outstanding activities of pharmacological or agronomic interest. The alkaloid family is one of the most relevant of these, given its production by a wide range of living beings, its structural variety, as well as the biological activities that have been discovered a long time ago.
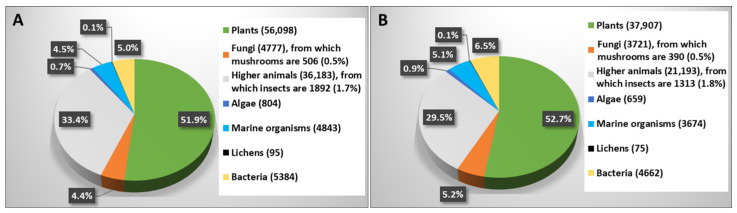
Alkaloids are a large group consisting of diverse subgroups of natural products that are most extensively studied in plants. Some examples of well-known alkaloids of a vegetal origin are morphine, which possesses common anesthetic and pain reliever activities [6]; caffeine, which is a stimulant in commonly consumed beverages [7]; or nicotine, which is an addictive constituent in tobacco [8]. Among the plant alkaloids, there is a large group produced by hundreds of species of Amaryllidaceae, for which their chemistry and biological activities have also been reported on in previous reviews [9]. These alkaloids have an assumed importance, not only for their chemistry, but also for their several biological activities [10,11,12,13,14,15,16]. Lycorin is the main Amaryllidaceae alkaloid, which has been known for a long time as lycorine, and has essentially been studied for its anticancer activity as well as for its natural and synthetic analogs and close isocarbostyryls [17,18,19,20]. However, several studies have shown the presence of alkaloids with promising medicinal properties in other types of organisms, including animals, insects (an animal subgroup), marine sources, bacteria, fungi, and mushrooms (one of the subgroups of fungi). Figure 1 provides an overview of the type of organisms involved in studies on alkaloids. From these data, it is possible to conclude that mushrooms are one of the least studied sources for alkaloids, which are only surpassed by lichens. Nevertheless, a sufficient number of scientific articles have reported the isolation and the biological activities of diverse mushroom alkaloids. The example of psilocybin and its metabolic product psilocin could be hilighted, which are two of the most studied hallucinogenic compounds from the psilocybin mushrooms [21].
Figure 1.
Distribution of references on alkaloids in the database SciFinder (A) without a time filter; (B) from 2002 to 30 May 2022. References were obtained using the keyword “alkaloid”, plus the corresponding keyword for each type of organism. The number of references for each item are shown in parentheses.
Thus, on the basis of these results, we report the biological and chemical characterization of mushroom alkaloids, as this source has been lesser studied than the others.
This review is focused on studies carried out on mushrooms over the last 20 years in relation to the alkaloids they produce. Considering Figure 1, 390 of the 506 references available in the literature for this topic, that is, 77%, were published throughout this period. This study intends to highlight the most significant developments found in the reviewed period, thereby giving rise to take perspective to carry out new research on this promising field. In some cases, the synthesis reported in the literature for some of the reviewed compounds will be highlighted. This is the case for laccarin, an alkaloid isolated at a low yield from the mushroom Laccaria vinaceoavellanea, which can become available through the enantioselective synthesis developed by Bower et al. (2007) [22].
The bibliography was selected from the database SciFinder by combining the keywords “alkaloid” and “mushroom”. The search was restricted to the period of 2002–2022. Additionally, some references were collected through complementary searches through SciFinder or Google Scholar. After a critical reading of the articles, 144 articles were selected and their main results and conclusions are included in this review.
The review is divided into subheadings considering the carbon skeleton of the reviewed compounds, in their chronological order of publication. Moreover, this review covers diverse structure-activity relationship (SAR) studies carried out during the reviewed period. These studies are generally based on the synthesis and evaluation of the bioactivity of a number of structural analogs, providing the best cases for the specific structural modifications that improve the activity levels. The study by Yuan et al. (2017) [23] represents a recent example of this kind of this study, and also provides the enantioselective synthesis of the already-known mushroom alkaloid lysergol.
2. New Alkaloids Found in Mushroom since 2002
Section 2 reports, in detail, the new alkaloids discovered in mushrooms during 2002–2022 (30 May). Given the larger number of compounds found for β-carbolines, pyrroloquinolines, pyrroles, and indoles, they have been grouped and described independently according to their carbon skeleton in Section 2.1, Section 2.2, Section 2.3 and Section 2.4. The alkaloids that have not been grouped are described in Section 2.5 in chronological order according to their year of discovery.
A structural consideration to take into account is that alkaloids are natural products whose nitrogen atom has basic properties. By extension, compounds that differ in this respect but are biogenetically related to them could be included [24], and this classification has been adopted in this review.
Thus, Table 1 shows the new alkaloids and related compounds produced by mushrooms discovered during the period covered by the review, together with their isolation source and the biological activities that were described for them.
Table 1.
Alkaloids and related compounds produced by mushrooms discovered in the period covered by the review (2002–2022).
| Alkaloid | Mushroom Source | Biological Activity | References |
|---|---|---|---|
| Subgroup: β-Carboline alkaloids (Figure 2) | |||
| 4-(Methylthio)canthin-6-one (5); 5-(methylthio)canthin-6-one (6); 9-(methylthio)canthin-6-one (7); 11-(methylthio)canthin-6-one (8); 2-methyl-β-carbolinium-1-propanoate (11) |
Boletus curtisii | - | [25] |
| Brunnein A (12) |
Cortinarius brunneus Different Hygrophorus spp. |
- | [26,27] |
| Brunnein B (13); brunnein C (14) | C. brunneus | - | [26] |
| C-1 diastereomer of brunnein B (15) | Cyclocybe cylindracea | Antioxidant | [28] |
| 10-Hydroxy-infractopicrin (17) | Cortinarius infractus | Inhibition of acetylcholinesterase | [29] |
| Metatacarboline family (19–25) | Mycena metata | Anticancer, for metatacarbolines D (23) and F (25) | [30,31] |
| 1-Acetyl-7-hydroxy-9H-pyrido [3,4-b]indole-3-carboxylic acid (27) | Sarcomyxa edulis | Anti-inflammatory | [32] |
| Subgroup: Pyrroloquinoline alkaloids (Figure 3) | |||
| Mycenarubin A (29) | Mycena haematopus, Mycena pelianthina and Mycena rosea | - | [33,34,35] |
| Mycenarubin B (30) | M. rosea | - | [33] |
| Mycenarubin D (31) | M. haematopus | Antibacterial | [36] |
| Mycenarubin E (32); mycenarubin F (33) | M. haematopus | - | [36] |
| Mycenarubin C (34) | M. rosea | - | [37] |
| Sanguinone A (35); sanguinone B (36); sanguinolentaquinone (37); decarboxydehydrosanguinone A (38) |
Mycena sanguinolenta | - | [38] |
| Haematopodin B (39) | M. haematopus | Antibacterial | [34,36] |
| Pelianthinarubin A (41); pelianthinarubin B (42) | M. pelianthina | - | [35] |
| Mycenaflavin A (43) | M. haematopus | Moderate antibacterial | [34] |
| Mycenaflavin B (44) | M. haematopus | Moderate antibacterial and cytotoxic | [34,39] |
| Mycenaflavin C (45); mycenaflavin D (46) | M. haematopus | - | [34] |
| Subgroup: Pyrrole alkaloids (Figure 4) | |||
| Inotopyrrole B (50) | Inonotus obliquus and Phlebopus portentosus | Neuroprotective against H2O2 damage | [40,41] |
| 2-[2-Formyl-5-(methoxymethyl)-1H-pyrrol-1-yl]acetic acid (51) | Leccinum extremiorientale | Low cytotoxic | [42] |
| 4-[2-Formyl-5-(hydroxymethyl)-1H-pyrrol-1-yl] butanoic acid (53) | Basidiomycetes-X, Grifola frondosa and L. extremiorientale | Hepatoprotective, low inhibition of α-glucosidase and low cytotoxic | [42,43,44,45] |
| Phlebopine A, also pyrrolefronine (54) | G. frondosa and P. portentosus | Inhibition of α-glucosidase, and mild neuroprotective against H2O2 damage | [41,45] |
| Phlebopine B (55); phlebopine C (56); 1-isopentyl-2-formyl-5-hydroxy-methylpyrrole (57) | P. portentosus | Moderate or mild neuroprotective against H2O2 damage | [41] |
| 2-[2-Formyl-5-(methoxymethyl)-1H-pyrrole-1-yl]propanoate (58) | P. portentosus | Inhibition of pancreatic lipase activity, and mild neuroprotective against H2O2 damage | [41,46] |
| 5-Hydroxymethyl-1-methyl-1H-pyrrole-2-carbaldehyde (59); 5-hydroxymethyl-1-ethyl-1H-pyrrole-2-carbaldehyde (60); 5-hydroxymethyl-1-acetic acid-1H-pyrrole-2-carbaldehyde (62) |
G. frondosa | Inhibition of α-glucosidase | [45] |
| Pyrrolezanthine (61) | G. frondosa | Anti-inflammatory and strong inhibition of α-glucosidase | [45,47] |
| 4-[2-formyl-5-(hydroxymethyl)-1H-pyrrol-1-yl] butanamide (63) | Basidiomycetes-X | Weak antioxidant | [43] |
| Subgroup: Indole alkaloids (Figure 6) | |||
| 5-Methoxy-4-methoxymethyl-2-methyl-1H-indole (75) | Tricholoma caligatum | - | [48] |
| 1-(1-β-Glucopyranosyl)-3-(methoxymethyl)-1H-indole (76); 1-(1-β-glucopyranosyl)-1H-indole-3-carbaldehyde (77) |
C. brunneus | - | [49] |
| Macrolepiotin (79) | Macrolepiota neomastoidea | - | [50] |
| 7-Methoxyindole-3-carboxylic acid methyl ester (80); 1-methylindole-3-carboxaldehyde (81) |
Phellinus linteus | - | [51] |
| 5-Hydroxyhypaphorine (82) | Astraeus odoratus | - | [52] |
| 4-(Ethoxymethyl)-1H-indole (85) | Tricholoma flavovirens | Plant growth | [53] |
| Corallocin C (87) | Hericium coralloides | Stimulation of neurite outgrowth | [54] |
| Terpendole N (88); terpendole O (89) | Pleurotus ostreatus | - | [55] |
| Subgroup: Miscellaneous alkaloids (Figures 7 and 8) | |||
| Dictyoquinazols A–C (92–94) | Dictyophora indusiata | Neuroprotective | [56] |
| Concavine (95) | Clitocybe concava | Weak antibacterial | [57] |
| Pyriferines A–C (96–98) | Pseudobaeospora pyrifera | - | [58] |
| Pycnoporin (99) | Pycnoporus cinnabarinus | Moderate antitumoral | [59] |
| Sinensine (100) | Ganoderma sinense | Protective against H2O2 oxidation | [60] |
| Sinensines B-D (101–103) | G. sinense | - | [61] |
| Sinensine E (104) | Ganoderma cochlear, Ganoderma luteomarginatum, and G. sinense | - | [61,62,63] |
| (+)-6S-Hydroxyganocochlearine A and (−)-6R-hydroxyganocochlearine A (105) | G. luteomarginatum | - | [62] |
| Ganocochlearine A (106) | Ganoderma australe, G. cochlear, and Ganoderma lucidum | Neuroprotective and anti-inflammatory | [64,65,66] |
| Ganocochlearine B (107) | G. cochlear | - | [64] |
| Ganocalicine A (108) | Ganoderma calidophilum | Anti-allergic | [67] |
| Ganocalicine B (109) | G. australe and G. calidophilum | - | [65,67] |
| Ganocochlearine C (110); ganocochlearine H (115) |
G. australe and G. cochlear | - | [63,65] |
| Ganocochlearines D-F (111–113); ganocochlearine I (116) |
G. cochlear | - | [63] |
| Lucidimine A (117); lucidimine D (120) | G. lucidum | - | [66,68] |
| Lucidimine B (118) | G. lucidum | Antioxidant and antiproliferative | [66,68,69] |
| Lucidimine C (119) | G. cochlear and G. lucidum | Antioxidant | [63,66,68,69] |
| Lucidimine E (121) | G. lucidum | Anti-inflammatory | [66] |
| Ganoapplanatumine A (122) | Ganoderma applanatum | - | [70] |
| Ganoapplanatumine B (123) | G. applanatum and G. cochlear | - | [63,70] |
| Australine (124) | G. australe | Neuroprotective | [65] |
| Erinacerins M–P (125–128) | Hericium erinaceus | Moderate cytotoxic | [71] |
| Erinacerin V (129) | Hericium sp. | - | [72] |
| Rosallin A (130) | Mycena rosella | Herbicidal | [73] |
| Rosallin B (131) | M. rosella | - | [73] |
| Consoramides A–C (132–134) | Irpex consors | - | [74] |
| Stereumamide A (135) | Stereum hirsutum | Antibacterial | [75] |
| Stereumamide B (136); stereumamide C (137) | S. hirsutum | - | [75] |
| Stereumamide D (138) | I. consors and S. hirsutum | Antibacterial | [74,75] |
2.1. β-Carboline Alkaloids
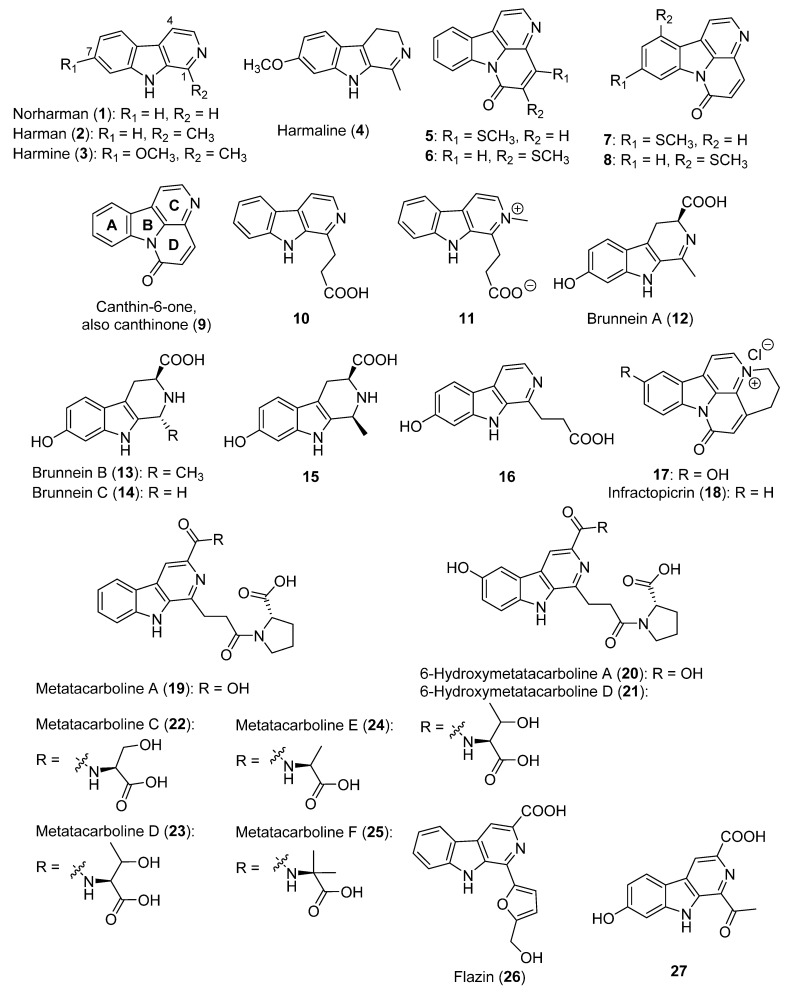
β-Carboline alkaloids are known for their various biological activities, including their antioxidant, antimicrobial, antiparasitic, antiviral, antitumor, hallucinogenic, and DNA intercalation activity, among others [30]. Norharman, and its methylated derivative harman (1 and 2, Figure 2), are among the most studied alkaloids from this family. They are normal endogenous body constituents that possess pharmacological properties, including cytotoxicity [76,77]. However, these two compounds might cause Parkinson’s and cancer [78]. These alkaloids have also been found in tobacco smoke and in other diverse plant species, as well as in food and drink [78,79,80]. Moreover, they are also produced by bacteria [77,81] and fungi [82]. Their occurrence in mushrooms has also been reported, and has been found in 27 species of the genus Hygrophorus [27] and in the Psilocybe species [83]. Harmine and harmaline (3 and 4, Figure 2) represent other known mushroom alkaloids with pharmacological properties [84,85].
Figure 2.
The structures of the β-carboline alkaloids isolated from mushrooms (1–27).
Canthin-6-one alkaloids 5–8 (Figure 2) were the first discovered β-carboline alkaloids in the review period and were isolated from the fruiting bodies of Boletus curtisii [25]. Canthin-6-one (or canthinone, 9, Figure 2) alkaloids are a subclass of β-carboline alkaloids that contain an additional D-ring [86]. Alkaloids 5–8 are characterized by the presence of a sulfur atom in their structure. In particular, they are close to canthin-6-one (9), but differ from it because of the presence of a thiomethyl group in different positions [25]. The same authors also reported the first isolation of canthin-6-one (9) outside of higher plants. Compound 9 has anti-fungal, anti-parasite, and cytotoxic properties [86,87]. As no activities were reported for thiomethylated alkaloids 5–8, extensive studies on their pharmacological activities would be of interest.
The harmane derivatives β-carboline-1-propanoic acid and 2-methyl-β-carbolinium-1-propanoate (10 and 11, Figure 2), the latter as a new compound, were also isolated from B. curtisii [25]. Compound 10 was also found in Cortinarius infractus [88] and in the plant kingdom [89,90,91], including its tentative identification in extracts from the matrix plants of the Ayahuasca tea beverage [92].
Three new compounds, named brunneins A–C (12–14, Figure 2), were isolated from Cortinarius brunneus [26]. Later, brunnein A (12) was also found in diverse Hygrophorus species [27]. The diastereomer of brunnein B (15, Figure 2) was also isolated from Cyclocybe cylindracea, and exhibited a marked antioxidant activity [28]. In addition, acid 16 (Figure 2) was isolated from C. brunneus, which was the first time from a non-vegetal source [26].
10-Hydroxy-infractopicrin (17, Figure 2) was isolated for the first time together with the already-known infractopicrin (18, Figure 2) from the toadstool C. infractus [29]. Both compounds 17 and 18 inhibited acetylcholinesterase with a higher selectivity than the reference drug galanthamine, thus they were suggested as potential drugs for Alzheimer’s disease.
A new family of 16 compounds, named metatacarbolines, was identified in the fruiting bodies of Mycena metata [30]. Each of these compounds is a β-carboline bonded to a specific amino acid, with the exception of metatacarboline A and 6-hydroxymetatacarboline A (19 and 20, Figure 2). 6-Hydroxymetatacarboline D (21, Figure 2) was the only isolated compound in this study [30], although a later study focused on the synthesis of some metatacarbolines. In particular, the syntheses of metatacarbolines A (19) and C–F (21–25, Figure 2) were reported with 40–75% overall yields [31] and their availability allowed for evaluating their anticancer activity. Metatacarbolines D (23) and F (25) showed a significant antiproliferative activity by arresting the cell cycle at the sub G0/G1 and G2/M phases of the cell cycle, respectively [31].
Flazin (26, Figure 2) was isolated from Suillus granulatus and Boletus umbriniporus for the first time from mushrooms [93]. It is the only reviewed alkaloid containing the β-carboline moiety joined with a furan ring.
The most recent β-carboline discovered in mushrooms (27, Figure 2) was isolated from Sarcomyxa edulis [32]. Compound 27 is the only reviewed β-carboline with a ketone group located in an exocyclic position. It showed a remarkable anti-inflammatory activity against lipopolysaccharide-induced NO [32].
2.2. Pyrroloquinoline Alkaloids
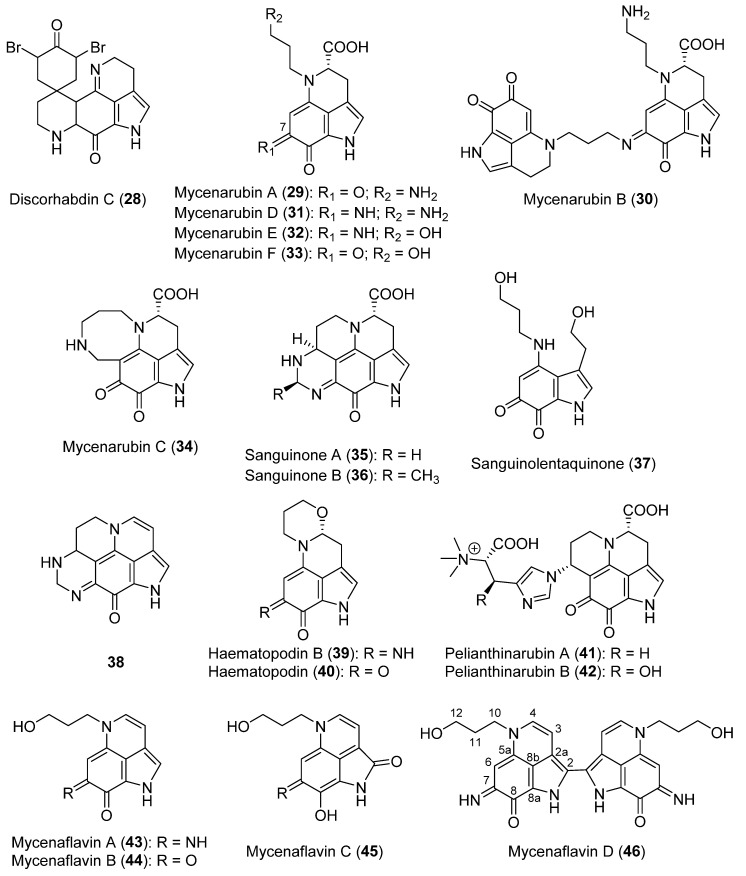
Pyrroloquinolines are a family of natural compounds mostly isolated from marine sponges, which gained interest with the discovery of the cytotoxic alkaloid discorhabdin C (28, Figure 3) in 1986 [94,95]. Diverse studies developed during 2002–2022 proved that mushrooms can also be sources of pyrroloquinolines, although a low number of studies on their bioactivities were performed. Many of the pyrroloquinoline alkaloids belong to the family of mycenarubins (29–34, Figure 3), which were discovered in 2007, with the isolation of mycenarubin A (29) [33]. Mycenarubin A was isolated together with its dimer mycenarubin B (30) from Mycena rosea, which represents the first occurrence of a dimeric pyrroloquinoline alkaloid in nature. The synthesis of mycenarubin A (29) was accomplished in 10 steps and produced a 21% total yield by Backenköhler et al. (2018) [39]. Later, mycenarrubins D–F (31–33) were isolated from Mycena haematopus [36]. Mycenarubin A (29) was also obtained from M. haematopus [34] and Mycena pelianthina, with the last species also being a source for the isolation of mycenarubin D (31) [35].
Figure 3.
The structures of discorhabdin C (28) and the pyrroloquinoline alkaloids isolated from mushrooms (29–46).
Mycenarubin D (31) showed an antibacterial activity against Azovibrio restrictus, Azoarcus tolulyticus, and Azospirillum brasilense, whereas mycenarubin A (29) was shown to be inactive as an antibacterial compound [34,37]. Thus, the presence of the C=NH unit at position 7 is a key group for the bioactivity of these pyrroloquinoline alkaloids.
Successively, mycenarrubin C (34, Figure 3) was isolated from M. rosea [37]. Compound 34 is a special pyrroloquinoline alkaloid with an eight-membered ring, which contains an additional C1 unit. The same authors also suggested that mycenarubin A (29) is the precursor of mycenarubin C (34).
Sanguinones A and B (35 and 36, Figure 3) were isolated from Mycena sanguinolenta, with sanguinone A (35) being the main metabolite [38]. The same article also reported the first isolation of sanguinolentaquinone (37, Figure 3), and the identification of decarboxydehydrosanguinone A (38, Figure 3) as an oxidative decarboxylation artifact of sanguinone A (35). The synthesis of 37 was later realized in eight steps and with a 28% total yield [39].
Haematopodin B (39, Figure 3) was isolated from M. haematopus, together with the already known haematopodin (40, Figure 3) [36]. The authors suggested that haematopodin (40) is the degradation product of haematopodin B (39). Haematopodin B (39) was shown to be as active as the reference antibiotic drug gentamicin against A. tolulyticus.
Pelianthinarubins A and B (41 and 42, Figure 3), two new pyrroloquinolines isolated from M. pelianthina, possess a more complex structure than the usual pyrroloquinoline alkaloids [35]. They might play a role in the chemical defense of M. pelianthina [35].
Mycenaflavins A–D (43–46, Figure 3) were first isolated from the fruiting bodies of M. haematopus, with mycenaflavin D (46) being the first dimeric pyrroloquinoline alkaloid with a C-C bridge between the two pyrroloquinoline units [34]. Compounds 43–45 differ from other pyrroloquinolines by possessing an additional double bond between C-3 and C-4, which generates a yellow color; whereas mycenaflavin D (46) is purple due to the extended conjugated π system [34]. The synthesis of mycenaflavin B (44) was achieved in eight steps and with a 15% total yield by Backenköhler et al. (2018) [39]. Alkaloid 44 showed a moderate cytotoxicity against fibroblast and melanoma cells [39]. The authors suggested that this bioactivity could be related to the planarity of the compound in relation to the possibility of DNA intercalation [39].
2.3. Pyrroles
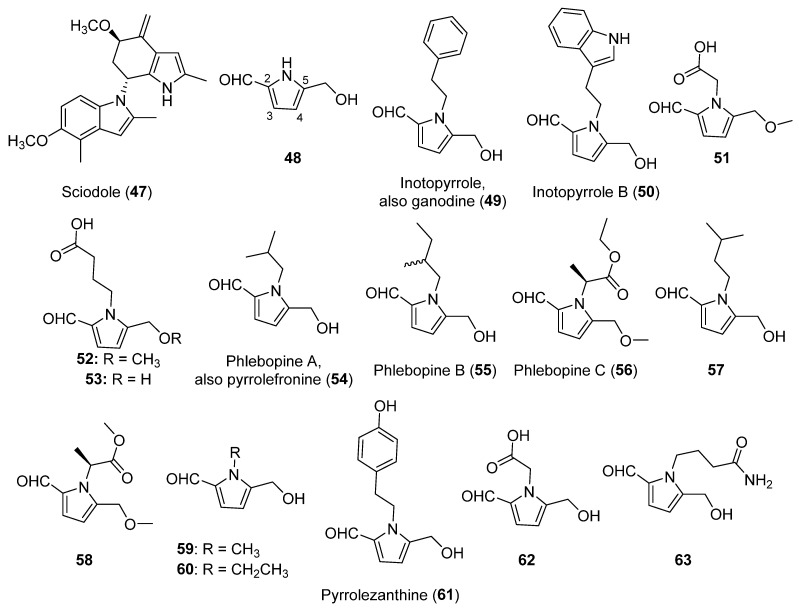
The structure of pyrroles, with a high electron density in their heteroaromatic ring, is of special interest when developing new bioactive drugs [96]. The alkaloids of this subgroup attract a great interest for their anticancer, antimicrobial, antiviral, antimalarial, antitubercular, anti-inflammatory, and enzyme inhibiting properties [97]. Indeed, according to the Scifinder database, 643 patents that used the term “pyrrole” in biological studies were issued, 550 of them since 2002. Before this date, diverse alkaloids including a pyrrole in their structure were known of mostly from a vegetal or marine origin. In mushrooms, the discovery of sciodole (47, Figure 4) from Tricholoma sciodes [98], an alkaloid containing both a pyrrole and indole moiety in its structure could be highlighted.
Figure 4.
The structures of the pyrrole alkaloids isolated from mushrooms (47–63).
It is worthy to note that pyrroles show acid properties, so they would not comply with the essential requirement to define them as alkaloids. However, pyrrolizidine alkaloids commonly accumulate as N-oxides, which are transformed into pyrrole derivatives during their metabolism [99]. This consideration makes it possible to find in the bibliography pyrrolic compounds cited as alkaloids by their authors, which is reviewed in this section.
From 2002, different pyrroles were discovered from mushrooms. All of them have an aldehyde function at C-2 and a primary hydroxyl or methoxy group at C-5, being structural derivatives of 5-(hydroxymethyl)-1H-pyrrole-2-carboxaldehyde (48, Figure 4). In fact, 48 was found for the first time from Inonotus obliquus in 2014 [100], and successively also from other mushroom species, as will be seen throughout this section.
Inotopyrrole (49, Figure 4), a benzyl derivative of 48 isolated from I. obliquus, was reported as a new mushroom compound [100]. However, its isolation and structure determination were previously reported when compound 49 was isolated from Ganoderma capense and named as ganodine [66,101]. Inotopyrrole B (50, Figure 4), a related compound formed by the bonding of the same pyrrole scaffold with an indole, was also found in I. obliquus [40]. Both inotopyrrole (49) and inotopyrrole B (50) were also isolated from the edible mushroom Phlebopus portentosus [41]. Structurally, inotopyrrole B (50) shares with the aforementioned sciodole (47, Figure 4) the particularity of presenting a pyrrole and indole moiety in its structure.
Three carboxylic acids (51-53, Figure 4) related to this family were isolated from the fruiting bodies of Leccinum extremiorientale, with 51 being a new compound. Compounds 51–53 showed a poor cytotoxicity [42]. Compound 53 had been already isolated from the plant Lycium chinense [44] and successively from the mushroom Basidiomycetes-X [43]. Compound 53 showed a remarkable hepatoprotective activity, suggesting that the carboxylic group of this pyrrole plays an important role in this biological activity [44].
Phlebopines A–C (54–56, Figure 4) were discovered in 2018 from P. portentosus [41], a species that also produces compounds 49 and 50. The absolute configuration of phlebopine B (55) was not identified. 1-Isopentyl-2-formyl-5-hydroxy-methylpyrrole and 2-[2-formyl-5-(methoxymethyl)-1H-pyrrole-1-yl]propanoate (57 and 58, Figure 4), which were previously found only from vegetal sources, were also reported as metabolites of P. portentosus [41,46]. Phlebopine C (56) and compound 58 are closely related, differing only in the length of the alkyl chain of their ester group. Compound 58 showed a relevant inhibitory activity towards pancreatic lipase [46].
The first isolation of pyrrolefronine from Grifola frondosa was reported by Chen et al. (2018) [45], although its structure corresponds with that of phlebopine A (54). Five other already-known pyrroles (48, 49, and 59–61, Figure 4) and acids 53 and 62 (Figure 4) were also isolated from G. frondosa [45]. Pyrrolezanthine (61), previously isolated from different vegetal species and later from the fermentation of a fungus with a plant [47], correspond with the phenolic form of inotopyrrole (49). An inhibitory activity against α-glucosidase was found for compounds 59–62, specially for compound 61 [45], which also showed anti-inflammatory effects [47].
4-[2-Formyl-5-(hydroxymethyl)-1H-pyrrol-1-yl] butanamide (63, Figure 4), the amide form of 52, was isolated together with the already-known 48 and the acid 53 from the edible Japanese mushroom Basidiomycetes-X. A weak antioxidant activity was described for 63 [43].
2.4. Indoles
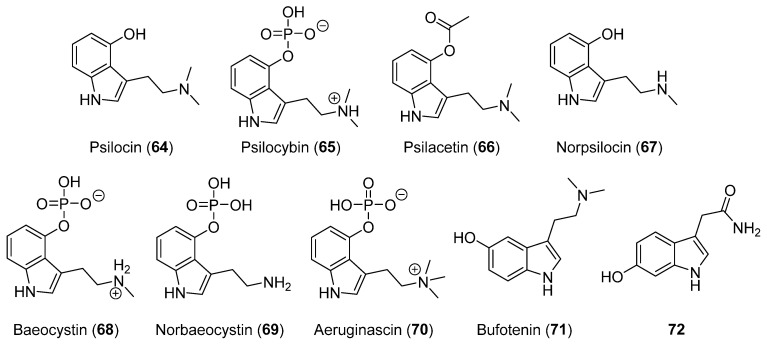
Indole alkaloids are of great relevance for drug development. In fact, some natural ones have been approved by the Food and Drug Administration (FDA), such as vincristine, vinblastine, vinorelbine, and vindesine for the treatment of leukemia, lymphoma, melanoma, breast cancer, and non-small cell lung cancer [102]. From a structural point of view, the indole scaffold corresponds to a pyrrole bonded to a benzene. However, unlike pyrrole compounds, for which there are not a remarkably high number of compounds identified in mushrooms, indoles are more abundant in these species. In fact, more than 140 compounds bearing an indole heterocycle were found in mushrooms, with the amino acid L-typtophan being the biogenic source of most of them [103]. Thus, structurally related indoles with endogenous activities such as 5-hydroxy-L-tryptophan, tryptamine, serotonin, melatonin, and bufotenin were identified in a diverse range of mushrooms [103].
Psilocin alkaloid, and its phosphorylated counterpart, psilocybin (64 and 65, Figure 5), are among the most studied indole metabolites produced by mushrooms. They are hallucinogens found in mushrooms of the genus Psilocybe, Panaeolus, Conocybe, Gymnopilus, Stropharia, Pluteus, and Panaeolina [21], which have been known of since the middle of the last century after their isolation from Psilocybe mexicana [104]. Both compounds have been extensively described in recent reviews [21,105,106].
Figure 5.
Already-known bioactive indole alkaloids produced by mushrooms (64–72).
Both compounds 64 and 65 have special relevance in therapeutic treatments due to their low toxicity and suitable physiological tolerance [21]. Because of these properties, throughout the period 2002–2022, they have continued to be the object of study in numerous investigations. The results are described in 280 articles and 101 patents that contain the term “psilocin”, as well as 924 articles and 176 patents for “psilocybin“, as can be found for this period in the Scifinder database. Indeed, psilocin-mushrooms containing psilocin (64), psilocybin (65), and psilacetin (66, Figure 5) have been suggested as viable chemotherapeutic agents against SARS-CoV-2 [107].
Norpsilocin, baeocystin, norbaeocystin, aeruginascin, and bufotenin (67–71, Figure 5) are other examples of indoles with psychoactive properties produced by mushrooms, although they have not been studied much other than psilocin or psilocybin [21,108]. The syntheses and biological evaluation of some of these were carried out by Sherwood et al. (2020) [108], and the antiviral activity of bufotenine (71) was reported [109]. Norpsilocin (67) was isolated for the first time in 2017 (from Psilocybe cubensis) and its psychoactive properties, with an agonist activity of the human 5-HT2A receptor close to that of psilocin (64), were also described [110]. Recently, effects in time estimation and cognition in in vivo assays for norpsilocin (67) were estimated, while both psilocin (64) and psilocybin (65) produced unspecific effects in these two parameters [111].
In addition to new pharmacological properties, studies of agronomic interest have also been developed with indolic compounds. In fact, the production of 6-hydroxy-1H-indole-3-acetamide (72, Figure 5), which is an already-known mushroom compound, was recently related to glyphosate resistance [112].
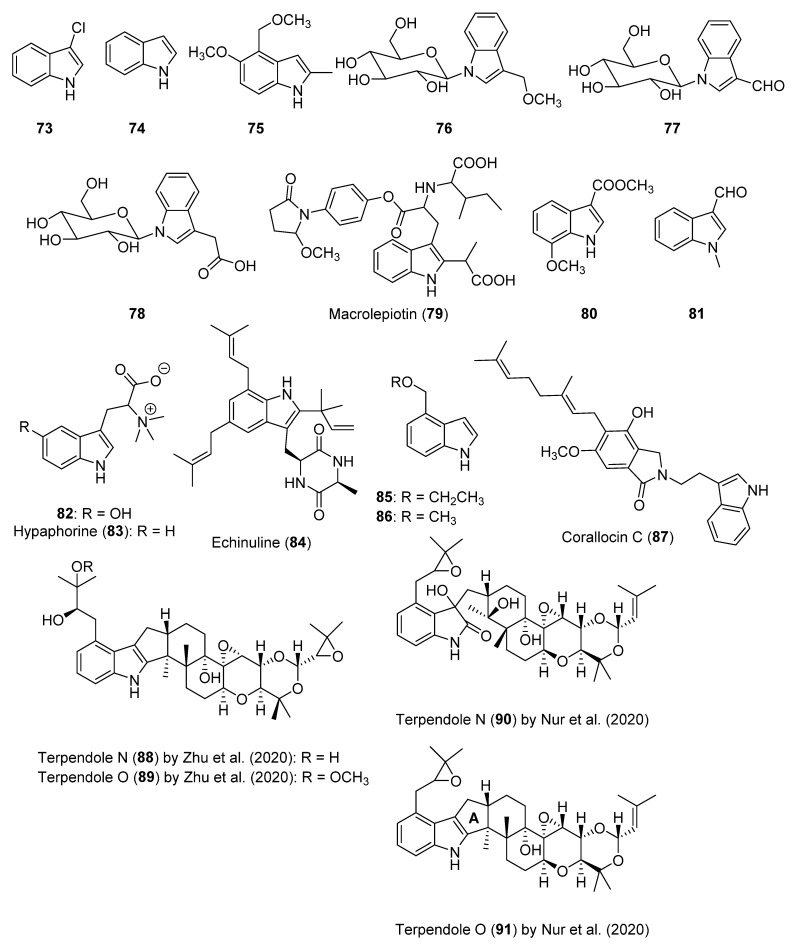
3-Chloroindole (73, Figure 6) was isolated from Hygrophorus paupertinus, the first time from a terrestrial organism, together with indole (74, Figure 6) and was identified as one of the compounds responsible for the fecal odor of this mushroom [113]. However, indole (74) is a bicyclic and heterocyclic aromatic compound, and not an alkaloid. 5-Methoxy-4-methoxymethyl-2-methyl-1H-indole (75, Figure 6) was only found in the volatile components of Tricholoma caligatum, by Fons et al. (2006) [48]. Its synthesis was accomplished in two steps and it achieved 17% global yield starting from 5-hydroxy-2-methylindole [114].
Figure 6.
The structures of the indoles and indole alkaloids isolated from mushrooms (73–91).
The three N-glucosylated indoles 76–78 (Figure 6) were isolated from the basidiomycete C. brunneus, with 76 and 77 being new compounds [49]. The endogenous role of compound 78 was investigated, and it was suggested that it may either act as an inactive transport or storage form of auxin (growth regulator), or that it is a detoxification product [49].
Macrolepiotin (79, Figure 6) was isolated from Macrolepiota neomastoidea, a poisonous mushroom that causes severe gastrointestinal symptoms [50].
7-Methoxyindole-3-carboxylic acid methyl ester and 1-methylindole-3-carboxaldehyde (80 and 81, Figure 6) were isolated from Phellinus linteus [51].
5-Hydroxyhypaphorine (82, Figure 6) was isolated for the first time from Astraeus odoratus, a species that also produces the betaine hypaphorine (83, Figure 6) [52].
Echinuline (84, Figure 6), an indole alkaloid previously isolated from filamentous fungi and vegetal species, was obtained for the first time from the basidiomycete Lentinus strigellus [115]. Alkaloid 84 showed cytotoxicity and damage to the alveolar walls and liver, and food and water containing this compound are refused by animals [116]. It belongs to the family of echinulins, alkaloids whose biosynthesis is currently under study [117,118].
4-(Ethoxymethyl)-1H-indole (85, Figure 6) was isolated together with its methoxylated derivative 86 (Figure 6) from Tricholoma flavovirens. Alkaloid 86 was an already-known indole previously found in other Tricholoma species. Both compounds have been shown to be active in plant growth bioassays [53].
Corallocin C (87, Figure 6) was isolated for the first time from Hericium coralloides. Compound 87 belongs to the family of corallocins, and has been characterized for containing an indole moiety. It showed a remarkable activity for stimulating neurite outgrowth [54].
At this point, it is worth mentioning the previously detailed isolation of inotopyrrole B (50, Figure 4) from I. obliquus, an alkaloid containing both an indole and a pyrrole moiety in its structure [40].
Terpendoles N and O (88 and 89, Figure 6) were isolated as new compounds from Pleurotus ostreatus [55]. The last time a new compound of the terpendole family was discovered was in 1999, when terpendole M was isolated from the fungus Neotyphodium lolii [119]. It should be noted that during the 2002–2022 period, new studies were carried out evaluating the bioactivity of some terpendoles [120,121,122,123,124,125,126]. Three new terpendoles produced by the fungus Volutella citronella, two of them named terpendoles N and O (90 and 91, Figure 6), were reported in another study [127], but their structures were different to those published by Zhu et al. (2020) [55]. In fact, the new structure for terpendole N differed significantly because the indole system contains an amide group. Compound 91 induced the inhibition of sterol O-acyltransferase isozymes, while 90 was not active [127]. Terpendoles N and O, with respect to most of the alkaloids cited in this review, presented up to eight rings, including two epoxides, and were the only compounds reviewed that contained an epoxide ring in their structure.
2.5. Miscellaneous Alkaloids
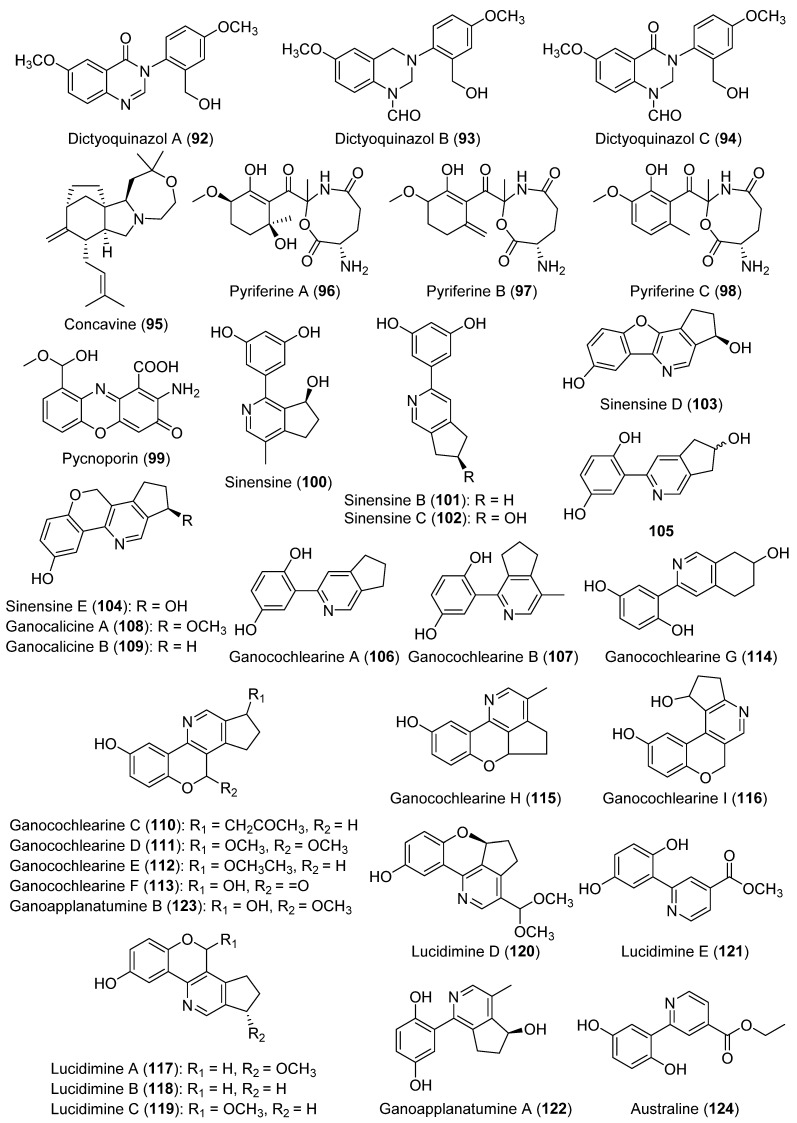
The family of dictyoquinazols was discovered in Dictyophora indusiata by Lee et al. (2002) [56]. Dictyoquinazols A–C (92–94, Figure 7) showed a neuroprotective potential against excitotoxicity in cultured mouse cortical neurons. They significantly protected the neurons from glutamate-induced neurotoxicity (at 5–10 µM) and from toxicity induced by N-methyl-D-aspartate (at 10–30 µM), although no antioxidant properties were found from the radical scavenging assays. Diverse synthetic strategies obtaining dictyoquinazols were later published [128,129,130]. In particular, the most recent one to synthesize dictyoquinazol A (92) [131] also allowed for the preparation of structural analogs of 92 with neuroprotective properties, which were used to carry out a SAR study whose conclusions will be reported in Section 3.
Figure 7.
The structures of the miscellaneous alkaloids 92–124.
Concavine (95, Figure 7), a new rearranged diterpene alkaloid, was isolated from Clitocybe concava, and showed a weak antibacterial activity against Bacillus cereus and Bacillus subtilis [57]. The total synthesis of compound 95 was accomplished in 16 steps and it achieved a 4.2% global yield [132], as well as the synthesis of diverse chlorinated analogs with an improved antibacterial activity [133].
Pyriferines A–C (96–98, Figure 7), characterized for containing a heterocyclic eight-membered ring, were isolated from the fruiting bodies of Pseudobaeospora pyrifera [58].
Pycnoporin (99, Figure 7), a new phenoxazone alkaloid, was isolated together with the already-known phenoxazones cinnabarin (also named polystictin), tramesanguin, and cinnabarinic acid from Pycnoporus cinnabarinus. Compound 99 showed a moderate antitumor activity [59].
The new alkaloid sinensine (100, Figure 7) was isolated from the fruiting bodies of Ganoderma sinense [60]. This compound was proven to be significantly active as a protecting agent against the injury induced by hydrogen peroxide oxidation on human umbilical cord endothelial cells (protective rate of 70.90% and EC50 = 6.2 mmol/L). Successively, sinensines B–E (101–104, Figure 7) were isolated from the same mushroom, although no studies on the bioactivity of these alkaloids were described [61]. Compounds 103 and 104 only differ in the number of carbon atoms of their oxygenated ring. More recently, sinensine E (104) was isolated together with the new alkaloid 105 (Figure 7) from Ganoderma luteomarginatum. Both compounds appeared to be a racemic mixture [62].
Several new alkaloids (106–124, Figure 7) were also achieved from the Ganoderma species and these findings will be detailed in the following paragraphs. Ganocochlearine A (106), the non-hydroxylated form of 105, was isolated together with ganocochlearine B (107) from Ganoderma cochlear [64]. Ganocochlearine A (106) was later isolated from Ganoderma australe, showing the protective activity of SH-SY5Y cells from glutamate-induced neural excitotoxicity and, consequently, its potential as a drug against neurodegenerative disorders [65]. Ganocochlearine A (106) was also later obtained from Ganoderma lucidum and exhibits remarkable neuroprotective (EC50 = 2.49 μM) and anti-inflammatory activities (IC50 = 4.68 μM) [66].
Two new alkaloids close to sinensine E (104), named ganocalicines A and B (108 and 109, Figure 7), were isolated from Ganoderma calidophilum [67]. Compounds 108 and 109, which are a methoxylated and non-hydroxylated form of sinensine E (104), respectively, were tested in anti-allergic assays. Alkaloid 108 showed its potential as a preventative or relieving drug against allergic symptoms: inhibitory effects on β-hexosaminidase activity (IC50 = 9.14 µM) and on the production of the allergic cytokine IL-4 and the lipid mediator LTB4 in antigen-stimulated RBL-2H3 cells (at 5–10 µM) [67].
Ganocochlearines C–I (110–116, Figure 7) are isolated from G. cochlear as racemic or scalemic mixtures [63].
Lucidimines A–D (117–120, Figure 7), four new alkaloids, were isolated from the fruiting bodies of G. lucidum [66,68], with lucidimine C (119) also being found in G. cochlear [63]. The total syntheses of lucidimines B (118) and C (119) was realized by Chen and Lan (2018) [69]. The antioxidant properties and relevant antiproliferative activity against MCF-7 cells (EC50 = 0.27) of compound 118 were also reported [69]. The poorer or null activities of compound 119 should be attributed to the presence of a methoxy group on the cyclopentene ring which 118 lacks. Lucidimine E (121, Figure 7) was successively isolated from the same mushroom and showed a significant anti-inflammatory activity [66].
Ganoapplanatumine A (122) and ganoapplanatumine B (123), the latter as a racemic mixture, were alkaloids obtained from Ganoderma applanatum [70]. Alkaloid 123 was also isolated from G. cochlear [63].
A new alkaloid, named australine (124, Figure 7), a disubstituted pyridine, and two new meroterpenoids, named australins A and B, were isolated together with five known compounds from the fruiting bodies of G. australe. The known compounds were identified as lingzhine C; ganocalicine B (109); and ganocochlearines A, C, and H (106, 110 and 115). Australine (124) and ganocochlearine A (106) and showed a significant protection ability against SH-SY5Y cells from glutamate-induced neural excitotoxicity at 10 µM [65]. Previously, a new tetrahydroxy pyrrolizidine alkaloid, named australine, was isolated from the seeds of Castanospermum australe and was shown to be a potent and specific inhibitor of amyloglucosidase [134]. However, the two alkaloids have a very different structure.
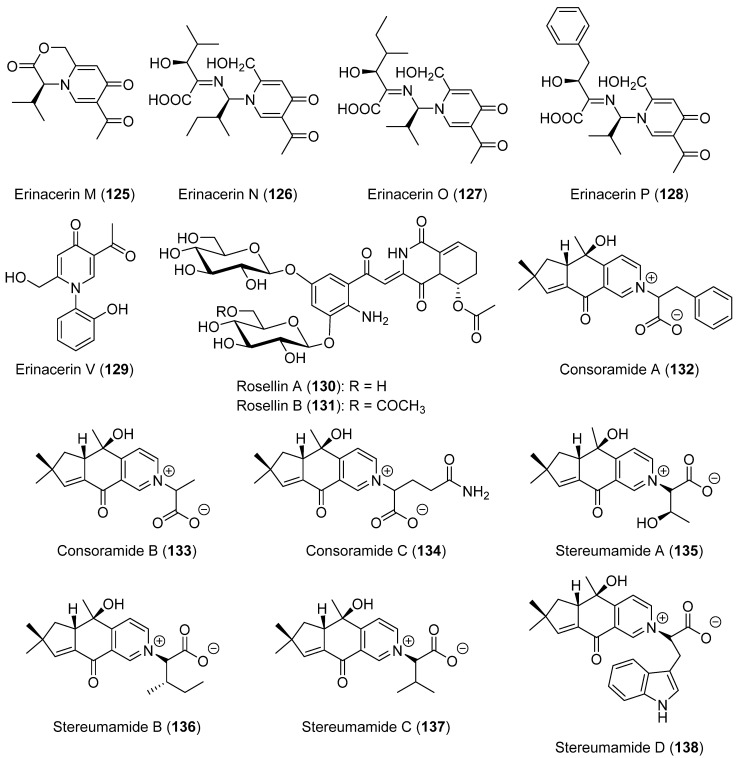
Erinacerins M–P (125–128, Figure 8) were isolated from the medicinal mushroom Hericium erinaceus [71]. They showed a moderate cytotoxic activity. Later, erinacerin V (129, Figure 8) was described as a new alkaloid purified from the mycelial culture of a unique North American edible Hericium mushroom [72].
Figure 8.
The structures of the miscellaneous alkaloids 125–138.
Rosellin A (130) and B (131) (Figure 8) were isolated as new glycosylated diketopiperazine alkaloids from the fruiting bodies of Mycena rosella, with 130 being obtained in a better yield [73]. Compound 130 showed a herbicidal activity, inducing strong bleaching of the leaves of Lepidium sativum [73].
Consoramides A–C (123–134, Figure 8) were isolated from Irpex consors as new zwitterionic alkaloids, together with different stereumamides, including stereumamide D (138) [74]. The closely related stereumamides A–D (135–138, Figure 8), which were the first example of a sesquiterpenes combined with α-amino acids to form quaternary ammonium hybrids, were previously isolated from Stereum hirsutum. Stereumamides A (135) and D (138) showed an antibacterial activity against Escherichia coli, Staphylococcus aureus, and Salmonella typhimurium, with minimum inhibitory concentration (MIC) values of 12.5–25.0 μg/mL [75].
3. Structure−Activity Relationship Studies
Throughout Section 2, more than 100 compounds discovered in 2002–2022 (Table 1) were described, as well as their bioactivity (if it has been evaluated). Section 3 focuses on the most relevant SAR results found assaying the activity of different interrelated compounds, or of the synthetic analogs of the reviewed alkaloids.
Regarding the β-carboline alkaloids, 16 analogs structurally related to harman alkaloids (Figure 2) were synthetized. These analogs presented diverse substituents at positions 1 or 9 (see 139, Figure 9), and it was found that both type of analogs had an improved broader spectrum of bactericidal activity. An improved activity was observed when the methyl or propyl groups were at C-1, whereas the benzyl group at position 9 could reduce it. On the other hand, all of the analogs showed an insecticidal activity, proving that the modifications applied did not generate a significant improvement in this context [135]. In a later study, a wide group of harman analogs were synthesized to improve the antibacterial activity of this alkaloid. This was achieved by diverse analogs, with 140 being the most active one, which also improved the activity of the positive control. Different SAR conclusions were obtained from this study. The methoxy group at C-6 (see 140, Figure 9) is beneficial for its antibacterial activity. Furthermore, it was concluded that the type of halogen substituents (CF3 > Br > Cl or CH3 > F or NO2), the position of the halogen atom (para > meta > ortho), and the kind of aromatic substituent R are significant for the antibacterial activity of the tested analogs [136].
Figure 9.
Related compounds and some synthetic structural analogs employed in SAR studies of the reviewed alkaloids 139–160.
Flazin (26, Figure 2) is a compound with a weak antiviral activity; thus, a wide collection of analogs to improve this activity were synthesized [137]. The results suggest that certain substituents at positions 3, 1′, and 5′ of flazin (see 141, Figure 9) might play a key role. The best result was obtained assaying flazinamide (141) (therapeutic index of 312.0, and EC50 = 0.38 µM). Therefore, the optimal combination is the one provided by the CONH2 group at C-3, an O-atom in position 1′, and the CH2OH group at C-5′.
SAR results were also obtained after testing the cytotoxicity of β-carboline alkaloids. Thus, the shift of the methoxy group of harmine (3, Figure 2) from C-7 to C-4 enhanced the cytotoxic activity; in addition, the substitution of C-1 is essential for achieving high activity levels [138]. Other authors have also reported the potential cholinesterase inhibitory activity of β-carbolines, and it was found that the quaternary ones are about one-sixth as potent as the reference alkaloid physostigmine [26]. A recent review on a wider overview on the bioactivities of β-carbolines and canthinones was recently published by Farouil et al. (2022) [86].
A complete report on SAR studies carried out on pyrrole compounds was published by Ahmad et al. (2018) [139]. As detailed in Section 2.3, this reviewed subgroup of compounds consists of derivatives of 5-(hydroxymethyl)-1H-pyrrole-2-carboxaldehyde (48, Figure 3). Compound 48 has moderate or low antifungal, antibacterial, and cytotoxic activities, as well as being inactive as an antioxidant or insecticidal compound [140]. It also showed moderate enzyme (α-glucosidase) inhibition [45]. On the other hand, some of the new pyrroles with different substituents on the nitrogen atom (49, 54, and 59–62, Figure 3) significantly improved this activity, especially compound 61. Thus, it could be pointed out that the higher substitution of this N atom favors the inhibition of the tested enzymes. Regarding the anti-proliferative activity against cancer cell lines, these substituted alkaloids did not show improved levels over 48 [45]. These results suggest that it cannot be generalized that the substitution on the N atom induces a general activity improvement, which is in accordance with the results observed for other pyrrole compounds reported in the literature [139].
Inotopyrroles (49 and 50, Figure 3) possess a remarkable neuroprotective activity, especially 50 [41]. On the other hand, alkaloids 54–58 (Figure 3) showed a lower activity. This result suggests that pyrroles bonded to another aromatic ring may improve this kind of pharmacological activity. It is worth highlighting that, in the case of 50, this aromatic ring is contained in an indole system. Indeed, pyrrolezanthine (61, Figure 3), the phenolic form of 49, has a strong inhibitory activity (IC50 = 28.65 µM) against mammalian α-glucosidase [45], as well as diverse anti-inflammatory effects, sometimes presenting different behaviors according to the concentration [47].
Regarding indole alkaloids, the antifungal activity of the new indoles described in Section 2.4 has not been tested. However, the indole moiety appeared essential for the antifungal activity, as reported for some of the analogs reviewed [141]. Thus, the study of the reviewed mushroom indoles and of new analogs in antifungal bioassays could be of interest. A wide overview of SAR conclusions obtained for indoles, covering many of the most relevant biological activities for the medical field, has been reported by Thanikachalam et al. (2019) [142]. A SAR study regarding the psychoactive activity of psilocybin (65, Figure 5) was carried out. In this study, 17 analogs containing different N,N-dialkyl substituents, and either a 4-hydroxy or 4-acetoxy group, were tested in in vivo bioassays. All of them were highly or moderately active, where bulkier N-alkyl groups and O-acetylation were found to affect the potency of the 5-HT receptors studied. It was also suggested that the O-acetylated compounds may be deacetylated in vivo, which make them act as prodrugs [143]. The SAR results reported by Sard et al. (2005) [144] found that the psilocybin analogs 1-methylpsilocybin (142) and 4-fluoro-N,N-dimethyltryptamine (143) (Figure 9) are potential efficient compounds for the treatment of obsessive compulsive disorders. Moreover, 1-methylpsilocin (144) would be of interest as it has been described as a selective agonist at the h5-HT2C receptor.
A SAR study was also performed on indoles 90 and 91 (Figure 6) related to the inhibitory activity of the sterol O-acyltransferase isozymes of terpendole compounds. Thus, it was concluded that the opening of the A-ring (see 91) had a negative effect, the presence of a hydroxyl group at the N-ring was not relevant, while the isoprenyl residue in the aromatic ring was not essential [127].
SAR conclusions were also described for the reviewed miscellaneous alkaloids. A group of analogs of dictyoquinazol A (92, Figure 7) were synthesized. Analogs 145–150 (Figure 9) equaled or improved the neuroprotective activities of dictyoquinazol A (92) against three injury stimuli (L-glutamate, H2O2, and staurosporine). The results showed that the methoxy groups linked to the benzene rings decreased the glutamate protection, but improved H2O2 protection; the modification of the heterocycle ring could improve H2O2 protection without compromising glutamate or staurosporine protection; and changing the hydroxyl group could improve glutamate protection, without compromising H2O2 or staurosporine protection [131].
Concavine (95, Figure 7) is an alkaloid with a weak antibacterial activity [57]. Diverse analogs were synthesized based on the incorporation of a chlorinated aromatic ring in its structure. This modification significantly improved its antibacterial activity [133]. Thus, 151 showed an antibiotic activity against B. subtilis, 152 and 153 against S. aureus, 154 against P. fluorescens, and 152 against E. coli (MIC = 6.25 µg/mL for all these cases) (Figure 9) [133]. The most active of the analogs reported was 155, followed by 156 (Figure 9), both characterized as being acyclic derivatives of concavine with MIC values always between 1.56–12.5 µg/mL for all of the bacterial species tested.
Cinnabarin (157) showed an antitumor activity with an IC50 value of 13 µM [59]. It should be noted that other phenoxazones, particularly Phx-1 and Phx-3 (159 and 160, Figure 9), are well-studied compounds for the development of anticancer drugs, as reviewed by Zorrilla et al. (2021) [3]. In the SAR study [59], pycnoporin (99, Figure 7) showed a moderate antitumor activity, whereas cinnabarinic acid (158, Figure 9) was not active. These compounds only differ in one substituent, allowing for concluding that the carboxyl group negatively affected the antitumor activity of this kind of phenoxazone, whereas the presence of the moiety -CH(OCH3)OH or -CH2OH at C-9 (see 158, Figure 9) could significantly improve this activity.
4. Conclusions
Here, the new alkaloids and related compounds produced by mushrooms since 2002 have been reviewed. Although mushrooms are a source that has not been studied as much as others in this context, it has been found that 114 new compounds with different structures (Table 1) have been isolated and identified. Different studies have shown the promising levels of bioactivity that many of them have, most of which are activities of pharmacological interest, such as antioxidant, anti-inflammatory, neuroprotective, antibacterial, and enzyme inhibition properties. This affords the opportunity to thoroughly explore these new compounds in future studies, in addition to the alkaloids that have been more studied, such as psilocin and its analogs. Furthermore, it is worth highlighting the low amount of references of studies on activities of agronomic interest, for example, aiming at exploring the phytotoxic potential of alkaloids produced by mushrooms. On the other hand, the development of new syntheses that allow for access to alkaloids in sufficient quantities for their study and to the improvement of their biological activity through structural modifications are also of high interest in this field.
For all of these reasons, mushrooms could be viewed as a source of potential active products, thereby potentially leading to further research on them.
Acknowledgments
J.G.Z. thanks the University of Cadiz for the postdoctoral support with the Margarita Salas fellowship (2021-067/PN/MS-RECUAL/CD), funded by the NextGenerationEU programme of the European Union. Antonio Evidente is associated to the Istituto di Chimica Biomolecolare, CNR, Pozzuoli, Italy.
Author Contributions
Conceptualization, J.G.Z. and A.E.; methodology, J.G.Z. and A.E.; software, J.G.Z.; validation, J.G.Z. and A.E.; formal analysis, J.G.Z.; investigation, J.G.Z.; resources, J.G.Z.; data curation, J.G.Z. and A.E.; writing—original draft preparation, J.G.Z. and A.E.; writing—review and editing, J.G.Z. and A.E.; visualization, J.G.Z. and A.E. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wieczorek P.P., Witkowska D., Jasicka-Misiak I., Poliwoda A., Oterman M., Zielińska K. Studies in Natural Products Chemistry. Volume 46. Elsevier; Amsterdam, The Netherlands: 2015. Bioactive alkaloids of hallucinogenic mushrooms; pp. 133–168. [Google Scholar]
- 2.Minkiewicz P., Iwaniak A., Darewicz M. BIOPEP-UWM database of bioactive peptides: Current opportunities. Int. J. Mol. Sci. 2019;20:5978. doi: 10.3390/ijms20235978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zorrilla J.G., Rial C., Cabrera D., Molinillo J.M.G., Varela R.M., Macías F.A. Pharmacological activities of aminophenoxazinones. Molecules. 2021;26:3453. doi: 10.3390/molecules26113453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pessione E., Cirrincione S. Bioactive molecules released in food by lactic acid bacteria: Encrypted peptides and biogenic amines. Front. Microbiol. 2016;7:876. doi: 10.3389/fmicb.2016.00876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen D.L., Wang B.W., Sun Z.C., Yang J.S., Xu X.D., Ma G.X. Natural nitrogenous sesquiterpenoids and their bioactivity: A review. Molecules. 2020;25:2485. doi: 10.3390/molecules25112485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wicks C., Hudlicky T., Rinner U. Alkaloids Chem. Biol. Vol. 86. 2021. Morphine alkaloids: History, biology, and synthesis; pp. 145–342. [DOI] [PubMed] [Google Scholar]
- 7.Barcelos R.P., Lima F.D., Carvalho N.R., Bresciani G., Royes L.F. Caffeine effects on systemic metabolism, oxidative-inflammatory pathways, and exercise performance. Nutr. Res. 2020;80:1–17. doi: 10.1016/j.nutres.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Prochaska J.J., Benowitz N.L. The past, present, and future of nicotine addiction therapy. Annu. Rev. Med. 2016;67:467–486. doi: 10.1146/annurev-med-111314-033712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kornienko A., Evidente A. Chemistry, biology, and medicinal potential of narciclasine and its congeners. Chem. Rev. 2008;108:1982–2014. doi: 10.1021/cr078198u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evidente A., Kireev A., Jenkins A., Romero A., Steelant W., Van Slambrouck S., Kornienko A. Biological evaluation of structurally diverse Amaryllidaceae alkaloids and their synthetic derivatives: Discovery of novel leads for anticancer drug design. Planta Med. 2009;75:501–507. doi: 10.1055/s-0029-1185340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schrader K.K., Avolio F., Andolfi A., Cimmino A., Evidente A. Ungeremine and its hemisynthesized analogues as bactericides against Flavobacterium columnare. J. Agric. Food Chem. 2013;61:1179–1183. doi: 10.1021/jf304586j. [DOI] [PubMed] [Google Scholar]
- 12.Schrader K.K., Andolfi A., Cantrell C.L., Cimmino A., Duke S.O., Osbrink W., Wedge D.E., Evidente A. A survey of phytotoxic microbial and plant metabolites as potential natural products for pest management. Chem. Biodivers. 2010;7:2261–2280. doi: 10.1002/cbdv.201000041. [DOI] [PubMed] [Google Scholar]
- 13.Van Goietsenoven G., Andolfi A., Lallemand B., Cimmino A., Lamoral-Theys D., Gras T., Abou-Donia A., Dubois J., Lefranc F., Mathieu V., et al. Amaryllidaceae alkaloids belonging to different structural subgroups display activity against apoptosis-resistant cancer cells. J. Nat. Prod. 2010;73:1223–1227. doi: 10.1021/np9008255. [DOI] [PubMed] [Google Scholar]
- 14.Masi M., Cala A., Tabanca N., Cimmino A., Green I.R., Bloomquist J.R., Van Otterlo W.A.L., Macias F.A., Evidente A. Alkaloids with activity against the Zika virus vector Aedes aegypti (L.)-crinsarnine and sarniensinol, two new crinine and mesembrine type alkaloids isolated from the South African plant Nerine sarniensis. Molecules. 2016;21:1432. doi: 10.3390/molecules21111432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masi M., Di Lecce R., Cimmino A., Evidente A. Advances in the chemical and biological characterization of Amaryllidaceae alkaloids and natural analogues isolated in the last decade. Molecules. 2020;25:5621. doi: 10.3390/molecules25235621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Govindaraju K., Ingels A., Hasan M.N., Sun D., Mathieu V., Masi M., Evidente A., Kornienko A. Synthetic analogues of the montanine-type alkaloids with activity against apoptosis-resistant cancer cells. Bioorg. Med. Chem. Lett. 2018;28:589–593. doi: 10.1016/j.bmcl.2018.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamoral-Theys D., Andolfi A., Van Goietsenoven G., Cimmino A., Le Calvé B., Wauthoz N., Mégalizzi V., Gras T., Bruyère C., Dubois J., et al. Lycorine, the main phenanthridine amaryllidaceae alkaloid, exhibits significant antitumor activity in cancer cells that display resistance to proapoptotic stimuli: An investigation of structure-activity relationship and mechanistic insight. J. Med. Chem. 2009;52:6244–6256. doi: 10.1021/jm901031h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evdokimov N.M., Lamoral-Theys D., Mathieu V., Andolfi A., Frolova L.V., Pelly S.C., van Otterlo W.A.L., Magedov I.V., Kiss R., Evidente A., et al. In search of a cytostatic agent derived from the alkaloid lycorine: Synthesis and growth inhibitory properties of lycorine derivatives. Bioorg. Med. Chem. 2011;19:7252–7261. doi: 10.1016/j.bmc.2011.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Goietsenoven G., Hutton J., Becker J., Lallemand B., Robert F., Lefranc F., Pirker C., Vandenbussche G., Van Antwerpen P., Evidente A., et al. Targeting of eEF1A with Amaryllidaceae isocarbostyrils as a strategy to combat melanomas. FASEB J. 2010;24:4575–4584. doi: 10.1096/fj.10-162263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Goietsenoven G., Mathieu V., Lefranc F., Kornienko A., Evidente A., Kiss R. Narciclasine as well as other Amaryllidaceae isocarbostyrils are promising GTP-ase targeting agents against brain cancers. Med. Res. Rev. 2013;33:439–455. doi: 10.1002/med.21253. [DOI] [PubMed] [Google Scholar]
- 21.Dinis-Oliveira R.J. Metabolism of psilocybin and psilocin: Clinical and forensic toxicological relevance. Drug Metab. Rev. 2017;49:84–91. doi: 10.1080/03602532.2016.1278228. [DOI] [PubMed] [Google Scholar]
- 22.Bower J.F., Riis-Johannessen T., Szeto P., Whitehead A.J., Gallagher T. Stereospecific construction of substituted piperidines. Synthesis of (-)-paroxetine and (+)-laccarin. Chem. Commun. 2007;2:728–730. doi: 10.1039/B617260A. [DOI] [PubMed] [Google Scholar]
- 23.Yuan H., Guo Z., Luo T. Synthesis of (+)-lysergol and its analogues to assess serotonin receptor activity. Org. Lett. 2017;19:624–627. doi: 10.1021/acs.orglett.6b03779. [DOI] [PubMed] [Google Scholar]
- 24.Moss G.P., Smith P.A.S., Tavernier D. Glossary of class names of organic compounds and reactivity intermediates based on structure (IUPAC Recommendations 1995) Pure Appl. Chem. 1995;67:1307–1375. doi: 10.1351/pac199567081307. [DOI] [Google Scholar]
- 25.Bröckelmann M.G., Dasenbrock J., Steffan B., Steglich W., Wang Y., Raabe G., Fleischhauer J. An unusual series of thiomethylated canthin-6-ones from the North American mushroom Boletus curtisii. Eur. J. Org. Chem. 2004;2004:4856–4863. doi: 10.1002/ejoc.200400519. [DOI] [Google Scholar]
- 26.Teichert A., Schmidt J., Porzel A., Arnold N., Wessjohann L. Brunneins A–C, β-carboline alkaloids from Cortinarius brunneus. J. Nat. Prod. 2007;70:1529–1531. doi: 10.1021/np070259w. [DOI] [PubMed] [Google Scholar]
- 27.Teichert A., Lübken T., Schmidt J., Kuhnt C., Huth M., Porzel A., Wessjohann L., Arnold N. Determination of β-carboline alkaloids in fruiting bodies of Hygrophorus spp. by liquid chromatography/electrospray ionisation tandem mass spectrometry. Phytochem. Anal. 2008;19:335–341. doi: 10.1002/pca.1057. [DOI] [PubMed] [Google Scholar]
- 28.Krüzselyi D., Vetter J., Ott P.G., Darcsi A., Béni S., Gömöry Á., Drahos L., Zsila F., Móricz Á.M. Isolation and structural elucidation of a novel brunnein-type antioxidant β-carboline alkaloid from Cyclocybe cylindracea. Fitoterapia. 2019;137:104180. doi: 10.1016/j.fitote.2019.104180. [DOI] [PubMed] [Google Scholar]
- 29.Geissler T., Brandt W., Porzel A., Schlenzig D., Kehlen A., Wessjohann L., Arnold N. Acetylcholinesterase inhibitors from the toadstool Cortinarius infractus. Bioorg. Med. Chem. 2010;18:2173–2177. doi: 10.1016/j.bmc.2010.01.074. [DOI] [PubMed] [Google Scholar]
- 30.Jaeger R.J.R., Lamshöft M., Gottfried S., Spiteller M., Spiteller P. HR-MALDI-MS imaging assisted screening of β-carboline alkaloids discovered from Mycena metata. J. Nat. Prod. 2013;76:127–134. doi: 10.1021/np300455a. [DOI] [PubMed] [Google Scholar]
- 31.Naveen B., Mudiraj A., Khamushavalli G., Babu P.P., Nagarajan R. Concise total synthesis of water soluble metatacarboline A, C, D, e and F and its anticancer activity. Eur. J. Med. Chem. 2016;113:167–178. doi: 10.1016/j.ejmech.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 32.Yao L., Lv J.-H., Pan M.-C., Xing L., Wang L.-P., Li C.-T., Liu S.-Y., Li Y. Two new compounds from edible mushroom Sarcomyxa edulis. Nat. Prod. Res. 2022:1–7. doi: 10.1080/14786419.2021.2023146. online ahead of print . [DOI] [PubMed] [Google Scholar]
- 33.Peters S., Spiteller P. Mycenarubins A and B, red pyrroloquinoline alkaloids from the mushroom Mycena rosea. Eur. J. Org. Chem. 2007;2007:1571–1576. doi: 10.1002/ejoc.200600826. [DOI] [Google Scholar]
- 34.Lohmann J.S., Wagner S., von Nussbaum M., Pulte A., Steglich W., Spiteller P. Mycenaflavin A, B, C, and D: Pyrroloquinoline alkaloids from the fruiting bodies of the mushroom Mycena haematopus. Chem. A Eur. J. 2018;24:8609–8614. doi: 10.1002/chem.201800235. [DOI] [PubMed] [Google Scholar]
- 35.Pulte A., Wagner S., Kogler H., Spiteller P. Pelianthinarubins A and B, red pyrroloquinoline alkaloids from the fruiting bodies of the mushroom Mycena pelianthina. J. Nat. Prod. 2016;79:873–878. doi: 10.1021/acs.jnatprod.5b00942. [DOI] [PubMed] [Google Scholar]
- 36.Peters S., Jaeger R.J.R., Spiteller P. Red pyrroloquinoline alkaloids from the mushroom Mycena haematopus. Eur. J. Org. Chem. 2008;2008:319–323. doi: 10.1002/ejoc.200700739. [DOI] [Google Scholar]
- 37.Himstedt R., Wagner S., Jaeger R.J.R., Lieunang Watat M., Backenköhler J., Rupcic Z., Stadler M., Spiteller P. Formaldehyde as a chemical defence agent of fruiting bodies of Mycena rosea and its role in the generation of the alkaloid mycenarubin C. ChemBioChem. 2020;21:1613–1620. doi: 10.1002/cbic.201900733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peters S., Spiteller P. Sanguinones A and B, blue pyrroloquinoline alkaloids from the fruiting bodies of the mushroom Mycena sanguinolenta. J. Nat. Prod. 2007;70:1274–1277. doi: 10.1021/np070179s. [DOI] [PubMed] [Google Scholar]
- 39.Backenköhler J., Reck B., Plaumann M., Spiteller P. Total Synthesis of mycenarubin A, sanguinolentaquinone and mycenaflavin B and their cytotoxic activities. Eur. J. Org. Chem. 2018;2018:2806–2816. doi: 10.1002/ejoc.201800417. [DOI] [Google Scholar]
- 40.Shan W.-G., Wang Y., Ma L.-F., Zhan Z.-J. A new pyrrole alkaloid from the mycelium of Inonotus obliquus. J. Chem. Res. 2017;41:392–393. doi: 10.3184/174751917X14967701766941. [DOI] [Google Scholar]
- 41.Sun Z., Hu M., Sun Z., Zhu N., Yang J., Ma G., Xu X. Pyrrole alkaloids from the edible mushroom Phlebopus portentosus with their bioactive activities. Molecules. 2018;23:1198. doi: 10.3390/molecules23051198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang N.-N., Huang S.-Z., Ma Q.-Y., Dai H.-F., Guo Z.-K., Yu Z.-F., Zhao Y.-X. A new pyrrole alkaloid from Leccinum extremiorientale. Chem. Nat. Compd. 2015;51:730–732. doi: 10.1007/s10600-015-1394-5. [DOI] [Google Scholar]
- 43.Sakamoto T., Nishida A., Wada N., Nakamura Y., Sato S., Konishi T., Matsugo S. Identification of a novel pyrrole alkaloid from the edible mushroom Basidiomycetes-X (Echigoshirayukidake) Molecules. 2020;25:4879. doi: 10.3390/molecules25214879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chin Y.-W., Lim S.W., Kim S.-H., Shin D.-Y., Suh Y.-G., Kim Y.-B., Kim Y.C., Kim J. Hepatoprotective pyrrole derivatives of Lycium chinense fruits. Bioorganic Med. Chem. Lett. 2003;13:79–81. doi: 10.1016/S0960-894X(02)00846-6. [DOI] [PubMed] [Google Scholar]
- 45.Chen S., Yong T., Xiao C., Su J., Zhang Y., Jiao C., Xie Y. Pyrrole alkaloids and ergosterols from Grifola frondosa exert anti-α-glucosidase and anti-proliferative activities. J. Funct. Foods. 2018;43:196–205. doi: 10.1016/j.jff.2018.02.007. [DOI] [Google Scholar]
- 46.Kim S.B., Chang B.Y., Hwang B.Y., Kim S.Y., Lee M.K. Pyrrole alkaloids from the fruits of Morus alba. Bioorg. Med. Chem. Lett. 2014;24:5656–5659. doi: 10.1016/j.bmcl.2014.10.073. [DOI] [PubMed] [Google Scholar]
- 47.Guan P., Wang X., Jiang Y., Dou N., Qu X., Liu J., Lin B., Han L., Huang X., Jiang C. The anti-inflammatory effects of jiangrines from Jiangella alba through inhibition of p38 and NF-κB signaling pathways. Bioorg. Chem. 2020;95:103507. doi: 10.1016/j.bioorg.2019.103507. [DOI] [PubMed] [Google Scholar]
- 48.Fons F., Rapior S., Fruchier A., Saviuc P., Bessière J.-M. Volatile composition of Clitocybe amoenolens, Tricholoma caligatum and Hebeloma radicosum. Cryptogam. Mycol. 2006;27:45–55. [Google Scholar]
- 49.Teichert A., Schmidt J., Porzel A., Arnold N., Wessjohann L. N-Glucosyl-1H-indole derivatives from Cortinarius brunneus (Basidiomycetes) Chem. Biodivers. 2008;5:664–669. doi: 10.1002/cbdv.200890062. [DOI] [PubMed] [Google Scholar]
- 50.Kim K.H., Park K.M., Choi S.U., Lee K.R. Macrolepiotin, a new indole alkaloid from Macrolepiota neomastoidea. J. Antibiot. 2009;62:335–338. doi: 10.1038/ja.2009.30. [DOI] [PubMed] [Google Scholar]
- 51.Samchai S., Seephonkai P., Kaewtong C. Two indole derivatives and phenolic compound isolated from mushroom Phellinus linteus. Chin. J. Nat. Med. 2011;9:173–175. doi: 10.3724/SP.J.1009.2011.00173. [DOI] [Google Scholar]
- 52.Arpha K., Phosri C., Suwannasai N., Mongkolthanaruk W., Sodngam S. Astraodoric acids A–D: New lanostane triterpenes from edible mushroom Astraeus odoratus and their anti-Mycobacterium tuberculosis H37Ra and cytotoxic activity. J. Agric. Food Chem. 2012;60:9834–9841. doi: 10.1021/jf302433r. [DOI] [PubMed] [Google Scholar]
- 53.Qiu W., Kobori H., Suzuki T., Choi J.-H., Deo V.K., Hirai H., Kawagishi H. A new compound from the mushroom Tricholoma flavovirens. Biosci. Biotechnol. Biochem. 2014;78:755–757. doi: 10.1080/09168451.2014.905174. [DOI] [PubMed] [Google Scholar]
- 54.Wittstein K., Rascher M., Rupcic Z., Löwen E., Winter B., Köster R.W., Stadler M. Corallocins A–C, nerve growth and brain-derived neurotrophic factor inducing metabolites from the mushroom Hericium coralloides. J. Nat. Prod. 2016;79:2264–2269. doi: 10.1021/acs.jnatprod.6b00371. [DOI] [PubMed] [Google Scholar]
- 55.Zhu L., Han X., Cui X., Li S., Qiao L., Chen R. Two new alkaloids from Pleurotus ostreatus (Jacq.: Pers.) Roll. Rec. Nat. Prod. 2020;14:319–325. doi: 10.25135/rnp.166.19.11.1480. [DOI] [Google Scholar]
- 56.Lee I.-K., Yun B.-S., Han G., Cho D.-H., Kim Y.-H., Yoo I.-D. Dictyoquinazols A, B, and C, new neuroprotective compounds from the mushroom Dictyophora indusiata. J. Nat. Prod. 2002;65:1769–1772. doi: 10.1021/np020163w. [DOI] [PubMed] [Google Scholar]
- 57.Arnone A., Bava A., Fronza G., Nasini G., Ragg E. Concavine, an unusual diterpenic alkaloid produced by the fungus Clitocybe concava. Tetrahedron Lett. 2005;46:8037–8039. doi: 10.1016/j.tetlet.2005.09.064. [DOI] [Google Scholar]
- 58.Quang D.N., Spiteller P., Porzel A., Schmidt J., Geissler T., Arnold N., Wessjohann L. Alkaloids from the mushroom Pseudobaeospora pyrifera, pyriferines A−C. J. Nat. Prod. 2008;71:1620–1622. doi: 10.1021/np800365f. [DOI] [PubMed] [Google Scholar]
- 59.Dias D.A., Urban S. HPLC and NMR studies of phenoxazone alkaloids from Pycnoporus Cinnabarinus. Nat. Prod. Commun. 2009;4:1934578X0900400. doi: 10.1177/1934578X0900400409. [DOI] [PubMed] [Google Scholar]
- 60.Liu C., Zhao F., Chen R. A novel alkaloid from the fruiting bodies of Ganoderma sinense Zhao, Xu et Zhang. Chinese Chem. Lett. 2010;21:197–199. doi: 10.1016/j.cclet.2009.07.023. [DOI] [Google Scholar]
- 61.Liu J.-Q., Wang C.-F., Peng X.-R., Qiu M.-H. New alkaloids from the fruiting bodies of Ganoderma sinense. Nat. Products Bioprospect. 2011;1:93–96. doi: 10.1007/s13659-011-0026-4. [DOI] [Google Scholar]
- 62.Li X.-C., Liu F., Su H.-G., Guo L., Zhou Q.-M., Huang Y.-J., Peng C., Xiong L. Two pairs of alkaloid enantiomers from Ganoderma luteomarginatum. Biochem. Syst. Ecol. 2019;86:103930. doi: 10.1016/j.bse.2019.103930. [DOI] [Google Scholar]
- 63.Wang X.-L., Dou M., Luo Q., Cheng L.-Z., Yan Y.-M., Li R.-T., Cheng Y.-X. Racemic alkaloids from the fungus Ganoderma cochlear. Fitoterapia. 2017;116:93–98. doi: 10.1016/j.fitote.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 64.Tian L., Wang X., Wang Y., Cheng Y. Two new alkaloids from Ganoderma cochlear. Natiral Prod. Res. Dev. 2015;27:1325–1328. doi: 10.16333/j.1001-6880.2015.08.001. [DOI] [Google Scholar]
- 65.Zhang J.-J., Dong Y., Qin F.-Y., Yan Y.-M., Cheng Y.-X. Meroterpenoids and alkaloids from Ganoderma australe. Nat. Prod. Res. 2019;35:3226–3232. doi: 10.1080/14786419.2019.1693565. [DOI] [PubMed] [Google Scholar]
- 66.Lu S.-Y., Peng X.-R., Dong J.-R., Yan H., Kong Q.-H., Shi Q.-Q., Li D.-S., Zhou L., Li Z.-R., Qiu M.-H. Aromatic constituents from Ganoderma lucidum and their neuroprotective and anti-inflammatory activities. Fitoterapia. 2019;134:58–64. doi: 10.1016/j.fitote.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 67.Huang S.Z., Cheng B.H., Ma Q.Y., Wang Q., Kong F.D., Dai H.F., Qiu S.Q., Zheng P.Y., Liu Z.Q., Zhao Y.-X. Anti-allergic prenylated hydroquinones and alkaloids from the fruiting body of Ganoderma calidophilum. RSC Adv. 2016;6:21139–21147. doi: 10.1039/C6RA01466F. [DOI] [Google Scholar]
- 68.Zhao Z.-Z., Chen H.-P., Feng T., Li Z.-H., Dong Z.-J., Liu J.-K. Lucidimine A-D, four new alkaloids from the fruiting bodies of Ganoderma lucidum. J. Asian Nat. Prod. Res. 2015;17:1160–1165. doi: 10.1080/10286020.2015.1119128. [DOI] [PubMed] [Google Scholar]
- 69.Chen Y., Lan P. Total syntheses and biological evaluation of the Ganoderma lucidum alkaloids lucidimines B and C. ACS Omega. 2018;3:3471–3481. doi: 10.1021/acsomega.8b00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luo Q., Yang X.-H., Yang Z.-L., Tu Z.-C., Cheng Y.-X. Miscellaneous meroterpenoids from Ganoderma applanatum. Tetrahedron. 2016;72:4564–4574. doi: 10.1016/j.tet.2016.06.019. [DOI] [Google Scholar]
- 71.Wang K., Bao L., Ma K., Liu N., Huang Y., Ren J., Wang W., Liu H. Eight new alkaloids with PTP1B and α-glucosidase inhibitory activities from the medicinal mushroom Hericium erinaceus. Tetrahedron. 2015;71:9557–9563. doi: 10.1016/j.tet.2015.10.068. [DOI] [Google Scholar]
- 72.Song X., Gaascht F., Schmidt-Dannert C., Salomon C.E. Discovery of antifungal and biofilm preventative compounds from mycelial cultures of a unique North American Hericium sp. fungus. Molecules. 2020;25:963. doi: 10.3390/molecules25040963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lohmann J.S., von Nussbaum M., Brandt W., Mülbradt J., Steglich W., Spiteller P. Rosellin A and B, two red diketopiperazine alkaloids from the mushroom Mycena rosella. Tetrahedron. 2018;74:5113–5118. doi: 10.1016/j.tet.2018.06.049. [DOI] [Google Scholar]
- 74.Kim J.-Y., Ki D.-W., Lee Y.-J., Ha L.S., Woo E.-E., Lee I.-K., Yun B.-S. Consoramides A–C, new zwitterionic alkaloids from the fungus Irpex consors. Mycobiology. 2021;49:434–437. doi: 10.1080/12298093.2021.1924926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Duan Y.-C., Feng J., Bai N., Li G.-H., Zhang K.-Q., Zhao P.-J. Four novel antibacterial sesquiterpene-α-amino acid quaternary ammonium hybrids from the mycelium of mushroom Stereum hirsutum. Fitoterapia. 2018;128:213–217. doi: 10.1016/j.fitote.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 76.Yang Y.J., Lee J.J., Jin C.M., Lim S.C., Lee M.K. Effects of harman and norharman on dopamine biosynthesis and L-DOPA-induced cytotoxicity in PC12 cells. Eur. J. Pharmacol. 2008;587:57–64. doi: 10.1016/j.ejphar.2008.03.050. [DOI] [PubMed] [Google Scholar]
- 77.Zheng L., Yan X., Han X., Chen H., Lin W., Lee F.S.C., Wang X. Identification of norharman as the cytotoxic compound produced by the sponge (Hymeniacidon perleve)-associated marine bacterium Pseudoalteromonas piscicida and its apoptotic effect on cancer cells. Biotechnol. Appl. Biochem. 2006;44:135. doi: 10.1042/BA20050176. [DOI] [PubMed] [Google Scholar]
- 78.Pfau W., Skog K. Exposure to β-carbolines norharman and harman. J. Chromatogr. B. 2004;802:115–126. doi: 10.1016/j.jchromb.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 79.Tsuchiya H., Hayashi H., Sato M., Shimizu H., Iinuma M. Quantitative analysis of all types of β-carboline alkaloids in medicinal plants and dried edible plants by high performance liquid chromatography with selective fluorometric detection. Phytochem. Anal. 1999;10:247–253. doi: 10.1002/(SICI)1099-1565(199909/10)10:5<247::AID-PCA465>3.0.CO;2-9. [DOI] [Google Scholar]
- 80.Zhang C.-X., Xi J., Zhao T.-P., Ma Y.-X., Wang X.-D. β-carbolines norharman and harman in vegetable oils in China. Food Addit. Contam. Part B. 2020;13:193–199. doi: 10.1080/19393210.2020.1759701. [DOI] [PubMed] [Google Scholar]
- 81.Kodani S., Imoto A., Mitsutani A., Murakami M. Isolation and identification of the antialgal compound from algicidal bacterium Pseudomonas sp. K44-1. J. Appl. Phycol. 2002;14:109–114. doi: 10.1023/A:1019533414018. [DOI] [Google Scholar]
- 82.Wrońska A.K., Boguś M.I. Harman and norharman, metabolites of the entomopathogenic fungus Conidiobolus coronatus (Entomophthorales), affect the serotonin levels and phagocytic activity of hemocytes, insect immunocompetent cells, in Galleria mellonella (Lepidoptera) Cell Biosci. 2019;9:29. doi: 10.1186/s13578-019-0291-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blei F., Dörner S., Fricke J., Baldeweg F., Trottmann F., Komor A., Meyer F., Hertweck C., Hoffmeister D. Simultaneous production of psilocybin and a cocktail of β-carboline monoamine oxidase inhibitors in “Magic” mushrooms. Chem. A Eur. J. 2020;26:729–734. doi: 10.1002/chem.201904363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khan F.A., Maalik A., Iqbal Z., Malik I. Recent pharmacological developments in β-carboline alkaloid “harmaline”. Eur. J. Pharmacol. 2013;721:391–394. doi: 10.1016/j.ejphar.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 85.Benzekri R., Bouslama L., Papetti A., Hammami M., Smaoui A., Limam F. Anti HSV-2 activity of Peganum harmala (L.) and isolation of the active compound. Microb. Pathog. 2018;114:291–298. doi: 10.1016/j.micpath.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 86.Farouil L., Sylvestre M., Fournet A., Cebrián-Torrejón G. Review on canthin-6-one alkaloids: Distribution, chemical aspects and biological activities. Eur. J. Med. Chem. Rep. 2022;5:100049. doi: 10.1016/j.ejmcr.2022.100049. [DOI] [Google Scholar]
- 87.Omosa L.K., Mbogo G.M., Korir E., Omole R., Seo E.-J., Yenesew A., Heydenreich M., Midiwo J.O., Efferth T. Cytotoxicity of fagaramide derivative and canthin-6-one from Zanthoxylum (Rutaceae) species against multidrug resistant leukemia cells. Nat. Prod. Res. 2021;35:579–586. doi: 10.1080/14786419.2019.1587424. [DOI] [PubMed] [Google Scholar]
- 88.Brondz I., Ekeberg D., Høiland K., Bell D.S., Annino A.R. The real nature of the indole alkaloids in Cortinarius infractus: Evaluation of artifact formation through solvent extraction method development. J. Chromatogr. A. 2007;1148:1–7. doi: 10.1016/j.chroma.2007.02.074. [DOI] [PubMed] [Google Scholar]
- 89.Wang N., Zhang J., Li Q., Xu H., Chen G., Li Z., Liu D., Yang X. Discovery of potent indoleamine 2,3-dioxygenase (IDO) inhibitor from alkaloids in Picrasma quassioides by virtual screening and in vitro evaluation. Fitoterapia. 2019;133:137–145. doi: 10.1016/j.fitote.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 90.Lumonadio L., Vanhaelen M. Indole alkaloids and quassin from Quassia africana. J. Nat. Prod. 1986;49:940. doi: 10.1021/np50047a037. [DOI] [Google Scholar]
- 91.Lai Z.-Q., Liu W.-H., Ip S.-P., Liao H.-J., Yi Y.-Y., Qin Z., Lai X.-P., Su Z.-R., Lin Z.-X. Seven alkaloids from Picrasma quassioides and their cytotoxic activities. Chem. Nat. Compd. 2014;50:884–888. doi: 10.1007/s10600-014-1106-6. [DOI] [Google Scholar]
- 92.Katchborian-Neto A., Santos W.T., Nicácio K.J., Corrêa J.O.A., Murgu M., Martins T.M.M., Gomes D.A., Goes A.M., Soares M.G., Dias D.F., et al. Neuroprotective potential of Ayahuasca and untargeted metabolomics analyses: Applicability to Parkinson’s disease. J. Ethnopharmacol. 2020;255:112743. doi: 10.1016/j.jep.2020.112743. [DOI] [PubMed] [Google Scholar]
- 93.Lee Y.-J., Hwang B.-S., Song J.-G., Kim D.-W., Woo E.-E., Lee I.-K., Yun B.-S. An indole alkaloid from the fruiting body of Boletus umbriniporus. Korean J. Mycol. 2015;43:68–70. doi: 10.4489/KJM.2015.43.1.68. [DOI] [Google Scholar]
- 94.Perry N.B., Blunt J.W., McCombs J.D., Munro M.H.G. Discorhabdin C, a highly cytotoxic pigment from a sponge of the genus Latrunculia. J. Org. Chem. 1986;51:5476–5478. doi: 10.1021/jo00376a096. [DOI] [Google Scholar]
- 95.Miyanaga A., Janso J.E., McDonald L., He M., Liu H., Barbieri L., Eustáquio A.S., Fielding E.N., Carter G.T., Jensen P.R., et al. Discovery and assembly-line biosynthesis of the lymphostin pyrroloquinoline alkaloid family of mTOR inhibitors in Salinispora bacteria. J. Am. Chem. Soc. 2011;133:13311–13313. doi: 10.1021/ja205655w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seipp K., Geske L., Opatz T. Marine pyrrole alkaloids. Mar. Drugs. 2021;19:514. doi: 10.3390/md19090514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gholap S.S. Pyrrole: An emerging scaffold for construction of valuable therapeutic agents. Eur. J. Med. Chem. 2016;110:13–31. doi: 10.1016/j.ejmech.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 98.Sterner O. The isolation and structure determination of sciodole, a new indole derivative from the fruit bodies of Tricholoma sciodes. Nat. Prod. Lett. 1994;4:9–14. doi: 10.1080/10575639408043885. [DOI] [Google Scholar]
- 99.Dewick P.M. Medicinal Natural Products: A Biosynthetic Approach. John Wiley & Sons; Hoboken, NJ, USA: 2009. Alkaloids; pp. 311–420. [Google Scholar]
- 100.Zhang L.-Y., Bai H.-B., Shan W.-G., Zhan Z.-J. A new Alkaloid from the mycelium of Inonotus obliquus. J. Chem. Res. 2014;38:245–246. doi: 10.3184/174751914X13944574433383. [DOI] [Google Scholar]
- 101.Yu J.G., Chen R.Y., Yao Z.X., Zhai Y.F., Yang S.L., Ma J.L. Studies on constituents of Ganoderma capense IV. The chemical structures of ganoine, ganodine and ganoderpurine. Acta Pharm. Sin. 1990;25:612–616. [PubMed] [Google Scholar]
- 102.Chadha N., Silakari O. Indoles as therapeutics of interest in medicinal chemistry: Bird’s eye view. Eur. J. Med. Chem. 2017;134:159–184. doi: 10.1016/j.ejmech.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 103.Homer J.A., Sperry J. Mushroom-derived indole alkaloids. J. Nat. Prod. 2017;80:2178–2187. doi: 10.1021/acs.jnatprod.7b00390. [DOI] [PubMed] [Google Scholar]
- 104.Hofmann A., Heim R., Brack A., Kobel H., Frey A., Ott H., Petrzilka T., Troxler F. Psilocybin und psilocin, zwei psychotrope wirkstoffe aus Mexikanischen rauschpilzen. Helv. Chim. Acta. 1959;42:1557–1572. doi: 10.1002/hlca.19590420518. [DOI] [Google Scholar]
- 105.Nichols D.E. Psilocybin: From ancient magic to modern medicine. J. Antibiot. 2020;73:679–686. doi: 10.1038/s41429-020-0311-8. [DOI] [PubMed] [Google Scholar]
- 106.Geiger H.A., Wurst M.G., Daniels R.N. DARK classics in chemical neuroscience: Psilocybin. ACS Chem. Neurosci. 2018;9:2438–2447. doi: 10.1021/acschemneuro.8b00186. [DOI] [PubMed] [Google Scholar]
- 107.Khan F.I., Hassan F., Lai D. In silico studies on psilocybin drug derivatives against SARS-CoV-2 and cytokine storm of human interleukin-6 receptor. Front. Immunol. 2022;12:794780. doi: 10.3389/fimmu.2021.794780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sherwood A.M., Halberstadt A.L., Klein A.K., McCorvy J.D., Kaylo K.W., Kargbo R.B., Meisenheimer P. Synthesis and biological evaluation of tryptamines found in hallucinogenic mushrooms: Norbaeocystin, baeocystin, norpsilocin, and aeruginascin. J. Nat. Prod. 2020;83:461–467. doi: 10.1021/acs.jnatprod.9b01061. [DOI] [PubMed] [Google Scholar]
- 109.Barboza C.M., Pimenta D.C., Vigerelli H., de Cássia Rodrigues da Silva A., Garcia J.G., Zamudio R.M., Castilho J.G., Montanha J.A., Roehe P.M., de Carvalho Ruthner Batista H.B. In vitro effects of bufotenine against RNA and DNA viruses. Brazilian J. Microbiol. 2021;52:2475–2482. doi: 10.1007/s42770-021-00612-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lenz C., Wick J., Hoffmeister D. Identification of ω-N-methyl-4-hydroxytryptamine (norpsilocin) as a Psilocybe natural product. J. Nat. Prod. 2017;80:2835–2838. doi: 10.1021/acs.jnatprod.7b00407. [DOI] [PubMed] [Google Scholar]
- 111.Popik P., Hogendorf A., Bugno R., Khoo S.Y.-S., Zajdel P., Malikowska-Racia N., Nikiforuk A., Golebiowska J. Effects of ketamine optical isomers, psilocybin, psilocin and norpsilocin on time estimation and cognition in rats. Psychopharmacology. 2022;239:1689–1703. doi: 10.1007/s00213-021-06020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li Z., Li X., Cui H., Zhao G., Zhai D., Chen J. Vegetative and fecundity fitness benefit found in a glyphosate-resistant Eleusine indica population caused by 5-enolpyruvylshikimate-3-phosphate synthase overexpression. Front. Plant Sci. 2021;12:776990. doi: 10.3389/fpls.2021.776990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wood W.F., Smith J., Wayman K., Largent D.L. Indole and 3-chloroindole: The source of the disagreeable odor of Hygrophorus paupertinus. Mycologia. 2003;95:807–808. doi: 10.1080/15572536.2004.11833039. [DOI] [PubMed] [Google Scholar]
- 114.Homer J.A., Sperry J. Synthesis of three Tricholoma-derived indoles via an ortho-quinone methide. Arkivoc. 2018;4:6–12. doi: 10.24820/ark.5550190.p010.475. [DOI] [Google Scholar]
- 115.Barros-Filho B.A., de Oliveira M.C.F., Mafezoli J., Barbosa F.G., Rodrigues-Filho E. Secondary metabolite production by the basidiomycete, Lentinus strigellus, under different culture conditions. Nat. Prod. Commun. 2012;7:1934578X1200700. doi: 10.1177/1934578X1200700620. [DOI] [PubMed] [Google Scholar]
- 116.Slack G.J., Puniani E., Frisvad J.C., Samson R.A., Miller J.D. Secondary metabolites from Eurotium species, Aspergillus calidoustus and A. insuetus common in Canadian homes with a review of their chemistry and biological activities. Mycol. Res. 2009;113:480–490. doi: 10.1016/j.mycres.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 117.Wohlgemuth V., Kindinger F., Xie X., Wang B.-G., Li S.-M. Two prenyltransferases govern a consecutive prenylation cascade in the biosynthesis of echinulin and neoechinulin. Org. Lett. 2017;19:5928–5931. doi: 10.1021/acs.orglett.7b02926. [DOI] [PubMed] [Google Scholar]
- 118.Nies J., Li S.-M. Prenylation and dehydrogenation of a C2-reversely prenylated diketopiperazine as a branching point in the biosynthesis of echinulin family alkaloids in Aspergillus ruber. ACS Chem. Biol. 2021;16:185–192. doi: 10.1021/acschembio.0c00874. [DOI] [PubMed] [Google Scholar]
- 119.Gatenby W.A., Munday-Finch S.C., Wilkins A.L., Miles C.O. Terpendole M, a novel indole−diterpenoid isolated from Lolium perenne infected with the endophytic fungus Neotyphodium lolii. J. Agric. Food Chem. 1999;47:1092–1097. doi: 10.1021/jf980767z. [DOI] [PubMed] [Google Scholar]
- 120.Nakazawa J., Yajima J., Usui T., Ueki M., Takatsuki A., Imoto M., Toyoshima Y.Y., Osada H. A novel action of terpendole E on the motor activity of mitotic kinesin Eg5. Chem. Biol. 2003;10:131–137. doi: 10.1016/S1074-5521(03)00020-6. [DOI] [PubMed] [Google Scholar]
- 121.Uchida R., Kim Y.-P., Namatame I., Tomoda H., Ōmura S. Sespendole, a new inhibitor of lipid droplet synthesis in macrophages, produced by Pseudobotrytis terrestris FKA-25. J. Antibiot. 2006;59:93–97. doi: 10.1038/ja.2006.13. [DOI] [PubMed] [Google Scholar]
- 122.Tarui Y., Chinen T., Nagumo Y., Motoyama T., Hayashi T., Hirota H., Muroi M., Ishii Y., Kondo H., Osada H., et al. Terpendole E and its derivative inhibit STLC- and GSK-1-Resistant Eg5. ChemBioChem. 2014;15:934–938. doi: 10.1002/cbic.201300808. [DOI] [PubMed] [Google Scholar]
- 123.Nagumo Y., Motoyama T., Hayashi T., Hirota H., Aono H., Kawatani M., Osada H., Usui T. Structure-activity relationships of terpendole E and its natural derivatives. ChemistrySelect. 2017;2:1533–1536. doi: 10.1002/slct.201602015. [DOI] [Google Scholar]
- 124.Zhao J.-C., Luan Z.-L., Liang J.-H., Cheng Z.-B., Sun C.-P., Wang Y.-L., Zhang M.-Y., Zhang T.-Y., Wang Y., Yang T.-M., et al. Drechmerin H, a novel 1(2), 2(18)-diseco indole diterpenoid from the fungus Drechmeria sp. as a natural agonist of human pregnane X receptor. Bioorg. Chem. 2018;79:250–256. doi: 10.1016/j.bioorg.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 125.Rahnama M., Maclean P., Fleetwood D.J., Johnson R.D. The LaeA orthologue in Epichloë festucae is required for symbiotic interaction with Lolium perenne. Fungal Genet. Biol. 2019;129:74–85. doi: 10.1016/j.fgb.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 126.Fernando K., Reddy P., Vassiliadis S., Spangenberg G.C., Rochfort S.J., Guthridge K.M. The known antimammalian and insecticidal alkaloids are not responsible for the antifungal activity of Epichloë endophytes. Plants. 2021;10:2486. doi: 10.3390/plants10112486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nur E.A.A., Kobayashi K., Amagai A., Ohshiro T., Tomoda H. New terpendole congeners, inhibitors of sterol O-acyltransferase, produced by Volutella citrinella BF-0440. Molecules. 2020;25:3079. doi: 10.3390/molecules25133079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Oh C.H., Song C.H. Total synthesis of neuroprotective dictyoquinazol A, B, and C. Synth. Commun. 2007;37:3311–3317. doi: 10.1080/00397910701489537. [DOI] [Google Scholar]
- 129.Xu L., Jiang Y., Ma D. Synthesis of 3-substituted and 2,3-disubstituted quinazolinones via Cu-catalyzed aryl amidation. Org. Lett. 2012;14:1150–1153. doi: 10.1021/ol300084v. [DOI] [PubMed] [Google Scholar]
- 130.Wangsahardja J., Marcolin G., Lizarme Y., Morris J., Hunter L. Concise synthesis of dictyoquinazol A via a dimerisation–cyclocondensation Sequence. Synlett. 2016;27:1237–1240. doi: 10.1055/s-0035-1561569. [DOI] [Google Scholar]
- 131.Lizarme Y., Wangsahardja J., Marcolin G.M., Morris J.C., Jones N.M., Hunter L. Synthesis and neuroprotective activity of dictyoquinazol A and analogues. Bioorg. Med. Chem. 2016;24:1480–1487. doi: 10.1016/j.bmc.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 132.Saint-Dizier F., Simpkins N.S. First total synthesis of concavine. Chem. Sci. 2017;8:3384–3389. doi: 10.1039/C6SC05627J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tang J., Jiang Y., Wang B., Shen Y. Synthesis and antimicrobial Activities of oxazepine and oxazocine derivatives. Zeitschrift für Naturforsch. C. 2014;69:283–290. doi: 10.5560/znc.2014-0029. [DOI] [PubMed] [Google Scholar]
- 134.Molyneux R.J., Benson M., Wong R.Y., Tropea J.E., Elbein A.D. Australine, a novel pyrrolizidine alkaloid glucosidase inhibitor from Castanospermum australe. J. Nat. Prod. 1988;51:1198–1206. doi: 10.1021/np50060a024. [DOI] [Google Scholar]
- 135.Zhang J., Li L., Dan W., Li J., Zhang Q., Bai H., Wang J. Synthesis and antimicrobial activities of 3-methyl-β-carboline derivatives. Nat. Prod. Commun. 2015;10:1934578X1501000. doi: 10.1177/1934578X1501000627. [DOI] [PubMed] [Google Scholar]
- 136.Dai J., Dan W., Ren S., Shang C., Wang J. Design, synthesis and biological evaluations of quaternization harman analogues as potential antibacterial agents. Eur. J. Med. Chem. 2018;160:23–36. doi: 10.1016/j.ejmech.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 137.Tang J.-G., Wang Y.-H., Wang R.-R., Dong Z.-J., Yang L.-M., Zheng Y.-T., Liu J.-K. Synthesis of analogues of flazin, in particular, flazinamide, as promising anti-HIV Agents. Chem. Biodivers. 2008;5:447–460. doi: 10.1002/cbdv.200890044. [DOI] [PubMed] [Google Scholar]
- 138.Peduto A., More V., de Caprariis P., Festa M., Capasso A., Piacente S., De Martino L., De Feo V., Filosa R. Synthesis and cytotoxic activity of new β-carboline derivatives. Mini-Rev. Med. Chem. 2011;11:486–491. doi: 10.2174/138955711795843383. [DOI] [PubMed] [Google Scholar]
- 139.Ahmad S., Alam O., Naim M.J., Shaquiquzzaman M., Alam M.M., Iqbal M. Pyrrole: An insight into recent pharmacological advances with structure activity relationship. Eur. J. Med. Chem. 2018;157:527–561. doi: 10.1016/j.ejmech.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 140.Xiao J., Zhang Q., Gao Y.-Q., Tang J., Zhang A., Gao J. Secondary metabolites from the endophytic Botryosphaeria dothidea of Melia azedarach and their antifungal, antibacterial, antioxidant, and cytotoxic activities. J. Agric. Food Chem. 2014;62:3584–3590. doi: 10.1021/jf500054f. [DOI] [PubMed] [Google Scholar]
- 141.Zhang M.-Z., Jia C.-Y., Gu Y.-C., Mulholland N., Turner S., Beattie D., Zhang W.-H., Yang G.-F., Clough J. Synthesis and antifungal activity of novel indole-replaced streptochlorin analogues. Eur. J. Med. Chem. 2017;126:669–674. doi: 10.1016/j.ejmech.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 142.Thanikachalam P.V., Maurya R.K., Garg V., Monga V. An insight into the medicinal perspective of synthetic analogs of indole: A review. Eur. J. Med. Chem. 2019;180:562–612. doi: 10.1016/j.ejmech.2019.07.019. [DOI] [PubMed] [Google Scholar]
- 143.Klein A.K., Chatha M., Laskowski L.J., Anderson E.I., Brandt S.D., Chapman S.J., McCorvy J.D., Halberstadt A.L. Investigation of the structure-activity relationships of psilocybin analogues. ACS Pharmacol. Transl. Sci. 2021;4:533–542. doi: 10.1021/acsptsci.0c00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sard H., Kumaran G., Morency C., Roth B.L., Toth B.A., He P., Shuster L. SAR of psilocybin analogs: Discovery of a selective 5-HT2C agonist. Bioorganic Med. Chem. Lett. 2005;15:4555–4559. doi: 10.1016/j.bmcl.2005.06.104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.