Abstract
Black garlic is obtained from fresh garlic (Allium sativum L.) that has been fermented for a period of time at a controlled high temperature (60–90°C) under controlled high humidity (80–90%). When compared with fresh garlic, black garlic does not release a strong offensive flavor owing to the reduced content of allicin. Enhanced bioactivity of black garlic compared with that of fresh garlic is attributed to its changes in physicochemical properties. Studies concerning the fundamental findings of black garlic, such as its production, bioactivity, and applications, have thus been conducted. Several types of black garlic products are also available in the market with a fair selling volume. In this article, we summarize the current knowledge of changes in the components, bioactivity, production, and applications of black garlic, as well as the proposed future prospects on their possible applications as a functional food product.
Keywords: black garlic, black garlic application, black garlic bioactivity, black garlic production, fermentation
1. Introduction
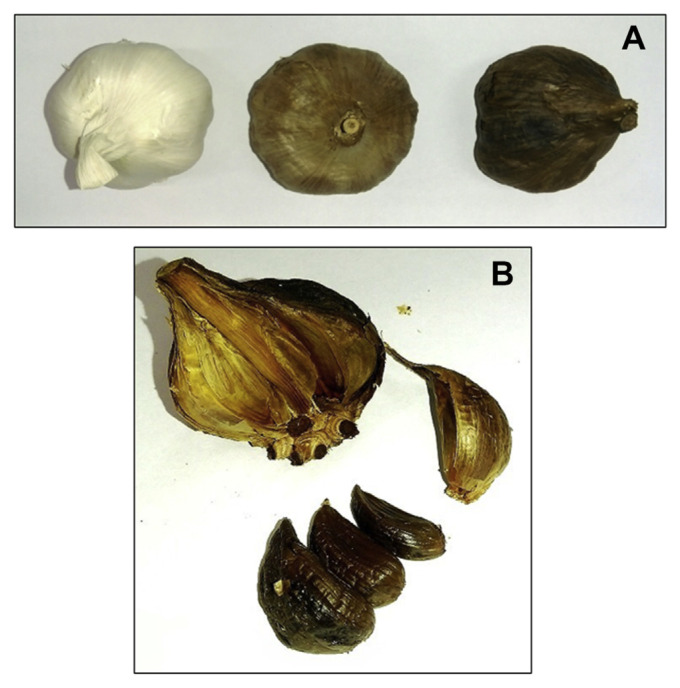
Black garlic (BG) is simply fresh garlic (Allium sativum L.) that has been fermented for a period of time at a high temperature under high humidity. The process turns garlic cloves dark, gives them a sweet taste, and alters their consistency to chewy and jelly-like (Figure 1). The duration of fermentation varies depending on cultures, manufacturers, and purposes [1].
Figure 1.
Black garlic. (A) Garlic during fermentation process (left to right). (B) Black garlic cloves.
The long history of the use of garlic in food and acute, chronic, and inhalation studies, although limited, reveals no credible adverse biological effects. Exact origins of BG are unknown and controversial. However, BG has long been consumed in South Korea, Japan, and Thailand for centuries, and was introduced into Taiwan and other countries around 10 years ago. In the past few years, high-end chefs have drawn much attention to BG, who have been using it to flavor chicken, fish, soup, and risotto [2].
When compared with fresh garlic, BG does not release a strong off-flavor due to the reduced content of allicin, which was converted into antioxidant compounds such as bioactive alkaloids and flavonoid compounds during the aging process [1]. The changes of physicochemical properties are the main reasons for enhanced bioactivity of BG compared with fresh garlic. Besides daily consumption, several studies have reported that BG extract demonstrates several functions, such as antioxidation, antiallergic, antidiabetes, anti-inflammation, and anticarcinogenic effects [3–7]. In 1990, Designer Foods Program listed garlic at the top of cancer-fighting candidates [8]. Although the Designer Foods Program no longer exists, scientists are still looking for what are now called bioactive components in different foods.
The two main focuses of this study are to summarize the current knowledge of the composition change, bioactivity, production, and applications of BG, and also to propose future prospects on their possible applications as a functional food product.
2. Nutritional content of garlic
The enhanced biological activity of BG when compared with fresh garlic lies in the conversion of phytochemical compounds during the fermentation process. In the following section, we will summarize the changes of garlic components between fresh garlic and BG.
2.1. Comparison of the components between fresh garlic and BG
Fresh garlic contains approximately 63% of water, 28% of carbohydrate (fructans), 2.3% of organosulfur compounds, 2% of proteins (alliinase), 1.2% of free amino acids (arginine), and 1.5% of fiber [9]. Nontreated fresh garlic also contains a high amount of γ-glutamylcysteines [10]. These compounds can be hydrolyzed and oxidized to form alliin, which accumulates naturally during the storage of garlic at a cool temperature. After processing, such as cutting, crushing, chewing, or dehydration, alliinase rapidly lyses the cytotoxic cysteine sulfoxides (alliin) to form cytotoxic and odoriferous alkyl alkane-thiosulfinates such as allicin [11]. Allicin contributes to the characteristic flavor and taste of garlic. Allicin and other thiosulfinates are immediately decomposed to other compounds such as diallyl sulfide, diallyl disulfide, and diallyl trisulfide, dithiins, and ajoene [11,12]. At the same time, γ-glutamylcysteines are converted to SAC through its catabolism pathway other than the alliin–allicin pathway [13]. SAC contributes to health benefits of garlic, such as its antidiabetic, antioxidant, and anti-inflammatory activities [14–16].
As for BG, during the thermal process, some chemical compounds from fresh garlic are converted into Amadori/Heyns compounds, which are key intermediate compounds of Maillard reaction [1]. The chemical compounds of aged BG (ABG) are complicated, and the quality of its products depends on the manufacturing process. Nevertheless, BG contains much more functional compounds such as SAC than fresh garlic.
The contents of chemical compounds of BG depend on the conditions during thermal processing. Some researchers reported that many valuable components within BG against diseases increased during the aging process, especially polyphenol, flavonoids, and some intermediates of Maillard reaction have been known as antioxidant agents [13,17]. Furthermore, the antioxidant activity of garlic varies across regions [18]; nevertheless, BG demonstrates significantly much higher biological activity, such as antioxidant properties, than fresh garlic [19].
Several studies have reported that water-soluble sugars, amino acids, total polyphenols, and flavonoids increased or decreased during thermal processing (Table 1) [13,20,21]. Three of Amadori and three of Heyns compounds in BG increased significantly—up to 40–100-fold higher than those in fresh garlic. In contrast, through the aging process for converting fresh garlic to BG, the amount of fructans decreased simultaneously, owing to the fact that fructose and glucose with some of amino acids play important roles in Maillard reaction in garlic processing.
Table 1.
Comparison between the components of black and fresh garlic.
| Components of black garlic compared with fresh garlic | Original concentration | |
|---|---|---|
| Water-soluble sugar | Increased 1.88–7.91-fold [1] | 450 mg/g |
| Polyphenol | Increased 4.19-fold [13] | 13.91 mg GAE/g |
| Flavonoid | Increased 4.77-fold [13] | 3.22 mg RE/g |
| Amadori & Heyns | Increased 40–100-fold [1] | 10 μg/g |
| Fructan | Decreased 0.15–0.01-fold [1] | 580 mg/g |
| Leucine | Increased 1.06-fold [13] | 58.62 mg/100 g |
| Isoleucine | Increased 1.67-fold [13] | 50.04 mg/100 g |
| Cysteine | Decreased 0.58-fold [13] | 81.06 mg/100 g |
| Phenylalanine | Increased 2.43-fold [13] | 55.64 mg/100 g |
| Tyrosine | Decreased 0.18-fold [13] | 449.95 mg/100 g |
GAE = gallic acid equivalents; RE = rutin equivalents.
3. BG processing
3.1. Effects of aging temperature on the quality of BG
It is well known that the aging period of garlic is shorter at higher temperatures [22]. In the case of aging process at 70°C, the speed of aging is two-fold faster than that at 60°C [23]. According to sensory evaluation, the quality of BG is better and its black color is homogeneous between 70°C and 80°C [23]. Even though BG is produced faster at 90°C, it produces nonideal tastes, such as bitter and sour tastes [23]. In the case of aging process at 60°C, the color of garlic was not completely black; thus, 60°C is also not an ideal condition for the aging process.
When the moisture content of garlic reaches 400–500 g/kg, BG can be suitable for eating because of its softness and elasticity. Ifmoisture content is about 350–400 g/kg, BG would be much drier and its elasticity would be poor. In particular, when moisture content goes below 350 g/kg, BG becomes too hard to eat [23]. Moreover, the aging speed of fresh garlic to BG is markedly slow when processed at 60°C. Although aging occurs smoothly at 80°C and 90°C, an adequate condition is relatively difficult to find because of its fluctuating phenol content and reducing sugar content [23].
Content of reducing sugar is also considered an important factor during the aging process. Some types of sugar and amino acids are required for Maillard reaction [24]. The reducing sugar content gradually increases at 60°C and 70°C during the whole process, which means that at these temperatures, the rate of formation of reducing sugar is faster than its rate of consumption. Although the content of reducing sugar increases at high temperatures, in the case of processing at 80°C and 90°C, ABG does not have an appropriate sweet flavor because of the consumption of a large amount of reducing sugar at high-temperature conditions [23]. Besides, reduction of amino acid content is also accelerated depending on the progress of Maillard reaction [23].
One of the main antioxidant compounds in BG is 5-hydroxymethylfurfural (5-HMF), and it is also an important intermediate product in Maillard reaction [23]. Regardless of temperature, the amount of 5-HMF is increased during the aging process. However, in the case of processing at 60°C, 5-HMF content increases very slowly during the whole process.
3.2. Effects of fermentation condition on the quality of BG
As we mentioned in the previous section, the quality of BG including its bioactivity and texture depends on the temperature during thermal processing. However, according to the discovery of Jung et al [25], fermented BG displays more effective bioactivity than ABG. In this section, we will summarize the quality of fermented BG and its potentials against several kinds of diseases.
Improvement of antioxidant activity will effectively prevent diabetes and its related complications [26,27]. Bioactivities of garlic such as antioxidant activity and hypoglycemic effect are already well known, and the antioxidant activity of garlic could be enhanced by processing. In recent years, Hien-Trung et al [28] discovered that the bioactivity of ginseng could be enhanced by yeast fermentation. Therefore, they hypothesized that the bioactivity of BG may also be enhanced by yeast fermentation.
According to Jung et al [25], yeast-fermented BG exhibited much better bioactivity against syndromes such as obesity, hyperlipidemia, nephropathy, and hepatopathy than ABG. For example, yeast-fermented garlic-treated mice demonstrated marked improvement in body weight, periovarian fat weight, adipocyte diameters, deposited abdominal fat pad thicknesses, serum total cholesterol, triglyceride, low-density lipoprotein (LDL) level, high-density lipoprotein (HDL) level, aspartate transaminase (AST), alanine transaminase (ALT), steatohepatitis, hepatocyte hypertrophy, serum blood urea nitrogen (BUN), and the number of abnormal kidney tubules compared with the high-fat diet (HFD)-treated controls. Furthermore, fermented BG 400 mg/kg and 200 mg/kg revealed significantly higher effects than ABG 400 mg/kg. In other words, fermented BG has more effective bioactivity against HFD-induced obesity, hyperlipidemia, nephropathy, and hepatopathy than ABG [25]. Therefore, the bioactivity of BG could be enhanced by yeast fermentation, and fermented BG may be more qualified to improve diabetes and its related complications. Owing to this reason, the components of fermented BG might be more or less different from those of ABG. However, the differences of the components between ABG and fermented BG have still not been investigated. Therefore, their component analysis should be required.
In summary, the aging period of BG is shorter at a high temperature; however, controlling the amount of some components might be difficult at a high temperature because their contents change rapidly during the aging process. Based on the results mentioned above, 70°C is considered the best condition for garlic aging. However, the quality of BG is affected by not only temperature, but also other factors such as humidity and fermentation [23,25]. Therefore, further investigations are also required.
4. Bioactivity of BG
Garlic is used for seasoning food, especially in Asian countries, and it has lots of health benefits [29]. However, the intense taste and smell of fresh raw garlic make it difficult for most people to appreciate it [30]. Therefore, different garlic formulations have been developed; ABG is one of the useful garlic types with an odorless character, produced by fermenting whole raw garlic at a controlled high temperature and under controlled high humidity [4,31]. Table 2 summarizes the current findings of bioactivity of BG.
Table 2.
Biological activities of black garlic.
| Biological activities | Biological effects | References |
|---|---|---|
| Antioxidant activity | ↓ Free radical (the trolox equivalent antioxidant capacity [TEAC], EDA, DPPH, and ABTS assay) in vitro ↑ SOD-like activity in vitro ↓ TBARS level in mice ↑ SOD, GSH-Px, and CAT activity |
[3,19,25,32,34–36] |
| Anticancer activity | ↑ Apoptosis in human leukemic U937 cells ↑ Cytotoxicity in human carcinoma A549 (lung carcinoma), MCF-7 (breast adenocarcinoma), AGS (stomach adenocarcinoma), and HepG2 (hepatocarcinoma) cells ↓ Tumor volume and weight in SGC-7901 human gastric cancer cells ↑ Apoptosis and cell cycle arrest in HT29 colon cancer cells |
[4,35–37] |
| Antiobesity activity | ↓ Body weight, abdominal fat weight, abdominal adipocyte diameters and abdominal fat pad thickness, triacylglyceride, LDL level, and ↑ HDL level in HFD-induced mice ↓ Weight gain and epididymal fat, triacylglyceride, and HDL level in HFD-induced rats |
[5,25] |
| Hepatoprotective activity | ↓ AST, ALT, ALP, and LDH levels and ↑ CYP2E1, glutathione S-transferase, quinone reductase, GSH-Px, glutathione reductase (GR), and CAT activities in ethanol-induced oxidative liver damage in rats ↓ Decreased ALT and AST levels in carbon tetrachloride and D-galactosamine-induced liver damage in rats, and in HFD-induced fatty liver and subsequent liver damage model in C57BL/6 mice |
[38,39] |
| Anti-inflammatory activity | ↓ ROS formation, VCAM-1, The human monocytic cell line (THP-1) monocyte adhesion, ICAM-1, and NF-κB in TNF-α-stimulated HUVECs ↓ Cell proliferation, cell cycle progress, ICAM-1, VCAM-1, NF-κB, and activator protein-1 (AP-1) in TNF-α-activated HESCs ↓ NO, TNF-α, prostaglandin E2 (PGE2), NO synthase, cyclooxygenase-2, and NF-κB in LPS-stimulated RAW 264.7 macrophages ↓ TNF-α and IL-6 in LPS-induced lethal shock in C57BL/6 mice |
[6,40–41,47–48] |
| Antiallergic activity | ↓ β-Hexosaminidase, TNF-α, PGE2, cyclooxygenase-2, and 5-LO in RBL-2H3 cells. ↓ PCA reaction on IgE-mediated passive cutaneous anaphylaxis reaction in mice |
[7] |
| Alleviating dyslipidemia | ↑ HDL-c level ↓ apo B |
[54] |
ABTS = 2,2-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid); ALP = alkaline phosphatase; ALT = alanine transaminase; AST = aspartate transaminase; CAT = catalase; DPPH = 1,1-diphenyl-2-picrylhydrazyl; EDA = electron-donating ability; GSH-Px = glutathione peroxidase; HDL = high-density lipoprotein; HDL-c = high-density lipoprotein cholesterol; HESC = human endometrial stromal cell; HFD = high-fat diet; HUVEC = human umbilical vein endothelial cell; ICAM-1 = intercellular cell adhesion molecule-1; IgE = immunoglobulin E; LDH = lactate dehydrogenase; LDL = low-density lipoprotein; 5-LO = 5-lipoxygenase; LPS = lipopolysaccharide; NF-κB = nuclear factor κB; PCA = passive cutaneous anaphylaxis; SOD = superoxide dismutase; TBARS = thiobarbituric acid reactive substances; TNF-α = tumor necrosis factor-α; VCAM-1 = vascular cell adhesion molecule-1.
4.1. Antioxidant activity
The antioxidant activity of garlic is affected by the ways of processing [42]. Alliin is an unstable compound in fresh garlic, which is converted into a stable compound, SAC, during the aging process and exhibits antioxidant activity [11,31,32]. Lee et al [32] reported that the decrease in the number of free radicals in ABG (59.2 ± 0.8 μmol/g wet weight) is more than that in garlic (13.3 ± 0.5 μmol/g wet weight), as revealed by the trolox equivalent antioxidant capacity (TEAC) assay in vitro [32]. Another study showed that 10 μg/mL of yeast-fermented BG had stronger antioxidant activity than BG, as detected by the in vitro electron-donating ability assay [25]. Fresh garlic undergoes 40 days of fermentation at 60–70°C and 85–95% relative humidity to produce BG. The BG extract had more than 10-fold increase in superoxide dismutase-like activity and scavenging activity against hydrogen peroxide compared with garlic extract in vitro [19]. Kim et al [34] showed that the formulation containing 10% of BG extract had higher radical scavenging activity than the formulation containing 10% (v/v) of garlic extract by 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2,2-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) assays in vitro. ABG was obtained from fresh garlic fermented at 80–90°C for 48–90 hours, at 70–80°C for another 48–60 hours, then at 60–70°C for 72–120 hours, and finally at 55–65°C for 72–120 hours. ABG also showed stronger antioxidant activity than fresh garlic by DPPH and 2,2-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) assays [3]. Seventy percent ethanol extract of BG had higher DPPH radical scavenging activity than 70% and 90% ethanol extracts of raw garlic and 90% ethanol extract of BG [35].
4.1.1. Extracts between fresh garlic and BG
Garlic and ABG were peeled off, mixed with 10 volumes of water, and then blended. Both garlic and ABG were extracted with water for 1 hour at 80°C and then centrifuged at 14,000g for 15 minutes [32]. Another yeast-fermented BG was extracted by heating with water twice under reflux at 80°C and the initial yield rate was 12.8%. Later, BG was fermented with Saccharomyces cerevisiae (KCTC 7910). After fermentation, the culture solutions were extracted by heating after removing the cells [25]. The BG obtained after 40-day fermentation was freeze dried and pulverized in 80% ethanol solution; the filtrate obtained was garlic extract [19]. The 10% BG formulation was obtained from BG that was blended with 10 volumes of water and then extracted at 80°C for 1 hour [34]. ABG was obtained from fresh garlic incubated at different temperatures for different hours. Then ABG was suspended in five volumes of distilled water. The suspended ABG was extracted in distilled water at 80–100°C for 2–6 hours [3]. The ethanol extract of BG was obtained by fermenting raw garlic at 75°C and 70% relative humidity for 4 weeks. BG was extracted with 70% or 90% ethanol two times for 6 hours or 12 hours at 50°C or 90°C [35].
4.1.2. Animal study
Male db/db (+/+) C57BL/KsL mice were divided into three groups. The control group was fed with an AIN-93G diet and an AIN-93G diet with 5% freeze-dried garlic or ABG for 7 weeks. At the end of experiment, mice were sacrificed and their livers were collected for further evaluation of the antioxidant activity of garlic and ABG. The analysis was conducted by measuring lipid peroxides and antioxidant enzymes in the liver. Garlic and ABG decreased the thiobarbituric acid reactive substance level and increased the activities of superoxide dismutase and glutathione peroxidase compared with the control group, but ABG further increased the catalase (CAT) activity [33].
4.2. Inhibition of cancer cell line growth
There are six characteristics of cancer during the multistep development of human tumors including sustained proliferative signaling, evaded growth suppressors, resisted cell death, enabled replicative immortality, induced angiogenesis, and activated invasion and metastasis. Therefore, functional food could block these six characteristics due to their anticancer ability [43].
The hexane extract of ABG (HEABG) had demonstrated its anticancer activity in human leukemic U937 cells. HEABG (2.5 μg/mL, 5 μg/mL, 7 μg/mL, and 10 μg/mL) inhibited cell growth by inducing the intrinsic pathway of apoptosis via upregulation of death receptor 4 and Fas ligand, and increasing the Bax/Bcl-2 protein expression ratio. HEABG also activated caspase-9 and caspase-3, and degraded poly(ADP-ribose)-polymerase in a concentration- and time-dependent manner. HEABG-inhibited cell growth also induced the extrinsic pathway of apoptosis via activated caspase-8, resulting in the truncated Bid expressed. HEABG showed its potential for anticancer ability by inducing caspase-dependent apoptosis through both intrinsic and extrinsic pathways in human leukemic U937 cells [4].
Another study showed that 70% ethanol extract of BG (500 μg/mL) caused cytotoxicity in human carcinoma A549 (lung carcinoma), MCF-7 (breast adenocarcinoma), AGS (stomach adenocarcinoma), and HepG2 (hepatocarcinoma) cells in a dose-dependent manner within 72 hours [35].
4.2.1. Human gastric cancer
The ABG extract (ABGE) was treated with 10 mg/mL, 50 mg/mL, and 100 mg/mL in SGC-7901 human gastric cancer cells and 100 mg/mL ABGE could induce apoptosis in the cell [36]. The authors further demonstrated the anticancer ability in the tumor-bearing mice model. The authors used male Kunming mice incubated with murine forestomach cells for 1 week and then treated with 200 mg/kg, 400 mg/kg, and 800 mg/kg ABGE by intraperitoneal injection. The results showed that ABGE decreased tumor volume and weight, and it also increased serum superoxide dismutases and glutathione peroxidase in the tumor-bearing mice model. The anticancer ability of ABGE may vary from its antioxidant activity [36].
4.2.2. Colon cancer
ABGE (20 mg/mL, 50 mg/mL, and 100 mg/mL) also exhibited anticancer ability in HT29 colon cancer cells. ABGE inhibited HT29 cell growth through apoptosis and cell cycle arrest via the phosphatidylinositol 3-kinaseprotein kinase B (PI3KAkt) signal transduction pathway. ABGE upregulated PTEN and downregulated Akt and p-Akt expression, and suppressed the mRNA and protein levels of the downstream target 70-kDa ribosomal protein S6 kinase 1 [37].
4.3. Antiobesity activity
Obesity is an inducer of other diseases such as type 2 diabetes, heart disease, liver disease, and the phenomena of liver damage, including hyperlipidemia[44], changes in liver weight, and serum AST and ALT levels [45].
Female Institute for Cancer Research (ICR) mice were fed with 45%/kcal of HFD for 28 days and then the mice were given 400 mg/kg BG and 100 mg/kg, 200 mg/kg, and 400 mg/kg of yeast-fermented BG for 63 days. BG and yeast-fermented BG significantly decreased body weight, abdominal fat weight, abdominal adipocyte diameters, and abdominal fat pad thickness compared with the HFD group. BG and yeast-fermented BG also decreased serum triacylglyceride and LDL levels, and increased serum HDL level compared with the HFD group. BG and yeast-fermented BG decreased serum AST, ALT, steatohepatitis, and hepatocyte diameters compared with the HFD group [25]. Male Sprague–Dawley rats were divided into four groups, and fed with normal food diet, HFD, and HFD + 0.5% or 1.5% BG extract for 5 weeks. The results showed that rats from the 1.5% BG extract group had decreased weight gain and epididymal fat compared with the HFD group. BG extract [1.5% (w/v)] also showed decreased triacylglyceride in the serum and liver and an increased HDL level in the serum [5].
4.4. Hepatoprotective function
Male Sprague–Dawley rats were fed with ethanol to induce oxidative liver damage. The mice were also given 100 mg/kg of ABG by oral gavage. The results showed that ABG decreased body weight and total fat pad weight. The plasma markers of liver function and injury, including AST, ALT, ALP, and LDH levels, were significantly decreased by ABG compared with those in the group treated with ethanol alone. ABG also increased CYP2E1 expression and the activities of glutathione S-transferase and quinone reductase were drug-metabolizing phase II enzymes and restored the thiobarbituric acid reactive substances, glutathione level, the activities of glutathione peroxidase, GR, and catalase in the liver [38].
Another study showed that 200 mg/kg ABG decreased ALT and AST levels in the liver in the carbon tetrachloride- and d-galactosamine-induced liver damage models of Sprague–Dawley rats. It also decreased the ALT and AST levels in HFD-induced fatty liver and subsequent liver damage model of C57BL/6 mice [39].
4.5. Immunomodulatory effect
Atherosclerosis is a chronic inflammatory disease of the arterial walls due to endothelial dysfunction, vascular inflammation, and formation of atheromatous plaque within the intima of the vessel wall. Atherosclerosis is also related to increased oxidative stress caused by vascular inflammation with various cytokines, including tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and interferon-γ, inducing endothelial activation via generation of reactive oxygen species and augmenting the expression of cell adhesion molecules on endothelial cells [41,46].
Previous researches showed that BG had antioxidant ability [31,32]. Dr Yoon’s group had been investigating the effect of different extraction methods of ABG in the TNF-α-stimulated human umbilical vein endothelial cell (HUVEC) model. Chloroform extract (30 μg/mL) of ABG was pretreated in TNF-α-stimulated HUVECs. This ABG extract inhibited reactive oxygen species formation and mRNA expression of vascular cell adhesion molecule-1 (VCAM-1), and reduced THP-1 monocyte adhesion to TNF-α-stimulated HUVECs. Chloroform extract of ABG also inhibited the activation of nuclear factor kappa B (NF-κB) transcription factor in TNF-α-stimulated HUVECs [40].
The compound 5-HMF was found in chloroform extract of ABG and treated in TNF-α-stimulated HUVECs. It suppressed total protein and mRNA expression of VCAM-1 and intercellular cell adhesion molecule-1 (ICAM-1) in TNF-α-induced cell surface. It also inhibited reactive oxygen species formation, THP-1 monocyte adhesion, and activation of NF-κB transcriptional factor in TNF-α-stimulated HUVECs [41]. HEABG (50 μg/mL) suppressed cell proliferation and cell cycle progress via extracellular signal–regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) pathways in TNF-α-activated human endometrial stromal cells isolated from patients with endometriosis. HEABG also had the potential to suppress the TNFα-induced ICAM-1 and VCAM-1 transcripts and protein expression via inhibition of the activation of NF-κB and AP-1 transcription factors [47].
Lipopolysaccharide (LPS) is an endotoxin that induces several cytokines, such as TNF-α, IL-1b, and IL-6, which are related to various inflammatory reactions [48]. There is another research in which the MTT assay showed that raw garlic extract was highly cytotoxic at concentrations over 250 μg/mL with or without LPS in the RAW 264.7 cells. Water extract of ABG (WEAGE) did not show significant cytotoxicity up to 2000 μg/mL. The result showed that WEAGE had less cytotoxicity than raw garlic extract. When WEAGE was added again at 15 hours before LPS was added to the cells, the results showed that WEAGE decreased the production of nitric oxide (NO), TNF-α, and prostaglandin-E2 in a dose-dependent manner in LPS-stimulated RAW 264.7 macrophages via downregulation of NO synthase and TNF-α mRNA expression, and cyclooxygenase-2 protein expression. Moreover, its anti-inflammatory mechanism decreased LPS-induced phosphorylation of JNK and p38MAPK, and inhibited the activation of NF-κB and phosphorylation in response to LPS-stimulated RAW 264.7 cells. The authors fed the C57BL/6 mice 120 mg/kg of WEAGE and raw garlic extract by oral gavage before injecting 20 mg/kg LPS (LPS-induced endotoxemia). WEAGE decreased the level of TNF-α and IL-6 in the serum against LPS-induced lethal shock in C57BL/6 mice [6].
4.6. Antiallergic action
More evidence showed that allergy diseases are influenced by environmental factors such as eating habits, stress, and living environment. In fact, number of allergy patients have been increasing in many countries [49].
Allergy is related to immunoglobulin E (IgE) antibodies and mast cells have to respond to the pathophysiology of anaphylaxis and other acute allergic reactions. Lots of evidence shows that IgE and mast cells have key roles in tissue remodeling that is associated with chronic allergic inflammation in asthma. Allergy is classified into five types. At type I allergy responses as anaphylactic type can be activated by the high-affinity IgE receptor (FcɛRI receptor) on the plasma membrane of mast and basophilic cells as an intragranular mediators such as histamine, arachidonic acid metabolites, proteases, serotonin, and heparin and it can release β-hexosaminidase, a general marker of degranulation. Therefore, mast cells have an important role in allergic reactions [50,51]. RBL-2H3 cells are used as a model for screening allergic reactions in vitro and passive cutaneous anaphylaxis as an animal model for screening IgE-mediated allergic responses [52,53]. Ethyl acetate extract of BG (2 mg/mL) inhibited the release of β-hexosaminidase and TNF-α that inhibited IgE-mediated allergic responses in RBL-2H3 cells. Moreover, BG10 was the active fraction from ethyl acetate extract of BG showing stronger inhibition of the release of β-hexosaminidase and TNF-α compared with other fractions. Furthermore, 50 μg/mL of BG10 inhibited the formation of prostaglandin E2 and leukotriene B4, and phosphorylation of Syk. BG10 also decreased the phosphorylation of cytosolic phospholipase A2 and 5-lipoxygenase, and the expression of cyclooxygenase-2 in RBL-2H3 cells. BG10 (66.7 mg/kg) given to mice by oral gavage for 1 hour decreased the passive cutaneous anaphylaxis reaction on IgE-mediated passive cutaneous anaphylaxis reaction in mice [7].
4.7. Reduction of blood lipid
Previous studies showed that BG improved serum lipid profiles such as total cholesterol, triglycerides, LDL, and HDL with mice fed with HFD [25].
Jung et al [54] showed that ABG could improve blood lipid profiles in patients with mild hypercholesterolemia. Sixty participants were divided into two groups. One was given 6 g ABG and the other was given placebo twice per day before a meal every morning and evening for 12 weeks. Although the ABG group did not show significant differences in triglyceride, LDL cholesterol, total cholesterol, or free fatty acid levels compared with the placebo group, ABG increased HDL cholesterol levels compared with the placebo group at the end of the study [54]. Serum apo B (atherogenic lipoprotein) is an independent and high predictive risk factor for coronary artery disease [55]. In conclusion, ABG supplement significantly decreased serum apo B [54].
4.8. Influences on memory and nervous systems
Monosodium glutamate (MSG) is well known, and has been used for seasoning all over the world due to its attractive umami taste [56]. However, some researchers reported that MSG might have adverse effect on various organs including Purkinje cells in the cerebellumand hippocampus [57,58]. The cerebellum and the hippocampus play an important role in the nervous system and the memory system, respectively. As it is, the brain is expected to be one of the organs most sensitive to the effects of MSG because of its high content of polyunsaturated fatty acids, high metabolism, and low antioxidant capacity, and the hard-to-replicate quality of its neuronal cells [59–61].
+Garlic has been known not only as a flavor enhancer, but also as a food that has high potential antioxidant activity. In particular, the antioxidant activity of BG is significantly higher (p < 0.05) than that of fresh garlic due to its higher polyphenol level and scavenging activity [56,62]. Some researchers investigated the effects of the ethanol extract of BG on the nervous and memory systems using a Wistar rat model with MSG [63,64]. According to Hermawati et al [63], BG-treated rats had significantly shorter escape latencies and path lengths than the control rats, both with or without MSG, in several trials of the nonvisible platform test of the Morris Water Maze (MWM) procedure. Furthermore, although the dosage of MSG may not be adequate to show significant reduction of the number of Purkinje cells, the combined administration of BG extract and MSG improved the reduction of the number of Purkinje cells compared with MSG only [64].
To sum up, BG might play an important role in improving some diseases as well as the functions of the memory and nervous systems due to its potential antioxidant activity. However, the current dosage of MSG may not be able to significantly reduce the number of Purkinje cells in the cerebella of rats. Therefore, further studies are required to discover whether a higher dosage of MSG can affect the number of Purkinje cells.
4.9. Influence of BG on TNF-α-related inflammation diseases
Accumulation of monocytes in the vessel wall is primarily induced by specific cell adhesion molecules such as VCAM-1, ICAM-1, and endothelial cell selection [40]. In particular, VCAM-1 is activated by cytokines such as TNF-α and IL-1 in the endothelium. This phenomenon could be attributed to atherosclerosis through the adhesion of monocytes in the endothelium. Furthermore, these cell adhesion molecules can result in endometriosis [42]. These are regulated by the expression of cytokines and chemokines.
Although TNF-α is well known as an inducer of expression of cell adhesion molecule, it is deeply involved in the inflammation reaction in humans. Following are the influences of BG extracts on TNF-α-related diseases.
Chloroform extract of BG could suppress cell adhesion molecules that are activated by TNF-α. Reactive oxygen species production, NF-κB activation, and adhesiveness to monocytes were also improved [40]. Moreover, 5-HMF, which is purified from BG, could also suppress cell adhesion molecules that are activated by TNF-α [41]. Furthermore, hexane extract of BG could reduce the expression of cell adhesion molecules such as ICAM-1 and VCAM-1 in TNF-α-activated endometrial stromal cells in humans.
In summary, hexane extract of BG could be effective in preventing and treating endometriosis in humans. Furthermore, chloroform extract of BG and 5-HMF could also be effective in preventing and treating atherosclerosis. However, the exact chemical compound which have bioactivity are still not investigated. Therefore, the compound analysis of them should be required.
5. Conclusion
Apparently, BG exhibits several advantages when compared with fresh garlic. Since garlic has long been consumed in the human society and has been recognized as one of the safe food substances, there will be no constraints for further invention of BG products for such functional food, food supplements, as well as medical purposes. A more systematic and efficient process for manufacturing BG is important since it is crucial to control the changes in metabolite levels during the fermentation process for industrial-level mass production.
Acknowledgments
This project was funded by the National Science Council, Taiwan (No. 104-2221-E-002-125-MY3). The authors would like to thank Mrs Qian-Wen Shang, who received her bachelor degree of English from National Chengchi University, for English editing.
Funding Statement
This project was funded by the National Science Council, Taiwan (No. 104-2221-E-002-125-MY3).
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
REFERENCES
- 1. Yuan H, Sun L, Chen M, Wang J. The comparison of the contents of sugar, Amadori, and Heyns compounds in fresh and black garlic. J Food Sci. 2016;81:C1662–8. doi: 10.1111/1750-3841.13365. [DOI] [PubMed] [Google Scholar]
- 2. Bradley C. New black magic: black garlic is new food sensation. Herald Times. Retrieved 2009-03-01, http://archive.is/http://www.heraldtimesonline.com/stories/2009/02/25/recipe.qp-1681035.sto. [Google Scholar]
- 3. Jeong YY, Ryu JH, Shin JH, Kang MJ, Kang JR, Han J, Kang D. Comparison of anti-oxidant and anti-inflammatory effects between fresh and aged black garlic extracts. Molecules. 2016;21:430. doi: 10.3390/molecules21040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Park C, Park S, Chung YH, Kim GY, Choi YW, Kim BW, Choi YH. Induction of apoptosis by a hexane extract of aged black garlic in the human leukemic U937 cells. Nutr Res Pract. 2014;8:132–7. doi: 10.4162/nrp.2014.8.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ha AW, Ying T, Kim WK. The effects of black garlic (Allium sativum) extracts on lipid metabolism in rats fed a high fat diet. Nutr Res Pract. 2015;9:30–6. doi: 10.4162/nrp.2015.9.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim MJ, Yoo YC, Kim HJ, Shin SK, Sohn EJ, Min AY, Sung NY, Kim MR. Aged black garlic exerts anti-inflammatory effects by decreasing no and proinflammatory cytokine production with less cytotoxicity in LPS-stimulated RAW 264.7 macrophages and LPS-induced septicemia mice. J Med Food. 2014;17:1057–63. doi: 10.1089/jmf.2013.3043. [DOI] [PubMed] [Google Scholar]
- 7. Yoo JM, Sok DE, Kim MR. Anti-allergic action of aged black garlic extract in RBL-2H3 cells and passive cutaneous anaphylaxis reaction in mice. J Med Food. 2014;17:92–102. doi: 10.1089/jmf.2013.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Theisen C. What ever happened to...? Looking back to years. J Natl Cancer Inst. 2001;93:1049–50. doi: 10.1093/jnci/93.14.1049. [DOI] [PubMed] [Google Scholar]
- 9. Santhosha SG, Jamuna P, Prabhavathi SN. Bioactive components of garlic and their physiological role in health maintenance: a review. Food Biosci. 2013;3:59–74. [Google Scholar]
- 10. Amagase H, Petesch BL, Matsuura H, Kasuga S, Itakura Y. Intake of garlic and its bioactive components. J Nutr. 2001;131:955S–62S. doi: 10.1093/jn/131.3.955S. [DOI] [PubMed] [Google Scholar]
- 11. Corzo-Martinez M, Corso N, Villamiel M. Biological properties of onions and garlic. Trends Food Sci Technol. 2007;18:609–25. [Google Scholar]
- 12. Amagase H. Clarifying the real bioactive constituents of garlic. J Nutr. 2006;131:955s–62s. doi: 10.1093/jn/136.3.716S. [DOI] [PubMed] [Google Scholar]
- 13. Choi S, Cha HS, Lee YS. Physicochemical and antioxidant properties of black garlic. Molecules. 2014;19:16811–23. doi: 10.3390/molecules191016811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saravanan G, Ponmurugan P. SAC improves streptozotocin-induced alterations of blood glucose, liver cytochrome P450 2E1, plasma antioxidant system, and adipocytes hormones in diabetic rats. Int J Endocrinol Metab. 2013;11:e10927. doi: 10.5812/ijem.10927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Colin GAL, Santana RA, Silva ICA, Chanez CME, Santamaria A, Maldonad PD. The antioxidant mechanisms underlying the aged garlic extract- and S-allylcystein-induced protection. Oxid Med Cell Longev. 2012;2012:1–16. doi: 10.1155/2012/907162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Colin GAL, Ali SF, Tune I, Santamaria A. On the antioxidant, neuroprotective and anti-inflammatory properties of S-allyl cysteine: an update. Neurochem Int. 2015;89:83–91. doi: 10.1016/j.neuint.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 17. Hwang IG, Kim HY, Woo KS, Lee J, Jeong HS. Biological activities of Maillard reaction products (MRPs) in a sugar–amino acid model system. J Food Chem. 2011;126:221–7. [Google Scholar]
- 18. Vokk R, Tedersoo E, Lougas T, Valgma K, Rosend J. Comparative study on anti-oxidant activity of garlic grown in different regions. Agro Res. 2014;12:821–4. [Google Scholar]
- 19. Sato E, Kohno M, Hamano H, Niwano Y. Increased antioxidative potency of garlic by spontaneous short-term fermentation. Plant Foods Hum Nutr. 2006;61:157–60. doi: 10.1007/s11130-006-0017-5. [DOI] [PubMed] [Google Scholar]
- 20. Gorinstein S, Leontowicz H, Leontowicz M, Namiesnik J, Najman K, Drzewiecki J, Cvikrova M, Martincova O, Katrich E, Trakhtenberg S. Comparison of the main bioactive compounds and antioxidant activities in garlic and white and red onions after treatment protocols. J Agric Food Chem. 2008;56:4418–26. doi: 10.1021/jf800038h. [DOI] [PubMed] [Google Scholar]
- 21. Ioannou I, Hafsa I, Hamdi S, Charbonnel C, Ghoul M. Review of the effects of food processing and formulation on flavonol and anthocyanin behavior. J Food Eng. 2012;111:208–17. [Google Scholar]
- 22. Toledano-Medina MA, Perez-Aparicio J, Moreno-Rojas R, Merinas-Amo T. Evolution of some physicochemical and antioxidant properties of black garlic whole bulbs and peeled cloves. J Food Chem. 2016;199:135–9. doi: 10.1016/j.foodchem.2015.11.128. [DOI] [PubMed] [Google Scholar]
- 23. Zhang X, Li N, Lu X, Liu P, Qiao X. Effects of temperature on the quality of black garlic. J Sci Food Agric. 2015;96:2366–72. doi: 10.1002/jsfa.7351. [DOI] [PubMed] [Google Scholar]
- 24. Hodge JE. Dehydrated foods, chemistry of browning reactions in model systems. J Agric Food Chem. 1953;1:928–43. [Google Scholar]
- 25. Jung YM, Lee SH, Lee DS, You MJ, Chung IK, Cheon WH, Kwon YS, Lee YJ, Ku SK. Fermented garlic protects diabetic, obese mice when fed a high-fat diet by antioxidant effects. Nutr Res. 2011;31:387–96. doi: 10.1016/j.nutres.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 26. Lean ME, Noroozi M, Kelly I, Burns J, Talwar D, Sattar N. Dietary flavonols protect diabetic human lymphocytes against oxidative damage to DNA. Diabetes. 1999;48:176–81. doi: 10.2337/diabetes.48.1.176. [DOI] [PubMed] [Google Scholar]
- 27. Sinclair AJ, Girling AJ, Gray L, Lunec J, Barnett AH. An investigation of the relationship between free radical activity and vitamin C metabolism in elderly diabetic subjects with retinopathy. J Gerontol. 1992;38:268–74. doi: 10.1159/000213339. [DOI] [PubMed] [Google Scholar]
- 28. Hien-Trung T, Han SJ, Kim SW, Lee YC, Kim DH. Bifidus fermentation increases hypolipidemic and hypoglycemic effects of red ginseng. J Microbial Biotechnol. 2007;17:1127–33. [PubMed] [Google Scholar]
- 29. Thomson M, Ali M. Garlic [Allium sativum]: a review of its potential use as an anti-cancer agent. Curr Cancer Drug Tar. 2003;3:67–81. doi: 10.2174/1568009033333736. [DOI] [PubMed] [Google Scholar]
- 30. Butt MS, Sultan MT, Butt MS, Iqbal J. Garlic: nature’s protection against physiological threats. Crit Rev Food Sci Nutr. 2009;49:538–51. doi: 10.1080/10408390802145344. [DOI] [PubMed] [Google Scholar]
- 31. Imai J, Ide N, Nagae S, Moriguchi T, Matsuura H, Itakura Y. Antioxidant and radical scavenging effects of aged garlic extract and its constituents. Planta Med. 1994;60:417–20. doi: 10.1055/s-2006-959522. [DOI] [PubMed] [Google Scholar]
- 32. Lee YM, Gweon OC, Seo YJ, Im J, Kang MJ, Kim MJ, Kim JI. Antioxidant effect of garlic and aged black garlic in animal model of type 2 diabetes mellitus. Nutr Res Pract. 2009;3:156–61. doi: 10.4162/nrp.2009.3.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sato E, Kohno M, Niwano Y. Increased level of tetrahydro-β-carbokine derivatives in short-term fermented garlic. Plant Food Hum J Nutr. 2006;61:175–8. doi: 10.1007/s11130-006-0028-2. [DOI] [PubMed] [Google Scholar]
- 34. Kim SH, Jung EY, Kang DH, Chang UJ, Hong YH, Suh HJ. Physical stability, antioxidative properties, and photoprotective effects of a functionalized formulation containing black garlic extract. J Photochem Photobiol B. 2012;117:104–10. doi: 10.1016/j.jphotobiol.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 35. Purev U, Chung MJ, Oh DH. Individual differences on immunostimulatory activity of raw and black garlic extract in human primary immune cells. Immunopharmacol Immunotoxicol. 2012;34:651–60. doi: 10.3109/08923973.2011.649288. [DOI] [PubMed] [Google Scholar]
- 36. Wang X, Jiao F, Wang QW, Wang J, Yang K, Hu RR, Liu HC, Wang HY, Wang YS. Aged black garlic extract induces inhibition of gastric cancer cell growth in vitro and in vivo. Mol Med Rep. 2012;5:66–72. doi: 10.3892/mmr.2011.588. [DOI] [PubMed] [Google Scholar]
- 37. Dong M, Yang G, Liu H, Liu X, Lin S, Sun D, Wang Y. Aged black garlic extract inhibits HT29 colon cancer cell growth via the PI3K/Akt signaling pathway. Biomed Rep. 2014;2:250–4. doi: 10.3892/br.2014.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim MH, Kim MJ, Lee JH, Han JI, Kim JH, Sok DE, Kim MR. Hepatoprotective effect of aged black garlic on chronic alcohol-induced liver injury in rats. J Med Food. 2011;14:732–8. doi: 10.1089/jmf.2010.1454. [DOI] [PubMed] [Google Scholar]
- 39. Shin JH, Lee CW, Oh SJ, Yun J, Kang MR, Han SB, Park H, Jung JC, Chung YH, Kang JS. Hepatoprotective effect of aged black garlic extract in rodents. Toxicol Res. 2014;30:49–54. doi: 10.5487/TR.2014.30.1.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee EN, Choi YW, Kim HK, Park JK, Kim HJ, Kim MJ, Lee HW, Kim KH, Bae SS, Kim BS, Yoon S. Chloroform extract of aged black garlic attenuates TNF-α-induced ROS generation, VCAM-1 expression, NF-κB activation and adhesiveness for monocytes in human umbilical vein endothelial cells. Phytother Res. 2011;25:92–100. doi: 10.1002/ptr.3230. [DOI] [PubMed] [Google Scholar]
- 41. Kim HK, Choi YW, Lee EN, Park JK, Kim SG, Park DJ, Kim BS, Lim YT, Yoon S. 5-Hydroxymethylfurfural from black garlic extract prevents TNF-α-induced monocytic cell adhesion to HUVECs by suppression of vascular cell adhesion molecule-1 expression, reactive oxygen species generation and NF-κb activation. Phytother Res. 2011;25:965–74. doi: 10.1002/ptr.3351. [DOI] [PubMed] [Google Scholar]
- 42. Queiroz YS, Ishimoto EY, Bastos DH, Sampaio GR, Torres EA. Garlic (Allium sativum L.) and ready-to-eat garlic products: in vitro antioxidant activity. Food Chem. 2009;115:371–4. [Google Scholar]
- 43. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 44. Yanovski SZ, Yanovski JA. Obesity. N Engl J Med. 2002;346:591–602. doi: 10.1056/NEJMra012586. [DOI] [PubMed] [Google Scholar]
- 45. Mukai M, Ozasa K, Hayashi K, Kawai K. Various S-GOT/S-GPT ratios in nonviral liver disorders and related physical conditions and life-style. Dig Dis Sci. 2002;47:549–55. doi: 10.1023/a:1017959801493. [DOI] [PubMed] [Google Scholar]
- 46. Lusis AJ. Atherosclerosis. Nature. 2000;407:233–41. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim KH, Park JK, Choi YW, Kim YH, Lee EN, Lee JR, Kim HS, Baek SY, Kim BS, Lee KS, Yoon S. Hexane extract of aged black garlic reduces cell proliferation and attenuates the expression of ICAM-1 and VCAM-1 in TNF-α-activated human endometrial stromal cells. Int J Mol Med. 2013;32:67–78. doi: 10.3892/ijmm.2013.1362. [DOI] [PubMed] [Google Scholar]
- 48. Bae GS, Kim MS, Jung WS, Seo SW, Yun SW, Kim SG, Park RK, Kim EC, Song HJ, Park SJ. Inhibition of lipopolysaccharide-induced inflammatory responses by piperine. Eur J Pharmacol. 2010;642:154–62. doi: 10.1016/j.ejphar.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 49. Wang DY. Risk factors of allergic rhinitis: genetic or environmental? Ther Clin Risk Manag. 2005;1:115–23. doi: 10.2147/tcrm.1.2.115.62907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Itoh T, Ohguchi K, Iinuma M, Nozawa Y, Akao Y. Inhibitory effect of xanthones isolated from the pericarp of Garcinia mangostana L. on rat basophilic leukemia RBL-2H3 cell degranulation. Bioorg Med Chem. 2008;16:4500–8. doi: 10.1016/j.bmc.2008.02.054. [DOI] [PubMed] [Google Scholar]
- 51. Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kemp SF, Lockey RF. Anaphylaxis: a review of causes and mechanisms. J Allergy Clin Immun. 2002;110:341–8. doi: 10.1067/mai.2002.126811. [DOI] [PubMed] [Google Scholar]
- 53. Justus DE, Saelinger C. Comparison of mouse strain skin sensitivities to anaphylactic mediators and susceptibility to passive cutaneous anaphylactic reactions. Infect Immun. 1976;13:413–6. doi: 10.1128/iai.13.2.413-416.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jung ES, Park SH, Choi EK, Ryu BH, Park BH, Kim DS, Kim YG, Chae SW. Reduction of blood lipid parameters by a 12-wk supplementation of aged black garlic: a randomized controlled trial. J Nutr. 2014;30:1034–9. doi: 10.1016/j.nut.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 55. Davidson MH. Apolipoprotein measurements: is more widespread use clinically indicated? Clin Cardiol. 2009;32:482–6. doi: 10.1002/clc.20559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Farombi EO, Onyema OO. Monosodium glutamate-induced oxidative damage and genotoxicity in the rat: modulatory role of vitamin C, vitamin E and quercetin. Hum Exp Toxicol. 2006;25:251–9. doi: 10.1191/0960327106ht621oa. [DOI] [PubMed] [Google Scholar]
- 57. Eweka AO, Om’Iniabohs FAE. Histological studies of the effects of monosodium glutamate on the cerebellum of adult Wistar rats. Internet J Neurol. 2007;8:1–5. [PMC free article] [PubMed] [Google Scholar]
- 58. Hashem HE, El-Din Safwat MD, Algaidi S. The effect of monosodium glutamate on the cerebellar cortex of male albino rats and the protective role of vitamin C (histological and immunohistochemical study) J Mol Histol. 2012;43:179–86. doi: 10.1007/s10735-011-9380-0. [DOI] [PubMed] [Google Scholar]
- 59.Blaylock RL. Excitotoxin: the taste that kills. Santa Fe: Health Press; 1997. [Google Scholar]
- 60. Singh P, Mann KA, Mangat HK, Kaur G. Prolonged glutamate excitotoxicity: effects on mitochondrial antioxidants and antioxidant enzymes. Mol Cell Biochem. 2003;243:139–45. doi: 10.1023/a:1021668314070. [DOI] [PubMed] [Google Scholar]
- 61. Noor N, Mourad I. Evaluation of antioxidant effect of Nigella sativa oil on monosodium glutamate-induced oxidative stress in rat brain. J Am Sci. 2010;6:13–9. [Google Scholar]
- 62. Kim J, Kang O, Gweon O. Comparison of phenolic acids and flavonoids in black garlic at different thermal processing steps. J Funct Foods. 2013;5:80–6. [Google Scholar]
- 63. Hermawati E, Sari DCR, Partadiredja G. The effects of black garlic ethanol extract on the spatial memory and estimated total number of pyramidal cells of the hippocampus of monosodium glutamate-exposed adolescent male Wistar rats. Anat Sci Int. 2015;90:275–86. doi: 10.1007/s12565-014-0262-x. [DOI] [PubMed] [Google Scholar]
- 64. Aminuddin M, Partadiredja G, Sari DCR. The effects of black garlic (Allium sativum L.) ethanol extract on the estimated total number of Purkinje cells and motor coordination of male adolescent Wistar rats treated with monosodium glutamate. Anat Sci Int. 2015;90:75–81. doi: 10.1007/s12565-014-0233-2. [DOI] [PubMed] [Google Scholar]