Abstract
The objectives of this study were to investigate the pH of common beverages and to evaluate the effects of common acidic beverages on the surface hardness and weight loss of human tooth specimens. A total of 106 beverages were conveniently purchased from supermarkets in Karachi, Pakistan. Prior to evaluation, beverages were refrigerated or stored at room temperature in accordance with the manufacturers’ recommendations. Beverages were categorized into six groups: ‘Sports and Energy drinks’, ‘Water’, ‘Fruit Juices and Drinks’, ‘Sodas’, ‘Milk and Flavored Milk’ and ‘Teas and Coffee’. Using a pH meter, the pH of each beverage was measured in triplicate at room temperature. In addition, the influence of five highly acidic beverages on the weight loss and surface hardness of human tooth specimens was evaluated using gravimetric analysis and the Vickers hardness tester, respectively. ‘Sports and Energy drinks’, ‘Fruits Juices and Drinks’ and ‘Sodas’ were the most acidic beverage categories, with a pH range of 3.00–5.00. A total of 33% of beverages tested in this study were highly acidic (pH less than 4.00), 29% of beverages were moderately acidic (pH 4.00–4.99) and 31% were mildly acidic (pH 5.00–6.99). Significant weight loss was observed in all immersed specimens compared to control counterparts (p < 0.05). Similarly, for surface hardness, five highly acidic beverages (Red Bull, Pepsi, Apple Cidra, Tang Mosambi and Tang Orange) significantly decreased the surface hardness of specimens (p < 0.05). The pH levels of commonly available beverages in Pakistan are highly acidic, which may encourage loss of minerals from teeth; hence, affecting their surface hardness.
Keywords: beverages, pH, dental erosion, surface hardness, weight loss
1. Introduction
Dental erosion is a localized, chronic, painless loss of tooth enamel and dentine that have been chemically etched away from the surface of a tooth [1]. Globally, dental erosion affects 20% to 45% of permanent and 30% to 50% of deciduous dentitions [2]. Dental erosion is multifactorial and occurs due to consistent exposure to acidic fluids without microbial involvement [3]. The presence of hydrogen ions interrelates proton-promoted dissolution of fluorapatite and hydroxyapatite crystals present in tooth enamel and dentine [3]. Dental erosion can be classified into two major types: extrinsic, due to exposure to acidic foods and beverages and certain medicines, and intrinsic, as a result of gastroesophageal reflux disease and vomiting [4,5].
A major cause of extrinsic dental erosion is the consumption of acidic foods and beverages [6]. Sugary and acidic beverages have cariogenic and acidogenic tendencies, causing erosion of enamel and dental caries [7]. Additionally, in patients with gastrointestinal disorders such as gastroesophageal reflux disease (GERD), gastric acid from the stomach [8] may lead to the loss of tooth structure, hypersensitivity and compromised esthetics.
In modern society, youth are more attracted to the consumption of carbonated drinks. A major dilemma of the modern fast-track lifestyle is the increased intake of readily available carbonated drinks and juices. Consumption of fruit juices has been popularized as a healthy alternative to other beverages; this is a common modern myth and is the reason many parents give their children commercially available fruit juices [9]. In a study conducted by de Almeida et al. [9], researchers analyzed commercial fruit juices available in Brazil; they concluded that these fruit juices have low pH levels and high sugar contents [10].
There is a positive relationship between intake of acidic beverages and dental erosion. The severity of dental erosion can be affected by multiple factors including the duration, frequency, time of exposure and temperature of the beverages [11,12,13,14,15,16]. Enamel dissolution occurs at a critical pH of 5.5; however, loss of minerals may start at an even higher pH [17,18,19]. A study by Bello et al. concluded that intake of juices and soft drinks among residents accounted for 51% of total fluid intake [20]. In addition, dental erosion poses a more serious problem among athletes. Over 35% of university athletes have suffered from dental erosion [21,22]. A National Dental Health Survey conducted by Dugmore et al. [23] reported that 59.7% of 12-year-old British children suffered dental erosion. El Aidi et al. [24] reported that the prevalence of tooth erosion among 12-year-old school-going children was 32.2%, which increased to 42.8%. Data regarding dental erosion in Pakistan are very scarce. In one study, researchers evaluated 12–14 year-old school-going children and reported an association between dietary habits and dental erosion. The major etiological factors in the occurrence of dental erosion included the pattern and frequency of consuming acidic beverages [25]. It is alarming that more than 80% of the highly educated representative group of the Pakistani population consume such beverages [26].
For assessing dental erosion, different examination standards and scoring systems are reported in the literature. Therefore, it is often difficult to compare the outcomes of various epidemiological studies [27]. With this perspective, the present study incorporated the most commonly used method for categorization of beverages as follows: extremely erosive drinks (pH = 2.00–3.00), erosive (pH = 3.00–4.00) and minimally erosive (pH = 4.00–6.00) [28]. Considering the erosive potential of various beverages, the aims of this study were to thoroughly examine the pH levels of the most commonly consumed beverages in Pakistan and to evaluate their effects on the weight loss and surface hardness of human teeth.
2. Materials and Methods
This study was conducted after obtaining ethical approval from the institutional review board (IRB) of the Dow University of Health Sciences, Karachi, Pakistan (IRB-1648/DUHS/Approval/27 June 2020).
2.1. Evaluation of pH of Beverages
A total of 106 conveniently available beverages were purchased from local supermarkets in Karachi, Pakistan. Beverages were stored at 27 ± 1 °C prior to evaluation. Tea and coffee products (n = 6) were prepared by adding 10 g of each powder to 100 mL of boiling water which was then cooled to room temperature. Powdered drinks (n = 3) were prepared according to the manufacturer’s instructions noted on the packaging. ‘Ready to drink’ beverages (n = 96) were shaken well prior to opening.
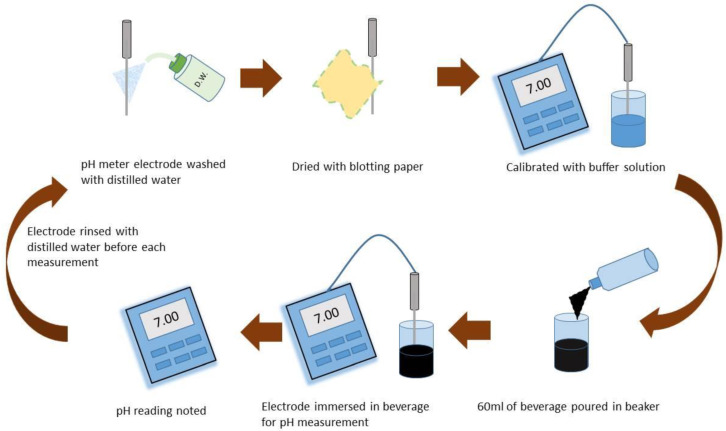
The pH of all beverages was determined using a pH meter (Model 720A, Thermoelectron Corp, Waltham, MA, USA). Measurements were taken immediately after removing the cork. The pH meter electrode was washed using distilled water and calibrated with HCL and NaOH as buffering solutions. Two beakers, ‘A’ and ‘B’, were filled with 100 mL distilled water and 60 mL of the experimental beverage, respectively. The electrode was first stirred in beaker A containing distilled water (pH 7.00). The pH electrode was then stirred in beaker ‘B’, containing an experimental beverage, and held stationary without touching the base or walls until stable readings were obtained on the pH meter. Three consecutive readings were recorded for each beverage. After each beverage testing, the beakers and the pH electrode were rinsed thoroughly with distilled water and dried with blotting paper before using them to test the next beverage (Figure 1).
Figure 1.
Schematic representation showing the procedure for pH measurement.
Based on pH measurements, five highly acidic beverages (L1 = Red Bull, L2 = Pepsi, L3 = Apple Cidra, L4 = Tang Mosambi and L5 = Tang Orange) were selected for further evaluation regarding their effects on tooth structure loss and surface hardness.
2.2. Specimen Preparation
Five premolars extracted for orthodontic purposes were collected from the Department of Oral Surgery, Dow University of Health Sciences (DUHS), Karachi, Pakistan. Informed consent was obtained from patients prior to using their extracted human teeth. The teeth were washed and disinfected using 0.5% chloramine T trihydrate solution (Permata Scientific, Sendirian Berhad, Johor Bahru, Malaysia). The teeth were then scaled using a dental scaler (Peizon Master 400,EMS, Nyon, Switzerland) to remove any calculus and debris and were carefully examined to rule out the presence of any caries, enamel hypoplasia, stains, restorations, cracks or other defects. Teeth with any pathological conditions were excluded.
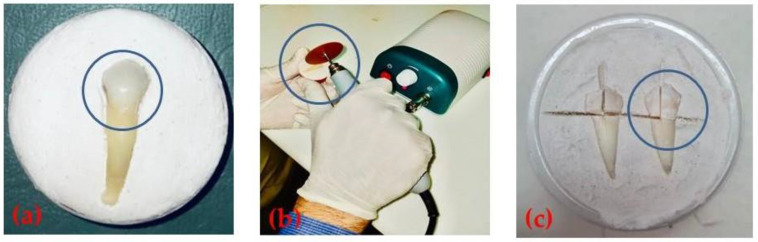
The cleaned and disinfected teeth were dried (Figure 2a) and longitudinally sectioned into two sections using a diamond cutting disc (MANI devices and instruments, Takenzawa, Japan) in a Micromotor (Figure 2b,c) (K-35 Cube 40,000 rpm, Seyang Micro Tech Co, Daegu, Korea). Each tooth section was labelled as follows: (A1, A2), (B1, B2), (C1, C2), (D1, D2) and (E1, E2). Subsequently, all sets of specimens were stored in distilled water until further experimentation. The thickness of each specimen was approximately 3 mm. Prior to testing, specimens were polished to remove debris, plaque and foreign particles. Briefly, samples were sequentially manually polished with silicon carbide papers of 600, 1200, 2500 and 4000 grit [29,30]. This was homogenous for all specimens. Moreover, the enamel surface of each specimen was exposed while the remaining part of the specimen was covered with nail varnish to simulate the oral environment.
Figure 2.
(a) Dried disinfected tooth. (b) Sectioning longitudinally using a microtome. (c) Cut into two longitudinal halves.
2.3. Weight Analysis of Specimens
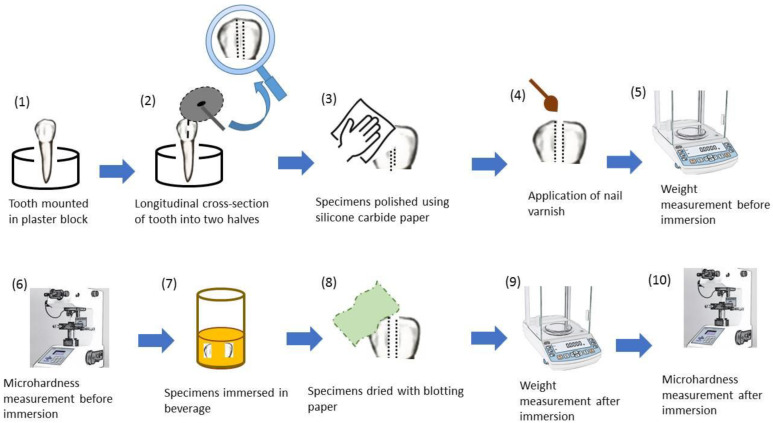
From each set, one specimen (A1, B1, C1, D1 and E1) was weighed for baseline readings before immersion into beverages. The specimens were dried with blotting papers for one hour at room temperature prior to recording the dry weight using an analytical balance (AL204 METTLER TOLEDO, Canada, accuracy 0.1 mg). The specimens (A1, B1, C1, D1 and E1) were then transferred to Falcon tubes (50 mL Conical Centrifuge Tubes, Fisher Scientific Co., Pittsburgh, PA, USA) containing 32.5 mL of a freshly opened beverage and labelled according to the name of the beverage (L1 to L5, respectively). Beverages were replaced every day until further analysis after the completion of a 7-day immersion period (Figure 3).
Figure 3.
A schematic diagram showing a complete representation of the experiment from tooth sectioning to measurement of surface hardness and weight.
2.4. Surface Hardness Testing
Surface hardness testing of specimens (A2, B2, C2, D2 and E2) was performed using the Vickers tester (ZHV Hardness Tester, ZwickRoell Indentec, Brierley Hill UK) at 100g load with a loading time of 15 s. Three indentations were performed for each specimen, which were then placed in beverages L1 to L5, respectively. After the 7-day immersion period, specimens were rinsed with distilled water, dried with blotting paper for one hour and analyzed using the surface hardness test (Figure 3).
2.5. Data Analysis
The mean and standard deviations for pH values of each beverage were recorded. One-way analysis of variance (ANOVA), including post hoc Tukey’s test, was conducted on the surface hardness and weight data to assess the difference between pre- and post- immersion tooth specimens.
3. Results
Twenty-one (n = 21) sports and energy drinks had pH values in the range of 3.04 (Holsten Black Grapes flavor) to 4.58 (Three Horses), with a mean and standard deviation of 3.81 and (0.00), respectively (Table 1). Water from five different companies (n = 5) had a pH range of 7.0 (Aquafina) to 7.63 (Nestle Pure Life), the mean and standard deviation of this group was 7.32 and (0.00), respectively (Table 2). Fruit juices and drinks (n = 33) had a pH range of 3.15 (Tang Mosambi) to 5.22 (Nestle Fruita Vitals Royal Mangoes), with a mean and standard deviation of 4.22 and (0.00), respectively (Table 3). Sodas (n = 16) had a pH range of 3.40 (Pepsi) to 4.59 (Pakola Cream Soda), with a mean and standard deviation of 3.77 and (0.00), respectively (Table 4). The average mean and standard deviation of the tea and coffee group was 5.99 and (0.01), respectively, with a pH range of 5.08 (Lipton Tea Yellow Label) to 6.88 (Nescafe Chilled Mocha) (Table 5). Milk products (n = 23) had a pH range of 6.27 (Go Fresh Coconut Milk Drink Plus Coconut Water Nata De CoCo Rose Flavor) to 7.87 (Nestle Milo), with a mean and standard deviation of 6.45 and (0.00), respectively (Table 6).
Table 1.
pH (mean and standard deviation) of sports drinks/energy drinks.
| S.No. | Sports Drinks/Energy Drinks | pH (Standard Deviation) | Batch No. |
|---|---|---|---|
| Erosive | |||
| 1 | Holsten Black Grapes Flavor | 3.04 (0.00) | 132A13 |
| 2 | Bavaria Holland Peach | 3.18 (0.00) | CLFB40612R |
| 3 | Bavaria Holland Pomegranate | 3.22 (0.00) | CLFB40612R |
| 4 | Bavaria Holland Mango Passion | 3.25 (0.00) | CLFB40612R |
| 5 | Bavaria Holland Strawberry | 3.30 (0.00) | CLFB 406 12R |
| 6 | Bavaria Holland Apple | 3.31 (0.00) | CLFB 406 12R |
| 7 | Walkers’ Ginger Beverage | 3.33 (0.00) | ** |
| 8 | Red Bull | 3.65 (0.00) | 8L01B07C |
| 9 | Activade Grapes | 3.83 (0.00) | ACT34 |
| 10 | Gatrorade Blue Bolt | 3.98 (0.01) | 14:20 P 071120 |
| Minimally Erosive | |||
| 11 | ** Malt Beverage Barbican | 4.01(0.00) | ** |
| 12 | Activade Berry Blue | 4.06 (0.00) | ACT32 |
| 13 | Activade Lemon Lime | 4.07 (0.00) | ACT31 |
| 14 | Gatrorade Tropical Fruit | 4.09 (0.00) | |
| 15 | Activade Orange | 4.14 (0.00) | |
| 16 | Gatrorade White Lightning | 4.15 (0.00) | B 13:16 P 081020 |
| 17 | Activade Fruit Punch | 4.15 (0.00) | ACT33 |
| 18 | Sting Energy Berry Blast | 4.17 (0.00) | P281120G58 |
| 19 | Sting Gold Rush | 4.20 (0.00) | P101120638 |
| 20 | **Malt 79 Murree Brewery | 4.42 (0.00) | 33H1 09:59 |
| 21 | Three Horses | 4.58 (0.00) | MRP17202 |
** Batch no. of beverage is not provided by the manufacturer.
Table 2.
pH (mean and standard deviation) of waters.
| pH of Waters | |||
|---|---|---|---|
| Waters | pH (Standard Deviation) | Batch No. | |
| Non Erosive | |||
| 22 | Aquafina | 7.0 (0.00) | 15:46 |
| 23 | Masafi Water | 7.1 (0.00) | ** |
| 24 | Mai Dubai | 7.33 (0.00) | ** |
| 25 | Nestle Pure Life Active Water | 7.58 (0.00) | 010215801A |
| 26 | Nestle Pure Life | 7.63 (0.00) | 028330621D |
** Batch no. of beverage is not provided by the manufacturer.
Table 3.
pH (mean and standard deviation) of fruit juices and drinks.
| pH of Fruit Juices and Drinks | |||
|---|---|---|---|
| Fruit Juices/Drinks | pH (Standard Deviation) | Batch No. | |
| Erosive | |||
| 27 | Tang Mosambi | 3.15 (0.01) | OTG 6301341 |
| 28 | Tang Orange Flavor | 3.16 (0.01) | |
| 29 | Tang Lemon and Pepper | 3.19 (0.00) | |
| 30 | Smart Choice Pineapple with Pulp | 3.49 (0.00) | ** |
| 31 | Lemonade (Active Foods) Mint Lemonade | 3.52 (0.00) | LIM 33 |
| 32 | Limonade (Active Foods) | 3.56 (0.00) | LIM02 |
| 33 | Smart Choice Peach with Pulp | 3.66 (0.00) | ** |
| 34 | Smart Choice Apple with Pulp Drink | 3.70 (0.00) | ** |
| 35 | Fruiti-O Guava Nectar | 3.91 (0.00) | 21004376L2 |
| 36 | Smile Lychee Flavor | 3.94 (0.00) | 21091291 L2 |
| 37 | Must Mango Fruit Drink | 3.96 (0.00) | 1008(16:02:07) |
| 38 | Fruiti-O Peach Fruit Drink | 4.00 (0.00) | 20031102009735368001 L2 |
| Minimally Erosive | |||
| 39 | Smile Apple | 4.04 (0.00) | ** |
| 40 | Smart Choice Red Grape | 4.12 (0.00) | ** |
| 41 | Fruitien Red Grapes Fruit Drink | 4.18 (0.00) | 20075:6L3E |
| 42 | Hemani Peach Drink with Basil Seeds | 4.20 (0.00) | EX007R2309 |
| 43 | Nestle Fruita Vitals Red Grapes | 4.24 (0.00) | 028015801L |
| 44 | Slice Mango Fruit Drink | 4.24 (0.00) | PX12B21:53 |
| 45 | Fruitien Pomegranate Nectar | 4.29 (0.00) | 0024826L |
| 46 | Nestle Fruita Vitals Pineapple | 4.50 (0.00) | 2.5E + 08 |
| 47 | Fruitien Joy Mango Fruit Drink | 4.52 (0.00) | 202892132L4 |
| 48 | Fruitien Pineapple Nectar | 4.54 (0.00) | ** |
| 49 | Hemani Cocktail Drink with Basil Seeds | 4.57 (0.00) | EX007R0103 |
| 50 | Nestle Fruita Vitals Peach Fruit Drink | 4.60 (0.00) | 018215801Z |
| 51 | Hemani Lychee Drink with Basil Seeds | 4.63 (0.00) | EX007R1202 |
| 52 | Anytime Green Apple Fruit Nectar | 4.66 (0.00) | 124(05:4248) |
| 53 | Hemani White Grapes with Basil Seeds | 4.77 (0.00) | ** |
| 54 | Nestle Fruita Vitals Apple Nectar | 4.82 (0.00) | 0309158010(13:59) |
| 55 | Nestle Fruita Vitals Kinnow Nectar | 4.95(0.00) | 031715803H(05:21) |
| 56 | Fruitien Chaunsa Mango Nectar | 5.02 (0.00) | 20276411L3E |
| 57 | Nestle Fruita Vitals Chaunsa Mango Nectar | 5.03 (0.00) | 031515802G |
| 58 | Nestle Fruita Vitals Guava Nectar | 5.10 (0.00) | 2.5E + 08 |
| 59 | Nestle Fruita Vitals Royal Mangoes | 5.22 (0.00) | 3.1E + 08 |
** Batch no. of beverage is not provided by the manufacturer.
Table 4.
pH (mean and standard deviation) of sodas.
| pH of Sodas | |||
|---|---|---|---|
| Soda | pH (Standard Deviation) | Batch No. | |
| Erosive | |||
| 60 | Pepsi | 3.40 (0.00) | P301120638 04:17 |
| 61 | Forest Club Soda | 3.47 (0.00) | ** |
| 62 | Pepsi Diet | 3.52 (0.00) | P061120GA 03:46 |
| 63 | Coca Cola Original Taste | 3.54 (0.00) | 1319L3 |
| 64 | Pakola (Lychee) | 3.55 (0.00) | ** |
| 65 | Pakola Lemon Lime | 3.61 (0.00) | 22106 |
| 66 | Pepsi Cola | 3.62 (0.00) | |
| 67 | Apple Sidra | 3.73 (0.00) | |
| 68 | Mirinda Orange Flavor | 3.78 (0.00) | P241120638 |
| 69 | Fanta Orange Flavor | 3.84 (0.00) | 0205M6PP36 |
| 70 | Mountain Dew | 3.87 (0.00) | P281020058 |
| 71 | Vimto Sparkling | 3.91 (0.01) | 661JLY21 |
| 72 | 7Up | 3.95 (0.00) | |
| 73 | Sprite | 3.96 (0.00) | |
| Minimally Erosive | |||
| 74 | 7Up Free | 4.06 (0.00) | P26102003A |
| 75 | Pakola Cream Soda | 4.59 (0.00) | 21APR217UMD |
** Batch no. of beverage is not provided by the manufacturer.
Table 5.
pH (mean and standard deviation) of teas and coffees.
| pH of Teas and Coffees | |||
|---|---|---|---|
| Teas and Coffees | pH (Standard Deviation) | Batch No. | |
| Minimally Erosive | |||
| 76 | Lipton Tea Yellow Label | 5.08 (0.01) | 5 |
| 77 | Tea Supreme | 5.11 (0.02) | 5 |
| 78 | Tapal Family Mixture | 5.31 (0.00) | 509234 |
| 79 | Tapal Danedar | 5.46 (0.00) | 513252 |
| 80 | Green Tea Lipton | 6.65 (0.01) | |
| 81 | Nescafe Chilled Latte | 6.70 (0.00) | 3.1E + 08 |
| 82 | Nescafe Coffee | 6.73 (0.01) | |
| 83 | Nescafe Chilled Mocha | 6.88 (0.00) | 029715801d |
** Batch no. of beverage is not provided by the manufacturer.
Table 6.
pH (mean and standard deviation) of milks and flavored milks.
| pH of Milks and Flavored Milks | |||
|---|---|---|---|
| Milks | pH (Standard Deviation) | Batch No. | |
| Minimally Erosive | |||
| 84 | Go Fresh Coconut Milk Drink plus Coconut Water with Nata De CoCo Rose Flavour | 6.27 (0.00) | RA189 2205J1626 |
| 85 | Go Fresh Coconut Milk Drink plus Coconut Water with Melon Flavor | 6.29(0.00) | RA189 2205J1809 |
| 86 | Day Fresh Milk Full Cream | 6.51 (0.00) | 0333P1B7 |
| 87 | Nurpur Full Cream Milk | 6.52 (0.00) | |
| 88 | Day Fresh Flavored Milk Banana | 6.53 (0.00) | 0186B1C4 |
| 89 | Day Fresh Flavored Milk Strawberry | 6.58 (0.00) | 0294S1B6 |
| 90 | Olpers Full Cream Milk | 6.60 (0.00) | |
| 91 | Oolala Flavored Milk Strawberry Shakarganj | 6.61 (0.00) | 13B(13:36:12) |
| 92 | Olpers Chocolate Flavored Milk | 6.65 (0.00) | 20(13:38:59) |
| 93 | Tarang (liquid tea whitener) | 6.65 (0.00) | 173(04:28:38) |
| 94 | Nestle Milk Pak Full Cream | 6.69 (0.00) | 032315801C |
| 95 | Day Fresh Flavored Milk Mango | 6.70 (0.00) | 0256M1C7 |
| 96 | Oolala Flavored Milk Badam and Zaffran | 6.71(0.00) | 125LFM(05:35:33) |
| 97 | Olpers Pro Cal Low Fat Milk | 6.72 (0.00) | D0233(19:26:43) |
| 98 | Day Fresh Flavored Milk Pista Zaffran | 6.73(0.00) | 0292Z1b7 |
| 99 | Nestle Nesvita | 6.77 (0.00) | 030915801U |
| 100 | Nurpur Flavored Milk Badam and Zaffran | 6.84 (0.00) | 32(06:41:56) |
| 101 | Pakola Chocolate Flavored Milk | 6.90 (0.00) | GS 12:44 B05 |
| 102 | Go Fresh Coconut Milk Drink with Chocolate | 6.93 (0.00) | |
| 103 | Pakola Flavored Milk Strawberry | 6.94 (0.00) | ** |
| 104 | Oolala Chocolate Flavored Milk Shakarganj | 6.97 (0.00) | 011(12:29:05) |
| Non Erosive | |||
| 105 | Pakola Double Delight Strawberry plus Vanilla | 7.07 (0.00) | ** |
| 106 | Milo Nestle | 7.87 (0.00) | 028515801U |
** Batch no. of beverage is not provided by the manufacturer.
Out of 106 drinks, 35 (33%) were found to be erosive with a pH value in the range of 3.00–3.99, and 31 (29%) beverages were minimally erosive (pH = 4.00–4.99). Similarly, 33 beverages were considered minimally erosive with pH values in the range of 5.00–6.99. None of the tested beverages were highly erosive. Water from all companies demonstrated a neutral pH range of 7.00–7.20, and five drinks had a pH range of 7.20–7.87.
In terms of weight analysis, significant weight loss was observed in all immersed specimens compared to control counterparts (p < 0.05) (Table 7). Similarly, for surface hardness, all five highly acidic beverages, namely Red Bull, Pepsi, Apple Cidra, Tang Mosambi and Tang Orange, significantly decreased the surface hardness of specimens (p < 0.05) (Table 8).
Table 7.
Weight of specimens before and after immersion in highly acidic beverages (mean and SD).
| Beverage Type | Red Bull | Pepsi | Apple Cidra | Tang Mosambi | Tang Orange |
|---|---|---|---|---|---|
|
Pre-immersion weight
(grams) |
0.18 (±0.00) | 0.08 (±0.00) | 0.10 (±0.00) | 0.12 (±0.00) | 0.16 (±0.01) |
|
Post-immersion Weight
(grams) |
0.11 (±0.00) | 0.04 (±0.00) | 0.05 (±0.00) | 0.07 (±0.00) | 0.11 (±0.00) |
| Weight Reduction in Specimens After Immersion (%) | 38.89 | 50.00 | 50.00 | 41.67 | 31.25 |
| p Value | p = 0.000 | p = 0.001 | p = 0.000 | p = 0.000 | p = 0.003 |
p < 0.05 indicates a statistically significant difference between pre- and post-immersion specimens.
Table 8.
Surface hardness of specimens before and after immersion in highly acidic beverages (mean and SD).
| Beverages Type | Red Bull | Pepsi | Apple Cidra | Tang Mosambi | Tang Orange |
|---|---|---|---|---|---|
|
Pre-immersion Hardness
(VHN) |
598 (±50) | 553 (±13) | 686 (±9) | 669 (±17) | 654 (±40) |
|
Post-immersion Hardness
(VHN) |
473 (±20) | 457 (±16) | 606 (±21) | 566 (±21) | 347 (±46) |
| Surface Hardness Reduction in Specimens After Immersion (%) | 21 | 17 | 12 | 15 | 47 |
| p Value | p = 0.001 | p = 0.001 | p = 0.003 | p = 0.003 | p = 0.001 |
p < 0.05 indicates a statistically significant difference between pre- and post-immersion specimens.
4. Discussion
The present study investigated pH levels of a wide variety of commonly used beverages. In addition, the erosive effects of various acidic beverages on dental hard tissues were determined by evaluating variations in the surface hardness and loss of minerals from human tooth specimens. The present study is the first of its kind to investigate a wide variety of beverages, including bottled waters, sports/energy drinks, fruit juices, carbonated sodas, flavored milks, coffees and teas; hence, it presents comprehensive and diverse data regarding the effects of these beverages on dental tissues. In contrast, previous studies [31,32] determined the pH of a limited number (maximum 14) of beverages and their effect on tooth erosion. Most of the beverages investigated in this study had an acidic pH. Highly acidic beverages significantly affected the weight loss and surface hardness of human tooth specimens (p < 0.05).
For the evaluation of beverages’ erosive potential, pH is considered the most important key factor [33,34]. In this study, we utilized an inverse logarithmic relationship reported by Larsen and Nyvad [28] to determine beverages’ pH and erosive potential. This method has also been employed by other researchers [35]. Beverage manufacturing companies do not usually print pH information on their product labels. Therefore, the present study, together with similar previous studies, provide invaluable data to health care workers and the general population.
Although the pH and resultant erosive potential of beverages have widely been reported in previous studies [21,33,35,36], it is difficult to compare these data with beverages available in Pakistan due to gross variations in manufacturing processes, temperature and equipment accuracy. For instance, beverages tested at a higher temperature exhibited lower pH values [37].
In terms of pH data, results in this study are not in agreement with previous studies conducted in Pakistan. For instance, Haq et al. [31] reported the pH values of Pepsi and Mountain Dew as 2.60 and 2.94, respectively; however, in this study the pH values for both drinks were observed as 3.47 and 3.87, respectively. This difference might be attributed to different scoring systems used in these studies. Similarly, in a previous study a sports drink (‘Sting’) exhibited a pH value of 3.0 [32]; in contrast, this study reported a pH value of 4.80 for the same drink. This variation in findings may be attributed to different methodologies and distinct pH testing methods. In this study, a pH electrode meter was used to evaluate the pH of various beverages with three consecutive readings; whereas their study used pH strip methods and performed experiments for 15 days. Four cycles were conducted each day at six-hour intervals.
This study clearly indicates that many of the commercially available, non-alcoholic beverages in Pakistan have the potential to cause dental erosion (Table 1, Table 2, Table 3, Table 4, Table 5 and Table 6). The increase in consumption of these beverages highlights a potentially serious oral and general health risk. Awareness of beverage pH is critical for designing preventive policies for patients susceptible to clinical erosion [38,39,40]. Minimization of erosive drinks (pH 3.00–3.99) and replacement with drinks having a pH of 4.00 or higher would be sensible recommendations for the prevention of erosion.
These data may be used as a reference for future research and may also provide health experts and individuals with an instant resource when advocating a healthy diet to and for consumers. Undoubtedly, these data regarding pH findings are significant and alarming for the health of human dentition.
The common methods used to analyze tooth erosion include surface hardness, scan electron microscopy, microradiography, chemical analysis, digital image analysis and atomic force microscopy [41]. In this study, we chose surface hardness (considered an effective approach to measure the change in a tooth’s surface microstructure), which is indirectly suggestive of the degree of demineralization. The findings of this study related to surface hardness clearly highlight the significant effect of all five acidic beverages on tooth structure (Table 8), which is in agreement with a previous study [42]. Jeong M.J et al. [42] evaluated the effect of four energy drinks, including ‘Red Bull’, on the surface hardness of tooth specimens; they observed a significant difference between the surface hardness values of pre- and post-immersion specimens.
The surface hardness of the Tang Orange treated specimen decreased more significantly compared to the other four tested beverages, although the pH values are not significantly different. This finding may be due to the presence of different ingredients in Tang Orange. It is a well-known fact that ingredients play a key role in dental erosion. For instance, the presence of citric acid and sugars in beverages has been considered a significant cause of dental erosion. Further studies with regard to the precise composition of beverages and scan electronic microscopic examination of beverage-treated specimens are warranted so as to discover the actual cause of such a finding [43,44].
There were significant differences among the hardness values of specimens. Since the specimens in our study were made from teeth of different patients, differences in the orientation of enamel rods, the orientation of the crystallites within the rods, the degree of demineralization and the presence of fluoride ions are expected. This might have led to distinct hardness values among the specimens evaluated in the study.
Weight loss of tooth structure is also considered a suitable method to predict the effect of beverages on erosion. Methew et al. [45] evaluated the effect of seven beverages (Pepsi, Red Bull, orange juice, apple juice, lemon juice, coffee and green tea) on the human tooth over a one-month period. They observed a significant loss of tooth structure with orange juice, Red Bull and Pepsi, which is in agreement with this study, as substantial tooth loss was evident with all five acidic beverages in our findings (Table 7). In another study, Bitri et al. [46] conducted an in vitro analysis to assess the erosive effect of some common drinks. The authors employed the weight-loss method and identified erosive effects in terms of dental hard tissue dissolution with all soft drinks evaluated. Weight loss in both of these studies cannot be compared, owing to methodological differences.
There are a few limitations in the present in vitro study. The buffering capacity and flushing action of saliva, which may potentially influence the erosive action of acidic beverages, was not simulated. In addition, morphological assessment to analyze the materials’ surface variations following acidic exposition were not investigated. Additionally, due to cultural and religious constraints, alcoholic beverages were not evaluated in this study.
Accordingly, despite the significant clinical relevance of this study, further research is essential to investigate erosion kinetics, surface corrosion, surface morphological analysis and diffusion in the solid state.
5. Conclusions
Under the limitations of this study, it was concluded that out of 106 beverages, the pH levels of 99 drinks in Pakistan were determined to be acidic, and hence, considered erosive. Moreover, five acidic beverages, namely Red Bull, Pepsi, Apple Cidra, Tang Mosambi and Tang Orange, clearly demonstrated erosive effects through the decreased surface hardness and weight loss of human tooth specimens.
Acknowledgments
The authors are grateful to Anser Bashir and Muhammad Rais Iqbal for their technical assistance.
Author Contributions
Conceptualization, N.K. and F.A.; methodology, H.Z., S.R., S.K. and T.F.; software, N.K.; validation, M.A.A., S.J.M. and H.Z.; formal analysis, M.S.Z.; investigation, S.K., T.F. and S.R.; resources, M.A.A. and S.J.M.; data curation, F.A.; writing—original draft preparation, D.H. and F.A.; writing—review and editing, D.H., N.K. and M.S.Z.; supervision, N.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the institutional review board (IRB) of the Dow University of Health Sciences, Karachi, Pakistan (IRB-1648/DUHS/Approval/27 June 2020).
Informed Consent Statement
Informed consent was obtained from all patients prior to using their extracted teeth.
Data Availability Statement
The data presented in this study are available on the request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zimmer S., Kirchner G., Bizhang M., Benedix M. Influence of various acidic beverages on tooth erosion. Evaluation by a new method. PLoS ONE. 2015;10:e0129462. doi: 10.1371/journal.pone.0129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schlueter N., Luka B. Erosive tooth wear. A review on global prevalence and on its prevalence in risk groups. Br. Dent. J. 2018;224:364–370. doi: 10.1038/sj.bdj.2018.167. [DOI] [PubMed] [Google Scholar]
- 3.Ganss C. Dental Erosion. Volume 20. Karger Publishers; London, UK: 2006. Definition of erosion and links to tooth wear; pp. 9–16. [DOI] [PubMed] [Google Scholar]
- 4.Jensdottir T., Arnadottir I., Thorsdottir I., Bardow A., Gudmundsson K., Theodors A. Relationship between dental erosion, soft drink consumption, and gastroesophageal reflux among Icelanders. Clin. Oral Investig. 2004;8:91–96. doi: 10.1007/s00784-003-0252-1. [DOI] [PubMed] [Google Scholar]
- 5.Yan F.R. Dental Erosion: Etiology, Diagnosis and Prevention. ADA Acad. Dent. Ther. Stomatol. 2011;31:75–84. [Google Scholar]
- 6.Quartarone E., Mustarelli P., Poggio J.C., Lombardini M. Surface kinetic roughening caused by dental erosion: An atomic force microscopy study. J. Appl. Phys. 2008;103:104702. doi: 10.1063/1.2927386. [DOI] [Google Scholar]
- 7.Ehlen L.A., Marshall T.A., Qian F., Wefel J.S., Warren J.J. Acidic beverages increase the risk of in vitro tooth erosion. Food Nutr. Res. 2008;28:299–303. doi: 10.1016/j.nutres.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hefferren J. Why is there and should there be more attention paid to dental erosion? Compend. Contin. Educ. Dent. 2004;25:4–8. [PubMed] [Google Scholar]
- 9.Bagde N.I., Tumane P. Studies on microbial flora of fruit juices and cold drinks. Asiat. J. Biotechnol. 2011;2:454–460. [Google Scholar]
- 10.De Almeida L.d.F.D., Abílio G.M.F., Cavalcante M.T., Castro R.D., Cavalcanti A.L. Cariogenic and erosive potential of industrialized fruit juices available in Brazil. Braz. J. Oral Sci. 2017;9:351–357. [Google Scholar]
- 11.Shellis R.P., Addy M. Interaction between attrition, abrasion and erosion in tooth wear. Monogr. Oral Sci. 2014;25:32–45. doi: 10.1159/000359936. [DOI] [PubMed] [Google Scholar]
- 12.Larsen M.J., Richards A. Flouride is unable to reduce dental erosion from soft drinks. Caries Res. 2002;36:75–80. doi: 10.1159/000057595. [DOI] [PubMed] [Google Scholar]
- 13.Cairns A.M., Watson M., Creanor S.L., Foye R.H. The pH and titratable acidity of a range of diluting drinks and their potential effect on Dental Erosion. J. Dent. 2002;30:313–317. doi: 10.1016/S0300-5712(02)00044-1. [DOI] [PubMed] [Google Scholar]
- 14.Joshi M., Joshi N., Kathariya R., Angadi P., Raikar S. Technique to Evaluate Dental Erosion: A systematic Review of Literature. J. Clin. Diagn. Res. 2016;10:1–7. doi: 10.7860/JCDR/2016/17996.8634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y.L., Chang H.H., Chang Y.C., Lu Y.C. Effects of fluoride and epigallocatechin gallate on soft-drink-induced dental erosion of enamel and root dentin. J. Formos. Med. Assoc. 2018;117:276–282. doi: 10.1016/j.jfma.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y.L., Chang C.C., Chi C.W., Chang H.H., Chiang Y.C., Chuang Y.C., Chang H.H., Huang G.F., Liao Y.S., Lin C.P. Erosive potential of soft drinks on human enamel: An in vitro study. J. Formos. Med. Assoc. 2014;113:850–856. doi: 10.1016/j.jfma.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Mathew T., Casamassimo P.S., Hayes J.R. Relationship between sports drinks and dental erosion in 304 university athletes in Columbus, Ohio, USA. Caries Res. 2002;36:281–287. doi: 10.1159/000063927. [DOI] [PubMed] [Google Scholar]
- 18.Harding M., Whelton H., O’ Mullane D., Cronin M. Dental erosion in 5-year-old Irish school children and associated factors: A pilot study. Community Dent. Health. 2003;20:165–170. [PubMed] [Google Scholar]
- 19.Coombes J.S. Sports drinks and dental erosion. Am. J. Dent. 2005;18:101–104. [PubMed] [Google Scholar]
- 20.Bello L., Al-Hammad N. Pattern of fluid consumption in a sample of Saudi Arabian adolescents aged 12–13 years. Int. J. Paediatr. Dent. 2006;16:168–173. doi: 10.1111/j.1365-263X.2006.00715.x. [DOI] [PubMed] [Google Scholar]
- 21.Seow W., Thong K. Erosive effects of common beverages on extracted premolar teeth. Aust. Dent. J. 2005;50:173–178. doi: 10.1111/j.1834-7819.2005.tb00357.x. [DOI] [PubMed] [Google Scholar]
- 22.Sirimaharaj V., Messer L.B., Morgan M. Acidic diet and dental erosion among athletes. Aust. Dent. J. 2002;47:228–236. doi: 10.1111/j.1834-7819.2002.tb00334.x. [DOI] [PubMed] [Google Scholar]
- 23.Dugmore C.R., Rock W.P. The prevalence of tooth erosion in 12-year-old children. Br. Dent. J. 2004;196:279–282. doi: 10.1038/sj.bdj.4811040. [DOI] [PubMed] [Google Scholar]
- 24.El Aidi H., Bronkhorst E.M., Truin G.J. A longitudinal study of tooth erosion in adolescents. Dent. Res. 2008;87:731–735. doi: 10.1177/154405910808700813. [DOI] [PubMed] [Google Scholar]
- 25.Shahbaz U., Quadir F., Hosein T. Determination of Prevalence of Dental Erosion in 12–14 Years School Children and Its Relationship with Dietary Habits. J. Coll. Physicians Surg. 2016;26:553–556. [PubMed] [Google Scholar]
- 26.Khan M.S., Nisar N., Naqvi S.A.A., Nawab F. Caffeine Consumption and Academic Performance among Medical Students of Dow University of Health Sciences (DUHS), Karachi, Pakistan. Annals. 2017;22:81–87. [Google Scholar]
- 27.Tschammler C., Simon A., Brockmann K., Röbl M., Wiegand A. Erosive tooth wear and caries experience in children and adolescents with obesity. J. Dent. 2019;83:77–86. doi: 10.1016/j.jdent.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Larsen M.J., Nyvad B. Enamel erosion by some soft drinks and orange juices relative to their pH, buffering effect and contents of calcium phosphate. Caries Res. 1999;33:81–87. doi: 10.1159/000016499. [DOI] [PubMed] [Google Scholar]
- 29.Amaya-Pajares S.P., Koi K., Watanabe H., da Costa J.B., Ferracane J.L. Development and maintenance of surface gloss of dental composites after polishing and brushing: Review of the literature. J. Esthet. Restor. Dent. 2022;34:15–41. doi: 10.1111/jerd.12875. [DOI] [PubMed] [Google Scholar]
- 30.Zipperian D.C. Metallographic Specimen Preparation Basics. [(accessed on 16 August 2021)]. Available online: www.metallographic.com.
- 31.Haq M.W., Batool M., Ahsan S.H., Lone M.A., Islam T. Dental Erosion; Influencing Factors & pH Analysis. Can. J. Appl. Sci. 2012;2:222–232. [Google Scholar]
- 32.Kazmi S., Mughal A., Habib M., Ayaz M., Tariq H., Khan A. Effects on the enamel due to the carbonated drinks-a SEM study. Pak. Oral Dent. J. 2016;36:221–225. [Google Scholar]
- 33.Cochrane N., Yuan Y., Walker G., Shen P., Chang C., Reynolds C. Erosive potential of sports beverages. Aust. Dent. J. 2012;57:359–364. doi: 10.1111/j.1834-7819.2012.01708.x. [DOI] [PubMed] [Google Scholar]
- 34.Jensdottir T., Holbrook P., Nauntofte B., Buchwald C., Bardow A. Immediate erosive potential of cola drinks and orange juices. J. Dent. Res. 2006;85:226–230. doi: 10.1177/154405910608500304. [DOI] [PubMed] [Google Scholar]
- 35.Reddy A., Norris D.F., Momeni S.S., Waldo B., Ruby J.D. The pH of beverages in the United States. J. Am. Dent. Assoc. 2013;147:255–263. doi: 10.1016/j.adaj.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hara A.T., Carvalho J.C., Zero D.T. Dental Erosion and Its Clinical Management. Springer International Publishing; Berlin/Heidelberg, Germany: 2015. Causes of dental erosion: Extrinsic factors; pp. 69–96. [Google Scholar]
- 37.Wang X., Lussi A. Assessment and management of dental erosion. Dent. Clin. N. Am. 2010;54:565–578. doi: 10.1016/j.cden.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Zero D.T., Lussi A. Erosion—Chemical and biological factors of importance to the dental practitioner. Int. Dent. J. 2005;55:285–290. doi: 10.1111/j.1875-595X.2005.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 39.Moynihan P.J. Dietary advice in dental practice. Br. Dent. J. 2002;193:563–568. doi: 10.1038/sj.bdj.4801628. [DOI] [PubMed] [Google Scholar]
- 40.Lussi A., Jaeggi T. Etiology and risk assessment. In: Lussi A., Jaeggi T., editors. Dental Erosion: Diagnosis, Risk Assessment, Prevention, Treatment. Quintessence; London, UK: 2011. pp. 37–53. [Google Scholar]
- 41.Ganess C., Klimek J., Schwartz N. A comparative profilometric in vitro study of the susceptibility of polished and natural human enamel and dentine surface to erosive demineralization. Arch. Oral Biol. 2000;45:897–902. doi: 10.1016/S0003-9969(00)00041-8. [DOI] [PubMed] [Google Scholar]
- 42.Jeong M.J., Jeong S.J., Son J.H., Chung S.K., Kim A., Kang E.J., Kim E.J., Kim H.I., Jang K.E., Cho M.H., et al. A study on the enamel erosion caused by energy drinks. J. Dent. Hyg. 2014;14:597–609. doi: 10.17135/jdhs.2014.14.4.597. [DOI] [Google Scholar]
- 43.Sauro S., Mannocci F., Piemontese M., Mongiorgi R. In situ enamel morphology evaluation after acidic soft drink consumption: Protection factor of contemporary toothpaste. Int. J. Dent. Hyg. 2008;6:188–192. doi: 10.1111/j.1601-5037.2008.00313.x. [DOI] [PubMed] [Google Scholar]
- 44.Von Fraunhofer J.A., Rogers M.M. Dissolution of dental enamel in soft drinks. Gen. Dent. 2004;52:308–312. [PubMed] [Google Scholar]
- 45.Mathew S., Luke A.M., Walia T., Masri A.G., Jamal H., Pawar A.M. Effect of fruit juices and other beverages on loss of tooth structure. Pesq. Bras. Odontoped. Clin. Integr. 2018;18:1–9. doi: 10.4034/PBOCI.2018.181.22. [DOI] [Google Scholar]
- 46.Bitri E., Petcu L., Mocanu G., Balaban D.P. In vitro assessment of erosive effects of some common soft drinks on dental hard tissues. Balk. J. Dent. Med. 2019;23:132–140. doi: 10.2478/bjdm-2019-0024. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on the request from the corresponding author.